Abstract
Advancing age is associated with progressive declines in physiological function that lead to overt chronic disease, frailty, and eventual mortality. Importantly, age-related physiological changes occur in cellularity, insulin-responsiveness, secretory profiles, and inflammatory status of adipose tissue, leading to adipose tissue dysfunction. Although the mechanisms underlying adipose tissue dysfunction are multifactorial, the consequences result in secretion of proinflammatory cytokines and chemokines, immune cell infiltration, an accumulation of senescent cells, and an increase in senescence-associated secretory phenotype (SASP). These processes synergistically promote chronic sterile inflammation, insulin resistance, and lipid redistribution away from subcutaneous adipose tissue. Without intervention, these effects contribute to age-related systemic metabolic dysfunction, physical limitations, and frailty. Thus adipose tissue dysfunction may be a fundamental contributor to the elevated risk of chronic disease, disability, and adverse health outcomes with advancing age.
Aging and the Adipose Organ
Aging is the leading risk factor for multiple chronic diseases and declines in physical function (60). In many cases, overt disease and disability are preceded by perturbations in metabolic homeostasis and inflammatory status. Adipose tissue is a dynamic organ system that plays a role in modulating systemic metabolism and inflammation (110, 118, 120). In early life, adipose tissue is highly adaptable to environmental changes due to its ability to rapidly alter its endocrine, inflammatory, and metabolic functions (86). This dynamic nature likely evolved as a means of responding to nutrient deprivation, cold-exposure, and/or infection. The rapidity and magnitude of these compensatory effects often vary by adipose depot and change with advancing age and health status. This depot-specific malleability can also promote deleterious effects when age-related changes occur in cellularity, insulin-responsiveness, secretory profiles, and inflammatory state of adipose tissue (118), which we will refer to as adipose tissue dysfunction in this review. The mechanisms responsible for adipose tissue dysfunction are multifactorial and can have secondary physiological effects on a variety of organ systems including liver, muscle, pancreas, and others. Perhaps more importantly, adipose tissue dysfunction is linked with dyslipidemia, metabolic declines, and chronic low-grade systemic inflammation (118), all of which are predictors of impaired physical function and frailty onset (82). Several dietary and pharmacological interventions that extend health span and longevity also delay or prevent adipose tissue dysfunction (37, 78, 111, 130). Furthermore, genetic manipulations that slow aging processes often prevent the pathophysiological mechanisms that promote visceral adiposity, ectopic lipid deposition, and metabolic disturbances (13, 15, 112, 114, 122). Given the role that age-related lipid redistribution plays in the development of systemic insulin resistance, chronic low-grade inflammation, and physical function declines (11, 92), it seems plausible that adipose tissue dysfunction may be a predictor of frailty and declining health span. Here, we will review the connections among adipose tissue dysfunction and systemic declines that lead to the onset of diabetes and frailty.
Mechanisms of Adipose Tissue Dysfunction with Aging
Adipose tissue is comprised of a variety of cell types. The density and function of these cells change throughout life (118). Preadipocytes, also known as adipose-derived stem cells, are one of the most abundant cell types in adipose tissue. Preadipocyte differentiation capacity often declines in aged rodents and humans (20, 57, 58, 61). This is particularly evident in subcutaneous depots where the vast majority of lipid is stored in healthy mammals (19, 118, 120). These perturbations likely contribute to increased central adiposity and ectopic lipid accumulation that are often observed with advancing age (38, 67, 84). Several studies have reported that age-related declines in preadipocyte differentiation are related to reduced CCAAT/enhancer-binding protein alpha (C/EBPα) and peroxisome proliferator-activated receptor gamma (PPARγ) expression and activity (53, 57, 101). Among the causes are increased activity of CCAAT/enhancer-binding protein beta liver-inhibitory protein (C/EBPβ-LIP) and CCAAT/enhancer-binding protein homologous protein (CHOP), which have been shown to form heterodimers with transcriptional regulators that drive adipogenesis (58, 98, 119), thereby impeding the differentiation cascade. Both C/EBPβ-LIP and CHOP expression are increased with aging in preadipocytes, adipocytes, and intact adipose tissue (58, 119) (FIGURE 1). C/EBPb-LIP and CHOP are induced by cellular stress responses such as metabolic dysfunction and inflammatory insults (58, 118, 119). Several reports indicate that the preservation and/or restoration of preadipocyte function in late life can prevent the secondary effects of adipose tissue dysfunction, including lipid redistribution and metabolic perturbations, possibly despite increased adiposity (5, 114, 129). A common theme in most of these models is that they are protected from low-grade inflammation, which undoubtedly plays a role in regulating the differentiation process (48, 54).
FIGURE 1.
Age-related adipose tissue dysfunction drives metabolic and functional declines leading to chronic disease and frailty
Advancing age induces adipose tissue dysfunction, including reduced function of preadipocytes, exacerbated secretion of pro-inflammatory cytokines and chemokines, increased infiltration of immune cells, elevated senescent cell burden, and an increase in the senescence-associated secretory phenotype (SASP). This age-related adipose tissue dysfunction leads to adipose redistribution, local and systemic insulin resistance, and chronic sterile inflammation. Without intervention this can induce systemic metabolic dysregulation and functional decline, eventually leading to diabetes and frailty.
Chronic low-grade inflammation is a hallmark of aging and is linked with the onset of diabetes and frailty (72, 90). Adipose tissue is believed to be a major contributor to systemic inflammation with advancing age (108, 127). Adipose-derived pro-inflammatory cytokines and chemokines arise from a variety of sources in aged mammals. Several reports indicate that preadipocytes can develop expression and secretory phenotypes reminiscent of activated macrophages (23, 25, 77, 133). This process is exacerbated by aging (19, 117), likely due to cellular stress responses brought about by lipotoxicity, hypoxia, and/or replicative arrest (45, 77, 118). Aging also promotes immune cell infiltration into adipose tissue (43, 76), although the magnitude of infiltration and phenotype of cells appear distinct from those observed with obesity (43) (FIGURE 2). Although lesser than what is observed with obesity, activated macrophages are increased in aged adipose tissue and likely contribute to the pro-inflammatory milieu (76). However, their contribution to the pro-inflammatory environment may be less than that of inflamed preadipocytes (118). Aging also leads to increased adipose tissue T-cell populations, particularly in visceral adipose tissue (76). The role of these cells in age-related adipose tissue inflammatory processes remains unresolved, but it is surmised they may dictate immune cell migration given their association with the size of fat-associated lymphoid clusters (43, 85).
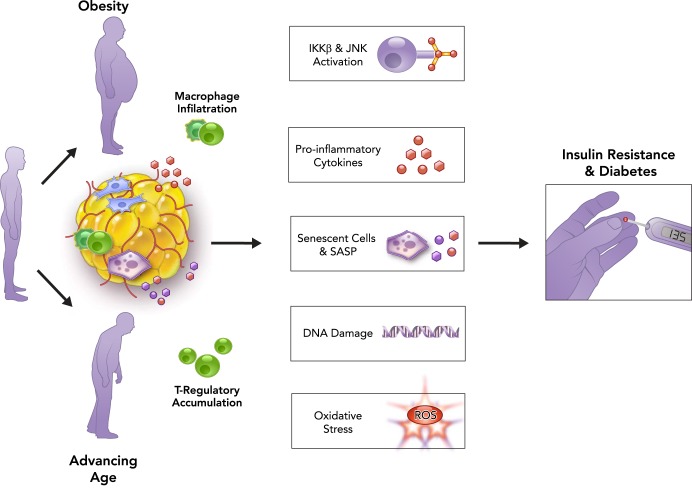
FIGURE 2.
Age and obesity synergistically and independently lead to adipose tissue dysfunction, ultimately resulting in metabolic dysfunction and diabetes
Advancing age and obesity are associated with adipose tissue dysfunction and a chronic subclinical pro-inflammatory state. The pathophysiological processes related to adipose tissue dysfunction in aging and obesity are somewhat independent: for example, although immune cell infiltration of adipose tissue occurs with both obesity and aging, macrophage infiltration is more prominent in obesity, whereas T-regulatory cell accumulation is more prominent in aging. The mechanisms underlying adipose tissue dysfunction drive cellular and molecular processes associated with accelerated aging, including activation of the kinases IκB kinase-β (IKKβ) and JUN NH2-terminal kinase (JNK), elevated pro-inflammatory cytokines, accumulation of senescent cells and increase in senescence-associated secretory phenotype (SASP), increased DNA damage, and oxidative stress. These biological aging processes result in local and systemic dysfunction, ultimately contributing to metabolic dysregulation, insulin resistance, and diabetes.
