The present work establishes that, when reaching to a target while standing, perturbations applied to the upper limb elicit a rapid response in lower limb muscles. Unlike voluntary movements, postural responses do not occur before corrections of the upper limb. We show the first evidence that corrective postural adjustments are modulated by upper limb behavioral context (target shape). Importantly, this indicates that postural responses take into account upper limb feedback for online control.
Keywords: posture, reflexes, task-dependent responses, upper limb
Abstract
An important aspect of motor control is the ability to perform tasks with the upper limbs while maintaining whole body balance. However, little is known about the coordination of upper limb voluntary and whole body postural control after mechanical disturbances that require both upper limb motor corrections to attain a behavioral goal and lower limb motor responses to maintain whole body balance. The present study identified the temporal organization of muscle responses and center of pressure (COP) changes following mechanical perturbations during reaching. Our results demonstrate that muscle responses in the upper limb are evoked first (∼50 ms), with lower limb muscle activity occurring immediately after, in as little as ∼60 ms after perturbation. Hand motion was immediately altered by the load, while COP changes occurred after ∼100 ms, when lower limb muscle activity was already present. Our secondary findings showed that both muscle activity and COP changes were influenced by behavioral context (by altering target shape, circle vs. rectangle). Voluntary and postural actions initially directed the hand toward the center of both target types, but after the perturbation upper limb and postural responses redirected the hand toward different spatial locations along the rectangle. Muscle activity was increased for both upper and lower limbs when correcting to the circle vs. the rectangle, and these differences emerged as early as the long-latency epoch (∼75–120 ms). Our results demonstrate that postural responses are rapidly and flexibly altered to consider the behavioral goal of the upper limb.
NEW & NOTEWORTHY The present work establishes that, when reaching to a target while standing, perturbations applied to the upper limb elicit a rapid response in lower limb muscles. Unlike voluntary movements, postural responses do not occur before corrections of the upper limb. We show the first evidence that corrective postural adjustments are modulated by upper limb behavioral context (target shape). Importantly, this indicates that postural responses take into account upper limb feedback for online control.
the fact that humans are bipedal means that many everyday activities require the ability to interact with objects with our upper limbs while maintaining whole body postural stability. For example, when we reach out toward a door knob while standing, predictable adjustments of posture occur. These adjustments serve to minimize the disturbance to balance and can also contribute to the reaching movement (Bouisset et al. 2000; Bouisset and Zattara 1987; Massion 1992). However, if the arm is unexpectedly bumped by a passerby when reaching, how are postural responses updated and integrated with the upper limb motor correction?
A possible strategy is that adjustments to posture are evoked in an organization similar to initiation of a voluntary movement. Postural adjustments typically begin 50–100 ms earlier than voluntary arm movements (Aruin and Latash 1995; Bouisset and Zattara 1987) and are tuned to the spatial and temporal requirements of the upper limb task (Aruin and Latash 1995; Horak et al. 1984; Leonard et al. 2009; Wing et al. 1997). A recent study found that, similar to voluntary movements, the “posture before arm” relationship was maintained during online corrections to reaching when subjects encountered a shift in the location of the spatial goal (a target jump) (Leonard et al. 2011). In this case, postural adjustments of the lower limb occurred ∼30 ms or more before upper limb corrective responses.
However, there are several key differences between corrections to a shift in a spatial goal and motor responses to mechanical disturbances to the body (upper limb). The most obvious is that mechanical disturbances are destabilizing and thus require a motor response, potentially to maintain balance but also to achieve the behavioral goal with the upper limb. As well, corrective responses related to unexpected changes to the body are faster than those for changes in the spatial goal, for either mechanical (Nashed et al. 2012, 2014) or visual (Brenner and Smeets 2003; Dimitriou et al. 2013) disturbances, suggesting that the motor system employs different processes for feedback related to the limb vs. the spatial goal (Scott 2016).
Importantly, goal-directed motor responses when the limb is mechanically disturbed begin at ∼60 ms (Nashed et al. 2012, 2014). Such responses are thought to be generated by transcortical feedback (Pruszynski et al. 2014), and their onset may be too rapid to allow for postural responses to occur beforehand. The alternative is that upper limb motor responses are delayed to allow for postural responses to be evoked first.
Therefore the first aim of the present study was to establish the onset of postural responses following a perturbation to the upper limb during goal-directed reaching. Coupling of these responses has been examined in a pair of studies, but with only one or two subjects. Mechanical perturbations applied during voluntary upper limb movement evoked lower limb muscle responses in two of the experimenters at latencies of 65–80 ms, after muscle responses in the upper limb at ~45 ms (Marsden et al., 1981, 1983). In the present study, we sought to fully characterize the timing of online upper limb and postural corrections by establishing the onset times of muscle activity and changes in the center of foot pressure after upper limb perturbations.
In addition to being remarkably fast, corrective responses to mechanical disturbances in the upper limb are also highly flexible. Several studies have highlighted that long- but not short-latency muscle responses can compensate for changes in background load (gain scaling) (Marsden et al. 1976; Matthews 1986; Pruszynski et al. 2009; Stein et al. 1995), express knowledge of limb dynamics (Kurtzer et al. 2009), and are influenced by verbal instruction (Capaday et al. 1994; Hagbarth 1967; Rothwell et al. 1980). These long-latency responses are also task dependent and can be modulated by features of the goal such as shape or size (Crevecoeur et al. 2013; Nashed et al. 2012) or the presence of obstacles (Nashed et al. 2014). Similarly, postural responses to whole body perturbations are also flexible. Mechanical perturbations, such as surface platform translations, can generate long-latency muscle responses in the lower limbs that reflect task-level control of the center of mass (Safavynia and Ting 2013; Welch and Ting 2008) and can lead to rapid reaching movements for stabilization that are tuned to the surrounding environment (Gage et al. 2007).
However, little is known about how task-dependent effects are incorporated into postural responses during online corrections of upper limb voluntary movements. In an early study, results presented for a single subject indicated that explicit instructions (“resist” or “do not resist” the upper limb perturbation) influenced postural responses after upper limb perturbations when the upper limbs were also engaged in a postural control task (holding a handle; Cordo and Nashner 1982). A recent study extended these findings to treadmill walking and determined that lower and upper limb muscle responses evoked from perturbations to handles held by subjects were influenced by instruction (Forero and Misiaszek 2014). However, the timing of these instruction-dependent differences is unclear, as muscle activity was averaged for 200 ms after response onset. In the upper limb, task-dependent features for online corrective responses emerge well within this 200-ms window (Crevecoeur et al. 2013; Nashed et al. 2012; Pruszynski et al. 2008).
Thus the second aim sought to identify 1) whether lower limb postural responses are altered by features of the task and 2) how rapidly these responses are initiated. We probed these questions by having participants perform standing reaches, and mechanical perturbations to the upper limb were applied on randomly selected trials. The goal target was also randomly presented as either a circle or a rectangle, as we have previously shown that corrective responses in the upper limb are influenced by the shape of the goal target (Nashed et al. 2012). Overall, we hypothesized that lower limb postural responses would be evoked after corrective responses in the upper limb and that both upper and lower limb responses would be task dependent, with larger motor responses for corrections to a circular target compared with a rectangular target.
METHODS
Participants
A total of 16 subjects (10 women, 6 men; 21–33 yr of age) participated in the present experiment. All participants were free from neurological or muscular disorders and provided written informed consent before participation in the study. The protocol was approved by the Research Ethics Board of Queen’s University. Experiments lasted between 2 and 2.5 h, and participants were compensated for their time.
Experimental Apparatus and Setup
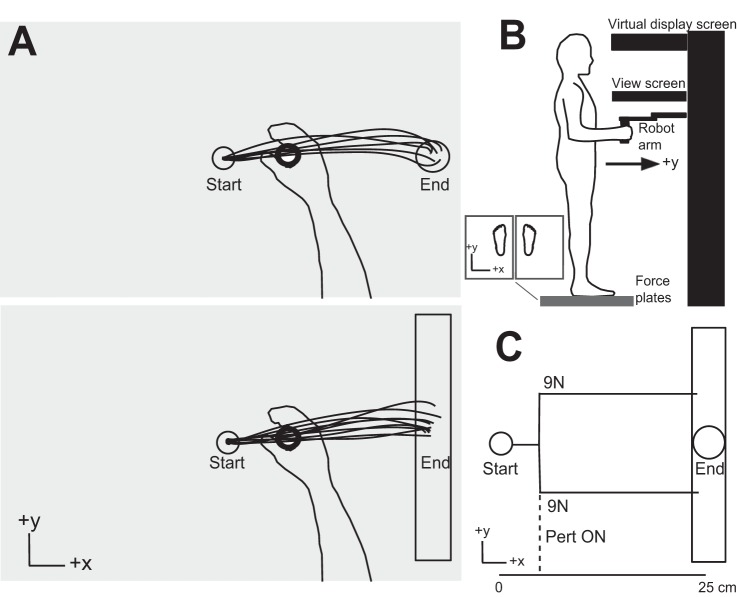
All experiments used an adjustable-height KINARM end-point robotic device (BKIN Technologies, Kingston, ON, Canada). The KINARM robots were adjusted vertically for each participant to allow him/her to stand and grasp the handle of the right robotic arm (with the participant’s elbow at ~90° and shoulder relaxed) and comfortably view the visual display that projected spatial targets and hand position (white circle, 0.5-cm diameter) down onto the workspace (Fig. 1). Participants stood barefoot on two force plates (TrueImpulse, Northern Digital, Waterloo, ON, Canada; 0.15-N resolution) that measured ground reaction forces (GRFs) and moments in the medial/lateral (x), anterior/posterior (y), and vertical (z) axes. Participants were instructed to stand comfortably with feet hip-distance apart and one foot on each force plate. Once a stance width was chosen, the outlines of the feet were marked on the force plates to ensure that participants stood in the same location for all trials. Subjects were instructed to stand straight, grasp the handle loosely (i.e., no weight bearing on handle), and stand with weight equally distributed between the feet. Initial standing position was visually inspected by the experimenter at the beginning of each trial, and participants were verbally prompted every few trials to ensure compliance. Kinematics of the handle (position and velocity), GRFs, and moments were sampled at 1 kHz and downsampled off-line to 500 Hz.
