Abstract
Recent human and animal studies investigating the roles of the genome, epigenome, and environmental cues have identified associations between offspring predisposition to life-long obesity/metabolic disease and epigenetic modifications such as DNA methylation. This review explores the mechanisms by which maternal exposures impair the health of not only the next generation but also potentially future generations of offspring.
The World Health Organization estimates that more than 1.9 billion people are overweight or obese globally and that number has more than doubled since 1980 (WHO, http://www.who.int/mediacentre/factsheets/fs311/en/). In addition to deleterious health effects to themselves, obesity and metabolic diseases in reproductive-age women put offspring at future risk for significant metabolic disturbance. For example, the American College of Obstetricians and Gynecologists reports that fetuses born to obese women are at increased risk of developing metabolic syndrome and childhood obesity (ACOG) (61). Although genetic variation plays a role in the development of obesity and metabolic disease, genome-wide estimates indicate that only ∼20% of the variation in susceptibility to developing obesity and metabolic disorders is genetic in origin (48, 88), leaving a large portion of the heritability unexplained. Together, these findings support the Developmental Origins of Health and Disease Hypothesis (DOHaD) (2), which states that environmental exposures early in embryonic and fetal life exert important influence on future disease development. David Barker was the first to propose this hypothesis after studying the adult health of children of pregnant mothers exposed to extreme famine during the Nazi occupation of The Netherlands during of the Dutch Hunger Winter. The offspring of these women had significantly higher fat deposition (33, 83) and lower levels of methylation of the gene encoding insulin-like growth factor 2 (IGF-2) at 60 years of age compared with those who were not exposed to prenatal famine (87). Although this phenomenon was due to maternal nutrient restriction, several more recent studies suggest that any perturbation to maternal diet as well as harsh environmental exposures can affect the next generation via epigenetic modifications. This review will focus on understanding the mechanisms by which maternal obesity in the mother affects not only the next generation but also potentially future generations of offspring.
Epigenetic Modifications
Intergenerational and transgenerational inheritance occurs when an environmental exposure or nutritional status of the mother alters the pattern of gene expression in a manner that persists into the next generation. This long-term, stable regulation of gene expression without any change in the gene sequences is termed epigenetic modification. Although the definition of epigenetics is frequently updated, three core features are always maintained, namely 1) absence of any alterations in the DNA sequence, 2) heritability, and 3) plasticity and reversibility. There are three major types of epigenetic modifications: DNA methylation, histone modification, and non-coding RNA.
In DNA methylation, DNA methyl transferases covalently transfer a methyl moiety to the 5′ position on a cytosine ring in a CpG dinucleotide (20). DNA methylation in the promoter region of a gene can cause gene silencing by inhibiting transcription factor binding or providing sites for methylated-DNA binding factors that recruit chromatin-inactivation complexes. Conversely, methylation in the body of a gene can activate transcription, but this mechanism is not well understood. DNA methylation is heritable because the maintenance methyl transferase DNA methyltransferase 1 (DNMT1) recognizes hemi-methylated CpGs generated during DNA replication (29) and dependably copies cytosine methylation from the parental to the daughter strand. Thus the methylation state is maintained throughout the life course and beyond if this maintenance occurs in meiosis (28). The epigenetic information carried by the sperm and oocyte genomes is largely erased during preimplantation development and reset in a lineage-specific fashion as the zygote undergoes differentiation. Later, during fetal development, a second wave of epigenetic erasure and reestablishment occurs specifically in primordial germ cells (9, 59, 64). Evidence from animal models indicates that persistent DNA methylation changes can be detected in sperm and oocytes of future generations, indicating that DNA methylation is not completely erased in primordial germ cells and post-fertilization (44, 71).
The mechanism responsible for active DNA demethylation and erasure is the subject of intense study. Recent studies have demonstrated that 5mC can be sequentially oxidized to 5-hydroxymethylcytosine (5hmC) (36, 43), 5-formylcytosine (5fC) and 5-carboxymethylcytosine (5caC) (32, 37) by the Tet dioxygenases (Tet1-3). A member of this family of proteins, Tet3, is highly expressed in oocytes and zygotes but is downregulated in two-cell-stage embryos. This presumed conversion of 5mC to 5hmC is impaired on silencing (93) or knockout (31) of maternal Tet3, leading to a delayed activation of key pluripotency factors in the paternal genome and partial embryonic lethality. These data suggest that loss of 5mC is at least in part due to Tet3-mediated oxidation. The molecular mechanisms by which 5hmC is removed to yield unmodified cytosine are still equivocal.
Chromatin can also undergo posttranslational covalent modifications at the NH2-terminal tails of histones. These modifications include methylation (39), acetylation (90), sumoylation (73), ubiquitination (74), and phosphorylation (47). Histones package DNA into chromosomes and modulate transcriptional activation and inactivation. Histone acetylation involves enzymatic addition of an acetyl group (COCH3) from acetyl coenzyme A. The process of histone acetylation regulates many cellular processes such as chromatin dynamics and transcription, gene silencing, apoptosis, differentiation, cell cycle progression, DNA repair and replication, and nuclear import. The enzymes involved in histone acetylation are called histone acetyltransferases (HATs), and they play a critical role in modulating histone H3 and H4 acetylation. Greater than 20 HATs have been discovered and are classified into five families: GNAT1, MYST, TAFII250, P300/CBP, and nuclear receptor coactivators such as ACTR (27). Histone deacetylaces (HDACs) are responsible for the hydrolytic removal of acetyl groups from histone lysine residues. Similar to HATs, HDACs play a critical role in various cellular processes involving histone H3 and H4. So far, at least four classes of HDACs have been identified. Class III HDACs represent the sirtuins SIRTs 1-7 (which all play a key role in energy homeostasis), require NAD+ cofactors, and include HDAC inhibition affects apoptosis, cell cycle arrest, and differentiation in cancer cells.
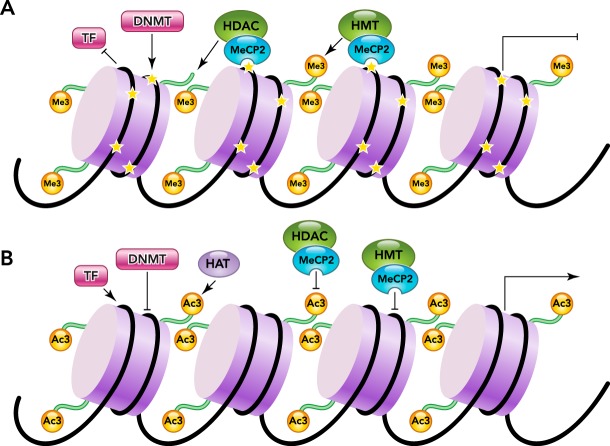
Another type of histone modification is methylation, which can be the transfer of one, two, or three methyl groups from S-adenosyl-l-methionine to lysine or arginine residues of histones by histone methyltransferases (HMTs) (FIGURE 1). In the cell nucleus, when histone methylation occurs, specific genes within the DNA are complexed with the histone, leading to activation or silencing (27). Several different histone methyltransferases exist, and they modify specific lysine or arginine residues. As one example, Ash1, ALL-1, MLL, ALR, Trx, SET1, SET7/9, and SMYD3 are HMTs that add methyl groups to histone H3 at lysine 4 (H3–K4) in mammalian cells, whereas SETDB1, Dim-5, ESET, G9a, SUV39-h1, SUV39-h2, and Eu-HMTase are HMTs that methylate histone H3 at lysine 9 (H3–K9) in mammalian cells, and polycomb group enzymes such as EZH2 and G9a catalyze methylation of histone H3 at lysine 27 (H3–K27) in mammalian cells (92). In contrast, arginine methylation of histones H3 and H4, which is mediated by a family of protein arginine methyltransferases (PRMTs), results in transcriptional activation. This family of PRMTs, consisting of two types, can catalyze single or dimethylation of arginine residues. Type II PRMTs are found to be strongly implicated in diseases like cancer (10).
