Abstract
PURPOSE
We aimed to evaluate the imaging features of solid pseudopapillary neoplasm (SPN) of the pancreas with an emphasis on radiologic-pathologic correlation.
METHODS
Ten patients (all female; mean age, 32 years) with histologic or cytologic diagnosis of SPN encountered between January 2007 and December 2013 were included in this study. Preoperative computed tomography (CT) images were reviewed for location, attenuation, enhancement pattern, margin, shape, size, morphology, presence of capsule and calcification. CT appearances were correlated with histopathologic findings.
RESULTS
Tumors in the distal pancreatic body and tail had a tendency to be larger (mean size 12.6 cm vs. 4.0 cm). Six of the nine tumors that were resected had a fibrous pseudocapsule at histology, five of which could be identified on CT scan. Eight lesions had mixed hypoenhancing solid components and cystic areas corresponding to tumor necrosis and hemorrhage. The two smallest lesions were purely solid and nonencapsulated. Varied patterns of calcification were seen in four tumors. Three of the four pancreatic tail tumors invaded the spleen. At a median follow-up of 53 months, there was no evidence of recurrence in the nine patients who underwent surgical resection of the tumor.
CONCLUSION
A mixed solid and cystic pancreatic mass in a young woman is suggestive of SPN. However, smaller lesions may be completely solid. Splenic invasion can occur in pancreatic tail SPNs; however, in this series it did not adversely affect the long-term outcome.
Solid pseudopapillary neoplasm (SPN) of the pancreas is a rare (1%–2% of exocrine pancreatic tumors) and usually benign disease (1). It was first described by Frantz in 1959 and has had a wide array of synonyms since then, including Gruber-Frantz tumor, solid and papillary epithelial neoplasm, solid-cystic-papillary epithelial neoplasm and adenocarcinoma of the pancreas in childhood (1, 2). It was eventually given its current name by the World Health Organization (WHO) in 1996 (3). SPNs are more often seen in nonCaucasian (especially Asian and African) young women and usually presents with vague abdominal discomfort or as a large abdominal mass (1, 4). A typical SPN is a well-encapsulated mass with solid and cystic components due to varying degrees of internal hemorrhage and necrosis (1, 5–7). However, atypical tumors can mimic adenocarcinoma, nonfunctioning islet cell tumor, cystadenomas or papillary cystadenocarcinoma. Most of the existing literature on SPNs is in the form of short case series and case reports from a surgical point of view with limited emphasis on their imaging features (4).
In this study, we reviewed the computed tomography (CT) imaging findings in 10 consecutive cases of SPN that were managed at our institution, with correlation to their clinical and pathologic features.
Methods
The study was performed with approval from the institutional review board. Waiver of informed consent was obtained. We retrospectively reviewed the radiologic studies of all patients with histologic or cytologic diagnosis of SPN managed at our institution between January 2007 and December 2013. Their clinical presentation was identified from the hospital electronic medical records and images reviewed on the hospital picture archiving and communication system (PACS).
There were 10 patients (all female) with a mean age of 32 years (median, 39 years; range, 9–60 years) at the time of presentation. Nine patients had a confirmed diagnosis made at surgical pathology, while six patients (including one who did not undergo surgery) had a preoperative diagnosis with endoscopic ultrasonography (EUS)-guided biopsy. All patients had CT scan performed on a 64-slice multidetector scanner. Four patients had multiphasic study for characterization of a pancreatic mass detected on a prior imaging study. Images were acquired on unenhanced as well as contrast-enhanced arterial (pancreatic) and portal venous phases. One patient had an additional five-minute delayed contrast-enhanced scan, which was performed under the instruction of the reporting radiologist. The images were acquired at slice thickness of 0.6 mm with 3 mm reconstructions in axial and coronal planes on a workstation: gantry rotation time 0.5 s, tube current for unenhanced phase 100 mAs, tube current for and contrast-enhanced phase 200 mAs, peak voltage 120 kVp. The Z-axis coverage of unenhanced and arterial (pancreatic) phase scans was from the domes of the diaphragm to the anterior superior iliac spines, while coverage of portal venous phase scans was to the ischial tuberosities. The arterial (pancreatic) phase images were acquired following intravenous injection of 100 mL of nonionic iodinated contrast (iohexol, Omnipaque 300, GE Healthcare) at a rate of 3 mL/s using the bolus tracking method. The portal venous phase images were acquired at 60 s from contrast injection. The remaining six patients had a single portal venous phase study with similar Z-axis coverage and image acquisition parameters. However, their images were reconstructed at 5 mm slice thickness. Two of them also had five-minute delayed scans under the instruction of the reporting radiologist. In these six patients, the decision was made to proceed with further evaluation by EUS or direct surgery without an additional multiphasic CT scan.