Fig. 1.
Experimental task setup. A: example traces of unperturbed reaching trajectories to the circle target (top) and the rectangle target (bottom). Either the circle or the rectangle target was presented (in a pseudorandomized order), and participants made reaching movements to the target. Perturbations were randomly applied in ~20% of trials. B: experimental apparatus; participants stood barefoot on 2 force platforms and grasped the end-point robotic handle. The robot was height adjustable, and the participant’s elbow joint was maintained at 90° (upper arm relative to forearm). Direct vision of the hand was occluded. Note that the orientation is different between the schematics in A and B. C: schematic of the perturbation loads applied to the handle. Loads were applied in the y-direction (9 N, orthogonal to reach direction) and displaced the upper limb either toward or away from the body. The perturbation was applied when the hand cursor reached 3 cm from the start target in the x-direction (direction of reach).
The Task
At the beginning of each trial, a red start target appeared (circular target, 1-cm diameter) directly in front of midline on the screen (Fig. 1A). Participants were instructed to bring the white dot representing their hand position into the start target (target turned green). After a random delay, an end target appeared at a distance of 25 cm to the right (x-direction) as either a circle (1.5-cm diameter) or a rectangle (1.5-cm width, 30-cm length). Participants were instructed to reach for the target as quickly and accurately as possible. In ~20% of trials, rapid perturbations (9 N, square pulse, 10-ms rise time) were applied when the hand had moved 3 cm (in the x-direction) from the start target (Fig. 1C). Perturbations were applied orthogonal to the reach direction, either the positive or the negative y-direction resulting in a perturbation “away” from or “toward” the body, respectively. Perturbation remained on for 1,500 ms or until the participants reached the end target and remained within the target for 500 ms. Participants were encouraged to reach the end targets within 300–550 ms during unperturbed trials, and this timing was loosened to 300–600 ms for perturbation trials. The end targets turned green only if participants reached the target in the appropriate amount of time. Otherwise, the end target remained red if the participants moved too slowly and turned blue if participants moved too quickly. During data analyses the maximum reach time was increased by 50 ms for each target/perturbation condition, and trials in which participants were unable to reach the end target within the new required time limits were excluded from analyses (overall percentages of trials excluded were 5% of unperturbed trials to the circle and 6% of unperturbed trials to the rectangle; 8% of trials that involved perturbations to the circle and 8% for perturbations to the rectangle).
Trials were presented in 4 blocks, each of which consisted of 50 trials of unperturbed reaches and 10 perturb trials (5 toward and 5 away), for each target type (circle and rectangle), for a total of 120 trials per block. Trials were presented in a random order for each block. To avoid fatigue, participants were required to rest in a seated position after each block (every ~15 min). Hand positions reported represent the actual position of the hand within the local coordinate system of the workspace. Target center was located at 18.6 cm from the front edge of the screen in the y-direction.
Muscle Recordings
Electromyographic (EMG) recordings were taken from 12 muscles with surface electrodes (Delsys, Boston, MA). The skin overlying the muscle was cleaned, and electrodes were placed on the muscle belly parallel to muscle fibers. The ground electrode was placed on the right patella. Muscle activity was collected from four muscles of the right upper limb: brachioradialis (elbow flexor), triceps lateralis (elbow extensor), anterior deltoid (flexion/medial rotation of shoulder), and posterior deltoid (extension/lateral rotation of shoulder). Muscle activity was collected bilaterally from the following lower limb muscles: long head biceps femoris (knee flexor), rectus femoris (knee extensor), tibialis anterior (ankle dorsiflexor), and gastrocnemius lateralis (ankle plantarflexor). Muscle activity was amplified (104), band-pass filtered (20–450 Hz), and digitally sampled at 1 kHz.
Data Analysis
All data analyses were performed in MATLAB (The MathWorks, Natick, MA). Kinematic data were low-pass filtered (20-Hz cutoff, dual pass, 4th-order Butterworth). EMG signals were band-pass filtered (25–250 Hz, dual pass, 6th-order Butterworth) and full-wave rectified. EMG signals were then normalized to their mean activity when resisting a 9-N static load at the hand, collected in an initial set of normalization trials (2 sets were collected, with the hand in the start position with the load directed “toward” the body in 1 set and the load directed “away” from the body in the other to correspond to the perturbation directions). All reaching trials were aligned to perturbation onset or, for unperturbed trials, the point in the reach where the perturbation would have occurred. As such, 0 ms corresponds to perturbation onset.
Center of Pressure Calculations
Center of pressure (COP) in the x (medial/lateral)- and y (anterior/posterior)-directions was calculated for each foot with the following equations:
where Fx, Fy, and Fz are GRFs in x-, y-, and z-directions, Mx and My are moments about the x- and y-axes, and d is the distance from the top to the center of the force plate (true origin, 0.0471 cm). The distance d is used to calculate the contribution of horizontal forces (Fx and Fy) to the horizontal moments generated about the y- and x-axes.
As two force plates were used, net COP was calculated from the COP from each limb to provide a measure of whole body COP movement. COPnet in the x- and y-directions was calculated as a weighted average of the Fz (vertical GRFs for each foot collected from each force plate) with the following equation from Winter et al. (1996):
Velocity parameters were calculated in each direction by differentiating COPnet x and COPnet y. COPnet speed was calculated by differentiating the resultant of COPnet movement in the x- and y-directions and dividing by the change in time. Hereafter, COP is used and should be considered to represent COPnet. COP positions are reported as the relative movement of COP from the beginning of the trial. As there were no spatial targets with which to align the COP movements, initial COP position was averaged over a 500-ms period of quiet stance (beginning at 1,000 ms before reach onset) and this baseline COP value was subtracted from the subsequent values of the trial to provide a relative displacement from quiet stance at the beginning of every trial.
Muscle Activity
We were particularly interested in identifying the onset time of task-related differences in feedback responses to the perturbation. Muscle activity was evaluated within previously defined epochs (Pruszynski et al. 2008) after perturbation onset for the upper limb: R1 = 20 to 45 ms, R2 = 45 to 75 ms, R3 = 75 to 105 ms, early voluntary (Vol) = 120 to 180 ms, and preperturbation (Pre) = −50 to 0 ms. Since lower limb latencies may be longer because of increased transmission time to the lower limb muscles, the R3 epoch was extended to 75–120 ms when considering lower limb responses. Previous work has shown that the earliest voluntary responses in the lower limbs to a visual stimuli occur between ~120 and 200 ms and these latencies are nearly doubled when the direction of movement is unpredictable (Nardone and Schieppati 1988). Thus we felt it conservative to assume that muscle activity recorded before 120 ms in the lower limbs did not contain voluntary influence from visual input about the moving limb.
Statistical Analyses
Receiver operator curves (ROCs) were used to determine the onset of perturbation-evoked differences in kinematics and EMG. The ROC curve represents the probability that an ideal observer could discriminate between two signals. Unperturbed reaches were compared with perturbed reaches (in each direction) for the circle target to determine the nominal perturbation response. Area under the ROC curve was calculated for each subject from the distribution of unperturbed and perturbed circle trials at each time step. Normalized EMG traces were smoothed with a zero-lag 5-ms moving average filter before calculation of the ROC curve to reduce variability. The onset of perturbation-related activity was identified when the ROC curve exceeded 0.25 or 0.75 (Green and Swets 1989). For muscle activity, the “knee” was found to determine onset by regressing back to the last time point where the curve crossed 0.50 (Pruszynski et al. 2008). ROC analyses were also used to determine the onset of task-related differences (circle vs. rectangle) in position and velocity of the hand and COP in the primary direction of perturbation (y-direction) when the ROC curve crossed. EMG data were not normally distributed (Kolmogorov-Smirnov 1-sided tests, P < 0.05); thus nonparametric comparisons were used. Kruskal-Wallis tests were used to compare ranked EMG onset times between muscle groups (unperturbed vs perturbation to the circle) in each direction. Preplanned comparisons with Wilcoxon signed-rank tests were used to determine differences in onsets between specific muscle groups.
Perturbation-evoked muscle activity was compared in each epoch (Pre, R1–R3, Vol) to determine task-related difference in muscle activity between corrections to the circle and the rectangle. Mean muscle activity was calculated for each participant within the specified epochs. To evaluate the muscle activity associated with the perturbation, the mean of control reaches (unperturbed reaches to circle/rectangle) was subtracted from the mean of the perturbation conditions for each participant, resulting in perturbation-evoked muscle activity between target conditions. EMG data were not normally distributed (Kolmogorov-Smirnov 1-sided tests, P < 0.05); thus nonparametric comparisons were used. Wilcoxon signed-rank tests were performed on the perturbation-evoked means for corrections to the circle vs. corrections to the rectangle within each epoch. Comparisons were performed for each perturbation direction (toward and away) on individual means from the six muscles that responded primarily to each direction (see results).
