Abstract
Hematopoietic stem cells (HSCs) have the ability to both self-renew and differentiate into all the mature blood cell lineages and thereby reconstitute the entire blood system. As such, HSCs are therapeutically valuable for treatment of hematological malignances and bone marrow failure. We recently showed that transient glucose elevation elicited dose-dependent effects on HSCs through elevated metabolic activity and subsequent ROS-mediated induction of Hypoxia Inducible Factor 1α (Hif1α). Platelet Derived Growth Factor B (pdgfb), a Hif1α-target, and its receptor, pdgfrb, were significantly upregulated in response to metabolic stimulation. While the function of PDGF-signaling is well established in vascular development, its role in hematopoiesis is less understood. Exposure to either a pan-PDGF inhibitor or a PDGFRβ-selective antagonist in the context of Hif1α stimulation blocked elevations in HSPC formation as determined by runx1;cmyb WISH and HSPC-reporter FACS analysis. Similar results were observed for morpholino knockdown of pdgfrb or dominant negative pdgfrb expression, indicating PDGFRβ signaling is a key downstream mediator of Hif1α-mediated induction of HSPCs. Notably, overexpression of pdgfb ligand enhanced HSPC numbers in the AGM at 36hpf and in the CHT at 48hpf. A survey of known PDGF-B/PDGFRβ regulatory targets by qPCR revealed a significant increase in inflammatory intermediates, including interleukin 6 (IL-6) and its receptor (IL-6R). MO-mediated knockdown of il6 or chemical inhibition of IL-6R antagonized the effect of pdgfb overexpression; furthermore, epistatic analysis of IL-6/IL-6R function confirmed activity downstream of Hif1α. Together these findings define a Hif1α-regulated signaling axis through PBFGB/PDGFRβ and IL-6/IL-6R that acts to control embryonic HSPC production.
INTRODUCTION
Hematopoietic stem cells (HSCs) possess the unique ability to both self-renew and differentiate into all the mature blood cell lineages and thereby reconstitute the entire blood system for a lifetime. In vertebrates, HSCs are initially generated during embryogenesis from specialized hemogenic endothelium in the ventral wall of the dorsal aorta, within an intraembryonic region termed the aorta-gonad-mesonephros (AGM) in mammals [1-4]. Runx1 is a transcription factor that is required for the production of definitive HSCs [5], controlling endothelial-to-hematopoietic transition (EHT) [6]. We previously determined that heightened glucose metabolism acts as an inductive trigger for production of Runx1+ HSCs during embryogenesis through metabolism-mediated ROS production and subsequent stabilization of Hypoxia inducible factor 1α (Hif1α) levels in vivo [7].
The HIF complex functions as the master regulator of the adaptive response to low oxygen concentration, or hypoxia. When oxygen levels in the cellular environment are sufficient, Hif1α is targeted for degradation by the von Hippel-Lindau tumor suppressor protein (VHL). In contrast, hypoxic stimuli – including both pathological and relative decreases in oxygen content-inhibits Hif1α degradation, allowing it to interact with Hif1β to activate transcription of hypoxia responsive genes, which affect a wide range of cellular processes including glucose metabolism, cellular proliferation, erythropoiesis, and angiogenesis [8]. Our prior studies indicated Hif1α was necessary for HSC production in zebrafish embryos [7]. While complete loss of Hif1a is embryonic lethal prior to the formation of definitive HSCs in the mouse [9], more recent analysis using conditional loss of Hif1a function confirms it likewise regulates HSC development in the mammalian embryo [10]. Nonetheless, the factor(s) downstream of Hif1α-regulation that influence embryonic HSPC production are unknown.
Platelet Derived Growth Factors (PDGFs) belong to family of regulatory factors that control cell growth and proliferation. PDGFs act through protein tyrosine kinase receptors, PDGFRα and PDGFRβ. Binding of PDGFs to cognate receptors results in dimerization and activation of receptor tyrosine kinase activity, leading to the initiation of downstream cytoplasmic signal transduction pathways including the Phosphatidylinositol 3-kinase (PI3K), Extracellular Signal-regulated kinase (ERK), and Proto-oncogene tyrosine-protein kinase Src to affect migration, proliferation, and differentiation of PDGF responsive cell types [11]. Previous studies have shown that PDGF-B expression is regulated by hypoxia in cardiovascular and neuronal cell types as well as breast cancer cells [12-14]; PDGF-B was further documented to be a direct target of Hif1α in experiments using HeLa cells [14]. While PDGF-B has an established role in vascular remodeling during later stages of development and in adult angiogenesis via recruitment of PDGFRβ expressing pericytes [11], its mechanistic impact on hematopoiesis is less clear.
PDGF-B or PDGFRb knockout embryos die at birth of cardiovascular dysfunction and organ specific hemorrhages, exhibiting both anemia and thrombocytopenia, which are currently thought to be secondary to defects in other organs, including the heart, placenta, vasculature and liver [15, 16]. Previous in vitro and in vivo studies have indicated a role of PDGF-B in erythropoiesis [17-19] and megakaryopoiesis [20], but stimulation required the presence of adherent stromal cells. PDGF has also been shown to act on platelets during wound healing, as well as stimulate pro-inflammatory cytokines from macrophages [21]. In mixed marrow cultures, incubation with recombinant PDGF-B enhanced colony formation of primitive hematopoietic precursors; however, it remains unclear whether PDGF directly affects hematopoietic function or indirectly stimulates HSPCs through the release of growth and differentiation factors from stromal cells [19]. Interestingly, irradiated wild-type mice can be reconstituted up to 4-12 months by grafting PDGF-B or PDGFRb-deficient hematopoietic cells [19], suggesting a non-cell autonomous function of PDGF-B signaling in adult HSC regulation. Finally, PDGF-B signaling in trophoblasts was more recently found to be a key component of the unique placental microenvironment that protects HSPCs from premature differentiation toward the erythroid lineage [22], indicating this growth factor pathway may exert additional hematopoietic regulatory impact during embryonic development.
PDGF/PDGFR signaling can stimulate the synthesis of various interleukins, including Interleukin-1 (IL-1β) and Interleukin 6 (IL-6), to affect cellular functions such as proliferation and migration in osteoblasts and smooth muscle cells [23]. IL-6 is a prominent pro-inflammatory cytokine produced during the immune response to infection [24] which acts by binding to the receptor complex made of the selective IL-6R (CD126) and the common signal transduction component gp130 (CD130). In vitro, IL-6, acting in synergy with IL-3, enhances the formation of multi-lineage blast cell colonies [25]. Consistent with these findings, IL-6 deficient mice show a reduction in the number of primitive hematopoietic colony forming progenitor cells, as well as decreased long term reconstituting stem cell potential in transplantation assays [26]. IL-6 along with Flt3, SCF, and Thrombopoietin, have been shown to enhance the proliferation of primitive hematopoietic stem and progenitor cells in vitro [27, 28], making it an intriguing target for Hif1α-mediated regulation of developmental HSPC production.
Here, we demonstrate a role for PDGF-B/PDGFRβ signaling downstream of Hif1α activation in the regulation of HSPC production during embryonic hematopoietic development. Inhibition of PDGFRβ signaling in the presence of chemical or genetic Hif1α stabilization attenuated its ability to enhance HSPC formation. In contrast, overexpression of pdgfb robustly increased HSPC production, even in the absence of HIF1α function. The effect of PDGF-B/PDGFRβ was mediated by IL-6/IL-6R activity, whereby loss of the ligand or receptor antagonism could block PDGF-induced HSPC expansion. Finally, IL-6 was both induced by and determined to function downstream of Hif1α stabilization. Together these findings uncover a signaling axis through PDGF-B/PDGFRβ and IL6/IL6R that regulates the scale of definitive HSPC formation via inflammatory signals in response to Hif1α stimulation.
METHODS
Zebrafish husbandry
Zebrafish were maintained according to Beth Israel Deaconess Medical Center IACUC-approved protocols. Tg(−6.0itga2b (CD41):eGFP [29], Tg(kdrl (flk1):dsRed), Tg(cmyb:eGFP), Tg(gata1:dsred) and Tg(EPV.Tp1CMmu.Hbb (Notch reporter):EGFP) lines were described previously [30, 31]. The pdgfrb mutant line was previously published [32]. The hsp70:ca-pdgfrb and hsp70:dn-pdgfrb lines were created as detailed below.
Chemical treatments and evaluation
Zebrafish embryos were exposed to compound-modifiers in E3 water in multi-well plates for durations noted. Compounds utilized were: DMPQ (10μM, Cayman), cobalt (II) chloride (CoCl2, 500μM, R&D Systems), DMOG (75μM, Cayman), AG1295 (10μM, Calbiochem), AG1296 (2.5μM, Cayman), and SC-144 (1μM, EMD Millipore). Whole-mount in situ hybridization (WISH) was performed as previously described [33]. Qualitative phenotypes for individual embryos (n≥20 embryos/condition, ≥2 replicate clutches) were scored as relatively high/medium/low in expression compared to sibling controls and graphically depicted as the percentage falling into each of the 3 phenotypic expression bins; “medium” expression was set as the most representative phenotype in the normal bell-curve distribution of each cohort of control embryos per experiment.
