Endothelin-1 (ET-1) is recognized as the body’s most potent endogenous vasoconstrictor, but the impact of this peptide on vascular function is not well understood. The present study revealed that the intra-arterial administration of ET-1 impaired both microvascular and conduit vessel function of the leg in young, healthy, humans. Studies employing vascular testing in patient cohorts that experience a disease-related increase in ET-1 should thus exercise caution, as ET-1 clearly impairs vascular function.
Keywords: vascular function, cardiovascular disease, flow-mediated dilation, endothelium
Abstract
Endothelin-1 (ET-1), a potent vasoconstrictor secreted by vascular endothelial cells, has been implicated in the pathophysiology of numerous cardiovascular diseases, yet the direct impact of ET-1 on vascular function remains unclear. Therefore, in seven young (23 ± 1 yr) healthy subjects, we investigated the effect of an intra-arterial infusion of ET-1 on reactive hyperemia (RH) and flow-mediated dilation (FMD) in the popliteal artery following 5 min of suprasystolic cuff occlusion. ET-1 infusion significantly attenuated basal leg blood flow (control: 62 ± 4 ml/min, ET-1: 47 ± 9 ml/min), RH [area-under-curve (AUC); control: 162 ± 15 ml, ET-1: 104 ± 16 ml], and peak RH (control: 572 ± 51 ml/min, ET-1: 412 ± 32 ml/min) (P < 0.05). Administration of ET-1 also reduced FMD (control: 2.4 ± 0.3%, ET-1: 0.5 ± 0.5%) and FMD normalized for shear rate (control: 10.5 × 10−4 ± 2.0 × 10−4%/s−1, ET-1: 0.9 × 10−4 ± 2.8 ×10−4%/s−1). These findings reveal that elevated levels of ET-1 have a significant impact on vascular function, indicating that studies employing RH and FMD as markers of microvascular function and nitric oxide bioavailability, respectively, should exercise caution, as ET-1 can impact these assessments by tipping the balance between vasodilation and vasoconstriction, in favor of the latter.
NEW & NOTEWORTHY Endothelin-1 (ET-1) is recognized as the body’s most potent endogenous vasoconstrictor, but the impact of this peptide on vascular function is not well understood. The present study revealed that the intra-arterial administration of ET-1 impaired both microvascular and conduit vessel function of the leg in young, healthy, humans. Studies employing vascular testing in patient cohorts that experience a disease-related increase in ET-1 should thus exercise caution, as ET-1 clearly impairs vascular function.
the vascular endothelium plays a primary role in the modulation and initiation of vasomotion, vascular permeability, angiogenesis, and inflammation (23). Healthy endothelial function is therefore an essential component of vascular homeostasis and control, largely dependent on the delicate balance between vasodilators and vasoconstrictors (55). When this balance is disrupted endothelial dysfunction results, characterized by inflammation, thrombosis, vasoconstriction, and atherosclerotic lesion formation (40). Endothelial dysfunction has often been attributed to impaired endothelial-dependent vasodilation, with the predominance of studies focusing on nitric oxide (NO)-mediated vasodilation (9, 17, 50). However, it is also likely that this vascular dysfunction is the consequence of a complex interaction between both endothelial-derived constricting and dilating factors (62).
In addition to NO, one of the key vasoactive substances produced and secreted by vascular endothelial cells is endothelin-1 (ET-1). Facilitated by two receptor subtypes, ETA and ETB (35, 54), ET-1 is recognized as the most potent endogenous vasoconstrictor (68) and is elevated in patients with cardiovascular disease (12, 39, 57). Our group has identified the importance of this vasoconstrictor pathway in the cardiovascular response to exercise in both the young (4, 64) and old (3), providing evidence that this pathway is key in the governing of vasomotion in the peripheral circulation. Furthermore, ET-1 has been suggested to markedly attenuate endothelium-dependent vasodilation, as assessed by acetylcholine-induced changes in resistance vessel blood flow (8). This is likely the consequence of a combination of greater ET-1 induced vasoconstriction and, perhaps to a lesser extent, ET-1-mediated changes in NO bioavailability (1, 36) that together “tip the balance” between vasodilators and vasoconstrictors in favor of the latter. Currently, the extent of ET-1’s contribution to vascular dysfunction remains poorly understood.
The flow-mediated dilation (FMD) test has emerged as a widely used, noninvasive assessment of endothelium-dependent conduit artery vasomotion, predicting the existence and extent of vascular pathology (14, 24, 59). Indeed, attenuated FMD has been observed in both preclinical and clinical cardiovascular disease states (25, 47, 53). However, the FMD test provides limited information about vascular function at the all important levels of the microcirculation. Measuring the reactive hyperemia (RH) induced by a period of cuff occlusion fills this void, providing an index of microvascular function that is complimentary to conduit vascular function, assessed via FMD. Like FMD, there is evidence that RH is independently correlated with the incidence of future cardiovascular events (15) and predictive of poor prognoses (14) in patients with cardiovascular disease, but, just as with FMD, the functional contribution of elevated ET-1 on RH is not yet known. The direct intra-arterial infusion of ET-1 into young healthy subjects would afford a simple, yet robust, paradigm in which to assess the impact of ET-1 on peripheral vascular function.
Therefore, with the intent to ultimately better understand the pathophysiology of cardiovascular disease, this study aimed to directly investigate the effect of the intra-arterial infusion of ET-1 on FMD and RH in young healthy individuals. As ET-1 is a potent vasoconstrictor, we hypothesized that in a young healthy population, where endogenous ET-1 plasma levels are typically low, intra-arterial infusion of ET-1 into the leg would tip the balance between vasodilation and vasoconstriction, significantly attenuating both ischemically induced vasodilation in resistance arterioles (i.e., RH), and endothelial-dependent vasodilation in conduit arteries (i.e., FMD).
METHODS
Subjects and General Procedures
Subjects.
Seven young healthy subjects (23 ± 1 yr; 6 men, 1 woman) participated in this study. All subjects were sedentary, nonsmokers, normotensive (<140/90 mmHg), and free of overt cardiovascular disease. Subjects were excluded from participation if they were taking any medications that would alter vascular responsiveness. Informed signed consent was obtained according to the University of California, San Diego, Human Subjects Protection Program requirements. Following the completion of health histories and physical examinations on all subjects, each reported to the laboratory in a fasted state (>8 h postprandial) and had refrained from caffeine and exercise before the study (>24 h).
Experimental protocol.
