Summary
Cancers are able to grow by subverting immune suppressive pathways, to prevent the malignant cells as being recognized as dangerous or foreign. This mechanism prevents the cancer from being eliminated by the immune system and allows disease to progress from a very early stage to a lethal state. Immunotherapies are newly developing interventions that modify the patient’s immune system to fight cancer, by either directly stimulating rejection-type processes or by blocking suppressive pathways. Extracellular adenosine generated by the ectonucleotidases CD39 and CD73 is a newly recognized “immune checkpoint mediator” that interferes with anti-tumor immune responses. In this review, we focus on CD39 and CD73 ectoenzymes and encompass aspects of the biochemistry of these molecules as well as detailing the distribution and function on immune cells. Effects of CD39 and CD73 inhibition in preclinical and clinical studies are discussed. Finally, we provide insights into potential clinical application of adenosinergic and other purinergic-targeting therapies and forecast how these might develop in combination with other anti-cancer modalities.
Keywords: T Cells, Cancer Inflammation, Tumor, Immunity, Immunotherapies Monocytes/Macrophages
Introduction
A major metabolite on which all life depends is adenosine triphosphate or ATP, which is well known and serves as the energy currency of the cell. This biochemical substance can be released at high levels from malignant cells, particularly in setting of damage from radiotherapy or chemotherapy. Extracellular ATP provokes inflammation by “purinergic signals” and plays a significant role in promoting anti-tumor responses. Conversely, hydrolysis of extracellular ATP by membrane-bound ectonucleotidases generates immunosuppressive adenosine, thus acting as a negative-feedback mechanism to prevent excessive inflammation and tissue damage.
Our colleagues, and we, have shown that tumors are proficient at converting ATP into adenosine, through the expression of the ectonucleotidases CD39 and CD73 on cancer cells, regulatory immune cells and the vasculature. These two ectonucleotidases, and others not discussed here, modulate purinergic signaling by scavenging largely proinflammatory, extracellular nucleotide mediators to generate immunosuppressive adenosine nucleosides. We hereafter detail the function of CD39 and CD73 in the context of tumorigenesis, discuss recent findings in experimental models and review the development of adenosine-targeting therapies in oncology.
Extracellular ATP release
Under normal physiological conditions, ATP is localized in the intracellular compartment, where concentrations vary from 1 to 10 mM (1), and is only present at negligible levels (10–100 nM) in the extracellular environment. In addition of its metabolic function, ATP also serves as an important extracellular signaling molecule (eATP) that triggers and then modulates several pathological effects in the settings of thrombosis and inflammation, such as chemotaxis (2), inflammasome activation (3) and platelet activation (4).
Importantly, eATP levels rise significantly in response to tissue-disturbing events, including inflammation, hypoxia or ischemia as well as in the setting of malignancy (5, 6). Regulated ATP release may occur independently of membrane damage and may be mediated through ATP binding cassette (ABC) transporters, vesicular release or channel-dependent mechanisms, these latter including pannexins (Panx) or hemichannel connexins (Conx) (6).
Of the Panx isoforms that have been identified so far, Panx1 has been the most studied, having an important role in the regulation of the blood flow, cell apoptosis and inflammasome activation (7, 8). Amongst the Conx hemichannels, Conx37 and Conx44 mediate ATP release from monocytes, neutrophils and endothelial cells during inflammatory conditions (9, 10). Release of ATP and ADP during inflammatory processes occurs also from platelet dense granules through an exocytosis-mediated mechanism (11).
Purinergic type-2 receptor signaling by ATP
Once in the extracellular milieu ATP mediates inflammatory effects upon binding to cell surface type 2 (P2) purinergic receptors (12); namely ligand-gated ion channel receptors (P2XR) and G-protein coupled receptors (P2YR). There are seven P2XR and eight P2YR, which are virtually expressed on all mammalian cells (13). Activation of these receptors can mediate acute processes that may influence metabolism, adhesion, activation or cell migration; or more protracted reactions, like those observed during chronic inflammatory responses (13).
By favoring transmembrane ion fluxes, P2XR are determinant in regulating intracellular ion concentrations, whereas P2YR control Ca2+ mobilization in immune cells, decrease the immunosuppressive effects of cAMP and, in the case of P2Y2 and P2Y14, promote phagocyte migration and activation (2, 14). In contrast to ionotropic P2XR that can be bound by ATP only, metabotropic P2YR can be also activated upon binding to other nucleotides such as UTP (P2Y2R and P2Y4R and to a lesser extent P2Y6R and P2Y11R), ADP (P2Y1R, P2Y11R and P2Y13R), UDP (P2Y6R), NADP (P2Y11R) and UDP glucose (P2Y14R).
Engagement of P2XR and P2YR results in the modulation of a wide range of innate and adaptive immune responses. Notably, while P2XR and P2YR can be present on monocytes and dendritic cells (DCs), lymphocytes express only P2XR. For instance, engagement of P2Y2R, P2Y6R and P2X7R results in activation of myeloid cells and induces chemotaxis in macrophages (2, 15, 16), activation of P2Y2R and P2X7R promotes DC activation and chemotaxis (17), and activation of P2Y11R, inhibits IL-12 and promotes IL-10 release by DCs (18) and has been linked to activation of granulocytes (19). When considering adaptive immunity, T cell activation has been found to result upon ATP binding to a number of P2XR, including P2X1R, P2X4R, P2X5R and P2X7R (20–23). P2X7R has been specifically associated with activation of both CD4 and CD8 effectors (21, 24, 25), iNKT cells (26), induction of apoptosis by Tregs (27, 28) and inhibition of Tr1 cell differentiation, as recently reported (29). While high ATP levels are associated with increased T cell effector function (25), low ATP doses play a trophic effect in the tumor microenvironment and may be associated with tumor growth promotion (30, 31) and metastatic spread (32).
Scavenging of eATP or other nucleotides and generation of adenosine and/or respective nucleosides
ATP and other nucleotides are rapidly hydrolyzed to generate adenosine monophosphate (AMP) and ultimately adenosine, which has opposing effects to those mediated through the P2 receptor signaling (33, 34). Extracellular nucleotide hydrolysis is governed by several families of ectonucleotidases (34), including the ecto-nucleoside triphosphate diphosphohydrolase (ENTPDases/genes are ENTPD), the prototype of this class of enzymes; the ecto-nucleotide pyrophosphate phosphodiesterases (E-NPPs), the NAD glycohydrolases; the CD38/NADase; alkaline phosphatases; adenylate kinase; the nucleoside diphosphate kinase; and the ecto-F1-Fo ATP synthases (35).
Amongst NTPDases, four (NTPDase 1,2,3 and 8) are located on the cell surface and exhibit a catalytic site facing the extracellular space; NTPDase 5 and 6 are located inside the cells and are secreted upon heterologous expression; NTPDase 4 and 7 are intracellularly located and they face the lumen of cytoplasmic organelles. These NTPDases differ with regard to their catalytic properties. While NTPDase 1 hydrolyzes ATP and ADP, NTPDase 3 and 8 have ATP as a preferential substrate (36) and NTPDase 2 hydrolyzes ATP only, being therefore classified as an ecto-ATPase (36). The prototype member of the NTPDase family is CD39, the rate-limiting enzyme in the cascade that catalyzes ATP and ADP into AMP, which is subsequently converted into adenosine by ecto-5’-nucleotidase, also known as CD73 (33). CD73-derived extracellular adenosine has a very short half-life (few seconds). It can be catabolized to inosine by membrane-bound adenosine deaminase, recaptured by equilibrative and concentrative nucleoside transporters or activate P1 purinergic receptors (33).
Adenosine receptor/type 1 purinergic receptor signaling
Extracellular adenosine can activate 4 distinct P1 purinergic receptors, namely A1, A2a, A2b and A3 adenosine receptors (37). All four receptors belong to the class A (rhodopsin-like) G protein-coupled receptor (GPCR) superfamily. A1, A2a and A2b protein sequences are highly conserved across mammalian species (over 80% identity) while A3 is more variable. Within the same species, homology between the different receptors is below 60% (38). In human, A1, A2a and A3 are considered as high affinity receptors for adenosine (Ki ranging from 100 to 300 nM) while A2b receptor has a lower affinity for adenosine (~15 uM) (37, 38).
A1 and A3 receptors are preferentially coupled to Gi/o proteins, inhibiting adenylate cyclase and cyclic AMP production. On the other hand, A2a and A2b receptors are generally Gs-coupled receptors that trigger intracellular cAMP accumulation. cAMP accumulation can activate both the canonical protein kinase A (PKA) and the non-canonical EPAC pathways (39, 40). In contrast to A2a receptors, A2b receptors can also couple to Gq proteins and trigger the phospholipase C pathway (40, 41). All four adenosine receptors have further been shown to induce mitogen-activated protein kinase (MAPK) and JNK pathways (41).
Biochemistry of CD39 and CD73
CD39 is the prototype ENTPD and was the first of the eight NTPDase enzymes to be cloned and sequenced (42, 43). The first ectoenzymes were purified as soluble ATP diphosphohydrolase (apyrase), which was isolated from potato tubers (44); and ATP diphosphohydrolases from porcine pancreas and bovine aorta that were found to share sequence homology with CD39 cDNA, isolated from human endothelial cells. It was also demonstrated that this NTPDase inhibits platelet aggregation in response to ADP, collagen and thrombin and that CD39 mRNA was present in several tissues, including placenta, lung, skeletal muscle and heart (45). Different CD39 splicing products have been also identified (45).
As do all NTPDases, CD39 has five highly conserved sequence domains, known as the ‘apyrase conserved regions’, which are involved in active site formation and extracellular nucleotide catalysis by phosphohydrolysis. ENTPD also shares common sequence motifs as well as secondary and tertiary structure similarities with members of the actin/HSP70/sugar kinase superfamily (46). CD39 is anchored to the cell membrane via two transmembrane domains that are essential for maintaining the catalytic activity and the specificity for the substrate (47, 48). CD39 undergoes functional modifications, including limited proteolysis and glycosylation, the latter being determinant in conferring catalytic activity to CD39 (49). CD39 N-terminal intracytoplasmic domain undergoes palmitoylation to enable the enzyme association with the lipid rafts (50–52). Experiments carried out to interfere with cholesterol levels via drugs, which are either depleting or sequestering membrane cholesterol, have shown strong inhibition of the CD39 ecto-enzymatic activity (52).
CD73 is a GPI-anchored enzyme that hydrolyses AMP into adenosine and inorganic phosphate (33, 53). CD73 also exists as a soluble form upon shedding of the GPI anchor, with similar activity to its membrane-bound form (54, 55). From a structural point of view, CD73 is organized in 3 domains: (i) one N-terminal domain containing metal binding sites, (ii) one C-terminal domain containing the catalytic site and (iii) one short alpha helix bridging the N and C terminal domains. CD73 glycoforms with different molecular weights and enzymatic activities have been described (56, 57). Functional CD73 consists of a non-covalently linked homodimer stabilized by hydrophobic interactions between adjacent C-terminal domains. CD73 homodimerization, as well as the binding of two zinc ions, are required for catalytic activity. CD73 homodimers exist in two conformations: open and closed (58, 59). Hydrolysis of AMP requires that the enzyme cycles through open and closed conformational states.
Ectonucleotidases and immunomodulation
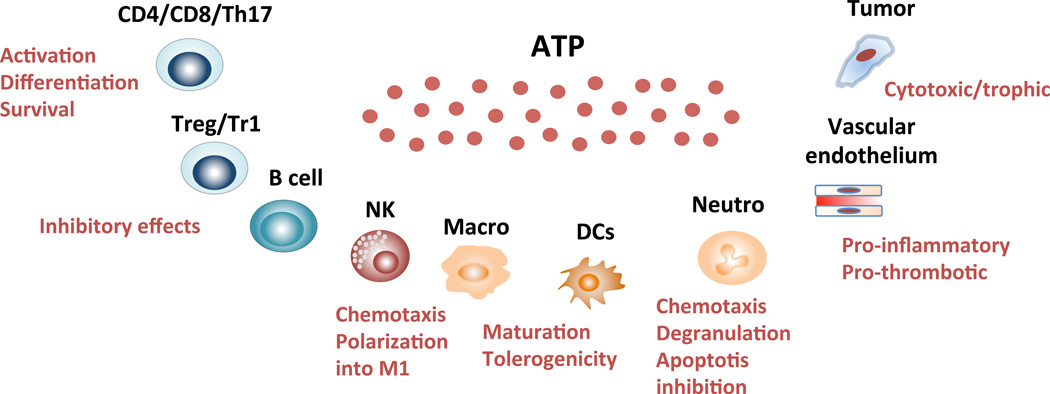
We hereafter review the functional properties of CD39 and CD73 on immune and vascular cells; with a focus on lymphoid, myeloid and endothelial cells.
In human peripheral blood, CD39 is constitutively expressed on >90% of B cells, >90% of monocytes, 20–30% of CD4+ T cells (including memory T cells and Tregs), <5% of CD8+ T cells and 2–5% of NK cells. Induction of CD39 is promoted upon exposure to pro-inflammatory cytokines (e.g. IL-6 and IL-27), oxidative stress and hypoxia, aryl hydrocarbon receptor (AhR) engagement by dietary compounds and endogenous ligands (Longhi, unpublished).
In human peripheral blood, CD73 is expressed on approximately 75% of B cells, 50% of CD8+ T cells, 10% of CD4+ T cells and 2–5% of NK cells (60). B cells, Tregs, Th17 cells, NK cells and myeloid-derived suppressor cells can co-express CD39 and CD73. As early as in 1977, reduced 5'-nucleotidase activity in blood lymphocytes was noted in patients with common variable immunodeficiency (CVI) and X-linked agamma-globulinemia (XLA) (60). Reduced CD73 expression in CVI and XLA patients is a consequence, rather than the cause, of defective lymphocyte development (60). Consistent with this notion, decreased levels of CD73 expression on lymphocytes has been documented in patients with HIV (61, 62) or infectious mononucleosis (63).
Effector T cells
Upon antigenic exposure T cells become activated as a result of a cascade of events involving the activation of the phospholipase C-ɣ, which generates inositol 1-4-5-triphosphate (IP3) and 1,2-diacylglycerol (DAG) from phosphatidylinositol 4,5 biphosphate (PIP2). This culminates in Ca2+ influx from the endoplasmic reticulum and subsequent re-uptake by the mitochondria and in ATP generation. In turn, ATP activates the mitogen-activated protein (MAP) kinase signaling that result in excessive T cell activation (64). Notably ATP released from activated T lymphocytes has been shown to have an autocrine effect through P2X7R (24, 25); interference with this pathway using oxidized ATP leads to diminished secretion of IL-2 and T cell proliferation (24) and is associated with amelioration of autoimmune type 1 diabetes and autoimmune encephalomyelitis in mice (65). Notably ATP release from activated T cells not only has an autocrine effect but also exerts paracrine effects on neighboring lymphocytes by inducing P2X7-P2X4-mediated Ca2+ waves and controlling their motility (66)
Within the T cell compartment, CD39 is chiefly expressed by CD4 lymphocytes, predominantly by the Treg subset (see below). There are, however, CD4+FOXP3− cells that also express CD39 and display a memory effector phenotype in humans (67). Polarization of naïve CD4 from CD39−/− mice to Th1 cells results in heightened IFNɣ levels that are, however, contained upon apyrase treatment. Apyrase treatment results also in the restoration of ectoenzymatic activity and adenosine generation (68). Induction of CD39 on CD4+ T cells has been reported being induced by age and contributing to impaired response to vaccines (69). Further, CD39+CD4+ T cells have been found to be prone to apoptosis and metabolic stress (69).
Earlier reports indicated CD39 as a marker for allo-sensitized CD8+ cytotoxic T cells distinct from CD8+CD39− cells that instead, mediate NK-like reactivity (70). CD3/CD28 stimulation of CD8 cells boosts the production of reactive oxygen species (ROS) by CD8 cells alongside the expression of CD39, the up-regulation of which is controlled by NADPH oxidase inhibition. Expression of CD39 in CD8 cells is instrumental in controlling IFNɣ production by CD8+CD39− cells through a mechanism requiring the engagement of A2A receptor (71). Expression of CD39 by cytotoxic CTLs has been also linked to the acquisition of immunosuppressive properties by these cells and regarded as a mechanism to keep the CTL expansion under control (72).
CD73 is expressed on a low proportion of circulating CD4+ T cells in healthy individuals, but this proportion significantly increases in patients with chronic inflammation, such as inflammatory bowel disease (IBD) (73). CD73+CD4+ T cells in IBD patients generally express CD45RO, indicative of a memory phenotype, and upon activation show a Th17 phenotype. Exogenous TNF-α (but not IFN-γ) dose-dependently increases CD73+CD4+ T cells in human PBMCs (62). CD73 is strongly induced by TGF-β on conventional CD4+ or CD8+ T cells (74). CD73 is also regulated by retinoic acid (75) and the active form of vitamin D (76).
Human peripheral CD8+ T cells expressing CD73 are essentially composed of naïve T cells, while a minority are memory CD8+ T cells (77). Upon activation, CD8+ T cells commonly downregulate CD73 expression, although this is not a universal phenomenon (62). In human solid tumors, CD73 is expressed at high levels on memory CD8+ T cells and is absent or low on terminally differentiated effector CD8+ T cells (78). It was proposed that CD73-derived adenosine and A2a regulate the transition from naïve/memory CD8+ T cells to effector cells by inhibition of Wnt signaling shutdown (78, 79).
Foxp3+ Tregs
CD4+CD25highFoxp3+ Tregs mediate immunotolerance by suppressing effector cell immunity. Tregs are highly sensitive to the effect of eATP that promotes death signaling through P2X7R engagement (28). ATP exerts an inhibitory role on the generation and function of these cells through activation of P2X7R and induces Treg conversion into effector Th17 cells (27).
Tregs mediate immunosuppression by a number of mechanisms, namely by modulating the function of antigen presenting cells (APCs), killing of target cells through granzyme and perforin, production and secretion of anti-inflammatory cytokines like IL-10 (80, 81), TGF-β (82, 83) and IL-35 (84), IL-2 deprivation from effector cells and metabolic disruption through ATP catalysis and ultimate generation of immunosuppressive adenosine (85). CD39 is constitutively expressed on murine Tregs in association with CD73 where it plays a central role in mediating immunosuppression (85). Therefore, Tregs from CD39−/− mice have impaired suppressive properties in vitro and fail to block allograft rejection in vivo (85). CD39+ Tregs ameliorate experimental colitis induced by T cell transfer (86), further supporting the role of CD39 in the Treg mechanism of suppression. Analysis of the CD4+CD39+ T cell pool in mice has, however, revealed the presence of two populations, one Foxp3+ and CD73bright, which represents bona fide Tregs; and another one Foxp3− that does not exert any suppression and is endowed with memory cell properties. These Foxp3-CD39+ cells secrete Th1, Th2 and Th17 cytokines and cause fast rejection of MHC-mismatched skin allografts (87).
A number of studies have suggested that CD39+ Tregs are somewhat specialized in suppressing IL-17 production (88, 89) and that the expression of CD39 prevents conversion into Th17 cells (67, 87). More recent evidence supports the concept that CD39 designates Th17 cells that have undergone immunosuppressive transformation, instead of alternatively preventing de novo transdifferentiation of Treg to Th17 (90).
A wide range of solid tumors and certain types of leukemia are associated with accumulation of Tregs expressing high levels of CD39. This feature results in accumulation of adenosine that promotes growth of tumor cells, angiogenesis, inhibition of Th1 cytokine production, adhesion of immune cells to endothelial cells and suppression of effector cells. Increased infiltration of Foxp3+ Tregs expressing CD39 is noted in experimental melanoma (91) and associated with suppression of NK cell-mediated anti-tumor immunity (92). In this later model, CD39 promotes growth of metastatic tumors that is inhibited upon administration of polyoxometalate 1 (POM1), a pharmacologic inhibitor of nucleoside triphosphate diphosphohydrolase activity (92). Heightened CD39 expression has been also reported in Tregs infiltrating colorectal cancer (93).
Increased levels of CD39+Foxp3+ Tregs are also found in human follicular lymphoma and linked to anergy of effector T cells due to high levels of adenosine (94). Treatment with A2A antagonists as well as ARL67156, an inhibitor of CD39 ectoenzymatic activity, results in an increase in IL-2 and IFNɣ secretion following CD3/CD28 stimulation (94), further supporting the evidence that CD39 plays a permissive role in the tumor setting.
