Combining the triple stimulation technique with the paired-pulse stimulation paradigm improves the consistency of short intracortical inhibition and facilitation and could be useful in research, but the interindividual variability precludes their utility for clinical practice. Our findings do not suggest that desynchronization of descending discharges following transcranial magnetic stimulation contributes to short intracortical inhibition or intracortical facilitation.
Keywords: transcranial magnetic stimulation, triple stimulation technique, corticospinal excitability, motor-evoked potential, spinal motor neurons
Abstract
The paired-pulse (PP) transcranial magnetic stimulation (TMS) paradigms allow the exploration of the motor cortex physiology. The triple stimulation technique (TST) improves conventional TMS by reducing effects of desynchronization of motor neuron discharges allowing a precise evaluation of the corticospinal conduction. The objective of our study was to explore PP TMS paradigms combined with the TST to study whether the desynchronization contributes to these phenomena and whether the combined TMS-TST protocol could improve the consistency of responses. We investigated the PP paradigms of short intracortical inhibition (SICI) with 2 ms interstimulus interval (ISI) and of intracortical facilitation (ICF) with 10 ms ISI in 22 healthy subjects applying either conventional TMS alone or combined with the TST protocol. The results of the PP paradigms combined with the TST of SICI and ICF do not differ from those with conventional TMS. However, combining the PP paradigm with the TST reduces their variability. These results speak against a contribution of the desynchronization of motor neuron discharges to the PP paradigms of SICI and ICF. Combining the PP TMS paradigm with the TST may improve their consistency, but the interindividual variability remains such that it precludes their utility for clinical practice.
NEW & NOTEWORTHY Combining the triple stimulation technique with the paired-pulse stimulation paradigm improves the consistency of short intracortical inhibition and facilitation and could be useful in research, but the interindividual variability precludes their utility for clinical practice. Our findings do not suggest that desynchronization of descending discharges following transcranial magnetic stimulation contributes to short intracortical inhibition or intracortical facilitation.
the paired-pulse (PP) paradigm of transcranial magnetic stimulation (TMS) is an established method to explore the physiology of the primary motor cortex and has contributed to our understanding of the presumed inhibitory and excitatory circuits in the healthy brain and their alterations in various disorders. These PP paradigms remain yet primarily applied in research since the inter- and intraindividual variability has limited their implementation in clinical practice (Chen et al. 2008). The variability concerns also motor-evoked potentials (MEPs) following single pulse TMS. Various factors contribute to this variability such as the number of recruited α-motor neurons that may vary depending on the cortical excitability (Wassermann 2002). A major source of variability is the desynchronization of the descending discharges (Rösler et al. 2002, 2008), which leads to a phase cancellation phenomenon whereby the positive and negative phases of the desynchronized action potentials cancel each other out and determine the MEPs. This desynchronization can be corrected for with the triple stimulation technique (TST) that, thereby, improves the accuracy in quantifying corticospinal conduction (Magistris et al. 1998). We may speculate that desynchronization contributes also to the conditioning effect in PP paradigms of short intracortical inhibition (SICI) and facilitation (ICF), since we detected MEPs at a conventionally considered subthreshold intensity (80% resting motor threshold) of the conditioning stimulus when applying the TST (unpublished data). This demonstrates spinal motor neuron discharges and raises the possibility that the conditioning stimulus may contribute to desynchronization (Rösler et al. 2002, 2008). The objectives of our investigation to combine the PP paradigms of SICI and ICF with the TST are to explore whether desynchronization contributes to SICI and ICF and whether the TST could reduce their variability. This combined PP-TST protocol could improve the accuracy in detecting changes in intracortical excitability and may potentially become useful in clinical practice for the diagnostic workup of various disorders. Although the development of the combined protocol required a certain technical expertise, an extended version of the commercially available TST protocol could ease its clinical implementation.
METHODS
Twenty-two healthy subjects (men, n = 15), aged 21–40 yr (mean age 27.6 ± 5.2 SD), gave their written informed consent to participate in the study. All were screened for TMS contraindications and declared no comorbidities or regular medication intake. All but one was right handed according to the Edinburgh Handedness Inventory (Oldfield 1971). The study conformed to the principles of the Declaration of Helsinki and was approved by the local ethics committee (protocol 311/11).