Subjects reported to the laboratory at 0800 on the experimental day and after 30 min of supine rest; two catheters [common femoral artery (CFA) and antecubital vein] were placed using sterile technique as previously reported (2). After catheter placement, subjects rested for 30 min, and then the control FMD trial was performed. Following the control FMD, ET-1 was infused intra-arterially using a constant speed infusion pump (Harvard Apparatus, Holliston, MA). Immediately before commencing the infusion, real-time blood flow was determined at the common femoral artery using the ultrasound Doppler, and infusion rate was blood flow-adjusted according to these “on the fly” blood flow values to ensure a similar effective drug concentration between subjects with differing leg blood flows. Due to the relatively slow ET-1 response kinetics (31), ET-1 was infused continuously at 40 pg/ml leg blood flow/min for 30–50 min until a plateau in vasoconstriction was attained, as determined by two consecutively unchanged femoral artery blood flow measurements taken every 5 min. The impact of a higher drug dose on this plateau was then assessed by a 5-min ET-1 infusion at double the initial dose. The initial drug dose was then resumed, and the ET-1 infusion FMD trial was performed with continuous ET-1 infusion for the duration of the FMD procedure. Due to the long-lasting effects of intra-arterial ET-1 infusion (15), the control FMD trial always preceded the ET-1 infusion FMD trial. To determine basal and end-infusion circulating plasma concentrations of ET-1, two venous blood samples were drawn from the antecubital catheter. One sample was drawn before control FMD trial and the other before ET-1 infusion FMD trial.
FMD and RH protocol.
Details of the FMD procedure have been described previously (27) and were performed in accordance with recently published guidelines (26, 61). Briefly, a pneumatic cuff was positioned on the lower right leg below the knee, proximal to the placement of the ultrasound Doppler probe on the popliteal artery (46). Following baseline measurements, the leg cuff was inflated to suprasystolic pressure (>250 mmHg) for 5 min. Full occlusion was documented by the loss of ultrasound spectra in vessels at the ankle, distal to the cuff. The cuff was then rapidly deflated, and measurements of popliteal artery diameter and blood velocity was obtained for 2 min. RH was assessed from these postocclusion measurements, and quantified as the cumulative popliteal artery blood flow [i.e., area under the curve (AUC)] for the 2-min period post cuff release.
ET-1 infusion.
ET-1 (Clinalfa; Merck Biosciences, Läufelfingen, Switzerland) was administered as a nonselective ETA and ETB receptor agonist. Previous studies have demonstrated that the constrictor response to ET-1 is principally mediated by the ETA receptor subtype (20, 45), promoting vasoconstriction through vascular smooth muscle activation. ET-1 was prepared at a concentration of 12.5 ng/ml 0.9% sterile saline and infused at a blood flow-adjusted rate of 40 pg−1·ml leg blood flow·min−1 (infusion rates 1.0–1.6 ml/min). The vascular effects of ET-1 vary widely according to dose and duration of drug administration (29), making dose-response curves difficult to characterize. Thus, for the present study, a dose of ET-1 was selected that has previously been reported to promote significant vasoconstriction without systemic effects (16, 58, 64). However, it should be noted that the impact of a higher drug dose on the ET-1-induced plateau in blood flow was then briefly assessed by a 5-min ET-1 infusion at double the initial dose.
Measurements
Ultrasound Doppler.
The ultrasound system (Logiq 7; GE Medical Systems, Milwaukee, WI) was equipped with a linear array transducer operating at an imaging frequency of 10 MHz, with a spatial resolution of 0.15 mm. Vessel lumen diameter was measured at a perpendicular angle along the central axis of the scanned area, where the best spatial resolution was achieved. The blood velocity profile was obtained using the same transducer with a Doppler frequency of 4.0–5.0 MHz, an insonation angle of 60° or less, a sample volume maximized according to vessel size, and a sample depth of 1.0–3.5 cm. In duplex mode, real-time ultrasound imaging and the pulse-wave velocity profile were viewed simultaneously. From artery diameter and mean blood velocity (Vmean), blood flow was calculated as: blood flow (ml/min) = Vmean · π · (vessel diameter/2)2 × 60. RH was quantified using blood flow over time (AUC) integrated with the use of commercially available software (SigmaPlot 8.0; Systat Software, Point Richmond, CA) using the trapezoidal rule and calculated as: ∑{yi[x(i+1) − xi] + (1/2)[y(i+1) − yi][x(i+1) − xi]}. Peak RH was identified as the highest postocclusion blood flow.
Ultrasound images and Doppler velocity waveforms were measured at rest for a 20-s period and at −4- to 16-s, 25- to 45-s, 55- to 75-s, and 85- to 105-s postcuff release. At all sample points, arterial diameter and angle-corrected, time-averaged, and intensity-weighted Vmean values were calculated using commercially available software (Logiq 7; GE). End-diastolic, ECG R-wave-gated images were collected via video output from the Logic 7 for offline analysis of popliteal artery vasodilation using automated edge-detection software (Medical Imaging Applications, Coralville, IA) that has been well-described and validated previously (44). FMD was calculated as the percent change from resting artery diameter to the largest diameter achieved during the 105 s of postinflation imaging.
Shear rate.
Shear stress has been identified as a mechanism that stimulates the vascular endothelium and results in subsequent vasodilation (51). However, as blood viscosity was not measured or expected to change, shear rate, a commonly used surrogate, was calculated by using the equation: shear rate (s−1) = 4 · Vmean(cm/s)/vessel diameter (cm) (19, 49, 66, 67). Cumulative shear rate was quantified using shear rate over time (AUC) (48, 52), integrated with the use of commercially available software (SigmaPlot 8.0; Systat Software) using the trapezoidal rule and calculated as: ∑{yi[x(i+1) − xi] + (1/2)[y(i+1) − yi][x(i+1) − xi]}. To mathematically normalize vasodilation for shear rate, FMD was divided by cumulative shear rate at the point of maximal vasodilation (52).
Blood pressure and heart rate.
Arterial blood pressure measurements were collected continually from within the femoral artery with the pressure transducer placed at the level of the catheter (Transpac IV; Abbot Laboratories). Mean arterial pressure (MAP; mmHg) was calculated as diastolic arterial pressure + (arterial pulse pressure × 0.33). Heart rate (HR) was monitored from a standard three-lead ECG as an integral component of the Doppler ultrasound system (Logiq 7; GE Medical Systems).
ET-1 concentration.
Plasma concentrations (pg/ml) of ET-1 were measured in duplicate and then averaged, utilizing a commercially available enzyme-linked immunosorbent assay (ELISA; R&D Systems, Minneapolis, MN).
Statistics.
Statistics were performed using commercially available software (SigmaStat 3.10; Systat Software). Repeated-measures ANOVA and paired t-tests were used to identify significant changes in measured variables within and between the control FMD and ET-1 infusion FMD trials, with the Bonferroni test used for post hoc analysis when a significant main effect was identified. All group data are expressed as means ± SE. Statistical significance was established at P < 0.05.
RESULTS
Subject characteristics are reported in Table 1. With seven subjects, the effect size was 1.9 for FMD and SD was 1.4 and power = 0.80 with an α of 0.05.
Table 1.
