Here we demonstrate that the Notch signaling pathway is essential for proliferation of stem cells in the mouse and human gastric corpus. We identify NOTCH1 and NOTCH2 as the predominant Notch receptors expressed in both mouse and human corpus and show that both receptors are required for corpus stem cell proliferation. We show that chronic Notch activation in corpus stem cells induces hyperproliferation and tissue hypertrophy, suggesting that Notch may drive gastric tumorigenesis.
Keywords: gastric organoids, gastric stem cells, SOX2, cellular differentiation, spasmolytic polypeptide expressing metaplasia (SPEM)
Abstract
The Notch signaling pathway is known to regulate stem cells and epithelial cell homeostasis in gastrointestinal tissues; however, Notch function in the corpus region of the stomach is poorly understood. In this study we examined the consequences of Notch inhibition and activation on cellular proliferation and differentiation and defined the specific Notch receptors functioning in the mouse and human corpus. Notch pathway activity was observed in the mouse corpus epithelium, and gene expression analysis revealed NOTCH1 and NOTCH2 to be the predominant Notch receptors in both mouse and human. Global Notch inhibition for 5 days reduced progenitor cell proliferation in the mouse corpus, as well as in organoids derived from mouse and human corpus tissue. Proliferation effects were mediated through both NOTCH1 and NOTCH2 receptors, as demonstrated by targeting each receptor alone or in combination with Notch receptor inhibitory antibodies. Analysis of differentiation by marker expression showed no change to the major cell lineages; however, there was a modest increase in the number of transitional cells coexpressing markers of mucous neck and chief cells. In contrast to reduced proliferation after pathway inhibition, Notch activation in the adult stomach resulted in increased proliferation coupled with reduced differentiation. These findings suggest that NOTCH1 and NOTCH2 signaling promotes progenitor cell proliferation in the mouse and human gastric corpus, which is consistent with previously defined roles for Notch in promoting stem and progenitor cell proliferation in the intestine and antral stomach.
NEW & NOTEWORTHY Here we demonstrate that the Notch signaling pathway is essential for proliferation of stem cells in the mouse and human gastric corpus. We identify NOTCH1 and NOTCH2 as the predominant Notch receptors expressed in both mouse and human corpus and show that both receptors are required for corpus stem cell proliferation. We show that chronic Notch activation in corpus stem cells induces hyperproliferation and tissue hypertrophy, suggesting that Notch may drive gastric tumorigenesis.
the gastric corpus epithelium is continuously renewed by stem cells that give rise to the various differentiated lineages of the stomach, including surface mucous cells, acid-secreting parietal cells, endocrine cells, mucous neck cells, and zymogenic chief cells. The Notch signaling pathway is known to be a key regulator of stem cells in the gastrointestinal tract, including the intestine and gastric antrum, regulating stem cell self-renewal and progenitor cell proliferation and differentiation (3, 5, 9, 19, 31, 32). Accordingly, pharmacologic Notch inhibition has been shown to reduce progenitor cell proliferation in the adult gastric corpus, although specific effects on corpus stem cells were not evaluated (19). Furthermore, the mechanism of Notch pathway function in the corpus, including the cellular basis and pathway components, is unknown.
Notch signaling involves ligand engagement with a Notch receptor on a neighboring cell, which induces proteolytic receptor cleavage to release an intracellular signaling fragment of the receptor, Notch intracellular domain (NICD), which translocates to the nucleus to form a transcriptional complex that activates target gene transcription (6). Notch regulation of the distal, antral region of the stomach is mediated through the NOTCH1 (N1) and NOTCH2 (N2) receptors, as demonstrated by studies that targeted these receptors with specific inhibitory antibodies (9). This study demonstrated that inhibiting both N1 and N2 signaling reduced epithelial cell proliferation in the antrum, similar to complete pathway inhibition using a pan-Notch inhibitor. In addition, global Notch inhibition as well as combined N1 and N2 inhibition altered cellular differentiation, with expansion of several different cell lineages and activation of corpus and intestinal cell markers suggesting loss of regional identity (9). Thus N1 and N2 function redundantly to regulate several aspects of antral epithelial cell homeostasis. Specific Notch receptor function in the corpus has not been defined.
Studies using genetic approaches to activate Notch signaling in the stomach via expression of NICD reinforced the conclusions from studies of Notch inhibition. Constitutive Notch activation in LGR5+ antral stem cells increased stem cell proliferation and function, decreased differentiation, and promoted antral gland fission and eventual tumorigenesis (5). Additionally, Notch activation in parietal progenitor cells of the developing stomach has been demonstrated to drive gastric adenoma formation in the corpus (19). Thus, in the stomach, Notch activation appears to promote proliferation and inhibit differentiation while Notch inhibition suppresses proliferation and promotes differentiation. However, Notch activation in adult corpus stem/progenitor cells has not yet been tested. Furthermore, there is nothing known about Notch function in the human corpus.
In this report, we describe a role for the Notch pathway to regulate stem/progenitor cells of both human and mouse gastric corpus. Using pharmacologic approaches to inhibit Notch, we demonstrate that Notch regulates gastric corpus epithelial cell proliferation through N1 and N2 signaling in adult mouse, and in human and mouse corpus organoids. We also utilized a Notch activation mouse model to target adult corpus stem and progenitor cells, showing that the Notch pathway is required to maintain adult corpus epithelial cell homeostasis.
MATERIALS AND METHODS
Mice.
Mice of both sexes, aged 2–5 mo, were used. CBF:H2B-Venus Notch reporter mice were used to identify cells undergoing active Notch signaling (26) (Jackson Laboratories no. 020942). ROSANotchIC (ROSANICD) (25) (Jackson Laboratories no. 008159) mice were used to activate Notch signaling in response to Cre recombinase. ROSA-CAG-LSL-tdTomato-WPRE (ROSATom) (23) (Jackson Laboratories no. 007909) mice were used for lineage tracing studies. Sox2-CreER (1) (Jackson Laboratories no. 017593) mice were used to activate genes in corpus stem cells by tamoxifen (TX) administration. CBF:H2B-Venus mice were on a mixed CD1 x FVB/N x C57BL/6 strain background; Sox2-CreER mice were on a C57BL/6 × 129/SvJae background, and ROSANICD and ROSATom mice were on a C57BL/6 background. Mice were housed in automated watered and ventilated cages and maintained on a 12-h light/dark cycle. Experimental procedures were approved by the University of Michigan Committee on the Use and Care of Animals.
Histological analysis of mouse and human tissue.
Mice were fasted overnight with ad libitum access to water before tissue collection. Stomachs were harvested and cut open along the greater curvature, and contents were rinsed in ice-cold PBS. In some experiments, mice were injected with 5-ethynyl-2′-deoxyuridine (EdU, 25 mg/kg; Invitrogen) 1.5 h before tissue collection. Human gastric tissue was obtained from five patients under Institutional Review Board-approved protocols at University of Michigan, with patient demographic information listed in Table 1. Tissues were processed for paraffin or frozen sections as previously described (5). Immunostaining was performed as previously described (18), using previously published primary antibodies to Ki67 (5), E-cadherin (5), H+,K+-ATPase alpha subunit (18), gastric intrinsic factor (IF; 9, 18), Muc5AC (5, 18), chromogranin A (18), green fluorescent protein (GFP; Alexa-488 conjugated; 5, 9), ghrelin (4), or Griffonia simplicifolia II (GSII) lectin (18). NOTCH1 (D1E11 no. 3608) or NOTCH2 (D76A6 no. 5732) immunostaining (rabbit monoclonals, 1:500; Cell Signaling Technology) was visualized via tyramide signal amplification according to manufacturer’s instructions (Invitrogen no. T20922). Imaging was performed using a Nikon E-800 fluorescence microscope or a Leica Inverted SP5X confocal microscope.
