Summary
Could improving the design of preclinical murine atherosclerosis studies help increase the success rate of cardiovascular clinical trials? In this Viewpoint, the authors advocate for a change from prevention to intervention study designs and rigorous lesion analyses which they argue will enhance the translational potential of murine atherosclerosis studies.
Keywords: Atherosclerosis, Translational Research, Study Design, Intervention Model
Clinical management of patients with Coronary Artery Disease (CAD) has made great progress by reducing risk factors like LDL cholesterol, hypertension, and diabetes. However, late-stage events associated with coronary atherosclerosis including myocardial infarction still account for nearly 16% of worldwide mortality and are forecasted to increase in prevalence.1 These events arise from three main processes – plaque rupture, plaque erosion, and shedding of calcific nodules. Plaque rupture accounts for the majority of CAD and typically occurs in vulnerable atherosclerotic lesions termed thin-capped fibroatheromas (TCFA),2 characterized by thin fibrous caps (<65 um) with high numbers of CD68+ macrophages relative to Acta2+ smooth muscle cells (SMC), large lipid-rich necrotic cores, and often evidence of intraplaque hemorrhage. Much of the difficulty in therapeutically targeting atherosclerosis can be attributed to: 1) the nearly ubiquitous prevalence of atherosclerosis development; 2) the slow, clinically-silent progression but acute and often catastrophic presentation of symptoms; and 3) most importantly, the difficulty in identifying patients at high-risk for plaque rupture or erosion and subsequent cardiovascular events. Therefore, clinical trials seeking to investigate novel CAD therapies are forced to enroll thousands of patients who already have manifestations of advanced CAD and spend hundreds of millions of dollars to conduct adequately powered phase III clinical trials to rigorously test therapeutic efficacy. And yet, cardiovascular disease trials have one of the lowest success rates of the major medical fields.3 Perhaps the lack of success can, in part, be attributed to suboptimal design of preclinical studies, which commonly study prevention of atherosclerosis development rather than interventional models and analyze simple parameters like lesion size and luminal lipid deposition to assess treatment effect. Although no animal models can perfectly recapitulate human disease, we believe there needs to be a greater emphasis on preclinical murine studies that better mimic therapeutic intervention in humans, rather than short-term prevention studies which may ultimately lead to inaccurate or inefficient translation in clinical trials. In this Viewpoint, we will discuss possible strategies for study design and lesion analysis that we, as basic scientists, should consider implementing to help improve the chance of successful translation of our research into clinical practice.
Perhaps some of the field’s collective reticence to studying late-stage murine lesions has been the controversy surrounding the occurrence of spontaneous plaque rupture. Despite the general assumption that plaque rupture is absent in murine atherosclerosis, several groups using rigorous post-mortem analyses have documented histological features of spontaneous plaque rupture in aged, hypercholesterolemic, genetically-engineered mice with or without high-fat diet feeding.4,5 But murine experimental models appear to share a similar challenge with CAD clinical trials, a low frequency of events. To overcome this challenge, several groups have attempted to induce plaque rupture in mice. These models employ extraordinary measures like mechanical disruption6, vascular casting7, and adenoviral delivery of p538 to induce rupture at a rate that allows for the use of a reasonable number of mice, but unlikely to reflect mechanisms that cause spontaneous plaque rupture in humans. Importantly, despite the lack of a reliable rupture model in mice, many of the cellular processes that are thought to control the transition from stable fibroatheroma to TCFA in humans (e.g., the accumulation of macrophages and T cells, the production of matrix-remodeling proteinases, the investment of contractile protein-expressing SMC in the fibrous cap, changes in the lipid and collagen content, cross-linking of extracellular matrix [ECM] components) do occur in murine atherosclerosis.