Subject characteristics
| Age, yr | 23 ± 1 |
| Height, cm | 175 ± 3 |
| Weight, kg | 69 ± 4 |
| Body mass index | 22 ± 1 |
| Heart rate, beats/min | 68 ± 5 |
| Mean arterial pressure, mmHg | 106 ± 2 |
| Triglycerides, mg/dl | 95 ± 17 |
| Total cholesterol, mg/dl | 160 ± 19 |
| HDL cholesterol, mg/dl | 42 ± 5 |
| LDL cholesterol, mg/dl | 99 ± 14 |
| Hemoglobin, g/dl | 15 ± 0.2 |
Values are means ± SE. HDL, high-density lipoprotein; LDL, low-density lipoprotein.
ET-1 Infusion Time
On average, ET-1 was infused for 40 ± 3 min to reach maximal ET-1-mediated vasoconstriction. As illustrated in Table 2, systemic plasma ET-1 levels were not elevated as a consequence of the intra-arterial infusion.
Table 2.
Impact of endothelin-1 infusion on cardiovascular parameters
| Preinfusion | End Infusion | |
|---|---|---|
| Heart rate, beats/min | 68 ± 5 | 71 ± 3 |
| Mean arterial pressure, mmHg | 106 ± 2 | 106 ± 4 |
| Popliteal artery diameter, cm | 0.59 ± 0.02 | 0.59 ± 0.02 |
| Popliteal artery mean blood velocity, cm/s | 26.2 ± 2.3 | 19.1 ± 3.4 |
| Popliteal blood flow, ml/min | 62 ± 4 | 47 ± 9* |
| Shear rate, s−1 | 26 ± 2 | 19 ± 3* |
| Systemic plasma (endothelin-1), pg/ml | 1.61 ± 0.13 | 1.65 ± 0.18 |
Values are means ± SE.
Significant difference between preinfusion and end infusion (P < 0.05).
HR, MAP, and Popliteal Artery Diameter
From preinfusion through the end of ET-1 infusion, HR, MAP, and popliteal artery diameter remained unchanged (P > 0.05; Table 2).
Reactive Hyperemia
Both RH (control: 162 ± 15 ml, ET-1: 104 ± 16 ml) and peak RH (control: 572 ± 51 ml/min, ET-1: 412 ± 32 ml/min) were significantly attenuated during the ET-1 infusion FMD trial compared with the control FMD trial (P < 0.05; Fig. 1).
Fig. 1.
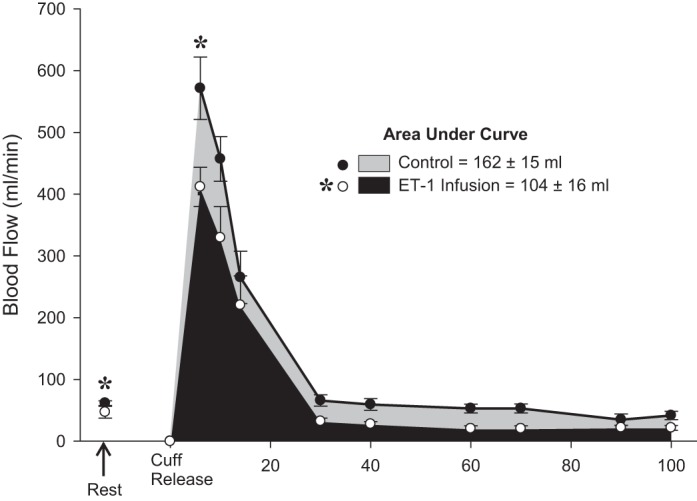
Resting blood flow and reactive hyperemia following cuff release. *Significant differences in resting blood flow, reactive hyperemia, and peak reactive hyperemia between control flow-mediated dilation (FMD) and endothelin-1 (ET-1) infusion FMD trials (P < 0.05).
Shear Rate
Resting shear rate was significantly attenuated during the ET-1 infusion FMD trial compared with the control FMD (P < 0.05; Table 2). However, following cuff release, shear rate AUC, assessed up to the point of maximal vasodilation, was not significantly different between the two trials (see Fig. 3B).
Fig. 3.
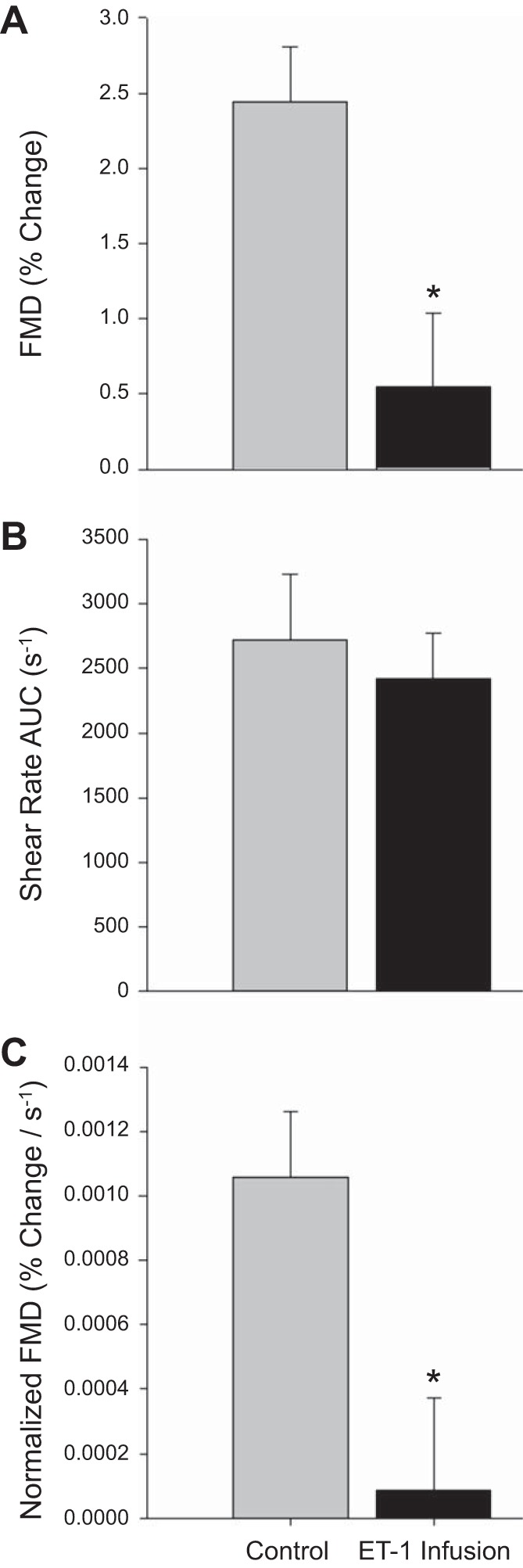
Flow-mediated dilation (FMD; %diameter change; A), shear rate (area under curve; B), and FMD normalized for shear rate [area under curve (AUC); C]. *Significant difference between control FMD and endothelin-1 (ET-1) infusion FMD trials (P < 0.05).