More recently, adipose tissue dysfunction has been linked to the accumulation of senescent cells (113, 114, 118, 129). Cellular senescence is an irreversible cell-cycle arrest that can be induced by telomere shortening, DNA damage, oncogene activation, and/or metabolic stress (18, 79, 87, 121). Senescent cells are highly pro-inflammatory and develop a phenotype described as the senescence-associated secretory phenotype (SASP) (29, 30). The SASP is comprised of cytokines, chemokines, matrix metalloproteinases, and growth factors, all of which can adversely affect the local microenvironment in adipose tissue by promoting preadipocyte inflammatory processes, inhibiting differentiation, and driving immune cell infiltration (118, 121, 129). The removal of senescent cells through genetic approaches has been found to preserve adipose tissue function, prevent age-related lipid redistribution, enhance vascular activity, and improve systemic metabolic parameters (4, 5, 99, 129). Additionally, pharmacological interventions that reduce senescent cell accumulation (senolytics) and/or suppress the SASP (SASP inhibitors) also elicit beneficial effects on preadipocyte differentiation capacity, adipose tissue insulin-sensitivity, and health span (129, 130, 132). Directly targeting senescent cells through the use of senolytic drugs has begun to show promise (22, 99, 131, 132) and may be the future for treating age-related chronic diseases, such as inflammatory disorders and diabetes (62, 91) (FIGURE 3).
FIGURE 3.
Synergistic effects of adipose tissue dysfunction, cellular senescence, and pro-inflammatory state leads to decline in physical function and frailty
Adipose tissue dysfunction and lipid redistribution, chronic sterile inflammation, and cellular senescence jointly contribute to muscle pathology and reduced contractile function, motor impairment, and physical dysfunction that may ultimately contribute to frailty. Co-incident insulin resistance and diabetes resulting from age-related adipose tissue dysfunction may further exacerbate declines and increase risk of frailty.
Adipose Tissue Dysfunction Promotes Metabolic Declines with Aging
Adipose tissue dysfunction is closely associated with lipid redistribution and chronic low-grade inflammation observed with advancing age (67, 83, 84). These conditions contribute to increased risk for metabolic perturbations such as insulin resistance, impaired glucose tolerance, and diabetes (83, 84). In U.S. adults over 65 years of age, diabetes prevalence varies from 22% to 33%, and the onset is clearly linked to regional adiposity, dyslipidemia, and inflammatory status (38, 63). The connections between diabetes and related comorbidities are well recognized (68, 116), although the pathophysiological mechanisms by which aging promotes insulin resistance and diabetes are only recently garnering attention. Interactions among aging, diabetes, and obesity are particularly interesting, since both diabetes and obesity are arguably conditions that elicit accelerated aging-like phenotypes (51, 81).
Obesity is a well known risk factor for progression of insulin resistance to diabetes, but aging, independently from obesity, may increase the risk of these chronic metabolic conditions through adipose tissue dysfunction, immunological changes, senescent cell accumulation, and pro-inflammatory pathways (70, 110, 118). Epidemiological evidence in middle-aged and older men or women reveals that even subclinical but elevated levels of circulating pro-inflammatory mediators, such as CRP, IL-6, and IL-1β, increase the risk of developing insulin resistance and diabetes (8, 49, 94, 95). Many of these relationships remain significant, although attenuated, after adjusting for obesity or body mass index (BMI). A series of examinations in middle-aged and older adults from the Atherosclerosis Risk in Communities (ARIC) Study support these findings (31, 32, 102). More recently, a case-cohort investigation from the ARIC found that, although older obese individuals had a sixfold greater risk of developing diabetes, the strength of the obesity-diabetes relation dropped by >50% after accounting for seven inflammatory markers: IL-6, CRP, orosoumucoid, sialic acid, white cell count, fibrinogen, and adiponectin (74). Collectively, these data implicate chronic, sterile inflammation in the interactions among obesity, insulin resistance, and diabetes in the context of aging in humans.
Mechanisms of Insulin Resistance and Diabetes with Aging
Mechanisms that underlie the clinical and epidemiological observations linking advancing age to insulin resistance and diabetes are multifactorial. Although mechanisms related to obesity may accelerate aging phenotypes in adipose tissue, aging per se can drive mechanistic pathways both independently and synergistically with obesity (3). For example, aging is associated with a pro-inflammatory state in metabolic tissues and upregulation of pro-inflammatory cytokines such as TNF-α, IL-6, and IL-1 family members, which can directly interfere with the insulin-signaling pathway and drive metabolic dysfunction (6, 55, 64, 73, 80, 88, 109). Although substantial evidence clearly implicates a mechanistic role for pro-inflammatory cytokines and the inflammasome on direct and indirect modulation of cellular and systemic insulin resistance, much of the in vivo work has been conducted in rodent models of obesity and diabetes (6, 52, 64, 80, 103, 109). Furthermore, several of the metabolic stressors that promote the common pathology of obesity, insulin resistance, and diabetes also activate the kinases IκB kinase-β (IKKβ) and JUN NH2-terminal kinase (JNK). These protein kinases are induced in response to inflammation and stress (104), and may be relevant to aging. However, investigations into mechanisms specific to age-related increases in pro-inflammatory status on adipose tissue dysfunction and insulin resistance have received less attention. This distinction could be important, since recent evidence suggests that, although chronic inflammation in dysfunctional adipose tissue is well documented in the pathophysiology of insulin resistance in obesity (128), age-related insulin resistance may be differentially regulated. Macrophage-driven inflammation is a key mechanism underlying insulin resistance in obesity (33, 89), but mechanisms contributing to insulin resistance with aging may differ physiologically. In a murine model, fat-resident regulatory T cells accumulate in dysfunctional adipose tissue with aging, but not in obesity, and mice deficient in these cells do not display age-associated insulin resistance but remain susceptible to obesity-associated insulin resistance and metabolic dysfunction (7). Visceral adipose tissue regulatory T cells may have a significant role in regulating local and systemic inflammation and metabolic processes differentially in obese vs. lean mice (35), and differential accumulation may be dependent on the presence of specific antigens and cytokines (notably IL-33) (66). Thus there may be distinct immune cell populations in visceral adipose tissue in aging from those in obesity. The processes driving metabolic dysregulation in aging and obesity may not be identical.
In an investigation into the shared cellular pathways of aging and obesity on metabolic dysregulation, Minamino et al. examined adipose tissues from genetically obese mice and found biomarkers of accelerated aging, including elevated levels of ROS and DNA damage, as well as higher expression of markers of cellular senescence, including senescence-associated β-galactosidase (SA β-gal), p53, and cyclin-dependent kinase inhibitor 1A (Cdkn1a) (81). Furthermore, p53 inhibition in these mice alleviated inflammation and improved insulin sensitivity, whereas transgenic overexpression of p53 and Cdkn1a, or p53 activation through telomere shortening, increased inflammation and insulin resistance in adipose tissue (81). Collectively, these data implicate cellular aging of adipose tissue, and in particular the p53 tumor suppressor pathway and adipose tissue cellular senescence in the regulation of insulin resistance (3). Cellular senescence may be central to the pathogenesis of age-related insulin resistance and diabetes (91, 118, 129), which could arguably precede immune cell infiltration.
