Our work exemplifies a novel role of neuropeptide Y (NPY) in regulating inflammation-induced tumorigenesis via two modalities: first by enhanced proliferation (PI3-K/pAkt), and second by downregulation of microRNA-375 (miR-375)-dependent apoptosis in intestinal epithelial cells. Our data establish the existence of a microRNA-mediated cross talk between enteric neurons producing NPY and intestinal epithelial cells, and the potential of neuropeptide-regulated miRNAs as potential therapeutic molecules for the management of inflammation-associated tumors in the gut.
Keywords: neuropeptide Y, inflammation-induced tumorigenesis, epithelial proliferation, miR-375
Abstract
We have demonstrated that neuropeptide Y (NPY), abundantly produced by enteric neurons, is an important regulator of intestinal inflammation. However, the role of NPY in the progression of chronic inflammation to tumorigenesis is unknown. We investigated whether NPY could modulate epithelial cell proliferation and apoptosis, and thus regulate tumorigenesis. Repeated cycles of dextran sodium sulfate (DSS) were used to model inflammation-induced tumorigenesis in wild-type (WT) and NPY knockout (NPY−/−) mice. Intestinal epithelial cell lines (T84) were used to assess the effects of NPY (0.1 µM) on epithelial proliferation and apoptosis in vitro. DSS-WT mice exhibited enhanced intestinal inflammation, polyp size, and polyp number (7.5 ± 0.8) compared with DSS-NPY−/− mice (4 ± 0.5, P < 0.01). Accordingly, DSS-WT mice also showed increased colonic epithelial proliferation (PCNA, Ki67) and reduced apoptosis (TUNEL) compared with DSS-NPY−/− mice. The apoptosis regulating microRNA, miR-375, was significantly downregulated in the colon of DSS-WT (2-fold, P < 0.01) compared with DSS-NPY−/−-mice. In vitro studies indicated that NPY promotes cell proliferation (increase in PCNA and β-catenin, P < 0.05) via phosphatidyl-inositol-3-kinase (PI3-K)-β-catenin signaling, suppressed miR-375 expression, and reduced apoptosis (increase in phospho-Bad). NPY-treated cells also displayed increased c-Myc and cyclin D1, and reduction in p21 (P < 0.05). Addition of miR-375 inhibitor to cells already treated with NPY did not further enhance the effects induced by NPY alone. Our findings demonstrate a novel regulation of inflammation-induced tumorigenesis by NPY-epithelial cross talk as mediated by activation of PI3-K signaling and downregulation of miR-375.
NEW & NOTEWORTHY Our work exemplifies a novel role of neuropeptide Y (NPY) in regulating inflammation-induced tumorigenesis via two modalities: first by enhanced proliferation (PI3-K/pAkt), and second by downregulation of microRNA-375 (miR-375)-dependent apoptosis in intestinal epithelial cells. Our data establish the existence of a microRNA-mediated cross talk between enteric neurons producing NPY and intestinal epithelial cells, and the potential of neuropeptide-regulated miRNAs as potential therapeutic molecules for the management of inflammation-associated tumors in the gut.
the link between chronic inflammation and cancer progression evolves from inflammation-induced generation of reactive oxygen species (ROS) that cause mutations in the tumor-suppressor genes leading to aberrations in cell cycle regulation and apoptosis (10, 22, 28, 39, 41). The inflammation-induced cancers are commonly associated with the digestive system, and include gastric cancer, hepatocellular carcinoma, and colitis-associated cancer (CAC). Clinical studies have demonstrated that patients with inflammatory bowel disease (IBD) are highly susceptible to CAC, and have worse prognosis compared with patients with sporadic colon cancer (2, 17, 43). Despite the similarity in location, the etiology of CAC is strikingly different from that of sporadic colon cancer (11, 16). CAC is mainly characterized by mutations in the tumor suppressor gene p53 (21). Inflammatory mediators especially cytokines play a major role in regulating inflammation-induced cancers as they have direct effects on epithelial signaling pathways like signal transducer and activator of transcription 3 (STAT3), mitogen activated protein kinase (MAPK), c-Jun NH2-terminal kinase (JNK), and nuclear factor-κB (NF-κB) that regulate cell survival, proliferation, migration, and apoptosis. Chronic inflammation also induces changes in microRNAs (miRNAs) that may regulate genes involved in tumor progression or suppression. miRNAs are small noncoding RNAs that regulate the expression of several target genes posttranscriptionally (3). It has been recently discovered that chronic inflammation can regulate epithelial miRNAs involved in cell cycle regulation, epithelial to mesenchymal transition, and apoptosis, etc., thus contributing to inflammation-associated cancers (22).
In addition to cytokines, the neuropeptides produced by the neurons in the gut (enteric nervous system or ENS) are powerful regulators of inflammatory responses (33), and neurogenic inflammation has been identified as a powerful component in inflammatory processes (7). However, the role and mechanism by which neuropeptides regulate the onset of CAC is not well defined. Neuropeptide Y (NPY) is an abundant 36-amino acid peptide that regulates diverse biological functions like food intake, anxiety, and sedation via five different G protein-coupled receptors (Y1, Y2, Y3, Y4, and Y5). We and others have demonstrated that NPY is an important regulator of inflammation (5, 6, 19, 44). We also demonstrated that NPY knockdown results in attenuated secretion of the pro-inflammatory cytokine TNF-α from enteric neurons of NPY knockout mice, establishing a regulatory cross talk between the enteric neuronal and immune compartments in the gut. However, the role of NPY in regulating the progression of chronic inflammation to tumorigenesis is not known. Here we investigated whether NPY could modulate epithelial cell proliferation and apoptosis, and thus regulate tumorigenesis via miRNA-based regulatory mechanisms.
MATERIALS AND METHODS
Animal models.
NPY knockout mouse (NPY−/−, stock no. 04545) and the corresponding WT mice on 129 S1/SvImJ backgrounds (stock no. 02448) were obtained from Jackson laboratories (Bar Harbor, ME). Mice were allowed standard chow and tap water ad libitum. All experiments were approved by the Emory University Institutional Animal Care and Use Committee.
Induction of inflammation-associated tumorigenesis.
Eight-week-old male and female WT and NPY−/− mice were administered 3 cycles of dextran sodium sulfate (3 cycles of 3% DSS in drinking water, alternating with 2 wk of regular drinking water in between each DSS cycle) (24). Body weight, stool consistency, and stool occult blood (Hemoccult SENSA; Beckman Coulter) was monitored both during the DSS and recovery phases to evaluate and compare the clinical scores between WT and NPY−/− mice. Mice were euthanized on day 64. The colon was carefully dissected longitudinally, and tumors were counted and tumor size (in mm of diameter) was measured under a dissecting microscope. Further, colons were flash frozen or paraffin fixed for histological evaluation by hematoxylin-eosin staining (H/E) or immunohistochemistry. The experimental groups of mice are shown in Table 1.
