Abstract
PURPOSE
We aimed to assess computed tomography (CT) findings of pulmonary tuberculosis (TB) and TB pleurisy in diabetes mellitus (DM) patients and to evaluate the effect of duration of DM on radiologic findings of pulmonary TB and TB pleurisy.
METHODS
Ninety-three consecutive patients diagnosed as active pulmonary TB with underlying DM were enrolled in our study. As a control group, 100 pulmonary TB patients without DM were randomly selected. TB patients with DM were subdivided into two subgroups depending on diabetes duration of ≥10 years or <10 years. Medical records and CT scans of the patients were retrospectively reviewed and compared.
RESULTS
Bilateral pulmonary involvement (odds ratio [OR]=2.39, P = 0.003), involvement of all lobes (OR=2.79, P = 0.013), and lymph node enlargement (OR=1.98, P = 0.022) were significantly more frequent CT findings among TB patients with DM compared with the controls. There were no statistically significant differences in CT findings of pulmonary TB depending on the duration of DM.
CONCLUSION
Bilateral pulmonary involvement, involvement of all lobes, and lymph node enlargement are significantly more common CT findings in TB patients with underlying DM than in patients without DM. Familiarity with the CT findings may be helpful to suggest prompt diagnosis of pulmonary TB in DM patients.
Tuberculosis (TB) is a major challenge among infectious diseases. In recent years, the prevalence of TB has been rising globally. According to 2014 Global Tuberculosis Report, 9 million people are estimated to have developed TB and 1.5 million deaths of TB patients were reported in 2013 (1). Diabetes mellitus (DM) is another major public health problem. The number of diabetic people worldwide is predicted to rise from 387 million in 2014 to 592 million in 2035 (2). Increased incidence of pulmonary TB among diabetes patients is well-known (3). According to a recent meta-analysis, diabetes patients have a three times higher risk of contracting TB than nondiabetics (4). Therefore, DM is a well-known risk factor for TB and might affect disease presentation and treatment response (5).
While upper lobe cavitary lesion is typical in adult pulmonary TB in immunocompetent hosts, lower lung zone disease, lymphadenopathy, and pleural effusions are common findings of TB in an immunocompromised host (6). There are several studies reporting radiologic findings of pulmonary TB in DM patients (7–14). However, few studies report computed tomography (CT) findings, and no previous study has been published describing the difference of radiologic manifestations depending on the duration of DM.
Considering the increasing prevalence of pulmonary TB in DM patients, the present study was conducted to assess the chest CT findings of TB in DM patients. In addition, the influence of duration of DM on radiologic manifestations of pulmonary TB and TB pleurisy was evaluated.
Methods
Diagnosis of pulmonary tuberculosis
Culture of Mycobacterium tuberculosis or polymerase chain reaction for Mycobacterium tuberculosis (TB-PCR) assay of either sputum or bronchoalveolar lavage (BAL) fluid was used for diagnosis of pulmonary TB. Otherwise, culture of the specimen obtained through percutaneous needle biopsy of the lung lesion revealed Mycobacterium tuberculosis. TB pleurisy was diagnosed by combination of pleural fluid adenosine deaminase (ADA) activity over 50 U/L and lymphocyte/neutrophil ratio of 0.75 or greater on pleural fluid analysis, regardless of presence of pulmonary parenchymal TB (15, 16).
Patients
This study was approved by the institutional review board, and the requirement for informed consent was waived due to retrospective data processing.
From January 2010 to December 2014, 1326 patients were confirmed as having pulmonary TB in our institution. Among them, 107 patients were excluded from the study. The exclusion criteria included diagnosis of human immunodeficiency virus infection and other immunodeficiency states, such as a recent diagnosis of a malignancy, current immunosuppressive therapy, radiotherapy, chronic use of corticosteroids, and microbiologically or serologically confirmed coinfection with other organisms.
A total of 129 patients had DM. Chest CT was performed in 93 patients who were grouped as the “TB with DM” group (65 males and 28 females; mean age, 61.6±11.5 years). The control group (n=100), labelled as the “TB without DM” group, were randomly selected from confirmed pulmonary TB patients without DM who met the inclusion and exclusion criteria, matching with the study group in terms of age and gender.
Additionally, the TB with DM group was subdivided into two subgroups depending on the duration of DM of ≥10 years or <10 years.
CT imaging
All CT images were obtained using a 64-slice multidetector CT (Brilliance 64; Philips Medical Systems) or a 256-slice multidetector CT (Somatom Definition Flash; Siemens Healthcare). The slice thickness of high-resolution CT was 1 mm and others were 3 mm. Contrast-enhanced studies were conducted following intravenous administration of 100 mL or 1.4 mL/kg of nonionic contrast material containing 300 mg iodine/mL. All chest CTs were performed from lung apices to lung bases.