Fluorescence Activated Cell Sorting
FACS analysis was performed using double transgenic Tg(flk1:dsRed;cmyb:GFP) or Tg(CD41:GFP;gata1:dsRed) reporter embryos as previously described [1]. Embryos (pools of 3-5 embryos per sample, ≥5 replicates) were dissociated, resuspended in 1×PBS, and analyzed on a BD FACSCanto II (BD Biosciences, San Jose, CA) in the presence of SYTOX Red Dead Cell Stain (5nM, Life Technologies, Waltham, MA). Data was analyzed using FlowJo X software (TreeStar, Ashland, OR). For isolation of endothelial (Flk1:dsRed+cMyb:GFP−) and HSPC (Flk1:dsRed+cMyb:GFP+) fractions, pooled embryos (n>1000) were sorted using FACSAria (BD Biosciences, San Jose, CA). After cell collection, RNA was extracted, treated with DNaseI (RNAqueous-Micro Total RNA isolation Kit, Life Technologies, Rockville, MD), and amplified with Ovation RNA Amplification System V2 (NuGEN).
Morpholino and mRNA Injection
Morpholinos to vhl and hif1a (GeneTools, Philomath, OR) were injected as described previously [7, 34]; MO sequences are listed in the Supplemental Methods. Briefly, each MO (dose: 0.2-0.4mM) was microinjected into 1-cell stage embryos and allowed to develop to the timepoint(s) of interest before processing with matched sibling controls for evaluation as above. For mRNA generation, pdgfb and il6 Coding Data Sequences were amplified by PCR (see primers pairs below) from IMAGE clones 6330609 and 40130735 respectively and cloned into pCS2+ (EcoRI/XbaI). mRNA was in vitro transcribed from NotI linearized constructs using the SP6 mMESSAGE mMACHINE kit (Life Technologies) and injected at the 1-cell stage. Mouse Pdgfb mRNA was injected at 25ng/μl, mouse Il6 mRNA was injected at 200ng/μl, and dnhif1 mRNA [35] was injected at 200ng/μl.
Pdgfb-
F: 5’ GATGGAATTCATGAATCGCTGCTGGGCG
R: 5’ GATGTCTAGACTAGGCTCCGAGGGTCTCC
Il6-
F: 5’ GATGGAATTCATGAAGTTCCTCTCTGCAAG
R: 5’ GATGTCTAGACTAGGTTTGCCGAGTAGATC
Generation and induction of the hsp70:ca-pdgfrb and hsp70:dn-pdgfrb expression constructs
Mouse pdgfrb Coding Data Sequence was amplified from the IMAGE clone 30060666 and cloned into the pENTR-D-TOPO vector (Invitrogen) using the following primers:
F 5’ CACCATGGGGCTTCCAGGAGTGATACCAG
R 5’ CTACAGGAAGCTGTCCTCTGCTTCAGCC
The D849V amino acid substitution used to generate the constitutively activating mutation (ca-pdgfrb), as described in murine Pdgfrb [36], was created by site-directed mutagenesis. The dn-pdgfrb construct, which possesses a truncation of the receptor tyrosine kinase domain, was generated by PCR amplification with the following primers and cloned into the pENTR-D-TOPO vector:
F 5’ CACCATGAAGAGTTCGACCATCAG
R 5’ CTATCTTCCTCCACACAGCAATG
The hsp70:ca-pdgfrb and hsp70:dn-pdgfrb constructs were generated using the multigateway LR Clonase (Invitrogen) Gateway reaction. The resulting plasmid was microinjected with tol2-transposase RNA into 1-cell-stage embryos [37]. Tg(hsp70:ca-pdgfrb) and WT sibling control embryos were heat-shocked at 27hpf by incubation in a 37°C water bath for 1 hour. Tg(hsp70:dn-pdgfrb) and WT sibling controls were heat shocked with the same conditions, and split for additional CoCl2 treatment following the incubation. Embryos were fixed at 48hpf for analysis and processed as above.
Microscopy
Fluorescent embryos were treated as above and imaged by fluorescence microscopy using a Zeiss Discovery V8/Axio Cam MRC and Axiovision LE software (Carl Zeiss), as previously described [34]. Cell counts were quantified using ImageJ (NIH). Two-tailed Student's t-tests were performed for investigational comparisons; one-tailed tests were run for statistical confirmation of WISH phenotypes. Data are presented as mean ± SEM, and p-values < 0.05 were considered significant.
Quantitative RT-PCR
qPCR was performed on cDNA isolated from pooled embryos at timepoints indicated (n=25 embryos/variable; see Supplemental Methods for qPCR primers) using ABI PRISM 7900HT (Invitrogen). Samples were run in technical triplicate with ≥3 biological replicates/condition. Ct values were determined using PCR Miner [38] and fold-change calculated by the ddCt or R0 method with tbp or B-actin as the reference gene.
Statistical Analyses
Two-tailed Student's t-tests were performed for statistical analysis and data presented, unless otherwise indicated. For confirmation of WISH results, one-tailed Student's t-tests were performed based on predicted outcome.
RESULTS
Hif1α stabilization enhances HSPC production via up-regulation of PDGF-B signaling
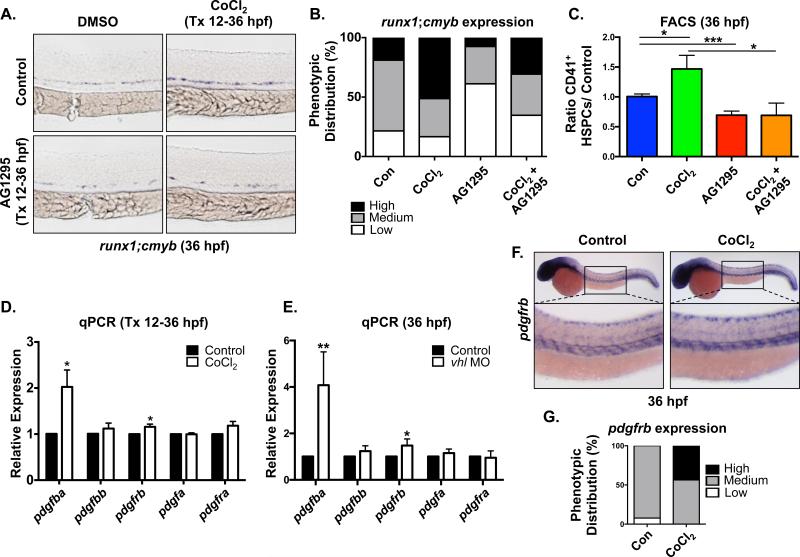
We previously identified platelet-derived growth factor (pdgf) as a potential target of Hif1α relevant to embryonic HSPC production by gene expression analysis [7]. To assess whether PDGF signaling functions downstream of Hif1α in developmental HSPC production, we first conducted modified epistasis experiments: zebrafish embryos were exposed to the pan-PDGF receptor inhibitor, AG1295 (10μM), in the presence or absence of the known Hif1α agonist CoCl2 (500μM) during the period of HSPC specification from 12-36hpf. Embryos treated with CoCl2 exhibited qualitatively stronger expression of runx1;cmyb in the ventral dorsal aorta (VDA) compared to age-match sibling controls as visualized by whole mount in situ hybridization (WISH), consistent with our prior analysis [7, 33] (Fig 1A,B). In contrast, the majority of embryos exposed to AG1295 displayed reduced runx1;cmyb expression in the VDA; furthermore, AG1295 blocked the CoCl2-mediated increase in the distribution of embryos with enhanced HSPC gene expression (Fig 1A,B). This effect was confirmed and quantified by flow cytometry using the Tg(−6.0itga2b(CD41):eGFP line (crossed to Tg(gata1:dsRed) to exclude thrombocytes), which has been previously shown to mark HSPCs with in vivo repopulating potential [7]. Exposure to AG1295 caused a 0.58-fold reduction (p<0.001) in CD41+;Gata− HSPCs (Fig 1C), and blocked the ability of CoCl2 to enhance HSPC numbers (p<0.05), suggesting that Hif1α influences HSPC development in part through PDGFR signaling.
Figure 1. Hif1α stabilization enhances HSPC production via PDGF activity.
(A) Embryonic exposure to the pan-PDGFR inhibitor AG1295 (10μM) (during HSC formation (12-36hpf) decreased runx1;cmyb WISH expression in the AGM and blocked the increase in runx1;cmyb expression due to exposure to Hif1α agonist (CoCl2, 500μM). Tx = treatment.
(B) Qualitative phenotypic distribution of embryos from panel 1A scored with low, medium or high runx1;cmyb expression in the AGM (n≥20 condition × 3 replicate clutches).
(C) FACS analysis confirmed that chemical stabilization of Hif1α enhanced HSCs, while inhibition of PDGFR decreased CD41+gata1− HSPCs at 36hpf in the presence of CoCl2 (*p<0.05, ***p<0.001, one-tailed t-test, n≥13 replicates/condition).
(D) RT-qPCR analysis showed that pdgfba and pdgfrb were significantly upregulated over baseline in CoCl2 treated embryos, whereas related family members were not affected (Tx 12-36hpf) (*p<0.05, two-tailed t-test, n≥3).
(E) In vhl MO injected embryos RT-qPCR analysis likewise indicated that only pdgfba and pdgfrb were significantly upregulated over matched controls (Tx 12-36hpf) (*p<0.05, **p<0.01, two-tailed t-test, n≥4).