Table 1.
Experimental groups of mice
| Mice | Vehicle group | DSS group |
|---|---|---|
| WT | Water | DSS |
| NPY−/− | Water | DSS |
NPY−/−, neuropeptide Y knockout; WT, wild type.
Clinical score.
Mice were monitored daily for changes in weight, occult blood, and diarrhea during the period of DSS administration, and clinical score was determined as described previously (4). The body weight gain during the recovery period was also monitored.
Histopathology.
Colons were paraffin-embedded after fixation in phosphate-buffered formalin. Tissue sections (8 μm) were stained with hematoxylin/eosin, and scanned images were evaluated for the percentage of mucosa harboring epithelial injury, inflammation, ulcers, polyps, and adenocarcinoma (neoplasia) by Aperio’s Image scope viewer (31) (Aperio, Vista, CA).
Immunohistochemistry.
Paraffin-embedded sections of colon were stained for Ki67 (marker of cell proliferation). A minimum of 10 crypts was counted for Ki67-positive cells per section.
Terminal deoxynucleotidyl transferase-mediated dUTP nick end labeling staining.
Immunofluorescent terminal deoxynucleotidyl transferase-mediated dUTP nick end labeling (TUNEL) staining was performed to measure apoptosis in colonic sections using the In Situ Cell Death Detection Kit as described by the manufacturer (Roche).
Total RNA/micro RNA (miRNA) extraction and quantitative real-time PCR.
Total RNA and miRNA were extracted using the RNeasy kit and miRNaesy kit (Qiagen), respectively. Real-time PCR was performed to determine the expression of miR-375 in mouse tissue and T84 epithelial cells.
Cell culture and transfection.
T84 epithelial cells were used for in vitro studies. Cells were cultured in DMEM: F12 (1:1) containing 2 mM glutamine and 10% fetal bovine serum (FBS). Cells were treated with NPY (0.1 µM) for 24 or 48 h (6). For miRNA inhibitor studies, cells were transfected with either negative control miRNA or miR-375 inhibitor (Life Technologies) using Lipofectamine RNAiMAX transfection reagent (Life Technologies, Grand Island, NY).
Western blotting.
Western blotting for assessing cellular proliferation, apoptosis, and cell cycle regulation was done using Ki67 (BD Transduction), apoptotic signaling kit, β-catenin, pAkt, c-Myc (Cell Signaling, Danvers, MA), PCNA (Abcam, Cambridge, MA), and mouse monoclonal anti-β-actin (Sigma-Aldrich, St. Louis, MO).
Statistical analysis.
The statistical analyses were performed using the GraphPad Prism software (GraphPad, San Diego, CA). Values are expressed as means ± SE. The data were analyzed using t-test or 1-way ANOVA followed by a Tukey’s post hoc test as applicable. P value < 0.05 was considered statistically significant in all analyses.
RESULTS
NPY−/− mice exhibit reduced tumorigenesis compared with WT mice.
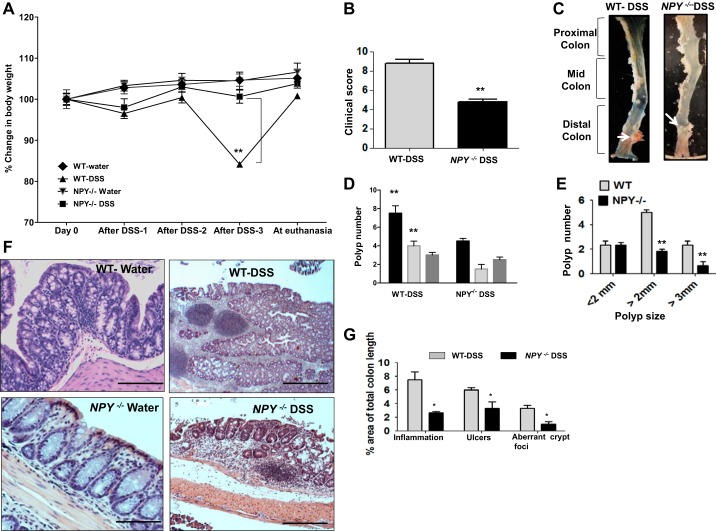
In the chronic DSS model of tumorigenesis [3 cycles of dextran sodium sulfate (3% DSS in drinking water), with a recovery period of 2 wk of regular water in between DSS cycles], we observed that NPY−/− mice presented with significantly attenuated inflammation, tumor incidence, and tumor size compared with WT mice. At the end of the experimental period NPY−/− mice recorded a net weight gain of ~3.8% (Fig. 1A, P < 0.01) compared with the WT mice. The clinical score (based on weight loss, occult blood, and diarrhea) was also higher in WT mice compared with the NPY−/− mice (Fig. 1B, P < 0.01). Analysis of the colon revealed that polyps were mainly localized in the distal and mid colons and absent from the proximal colon. WT mice had significantly higher number of polyps (7.5 ± 0.8) compared with NPY−/− mice displayed in the representative photos (4.0 ± 0.3, P < 0.01, Fig. 1, C and D). Analysis of the polyp sizes revealed that the majority of polyps in WT mice were larger than 2 mm in diameter compared with NPY−/− mice (Fig. 1E, P < 0.01). Further histopathological analysis by hematoxylin-eosin staining demonstrated higher incidence of high-grade dysplasia and adenocarcinoma in WT mice compared with NPY−/− mice, which exhibited low-grade dysplasia (Fig. 1F). In addition, a quantitative measure of histopathology was obtained by scanning H/E stained slides, and the area of mucosa displaying inflammation, ulcers, and abnormal crypt foci was also calculated as a percentage of the total colon area by image scope software (Aperio). We observed that WT mice exhibited more inflammation, ulceration, and aberrant crypt foci as represented in Fig. 1G (P < 0.05). Collectively, these results indicate that NPY−/− mice show less susceptibility to inflammation-induced tumorigenesis than WT mice.
Fig. 1.
NPY−/− mice exhibit reduced tumorigenesis compared with WT mice. Eight-week-old male and female WT and NPY−/− mice were administered repeated cycles of dextran sodium sulfate (3 cycles of % DSS alternating with 2 wk of regular water in between each DSS cycle) to induce inflammation-induced cancer. Body weight, stool consistency, and stool occult blood (Hemoccult SENSA; Beckman Coulter) were monitored during the DSS treatment and recovery phases to evaluate and compare the clinical score between WT and NPY−/− mice. Upon euthanasia, colon was cut open longitudinally, tumors were counted, and tumor size (diameter) was measured under a dissecting microscope. Further colons were paraffin fixed for histological evaluation. Tissue sections (8 μm) were stained with hematoxylin/eosin, and scanned images were evaluated for the percentage of mucosa harboring epithelial injury, inflammation, ulcers, polyps, and adenocarcinoma (neoplasia) by Aperio’s Image scope viewer (Aperio, Vista, CA). Body weight changes in water and DSS-treated groups of WT and NPY−/− mice during the experimental period (A), inflammatory score (B), representative distal colons from DSS-treated WT and NPY −/− mice showing large polyp in the WT (C), total polyp number (D), and polyp number vs. polyp size (E). F: representative colonic sections from mice stained with hematoxylin-eosin. G represents Aperio score. Values are means ± SE; n = 8. *P < 0.05, **P < 0.01. Scale bar, 20 μm; ×20.