Murine FoxP3+ Tregs co-express CD39 and CD73 (85). In support of a role for CD73 on mouse Treg in tumor development, reconstitution of Treg-depleted mice with CD73-deficient Tregs failed to promote tumor growth, in contrast to wild type Tregs (95). In humans, only 1–5% of circulating FOXP3+ CD4+ T cells expresses CD73. Compared to conventional T cells, CD73 is enriched intracellularly in human FOXP3+ T cells (96, 97). Interestingly, human FOXP3+ T cells also express lower levels of CD26-adenosine deaminase compared to conventional T cells (96), revealing a decreased ability to degrade adenosine. It has been proposed that CD73 surface expression on human Tregs is induced upon activation (98). In support of this, proliferating ICOS+ Tregs in melanoma patients treated with high-dose IL-2 have been shown to co-express high levels of both CD73 and CD39 (99). Also in support of a role for CD73 on human Tregs, inhibition of CD73 reduces the suppressive capacity of human Tregs during in vitro mixed lymphocyte reactions (96). However, exogenous 5’AMP used in vitro may directly modulate T cell function through A1 receptor (100). While the role of CD73 on human Tregs remains controversial, it is noteworthy that humans harboring CD73 loss-of-function mutations (101) do not develop autoimmune disorders associated with Treg deficiencies (102). It is generally viewed that human Tregs can generate adenosine through paracrine interactions with neighboring CD73-expressing cells or tumor-derived exosomes (98, 103).
Adenosine in turn modulates Tregs function. Activation of A2a receptor on Tregs promotes Treg proliferation, CTLA-4 and PD-1 expression, and enhances immunosuppressive functions (104)–(105). A2b receptor activation may also promote Treg function (106). A2b-deficient mice fail to induce Tregs after endotoxin-induced inflammation, and A2b gene expression is upregulated in Treg following TCR activation (107). Another mechanism by which adenosine regulates Treg function is by promoting their interactions with DCs. Adenosine favors Tregs/DCs clustering via A2a-Epac1-Rap1–dependent pathway, thereby rendering DCs less stimulatory (108) – see later.
T regulatory type 1 cells (Tr1)
T regulatory type 1 (Tr1) cells represent a subset of regulatory lymphocytes characterized by secretion of IL-10, negativity for Foxp3 and expression of CD49b and LAG-3 in both mice and human (109, 110). At variance with Foxp3+ Tregs, Tr1 cells play a specific role during the resolution of or during chronic phases of inflammation (109). Tr1 cells mediate suppression predominantly through secretion of IL-10 and TGF-β, though a cell contact dependent mechanism has been also described and found to be associated with the expression of CTLA-4 and PD1 (111, 112). Since their first description, Tr1 cells have been proven to be determinant in the maintenance of tolerance and immune homeostasis during hematopoietic stem cell transplantation and autoimmune disorders. Tr1 can be differentiated from CD4 cells upon exposure to IL-27 through a mechanism involving AhR and Stat3 (113–116). Antagonist effects of eATP and CD39 on Tr1 cells have been recently demonstrated by Mascanfroni et al, who reported inhibition of Tr1 cell differentiation as result of exposure to eATP and hypoxia. In contrast, CD39 contributes to Tr1 suppressive activity via generation of adenosine in tandem with CD73 expressed by adjacent effector cells and APCs (29, 117). Tr1 cells can be induced by the tumor microenvironment (118) and act as potent suppressor of tumor immunity possibly as result of high levels of CD39 and CD73 and consequent generation of adenosine as shown in colorectal cancer (93) and head and neck squamous cell carcinoma (119–121).
Th17 cells
Th17 cells are a lymphocyte subset developing independently of Th1 and Th2 subsets, upon exposure of CD4 cells to IL-6, TGF-β or IL-21 in mouse; IL-6, TGF-β and IL-1β in human (122, 123). ATP that can be generated by commensal bacteria has been shown to favor the differentiation of Th17 cells in the lamina propria in a colitis model (124). Further evidence has been provided by human studies where IL-17 induced P2X7R activation boosted Th17 cell conversion at the expenses of Tregs (125). ATP pro-inflammatory effects can also synergize with those mediated by Toll-like receptor ligands to induce IL-23 and IL-1β from cultured monocytes to promote Th17 cell differentiation (126). ATP was also found to promote survival of Th17 cells by increasing the expression of IL-1β through ASC-HLRP3-dependent caspase 8 activation (127).
The link between ATP and pro-inflammatory Th17 cells has also been recently confirmed by studies showing that activation of P2X7R through ATP favors a cytokine milieu promoting Th17 cells in tissue from metabolically unhealthy obese donors, as compared to healthy obese and lean donors (128).
In contrast to the pro-inflammatory effects of ATP, expression of CD39 has been found to confer immunosuppressive properties upon Th17 cells. Exposure of IL-6 and TGF-β induces Stat3 and down-regulates Gfi, both events being relevant for the induction of CD39 and CD73 by Th17 cells (129). Accordingly, increased ectonucleotidase levels are associated with augmented generation of adenosine and with suppression by Th17 cells of CD4 and CD8 anti-tumor immunity (129). While examining the phenotype of tumor infiltrating Th17 cells in murine models of thymoma, melanoma, lung and colon carcinoma, intratumoral CD4+ Rorɣt+ cells were found to be positive for both CD39 and CD73. Adoptive transfer of wild type cells enhanced tumor growth, an effect that was not observed when adoptive transfer of CD39−/− cells was carried out (129). Importantly, in breast cancer patients, accumulation ectonucleotidase-expressing Th17 cells were shown to predict poor outcome and to confer a negative prognostic value to CD8+ T cells infiltration (130). Of note, Th17 cells generated in the absence of TGF-b1, which lack CD39 and CD73, not only fail to promote tumor growth, but are also endowed with anti-tumor functions (131). Th17 cells generated in the presence of IL-23, TGF-b3 and IL-1b do not express CD73 and CD39 (132). Extracellular adenosine also acts in an autocrine fashion to stabilize Th17 cell differentiation by favoring the expression of stem cell-related transcription factors such as tcf-7 and lef-1 and by restraining the acquisition of Th1 effector molecules such as granzyme B and IFNɣ (78). Besides, activation of A2b receptors on dendritic cells (DCs) was shown to promote differentiation of IL-17 producing T cells and their expression of IL-23R and RORγt (133).
We have shown that expression of CD39 marks Th17 cells endowed with suppressor activity (90). In contrast with prototypical Th17, these suppressor Th17 cells (supTh17) exert suppressive function and generate adenosine and other purine derivatives (90). supTh17 can be detected in the circulation and lamina propria of healthy individuals and are diminished in Crohn’s patients. Recent data have confirmed these findings by showing that expression of CD39 by in vitro generated Th17 cells is associated with the ability of these cells to produce IL-10 (132) and to limit colon damage in vivo. Interestingly CD39 expression also conferred those cell the capacity to resist to ATP-induced cell death.
Lack or low levels of CD39 by Th17 cells have been reported also in the context of other autoimmune conditions, like autoimmune liver diseases, (134) in which defective CD39 and consequent impaired adenosine generation, favors persistence of Th17 cells at an inflammatory state.
γδ T cells
CD73 and CD39 can be expressed on γδ T cells, which constitute a prevalent T cell population in epithelial tissues. In contrast to classical αβ T cells, γδ T cells recognize diverse antigens, including non-classical MHC molecules, heat shock proteins and lipids (135). In tumors, γδ T cells can display cytotoxic or regulatory functions. Regulatory gd T cells can express latency-associated peptide (LAP), which is converted to TGF-β1 by thrombospondin-1 (TSP-1) (136).
In mice, CD73 is expressed on more than 90% of peripheral γδ T cells (137). CD73 is expressed in a TCR-ligand inducible manner and its expression associated with enrichment of transcription factors linked to effector function (137). Yet, studies in CD73-deficient mice suggest that CD73 is dispensable for γδ T cell development (138), but may play a role in their regulatory functions (139, 140). In contrast, TCR-dependent ATP release and autocrine stimulation of P2X7R on immature thymocytes was shown to promote γδ T cell development (141).
Recently, CD39 was identified as a marker of mouse regulatory γδ T cells (142). In murine lymph nodes, a population of CD25+CD39+ γδ T cells was identified and shown to suppress αβ T cell proliferation in vitro in a mechanism dependent on IL-10 and dendritic cells. Interestingly, chronic TCR stimulation + IL-2 for 10 days was sufficient to induce CD39 expression on γδ T cells and to endow them with immunosuppressive functions (142). These results are supported by another report showing that activated murine γδ T cells co-express CD73 and CD39 and display immunosuppressive functions, while most resting γδ T cells do not constitutively express CD39, with the exception of liver γδ T cells (143). In the context of cancers, γδ T cells infiltrating murine pancreatic tumors selectively upregulate CD39 together with other immunosuppressive factors, and support tumorigenesis by restraining αβ T cell immunosurveillance (144).
In human, Vγ9Vδ2 T cells, whose function is to detect self and pathogen-associated phosphoantigens (pAgs), do not express CD73 nor CD39, but can upregulate CD39 upon TCR stimulation (145). It was proposed that CD39 upregulation upon γδ TCR stimulation acts as a feedback mechanism to desensitize cells to self and microbial pAg. Interestingly, CD39 was shown to dephosphorylate pAgs, rendering them inactive at stimulating γδ T cells (145).
NK and NKT cells
NK cells are an innate immune subset involved in vascular injury and in anti-tumor defense. These cells are subjected to the effects of ATP through activation of P2 receptors. Human NK cells express P2X1R, P2X4R, P2X5R, P2X6R and P2X7R as well as a number of P2YR, including P2Y1R, P2Y2R, P2Y4R, P2Y6R, P2Y11R, P2Y12R, P2Y13R and P2Y14R (146). There is evidence that CX3CL1 induced NK cell chemotaxis and cytotoxicity are modulated through activation of P2Y11R, suggesting inhibition of this receptor as a way to control NK cell-mediated damage.
Absence of CD39 has been associated with the abrogation of IFN-γ secretion by NK cells and subsequent protection from liver damage in mice with ischemia/reperfusion injury (147). Further, CD39 deletion has been shown to be protective in the context of Con A hepatitis, induced by NKT cells (26). Additional protective effects of CD39 deletion have been demonstrated in the context of iNKT cell-mediated hyperoxic acute lung injury (148), where CD39−/− mice appear to tolerate hyperoxia as a consequence of iNKT cell auto-depletion, when compared to wild type mice that develop severe lung injury.
In the tumor setting expression of CD39 with consequent ATP hydrolysis and adenosine generation compromises anti-tumor immune responses, including those that may be mediated by NK cells. Therefore, interference with CD39 using CD39 inhibitors or blocking antibodies might represent a strategy to keep cell-mediated immunosuppression under control in the tumor setting (149).
Expression of CD73 is virtually absent from circulating human and mouse NK cells in healthy individuals. Tumor-infiltrating NK cells, however, can express significant levels of CD73 (150). Interestingly upon exposure to mesenchymal stromal cells (MSC), human NK cells also upregulate CD73 (151). Thus, upon encounter with environmental factors, NK cells can acquire CD73 expression and exert immunosuppressive function by production of adenosine. In a recent report, human NK cells were also shown to produce adenosine via a CD38-mediated pathway (152). A2a is the predominant adenosine receptor expressed by NK cells and its expression has been shown to be augmented in pathological conditions (153). Stimulation of A2a on NK cells strongly suppress NK cell activation and cytotoxic functions (154–156). In the context of tumor, accumulation of CD73-derived adenosine and subsequent A2a-mediated suppression of NK cell anti-tumor activity has been shown to be a pivotal mechanism for the development of metastasis (154, 157, 158).
B cells
CD39 was initially described as a B lymphocyte activation marker (42). Global deletion of CD39, as in CD39−/− mice, does not alter the B cell number in the peripheral blood and in the spleen. CD39−/− mice, however, exhibit impaired B cell memory responses to T dependent antigens, suggesting that CD39 may contribute to the affinity maturation of antibody response as well as post-germinal center B cell differentiation (159). Human B cells have been found to co-express CD39 and CD73 and express A1, A2 and A3 adenosine receptors (160). The A3 receptor was found to be specifically responsible for autocrine signaling and self-regulation. Generation of AMP and adenosine by this CD39+ CD73+ B cell subset is linked to control over CD4 and CD8 effector immunity (160). Further studies have characterized the phenotype and functionality of CD39high human regulatory B cells (Bregs) and show that these cells display high proliferative capacity while operating suppression through adenosine generation and IL-10 secretion (161).
CD73 is broadly expressed in human peripheral blood B cells (160, 162). CD73 is also found on a subset of IgD+ human germinal center (GC) B cells and on a population of memory B cells (IgM+ and isotype switched) (163, 164), while plasma cells are negative for CD73. In mice, most peripheral blood and secondary lymphoid tissue B cells are negative for CD73. Mouse CD73 is rather restricted to specific B cell subsets, such as mature class-switched and GC B cells (163), and on subsets of memory B cells, B-1 cells, marginal zone (MZ) B cells, and B cells with regulatory or suppressive function (163, 165). While CD73 is considered a marker of class-switched memory B cells (166–168), CD73 can also be expressed on memory B cells that developed outside of the GC, such as in the context of an extra-follicular reaction (169).
CD73 likely regulates GC formation (162, 164, 170, 171). Within the GC, CD73 can be found on B cells, T follicular helper cells (TFH) (172) and follicular dendritic cells (FDC), where it was shown to promote B cells adhesion to FDC (164, 173). CD73 was also shown to promote class switch recombination (CSR) in vitro. When combined with BCR and TLR agonists, adenosine significantly increases differentiation of B cells towards class-switched B cells. Consistent with a role for CD73 in CSR, B cells from CVI patients show very low CD73 expression (163), and CD73 on neonate B cells gradually increases during the first 6 months of life, correlating with their ability to make IgG. In vivo immunization of CD73-deficient mice, however, revealed only slightly delayed or normal isotype switched responses (172, 174).
CD73 expression has been shown to promote Breg function. CD73 expression indeed defines a population of murine B-1 and human IL-10-producing B cells with immunosuppressive activity towards T cells (161, 165). Notably, adenosine-producing B cells produce significantly more IL-10 and IL-6, and activation of A1 and A2a receptors promoted expansion and functions of adenosine-producing B cells.
Myeloid cells
Dendritic cells (DC)
DCs shape adaptive immunity through antigen presentation and modulation of T cell activation. The effects of eATP on monocyte derived DCs have been demonstrated in a number of investigations, both in the human and the experimental setting. ATP has been found to promote human DC maturation, as indicated by up-regulation of CD80, CD83 and CD86 (175) costimulatory molecules as well as the secretion of IL-12 (176), these effects being modulated upon activation of P2Y11R (176). The role of P2Y11R in the modulation of DC activation has been proposed by Schnurr et al, who has shown that exposure of human DCs to eATP gradients inhibit their migratory capacity in a dose dependent manner (177).
Studies of microarray analysis of ATP stimulated human DCs has revealed induction of thrombospondin 1 and indoleamine 2.3-dioxygenase (IDO); two immunosuppressive genes, suggesting an important role for ATP in promoting immunotolerance (178). This specific role of eATP in favoring immunotolerance by conferring immunosuppressive properties to DCs, has been also supported by data showing no impacts of ATP on IL-10 but rather selectively dampening pro-inflammatory cytokine secretion (179). In the context of tumor microenvironment, recent studies have proposed a role for eATP in the activation of the NLRP3 inflammasome in DCs through P2X7R (180, 181).
DCs can also express CD39 and in the absence of it they become unresponsive to ATP (182). CD39−/− mice do harbor major defects in DC formation, antigen presentation, and response to haptens. It has been proposed that defective functionality of CD39−/− DCs resides in the diminished ability of these cells in starting and maintaining cell-to-cell contact and that CD39 on DCs is translocated to the immunological synapse during antigen presentation to favor cellular contact signaling (68). Recent investigations demonstrated the importance of CD39 on DCs exposed to IL-27 immunomodulation. Based on these data, IL-27 can promote tolerance by dampening Th1 and Th17 immunity through up-regulation of CD39 on DCs and consequent decrease in ATP concentration and NLRP3 inflammasome activation (117).
CD73-derived adenosine also promotes aberrant differentiation of DCs. Specifically, activation of A2b receptor on DCs promotes a tolerogenic phenotype characterized by increased production of IL-6, IL-10, VEGF, and IL-8 and expression of immunosuppressive proteins like IDO, TGF-β, arginase 2 and COX2 (183). Highlighting the impact of adenosine on tumor DCs, injection of adenosine-treated DCs into tumor-bearing mice was shown to increase tumor growth. A2b receptor signaling can thus endow DCs with pro-tumorigenic effects. In support of a role for A2b in regulating DC-mediated anti-tumor immunity, A2b blockade promoted DC activation in murine bladder tumors (184).
Monocytes
Ectoenzymes can also regulate leukocyte trafficking (185) and CD39 is specifically regarded as a key molecule in the control of neutrophil accumulation under hypoxic conditions (186). ATP and the purinergic signaling can also impact monocyte functionality as ATP acts as chemotactic factor to monocytes in addition to activating the NF-kB signaling pathway and favoring the polarization into M1 monocytes. These effects are mediated through P2X1R, P2X2R and P2X4R. M2 monocytes, which are characterized by lower production of TNF-α and higher levels of IL-10, arginase 1 and VEGF can be induced by adenosine signaling (187, 188) through activation of A2A and A2B receptors (189, 190).
CD39 is the predominant ecto-NTPDase expressed by monocytes/macrophages. There is evidence that presence of CD39 on monocytes regulates the sequestration of these cells in the ischemic cerebral tissue and inhibits their chemotaxis, adhesion and ability to transmigrate (191). That CD39 plays a key role in self-limiting the activation process, was also supported by studies conducted in the setting of sepsis, in which lack of CD39 expression by macrophages resulted in the inability of these cells to transition to a regulatory state and in continuous production of inflammatory cytokines (192).
Regarding CD73, less than 5% of circulating CD14+ monocytes express this ectonucleotidase in healthy individuals. Tissue macrophages, however, can express both CD39 and CD73 (193). Activation of macrophages through TLR induces the release ATP, which gets rapidly hydrolyzed by CD39 on macrophages (192). Regulation of CD39 and CD73 levels in turn regulates adenosine production. Zanin et al. demonstrated that human macrophages with a pro-inflammatory phenotype (i.e. M1 macrophages) express lower levels of CD39 and CD73 compared to macrophages with an anti-inflammatory phenotype (i.e. M2 macrophages) (194). In contrast, Eichin et al. demonstrated that CD73 expression on human macrophages is only achieved after polarization with LPS and TNF-α, and that exogenous AMP or CD73 inhibition has no effect of macrophage polarization (195). In mice, treatment with the CD73 inhibitor APCP has been shown to enhance M1 macrophage predominance and to reduce Th2 responses (196). CD73-deficient mice have also been reported to show decreased M2 tumor-associated macrophages (197). Taken together, these studies strongly suggest that macrophages regulate inflammation through ATP catabolism (194). In turn, adenosine modulates macrophage function. Adenosine has been shown to promote expression of M2 markers (198) and to enhance IL-10-induced STAT-3 activation in macrophages (199).
Neutrophils
Neutrophils secrete ATP and are subjected to its effect upon engagement of the P2Y1R, P2Y2R, P2Y4R, P2Y6R, P2Y11R and P2Y14R and the P2X1R, P2X4R, P2X5R and P2X7R subtypes. Induction of neutrophil chemotaxis is induced through P2Y2R and A3 receptor activation (2). There is also evidence that ATP can act in concert with IL-8, a major neutrophil chemoattractant to favor human neutrophil migration, an effect likely to be mediated upon engagement of P2Y2R (200). Further, eATP triggers production of ROS through P2X7R (201), degranulation as well as aggregation of human neutrophils (202). ATP can also exert concentration-dependent anti-apoptotic effects on neutrophils and this was found to be mediated through P2Y11R activation (203).
Neutrophils co-express CD39, CD73 and all four adenosine receptors (204). Therefore, neutrophils have the capacity to generate adenosine and receive an autocrine feedback through adenosine receptor stimulation. While unstimulated neutrophils continuously produce and secrete low amount of adenosine, activated neutrophils can be a major source of tissue adenosine in inflammatory microenvironment (204–206). Not only do neutrophils can directly secrete adenosine and inhibit its degradation, they also release ATP upon activation which is then process by CD39 and CD73 to adenosine (9).