(F) WISH analysis of wild-type embryos at 36hpf indicated pdgfrb expression throughout the trunk of the embryo, including enrichment in the region of the VDA; pdgfrb expression was notably increased by CoCl2 exposure.
(G) Qualitative phenotypic distribution of embryos from panel 1F scored with low, medium or high pdgfrb expression in the AGM (n≥20 condition × 2 replicate clutches).
To determine which PDGF family members are the primary targets of Hif1α in this context, embryos were exposed to CoCl2 during HSPC specification and PDGF-related gene expression was examined by whole embryo RT-qPCR. CoCl2 treatment significantly increased gene expression of pdgfba (p<0.05; the gene encoding the PDGFb ligand is duplicated in zebrafish: pdgfba and pdgfbb) and pdgfrb (p<0.05); notably, expression of related factors were not statistically altered (Fig 1D). As confirmation of regulatory specificity, embryos were either injected with a previously published MO targeting Von Hippel-Lindau (VHL), an E3 ubiquitin ligase that normally targets hydroxylated Hif1α to the proteasome for degradation when oxygenation is sufficient, or exposed to DMOG (75μM), a prolyl hydroxylase inhibitor that leads to Hif1α stabilization and increased runx1 expression [7]. Each treatment resulted in increased expression of pdgfba (p<0.01) and pdgfrb (p<0.05) by RT-qPCR (Fig 1E and Fig S1A), but no significant differences in closely related family members. WISH analysis indicated that pdgfrb is expressed in the trunk region encompassing the VDA at 36hpf, consistent with prior observations [39]; furthermore, increased local expression of pdgfrb was documented in embryos exposed to CoCl2 (Fig 1F,G). RT-qPCR analysis of sorted cell populations from Tg(flk1:dsRed;cmyb:GFP) embryos at 36hpf confirmed that pdgfba and pdgfrb are expressed in both Flk1+;cMyb− endothelium and Flk1+;cMyb+ HSPCs (Fig S1B), with each also found in the negative fraction. To identify which cell types may be responding to Hif1α regulation through PDGFRβ signaling, we compared expression levels of pdgfba and pdgfrb in sorted cell populations from flk1:dsRed;cmyb:GFP embryos that had been treated with CoCl2 (12-36hpf) to matched DMSO controls. As expected, the established Hif1α target erythropoietin receptor (epor) was robustly induced following treatment. RT-qPCR analysis showed that expression of pdgfrb was increased in all sorted fractions (Fig S1C); interestingly, pdgfba expression was increased specifically in the Flk+cMyb− endothelial population, similar to that seen for runx1, implying PDGF activity may directly impact hematovascular commitment and/or subsequent HSPC production downstream of Hif1α stimulation [7]. As PDGF signaling impacts vascular remodeling [40], we assessed the effect of AG1295 treatment, as well as a targeted PDGFRβ-selective inhibitor, DMPQ (10μM), on Notch-dependent hemogenic specification. No differences were observed in flk1 (endothelial) or ephrinb2a (arterial) expression at 36hpf at the doses utilized (Fig S1D,E); in contrast, while AG1295 dramatically reduced gfp expression in the VDA using the Notch reporter line, Tg(EPV.Tp1CMmu.Hbb:EGFP) [31], DMPQ had no effect on hematovascular commitment (Fig S1F,G). Together, these data suggest that Hif1α stabilization can enhance HSPC production, independent of hemogenic specification, via the up-regulation of pdgfba and pdgfrb expression
PDGFRβ signaling is required downstream of Hif1α to control AGM HSPC production
To determine if PDGFRβ activity was necessary for HPSC formation in the VDA, we employed a gene knockdown approach using a previously validated pdgfrb morpholino [41]; embryos injected with pdgfrb MO had no gross aortic phenotype at 36hpf at the doses utilized as determined by flk1 expression, consistent with DMPQ exposure (Fig S1D) and prior reports [32](Fig S2A). In contrast, runx1;cmyb staining was reduced but not eliminated in half of the embryos examined (Fig S2B,C). Similar findings were observed using a recently described pdgfrb mutant [32]; however, while homozygous embryos did not exhibit a strong HSPC phenotype, the majority of heterozygous siblings showed reduced runx1;cmyb expression in the VDA (Fig S2D,E). This data suggests potential compensatory interactions are elicited by complete pdgfrb loss (perhaps by the closely related pdgfra) and highlights that dosage-dependent PDGFRβ regulation alone is active in, but not essential for, developmental HSPC specification.
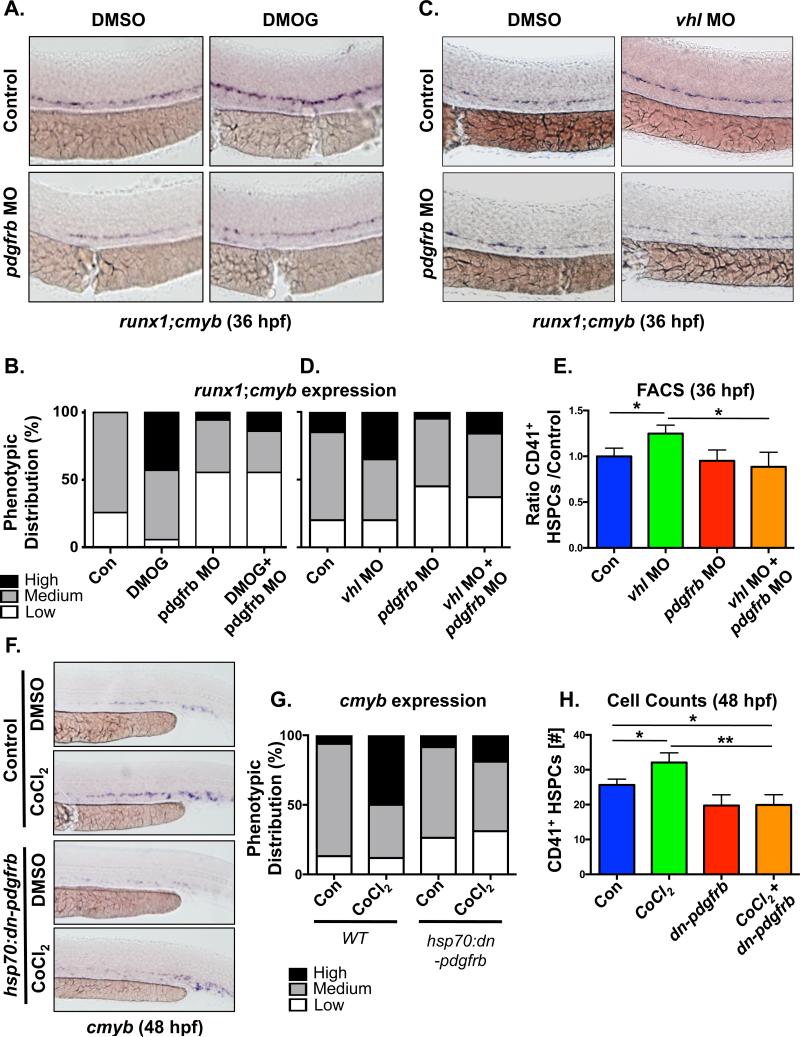
As Hif1α stimulation specifically stimulated pdgfrb expression, we next sought to determine if PDGFRβ signaling was required to mediate its effect on HSPC numbers using modified epistasis analysis. Stabilization of Hif1α via DMOG treatment increased runx1;cmyb staining in control embryos, consistent with our prior analysis [7]; in contrast, DMOG was unable to elicit elevated expression in pdgfrb morphants (Fig 2A,B). Similar observations were seen for chemical inhibition of PDGFRb using DMPQ in the setting of Hif1a stimulation (Fig S2F,G). The requirement of PDGFRβ activity downstream of Hif1α was further validated using the vhl MO. Consistent with stabilized Hif1α levels in the absence of VHL function and our prior observations [7], vhl morphant embryos had enhanced runx1;cmyb expression in the VDA; however, this effect was also not observed in pdgfrb morphants (Fig 2C,D). To confirm and quantify these findings, FACS for CD41:GFP was performed at 36hpf, and demonstrated that the significant increase in HSPCs caused by loss of VHL-mediated Hif1α regulation (p<0.05) was blocked by pdgfrb knockdown (p<0.05) (Fig 2E). Finally, to ensure the effects on HSPC numbers were not due to embryonic toxicity impacting aortic specification and/or function, and to further confirm the specificity of the role of pdgfrb, a dominant negative construct was employed. Heat shock induction at 28hpf, after hemogenic endothelial specification and the onset of circulation, had modest impact alone, yet dramatically blocked the impact of CoCl2 stimulation by WISH at 48hpf (Fig 2F,G). This effect was confirmed by CD41+ HSPC cell counts, whereby expression of dn-pdgfrb significantly blocked the ability of CoCl2-mediated Hif1α activation to increase HSPC numbers (Fig 2H). Together, these findings indicate that PDGFRβ is a functionally relevant target of Hif1α in embryonic HSPC regulation.
Fig 2. PDGFRβ signaling acts downstream of Hif1α to control AGM HSPC production.
(A) Morpholino knockdown of pdgfrb attenuated the increase in runx1;cmyb expression in embryos treated with the Hif1α agonist DMOG (75μM).
(B) Qualitative phenotypic distribution of embryos from panel 2A (n≥20/condition × 3 replicate clutches).