We first examined the corrective responses for reaches to the circle target to determine the nominal postural responses associated with each direction of upper limb perturbation. We were particularly interested in the evolution of upper and lower limb kinematics and muscle activity to establish the timeline of the relative corrective response onsets. Second, we compared the perturbation responses between the two target conditions, circle and rectangle, to determine the influence of target shape on the response magnitudes.
RESULTS
Whole Body Postural Responses to Upper Limb Reaching and Perturbations
Kinematics.
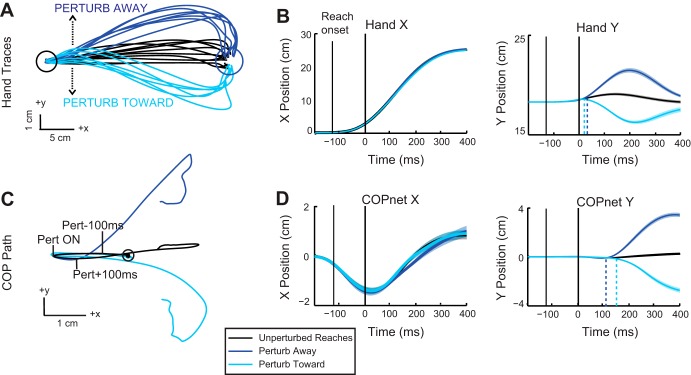
Representative hand and COP traces for reaches to the circular target for a single subject are shown in Fig. 2. The trajectories of the hand during unperturbed reaches to the circle are illustrated by the black traces, which show straight movements to the target with a slight upward curvature. Mean movement time for unperturbed reaches to the circle was 411 ms (standard deviation = 29 ms). In trials with a perturbation, hand path deviated from this nominal trajectory almost immediately following perturbation onset (Fig. 2A, light blue and dark blue traces). Changes in hand path occurred primarily in the direction of the perturbation (along the y- or anterior-posterior direction) with almost no changes occurring in the direction of reach progression (x- or medial-lateral direction; Fig. 2B). ROC analyses determined that perturbation-related differences in hand trajectory in the y-direction occurred at 45 ms following perturb away and 43 ms following perturb toward. Earlier deviations were observed for the velocity of the hand in the y-direction with latencies of 11 ms for perturb away and 12 ms for perturb toward. When the perturbation was present, movement times were significantly decreased for the perturb away condition compared with unperturbed reach times (perturb mean = 393 ms, t15 = –3.04, P = 0.04) but not significantly different for the perturb toward (perturb mean = 430 ms, t15 = –2.49, P = 0.12).
Fig. 2.
Kinematic traces when reaching to the circular target. A: hand trajectories for a representative participant depicting control reaches to the circular target (black traces) and reaching trials when the upper limb was perturbed away (dark blue traces) or toward (light blue traces) the body. B: group mean (solid trace) and SE (shaded area) of hand trajectories in the x (medial/lateral)- and y (anterior/posterior)-directions (color scheme as in A). All trials are aligned to perturbation onset (0 ms). Dashed lines represent onset of perturbation response calculated from ROC analyses. C: mean COP traces from the representative participant in A for reaches with and without a perturbation (color scheme as in A). Circle indicates the initial starting position of the COP, and tick marks indicate perturbation onset (Pert ON) and 100 ms before and after perturbation onset. D: group mean (solid trace) and SE (shaded area) COP trajectories in the x (medial/lateral)- and y (anterior/posterior)-directions aligned to perturbation onset (color scheme as in A). Dashed lines represent onset of perturbation response.
The COP paths associated with reaches to the circle are shown in Fig. 2C. COP for unperturbed reaches is represented by the black trace. COP initially moved in the negative x (leftward)-direction before reversing and moving to the right. The initial leftward COP movement occurred before reach onset (see Fig. 2, B and D) and therefore reflects an anticipatory postural adjustment. For trials containing a perturbation, the COP deviated from its nominal path but not until after the perturbation was applied to the upper limb (Fig. 2C, light and dark blue traces). Similar to the upper limb, the primary deviation occurred in the y-direction, with little change observed in the x-direction (Fig. 2D). The initial COP deviations were in the same direction as the upper limb perturbation, with perturb away evoking anterior displacement of the hand and COP and perturb toward evoking posterior displacement. Perturbation-related differences in COP occurred later than in the hand. Critically, changes in COP following upper limb perturbations were much later than observed changes in hand motion. Onset latencies of COP differences in the y-direction were 150 ms after perturb away and 175 ms after perturb toward. Differences were also observed in COP y velocity and occurred slightly earlier than perturbation-evoked changes in COP position. COP y velocity diverged at 115 ms after perturb away onset and 134 ms after perturb toward onset.
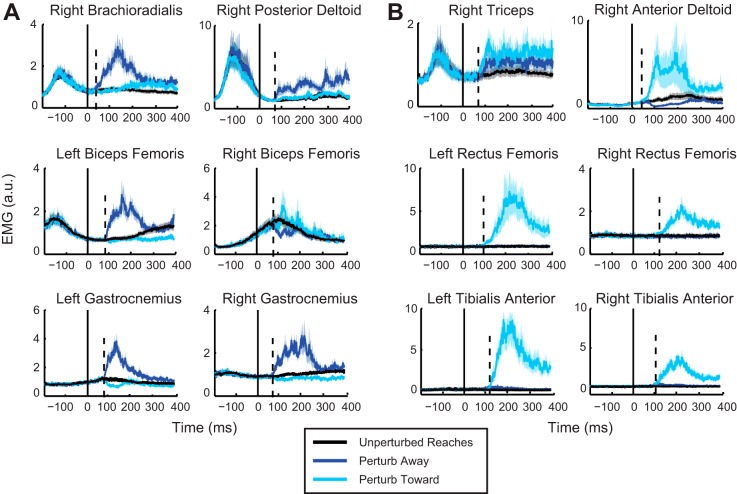
Muscle activity: unperturbed reaches.
Of the 12 muscles recorded, only a few were nominally active during unperturbed reaches to the targets. In the upper limb we saw large initial bursts from brachioradialis, posterior deltoid, and triceps but little initial activity in the anterior deltoid (Fig. 3; group means shown). In the lower limbs only the biceps femoris muscle showed consistent anticipatory activity. The left biceps femoris showed a consistent activation across participants at ~200 ms before reach onset. This was accompanied by a suppression of the right biceps femoris in most participants. As the reach progressed, the pattern of activation for the biceps femoris reversed: left side activity decreased and right side activity increased. This pattern of activation is consistent with initial loading of the left leg and unloading of the right leg, followed by unloading the left and shifting the weight onto the right leg. Initial activation of the left biceps femoris occurs ~50 ms before the initial leftward motion of the COP, which, along with the subsequent loading/unloading pattern of muscle activation, suggests that the biceps femoris activity contributes to the left-to-right COP path during the reach.
Fig. 3.
A: group mean (solid trace) and SE (shaded area) of EMG for the 6 muscles that responded primarily to the away perturbation direction. Responses for all muscles are shown for both perturbation directions, with activity for perturb away in dark blue and perturb toward in light blue. All trials are aligned to perturbation onset (0 ms). Dashed line represents the onset of the perturbation response as calculated from ROC analyses. B: group mean and SE of EMG for the 6 muscles that responded primarily to the toward perturbation direction (color scheme as in A). a.u., Arbitrary units.
Perturbation responses: reaches to circular target.
Six of the twelve muscles responded primarily to perturb away (group means represented in Fig. 3A): brachioradialis, posterior deltoid, biceps femoris (right and left), and gastrocnemius (right and left). The remaining six muscles responded to perturb toward (Fig. 3B): triceps lateralis, anterior deltoid, rectus femoris (right and left), and tibialis anterior (right and left). In the lower limbs, the general pattern of activation served to oppose the perturbation direction. Muscle activation of the biceps femoris and gastrocnemius tends to move the body backward or, in this case, counteracts the forward perturbation to stabilize the body. The reverse was seen for the perturb toward, where the tibialis anterior and rectus femoris activation, which tends to move the body forward, serves to oppose the backward perturbation. The following response onsets are reported for each muscle for their corresponding perturbation direction.
upper limb responses.
The upper limb muscles displayed the earliest discernible response to the perturbation, with a mean onset latency of 53 ms (range of individual mean onsets: 29–85 ms) in brachioradialis and 74 ms (55–113 ms) in posterior deltoid (perturb away) and 58 ms (35–85 ms) in triceps and 52 ms (25–93 ms) in anterior deltoid (perturb toward).
lower limb responses.
The left biceps femoris muscle activity (knee flexors) increased after the upper limb perturbation at a latency of 85 ms (55–143 ms) after perturb onset. The right biceps femoris muscle displayed a less consistent response across subjects, with 3 of 16 participants showing an increase in activity to perturb away with a mean onset of 88 ms (60–105 ms). Three other participants exhibited the opposite pattern, with an inhibition after perturb away with a mean onset of 78 ms (75–82 ms). Ten participants showed no significant change from unperturbed activation in response to either perturbation direction. The ankle plantarflexors were activated slightly earlier than knee flexor inhibition, with a burst of activity in the left and right gastrocnemius muscles at 78 ms (55–95 ms) and 79 ms (59–123 ms), respectively. Left rectus femoris displayed a response time of 94 ms (75–121 ms) after the perturbation, whereas the right rectus femoris responded at ~110 ms (66–150 ms) (but only in 8 of 16 participants). The left and right tibialis anterior had similar response times at 110 ms (98–142 ms) and 111 ms (62–131 ms), respectively.