FIGURE 1.
Interactions of DNA methylation and histone modifications
A: closed chromatin has CpG methylation (46) generated by DNA methyltransferase (DNMT) and histone methylation at H3K9, H4K20, and H2A/H4R3, with recruitment of histone deacetylase (HDAC) and methyltransferase (HMT) by methylated CpG by means of MeCP2. Transcription factors (TFs) are inhibited from binding, and gene expression is silenced. B: open, unmethylated chromatin does not recruit HDAC and HMT, and H4K3 methylation blocks DNMT binding. TFs bind unmethylated chromatin, recruiting histone acetyltransferase (HAT), which promotes transcriptional activation.
Histone demethylation occurs via two major families of demethylases: lysine-specific demethylase 1 (LSD1) and Jumonji domain containing histone demethylases (JMJD2, JMJD3/UTX and JARIDs). These enzymes act on specific amino acid residues and single as well as multimethylated sites. As an example, LSD1 demethylates histone H3 at either the mono- or dimethylated lysine 4, whereas JARID acts on trimethylated lysine 4. JMJD3 and UTX demethylate H3 at the di- and trimethylated lysine 27, whereas JMJD1 demethylated mono- and dimethylated lysine 9 and JMJD2 catalyzed the demethylation of trimethylated lysine 9 (67). Inhibition of histone demethylases may lead to histone remethylation at specific residues important for chromatin dynamics and gene expression. Although no biochemical mechanisms have determined how chromatin modifications may be transferred intergeneratinally, transmission of sperm-specific chromatin modifications through several rounds of replication has been reported in C. elegans (25). Whether this occurs in mammals is largely unknown.
There is solid data to suggest that noncoding RNAs play a role in transgenerational silencing (22). There are several classes of small noncoding RNA (sncRNA), including miRNAs (hairpin RNAs with imperfect complementarity to multiple targets), siRNAs (with perfect complementarity to targets) (17) and PIWI-interacting RNAs (piRNAs) (4, 57) [which specialize in targeting transposon transcription in germ cells and are of particular interest for gamete-mediated transgenerational epigenetic inheritance (3)]. In addition, given the unique sequence identity of sncRNAs, they play a key nuclear epigenetic role by tagging specific loci for either histone (19, 52, 83) or DNA methylation. The fact that sncRNA can be released from one cell and be systematically targeted (54, 62, 68) creates an opportunity for delivering signals from a tissue that senses exposure to germ cells. For example, the siRNA effector molecule the Argonaut 1 (Ago1) protein interacts with the H3K9 methyltransferase Clr4, recruiting it to the locus, resulting in silencing through histone H3K9 methylation (19, 52, 83).
Epigenetic Inheritance
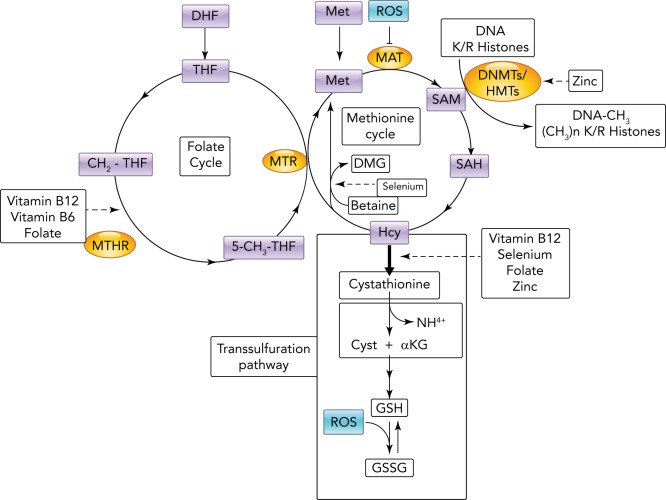
Numerous studies support the potentially widespread influence of epigenetic modifications on intergenerational inheritance. For example, oxidative stress during pregnancy may establish a specific epigenetic pattern that contributes to the early origins of adult vascular disease (33), and evidence suggests that reactive oxygen species (ROS) in the mother can generate epigenetic changes in the fetus (34). ROS contain one or more unpaired electrons that are reactive and modify lipids, proteins, or nucleic acids. Excess ROS during fetal development can modify the epigenome via deamination of nitrogenous bases leading to a loss of methylation sites in the DNA (epimutations). In addition, S-adenosylmethionine (SAM) is the main substrate for DNMTs and histone methyltransferases (HMT); however, SAM is also used to make glutathione, a ROS scavenger, and thus ROS can also induce global epigenetic changes by triggering the synthesis of glutathione. This activation of the transsulfuration pathway in turn competes directly with the DNMTs and HMTs for methyl donors (84) (FIGURE 2).
FIGURE 2.
Metabolic influence of oxidative stress on DNA and histone methylation
To maintain the correct amount of S-adenosylmethionine (SAM), the main substrate used by DNA methyltransferases (DNMTs) and histone methyltransferases (HMTs), homocysteine (Hcy) produced by methylation reactions must be recycled for continuous synthesis of SAM. This occurs through interplay between the folate and methionine cycles. An excess of reactive oxygen species (ROS) can inhibit methyl adenosyltransferase and increase demand for reduced glutathione produced in the transsulfuration pathway that competes for homocysteine, limiting Hcy entry into the methionine cycle. Note that several reactions implicated directly and indirectly in the synthesis of SAM require micronutrients as enzyme cofactors (arrow dashed lines). 5-CH3THF, methyl-tetrahydrofolate; aKG, alfa-ketogluta-rate; CH2THF, methylene-tetrahydrofolate; Cys, cysteine; DHF, dihydrofolate; DMG, di-methylglyoxime; DNMTs, DNA methyltrans-ferase; GSSG, glutathione oxidized; MAT, S-methionine adenosyltransferase; Met, methionine; MTHR, methylene-tetrahydrofolate reductase; MTR, 5-methyltetrahydrofolate-homocysteine methyltransferase; SAH, S-adenosylhomo-cysteine; THF, tetrahydrofolate.