Aging, Physical Function, and Frailty
Aging is characterized by the diminished capacity to respond to stressors and return to homeostasis. As such, aging is the major risk factor for a myriad of diseases and interrelated chronic geriatric conditions, including frailty. Frailty is a geriatric syndrome associated with increased vulnerability following a stress, which increases the risk of adverse outcomes, such as falls, delirium, and disability (1, 27, 41, 42, 106). It is estimated that roughly 25-50% of persons over the age of 85 years are frail (27, 106), which may have substantial consequences not only for human health but also costs associated with the burden of long-term care. A clinical definition of frailty is not fully agreed upon, but most working definitions of the frailty phenotype include declines in physical function and body composition, including unintentional weight loss or loss of muscle mass, muscle weakness, exhaustion or poor endurance, slow walking speed, and low physical activity (41), which result in reduced overall physiological reserve. Perhaps causally related are the age-related changes in muscle pathology that often result in motor unit impairments (declines in contractile velocity and force production) and declines in physical function needed for mobility, balance, strength, and endurance (97). The preservation of physical function is critically important for independent living, and age-related declines in physical function are predictive of high rates of disability and mortality (46, 47, 71, 115). Progressive declines in physical function are principal factors associated with frailty, and there appears to be shared etiology between development of age-related physical dysfunction and frailty.
Epidemiological evidence indicates a link between inflammation and declines in physical function and/or frailty. For example, serum IL-6 is independently related to functional status and impaired walking ability in older adults (14, 34). Furthermore, inflammatory cytokines are associated with several of the individual components of frailty in older adults, including physical dysfunction, and associations between frailty syndrome and inflammation persist even in the absence of common age-related diseases (126). Indeed, a pro-inflammatory state is hypothesized to be at the center of a dynamic model of declines in physical function and physiological processes leading to frailty (40). Given that adipose tissue is a rich source of inflammatory cytokines, it is plausible that age-related adipose tissue dysfunction could be one factor contributing to the systemic pro-inflammatory state associated with these functional declines. Although limited epidemiological evidence exists describing the role of adipose tissue dysfunction in the relationship between chronic, sterile inflammation and physical dysfunction in the absence of obesity or metabolic decline, redistribution of lipid, particularly infiltration into muscle, is associated with motor impairment and declining physical function in older adults (11, 125).
Mechanisms of Physical Dysfunction and Frailty with Aging
The mechanisms by which age-related adipose tissue dysfunction and pro-inflammatory status promote declines in physical function and frailty are incompletely understood. Despite extensive examination of the biomechanical and biological mechanisms linking adipose tissue to declines in physical function, the overwhelming emphasis has previously been placed on the role of excess adiposity, obesity, and sarcopenic obesity (9, 21, 123), and not age-related adipose tissue dysfunction, as emphasized in this review. This distinction is important because, although there are strong associations between greater adiposity and decline in physical function in older adults, these are not entirely explained by potential covariates, including physical activity, chronic diseases, lower muscle mass, or even age. This suggests that there may be effects of adipose tissue itself on the molecular, structural, and metabolic properties of muscle contributing to functional decline (24).
Redistribution of lipid due to adipose tissue dysfunction may lead to age-related functional declines. As previously indicated, advancing age results in an increase in lipid infiltration into muscle, including intermuscular adipocytes along with intramuscular (within muscle but between fibers) and intramyocellular lipid accumulation (10), which have been implicated in functional impairments in older adults (11, 125). There is a strong inverse relationship between the number and size of myofiber lipid droplets and loss of contraction speed and power generation of single muscle fibers from older adults in vitro, and these likely contribute to reduced muscle force and power development observed in the same subjects in vivo (24). Thus there are links between amount and location of fat and muscle function at the cellular and systemic levels in older adults, and these impairments could limit functional ability important for mobility (124). Such effects on function are further compounded when accompanied by sedentary behavior, since muscular lipid redistribution and physical inactivity can exacerbate lipotoxicity and blunt anabolic signaling within muscles (50).
Additionally, several studies in rodents and culture systems suggest that pro-inflammatory cytokines may be implicated in muscle atrophy and/or functional declines. For instance, TNF-α, IL-1β, and IL-6 detected in skeletal muscle can predict declines in grip strength, locomotor ability, and endurance in aged animals (56). Additional studies report that chronic inflammatory disease states lead to pronounced catabolic effects in various tissues, leading to skeletal muscle atrophy (107, 134). Additionally, chronic activation of nuclear factor-κB (NF-κB), a transcription factor central to the classical signaling pathway inducing expression of pro-inflammatory cytokines, can promote muscle atrophy (17), whereas targeted ablation of an NF-κB-activating enzyme can improve muscle strength, mass, and regeneration (86). Systemically, low-grade and persistent inflammation can also interfere with anabolic signaling since IL-6 and TNF-α can downregulate insulin, IGF-1, and erythropoietin signaling, in addition to protein synthesis after a meal or exercise (39). Key insights into the role of inflammatory cytokines in functional decline and frailty can be gleaned from a frail mouse model developed from targeted depletion of IL-10, an important anti-inflammatory cytokine. These mice have increased basal circulating cytokines, including IL-6, IL-1β, and TNF-α, as well as muscle weakness, altered skeletal muscle gene expression, endocrine changes, IGF-1 dysregulation, and increased mortality (65). However, inflammatory dysregulation in this model occurs with much earlier onset, possibly in utero, than would be expected in humans, thereby complicating the translation of findings from this model to older adults (82).
There also may be synergistic effects of inflammation and lipid redistribution on skeletal muscle and possibly physical dysfunction and frailty. In human primary cell culture experiments, the secretory profile of visceral adipose tissue obtained from obese individuals induced a gene and protein expression profile in co-cultured myocytes indicative of muscle atrophy and suppression of key contractile elements. These myocytes had increased secretion of cytokines and chemokines, including IL-1β and IL-6, leading to the conclusion that adipocytes support a pro-inflammatory milieu that is harmful to muscle and may lead to reduced contractile properties and possibly functional decline (93). Although this investigation indicates a unique interaction among adipocytes, inflammation, and muscle, these findings were performed in the context of obesity, not aging, and muscle function was inferred but not measured (59). Thus there may be a direct link between pro-inflammatory secretory profiles from dysfunctional adipose tissue and skeletal muscle, possibly contributing to motor impairment and physical dysfunction, but this area warrants further investigation.
Finally, cellular senescence and the related SASP have been proposed to play an essential role in the development of age-related functional decline and, in particular, the frailty syndrome (69). This contribution could be related to pro-inflammatory signaling cascades, limited capacity to regenerate damaged or atrophied tissues due to restricted regenerative ability of stem cell pools, and impaired tissue functionality (28, 69, 121, 129). Recently, Schafer et al. provided evidence linking cellular senescence to functional decline, demonstrating an association between increased senescent cell biomarkers, the SASP, and worsened physical and metabolic health following a “fast-food diet” (100). Furthermore, exercise prevented the accumulation of senescent cells and alleviated the functional declines that accompanied the diet, providing evidence supporting the value of therapeutic approaches that suppress cellular senescence and the SASP on functional health outcomes.
Relationships Between Diabetes and Frailty
Evidence suggests there may be a link between diabetes and frailty with advancing age. The prevalence of both metabolic dysfunction and frailty increases with advancing age. Interestingly, diabetes and frailty are independently predictive of mortality, but mortality risk is even greater with co-occurrence of frailty and diabetes (16). Moreover, diabetes and frailty may share many pathophysiological mechanisms, including low-grade systemic inflammation, cytokine dysregulation, senescent cell accumulation, and adipose tissue dysfunction. Metabolic dysregulation and insulin resistance in skeletal muscle may exacerbate functional decline and frailty (26). Skeletal muscle is a key peripheral organ responsible for glucose uptake and utilization, and glucose disposal is impaired with insulin resistance (44). This is complicated by the age-related loss of muscle mass. Furthermore, insulin inhibits catabolism in skeletal muscle (2). However, anabolic resistance develops with advancing age and may further affect muscle mass, muscle function, and whole-body glucose homeostasis (96). With the frequent co-occurrence of obesity and insulin resistance and the associated subclinical inflammatory state, these effects are further compounded. In addition to the shared mechanisms already described, possible causes of dysfunction may be related to macrophage infiltration (36), imbalance between M1 and M2 macrophages (12, 75), activation of cellular stress signaling pathways (105), and inflammatory and molecular pathways related to carbohydrate and protein metabolism within muscle (26).