Relative onset times.
After perturb toward, anterior deltoid onset time was significantly earlier than all four lower limb muscles (left rectus femoris, P = 1.2E−4; right rectus femoris, P = 0.0078; left tibialis anterior, P = 4.9E−4; right tibialis anterior, P = 4.9E−4). Triceps lateralis onset was significantly earlier than three of four lower limb muscles (left rectus femoris, P = 0.002; left tibialis anterior, P = 0.0039; right tibialis anterior, P = 0.0039; but not right rectus femoris, P = 0.13).
Relative onset times: EMG and kinematics.
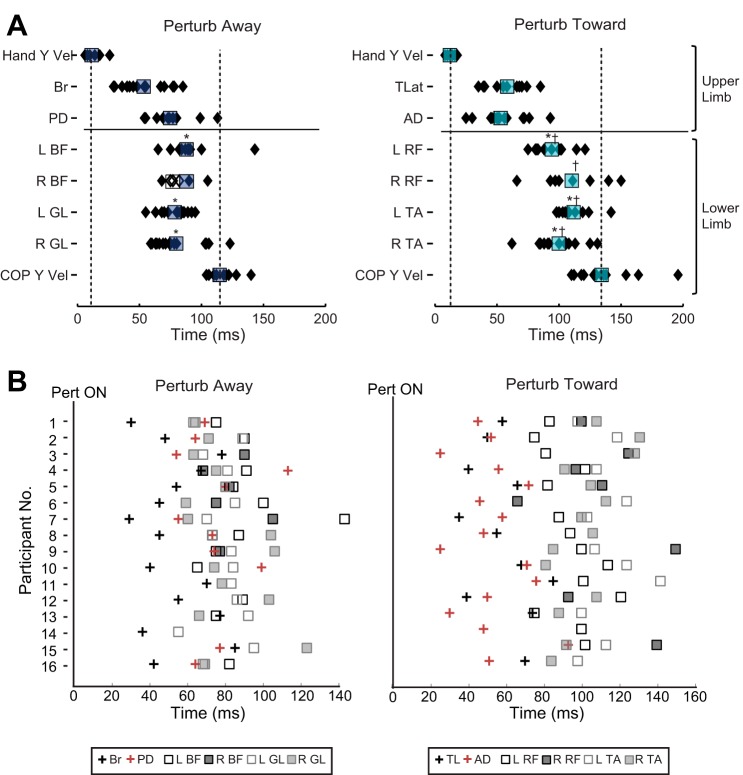
The relative onset times of upper and lower limb kinematics and muscle activity related to each perturbation direction are illustrated in Fig. 4. Change in upper limb muscle responses occurred after the initial change in hand velocity (Fig. 4A). In contrast, lower limb muscle activity occurred before any changes to in COP position or velocity.
Fig. 4.
Temporal evolution of perturbation-evoked responses. A: illustration of the mean (squares) and individual (diamonds) onset times of perturbation responses in kinematics and EMG for each perturbation direction: away (dark blue) and toward (light blue). Onset times are calculated with respect to perturbation onset (0 ms). For right biceps femoris (BF), filled symbols represent an increase in activity and open symbols represent a decrease in activity after perturbation. Symbols above the lower limb muscles indicate which mean onsets are significantly different from brachioradialis (Br) (*) for perturb away and triceps lateralis (TL) (*) and anterior deltoid (AD) (†) for perturb toward. B: relative onset times for all muscles for individual participants. Onset times are calculated with respect to perturbation onset (0 ms). PD, posterior deltoid; GL, gastrocnemius lateralis; TLat, triceps lateralis; RF, rectus femoris; TA, tibialis anterior; L, left; R, right.
Looking individually across participants, almost all (15/16) show that a response in at least one upper limb muscle was evoked before (or simultaneous to) lower limb muscle onset (Fig. 4B). In one subject in each direction (participant 13, perturb away; participant 15, perturb toward) lower limb muscles were activated before any recorded upper limb muscle responses.
Task-Dependent Differences: Influence of Target Shape
Unperturbed reaches.
Reaching movements to the rectangle tended to be directed toward the middle of the rectangle near the same spatial location as the circular target. Hand position at the end of the reach spanned a greater range for the rectangle compared with the circle, reflecting that subjects took advantage of the target shape, minimizing corrections along the long axis of the target (see Nashed et al. 2012).
The initial trajectory over the first 3 cm was statistically different between the rectangular and circular targets for 7 of the 16 subjects (determined by fluctuation over ROC > 0.75 or < 0.25). In general, these subjects tended to reach slightly away for the rectangle relative to the hand paths to reach to the circle. There was no statistical difference in the initial trajectories for 9 of the 16 subjects between the two target shapes. To compare responses, it is important that initial kinematics be consistent across the two tasks; therefore subsequent analyses were focused on these nine participants with similar reach kinematics between the target conditions. For this subgroup unperturbed movement times were not different between reaches to the two targets (mean circle = 398 ms, rectangle = 402 ms, t8 = −0.74, P = 2.39).
Figure 5 illustrates a subject who generated similar initial reach trajectories for reaches to the circular or the rectangular target. For the subset of nine subjects, end-point positions were more variable for reaches to the rectangle than to the circle [standard deviation (SD) circle = 0.23 cm, rectangle = 0.99 cm, t8 = −4.89, P = 0.0012]. Similarly, COP trajectories overlapped during reaches to the circle and rectangle (Fig. 5B). Despite more variability in the hand end-point position, the end-point position of the COP was consistent between targets, with no significant difference in SD (SD circle = 0.42 cm, rectangle = 0.42 cm, t8 = −0.59, P = 0.56).
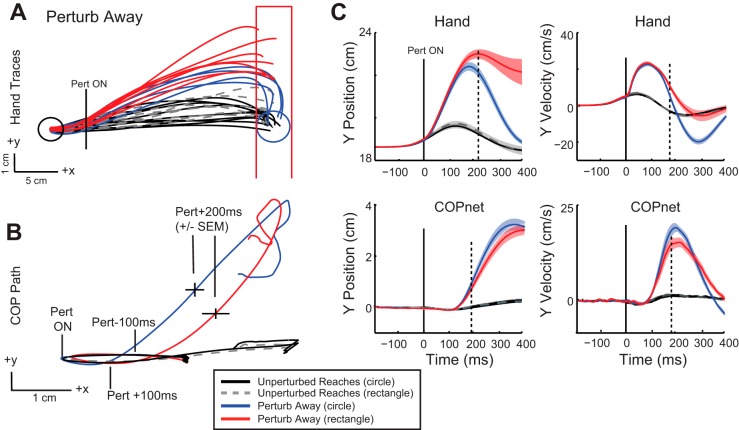
Fig. 5.
Kinematic traces when reaching to the circle and rectangle target for the perturb away condition. A: hand trajectories for a representative participant depicting unperturbed reaches to the circle target (black traces) and the rectangle target (gray dashed traces). Reach trajectories are also illustrated after perturb away for reaches to the circle (blue traces) and the rectangle (red traces). B: mean COPnet traces from the same representative participant for unperturbed and perturbed reaching trials to circle and rectangle (color scheme as in A). Circle indicates the initial starting position of the COP, and tick marks indicate perturbation onset (Pert ON), 100 ms before and after perturbation onset, and 200 ms after perturbation onset (±SE). C: group mean (solid trace) and SE (shaded area) of hand and COPnet trajectories and velocity in the y-direction aligned to perturbation onset (0 ms). Color scheme as in A. Solid vertical line indicates perturbation onset; dashed vertical lines indicate deviation of red and blue perturb traces determined by ROC analyses.
Perturbation responses.
end-point position: upper limb.
Figures 5 and 6 illustrate exemplar reach trajectories after perturbations away and toward the body, respectively. After perturbations to the hand, participants made rapid, rigorous corrections to the circle and exhibited smaller corrective responses when reaching to the rectangular target. End-point position of the hand (y-direction) was significantly different for the circle compared with the rectangle after perturbations in either direction (perturb away: circle = 18.84 cm, rectangle = 21.95 cm, t8 = −3.9, P = 0.005; perturb toward: circle = 18.5 cm, rectangle = 14.3 cm, t8 = 5.8, P = 0.0004). The end position of the hand was directed toward the end of the rectangle, depending on the direction of the perturbation (Fig. 5A perturb away; Fig. 6A perturb toward). The variability of end-point position was greater for corrections to the rectangle (SD = 1.66 cm) compared with the circle (SD = 0.31 cm, t8 −5.3, P = 0.0007) for perturb away. Similar differences were found for the perturb toward condition (rectangle SD = 1.46 cm, circle SD = 0.29, t8 = 7.7, P < 0.0001). Movement times were similar between target conditions for both perturbation directions (perturb away: mean circle = 381 ms, mean rectangle = 393 ms, t8 = −1.41, P = 0.98; perturb toward: circle = 416 ms, rectangle = 390 ms, t8 = 3.09, P = 0.07).