We explored the PP-TMS paradigms of ICF and SICI in both the conventional way with TMS alone and combined with the TST. For each condition [single pulse, inhibitory PP with 2 ms interstimulus interval (ISI), facilitatory PP with 10 ms ISI] 12 stimuli were given in a randomized order with each method (TMS and the TST). The number of 12 stimuli was chosen in accordance with the recommendations of the International Federation of Clinical Neurophysiology (IFCN) (Rossini et al. 2015) of 8–10 trials and with a small study that found no difference in intersession variability for ICF and SICI with 10, 15, or 20 trials (Boroojerdi et al. 2000). We explored the motor cortex of the left hemisphere in right-handed participants and the right in a single left-handed participant.
EMG recordings.
We obtained EMG recordings from the abductor digiti minimi (ADM muscle) using surface electrodes in a belly-tendon montage. Signals were amplified, band-pass filtered (1–5 kHz), and then sampled at a rate of 25 kHz and stored for off-line analysis. We applied a Fourier transformation analysis of the digitalized signal to identify potential 50-Hz artifacts. A custom made acquisition and preprocessing software allowed then their complete removal without altering the signal components (custom made acquisition and preprocessing software coded on LabVIEW 12.0f3 by Sci-Consulting; postprocessing software coded on LabVIEW 8.6 by N. Dang, National Institutes of Health, Bethesda, MD).
Fingers II to V of the participants were taped together, and their hand was placed over a cushion.
Transcranial magnetic stimulation.
TMS stimuli were applied with a figure-of-eight handheld coil (70 mm) over the hand motor cortex, using a Magstim bistim2 stimulator (Magstim, Spring Gardens, Whitland, UK). The localization of the optimal cortical stimulation spot (motor hot spot) for the ADM was determined in accordance with the guidelines of IFCN (Groppa et al. 2012) as follows: stimuli of the presumed motor hot spot were followed by stimuli of the surrounding scalp until the area with the largest MEP was detected and marked on the cap. Resting motor threshold (RMT) was determined using the maximum likelihood threshold hunting procedure described by Awiszus (2003) (Motor Threshold Assessment Tool, version 2.0: http://www.clinicalresearcher.org/software.htm). Motor evoked potentials of peak-to-peak amplitude of >50 μV were considered valid responses and fed back accordingly to the software.
Triple stimulation technique.
The TST has been developed by Magistris et al. (1998). TST links, through the collision technique, the peripheral with the central conduction (Magistris et al. 1999). This allows to correct the desynchronization of the motor neuron discharges and to quantify precisely the corticospinal conduction. The TST consists of a succession of three stimuli: a first TMS pulse over the cortical motor cortex, followed by a supramaximal stimulation of the ulnar nerve over the wrist, and finally by a supramaximal stimulation of the brachial plexus at the Erb’s point. In healthy subjects the first TMS pulse is believed to activate all the cortical and spinal motor neurons, if supramaximal stimulation intensity is used. Desynchronized descending discharges elicited by TMS and the ascending action potentials elicited by the ulnar nerve stimulation cancel each other out through a collision. Next, the third brachial plexus stimulation will elicit synchronized discharges and be able to evoke a maximal compound muscle action potential. In case of pathological conditions affecting the cortical motor neurons and/or the corticospinal tract, or in case of submaximal stimulation intensity, there is only a reduced number of descending discharges that collide with the corresponding number of ascending action potentials from the ulnar nerve stimulation. The remaining ascending action potentials will collide with the descending discharges from the brachial plexus stimulation, which results in a smaller muscle response that equals the reduced number of corticospinal fibers originally brought to discharge with TMS. Therefore, the TST generates a “blueprint” of corticospinal conduction by “replacing” the desynchronized TMS discharges by synchronous action potentials from the brachial plexus stimulation. Accordingly, the size of the TST response depends on the amount of spinal motor neurons driven to discharge by the TMS (Magistris et al. 1998). The delays between the three stimulations were the following: delay I = “minimal MEP latency” − “M wavewrist latency.” Delay II = “M waveErb latency” − M wavewrist latency (Magistris et al. 1998). The trigger for the three stimuli was given using a commercially available TST software package for the Nicolet Viking apparatus (Nicolet Biomedical, Madison, WI). Stimuli to the ulnar nerve were given using a bipolar stimulation electrode taped over the ulnar nerve. Our electrode consisted of an anode and a cathode (both of diameter 0.8 cm) separated by a distance of 2 cm. The cathode was placed 8 cm proximally to the active electrode over the ADM muscle belly and the anode proximal to the cathode. The stimuli at the brachial plexus were given with a handheld monopolar cathode (0.8 cm diameter), and an anode electrode of 80 cm2 of surface taped over the scapula. To determine the intensity of the supramaximal stimulation, the intensities were gradually increased up to a point where further increases were not reflected in an increase in size of the registered MEPs, for both ulnar and brachial plexus stimulation.