Table 1.
Patient information for human tissue samples used in current study
| Patient | Age | Gender | Sample Type |
|---|---|---|---|
| H29 | 59 | M | Surgical |
| H36 | 56 | M | Surgical |
| H45 | 48 | F | Biopsy |
| H52 | 48 | M | Surgical |
| H59 | 25 | M | Biopsy |
Human gastric corpus tissue was collected as surgical resections or biopsies from five patients at the University of Michigan under Institutional Review Board-approved protocols. Patient demographic information related to age and gender is included. M, male; F, female.
Gastric gland immunostaining and imaging.
Corpus tissue from CBF:H2B-Venus mice was cut into ~2 mm3 pieces and incubated in 15 mM EDTA/Dulbecco’s PBS (DPBS) for 1 h at 4°C on a rocking platform. Tissue was vortexed for 2 min to release glands. Underlying tissue fragments were removed, and glands were pelleted at 150 g for 10 min. For whole mount staining, glands were resuspended in 500-μl 4% paraformaldehyde (PFA) for 10 min on ice, pelleted at 350 g for 5 min, and resuspended in 500-μl blocking solution [20% goat serum, 1% BSA in 0.3% Tris PBS (TPBS)] for 1 h at room temperature. Glands were incubated in rabbit anti-GFP conjugated to Alexa-488 (1:200; Invitrogen) overnight at 4°C, washed in DPBS, mounted on slides as previously described (5), and imaged using a Leica Inverted SP5X confocal microscope.
Mouse and human gastric organoid culture.
Organoid cultures were initiated as described (5, 9). For mouse organoids, isolated corpus glands were pelleted at 150 g for 10 min at 4°C and resuspended in 200 μl of organoid culture media [50% L-WRN conditioned media, 20% FBS, 1X penicillin-streptomycin (Pen-Strep), 2 mM l-glutamine, 1X Fungizone, 1X gentamicin, Y-27532 (10 μM, Tocris) in Advanced DMEM/F12]. Aliquots were diluted in Matrigel at a 1:4 ratio before plating in a prewarmed 24-well plate and overlaying with 500-μl culture media (5). Human corpus organoid cultures were initiated as previously described for human antrum (9). For both mouse and human corpus organoids, media was replaced every other day, and cultures were maintained via passaging once (human) or twice (mouse) per week. Studies were performed in established organoid lines that had been passaged at least three times before analysis as detailed in figure legends.
Notch inhibition or activation.
For in vivo Notch inhibition experiments, C57BL/6 mice were treated with the gamma-secretase inhibitor (GSI) dibenzazepine (DBZ, 30 μmol/kg ip; SYNCOM, Groningen, The Netherlands) or vehicle once per day for 5 days, with stomachs collected the next day as previously described (5, 9). Experiments targeting either NOTCH1 (N1), NOTCH2 (N2), or both receptors with humanized IgG1 neutralizing monoclonal antibodies specific for each receptor (34) or an irrelevant control IgG1 antibody interacting with herpes simplex virus gD protein (Gd) were performed as previously described (9). For in vitro Notch inhibition in corpus organoids, the GSI N-[N-(3,5-difluorophenacetyl-l-alanyl)]-(S)-phenylglycine-t-butyl ester (DAPT, 10 μM; EMD4Biosciences, Gibbstown, NJ) or anti-Gd, anti-N1, and/or anti-N2 inhibitory antibodies (10 μg/ml) were added to organoid culture media and renewed every other day for 5 days as previously described (9).
For Notch activation experiments in SOX2+ corpus stem cells, control (ROSANICD) and Sox2-CreER; ROSANICD mice were treated with TX (100 or 200 mg/kg in corn oil via oral gavage; Sigma), and stomachs were collected either 1-day or 3-mo post-TX. For a 1-day chase, animals were administered a single dose of 200 mg/kg TX, and stomachs were harvested the next day. For a 3-mo chase, animals were administered 100 mg/kg TX daily for 5 days. Notch activation was also assessed in vitro by treating established corpus organoids (ROSANICD and Sox2-CreER; ROSANICD) with (Z)-4-hydroxytamoxifen (4-OHT, 1 μM; Sigma). Organoid size in ROSANICD and Sox2-CreER; ROSANICD cultures was measured 24-h postpassage and daily after 4-OHT addition to the culture media.
Whole mount staining of gastric organoids.
Epithelial cell proliferation in mouse and human corpus organoids was assessed via whole mount staining of EdU incorporation after 5 days of vehicle or DAPT treatment. EdU (10 μM; Invitrogen) was added to organoid culture media for 2 h. Afterward, media were aspirated, and organoids were collected in 1X DPBS. Organoids were pelleted at 300 g for 5 min, washed in DPBS, and resuspended in 200-μl 2% PFA for 30 min at 4°C. Organoids were pelleted, washed in DPBS, and resuspended in 200-μl 0.5% TPBS for 20 min at room temperature. Organoids were pelleted and resuspended in 150-μl EdU reaction cocktail for 30 min at room temperature according to manufacturer’s instructions (Invitrogen). Organoids were washed 3 × 5 min in DPBS and mounted on slides using Prolong Gold with 4′,6-diamidino-2-phenylindole (DAPI). Imaging was performed using a Leica Inverted SP5X confocal microscope.
Gene expression analysis.
RNA was isolated from mouse gastric corpus tissue by homogenization in lysis buffer (RLT; Qiagen) with β-mercaptoethanol (10 μl/ml) or from mouse and human corpus organoids by passing organoids through a syringe with a 25-gauge needle 20 times, followed by DNase I treatment and purification using the RNeasy Mini Kit (Qiagen). RNA was isolated from human corpus tissue by homogenization in Trizol (Invitrogen), followed by DNase I treatment and purification as described above. cDNA was prepared from 500-ng total RNA, and quantitative PCR was performed as previously described, using published mouse and human primer sets to mouse Notch1–4 (9), human NOTCH1–4 (9), Atp4a (18), Chga (18), Gif (18), and Muc5ac (18), with Gapdh (mouse; 18) or ACTB (human; 9) as an internal reference.
Morphometrics.
Morphometric analysis was performed in a blinded manner using ImageJ software (1.43u; W. Rasband, National Institutes of Health). For analysis of cellular proliferation, the number of Ki67+ cells per epithelial area (μm2) was quantified from at least three images per animal (n = 3–7 mice). For analysis of GSII+/IF+ transitional cells, the number of double-stained cells per corpus gland was quantified in vehicle- (294 glands, n = 5 mice), DBZ- (235 glands, n = 5 mice), anti-Gd- (83 glands, n = 3 mice), anti-N1/anti-N2- (150 glands, n = 8 mice), anti-N1- (180 glands, n = 6 mice), and anti-N2-treated (241 glands, n = 7 mice) animals.
Statistics.
GraphPad Prism was used for statistical analysis of quantitative data sets. Data are presented as means ± SE and were analyzed using Student’s t-test, one- or two-way ANOVA with Dunnett’s post hoc analysis. P < 0.05 was considered statistically significant.