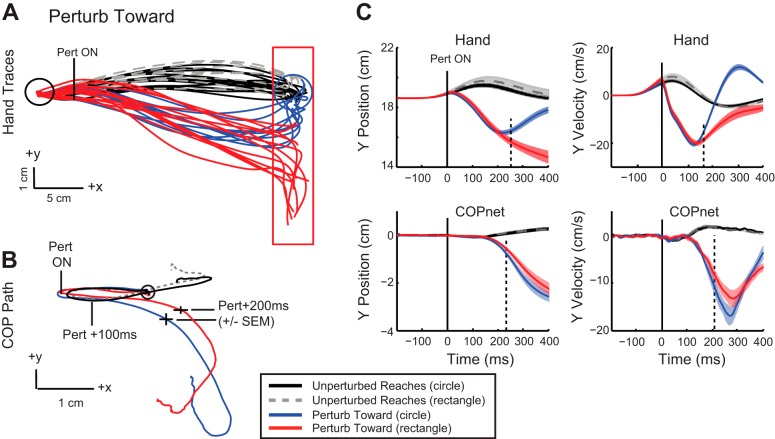
Fig. 6.
Kinematic traces for perturb toward condition. Color scheme as in Fig. 5. A: hand trajectories for a representative participant depicting both unperturbed and perturbed reaches to the circular and rectangular targets. B: mean COPnet traces from the same representative participant. Circle indicates the initial starting position, and tick marks indicate perturbation onset, 100 ms before and after perturbation onset, and 200 ms after perturbation onset (±SE). C: group mean (solid trace) and SE (shaded area) of hand and COPnet trajectories and velocity. Dashed vertical lines indicate deviation of red and blue perturb traces determined by ROC analyses.
end-point position: lower limb.
COP trajectories displayed small but significant target-dependent differences in position when the hand reached the end target after a perturbation. In general, final COP position mirrored the responses seen in the upper limb, with more eccentrically located positions for corrections to the rectangle vs. the circle after either perturbation direction. COP y position was 2.2 cm from its initial position when the hand corrected to the circle and 2.4 cm for corrections to the rectangle (t8 = −2.9, P = 0.01; circle vs. rectangle) after perturb away. For perturb toward, final COP y position was −2.5 cm from its initial position for corrections to the circle and −2.7 cm for corrections to the rectangle (t8 = 4.7, P = 0.002; circle vs. rectangle).
While the peak differences in hand position between target conditions occurred when the hand cursor reached the end target, peak difference in COP occurred between 200 and 400 ms after perturbation onset (Fig. 5B perturb away; Fig. 6B perturb toward). Within this earlier time epoch, peak COP displacement was greater for the circle condition compared with the rectangle condition for perturb away (circle = 3.7 cm, rectangle = 3.4 cm, t8 = 4.8, P = 0.0004) and perturb toward (circle = −2.8 cm, rectangle = −2.5 cm, t8 = 3.7, P = 0.003). Larger excursions of the COP reflect more rigorous corrections to the circle compared with the rectangle, as COP movements generated are likely generated from greater ankle muscle activity in the circle condition (presented below). This effect is mirrored in the velocity traces, as greater COP velocity in the y-direction is quickly achieved for corrections to the circle compared with the rectangle (Fig. 5C perturb away; Fig. 6C perturb toward).
Onset of target-dependent differences.
upper limb.
ROC analysis was used to identify the onset time of target-dependent differences in corrective responses. These onsets were detectable in most of the nine subjects. Differences in hand position in the y-direction occurred at ~224 ms (168–270 ms, n = 8/9; Fig. 5C) after perturb away and ~246 ms (172–342 ms, n = 9/9; Fig. 6C) after the perturb toward. Differences in hand y velocity occurred earlier, ~192 ms (126–360 ms, n = 9/9; Fig. 5C) after perturb away and ~172 ms (134–222 ms, n = 8/9; Fig. 6C) after perturb toward.
lower limb.
Differences in COP tended to be small and transient, making it difficult to identify onset of target-dependent changes for some subjects. For those that were identified, COP y position diverged at ~198 ms (160–248 ms, n = 6/9; Fig. 5C) after perturb away and at ~238 ms (216–264 ms, n = 4/9; Fig. 6C) after perturb toward. Similar to the upper limb, changes in COP y velocity emerged slightly earlier, at ~190 ms (152–206 ms, n = 6/9; Fig. 5C) after perturb away and at ~204 ms (190–211 ms, n = 4/9; Fig. 6C) after perturbation onset. Looking individually across participants for whom velocity differences were determined for both the hand and COP, hand onset minus COP onset values were on average −30 ms (5/9 perturb away, 4/9 perturb toward), with a range from 0 ms to −72 ms. This indicates that differences in velocity between the two targets occurred earlier for the hand compared with the COP (or at the same time).
Perturbation responses: muscle activity.
Figure 7 illustrates representative perturbation-evoked muscle responses for an exemplar participant reaching to the circle or the rectangle. In general, muscle activity was greater after perturbation (in either direction) for corrections to the circle compared with the rectangle target. The transient and distributed nature of EMG changes made it difficult to determine the onset time of task-dependent responses for individual muscles in each participant with ROC analyses. Therefore, we analyzed the epoch within which task-dependent differences in muscle activity could be observed for the group. We compared mean perturbation-evoked muscle activity in previously defined epochs of the stretch responses. Unperturbed muscle activity was subtracted from perturbation-evoked muscle activity and compared across target conditions in each epoch: Pre (−50 to 0 ms), R1 (25 to 45 ms), R2 (45 to 75 ms), R3 [75 to 110 ms (upper limb), 75 to 119 ms (lower limb)], and Vol (120 to 180 ms).
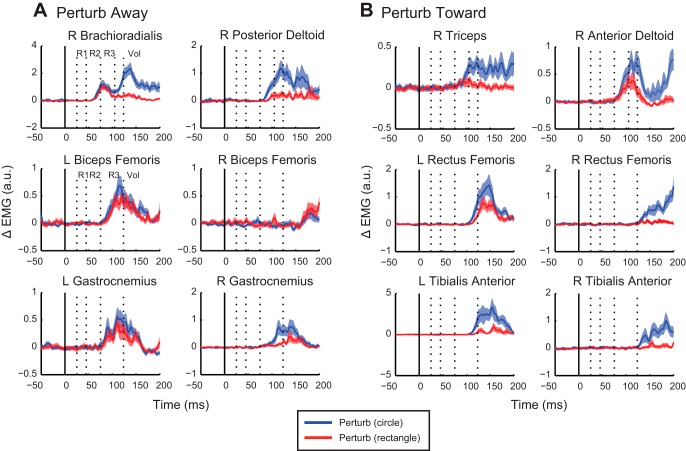
Fig. 7.
Task-dependent differences in perturbation-evoked muscle activity for an exemplar participant. A: mean (solid traces) and SE (shaded area) of EMG for the 6 muscles that responded primarily to the away perturbation direction. Perturbation-evoked muscle responses are represented as ΔEMG (perturbed − unperturbed activity) for corrections to the circle (blue) and to the rectangle (red). Solid vertical line indicates perturbation onset; dotted vertical lines indicate epochs of the stretch response (R1–R3) and early voluntary epoch (Vol). B: mean (solid traces) and SE (shaded area) of EMG for the 6 muscles that responded primarily to the toward perturbation direction.
Circle vs. rectangle: perturb away.
After perturb away, significant target-dependent differences were found for the brachioradialis muscle, which showed greater activation during corrections to the circle vs. the rectangle within the R3 (P = 0.027) and Vol (P = 0.0039) epochs (significant epochs are indicated in Fig. 8). Posterior deltoid exhibited significant differences in the early Vol epoch only (P = 0.004). Significant target-dependent differences were found in lower limb muscles, with muscle activity elevated for corrections to the circle. These differences emerged for the left biceps femoris within the early Vol epoch (P = 0.039), during the R3 (P = 0.0039) and Vol (P = 0.0039) epochs for the right gastrocnemius, and during the early Vol epoch (P = 0.004) for the left gastrocnemius.
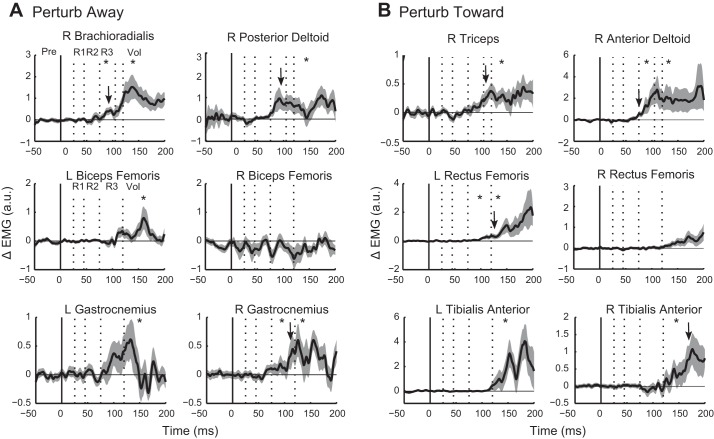
Fig. 8.
Group differences in perturbation-evoked activity between circle and rectangle corrections. A: group mean (solid trace) and SE (shaded area) of the differential signal (circle − rectangle) after perturb away. Positive values indicate greater activity for corrections to the circle. Solid vertical line indicates perturbation onset; dotted vertical lines indicate epochs of the stretch response (R1–R3) and early voluntary epoch (Vol). B: group mean and SE of the differential signal after perturb toward. *P < 0.05, Wilcoxon test. Downward arrows represent the onset of significant positive deflection of the curve from 0 as identified by running Wilcoxon signed-rank test (P < 0.05 for at least 15 ms).