The clinical presentation, level of tumor markers (when available), surgical findings and postoperative follow-up in relevant cases were recorded. The CT scans were reviewed for tumor location within the pancreas, patterns of mass effect on adjoining fat planes and structures, attenuation and enhancement pattern relative to the surrounding pancreatic parenchyma on each phase of scanning by two radiologists (with 10 and 5 years of experience in abdominal CT). The lesion margin (well or ill-defined), shape (round, ovoid, or lobulated), size (longest dimension in any axis), tumor texture (homogeneous or heterogeneous) and internal morphology (solid, cystic, or mixed; presence of septations) were evaluated. The presence of a capsule or calcification was noted separately. In patients who had surgical excision of the tumor, the radiologic findings were correlated with the histologic findings, which were reviewed together with a senior pathologist.
Results
The most common presentation was upper abdominal pain, as seen in five patients. In three patients, the tumor was detected incidentally on a CT scan performed for other indications. One patient presented with left ureteric colic and another one with a palpable abdominal mass (Table 1). Serum carcinoembryonic antigen, cancer antigen 19.9 (CA 19-9), and alpha-fetoprotein tumor marker levels were available in seven out of 10 patients; they were within normal limits except in Patient no. 10 who had slightly elevated CA19-9. In the six patients who underwent EUS-guided biopsy, the diagnosis of SPN was made on cytology and the inference was consistent with surgical pathology. Nine patients underwent surgical resection of the pancreatic tumor, while one patient declined surgical treatment. In patients who underwent surgery, there was no recurrence on imaging and clinical follow-up (median follow-up, 53 months) (Table 1).
Table 1.
Clinical features of 10 cases of SPNs
| No/Sex/Age (yrs) | Clinical presentation | Diagnosis | Management | Outcome |
|---|---|---|---|---|
| 1/F/42 | Incidental finding | EUS biopsy and surgical pathology | Distal pancreatectomy and splenectomy | No recurrence at 96 months |
| 2/F/27 | Upper abdominal mass | Surgical pathology | Distal pancreatectomy and splenectomy | No recurrence at 85 months |
| 3/F/49 | Upper abdominal pain | EUS biopsy and surgical pathology | Distal pancreatectomy and splenectomy | No recurrence at 76 months |
| 4/F/24 | Upper abdominal pain | EUS biopsy and surgical pathology | Median pancreatectomy | Complicated by pancreaticojejunostomy fistula No recurrence at 63 months |
| 5/F/13 | Upper abdominal pain | Surgical pathology | Distal pancreatectomy and splenectomy | No recurrence at 58 months |
| 6/F/9 | Upper abdominal pain | Surgical pathology | Distal pancreatectomy and splenectomy | No recurrence at 48 months |
| 7/F/37 | Ureteric colic | Surgical pathology | Whipple’s | No recurrence at 42 months |
| 8/F/53 | Incidental finding | EUS biopsy and surgical pathology | Pylorus-preserving pancreaticoduodenectomy | No recurrence at 37 months |
| 9/F/60 | Upper abdominal pain | EUS biopsy and surgical pathology | Whipple’s | Complicated by bleeding pancreaticojejunostomy No recurrence at 26 months |
| 10/F/42 | Incidental finding | EUS biopsy | Conservative treatment | No progression at 23 months |
SPN, solid pseudopapillary neoplasm; F, female; EUS, endoscopic ultrasonography.