Studies of human birth cohorts have revealed associations between maternal exposures and epigenetic modifications in the offspring (26, 86). One study demonstrated that children whose mothers took folic acid had higher methylation in the differentially methylated region (DMR) of the IGF2 gene than those whose mothers did not take folic acid (35). This work also revealed an inverse association between IGF2 methylation and birth weight, suggesting that periconceptional folic acid consumption affects the intra-uterine programming of growth and development of the child, with consequences for health and disease throughout life (35). These studies suggest that changes in maternal diet before conception and throughout the gestational period in human development can affect the predisposition of the offspring to develop diabetes, obesity, and metabolic syndrome in adult life.
Obesity and Offspring Epigenetic Changes
Several lines of evidence in humans and model organisms support the notion that early life exposures to obesity, defined as before conception as a gamete, and after conception and thoughout gestation as a fetus can alter the nuclear epigenome of the exposed offspring (F1) and promote metabolic disease. This phenomenon, also known as developmental programing of a fetus, occurs when the expected developmental pattern is disturbed by inappropriately timed signals that redirect the fetus on an altered developmental trajectory, leading to disease in adult life (48). Several studies have shown that maternal obesity induced by a high-fat/high-sucrose diet can program offspring for cardiovascular disease, obesity, metabolic syndrome, diabetes, and disorders of the hypothalamic/pituitary/adrenal axis (2, 24, 66, 97). A few studies have linked this programing to distinct epigenetic alterations, as highlighted next.
Nathanielsz et al. demonstrated that maternal obesity via a high-fat, high-energy diet results in significant disturbance of the fetal methionine cycle in baboons (56). Since the methionine cycle provides methyl groups to several metabolic processes, this is one mechanism by which an abnormal nutritional environment may contribute to transgenerational inheritance of metabolic disease. In another study, Desai et al. exposed pregnant and lactating rats to a high-fat diet. Despite consuming regular chow after weaning, the resulting offspring were significantly heavier than controls as adults. On day 1 of life, the pups had significantly decreased expression of the energy sensors Hes1 and Mash1, increased expression of appetite/satiety neuropeptides in the hypothalamus, and decreased expression of DNMT1. At 6 mo of age, adult males from the overfed mothers had significantly increased expression of energy sensors and decreased expression of histone deacetylases. These results suggest that epigenetic changes in the pups altered gene expression and affected adult neuronal differentiation, leading to overeating and obesity (18).
Transgenerational Inheritance
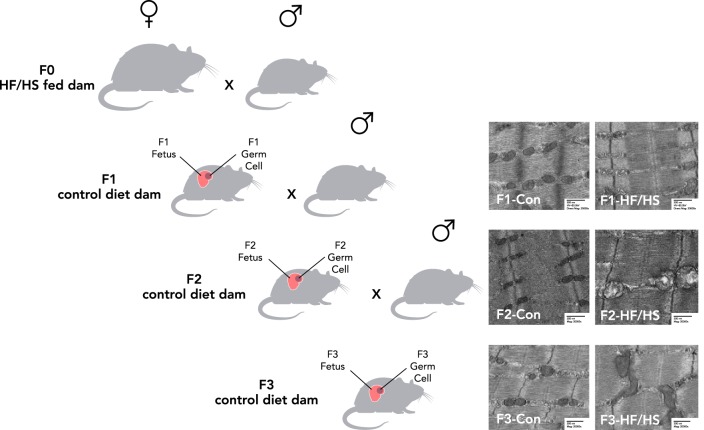
The majority of studies investigating DOHaD have focused on intergenerational transmission of risk of metabolic dysfunction. In this case, risk is directly transmitted to the offspring through metabolic programing of the fetus (86) while in utero. In such studies in sheep (91), pigs (8), macaques (82), mice (40), rats (6), and fruitflies (58), the mothers were under- or overfed during pregnancy, and effects on the next generation were observed (Table 1). However, increasing evidence indicates that metabolic risk also can be transmitted transgenerationally. This type of inheritance occurs when phenotypes acquired from an initial exposure (in the F0) are transmitted to the next generations (F1, F2, and F3) in the absence of any continued exposure (76, 77). Although evidence supports transmission of disease risk through both the maternal (60) and paternal germlines (21, 81), recent data suggest a stronger association between maternal metabolic characteristics and fetal outcomes (55). To be considered transgenerational after maternal exposure, the inherited traits must be apparent in the F3 generation because the F1 embryo and F2 primordial germ cells are directly exposed to a given environmental factor in utero. In such cases, a change must occur in the germline to allow the effect to persist through three generations (FIGURE 3).
Table 1.
Examples of maternal exposures leading to obesity in the offspring and epigenetic ramifications
| Maternal Exposure | Type of Transmission | Fetal Manifestation | Type of Animal | Reference |
|---|---|---|---|---|
| High-fat/high-energy diet | Intergeneration | ↓ Methionine cycles in fetal serum infers epigenetics involved | Baboon | 56 |
| High-fat diet | Intergenerational | ↓ DMNT1/SIRT1/HDAC | Rat | 18 |
| ↑ Satiety/appetite peptide AgRP/POMC leads to hyperphagia/obeseyy | ||||
| PAHs | Intergenerational | ↓ Methylation of PPARα leading to ↑ PPARα, C/EBP, Cox2, FAS expression leads to fetal obesity and body fat | Mice | 96 |
| Famine or caloric deprivation | Intergenerational | ↓ IGF2 and INS methylation leading to increased expression of obesity and HTN in adulthood | Human | 33, 83, 87 |
| Folic acid | Intergenerational | ↓???↑ H19 methylation in offspring of mothers not taking FA; normal in FA-taking mothers | Human | 35 |
| High-fat/high-sucrose diet | Transgenerational | Abnormal mitochondrial function in F0 oocytes, F1, F2 and F3 tissues; decreased mtDNA complex proteins I–IV in F1–F2 leads to metabolic dysfunction | Mouse | 69 |
FIGURE 3.
Transgenerational transmission
To be considered transgenerational transmission after a maternal exposure such as a high-fat/high-sugar (HF/HS) diet in this example, the inherited traits must be apparent in the F3 generation since the F1 embryo and F2 primordial germ cells are directly exposed to a given environmental factor in utero. In such cases, a change must occur in the germline to allow the effect to persist through three generations. As an example, EM images are shown of skeletal muscle of three generations of mice following the F0 mother's exposure to a HF/HS diet (69).
Endocrine-Disrupting Chemicals, Epigenetics, and Obesity in Offspring
Although the major contributing factors to obesity are high caloric intake, a sedentary lifestyle, and genetic predisposition, recent studies strongly suggest a role for exposure to environmental obesogens, such as endocrine-disrupting chemicals (EDCs) (30). EDCs, which are found in plastics, personal care products, household products, and our diets, alter hormonal pathways that regulate lipid metabolism and stimulate adipocyte differentiation (38, 45). Studies in animals are revealing that exposure to EDCs is associated with epigenetic modifications and obesogenic outcomes. In one study, pregnant dams were exposed to an air-borne mixture of polyaromatic hydrocarbons (PAH) at concentrations equivalent to PAHs measured in pregnant women (96). Gestational PAH exposure was associated with development of adiposity in the F1 and F2 generation, with long-lasting effects on body weight, fat mass, and adipocyte size. These effects were found to be due to increased expression of the genes encoding peroxisome proliferation-activated receptor gamma (PPARγ; a regulator of fatty acid storage and glucose metabolism) and other adipogenic genes, and decreased DNA methylation in the promoter of PPARγ (96).