Summary
Adipose tissue is a dynamic organ system responsible for energy storage and nutrient sensing, but it also has a broad range of endocrine and immunological functions. With aging, adipose tissue undergoes dramatic changes in functional capacity, which is implicated in systemic pro-inflammatory status and several other biological hallmarks of aging, including cellular senescence, mitochondrial dysfunction, metabolic declines, and dysregulated nutrient sensing pathways. This cascade of molecular and cellular changes in adipose tissue can drive systemic pathophysiological processes that result in age-related chronic disease such as diabetes, as well as declines in physical function and frailty. In conclusion, adipose tissue dysfunction may be a fundamental contributor to the elevated risk of chronic disease, disability, and adverse health outcomes with advancing age. These intimate relationships make therapeutic targeting of adipose tissue an attractive approach to combating age-related diseases and disability as a single entity as opposed to targeting individual conditions one-at-a-time.
Footnotes
This work was supported National Institute on Aging Grants K99 AG-51661 (M.B.S), R01 AG-13925 (J.L.K), T32 AG-33534 (J.N.J), and P30 AG-21332 (J.N.J and B.J.N); The Connor Group; and the Ted Nash, Noaber, and Glenn Foundations.
No conflicts of interest, financial or otherwise, are declared by the author(s).
Author contributions: M.B.S. and J.N.J. drafted manuscript; M.B.S., J.N.J., B.J.N., and J.L.K. edited and revised manuscript; J.N.J. prepared figures; M.B.S., J.N.J., B.J.N., and J.L.K. approved final version of manuscript.
References
- 1.Abbatecola AM, Olivieri F, Corsonello A, Strollo F, Fumagalli A, Lattanzio F. Frailty and safety: the example of diabetes. Drug Saf 35, Suppl 1: 63–71, 2012. [DOI] [PubMed] [Google Scholar]
- 2.Abdulla H, Smith K, Atherton PJ, Idris I. Role of insulin in the regulation of human skeletal muscle protein synthesis and breakdown: a systematic review and meta-analysis. Diabetologia 59: 44–55, 2016. [DOI] [PubMed] [Google Scholar]
- 3.Ahima RS. Connecting obesity, aging and diabetes. Nat Med 15: 996–997, 2009. [DOI] [PubMed] [Google Scholar]
- 4.Baker DJ, Childs BG, Durik M, Wijers ME, Sieben CJ, Zhong J, Saltness RA, Jeganathan KB, Verzosa GC, Pezeshki A, Khazaie K, Miller JD, van Deursen JM. Naturally occurring p16(Ink4a)-positive cells shorten healthy lifespan. Nature 530: 184–189, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baker DJ, Wijshake T, Tchkonia T, LeBrasseur NK, Childs BG, van de Sluis B, Kirkland JL, van Deursen JM. Clearance of p16Ink4a-positive senescent cells delays ageing-associated disorders. Nature 479: 232–236, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ballak DB, Stienstra R, Tack CJ, Dinarello CA, van Diepen JA. IL-1 family members in the pathogenesis and treatment of metabolic disease: focus on adipose tissue inflammation and insulin resistance. Cytokine 75: 280–290, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bapat SP, Myoung Suh J, Fang S, Liu S, Zhang Y, Cheng A, Zhou C, Liang Y, LeBlanc M, Liddle C, Atkins AR, Yu RT, Downes M, Evans RM, Zheng Y. Depletion of fat-resident Treg cells prevents age-associated insulin resistance. Nature 528: 137–141, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Barzilay JI, Abraham L, Heckbert SR, Cushman M, Kuller LH, Resnick HE, Tracy RP. The relation of markers of inflammation to the development of glucose disorders in the elderly: the Cardiovascular Health Study. Diabetes 50: 2384–2389, 2001. [DOI] [PubMed] [Google Scholar]
- 9.Baumgartner RN, Wayne SJ, Waters DL, Janssen I, Gallagher D, Morley JE. Sarcopenic obesity predicts instrumental activities of daily living disability in the elderly. Obes Res 12: 1995–2004, 2004. [DOI] [PubMed] [Google Scholar]
- 10.Beaufrere B, Morio B. Fat and protein redistribution with aging: metabolic considerations. Eur J Clin Nutr 54, Suppl 3: S48–S53, 2000. [DOI] [PubMed] [Google Scholar]
- 11.Beavers KM, Beavers DP, Houston DK, Harris TB, Hue TF, Koster A, Newman AB, Simonsick EM, Studenski SA, Nicklas BJ, Kritchevsky SB. Associations between body composition and gait-speed decline: results from the Health, Aging, and Body Composition Study. Am J Clin Nutr 97: 552–560, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Beenakker KG, Westendorp RG, de Craen AJ, Slagboom PE, van Heemst D, Maier AB. Pro-inflammatory capacity of classically activated monocytes relates positively to muscle mass and strength. Aging Cell 12: 682–689, 2013. [DOI] [PubMed] [Google Scholar]
- 13.Berryman DE, List EO, Palmer AJ, Chung MY, Wright-Piekarski J, Lubbers E, O'Connor P, Okada S, Kopchick JJ. Two-year body composition analyses of long-lived GHR null mice. J Gerontol A Biol Sci Med Sci 65: 31–40, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Blain H, Jaussent A, Beziat S, Dupuy AM, Bernard PL, Mariano-Goulart D, Cristol JP, Sultan C, Picot MC. Low serum IL-6 is associated with high 6-minute walking performance in asymptomatic women aged 20 to 70years. Exp Gerontol 47: 143–148, 2012. [DOI] [PubMed] [Google Scholar]
- 15.Bluher M, Kahn BB, Kahn CR. Extended longevity in mice lacking the insulin receptor in adipose tissue. Science 299: 572–574, 2003. [DOI] [PubMed] [Google Scholar]
- 16.Cacciatore F, Testa G, Galizia G, Della-Morte D, Mazzella F, Langellotto A, Pirozzi G, Ferro G, Gargiulo G, Ferrara N, Rengo F, Abete P. Clinical frailty and long-term mortality in elderly subjects with diabetes. Acta Diabetol 50: 251–260, 2013. [DOI] [PubMed] [Google Scholar]
- 17.Cai D, Frantz JD, Tawa NE Jr, Melendez PA, Oh BC, Lidov HG, Hasselgren PO, Frontera WR, Lee J, Glass DJ, Shoelson SE. IKKbeta/NF-kappaB activation causes severe muscle wasting in mice. Cell 119: 285–298, 2004. [DOI] [PubMed] [Google Scholar]
- 18.Campisi J. Senescent cells, tumor suppression, and organismal aging: good citizens, bad neighbors. Cell 120: 513–522, 2005. [DOI] [PubMed] [Google Scholar]
- 19.Cartwright MJ, Schlauch K, Lenburg ME, Tchkonia T, Pirtskhalava T, Cartwright A, Thomou T, Kirkland JL. Aging, depot origin, and preadipocyte gene expression. J Gerontol A Biol Sci Med Sci 65: 242–251, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Caso G, McNurlan MA, Mileva I, Zemlyak A, Mynarcik DC, Gelato MC. Peripheral fat loss and decline in adipogenesis in older humans. Metab Clin Exp 62: 337–340, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cesari M, Kritchevsky SB, Baumgartner RN, Atkinson HH, Penninx BW, Lenchik L, Palla SL, Ambrosius WT, Tracy RP, Pahor M. Sarcopenia, obesity, and inflammation: results from the Trial of Angiotensin Converting Enzyme Inhibition and Novel Cardiovascular Risk Factors Study. Am J Clin Nutr 82: 428–434, 2005. [DOI] [PubMed] [Google Scholar]
- 22.Chang J, Wang Y, Shao L, Laberge RM, Demaria M, Campisi J, Janakiraman K, Sharpless NE, Ding S, Feng W, Luo Y, Wang X, Aykin-Burns N, Krager K, Ponnappan U, Hauer-Jensen M, Meng A, Zhou D. Clearance of senescent cells by ABT263 rejuvenates aged hematopoietic stem cells in mice. Nat Med 22: 78–83, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Charriere G, Cousin B, Arnaud E, Andre M, Bacou F, Penicaud L, Casteilla L. Preadipocyte conversion to macrophage. Evidence of plasticity. J Biol Chem 278: 9850–9855, 2003. [DOI] [PubMed] [Google Scholar]