The mean long-axis tumor diameter was 6.8 cm (range, 2.6 – 21.4 cm) (Table 2). There was a slight predilection for the lesions to be located in the distal body and tail of the pancreas (Table 2, Fig. 1). The tumors in this location were the largest (measuring up to 21 cm in diameter) and they presented with upper abdominal pain or with a palpable lump. All 10 tumors had well-defined margins at the CT scan, with a capsule demonstrable in five of them (Figs. 1, 2). The capsule was complete in three cases and partial in the remaining two cases.
Table 2.
Imaging features of 10 cases of SPNs
| No | Phases | Location in pancreas | Size (cm) | Shape | Margins | Capsule | Internal morphology | Enhancement pattern and texture of solid components | Calcification | Parenchymal atrophy and ductal dilatation | Local invasion and distant metastasis |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Unenhanced, Art, PV, delayed | Proximal body | 4.0 | Round | Well-defined | Complete | Predominantly solid | Heterogeneous iso-hypoattenuating on all phases | No | No | No |
| 2 | PV | Distal body and tail | 21.4 | Lobulated | Well-defined | Partial | Equally solid and cystic (Peripheral solid, central cystic) | Heterogeneous hypoattenuating | No | No | Splenic invasion |
| 3 | PV, delayed | Tail | 12.2 | Lobulated | Well-defined | No | Predominantly cystic (Peripheral solid, central cystic with thick septations) | Heterogeneous isoattenuating on all phases | No | No | Splenic invasion |
| 4 | PV | Neck | 3.3 | Oval | Well-defined | No | Predominantly solid | Homogeneous hypoattenuating | No | No | No |
| 5 | PV | Distal body and tail | 9.1 | Lobulated | Well-defined | Complete | Predominantly solid | Heterogeneous hypoattenuating | No | No | No |
| 6 | PV, delayed | Distal body and tail | 7.7 | Lobulated | Well-defined | Partial | Predominantly solid | Heterogeneous hypoattenuating on all phases | No | No | Splenic invasion |
| 7 | Unenhanced, Art, PV | Head | 6.0 | Lobulated | Well-defined | No | Predominantly solid | Heterogeneous hypoattenuating on all phases | Peripheral | Ductal dilatation upstream to the mass | No |
| 8 | CT thorax | Uncinate process | 2.7 | Oval | Well-defined | No | Purely solid | Homogeneous hypoattenuating | Single focal | No | No |
| 9 | Unenhanced, Art, PV | Neck | 5.6 | Oval | Well-defined | Complete | Predominantly solid | Heterogeneous hypoattenuating on all phases | Eccentric dense | No | No |
| 10 | Unenhanced, Art, PV | Proximal body | 2.6 | Lobulated | Well-defined | No | Purely solid | Isodense on non-contrast images. Homogeneous hypoattenuating on post-contrast phases | Faint amorphous | No | No |
SPN, solid pseudopapillary neoplasm; Art, arterial (pancreatic); PV, portal venous.
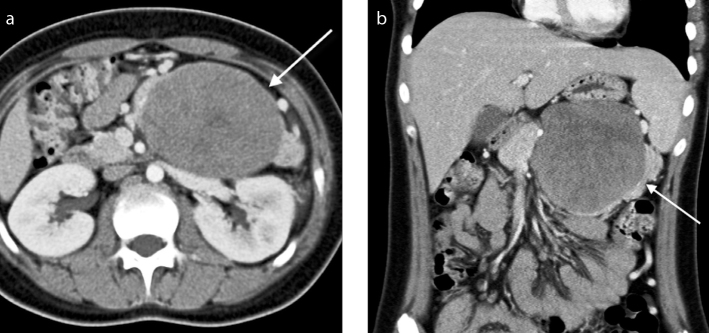
Figure 1. a, b.
A 13-year-old girl who presented with upper abdominal pain. Axial (a) and coronal (b) contrast-enhanced CT images in the portal venous phase demonstrate a large mass in the distal body and tail of the pancreas (arrow). It is well-defined with lobulated margins and a pseudocapsule. It is predominantly solid with hypoattenuating solid components.