(C) Knockdown of vhl increased runx1;cmyb WISH expression in the AGM at 36hpf, while co-injection with the pdgfrb MO blocked this effect.
(D) Qualitative phenotypic distribution of embryos from panel 2D (n≥20/condition × 2 replicate clutches).
(E) FACS analysis for CD41+(Gata1−) HSPCs following injection of MOs to vhl and pdgfrb alone and combined confirmed a reduced impact for Hif1α stabilization with loss of PDGF signaling (*p<0.05, one-tailed t-test, n≥7).
(F) Ectopic activation of a dominant negative pdgfrb transgene, Tg(hsp70:dn-pdgfrb), at 27hpf (37°C for 1 hour) diminished the impact of CoCl2 stimulation on cmyb expression at 48hpf.
(G) Qualitative phenotypic distribution of embryos from panel S2G (n≥20/condition × 4 replicate clutches).
(H) CD41+ HSPC cell counts at 48hpf confirmed the effect of loss of PDGFRB signaling, mediated by induction of a dominant negative receptor, on Hif1α activation by CoCl2 (*p<0.05, **p<0.01, one-tailed t-test, n≥8).
pdgfb overexpression increases HSPC production in the absence of Hif1α
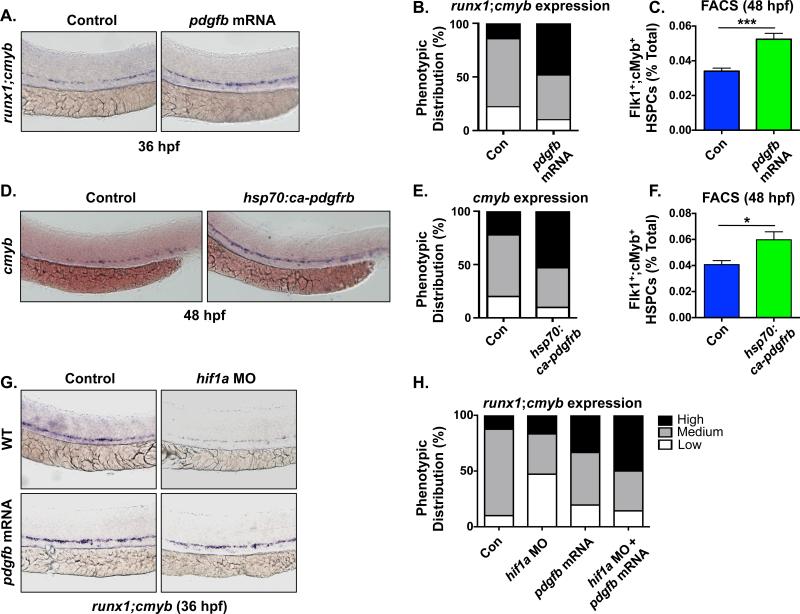
To next determine if PDGF signaling plays an instructive role in embryonic HSPC production in the AGM, we overexpressed murine pdgfb mRNA in the zebrafish embryo. Embryos injected with 25ng/μL pdgfb mRNA exhibited enhanced runx1;cmyb expression (Fig 3A,B) and increased Flk1+;cMyb+ HSPC counts in the AGM at 36hpf (Control: 6±1.5 cells/AGM, pdgfb mRNA: 10.4±2.2, n≥15/condition, p<0.0001) (Fig S3A,B). Furthermore, this effect was sustained at 48hpf as assessed by cmyb expression in the CHT (Fig S3C,D) and quantified by Flk1+;cMyb+ flow cytometry (1.54-fold increase vs. control, p<0.0001) (Fig 3C). Analysis of embryos expressing Tg(−6.0itga2b(CD41):eGFP confirmed a significant increase in CD41:GFP+ HSPCs in the CHT following overexpression of murine pdgfb (Control: 13.1±7.4 cells/CHT, pdgfb mRNA: 18.9±9.8, n≥35/condition, p<0.0053) (Fig S3E,F). As PDGF-B could stimulate multiple PDGFRs, a heat shock inducible construct was created to investigate the effect of specific upregulation of PDGFRβ activity. Induction of constitutively active pdgfrb at 27hpf mimicked the effects of pdgfb overexpression, enhancing cmyb expression at 48hpf (Fig 3D,E) and increasing the total number of Flk1+cMyb+ HSPCs as determined by FACS analysis (p<0.05) (Fig 3F). Finally, to assess whether increased PDGF-B/PDGFRβ signaling could rescue the effect of reduced Hif1α activity on HSPC production in the VDA a modified epistasis experiment was conducted. Consistent with our prior observations [7], runx1;cmyb expression was reduced in hif1α morphants. Overexpression of pdgfb mRNA in hif1α morphant embryos enhanced the proportion of embryos showing elevated runx1;cmyb levels (Fig 3G,H). Together these data indicate that PDGF-B/PDGFRβ receptor stimulation is functionally conserved across vertebrate species and is sufficient to increase HSC induction from the hemogenic endothelium.
Fig 3. PDGF-B stimulation increases developmental HSPC numbers.
(A) Overexpression of pdgfb by mRNA injection enhanced runx1;cmyb expression in the VDA by WISH at 36hpf.
(B) Qualitative phenotypic distribution of embryos from panel 3A (n≥20/condition × 5 replicate clutches).
(C) FACS analysis confirmed that pdgfb overexpression increased Flk1:dsRed+cMyb:GFP+ HSPCs at 48hpf (1.54 fold increase, ***p≤0.0001, two-tailed t test, n≥5 replicates/condition).
(D) WISH analysis showed enhanced cmyb expression at 48hpf following ectopic activation of PDGFRβ signaling using hsp70:ca-pdgfrb induced at 27hpf (37°C for 1 hour)
(E) Qualitative phenotypic distribution of embryos from panel 3D (n≥20/condition × 5 replicate clutches).
(F) FACS analysis confirmed a significant increase in Flk1+cMyb+ HSPCs (48hpf) following PDGFRβ activation mediated by induced expression of ca-pdgfrb (1.47 fold increase, *p<0.05, two-tailed t test, n≥5 replicates/condition).
(G) Overexpression of pdgfb ligand rescued the reduction in runx1;cmyb expression found in hif1a morphants at 36hpf.
(H) Qualitative phenotypic distribution of embryos from panel 3G (n≥20/condition × 3 replicate clutches).
IL-6 is required downstream of PDGFRβ to stimulate AGM HSPC expansion
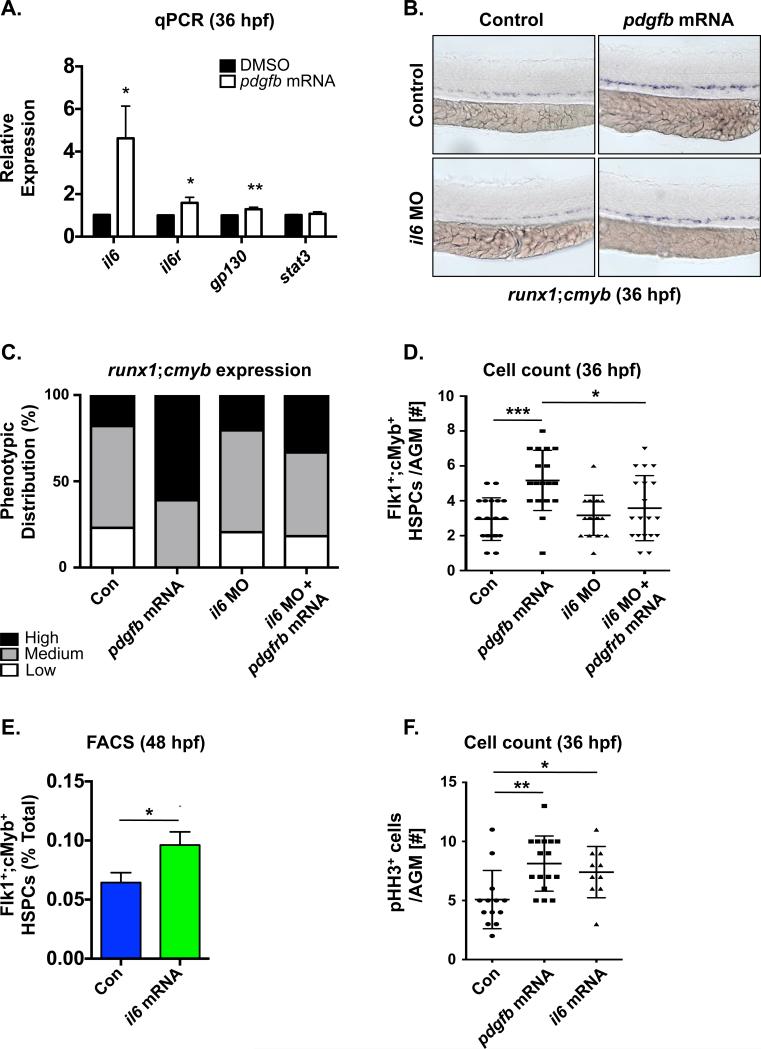
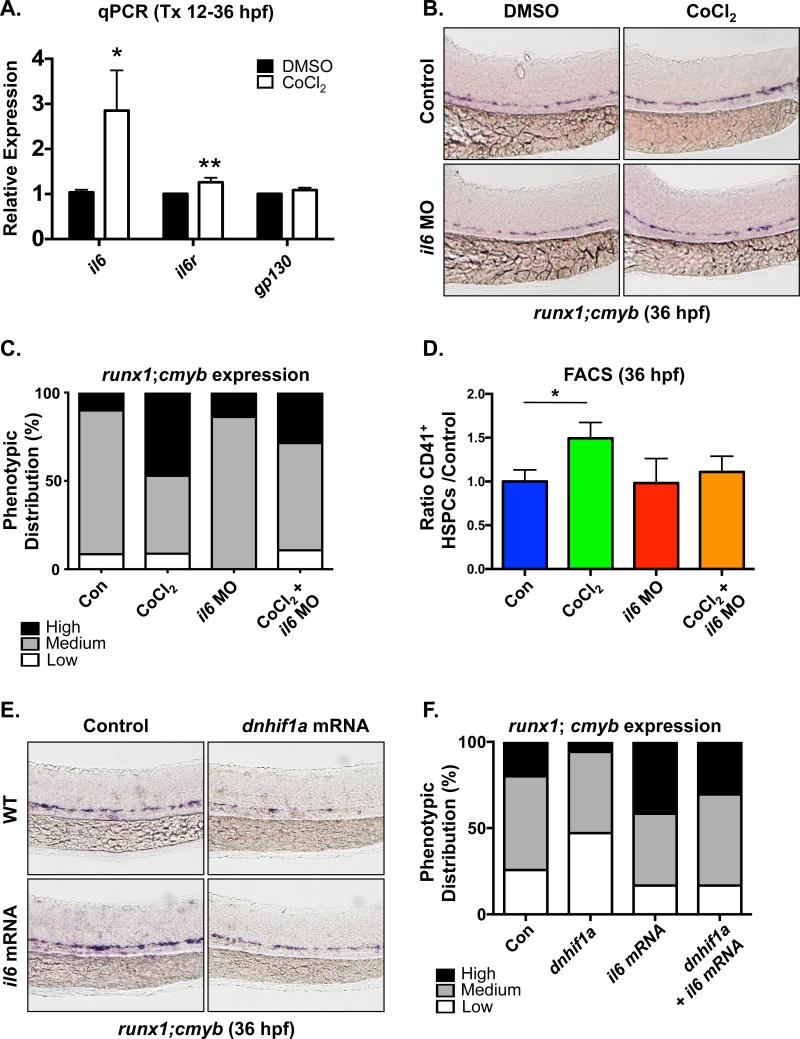
To understand the mechanism by which PDGF-B/PDGFRβ signaling regulates HSPC formation, we examined previously identified PDGF-B regulatory targets known to impact vascular and/or hematopoietic cell types [42]. Expression of inflammatory mediators il1b, il6, mmp2 and mmp9 were increased in 36hpf embryos injected with pdgfb mRNA, with il6 was the most strongly up-regulated (4.6-fold change; p<0.05) (Fig 4A and Fig S4A). In addition, pdgfb overexpression also significantly increased expression of the il6 receptor (il6r) and its co-receptor, gp130 (il6st) (Fig 4A), suggesting that IL-6 signaling could be a functionally relevant target of PDGF-B/PDGFRβ in regulation of embryonic HSPC production. RT-qPCR analysis of sorted cell fractions of wild-type embryos at 36hpf showed that il6r and gp130 are relatively enriched in Flk1+;cMyb− endothelium and Flk1+;cMyb+ HSCs (Fig S4B) at this stage of development. Modified epistasis analysis