Increased proliferation in WT mice accounts for enhanced tumorigenesis compared with NPY−/− mice.
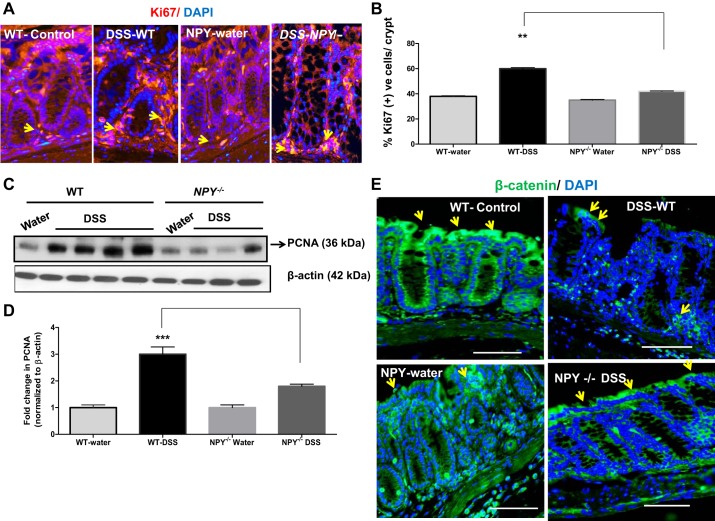
Balance between cellular proliferation and apoptosis is the key to intestinal homeostasis; however, during tumorigenesis proliferation overrides the apoptotic responses. To understand the mechanism by which NPY promotes tumor growth, we next assessed epithelial proliferation (ki67 marker) in the distal colonic sections from DSS–treated WT and NPY−/− mice. The number of ki67 positive (+ve) cells per crypt (red) was counted and expressed as percent of total number of DAPI + ve (blue) cells. There was a significant increase in percent ki67 cells/crypt in WT mice, indicating enhanced proliferation compared with DSS-NPY−/− mice (Fig. 2A, P < 0.01). These results are graphically represented in Fig. 2B. In addition to ki67, we also assessed another proliferative marker, viz, proliferating cell nuclear antigen (PCNA) by Western blotting. We observed significantly higher levels of PCNA in WT mice compared with NPY−/− mice (Fig. 2C). These results are graphically represented after densitometric analysis of blots where we observed approximately a threefold increase in PCNA from WT mice (Fig. 2D, P < 0.001). These findings suggest that NPY-induced intestinal epithelial cell proliferation might have enhanced the tumor growth in WT mice compared with NPY−/− mice.
Fig. 2.
WT mice exhibit increased proliferation compared with NPY−/− mice. Inflammation-induced tumors were modeled in WT and NPY−/− mice as described in materials and methods. Upon euthanasia, the colons were fixed in paraffin, and tissue sections were immunostained for proliferative markers Ki67 or β-catenin. The percent of Ki67 positive cells per crypt were calculated based on total cells enumerated from DAPI nuclear staining. Images were captured using a Zeiss fluorescence microscope. Distal colon from WT and NPY−/− mice was also probed for proliferating cell nuclear antigen (PCNA) by Western blotting. A: representative colonic sections with Ki67 immunostaining; arrows represent Ki67 + DAPI positive cells. B: graphical representation of %Ki67 positive cells per crypt. C: Western blots depicting the PCNA levels in mice. D represents the histogram. E: representative colonic sections from mice to depict changes in localization of β-catenin (green) and DAPI (blue) by immunostaining. Magnification, ×20. Values are means ± SE; n = 3–4. Significant differences in WT-DSS compared with NPY−/−-DSS group: **P < 0.01, ***P < 0.001. Scale bar, 20 μm.
Loss of membrane-bound β-catenin and increase in its nuclear translocation are hallmarks of tumors (27). Nuclear translocation of β-catenin triggers the transcription of several cell proliferative genes like c-Myc (myelocytomatosis oncogene) and cyclin D1 (30) that mediate enhanced proliferation in tumors. Immunostaining revealed a significant change in the localization of β-catenin in DSS-treated groups of mice. In the control mice in both groups, β-catenin (green) was more membrane-bound and highly expressed in the apical surface (represented by yellow arrows, Fig. 2E). However, upon DSS treatment WT mice displayed a loss of β-catenin from apical surfaces (yellow arrows), whereas in NPY−/− mice there was relatively reduced loss of β-catenin from apical surfaces. However, we have observed that WT mice exhibited more severe colitis with loss of the epithelial surface, which could account for significantly less staining in the apical area, and thereby changes in localization. These data suggest that NPY might be activating β-catenin signaling pathways, thus contributing to increased proliferation and tumorigenesis in WT mice.
Reduction of apoptosis in WT mice is associated with downregulation of microRNA miR-375.
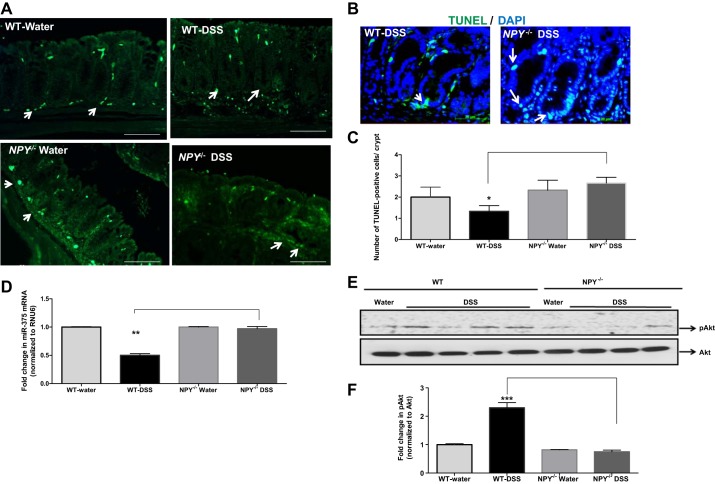
As apoptosis is a key limiting factor in tumorigenesis, we next investigated apoptosis in the colonic sections from WT and NPY−/− mice by TUNEL staining (Fig. 3A). There was a decrease in the number of TUNEL-positive cells per crypt in DSS-WT mice compared with DSS-NPY−/− mice (Fig. 3, B and C, P < 0.05). Taken together these data indicate that an increase in proliferation (from Fig. 2) coupled with reduction in apoptosis (Fig. 3) contributed to enhanced tumorigenesis in the WT mice compared with NPY−/− mice.