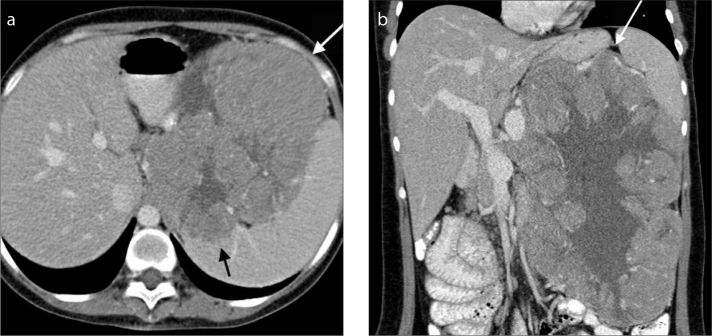
Figure 2. a, b.
A 27-year-old woman who presented with an upper abdominal mass. Axial (a) and coronal (b) contrast-enhanced CT images in the portal venous phase demonstrate a large mass in the distal body and tail of the pancreas (white arrow). It is well-defined with lobulated margins, a partial pseudocapsule and characteristic peripheral hypoattenuating solid and central cystic components. There is loss of the fat plane between the mass and the spleen, with foci of splenic invasion (black arrow).
Eight lesions were of mixed solid-cystic morphology (Fig. 2), while two were purely solid (Fig. 3). Of eight lesions with mixed morphology, six were predominantly solid, one had equal solid and cystic components, and one was predominantly cystic (Table 2). In mixed-type tumors, the cystic components were centrally located. One of them had thick enhancing septations.
Figure 3.
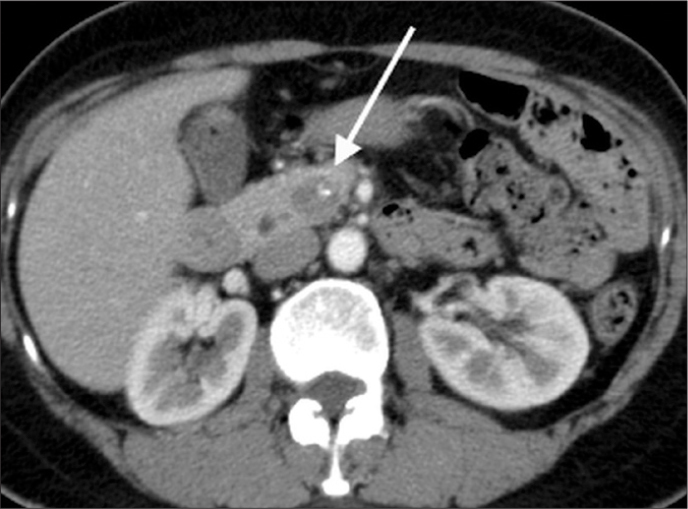
A 53-year-old woman who had an incidental finding of a pancreatic mass on CT scan of the thorax. Axial contrast-enhanced CT image in the portal venous phase demonstrates a small mass in the uncinate process of the pancreas (arrow). It is well-defined and purely solid with no discernible pseudocapsule. A single focal eccentric calcification is noted.
In the four available unenhanced studies, the solid component of SPN was isodense to the rest of the pancreas in one tumor and hypodense in the rest. All tumors enhanced poorly compared with the rest of the gland. The enhancement pattern was heterogeneous in the seven larger tumors while the three smaller tumors (around 3 cm in size) showed more homogeneous enhancement. Calcification was seen in four tumors, with varied appearances ranging from peripheral to coarse, faint amorphous, and single focal calcification (Fig. 3).
Three tumors unequivocally invaded the spleen (Fig. 2), which was subsequently confirmed at surgery. These patients had radiologic evidence of portosystemic shunting, due to compression of the splenic vein. However, there was no overt vascular invasion.
Only one of the large tumors in the pancreatic head had mass effect upon the adjacent pancreatic duct with upstream dilatation of the main pancreatic duct. There was no pancreatic parenchymal atrophy, peripancreatic fat infiltration, regional lymphadenopathy or distant metastasis in any of the patients.