- 24.Choi SJ, Files DC, Zhang T, Wang ZM, Messi ML, Gregory H, Stone J, Lyles MF, Dhar S, Marsh AP, Nicklas BJ, Delbono O. Intramyocellular lipid and impaired myofiber contraction in normal weight and obese older adults. J Gerontol A Biol Sci Med Sci 71: 557–564, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chung S, Lapoint K, Martinez K, Kennedy A, Boysen Sandberg M, McIntosh MK. Preadipocytes mediate lipopolysaccharide-induced inflammation and insulin resistance in primary cultures of newly differentiated human adipocytes. Endocrinology 147: 5340–5351, 2006. [DOI] [PubMed] [Google Scholar]
- 26.Cleasby ME, Jamieson P, Atherton PJ. Insulin resistance and sarcopenia: mechanistic links between common co-morbidities. J Endocrinol 229: 67–81, 2016. [DOI] [PubMed] [Google Scholar]
- 27.Clegg A, Young J, Iliffe S, Rikkert MO, Rockwood K. Frailty in elderly people. Lancet 381: 752–762, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Collado M, Blasco MA, Serrano M. Cellular senescence in cancer and aging. Cell 130: 223–233, 2007. [DOI] [PubMed] [Google Scholar]
- 29.Coppe JP, Desprez PY, Krtolica A, Campisi J. The senescence-associated secretory phenotype: the dark side of tumor suppression. Ann Rev Pathol 5: 99–118, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Coppe JP, Patil CK, Rodier F, Sun Y, Munoz DP, Goldstein J, Nelson PS, Desprez PY, Campisi J. Senescence-associated secretory phenotypes reveal cell-nonautonomous functions of oncogenic RAS and the p53 tumor suppressor. PLoS Biol 6: 2853–2868, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Duncan BB, Schmidt MI, Chambless LE, Folsom AR, Carpenter M, Heiss G. Fibrinogen, other putative markers of inflammation, and weight gain in middle-aged adults: the ARIC study. Atherosclerosis Risk in Communities. Obes Res 8: 279–286, 2000. [DOI] [PubMed] [Google Scholar]
- 32.Duncan BB, Schmidt MI, Pankow JS, Ballantyne CM, Couper D, Vigo A, Hoogeveen R, Folsom AR, Heiss G, Atherosclerosis Risk in Communities. Low-grade systemic inflammation and the development of Type 2 diabetes: the atherosclerosis risk in communities study. Diabetes 52: 1799–1805, 2003. [DOI] [PubMed] [Google Scholar]
- 33.Ferrante AW., Jr Macrophages, fat, and the emergence of immunometabolism. J Clin Invest 123: 4992–4993, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ferrucci L, Harris TB, Guralnik JM, Tracy RP, Corti MC, Cohen HJ, Penninx B, Pahor M, Wallace R, Havlik RJ. Serum IL-6 level and the development of disability in older persons. J Am Geriatr Soc 47: 639–646, 1999. [DOI] [PubMed] [Google Scholar]
- 35.Feuerer M, Herrero L, Cipolletta D, Naaz A, Wong J, Nayer A, Lee J, Goldfine AB, Benoist C, Shoelson S, Mathis D. Lean, but not obese, fat is enriched for a unique population of regulatory T cells that affect metabolic parameters. Nat Med 15: 930–939, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fink LN, Costford SR, Lee YS, Jensen TE, Bilan PJ, Oberbach A, Bluher M, Olefsky JM, Sams A, Klip A. Pro-inflammatory macrophages increase in skeletal muscle of high fat-fed mice and correlate with metabolic risk markers in humans. Obesity (Silver Spring) 22: 747–757, 2014. [DOI] [PubMed] [Google Scholar]
- 37.Fontana L, Partridge L. Promoting health and longevity through diet: from model organisms to humans. Cell 161: 106–118, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fox CS, Massaro JM, Hoffmann U, Pou KM, Maurovich-Horvat P, Liu CY, Vasan RS, Murabito JM, Meigs JB, Cupples LA, D'Agostino RB Sr, O'Donnell CJ. Abdominal visceral and subcutaneous adipose tissue compartments: association with metabolic risk factors in the Framingham Heart Study. Circulation 116: 39–48, 2007. [DOI] [PubMed] [Google Scholar]
- 39.Franceschi C, Campisi J. Chronic inflammation (inflammaging) and its potential contribution to age-associated diseases. J Gerontol A Biol Sci Med Sci 69, Suppl 1: S4–S9, 2014. [DOI] [PubMed] [Google Scholar]
- 40.Fried LP, Tangen CM, Walston J, Newman AB, Hirsch C, Gottdiener J, Seeman T, Tracy R, Kop WJ, Burke G, McBurnie MA. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci 56: 146–156, 2001. [DOI] [PubMed] [Google Scholar]
- 41.Fried LP, Tangen CM, Walston J, Newman AB, Hirsch C, Gottdiener J, Seeman T, Tracy R, Kop WJ, Burke G, McBurnie MA, Cardiovascular Health Study Collaborative Research Group. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci 56: 146–156, 2001. [DOI] [PubMed] [Google Scholar]
- 42.Fried LP, Xue QL, Cappola AR, Ferrucci L, Chaves P, Varadhan R, Guralnik JM, Leng SX, Semba RD, Walston JD, Blaum CS, Bandeen-Roche K. Nonlinear multisystem physiological dysregulation associated with frailty in older women: implications for etiology and treatment. J Gerontol A Biol Sci Med Sci 64: 1049–1057, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Garg SK, Delaney C, Shi H, Yung R. Changes in adipose tissue macrophages and T cells during aging. Crit Rev Immunol 34: 1–14, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gumbiner B, Thorburn AW, Ditzler TM, Bulacan F, Henry RR. Role of impaired intracellular glucose metabolism in the insulin resistance of aging. Metabolism 41: 1115–1121, 1992. [DOI] [PubMed] [Google Scholar]
- 45.Guo W, Pirtskhalava T, Tchkonia T, Xie W, Thomou T, Han J, Wang T, Wong S, Cartwright A, Hegardt FG, Corkey BE, Kirkland JL. Aging results in paradoxical susceptibility of fat cell progenitors to lipotoxicity. Am J Physiol Endocrinol Metab 292: E1041–E1051, 2007. [DOI] [PubMed] [Google Scholar]
- 46.Guralnik JM, Ferrucci L, Pieper CF, Leveille SG, Markides KS, Ostir GV, Studenski S, Berkman LF, Wallace RB. Lower extremity function and subsequent disability: consistency across studies, predictive models, and value of gait speed alone compared with the short physical performance battery. J Gerontol A Biol Sci Med Sci 55: 221–231, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Guralnik JM, Ferrucci L, Simonsick EM, Salive ME, Wallace RB. Lower-extremity function in persons over the age of 70 years as a predictor of subsequent disability. N Engl J Med 332: 556–561, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gustafson B, Gogg S, Hedjazifar S, Jenndahl L, Hammarstedt A, Smith U. Inflammation and impaired adipogenesis in hypertrophic obesity in man. Am J Physiol Endocrinol Metab 297: E999–E1003, 2009. [DOI] [PubMed] [Google Scholar]
- 49.Han TS, Sattar N, Williams K, Gonzalez-Villalpando C, Lean ME, Haffner SM. Prospective study of C-reactive protein in relation to the development of diabetes and metabolic syndrome in the Mexico City Diabetes Study. Diabetes Care 25: 2016–2021, 2002. [DOI] [PubMed] [Google Scholar]