Fig. 3.
Reduced apoptosis in WT mice is associated with downregulation of micro RNA-375. Inflammation-induced tumors were modeled in WT and NPY−/− mice as described in materials and methods. Upon euthanasia, the colons were fixed in paraffin, tissue sections were immunostained to evaluate apoptosis using the TUNEL kit (Roche), and DAPI staining was also done. Images were captured using a Zeiss fluorescence microscope. microRNA was extracted from the tumors of mice using miRNesy kit (Qiagen), and expression of miR-375 was assessed by real-time PCR. A: representative colonic sections with TUNEL immunostaining; arrows represent TUNEL positive cells. B: the number of TUNEL-positive cells was evaluated per crypt, and the histogram is represented in C. Changes in miR-375 levels in mice (D); levels of miR-375 target protein pAKt in mouse tissue (E), and the corresponding histogram (F). Magnification 20×. Values are means ± SE; n = 3–4, Significant differences in WT-DSS compared with NPY−/−-DSS group:*P < 0.05, **P < 0.01, ***P < 0.001. Scale bar 20, μm.
Apoptosis-promoting microRNAs (miRNAs) are usually downregulated in cancer, and hence we investigated if reduced apoptosis was linked to miRNA downregulation. We extracted the miRNA from the colon of DSS-treated WT and NPY−/− mice and performed quantitative real-time PCR analysis of commonly downregulated miRNAs related to apoptosis. We observed that miR-375 was significantly downregulated in DSS-WT mice (2-fold) compared with DSS-NPY−/− mice (Fig. 3D, P < 0.01). Further analysis of the putative targets of miR-375 by a web-based tool (Target scan) indicated that targets of miR-375 include several signaling molecules that regulate survival, proliferation, and apoptosis like pAkt, caspase-3, and Bcl-2. We found that in accordance with miR-375 downregulation, the survival target pAkt (Fig. 3, E and F, P < 0.001) was upregulated in DSS-WT mice compared with DSS-NPY−/− mice.
NPY-induced epithelial cell proliferation involves β-catenin activation via phosphatidyl-inositol-3-kinase.
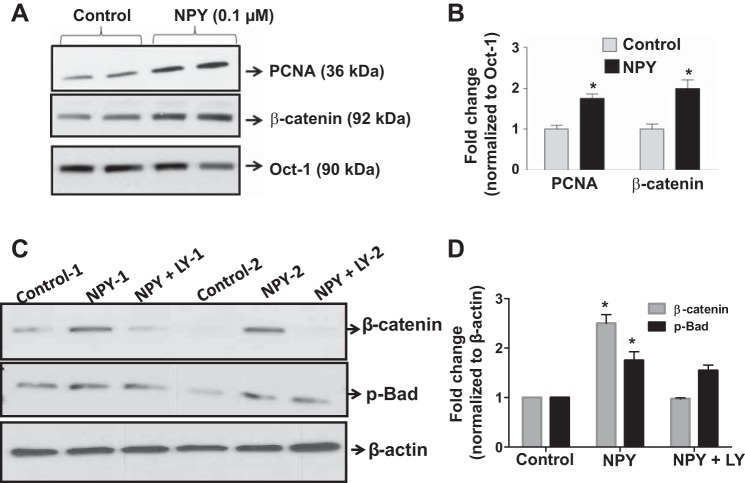
We have previously shown that NPY promotes epithelial cell survival via phosphatidyl-inositol-3-kinase (PI3-K) signaling pathway (6). Here we assessed if NPY promoted intestinal epithelial cell (IEC) proliferation. We observed that nuclear extracts from NPY-treated (0.1 µM) epithelial cells exhibited enhanced proliferation markers like nuclear PCNA (proliferating cell nuclear antigen) and β-catenin (Fig. 4, A and B, P < 0.05). We also found that NPY-treated cells exhibited a significant upregulation of the anti-apoptotic marker phospho-Bad. Further studies revealed that NPY-induced effects on proliferation via β-catenin were sensitive to PI3-K inhibition (Fig. 4, C and D, P < 0.05). These data suggest that NPY-induced PI3-kinase-β-catenin signaling possibly underlies the enhanced susceptibility to tumorigenesis, as observed in WT mice.
Fig. 4.
NPY-induced epithelial cell proliferation involves β-catenin activation via phosphatidyl-inositol-3-kinase. T84 cells were treated with NPY (0.1 µM) for 24 h. The nuclear fraction was probed for β-catenin and PCNA by Western blotting; Oct-1 was used as loading control. In a separate experiment T84 cells were treated with LY 294002, a phosphatidyl-inositol-3-kinase (PI3-K) inhibitor (20 µM), and probed for β-catenin and phospho-Bad. A and B: representative Western blots and corresponding histograms depicting changes in β-catenin and PCNA in control and NPY-treated cells. C and D: representative Western blots and the corresponding histograms depicting alterations in β-catenin and phospho-Bad with LY 294002 treatment. Values are means ± SE; n = 4. *P < 0.05.
NPY enhances cellular proliferation via downregulation of miR-375, and supplementation of a miR-375 inhibitor results in synergistic effects.
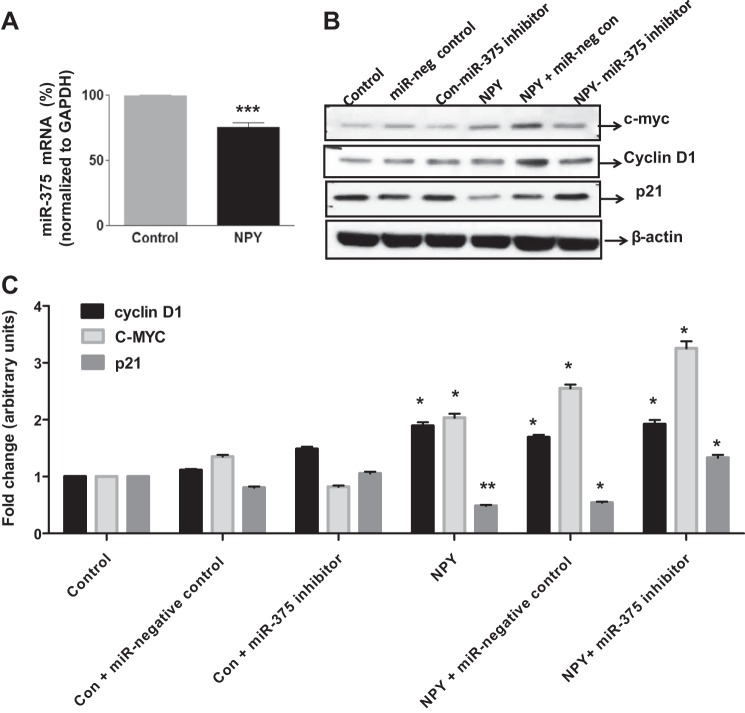
To decipher the probable regulatory mechanisms behind NPY-induced proliferation, we treated T84 cells with NPY (0.1 µM) and assessed miR-375 expression. In addition, we also transfected the cells with miR-375 inhibitor (30 nmol) and assessed various markers of cellular proliferation (c-Myc cyclin D1) and cell cycle arrest (p21/waf1/cip1 or commonly p21) by Western blotting. We observed that NPY suppressed miR-375 expression by 23% (Fig. 5A, P < 0.001). We observed that addition of NPY resulted in enhanced c-Myc and Cyclin D1, the downstream targets of β-catenin mediating enhanced proliferation, compared with control. NPY also induced the downregulation of p21 that mediates cell cycle arrest compared with control (Fig. 5, B and C, P < 0.05). However, we observed that supplementing the NPY-treated cells with a miR-375 inhibitor did not further enhance these effects compared with cells treated with NPY alone, probably due to the inhibitory effect of NPY on miR-375 mRNA itself (Fig. 5A) which is upstream of the action of miR-inhibitor. Thus, taken together, our in vitro data suggest that NPY-induced cellular proliferation involves PI3-K-β-catenin signaling on one hand and downregulation of miR-375 on the other.