Flow-Mediated Dilation
A statistically significant vasodilation was observed postcuff release in the control FMD trial (P < 0.05). However, dilation during the ET-1 infusion trial did not achieve statistical significance (P = 0.35). Consistent with the literature, peak dilation occurred, on average, between 50 and 70 s following cuff release (7, 18) and time to peak dilation was not different between trials. Popliteal artery diameter increased significantly during the FMD test in the control trial but did not change from baseline values in the ET-1 trial (Fig. 2). Similarly, FMD, expressed as a relative change, revealed a significant attenuation during the ET-1 infusion trial (control: 2.4 ± 0.3%, ET-1 infusion: 0.5 ± 0.5%; P < 0.05; Fig. 3A). When FMD was normalized for the shear rate (AUC), the ET-1 infusion FMD trial remained significantly attenuated compared with the control FMD (control: 10.5 × 10−4 ± 2.0 × 10−4, ET-1 infusion: 0.9 × 10−4 ± 2.8 × 10−4% Δ/s−1; P < 0.05; Fig. 3C).
Fig. 2.
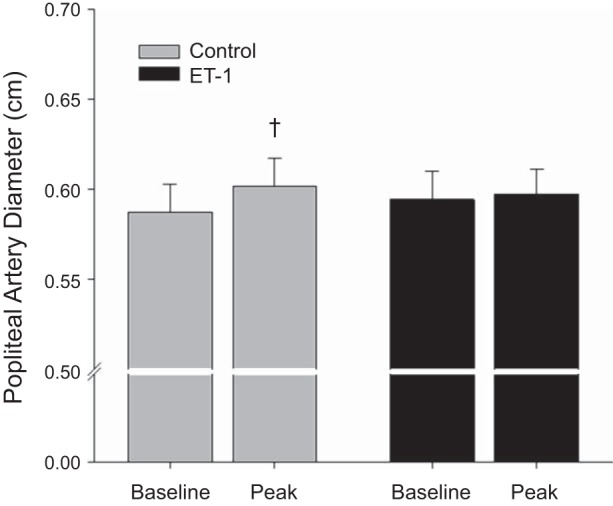
Baseline and peak change in popliteal artery diameter values during the flow-mediated dilation (FMD) test during control and endothelin-1 (ET-1) trials. †Significant change from baseline (P < 0.05).
DISCUSSION
The primary findings of this study are that the exogenous infusion of ET-1 significantly attenuated RH and FMD in young healthy individuals. Of note, there was a significant reduction in basal blood flow with no significant effect on basal popliteal artery diameter. In combination, these data suggest that ET-1 plays a significant vasoconstrictive role in the resistance vascular bed, while likely contributing more as a disruptive agent on endothelial function in the conduit artery, with the latter effect negatively impacting FMD. Accordingly, studies employing RH and FMD as markers of microvascular function and NO bioavailability, respectively, in cardiovascular disease patients should exercise caution, as ET-1, often elevated in this population, can impact these assessments by tipping the balance between vasodilation and vasoconstriction, in favor of the latter. Indeed, failure to recognize the impact of ET-1-mediated vasoconstriction on RH and FMD measurements could, therefore, lead to erroneous conclusions regarding endothelial health in patients with cardiovascular disease.
ET-1-Modulated Vasoconstriction
The overall cardiovascular effect of endogenous ET-1 depends on the balance between ETA and ETB receptor-mediated responses. Located on endothelial cells, the ETB receptor primarily elicits a NO-mediated vasodilation, whereas ET-1’s vasoconstrictive effects are derived from both the ETA and ETB receptors located on the vascular smooth muscle cells (30). Combined ETA/ETB receptor antagonist studies have revealed that the predominant physiological effect of ET-1 is vasoconstriction (28), establishing the importance of this endothelial-derived peptide in providing long-lasting increases in vascular tone in response to stimuli such as increases in pulsatile stretch (42), shear stress (43), hypoxia (37), and a reduction in pH. Since these physical and chemical stimuli are among the host of changes that take place within the skeletal muscle during exercise (41), our group has recently undertaken a series of studies to evaluate the importance of the ET-1 pathway in the regulation of skeletal muscle blood flow and arterial blood pressure during knee-extensor exercise. Through the intra-arterial administration of ET-1 and ETA receptor inhibitors, these studies identified vasoconstriction via the ETA receptor as an important mechanism for both the restraint of blood flow in the exercising limb and maintenance of arterial blood pressure in young (4, 64) and older (3) healthy adults. While these previous studies provided new information regarding the importance of this pathway in the cardiovascular response to exercise, it left unanswered the important question of whether ET-1-mediated vasoconstriction influences peripheral vascular function.
In the present study, ET-1-induced vasoconstriction, documented by the reduction in blood flow, reached a plateau, on average, following 40 min of infusion, in agreement with previous work from our group (64). Interestingly, the infusion of exogenous ET-1 did not significantly alter basal popliteal artery diameter (Table 2 and Fig. 2), while the attenuation in basal blood flow and RH (Fig. 1) revealed a significant vasoconstrictive effect upon the resistance vessels further downstream. These data are in agreement with previous findings that revealed an ET-1-mediated attenuation in endothelial-dependent vasodilation in resistance vessels, as elicited by intra-arterial infusions of acetylcholine (10, 11, 38). However, the current data extend this knowledge of ET-1’s vascular consequence to larger conduit arteries (Table 2), revealing that ET-1 plays a less significant vasoconstrictive role in conduit arteries when compared with the resistance vasculature (Fig. 1).
Huang et al. (33) recently reported the clinical significance of RH, documenting that a lower RH is associated with increased cardiovascular risk in patients with peripheral artery disease. The current data clearly exhibit the negative impact of ET-1 on RH and raise the interesting possibility that elevated ET-1, as often found in diseased populations (12, 39, 57), could be a primary cause of decreased RH. Certainly, further research in this particular area is necessary to elucidate the clinical relevance of this potential link among ET-1, RH, and cardiovascular risk.