- 50.Haran PH, Rivas DA, Fielding RA. Role and potential mechanisms of anabolic resistance in sarcopenia. J Cachexia Sarcopenia Muscle 3: 157–162, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Horvath S, Erhart W, Brosch M, Ammerpohl O, von Schonfels W, Ahrens M, Heits N, Bell JT, Tsai PC, Spector TD, Deloukas P, Siebert R, Sipos B, Becker T, Rocken C, Schafmayer C, Hampe J. Obesity accelerates epigenetic aging of human liver. Proc Natl Acad Sci USA 111: 15538–15543, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hotamisligil GS, Shargill NS, Spiegelman BM. Adipose expression of tumor necrosis factor-alpha: direct role in obesity-linked insulin resistance. Science 259: 87–91, 1993. [DOI] [PubMed] [Google Scholar]
- 53.Hotta K, Bodkin NL, Gustafson TA, Yoshioka S, Ortmeyer HK, Hansen BC. Age-related adipose tissue mRNA expression of ADD1/SREBP1, PPARgamma, lipoprotein lipase, and GLUT4 glucose transporter in rhesus monkeys. J Gerontol A Biol Sci Med Sci 54: 183–188, 1999. [DOI] [PubMed] [Google Scholar]
- 54.Isakson P, Hammarstedt A, Gustafson B, Smith U. Impaired preadipocyte differentiation in human abdominal obesity: role of Wnt, tumor necrosis factor-alpha, and inflammation. Diabetes 58: 1550–1557, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Jager J, Gremeaux T, Cormont M, Le Marchand-Brustel Y, Tanti JF. Interleukin-1beta-induced insulin resistance in adipocytes through down-regulation of insulin receptor substrate-1 expression. Endocrinology 148: 241–251, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Justice JN, Gioscia-Ryan RA, Johnson LC, Battson ML, de Picciotto NE, Beck HJ, Jiang H, Sindler AL, Bryan NS, Enoka RM, Seals DR. Sodium nitrite supplementation improves motor function and skeletal muscle inflammatory profile in old male mice. J Appl Physiol 118: 163–169, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Karagiannides I, Tchkonia T, Dobson DE, Steppan CM, Cummins P, Chan G, Salvatori K, Hadzopoulou-Cladaras M, Kirkland JL. Altered expression of C/EBP family members results in decreased adipogenesis with aging. Am J Physiol Regul Integr Comp Physiol 280: R1772–R1780, 2001. [DOI] [PubMed] [Google Scholar]
- 58.Karagiannides I, Thomou T, Tchkonia T, Pirtskhalava T, Kypreos KE, Cartwright A, Dalagiorgou G, Lash TL, Farmer SR, Timchenko NA, Kirkland JL. Increased CUG triplet repeat-binding protein-1 predisposes to impaired adipogenesis with aging. J Biol Chem 281: 23025–23033, 2006. [DOI] [PubMed] [Google Scholar]
- 59.Kelley DE, Goodpaster BH. Stewing in not-so-good juices: interactions of skeletal muscle with adipose secretions. Diabetes 64: 3055–3057, 2015. [DOI] [PubMed] [Google Scholar]
- 60.Kirkland JL. Translating advances from the basic biology of aging into clinical application. Exp Gerontol 48: 1–5, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kirkland JL, Hollenberg CH, Gillon WS. Age, anatomic site, and the replication and differentiation of adipocyte precursors. Am J Physiol Cell Physiol 258: C206–C210, 1990. [DOI] [PubMed] [Google Scholar]
- 62.Kirkland JL, Tchkonia T. Clinical strategies and animal models for developing senolytic agents. Exp Gerontol 68: 19–25, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kirkman MS, Briscoe VJ, Clark N, Florez H, Haas LB, Halter JB, Huang ES, Korytkowski MT, Munshi MN, Odegard PS, Pratley RE, Swift CS. Diabetes in older adults. Diabetes Care 35: 2650–2664, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Klover PJ, Zimmers TA, Koniaris LG, Mooney RA. Chronic exposure to interleukin-6 causes hepatic insulin resistance in mice. Diabetes 52: 2784–2789, 2003. [DOI] [PubMed] [Google Scholar]
- 65.Ko F, Yu Q, Xue QL, Yao W, Brayton C, Yang H, Fedarko N, Walston J. Inflammation and mortality in a frail mouse model. Age 34: 705–715, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kolodin D, van Panhuys N, Li C, Magnuson AM, Cipolletta D, Miller CM, Wagers A, Germain RN, Benoist C, Mathis D. Antigen- and cytokine-driven accumulation of regulatory T cells in visceral adipose tissue of lean mice. Cell Metab 21: 543–557, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kuk JL, Saunders TJ, Davidson LE, Ross R. Age-related changes in total and regional fat distribution. Ageing Res Rev 8: 339–348, 2009. [DOI] [PubMed] [Google Scholar]
- 68.Leahy JL. Pathogenesis of Type 2 diabetes mellitus. Arch Med Res 36: 197–209, 2005. [DOI] [PubMed] [Google Scholar]
- 69.LeBrasseur NK, Tchkonia T, Kirkland JL. Cellular senescence and the biology of aging, disease, and frailty. Nestle Nutr Inst Workshop Ser 83: 11–18, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Licastro F, Candore G, Lio D, Porcellini E, Colonna-Romano G, Franceschi C, Caruso C. Innate immunity and inflammation in ageing: a key for understanding age-related diseases. Immun Ageing 2: 8, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lindle RS, Metter EJ, Lynch NA, Fleg JL, Fozard JL, Tobin J, Roy TA, Hurley BF. Age and gender comparisons of muscle strength in 654 women and men aged 20–93 yr. J Appl Physiol 83: 1581–1587, 1997. [DOI] [PubMed] [Google Scholar]
- 72.Lopez-Otin C, Blasco MA, Partridge L, Serrano M, Kroemer G. The hallmarks of aging. Cell 153: 1194–1217, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lorenzo M, Fernandez-Veledo S, Vila-Bedmar R, Garcia-Guerra L, De Alvaro C, Nieto-Vazquez I. Insulin resistance induced by tumor necrosis factor-alpha in myocytes and brown adipocytes. J Anim Sci 86: 94–104, 2008. [DOI] [PubMed] [Google Scholar]
- 74.Luft VC, Schmidt MI, Pankow JS, Couper D, Ballantyne CM, Young JH, Duncan BB. Chronic inflammation role in the obesity-diabetes association: a case-cohort study. Diabetol Metab Syndr 5: 31, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Lumeng CN, Bodzin JL, Saltiel AR. Obesity induces a phenotypic switch in adipose tissue macrophage polarization. J Clin Invest 117: 175–184, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lumeng CN, Liu J, Geletka L, Delaney C, Delproposto J, Desai A, Oatmen K, Martinez-Santibanez G, Julius A, Garg S, Yung RL. Aging is associated with an increase in T cells and inflammatory macrophages in visceral adipose tissue. J Immunol 187: 6208–6216, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Mack I, BelAiba RS, Djordjevic T, Gorlach A, Hauner H, Bader BL. Functional analyses reveal the greater potency of preadipocytes compared with adipocytes as endothelial cell activator under normoxia, hypoxia, and TNFalpha exposure. Am J Physiol Endocrinol Metab 297: E735–E748, 2009. [DOI] [PubMed] [Google Scholar]
- 78.Martin-Montalvo A, Mercken EM, Mitchell SJ, Palacios HH, Mote PL, Scheibye-Knudsen M, Gomes AP, Ward TM, Minor RK, Blouin MJ, Schwab M, Pollak M, Zhang Y, Yu Y, Becker KG, Bohr VA, Ingram DK, Sinclair DA, Wolf NS, Spindler SR, Bernier M, de Cabo R. Metformin improves healthspan and lifespan in mice. Nat Commun 4: 2192, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Martin-Ruiz C, Saretzki G, Petrie J, Ladhoff J, Jeyapalan J, Wei W, Sedivy J, von Zglinicki T. Stochastic variation in telomere shortening rate causes heterogeneity of human fibroblast replicative life span. J Biol Chem 279: 17826–17833, 2004. [DOI] [PubMed] [Google Scholar]