RESULTS
Active Notch signaling occurs in the gastric corpus epithelium.
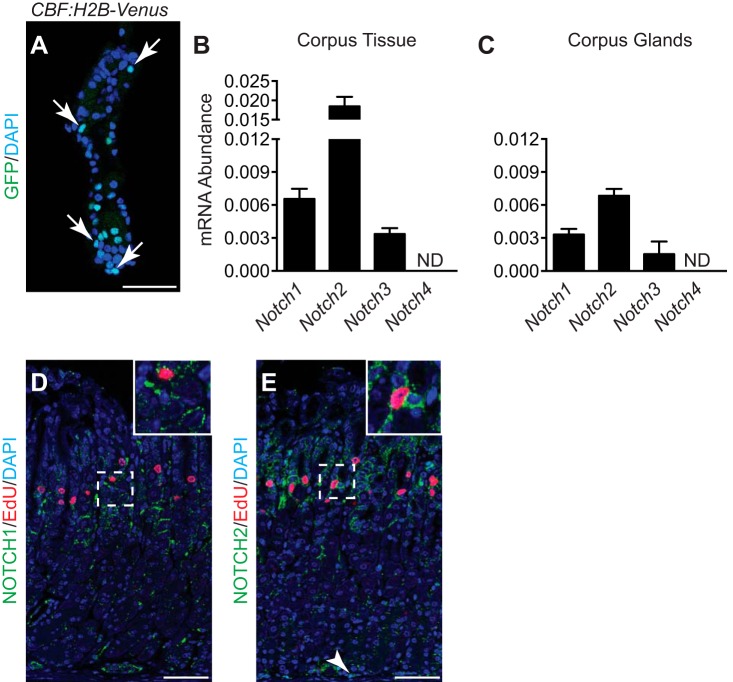
We sought to determine whether Notch signaling was active in the adult mouse gastric corpus epithelium using the CBF:H2B-Venus Notch reporter mouse, which provides a fluorescent cell readout of Notch signaling (26). Analysis of isolated corpus glands from this mouse revealed multiple Venus+ cells present in the glandular epithelium (Fig. 1A). We next characterized expression of the four Notch receptors by quantitative real-time PCR (qRT-PCR) analysis of RNA isolated from full-thickness corpus tissue (Fig. 1B) and corpus glands (Fig. 1C). This analysis showed receptor expression in both full-thickness and glandular tissue, with N1 and N2 receptors most abundant. Recent studies showed that N1 and N2 were the key receptors regulating epithelial cell proliferation in the antral stomach (9). Accordingly, immunostaining of corpus tissue revealed that N1 and N2 were expressed in the stem/progenitor cell isthmus region of the corpus. Costaining for the proliferation marker EdU showed colocalization of N1 and N2 in some EdU+ cells, suggesting Notch signaling in adult corpus stem or progenitor cells (Fig. 1, D and E). In addition to Notch receptor expression in the progenitor isthmus region, cells at the gland base expressed N2 (Fig. 1E, arrowhead), consistent with GFP+ cells observed at the base of isolated corpus glands from the CBF:H2B-Venus Notch reporter mouse (Fig. 1A). Overall, N1 staining was less than N2, and N1 expression at the gland base was not apparent.
Fig. 1.
Active Notch signaling occurs in the mouse corpus epithelium. A: GFP immunostaining of an isolated corpus gland from the CBF:H2B-Venus Notch reporter mouse. Multiple GFP+ nuclei are present (arrows), indicating epithelial cells undergoing active Notch signaling. B and C: qRT-PCR analysis of Notch1–4 receptors in full-thickness corpus tissue (B) or corpus glands (C). ND, not detected. Data are presented as means ± SE (n = 4–5 mice/group). D and E: coimmunostaining of NOTCH1 (green) and EdU (red; D) or NOTCH2 (green) and EdU (red; E). White dashed boxes are magnified in the insets. Arrowhead indicates NOTCH2+ epithelial cells at the base of the corpus glands. Scale bars: 50 μm.
Notch regulates gastric epithelial cell proliferation.
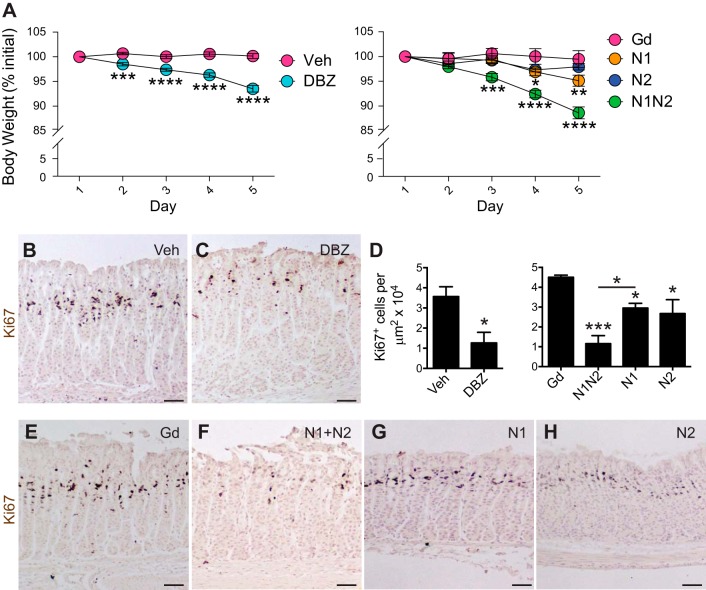
To test the function of N1 and N2 in regulating corpus epithelial cell homeostasis, we treated mice with inhibitory antibodies that selectively target N1 or N2 and compared those results to the effects of pan-Notch inhibition with the gamma-secretase inhibitor DBZ. For these experiments, we analyzed mice after 5 days of DBZ or antibody treatment. This short time point was necessary, as pan-Notch inhibition results in rapid weight loss and animal morbidity in both DBZ-treated and anti-N1/anti-N2-treated animals due to intestinal failure (31, 32) (Fig. 2A). It is noteworthy that anti-N1-treated animals exhibited a more modest, yet significant reduction in body weight compared with anti-Gd control-treated animals by day 4, while anti-N2-treated animals did not lose weight, suggesting that N1 is the primary receptor that mediates morbidity.
Fig. 2.
Notch inhibition reduces progenitor cell proliferation in the corpus epithelium. A: body weight (calculated as % initial weight) was measured in vehicle- (n = 26), DBZ- (n = 28), Gd- (n = 4), N1- (n = 7), N2- (n = 7), and N1/N2-treated (n = 7) animals each day before tissue collection on day 6. Data are presented as means ± SE *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001 vs. control using two-way ANOVA. B, C, and E–H: paraffin sections from each group in A were stained for Ki67 to examine cellular proliferation. Hematoxylin was used as a nuclear counterstain. D: morphometric quantification of Ki67+ cells. Data are presented as means ± SE (n = 3–5 mice/group). *P < 0.05 vs. vehicle using Student’s t-test; *P < 0.05, ***P < 0.001 vs. Gd using one-way ANOVA. Scale bars: 50 μm.