Fig. 5.
NPY-induced cellular proliferation is further enhanced by inhibition of miR-375 inhibitor. T84 cells were treated with NPY, and miRNA was extracted using Qiagen miRNAesy kit. The expression of miR-375 was assessed by real-time PCR. In a separate experiment, T84 cells were transfected with negative control miR-375 or miR-375 inhibitor (30 nM) using lipofectamine RNAiMAX, followed by treatment with NPY (0.1 µM) for 24 h. The lysates were probed for proliferation cell cycle regulators and apoptotic markers by Western blotting. A: miR-375 expression in control and NPY treated cells. B: Western blots for c-Myc p21, and Cyclin D1. C: the corresponding histograms. Values are means ± SE; n = 4. *P < 0.05, **P < 0.01, ***P < 0.001.
Collectively our data suggest that NPY from enteric neurons can promote epithelial cell survival via PI3-K/pAkt, and enhance cellular proliferation via β-catenin and its downstream targets like c-Myc and cyclin D1. In addition, NPY also reduces apoptosis via downregulation of p21, the mediator of cell cycle arrest, and also by upregulation of the anti-apoptotic phospho-Bad. Thus NPY facilitates tumorigenesis by upregulating survival and proliferative signals, and by downregulating apoptotic signals. The proposed working model for possible NPY-epithelial interactions that facilitate tumorigenesis is represented in Fig. 6.
Fig. 6.
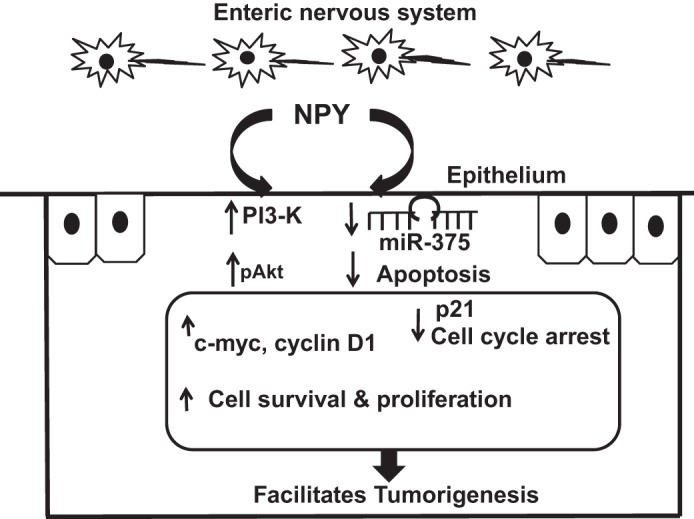
Modeling NPY-epithelial cross talk that favors cell survival and proliferation and inhibits apoptosis. NPY released from the enteric neurons can activate phosphatidyl-inositol-3-kinase (PI3-K) pathway and increase pAkt signals leading to cell survival, and proliferation via c-Myc and cyclin D1. In addition, NPY also downregulates miR-375, thereby reducing apoptosis. Furthermore, NPY also inhibits p21, which mediates cell cycle arrest (G1 to the synthetic S phase). Taken together, NPY-induced enhancement in cell survival and proliferation with a concomitant downregulation of apoptosis facilitates inflammation-induced tumorigenesis.
DISCUSSION
NPY has been reported to have bimodal roles in the tumorigenesis of prostate and breast cancers, and Ewing’s sarcoma. However, its role in inflammation-induced tumorigenesis is unknown. A classic example of inflammation-induced tumor is colitis-associated cancer which is triggered by mutations in the tumor suppressor p53 (29, 36) that regulates cell cycle arrest (G1-S phase) and activates the intrinsic apoptotic machinery.
In the current study we have highlighted the role of enteric neuropeptide NPY-epithelial cross talk in favoring inflammation-induced tumors using repeated DSS cycle model. Here we demonstrate that NPY−/− mice have reduced susceptibility to inflammation-associated tumor, consistent with our earlier observations that NP−/− mice are resistant to inflammation (5). NPY−/− mice were characterized by reduced inflammation and did not exhibit reduction in body weight at the end of the DSS cycle. It is to be noted here that this phenomenon is not related to the genetic deletion of NPY; as it has been demonstrated that genetic deletion of NPY does not affect food intake or the body weight under normal conditions as demonstrated by several studies (13, 14). In addition, NPY−/− mice were characterized by reduced tumor burden and tumor size in comparison to WT mice. To further address if reduced inflammation is the only protective feature in NPY−/− mice, we next investigated the role of NPY in regulating survival, proliferation, and apoptosis of epithelial cells. We found significantly higher proliferation rates (Ki67, PCNA) and reduced apoptosis (TUNEL) in WT mice, suggesting that NPY plays a role in regulating intestinal epithelial cell proliferation and apoptosis. Thus we observed that NPY-induced epithelial effects (in addition to inflammation) are also crucial determinants in the transition from a chronic inflammatory state to tumorigenesis. Further in vitro studies on epithelial cells demonstrated that NPY promotes cellular survival and proliferation via PI3-K and β-catenin, respectively. Interestingly we observed from immunostaining that NPY−/− mice displayed more apical β-catenin even after inflammation-induced tumorigenesis compared with WT mice. However, we observed that WT mice exhibited more severe colitis with loss of the epithelial surface, which could justify/account for reduction in β-catenin staining in the apical area. We have previously demonstrated that WT mice have increased permeability compared with NPY−/− mice (6); hence it is also speculated that NPY-induced β-catenin nuclear translocation may also decrease its interaction with cytoskeletal proteins like E-cadherin (8, 40), thus accounting for increased epithelial permeability in WT mice.