Both ATP and adenosine have a pivotal role for neutrophil chemotaxis to inflammatory sites. It was demonstrated that ATP release and conversion into adenosine by migrating neutrophils is polarized to guide neutrophils migration towards the inflammatory site (2). This phenomenon was shown to be dependent on polarized expression of CD73, CD39 and local activation of P2Y2 and A3 receptors by ATP and adenosine, respectively (2). In support for a major role of adenosine in neutrophil chemotaxis, both A3 and CD39 deficient mice displays impaired neutrophil recruitment to sites of inflammation (207, 208). A1 and A2a receptors have also been involved in neutrophils migration. Similarly to A3, A1 activation on neutrophils was shown to promote chemotaxis (209). A2a receptor signaling was shown to broadly inhibit neutrophil activation while still contributing to directional neutrophil chemotaxis by blocking backward migration of extravasated neutrophils toward the endothelium (204). In contrast, A2b receptor activation on neutrophil blocked their migration across epithelial and endothelial barriers (210, 211). Furthermore, in addition to its involvement in neutrophils chemotaxis and migration, adenosine has been shown to modulate various neutrophils functions including adhesion and transmigration, the release of inflammatory mediators, phagocytosis, degranulation and oxidative burst (reviewed in (204)). In many situations, A2a and A2b receptor engagement have an immunosuppressive action on neutrophils while A1 and A3 stimulation promotes neutrophil pro-inflammatory activities (204).
Monocytic (M-) and polymorphonuclear (PMN-) myeloid-derived suppressor cells (MDSC)
MDSC are immature myeloid cells that accumulate in cancer patients. Importantly, the presence of MDSCs in human tumors is correlated with decreased efficacy of anti-PD-1 therapies and other immunotherapies (212). MDSCs are largely regulated by STAT signaling, with a predominant role for STAT-3 (213). In mice, CD39 and CD73 can be co-expressed by MDSCs and A2b receptor can promote their expansion with a preferential accumulation of PMN-MDSCs (214). Further in support of a role for A2b in MDSC development, blockade of A2b decreases the accumulation of tumor infiltrating MDSCs in melanoma-bearing mice (215). Inversely, administration of an A2b specific agonist promotes infiltration of MDSCs in tumor-bearing mice (216). TGF-β signaling has been recently reported to be determinant in the differentiation of MDSCs into protumorigenic terminally differentiated myeloid mononuclear cells (TDMMCs) that express high levels of CD39, CD73 and produce adenosine (217). Myeloid cell-specific disruption of TGF-β signaling resulted in reduced accumulation of TDMMCs, increased T cell infiltration, reduced blood vessel density which limited the progression of Lewis lung carcinoma and spontaneous mammary carcinoma (217).
Few studies have evaluated CD39 and CD73 expression in human MDSCs. A recent analysis of MDSCs in colorectal cancer patients revealed expression of both CD39 and CD73 in PMN-MDSCs and lower CD73 expression in M-MDSCs (218). Notably, CD39 and CD73 were expressed at higher levels in MDSCs from cancer patients compared to healthy volunteers (218). Thus, macrophages and MDSCs that infiltrate tumors are likely an important source of extracellular adenosine that contributes to tumor immune escape.
Ectonucleotidases and vascular regulation
ATP is known to modulate a variety of processes linked to endothelial cell activation, including vascular inflammation and thrombosis (219). ATP increases the intracellular levels of Ca2+, induces cytoskeletal rearrangements and stimulates the phosphorylation of proteins like focal adhesion kinase (FAK), paxillin, proline-rich tyrosine kinase 2 (Pyk2) and p38 MAP kinase. Activation of endothelial cells by extracellular nucleotides induces cytoskeletal rearrangements and increases cell motility (220). ATP is also released by endothelial cells during changes in flow or after exposure to hypoxic conditions (221); it activates P2YR and this promotes release of NO and consequent vessel relaxation. ATP is then hydrolyzed into adenosine that will also result in vasodilation upon engagement of P1 receptors.
CD39 is the major NTPDase expressed on endothelial cells and the associated vascular smooth muscle and plays a key role to mitigate thrombotic and inflammatory events (222). There is evidence that in Apo-E deficient mice, a model of atherosclerosis, CD39 is mainly expressed in atheroprotective areas as well as in regions of stable flow (223). Situations causing disturbed flow determine partial suppression of CD39 expression on endothelial cells. Further, the same study has provided evidence that unidirectional laminar sheer stress is associated with increase in CD39 expression in human endothelial cells, with consequent atheroprotective effects (223). An indirect confirmation of the anti-atherogenic effects of CD39 expression has been provided by Hot and colleagues, who showed that simvastatin, in addition to reducing IL-17 induced pro-inflammatory cytokines, boosted also CD39 expression on human endothelial cells (224). In the tumor setting, expression of CD39 by the vasculature, particularly endothelial cells could promote tumor growth by scavenging eATP (225).
Using CD73 deficient mice, landmark studies by L. Thompson (138) have demonstrated the pivotal role of CD73-derived adenosine for the maintenance of vascular endothelium barrier function, especially in hypoxic conditions. Under hypoxia, CD73 gene-targeted mice develop vascular leakage in multiple organs associated with strong immune infiltrates surrounding vessels (138). This strong, hypoxia-associated phenotype is explained by the fact that CD73 expression is upregulated by HIF transcription factors in various cell types including endothelial cells (138, 186, 226). Subsequent activation of A2b receptor in response to hypoxia maintains endothelial barrier function. Accordingly, a recent study by Eckle et al. demonstrated that when associated to hypoxia, a global A2b deficiency or injection of a specific A2b inhibitor phenocopy the hypoxia-induced vascular leakage observed in CD73-deficient mice (211). Therefore, CD73-derived adenosine and subsequent A2b receptor activation is a crucial pathway for the maintenance of the vascular endothelial barrier function (227). The involvement of endothelial cell-specific CD73 and A2b has been demonstrated in vitro (211, 228, 229).
Interestingly, the barrier-protecting function of CD73-derived adenosine does not seem to be applicable to all types of endothelial surfaces. Indeed, recent studies by the group of M. Bynoe indicate that the CD73-adenosine pathway rather favors the permeability and the entrance of immune cells at the blood brain barrier (BBB) and blood-CSF barrier (230). Using a murine model of EAE, this group demonstrated that CD73-derived adenosine and A2a receptor signaling is required for immune cell homing to the central nervous system and induction of EAE (231–233).
Notably, a similar CD73 / A2a dependent pathway was shown to drive the recruitment of M2 macrophages in the CNS following spinal cord injury (234). In other studies, in vivo stimulation of A1 and A2a receptors using specific agonists was shown to transiently increase BBB barrier permeability to macromolecules including antibodies or chemotherapeutic agents (235, 236), through both the paracellular and transcellular pathways (235, 237).
Surprisingly, recent studies indicate that CD73 barrier-promoting function is not active in the lymphatic endothelium, despite high expression of CD73 (228). The role of lymphatic CD73 thus remains unclear. In the vascular endothelium, CD73 has been involved in lymphocyte adhesion and transmigration through endothelial barriers (238, 239). Lymphocytes adhering to CD73+ vascular endothelial cells can block CD73 enzymatic activity, thereby favoring their transmigration across the endothelium (240). In human lymphatic endothelium, CD73 is expressed on afferent but not efferent lymphatic (241). In murine lymph nodes, CD73 is not expressed in lymphatic sinuses but highly expressed on High Endothelial Venules (HEV) (242). Using CD73 deficient mice subcutaneously injected with WT or CD73 deficient lymphocytes, it was demonstrated that lymphatic CD73 is not involved in leukocyte trafficking to lymph nodes in resting or inflammatory conditions (241).
In contrast, lymphocyte migration from the bloodstream to lymph nodes through HEV was enhanced in CD73-deficent mice and not dependent on the expression of CD73 on lymphocytes suggesting that HEV-derived CD73 is modulating lymphocyte trafficking to lymph nodes (242). This effect was reversed using a specific A2b agonist and A2b was shown to be the predominant adenosine receptor expressed by HEV (242). Thus, local adenosine production by CD73+ HEV followed by autocrine stimulation of A2b seems to be a mechanism restricting lymphocyte extravasation from the bloodstream into lymph nodes.
In humans, CD73 loss-of-function mutations have been described and linked to a rare autosomal-recessive disorder causing ectopic calcification of joint and arteries (CALJA). Initially identified in nine individuals from three unrelated families in 2011, arterial calcification due to CD73 deficiency (ACDC) has been show to specifically target lower extremity arteries, as well as of the hand and foot joint capsules (101, 243). Recently, a second report from an independent Chinese group, expanded the clinical portrait of ACDC identifying novel NT5E loss-of-function mutations and showing that calcification could also occurred in upper extremity arteries (244). From a mechanistic point of view, CD73-derived adenosine was show to repress the expression of tissue non-specific alkaline phosphatase (TNAP), thereby maintaining a high inorganic pyrophosphate / phosphate (PPi/Pi) ratio to inhibit mineralization. In patients with CD73 mutations, TNAP is overexpressed, thus promoting calcification (101). In accordance with these observations, a recent report by Li and colleagues described the development joint calcification in 11 to 13 month old CD73-deficient mice fed with a diet rich in phosphate and low in magnesium (245). Unlike ACDC patients, however, no arterial ectopic calcification was observed in CD73-deficient mice.
Purinergic signaling and cancer
The purinergic signaling system has considerable effects on tumor growth, survival and progression, by impacting not only the tumor itself but also the immune responses and the microenvironment associated with it (5).
In solid tumors, ATP is abundantly released in the extracellular space where its concentration can reach a few hundred micromole per liter, a concentration more than a thousand time higher than in healthy tissues (5, 246). This phenomenon is mainly due to cell death in the tumor core, and to metabolic or hypoxic stress and pro-inflammatory signals that stimulate active export of ATP by connexins and pannexin channels expressed by immune and endothelial cells (6, 247). In the tumor microenvironment (TME), eATP acts as a danger signal involved in the recruitment of innate immune cells and in the priming of anti-tumor immunity through the activation of P2X and P2Y receptors (180, 248, 249). In support for a pivotal role of P2 purinergic receptors in the initiation of anti-tumor immunity, ATP release in the TME is considered as one of the three hallmarks (with calreticulin exposure and HMGB1 secretion) of the immunogenic cell death (ICD) process (6). However, in the TME, extracellular ATP is most often degraded into immunosuppressive adenosine via the concerted enzymatic activity of CD39 and CD73 (40, 250–252). As a consequence, in various solid tumors, adenosine levels can reach micromolar concentrations (253–255) and dampen anti-tumor immunity through the activation of P1 receptors (mainly A2a and A2b receptors) on immune cells. Moreover, while high concentrations of ATP have been associated to enhanced anti-tumor immunity and to cytotoxicity on tumor cells, low ATP levels due to excessive ectonucleotidase activity can, on the contrary, support tumor growth.
P2XR and P2YR are widely distributed in tumor tissues and it is well established that virtually all cancer cell lines respond to extracellular nucleotides, including ATP (5, 256). Most tumors express P2X7R, the activation of which has been classically linked to cytotoxic effects, although growing evidence has also associated the stimulation of this ATP receptor with tumor growth (5, 247). While high ATP concentrations trigger cytotoxic P2X7R responses, low dose ATP results in P2X7R-mediated trophic effects (31, 257–260). There is also evidence that both cancer cells and other cells present in the tumor microenvironment may be resistant to high ATP concentration (261), this resulting, at least in some cancer type, from P2X7R uncoupling from intracellular death pathways (30). Stimulation of P2X7R with low ATP concentrations results in oxidative phosphorylation and aerobic glycolysis, both events being associated with an overall increase in the intracellular ATP content (31) and consequent gain in proliferative advantage by P2X7R expressing cells. P2X7R stimulation is also associated with the activation of intracellular pathways, like NFATc1, Erk, Akt and HIF-1α (262–264); however, lack of P2X7R impairs allogeneic responses (265, 266) and promotes tumor metastasis (267, 268).
The impact of ATP signaling on cancer tissue is also mediated through P2YR, particularly P2Y1R and P2Y2R, the simulation of which has been found to promote tumor growth. In the setting of prostate and breast cancers, P2YR activation is associated with invasiveness, metastatic niche formation and spreading (269, 270). Blockade of P2Y2R has favorable effects in reducing metastatic dissemination, although this strategy in some cells may favor tumor growth, like in the case of nasopharyngeal carcinoma and human colon carcinoma (271, 272). Both solid tumors (273, 274) and certain types of leukemia (94, 275) express the ectoenzymes responsible for conversion of ATP into immunosuppressive adenosine, that in turn promotes growth of tumor cells, inhibits Th1 cell immunity and contributes to the suppression of effector T cells.
CD39 in tumor immunity
Our groups have been amongst the first to demonstrate the critical role of CD39 and CD73 in the regulation of anti-tumor immunity.
We have demonstrated that CD39 expression by Tregs plays a permissive role in a mouse model of hepatic metastasis, developed through portal vein infusion of luciferase-expressing melanoma B16/F10 cells and MCA-38 colon cancer cells into wild type and CD39−/− mice (92). Growth of melanoma metastatic tumors was strongly inhibited in CD39−/− mice or in chimeric mice reconstituted with CD39−/− bone marrow derived cells (92). CD39+ Tregs inhibited NK cell anti-tumor immunity both in vitro and in vivo. Using bone marrow chimeras, it was demonstrated that both hematopoietic and non-hematopoietic expression of CD39 contributed to tumor immune escape. CD39-expression on Treg was essential for tumor growth thereby supporting the critical linked functionality of CD73-derived adenosine for the immunosuppressive impacts.
We also have observed rapid growth of colorectal MC-26 cell line derived hepatic metastases in CD39 overexpressing transgenic mice (276). Accordingly, intrasplenic injection of MC-26 cells produced liver metastases that were significantly larger in CD39 overexpressing animals, further emphasizing the role of CD39 in promoting metastatic tumor growth (276). In other in vitro studies where we studied Luc-B16/F10 melanoma cells, we observed that ATP-induced alterations in tumor cell growth were nullified following treatment with apyrase, a soluble form of CD39 and that this effect was dose dependent (225). In additional experiments in which Luc-B16/F10 cells were co-cultured with liver sinusoidal endothelial cells (LSEC) and exposed to ATP, we demonstrated that expression of CD39 by LSEC counterbalances the inhibitory effect exhibited by ATP on tumor growth (225).
Treatment with POM1, a pharmacological CD39 inhibitor, was also shown to significantly limits the tumor growth (276). Deletion of CD39 or direct inhibition also potently suppresses tumor angiogenesis in several models tested to date (92, 225, 277). Genetic deletion of CD39 with abrogation of angiogenesis results in decreased growth of implanted LLC and B16-F10 tumors while inhibiting development of pulmonary metastases (277). Decreases in Cd39-null endothelial cell adhesion and integrin dysfunction can be linked to decreased activation of focal adhesion kinase and extracellular signaling-regulated kinase-1 and -2 in these cellular models (277, 278).
Collectively all these studies support an inverse correlation between expression of CD39 by tumor cells, tumor infiltrating lymphocytes or tumor endothelial cells and anti-cancer effector immunity or angiogenesis. Our data indicates CD39 to be a possible target in tumor immunotherapy and at the same time to provide anti-angiogenesis modalities.
CD73 in tumor immunity
Building on the landmark study of Sitkovsky and colleagues which demonstrated tumor rejection in A2a-deficient mice (253), we and others investigated the phenotype of CD73-deficient mice challenged with various types of tumor models (95, 197, 279, 280). Host CD73 deficiency results in delayed tumor growth in multiple syngeneic transplantable tumor models including ovalbumin-expressing MC38 colon cancer, EG7 lymphoma, AT-3 mammary tumors, ID8 ovarian tumors, B16F10 melanoma and TRAMP-C1 prostate tumors (197, 279, 279, 280). CD73-deficient mice are also resistant to lung metastasis development following tail-vein injections of B16F10 melanoma and TRAMP-C1 prostate cancer cells (95, 279).
Depending on the models, the tumor-protective effect of CD73 loss was dependent on CD8+ T cells, NK cells and IFNɣ secretion (95, 279, 280). Using bone marrow chimeras, it was demonstrated that both hematopoietic and non-hematopoietic expression of CD73 contributed to tumor immune escape (279, 280). Interestingly, amongst hematopoietic cells, CD73-expression on Treg was essential for tumor growth thereby supporting the critical function of CD73-derived adenosine for the immunosuppressive function of Tregs (279, 280). With regards to the non-hematopoietic compartment, CD73 expression was detected on tumor-associated endothelial cells and associated with reduced ICAM-1 expression thus suggesting that endothelium-derived CD73 may limit T cell homing to tumors (280). Further in support for a role of endothelial cell-derived CD73 in anti-tumor immunity, the accumulation of adoptively transferred CD8+ T cells was enhanced in both tumor and tumor-draining lymph nodes of CD73-deficient mice. Of note, additional results suggest that endothelium derived CD73 may also regulates tumor cell migration across vascular endothelium. In support of that, diminished lung metastasis of B16F10 tumor cells is maintained in CD73-deficient mice depleted in NK cells, T cells or adoptively transferred with WT hematopoietic cells (95).
Autochthonous vs. transplanted tumor models
The biology of autochthonous tumors can differ substantially from that of transplanted cancers. This relates in part to different cellular interactions between tumor cells and stromal cells. When evaluating tumorigenesis in gene-targeted mice in situ, potential tumor cell-intrinsic functions of the targeted gene may be missed when using transplanted tumors. We have observed that CD39-deficient mice show decreased tumor growth and metastasis of transplanted tumors (276), while the deletion of CD39 favors the development of induced and spontaneous autochthonous liver cancer (281). Loss of CD39 was found to be associated with a high concentration of extracellular nucleotides that facilitated proliferation of hepatocytes, suppressed autophagy and impacted the glycolytic metabolism of these cells, shifting towards the Warburg phenotype. Of note, while ATP could boost both Ras-mitogen activated protein kinase (MAPK) and mammalian target of rapamycin (mTOR) pathways, rapamycin could interfere with both and restore normal purinergic signaling-mediated responses in hepatocytes (281).
CD73-deficient mice have been shown to be resistant to autochthonous tumor formation. Accordingly, CD73 deficiency suppressed prostate tumorigenesis in TRAMP transgenic mice and delayed the formation of 3-methylcholanthrene (MCA)-induced fibrosarcomas (279). Suppression of (MCA)-induced fibrosarcomas by CD73 deficiency relied upon IFN-γ, natural killer (NK) cells, and CD8+ T cells.
CD39 in human cancers
Expression of CD39 by human tumors and infiltrating immune cells has been widely described and, in some studies, it has been proposed as a prognostic marker. CD39 has been found to be elevated in pancreatic cancer and is preferentially expressed by the vasculature and stromal elements (282). High-tissue CD39 mRNA levels correlated with better long-term survival after tumor resection (282). A subsequent investigation conducted in the setting of human rectal carcinoma revealed heightened CD39 expression both in primary tumors and metastases, although it was proposed that the combination of both CD39 and CD73, rather than CD39 alone, could provide a better prognostic value (283).
Additional studies highlighted high expression of CD39 in myeloid cells obtained from human ovarian cancer tissue. These CD39+ expressing cells were able to suppress effector CD4 cells via adenosine generation, this phenomenon being abrogated by CD39 blockade (284). In the setting of hematological malignancies, CD39+ CD4+ and CD8+ cells are over-represented in follicular lymphoma as compared to normal or reactive lymph nodes or normal peripheral blood (94). Expression of CD39 is associated with generation of adenosine that has inhibitory role on effector anti-tumor immunity.
In patients with chronic lymphocytic leukemia (CLL), high CD39 expression was observed in both CD4 and CD8 cells and found to be associated with an advanced disease stage (285). In another study by Perry et al, CD4+CD39+ cells were higher in CLL patients who warranted therapeutic intervention and in those with non mutated immunoglobulin heavy chain variable region gene (286).
CD73 in human cancers
Expression of CD73 in the tumor microenvironment (TME) of various types of cancer is at least partly driven by hypoxia and activation of HIF transcription factors (255, 287, 288). Tumor signaling pathways, such as WNT (289), epithelial-to-mesenchymal transition (EMT) (290–292), mutations in TP53 (293) or KRAS (294), TGF-β (292) and loss of estrogen receptor signaling (335) have also been associated with increased expression of CD73. As a consequence, CD73-derived adenosine accumulates in the TME and exerts multiple immunosuppressive actions to dampens anti-tumor immunity (250). CD73 expression and its association with clinico-pathological characteristics has been studies in several types of human cancers, including melanoma, breast cancer, acute lymphocytic leukemia, chronic lymphocytic leukemia, glioblastoma, head & neck cancers, high grade serous ovarian cancer, endometrial cancer, colorectal, prostate, bladder cancer, gastric cancer, kidney cancer and pancreatic carcinomas (extensively reviewed in (251, 295). In general, these studies demonstrated that high levels of CD73 expression in the TME are associated with worse clinical outcomes.