Images
Chest CT data were obtained from the picture archive communication system (PACS) and all images were reviewed on PACS monitors by two radiologists with 20 years and 3 years of image interpretation experience. The two radiologists retrospectively reviewed the CT images and reached a consensus through discussion. The images were interpreted blinded as to the DM status of the patients.
The CT images of each case were reviewed with the focus on the following features: bilaterality, location of lung parenchymal involvement, presence of consolidation (defined as dense opacification without forming mass), mass (round shaped opacity >3 cm in diameter), nodule (≤3 cm in diameter, regardless of margin), cavity (presence of air in the consolidation or mass/nodule), pleural effusion, and lymph node enlargement (≥10 mm in short axis diameter). Furthermore, presence of centrilobular nodule, location and multiplicity of cavities, location of enlarged lymph nodes classified as mediastinal, hilar, or both, were also assessed. The typical or atypical location of pulmonary TB was confined to the pulmonary parenchymal involvement, excluding patients with TB pleurisy without parenchymal lesions. Typical location was defined as pulmonary parenchymal involvement at apical and posterior segments of the upper lobes and/or superior segment of lower lobes. Atypical location was defined as basal segments of the lower lobes and/or anterior segment of the upper lobes and/or middle lobes (17).
Statistical analysis
Demographic and laboratory features between the groups and subgroups were compared using the independent samples t-test for continuous variables. Differences in the CT imaging features between the groups were analyzed using Chi-square analysis and Fisher’s exact test for categorical variables. CT findings with P < 0.1 on chi-square test were entered into the multivariable binary logistic regression analysis (backward conditional model), adjusting for age and gender. The odds ratio (OR) and its 95% confidence interval (CI) were calculated. P < 0.05 was taken as indicative of a statistically significant difference. The data were analyzed using SPSS version 22.0 (IBM Corp.).
Results
The demographic characteristics of the TB with DM and TB without DM groups are presented in Table 1. Age and gender did not show statistically significant differences between the two groups. However, a larger number of patients in the TB with DM group had other comorbidities, such as hypertension, chronic renal disease, heart failure, liver cirrhosis, cardiac arrhythmia, compared with the control group (P < 0.001). Adenosine deaminase (ADA) values of pleural fluid between the two groups were not significantly different (P = 0.389). All the TB pleurisy were exudates based on Light’s criteria (18).
Table 1.
Demographic, clinical, and laboratory features of pulmonary TB patients with or without DM
| Characteristics | TB with DM (n=93) | TB without DM (n=100) | P |
|---|---|---|---|
| Age (years)a | 61.6±11.5 (33–89) | 60.9±10.9 (33–88) | 0.735 |
|
| |||
| Gender (male/female) | 65/28 | 66/34 | 0.563 |
|
| |||
| Comorbidity, n (%)b | 61 (65.6) | 28 (28.0) | <0.001 |
|
| |||
| CT | 0.052 | ||
| Unenhanced CT | 5 | 15 | |
| High-resolution CT | 27 | 20 | |
| Enhanced CT | 61 | 65 | |
|
| |||
| TB diagnosis | 0.026 | ||
| Culture | 62 | 85 | |
| TB-PCR | 10 | 5 | |
| Tissue culture | 13 | 5 | |
| ADA | 8 | 5 | |
|
| |||
| ADA (U/L)a,c | 71.0±33.9 | 79.1±24.9 | 0.389 |
|
| |||
| HbA1c (%)a,d | 8.0±1.9 | N/A | N/A |
TB, tuberculosis; DM, diabetes mellitus; CT, computed tomography; TB-PCR, polymerase chain reaction for Mycobacterium tuberculosis; ADA, adenosine deaminase; HbA1c, hemoglobin A1c; N/A, not applicable.
Mean±SD (range).
Presence of at least one comorbidity. Data in parentheses are percentages.
Assessed in case of TB pleurisy (Patients with DM ≥10 years, n=7; patients with DM <10 years, n=9).
Normal range of HbA1c: 4.1%–5.6%.
The CT findings of the TB with DM group (Figs. 1–3) and the TB without DM group (Fig. 4) are summarized in Table 2.
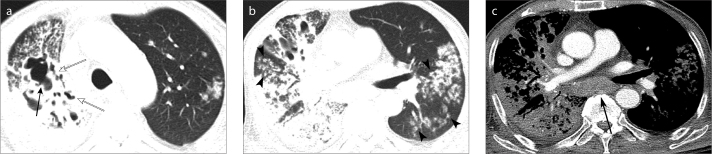
Figure 1. a–c.
An 80-year-old man with underlying diabetes mellitus (DM) and active pulmonary tuberculosis (TB). Enhanced computed tomography (CT) scans show bilateral and all lobar involvement of active pulmonary TB. Axial images with lung window setting (a, b) show multifocal patchy consolidations with cavitary (solid arrow) and bronchiectatic change (open arrow) in the right upper, middle, and lower lobes. Multiple nodules (arrowheads) are noted in bilateral lungs, all lobes. Axial image with soft-tissue window setting (c) shows large lymph nodes in the subcarinal area (arrow). A small amount of right pleural effusion is also noted.