Analysis of corpus epithelial cell proliferation by quantification of Ki67+ cells revealed a significant reduction in cell number with combined anti-N1/anti-N2 treatment, similar to pan-Notch inhibition with DBZ (Fig. 2, B–H). Individual anti-N1 or anti-N2 treatment also resulted in a significant reduction in corpus epithelial cell proliferation, but to a lesser extent than combined treatment, suggesting redundant receptor function to regulate proliferation. Although morbidity of DBZ- and anti-N1/anti-N2-treated animals precluded analysis beyond 5 days, we analyzed anti-N1- and anti-N2-treated mice at longer time periods postantibody treatment, showing that cellular proliferation had returned to baseline levels at 2 and 4 wk (data not shown).
Notch is required for mouse corpus organoid growth.
We established organoids from the CBF:H2B-Venus Notch reporter mouse and observed Venus+ epithelial cells (Fig. 3A), suggesting active Notch signaling, even though Notch ligand is not included in the culture media. Gene expression analysis revealed that similar to corpus tissue, N1 and N2 were the predominant receptors expressed in organoids (Fig. 3B). To test Notch regulation of corpus organoid growth, we treated established organoid cultures with the pan-Notch inhibitor DAPT (Fig. 3, C–F) or Notch receptor inhibitory antibodies (Fig. 3, G–J). DAPT and anti-N1/anti-N2 treatment caused a similar reduction in organoid growth, with reduced epithelial cell proliferation observed in DAPT-treated organoids (Fig. 3, E and F). Individual receptor targeting mimicked what was observed in vivo, with significant growth reduction observed with either anti-N1 or anti-N2 but not as severe as with pan-Notch inhibition or combined receptor targeting (Fig. 3K). These data suggest that corpus organoid growth requires N1 and N2 signaling.
Fig. 3.
Notch is required for growth and proliferation of mouse corpus organoids. A: whole mount GFP (green)/E-cadherin (red) coimmunostaining of a corpus organoid established from the CBF:H2B-Venus Notch reporter mouse. DAPI (blue) used as nuclear counterstain. B: qRT-PCR analysis of Notch1–4 receptors in corpus organoids (n = 3 independent organoid lines). ND, not detected. C, D, and G–J: morphology of corpus organoids after Notch inhibition with DAPT (C and D) or Notch receptor inhibitory antibodies (G–J). E and F: whole mount EdU (red) staining of mouse corpus organoids after Notch inhibition with DAPT. DAPI used as nuclear counterstain. K: organoid size was measured in control or Notch-inhibited organoid cultures. Data are presented as means ± SE (n = 3 technical replicates from 1 organoid line). ****P < 0.0001. Scale bars: 50 (A, E, and F) or 250 μm (C, D, and G–J).
Notch regulates human corpus stem cell function.
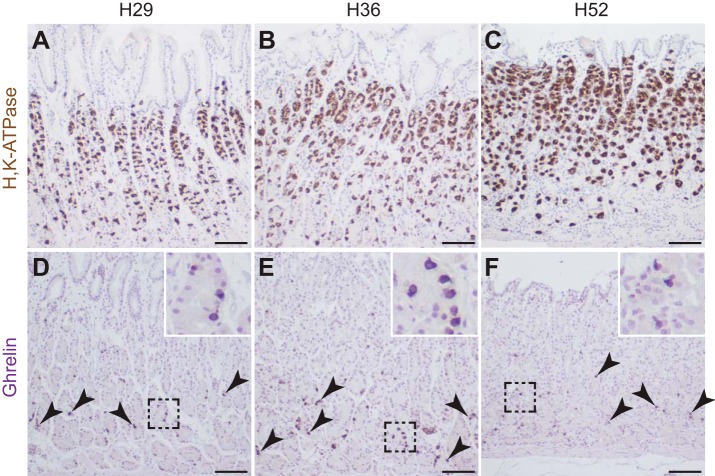
We next examined Notch pathway regulation of human corpus organoid cultures, taking advantage of the design of the inhibitory antibodies to recognize both mouse and human Notch receptors (34). Human surgical specimens and biopsies were used to generate corpus organoid lines (Table 1), with corpus tissue identity confirmed in the surgical samples by immunohistochemistry for H+,K+-ATPase to identify parietal cells (Fig. 4, A–C) and ghrelin (Fig. 4, D–F, arrowheads), a hormone that is expressed in the corpus but not the antrum of the human stomach (4).
Fig. 4.
Histological analysis of human gastric tissue. Paraffin sections were immunostained for H,K-ATPase (A–C) or Ghrelin (D–F) to verify corpus identity of human surgical tissue samples. Arrowheads indicate Ghrelin+ cells, with boxed areas magnified in insets. Scale bars: 100 μm.
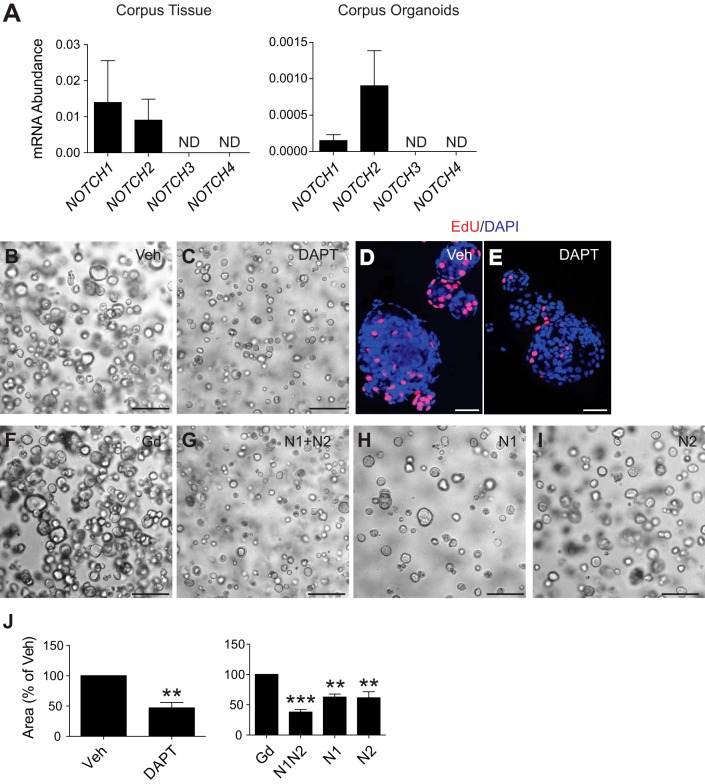
Gene expression analysis showed that NOTCH1 and NOTCH2 were the predominant Notch receptors expressed in both full-thickness human corpus tissue and corpus organoids (Fig. 5A). Treatment of three independent established human corpus organoid lines with DAPT (Fig. 5, B–E) or anti-N1/anti-N2 receptor-targeting antibodies significantly reduced growth and proliferation in culture, while individual receptor targeting reduced organoid size, but to a lesser extent (Fig. 5, F–J), suggesting that similar to our observations in mouse, signaling through both N1 and N2 supports human corpus stem cell function.
Fig. 5.
Human corpus organoid growth is regulated by Notch signaling. A: gene expression of NOTCH1–4 receptors in full-thickness human corpus tissue (n = 3 patients) or human corpus organoids (n = 3 independent lines: H45, H52, H59). ND, not detected. B, C, and F–I: morphology of human corpus organoids after Notch inhibition with DAPT (B and C) or Notch receptor inhibitory antibodies (F–I). D and E: whole mount EdU (red) staining of human corpus organoids after Notch inhibition with DAPT. DAPI used as nuclear counterstain. J: organoid size was measured from control or Notch-inhibited organoids. Data are presented as means ± SE (n = 3 independent lines: H45, H52, H59). **P < 0.01, ***P < 0.001 vs. control. Scale bars: 50 (D and E) or 100 μm (B, C, and F–I).