Other studies have identified CD73 as a marker of good prognosis, including in low grade breast cancers (296) and endometrial carcinomas (297). In endometrial cancer, loss of CD73 expression in endometrial tumors was show to disrupt the endometrial epithelial barrier integrity thereby favoring tumor progression (297). This observation is in accordance with the well-described barrier-protecting role of CD73-derived adenosine in vascular endothelium or intestinal epithelium. In endometrial epithelium, barrier-promoting function of CD73-derived adenosine appears to be dependent on A1 receptor (297). Whether CD73 acts as a tumor suppressor in the development of other types of cancers is an important question that remains to be addressed.
Interestingly, recent data suggest that plasma concentrations of soluble CD73 (sCD73) are higher in cancer patients compared to healthy individuals (298). Increased sCD73 levels in blood were also reported in patients with acute inflammatory pancreatitis (299). Upregulation of sCD73 levels in blood might therefore reflect tissue/tumor inflammation. Another hypothesis is that upregulation of sCD73 reflects tissue/tumor hypoxia. In support of the latter, it was recently shown that blood adenosine and sCD73 levels are induced in healthy individuals exposed to high altitude, which triggers tissue hypoxia (300). Interestingly, production of sCD73 and activation of A2b in erythrocytes was shown to increase oxygen delivery and adaptation to hypoxia. Activation of A2b in erythrocytes activates AMPK, which in turn increases 2,3-bisphosphoglycerate (2,3-BPG) levels, a metabolic byproduct of glycolysis that regulates hemoglobin-oxygen affinity (300). Whether tumors can make use the sCD73-A2b axis to metabolically adapt to hypoxia remains unknown.
Targeting ectonucleotidases – experimental and clinical studies
Given the central role of CD39 in down-modulating effector anti-tumor immunity through generation of adenosine, strategies targeting this ectonucleotidase would represent a promising approach in cancer immunotherapy. Targeting immunosuppressive adenosine by inhibiting CD39 may restore anti-tumor responses or boost the efficacy of other anti-cancer therapies. Blockade of CD39 activity can restore CD8 and NK cell cytotoxic activity after co-culture of PBMCs with melanoma cells in vitro (301). Further, in the context of follicular lymphoma, treatment with the CD39 inhibitor ARL6715 or with specific A2A and A2B receptor antagonists resulted in partial overcome of T-cell hypo-responsiveness to stimulation (94).
The efficacy of POM1 in blocking CD39 activity has been shown in experimental animal models showing that POM1 injection for 10 days could limit B16 melanoma and MCA-38 colonic tumor growth (92). Notably the effect obtained in the presence of POM1 was comparable to the phenotype observed in CD39−/− mice (92). No signs of toxicity were reported during the course of treatment (92).
Attempts to curb the immunosuppressive effects of adenosine while boosting anti-tumor responses have been directed at developing anti-CD39 monoclonal antibodies. Two anti-CD39 monoclonal antibodies, BY40 and BA54G, have been proposed to block CD39 enzymatic activity (302), whereas our own candidate monoclonal antibodies seem to decrease levels of expression of CD39 on target cells. Likewise, the BY40 monoclonal antibody could induce down-modulation of CD39 on the surface of YT2C2 NK cell line as well as in circulating Tregs from HIV negative controls and to decrease CD39 ATPase activity on primary monocytes in vitro (303). Clinical efficacy of these anti-CD39 monoclonal antibodies has not been not evaluated to date.
It has been reported that an antisense to Epstein Barr virus LMP1 gene can substantially modulate the expression of CD39 in the B95.8 cell line, suggesting that control CD39 expression could be also achieved by CD39 specific antisense modalities (304).
With regards to CD73, early observations by L.F. Thompson et al. revealed that immobilized anti-CD73 mAbs could enhance pro-mitogenic effects of anti-CD3 mAb on human T cells (305). Interestingly, this effect was substrate-independent, reminiscent of what has been observed following cross-linking of other GPI-anchored proteins, such as CD90, Ly-6A/E, CD48 and CD59 (306). Work by Blay and colleagues subsequently identified extracellular adenosine as a potent inhibitor of CD3+ T cells and anti-tumor activity (307). A few years later, Sitkovsky and colleagues uncovered the pivotal role of adenosine A2a receptor in suppressing inflammation in mice and demonstrated that A2a-deficient mice developed increased anti-tumor immunity (253, 308). Building on the observation that CD73-deficient mice also have increased anti-tumor immunity, proof-of-concept studies demonstrated the potential of anti-CD73 therapy for cancer immunotherapy (95, 197, 273, 274, 279, 280). Blocking CD73 on host and tumor cells was shown to alleviate adenosine-mediated immunosuppression and to reduce metastasis (197, 309). One study demonstrated a role for B cells in the therapeutic activity of CD73 blockade (310).
Targeting CD73 has also been shown to suppress tumorigenesis independently of its immune-stimulating effects. For instance, gene-silencing of CD73 in human tumor cells is associated with decreased expression of anti-apoptotic proteins (291, 311). Treatment of MDA-MB-231 tumors in SCID mice with anti-CD73 mAb or APCP also significantly delays tumor growth (312, 313) and metastasis (314). CD73 expression in tumor cells also significantly increases their metastatic potential through a mechanism only partly dependent on immunosuppression(154, 273). Activation of A2b receptors on tumor cells is likely an important mechanism by which CD73-adenosine promotes metastasis (154, 273). Recently, A2b receptor signaling in tumor cells was shown to promote ERK activation and FXYD5 expression, a regulator of cell–cell adhesion (315). Other studies have linked A2b signaling to decreased cell adhesion through modulation of Rap1 localization (316). Expression of A2b receptor on tumor cells is at least partly regulated by the transcription factor FOS-related antigen 1 (FRA-1) (317).
Targeted blockade of CD73 also suppresses tumor angiogenesis. Anti-CD73 mAb therapy was indeed shown to reduce vascular endothelial cells present in 4T1.2 tumors (318, 319). Both enzymatic and non-enzymatic functions of CD73 appear to promote tumor angiogenesis. Adenosine-dependent CD73 activity stimulated VEGF production and focal adhesion kinase (FAK) signaling, while adenosine-independent CD73 activity promoted the formation of capillary-like structures by endothelial cells (318). Others have also suggested a role for CD73 in promoting tumor cell adhesion to extracellular matrix proteins. For instance, CD73 was shown to promote melanoma cell adhesion to Tenascin-C independently of its enzymatic activity (320, 321).
A first-in-class therapeutic anti-CD73 mAb, MEDI9447, is currently being evaluated in Phase 1 clinical trial in cancer patients. MEDI9447 binds a discontinuous epitope within the N-terminal domain of CD73, which results in non-competitive inhibition its enzymatic activity (322). MEDI9447 was shown to inhibit both membrane-bound and sCD73. Data from MedImmune suggest that MEDI9447 induces bridging or cross-linking of CD73 dimers, restricting its necessary conformational change (322). Intriguingly, MEDI9447 also blocks surface-bound CD73 through monovalent interactions, presumably through steric hindrance preventing proper rotation. Although MEDI9447 can block sCD73 and surface-bound CD73, it remains unknown which form is preferentially inhibited in the tumor microenvironment. Bristol Myers Squibb (BMS) also recently reported the development of an anti-CD73 mAb that inhibits enzymatic activity and provoke cellular internalization (323). Intriguingly, BMS’s anti-CD73 mAb consists of a human IgG2-IgG1 hybrid antibody with effector function eliminated by mutations of the Fc. It is proposed that the IgG2 portion of this mAb drives superior internalization. Both MedImmune and BMS reported that combined administration of anti-CD73 mAb and PD-1 blockade resulted in improved anti-tumor activity over either single therapy in syngeneic tumor models (309, 323). Innate Pharma also reported a research program around anti-CD73 antibodies. Data presented showed that anti-CD73 mAbs inhibited enzymatic activity could effectively reverse AMP-mediated T cell suppression in vitro (324). Corvus Pharmaceuticals and Arcus Biosciences have also recently disclosed CD73 inhibitor programs.
Targeting adenosine receptors
An alternative approach to targeting CD39 or CD73 is to target adenosine receptors. As mentioned above, A2a is expressed and exerts immunosuppressive activities on various immune subsets. Targeting A2a is thus an attracting strategy to enhance anti-tumor immune responses. Several studies have shown that administration of A2a antagonists can enhance anti-tumor immunity in pre-clinical studies (154, 157, 325, 326). Of note, J. Linden and colleagues have reported that persistent blockade of A2a receptors can be detrimental to anti-tumor T cells, leading to increased activation induced cell death associated with decreased expression of IL-7R (327, 328). Proper dose scheduling of A2a antagonists may therefore be critical to achieving optimal results in the clinic. At least two A2a antagonists are currently in Phase 1 clinical trials in oncology (NCT02403193 and NCT02655822).
Targeting both tumor and host-derived A2b receptor has also been shown to decrease tumor growth and metastasis and to promote anti-tumor immunity (154, 184, 215, 216, 315, 329). Activation of A2b on tumor cells enhances their metastatic potential, while activation of A2b on myeloid cells promote tumor tolerance (154, 184, 215, 216, 315, 316). Of interest, similarly to CD73, A2b gene expression correlates with poor survival in TNBC (315, 317) suggesting that the CD73-A2b axis could be a target for TNBC (330).
Combinational therapies
Targeting multiple immunosuppressive pathways can synergistically enhance anti-tumor immune responses.
As immune cells infiltrating the tumor co-express CD39 in association with other co-inhibitory molecules (e.g. CTLA4 and PDL1), a combinational approach targeting both CD39, CD73 and co-inhibitory molecules has been proposed with the aim to control the immunosuppressive potential of adenosine signaling while minimizing the side effects of anti-CTLA4 and anti-PD1 blockade (250, 251, 288, 295, 302, 330, 331).
Inhibition of adenosine signaling has been shown to synergize with anti-PD-1 or anti CTLA-4 mAbs in preclinical studies (158, 325, 326, 332, 333). Phase 1 clinical trials evaluating CD73 or A2a blockade in combination with PD-1/PD-L1 inhibitors are currently being conducted (NCT02503774 and NCT02655822). Targeting A2a or CD73 in combination with adoptive cell therapy is another promising combination (280). Accumulating evidence suggest that chemotherapy (6) and radiotherapy (334) can also synergize with immunotherapies. Consistent with this notion, inhibition of CD73, A2a or A2b has been shown to enhance the activity of chemotherapy (215, 315, 335). Likewise, CD73 blockade has been shown to augment the efficacy of radiotherapy (336, 337).
Dual targeting of CD73 and A2a receptor
Recently, Young et al. (338) reported on the generation of gene-targeted mice deficient for both CD73 and A2a. Mice deficient in both CD73 and A2a were found to be more resistant to transplanted tumors and carcinogenesis than single knockout (KO) mice. This study revealed for the first time non-redundant roles for host A2a and CD73 in tumor immunity, and suggests that combinatorial therapies may have synergistic activity. Interestingly, tumors in double KO mice showed increased CD8+ T cell distribution within the core of tumors compared to single KO mice. Notably, the authors demonstrated a significant upregulation of CD73 expression in A2a-deficient mice, in both tumor cells and endothelial cells. The authors proposed that this upregulation of CD73 might contribute to resistance to monotherapy with A2a antagonism. Consistent with the phenotype of double KO mice, dual therapy with anti-CD73 mAb and A2A antagonist (SCH58261) showed greater anti-tumor activity than either single agent. Surprisingly, however, combining A2a antagonism with the CD73 inhibitor APCP revealed no increased therapeutic response. As suggested by the authors, this may reflect multi-functionality of CD73 beyond ectonucleotidase activity. Using a tool anti-CD73 mAb (clone TY/23) and a novel anti-CD73 mAb (clone 2C5) cross-reactive to mouse and human CD73, the authors also showed that therapeutic activity of these mAbs is dependent on Fc receptor binding. Interestingly, anti-CD73 mAbs currently in clinical trials have demonstrated anti-tumor activity independent of FcR in mice. Both MedImmune’s MEDI9947 and BMS’ IgG2-IgG1 hybrid mAb lack Fc function (322, 323).
Conclusions
This review has addressed the basic biology of co inhibitory pathway associated with purinergic signaling with the focus on CD39 and CD73, extracellular nucleotide scavenging and adenosine generation. We have discussed how this basic knowledge with the innovative insights now being gained into regulatory cell functionality and vascular biology are being translated to therapy for the common solid and cellular tumors.
Key questions that remain unaddressed include defining the unique and overlapping effects of targeting CD39, CD73 and adenosine receptors; determining the consequences of targeting CD39, CD73 or adenosine receptors on extracellular ATP levels per se; evaluating the activity of dual targeting of CD39 and CD73; developing reliable methods to measure extracellular adenosine levels in the tumor microenviroment; and identifying biomarkers to select patient for treatment with purinergic-targeted therapies.
We are hopeful of the ultimate clinical application of biological therapies targeting CD73 and CD39 that can be used in conjunction with anti-adenosinergic treatments to augment efficacy of other checkpoint blockade inhibitors. It is our prediction that these select purinergic treatments will be used as adjunctive measures with more standard chemotherapies, radiation and surgery.
Figure 1. Effect of ATP on immune cells and vascular endothelium within tumors.
ATP impacts a variety of cells upon engagement of P2 receptors and results in the initiation of those pathways predominantly resulting in heightened inflammation and regulatory cell inhibition. Further, ATP effects result in T cell activation and promote Th17 cell differentiation upon induction of IL-23 and IL-1b from myeloid cells and monocyte-macrophages.
ATP inhibits Tregs, Tr1 and B cells, whilst favoring chemotaxis of NK cells and neutrophils, M1-type macrophage deviation and DC maturation. ATP also modulates a variety of processes linked to the activation of endothelial cells, inflammation and platelet-mediated thrombosis. In the tumor microenvironment, boluses of high extracellular concentrations of ATP as with tumor necrosis act as “danger signals”, resulting in the recruitment of innate immune cells and boosting anti-tumor cell immunity. In contrast, for unclear reasons, low concentrations and repeated exposure to ATP may be paradoxically associated with immunosuppressive manifestations, trophic effects and enhanced tumor growth (339).
Figure 2. Effect of CD39/CD73 expression and adenosine generation within tumors.
In response to hypoxia and other factors (see Table 1), cells in the tumor microenvironment acquire or increase adenosine-generating capabilities, largely through the expression of CD39 and CD73 ectonucleotidases and scavenging of extracellular ATP. Cells expressing CD39 and/or CD73 include tumor cells, cancer-associated fibroblasts (CAF), endothelial cells, Foxp3+ Tregs, Tr1 cells, Th17 cells, γδ T cells, NK cells, invariant (i)NKT cells, effector and memory T cells, B regulatory cells (Breg), myeloid-derived suppressor cells (MDSC), macrophages and neutrophils, inter alia.
CD39 and CD73 expression results in the accumulation of micromolar concentrations of adenosine capable of activating both high-affinity A2a and low-affinity A2b receptors. This molecular mechanism in turn increases tumor cell survival and metastasis, promotes angiogenesis, prevents thrombosis, increases fibrosis, enhances the suppressive function of Tregs, Tr1, macrophages and MDSC. This effect also promotes antigen tolerance, inhibits lymphocyte effector function and prevents memory T cells from differentiation into effector cells and all of these factors facilitate tumor growth.
Table 1.
CD39, CD73 and A2 adenosine receptors as cancer targets.
| Target expression | Target activity | Pre-clinical and clinical studies |
CD39/ENTPD1
|
|
|
CD73/ecto-5’-nucleotidase
|
|
|
A2a adenosine receptor
|
|
|
A2b adenosine receptor
|
|
|
Acknowledgments
This work has been supported by the National Institute of Health grants P01 HL107152 and R21 CA164970 to SCR, by Ben and Rose Cole Charitable PRIA foundation and by Leona M. and Harry B. Helmsley Charitable Trust 281574.5069091.0010 award to SCR through the pilot grant funding program of the Harvard Institute of Translational Immunology.
JS has support from the Canadian Institute of Health Research, the Susan G. Komen Foundation, the Terry Fox Research Institute, the Leukemia and Lymphoma Society of Canada, and the Famille Jean-Guy Sabourin Research Chair in Pharmacology. BA is supported by a MITACS Elevate fellowship.