ET-1 and Endothelial Dysfunction
Although there have been several studies documenting an improvement in endothelial-mediated blood flow with the infusion of ET-1 receptor antagonists (5, 6, 8, 10, 28), this is the first study to observe the direct effect of intra-arterial infusion of ET-1 on FMD. A major finding of this study was that the infusion of ET-1, although not affecting basal vessel diameter, did attenuate FMD (Figs. 2 and 3, A and C), without altering the major stimulus, shear stress (Fig. 3B). This provides evidence that elevated levels of ET-1 have a functional effect on conduit arteries, attenuating the mechanisms driving FMD. In line with these observations, Casey et al. (13) demonstrated that FMD was inversely related to endogenous plasma concentrations of ET-1 in coronary artery disease patients, suggesting ET-1 may have an inhibitory role in FMD. Although the current study is the first to document a direct consequence of intra-arterial infusion of exogenous ET-1 on FMD in young healthy individuals, other studies have investigated the direct and indirect effects of ET-1 on endothelial function, and specifically NO bioavailability (34). By receptor-mediated processes, ET-1 has been implicated in the lowering of NO production [caveolin-1-mediated inhibition of endothelial nitric oxide synthase (eNOS) activity] (36) or increasing NO degradation (via formation of reactive oxygen species) (1). Furthermore, Spieker et al. (56) observed a marked decrease in vascular function, as measured by radial artery FMD, after a 3-min mental stress task, a response that was prevented following intra-arterial infusion of an endothelin-A receptor antagonist (BQ-123). However, only blocking the endothelin-A receptors, as performed in the study of Spieker et al. (56), means that endothelin-B receptors, known to provoke vasodilation via a NO-mediated mechanism (63), may have been more stimulated by a given ET-1 concentration, making the findings a somewhat difficult to interpret. Regardless, by its multiple mechanisms, the intra-arterial infusion of ET-1 in the current study may have altered the contribution of NO to FMD. Germane to many preclinical and clinical cardiovascular disease populations that often exhibit both an attenuated FMD and elevated ET-1 levels (25, 47, 53), we speculate that although decreased FMD may indeed be related to a decrease in NO in these populations, this may also be, to some extent, attributable to elevated ET-1 levels. However, we recognize that establishing a causal role between infused ET-1 and reduced NO bioavailability is beyond the scope of the present study and suggest that additional investigations in both healthy and patient populations are warranted to explore this interesting possibility.
Classically, endothelial dysfunction has been attributed to a decrease in NO by a disturbance in its precursors and governing mechanisms. Indeed, the current data suggest that elevated levels of ET-1 have a considerable effect on NO bioavailability and therefore contribute to endothelial dysfunction. Donato et al. (21) revealed that with advancing age, eNOS expression and activation were not different from the young but interestingly found significantly elevated levels of endothelial cell ET-1 expression and associated endothelial dysfunction in the aged group. Their data suggest that despite the preservation of the key enzyme eNOS, ET-1 was associated with a dysfunctional endothelium. This underscores ET-1’s important role in the continuum of endothelial dysfunction and subsequent vascular disease.
The observed reduction in FMD following ET-1 administration raises the question of whether this response is unique to the ET-1 pathway or if vasoconstriction of any origin would impair the FMD response. Although the present study was not designed to address this issue but rather was focused upon ET-1 which is elevated in patients with cardiovascular disease (12, 39, 57), it is noteworthy that others have reported a similar reduction in FMD following experimental manipulation of the α-adrenergic pathway. Indeed, several studies to date have identified the ability of acute increases in sympathetic nervous system activity to markedly reduce brachial artery FMD (22, 32, 60), implicating this pathway as an additional, important mediator of endothelium-dependent vasodilation. Taken together, these previous and current findings build a strong case for the modulatory influence of vasoconstrictors on the FMD response in young, healthy adults. However, such results also raise the question of whether this relationship should be considered when assessing vascular function in patient populations characterized by increased activity of these vasoconstricting pathways.
Experimental Considerations
It is acknowledged that a complete dose-response relationship was not performed for ET-1; however, doses were chosen based on previous studies that have identified the highest attainable dose that may be administered while minimizing the risk of drug spillover into the systemic circulation (64) (Table 2), a consequence that could alter systemic pressure and cloud the interpretation of blood flow and vascular responses (16, 58). While infusion of ET-1 upstream from the site of measurement should theoretically create favorable conditions for ET-1 to bind with ETA/ETB receptors throughout the arterial tree of the leg, the exact site of binding within the lower limb circulation is unknown. However, the attenuated FMD in the presence of the infused ET-1, with no change in shear rate, is supportive of a local effect of the ET-1 in the popliteal artery itself. It is also acknowledged that antagonist drug infusions were not undertaken to examine whether ETA/ETB receptor inhibition could block the effect of ET-1 on FMD or RH and also recognized that endothelium-independent responses to ET-1 were not determined.
Conclusion
The results of this study provide new insight into the impact of ET-1 on blood flow control as measured by RH and vascular function as measured by FMD. Specifically, intra-arterial infusion of exogenous ET-1 in young, apparently healthy individuals caused a significant attenuation in basal blood flow and in RH, without affecting basal conduit artery diameter. However, locally elevated ET-1 levels significantly attenuated FMD. These data suggest that ET-1 plays a significant vasoconstrictive role in the resistance vascular bed, while contributing more as an endothelial dysfunction/disruptive agent in the conduit artery. Moreover, cautious interpretation of FMD should be exercised as ET-1, often elevated in preclinical and clinical cardiovascular disease patient populations, can indeed affect peripheral vascular function by tipping the delicate vasoactive balance in favor of vasoconstriction.
GRANTS
This work was funded National Heart, Lung, and Blood Institute Grants HL-091830, and HL-118313 and the U.S. Department of Veterans Affairs Grants RX000182, RX001433, and RX001418.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
S.K.N., D.W.W., and R.S.R. performed experiments; S.K.N., J.Z., D.W.W., and R.S.R. analyzed data; S.K.N., J.Z., D.W.W., and R.S.R. interpreted results of experiments; S.K.N., D.W.W., and R.S.R. prepared figures; S.K.N., D.W.W., and R.S.R. drafted manuscript; S.K.N., J.Z., D.W.W., and R.S.R. edited and revised manuscript; S.K.N., J.Z., D.W.W., and R.S.R. approved final version of manuscript.
ACKNOWLEDGMENTS
All experiments were performed at the Human Physiology Laboratory, Division of Physiology, University of California San Diego.