On gross examination of the pathologic specimens, areas of tumor necrosis and hemorrhagic degeneration corresponded to cystic areas on imaging (Fig. 4). A fibrous pseudocapsule was present in six cases. Five of them were identified on imaging except in one case (case no. 4) where a fibrous pseudocapsule partially surrounding the tumor could only be seen at microscopy. In two cases with splenic invasion, breach of the pseudocapsule was visible, both on CT and histology. There was no pseudocapsule in the third tumor with splenic invasion. On microscopy, there were sheets of tumor cells interspersed with pseudopapillae of cells around fibrovascular cores in varying proportions, forming a solid-pseudopapillary pattern (Fig. 4). Mitotic figures were rarely seen. Calcification of the hyalinized stroma was seen in cases that demonstrated calcification on CT. Extensive dystrophic calcification was detected in Patient no. 9, visualized as coarse calcification on imaging. No lymphovascular or perineural invasion was seen in any of the cases.
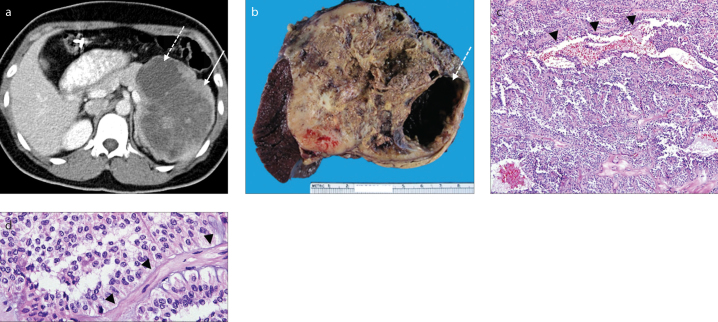
Figure 4. a–d.
A 49-year-old woman who presented with upper abdominal pain. Axial contrast-enhanced CT image (a) in the portal venous phase demonstrates a large well-defined lobulated mass in the pancreatic tail with mixed solid (solid arrow) and cystic (dashed arrow) components. Polypectomy clips are incidentally noted in the transverse colon. Gross pathologic specimen (b) shows a circumscribed tumor with a variegated tan cut surface and areas of cystic change (dashed arrow) corresponding to those seen on CT. Medium power microscopy (c) demonstrates tumor cells loosely arranged around vessels (arrowheads; Hematoxylin and Eosin [HE] staining, original magnification ×100). High power microscopy (d) demonstrates relatively bland viable tumor cells arranged around a fibrovascular core (arrowheads; HE staining, original magnification ×400).
Discussion
In our study, SPNs demonstrated a fairly similar set of imaging findings at CT scan reflective of the histopathologic changes within the tumor. They tend to be large, well-defined, isodense to normal pancreas on unenhanced images and heterogeneously hypoenhancing on postcontrast images with predominantly (80%) mixed solid-cystic morphology. Half of them had a demonstrable pseudocapsule, 40% had internal calcification, while 30% showed splenic invasion. Meanwhile, the occasional smaller tumors were more homogeneous and solid with no pseudocapsule identified at CT scan. Although not pathognomonic, in the appropriate clinical context these imaging findings are reasonably suggestive of the diagnosis.
In a large review of 718 reported cases of SPN from published literature, Papavramidis et al. (4) reported a female to male ratio of 10:1 and a mean age of 22 years (4). All patients in our series were female, although the mean age of presentation was slightly older at 32 years. Although commonly associated with young women, this condition has also been reported in older and pediatric patients (4, 8). The oldest patient in our series was in the seventh decade of her life. Two of our patients (20%) were in the pediatric age group (9 and 13 years).
SPNs may appear in any part of the pancreas, but are more likely to be found in the distal body and tail as also noted in our study (4). These tumors were also larger in size than those found elsewhere in the pancreas (mean size, 12.6 cm vs. 4.0 cm). Their location may have allowed them to assume a larger size before manifesting clinically.
We found two patterns of imaging features in this series. In general, larger tumors followed the classic CT description of SPNs in the literature, presenting as large well-encapsulated masses with variable solid and cystic components (1, 5–7). As documented in the literature, we also found the cystic components to be more centrally located, while the solid components were more peripheral. The solid components are known to be tumors hypoattenuating on both unenhanced and contrast-enhanced pancreatic and portal venous phases (Fig. 2) (5). Meanwhile, the two smaller lesions (less than 3 cm) in our series were purely solid and nonencapsulated (Fig. 3) and located in the proximal pancreas. This is consistent with a previous study, which found smaller SPNs more likely to be purely solid with sharp margins and lacking a capsule (9). However, they did not find any difference in the location of such solid small SPNs. These atypical SPNs may be difficult to differentiate from other solid pancreatic tumors.