Figure 2.
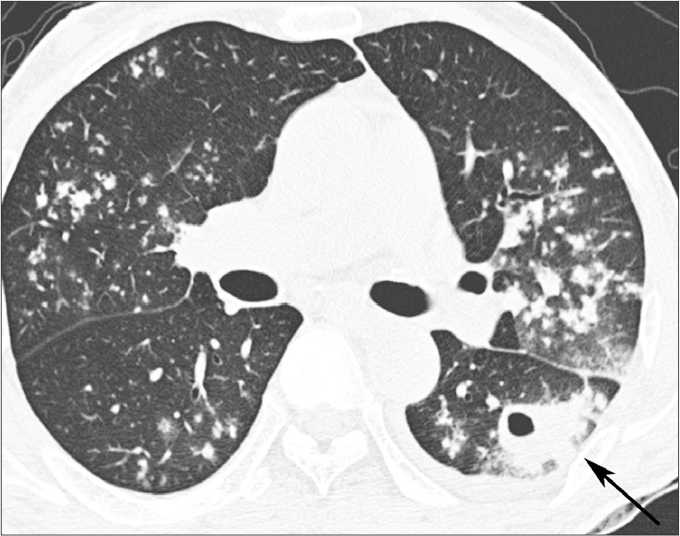
A 59-year-old man with underlying DM and active pulmonary TB. High-resolution CT image shows bilateral lung involvement of active pulmonary TB. It shows multiple small nodules in bilateral upper lobes and superior segments of bilateral lower lobes. A cavitary mass (arrow) is located in the superior segment of the left lower lobe. A small amount of the left pleural effusion is noted.
Figure 3.
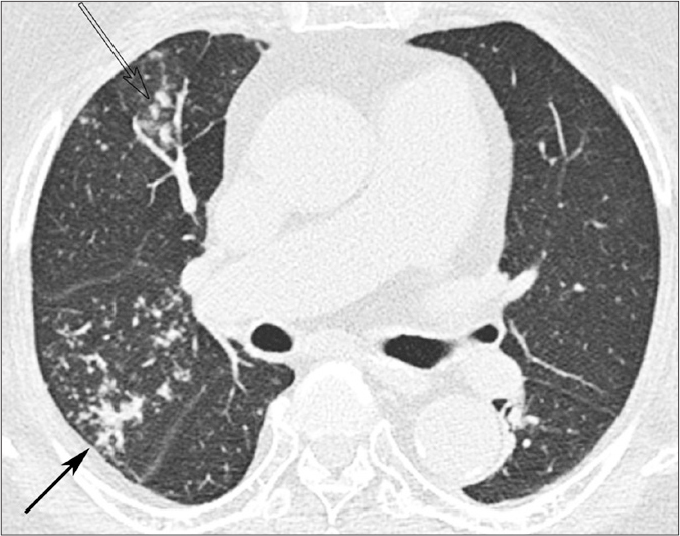
A 79-year-old woman with underlying DM and active pulmonary TB. High-resolution CT shows isolated atypical location involvement of active pulmonary TB. It shows multiple centrilobular nodules with nodular clustering and tree-in-bud appearance in the anterior segment of right upper lobe (open arrow) and the right middle lobe (solid arrow).
Figure 4.
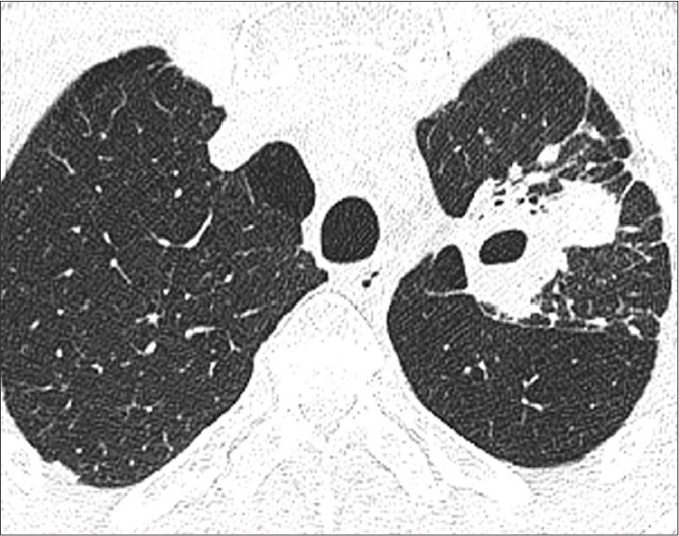
A 63-year-old man without underlying DM, confirmed as active pulmonary TB. High-resolution CT scan shows cavitary mass with air-fluid level in the apicoposterior segment of the left upper lobe, which is a typical location of active pulmonary TB.