Paired-pulse paradigms.
PP paradigms were originally described by Kujirai et al. (1993) as inhibiting or facilitating MEPs depending on the ISI. They consist of a pair of TMS stimuli, a conditioning stimulus (CS) preceding a test stimulus (TS) given over the motor cortex hot spot of the target muscle. For short ISI (1–5 ms) the evoked MEPs are smaller compared with the MEPs of a TS (single TMS stimulus, this paradigm is called SICI), whereas for longer ISI (10–15 ms) the MEPs are bigger (this paradigm is called ICF) (Fig. 1). In our study the CS was given at 80% of the RMT, the TS at 120% of the RMT, and the ISI was either 2 ms for SICI or 10 ms for ICF. We continuously monitored muscle activity (Stinear and Byblow 2004) while reminding participants to relax when necessary, and finally had to exclude just a few trials due to ongoing EMG activity.
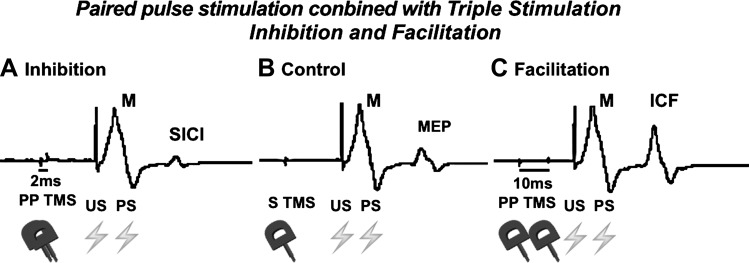
Fig. 1.
Three single trials of the same subject. The sequences of stimulation are indicated by symbols of transcranial magnetic stimulation (TMS) (coils) and electric stimulation (thunders). The first negative deflection arises from the ulnar nerve stimulation. The second negative deflection arises from the Erb’s stimulation and is the response of interest. In case of 2 ms interstimulus interval (ISI) these responses are inhibited (A) while with 10 ms ISI they are facilitated (C) compared with the response of a single TMS stimulus (B). PP, paired pulse; US, ulnar nerve stimulation; PS, brachial plexus stimulation; SICI, short intracortical inhibition; ICF, intracortical facilitation; MEP, motor-evoked potential; M, maximal muscle response.
To compare the variability of the different measures, the coefficient of variation (CV) was computed as the SD divided by mean, as previously done with TMS by Kiers et al. (1993).
Statistical analysis.
For each of the two methods (TST, TMS), the parameter of interest, peak-to-peak amplitude or its CV, was first explained by the conditions (3 modalities: single, SICI, and ICF) using univariate linear regression models taking into account the repeated-measure design. The effect of the method on the dependent variables was also compared for each of the three conditions using simple linear regression.
Next, the dependent variable was explained using multiple linear regression models with condition and method (TST vs. TMS) as the independent variables along with their interaction while taking into account the repeated-measure design. All statistics were performed with Stata version 14.1.
P values <0.05 were considered statistically significant.
RESULTS
We determined the mean resting motor threshold at 46.8 ± 8.9 SD of the maximum stimulator output (MSO). Magnetic stimuli were given with a mean intensity of 37.7 ± 7.0 SD of MSO for the CS and 56.3 ± 11.0 SD of MSO for the TS. The mean electric stimulation intensities were at the ulnar nerve of 121.8 ± 39.6 (SD) V and at the brachial plexus of 160.2 ± 73.6 (SD) V. The mean first delays between the cortical and the ulnar stimulation were 16.8 ± 2.3 (SD) ms and the mean second delays between the ulnar and the brachial stimulation were 9.1 ± 1.5 (SD) ms.