In other work, maternal exposure to the organochlorine pesticides DDT and methoxychlor during pregnancy led to normal F1 pups but obesity in the F3 generation, suggesting a transgenerational effect (76). This transmission was confirmed with genome-wide DNA methylation analysis, which revealed differentially methylated regions of genes that are associated with obesogenesis (50, 51). Subsequent work revealed that gestational exposure to several other EDCs, such as bisphenol A and the phthalate plasticizers diethyl hexyl phthalate and dibutyl phthalate, all resulted in a similar F3 obese phenotype (78). This phenotype correlated with differential methylation in genes involved in obesity-related metabolic pathways, although each EDC or mixture resulted in a unique pattern of altered methylation in the F3 generation.
A Role for Mitochondria
Despite reports of both maternal and paternal intergenerational inheritance, recent studies suggest a stronger relationship between maternal obesity and metabolic disorders in offspring. This may be explained, in part, by the fact that mitochondria are maternally inherited. Mitochondria perform vital cellular tasks such as generation of adenosine triphosphate via the electron transport chain, and they are essential for regulation of metabolism, apoptosis, cell signaling, and numerous biochemical pathways. Although the exact mechanisms are unclear (53), mitochondrial dysfunction is associated with metabolic disorders such as obesity, insulin resistance, and diabetes (14, 15, 42). Thus it is reasonable to hypothesize that exposure to an abnormal maternal metabolic environment causes formation of dysfunctional mitochondria, which are then inherited by the offspring.
Our laboratory demonstrated in a mouse model that high-fat/high-sucrose diet-induced metabolic syndrome alters oocyte mitochondrial morphology and impairs oocyte mitochondrial function (7, 49, 65). Importantly, offspring born to obese mothers developed characteristics of metabolic syndrome (41). These data suggest that alterations to oocyte mitochondrial morphology and function may affect offspring health. In a recent study, we fed F0 dams a high-fat/high-sucrose diet for 8 wk and then mated them to males that had been fed a control chow diet. All offspring were also fed a control chow diet. We used transmission electron microscopy to examine mitochondria in muscle cells of the F1 pups and found them to be morphologically abnormal; they were larger, swollen, and contained disarrayed cristae. We also found that oxygen consumption was significantly decreased in skeletal muscle mitochondria and that expression of electron transport chain proteins was decreased. These impaired mitochondria were transmitted to the skeletal muscle and germ cells of both the F2 and F3 generation, thus demonstrating transgenerational inheritance. These data suggest that impaired mitochondria of oocyte origin were capable of influencing mitochondrial dysfunction throughout the entire organism for three generations (69). One possible explanation for this transgenerational inheritance of dysfunctional mitochondria is epigenetic modifications to nuclear DNA encoding mitochondrial proteins. Over 90% of the 4,900 mitochondrial proteins are encoded by nuclear DNA. Heritable silencing of some of these genes could lead to the phenotype we have identified.
Alternatively, this transgenerational inheritance could reflect alterations to the mitochondrial DNA (mtDNA). In humans, mtDNA is ∼16,500 base pairs and encodes 37 genes, all of which are essential for normal mitochondrial function, and 13 of which encode enzymes involved in oxidative phosphorylation and the electron transport chain. Because it does not contain histones, it was long thought that mtDNA could not be epigenetically modified. However, this dogma was first challenged with the identification and characterization of DNMT1 in mitochondria from mouse embryonic fibroblasts and colon cancer cells (75). Soon after, DNMT3a was identified in mitochondrial fractions in mouse and human central nervous system tissue (11). Since those initial discoveries, both 5-methylcytosine and 5-hydroxymethylcytosine modifications have been detected in mtDNA by bisulfite genomic sequencing, leading to the speculation that epigenetic modification of mtDNA is prevalent.
Although it might be attractive to consider that transmission of abnormal mitochondria may be the main cause of this metabolic dysfunction in offspring, several studies have also found other cellular difference in the germ cells of obese mothers, such as increased RNA (95) and endoplasmic reticulum stress (94). In recent work from our laboratory, we determined that anti-oxidants can partially reverse the effects of the high-fat diet on mitochondrial function by rescuing mitochondrial electron transport dysfunction; however, these effects only partially rescue the full oocyte phenotype. This suggests that the mitochondrial dysfunction may be only one cause of the transgenerational transmission (5). More high-quality studies are needed to determine which developmental effects on the oocyte lead to this pattern of transmission.
A Potential Role for Extracellular Vesicles in Transgenerational Transmission
Several new studies suggest that somatic cells can communicate their exposures via contents in extracellular vesicles (EVs) or exosomes (85). EVs are extremely small (0.04- to 1-nm diameter) lipid-enclosed vesicles that have been identified in most cell types and detected in all biological fluids including blood, urine, breast milk, semen, and amniotic fluid (63). EV cargo consists of lipid, protein, and nucleic acids. The contents are thought to be selectively packaged, and some EVs contain proteins, functional RNAs, and RNAs enriched for specific microRNAs (miRNAs), all of which can function in posttranscriptional gene silencing. This cargo is deposited by fusion with or endocytosis into the target cell (12), providing a way for somatic cells to directly trigger cellular response in other near or distant cells, possibly including germ cells. Studies have shown that the content of EVs is altered in response to environmental conditions, and, in this fashion, EVs may be capable of transmitting information in the form of miRNAs to the germline (13, 72, 79). A recent study in humans revealed that EVs secreted by adipocytes from obese individuals contain differentially expressed miRNAs that targeted mRNAs involved in obesogenesis (23). This novel and exciting method of intracellular communication may facilitate epigenetic transgenerational transmission of information about maternal or paternal dietary or metabolic state. This type of EV transfer from somatic cells to germ cells has been suggested by many but demonstrated by only a few studies reporting delivery of miRNAs to sperm and oocytes (1, 16, 70, 80, 89). Definitive studies testing this hypothesis have yet to be performed.
Conclusions
Recent research in DOHaD has yielded exciting results and elucidated new research avenues, but we still have much to learn about the complex interactions between the genome, epigenome, and environmental cues. It is exceedingly difficult to isolate the contribution of each of these factors and to track that contribution across generations, especially in humans. Nonetheless, both animal studies and human birth cohort studies investigating pre- and periconceptional gestational exposures have identified associations between offspring predisposition to life-long obesity and metabolic disease and epigenetic modifications such as DNA methylation. Research should now be focused on confirming the mechanisms of transgenerational effects and identifying targets that could be modified to prevent further transmission of metabolic dysfunction to future generation.
ACKNOWLEDGMENTS
This work was supported by National Institute of Child Health and Human Development Grant R01 HD-083895 to K.H.M.
Footnotes
No conflicts of interest, financial or otherwise, are declared by the author(s).