- 80.McGillicuddy FC, Harford KA, Reynolds CM, Oliver E, Claessens M, Mills KH, Roche HM. Lack of interleukin-1 receptor I (IL-1RI) protects mice from high-fat diet-induced adipose tissue inflammation coincident with improved glucose homeostasis. Diabetes 60: 1688–1698, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Minamino T, Orimo M, Shimizu I, Kunieda T, Yokoyama M, Ito T, Nojima A, Nabetani A, Oike Y, Matsubara H, Ishikawa F, Komuro I. A crucial role for adipose tissue p53 in the regulation of insulin resistance. Nat Med 15: 1082–1087, 2009. [DOI] [PubMed] [Google Scholar]
- 82.Mohler MJ, Fain MJ, Wertheimer AM, Najafi B, Nikolich-Zugich J. The frailty syndrome: clinical measurements and basic underpinnings in humans and animals. Exp Gerontol 54: 6–13, 2014. [DOI] [PubMed] [Google Scholar]
- 83.Morley JE. The metabolic syndrome and aging. J Gerontol A Biol Sci Med Sci 59: 139–142, 2004. [DOI] [PubMed] [Google Scholar]
- 84.Morley JE, Sinclair A. The metabolic syndrome in older persons: a loosely defined constellation of symptoms or a distinct entity? Age Ageing 38: 494–497, 2009. [DOI] [PubMed] [Google Scholar]
- 85.Moro K, Yamada T, Tanabe M, Takeuchi T, Ikawa T, Kawamoto H, Furusawa J, Ohtani M, Fujii H, Koyasu S. Innate production of T(H)2 cytokines by adipose tissue-associated c-Kit(+)Sca-1(+) lymphoid cells. Nature 463: 540–544, 2010. [DOI] [PubMed] [Google Scholar]
- 86.Mourkioti F, Kratsios P, Luedde T, Song YH, Delafontaine P, Adami R, Parente V, Bottinelli R, Pasparakis M, Rosenthal N. Targeted ablation of IKK2 improves skeletal muscle strength, maintains mass, and promotes regeneration. J Clin Invest 116: 2945–2954, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Narita M, Nunez S, Heard E, Lin AW, Hearn SA, Spector DL, Hannon GJ, Lowe SW. Rb-mediated heterochromatin formation and silencing of E2F target genes during cellular senescence. Cell 113: 703–716, 2003. [DOI] [PubMed] [Google Scholar]
- 88.Nieto-Vazquez I, Fernandez-Veledo S, Kramer DK, Vila-Bedmar R, Garcia-Guerra L, Lorenzo M. Insulin resistance associated to obesity: the link TNF-alpha. Arch Physiol Biochem 114: 183–194, 2008. [DOI] [PubMed] [Google Scholar]
- 89.Osborn O, Olefsky JM. The cellular and signaling networks linking the immune system and metabolism in disease. Nat Med 18: 363–374, 2012. [DOI] [PubMed] [Google Scholar]
- 90.Paganelli R, Di Iorio A, Cherubini A, Lauretani F, Mussi C, Volpato S, Abate M, Abate G, Ferrucci L. Frailty of older age: the role of the endocrine–immune interaction. Curr Pharm Design 12: 3147–3159, 2006. [DOI] [PubMed] [Google Scholar]
- 91.Palmer AK, Tchkonia T, LeBrasseur NK, Chini EN, Xu M, Kirkland JL. Cellular senescence in Type 2 diabetes: a therapeutic opportunity. Diabetes 64: 2289–2298, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Pararasa C, Bailey CJ, Griffiths HR. Ageing, adipose tissue, fatty acids and inflammation. Biogerontology 16: 235–248, 2015. [DOI] [PubMed] [Google Scholar]
- 93.Pellegrinelli V, Rouault C, Rodriguez-Cuenca S, Albert V, Edom-Vovard F, Vidal-Puig A, Clement K, Butler-Browne GS, Lacasa D. Human adipocytes induce inflammation and atrophy in muscle cells during obesity. Diabetes 64: 3121–3134, 2015. [DOI] [PubMed] [Google Scholar]
- 94.Pradhan AD, Cook NR, Buring JE, Manson JE, Ridker PM. C-reactive protein is independently associated with fasting insulin in nondiabetic women. Arterioscler Thromb Vasc Biol 23: 650–655, 2003. [DOI] [PubMed] [Google Scholar]
- 95.Pradhan AD, Manson JE, Rifai N, Buring JE, Ridker PM. C-reactive protein, interleukin 6, and risk of developing Type 2 diabetes mellitus. JAMA 286: 327–334, 2001. [DOI] [PubMed] [Google Scholar]
- 96.Rasmussen BB, Fujita S, Wolfe RR, Mittendorfer B, Roy M, Rowe VL, Volpi E. Insulin resistance of muscle protein metabolism in aging. FASEB J 20: 768–769, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Reid KF, Fielding RA. Skeletal muscle power: a critical determinant of physical functioning in older adults. Exerc Sport Sci Rev 40: 4–12, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Ron D, Habener JF. CHOP, a novel developmentally regulated nuclear protein that dimerizes with transcription factors C/EBP and LAP and functions as a dominant-negative inhibitor of gene transcription. Genes Dev 6: 439–453, 1992. [DOI] [PubMed] [Google Scholar]
- 99.Roos CM, Zhang B, Palmer AK, Ogrodnik MB, Pirtskhalava T, Thalji NM, Hagler M, Jurk D, Smith LA, Casaclang-Verzosa G, Zhu Y, Schafer MJ, Tchkonia T, Kirkland JL, Miller JD. Chronic senolytic treatment alleviates established vasomotor dysfunction in aged or atherosclerotic mice. Aging Cell 15: 973–977, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Schafer MJ, White TA, Evans G, Tonne JM, Verzosa GC, Stout MB, Mazula DL, Palmer AK, Baker DJ, Jensen MD, Torbenson MS, Miller JD, Ikeda Y, Tchkonia T, van Deursen JM, Kirkland JL, LeBrasseur NK. Exercise prevents diet-induced cellular senescence in adipose tissue. Diabetes 65: 1606–1615, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Schipper BM, Marra KG, Zhang W, Donnenberg AD, Rubin JP. Regional anatomic and age effects on cell function of human adipose-derived stem cells. Ann Plastic Surg 60: 538–544, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Schmidt MI, Duncan BB, Sharrett AR, Lindberg G, Savage PJ, Offenbacher S, Azambuja MI, Tracy RP, Heiss G. Markers of inflammation and prediction of diabetes mellitus in adults (Atherosclerosis Risk in Communities study): a cohort study. Lancet 353: 1649–1652, 1999. [DOI] [PubMed] [Google Scholar]
- 103.Senn JJ, Klover PJ, Nowak IA, Mooney RA. Interleukin-6 induces cellular insulin resistance in hepatocytes. Diabetes 51: 3391–3399, 2002. [DOI] [PubMed] [Google Scholar]
- 104.Shoelson SE, Lee J, Goldfine AB. Inflammation and insulin resistance. J Clin Invest 116: 1793–1801, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Sishi B, Loos B, Ellis B, Smith W, du Toit EF, Engelbrecht AM. Diet-induced obesity alters signalling pathways and induces atrophy and apoptosis in skeletal muscle in a prediabetic rat model. Exp Physiol 96: 179–193, 2011. [DOI] [PubMed] [Google Scholar]
- 106.Song X, Mitnitski A, Rockwood K. Prevalence and 10-year outcomes of frailty in older adults in relation to deficit accumulation. J Am Geriatr Soc 58: 681–687, 2010. [DOI] [PubMed] [Google Scholar]
- 107.Spate U, Schulze PC. Proinflammatory cytokines and skeletal muscle. Curr Opin Clin Nutr Metab Care 7: 265–269, 2004. [DOI] [PubMed] [Google Scholar]
- 108.Starr ME, Evers BM, Saito H. Age-associated increase in cytokine production during systemic inflammation: adipose tissue as a major source of IL-6. J Gerontol A Biol Sci Med Sci 64: 723–730, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Stienstra R, Joosten LA, Koenen T, van Tits B, van Diepen JA, van den Berg SA, Rensen PC, Voshol PJ, Fantuzzi G, Hijmans A, Kersten S, Muller M, van den Berg WB, van Rooijen N, Wabitsch M, Kullberg BJ, van der Meer JW, Kanneganti T, Tack CJ, Netea MG. The inflammasome-mediated caspase-1 activation controls adipocyte differentiation and insulin sensitivity. Cell Metab 12: 593–605, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Stout M, Tchkonia T, Kirkland J. The aging adipose organ: lipid redistribution, inflammation, and cellular senescence. In: Adipose Tissue and Adipokines in Health and Disease, edited by Fantuzzi G, Braunschweig C. New York: Humana, 201469–80. [Google Scholar]