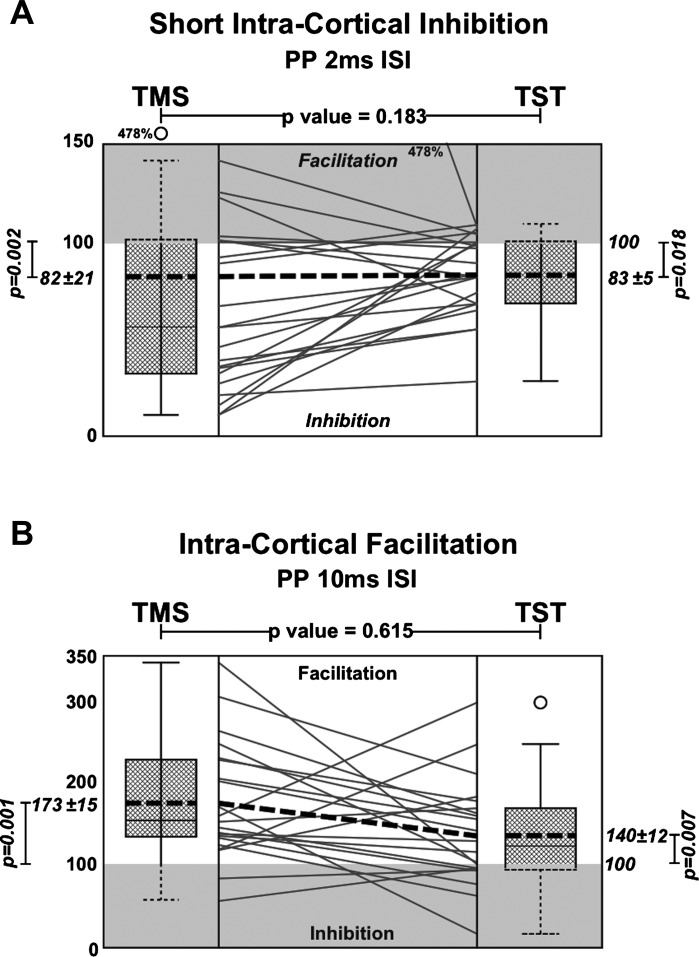
In both methods, conventional TMS alone and TMS combined with the TST protocol, the conditioned MEP amplitude responses were increased with an ISI of 10 ms. The mean peak-to-peak amplitude ratio (mean of the PP MEPs/mean of the single pulse MEPs) was 173.4 ± 14.9% for the TMS [linear regression analysis conditioned vs. unconditioned MEPs, F(1,21) = 16.19, P = 0.001] and 140.3 ± 12.3% for the TST [linear regression analysis conditioned vs. unconditioned, F(1,21) = 8.89, P = 0.007].
With an ISI of 2 ms the conditioned MEP amplitudes were decreased with both methods. The mean amplitude ratio was 82.1 ± 20.7 for the TMS [linear regression analysis conditioned vs. unconditioned MEPs, F(1,21) = 12.27, P = 0.002] and 83 ± 4.6 for the TST [F(1,21) = 6.57, P = 0.018]. However, with both methods and with each ISI, there were subjects showing inhibition and facilitation when the opposite was expected: seven subjects with TMS and five with TST showed facilitation with ISI of 2 ms and two subjects with TMS and eight with TST showed inhibition with ISI of 10 ms.
Linear regression analysis comparing the two methods showed no significant difference between them in detecting facilitation [TMS vs. TST, F(5, 21) = 8.10, P = 0.615] or inhibition [TMS vs. TST, F(5, 21) = 8.10, P = 0.183] (Fig. 2).
Fig. 2.
Results of the PP stimulation paradigms. A: results of SICI with an ISI of 2 ms. B: results of ICF with an ISI of 10 ms with TMS and triple stimulation technique (TST). Box plots show the percentage of the conditioned amplitude (expressed as the ratio between the mean conditioned responses and the mean single pulse stimulation). Bottom of the box plots represent the 25th percentile and the top the 75th percentile. Bars represents the maximum/minimum values. Outliers [<1st quartile – 1.5 interquartile range (IQR) or >3rd quartile + 1.5 IQR] are represented by dots. The broken lines inside the box plots represent the mean, the solid lines represent the median. In the boxplot in Fig. 1A for TST these two values are superimposed. In the central area each line represents a participant’s response, and the broken line represents the group mean. P values are provided for the comparisons as indicated (TMS vs. TST, conditioned vs. unconditioned) for each method [SICI (A) or ICF (B)]. For better clarity, in unshaded and shaded areas of expected and contrary responses correspondingly (facilitation in the 2-ms ISI-SICI and vice versa).
There was a significant reduction of the CV with TST for single stimulation [from 0.62 ± 0.38 with TMS to 0.36 ± 0.22 with TST (−42%), P = 0.017] and SICI [0.73 ± 0.26 to 0.30 ± 0.18 (−59%), P < 0.000] but not for ICF [0.53 ± 0.35 to 0.35 ± 0.22 (−34%), P = 0.069] (see Table 1).
Table 1.