REFERENCES
- 1.Amiri F, Virdis A, Neves MF, Iglarz M, Seidah NG, Touyz RM, Reudelhuber TL, Schiffrin EL. Endothelium-restricted overexpression of human endothelin-1 causes vascular remodeling and endothelial dysfunction. Circulation 110: 2233–2240, 2004. doi: 10.1161/01.CIR.0000144462.08345.B9. [DOI] [PubMed] [Google Scholar]
- 2.Barden J, Lawrenson L, Poole JG, Kim J, Wray DW, Bailey DM, Richardson RS. Limitations to vasodilatory capacity and. VO2 max in trained human skeletal muscle. Am J Physiol Heart Circ Physiol 292: H2491–H2497, 2007. doi: 10.1152/ajpheart.01396.2006. [DOI] [PubMed] [Google Scholar]
- 3.Barrett-O’Keefe Z, Ives SJ, Trinity JD, Morgan G, Rossman MJ, Donato AJ, Runnels S, Morgan DE, Gmelch BS, Bledsoe AD, Richardson RS, Wray DW. Endothelin-A-mediated vasoconstriction during exercise with advancing age. J Gerontol A Biol Sci Med Sci 70: 554-556, 2015. doi: 10.1093/gerona/glu065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barrett-O’Keefe Z, Ives SJ, Trinity JD, Morgan G, Rossman MJ, Donato AJ, Runnels S, Morgan DE, Gmelch BS, Bledsoe AD, Richardson RS, Wray DW. Taming the “sleeping giant”: the role of endothelin-1 in the regulation of skeletal muscle blood flow and arterial blood pressure during exercise. Am J Physiol Heart Circ Physiol 304: H162–H169, 2013. doi: 10.1152/ajpheart.00603.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barton M, d’Uscio LV, Shaw S, Meyer P, Moreau P, Lüscher TF. ET(A) receptor blockade prevents increased tissue endothelin-1, vascular hypertrophy, and endothelial dysfunction in salt-sensitive hypertension. Hypertension 31: 499–504, 1998. doi: 10.1161/01.HYP.31.1.499. [DOI] [PubMed] [Google Scholar]
- 6.Berger R, Stanek B, Hülsmann M, Frey B, Heher S, Pacher R, Neunteufl T. Effects of endothelin a receptor blockade on endothelial function in patients with chronic heart failure. Circulation 103: 981–986, 2001. doi: 10.1161/01.CIR.103.7.981. [DOI] [PubMed] [Google Scholar]
- 7.Black MA, Cable NT, Thijssen DH, Green DJ. Importance of measuring the time course of flow-mediated dilatation in humans. Hypertension 51: 203–210, 2008. doi: 10.1161/HYPERTENSIONAHA.107.101014. [DOI] [PubMed] [Google Scholar]
- 8.Böhm F, Ahlborg G, Pernow J. Endothelin-1 inhibits endothelium-dependent vasodilatation in the human forearm: reversal by ETA receptor blockade in patients with atherosclerosis. Clin Sci (Lond) 102: 321–327, 2002. doi: 10.1042/cs1020321. [DOI] [PubMed] [Google Scholar]
- 9.Cai H, Harrison DG. Endothelial dysfunction in cardiovascular diseases: the role of oxidant stress. Circ Res 87: 840–844, 2000. doi: 10.1161/01.RES.87.10.840. [DOI] [PubMed] [Google Scholar]
- 10.Cardillo C, Campia U, Kilcoyne CM, Bryant MB, Panza JA. Improved endothelium-dependent vasodilation after blockade of endothelin receptors in patients with essential hypertension. Circulation 105: 452–456, 2002. doi: 10.1161/hc0402.102989. [DOI] [PubMed] [Google Scholar]
- 11.Cardillo C, Kilcoyne CM, Cannon RO III, Panza JA. Interactions between nitric oxide and endothelin in the regulation of vascular tone of human resistance vessels in vivo. Hypertension 35: 1237–1241, 2000. doi: 10.1161/01.HYP.35.6.1237. [DOI] [PubMed] [Google Scholar]
- 12.Cardillo C, Kilcoyne CM, Waclawiw M, Cannon RO III, Panza JA. Role of endothelin in the increased vascular tone of patients with essential hypertension. Hypertension 33: 753–758, 1999. doi: 10.1161/01.HYP.33.2.753. [DOI] [PubMed] [Google Scholar]
- 13.Casey DP, Nichols WW, Conti CR, Braith RW. Relationship between endogenous concentrations of vasoactive substances and measures of peripheral vasodilator function in patients with coronary artery disease. Clin Exp Pharmacol Physiol 37: 24–28, 2010. [DOI] [PubMed] [Google Scholar]
- 14.Celermajer DS, Sorensen KE, Spiegelhalter DJ, Georgakopoulos D, Robinson J, Deanfield JE. Aging is associated with endothelial dysfunction in healthy men years before the age-related decline in women. J Am Coll Cardiol 24: 471–476, 1994. doi: 10.1016/0735-1097(94)90305-0. [DOI] [PubMed] [Google Scholar]
- 15.Clarke JG, Larkin SW, Benjamin N, Keogh BE, Chester A, Davies GJ, Taylor KM, Maseri A. Endothelin-1 is a potent long-lasting vasoconstrictor in dog peripheral vasculature in vivo. J Cardiovasc Pharmacol 13, Suppl 5: S211–S212, 1989. doi: 10.1097/00005344-198900135-00061. [DOI] [PubMed] [Google Scholar]
- 16.Cockcroft JR, Clarke JG, Webb DJ. The effect of intra-arterial endothelin on resting blood flow and sympathetically mediated vasoconstriction in the forearm of man. Br J Clin Pharmacol 31: 521–524, 1991. doi: 10.1111/j.1365-2125.1991.tb05573.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cooke JP, Dzau VJ. Nitric oxide synthase: role in the genesis of vascular disease. Annu Rev Med 48: 489–509, 1997. doi: 10.1146/annurev.med.48.1.489. [DOI] [PubMed] [Google Scholar]
- 18.Corretti MC, Anderson TJ, Benjamin EJ, Celermajer D, Charbonneau F, Creager MA, Deanfield J, Drexler H, Gerhard-Herman M, Herrington D, Vallance P, Vita J, Vogel R; International Brachial Artery Reactivity Task Force . Guidelines for the ultrasound assessment of endothelial-dependent flow-mediated vasodilation of the brachial artery: a report of the International Brachial Artery Reactivity Task Force. J Am Coll Cardiol 39: 257–265, 2002. doi: 10.1016/S0735-1097(01)01746-6. [DOI] [PubMed] [Google Scholar]
- 19.de Groot PC, Poelkens F, Kooijman M, Hopman MT. Preserved flow-mediated dilation in the inactive legs of spinal cord-injured individuals. Am J Physiol Heart Circ Physiol 287: H374–H380, 2004. doi: 10.1152/ajpheart.00958.2003. [DOI] [PubMed] [Google Scholar]
- 20.Deng LY, Li JS, Schiffrin EL. Endothelin receptor subtypes in resistance arteries from humans and rats. Cardiovasc Res 29: 532–535, 1995. doi: 10.1016/S0008-6363(96)88530-0. [DOI] [PubMed] [Google Scholar]