Author contributions: K.J., J.S., and K.H.M. drafted manuscript; K.H.M. conception and design of research; K.H.M. edited and revised manuscript; K.H.M. approved final version of manuscript; K.H.M. prepared figures.
References
- 1.Al-Dossary AA, Strehler EE, Martin-Deleon PA. Expression and secretion of plasma membrane Ca2+-ATPase 4a (PMCA4a) during murine estrus: association with oviductal exosomes and uptake in sperm. PLos One 8: e80181, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barker DJ. The origins of the developmental origins theory. J Intern Med 261: 412–417, 2007. [DOI] [PubMed] [Google Scholar]
- 3.Bateson P, Gluckman P. Plasticity and robustness in development and evolution. Int J Epidemiol 41: 219–223, 2012. [DOI] [PubMed] [Google Scholar]
- 4.Benton MC, Johnstone A, Eccles D, Harmon B, Hayes MT, Lea RA, Griffiths L, Hoffman EP, Stubbs RS, Macartney-Coxson D. An analysis of DNA methylation in human adipose tissue reveals differential modification of obesity genes before and after gastric bypass and weight loss. Genome Biol 16: 8, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boots CE, Boudoures A, Zhang W, Drury A, Moley KH. Obesity-induced oocyte mitochondrial defects are partially prevented and rescued by supplementation with co-enzyme Q10 in a mouse model. Hum Reprod 31: 2090–2097, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Borengasser SJ, Zhong Y, Kang P, Lindsey F, Ronis MJ, Badger TM, Gomez-Acevedo H, Shankar K. Maternal obesity enhances white adipose tissue differentiation and alters genome-scale DNA methylation in male rat offspring. Endocrinology 154: 4113–4125, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Boudoures AL, Chi M, Thompson A, Zhang W, Moley KH. The effects of voluntary exercise on oocyte quality in a diet-induced obese murine model. Reproduction 151: 261–270, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Braunschweig M, Jagannathan V, Gutzwiller A, Bee G. Investigations on transgenerational epigenetic response down the male line in F2 pigs. PLos One 7: e30583, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cantone I, Fisher AG. Epigenetic programming and reprogramming during development. Nat Struct Mol Biol 20: 282–289, 2013. [DOI] [PubMed] [Google Scholar]
- 10.Chen D, Ma H, Hong H, Koh SS, Huang SM, Schurter BT, Aswad DW, Stallcup MR. Regulation of transcription by a protein methyltransferase. Science 284: 2174–2177, 1999. [DOI] [PubMed] [Google Scholar]
- 11.Chestnut BA, Chang Q, Price A, Lesuisse C, Wong M, Martin LJ. Epigenetic regulation of motor neuron cell death through DNA methylation. J Neurosci 31: 16619–16636, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cocucci E, Meldolesi J. Ectosomes and exosomes: shedding the confusion between extracellular vesicles. Trends Cell Biol 25: 364–372, 2015. [DOI] [PubMed] [Google Scholar]
- 13.Cossetti C, Lugini L, Astrologo L, Saggio I, Fais S, Spadafora C. Soma-to-germline transmission of RNA in mice xenografted with human tumour cells: possible transport by exosomes. PLos One 9: e101629, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Crescenzo R, Bianco F, Mazzoli A, Giacco A, Liverini G, Iossa S. Mitochondrial efficiency and insulin resistance. Front Physiol 5: 512, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Crescenzo R, Bianco F, Mazzoli A, Giacco A, Liverini G, Iossa S. A possible link between hepatic mitochondrial dysfunction and diet-induced insulin resistance. Eur J Nutr 55: 1–6, 2016. [DOI] [PubMed] [Google Scholar]
- 16.da Silveira JC, Veeramachaneni DN, Winger QA, Carnevale EM, Bouma GJ. Cell-secreted vesicles in equine ovarian follicular fluid contain miRNAs and proteins: a possible new form of cell communication within the ovarian follicle. Biol Reprod 86: 71, 2012. [DOI] [PubMed] [Google Scholar]
- 17.Dayeh T, Volkov P, Salo S, Hall E, Nilsson E, Olsson AH, Kirkpatrick CL, Wollheim CB, Eliasson L, Ronn T, Bacos K, Ling C. Genome-wide DNA methylation analysis of human pancreatic islets from type 2 diabetic and non-diabetic donors identifies candidate genes that influence insulin secretion. PLoS Genet 10: e1004160, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Desai M, Han G, Ross MG. Programmed hyperphagia in offspring of obese dams: altered expression of hypothalamic nutrient sensors, neurogenic factors and epigenetic modulators. Appetite 99: 193–199, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dominguez-Salas P, Moore SE, Baker MS, Bergen AW, Cox SE, Dyer RA, Fulford AJ, Guan Y, Laritsky E, Silver MJ, Swan GE, Zeisel SH, Innis SM, Waterland RA, Prentice AM, Hennig BJ. Maternal nutrition at conception modulates DNA methylation of human metastable epialleles. Nat Commun 5: 3746, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Drahovsky D, Morris NR. Mechanism of action of rat liver DNA methylase. I. Interaction with double-stranded methyl-acceptor DNA. J Mol Biol 57: 475–489, 1971. [DOI] [PubMed] [Google Scholar]
- 21.Dunn GA, Bale TL. Maternal high-fat diet effects on third-generation female body size via the paternal lineage. Endocrinology 152: 2228–2236, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Feinberg AP, Irizarry RA. Evolution in health and medicine Sackler colloquium: stochastic epigenetic variation as a driving force of development, evolutionary adaptation, and disease. Proc Natl Acad Sci USA 107, Suppl 1: 1757–1764, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ferrante SC, Nadler EP, Pillai DK, Hubal MJ, Wang Z, Wang JM, Gordish-Dressman H, Koeck E, Sevilla S, Wiles AA, Freishtat RJ. Adipocyte-derived exosomal miRNAs: a novel mechanism for obesity-related disease. Pediatr Res 77: 447–454, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Frias AE, Morgan TK, Evans AE, Rasanen J, Oh KY, Thornburg KL, Grove KL. Maternal high-fat diet disturbs uteroplacental hemodynamics and increases the frequency of stillbirth in a nonhuman primate model of excess nutrition. Endocrinology 152: 2456–2464, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gaydos LJ, Wang W, Strome Gene repression S. H3K27me and PRC2 transmit a memory of repression across generations and during development. Science 345: 1515–1518, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Grandjean P, Barouki R, Bellinger DC, Casteleyn L, Chadwick LH, Cordier S, Etzel RA, Gray KA, Ha EH, Junien C, Karagas M, Kawamoto T, Paige Lawrence B, Perera FP, Prins GS, Puga A, Rosenfeld CS, Sherr DH, Sly PD, Suk W, Sun Q, Toppari J, van den Hazel P, Walker CL, Heindel JJ. Life-long implications of developmental exposure to environmental stressors: new perspectives. Endocrinology 156: 3408–3415, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Greer EL, Shi Y. Histone methylation: a dynamic mark in health, disease and inheritance. Nat Rev Gen 13: 343–357, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gruenbaum Y, Cedar H, Razin A. Substrate and sequence specificity of a eukaryotic DNA methylase. Nature 295: 620–622, 1982. [DOI] [PubMed] [Google Scholar]