Recently there has been an increased appreciation regarding the role of microRNAs (miRNA) in regulating inflammatory diseases and cancers (29). Of these, miR-375 has been demonstrated to play a crucial regulatory role in colorectal (9, 42), gastric (32, 35), pancreatic (37, 45), head and neck cancers (18, 25). Downregulation of miR-375 in these cancers has been attributed to reduced apoptosis and enhanced proliferation resulting in carcinoma. miR-375 is a tumor-suppressing miRNA that has an apoptosis-promoting role in various cancers including colorectal cancer (15). In addition, miR-375 has been demonstrated to induce cell cycle arrest in epithelial cells (23, 37, 42). Most recent studies also indicate that miR-375 also plays a role in epithelial-mesenchymal transition (20). The targets of miR375 include several genes that regulate apoptosis and cell survival, like caspase, Akt, Bcl-2, and PDK1. Interestingly it has also been demonstrated that miR-375 can also impair dendrite formation during the late stages of neuronal development via targeting the neuronal RNA-binding protein HuD that modulates neuronal differentiation, regeneration, and plasticity and cytoskeletal reorganization (1).
Our in vivo studies demonstrated a twofold downregulation of miR-375 in WT mice, with a corresponding upregulation of pAkt, a target of miR-375. These results indicate that NPY-induced miR-375 plays a role in promoting epithelial cell proliferation and reducing apoptosis. Our studies using epithelial cells in vitro indicated that NPY upregulates PI3-kinase/pAkt signaling and thereby increases cell survival. Further we also observed that NPY induces nuclear translocation of β-catenin and upregulates its downstream targets like c-Myc and cyclin D1. Interestingly, we also found that NPY modestly downregulates miR-375 (23%) in epithelia and inhibits cell cycle arrest protein p21. However we observed that addition of miR-375 inhibitor to cells already treated with NPY did not further enhance the effects induced by NPY alone, suggestive of the upstream transcriptional downregulation of miR-375 by NPY. Taken together, our data suggest that NPY-induced effects on PI3-K and miR-375 are responsible for fine tuning the signaling pathways toward cellular proliferation and inhibiting apoptosis.
In addition to the effects of NPY on proliferation and apoptosis as discussed in the regulation of tumorigenesis in this paper, we also remind the readers that NPY has well-documented angiogenic effects through VEGF (34). In addition, hypoxia (38) and the changes in expression of the various NPY receptors has also been reported to regulate the angiogenic activity of NPY (26). However, the contribution of these factors remains to be pursued in future studies and is beyond the scope of our current manuscript.
In conclusion, our work exemplifies a novel role of NPY in regulating inflammation-induced tumorigenesis via miRNA-dependent regulation of epithelial cell survival, proliferation, and apoptosis. Moreover the current study highlights the role of neuropeptide-regulated miRNAs in regulating epithelial proliferation, which plays a key role in maintaining epithelial homeostasis and tumor suppression. Perhaps most importantly, our data establish the existence of a microRNA-mediated cross talk between enteric neurons producing NPY and intestinal epithelial cells and the potential of neuropeptides and associated miRNAs as potential therapeutic molecules. Understanding these regulatory pathways could provide insight into the management of cancer arising from chronic inflammatory diseases of the gut.
GRANTS
We thank the funding agencies for the financial support: Crohn’s and Colitis Foundation of America (Career Development Award to B. Chandrasekharan) and National Institute of Diabetes and Digestive and Kidney Diseases Grant NIH-RO1-DK080684 and Veterans Affairs Merit Award (to Shanthi Srinivasan).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
S.J. and B.C. performed experiments; S.J., S.S., and B.C. analyzed data; S.J., S.S., and B.C. interpreted results of experiments; S.J., S.S., and B.C. edited and revised manuscript; S.S. and B.C. approved final version of manuscript; B.C. prepared figures; B.C. drafted manuscript.
REFERENCES
- 1.Abdelmohsen K, Hutchison ER, Lee EK, Kuwano Y, Kim MM, Masuda K, Srikantan S, Subaran SS, Marasa BS, Mattson MP, Gorospe M. miR-375 inhibits differentiation of neurites by lowering HuD levels. Mol Cell Biol 30: 4197–4210, 2010. doi: 10.1128/MCB.00316-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Adams SV, Ahnen DJ, Baron JA, Campbell PT, Gallinger S, Grady WM, LeMarchand L, Lindor NM, Potter JD, Newcomb PA. Survival after inflammatory bowel disease-associated colorectal cancer in the Colon Cancer Family Registry. World J Gastroenterol 19: 3241–3248, 2013. doi: 10.3748/wjg.v19.i21.3241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bartel DP. MicroRNAs: target recognition and regulatory functions. Cell 136: 215–233, 2009. doi: 10.1016/j.cell.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Castaneda FE, Walia B, Vijay-Kumar M, Patel NR, Roser S, Kolachala VL, Rojas M, Wang L, Oprea G, Garg P, Gewirtz AT, Roman J, Merlin D, Sitaraman SV. Targeted deletion of metalloproteinase 9 attenuates experimental colitis in mice: central role of epithelial-derived MMP. Gastroenterology 129: 1991–2008, 2005. doi: 10.1053/j.gastro.2005.09.017. [DOI] [PubMed] [Google Scholar]