References
- 1.Trautmann A. Extracellular ATP in the immune system: more than just a “danger signal. ”. Sci Signal. 2009;2:pe6. doi: 10.1126/scisignal.256pe6. [DOI] [PubMed] [Google Scholar]
- 2.Chen Y, Corriden R, Inoue Y, et al. ATP release guides neutrophil chemotaxis via P2Y2 and A3 receptors. Science. 2006;314:1792–1795. doi: 10.1126/science.1132559. [DOI] [PubMed] [Google Scholar]
- 3.Latz E. The inflammasomes: mechanisms of activation and function. Curr Opin Immunol. 2010;22:28–33. doi: 10.1016/j.coi.2009.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Eltzschig HK, Sitkovsky MV, Robson SC. Purinergic Signaling during Inflammation. N. Engl. J. Med. 2012;367:2322–2333. doi: 10.1056/NEJMra1205750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Di Virgilio F, Adinolfi E. Extracellular purines, purinergic receptors and tumor growth. Oncogene. 2016 doi: 10.1038/onc.2016.206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kroemer G, Galluzzi L, Kepp O, Zitvogel L. Immunogenic Cell Death in Cancer Therapy. Annu. Rev. Immunol. 2013;31:51–72. doi: 10.1146/annurev-immunol-032712-100008. [DOI] [PubMed] [Google Scholar]
- 7.Silverman WR, de Rivero Vaccari JP, Locovei S, et al. The pannexin 1 channel activates the inflammasome in neurons and astrocytes. J Biol Chem. 2009;284:18143–18151. doi: 10.1074/jbc.M109.004804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sridharan M, Adderley SP, Bowles EA, et al. Pannexin 1 is the conduit for low oxygen tension-induced ATP release from human erythrocytes. Am J Physiol Heart Circ Physiol. 2010;299:H1146–H1152. doi: 10.1152/ajpheart.00301.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Eltzschig HK, Eckle T, Mager A, et al. ATP release from activated neutrophils occurs via connexin 43 and modulates adenosine-dependent endothelial cell function. Circ. Res. 2006;99:1100–1108. doi: 10.1161/01.RES.0000250174.31269.70. [DOI] [PubMed] [Google Scholar]
- 10.Robertson J, Lang S, Lambert PA, Martin PE. Peptidoglycan derived from Staphylococcus epidermidis induces Connexin43 hemichannel activity with consequences on the innate immune response in endothelial cells. Biochem J. 2010;432:133–143. doi: 10.1042/BJ20091753. [DOI] [PubMed] [Google Scholar]
- 11.Rendu F, Brohard-Bohn B. The platelet release reaction: granules’ constituents, secretion and functions. Platelets. 2001;12:261–273. doi: 10.1080/09537100120068170. [DOI] [PubMed] [Google Scholar]
- 12.Burnstock G, Knight GE. Cellular distribution and functions of P2 receptor subtypes in different systems. Int Rev Cytol. 2004;240:31–304. doi: 10.1016/S0074-7696(04)40002-3. [DOI] [PubMed] [Google Scholar]
- 13.Burnstock G. Purinergic signaling and vascular cell proliferation and death. Arter. Thromb Vasc Biol. 2002;22:364–373. doi: 10.1161/hq0302.105360. [DOI] [PubMed] [Google Scholar]
- 14.Barrett MO, Sesma JI, Ball CB, Jayasekara PS, Jacobson KA, Lazarowski ER, Harden TK. A selective high-affinity antagonist of the P2Y14 receptor inhibits UDP-glucose-stimulated chemotaxis of human neutrophils. Mol Pharmacol. 2013;84:41–49. doi: 10.1124/mol.113.085654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pelegrin P, Barroso-Gutierrez C, Surprenant A. P2X7 receptor differentially couples to distinct release pathways for IL-1beta in mouse macrophage. J Immunol. 2008;180:7147–7157. doi: 10.4049/jimmunol.180.11.7147. [DOI] [PubMed] [Google Scholar]
- 16.Garcia RA, Yan M, Search D, et al. P2Y6 receptor potentiates pro-inflammatory responses in macrophages and exhibits differential roles in atherosclerotic lesion development. PLoS One. 2014;9:e111385. doi: 10.1371/journal.pone.0111385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Idzko M, Dichmann S, Ferrari D, et al. Nucleotides induce chemotaxis and actin polymerization in immature but not mature human dendritic cells via activation of pertussis toxin-sensitive P2y receptors. Blood. 2002;100:925–932. doi: 10.1182/blood.v100.3.925. [DOI] [PubMed] [Google Scholar]
- 18.Marteau F, Communi D, Boeynaems JM, Suarez Gonzalez N. Involvement of multiple P2Y receptors and signaling pathways in the action of adenine nucleotides diphosphates on human monocyte-derived dendritic cells. J Leukoc Biol. 2004;76:796–803. doi: 10.1189/jlb.0104032. [DOI] [PubMed] [Google Scholar]
- 19.Moreschi I, Bruzzone S, Bodrato N, et al. NAADP+ is an agonist of the human P2Y11 purinergic receptor. Cell Calcium. 2008;43:344–355. doi: 10.1016/j.ceca.2007.06.006. [DOI] [PubMed] [Google Scholar]
- 20.Woehrle T, Yip L, Elkhal A, et al. Pannexin-1 hemichannel-mediated ATP release together with P2X1 and P2X4 receptors regulate T-cell activation at the immune synapse. Blood. 2010;116:3475–3484. doi: 10.1182/blood-2010-04-277707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang L, Jacobsen SE, Bengtsson A, Erlinge D. P2 receptor mRNA expression profiles in human lymphocytes, monocytes and CD34+ stem and progenitor cells. BMC Immunol. 2004;5:16. doi: 10.1186/1471-2172-5-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Overes IM, Rijke B de, Horssen-Zoetbrood A van, et al. Expression of P2X5 in lymphoid malignancies results in LRH-1-specific cytotoxic T-cell-mediated lysis. Br J Haematol. 2008;141:799–807. doi: 10.1111/j.1365-2141.2008.07125.x. [DOI] [PubMed] [Google Scholar]
- 23.Tsukimoto M, Tokunaga A, Harada H, Kojima S. Blockade of murine T cell activation by antagonists of P2Y6 and P2X7 receptors. Biochem Biophys Res Commun. 2009;384:512–518. doi: 10.1016/j.bbrc.2009.05.011. [DOI] [PubMed] [Google Scholar]
- 24.Schenk U, Westendorf AM, Radaelli E, et al. Purinergic control of T cell activation by ATP released through pannexin-1 hemichannels. Sci Signal. 2008;1:ra6. doi: 10.1126/scisignal.1160583. [DOI] [PubMed] [Google Scholar]
- 25.Yip L, Woehrle T, Corriden R, et al. Autocrine regulation of T-cell activation by ATP release and P2X7 receptors. FASEB J. 2009;23:1685–1693. doi: 10.1096/fj.08-126458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Beldi G, Wu Y, Banz Y, et al. Natural killer T cell dysfunction in CD39-null mice protects against concanavalin A-induced hepatitis. Hepatology. 2008;48:841–852. doi: 10.1002/hep.22401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schenk U, Frascoli M, Proietti M, et al. ATP inhibits the generation and function of regulatory T cells through the activation of purinergic P2X receptors. Sci Signal. 2011;4:ra12. doi: 10.1126/scisignal.2001270. [DOI] [PubMed] [Google Scholar]
- 28.Aswad F, Kawamura H, Dennert G. High sensitivity of CD4+CD25+ regulatory T cells to extracellular metabolites nicotinamide adenine dinucleotide and ATP: a role for P2X7 receptors. J Immunol. 2005;175:3075–3083. doi: 10.4049/jimmunol.175.5.3075. [DOI] [PubMed] [Google Scholar]
- 29.Mascanfroni ID, Takenaka MC, Yeste A, et al. Metabolic control of type 1 regulatory T cell differentiation by AHR and HIF1-alpha. Nat Med. 2015;21:638–646. doi: 10.1038/nm.3868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Raffaghello L, Chiozzi P, Falzoni S, Di Virgilio F, Pistoia V. The P2X7 receptor sustains the growth of human neuroblastoma cells through a substance P-dependent mechanism. Cancer Res. 2006;66:907–914. doi: 10.1158/0008-5472.CAN-05-3185. [DOI] [PubMed] [Google Scholar]
- 31.Adinolfi E, Callegari MG, Ferrari D, et al. Basal activation of the P2X7 ATP receptor elevates mitochondrial calcium and potential, increases cellular ATP levels, and promotes serum-independent growth. Mol Biol Cell. 2005;16:3260–3272. doi: 10.1091/mbc.E04-11-1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Qiu Y, Li WH, Zhang HQ, Liu Y, Tian XX, Fang WG. P2X7 mediates ATP-driven invasiveness in prostate cancer cells. PLoS One. 2014;9:e114371. doi: 10.1371/journal.pone.0114371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yegutkin GG. Nucleotide- and nucleoside-converting ectoenzymes: Important modulators of purinergic signalling cascade. Biochim. Biophys. Acta BBA - Mol. Cell Res. 2008;1783:673–694. doi: 10.1016/j.bbamcr.2008.01.024. [DOI] [PubMed] [Google Scholar]
- 34.Yegutkin GG. Enzymes involved in metabolism of extracellular nucleotides and nucleosides: functional implications and measurement of activities. Crit. Rev. Biochem. Mol. Biol. 2014;49:473–497. doi: 10.3109/10409238.2014.953627. [DOI] [PubMed] [Google Scholar]
- 35.Moser TL, Kenan DJ, Ashley TA, et al. Endothelial cell surface F1-F0 ATP synthase is active in ATP synthesis and is inhibited by angiostatin. Proc Natl Acad Sci U A. 2001;98:6656–6661. doi: 10.1073/pnas.131067798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kukulski F, Levesque SA, Lavoie EG, et al. Comparative hydrolysis of P2 receptor agonists by NTPDases 1, 2, 3 and 8. Purinergic Signal. 2005;1:193–204. doi: 10.1007/s11302-005-6217-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fredholm BB, IJzerman AP, Jacobson KA, Linden J, Müller CE. International Union of Basic and Clinical Pharmacology. LXXXI. Nomenclature and Classification of Adenosine Receptors—An Update. Pharmacol. Rev. 2011;63:1–34. doi: 10.1124/pr.110.003285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Müller CE, Jacobson KA. Recent developments in adenosine receptor ligands and their potential as novel drugs. Biochim. Biophys. Acta BBA - Biomembr. 2011;1808:1290–1308. doi: 10.1016/j.bbamem.2010.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Milne GR, Palmer TM. Anti-Inflammatory and Immunosuppressive Effects of the A2A Adenosine Receptor. Sci. World J. 2011;11:320–339. doi: 10.1100/tsw.2011.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Antonioli L, Blandizzi C, Pacher P, Haskó G. Immunity, inflammation and cancer: a leading role for adenosine. Nat. Rev. Cancer. 2013;13:842–857. doi: 10.1038/nrc3613. [DOI] [PubMed] [Google Scholar]
- 41.Schulte G, Fredholm BB. Signalling from adenosine receptors to mitogen-activated protein kinases. Cell. Signal. 2003;15:813–827. doi: 10.1016/s0898-6568(03)00058-5. [DOI] [PubMed] [Google Scholar]
- 42.Maliszewski CR, Delespesse GJ, Schoenborn MA, et al. The CD39 lymphoid cell activation antigen. Molecular cloning and structural characterization. J Immunol. 1994;153:3574–3583. [PubMed] [Google Scholar]
- 43.Chadwick BP, Frischauf AM. Cloning and mapping of a human and mouse gene with homology to ecto-ATPase genes. Mamm Genome. 1997;8:668–672. doi: 10.1007/s003359900534. [DOI] [PubMed] [Google Scholar]
- 44.Handa M, Guidotti G. Purification and cloning of a soluble ATP-diphosphohydrolase (apyrase) from potato tubers (Solanum tuberosum) Biochem Biophys Res Commun. 1996;218:916–923. doi: 10.1006/bbrc.1996.0162. [DOI] [PubMed] [Google Scholar]
- 45.Kaczmarek E, Koziak K, Sevigny J, et al. Identification and characterization of CD39/vascular ATP diphosphohydrolase. J Biol Chem. 1996;271:33116–33122. doi: 10.1074/jbc.271.51.33116. [DOI] [PubMed] [Google Scholar]
- 46.Kirley TL, Crawford PA, Smith TM. The structure of the nucleoside triphosphate diphosphohydrolases (NTPDases) as revealed by mutagenic and computational modeling analyses. Purinergic Signal. 2006;2:379–389. doi: 10.1007/s11302-005-5301-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Grinthal A, Guidotti G. CD39, NTPDase 1, is attached to the plasma membrane by two transmembrane domains. Why? Purinergic Signal. 2006;2:391–398. doi: 10.1007/s11302-005-5907-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Grinthal A, Guidotti G. Transmembrane domains confer different substrate specificities and adenosine diphosphate hydrolysis mechanisms on CD39, CD39L1, and chimeras. Biochemistry (Mosc.) 2002;41:1947–1956. doi: 10.1021/bi015563h. [DOI] [PubMed] [Google Scholar]
- 49.Zhong X, Malhotra R, Woodruff R, Guidotti G. Mammalian plasma membrane ecto-nucleoside triphosphate diphosphohydrolase 1, CD39, is not active intracellularly. The N-glycosylation state of CD39 correlates with surface activity and localization. J Biol Chem. 2001;276:41518–41525. doi: 10.1074/jbc.M104415200. [DOI] [PubMed] [Google Scholar]
- 50.Koziak K, Kaczmarek E, Kittel A, et al. Palmitoylation targets CD39/endothelial ATP diphosphohydrolase to caveolae. J Biol Chem. 2000;275:2057–2062. doi: 10.1074/jbc.275.3.2057. [DOI] [PubMed] [Google Scholar]
- 51.Kittel A, Kaczmarek E, Sevigny J, Lengyel K, Csizmadia E, Robson SC. CD39 as a caveolar-associated ectonucleotidase. Biochem Biophys Res Commun. 1999;262:596–599. doi: 10.1006/bbrc.1999.1254. [DOI] [PubMed] [Google Scholar]
- 52.Papanikolaou A, Papafotika A, Murphy C, et al. Cholesterol-dependent lipid assemblies regulate the activity of the ecto-nucleotidase CD39. J Biol Chem. 2005;280:26406–26414. doi: 10.1074/jbc.M413927200. [DOI] [PubMed] [Google Scholar]
- 53.Robson SC, Sévigny J, Zimmermann H. The E-NTPDase family of ectonucleotidases: Structure function relationships and pathophysiological significance. Purinergic Signal. 2006;2:409. doi: 10.1007/s11302-006-9003-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Airas L, Niemelä J, Salmi M, Puurunen T, Smith DJ, Jalkanen S. Differential Regulation and Function of CD73, a Glycosyl-Phosphatidylinositol–linked 70-kD Adhesion Molecule, on Lymphocytes and Endothelial Cells. J. Cell Biol. 1997;136:421–431. doi: 10.1083/jcb.136.2.421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yegutkin G, Bodin P, Burnstock G. Effect of shear stress on the release of soluble ecto-enzymes ATPase and 5′-nucleotidase along with endogenous ATP from vascular endothelial cells. Br. J. Pharmacol. 2000;129:921–926. doi: 10.1038/sj.bjp.0703136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Fini C, Talamo F, Cherri S, Coli M, Floridi A, Ferrara L, Scaloni A. Biochemical and mass spectrometric characterization of soluble ecto-5’-nucleotidase from bull seminal plasma. Biochem. J. 2003;372:443–451. doi: 10.1042/BJ20021687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Buschette-Brambrink S, Gutensohn W. Human Placental Ecto-5′-nucleotidase: Isoforms and Chemical Crosslinking Products of the Membrane-Bound and Isolated Enzyme. Biol. Chem. Hoppe. Seyler. 2009;370:67–74. doi: 10.1515/bchm3.1989.370.1.67. [DOI] [PubMed] [Google Scholar]
- 58.Heuts DPHM, Weissenborn MJ, Olkhov RV, Shaw AM, Gummadova J, Levy C, Scrutton NS. Crystal Structure of a Soluble Form of Human CD73 with Ecto-5′-Nucleotidase Activity. Chem Bio Chem. 2012;13:2384–2391. doi: 10.1002/cbic.201200426. [DOI] [PubMed] [Google Scholar]
- 59.Knapp K, Zebisch M, Pippel J, El-Tayeb A, Müller CE, Sträter N. Crystal Structure of the Human Ecto-5′-Nucleotidase (CD73): Insights into the Regulation of Purinergic Signaling. Structure. 2012;20:2161–2173. doi: 10.1016/j.str.2012.10.001. [DOI] [PubMed] [Google Scholar]
- 60.Resta R, Yamashita Y, Thompson LF. Ecto-enzyme and signaling functions of lymphocyte CD73. Immunol. Rev. 1998;161:95–109. doi: 10.1111/j.1600-065x.1998.tb01574.x. [DOI] [PubMed] [Google Scholar]
- 61.Salazar-Gonzalez JF, Moody DJ, Giorgi JV, Martinez-Maza O, Mitsuyasu RT, Fahey JL. Reduced ecto-5’-nucleotidase activity and enhanced OKT10 and HLA-DR expression on CD8 (T suppressor/cytotoxic) lymphocytes in the acquired immune deficiency syndrome: evidence of CD8 cell immaturity. J. Immunol. Baltim. Md 1950. 1985;135:1778–1785. [PubMed] [Google Scholar]
- 62.Tóth I, Le AQ, Hartjen P, et al. Decreased frequency of CD73+CD8+ T cells of HIV-infected patients correlates with immune activation and T cell exhaustion. J. Leukoc. Biol. 2013;94:551–561. doi: 10.1189/jlb.0113018. [DOI] [PubMed] [Google Scholar]
- 63.Quagliata F, Faig D, Conklyn M, Silber R. Studies on the lymphocyte 5’-nucleotidase in chronic lymphocytic leukemia, infectious mononucleosis, normal subpopulations, and phytohemagglutinin-stimulated cells. Cancer Res. 1974;34:3197–3202. [PubMed] [Google Scholar]
- 64.Porcellini S, Traggiai E, Schenk U, et al. Regulation of peripheral T cell activation by calreticulin. J Exp Med. 2006;203:461–471. doi: 10.1084/jem.20051519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lang PA, Merkler D, Funkner P, et al. Oxidized ATP inhibits T-cell-mediated autoimmunity. Eur J Immunol. 2010;40:2401–2408. doi: 10.1002/eji.200939838. [DOI] [PubMed] [Google Scholar]
- 66.Wang CM, Ploia C, Anselmi F, Sarukhan A, Viola A. Adenosine triphosphate acts as a paracrine signaling molecule to reduce the motility of T cells. EMBO J. 2014;33:1354–1364. doi: 10.15252/embj.201386666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Dwyer KM, Hanidziar D, Putheti P, et al. Expression of CD39 by human peripheral blood CD4+ CD25+ T cells denotes a regulatory memory phenotype. Am J Transpl. 2010;10:2410–2420. doi: 10.1111/j.1600-6143.2010.03291.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Dwyer KM, Deaglio S, Gao W, Friedman D, Strom TB, Robson SC. CD39 and control of cellular immune responses. Purinergic Signal. 2007;3:171–180. doi: 10.1007/s11302-006-9050-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Fang F, Yu M, Cavanagh MM, Hutter Saunders J, Qi Q, Ye Z, Le Saux S, et al. Expression of CD39 on Activated T Cells Impairs their Survival in Older Individuals. Cell Rep. 2016;14:1218–1231. doi: 10.1016/j.celrep.2016.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Gouttefangeas C, Mansur I, Schmid M, et al. The CD39 molecule defines distinct cytotoxic subsets within alloactivated human CD8-positive cells. Eur J Immunol. 1992;22:2681–2685. doi: 10.1002/eji.1830221031. [DOI] [PubMed] [Google Scholar]
- 71.Bai A, Moss A, Rothweiler S, Longhi MS, Wu Y, Junger WG, Robson SC. NADH oxidase-dependent CD39 expression by CD8(+) T cells modulates interferon gamma responses via generation of adenosine. Nat Commun. 2015;6:8819. doi: 10.1038/ncomms9819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Noble A, Mehta H, Lovell A, Papaioannou E, Fairbanks L. IL-12 and IL-4 activate a CD39-dependent intrinsic peripheral tolerance mechanism in CD8(+) T cells. Eur J Immunol. 2016;46:1438–1448. doi: 10.1002/eji.201545939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Doherty GA, Bai A, Hanidziar D, et al. CD73 is a phenotypic marker of effector memory Th17 cells in inflammatory bowel disease. Eur. J. Immunol. 2012;42:3062–3072. doi: 10.1002/eji.201242623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Regateiro FS, Howie D, Nolan KF, Agorogiannis EI, Greaves DR, Cobbold SP, Waldmann H. Generation of anti-inflammatory adenosine byleukocytes is regulated by TGF-β. Eur. J. Immunol. 2011;41:2955–2965. doi: 10.1002/eji.201141512. [DOI] [PubMed] [Google Scholar]
- 75.Francois V, Shehade H, Acolty V, Preyat N, Delrée P, Moser M, Oldenhove G. Intestinal immunopathology is associated with decreased CD73-generated adenosine during lethal infection. Mucosal Immunol. 2015;8:773–784. doi: 10.1038/mi.2014.108. [DOI] [PubMed] [Google Scholar]
- 76.Mann EH, Chambers ES, Chen Y-H, Richards DF, Hawrylowicz CM. 1α,25-dihydroxyvitamin D3 acts via transforming growth factor-β to up-regulate expression of immunosuppressive CD73 on human CD4+ Foxp3− T cells. Immunology. 2015;146:423–431. doi: 10.1111/imm.12519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Murata K, Tsukahara T, Emori M, et al. Identification of a novel human memory T-cell population with the characteristics of stem-like chemo-resistance. Oncoimmunology. 2016;5:e1165376. doi: 10.1080/2162402X.2016.1165376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Flores-Santibáñez F, Fernández D, Meza D, et al. CD73-mediated adenosine production promotes stem cell-like properties in mouse Tc17 cells. Immunology. 2015;146:582–594. doi: 10.1111/imm.12529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Bono MR, Fernández D, Flores-Santibáñez F, Rosemblatt M, Sauma D. CD73 and CD39 ectonucleotidases in T cell differentiation: Beyond immunosuppression. FEBS Lett. 2015;589:3454–3460. doi: 10.1016/j.febslet.2015.07.027. [DOI] [PubMed] [Google Scholar]