demonstrated that MO-mediated knockdown of il6 only modestly impacted runx1 expression in the VDA at 36hpf (Fig 4B,C), consistent with its low baseline level of expression (Fig S4B). However, pdgfb overexpression could no longer increase runx1;cmyb expression in the AGM in the absence of IL-6 function mediated either by MO knockdown (Fig 4B,C) or chemical inhibition of IL-6R/gp130 with the chemical antagonist SC-144 (Fig S4C,D). These findings were confirmed and quantified by Flk1+;cMyb+ cell counts in the VDA at 36hpf (Control: 2.9±1.2 cells/AGM, pdgfb mRNA: 5.1±1.7, il6 MO: 3.5±1.7, pdgfb mRNA+il6 MO: 3.6±1.9, n≥15/condition, *p<0.05, ***p<0.0005) (Fig 4D) and together indicate IL-6 activity is a functionally relevant mediator of PDGFRB stimulation on HSPC development. In support of that conclusion, overexpression of il6 mRNA increased runx1 expression in the AGM at 36hpf (Fig S4E,F) as well as the percentage of Flk1+;cMyb+ HSPCs at 48hpf as assessed by FACS (1.49-fold vs. control, p≤0.02) (Fig 4E). Furthermore, il6 mRNA significantly elevated the number of phospho-histone H3 (pH3)-positive cells in the VDA region, similar to that seen with pdgfb injection (Control: 5.1±2.5 cells/AGM, pdgfb mRNA: 7.9±2.5, p≤0.014; il6 mRNA: 7.4±2.2, p≤0.002, n≥10/condition) (Fig 4F). Altogether, these data indicate that IL-6 functions downstream of PDGF-B/PDGFRβ to increase HSPC production in the developing embryo via proliferative expansion.
Fig 4. IL-6 acts downstream of PDGFRβ signaling to stimulate AGM HSPC production.
(A) qPCR analysis showed il6 and its’ receptor and co-receptor, il6R and gp130, are upregulated in pdgfb mRNA-injected embryos (*p<0.05, **p<0.01, two-tailed t-test, n≥3).
(B) Morpholino knockdown of il6 blocked the ability of pdgfb mRNA to increase runx1;cmyb expression in the AGM.
(C) Qualitative phenotypic distribution of embryos from panel 4B (n≥20/condition × 3 replicate clutches).
(D) Absolute counts of Flk1:dsRed+cMyb:GFP+ HSPCs from embryos overexpressing pdgfb were significantly reduced with MO-mediated loss of il6 (***p<0.0005, *p<0.05, two-tailed t-test, n≥15/condition).
(E) FACS analysis for Flk1+cMyb+ HSPCs at 48hpf showed that overexpression of il6 significantly increases HSPCs (1.49-fold vs. control, *p<0.02, two-tailed t-test, n≥5 replicates/condition).
(F) Absolute cell counts of phospho histone H3 expressing (pHH3+) cells in the VDA were increased in both pdgfb and il6 mRNA-injected embryos compared to matched sibling controls (*p<0.05, **p<0.01, two-tailed t-test, n ≥10/condition).
IL-6 signaling acts downstream of Hif1α to increase AGM HSPC production
Our previous studies indicated that elevated glucose metabolism caused ROS-mediated Hif1α stabilization, leading to increased developmental HSPC specification and proliferative expansion [7]. To further strengthen the regulatory network connecting Hif1α and PDGFRβ signaling to HSC regulation via IL-6, we directly examined the role of IL-6 signaling downstream of Hif1α induction on embryonic HSPC formation. Hif1α stabilization with CoCl2 (12-36hpf) significantly up-regulated the expression of il6 (p<0.05) and its receptor il6r (p<0.01) by RT-qPCR (Fig 5A); furthermore, analysis of sorted HSPC fractions showed the predominant responses were found in the Flk1+;cMyb− endothelial and Flk1+;cMyb+ HSC populations (Fig S5A), similar to that seen for pdgfb and pdgfrb. Modified epistasis experiments indicated that blocking IL-6 signaling using either the il6 MO or SC-144 partially suppressed the effect of CoCl2 on runx1;cmyb expression in the AGM (Fig 5B,C and S5B,C). This finding was confirmed and quantified by CD41+ HSPC analysis using FACS: whereas CoCl2 normally induced HSPC numbers, this effect was absent in the context of il6 knockdown (Fig 5D), suggesting that IL-6 signaling acts to further mediate the effect of Hif1α on embryonic HSPC number. Finally, to determine whether IL-6 signaling is sufficient to increase HSPC induction in the absence of Hif1α activity, similarly to that observed for PDGF-B, embryos were co-injected with dominant-negative hif1a (dnhif1a) [35] and il6 mRNA. Inhibition of Hif1α function decreased runx1;cmyb expression in the AGM, consistent with prior analysis [7]; importantly, overexpression of il6 mRNA was able to restore runx1;cmyb expressing HSPCs to levels seen in controls (Fig 5E,F), indicating that IL-6 signaling functions downstream of Hif1α to influence HSPC production. Collectively, our data reveal a PDGF-associated signaling network connecting Hif1α-stabilization to IL-6 signaling that provides a method to regulate HSPC production through inflammatory cascades in response to environmental stimuli during development.
Fig 5. IL-6 signaling functions downstream of Hif1α in AGM HSPC regulation.
(A) RT-qPCR analysis demonstrated that expression of il6 and its receptor il6R are increased by CoCl2 exposure (*p<0.05, **p<0.01, one-tailed t-test, n≥3).
(B) Morpholino knockdown of il6 diminished the ability of CoCl2 to stimulate runx1;cmyb expression in the VDA over that seen in controls as determined by WISH analysis at 36hpf.
(C) Qualitative phenotypic distribution of embryos from panel 5B (n≥20/condition × 3 replicate clutches).