Notch inhibition induces expansion of mucous neck-zymogenic transition cells.
To address cellular differentiation, we measured differentiated cell markers in DBZ-, anti-N1-, anti-N2-, and anti-N1/anti-N2-treated mice. Analysis of marker gene expression by qRT-PCR showed similar expression of Atp4a (parietal), Chga (enteroendocrine), Gif (chief), and Muc5ac (surface mucous) in DBZ- and Notch receptor antibody-treated mice compared with controls (Fig. 6). Immunostaining showed no apparent changes in cells expressing markers of parietal (H+,K+-ATPase alpha), enteroendocrine (Chromogranin A), or surface mucous (MUC5AC) cells (Fig. 6). However, analysis of the mucous neck-zymogenic lineage via coimmunostaining for GSII lectin and intrinsic factor revealed an apparent increase in the number of transition cells that coexpress markers of both cell types after Notch inhibition (Fig. 6, I and J). Quantitative measure of numbers of GSII+/IF+ costained cells showed a significant increase in DBZ- (Fig. 7, A–C) and anti-N1/anti-N2 antibody-treated mice (Fig. 7, D, E, and H). Treatment with anti-N1 or anti-N2 receptor-targeting antibodies alone did not affect the number of these cells (Fig. 7, F and G).
Fig. 6.
Notch inhibition does not affect expression of corpus epithelial cell differentiation markers. Immunohistochemical and gene expression analysis of corpus differentiated cell lineages, including parietal (A–D), enteroendocrine (E–H), mucous neck/zymogenic (I–L), and surface mucous (M–P) cells after Notch inhibition with DBZ or Notch receptor inhibitory antibodies. Data are presented as means ± SE (n = 4 mice/group). Scale bars: 50 μm.
Fig. 7.
Notch inhibition increases GSII+/IF+ transitional cells in the gastric corpus. Paraffin sections stained for GSII (green) and intrinsic factor (red) in DBZ-treated (A and B) or Notch receptor inhibitory antibody-treated (D–G) mice. DAPI used as nuclear counterstain. Arrows indicate GSII+/IF+ cells. C and H: morphometric quantification of GSII+/IF+ transitional cells in DBZ-treated (C) or Notch receptor inhibitory antibody-treated (H) mice. Data are presented as means ± SE (n = 3–8 mice/group). **P < 0.01 vs. control. Scale bars: 50 μm.
Notch activation in corpus stem cells increases stem/progenitor cell proliferation and induces tissue hypertrophy.
We next used a genetic approach to test whether Notch activation in adult corpus stem cells would perturb gastric epithelial cell homeostasis. The Sox2-CreER mouse strain has been previously described to be expressed in various corpus epithelial cell lineages, including active stem cells (1). We confirmed expression of Sox2-CreER in adult corpus by lineage tracing analysis in Sox2-CreER; ROSATom mice (Fig. 8A), which showed scattered cells throughout the corpus epithelium 1-day post-TX (Fig. 8B) and full lineage stripes at 3-mo post-TX (Fig. 8C). Costaining with the proliferation marker EdU revealed that a proportion of labeled cells 1-day post-TX were actively proliferating, confirming SOX2 expression in corpus stem/progenitor cells. Analysis of Sox2-CreER; ROSANICD mice 3-mo post-TX treatment revealed a phenotype consistent with Notch activation, with localized areas of gland hypertrophy (Fig. 8, D and E), increased epithelial cell proliferation (Fig. 8, F and G), and reduced cellular differentiation (Fig. 8, H and I). It is noteworthy that these cellular changes occurred in a mosaic manner, consistent with the patchy recombination observed via nuclear GFP staining to identify ROSANICD (data not shown). Overall, the findings reveal a key role for Notch to stimulate adult corpus epithelial cell proliferation.
Fig. 8.
SOX2+ adult corpus stem cells are regulated by Notch signaling. A: schematic of mouse genetic model. Sox2 drives expression of a tamoxifen-regulated CreER, which recombines loxP sites (white triangles), leading to excision of a STOP cassette and activation of tdTomato or NICD expression. ires, Internal ribosome entry site; nEGFP, nuclear localized enhanced GFP. B and C: frozen tissue sections from Sox2-CreER; ROSATom mice were examined for native tdTomato fluorescence 1-day or 3-mo post-TX treatment. Costaining with EdU (green, arrowheads) in B identified Tomato+ proliferating cells that are magnified in the inset. D–I: paraffin sections from ROSANICD or Sox2-CreER; ROSANICD mice 3-mo post-TX treatment were stained for hematoxylin and eosin (H&E; D and E), Ki67 (F and G), and H,K-ATPase (H and I). Scale bars: 50 (B and C) or 100 μm (D–I).
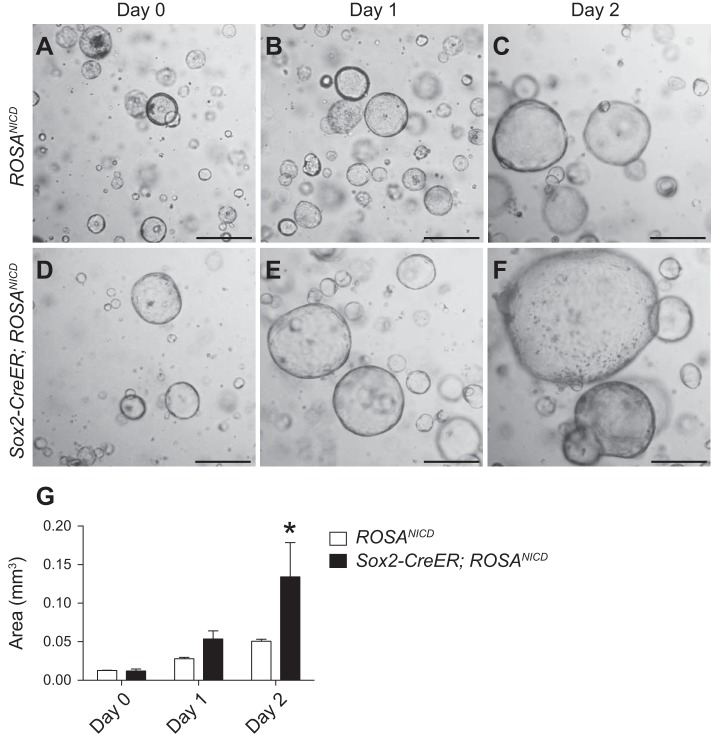
We tested Notch regulation of corpus stem cell function by establishing corpus organoids from ROSANICD and Sox2-CreER; ROSANICD mice and activating NICD in vitro (Fig. 9). Two days after 4-OHT activation of Cre recombinase, organoids from Sox2-CreER; ROSANICD mice were significantly larger compared with ROSANICD organoids (Fig. 9G), suggesting that Notch activation increases cell proliferation to accelerate organoid growth.
Fig. 9.
Notch activation in SOX2+ corpus stem cells stimulates corpus organoid growth. Corpus organoid cultures were initiated from ROSANICD control (A–C) or Sox2-CreER; ROSANICD (D–F) mice and treated with 1 μM 4-OHT for 48 h to activate NICD. Day 0 indicates 24-h postpassage, before 4-OHT treatment. G: measurement of corpus organoid size from ROSANICD (white bars) and Sox2-CreER; ROSANICD (black bars) mice. Data are presented as means ± SE (n = 3 independent organoid lines/group). *P < 0.05 vs. ROSANICD. Scale bars: 500 μm.