- 5.Chandrasekharan B, Bala V, Kolachala VL, Vijay-Kumar M, Jones D, Gewirtz AT, Sitaraman SV, Srinivasan S. Targeted deletion of neuropeptide Y (NPY) modulates experimental colitis. PLoS One 3: e3304, 2008. doi: 10.1371/journal.pone.0003304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chandrasekharan B, Jeppsson S, Pienkowski S, Belsham DD, Sitaraman SV, Merlin D, Kokkotou E, Nusrat A, Tansey MG, Srinivasan S. Tumor necrosis factor-neuropeptide Y cross talk regulates inflammation, epithelial barrier functions, and colonic motility. Inflamm Bowel Dis 19: 2535–2546, 2013. doi: 10.1097/01.MIB.0000437042.59208.9f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chandrasekharan B, Nezami BG, Srinivasan S. Emerging neuropeptide targets in inflammation: NPY and VIP. Am J Physiol Gastrointest Liver Physiol 304: G949–G957, 2013. doi: 10.1152/ajpgi.00493.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chaw SY, Majeed AA, Dalley AJ, Chan A, Stein S, Farah CS. Epithelial to mesenchymal transition (EMT) biomarkers--E-cadherin, beta-catenin, APC and Vimentin--in oral squamous cell carcinogenesis and transformation. Oral Oncol 48: 997–1006, 2012. doi: 10.1016/j.oraloncology.2012.05.011. [DOI] [PubMed] [Google Scholar]
- 9.Christensen LL, Holm A, Rantala J, Kallioniemi O, Rasmussen MH, Ostenfeld MS, Dagnaes-Hansen F, Øster B, Schepeler T, Tobiasen H, Thorsen K, Sieber OM, Gibbs P, Lamy P, Hansen TF, Jakobsen A, Riising EM, Helin K, Lubinski J, Hagemann-Madsen R, Laurberg S, Ørntoft TF, Andersen CL. Functional screening identifies miRNAs influencing apoptosis and proliferation in colorectal cancer. PLoS One 9: e96767, 2014. doi: 10.1371/journal.pone.0096767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cooks T, Pateras IS, Tarcic O, Solomon H, Schetter AJ, Wilder S, Lozano G, Pikarsky E, Forshew T, Rosenfeld N, Harpaz N, Itzkowitz S, Harris CC, Rotter V, Gorgoulis VG, Oren M. Mutant p53 prolongs NF-κB activation and promotes chronic inflammation and inflammation-associated colorectal cancer. Cancer Cell 23: 634–646, 2013. doi: 10.1016/j.ccr.2013.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Danese S, Malesci A, Vetrano S. Colitis-associated cancer: the dark side of inflammatory bowel disease. Gut 60: 1609–1610, 2011. doi: 10.1136/gutjnl-2011-300953. [DOI] [PubMed] [Google Scholar]
- 12.Deng L, Zhou JF, Sellers RS, Li JF, Nguyen AV, Wang Y, Orlofsky A, Liu Q, Hume DA, Pollard JW, Augenlicht L, Lin EY. A novel mouse model of inflammatory bowel disease links mammalian target of rapamycin-dependent hyperproliferation of colonic epithelium to inflammation-associated tumorigenesis. Am J Pathol 176: 952–967, 2010. doi: 10.2353/ajpath.2010.090622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Erickson JC, Clegg KE, Palmiter RD. Sensitivity to leptin and susceptibility to seizures of mice lacking neuropeptide Y. Nature 381: 415–421, 1996. doi: 10.1038/381415a0. [DOI] [PubMed] [Google Scholar]
- 14.Erickson JC, Hollopeter G, Palmiter RD. Attenuation of the obesity syndrome of ob/ob mice by the loss of neuropeptide Y. Science 274: 1704–1707, 1996. doi: 10.1126/science.274.5293.1704. [DOI] [PubMed] [Google Scholar]
- 15.Faltejskova P, Svoboda M, Srutova K, Mlcochova J, Besse A, Nekvind ova J, Radova L, Fabian P, Slaba K, Kiss I, Vyzula R, Slaby O. Identification and functional screening of microRNAs highly deregulated in colorectal cancer. J Cell Mol Med 16: 2655–2666, 2012. doi: 10.1111/j.1582-4934.2012.01579.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fodde R, Kuipers J, Rosenberg C, Smits R, Kielman M, Gaspar C, van Es JH, Breukel C, Wiegant J, Giles RH, Clevers H. Mutations in the APC tumour suppressor gene cause chromosomal instability. Nat Cell Biol 3: 433–438, 2001. doi: 10.1038/35070129. [DOI] [PubMed] [Google Scholar]
- 17.Harpaz N, Ward SC, Mescoli C, Itzkowitz SH, Polydorides AD. Precancerous lesions in inflammatory bowel disease. Best Pract Res Clin Gastroenterol 27: 257–267, 2013. doi: 10.1016/j.bpg.2013.03.014. [DOI] [PubMed] [Google Scholar]
- 18.Harris T, Jimenez L, Kawachi N, Fan JB, Chen J, Belbin T, Ramnauth A, Loudig O, Keller CE, Smith R, Prystowsky MB, Schlecht NF, Segall JE, Childs G. Low-level expression of miR-375 correlates with poor outcome and metastasis while altering the invasive properties of head and neck squamous cell carcinomas. Am J Pathol 180: 917–928, 2012. doi: 10.1016/j.ajpath.2011.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hassani H, Lucas G, Rozell B, Ernfors P. Attenuation of acute experimental colitis by preventing NPY Y1 receptor signaling. Am J Physiol Gastrointest Liver Physiol 288: G550–G556, 2005. doi: 10.1152/ajpgi.00182.2004. [DOI] [PubMed] [Google Scholar]
- 20.Hong S, Noh H, Teng Y, Shao J, Rehmani H, Ding HF, Dong Z, Su SB, Shi H, Kim J, Huang S. SHOX2 is a direct miR-375 target and a novel epithelial-to-mesenchymal transition inducer in breast cancer cells. Neoplasia 16: 279–90.e1, 2014. doi: 10.1016/j.neo.2014.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Itzkowitz SH, Yio X. Inflammation and cancer IV. Colorectal cancer in inflammatory bowel disease: the role of inflammation. Am J Physiol Gastrointest Liver Physiol 287: G7–G17, 2004. doi: 10.1152/ajpgi.00079.2004. [DOI] [PubMed] [Google Scholar]
- 22.Josse C, Bouznad N, Geurts P, Irrthum A, Huynh-Thu VA, Servais L, Hego A, Delvenne P, Bours V, Oury C. Identification of a microRNA landscape targeting the PI3K/Akt signaling pathway in inflammation-induced colorectal carcinogenesis. Am J Physiol Gastrointest Liver Physiol 306: G229–G243, 2014. doi: 10.1152/ajpgi.00484.2012. [DOI] [PubMed] [Google Scholar]
- 23.Jung HM, Phillips BL, Chan EK. miR-375 activates p21 and suppresses telomerase activity by coordinately regulating HPV E6/E7, E6AP, CIP2A, and 14-3-3ζ. Mol Cancer 13: 80, 2014. doi: 10.1186/1476-4598-13-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kathiria AS, Neumann WL, Rhees J, Hotchkiss E, Cheng Y, Genta RM, Meltzer SJ, Souza RF, Theiss AL. Prohibitin attenuates colitis-associated tumorigenesis in mice by modulating p53 and STAT3 apoptotic responses. Cancer Res 72: 5778–5789, 2012. doi: 10.1158/0008-5472.CAN-12-0603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kinoshita T, Hanazawa T, Nohata N, Okamoto Y, Seki N. The functional significance of microRNA-375 in human squamous cell carcinoma: aberrant expression and effects on cancer pathways. J Hum Genet 57: 556–563, 2012. doi: 10.1038/jhg.2012.75. [DOI] [PubMed] [Google Scholar]