However, many preclinical studies limit their analysis to plaque burden as determined by the cross sectional area of isolated lesions in the aortic root or brachiocephalic artery and/or en face analysis of lipid deposition, which is unable to distinguish fatty streaks from more advanced lesions. Furthermore, it has become increasingly clear that lesion size is not a suitable surrogate for lesion stability, which is more accurately the integral of the stabilizing and destabilizing processes at work within the lesion. Therefore, novel therapeutics should seek to modulate lesion stability by either inhibiting processes involved in destabilization (e.g., lipid lowering with statins or PCSK9 inhibitors) or inducing those that augment stability (e.g., enhancing the production of protective ECM components within the fibrous cap). Although the ECM-producing cells within the fibrous cap have been assumed to be primarily SMC-derived, only recently have rigorous lineage-tracing studies from our lab9,10 and others11 provided compelling evidence that SMC play a critical role in lesion pathogenesis. However, contrary to dogma, they may not always perform beneficial roles. By employing SMC lineage-tracing Myh11 ERT2Cre eYFP ApoE−/− mice fed 18 weeks of Western-diet (WD) to induce advanced atherosclerotic lesions, we have shown that: 1) >80% of SMC-derived cells lack detectable Acta2 expression; and 2) >30% of Lgals3+ cells are SMC-derived in both mouse and human lesions.9 By combining lineage-tracing with simultaneous SMC-specific gene knockout (KO) of pluripotency factors Klf4 or Oct4, we have shown that SMC can play a critical role in the pathogenesis of late-stage lesions, which can be either atheroprotective or atheropromoting depending on the nature of their phenotypic transitions. For example, Klf4-dependent transitions, including formation of SMC-derived macrophage-marker+ foam cells,9 exacerbated lesion pathogenesis whereas Oct4-dependent transitions were atheroprotective, being required for migration and stable investment of SMC into the fibrous cap.10 Taken together, these results highlight the critical importance of identifying factors and mechanisms that promote plaque stabilizing “atheroprotective changes” within SMC and other major cell types within lesions, and therapeutic approaches that can induce such changes. Findings also highlight the importance of lineage tracing in atherosclerosis. Due to significant plasticity in multiple lesion cell types, there is substantial ambiguity in cell identification when using traditional markers (e.g., Acta2, CD68, CD31), which can only be overcome by the use of rigorous lineage tracing techniques.
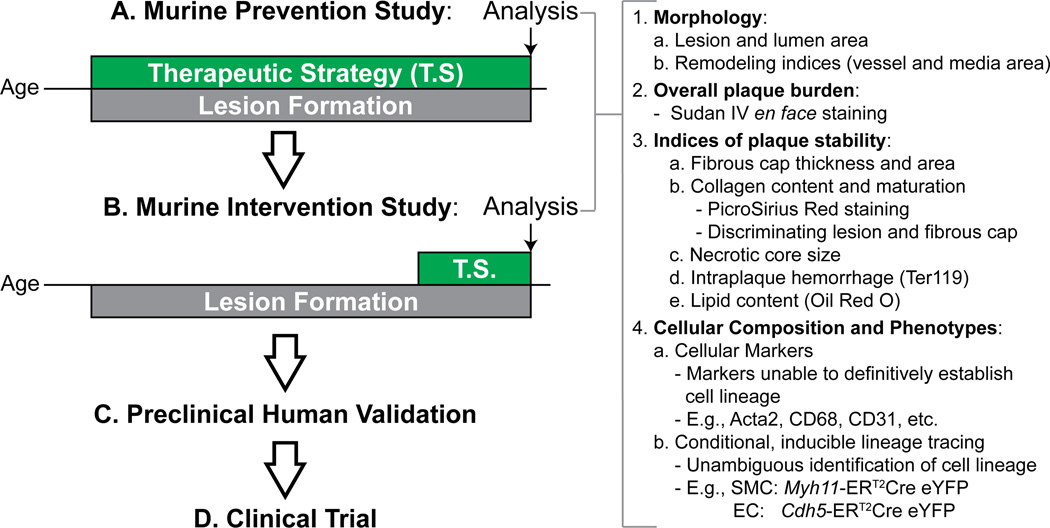
In addition to studying more relevant pathologic parameters, scientists should consider implementing more relevant study designs. Currently, the overwhelming majority of atherosclerosis studies implement models of prevention. Namely, the researcher deletes gene x or provides drug y prior to – or in the very early stages of – lesion formation, and therefore the therapeutic agent or genetic manipulation is given to young, healthy mice and is present throughout lesion development (Figure 1). With these prevention models, we have learned a great deal about the steps of atherosclerosis development – determining the key cellular players and identifying thousands of genes that can alter lesion accumulation – but unfortunately this has translated to very few novel therapies. Therefore, although prevention models may be suitable for assessing therapeutic feasibility, we advocate for additional interventional studies prior to initiation of clinical trials. Here, mice are treated with the therapy or gene knockout only after they have established advanced atherosclerosis. By providing the therapy at this stage and then assessing for changes in the indices of lesion stability as described above, we