(D) FACS analysis confirmed that CoCl2 was unable to enhance the number of CD41+ HSPCs in the presence of il6 knockdown (*p<0.05, one-tailed t-test, n≥4).
(E) Injection of dnhif1a mRNA decreased runx1;cmyb expression in the VDA at 36hpf, which could be partially ameliorated by overexpression of il6.
(F) Qualitative phenotypic distribution of embryos from panel 5E (n≥20/condition × 3 replicate clutches.
DISCUSSION
The generation and production of HSCs from hemogenic endothelium is an intricate and multifaceted process involving multiple signaling mechanisms and molecular inputs that are spatially as well as temporally regulated [43, 44]. It is becoming increasingly clear that environmental and external factors can act upon and feed into the normal process of HSC generation, given that the developing embryo is exposed to fluctuating levels of oxygen, nutrients and energy supply [7, 45]. The importance of the hypoxic sensor Hif1α is now well documented in the adult BM niche, where local hypoxia and resultant Hif1α levels serve a key physiological mechanism to regulate HSC number and intracellular damage by maintaining low metabolic rate and HSC cell cycle quiescence [46]. Interestingly, we previously showed that Hif1α can also regulate embryonic HSC numbers and function, in this case by mediating the metabolic response to glucose metabolism to control induction of HSCs from the hemogenic endothelium and their subsequent proliferative expansion in the embryo [7]; a role for Hif1α as an inductive rather than quiescent factor was similarly demonstrated in mammalian embryos [10]. While Hif1α has been long established to act as a physiological sensor to regulate downstream targets such as Erythropoietin (Epo) and Vascular Endothelial Growth Factor (VEGF) to maintain erythroid homeostasis and vascular remodeling in response to hypoxia [47], the functional targets of Hif1α that could act to stimulate HSPC production in an expansive niche remain to be identified. In this paper, we show a novel mechanism of action mediated by Hif1α, which through regulation of PDGFRβ signaling, ensures proper production of HSCs through regulation of proproliferative inflammatory signaling during periods of metabolic stimulation throughout embryonic development.
Expression of PDGF-B and its receptor is induced by glucose exposure and the subsequent increase in metabolic activity, including ROS production, [7] as well as direct chemical and/or genetic-mediated Hif1α stabilization. PDGF-B has previously been shown to promote the ability of primitive hematopoietic precursor cells to form multilineage colonies in culture, as well as the ex vivo expansion of CD34+ human cord blood cells, although it has been unclear whether the expansion is a result of direct stimulation or secondary to autologous effects on stromal supporting cells [21, 48]. Our results show that PDGF-B signaling is necessary for the Hif1α-mediated induction of HSCs, and that overexpression of PDGF-B is sufficient to stimulate increased HSPC production during hematopoietic development in vivo. Recently, PDGF-B activity in trophoblasts was found to be a vital component of the placental niche where it functions to protect HSPCs from premature differentiation into red blood cells by suppressing the production of EPO [22]; this data together with our own presented here suggests that PDGFB may be playing a role in regulating the balance between HSPC maintenance and differentiation during development. In our studies, we further identify IL-6 signaling downstream of PDGFRβ activity in mediating the effect of metabolic induction of HSCs, as the effect of PDGF-B overexpression on HSPCs number can be attenuated by morpholino knockdown of IL-6. Inflammatory cytokines have been well characterized to activate the mobilization, proliferation, and differentiation of HSCs to ensure an adequate response to infection or injury during demand-driven hematopoiesis [49, 50], and have also recently been shown to play an important role in embryonic HSC development [51-54]. We have identified IL-6 as an additional inflammatory factor that can influence magnitude of developmental HSC production under the control of the Hif1α-PDGFRβ signaling axis. Interestingly, hypoxia has been shown previously to induce the expression of cytokines and proinflammatory mediators, including IL-6 [55, 56]. Interestingly, studies involving murine marrow cells cultured ex vivo had documented that IL-6 in combination with physiologically low oxygen concentration (1%) improved the maintenance of primitive HSC subpopulations [57] and more recent studies have reported that addition of IL-6 to hypoxia mimicking culture conditions can enhance in vivo long term reconstituting hematopoietic stem cell potential [58]. As there exist many regulatory inputs that converge to influence hematopoiesis, IL-6 may serve as a regulatory node where key environmental stimulus signal into to ensure right number of HSC production and balance of hematopoiesis. In our studies, pdgfb expression was found to be increased specifically in the endothelial cells in response to CoCl2 mediated Hif1α stabilization, while pdgfrb was found upregulated in both the endothelial as well as hemogenic endothelial/HSC population. IL-6 and IL-6R were similarly upregulated in both the endothelium and HSPCs in response to CoCl2. While significant further investigation involving cell-type specific studies is warranted, we propose a possible mechanism of action whereby PDGF-B, acting through both an autocrine and paracrine mechanism, can influence IL-6/IL-6R signaling to thereby regulate the overall production of HSPCs.
While mechanistic investigations into how growth factor signaling involving PDGF-B can lead to the activation of IL-6 were beyond the scope of this study, there are many other reports that provide insight into the myriad of possible routes of regulation. In osteoblastic cells, Protein Kinase C (PKC) activation of members of the activator protein-1 (AP-1) complex was determined as having a direct role in the PDGF-B induction of IL-6 [23]. In contrast in glioma-initiating cells, a PDGF-driven signaling axis involving NO-dependent inhibitor of differentiation 4 (ID4) has been shown to promote JAGGED1–NOTCH activity [59]. Related, both direct and non-canonical regulation of IL-6 expression by Notch has been previously reported [60, 61]. As it is well established that generation of HSCs requires multiple Notch signaling inputs [62], it will be interesting to investigate whether Notch signaling may be a part of the Hif1α-PDGFRβ signaling axis in regard to the ability of IL-6 to enhance production of HSPCs. Furthermore, while we report increased proliferation as assessed by pHH3+ cell counts following pdgfb and il6 overexpression, whether we are affecting hemogenic endothelial competence for HSC generation or the proliferation of specified HSCs is complicated by the fact that both functions are contemporaneous. Future studies involving live cell imaging using transgenic reporters labeling endothelial and hematopoietic populations may help clarify the exact process in HSPC development that is altered by the Hif1α-PDGFRβ-IL-6 signaling axis.
In summary, we have shown that during development, PDGFRβ can function downstream of Hif1α signaling to regulate the scale of definitive HSPC formation via IL-6 mediated inflammatory regulation. We anticipate that further understanding of exactly how environmental and physiological inputs can induce cell signaling events, in this case metabolic induction of PDGFRβ signaling, to affect the normal course of HSPC production in vivo will provide new insights into mechanisms to improve in vitro generation or expansion of HSCs for research and therapeutic purposes.
Supplementary Material
ACKNOWLEDGEMENTS
We thank the BIDMC Flow Cytometry Core Facility, the Children's Hospital Genomics Core Facility, and HSCI Center for Stem Cell Bioinformatics for technical support. We thank N. Lawson (University of Massachusetts Medical School) for the pdgfrb mutant line. This investigation was supported by 1R01DK098241-01A1 (TEN).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
AUTHOR CONTRIBUTIONS
SL, VE, WK, IMF, LNT and SYL performed embryo exposures, MO injections, and in situ hybridizations. SL, MC and WK conducted FACS analysis and sorting. SL, VE and WK ran qPCR analysis and performed fluorescence microscopy. SL, VE and TEN designed experiments, evaluated results and wrote the manuscript. All authors reviewed and edited the manuscript. The authors have no conflicts of interest to declare.