- 26.Kitlinska J, Abe K, Kuo L, Pons J, Yu M, Li L, Tilan J, Everhart L, Lee EW, Zukowska Z, Toretsky JA. Differential effects of neuropeptide Y on the growth and vascularization of neural crest-derived tumors. Cancer Res 65: 1719–1728, 2005. doi: 10.1158/0008-5472.CAN-04-2192. [DOI] [PubMed] [Google Scholar]
- 27.Kolegraff K, Nava P, Helms MN, Parkos CA, Nusrat A. Loss of desmocollin-2 confers a tumorigenic phenotype to colonic epithelial cells through activation of Akt/β-catenin signaling. Mol Biol Cell 22: 1121–1134, 2011. doi: 10.1091/mbc.E10-10-0845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kraus S, Arber N. Inflammation and colorectal cancer. Curr Opin Pharmacol 9: 405–410, 2009. doi: 10.1016/j.coph.2009.06.006. [DOI] [PubMed] [Google Scholar]
- 29.Kukitsu T, Takayama T, Miyanishi K, Nobuoka A, Katsuki S, Sato Y, Takimoto R, Matsunaga T, Kato J, Sonoda T, Sakamaki S, Niitsu Y. Aberrant crypt foci as precursors of the dysplasia-carcinoma sequence in patients with ulcerative colitis. Clin Cancer Res 14: 48–54, 2008. doi: 10.1158/1078-0432.CCR-07-1835. [DOI] [PubMed] [Google Scholar]
- 30.Larriba MJ, González-Sancho JM, Barbáchano A, Niell N, Ferrer-Mayorga G, Muñoz A. Vitamin D Is a Multilevel Repressor of Wnt/b-Catenin Signaling in Cancer Cells. Cancers (Basel) 5: 1242–1260, 2013. doi: 10.3390/cancers5041242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lee SJ, Leoni G, Neumann PA, Chun J, Nusrat A, Yun CC. Distinct phospholipase C-β isozymes mediate lysophosphatidic acid receptor 1 effects on intestinal epithelial homeostasis and wound closure. Mol Cell Biol 33: 2016–2028, 2013. doi: 10.1128/MCB.00038-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liu Y, Xing R, Zhang X, Dong W, Zhang J, Yan Z, Li W, Cui J, Lu Y. miR-375 targets the p53 gene to regulate cellular response to ionizing radiation and etoposide in gastric cancer cells. DNA Repair (Amst) 12: 741–750, 2013. doi: 10.1016/j.dnarep.2013.06.002. [DOI] [PubMed] [Google Scholar]
- 33.Margolis KG, Stevanovic K, Karamooz N, Li ZS, Ahuja A, D’Autréaux F, Saurman V, Chalazonitis A, Gershon MD. Enteric neuronal density contributes to the severity of intestinal inflammation. Gastroenterology 141: 588–598e.2, 2011. doi: 10.1053/j.gastro.2011.04.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Medeiros PJ, Jackson DN. Neuropeptide Y Y5-receptor activation on breast cancer cells acts as a paracrine system that stimulates VEGF expression and secretion to promote angiogenesis. Peptides 48: 106–113, 2013. doi: 10.1016/j.peptides.2013.07.029. [DOI] [PubMed] [Google Scholar]
- 35.Miao L, Liu K, Xie M, Xing Y, Xi T. miR-375 inhibits Helicobacter pylori-induced gastric carcinogenesis by blocking JAK2-STAT3 signaling. Cancer Immunol Immunother 63: 699–711, 2014. doi: 10.1007/s00262-014-1550-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Santiago C, Pagán B, Isidro AA, Appleyard CB. Prolonged chronic inflammation progresses to dysplasia in a novel rat model of colitis-associated colon cancer. Cancer Res 67: 10766–10773, 2007. doi: 10.1158/0008-5472.CAN-07-1418. [DOI] [PubMed] [Google Scholar]
- 37.Song SD, Zhou J, Zhou J, Zhao H, Cen JN, Li DC. MicroRNA-375 targets the 3-phosphoinositide-dependent protein kinase-1 gene in pancreatic carcinoma. Oncol Lett 6: 953–959, 2013. doi: 10.3892/ol.2013.1510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tilan JU, Lu C, Galli S, Izycka-Swieszewska E, Earnest JP, Shabbir A, Everhart LM, Wang S, Martin S, Horton M, Mahajan A, Christian D, O’Neill A, Wang H, Zhuang T, Czarnecka M, Johnson MD, Toretsky JA, Kitlinska J. Hypoxia shifts activity of neuropeptide Y in Ewing sarcoma from growth-inhibitory to growth-promoting effects. Oncotarget 4: 2487–2501, 2013. doi: 10.18632/oncotarget.1604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Toledano MB. The guardian recruits cops: the p53-p21 axis delegates prosurvival duties to the Keap1-Nrf2 stress pathway. Mol Cell 34: 637–639, 2009. doi: 10.1016/j.molcel.2009.06.005. [DOI] [PubMed] [Google Scholar]
- 40.van Dekken H, Wink JC, Vissers KJ, Franken PF, Ruud Schouten W, J Hop WC, Kuipers EJ, Fodde R, Janneke van der Woude C. Wnt pathway-related gene expression during malignant progression in ulcerative colitis. Acta Histochem 109: 266–272, 2007. doi: 10.1016/j.acthis.2007.02.007. [DOI] [PubMed] [Google Scholar]
- 41.Waldner MJ, Neurath MF. Cytokines in colitis associated cancer: potential drug targets? Inflamm Allergy Drug Targets 7: 187–194, 2008. doi: 10.2174/187152808785748137. [DOI] [PubMed] [Google Scholar]
- 42.Wang Y, Tang Q, Li M, Jiang S, Wang X. MicroRNA-375 inhibits colorectal cancer growth by targeting PIK3CA. Biochem Biophys Res Commun 444: 199–204, 2014. doi: 10.1016/j.bbrc.2014.01.028. [DOI] [PubMed] [Google Scholar]
- 43.Watanabe T, Konishi T, Kishimoto J, Kotake K, Muto T, Sugihara K; Japanese Society for Cancer of the Colon and Rectum . Ulcerative colitis-associated colorectal cancer shows a poorer survival than sporadic colorectal cancer: a nationwide Japanese study. Inflamm Bowel Dis 17: 802–808, 2011. doi: 10.1002/ibd.21365. [DOI] [PubMed] [Google Scholar]
- 44.Wheway J, Mackay CR, Newton RA, Sainsbury A, Boey D, Herzog H, Mackay F. A fundamental bimodal role for neuropeptide Y1 receptor in the immune system. J Exp Med 202: 1527–1538, 2005. doi: 10.1084/jem.20051971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhou J, Song S, He S, Zhu X, Zhang Y, Yi B, Zhang B, Qin G, Li D. MicroRNA-375 targets PDK1 in pancreatic carcinoma and suppresses cell growth through the Akt signaling pathway. Int J Mol Med 33: 950–956, 2014. doi: 10.3892/ijmm.2014.1638. [DOI] [PubMed] [Google Scholar]