The origin and histogenesis of SPN is still unknown, although it is thought to originate from totipotential stem cells with the capacity for both endocrine and exocrine differentiation (10). These tumors are also often texturally fairly soft; this accounts for the rarity of ductal dilatation and jaundice even for tumors arising in the pancreatic head (4, 11). A fibrous pseudocapsule delineates the tumor from the rest of the pancreas, giving a well-circumscribed appearance on gross pathology (12, 14). Larger lesions tend to outgrow their blood supply (11) and undergo cystic degeneration and hemorrhage, which are seen as internal heterogeneity on CT (4, 11–14). These gross pathologic features were reflected in our CT imaging findings (Fig. 4). Under the microscope, the tumor was composed of uniform epithelioid tumor cells, arranged in nests and pseudopapillary structures as viable tumor cells remained attached to hyalinized vascular cores (Fig. 4), while other cells further from the blood supply underwent degenerative changes (11–14). It is due to this appearance that the tumor acquires its name. The tumor cells sometimes contain periodic acid-Schiff positive and diastase-resistant globules. Areas of cystic change may be present, particularly in larger tumors, accompanied by reactive changes such as the accumulation of cholesterol clefts (11–14). In smaller tumors, solid sheets of cells predominate, with hemorrhage and cystic change being rare. This explains the corresponding solid appearance on imaging (4, 11–13). The degenerate tumor tissue can undergo dystrophic calcification (14). Between 29% and 65% of SPNs are reported to have calcification (5, 11). Calcification of the fibrous pseudocapsule is not uncommon. Buetow et al. (5) described peripheral calcification as a feature of SPNs. However, other patterns such as coarse central (from dystrophic calcification), stippled, and eggshell calcifications have also been reported (15, 16). A variety of patterns of calcifications were noted in our study as well.
Local invasion of the spleen was observed on imaging and confirmed at surgery in three of the four pancreatic tail lesions in our series. Two of the lesions were partially encapsulated, with the capsule not visualized along the margins of splenic invasion. These corresponded to foci of capsular breach on pathology. These lesions were also large, measuring between 7.7 and 21.4 cm. There is limited data on the incidence of splenic invasion in SPNs, although it has been described as an infrequent feature, which may be associated with an increased potential for malignant behavior (15, 17). In Papavramidis’s review, it was only seen in 17 of 497 adequately described cases (3.4%) (4).
None of our cases had metastases on imaging and/or clinical follow-up. Metastases, most commonly to the liver, have been reported in 5%–15% of cases (11). Angioinvasion, perineural invasion, deep invasion of the surrounding pancreatic parenchyma as well as large size, cellular or nuclear atypia, high mitotic rate and extensive necrosis have been reported to increase the malignant potential of SPNs (11, 17). However, in the absence of these histologic features, metastases can still rarely occur (18). SPNs are thus classified as lesions of uncertain malignant potential in the latest WHO classification (18).
While there may be overlap between the CT appearances of SPN seen in this study and of other pancreatic tumors described in the literature, we believe that a reasonable differential diagnosis is possible in the appropriate clinical context. The presence of solid enhancing components and absence of stigmata of pancreatitis differentiates the large solid-cystic SPNs from pancreatic pseudocysts and walled-off collections (19), while the younger age at presentation may help in distinguishing them from mucinous cystic neoplasms of the pancreas (15, 20). With early hyperenhancement in smaller lesions and lack of solid components in the larger ones, serous cystadenomas are unlikely to mimic SPNs (11, 15, 21). Compared with adenocarcinomas, SPNs have well-defined margins with no ductal dilatation or upstream pancreatic atrophy. The typical early enhancing neuroendocrine tumors of the pancreas would rarely cause a diagnostic dilemma; however, larger necrotic ones with poor enhancement and normal biochemical markers may be difficult to distinguish from SPNs (11).