Table 2.
CT findings in pulmonary TB patients with or without DM
| CT findings | TB with DM (n=93) | TB without DM (n=100) | P |
|---|---|---|---|
| Bilaterality | 58 (62.4) | 41 (41.0) | 0.003 |
|
| |||
| All lobes | 22 (23.7) | 10 (10.0) | 0.010 |
|
| |||
| Atypical location only | 8 (8.9) | 3 (3.2) | 0.103 |
|
| |||
| Involvement of atypical locationa | 53 (58.9) | 55 (58.5) | 0.957 |
|
| |||
| TB pleurisy onlyb | 3 (3.2) | 6 (6.0) | 0.504 |
|
| |||
| Consolidation | 53 (57.0) | 65 (65.0) | 0.254 |
|
| |||
| Mass | 24 (25.8) | 16 (16.0) | 0.094 |
|
| |||
| Nodule | 78 (83.9) | 74 (74.0) | 0.091 |
| Centrilobular nodule | 71 (91.0) | 65 (87.8) | 0.082 |
|
| |||
| Cavity | 44 (47.3) | 37 (37.0) | 0.153 |
| Atypical location | 11 (25.0) | 6 (16.2) | 0.334 |
| Multiple cavities | 15 (34.1) | 9 (24.3) | 0.134 |
|
| |||
| Pleural effusion | 39 (41.9) | 41 (41.0) | 0.901 |
|
| |||
| TB pleurisy | 16 (17.2) | 24 (24.0) | 0.253 |
|
| |||
| Lymph node enlargement | 48 (51.6) | 35 (35.0) | 0.018 |
| Mediastinum | 19 (39.6) | 12 (34.3) | |
| Hilum | 8 (16.7) | 6 (17.1) | |
| Both | 21 (43.8) | 17 (48.6) | |
Data are presented as number of patients, with percentage in parentheses.
CT, computed tomography; TB, tuberculosis; DM, diabetes mellitus.
Atypical location was defined as basal segments of the lower lobes and/or anterior segment of the upper lobes and/or middle lobes, and was confined to the pulmonary parenchymal lesion, excluded TB pleurisy.
TB pleurisy without pulmonary parenchymal lesion.
In the TB with DM group, bilateral pulmonary involvement (Figs. 1 and 2) was found in 58 patients (62.4%) and involvement of all lobes (Fig. 1a, 1b) was found in 22 patients (23.7%). These were significantly more frequent compared with the control group (P = 0.003 and P = 0.010, respectively). Furthermore, lymph node enlargement (Fig. 1c) was more common in the TB with DM group than in the control group (51.6% [48/93] vs. 35.0% [35/100], P = 0.018).
Masses were more frequently identified in the TB with DM group than in the control group (25.8% vs. 16.0%, P = 0.094). The mean size of masses were 4.3±0.3 cm in the TB with DM group and 3.9±0.2 cm in the control group (P = 0.310). The nodules and centrilobular nodules were also more common in the TB with DM group than in the control group (nodule, 83.9% vs. 74.0%, P = 0.091; centrilobular nodule, 91.0% vs. 87.8%, P = 0.082). However, the differences did not reach statistical significance.
Isolated atypical location involvement (Fig. 3), presence of cavity, atypical location of cavity, and multiple cavities were more frequently observed in the TB with DM group than in the control group; however, this difference was not statistically significant. In addition, the two groups showed no differences regarding the frequency of atypical location involvement, consolidation, pleural effusion, and TB pleurisy on CT.
Multivariable binary logistic regression analysis showed that bilateral pulmonary involvement (OR, 2.39; 95% CI, 1.34–4.25; P = 0.003), pulmonary involvement of all lobes (OR, 2.79; 95% CI, 1.24–6.27; P = 0.013), and lymph node enlargement (OR, 1.98; 95% CI, 1.10–3.55; P = 0.022) were significantly more frequent in the TB with DM group than in the control group. However, there was no significant difference between groups in terms of mass, nodule, and centrilobular nodules (P = 0.096, P = 0.096, P = 0.086, respectively).
The TB with DM group was subdivided into two subgroups depending on the duration of DM: DM ≥10 years (n=45) and DM <10 years (n=48). The mean duration of DM was 18.8±8.5 years in patients with DM ≥10 years and 3.2±2.9 years in patients with DM ≥10 years (Table 3). The mean age of patients was 64.0±10.4 years in patients with DM ≥10 years and 64.0±10.4 years in patients with DM <10 years (P = 0.035). No significant differences between these two groups were observed regarding gender, presence of comorbidity, CT technique, and diagnostic method of TB. The ADA value of pleural effusion was evaluated in seven patients with DM ≥10 years and nine patients with DM <10 years, and no significant difference was found (P = 0.590). Serum hemoglobin A1c (HbA1c) values were present for all patients with DM, and no significant difference was found between groups of patients with ≥10 years and <10 years of DM duration (P = 0.555).