Paired-pulse stimulation responses of short intracortical inhibition, intracortical facilitation, and control stimulation
| TMS, mV | TST, mV | P | CV (TMS) | CV (TST) | P | |
|---|---|---|---|---|---|---|
| Inhibition | 0.90 ± 1.24 | 1.60 ± 1.52 | 0.050 | 0.73 ± 0.26 | 0.30 ± 0.18 | <0.000 |
| Control | 1.48 ± 1.60 | 1.97 ± 1.69 | 0.252 | 0.62 ± 0.38 | 0.36 ± 0.22 | 0.017 |
| Facilitation | 2.31 ± 2.20 | 2.67 ± 2.24 | 0.435 | 0.53 ± 0.35 | 0.35 ± 0.22 | 0.069 |
Values represent means ± SD; n = 22 subjects. TMS, transcranial magnetic stimulation; TST, triple stimulation technique. Effects of conditions and method on the amplitude and its coefficient of variation (CV) are shown. P values are obtained from multiple linear regression taking into account the repeated-measure design.
DISCUSSION
The objectives of our study were to explore the physiology underlying the PP-TMS paradigms of ICF and SICI with the TST (Magistris et al. 1998) and to determine whether this combined PP-TST protocol could reduce their variability. The TST increases the accuracy in quantifying corticospinal conduction by correcting for the desynchronization of the descending discharges following TMS, which reduces the variability of MEPs and leads, thereby, to more consistent results (Rösler et al. 2008). The principal findings of our study are that the TST confirms the inhibition and facilitation of MEPs in the PP paradigms. Combining PP with TST reduces the variability of SICI by −59% and ICF, although not significantly, by −34%, but, in some participants, there are effects opposite to the expected, such as facilitation with the inhibitory protocol and vice versa, which need to be further explored.
The concept of the PP paradigms of ICF and SICI (Kujirai et al. 1993) has contributed to a better understanding of the physiology of the primary motor cortex and the pathophysiology of various disorders. However, the intra- and interindividual variability (Boroojerdi et al. 2000; López-Alonso et al. 2014; Orth et al. 2003; Wassermann 2002) remains a major issue precluding their clinical application (see also the consensus of the IFCN; Chen et al. 2008). Given that the combined PP-TST protocol confirms comparable effects of inhibition and facilitation as with the conventional PP-TMS paradigms, the desynchronization of descending and of spinal motor neuron discharges may not contribute. This is another argument in favor of intracortical mechanisms presumed to be mediated by a population of inhibitory interneurons acting on motor neurons (Chen et al. 2008; Reis et al. 2008; Rossini et al. 2015). There is support for this mechanism by the observation that transcranial electrical stimulation, which activates directly the (corticospinal) axons of the motor neurons, does not induce a response inhibition in contrast to TMS, which presumably activates the cortical interneurons (Kujirai et al. 1993). Further support for an intracortical origin comes from cervical recordings showing an inhibition of late descending I waves in SICI (Di Lazzaro et al. 1998b; Nakamura et al. 1997). Our results are in line with another PP-TST paradigm of inhibition (Mall et al. 2001) that has been postulated to result from a reduced number of recruited spinal motor neurons possibly due to an inhibition of the late I waves (Di Lazzaro et al. 1998b; Nakamura et al. 1997).
In ICF, cervical cord recordings show a similar pattern of early and late I descending waves as with a single TMS pulse (Di Lazzaro et al. 2006). This is intriguing since ICF appears not to result in or be mediated by a change of the descending volleys. This could point to spinal mechanisms, as supported by our observation when applying TST of MEPs at a conditioning stimulus intensity of 80% RMT, which indicates spinal motor neuron discharges (unpublished observations). Thus, the conditioning stimulus could prime the spinal motor neurons and lead to more synchronous discharges upon the subsequent test stimulus. This synchronization of spinal motor neuron discharges is presumed to underlie facilitation by voluntary muscle contraction (Rossini et al. 2015). Further support for a synchronization mechanism in ICF and facilitation during voluntary activity comes from epidural recordings of descending volleys, which are not different following single pulse TMS at rest or during contraction (Di Lazzaro et al. 1998a) as in ICF (Di Lazzaro et al. 2006). Synchronization of spinal motor neuron discharges could explain why TST does not further reduce the variability of MEPs during muscle contraction compared with TMS (Rösler et al. 2008) as we observed with ICF. Besides synchronization, facilitation could result from repetitive spinal motor neuron discharges that have been postulated to increase with stimulation intensity and muscle contraction (Z'Graggen et al. 2008). This could be another potential mechanism in ICF that we currently explore by an extended TST protocol (Z'Graggen et al. 2008).