- 29.Gruenbaum Y, Stein R, Cedar H, Razin A. Methylation of CpG sequences in eukaryotic DNA. FEBS Lett 124: 67–71, 1981. [DOI] [PubMed] [Google Scholar]
- 30.Grun F, Blumberg B. Environmental obesogens: organotins and endocrine disruption via nuclear receptor signaling. Endocrinology 147: S50–55, 2006. [DOI] [PubMed] [Google Scholar]
- 31.Gu TP, Guo F, Yang H, Wu HP, Xu GF, Liu W, Xie ZG, Shi L, He X, Jin SG, Iqbal K, Shi YG, Deng Z, Szabo PE, Pfeifer GP, Li J, Xu GL. The role of Tet3 DNA dioxygenase in epigenetic reprogramming by oocytes. Nature 477: 606–610, 2011. [DOI] [PubMed] [Google Scholar]
- 32.He YF, Li BZ, Li Z, Liu P, Wang Y, Tang Q, Ding J, Jia Y, Chen Z, Li L, Sun Y, Li X, Dai Q, Song CX, Zhang K, He C, Xu GL. Tet-mediated formation of 5-carboxylcytosine and its excision by TDG in mammalian DNA. Science 333: 1303–1307, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Heijmans BT, Tobi EW, Stein AD, Putter H, Blauw GJ, Susser ES, Slagboom PE, Lumey LH. Persistent epigenetic differences associated with prenatal exposure to famine in humans. Proc Natl Acad Sci USA 105: 17046–17049, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hitchler MJ, Domann FE. An epigenetic perspective on the free radical theory of development. Free Radic Biol Med 43: 1023–1036, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hoyo C, Murtha AP, Schildkraut JM, Jirtle RL, Demark-Wahnefried W, Forman MR, Iversen ES, Kurtzberg J, Overcash F, Huang Z, Murphy SK. Methylation variation at IGF2 differentially methylated regions and maternal folic acid use before and during pregnancy. Epigenetics 6: 928–936, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ito S, D'Alessio AC, Taranova OV, Hong K, Sowers LC, Zhang Y. Role of Tet proteins in 5mC to 5hmC conversion, ES-cell self-renewal and inner cell mass specification. Nature 466: 1129–1133, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ito S, Shen L, Dai Q, Wu SC, Collins LB, Swenberg JA, He C, Zhang Y. Tet proteins can convert 5-methylcytosine to 5-formylcytosine and 5-carboxylcytosine. Science 333: 1300–1303, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Janesick A, Blumberg B. Minireview: PPARgamma as the target of obesogens. J Steroid Biochem Mol Biol 127: 4–8, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jenuwein T. Re-SET-ting heterochromatin by histone methyltransferases. Trends Cell Biol 11: 266–273, 2001. [DOI] [PubMed] [Google Scholar]
- 40.Jousse C, Parry L, Lambert-Langlais S, Maurin AC, Averous J, Bruhat A, Carraro V, Tost J, Letteron P, Chen P, Jockers R, Launay JM, Mallet J, Fafournoux P. Perinatal undernutrition affects the methylation and expression of the leptin gene in adults: implication for the understanding of metabolic syndrome. FASEB J 25: 3271–3278, 2011. [DOI] [PubMed] [Google Scholar]
- 41.Jungheim ES, Moley KH. Current knowledge of obesity's effects in the pre- and periconceptional periods and avenues for future research. Am J Obstet Gynecol 203: 525–530, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kelley DE, He J, Menshikova EV, Ritov VB. Dysfunction of mitochondria in human skeletal muscle in Type 2 diabetes. Diabetes 51: 2944–2950, 2002. [DOI] [PubMed] [Google Scholar]
- 43.Kriaucionis S, Heintz N. The nuclear DNA base 5-hydroxymethylcytosine is present in Purkinje neurons and the brain. Science 324: 929–930, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lane N, Dean W, Erhardt S, Hajkova P, Surani A, Walter J, Reik W. Resistance of IAPs to methylation reprogramming may provide a mechanism for epigenetic inheritance in the mouse. Genesis 35: 88–93, 2003. [DOI] [PubMed] [Google Scholar]
- 45.Legler J. An integrated approach to assess the role of chemical exposure in obesity. Obesity (Silver Spring) 21: 1084–1085, 2013. [DOI] [PubMed] [Google Scholar]
- 46.Leung W, Shaffer CD, Reed LK, Smith ST, Barshop W, Dirkes W, Dothager M, Lee P, Wong J, Xiong D, Yuan H, Bedard JE, Machone JF, Patterson SD, Price AL, Turner BA, Robic S, Luippold EK, McCartha SR, Walji TA, Walker CA, Saville K, Abrams MK, Armstrong AR, Armstrong W, Bailey RJ, Barberi CR, Beck LR, Blaker AL, Blunden CE, Brand JP, Brock EJ, Brooks DW, Brown M, Butzler SC, Clark EM, Clark NB, Collins AA, Cotteleer RJ, Cullimore PR, Dawson SG, Docking CT, Dorsett SL, Dougherty GA, Downey KA, Drake AP, Earl EK, Floyd TG, Forsyth JD, Foust JD, Franchi SL, Geary JF, Hanson CK, Harding TS, Harris CB, Heckman JM, Holderness HL, Howey NA, Jacobs DA, Jewell ES, Kaisler M, Karaska EA, Kehoe JL, Koaches HC, Koehler J, Koenig D, Kujawski AJ, Kus JE, Lammers JA, Leads RR, Leatherman EC, Lippert RN, Messenger GS, Morrow AT, Newcomb V, Plasman HJ, Potocny SJ, Powers MK, Reem RM, Rennhack JP, Reynolds KR, Reynolds LA, Rhee DK, Rivard AB, Ronk AJ, Rooney MB, Rubin LS, Salbert LR, Saluja RK, Schauder T, Schneiter AR, Schulz RW, Smith KE, Spencer S, Swanson BR, Tache MA, Tewilliager AA, Tilot AK, VanEck E, Villerot MM, et al. Drosophila muller f elements maintain a distinct set of genomic properties over 40 million years of evolution. G3 (Bethesda) 5: 719–740, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lin S, Yuan ZF, Han Y, Marchione DM, Garcia BA. Preferential phosphorylation on old histones during early mitosis in human cells. J Biol Chem 291: 15342–15357, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Locke AE, Kahali B, Berndt SI, Justice AE, Pers TH, Day FR, Powell C, Vedantam S, Buchkovich ML, Yang J, Croteau-Chonka DC, Esko T, Fall T, Ferreira T, Gustafsson S, Kutalik Z, Luan J, Magi R, Randall JC, Winkler TW, Wood AR, Workalemahu T, Faul JD, Smith JA, Hua Zhao J, Zhao W, Chen J, Fehrmann R, Hedman AK, Karjalainen J, Schmidt EM, Absher D, Amin N, Anderson D, Beekman M, Bolton JL, Bragg-Gresham JL, Buyske S, Demirkan A, Deng G, Ehret GB, Feenstra B, Feitosa MF, Fischer K, Goel A, Gong J, Jackson AU, Kanoni S, Kleber ME, Kristiansson