- 111.Stout MB, Steyn FJ, Jurczak MJ, Camporez JG, Zhu Y, Hawse JR, Jurk D, Palmer AK, Xu M, Pirtskhalava T, Evans GL, de Souza Santos R, Frank AP, White TA, Monroe DG, Singh RJ, Casaclang-Verzosa G, Miller JD, Clegg DJ, LeBrasseur NK, von Zglinicki T, Shulman GI, Tchkonia T, Kirkland JL.. 17Alpha-estradiol alleviates age-related metabolic and inflammatory dysfunction in male mice without inducing feminization. J Gerontol A Biol Sci Med Sci. In press. [DOI] [PMC free article] [PubMed]
- 112.Stout MB, Swindell WR, Zhi X, Rohde K, List EO, Berryman DE, Kopchick JJ, Gesing A, Fang Y, Masternak MM. Transcriptome profiling reveals divergent expression shifts in brown and white adipose tissue from long-lived GHRKO mice. Oncotarget 6: 26702–26715, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Stout MB, Tchkonia T, Kirkland JL. Growth hormone in adipose dysfunction and senescence. Oncotarget 6: 10667–10668, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Stout MB, Tchkonia T, Pirtskhalava T, Palmer AK, List EO, Berryman DE, Lubbers ER, Escande C, Spong A, Masternak MM, Oberg AL, LeBrasseur NK, Miller RA, Kopchick JJ, Bartke A, Kirkland JL. Growth hormone action predicts age-related white adipose tissue dysfunction and senescent cell burden in mice. Aging (Milano) 6: 575–586, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Studenski S, Perera S, Patel K, Rosano C, Faulkner K, Inzitari M, Brach J, Chandler J, Cawthon P, Connor EB, Nevitt M, Visser M, Kritchevsky S, Badinelli S, Harris T, Newman AB, Cauley J, Ferrucci L, Guralnik J. Gait speed and survival in older adults. JAMA 305: 50–58, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Stumvoll M, Goldstein BJ, van Haeften TW. Type 2 diabetes: principles of pathogenesis and therapy. Lancet 365: 1333–1346, 2005. [DOI] [PubMed] [Google Scholar]
- 117.Tchkonia T, Lenburg M, Thomou T, Giorgadze N, Frampton G, Pirtskhalava T, Cartwright A, Cartwright M, Flanagan J, Karagiannides I, Gerry N, Forse RA, Tchoukalova Y, Jensen MD, Pothoulakis C, Kirkland JL. Identification of depot-specific human fat cell progenitors through distinct expression profiles and developmental gene patterns. Am J Physiol Endocrinol Metab 292: E298–E307, 2007. [DOI] [PubMed] [Google Scholar]
- 118.Tchkonia T, Morbeck DE, Von Zglinicki T, Van Deursen J, Lustgarten J, Scrable H, Khosla S, Jensen MD, Kirkland JL. Fat tissue, aging, and cellular senescence. Aging Cell 9: 667–684, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Tchkonia T, Pirtskhalava T, Thomou T, Cartwright MJ, Wise B, Karagiannides I, Shpilman A, Lash TL, Becherer JD, Kirkland JL. Increased TNFalpha and CCAAT/enhancer-binding protein homologous protein with aging predispose preadipocytes to resist adipogenesis. Am J Physiol Endocrinol Metab 293: E1810–E1819, 2007. [DOI] [PubMed] [Google Scholar]
- 120.Tchkonia T, Thomou T, Zhu Y, Karagiannides I, Pothoulakis C, Jensen MD, Kirkland JL. Mechanisms and metabolic implications of regional differences among fat depots. Cell Metab 17: 644–656, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Tchkonia T, Zhu Y, van Deursen J, Campisi J, Kirkland JL. Cellular senescence and the senescent secretory phenotype: therapeutic opportunities. J Clin Invest 123: 966–972, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Um SH, Frigerio F, Watanabe M, Picard F, Joaquin M, Sticker M, Fumagalli S, Allegrini PR, Kozma SC, Auwerx J, Thomas G. Absence of S6K1 protects against age- and diet-induced obesity while enhancing insulin sensitivity. Nature 431: 200–205, 2004. [DOI] [PubMed] [Google Scholar]
- 123.Vincent HK, Mathews A. Obesity and mobility in advancing age: mechanisms and interventions to preserve independent mobility. Curr Obes Rep 2: 275–283, 2013. [Google Scholar]
- 124.Visser M, Goodpaster BH, Kritchevsky SB, Newman AB, Nevitt M, Rubin SM, Simonsick EM, Harris TB. Muscle mass, muscle strength, and muscle fat infiltration as predictors of incident mobility limitations in well-functioning older persons. J Gerontol A Biol Sci Med Sci 60: 324–333, 2005. [DOI] [PubMed] [Google Scholar]
- 125.Visser M, Kritchevsky SB, Goodpaster BH, Newman AB, Nevitt M, Stamm E, Harris TB. Leg muscle mass and composition in relation to lower extremity performance in men and women aged 70 to 79: the health, aging and body composition study. J Am Geriatr Soc 50: 897–904, 2002. [DOI] [PubMed] [Google Scholar]
- 126.Walston J, McBurnie MA, Newman A, Tracy RP, Kop WJ, Hirsch CH, Gottdiener J, Fried LP, Cardiovascular Health Study. Frailty and activation of the inflammation and coagulation systems with and without clinical comorbidities: results from the Cardiovascular Health Study. Arch Intern Med 162: 2333–2341, 2002. [DOI] [PubMed] [Google Scholar]
- 127.Wu D, Ren Z, Pae M, Guo W, Cui X, Merrill AH, Meydani SN. Aging up-regulates expression of inflammatory mediators in mouse adipose tissue. J Immunol 179: 4829–4839, 2007. [DOI] [PubMed] [Google Scholar]
- 128.Xu H, Barnes GT, Yang Q, Tan G, Yang D, Chou CJ, Sole J, Nichols A, Ross JS, Tartaglia LA, Chen H. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J Clin Invest 112: 1821–1830, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Xu M, Palmer AK, Ding H, Weivoda MM, Pirtskhalava T, White TA, Sepe A, Johnson KO, Stout MB, Giorgadze N, Jensen MD, LeBrasseur NK, Tchkonia T, Kirkland JL. Targeting senescent cells enhances adipogenesis and metabolic function in old age. Elife 4: e12997, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Xu M, Tchkonia T, Ding H, Ogrodnik M, Lubbers ER, Pirtskhalava T, White TA, Johnson KO, Stout MB, Mezera V, Giorgadze N, Jensen MD, LeBrasseur NK, Kirkland JL. JAK inhibition alleviates the cellular senescence-associated secretory phenotype and frailty in old age. Proc Natl Acad Sci USA 112: 6301–6310, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Zhu Y, Tchkonia T, Fuhrmann-Stroissnigg H, Dai HM, Ling YY, Stout MB, Pirtskhalava T, Giorgadze N, Johnson KO, Giles CB, Wren JD, Niedernhofer LJ, Robbins PD, Kirkland JL. Identification of a novel senolytic agent, navitoclax, targeting the Bcl-2 family of anti-apoptotic factors. Aging Cell 15: 428–435, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Zhu Y, Tchkonia T, Pirtskhalava T, Gower AC, Ding H, Giorgadze N, Palmer AK, Ikeno Y, Hubbard GB, Lenburg M, O'Hara SP, LaRusso NF, Miller JD, Roos CM, Verzosa GC, LeBrasseur NK, Wren JD, Farr JN, Khosla S, Stout MB, McGowan SJ, Fuhrmann-Stroissnigg H, Gurkar AU, Zhao J, Colangelo D, Dorronsoro A, Ling YY, Barghouthy AS, Navarro DC, Sano T, Robbins PD, Niedernhofer LJ, Kirkland JL. The Achilles' heel of senescent cells: from transcriptome to senolytic drugs. Aging Cell 14: 644–658, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Zhu Y, Tchkonia T, Stout MB, Giorgadze N, Wang L, Li PW, Heppelmann CJ, Bouloumie A, Jensen MD, Bergen HR 3rd, Kirkland JL. Inflammation and the depot-specific secretome of human preadipocytes. Obesity (Silver Spring) 23: 989–999, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Zoico E, Roubenoff R. The role of cytokines in regulating protein metabolism and muscle function. Nutr Rev 60: 39–51, 2002. [DOI] [PubMed] [Google Scholar]