Our study confirms the intra- and interindividual variability of conditioning effects in the PP stimulation paradigms even opposite to the expected (see Fig. 2) (Boroojerdi et al. 2000; Du et al. 2014; López-Alonso et al. 2014; Maeda et al. 2002; Murase et al. 2015; Orth et al. 2003; Peinemann et al. 2001; Wassermann et al. 2001; Wassermann 2002) and may even differ between the stimulation methods (TMS or TST). Longitudinal studies investigating inter- and intraindividual variability (Du et al. 2014; Maeda et al. 2002) demonstrate reproducibility of SICI, which could correspond to an individual trait. Much remains yet undetermined, and further investigations are needed. Our results are in line with the third of the variability that may be attributed to desynchronization of the descending volleys and can be corrected for by TST (Magistris et al. 1998; Rösler et al. 2002, 2008). This is relevant, since TST, by correcting for desynchronization, is the first method that has proven the possibility of TMS to activate the complete pool of cortical motor neurons projecting to a target muscle (Magistris et al. 1998). For this reason, (supramaximal) TST has become the clinical reference for assessing corticospinal conduction. However, TST indicates that submaximal stimulation activates a variable number of motor neurons that contribute to the variability (Rösler et al. 2008).
Limitations of this study could derive from the technical complexity combining both the TST and PP protocols, which can be mastered. Although the lack of preliminary data precluded a power computation, our study population matches largely similar studies and follows IFCN recommendations (Groppa et al. 2012; Rossini et al. 2015). A true physical and mental resting condition poses a challenge and depends on the compliance. We cannot rule out that plexus stimulation could be felt as unpleasant and may have caused some tension, particularly in more anxious participants (Wassermann et al. 2001), but most reported to habituate. This may have been a reason that no participant withdrew. Few declined to participate, who may have anxiety-related traits, and a selection bias cannot be excluded.
We conclude that desynchronization does not to contribute to SICI and ICF but explains about one-third of the variability of single pulse (unconditioned) and conditioned MEPs. Combining the PP stimulation protocol with TST improves the accuracy of measures, but interindividual variability in both SICI and ICF precludes yet the clinical applicability, although an IFCN consensus recognizes the potential diagnostic utility in various disorders (Chen et al. 2008). Nevertheless, better accuracy in evaluating cortical excitability could serve the research in amyotrophic lateral sclerosis (Geevasinga et al. 2014; Vucic et al. 2013), dystonia (Chen et al. 2008), Parkinson disease (Benninger 2013), and other pathologies, but these findings apply to healthy subjects and may differ in patients.
GRANTS
This work was funded, in part, by a grant from the Baasch-Medicus Foundation (to D. H. Benninger).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
L.C. and M.A.S. performed experiments; L.C. and D.H.B. analyzed data; L.C., M.A.S., F.R.H., and D.H.B. interpreted results of experiments; L.C. and D.H.B. prepared figures; L.C., M.A.S., F.R.H., and D.H.B. drafted manuscript; L.C., M.A.S., F.R.H., and D.H.B. edited and revised manuscript; L.C., M.A.S., F.R.H., and D.H.B. approved final version of manuscript.