- 21.Donato AJ, Gano LB, Eskurza I, Silver AE, Gates PE, Jablonski K, Seals DR. Vascular endothelial dysfunction with aging: endothelin-1 and endothelial nitric oxide synthase. Am J Physiol Heart Circ Physiol 297: H425–H432, 2009. doi: 10.1152/ajpheart.00689.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dyson KS, Shoemaker JK, Hughson RL. Effect of acute sympathetic nervous system activation on flow-mediated dilation of brachial artery. Am J Physiol Heart Circ Physiol 290: H1446–H1453, 2006. doi: 10.1152/ajpheart.00771.2005. [DOI] [PubMed] [Google Scholar]
- 23.Esper RJ, Nordaby RA, Vilariño JO, Paragano A, Cacharrón JL, Machado RA. Endothelial dysfunction: a comprehensive appraisal. Cardiovasc Diabetol 5: 4, 2006. doi: 10.1186/1475-2840-5-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Faulx MD, Wright AT, Hoit BD. Detection of endothelial dysfunction with brachial artery ultrasound scanning. Am Heart J 145: 943–951, 2003. doi: 10.1016/S0002-8703(03)00097-8. [DOI] [PubMed] [Google Scholar]
- 25.Gokce N. Clinical Manifestations of Endothelial Dysfunction. Philadelphia, PA: Lippincott Williams & Wilkins, 2002, p. 685–706. [Google Scholar]
- 26.Harris RA, Nishiyama SK, Wray DW, Richardson RS. Ultrasound assessment of flow-mediated dilation. Hypertension 55: 1075–1085, 2010. doi: 10.1161/HYPERTENSIONAHA.110.150821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Harris RA, Nishiyama SK, Wray DW, Tedjasaputra V, Bailey DM, Richardson RS. The effect of oral antioxidants on brachial artery flow-mediated dilation following 5 and 10 min of ischemia. Eur J Appl Physiol 107: 445–453, 2009. doi: 10.1007/s00421-009-1147-x. [DOI] [PubMed] [Google Scholar]
- 28.Haynes WG, Ferro CJ, O’Kane KP, Somerville D, Lomax CC, Webb DJ. Systemic endothelin receptor blockade decreases peripheral vascular resistance and blood pressure in humans. Circulation 93: 1860–1870, 1996. doi: 10.1161/01.CIR.93.10.1860. [DOI] [PubMed] [Google Scholar]
- 29.Haynes WG, Strachan FE, Webb DJ. Endothelin ETA and ETB receptors cause vasoconstriction of human resistance and capacitance vessels in vivo. Circulation 92: 357–363, 1995. doi: 10.1161/01.CIR.92.3.357. [DOI] [PubMed] [Google Scholar]
- 30.Haynes WG, Webb DJ. Endothelin as a regulator of cardiovascular function in health and disease. J Hypertens 16: 1081–1098, 1998. doi: 10.1097/00004872-199816080-00001. [DOI] [PubMed] [Google Scholar]
- 31.Haynes WG, Webb DJ. Endothelin: a long-acting local constrictor hormone. Br J Hosp Med 47: 340–349, 1992. [PubMed] [Google Scholar]
- 32.Hijmering ML, Stroes ES, Olijhoek J, Hutten BA, Blankestijn PJ, Rabelink TJ. Sympathetic activation markedly reduces endothelium-dependent, flow-mediated vasodilation. J Am Coll Cardiol 39: 683–688, 2002. doi: 10.1016/S0735-1097(01)01786-7. [DOI] [PubMed] [Google Scholar]
- 33.Huang AL, Silver AE, Shvenke E, Schopfer DW, Jahangir E, Titas MA, Shpilman A, Menzoian JO, Watkins MT, Raffetto JD, Gibbons G, Woodson J, Shaw PM, Dhadly M, Eberhardt RT, Keaney JF Jr, Gokce N, Vita JA. Predictive value of reactive hyperemia for cardiovascular events in patients with peripheral arterial disease undergoing vascular surgery. Arterioscler Thromb Vasc Biol 27: 2113–2119, 2007. doi: 10.1161/ATVBAHA.107.147322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Iglarz M, Clozel M. Mechanisms of ET-1-induced endothelial dysfunction. J Cardiovasc Pharmacol 50: 621–628, 2007. doi: 10.1097/FJC.0b013e31813c6cc3. [DOI] [PubMed] [Google Scholar]
- 35.Just A, Olson AJ, Arendshorst WJ. Dual constrictor and dilator actions of ET(B) receptors in the rat renal microcirculation: interactions with ET(A) receptors. Am J Physiol Renal Physiol 286: F660–F668, 2004. doi: 10.1152/ajprenal.00368.2003. [DOI] [PubMed] [Google Scholar]
- 36.Karaa A, Kamoun WS, Clemens MG. Oxidative stress disrupts nitric oxide synthase activation in liver endothelial cells. Free Radic Biol Med 39: 1320–1331, 2005. doi: 10.1016/j.freeradbiomed.2005.06.014. [DOI] [PubMed] [Google Scholar]
- 37.Kourembanas S, Marsden PA, McQuillan LP, Faller DV. Hypoxia induces endothelin gene expression and secretion in cultured human endothelium. J Clin Invest 88: 1054–1057, 1991. doi: 10.1172/JCI115367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Krum H, Cranswick N, Pellizzer AM. Effect of endothelin-1 on endothelium-derived vascular responsiveness in man. Clin Sci (Lond) 95: 151–156, 1998. doi: 10.1042/cs0950151. [DOI] [PubMed] [Google Scholar]
- 39.Lerman A, Edwards BS, Hallett JW, Heublein DM, Sandberg SM, Burnett JC Jr. Circulating and tissue endothelin immunoreactivity in advanced atherosclerosis. N Engl J Med 325: 997–1001, 1991. doi: 10.1056/NEJM199110033251404. [DOI] [PubMed] [Google Scholar]
- 40.Levine GN, Keaney JF Jr, Vita JA. Cholesterol reduction in cardiovascular disease. Clinical benefits and possible mechanisms. N Engl J Med 332: 512–521, 1995. doi: 10.1056/NEJM199502233320807. [DOI] [PubMed] [Google Scholar]
- 41.Lüscher TF, Barton M. Endothelins and endothelin receptor antagonists: therapeutic considerations for a novel class of cardiovascular drugs. Circulation 102: 2434–2440, 2000. doi: 10.1161/01.CIR.102.19.2434. [DOI] [PubMed] [Google Scholar]
- 42.Macarthur H, Warner TD, Wood EG, Corder R, Vane JR. Endothelin-1 release from endothelial cells in culture is elevated both acutely and chronically by short periods of mechanical stretch. Biochem Biophys Res Commun 200: 395–400, 1994. doi: 10.1006/bbrc.1994.1462. [DOI] [PubMed] [Google Scholar]
- 43.Malek A, Izumo S. Physiological fluid shear stress causes downregulation of endothelin-1 mRNA in bovine aortic endothelium. Am J PhysioCell Physiol 263: C389–C396, 1992. [DOI] [PubMed] [Google Scholar]
- 44.Mancini GB, Yeoh E, Abbott D, Chan S. Validation of an automated method for assessing brachial artery endothelial dysfunction. Can J Cardiol 18: 259–262, 2002. [PubMed] [Google Scholar]