Following successful surgical resection, we did not encounter any distant metastasis or local recurrence in the follow-up period.
The main limitations of our study are its retrospective nature and selection bias. SPN cases that did not undergo biopsy or surgery have been excluded from the study. The study is also of a descriptive nature with limited scope for statistical analysis.
In conclusion, CT imaging features of SPNs correlate well with their histopathologic findings. The diagnosis should be considered whenever a mixed solid and cystic pancreatic mass is found in a young woman. Smaller lesions may be completely solid and present a diagnostic challenge. Nevertheless imaging features can help in generating a reasonable differential diagnosis. Splenic invasion can occur in pancreatic tail SPNs; however, in the current series this did not adversely affect the long-term outcome.
Main points.
CT imaging features of pancreatic solid pseudopapillary neoplasms (SPNs) correlate well with their pathologic findings.
A well-defined mixed solid-cystic pancreatic mass in a young woman should raise the suspicion of SPN.
Small SPNs may not have the pathognomonic imaging features and can be purely solid.
In the appropriate clinical context, CT is highly likely to provide a differential diagnosis of SPN.
Footnotes
Conflict of interest disclosure
The authors declared no conflicts of interest.
References
- 1.Coleman KM, Doherty MC, Bigler SA. Solid-pseudopapillary tumor of the pancreas. Radiographics. 2003;23:1644–1648. doi: 10.1148/rg.236035006. https://doi.org/10.1148/rg.236035006. [DOI] [PubMed] [Google Scholar]
- 2.Frantz VK. Tumors of the pancreas. In: Rosai J, Sorbin L, editors. Atlas of tumor pathology, Section VII, fasc. 27 and 28. Washington, DC: Armed Forces Institute of Pathology; 1959. pp. 32–33. [Google Scholar]
- 3.Kloppel G, Solcia E, Longnecker DS, et al. WHO international histological classification of tumors. 2nd ed. Berlin, Heidelberg, New York: Springer; 1996. Histological typing of tumors of the exocrine pancreas; p. 14. https://doi.org/10.1007/978-3-642-61024-0. [Google Scholar]
- 4.Papavramidis T, Papavramidis S. Solid pseudopapillary tumors of the pancreas: review of 718 patients reported in English literature. J Am Coll Surg. 2005;200:965–972. doi: 10.1016/j.jamcollsurg.2005.02.011. https://doi.org/10.1016/j.jamcollsurg.2005.02.011. [DOI] [PubMed] [Google Scholar]
- 5.Buetow PC, Buck JL, Pantongrag-Brown L, Beck KG, Ros PR, Adair CF. Solid and papillary epithelial neoplasm of the pancreas: imaging-pathologic correlation on 56 cases. Radiology. 1996;199:707–711. doi: 10.1148/radiology.199.3.8637992. https://doi.org/10.1148/radiology.199.3.8637992. [DOI] [PubMed] [Google Scholar]
- 6.Dong PR, Lu DS, Degregario F, Fell SC, Au A, Kadell BM. Solid and papillary neoplasm of the pancreas: radiological-pathological study of five cases and review of the literature. Clin Radiol. 1996;51:702–705. doi: 10.1016/s0009-9260(96)80242-x. https://doi.org/10.1016/S0009-9260(96)80242-X. [DOI] [PubMed] [Google Scholar]
- 7.Kehagias D, Smyrniotis V, Kalovidouris A, et al. Cystic tumors of the pancreas: preoperative imaging, diagnosis, and treatment. Int Surg. 2002;87:171–174. [PubMed] [Google Scholar]
- 8.Bouassida M, Mighri MM, Bacha D, et al. Solid pseudopapillary neoplasm of the pancreas in an old man: age does not matter. Pan Afr Med J. 2012;13:8. [PMC free article] [PubMed] [Google Scholar]