- 80.Asseman C, Mauze S, Leach MW, Coffman RL, Powrie F. An essential role for interleukin 10 in the function of regulatory T cells that inhibit intestinal inflammation. J Exp Med. 1999;190:995–1004. doi: 10.1084/jem.190.7.995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Hara M, Kingsley CI, Niimi M, et al. IL-10 is required for regulatory T cells to mediate tolerance to alloantigens in vivo. J Immunol. 2001;166:3789–3796. doi: 10.4049/jimmunol.166.6.3789. [DOI] [PubMed] [Google Scholar]
- 82.Nakamura K, Kitani A, Strober W. Cell contact-dependent immunosuppression by CD4(+)CD25(+) regulatory T cells is mediated by cell surface-bound transforming growth factor beta. J Exp Med. 2001;194:629–644. doi: 10.1084/jem.194.5.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Powrie F, Carlino J, Leach MW, Mauze S, Coffman RL. A critical role for transforming growth factor-beta but not interleukin 4 in the suppression of T helper type 1-mediated colitis by CD45RB(low) CD4+ T cells. J Exp Med. 1996;183:2669–2674. doi: 10.1084/jem.183.6.2669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Collison LW, Workman CJ, Kuo TT, et al. The inhibitory cytokine IL-35 contributes to regulatory T-cell function. Nature. 2007;450:566–569. doi: 10.1038/nature06306. [DOI] [PubMed] [Google Scholar]
- 85.Deaglio S, Dwyer KM, Gao W, et al. Adenosine generation catalyzed by CD39 and CD73 expressed on regulatory T cells mediates immune suppression. J. Exp. Med. 2007;204:1257–1265. doi: 10.1084/jem.20062512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Gibson DJ, Elliott L, McDermott E, et al. Heightened Expression of CD39 by Regulatory T Lymphocytes Is Associated with Therapeutic Remission in Inflammatory Bowel Disease. Inflamm Bowel Dis. 2015;21:2806–2814. doi: 10.1097/MIB.0000000000000566. [DOI] [PubMed] [Google Scholar]
- 87.Zhou Q, Yan J, Putheti P, et al. Isolated CD39 expression on CD4+ T cells denotes both regulatory and memory populations. Am J Transpl. 2009;9:2303–2311. doi: 10.1111/j.1600-6143.2009.02777.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Fletcher JM, Lonergan R, Costelloe L, et al. CD39+Foxp3+ regulatory T Cells suppress pathogenic Th17 cells and are impaired in multiple sclerosis. J Immunol. 2009;183:7602–7610. doi: 10.4049/jimmunol.0901881. [DOI] [PubMed] [Google Scholar]
- 89.Grant CR, Liberal R, Holder BS, et al. Dysfunctional CD39(POS) regulatory T cells and aberrant control of T-helper type 17 cells in autoimmune hepatitis. Hepatology. 2014;59:1007–1015. doi: 10.1002/hep.26583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Longhi MS, Moss A, Bai A, et al. Characterization of human CD39+ Th17 cells with suppressor activity and modulation in inflammatory bowel disease. PLoS One. 2014;9:e87956. doi: 10.1371/journal.pone.0087956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Umansky V, Shevchenko I, Bazhin AV, Utikal J. Extracellular adenosine metabolism in immune cells in melanoma. Cancer Immunol Immunother. 2014;63:1073–1080. doi: 10.1007/s00262-014-1553-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Sun X, Wu Y, Gao W, et al. CD39/ENTPD1 expression by CD4+Foxp3+ regulatory T cells promotes hepatic metastatic tumor growth in mice. Gastroenterology. 2010;139:1030–1040. doi: 10.1053/j.gastro.2010.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Scurr M, Ladell K, Besneux M, et al. Highly prevalent colorectal cancer-infiltrating LAP(+) Foxp3(−) T cells exhibit more potent immunosuppressive activity than Foxp3(+) regulatory T cells. Mucosal Immunol. 2014;7:428–439. doi: 10.1038/mi.2013.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Hilchey SP, Kobie JJ, Cochran MR, et al. Human follicular lymphoma CD39+-infiltrating T cells contribute to adenosine-mediated T cell hyporesponsiveness. J Immunol. 2009;183:6157–6166. doi: 10.4049/jimmunol.0900475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Stagg J, Divisekera U, Duret H, Sparwasser T, Teng MWL, Darcy PK, Smyth MJ. CD73-Deficient Mice Have Increased Antitumor Immunity and Are Resistant to Experimental Metastasis. Cancer Res. 2011;71:2892–2900. doi: 10.1158/0008-5472.CAN-10-4246. [DOI] [PubMed] [Google Scholar]
- 96.Mandapathil M, Hilldorfer B, Szczepanski MJ, et al. Generation and Accumulation of Immunosuppressive Adenosine by Human CD4+CD25highFOXP3+ Regulatory T Cells. J. Biol. Chem. 2010;285:7176–7186. doi: 10.1074/jbc.M109.047423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Burton CT, Westrop SJ, Eccles-James I, Boasso A, Nelson MR, Bower M, Imami N. Altered phenotype of regulatory T cells associated with lack of human immunodeficiency virus (HIV)-1-specific suppressive function. Clin. Exp. Immunol. 2011;166:191–200. doi: 10.1111/j.1365-2249.2011.04451.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Schuler PJ, Saze Z, Hong C-S, et al. Human CD4+CD39+ regulatory T cells produce adenosine upon co-expression of surface CD73 or contact with CD73+ exosomes or CD73+ cells. Clin. Exp. Immunol. 2014;177:531–543. doi: 10.1111/cei.12354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Sim GC, Martin-Orozco N, Jin L, et al. IL-2 therapy promotes suppressive ICOS+ Treg expansion in melanoma patients. J. Clin. Invest. 2014;124:99–110. doi: 10.1172/JCI46266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Rittiner JE, Korboukh I, Hull-Ryde EA, Jin J, Janzen WP, Frye SV, Zylka MJ. AMP is an adenosine A1 receptor agonist. J. Biol. Chem. 2012;287:5301–5309. doi: 10.1074/jbc.M111.291666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.St. Hilaire C, Ziegler SG, Markello TC, et al. NT5E Mutations and Arterial Calcifications. N. Engl. J. Med. 2011;364:432–442. doi: 10.1056/NEJMoa0912923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Rudensky AY. Regulatory T cells and Foxp3. Immunol. Rev. 2011;241:260–268. doi: 10.1111/j.1600-065X.2011.01018.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Clayton A, Al-Taei S, Webber J, Mason MD, Tabi Z. Cancer Exosomes Express CD39 and CD73, Which Suppress T Cells through Adenosine Production. J. Immunol. 2011;187:676–683. doi: 10.4049/jimmunol.1003884. [DOI] [PubMed] [Google Scholar]
- 104.Ohta A, Kini R, Ohta A, Subramanian M, Madasu M, Sitkovsky M. The development and immunosuppressive functions of CD4+ CD25+ FoxP3+ regulatory T cells are under influence of the adenosine-A2A adenosine receptor pathway. Front. Immunol. 2012;3 doi: 10.3389/fimmu.2012.00190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Kinsey GR, Huang L, Jaworska K, et al. Autocrine adenosine signaling promotes regulatory T cell-mediated renal protection. J. Am. Soc. Nephrol. JASN. 2012;23:1528–1537. doi: 10.1681/ASN.2012010070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Ehrentraut H, Westrich JA, Eltzschig HK, Clambey ET. Adora2b Adenosine Receptor Engagement Enhances Regulatory T Cell Abundance during Endotoxin-Induced Pulmonary Inflammation. PLOS ONE. 2012;7:e32416. doi: 10.1371/journal.pone.0032416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Romio M, Reinbeck B, Bongardt S, Hüls S, Burghoff S, Schrader J. Extracellular purine metabolism and signaling of CD73-derived adenosine in murine Treg and Teff cells. Am. J. Physiol. - Cell Physiol. 2011;301:C530–C539. doi: 10.1152/ajpcell.00385.2010. [DOI] [PubMed] [Google Scholar]
- 108.Ring S, Pushkarevskaya A, Schild H, et al. Regulatory T cell-derived adenosine induces dendritic cell migration through the Epac-Rap1 pathway. J. Immunol. Baltim. Md 1950. 2015;194:3735–3744. doi: 10.4049/jimmunol.1401434. [DOI] [PubMed] [Google Scholar]
- 109.Groux H, O’Garra A, Bigler M, Rouleau M, Antonenko S, Vries JE de, Roncarolo MG. A CD4+ T-cell subset inhibits antigen-specific T-cell responses and prevents colitis. Nature. 1997;389:737–742. doi: 10.1038/39614. [DOI] [PubMed] [Google Scholar]
- 110.Gagliani N, Magnani CF, Huber S, et al. Coexpression of CD49b and LAG-3 identifies human and mouse T regulatory type 1 cells. Nat Med. 2013;19:739–746. doi: 10.1038/nm.3179. [DOI] [PubMed] [Google Scholar]
- 111.Akdis M, Verhagen J, Taylor A, et al. Immune responses in healthy and allergic individuals are characterized by a fine balance between allergen-specific T regulatory 1 and T helper 2 cells. J Exp Med. 2004;199:1567–1575. doi: 10.1084/jem.20032058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Bacchetta R, Sartirana C, Levings MK, Bordignon C, Narula S, Roncarolo MG. Growth and expansion of human T regulatory type 1 cells are independent from TCR activation but require exogenous cytokines. Eur J Immunol. 2002;32:2237–2245. doi: 10.1002/1521-4141(200208)32:8<2237::AID-IMMU2237>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 113.Awasthi A, Carrier Y, Peron JP, et al. A dominant function for interleukin 27 in generating interleukin 10-producing anti-inflammatory T cells. Nat Immunol. 2007;8:1380–1389. doi: 10.1038/ni1541. [DOI] [PubMed] [Google Scholar]
- 114.Stumhofer JS, Silver JS, Laurence A, et al. Interleukins 27 and 6 induce STAT3-mediated T cell production of interleukin 10. Nat Immunol. 2007;8:1363–1371. doi: 10.1038/ni1537. [DOI] [PubMed] [Google Scholar]
- 115.Fitzgerald DC, Zhang GX, El-Behi M, et al. Suppression of autoimmune inflammation of the central nervous system by interleukin 10 secreted by interleukin 27-stimulated T cells. Nat Immunol. 2007;8:1372–1379. doi: 10.1038/ni1540. [DOI] [PubMed] [Google Scholar]
- 116.Apetoh L, Quintana FJ, Pot C, et al. The aryl hydrocarbon receptor interacts with c-Maf to promote the differentiation of type 1 regulatory T cells induced by IL-27. Nat Immunol. 2010;11:854–861. doi: 10.1038/ni.1912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Mascanfroni ID, Yeste A, Vieira SM, et al. IL-27 acts on DCs to suppress the T cell response and autoimmunity by inducing expression of the immunoregulatory molecule CD39. Nat Immunol. 2013;14:1054–1063. doi: 10.1038/ni.2695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Bergmann C, Strauss L, Zeidler R, Lang S, Whiteside TL. Expansion and characteristics of human T regulatory type 1 cells in co-cultures simulating tumor microenvironment. Cancer Immunol Immunother. 2007;56:1429–1442. doi: 10.1007/s00262-007-0280-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Mandapathil M, Szczepanski M, Harasymczuk M, et al. CD26 expression and adenosine deaminase activity in regulatory T cells (Treg) and CD4+ T effector cells in patients with head and neck squamous cell carcinoma. Oncoimmunology. 2012;1:659–669. doi: 10.4161/onci.20387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Mandapathil M, Szczepanski MJ, Szajnik M, et al. Adenosine and Prostaglandin E2 Cooperate in the Suppression of Immune Responses Mediated by Adaptive Regulatory T Cells. J. Biol. Chem. 2010;285:27571–27580. doi: 10.1074/jbc.M110.127100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Mandapathil M, Szczepanski MJ, Szajnik M, et al. Increased Ectonucleotidase Expression and Activity in Regulatory T Cells of Patients with Head and Neck Cancer. Clin. Cancer Res. 2009;15:6348–6357. doi: 10.1158/1078-0432.CCR-09-1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Miossec P, Korn T, Kuchroo VK. Interleukin-17 and type 17 helper T cells. N Engl J Med. 2009;361:888–898. doi: 10.1056/NEJMra0707449. [DOI] [PubMed] [Google Scholar]
- 123.Korn T, Bettelli E, Oukka M, Kuchroo VK. IL-17 and Th17 Cells. Annu Rev Immunol. 2009;27:485–517. doi: 10.1146/annurev.immunol.021908.132710. [DOI] [PubMed] [Google Scholar]
- 124.Atarashi K, Nishimura J, Shima T, et al. ATP drives lamina propria T(H)17 cell differentiation. Nature. 2008;455:808–812. doi: 10.1038/nature07240. [DOI] [PubMed] [Google Scholar]
- 125.Younas M, Hue S, Lacabaratz C, et al. IL-7 modulates in vitro and in vivo human memory T regulatory cell functions through the CD39/ATP axis. J Immunol. 2013;191:3161–3168. doi: 10.4049/jimmunol.1203547. [DOI] [PubMed] [Google Scholar]
- 126.Paustian C, Taylor P, Johnson T, et al. Extracellular ATP and Toll-like receptor 2 agonists trigger in human monocytes an activation program that favors T helper 17. PLoS One. 2013;8:e54804. doi: 10.1371/journal.pone.0054804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Martin BN, Wang C, Zhang CJ, et al. T cell-intrinsic ASC critically promotes T(H)17-mediated experimental autoimmune encephalomyelitis. Nat Immunol. 2016;17:583–592. doi: 10.1038/ni.3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Pandolfi JB, Ferraro AA, Sananez I, et al. ATP-Induced Inflammation Drives Tissue-Resident Th17 Cells in Metabolically Unhealthy Obesity. J Immunol. 2016;196:3287–3296. doi: 10.4049/jimmunol.1502506. [DOI] [PubMed] [Google Scholar]
- 129.Chalmin F, Mignot G, Bruchard M, et al. Stat3 and Gfi-1 Transcription Factors Control Th17 Cell Immunosuppressive Activity via the Regulation of Ectonucleotidase Expression. Immunity. 2012;36:362–373. doi: 10.1016/j.immuni.2011.12.019. [DOI] [PubMed] [Google Scholar]
- 130.Thibaudin M, Chaix M, Boidot R, et al. Human ectonucleotidase-expressing CD25high Th17 cells accumulate in breast cancer tumors and exert immunosuppressive functions. OncoImmunology. 2016;5:e1055444. doi: 10.1080/2162402X.2015.1055444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Chatterjee S, Thyagarajan K, Kesarwani P, et al. Reducing CD73 expression by IL1β-Programmed Th17 cells improves immunotherapeutic control of tumors. Cancer Res. 2014;74:6048–6059. doi: 10.1158/0008-5472.CAN-14-1450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Fernandez D, Flores-Santibanez F, Neira J, et al. Purinergic Signaling as a Regulator of Th17 Cell Plasticity. PLoS One. 2016;11:e0157889. doi: 10.1371/journal.pone.0157889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Wilson JM, Kurtz CC, Black SG, Ross WG, Alam MS, Linden J, Ernst PB. The A2B Adenosine Receptor Promotes Th17 Differentiation via Stimulation of Dendritic Cell IL-6. J. Immunol. 2011;186:6746–6752. doi: 10.4049/jimmunol.1100117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Liberal R, Grant CR, Ma Y, et al. CD39 mediated regulation of Th17-cell effector function is impaired in juvenile autoimmune liver disease. J Autoimmun. 2016;72:102–112. doi: 10.1016/j.jaut.2016.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Carding SR, Egan PJ. Gammadelta T cells: functional plasticity and heterogeneity. Nat. Rev. Immunol. 2002;2:336–345. doi: 10.1038/nri797. [DOI] [PubMed] [Google Scholar]
- 136.Rezende RM, Cunha AP da, Kuhn C, et al. Identification and characterization of latency-associated peptide-expressing γδ T cells. Nat. Commun. 2015;6:8726. doi: 10.1038/ncomms9726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Coffey F, Lee S-Y, Buus TB, et al. The TCR ligand-inducible expression of CD73 marks γδ lineage commitment and a metastable intermediate in effector specification. J. Exp. Med. 2014;211:329–343. doi: 10.1084/jem.20131540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Thompson LF, Eltzschig HK, Ibla JC, Wiele CJVD, Resta R, Morote-Garcia JC, Colgan SP. Crucial Role for Ecto-5′-Nucleotidase (CD73) in Vascular Leakage during Hypoxia. J. Exp. Med. 2004;200:1395–1405. doi: 10.1084/jem.20040915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Liang D, Zuo A, Zhao R, et al. CD73 Expressed on γδ T Cells Shapes Their Regulatory Effect in Experimental Autoimmune Uveitis. PloS One. 2016;11:e0150078. doi: 10.1371/journal.pone.0150078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Liang D, Zuo A, Shao H, Chen M, Kaplan HJ, Sun D. Roles of the Adenosine Receptor and CD73 in the Regulatory Effect of γδ T Cells. PLOS ONE. 2014;9:e108932. doi: 10.1371/journal.pone.0108932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Frascoli M, Marcandalli J, Schenk U, Grassi F. Purinergic P2X7 Receptor Drives T Cell Lineage Choice and Shapes Peripheral γδ Cells. J. Immunol. 2012;189:174–180. doi: 10.4049/jimmunol.1101582. [DOI] [PubMed] [Google Scholar]
- 142.Otsuka A, Hanakawa S, Miyachi Y, Kabashima K. CD39: A new surface marker of mouse regulatory γδ T cells. J. Allergy Clin. Immunol. 2013;132:1448–1451. doi: 10.1016/j.jaci.2013.05.037. [DOI] [PubMed] [Google Scholar]
- 143.Ujiie H, Shevach EM. γδ T Cells Protect the Liver and Lungs of Mice from Autoimmunity Induced by Scurfy Lymphocytes. J. Immunol. Baltim. Md 1950. 2016;196:1517–1528. doi: 10.4049/jimmunol.1501774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Daley D, Zambirinis CP, Seifert L, et al. γδ T Cells Support Pancreatic Oncogenesis by Restraining αβ T Cell Activation. Cell. 2016;166:1485.e15–1499.e15. doi: 10.1016/j.cell.2016.07.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Gruenbacher G, Gander H, Rahm A, Idzko M, Nussbaumer O, Thurnher M. Ecto-ATPase CD39 Inactivates Isoprenoid-Derived Vγ9Vδ2 T Cell Phosphoantigens. Cell Rep. 2016;16:444–456. doi: 10.1016/j.celrep.2016.06.009. [DOI] [PubMed] [Google Scholar]
- 146.Gorini S, Callegari G, Romagnoli G, et al. ATP secreted by endothelial cells blocks CX(3)CL 1-elicited natural killer cell chemotaxis and cytotoxicity via P2Y(1)(1) receptor activation. Blood. 2010;116:4492–4500. doi: 10.1182/blood-2009-12-260828. [DOI] [PubMed] [Google Scholar]
- 147.Beldi G, Banz Y, Kroemer A, et al. Deletion of CD39 on natural killer cells attenuates hepatic ischemia/reperfusion injury in mice. Hepatology. 2010;51:1702–1711. doi: 10.1002/hep.23510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Nowak-Machen M, Schmelzle M, Hanidziar D, et al. Pulmonary natural killer T cells play an essential role in mediating hyperoxic acute lung injury. Am J Respir Cell Mol Biol. 2013;48:601–609. doi: 10.1165/rcmb.2012-0180OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Bastid J, Regairaz A, Bonnefoy N, et al. Inhibition of CD39 enzymatic function at the surface of tumor cells alleviates their immunosuppressive activity. Cancer Immunol Res. 2015;3:254–265. doi: 10.1158/2326-6066.CIR-14-0018. [DOI] [PubMed] [Google Scholar]
- 150.Buisseret L, Garaud S, Allard B, et al. Abstract 3361: CD73 expression on tumor-infiltrating breast cancer leukocytes. Cancer Res. 2015;75:3361–3361. [Google Scholar]
- 151.Chatterjee D, Tufa DM, Baehre H, Hass R, Schmidt RE, Jacobs R. Natural killer cells acquire CD73 expression upon exposure to mesenchymal stem cells. Blood. 2014;123:594–595. doi: 10.1182/blood-2013-09-524827. [DOI] [PubMed] [Google Scholar]
- 152.Morandi F, Horenstein AL, Chillemi A, et al. CD56brightCD16− NK Cells Produce Adenosine through a CD38-Mediated Pathway and Act as Regulatory Cells Inhibiting Autologous CD4+ T Cell Proliferation. J. Immunol. 2015;195:965–972. doi: 10.4049/jimmunol.1500591. [DOI] [PubMed] [Google Scholar]