Table 3.
Demographic, clinical, and laboratory features of pulmonary TB patients based on duration of DM
| Characteristics | DM ≥10 years (n=45) | DM <10 years (n=48) | P |
|---|---|---|---|
| Age (year)a | 64.0±10.4 (39–89) | 59.4±13.2 (33–87) | 0.035 |
|
| |||
| Gender (male/female) | 31/14 | 34/14 | 0.838 |
|
| |||
| Comorbidity, n (%) | 31 (68.9) | 30 (62.5) | 0.517 |
|
| |||
| CT | 0.724 | ||
| Unenhanced CT | 3 | 2 | |
| High-resolution CT | 14 | 13 | |
| Enhanced CT | 28 | 33 | |
|
| |||
| TB diagnosis | 0.653 | ||
| Culture | 29 | 33 | |
| TB-PCR | 6 | 4 | |
| Tissue culture | 5 | 8 | |
| ADA | 5 | 3 | |
|
| |||
| ADA (U/L)a,b | 65.5±33.0 | 75.2±26.2 | 0.590 |
|
| |||
| HbA1c (%)a,c | 8.2±1.7 | 7.9±2.1 | 0.555 |
|
| |||
| DM duration (years)a | 18.8±8.5 | 3.2±2.9 | <0.001 |
DM, diabetes mellitus; CT, computed tomography; TB, tuberculosis; TB-PCR, polymerase chain reaction for Mycobacterium tuberculosis; ADA, adenosine deaminase; HbA1c, hemoglobin A1c.
Values correspond to mean±SD (range).
Assessed in case of TB pleurisy (Patients with DM ≥10 years, n=7; patients with DM <10 years, n=9).
Normal range of HbA1c: 4.1%–5.6%.
The CT findings of pulmonary TB in patients with ≥10 years and <10 years of DM are presented in Table 4. Bilateral pulmonary involvement was identified in 71.1% of patients with DM ≥10 years and in 54.2% of patients with DM <10 years (P = 0.092). However, there was no significant difference in CT findings of pulmonary TB depending on the duration of DM. Multivariable binary logistic regression analysis showed that bilateral pulmonary involvement was not an independent contributor to predict long-standing DM in pulmonary TB patients (OR, 2.08; 95% CI, 0.89–4.92; P = 0.094).
Table 4.
CT findings of the pulmonary tuberculosis patients based on duration of DM
| CT findings | DM ≥10 years (n=45) | DM <10 years (n=48) | P |
|---|---|---|---|
| Bilaterality | 32 (71.1) | 26 (54.2) | 0.092 |
|
| |||
| All lobes | 12 (26.7) | 10 (20.8) | 0.508 |
|
| |||
| Atypical location onlya | 2 (4.4) | 6 (13.3) | 0.268 |
|
| |||
| Involvement of atypical locationa | 26 (57.8) | 27 (60.0) | 0.834 |
|
| |||
| TB pleurisy onlyb | 0 (0) | 3 (6.3) | 0.243 |
|
| |||
| Consolidation | 27 (60.0) | 26 (54.2) | 0.570 |
|
| |||
| Mass | 14 (31.1) | 10 (20.8) | 0.263 |
|
| |||
| Nodule | 39 (86.7) | 39 (81.3) | 0.478 |
| Centrilobular nodule | 37 (94.9) | 34 (87.2) | 0.197 |
|
| |||
| Cavity | 23 (51.1) | 21 (43.8) | 0.483 |
| Atypical location | 4 (17.4) | 7 (33.3) | 0.222 |
| Multiple cavities | 7 (15.6) | 8 (16.7) | 0.884 |
| Pleural effusion | 19 (42.2) | 20 (41.7) | 0.960 |
|
| |||
| TB pleurisy | 7 (15.6) | 9 (18.8) | 0.683 |
|
| |||
| Lymph node enlargement | 27 (60.0) | 21 (43.8) | 0.146 |
| Mediastinum | 8 (29.6) | 11 (52.4) | |
| Hilum | 6 (22.2) | 2 (9.5) | |
| Both | 13 (48.1) | 8 (38.1) | |
Data are presented as number of patients, with percentage in parentheses.
CT, computed tomography; DM, diabetes mellitus; TB, tuberculosis.
Atypical location was defined as basal segments of the lower lobes and/or anterior segment of the upper lobes and/or middle lobes, and was confined to the pulmonary parenchymal lesion, excluded TB pleurisy.
TB pleurisy without pulmonary parenchymal lesion.