DISCUSSION
In the present study, we demonstrate that the Notch signaling pathway regulates epithelial cell homeostasis in the mouse and human gastric corpus. Our findings suggest a fundamental role for Notch signaling to promote stem/progenitor cell proliferation. Our observation that global Notch inhibition reduced corpus epithelial cell proliferation in adult mice follows similar studies reported by Kim and Shivdasani (19). Here we extend this finding to define the key Notch receptors mediating this effect. Treatment of adult mice with receptor-specific inhibitory antibodies revealed that corpus epithelial cell proliferation is mediated primarily through N1 and N2 signaling. Combined receptor inhibition reduced proliferation to a similar extent as global Notch inhibition with DBZ, while individual N1 and N2 blockade had an intermediate effect, suggesting that these two receptors function additively. N1 and N2 receptor expression in epithelial cells in the stem/progenitor cell zone of the mouse corpus is consistent with our observed requirement for both receptors to support gastric corpus epithelial cell proliferation. The finding that N1 and N2 are the primary Notch receptors promoting proliferation in the gastric corpus is consistent with previous studies demonstrating N1 and N2 as the key receptors regulating proliferation in the antral stomach (9) and the intestine (3, 29). In the intestine, genetic studies have demonstrated that N1 is the primary Notch receptor regulating active stem cells (3). Whether N1 may play a similar role in the gastric corpus will be an important point to pursue further once specific corpus stem cell markers are described. Although several studies have identified genetic Cre drivers that are expressed in corpus stem cells, including active stem cells responsible for replenishing the epithelium (1, 24, 27), as well as markers that label “reserve” stem cells that expand upon injury (11, 30), these Cre drivers are also expressed in differentiated cells; thus a specific marker for the active stem cell in the corpus remains to be described. How the Notch pathway regulates the various corpus stem cell populations during normal tissue homeostasis and neoplastic transformation will be crucial to our understanding of stem cell-driven gastric diseases such as cancer.
Our observation that Notch is required for corpus organoid growth via N1 and N2 is consistent with our in vivo findings on proliferation and demonstrates that signaling self-organizes within the epithelial cell compartment when gastric glands are cultured. Analysis of Notch reporter mice revealed pathway activity in both isolated corpus glands and in organoids, consistent with active Notch signaling in selected epithelial cells in the adult mouse stomach. These findings suggest that in vivo Notch function results from ligand-receptor interactions between neighboring epithelial cells, a function consistent with the membrane localization for both ligand and receptor and the known signaling mechanisms for this pathway (6). Future studies to identify Notch ligand expression in the stomach will further our understanding of Notch function in the stomach, including definition of key niche cells supporting gastric stem/progenitor cells in the adult corpus.
Our studies of corpus organoids suggest that Notch regulation of stem/progenitor cell proliferation is a fundamental property of gastric cellular homeostasis. Similar results were obtained with both human and mouse corpus organoids; N1 and N2 were the most abundantly expressed Notch receptors, and both N1 and N2 were required for organoid growth in culture. These similarities suggest that the mouse is a useful model to describe fundamental mechanisms of Notch regulation of the mammalian stomach.
Our studies also showed that activating Notch signaling in the adult corpus promotes proliferation and blocks differentiation. NICD expression was activated with the inducible Sox2-CreER driver, leading to increased epithelial cell proliferation, tissue hypertrophy, and reduced cellular differentiation in vivo, as well as increased organoid growth in vitro. These outcomes are consistent with a report showing hypertrophy and increased proliferation with prenatal Notch activation in parietal progenitor cells using the constitutive Atp4b-Cre driver (19), as well as a report demonstrating that Notch promotes proliferation of adult LGR5+ antral stem cells with the inducible Lgr5-GFP-CreERT2 driver (5). Notch-induced tissue hypertrophy is consistent with reports of increased Notch expression in human gastric cancer associated with poor prognosis (2, 7, 20, 33). Increased expression of NOTCH1 and NOTCH2 Notch receptors, as well as the JAG1 Notch ligand, has been linked to increased morbidity associated with gastric cancer (2, 12, 35). Furthermore, in vitro studies in human gastric adenocarcinoma cell lines with increased NOTCH1 expression demonstrate that Notch inhibition can reduce cancer growth (21). Further understanding of Notch mechanisms regulating homeostasis and promoting hyperproliferative disease may help identify potential therapeutic targets for gastric cancer.
We have previously shown that the Notch pathway regulates epithelial cell differentiation in the mouse gastric antrum, where inhibition of Notch-induced differentiation of mucous and endocrine cell lineages (5, 9) and Notch activation in LGR5+ antral stem cells repressed differentiation (5). In our current study, we find similar outcomes with regard to Notch activation, in that induction of NICD expression in the adult corpus repressed cellular differentiation. However, Notch inhibition with DBZ or Notch receptor inhibitory antibodies did not alter differentiated marker gene expression in the corpus. These results are not unexpected because the lifespan of most corpus lineages lasts from several weeks (mucous neck and endocrine cells; 16, 17) to several months (parietal and chief cells; 14, 16), a time frame which vastly exceeds our 5-day Notch inhibition treatment regimen. Surprisingly, we did not detect any alterations to expression of surface mucous cell markers in the corpus, despite a reported lifespan of 3–5 days for this cell type (15). We previously showed that blocking Notch in the gastric antrum induced differentiation of all antral lineages, including the surface mucous cell population (5, 9). In the corpus, it is possible that differentiation of the surface mucous cell lineage occurs via a Notch-independent mechanism, possibly through a progenitor cell that is committed to formation of this lineage. It has not been definitively shown whether in the corpus a single population of actively cycling stem cells exists and forms all differentiated lineages or whether multiple stem and progenitor cell populations regulate differentiation of each lineage. Identification of these potential stem cell populations will be crucial for studying pathway regulation of corpus epithelial cell differentiation in future studies. Furthermore, Notch inhibition approaches that specifically target the gastric corpus while sparing the intestine will allow more long-term analysis of Notch pathway regulation of differentiation.
Our Notch inhibition studies, however, did reveal a significant increase in the number of GSII+/IF+ transitional cells in both DBZ- and anti-N1/anti-N2-treated animals. GSII+/IF+ transitional cells in the gastric corpus are a relatively rare cell population that occurs as mucous neck cells differentiate into zymogenic chief cells (16, 28). Interestingly, expansion of the mucous neck cell lineage and increased numbers of GSII+/IF+ costained cells also occurs in spasmolytic polypeptide-expressing metaplasia (SPEM), which is a consequence of parietal cell loss due to acute or chronic damage (8, 10, 13, 22). In our study, however, we did not observe SPEM-associated parietal or chief cell loss, based on normal marker expression of these cell types (Fig. 6). Rather, it is possible that Notch inhibition induces the accumulation of GSII+/IF+ costained cells as a cellular remodeling mechanism in the corpus, similar to what we have previously reported for the gastric antrum (9), where Notch inhibition increased expression of GSII+/IF+ costained cells at the antral gland base. Cellular remodeling as a consequence of Notch inhibition has also been reported in intestine, with expansion of cells that coexpress goblet cell (MUC2) and Paneth cell (MMP7) markers in the crypt base (3, 32). Taken together, our findings suggest that the Notch pathway is necessary for maintaining proper lineage specification in the gastrointestinal tract and that perturbation in the Notch pathway can activate a cellular remodeling mechanism that leads to alterations in epithelial cell differentiation.