- 45.Merkus D, Houweling B, Mirza A, Boomsma F, van den Meiracker AH, Duncker DJ. Contribution of endothelin and its receptors to the regulation of vascular tone during exercise is different in the systemic, coronary and pulmonary circulation. Cardiovasc Res 59: 745–754, 2003. doi: 10.1016/S0008-6363(03)00479-6. [DOI] [PubMed] [Google Scholar]
- 46.Nishiyama SK, Walter Wray D, Berkstresser K, Ramaswamy M, Richardson RS. Limb-specific differences in flow-mediated dilation: the role of shear rate. J Appl Physiol (1985) 103: 843–851, 2007. doi: 10.1152/japplphysiol.00273.2007. [DOI] [PubMed] [Google Scholar]
- 47.Panza JA, Quyyumi AA, Brush JE Jr, Epstein SE. Abnormal endothelium-dependent vascular relaxation in patients with essential hypertension. N Engl J Med 323: 22–27, 1990. doi: 10.1056/NEJM199007053230105. [DOI] [PubMed] [Google Scholar]
- 48.Parker BA, Smithmyer SL, Jarvis SS, Ridout SJ, Pawelczyk JA, Proctor DN. Evidence for reduced sympatholysis in leg resistance vasculature of healthy older women. Am J Physiol Heart Circ Physiol 292: H1148–H1156, 2007. doi: 10.1152/ajpheart.00729.2006. [DOI] [PubMed] [Google Scholar]
- 49.Parker BA, Trehearn TL, Meendering JR. Pick your Poiseuille: normalizing the shear stimulus in studies of flow-mediated dilation. J Appl Physiol (1985) 107: 1357–1359, 2009. doi: 10.1152/japplphysiol.91302.2008. [DOI] [PubMed] [Google Scholar]
- 50.Pober JS, Min W, Bradley JR. Mechanisms of endothelial dysfunction, injury, and death. Annu Rev Pathol 4: 71–95, 2009. doi: 10.1146/annurev.pathol.4.110807.092155. [DOI] [PubMed] [Google Scholar]
- 51.Pohl U, Holtz J, Busse R, Bassenge E. Crucial role of endothelium in the vasodilator response to increased flow in vivo. Hypertension 8: 37–44, 1986. doi: 10.1161/01.HYP.8.1.37. [DOI] [PubMed] [Google Scholar]
- 52.Pyke KE, Tschakovsky ME. The relationship between shear stress and flow-mediated dilatation: implications for the assessment of endothelial function. J Physiol 568: 357–369, 2005. doi: 10.1113/jphysiol.2005.089755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ross R. The pathogenesis of atherosclerosis--an update. N Engl J Med 314: 488–500, 1986. doi: 10.1056/NEJM198602203140806. [DOI] [PubMed] [Google Scholar]
- 54.Sakurai T, Yanagisawa M, Takuwa Y, Miyazaki H, Kimura S, Goto K, Masaki T. Cloning of a cDNA encoding a non-isopeptide-selective subtype of the endothelin receptor. Nature 348: 732–735, 1990. doi: 10.1038/348732a0. [DOI] [PubMed] [Google Scholar]
- 55.Spieker LE, Flammer AJ, Lüscher TF. The vascular endothelium in hypertension. Handb Exp Pharmacol 176: 249–283, 2006. doi: 10.1007/3-540-36028-X_8. [DOI] [PubMed] [Google Scholar]
- 56.Spieker LE, Hürlimann D, Ruschitzka F, Corti R, Enseleit F, Shaw S, Hayoz D, Deanfield JE, Lüscher TF, Noll G. Mental stress induces prolonged endothelial dysfunction via endothelin-A receptors. Circulation 105: 2817–2820, 2002. doi: 10.1161/01.CIR.0000021598.15895.34. [DOI] [PubMed] [Google Scholar]
- 57.Stewart DJ, Cernacek P, Costello KB, Rouleau JL. Elevated endothelin-1 in heart failure and loss of normal response to postural change. Circulation 85: 510–517, 1992. doi: 10.1161/01.CIR.85.2.510. [DOI] [PubMed] [Google Scholar]
- 58.Strachan FE, Newby DE, Sciberras DG, McCrea JB, Goldberg MR, Webb DJ. Repeatability of local forearm vasoconstriction to endothelin-1 measured by venous occlusion plethysmography. Br J Clin Pharmacol 54: 386–394, 2002. doi: 10.1046/j.1365-2125.2002.01653.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Takase B, Uehata A, Akima T, Nagai T, Nishioka T, Hamabe A, Satomura K, Ohsuzu F, Kurita A. Endothelium-dependent flow-mediated vasodilation in coronary and brachial arteries in suspected coronary artery disease. Am J Cardiol 82: 1535–1539, 1998. doi: 10.1016/S0002-9149(98)00702-4. [DOI] [PubMed] [Google Scholar]
- 60.Thijssen DH, Atkinson CL, Ono K, Sprung VS, Spence AL, Pugh CJ, Green DJ. Sympathetic nervous system activation, arterial shear rate, and flow-mediated dilation. J Appl Physiol (1985) 116: 1300–1307, 2014. doi: 10.1152/japplphysiol.00110.2014. [DOI] [PubMed] [Google Scholar]
- 61.Thijssen DH, Black MA, Pyke KE, Padilla J, Atkinson G, Harris RA, Parker B, Widlansky ME, Tschakovsky ME, Green DJ. Assessment of flow-mediated dilation in humans: a methodological and physiological guideline. Am J Physiol Heart Circ Physiol 300: H2–H12, 2011. doi: 10.1152/ajpheart.00471.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Thijssen DH, Rongen GA, Smits P, Hopman MT. Physical (in)activity and endothelium-derived constricting factors: overlooked adaptations. J Physiol 586: 319–324, 2008. doi: 10.1113/jphysiol.2007.145698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Verhaar MC, Strachan FE, Newby DE, Cruden NL, Koomans HA, Rabelink TJ, Webb DJ. Endothelin-A receptor antagonist-mediated vasodilatation is attenuated by inhibition of nitric oxide synthesis and by endothelin-B receptor blockade. Circulation 97: 752–756, 1998. doi: 10.1161/01.CIR.97.8.752. [DOI] [PubMed] [Google Scholar]
- 64.Wray DW, Nishiyama SK, Donato AJ, Sander M, Wagner PD, Richardson RS. Endothelin-1-mediated vasoconstriction at rest and during dynamic exercise in healthy humans. Am J Physiol Heart Circ Physiol 293: H2550–H2556, 2007. doi: 10.1152/ajpheart.00867.2007. [DOI] [PubMed] [Google Scholar]
- 66.Wray DW, Uberoi A, Lawrenson L, Richardson RS. Evidence of preserved endothelial function and vascular plasticity with age. Am J Physiol Heart Circ Physiol 290: H1271–H1277, 2006. doi: 10.1152/ajpheart.00883.2005. [DOI] [PubMed] [Google Scholar]
- 67.Wray DW, Uberoi A, Lawrenson L, Richardson RS. Heterogeneous limb vascular responsiveness to shear stimuli during dynamic exercise in humans. J Appl Physiol (1985) 99: 81–86, 2005. doi: 10.1152/japplphysiol.01285.2004. [DOI] [PubMed] [Google Scholar]
- 68.Yanagisawa M, Kurihara H, Kimura S, Tomobe Y, Kobayashi M, Mitsui Y, Yazaki Y, Goto K, Masaki T. A novel potent vasoconstrictor peptide produced by vascular endothelial cells. Nature 332: 411–415, 1988. doi: 10.1038/332411a0. [DOI] [PubMed] [Google Scholar]