K, Lim U, Lotay V, Mangino M, Mateo Leach I, Medina-Gomez C, Medland SE, Nalls MA, Palmer CD, Pasko D, Pechlivanis S, Peters MJ, Prokopenko I, Shungin D, Stancakova A, Strawbridge RJ, Ju Sung Y, Tanaka T, Teumer A, Trompet S, van der Laan SW, van Setten J, Van Vliet-Ostaptchouk JV, Wang Z, Yengo L, Zhang W, Isaacs A, Albrecht E, Arnlov J, Arscott GM, Attwood AP, Bandinelli S, Barrett A, Bas IN, Bellis C, Bennett AJ, Berne C, Blagieva R, Bluher M, Bohringer S, Bonnycastle LL, Bottcher Y, Boyd HA, Bruinenberg M, Caspersen IH, Ida Chen YD, Clarke R, Daw EW, de Craen AJ, Delgado G, Dimitriou M, et al. Genetic studies of body mass index yield new insights for obesity biology. Nature 518: 197–206, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Luzzo KM, Wang Q, Purcell SH, Chi M, Jimenez PT, Grindler N, Schedl T, Moley KH. High fat diet induced developmental defects in the mouse: oocyte meiotic aneuploidy and fetal growth retardation/brain defects. PLos One 7: e49217, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Manikkam M, Haque MM, Guerrero-Bosagna C, Nilsson EE, Skinner MK. Pesticide methoxychlor promotes the epigenetic transgenerational inheritance of adult-onset disease through the female germline. PLos One 9: e102091, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Manikkam M, Tracey R, Guerrero-Bosagna C, Skinner MK. Plastics derived endocrine disruptors (BPA, DEHP and DBP) induce epigenetic transgenerational inheritance of obesity, reproductive disease and sperm epimutations. PLos One 8: e55387, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Martino D, Loke YJ, Gordon L, Ollikainen M, Cruickshank MN, Saffery R, Craig JM. Longitudinal, genome-scale analysis of DNA methylation in twins from birth to 18 months of age reveals rapid epigenetic change in early life and pair-specific effects of discordance. Genome Biol 14: R42, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Montgomery MK, Turner N. Mitochondrial dysfunction and insulin resistance: an update. Endocr Connect 4: R1–R15, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Morales E, Groom A, Lawlor DA, Relton CL. DNA methylation signatures in cord blood associated with maternal gestational weight gain: results from the ALSPAC cohort. BMC Res Notes 7: 278, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Murrin CM, Kelly GE, Tremblay RE, Kelleher CC. Body mass index and height over three generations: evidence from the Lifeways cross-generational cohort study. BMC Public Health 12: 81, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Nathanielsz PW, Yan J, Green R, Nijland M, Miller JW, Wu G, McDonald TJ, Caudill MA. Maternal obesity disrupts the methionine cycle in baboon pregnancy. Physiol Rep 3: e12564, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Nilsson E, Jansson PA, Perfilyev A, Volkov P, Pedersen M, Svensson MK, Poulsen P, Ribel-Madsen R, Pedersen NL, Almgren P, Fadista J, Ronn T, Klarlund Pedersen B, Scheele C, Vaag A, Ling C. Altered DNA methylation and differential expression of genes influencing metabolism and inflammation in adipose tissue from subjects with Type 2 diabetes. Diabetes 63: 2962–2976, 2014. [DOI] [PubMed] [Google Scholar]
- 58.Ost A, Lempradl A, Casas E, Weigert M, Tiko T, Deniz M, Pantano L, Boenisch U, Itskov PM, Stoeckius M, Ruf M, Rajewsky N, Reuter G, Iovino N, Ribeiro C, Alenius M, Heyne S, Vavouri T, Pospisilik JA. Paternal diet defines offspring chromatin state and intergenerational obesity. Cell 159: 1352–1364, 2014. [DOI] [PubMed] [Google Scholar]
- 59.Oswald J, Engemann S, Lane N, Mayer W, Olek A, Fundele R, Dean W, Reik W, Walter J. Active demethylation of the paternal genome in the mouse zygote. Curr Biol 10: 475–478, 2000. [DOI] [PubMed] [Google Scholar]
- 60.Ponzio BF, Carvalho MH, Fortes ZB, do Carmo Franco M. Implications of maternal nutrient restriction in transgenerational programming of hypertension and endothelial dysfunction across F1–F3 offspring. Life Sci 90: 571–577, 2012. [DOI] [PubMed] [Google Scholar]
- 61.Practice Committee of the American Society for Reproductive Medicine. Obesity and reproduction: a committee opinion. Fertil Steril 104: 1116–1126, 2015. [DOI] [PubMed] [Google Scholar]
- 62.Quilter CR, Cooper WN, Cliffe KM, Skinner BM, Prentice PM, Nelson L, Bauer J, Ong KK, Constancia M, Lowe WL, Affara NA, Dunger DB. Impact on offspring methylation patterns of maternal gestational diabetes mellitus and intrauterine growth restraint suggest common genes and pathways linked to subsequent Type 2 diabetes risk. FASEB J 28: 4868–4879, 2014. [DOI] [PubMed] [Google Scholar]
- 63.Raposo G, Stoorvogel W. Extracellular vesicles: exosomes, microvesicles, friends. J Cell Biol 200: 373–383, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Reik W, Dean W, Walter J. Epigenetic reprogramming in mammalian development. Science 293: 1089–1093, 2001. [DOI] [PubMed] [Google Scholar]
- 65.Reynolds KA, Boudoures AL, Chi MM, Wang Q, Moley KH. Adverse effects of obesity and/or high-fat diet on oocyte quality and metabolism are not reversible with resumption of regular diet in mice. Reprod Fertil Dev 27: 716–724, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Rogers LK, Velten M. Maternal inflammation, growth retardation, and preterm birth: insights into adult cardiovascular disease. Life Sci 89: 417–421, 2011. [DOI] [PubMed] [Google Scholar]
- 67.Rotili D, Mai A. Targeting histone demethylases: a new avenue for the fight against cancer. Genes Cancer 2: 663–679, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ruchat SM, Houde AA, Voisin G, St-Pierre J, Perron P, Baillargeon JP, Gaudet D, Hivert MF, Brisson D, Bouchard L. Gestational diabetes mellitus epigenetically affects genes predominantly involved in metabolic diseases. Epigenetics 8: 935–943, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Saben JL, Boudoures AL, Asghar Z, Thompson A, Drury A, Zhang W, Chi M, Cusumano A, Scheaffer S, Moley KH. Maternal metabolic syndrome programs mitochondrial dysfunction via germline changes across three generations. Cell Rep 16: 1–8, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Sang Q, Yao Z, Wang H, Feng R, Wang H, Zhao X, Xing Q, Jin L, He L, Wu L, Wang L. Identification of microRNAs in human follicular fluid: characterization of microRNAs that govern steroidogenesis in vitro and are associated with polycystic ovary syndrome in vivo. J Clin Endocrinol Metab 98: 3068–3079, 2013. [DOI] [PubMed] [Google Scholar]