Circle vs. rectangle: perturb toward.
For illustration purposes, the onset of target-dependent differences for all muscles is plotted as the difference between the circle and rectangle target conditions in Fig. 8. Perturbation-evoked muscle activity for the rectangle was subtracted from perturbation-evoked muscle activity for the circle. Positive deflections of the curves reflect greater muscle activity evoked for corrections to the circle. To identify the onset of the positive deflections, we applied a running Wilcoxon signed-rank test to the difference signals of all nine participants at each point in time to determine when the distribution was significantly different from a mean of zero (similar to a running t-test; Wilcoxon test was used because of nonparametric EMG data, but the use of a running t-test produced identical results). The onsets were determined as the initial time point when the P values fell below 0.05 and remained below for at least 15 ms. The onsets of task-dependent differences in the arm muscles were identified at 83 ms, 103 ms, 112 ms, and 75 ms for the brachioradialis, posterior deltoid, triceps, and anterior deltoid muscles, respectively. Signals from the lower limb muscles were more variable, and the onsets were more difficult to determine. Onsets were identified at 110 ms, 131 ms, and 174 ms for the right gastrocnemius, left rectus femoris, and right tibialis anterior, respectively. Both the left gastrocnemius and the left tibialis anterior showed similar troughs in their P values at approximately the same time as their right counterparts, but the P values either did not fall below 0.05 or did not remain below 0.05 for 15 ms.
No changes in preperturbation activity were found between target conditions in either perturbation direction, indicating that differences in anticipatory muscle activity or cocontraction did not account for the target-dependent differences observed. If participants were cocontracting or changing their anticipatory muscle activity between target conditions, baseline differences in muscle activity would emerge regardless of perturbation direction, since participants could not anticipate the occurrence or direction of perturbation.
DISCUSSION
The present study established that, when reaching to a target while standing, perturbations applied to the upper limb elicit a rapid and distributed response in lower limb muscles in as little as ~60 ms, with changes in the COP observed after 100 ms. Furthermore, lower limb corrective responses were influenced by whether the target was a small circle or a long rectangle in as little as ~110 ms, with target-dependent changes in the COP observed at ~200 ms. These results highlight that task-dependent corrective responses in the upper limb rapidly elicit task-dependent postural responses to maintain whole body equilibrium.
There are limitations to the present paradigm that made it difficult to determine the onset of target-related differences in both the upper and lower limb muscles for individual participants. In general, the responses were more subtle and less consistent across muscles and participants than in our previous work using an exoskeleton robotic arm in a seated position (Kurtzer et al. 2009; Nashed et al. 2012, 2014; Pruszynski et al. 2008). This may be due to the fact that standing and grasping a handle largely increases the degrees of freedom, thus increasing the number of behavioral strategies that could be employed. This would impact our ability to detect distributed and subtle changes in muscle activity between the two target conditions. Lower limb responses in particular may have further increased variability due to differing postural strategies (using different muscles) between participants. For example, in response to whole body perturbations, participants can utilize a hip or ankle strategy (or a blend of the two), each using different muscle activation patterns (Horak and Nashner 1986). In addition, muscles in the back and abdomen were not measured in the present paradigm, and these muscles could have contributed to postural adjustments. Finally, we randomized the presentation of each target type to minimize baseline differences in hand kinematics (confirmed with pilot testing). However, randomization may have decreased the magnitude of the difference between corrective responses from trial to trial (Orban de Xivry 2013).
Characterization of Anticipatory Postural Activity
Anticipatory postural adjustments typically occur ~50–100 ms before a voluntary movement of the upper limb (Aruin and Latash 1995; Bouisset et al. 2000; Bouisset and Zattara 1987). In the present paradigm we did not observe much anticipatory activation in the lower limb muscles before reach onset. This is not surprising, as the muscles we examined were specifically targeted for their contribution to the large anterior-posterior corrective responses examined in this study. Thus we did not record from muscles that would likely have contributed to the anticipatory medial-lateral weight shift (i.e., adductors at the hips). Nevertheless, we did see consistent anticipatory activity in the biceps femoris muscles (knee flexors). The left biceps femoris muscle displayed an anticipatory burst of activity at ~200 ms before reach onset. This was often accompanied by an inhibition of the right biceps femoris and then followed by a reversal in activation as the reach progressed (left inhibition and right activation). This pattern of muscle activity is consistent with a loading/unloading response that would account for the anticipatory changes that we observed in the COP—a shift to the left as the left leg was loaded before reach initiation followed by rightward motion of the COP as the reach was initiated.
Characterization of Perturbation Responses
The timing of muscle activity and COP changes shows that feedback from the upper limb after perturbation likely drives the lower limb postural responses. Although motion of the upper limb was immediately modified by the mechanical perturbation in the present study, changes in the COP did not occur for almost 100 ms (Fig. 2). The delay suggests that the applied loads to the upper limb did not immediately transfer through the body to alter the forces between the foot and the ground and were instead “absorbed” within the body. This effect was seen previously for perturbations applied to the arm while subjects were engaged in a postural task (holding a handle) while standing (Cordo and Nashner 1982). Importantly, this suggests that the responses in the lower limbs were not caused by direct stretch of the muscle but were indirectly evoked by input from the upper limb. In the present paradigm, at ~100 ms after the perturbation the COP moved in the direction of the perturbation (i.e., anterior for perturb away), most likely to oppose the motion of the arm, assist in stabilization, and drive the corrective movement of the arm to the target. Muscle activity in the lower limb was observed at roughly 70 ms (i.e., before COP changes), suggesting that changes in COP were generated through active muscle control. Specifically, for the perturb away condition, the large bilateral activation of the gastrocnemius muscle after the perturbation causes plantarflexion about the ankle and served to drive the COP forward under the feet. In contrast, after the perturb away, tibialis anterior activation causes dorsiflexion, which moved the COP backward. In this paradigm, the EMG-COP coupling reflects a strategy to maintain balance against the perturbation and assist in the corrective response of the upper limb, the latter being a postural strategy described previously for voluntary (Aruin and Latash 1995; Leonard et al. 2009) and corrective (Leonard et al. 2011) upper limb movements during standing.
Task-dependent differences in COP were characterized by greater excursions for corrections to the circular vs the rectangular target, seen as higher peak COP and greater COP velocity traces (Fig. 5). The COP changes were evoked by the task-dependent differences in lower limb muscle activity that emerged before 200 ms (within 75–180 ms). Greater muscle activity was generated (in both upper and lower limbs) for corrections to the circular target, where more rigorous corrective movements were required to overcome the applied force and correct back to the target. The increased lower limb muscle activity generated greater dorsi- or plantarflexor torque, which resulted in greater excursions of the COP. This is contrasted with the rectangular target, where the upper limb was corrected to more distal areas of the target, which required less muscle activity from both the upper and lower limbs and, as a result, smaller COP excursions.
Organization of Muscle Responses to Mechanical Perturbations
Motor responses to mechanical loads applied to the body typically have distinct latencies due to variations in the transmission times to and from the spinal cord and brain. A mechanical perturbation to the upper limb generates several phases of activity in shoulder and elbow muscles, including a short-latency response at 25–45 ms that is generated by the spinal cord, is relatively small, and scales with the size of the disturbance (Marsden et al. 1976; Matthews 1986). This is followed by a long-latency motor response that occurs at ~60–70 ms and is task dependent (Scott et al. 2015). Mechanical disturbances to whole body posture (translations and rotations of the support surface) also elicit phasic responses in distal lower limb muscles, although at longer latencies than for the upper limb because of longer transmission times. For the calf muscles, there is a short-latency response at 45–65 ms generated by spinal feedback (Diener et al. 1984; Nashner 1976) and long-latency responses at ~90–100 ms that are task dependent (Diener et al. 1984; Horak and Nashner 1986; Nashner 1976).
In this study, the coupling of lower limb postural responses with disturbances of the upper limb resulted in a blending of the two transmission times, with sensory transmission related to the upper limb and motor response related to the lower limb. This predicts that spinal or segmental feedback responses in the lower limb could be as short as ~35 ms (shortest postural response time less ~10 ms for shorter sensory transmission distance from the upper limb). Similarly, this would predict transcortical feedback responses could be as short as ~65–70 ms, given that the minimum transmission time for a transcortical loop from the distal lower limb muscles is ~75–80 ms (Petersen et al. 1998). In the present study mean lower limb onset times fell between 78 and 111 ms, which suggests that these responses could have been generated from supraspinal feedback.
Organization of Upper and Lower Limb Responses: Do the Arms Wait for the Legs?
To fully characterize responses to upper limb perturbations when standing, we sought to identify the relative timing between the upper limb corrective response and the initiation of postural changes. This is a particularly pertinent question in light of the fact that after a target shift during reaching postural adjustments preceded online corrections to the upper limb, similar to the organization of voluntary arm movements (Leonard et al. 2011). In seated paradigms, shifts of the end target during reaching evoke a rapid, “automatic” response toward the new target location, with kinematic changes as early as 110–125 ms (Day and Lyon 2000; Pruszynski et al. 2016) and EMG responses as early as 90–110 ms (Dimitriou et al. 2013; Pruszynski et al. 2016). In the study by Leonard and colleagues, corrections to upper limb velocity emerged at ~180–190 ms after target shift, which suggests that when standing upper limb corrections are delayed to allow postural responses to be generated first.