Overall, we report a previously unknown role for the Notch signaling pathway to support both mouse and human corpus stem cell homeostasis via N1 and N2. Future studies to identify the long-term consequences of Notch-mediated alterations in cellular differentiation will provide insight into how the Notch pathway may be targeted for treatment of precancerous metaplastic conditions in the human stomach.
GRANTS
E. S. Demitrack was supported by National Institutes of Health (NIH) Grants F32-DK-093349, T32-HD-007505, and UL1-TR-000433 and an American Association for Cancer Research-Debbie’s Dream Foundation Career Development Award. G. B. Gifford was supported by NIH Grants T32-GM-0008322 and T32-DK-094775. The research was funded by NIH Grants P01-DK-06041 and P50-CA-130810, American Gastroenterological Association Research Foundation project awards to L. C. Samuelson, and core support from NIH Grant P30-DK-34933 to the Michigan Gastrointestinal Research Center and NIH Support Grant P30-CA-6592 to the University of Michigan Cancer Center.
DISCLOSURES
C. W. Siebel is an employee of Genentech, Inc., and owns shares of Roche. All other authors declare no conflicts of interest.
AUTHOR CONTRIBUTIONS
E.S.D., G.B.G., T.M.K., and N.H. performed experiments; E.S.D., G.B.G., T.M.K., N.H., and L.C.S. analyzed data; E.S.D., G.B.G., T.M.K., N.H., and L.C.S. interpreted results of experiments; E.S.D. and G.B.G. prepared figures; E.S.D., G.B.G., and L.C.S. drafted manuscript; E.S.D., G.B.G., T.M.K., N.H., A.T., D.K.T., C.W.S., and L.C.S. edited and revised manuscript; E.S.D., G.B.G., T.M.K., N.H., A.T., D.K.T., C.W.S., and L.C.S. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Dr. Thaddeus Stappenbeck for L-WRN cells, Christopher Altheim for generating L-WRN conditioned media, and Erin Collin for maintaining mouse colonies.
REFERENCES
- 1.Arnold K, Sarkar A, Yram MA, Polo JM, Bronson R, Sengupta S, Seandel M, Geijsen N, Hochedlinger K. Sox2(+) adult stem and progenitor cells are important for tissue regeneration and survival of mice. Cell Stem Cell 9: 317–329, 2011. doi: 10.1016/j.stem.2011.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bauer L, Takacs A, Slotta-Huspenina J, Langer R, Becker K, Novotny A, Ott K, Walch A, Hapfelmeier A, Keller G. Clinical significance of NOTCH1 and NOTCH2 expression in gastric carcinomas: an immunohistochemical study. Front Oncol 5: 94, 2015. doi: 10.3389/fonc.2015.00094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Carulli AJ, Keeley TM, Demitrack ES, Chung J, Maillard I, Samuelson LC. Notch receptor regulation of intestinal stem cell homeostasis and crypt regeneration. Dev Biol 402: 98–108, 2015. doi: 10.1016/j.ydbio.2015.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Choi E, Roland JT, Barlow BJ, O’Neal R, Rich AE, Nam KT, Shi C, Goldenring JR. Cell lineage distribution atlas of the human stomach reveals heterogeneous gland populations in the gastric antrum. Gut 63: 1711–1720, 2014. doi: 10.1136/gutjnl-2013-305964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Demitrack ES, Gifford GB, Keeley TM, Carulli AJ, VanDussen KL, Thomas D, Giordano TJ, Liu Z, Kopan R, Samuelson LC. Notch signaling regulates gastric antral LGR5 stem cell function. EMBO J 34: 2522–2536, 2015. doi: 10.15252/embj.201490583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Demitrack ES, Samuelson LC. Notch regulation of gastrointestinal stem cells. J Physiol 594: 4791–4803, 2016. doi: 10.1113/JP271667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Du X, Cheng Z, Wang YH, Guo ZH, Zhang SQ, Hu JK, Zhou ZG. Role of Notch signaling pathway in gastric cancer: a meta-analysis of the literature. World J Gastroenterol 20: 9191–9199, 2014. doi: 10.3748/wjg.v20.i27.9191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fox JG, Li X, Cahill RJ, Andrutis K, Rustgi AK, Odze R, Wang TC. Hypertrophic gastropathy in Helicobacter felis-infected wild-type C57BL/6 mice and p53 hemizygous transgenic mice. Gastroenterology 110: 155–166, 1996. doi: 10.1053/gast.1996.v110.pm8536852. [DOI] [PubMed] [Google Scholar]
- 9.Gifford GB, Demitrack ES, Keeley TM, Tam A, La Cunza N, Dedhia PH, Spence JR, Simeone DM, Saotome I, Louvi A, Siebel CW, Samuelson LC. Notch1 and Notch2 receptors regulate mouse and human gastric antral epithelial cell homoeostasis. Gut (March 1, 2016). doi: 10.1136/gutjnl-2015-310811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Goldenring JR, Ray GS, Coffey RJ Jr, Meunier PC, Haley PJ, Barnes TB, Car BD. Reversible drug-induced oxyntic atrophy in rats. Gastroenterology 118: 1080–1093, 2000. doi: 10.1016/S0016-5085(00)70361-1. [DOI] [PubMed] [Google Scholar]
- 11.Hayakawa Y, Ariyama H, Stancikova J, Sakitani K, Asfaha S, Renz BW, Dubeykovskaya ZA, Shibata W, Wang H, Westphalen CB, Chen X, Takemoto Y, Kim W, Khurana SS, Tailor Y, Nagar K, Tomita H, Hara A, Sepulveda AR, Setlik W, Gershon MD, Saha S, Ding L, Shen Z, Fox JG, Friedman RA, Konieczny SF, Worthley DL, Korinek V, Wang TC. Mist1 expressing gastric stem cells maintain the normal and neoplastic gastric epithelium and are supported by a perivascular stem cell niche. Cancer Cell 28: 800–814, 2015. doi: 10.1016/j.ccell.2015.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hsu KW, Hsieh RH, Huang KH, Fen-Yau Li A, Chi CW, Wang TY, Tseng MJ, Wu KJ, Yeh TS. Activation of the Notch1/STAT3/Twist signaling axis promotes gastric cancer progression. Carcinogenesis 33: 1459–1467, 2012. doi: 10.1093/carcin/bgs165. [DOI] [PubMed] [Google Scholar]
- 13.Huh WJ, Khurana SS, Geahlen JH, Kohli K, Waller RA, Mills JC. Tamoxifen induces rapid, reversible atrophy, and metaplasia in mouse stomach. Gastroenterology 142: 21–24.e7, 2012. doi: 10.1053/j.gastro.2011.09.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Karam SM. Dynamics of epithelial cells in the corpus of the mouse stomach. IV. Bidirectional migration of parietal cells ending in their gradual degeneration and loss. Anat Rec 236: 314–332, 1993. doi: 10.1002/ar.1092360205. [DOI] [PubMed] [Google Scholar]
- 15.Karam SM, Leblond CP. Dynamics of epithelial cells in the corpus of the mouse stomach. II. Outward migration of pit cells. Anat Rec 236: 280–296, 1993. doi: 10.1002/ar.1092360203. [DOI] [PubMed] [Google Scholar]