- 153.Wallace KL, Linden J. Adenosine A2A receptors induced on iNKT and NK cells reduce pulmonary inflammation and injury in mice with sickle cell disease. Blood. 2010;116:5010–5020. doi: 10.1182/blood-2010-06-290643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Beavis PA, Divisekera U, Paget C, et al. Blockade of A2A receptors potently suppresses the metastasis of CD73+ tumors. Proc. Natl. Acad. Sci. U. S. A. 2013;110:14711–14716. doi: 10.1073/pnas.1308209110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Häusler SFM, Barrio IM del, Strohschein J, et al. Ectonucleotidases CD39 and CD73 on OvCA cells are potent adenosine-generating enzymes responsible for adenosine receptor 2A-dependent suppression of T cell function and NK cell cytotoxicity. Cancer Immunol. Immunother. 2011;60:1405–1418. doi: 10.1007/s00262-011-1040-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Raskovalova T, Lokshin A, Huang X, Jackson EK, Gorelik DE. Adenosine-mediated inhibition of cytotoxic activity and cytokine production by IL-2/NKp46-Activated NK cells. Immunol. Res. 2006;36:91–99. doi: 10.1385/IR:36:1:91. [DOI] [PubMed] [Google Scholar]
- 157.Hatfield SM, Kjaergaard J, Lukashev D, et al. Immunological mechanisms of the antitumor effects of supplemental oxygenation. Sci. Transl. Med. 2015;7:277ra30–277ra30. doi: 10.1126/scitranslmed.aaa1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Mittal D, Young A, Stannard K, et al. Antimetastatic Effects of Blocking PD-1 and the Adenosine A2A Receptor. Cancer Res. 2014;74:3652–3658. doi: 10.1158/0008-5472.CAN-14-0957. [DOI] [PubMed] [Google Scholar]
- 159.Dwyer KM, Deaglio S, Gao W, Friedman D, Strom TB, Robson SC. CD39 and control of cellular immune responses. Purinergic Signal. 2007;3:171–180. doi: 10.1007/s11302-006-9050-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Saze Z, Schuler PJ, Hong C-S, Cheng D, Jackson EK, Whiteside TL. Adenosine production by human B cells and B cell–mediated suppression of activated T cells. Blood. 2013;122:9–18. doi: 10.1182/blood-2013-02-482406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Figueiró F, Muller L, Funk S, Jackson EK, Battastini AMO, Whiteside TL. Phenotypic and functional characteristics of CD39(high) human regulatory B cells (Breg) Oncoimmunology. 2016;5:e1082703. doi: 10.1080/2162402X.2015.1082703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Thomson LF, Ruedi JM, Glass A, et al. Production and characterization of monoclonal antibodies to the glycosyl phosphatidylinositol-anchored lymphocyte differentiation antigen ecto-5’-nucleotidase (CD73) Tissue Antigens. 1990;35:9–19. doi: 10.1111/j.1399-0039.1990.tb01750.x. [DOI] [PubMed] [Google Scholar]
- 163.Schena F, Volpi S, Faliti CE, et al. Dependence of Immunoglobulin Class Switch Recombination in B Cells on Vesicular Release of ATP and CD73 Ectonucleotidase Activity. Cell Rep. 2013;3:1824–1831. doi: 10.1016/j.celrep.2013.05.022. [DOI] [PubMed] [Google Scholar]
- 164.Airas L, Jalkanen S. CD73 mediates adhesion of B cells to follicular dendritic cells. Blood. 1996;88:1755–1764. [PubMed] [Google Scholar]
- 165.Kaku H, Cheng KF, Al-Abed Y, Rothstein TL. A novel mechanism of B cell-mediated immune suppression through CD73 expression and adenosine production. J. Immunol. Baltim. Md 1950. 2014;193:5904–5913. doi: 10.4049/jimmunol.1400336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Taylor JJ, Pape KA, Jenkins MK. A germinal center-independent pathway generates unswitched memory B cells early in the primary response. J. Exp. Med. 2012;209:597–606. doi: 10.1084/jem.20111696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Anderson SM, Tomayko MM, Ahuja A, Haberman AM, Shlomchik MJ. New markers for murine memory B cells that define mutated and unmutated subsets. J. Exp. Med. 2007;204:2103–2114. doi: 10.1084/jem.20062571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Tomayko MM, Steinel NC, Anderson SM, Shlomchik MJ. Cutting edge: Hierarchy of maturity of murine memory B cell subsets. J. Immunol. Baltim. Md 1950. 2010;185:7146–7150. doi: 10.4049/jimmunol.1002163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Sweet RA, Cullen JL, Shlomchik MJ. Rheumatoid factor B cell memory leads to rapid, switched antibody-forming cell responses. J. Immunol. Baltim. Md 1950. 2013;190:1974–1981. doi: 10.4049/jimmunol.1202816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Clark EA, Grabstein KH, Shu GL. Cultured human follicular dendritic cells. Growth characteristics and interactions with B lymphocytes. J. Immunol. Baltim. Md 1950. 1992;148:3327–3335. [PubMed] [Google Scholar]
- 171.Clark EA, Grabstein KH, Gown AM, Skelly M, Kaisho T, Hirano T, Shu GL. Activation of B lymphocyte maturation by a human follicular dendritic cell line, FDC-1. J. Immunol. Baltim. Md 1950. 1995;155:545–555. [PubMed] [Google Scholar]
- 172.Conter LJ, Song E, Shlomchik MJ, Tomayko MM. CD73 Expression Is Dynamically Regulated in the Germinal Center and Bone Marrow Plasma Cells Are Diminished in Its Absence. PLOS ONE. 2014;9:e92009. doi: 10.1371/journal.pone.0092009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Airas L. CD73 and adhesion of B-cells to follicular dendritic cells. Leuk. Lymphoma. 1998;29:37–47. doi: 10.3109/10428199809058380. [DOI] [PubMed] [Google Scholar]
- 174.Chrobak P, Charlebois R, Rejtar P, El Bikai R, Allard B, Stagg J. CD73 plays a protective role in collagen-induced arthritis. J. Immunol. Baltim. Md 1950. 2015;194:2487–2492. doi: 10.4049/jimmunol.1401416. [DOI] [PubMed] [Google Scholar]
- 175.Berchtold S, Ogilvie AL, Bogdan C, Muhl-Zurbes P, Ogilvie A, Schuler G, Steinkasserer A. Human monocyte derived dendritic cells express functional P2X and P2Y receptors as well as ecto-nucleotidases. FEBS Lett. 1999;458:424–428. doi: 10.1016/s0014-5793(99)01197-7. [DOI] [PubMed] [Google Scholar]
- 176.Wilkin F, Duhant X, Bruyns C, Suarez-Huerta N, Boeynaems JM, Robaye B. The P2Y11 receptor mediates the ATP-induced maturation of human monocyte-derived dendritic cells. J Immunol. 2001;166:7172–7177. doi: 10.4049/jimmunol.166.12.7172. [DOI] [PubMed] [Google Scholar]
- 177.Schnurr M, Toy T, Stoitzner P, et al. ATP gradients inhibit the migratory capacity of specific human dendritic cell types: implications for P2Y11 receptor signaling. Blood. 2003;102:613–620. doi: 10.1182/blood-2002-12-3745. [DOI] [PubMed] [Google Scholar]
- 178.Marteau F, Gonzalez NS, Communi D, Goldman M, Boeynaems J-M, Communi D. Thrombospondin-1 and indoleamine 2,3-dioxygenase are major targets of extracellular ATP in human dendritic cells. Blood. 2005;106:3860–3866. doi: 10.1182/blood-2005-05-1843. [DOI] [PubMed] [Google Scholar]
- 179.Chadet S, Ivanes F, Benoist L, et al. Hypoxia/Reoxygenation Inhibits P2Y11 Receptor Expression and Its Immunosuppressive Activity in Human Dendritic Cells. J Immunol. 2015;195:651–660. doi: 10.4049/jimmunol.1500197. [DOI] [PubMed] [Google Scholar]
- 180.Ghiringhelli F, Apetoh L, Tesniere A, et al. Activation of the NLRP3 inflammasome in dendritic cells induces IL-1β–dependent adaptive immunity against tumors. Nat. Med. 2009;15:1170–1178. doi: 10.1038/nm.2028. [DOI] [PubMed] [Google Scholar]
- 181.Aymeric L, Apetoh L, Ghiringhelli F, et al. Tumor cell death and ATP release prime dendritic cells and efficient anticancer immunity. Cancer Res. 2010;70:855–858. doi: 10.1158/0008-5472.CAN-09-3566. [DOI] [PubMed] [Google Scholar]
- 182.Burch LH, Picher M. E-NTPDases in human airways: Regulation and relevance for chronic lung diseases. Purinergic Signal. 2006;2:399–408. doi: 10.1007/s11302-006-9001-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Novitskiy SV, Ryzhov S, Zaynagetdinov R, et al. Adenosine receptors in regulation of dendritic cell differentiation and function. Blood. 2008;112:1822–1831. doi: 10.1182/blood-2008-02-136325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Cekic C, Sag D, Li Y, Theodorescu D, Strieter RM, Linden J. Adenosine A2B Receptor Blockade Slows Growth of Bladder and Breast Tumors. J. Immunol. 2012;188:198–205. doi: 10.4049/jimmunol.1101845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Salmi M, Jalkanen S. Cell-surface enzymes in control of leukocyte trafficking. Nat Rev Immunol. 2005;5:760–771. doi: 10.1038/nri1705. [DOI] [PubMed] [Google Scholar]
- 186.Eltzschig HK, Ibla JC, Furuta GT, et al. Coordinated adenine nucleotide phosphohydrolysis and nucleoside signaling in posthypoxic endothelium: role of ectonucleotidases and adenosine A2B receptors. J. Exp. Med. 2003;198:783–796. doi: 10.1084/jem.20030891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Ferrante CJ, Pinhal-Enfield G, Elson G, Cronstein BN, Hasko G, Outram S, Leibovich SJ. The adenosine-dependent angiogenic switch of macrophages to an M2-like phenotype is independent of interleukin-4 receptor alpha (IL-4Ralpha) signaling. Inflammation. 2013;36:921–931. doi: 10.1007/s10753-013-9621-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Majumdar S, Aggarwal BB. Adenosine suppresses activation of nuclear factor-kappaB selectively induced by tumor necrosis factor in different cell types. Oncogene. 2003;22:1206–1218. doi: 10.1038/sj.onc.1206184. [DOI] [PubMed] [Google Scholar]
- 189.Elson G, Eisenberg M, Garg C, Outram S, Ferrante CJ, Hasko G, Leibovich SJ. Induction of murine adenosine A(2A) receptor expression by LPS: analysis of the 5’ upstream promoter. Genes Immun. 2013;14:147–153. doi: 10.1038/gene.2012.60. [DOI] [PubMed] [Google Scholar]
- 190.Murphree LJ, Sullivan GW, Marshall MA, Linden J. Lipopolysaccharide rapidly modifies adenosine receptor transcripts in murine and human macrophages: role of NF-kappaB in A(2A) adenosine receptor induction. Biochem J. 2005;391:575–580. doi: 10.1042/BJ20050888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Hyman MC, Petrovic-Djergovic D, Visovatti SH, et al. Self-regulation of inflammatory cell trafficking in mice by the leukocyte surface apyrase CD39. J Clin Invest. 2009;119:1136–1149. doi: 10.1172/JCI36433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Cohen HB, Briggs KT, Marino JP, Ravid K, Robson SC, Mosser DM. TLR stimulation initiates a CD39-based autoregulatory mechanism that limits macrophage inflammatory responses. Blood. 2013;122:1935–1945. doi: 10.1182/blood-2013-04-496216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Hamidzadeh K, Mosser DM. Purinergic Signaling to Terminate TLR Responses in Macrophages. Front. Immunol. 2016;7:74. doi: 10.3389/fimmu.2016.00074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Zanin RF, Braganhol E, Bergamin LS, et al. Differential Macrophage Activation Alters the Expression Profile of NTPDase and Ecto-5′-Nucleotidase. PLOS ONE. 2012;7:e31205. doi: 10.1371/journal.pone.0031205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Eichin D, Laurila JP, Jalkanen S, Salmi M. CD73 Activity is Dispensable for the Polarization of M2 Macrophages. PloS One. 2015;10:e0134721. doi: 10.1371/journal.pone.0134721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Ponce NE, Sanmarco LM, Eberhardt N, García MC, Rivarola HW, Cano RC, Aoki MP. CD73 Inhibition Shifts Cardiac Macrophage Polarization toward a Microbicidal Phenotype and Ameliorates the Outcome of Experimental Chagas Cardiomyopathy. J. Immunol. Baltim. Md 1950. 2016;197:814–823. doi: 10.4049/jimmunol.1600371. [DOI] [PubMed] [Google Scholar]
- 197.Yegutkin GG, Marttila-Ichihara F, Karikoski M, et al. Altered purinergic signaling in CD73-deficient mice inhibits tumor progression. Eur. J. Immunol. 2011;41:1231–1241. doi: 10.1002/eji.201041292. [DOI] [PubMed] [Google Scholar]
- 198.Csóka B, Selmeczy Z, Koscsó B, et al. Adenosine promotes alternative macrophage activation via A2A and A2B receptors. FASEB J. 2012;26:376–386. doi: 10.1096/fj.11-190934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Koscsó B, Csóka B, Kókai E, et al. Adenosine augments IL-10-induced STAT3 signaling in M2c macrophages. J. Leukoc. Biol. 2013;94:1309–1315. doi: 10.1189/jlb.0113043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Kukulski F, Ben Yebdri F, Lecka J, Kauffenstein G, Levesque SA, Martin-Satue M, Sevigny J. Extracellular ATP and P2 receptors are required for IL-8 to induce neutrophil migration. Cytokine. 2009;46:166–170. doi: 10.1016/j.cyto.2009.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Suh BC, Kim JS, Namgung U, Ha H, Kim KT. P2X7 nucleotide receptor mediation of membrane pore formation and superoxide generation in human promyelocytes and neutrophils. J Immunol. 2001;166:6754–6763. doi: 10.4049/jimmunol.166.11.6754. [DOI] [PubMed] [Google Scholar]
- 202.Seifert R, Wenzel K, Eckstein F, Schultz G. Purine and pyrimidine nucleotides potentiate activation of NADPH oxidase and degranulation by chemotactic peptides and induce aggregation of human neutrophils via G proteins. Eur J Biochem. 1989;181:277–285. doi: 10.1111/j.1432-1033.1989.tb14722.x. [DOI] [PubMed] [Google Scholar]
- 203.Vaughan KR, Stokes L, Prince LR, et al. Inhibition of neutrophil apoptosis by ATP is mediated by the P2Y11 receptor. J Immunol. 2007;179:8544–8553. doi: 10.4049/jimmunol.179.12.8544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Barletta KE, Ley K, Mehrad B. Regulation of neutrophil function by adenosine. Arterioscler. Thromb. Vasc. Biol. 2012;32:856–864. doi: 10.1161/ATVBAHA.111.226845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Waeg G van, Van den Berghe G. Purine catabolism in polymorphonuclear neutrophils. Phorbol myristate acetate-induced accumulation of adenosine owing to inactivation of extracellularly released adenosine deaminase. J. Clin. Invest. 1991;87:305–312. doi: 10.1172/JCI114987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Kitakaze M, Hori M, Morioka T, et al. Attenuation of ecto-5’-nucleotidase activity and adenosine release in activated human polymorphonuclear leukocytes. Circ. Res. 1993;73:524–533. doi: 10.1161/01.res.73.3.524. [DOI] [PubMed] [Google Scholar]
- 207.Inoue Y, Chen Y, Hirsh MI, Yip L, Junger WG. A3 and P2Y2 receptors control the recruitment of neutrophils to the lungs in a mouse model of sepsis. Shock Augusta Ga. 2008;30:173–177. doi: 10.1097/shk.0b013e318160dad4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Corriden R, Chen Y, Inoue Y, Beldi G, Robson SC, Insel PA, Junger WG. Ecto-nucleoside triphosphate diphosphohydrolase 1 (E-NTPDase1/CD39) regulates neutrophil chemotaxis by hydrolyzing released ATP to adenosine. J. Biol. Chem. 2008;283:28480–28486. doi: 10.1074/jbc.M800039200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Cronstein BN, Daguma L, Nichols D, Hutchison AJ, Williams M. The adenosine/neutrophil paradox resolved: human neutrophils possess both A1 and A2 receptors that promote chemotaxis and inhibit O2 generation, respectively. J. Clin. Invest. 1990;85:1150–1157. doi: 10.1172/JCI114547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Rosenberger P, Schwab JM, Mirakaj V, et al. Hypoxia-inducible factor-dependent induction of netrin-1 dampens inflammation caused by hypoxia. Nat. Immunol. 2009;10:195–202. doi: 10.1038/ni.1683. [DOI] [PubMed] [Google Scholar]
- 211.Eckle T, Faigle M, Grenz A, Laucher S, Thompson LF, Eltzschig HK. A2B adenosine receptor dampens hypoxia-induced vascular leak. Blood. 2008;111:2024–2035. doi: 10.1182/blood-2007-10-117044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Haas N de, Koning C de, Spilgies L, Vries IJM de, Hato SV. Improving cancer immunotherapy by targeting the STATe of MDSCs. Oncoimmunology. 2016;5:e1196312. doi: 10.1080/2162402X.2016.1196312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Vasquez-Dunddel D, Pan F, Zeng Q, et al. STAT3 regulates arginase-I in myeloid-derived suppressor cells from cancer patients. J. Clin. Invest. 2013;123:1580–1589. doi: 10.1172/JCI60083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Ryzhov S, Novitskiy SV, Goldstein AE, et al. Adenosinergic Regulation of the Expansion and Immunosuppressive Activity of CD11b+Gr1+ Cells. J. Immunol. 2011;187:6120–6129. doi: 10.4049/jimmunol.1101225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Iannone R, Miele L, Maiolino P, Pinto A, Morello S. Blockade of A2b Adenosine Receptor Reduces Tumor Growth and Immune Suppression Mediated by Myeloid-Derived Suppressor Cells in a Mouse Model of Melanoma. Neoplasia. 2013;15:1400. doi: 10.1593/neo.131748. IN10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Sorrentino C, Miele L, Porta A, Pinto A, Morello S. Myeloid-derived suppressor cells contribute to A2B adenosine receptor-induced VEGF production and angiogenesis in a mouse melanoma model. Oncotarget. 2015;6:27478–27489. doi: 10.18632/oncotarget.4393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Ryzhov SV, Pickup MW, Chytil A, et al. Role of TGF-β Signaling in Generation of CD39+CD73+ Myeloid Cells in Tumors. J. Immunol. 2014;193:3155–3164. doi: 10.4049/jimmunol.1400578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Limagne E, Euvrard R, Thibaudin M, et al. Accumulation of MDSC and Th17 Cells in Patients with Metastatic Colorectal Cancer Predicts the Efficacy of a FOLFOX-Bevacizumab Drug Treatment Regimen. Cancer Res. 2016;76:5241–5252. doi: 10.1158/0008-5472.CAN-15-3164. [DOI] [PubMed] [Google Scholar]
- 219.Burnstock G. Purinergic Signalling and Endothelium. Curr. Vasc. Pharmacol. 2016;14:130–145. doi: 10.2174/1570161114666151202204948. [DOI] [PubMed] [Google Scholar]
- 220.Kaczmarek E, Erb L, Koziak K, et al. Modulation of endothelial cell migration by extracellular nucleotides: involvement of focal adhesion kinase and phosphatidylinositol 3-kinase-mediated pathways. Thromb Haemost. 2005;93:735–742. doi: 10.1267/THRO05040735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Lohman AW, Billaud M, Isakson BE. Mechanisms of ATP release and signalling in the blood vessel wall. Cardiovasc. Res. 2012;95:269–280. doi: 10.1093/cvr/cvs187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Kanthi YM, Sutton NR, Pinsky DJ. CD39: Interface Between Vascular Thrombosis and Inflammation. Curr. Atheroscler. Rep. 2014;16:425. doi: 10.1007/s11883-014-0425-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Kanthi Y, Hyman MC, Liao H, et al. Flow-dependent expression of ectonucleotide tri(di)phosphohydrolase-1 and suppression of atherosclerosis. J Clin Invest. 2015;125:3027–3036. doi: 10.1172/JCI79514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Hot A, Lavocat F, Lenief V, Miossec P. Simvastatin inhibits the pro-inflammatory and pro-thrombotic effects of IL-17 and TNF-alpha on endothelial cells. Ann Rheum Dis. 2013;72:754–760. doi: 10.1136/annrheumdis-2012-201887. [DOI] [PubMed] [Google Scholar]
- 225.Feng L, Sun X, Csizmadia E, et al. Vascular CD39/ENTPD1 directly promotes tumor cell growth by scavenging extracellular adenosine triphosphate. Neoplasia. 2011;13:206–216. doi: 10.1593/neo.101332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Synnestvedt K, Furuta GT, Comerford KM, et al. Ecto-5′-nucleotidase (CD73) regulation by hypoxia-inducible factor-1 mediates permeability changes in intestinal epithelia. J. Clin. Invest. 2002;110:993–1002. doi: 10.1172/JCI15337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Eckle T, Krahn T, Grenz A, et al. Cardioprotection by Ecto-5′-Nucleotidase (CD73) and A2B Adenosine Receptors. Circulation. 2007;115:1581–1590. doi: 10.1161/CIRCULATIONAHA.106.669697. [DOI] [PubMed] [Google Scholar]
- 228.Yegutkin GG, Auvinen K, Rantakari P, et al. Ecto-5′-nucleotidase/CD73 enhances endothelial barrier function and sprouting in blood but not lymphatic vasculature. Eur. J. Immunol. 2015;45:562–573. doi: 10.1002/eji.201444856. [DOI] [PubMed] [Google Scholar]