REFERENCES
- 1.Bertrand JY, Chi NC, Santoso B, Teng S, Stainier DY, Traver D. Haematopoietic stem cells derive directly from aortic endothelium during development. Nature. 2010;464(7285):108–11. doi: 10.1038/nature08738. Epub 2010/02/16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chen AT, Zon LI. Zebrafish blood stem cells. Journal of cellular biochemistry. 2009;108(1):35–42. doi: 10.1002/jcb.22251. Epub 2009/07/01. [DOI] [PubMed] [Google Scholar]
- 3.Dzierzak E, Speck NA. Of lineage and legacy: the development of mammalian hematopoietic stem cells. Nature immunology. 2008;9(2):129–36. doi: 10.1038/ni1560. Epub 2008/01/22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kissa K, Herbomel P. Blood stem cells emerge from aortic endothelium by a novel type of cell transition. Nature. 2010;464(7285):112–5. doi: 10.1038/nature08761. Epub 2010/02/16. [DOI] [PubMed] [Google Scholar]
- 5.North TE, de Bruijn MF, Stacy T, Talebian L, Lind E, Robin C, et al. Runx1 expression marks long-term repopulating hematopoietic stem cells in the midgestation mouse embryo. Immunity. 2002;16(5):661–72. doi: 10.1016/s1074-7613(02)00296-0. Epub 2002/06/07. [DOI] [PubMed] [Google Scholar]
- 6.Chen MJ, Yokomizo T, Zeigler BM, Dzierzak E, Speck NA. Runx1 is required for the endothelial to haematopoietic cell transition but not thereafter. Nature. 2009;457(7231):887–91. doi: 10.1038/nature07619. Epub 2009/01/09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Harris JM, Esain V, Frechette GM, Harris LJ, Cox AG, Cortes M, et al. Glucose metabolism impacts the spatiotemporal onset and magnitude of HSC induction in vivo. Blood. 2013;121(13):2483–93. doi: 10.1182/blood-2012-12-471201. Epub 2013/01/24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Simon MC, Keith B. The role of oxygen availability in embryonic development and stem cell function. Nature reviews Molecular cell biology. 2008;9(4):285–96. doi: 10.1038/nrm2354. Epub 2008/02/21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kotch LE, Iyer NV, Laughner E, Semenza GL. Defective vascularization of HIF-1alpha-null embryos is not associated with VEGF deficiency but with mesenchymal cell death. Developmental biology. 1999;209(2):254–67. doi: 10.1006/dbio.1999.9253. Epub 1999/05/18. [DOI] [PubMed] [Google Scholar]
- 10.Imanirad P, Solaimani Kartalaei P, Crisan M, Vink C, Yamada-Inagawa T, de Pater E, et al. HIF1alpha is a regulator of hematopoietic progenitor and stem cell development in hypoxic sites of the mouse embryo. Stem cell research. 2014;12(1):24–35. doi: 10.1016/j.scr.2013.09.006. Epub 2013/10/22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rolny C, Nilsson I, Magnusson P, Armulik A, Jakobsson L, Wentzel P, et al. Platelet-derived growth factor receptor-beta promotes early endothelial cell differentiation. Blood. 2006;108(6):1877–86. doi: 10.1182/blood-2006-04-014894. Epub 2006/05/13. [DOI] [PubMed] [Google Scholar]
- 12.Kelly BD, Hackett SF, Hirota K, Oshima Y, Cai Z, Berg-Dixon S, et al. Cell type-specific regulation of angiogenic growth factor gene expression and induction of angiogenesis in nonischemic tissue by a constitutively active form of hypoxia-inducible factor 1. Circulation research. 2003;93(11):1074–81. doi: 10.1161/01.RES.0000102937.50486.1B. Epub 2003/10/25. [DOI] [PubMed] [Google Scholar]
- 13.Zhang SX, Gozal D, Sachleben LR, Jr., Rane M, Klein JB, Gozal E. Hypoxia induces an autocrine-paracrine survival pathway via platelet-derived growth factor (PDGF)-B/PDGF-beta receptor/phosphatidylinositol 3-kinase/Akt signaling in RN46A neuronal cells. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2003;17(12):1709–11. doi: 10.1096/fj.02-1111fje. Epub 2003/09/06. [DOI] [PubMed] [Google Scholar]
- 14.Schito L, Rey S, Tafani M, Zhang H, Wong CC, Russo A, et al. Hypoxia-inducible factor 1-dependent expression of platelet-derived growth factor B promotes lymphatic metastasis of hypoxic breast cancer cells. Proceedings of the National Academy of Sciences of the United States of America. 2012;109(40):E2707–16. doi: 10.1073/pnas.1214019109. Epub 2012/09/27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Soriano P. Abnormal kidney development and hematological disorders in PDGF beta-receptor mutant mice. Genes & development. 1994;8(16):1888–96. doi: 10.1101/gad.8.16.1888. Epub 1994/08/15. [DOI] [PubMed] [Google Scholar]
- 16.Leveen P, Pekny M, Gebre-Medhin S, Swolin B, Larsson E, Betsholtz C. Mice deficient for PDGF B show renal, cardiovascular, and hematological abnormalities. Genes & development. 1994;8(16):1875–87. doi: 10.1101/gad.8.16.1875. Epub 1994/08/15. [DOI] [PubMed] [Google Scholar]
- 17.Keutzer JC, Sytkowski AJ. Regulated production of a pleiotropic cytokine-platelet-derived growth factor--by differentiating erythroid cells in vitro and in vivo. Proceedings of the National Academy of Sciences of the United States of America. 1995;92(11):4967–71. doi: 10.1073/pnas.92.11.4967. Epub 1995/05/23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dainiak N, Davies G, Kalmanti M, Lawler J, Kulkarni V. Platelet-derived growth factor promotes proliferation of erythropoietic progenitor cells in vitro. The Journal of clinical investigation. 1983;71(5):1206–14. doi: 10.1172/JCI110869. Epub 1983/05/01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kaminski WE, Lindahl P, Lin NL, Broudy VC, Crosby JR, Hellstrom M, et al. Basis of hematopoietic defects in platelet-derived growth factor (PDGF)-B and PDGF beta-receptor null mice. Blood. 2001;97(7):1990–8. doi: 10.1182/blood.v97.7.1990. Epub 2001/03/27. [DOI] [PubMed] [Google Scholar]
- 20.Yang M, Chesterman CN, Chong BH. Recombinant PDGF enhances megakaryocytopoiesis in vitro. British journal of haematology. 1995;91(2):285–9. doi: 10.1111/j.1365-2141.1995.tb05291.x. Epub 1995/10/01. [DOI] [PubMed] [Google Scholar]
- 21.Yan XQ, Brady G, Iscove NN. Platelet-derived growth factor (PDGF) activates primitive hematopoietic precursors (pre-CFCmulti) by up-regulating IL-1 in PDGF receptor-expressing macrophages. J Immunol. 1993;150(6):2440–8. Epub 1993/03/15. [PubMed] [Google Scholar]
- 22.Chhabra A, Lechner AJ, Ueno M, Acharya A, Van Handel B, Wang Y, et al. Trophoblasts regulate the placental hematopoietic niche through PDGF-B signaling. Developmental cell. 2012;22(3):651–9. doi: 10.1016/j.devcel.2011.12.022. Epub 2012/03/06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Franchimont N, Durant D, Rydziel S, Canalis E. Platelet-derived growth factor induces interleukin-6 transcription in osteoblasts through the activator protein-1 complex and activating transcription factor-2. The Journal of biological chemistry. 1999;274(10):6783–9. doi: 10.1074/jbc.274.10.6783. Epub 1999/02/26. [DOI] [PubMed] [Google Scholar]
- 24.Moshage H. Cytokines and the hepatic acute phase response. The Journal of pathology. 1997;181(3):257–66. doi: 10.1002/(SICI)1096-9896(199703)181:3<257::AID-PATH756>3.0.CO;2-U. Epub 1997/03/01. [DOI] [PubMed] [Google Scholar]
- 25.Ikebuchi K, Wong GG, Clark SC, Ihle JN, Hirai Y, Ogawa M. Interleukin 6 enhancement of interleukin 3-dependent proliferation of multipotential hemopoietic progenitors. Proceedings of the National Academy of Sciences of the United States of America. 1987;84(24):9035–9. doi: 10.1073/pnas.84.24.9035. Epub 1987/12/01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bernad A, Kopf M, Kulbacki R, Weich N, Koehler G, Gutierrez-Ramos JC. Interleukin-6 is required in vivo for the regulation of stem cells and committed progenitors of the hematopoietic system. Immunity. 1994;1(9):725–31. doi: 10.1016/s1074-7613(94)80014-6. Epub 1994/12/01. [DOI] [PubMed] [Google Scholar]
- 27.Zandstra PW, Conneally E, Petzer AL, Piret JM, Eaves CJ. Cytokine manipulation of primitive human hematopoietic cell self-renewal. Proceedings of the National Academy of Sciences of the United States of America. 1997;94(9):4698–703. doi: 10.1073/pnas.94.9.4698. Epub 1997/04/29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Henschler R, Brugger W, Luft T, Frey T, Mertelsmann R, Kanz L. Maintenance of transplantation potential in ex vivo expanded CD34(+)-selected human peripheral blood progenitor cells. Blood. 1994;84(9):2898–903. Epub 1994/11/01. [PubMed] [Google Scholar]