obtain a better prediction of how the intervention will impact the processes regulating late-stage lesion vulnerability and, hopefully, gain better insight into its impact on advanced human lesions. Clearly, different processes are at work during lesion development versus late-stage disease. For example, elegant studies from Filip Swirski’s group12 have shown that monocyte recruitment drives early lesion macrophage accumulation but that as the lesion matures the accumulation becomes a function of local macrophage proliferation. These fundamental biological differences between disease stages may indeed provide one possible explanation for the poor translation of prevention studies to patients with advanced disease. It should be noted that idea of implementing interventional models to the study of atherosclerosis is not novel. Indeed, there are many excellent examples in the literature using both pharmacologic (e.g., delivering collagen IV-targeted nanoparticles containing pro-resolving peptide, Ac2–26)13 and genetic (e.g., SMC-specific KO of Akt1 after 16 weeks of WD)14 interventions validating its importance to the field – but we feel that it remains heavily underutilized. Another study design that has been used to study the processes critical in late-stage atherosclerosis are regression models. These are variations of the intervention model in which atherosclerosis is induced by chronic hypercholesterolemia but then regressed by either normalizing specific lipid components or transplanting diseased vessels into healthy organisms. These models have been helpful to identify of processes that may be reversible in advanced atherosclerosis like CCR7-mediated macrophage egress15 or reverse cholesterol transport and may prove valuable when investigating strategies to encourage these processes.
Figure 1. Proposed modifications to preclinical pipeline for experimental atherosclerosis studies.
Prevention Studies may represent a good proof-of-principle for a novel therapeutic or gene knockout but in the setting of atherosclerosis may not translate to older patients with established disease (A). Instead, we should implement Intervention Studies, which we argue will better predict the effect of a therapeutic strategy for treating humans with advanced atherosclerotic lesions. Both of these approaches should analyze parameters that not only provide information about lesion size but also investigate multiple key cellular processes implicated in lesion vulnerability in humans (B). In addition, it is critical to identify innovative ways for validating results in preclinical animal studies to the extent possible including use of classic immunohistological analyses of human autopsy specimens as well as large-scale genomic approaches (C). Of course, the final validation of new CVD therapies will require well designed Clinical Trials (D).
In summary, we hope to motivate investigators to reevaluate the way we apply mouse models to the study of atherosclerosis. With the residual risk for atherosclerotic complications that remains despite current standard of care, there exists a critical need for therapies that not only focus on limiting destabilization but also seek to promote better inflammatory resolution, healing, and overall plaque stabilization. We believe that the first step toward achieving this goal is to begin studying potential therapeutics using interventional models in mice and to rigorously characterize their effect on processes critical in maintaining lesion stability. While no animal model completely recapitulates human disease we can and need to do a better job of matching our experimental animal model designs to the unmet clinical needs.
Supplementary Material
Acknowledgments
Sources of funding: This work was supported by R01 grants HL057353, HL098538, and HL087867 to G.K. Owens, and the American Heart Association 15SDG25860021 for D. Gomez.
Footnotes
Disclosure: None.