Discussion
In our study, bilateral pulmonary involvement, involvement of all lobes, and lymph node enlargement were significantly more common CT findings in TB patients with underlying DM than in patients without DM. As it is known, the most common form of pulmonary TB in adults is post-primary or reactivation TB. Typical CT findings of reactivation of pulmonary TB include centrilobular small nodules, branching linear opacities, patchy consolidation, and cavitation (19–21). Post-primary TB most commonly involves the upper lobes and the superior segments of the lower lobes (25, 26). In addition, pleural effusion may be the sole imaging manifestation of TB. Thus, we included patients diagnosed as having TB pleurisy with no pulmonary parenchymal lesion.
Several studies suggested that pulmonary TB in diabetic patients may be more likely to present atypical patterns and distributions in radiologic images (7–9, 22–24). In our study, the most common isolated atypical location was the right middle lobe. Pulmonary TB with isolated atypical location was identified in 8.9% of patients in the TB with DM group and 3.2% in the control group; however, these differences did not reach statistical significance. In previous studies, the prevalence of pulmonary TB solely in the lower lung zone was reported to range from 5% to 20% (9). Also DM was thought to be the only significant condition to predispose disease of the lower lung zone in a previous study (17); however, we found no statistically significant differences in the feature of isolated atypical location involvement between diabetics and nondiabetics.
The frequency of atypical location involvement with or without the typical location was similar between the two groups (58.9% vs. 58.5%). Previous studies documented 23%–48% of lower lung field involvement (7, 8, 24). Atypical location of TB on imaging can lead to a misdiagnosis. In fact, patients with the lower lung field TB have often been misdiagnosed as cases of pneumonia, carcinoma, or lung abscess (10). Familiarity with the atypical locations of pulmonary TB is crucial to avoid delayed diagnosis and provide an appropriate treatment of TB.
Bilateral lung involvement was observed in 62.4% of patients in the TB with DM group and 41% of patients in the control group. This CT finding was significantly more frequent in TB patients with DM than without DM (OR, 2.39). Several previous studies documented that 18%–47% of patients showed bilateral involvement of pulmonary TB in DM patients, in contrast to our study (10, 11). The reason for this discrepancy may be that previous studies were based on chest radiography findings, rather than CT findings. CT is a more sensitive modality to identify subtle lung parenchymal abnormalities (27).
A higher frequency of multilobar involvement of pulmonary TB in DM patients has been described (12). In our study, the TB with DM group showed a significantly higher frequency of all lobar involvement than the control group (23.7% vs. 10.0%). All lobar involvement was an independent contributor to predict the presence of DM in pulmonary TB patients (OR, 2.79).
A higher frequency of cavities in diabetic TB patients compared with nondiabetic TB patients has been suggested in several studies (7, 8, 23). Shaikh et al. (7) reported cavitary lung lesions in 50.8% of diabetic TB patients compared with 39.0% of nondiabetic TB patients. Perez-Guzman et al. (8) suggested increase in cavities in the lower lung fields and multiple cavities in diabetics. However, some other studies reported no radiologic differences in frequency of cavities between diabetic and nondiabetic TB patients (13). In our study, the presence of cavities, multiple cavities, and atypical location of cavities were more commonly observed in DM patients, but there were no statistically significant differences.
Mass, nodule, and centrilobular nodule demonstrated higher prevalence in DM patients compared with the control group, although the differences did not reach statistical significance on multiple logistic regression analysis. The result of our study is valuable in that no previous studies mentioned these CT findings in TB patients with DM.
Lymphadenopathy was identified significantly more commonly in DM patients than in the control group (51.6% vs. 35.0%; OR, 1.98). It is notable that relatively large number of patients demonstrated lymph node enlargement. A previous study, based on simple chest radiography, found lymphadenopathy in only 5% of TB patients with DM (11).
The next topic was the effect of duration of DM on CT findings of pulmonary TB. Jabbar et al. (11) suggested that the prevalence of TB increases with the duration of DM, and the highest prevalence is seen 10 years after the diagnosis. In our study, prevalence of bilateral lung involvements was higher among patients with ≥10 years of DM than those with <10 years of DM (71.1% vs. 54.2%). However, there were no significant differences in other CT findings of pulmonary TB depending on the duration of DM. Nevertheless, our study is meaningful because this is the first study to compare CT findings of pulmonary TB depending on patient’s duration of DM.
Our study has several limitations. First, due to the retrospective nature of our study, CT examinations were not performed using the same protocol in all patients. However, no statistically significant differences were observed between the groups. Second, there is a possibility of selection bias: patients with pulmonary TB with typical location might have been excluded, as these patients were not required to have a chest CT. However, the purpose of this study was to evaluate the CT findings of pulmonary TB in DM patients showing atypical findings to help suspect TB in DM cohorts. Third, although we maximally excluded immunodeficiency states, the presence of comorbidities between the two groups was significantly different. Inclusion of DM patients with renal disease was inevitable, because chronic renal failure is strongly associated with long-standing DM. Fourth, association between DM control and CT findings was not evaluated in the present study. A previous study reported that glycemic control based on HbA1c influenced chest radiography findings of pulmonary TB in DM patients (14). Further CT based research is needed to address this issue. Finally, while the finding of enlarged lymph nodes with central low attenuation representing caseous necrosis is a well-known imaging feature of active TB on CT (26), our study did not evaluate this finding. We look forward to conducting a further study concerning necrosis in lymph nodes of TB patients with DM.