- 29.Lin HF, Traver D, Zhu H, Dooley K, Paw BH, Zon LI, et al. Analysis of thrombocyte development in CD41-GFP transgenic zebrafish. Blood. 2005;106(12):3803–10. doi: 10.1182/blood-2005-01-0179. Epub 2005/08/16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.North TE, Babu IR, Vedder LM, Lord AM, Wishnok JS, Tannenbaum SR, et al. PGE2-regulated wnt signaling and N-acetylcysteine are synergistically hepatoprotective in zebrafish acetaminophen injury. Proceedings of the National Academy of Sciences of the United States of America. 2010;107(40):17315–20. doi: 10.1073/pnas.1008209107. Epub 2010/09/22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Parsons MJ, Pisharath H, Yusuff S, Moore JC, Siekmann AF, Lawson N, et al. Notch-responsive cells initiate the secondary transition in larval zebrafish pancreas. Mechanisms of development. 2009;126(10):898–912. doi: 10.1016/j.mod.2009.07.002. Epub 2009/07/15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kok FO, Shin M, Ni CW, Gupta A, Grosse AS, van Impel A, et al. Reverse genetic screening reveals poor correlation between morpholino-induced and mutant phenotypes in zebrafish. Developmental cell. 2015;32(1):97–108. doi: 10.1016/j.devcel.2014.11.018. Epub 2014/12/24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kwan W, Cortes M, Frost I, Esain V, Theodore LN, Liu SY, et al. The Central Nervous System Regulates Embryonic HSPC Production via Stress-Responsive Glucocorticoid Receptor Signaling. Cell stem cell. 2016 doi: 10.1016/j.stem.2016.06.004. Epub 2016/07/19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.North TE, Goessling W, Walkley CR, Lengerke C, Kopani KR, Lord AM, et al. Prostaglandin E2 regulates vertebrate haematopoietic stem cell homeostasis. Nature. 2007;447(7147):1007–11. doi: 10.1038/nature05883. Epub 2007/06/22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Elks PM, van Eeden FJ, Dixon G, Wang X, Reyes-Aldasoro CC, Ingham PW, et al. Activation of hypoxia-inducible factor-1alpha (Hif-1alpha) delays inflammation resolution by reducing neutrophil apoptosis and reverse migration in a zebrafish inflammation model. Blood. 2011;118(3):712–22. doi: 10.1182/blood-2010-12-324186. Epub 2011/05/11. [DOI] [PubMed] [Google Scholar]
- 36.Magnusson PU, Looman C, Ahgren A, Wu Y, Claesson-Welsh L, Heuchel RL. Platelet-derived growth factor receptor-beta constitutive activity promotes angiogenesis in vivo and in vitro. Arteriosclerosis, thrombosis, and vascular biology. 2007;27(10):2142–9. doi: 10.1161/01.ATV.0000282198.60701.94. Epub 2007/07/28. [DOI] [PubMed] [Google Scholar]
- 37.Kawakami K, Takeda H, Kawakami N, Kobayashi M, Matsuda N, Mishina M. A transposon-mediated gene trap approach identifies developmentally regulated genes in zebrafish. Developmental cell. 2004;7(1):133–44. doi: 10.1016/j.devcel.2004.06.005. Epub 2004/07/09. [DOI] [PubMed] [Google Scholar]
- 38.Zhao S, Fernald RD. Comprehensive algorithm for quantitative real-time polymerase chain reaction. Journal of computational biology : a journal of computational molecular cell biology. 2005;12(8):1047–64. doi: 10.1089/cmb.2005.12.1047. Epub 2005/10/26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang Y, Pan L, Moens CB, Appel B. Notch3 establishes brain vascular integrity by regulating pericyte number. Development. 2014;141(2):307–17. doi: 10.1242/dev.096107. Epub 2013/12/07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Betsholtz C, Karlsson L, Lindahl P. Developmental roles of platelet-derived growth factors. BioEssays : news and reviews in molecular, cellular and developmental biology. 2001;23(6):494–507. doi: 10.1002/bies.1069. Epub 2001/06/01. [DOI] [PubMed] [Google Scholar]
- 41.Wiens KM, Lee HL, Shimada H, Metcalf AE, Chao MY, Lien CL. Platelet-derived growth factor receptor beta is critical for zebrafish intersegmental vessel formation. PloS one. 2010;5(6):e11324. doi: 10.1371/journal.pone.0011324. Epub 2010/07/02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wu E, Palmer N, Tian Z, Moseman AP, Galdzicki M, Wang X, et al. Comprehensive dissection of PDGF-PDGFR signaling pathways in PDGFR genetically defined cells. PloS one. 2008;3(11):e3794. doi: 10.1371/journal.pone.0003794. Epub 2008/11/26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Clements WK, Traver D. Signalling pathways that control vertebrate haematopoietic stem cell specification. Nature reviews Immunology. 2013;13(5):336–48. doi: 10.1038/nri3443. Epub 2013/04/27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kim AD, Stachura DL, Traver D. Cell signaling pathways involved in hematopoietic stem cell specification. Experimental cell research. 2014;329(2):227–33. doi: 10.1016/j.yexcr.2014.10.011. Epub 2014/12/03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.North TE, Goessling W, Peeters M, Li P, Ceol C, Lord AM, et al. Hematopoietic stem cell development is dependent on blood flow. Cell. 2009;137(4):736–48. doi: 10.1016/j.cell.2009.04.023. Epub 2009/05/20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Takubo K, Goda N, Yamada W, Iriuchishima H, Ikeda E, Kubota Y, et al. Regulation of the HIF-1alpha level is essential for hematopoietic stem cells. Cell stem cell. 2010;7(3):391–402. doi: 10.1016/j.stem.2010.06.020. Epub 2010/09/02. [DOI] [PubMed] [Google Scholar]
- 47.Semenza GL. Hypoxia-inducible factors in physiology and medicine. Cell. 2012;148(3):399–408. doi: 10.1016/j.cell.2012.01.021. Epub 2012/02/07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Su RJ, Zhang XB, Li K, Yang M, Li CK, Fok TF, et al. Platelet-derived growth factor promotes ex vivo expansion of CD34+ cells from human cord blood and enhances long-term culture-initiating cells, non-obese diabetic/severe combined immunodeficient repopulating cells and formation of adherent cells. British journal of haematology. 2002;117(3):735–46. doi: 10.1046/j.1365-2141.2002.03500.x. Epub 2002/05/25. [DOI] [PubMed] [Google Scholar]
- 49.Schuettpelz LG, Link DC. Regulation of hematopoietic stem cell activity by inflammation. Frontiers in immunology. 2013;4:204. doi: 10.3389/fimmu.2013.00204. Epub 2013/07/25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Takizawa H, Boettcher S, Manz MG. Demand-adapted regulation of early hematopoiesis in infection and inflammation. Blood. 2012;119(13):2991–3002. doi: 10.1182/blood-2011-12-380113. Epub 2012/01/17. [DOI] [PubMed] [Google Scholar]
- 51.Li Y, Esain V, Teng L, Xu J, Kwan W, Frost IM, et al. Inflammatory signaling regulates embryonic hematopoietic stem and progenitor cell production. Genes & development. 2014;28(23):2597–612. doi: 10.1101/gad.253302.114. Epub 2014/11/15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sawamiphak S, Kontarakis Z, Stainier DY. Interferon gamma signaling positively regulates hematopoietic stem cell emergence. Developmental cell. 2014;31(5):640–53. doi: 10.1016/j.devcel.2014.11.007. Epub 2014/12/10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Espin-Palazon R, Stachura DL, Campbell CA, Garcia-Moreno D, Del Cid N, Kim AD, et al. Proinflammatory signaling regulates hematopoietic stem cell emergence. Cell. 2014;159(5):1070–85. doi: 10.1016/j.cell.2014.10.031. Epub 2014/11/25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.He Q, Zhang C, Wang L, Zhang P, Ma D, Lv J, et al. Inflammatory signaling regulates hematopoietic stem and progenitor cell emergence in vertebrates. Blood. 2015;125(7):1098–106. doi: 10.1182/blood-2014-09-601542. Epub 2014/12/30. [DOI] [PubMed] [Google Scholar]
- 55.Tamm M, Bihl M, Eickelberg O, Stulz P, Perruchoud AP, Roth M. Hypoxia-induced interleukin-6 and interleukin-8 production is mediated by platelet-activating factor and platelet-derived growth factor in primary human lung cells. American journal of respiratory cell and molecular biology. 1998;19(4):653–61. doi: 10.1165/ajrcmb.19.4.3058. Epub 1998/10/08. [DOI] [PubMed] [Google Scholar]
- 56.Yan SF, Tritto I, Pinsky D, Liao H, Huang J, Fuller G, et al. Induction of interleukin 6 (IL-6) by hypoxia in vascular cells. Central role of the binding site for nuclear factor-IL-6. The Journal of biological chemistry. 1995;270(19):11463–71. doi: 10.1074/jbc.270.19.11463. Epub 1995/05/12. [DOI] [PubMed] [Google Scholar]
- 57.Kovacevic-Filipovic M, Petakov M, Hermitte F, Debeissat C, Krstic A, Jovcic G, et al. Interleukin-6 (IL-6) and low O(2) concentration (1%) synergize to improve the maintenance of hematopoietic stem cells (pre-CFC). Journal of cellular physiology. 2007;212(1):68–75. doi: 10.1002/jcp.21003. Epub 2007/02/22. [DOI] [PubMed] [Google Scholar]
- 58.Duchez P, Rodriguez L, Chevaleyre J, Lapostolle V, Vlaski M, Brunet de la Grange P, et al. Interleukin-6 enhances the activity of in vivo long-term reconstituting hematopoietic stem cells in “hypoxic-like” expansion cultures ex vivo. Transfusion. 2015;55(11):2684–91. doi: 10.1111/trf.13175. Epub 2015/05/28. [DOI] [PubMed] [Google Scholar]
- 59.Jeon HM, Kim SH, Jin X, Park JB, Joshi K, Nakano I, et al. Crosstalk between glioma-initiating cells and endothelial cells drives tumor progression. Cancer research. 2014;74(16):4482–92. doi: 10.1158/0008-5472.CAN-13-1597. Epub 2014/06/26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wongchana W, Palaga T. Direct regulation of interleukin-6 expression by Notch signaling in macrophages. Cellular & molecular immunology. 2012;9(2):155–62. doi: 10.1038/cmi.2011.36. Epub 2011/10/11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Jin S, Mutvei AP, Chivukula IV, Andersson ER, Ramskold D, Sandberg R, et al. Non-canonical Notch signaling activates IL-6/JAK/STAT signaling in breast tumor cells and is controlled by p53 and IKKalpha/IKKbeta. Oncogene. 2013;32(41):4892–902. doi: 10.1038/onc.2012.517. Epub 2012/11/28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Butko E, Pouget C, Traver D. Complex regulation of HSC emergence by the Notch signaling pathway. Developmental biology. 2016;409(1):129–38. doi: 10.1016/j.ydbio.2015.11.008. Epub 2015/11/21. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.