REFERENCES
- Awiszus F. TMS and threshold hunting. Suppl Clin Neurophysiol 56: 13–23, 2003. doi: 10.1016/S1567-424X(09)70205-3. [DOI] [PubMed] [Google Scholar]
- Benninger DH. Parkinson’s disease. Handb Clin Neurol 116: 469–483, 2013. doi: 10.1016/B978-0-444-53497-2.00037-1. [DOI] [PubMed] [Google Scholar]
- Boroojerdi B, Kopylev L, Battaglia F, Facchini S, Ziemann U, Muellbacher W, Cohen LG. Reproducibility of intracortical inhibition and facilitation using the paired-pulse paradigm. Muscle Nerve 23: 1594–1597, 2000. doi:. [DOI] [PubMed] [Google Scholar]
- Chen R, Cros D, Curra A, Di Lazzaro V, Lefaucheur JP, Magistris MR, Mills K, Rösler KM, Triggs WJ, Ugawa Y, Ziemann U. The clinical diagnostic utility of transcranial magnetic stimulation: report of an IFCN committee. Clin Neurophysiol 119: 504–532, 2008. doi: 10.1016/j.clinph.2007.10.014. [DOI] [PubMed] [Google Scholar]
- Di Lazzaro V, Pilato F, Oliviero A, Dileone M, Saturno E, Mazzone P, Insola A, Profice P, Ranieri F, Capone F, Tonali PA, Rothwell JC. Origin of facilitation of motor-evoked potentials after paired magnetic stimulation: direct recording of epidural activity in conscious humans. J Neurophysiol 96: 1765–1771, 2006. doi: 10.1152/jn.00360.2006. [DOI] [PubMed] [Google Scholar]
- Di Lazzaro V, Restuccia D, Oliviero A, Profice P, Ferrara L, Insola A, Mazzone P, Tonali P, Rothwell JC. Effects of voluntary contraction on descending volleys evoked by transcranial stimulation in conscious humans. J Physiol 508: 625–633, 1998a. doi: 10.1111/j.1469-7793.1998.625bq.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Lazzaro V, Restuccia D, Oliviero A, Profice P, Ferrara L, Insola A, Mazzone P, Tonali P, Rothwell JC. Magnetic transcranial stimulation at intensities below active motor threshold activates intracortical inhibitory circuits. Exp Brain Res 119: 265–268, 1998b. doi: 10.1007/s002210050341. [DOI] [PubMed] [Google Scholar]
- Du X, Summerfelt A, Chiappelli J, Holcomb HH, Hong LE. Individualized brain inhibition and excitation profile in response to paired-pulse TMS. J Mot Behav 46: 39–48, 2014. doi: 10.1080/00222895.2013.850401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geevasinga N, Menon P, Yiannikas C, Kiernan MC, Vucic S. Diagnostic utility of cortical excitability studies in amyotrophic lateral sclerosis. Eur J Neurol 21: 1451–1457, 2014. doi: 10.1111/ene.12422. [DOI] [PubMed] [Google Scholar]
- Groppa S, Oliviero A, Eisen A, Quartarone A, Cohen LG, Mall V, Kaelin-Lang A, Mima T, Rossi S, Thickbroom GW, Rossini PM, Ziemann U, Valls-Solé J, Siebner HR. A practical guide to diagnostic transcranial magnetic stimulation: report of an IFCN committee. Clin Neurophysiol 123: 858–882, 2012. doi: 10.1016/j.clinph.2012.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiers L, Cros D, Chiappa KH, Fang J. Variability of motor potentials evoked by transcranial magnetic stimulation. Electroencephalogr Clin Neurophysiol 89: 415–423, 1993. doi: 10.1016/0168-5597(93)90115-6. [DOI] [PubMed] [Google Scholar]
- Kujirai T, Caramia MD, Rothwell JC, Day BL, Thompson PD, Ferbert A, Wroe S, Asselman P, Marsden CD. Corticocortical inhibition in human motor cortex. J Physiol 471: 501–519, 1993. doi: 10.1113/jphysiol.1993.sp019912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- López-Alonso V, Cheeran B, Río-Rodríguez D, Fernández-Del-Olmo M. Inter-individual variability in response to non-invasive brain stimulation paradigms. Brain Stimulat 7: 372–380, 2014. doi: 10.1016/j.brs.2014.02.004. [DOI] [PubMed] [Google Scholar]
- Maeda F, Gangitano M, Thall M, Pascual-Leone A. Inter- and intra-individual variability of paired-pulse curves with transcranial magnetic stimulation (TMS). Clin Neurophysiol 113: 376–382, 2002. doi: 10.1016/S1388-2457(02)00008-1. [DOI] [PubMed] [Google Scholar]
- Magistris MR, Rösler KM, Truffert A, Landis T, Hess CW. A clinical study of motor evoked potentials using a triple stimulation technique. Brain 122: 265–279, 1999. doi: 10.1093/brain/122.2.265. [DOI] [PubMed] [Google Scholar]
- Magistris MR, Rösler KM, Truffert A, Myers JP. Transcranial stimulation excites virtually all motor neurons supplying the target muscle. A demonstration and a method improving the study of motor evoked potentials. Brain 121: 437–450, 1998. doi: 10.1093/brain/121.3.437. [DOI] [PubMed] [Google Scholar]