If the motor system organizes responses to mechanical perturbations in a similar way, we would expect to see upper limb responses delayed after a perturbation to allow for postural responses to occur in advance. Our data show minimal delays in generation of upper limb motor responses when standing. Admittedly, fewer than half of the subjects showed muscle onsets in the upper limbs within the 25–45 ms short-latency epoch (7 subjects for anterior and 6 subjects for posterior perturbations). In fact, mean onsets for upper limb muscles for the group ranged from 52 to 79 ms. However, we saw a similar lack of short-latency responses in our previous paradigm when subjects were seated (Nashed et al. 2012), which suggests that the absence of the short-latency response is due to other factors and not simply a delay in the upper limb response to allow postural responses to occur. For instance, the applied load used in both studies generated a relatively small deviation of the hand during reaching. This allowed participants to successfully complete the task goal. The use of a larger load might have evoked a more consistent short-latency response, given that these early responses scale directly with load size (Stein et al. 1995), but the subjects would likely not be able to complete the behavioral task.
Importantly, our key observation was that upper limb responses occurred before lower limb muscle onset in almost all subjects. As highlighted in Fig. 4, the onset of at least one arm muscle preceded lower limb muscle onsets in all but two participants (and only in 1 direction for each of these participants). This fits with earlier case presentations of two subjects performing thumb and upper limb movements while standing. Perturbation to the moving digit or limb evoked local muscle responses at ~45 ms, while postural lower limb muscle responses were elicited between 65 and 110 ms in the two subjects (Marsden et al. 1981, 1983). This organization suggests that, unlike voluntary reaching, postural responses to maintain balance do not delay motor responses to the upper limb after mechanical disturbances.
Examination of task-dependent changes in motor corrections also highlighted that upper limb motor responses occurred before lower limb postural responses. The earliest differences in the upper limb were found within the R3 (long latency) epoch, with onset times identified at 75 and 82 ms for the anterior deltoid and brachioradialis muscles. This timing is slightly delayed compared with the same task in a seated paradigm, where task-dependent differences were consistently found within the R2 epoch and onsets emerged as early as 60–70 ms (Nashed et al. 2012). It may be that the change in posture (from seated to standing) delayed the task-dependent changes to upper limb muscles to allow for closer temporal coupling with lower limb muscle onsets, which were also observed within the R3 epoch. However, although lower limb differences were found in R3, the onset of the difference emerged toward the later part of the epoch (110 ms for the right gastrocnemius muscle) or just outside the epoch (131 ms for the left rectus femoris). Furthermore, considering behavior at the individual participant level, task-dependent changes in the COP were detected at the same time as, or after, the onset of differences in hand velocity. On average there was a 30-ms delay between hand and COP, with a range of 0–74 ms across individuals. Interestingly, compared with the seated paradigm, hand velocity differences actually emerged slightly earlier [172–190 ms in the present study compared with 184–196 ms in seated subjects (Nashed et al. 2012)], which would suggest that the small changes in EMG onset times between standing and seated paradigms did not have a substantial impact on hand motion.
How Are Upper and Lower Limb Corrective Responses Coordinated?
The traditional view for coupling voluntary and postural control is that cortical areas generate voluntary commands while brain stem areas produce the associated postural responses. Certainly there is much evidence to support the role of the brain stem in coordinating upper and lower limb responses (Luccarini et al. 1990; Schepens and Drew 2006). More recently, it has been suggested that the cortex may also provide contributions to postural adjustments during stepping and reaching (MacKinnon et al. 2007; Yakovenko and Drew 2009). There is evidence that cortical areas are involved in the task dependence of the long-latency response following mechanical perturbation to the upper limb (Evarts and Tanji 1976; Pruszynski et al. 2011, 2014). Primary motor and premotor (SMA and PMd) cortical areas are also associated with simple upper and lower limb coordination tasks (Byblow et al. 2007; Debaere et al. 2001; Nakagawa et al. 2016). Furthermore, M1, PMd, and parietal area 5 show responses to mechanical disturbances of the upper limb as quickly as 25 ms or less (Omrani et al. 2016). Therefore, the cortex could play a key role in coordinating both the upper limb and lower limb postural corrective responses. The present paradigm was not designed to determine the neural structures involved. Nevertheless, future work should utilize the timing established in the present study to probe these responses by using cortical stimulation (i.e., transcranial magnetic stimulation) to investigate the involvement of cortical areas in postural responses during upper limb corrections.
Conclusions
In this study we show that, when reaching to a target while standing, perturbations applied to the upper limb elicit a rapid response in lower limb muscles in as little as ~60–70 ms, with changes in the COP observed after 100 ms. These lower limb corrective responses were influenced by target shape, with differences in muscle activity arising in as little as ~110 ms and target-dependent changes in the COP observed at ~200 ms. These results highlight that task-dependent feedback is engaged in the upper limb to achieve the behavioral goal and, importantly, that similar feedback processes are used to elicit task-appropriate postural responses of the lower limb either at the same time as or slightly after upper limb corrections.
GRANTS
This work was supported by the Ontario Research Fund—Research Excellence Grant. C. R. Lowrey was supported by a salary award from the Canadian Institutes of Health Research (CIHR). S. H. Scott was supported by a GSK-CIHR chair in Neuroscience.
DISCLOSURES
S. H. Scott is associated with BKIN Technologies, which commercializes the KINARM device used in this study. The remaining authors declare no competing financial interests.
AUTHOR CONTRIBUTIONS
C.R.L. and J.Y.N. performed experiments; C.R.L., J.Y.N., and S.H.S. analyzed data; C.R.L. and S.H.S. interpreted results of experiments; C.R.L. prepared figures; C.R.L. drafted manuscript; C.R.L., J.Y.N., and S.H.S. edited and revised manuscript; C.R.L., J.Y.N., and S.H.S. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Kim Moore and Justin Peterson for their expert technical support and members of the LIMB Lab for their helpful comments and feedback on this work.
REFERENCES
- Aruin AS, Latash ML. Directional specificity of postural muscles in feed-forward postural reactions during fast voluntary arm movements. Exp Brain Res 103: 323–332, 1995. doi: 10.1007/BF00231718. [DOI] [PubMed] [Google Scholar]
- Bouisset S, Richardson J, Zattara M. Are amplitude and duration of anticipatory postural adjustments identically scaled to focal movement parameters in humans? Neurosci Lett 278: 153–156, 2000. doi: 10.1016/S0304-3940(99)00912-X. [DOI] [PubMed] [Google Scholar]
- Bouisset S, Zattara M. Biomechanical study of the programming of anticipatory postural adjustments associated with voluntary movement. J Biomech 20: 735–742, 1987. doi: 10.1016/0021-9290(87)90052-2. [DOI] [PubMed] [Google Scholar]
- Brenner E, Smeets JB. Fast corrections of movements with a computer mouse. Spat Vis 16: 365–376, 2003. doi: 10.1163/156856803322467581. [DOI] [PubMed] [Google Scholar]
- Byblow WD, Coxon JP, Stinear CM, Fleming MK, Williams G, Müller JF, Ziemann U. Functional connectivity between secondary and primary motor areas underlying hand-foot coordination. J Neurophysiol 98: 414–422, 2007. doi: 10.1152/jn.00325.2007. [DOI] [PubMed] [Google Scholar]
- Capaday C, Forget R, Milner T. A re-examination of the effects of instruction on the long-latency stretch reflex response of the flexor pollicis longus muscle. Exp Brain Res 100: 515–521, 1994. doi: 10.1007/BF02738411. [DOI] [PubMed] [Google Scholar]
- Cordo PJ, Nashner LM. Properties of postural adjustments associated with rapid arm movements. J Neurophysiol 47: 287–302, 1982. [DOI] [PubMed] [Google Scholar]
- Crevecoeur F, Kurtzer I, Bourke T, Scott SH. Feedback responses rapidly scale with the urgency to correct for external perturbations. J Neurophysiol 110: 1323–1332, 2013. doi: 10.1152/jn.00216.2013. [DOI] [PubMed] [Google Scholar]
- Day BL, Lyon IN. Voluntary modification of automatic arm movements evoked by motion of a visual target. Exp Brain Res 130: 159–168, 2000. doi: 10.1007/s002219900218. [DOI] [PubMed] [Google Scholar]