- 9.Baek JH, Lee JM, Kim SH, et al. Small (<or=3 cm) solid pseudopapillary tumors of the pancreas at multiphasic multidetector CT. Radiology. 2010;257:97–106. doi: 10.1148/radiol.10092089. https://doi.org/10.1148/radiol.10092089. [DOI] [PubMed] [Google Scholar]
- 10.Liu X, Rauch TM, Siegal GP, Jhala N. Solid-pseudopapillary neoplasm of the pancreas: Three cases with a literature review. Appl Immunohistochem Mol Morphol. 2006;14:445–453. doi: 10.1097/01.pai.0000194763.86513.e4. https://doi.org/10.1097/01.pai.0000194763.86513.e4. [DOI] [PubMed] [Google Scholar]
- 11.Ganeshan DM, Paulson E, Tamm EP, Taggart MW, Balachandran A, Bhosale P. Solid pseudo-papillary tumors of the pancreas: current update. Abdom Imaging. 2013;38:1373–1382. doi: 10.1007/s00261-013-0015-7. https://doi.org/10.1007/s00261-013-0015-7. [DOI] [PubMed] [Google Scholar]
- 12.Santini D, Poli F, Lega S. Solid-papillary tumors of the pancreas: histopathology. JOP. 2006;7:131–136. [PubMed] [Google Scholar]
- 13.Yao X, Ji Y, Zeng M, Rao S, Yang B. Solid pseudopapillary tumor of the pancreas: cross-sectional imaging and pathologic correlation. Pancreas. 2010;39:486–491. doi: 10.1097/MPA.0b013e3181bd6839. https://doi.org/10.1097/MPA.0b013e3181bd6839. [DOI] [PubMed] [Google Scholar]
- 14.Zaher I. Chakhachiro and Ghazi Zaatari. Solid-pseudopapillary neoplasm: a pancreatic enigma. Arch Pathol Lab Med. 2009;133:1989–1993. doi: 10.5858/133.12.1989. [DOI] [PubMed] [Google Scholar]
- 15.Choi JY, Kim MJ, Kim JH, et al. Solid pseudopapillary tumor of the pancreas: typical and atypical manifestations. AJR Am J Roentgenol. 2006;187:W178–186. doi: 10.2214/AJR.05.0569. https://doi.org/10.2214/AJR.05.0569. [DOI] [PubMed] [Google Scholar]
- 16.Lack EE. Pathology of the pancreas, gallbladder, extrahepatic biliary tract, and ampullary region. New York: Oxford University Press; 2001. p. 281. [Google Scholar]
- 17.Nishihara K, Nagoshi M, Tsuneyoshi M, Yamaguchi K, Hayashi I. Papillary cystic tumors of the pancreas. Assessment of their malignant potential. Cancer. 1993;71:82–92. doi: 10.1002/1097-0142(19930101)71:1<82::aid-cncr2820710114>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 18.Klöppel G, Hruban R, Lüttges J, Kern S, Klimstra D, Adler G. Tumours of the Exocrine Pancreas. In: Bosman FT, Carnerio F, Hruban RH, Theise ND, editors. WHO classification of tumors of the digestive system. 4th ed. WHO press; 2010. pp. 246–248. [Google Scholar]
- 19.Sahani DV, Kalva SP, Fischman AJ, et al. Detection of liver metastases from adenocarcinoma of the colon and pancreas: comparison of mangafodipir trisodium-enhanced liver MRI and whole-body FDG PET. AJR Am J Roentgenol. 2005;185:239–46. doi: 10.2214/ajr.185.1.01850239. https://doi.org/10.2214/ajr.185.1.01850239. [DOI] [PubMed] [Google Scholar]
- 20.Thompson LD, Becker RC, Przygodzki RM, Adair CF, Heffess CS. Mucinous cystic neoplasm (mucinous cystadenocarcinoma of low-grade malignant potential) of the pancreas: a clinicopathologic study of 130 cases. Am J Surg Pathol. 1999;23:1–16. doi: 10.1097/00000478-199901000-00001. https://doi.org/10.1097/00000478-199901000-00001. [DOI] [PubMed] [Google Scholar]
- 21.Choi JY, Kim MJ, Lee JY, et al. Typical and atypical manifestations of serous cystadenoma of the pancreas: imaging findings with pathologic correlation. AJR Am J Roentgenol. 2009;193:136–142. doi: 10.2214/AJR.08.1309. https://doi.org/10.2214/AJR.08.1309. [DOI] [PubMed] [Google Scholar]