- Mall V, Glocker FX, Fietzek U, Heinen F, Berweck S, Korinthenberg R, Rösler KM. Inhibitory conditioning stimulus in transcranial magnetic stimulation reduces the number of excited spinal motor neurons. Clin Neurophysiol 112: 1810–1813, 2001. doi: 10.1016/S1388-2457(01)00638-1. [DOI] [PubMed] [Google Scholar]
- Murase N, Cengiz B, Rothwell JC. Inter-individual variation in the after-effect of paired associative stimulation can be predicted from short-interval intracortical inhibition with the threshold tracking method. Brain Stimulat 8: 105–113, 2015. doi: 10.1016/j.brs.2014.09.010. [DOI] [PubMed] [Google Scholar]
- Nakamura H, Kitagawa H, Kawaguchi Y, Tsuji H. Intracortical facilitation and inhibition after transcranial magnetic stimulation in conscious humans. J Physiol 498: 817–823, 1997. doi: 10.1113/jphysiol.1997.sp021905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia 9: 97–113, 1971. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Orth M, Snijders AH, Rothwell JC. The variability of intracortical inhibition and facilitation. Clin Neurophysiol 114: 2362–2369, 2003. doi: 10.1016/S1388-2457(03)00243-8. [DOI] [PubMed] [Google Scholar]
- Peinemann A, Lehner C, Conrad B, Siebner HR. Age-related decrease in paired-pulse intracortical inhibition in the human primary motor cortex. Neurosci Lett 313: 33–36, 2001. doi: 10.1016/S0304-3940(01)02239-X. [DOI] [PubMed] [Google Scholar]
- Reis J, Swayne OB, Vandermeeren Y, Camus M, Dimyan MA, Harris-Love M, Perez MA, Ragert P, Rothwell JC, Cohen LG. Contribution of transcranial magnetic stimulation to the understanding of cortical mechanisms involved in motor control. J Physiol 586: 325–351, 2008. doi: 10.1113/jphysiol.2007.144824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rösler KM, Petrow E, Mathis J, Arányi Z, Hess CW, Magistris MR. Effect of discharge desynchronization on the size of motor evoked potentials: an analysis. Clin Neurophysiol 113: 1680–1687, 2002. doi: 10.1016/S1388-2457(02)00263-8. [DOI] [PubMed] [Google Scholar]
- Rösler KM, Roth DM, Magistris MR. Trial-to-trial size variability of motor-evoked potentials. A study using the triple stimulation technique. Exp Brain Res 187: 51–59, 2008. doi: 10.1007/s00221-008-1278-z. [DOI] [PubMed] [Google Scholar]
- Rossini PM, Burke D, Chen R, Cohen LG, Daskalakis Z, Di Iorio R, Di Lazzaro V, Ferreri F, Fitzgerald PB, George MS, Hallett M, Lefaucheur JP, Langguth B, Matsumoto H, Miniussi C, Nitsche MA, Pascual-Leone A, Paulus W, Rossi S, Rothwell JC, Siebner HR, Ugawa Y, Walsh V, Ziemann U. Non-invasive electrical and magnetic stimulation of the brain, spinal cord, roots and peripheral nerves: Basic principles and procedures for routine clinical and research application. An updated report from an I.F.C.N. Committee. Clin Neurophysiol 126: 1071–1107, 2015. doi: 10.1016/j.clinph.2015.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stinear CM, Byblow WD. Elevated threshold for intracortical inhibition in focal hand dystonia. Mov Disord 19: 1312–1317, 2004. doi: 10.1002/mds.20160. [DOI] [PubMed] [Google Scholar]
- Vucic S, Ziemann U, Eisen A, Hallett M, Kiernan MC. Transcranial magnetic stimulation and amyotrophic lateral sclerosis: pathophysiological insights. J Neurol Neurosurg Psychiatry 84: 1161–1170, 2013. doi: 10.1136/jnnp-2012-304019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wassermann EM. Variation in the response to transcranial magnetic brain stimulation in the general population. Clin Neurophysiol 113: 1165–1171, 2002. doi: 10.1016/S1388-2457(02)00144-X. [DOI] [PubMed] [Google Scholar]
- Wassermann EM, Greenberg BD, Nguyen MB, Murphy DL. Motor cortex excitability correlates with an anxiety-related personality trait. Biol Psychiatry 50: 377–382, 2001. doi: 10.1016/S0006-3223(01)01210-0. [DOI] [PubMed] [Google Scholar]
- Z'Graggen WJ, Humm AM, Oppliger-Bachmann S, Hosang M, Rösler KM. Repetitive spinal motor neuron discharges following single transcranial magnetic stimulation: relation to dexterity. Exp Brain Res 188: 579–587, 2008. doi: 10.1007/s00221-008-1389-6. [DOI] [PubMed] [Google Scholar]