- Debaere F, Swinnen SP, Béatse E, Sunaert S, Van Hecke P, Duysens J. Brain areas involved in interlimb coordination: a distributed network. Neuroimage 14: 947–958, 2001. doi: 10.1006/nimg.2001.0892. [DOI] [PubMed] [Google Scholar]
- Diener HC, Dichgans J, Bootz F, Bacher M. Early stabilization of human posture after a sudden disturbance: influence of rate and amplitude of displacement. Exp Brain Res 56: 126–134, 1984. doi: 10.1007/BF00237448. [DOI] [PubMed] [Google Scholar]
- Dimitriou M, Wolpert DM, Franklin DW. The temporal evolution of feedback gains rapidly update to task demands. J Neurosci 33: 10898–10909, 2013. doi: 10.1523/JNEUROSCI.5669-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evarts EV, Tanji J. Reflex and intended responses in motor cortex pyramidal tract neurons of monkey. J Neurophysiol 39: 1069–1080, 1976. [DOI] [PubMed] [Google Scholar]
- Forero J, Misiaszek JE. Balance-corrective responses to unexpected perturbations at the arms during treadmill walking. J Neurophysiol 112: 1790–1800, 2014. doi: 10.1152/jn.00719.2013. [DOI] [PubMed] [Google Scholar]
- Gage WH, Zabjek KF, Hill SW, McIlroy WE. Parallels in control of voluntary and perturbation-evoked reach-to-grasp movements: EMG and kinematics. Exp Brain Res 181: 627–637, 2007. doi: 10.1007/s00221-007-0959-3. [DOI] [PubMed] [Google Scholar]
- Green DM, Swets JA. Signal Detection Theory and Psychophysics. Los Altos Hills, CA: Peninsula, 1989. [Google Scholar]
- Hagbarth KE. EMG studies of stretch reflexes in man. Electroencephalogr Clin Neurophysiol 22: 25, 1967. [PubMed] [Google Scholar]
- Horak FB, Esselman P, Anderson ME, Lynch MK. The effects of movement velocity, mass displaced, and task certainty on associated postural adjustments made by normal and hemiplegic individuals. J Neurol Neurosurg Psychiatry 47: 1020–1028, 1984. doi: 10.1136/jnnp.47.9.1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horak FB, Nashner LM. Central programming of postural movements: adaptation to altered support-surface configurations. J Neurophysiol 55: 1369–1381, 1986. [DOI] [PubMed] [Google Scholar]
- Kurtzer I, Pruszynski JA, Scott SH. Long-latency responses during reaching account for the mechanical interaction between the shoulder and elbow joints. J Neurophysiol 102: 3004–3015, 2009. doi: 10.1152/jn.00453.2009. [DOI] [PubMed] [Google Scholar]
- Leonard JA, Brown RH, Stapley PJ. Reaching to multiple targets when standing: the spatial organization of feedforward postural adjustments. J Neurophysiol 101: 2120–2133, 2009. doi: 10.1152/jn.91135.2008. [DOI] [PubMed] [Google Scholar]
- Leonard JA, Gritsenko V, Ouckama R, Stapley PJ. Postural adjustments for online corrections of arm movements in standing humans. J Neurophysiol 105: 2375–2388, 2011. doi: 10.1152/jn.00944.2010. [DOI] [PubMed] [Google Scholar]
- Luccarini P, Gahery Y, Pompeiano O. Cholinoceptive pontine reticular structures modify the postural adjustments during the limb movements induced by cortical stimulation. Arch Ital Biol 128: 19–45, 1990. [PubMed] [Google Scholar]
- MacKinnon CD, Bissig D, Chiusano J, Miller E, Rudnick L, Jager C, Zhang Y, Mille ML, Rogers MW. Preparation of anticipatory postural adjustments prior to stepping. J Neurophysiol 97: 4368–4379, 2007. doi: 10.1152/jn.01136.2006. [DOI] [PubMed] [Google Scholar]
- Marsden CD, Merton PA, Morton HB. Stretch reflex and servo action in a variety of human muscles. J Physiol 259: 531–560, 1976. doi: 10.1113/jphysiol.1976.sp011481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsden CD, Merton PA, Morton HB. Human postural responses. Brain 104: 513–534, 1981. doi: 10.1093/brain/104.3.513. [DOI] [PubMed] [Google Scholar]
- Marsden CD, Merton PA, Morton HB. Rapid postural reactions to mechanical displacement of the hand in man. Adv Neurol 39: 645–659, 1983. [PubMed] [Google Scholar]
- Massion J. Movement, posture and equilibrium: interaction and coordination. Prog Neurobiol 38: 35–56, 1992. doi: 10.1016/0301-0082(92)90034-C. [DOI] [PubMed] [Google Scholar]
- Matthews PB. Observations on the automatic compensation of reflex gain on varying the pre-existing level of motor discharge in man. J Physiol 374: 73–90, 1986. doi: 10.1113/jphysiol.1986.sp016066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagawa K, Kawashima S, Mizuguchi N, Kanosue K. Difference in activity in the supplementary motor area depending on limb combination of hand-foot coordinated movements. Front Hum Neurosci 10: 499, 2016. doi: 10.3389/fnhum.2016.00499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nardone A, Schieppati M. Postural adjustments associated with voluntary contraction of leg muscles in standing man. Exp Brain Res 69: 469–480, 1988. doi: 10.1007/BF00247301. [DOI] [PubMed] [Google Scholar]
- Nashed JY, Crevecoeur F, Scott SH. Influence of the behavioral goal and environmental obstacles on rapid feedback responses. J Neurophysiol 108: 999–1009, 2012. doi: 10.1152/jn.01089.2011. [DOI] [PubMed] [Google Scholar]
- Nashed JY, Crevecoeur F, Scott SH. Rapid online selection between multiple motor plans. J Neurosci 34: 1769–1780, 2014. doi: 10.1523/JNEUROSCI.3063-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nashner LM. Adapting reflexes controlling the human posture. Exp Brain Res 26: 59–72, 1976. doi: 10.1007/BF00235249. [DOI] [PubMed] [Google Scholar]
- Omrani M, Murnaghan CD, Pruszynski JA, Scott SH. Distributed task-specific processing of somatosensory feedback for voluntary motor control. Elife 5: e13141, 2016. doi: 10.7554/eLife.13141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orban de Xivry JJ. Trial-to-trial reoptimization of motor behavior due to changes in task demands is limited. PLoS One 8: e66013, 2013. doi: 10.1371/journal.pone.0066013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen N, Christensen LO, Morita H, Sinkjaer T, Nielsen J. Evidence that a transcortical pathway contributes to stretch reflexes in the tibialis anterior muscle in man. J Physiol 512: 267–276, 1998. doi: 10.1111/j.1469-7793.1998.267bf.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pruszynski JA, Johansson RS, Flanagan JR. A rapid tactile-motor reflex automatically guides reaching toward handheld objects. Curr Biol 26: 788–792, 2016. doi: 10.1016/j.cub.2016.01.027. [DOI] [PubMed] [Google Scholar]
- Pruszynski JA, Kurtzer I, Lillicrap TP, Scott SH. Temporal evolution of “automatic gain-scaling”. J Neurophysiol 102: 992–1003, 2009. doi: 10.1152/jn.00085.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pruszynski JA, Kurtzer I, Nashed JY, Omrani M, Brouwer B, Scott SH. Primary motor cortex underlies multi-joint integration for fast feedback control. Nature 478: 387–390, 2011. doi: 10.1038/nature10436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pruszynski JA, Kurtzer I, Scott SH. Rapid motor responses are appropriately tuned to the metrics of a visuospatial task. J Neurophysiol 100: 224–238, 2008. doi: 10.1152/jn.90262.2008. [DOI] [PubMed] [Google Scholar]
- Pruszynski JA, Omrani M, Scott SH. Goal-dependent modulation of fast feedback responses in primary motor cortex. J Neurosci 34: 4608–4617, 2014. doi: 10.1523/JNEUROSCI.4520-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothwell JC, Traub MM, Marsden CD. Influence of voluntary intent on the human long-latency stretch reflex. Nature 286: 496–498, 1980. doi: 10.1038/286496a0. [DOI] [PubMed] [Google Scholar]
- Safavynia SA, Ting LH. Long-latency muscle activity reflects continuous, delayed sensorimotor feedback of task-level and not joint-level error. J Neurophysiol 110: 1278–1290, 2013. doi: 10.1152/jn.00609.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schepens B, Drew T. Descending signals from the pontomedullary reticular formation are bilateral, asymmetric, and gated during reaching movements in the cat. J Neurophysiol 96: 2229–2252, 2006. doi: 10.1152/jn.00342.2006. [DOI] [PubMed] [Google Scholar]
- Scott SH. A Functional taxonomy of bottom-up sensory feedback processing for motor actions. Trends Neurosci 39: 512–526, 2016. doi: 10.1016/j.tins.2016.06.001. [DOI] [PubMed] [Google Scholar]
- Scott SH, Cluff T, Lowrey CR, Takei T. Feedback control during voluntary motor actions. Curr Opin Neurobiol 33: 85–94, 2015. doi: 10.1016/j.conb.2015.03.006. [DOI] [PubMed] [Google Scholar]
- Stein RB, Hunter IW, Lafontaine SR, Jones LA. Analysis of short-latency reflexes in human elbow flexor muscles. J Neurophysiol 73: 1900–1911, 1995. [DOI] [PubMed] [Google Scholar]
- Welch TD, Ting LH. A feedback model reproduces muscle activity during human postural responses to support-surface translations. J Neurophysiol 99: 1032–1038, 2008. doi: 10.1152/jn.01110.2007. [DOI] [PubMed] [Google Scholar]
- Wing AM, Flanagan JR, Richardson J. Anticipatory postural adjustments in stance and grip. Exp Brain Res 116: 122–130, 1997. doi: 10.1007/PL00005732. [DOI] [PubMed] [Google Scholar]
- Winter DA, Prince F, Frank JS, Powell C, Zabjek KF. Unified theory regarding A/P and M/L balance in quiet stance. J Neurophysiol 75: 2334–2343, 1996. [DOI] [PubMed] [Google Scholar]
- Yakovenko S, Drew T. A motor cortical contribution to the anticipatory postural adjustments that precede reaching in the cat. J Neurophysiol 102: 853–874, 2009. doi: 10.1152/jn.00042.2009. [DOI] [PubMed] [Google Scholar]