In conclusion, bilateral pulmonary involvement, pulmonary involvement of all lobes, and lymph node enlargement were significantly more frequent CT findings in TB patients with DM compared with nondiabetic TB patients. CT findings of pulmonary TB did not differ significantly depending on the duration of DM. Familiarity with CT findings of pulmonary TB in DM patients may be helpful in terms of a prompt diagnosis and proper treatment.
Main points.
Bilateral pulmonary involvement, pulmonary involvement of all lobes, and lymph node enlargement were significantly more frequent CT findings in tuberculosis (TB) patients with diabetes mellitus (DM) compared with TB patients without DM.
There were no statistically significant differences in the CT findings of pulmonary TB did not display significant differences depending on the duration of DM.
To know the CT findings of pulmonary TB in diabetes patients may be helpful in terms of suggesting prompt diagnosis and proper treatment.
Acknowledgements
We would like to express special thanks to Young Soo Joo for help on statistical analyses.
Footnotes
Conflict of interest disclosure: The authors declared no conflicts of interest.
References
- 1.World Health Organization. Global Tuberculosis Report. Geneva: World Health Organization; 2013. [Google Scholar]
- 2.International Diabetes Federation. Diabetes Atlas. 6th ed. Brussels: International Diabetes Federation; 2014. [Google Scholar]
- 3.Baghaei P, Marjani M, Javanmard P, Tabarsi P, Masjedi MR. Diabetes mellitus and tuberculosis facts and controversies. J Diabetes Metab Disord. 2013;12:58. doi: 10.1186/2251-6581-12-58. https://doi.org/10.1186/2251-6581-12-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jeon CY, Murray MB. Diabetes mellitus increases the risk of active tuberculosis: a systematic review of 13 observational studies. PLoS Med. 2008;5:e152. doi: 10.1371/journal.pmed.0050152. https://doi.org/10.1371/journal.pmed.0050181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dixon B. Diabetes and tuberculosis: an unhealthy partnership. Lancet Infect Dis. 2007;7:444. doi: 10.1016/S1473-3099(07)70144-5. https://doi.org/10.1016/S1473-3099(07)70144-5. [DOI] [PubMed] [Google Scholar]
- 6.Rozenshtein A, Hao F, Starc MT, Pearson GDN. Radiographic appearance of pulmonary tuberculosis: dogma disproved. AJR Am J Roentgenol. 2015;204:974–978. doi: 10.2214/AJR.14.13483. https://doi.org/10.2214/AJR.14.13483. [DOI] [PubMed] [Google Scholar]
- 7.Shaikh MA, Singla R, Khan NB, Sharif NS, Saigh MO. Does diabetes alter the radiological presentation of pulmonary tuberculosis. Saudi Med J. 2003;24:278–281. [PubMed] [Google Scholar]
- 8.Perez-Guzman C, Torres-Cruz A, Villarreal-Velarde H, Salazar-Lezama M, Vargas M. Atypical radiological images of pulmonary tuberculosis in 192 diabetic patients: a comparative study. Int J Tuberc Lung Dis. 2001;5:455–461. [PubMed] [Google Scholar]
- 9.Hadlock F, Park S, Awe R, Rivera M. Unusual radiographic findings in adult pulmonary tuberculosis. AJR Am J Roentgenol. 1980;134:1015–1018. doi: 10.2214/ajr.134.5.1015. https://doi.org/10.2214/ajr.134.5.1015. [DOI] [PubMed] [Google Scholar]
- 10.Patel AK, Rami KC, Ghanchi FD. Radiological presentation of patients of pulmonary tuberculosis with diabetes mellitus. Lung India. 2011;28:70. doi: 10.4103/0970-2113.76308. https://doi.org/10.4103/0970-2113.76308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jabbar A, Hussain SF, Khan AA. Clinical characteristics of pulmonary tuberculosis in adult Pakistani patients with co-existing diabetes mellitus. East Mediterr Health J. 2006;12:522–527. [PubMed] [Google Scholar]
- 12.Umut S, Tosun GA, Yildirim N. Radiographic location of pulmonary tuberculosis in diabetic patients. Chest. 1994;106:326. https://doi.org/10.1378/chest.106.1.326a. [PubMed] [Google Scholar]