References
- 1.GBD 2015 DALYs and HALE Collaborators. Global, regional, and national disability-adjusted life-years (DALYs) for 315 diseases and injuries and healthy life expectancy (HALE), 1990–2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet. 2016;388:1603–1658. doi: 10.1016/S0140-6736(16)31460-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kolodgie FD, Virmani R, Burke AP, Farb A, Weber DK, Kutys R, Finn AV, Gold HK. Pathologic assessment of the vulnerable human coronary plaque. Heart. 2004;90:1385–1391. doi: 10.1136/hrt.2004.041798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hay M, Thomas DW, Craighead JL, Economides C, Rosenthal J. Clinical development success rates for investigational drugs. Nat Biotechnol. 2014;32:40–51. doi: 10.1038/nbt.2786. [DOI] [PubMed] [Google Scholar]
- 4.Johnson JL, Jackson CL. Atherosclerotic plaque rupture in the apolipoprotein E knockout mouse. Atherosclerosis. 2001;154:399–406. doi: 10.1016/s0021-9150(00)00515-3. [DOI] [PubMed] [Google Scholar]
- 5.Calara F, Silvestre M, Casanada F, Yuan N, Napoli C, Palinski W. Spontaneous plaque rupture and secondary thrombosis in apolipoprotein E-deficient and LDL receptor-deficient mice. J Pathol. 2001;195:257–263. doi: 10.1002/path.915. [DOI] [PubMed] [Google Scholar]
- 6.Bentzon JF, Sondergaard CS, Kassem M, Falk E. Smooth muscle cells healing atherosclerotic plaque disruptions are of local, not blood, origin in apolipoprotein E knockout mice. Circulation. 2007;116:2053–2061. doi: 10.1161/CIRCULATIONAHA.107.722355. [DOI] [PubMed] [Google Scholar]
- 7.Sasaki T, Kuzuya M, Nakamura K, Cheng XW, Shibata T, Sato K, Iguchi A. A simple method of plaque rupture induction in apolipoprotein E-deficient mice. Arterioscler Thromb Vasc Biol. 2006;26:1304–1309. doi: 10.1161/01.ATV.0000219687.71607.f7. [DOI] [PubMed] [Google Scholar]
- 8.Thusen von der JH. Induction of Atherosclerotic Plaque Rupture in Apolipoprotein E−/− Mice After Adenovirus-Mediated Transfer of p53. Circulation. 2002;105:2064–2070. doi: 10.1161/01.cir.0000015502.97828.93. [DOI] [PubMed] [Google Scholar]
- 9.Shankman LS, Gomez D, Cherepanova OA, Salmon M, Alencar GF, Haskins RM, Swiatlowska P, Newman AAC, Greene ES, Straub AC, Isakson B, Randolph GJ, Owens GK. KLF4-dependent phenotypic modulation of smooth muscle cells has a key role in atherosclerotic plaque pathogenesis. Nat Med. 2015;21:628–637. doi: 10.1038/nm.3866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cherepanova OA, Gomez D, Shankman LS, Swiatlowska P, Williams J, Sarmento OF, Alencar GF, Hess DL, Bevard MH, Greene ES, Murgai M, Turner SD, Geng Y-J, Bekiranov S, Connelly JJ, Tomilin A, Owens GK. Activation of the pluripotency factor OCT4 in smooth muscle cells is atheroprotective. Nat Med. 2016;22:657–665. doi: 10.1038/nm.4109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Feil S, Fehrenbacher B, Lukowski R, Essmann F, Schulze-Osthoff K, Schaller M, Feil R. Transdifferentiation of vascular smooth muscle cells to macrophage-like cells during atherogenesis. Circ Res. 2014;115:662–667. doi: 10.1161/CIRCRESAHA.115.304634. [DOI] [PubMed] [Google Scholar]
- 12.Robbins CS, Hilgendorf I, Weber GF, Theurl I, Iwamoto Y, Figueiredo J-L, Gorbatov R, Sukhova GK, Gerhardt LMS, Smyth D, Zavitz CCJ, Shikatani EA, Parsons M, van Rooijen N, Lin HY, Husain M, Libby P, Nahrendorf M, Weissleder R, Swirski FK. Local proliferation dominates lesional macrophage accumulation in atherosclerosis. Nat Med. 2013;19:1166–1172. doi: 10.1038/nm.3258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fredman G, Kamaly N, Spolitu S, Milton J, Ghorpade D, Chiasson R, Kuriakose G, Perretti M, Farokhzad O, Farokzhad O, Tabas I. Targeted nanoparticles containing the proresolving peptide Ac2–26 protect against advanced atherosclerosis in hypercholesterolemic mice. Sci Transl Med. 2015;7:275ra20–275ra20. doi: 10.1126/scitranslmed.aaa1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rotllan N, Wanschel AC, Fernandez-Hernando A, Salerno AG, Offermanns S, Sessa WC, Fernandez-Hernando C. Genetic Evidence Supports a Major Role for Akt1 in VSMCs During Atherogenesis. Circ Res. 2015;116:1744–1752. doi: 10.1161/CIRCRESAHA.116.305895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Feig JE, Shang Y, Rotllan N, Vengrenyuk Y, Wu C, Shamir R, Torra IP, Fernandez-Hernando C, Fisher EA, Garabedian MJ. Statins promote the regression of atherosclerosis via activation of the CCR7-dependent emigration pathway in macrophages. PLoS ONE. 2011;6:e28534. doi: 10.1371/journal.pone.0028534. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.