- 71.Seisenberger S, Andrews S, Krueger F, Arand J, Walter J, Santos F, Popp C, Thienpont B, Dean W, Reik W. The dynamics of genome-wide DNA methylation reprogramming in mouse primordial germ cells. Mol Cell 48: 849–862, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sharma A. Bioinformatic analysis revealing association of exosomal mRNAs and proteins in epigenetic inheritance. J Theor Biol 357: 143–149, 2014. [DOI] [PubMed] [Google Scholar]
- 73.Shiio Y, Eisenman RN. Histone sumoylation is associated with transcriptional repression. Proc Natl Acad Sci USA 100: 13225–13230, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Shilatifard A. Chromatin modifications by methylation and ubiquitination: implications in the regulation of gene expression. Annu Rev Biochem 75: 243–269, 2006. [DOI] [PubMed] [Google Scholar]
- 75.Shock LS, Thakkar PV, Peterson EJ, Moran RG, Taylor SM. DNA methyltransferase 1, cytosine methylation, and cytosine hydroxymethylation in mammalian mitochondria. Proc Natl Acad Sci USA 108: 3630–3635, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Skinner MK. Endocrine disruptor induction of epigenetic transgenerational inheritance of disease. Mol Cell Endocrinol 398: 4–12, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Skinner MK. Environmental epigenetic transgenerational inheritance and somatic epigenetic mitotic stability. Epigenetics 6: 838–842, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Skinner MK, Guerrero-Bosagna C. Role of CpG deserts in the epigenetic transgenerational inheritance of differential DNA methylation regions. BMC Genomics 15: 692, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Smythies J, Edelstein L, Ramachandran V. Molecular mechanisms for the inheritance of acquired characteristics-exosomes, microRNA shuttling, fear and stress: Lamarck resurrected? Front Genet 5: 133, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Sohel MM, Hoelker M, Noferesti SS, Salilew-Wondim D, Tholen E, Looft C, Rings F, Uddin MJ, Spencer TE, Schellander K, Tesfaye D. Exosomal and non-exosomal transport of extra-cellular microRNAs in follicular fluid: implications for bovine oocyte developmental competence. PLos One 8: e78505, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Song Y, Wu N, Wang S, Gao M, Song P, Lou J, Tan Y, Liu K. Transgenerational impaired male fertility with an Igf2 epigenetic defect in the rat are induced by the endocrine disruptor p,p′-DDE. Hum Reprod 2 9: 2512–2521, 2014. [DOI] [PubMed] [Google Scholar]
- 82.Suter M, Bocock P, Showalter L, Hu M, Shope C, McKnight R, Grove K, Lane R, Aagaard-Tillery K. Epigenomics: maternal high-fat diet exposure in utero disrupts peripheral circadian gene expression in nonhuman primates. FASEB J 25: 714–726, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Tobi EW, Slagboom PE, van Dongen J, Kremer D, Stein AD, Putter H, Heijmans BT, Lumey LH. Prenatal famine and genetic variation are independently and additively associated with DNA methylation at regulatory loci within IGF2/H19. PLos One 7: e37933, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ulrey CL, Liu L, Andrews LG, Tollefsbol TO. The impact of metabolism on DNA methylation. Hum Mol Genet 14, Spec No 1: R139–R147, 2005. [DOI] [PubMed] [Google Scholar]
- 85.Valadi H, Ekstrom K, Bossios A, Sjostrand M, Lee JJ, Lotvall JO. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat Cell Biol 9: 654–659, 2007. [DOI] [PubMed] [Google Scholar]
- 86.van Dijk SJ, Molloy PL, Varinli H, Morrison JL, Muhlhausler BS, Members of EpiSCOPE . Epigenetics and human obesity. Int J Obes (Lond) 39: 85–97, 2015. [DOI] [PubMed] [Google Scholar]
- 87.Veenendaal MV, Painter RC, de Rooij SR, Bossuyt PM, van der Post JA, Gluckman PD, Hanson MA, Roseboom TJ. Transgenerational effects of prenatal exposure to the 1944-45 Dutch famine. BJOG 120: 548–553, 2013. [DOI] [PubMed] [Google Scholar]
- 88.Visscher PM, Brown MA, McCarthy MI, Yang J. Five years of GWAS discovery. Am J Hum Genet 90: 7–24, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Vojtech L, Woo S, Hughes S, Levy C, Ballweber L, Sauteraud RP, Strobl J, Westerberg K, Gottardo R, Tewari M, Hladik F. Exosomes in human semen carry a distinctive repertoire of small non-coding RNAs with potential regulatory functions. Nucleic Acids Res 42: 7290–7304, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Wade PA, Pruss D, Wolffe AP. Histone acetylation: chromatin in action. Trends Biochem Sci 22: 128–132, 1997. [DOI] [PubMed] [Google Scholar]
- 91.Williams-Wyss O, Zhang S, MacLaughlin SM, Kleemann D, Walker SK, Suter CM, Cropley JE, Morrison JL, Roberts CT, McMillen IC. Embryo number and periconceptional undernutrition in the sheep have differential effects on adrenal epigenotype, growth, and development. Am J Physiol Endocrinol Metab 307: E141–E150, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Wood A. Posttranslational modifications of histones by methylation. In: Advances in Protein Chemistry 67, edited by Conaway J, Conaway R. Amsterdam: Elsevier, 2004201–222. [DOI] [PubMed] [Google Scholar]
- 93.Wossidlo M, Nakamura T, Lepikhov K, Marques CJ, Zakhartchenko V, Boiani M, Arand J, Nakano T, Reik W, Walter J. 5-Hydroxymethylcytosine in the mammalian zygote is linked with epigenetic reprogramming. Nat Commun 2: 241, 2011. [DOI] [PubMed] [Google Scholar]
- 94.Wu LL, Russell DL, Wong SL, Chen M, Tsai TS, St John JC, Norman RJ, Febbraio MA, Carroll J, Robker RL. Mitochondrial dysfunction in oocytes of obese mothers: transmission to offspring and reversal by pharmacological endoplasmic reticulum stress inhibitors. Development 142: 681–691, 2015. [DOI] [PubMed] [Google Scholar]
- 95.Xie F, Anderson CL, Timme KR, Kurz SG, Fernando SC, Wood JR. Obesity-dependent increases in oocyte mRNAs are associated with increases in proinflammatory signaling and gut microbial abundance of Lachnospiraceae in female mice. Endocrinology 157: 1630–1643, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Yan Z, Zhang H, Maher C, Arteaga-Solis E, Champagne FA, Wu L, McDonald JD, Yan B, Schwartz GJ, Miller RL. Prenatal polycyclic aromatic hydrocarbon, adiposity, peroxisome proliferator-activated receptor (PPAR) gamma methylation in offspring, grand-offspring mice. PLos One 9: e110706, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Yogev Y, Catalano PM. Pregnancy and obesity. Obstet Gynecol Clin North Am 36: 285–300, viii, 2009. [DOI] [PubMed] [Google Scholar]