- 13.Morris JT, Seaworth BJ, McAllister CK. Pulmonary tuberculosis in diabetics. Chest. 1992;102:539–541. doi: 10.1378/chest.102.2.539. https://doi.org/10.1378/chest.102.2.539. [DOI] [PubMed] [Google Scholar]
- 14.Chiang CY, Lee JJ, Chien ST, et al. Glycemic control and radiographic manifestations of tuberculosis in diabetic patients. PloS one. 2014;9:e93397. doi: 10.1371/journal.pone.0093397. https://doi.org/10.1371/journal.pone.0093397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jeon D. Tuberculous pleurisy: an update. Tuberc Respir Dis. 2014;76:153–159. doi: 10.4046/trd.2014.76.4.153. https://doi.org/10.4046/trd.2014.76.4.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Burgess LJ, Maritz FJ, Roux IL, Taljaard JJF. Combined use of pleural adenosine deaminase with lymphocyte/neutrophil ratio: increased specificity for the diagnosis of tuberculous pleuritis. Chest. 1996;109:414–419. doi: 10.1378/chest.109.2.414. https://doi.org/10.1378/chest.109.2.414. [DOI] [PubMed] [Google Scholar]
- 17.Ikezoe J, Takeuchi N, Johkoh T, et al. CT appearance of pulmonary tuberculosis in diabetic and immunocompromised patients: comparison with patients who had no underlying disease. AJR Am J Roentgenol. 1992;159:1175–1179. doi: 10.2214/ajr.159.6.1442377. https://doi.org/10.2214/ajr.159.6.1442377. [DOI] [PubMed] [Google Scholar]
- 18.Bernard JR, Thomas FO, Cragun WH. The serum-effusion albumin gradient in the evaluation of pleural effusions. Chest. 1990;98:546–549. doi: 10.1378/chest.98.3.546. https://doi.org/10.1378/chest.98.3.546. [DOI] [PubMed] [Google Scholar]
- 19.Im JG, Itoh H, Lee KS, Han MC. CT-pathology correlation of pulmonary tuberculosis. Crit Rev Diagn Imaging. 1995;36:227–285. [PubMed] [Google Scholar]
- 20.Lee KS, Song KS, Lim TH, Kim PN, Kim I, Lee BH. Adult-onset pulmonary tuberculosis: findings on chest radiographs and CT scans. AJR Am J Roentgenol. 1993;160:753–758. doi: 10.2214/ajr.160.4.8456658. https://doi.org/10.2214/ajr.160.4.8456658. [DOI] [PubMed] [Google Scholar]
- 21.Lee K, Im J. CT in adults with tuberculosis of the chest: characteristic findings and role in management. AJR Am J Roentgenol. 1995;164:1361–1367. doi: 10.2214/ajr.164.6.7754873. https://doi.org/10.2214/ajr.164.6.7754873. [DOI] [PubMed] [Google Scholar]
- 22.Perez-Guzman C, Torres-Cruz A, Villarreal-Velarde H, Vargas MH. Progressive age-related changes in pulmonary tuberculosis images and the effect of diabetes. Am J Respir Crit Care Med. 2000;162:1738–1740. doi: 10.1164/ajrccm.162.5.2001040. https://doi.org/10.1164/ajrccm.162.5.2001040. [DOI] [PubMed] [Google Scholar]
- 23.Alavi SM, Khoshkho MM, Salmanzadeh S, Eghtesad M. Comparison of epidemiological, clinical, laboratory and radiological features of hospitalized diabetic and non-diabetic patients with pulmonary tuberculosis at razi hospital in ahvaz. Jundishapur J Microbiol. 2014;7:e12447. doi: 10.5812/jjm.12447. https://doi.org/10.5812/jjm.12447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bacakoglu F, Basoglu OK, Cok G, Sayiner A, Ates M. Pulmonary tuberculosis in patients with diabetes mellitus. Respiration. 2001;68:595–600. doi: 10.1159/000050578. https://doi.org/10.1159/000050578. [DOI] [PubMed] [Google Scholar]
- 25.Woodring JH, Vandiviere H, Fried A, Dillon M, Williams T, Melvin I. Update: the radiographic features of pulmonary tuberculosis. AJR Am J Roentgenol. 1986;146:497–506. doi: 10.2214/ajr.146.3.497. https://doi.org/10.2214/ajr.146.3.497. [DOI] [PubMed] [Google Scholar]
- 26.Jeong YJ, Lee KS. Pulmonary tuberculosis: up-to-date imaging and management. AJR Am J Roentgenol. 2008;191:834–844. doi: 10.2214/AJR.07.3896. https://doi.org/10.2214/AJR.07.3896. [DOI] [PubMed] [Google Scholar]
- 27.Kim WS, Moon WK, Kim IO, et al. Pulmonary tuberculosis in children: evaluation with CT. AJR Am J Roentgenol. 1997;168:1005–1009. doi: 10.2214/ajr.168.4.9124105. https://doi.org/10.2214/ajr.168.4.9124105. [DOI] [PubMed] [Google Scholar]