- 16.Karam SM, Leblond CP. Dynamics of epithelial cells in the corpus of the mouse stomach. III. Inward migration of neck cells followed by progressive transformation into zymogenic cells. Anat Rec 236: 297–313, 1993. doi: 10.1002/ar.1092360204. [DOI] [PubMed] [Google Scholar]
- 17.Karam SM, Leblond CP. Dynamics of epithelial cells in the corpus of the mouse stomach. V. Behavior of entero-endocrine and caveolated cells: general conclusions on cell kinetics in the oxyntic epithelium. Anat Rec 236: 333–340, 1993. doi: 10.1002/ar.1092360206. [DOI] [PubMed] [Google Scholar]
- 18.Keeley TM, Samuelson LC. Cytodifferentiation of the postnatal mouse stomach in normal and Huntingtin-interacting protein 1-related-deficient mice. Am J Physiol Gastrointest Liver Physiol 299: G1241–G1251, 2010. doi: 10.1152/ajpgi.00239.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kim TH, Shivdasani RA. Notch signaling in stomach epithelial stem cell homeostasis. J Exp Med 208: 677–688, 2011. doi: 10.1084/jem.20101737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee HW, Kim SJ, Choi IJ, Song J, Chun KH. Targeting Notch signaling by γ-secretase inhibitor I enhances the cytotoxic effect of 5-FU in gastric cancer. Clin Exp Metastasis 32: 593–603, 2015. doi: 10.1007/s10585-015-9730-5. [DOI] [PubMed] [Google Scholar]
- 21.Li LC, Peng Y, Liu YM, Wang LL, Wu XL. Gastric cancer cell growth and epithelial-mesenchymal transition are inhibited by γ-secretase inhibitor DAPT. Oncol Lett 7: 2160–2164, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu Z, Demitrack ES, Keeley TM, Eaton KA, El-Zaatari M, Merchant JL, Samuelson LC. IFNγ contributes to the development of gastric epithelial cell metaplasia in Huntingtin interacting protein 1 related (Hip1r)-deficient mice. Lab Invest 92: 1045–1057, 2012. doi: 10.1038/labinvest.2012.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Madisen L, Zwingman TA, Sunkin SM, Oh SW, Zariwala HA, Gu H, Ng LL, Palmiter RD, Hawrylycz MJ, Jones AR, Lein ES, Zeng H. A robust and high-throughput Cre reporting and characterization system for the whole mouse brain. Nat Neurosci 13: 133–140, 2010. doi: 10.1038/nn.2467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Matsuo J, Kimura S, Yamamura A, Koh CP, Hossain MZ, Heng DL, Kohu K, Voon DC, Hiai H, Unno M, So JB, Zhu F, Srivastava S, Teh M, Yeoh KG, Osato M, Ito Y. Identification of stem cells in the epithelium of the stomach corpus and antrum of mice. Gastroenterology 152: 218–231.e14, 2017. doi: 10.1053/j.gastro.2016.09.018. [DOI] [PubMed] [Google Scholar]
- 25.Murtaugh LC, Stanger BZ, Kwan KM, Melton DA. Notch signaling controls multiple steps of pancreatic differentiation. Proc Natl Acad Sci U S A 100: 14920–14925, 2003. doi: 10.1073/pnas.2436557100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nowotschin S, Xenopoulos P, Schrode N, Hadjantonakis AK. A bright single-cell resolution live imaging reporter of Notch signaling in the mouse. BMC Dev Biol 13: 15, 2013. doi: 10.1186/1471-213X-13-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Powell AE, Wang Y, Li Y, Poulin EJ, Means AL, Washington MK, Higginbotham JN, Juchheim A, Prasad N, Levy SE, Guo Y, Shyr Y, Aronow BJ, Haigis KM, Franklin JL, Coffey RJ. The pan-ErbB negative regulator Lrig1 is an intestinal stem cell marker that functions as a tumor suppressor. Cell 149: 146–158, 2012. doi: 10.1016/j.cell.2012.02.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ramsey VG, Doherty JM, Chen CC, Stappenbeck TS, Konieczny SF, Mills JC. The maturation of mucus-secreting gastric epithelial progenitors into digestive-enzyme secreting zymogenic cells requires Mist1. Development 134: 211–222, 2007. doi: 10.1242/dev.02700. [DOI] [PubMed] [Google Scholar]
- 29.Riccio O, van Gijn ME, Bezdek AC, Pellegrinet L, van Es JH, Zimber-Strobl U, Strobl LJ, Honjo T, Clevers H, Radtke F. Loss of intestinal crypt progenitor cells owing to inactivation of both Notch1 and Notch2 is accompanied by derepression of CDK inhibitors p27Kip1 and p57Kip2. EMBO Rep 9: 377–383, 2008. doi: 10.1038/embor.2008.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stange DE, Koo BK, Huch M, Sibbel G, Basak O, Lyubimova A, Kujala P, Bartfeld S, Koster J, Geahlen JH, Peters PJ, van Es JH, van de Wetering M, Mills JC, Clevers H. Differentiated Troy+ chief cells act as reserve stem cells to generate all lineages of the stomach epithelium. Cell 155: 357–368, 2013. doi: 10.1016/j.cell.2013.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.van Es JH, van Gijn ME, Riccio O, van den Born M, Vooijs M, Begthel H, Cozijnsen M, Robine S, Winton DJ, Radtke F, Clevers H. Notch/gamma-secretase inhibition turns proliferative cells in intestinal crypts and adenomas into goblet cells. Nature 435: 959–963, 2005. doi: 10.1038/nature03659. [DOI] [PubMed] [Google Scholar]
- 32.VanDussen KL, Carulli AJ, Keeley TM, Patel SR, Puthoff BJ, Magness ST, Tran IT, Maillard I, Siebel C, Kolterud Å, Grosse AS, Gumucio DL, Ernst SA, Tsai YH, Dempsey PJ, Samuelson LC. Notch signaling modulates proliferation and differentiation of intestinal crypt base columnar stem cells. Development 139: 488–497, 2012. doi: 10.1242/dev.070763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wu X, Liu W, Tang D, Xiao H, Wu Z, Chen C, Yao X, Liu F, Li G. Prognostic values of four Notch receptor mRNA expression in gastric cancer. Sci Rep 6: 28044, 2016. doi: 10.1038/srep28044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wu Y, Cain-Hom C, Choy L, Hagenbeek TJ, de Leon GP, Chen Y, Finkle D, Venook R, Wu X, Ridgway J, Schahin-Reed D, Dow GJ, Shelton A, Stawicki S, Watts RJ, Zhang J, Choy R, Howard P, Kadyk L, Yan M, Zha J, Callahan CA, Hymowitz SG, Siebel CW. Therapeutic antibody targeting of individual Notch receptors. Nature 464: 1052–1057, 2010. doi: 10.1038/nature08878. [DOI] [PubMed] [Google Scholar]
- 35.Yeh TS, Wu CW, Hsu KW, Liao WJ, Yang MC, Li AF, Wang AM, Kuo ML, Chi CW. The activated Notch1 signal pathway is associated with gastric cancer progression through cyclooxygenase-2. Cancer Res 69: 5039–5048, 2009. doi: 10.1158/0008-5472.CAN-08-4021. [DOI] [PubMed] [Google Scholar]