- 229.Lennon PF, Taylor CT, Stahl GL, Colgan SP. Neutrophil-derived 5′-Adenosine Monophosphate Promotes Endothelial Barrier Function via CD73-mediated Conversion to Adenosine and Endothelial A2B Receptor Activation. J. Exp. Med. 1998;188:1433–1443. doi: 10.1084/jem.188.8.1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Bynoe MS, Viret C, Yan A, Kim D-G. Adenosine receptor signaling: a key to opening the blood–brain door. Fluids Barriers CNS. 2015;12:20. doi: 10.1186/s12987-015-0017-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Mills JH, Thompson LF, Mueller C, et al. CD73 is required for efficient entry of lymphocytes into the central nervous system during experimental autoimmune encephalomyelitis. Proc. Natl. Acad. Sci. 2008;105:9325–9330. doi: 10.1073/pnas.0711175105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Mills JH, Kim D-G, Krenz A, Chen J-F, Bynoe MS. A2A Adenosine Receptor Signaling in Lymphocytes and the Central Nervous System Regulates Inflammation during Experimental Autoimmune Encephalomyelitis. J. Immunol. 2012;188:5713–5722. doi: 10.4049/jimmunol.1200545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Mills JH, Alabanza LM, Mahamed DA, Bynoe MS. Extracellular adenosine signaling induces CX3CL1 expression in the brain to promote experimental autoimmune encephalomyelitis. J. Neuroinflammation. 2012;9:193. doi: 10.1186/1742-2094-9-193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Shechter R, Miller O, Yovel G, et al. Recruitment of Beneficial M2 Macrophages to Injured Spinal Cord Is Orchestrated by Remote Brain Choroid Plexus. Immunity. 2013;38:555–569. doi: 10.1016/j.immuni.2013.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Carman AJ, Mills JH, Krenz A, Kim D-G, Bynoe MS. Adenosine Receptor Signaling Modulates Permeability of the Blood–Brain Barrier. J. Neurosci. 2011;31:13272–13280. doi: 10.1523/JNEUROSCI.3337-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Kim D-G, Bynoe MS. A2A adenosine receptor modulates drug efflux transporter P-glycoprotein at the blood-brain barrier. J. Clin. Invest. 2016;126:1717–1733. doi: 10.1172/JCI76207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Kim D-G, Bynoe MS. A2A Adenosine Receptor Regulates the Human Blood-Brain Barrier Permeability. Mol. Neurobiol. 2014;52:664–678. doi: 10.1007/s12035-014-8879-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Airas L, Hellman J, Salmi M, Bono P, Puurunen T, Smith DJ, Jalkanen S. CD73 is involved in lymphocyte binding to the endothelium: characterization of lymphocyte-vascular adhesion protein 2 identifies it as CD73. J. Exp. Med. 1995;182:1603–1608. doi: 10.1084/jem.182.5.1603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Airas L, Niemelä J, Jalkanen S. CD73 Engagement Promotes Lymphocyte Binding to Endothelial Cells Via a Lymphocyte Function-Associated Antigen-1-Dependent Mechanism. J. Immunol. 2000;165:5411–5417. doi: 10.4049/jimmunol.165.10.5411. [DOI] [PubMed] [Google Scholar]
- 240.Henttinen T, Jalkanen S, Yegutkin GG. Adherent Leukocytes Prevent Adenosine Formation and Impair Endothelial Barrier Function by Ecto-5′-nucleotidase/CD73-dependent Mechanism. J. Biol. Chem. 2003;278:24888–24895. doi: 10.1074/jbc.M300779200. [DOI] [PubMed] [Google Scholar]
- 241.Ålgars A, Karikoski M, Yegutkin GG, Stoitzner P, Niemelä J, Salmi M, Jalkanen S. Different role of CD73 in leukocyte trafficking via blood and lymph vessels. Blood. 2011;117:4387–4393. doi: 10.1182/blood-2010-11-321646. [DOI] [PubMed] [Google Scholar]
- 242.Takedachi M, Qu D, Ebisuno Y, et al. CD73-Generated Adenosine Restricts Lymphocyte Migration into Draining Lymph Nodes. J. Immunol. 2008;180:6288–6296. doi: 10.4049/jimmunol.180.9.6288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Markello TC, Pak LK, St. Hilaire C, et al. Vascular pathology of medial arterial calcifications in NT5E deficiency: Implications for the role of adenosine in pseudoxanthoma elasticum. Mol. Genet. Metab. 2011;103:44–50. doi: 10.1016/j.ymgme.2011.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Zhang Z, He J-W, Fu W-Z, Zhang C-Q, Zhang Z-L. Calcification of joints and arteries: second report with novel NT5E mutations and expansion of the phenotype. J. Hum. Genet. 2015;60:561–564. doi: 10.1038/jhg.2015.85. [DOI] [PubMed] [Google Scholar]
- 245.Li Q, Price TP, Sundberg JP, Uitto J. Juxta-articular joint-capsule mineralization in CD73 deficient mice: Similarities to patients with NT5E mutations. Cell Cycle. 2014;13:2609–2615. doi: 10.4161/15384101.2014.943567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Pellegatti P, Raffaghello L, Bianchi G, Piccardi F, Pistoia V, Virgilio FD. Increased Level of Extracellular ATP at Tumor Sites: In Vivo Imaging with Plasma Membrane Luciferase. PLOS ONE. 2008;3:e2599. doi: 10.1371/journal.pone.0002599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Virgilio FD. Purines, Purinergic Receptors, and Cancer. Cancer Res. 2012;72:5441–5447. doi: 10.1158/0008-5472.CAN-12-1600. [DOI] [PubMed] [Google Scholar]
- 248.Aymeric L, Apetoh L, Ghiringhelli F, et al. Tumor Cell Death and ATP Release Prime Dendritic Cells and Efficient Anticancer Immunity. Cancer Res. 2010;70:855–858. doi: 10.1158/0008-5472.CAN-09-3566. [DOI] [PubMed] [Google Scholar]
- 249.Michaud M, Martins I, Sukkurwala AQ, et al. Autophagy-Dependent Anticancer Immune Responses Induced by Chemotherapeutic Agents in Mice. Science. 2011;334:1573–1577. doi: 10.1126/science.1208347. [DOI] [PubMed] [Google Scholar]
- 250.Allard B, Beavis PA, Darcy PK, Stagg J. Immunosuppressive activities of adenosine in cancer. Curr. Opin. Pharmacol. 2016;29:7–16. doi: 10.1016/j.coph.2016.04.001. [DOI] [PubMed] [Google Scholar]
- 251.Allard D, Allard B, Gaudreau P-O, Chrobak P, Stagg J. CD73–adenosine: a next-generation target in immuno-oncology. Immunotherapy. 2016;8:145–163. doi: 10.2217/imt.15.106. [DOI] [PubMed] [Google Scholar]
- 252.Antonioli L, Pacher P, Vizi ES, Haskó G. CD39 and CD73 in immunity and inflammation. Trends Mol. Med. 2013;19:355–367. doi: 10.1016/j.molmed.2013.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Ohta A, Gorelik E, Prasad SJ, et al. A2A adenosine receptor protects tumors from antitumor T cells. Proc. Natl. Acad. Sci. 2006;103:13132–13137. doi: 10.1073/pnas.0605251103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Blay J, White TD, Hoskin DW. The extracellular fluid of solid carcinomas contains immunosuppressive concentrations of adenosine. Cancer Res. 1997;57:2602–2605. [PubMed] [Google Scholar]
- 255.Hatfield SM, Kjaergaard J, Lukashev D, et al. Systemic oxygenation weakens the hypoxia and hypoxia inducible factor 1α-dependent and extracellular adenosine-mediated tumor protection. J. Mol. Med. 2014;92:1283–1292. doi: 10.1007/s00109-014-1189-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Burnstock G, Di Virgilio F. Purinergic signalling and cancer. Purinergic Signal. 2013;9:491–540. doi: 10.1007/s11302-013-9372-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Di Virgilio F, Bronte V, Collavo D, Zanovello P. Responses of mouse lymphocytes to extracellular adenosine 5’-triphosphate (ATP). Lymphocytes with cytotoxic activity are resistant to the permeabilizing effects of ATP. J Immunol. 1989;143:1955–1960. [PubMed] [Google Scholar]
- 258.Surprenant A, Rassendren F, Kawashima E, North RA, Buell G. The cytolytic P2Z receptor for extracellular ATP identified as a P2X receptor (P2X7) Science. 1996;272:735–738. doi: 10.1126/science.272.5262.735. [DOI] [PubMed] [Google Scholar]
- 259.Baricordi OR, Ferrari D, Melchiorri L, et al. An ATP-activated channel is involved in mitogenic stimulation of human T lymphocytes. Blood. 1996;87:682–690. [PubMed] [Google Scholar]
- 260.Adinolfi E, Melchiorri L, Falzoni S, et al. P2X7 receptor expression in evolutive and indolent forms of chronic B lymphocytic leukemia. Blood. 2002;99:706–708. doi: 10.1182/blood.v99.2.706. [DOI] [PubMed] [Google Scholar]
- 261.Bianchi G, Vuerich M, Pellegatti P, et al. ATP/P2X7 axis modulates myeloid-derived suppressor cell functions in neuroblastoma microenvironment. Cell Death Dis. 2014;5:e1135. doi: 10.1038/cddis.2014.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Amoroso F, Capece M, Rotondo A, et al. The P2X7 receptor is a key modulator of the PI3K/GSK3beta/VEGF signaling network: evidence in experimental neuroblastoma. Oncogene. 2015;34:5240–5251. doi: 10.1038/onc.2014.444. [DOI] [PubMed] [Google Scholar]
- 263.Adinolfi E, Callegari MG, Cirillo M, et al. Expression of the P2X7 receptor increases the Ca2+ content of the endoplasmic reticulum, activates NFATc1, and protects from apoptosis. J Biol Chem. 2009;284:10120–10128. doi: 10.1074/jbc.M805805200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Tafani M, Schito L, Pellegrini L, et al. Hypoxia-increased RAGE and P2X7R expression regulates tumor cell invasion through phosphorylation of Erk1/2 and Akt and nuclear translocation of NF-{kappa}B. Carcinogenesis. 2011;32:1167–1175. doi: 10.1093/carcin/bgr101. [DOI] [PubMed] [Google Scholar]
- 265.Wilhelm K, Ganesan J, Muller T, et al. Graft-versus-host disease is enhanced by extracellular ATP activating P2X7R. Nat Med. 2010;16:1434–1438. doi: 10.1038/nm.2242. [DOI] [PubMed] [Google Scholar]
- 266.Vergani A, Tezza S, D’Addio F, et al. Long-term heart transplant survival by targeting the ionotropic purinergic receptor P2X7. Circulation. 2013;127:463–475. doi: 10.1161/CIRCULATIONAHA.112.123653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Adinolfi E, Capece M, Franceschini A, et al. Accelerated tumor progression in mice lacking the ATP receptor P2X7. Cancer Res. 2015;75:635–644. doi: 10.1158/0008-5472.CAN-14-1259. [DOI] [PubMed] [Google Scholar]
- 268.Hofman P, Cherfils-Vicini J, Bazin M, et al. Genetic and pharmacological inactivation of the purinergic P2RX7 receptor dampens inflammation but increases tumor incidence in a mouse model of colitis-associated cancer. Cancer Res. 2015;75:835–845. doi: 10.1158/0008-5472.CAN-14-1778. [DOI] [PubMed] [Google Scholar]
- 269.Li WH, Qiu Y, Zhang HQ, Liu Y, You JF, Tian XX, Fang WG. P2Y2 receptor promotes cell invasion and metastasis in prostate cancer cells. Br J Cancer. 2013;109:1666–1675. doi: 10.1038/bjc.2013.484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Joo YN, Jin H, Eun SY, Park SW, Chang KC, Kim HJ. P2Y2R activation by nucleotides released from the highly metastatic breast cancer cell MDA-MB-231 contributes to pre-metastatic niche formation by mediating lysyl oxidase secretion, collagen crosslinking, and monocyte recruitment. Oncotarget. 2014;5:9322–9334. doi: 10.18632/oncotarget.2427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Yang G, Zhang S, Zhang Y, et al. The inhibitory effects of extracellular ATP on the growth of nasopharyngeal carcinoma cells via P2Y2 receptor and osteopontin. J Exp Clin Cancer Res. 2014;33:53. doi: 10.1186/1756-9966-33-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Coutinho-Silva R, Stahl L, Cheung KK, Campos NE de, Oliveira Souza C de, Ojcius DM, Burnstock G. P2X and P2Y purinergic receptors on human intestinal epithelial carcinoma cells: effects of extracellular nucleotides on apoptosis and cell proliferation. Am J Physiol Gastrointest Liver Physiol. 2005;288:G1024–G1035. doi: 10.1152/ajpgi.00211.2004. [DOI] [PubMed] [Google Scholar]
- 273.Stagg J, Divisekera U, McLaughlin N, et al. Anti-CD73 antibody therapy inhibits breast tumor growth and metastasis. Proc. Natl. Acad. Sci. 2010;107:1547–1552. doi: 10.1073/pnas.0908801107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Jin D, Fan J, Wang L, et al. CD73 on Tumor Cells Impairs Antitumor T-Cell Responses: A Novel Mechanism of Tumor-Induced Immune Suppression. Cancer Res. 2010;70:2245–2255. doi: 10.1158/0008-5472.CAN-09-3109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Serra S, Horenstein AL, Vaisitti T, et al. CD73-generated extracellular adenosine in chronic lymphocytic leukemia creates local conditions counteracting drug-induced cell death. Blood. 2011;118:6141–6152. doi: 10.1182/blood-2011-08-374728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Kunzli BM, Bernlochner MI, Rath S, et al. Impact of CD39 and purinergic signalling on the growth and metastasis of colorectal cancer. Purinergic Signal. 2011;7:231–241. doi: 10.1007/s11302-011-9228-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Jackson SW, Hoshi T, Wu Y, et al. Disordered purinergic signaling inhibits pathological angiogenesis in cd39/Entpd1-null mice. Am. J. Pathol. 2007;171:1395–1404. doi: 10.2353/ajpath.2007.070190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Goepfert C, Sundberg C, Sévigny J, Enjyoji K, Hoshi T, Csizmadia E, Robson S. Disordered cellular migration and angiogenesis in cd39-null mice. Circulation. 2001;104:3109–3115. doi: 10.1161/hc5001.100663. [DOI] [PubMed] [Google Scholar]
- 279.Stagg J, Beavis PA, Divisekera U, Liu MCP, Möller A, Darcy PK, Smyth MJ. CD73-Deficient Mice Are Resistant to Carcinogenesis. Cancer Res. 2012;72:2190–2196. doi: 10.1158/0008-5472.CAN-12-0420. [DOI] [PubMed] [Google Scholar]
- 280.Wang L, Fan J, Thompson LF, Zhang Y, Shin T, Curiel TJ, Zhang B. CD73 has distinct roles in nonhematopoietic and hematopoietic cells to promote tumor growth in mice. J. Clin. Invest. 2011;121:2371–2382. doi: 10.1172/JCI45559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Sun X, Han L, Seth P, et al. Disordered purinergic signaling and abnormal cellular metabolism are associated with development of liver cancer in Cd39/ENTPD1 null mice. Hepatology. 2013;57:205–216. doi: 10.1002/hep.25989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Kunzli BM, Berberat PO, Giese T, et al. Upregulation of CD39/NTPDases and P2 receptors in human pancreatic disease. Am J Physiol Gastrointest Liver Physiol. 2007;292:G223–G230. doi: 10.1152/ajpgi.00259.2006. [DOI] [PubMed] [Google Scholar]
- 283.Zhang B, Cheng B, Li FS, et al. High expression of CD39/ENTPD1 in malignant epithelial cells of human rectal adenocarcinoma. Tumour Biol. 2015;36:9411–9419. doi: 10.1007/s13277-015-3683-9. [DOI] [PubMed] [Google Scholar]
- 284.Montalbán Del Barrio I, Penski C, Schlahsa L, et al. Adenosine-generating ovarian cancer cells attract myeloid cells which differentiate into adenosine-generating tumor associated macrophages - a self-amplifying, CD39- and CD73-dependent mechanism for tumor immune escape. J. Immunother. Cancer. 2016;4:49. doi: 10.1186/s40425-016-0154-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Pulte D, Furman RR, Broekman MJ, et al. CD39 expression on T lymphocytes correlates with severity of disease in patients with chronic lymphocytic leukemia. Clin Lymphoma Myeloma Leuk. 2011;11:367–372. doi: 10.1016/j.clml.2011.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Perry C, Hazan-Halevy I, Kay S, et al. Increased CD39 expression on CD4(+) T lymphocytes has clinical and prognostic significance in chronic lymphocytic leukemia. Ann Hematol. 2012;91:1271–1279. doi: 10.1007/s00277-012-1425-2. [DOI] [PubMed] [Google Scholar]
- 287.Vaupel P, Mayer A. Hypoxia-Driven Adenosine Accumulation: A Crucial Microenvironmental Factor Promoting Tumor Progression. Adv. Exp. Med. Biol. 2016;876:177–183. doi: 10.1007/978-1-4939-3023-4_22. [DOI] [PubMed] [Google Scholar]
- 288.Sitkovsky MV, Hatfield S, Abbott R, Belikoff B, Lukashev D, Ohta A. Hostile, Hypoxia–A2-Adenosinergic Tumor Biology as the Next Barrier to Overcome for Tumor Immunologists. Cancer Immunol. Res. 2014;2:598–605. doi: 10.1158/2326-6066.CIR-14-0075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.Spychala J, Kitajewski J. Wnt and β-catenin signaling target the expression of ecto-5′-nucleotidase and increase extracellular adenosine generation. Exp. Cell Res. 2004;296:99–108. doi: 10.1016/j.yexcr.2003.11.001. [DOI] [PubMed] [Google Scholar]
- 290.Xiong L, Wen Y, Miao X, Yang Z. NT5E and FcGBP as key regulators of TGF-1-induced epithelial-mesenchymal transition (EMT) are associated with tumor progression and survival of patients with gallbladder cancer. Cell Tissue Res. 2014;355:365–374. doi: 10.1007/s00441-013-1752-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Turcotte M, Spring K, Pommey S, et al. CD73 Is Associated with Poor Prognosis in High-Grade Serous Ovarian Cancer. Cancer Res. 2015;75:4494–4503. doi: 10.1158/0008-5472.CAN-14-3569. [DOI] [PubMed] [Google Scholar]
- 292.Leclerc BG, Charlebois R, Chouinard G, Allard B, Pommey S, Saad F, Stagg J. CD73 Expression Is an Independent Prognostic Factor in Prostate Cancer. Clin. Cancer Res. 2016;22:158–166. doi: 10.1158/1078-0432.CCR-15-1181. [DOI] [PubMed] [Google Scholar]
- 293.Lawrence RT, Perez EM, Hernández D, et al. The Proteomic Landscape of Triple-Negative Breast Cancer. Cell Rep. 2015;11:630–644. doi: 10.1016/j.celrep.2015.03.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.Sunaga N, Shames DS, Girard L, et al. Knockdown of Oncogenic KRAS in Non–Small Cell Lung Cancers Suppresses Tumor Growth and Sensitizes Tumor Cells to Targeted Therapy. Mol. Cancer Ther. 2011;10:336–346. doi: 10.1158/1535-7163.MCT-10-0750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.Antonioli L, Yegutkin GG, Pacher P, Blandizzi C, Haskó G. Anti-CD73 in Cancer Immunotherapy: Awakening New Opportunities. Trends Cancer. 2016;2:95–109. doi: 10.1016/j.trecan.2016.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.Supernat A, Markiewicz A, Welnicka-Jaskiewicz M, et al. CD73 expression as a potential marker of good prognosis in breast carcinoma. Appl. Immunohistochem. Mol. Morphol. AIMM Off. Publ. Soc. Appl. Immunohistochem. 2012;20:103–107. doi: 10.1097/pai.0b013e3182311d82. [DOI] [PubMed] [Google Scholar]
- 297.Bowser JL, Blackburn MR, Shipley GL, Molina JG, Dunner K, Broaddus RR. Loss of CD73-mediated actin polymerization promotes endometrial tumor progression. J. Clin. Invest. 126:220–238. doi: 10.1172/JCI79380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298.Huang Q, Durham NM, Sult E, et al. Abstract 1538: Levels and enzyme activity of CD73 in primary samples from cancer patients. Cancer Res. 2015;75:1538–1538. [Google Scholar]
- 299.Maksimow M, Kyhälä L, Nieminen A, et al. Early prediction of persistent organ failure by soluble CD73 in patients with acute pancreatitis*. Crit. Care Med. 2014;42:2556–2564. doi: 10.1097/CCM.0000000000000550. [DOI] [PubMed] [Google Scholar]
- 300.Liu H, Zhang Y, Wu H, et al. Beneficial Role of Erythrocyte Adenosine A2B Receptor-Mediated AMP-Activated Protein Kinase Activation in High-Altitude Hypoxia. Circulation. 2016;134:405–421. doi: 10.1161/CIRCULATIONAHA.116.021311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301.Shen L, Sundstedt A, Ciesielski M, et al. Tasquinimod modulates suppressive myeloid cells and enhances cancer immunotherapies in murine models. Cancer Immunol Res. 2015;3:136–148. doi: 10.1158/2326-6066.CIR-14-0036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302.Bastid J, Cottalorda-Regairaz A, Alberici G, Bonnefoy N, Eliaou JF, Bensussan A. ENTPD1/CD39 is a promising therapeutic target in oncology. Oncogene. 2013;32:1743–1751. doi: 10.1038/onc.2012.269. [DOI] [PubMed] [Google Scholar]
- 303.Nikolova M, Carriere M, Jenabian MA, et al. CD39/adenosine pathway is involved in AIDS progression. PLoS Pathog. 2011;7:e1002110. doi: 10.1371/journal.ppat.1002110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304.Masciarelli S, Mattioli B, Galletti R, Samoggia P, Chichiarelli S, Mearini G, Mattia E. Antisense to Epstein Barr Virus-encoded LMP1 does not affect the transcription of viral and cellular proliferation-related genes, but induces phenotypic effects on EBV-transformed B lymphocytes. Oncogene. 2002;21:4166–4170. doi: 10.1038/sj.onc.1205515. [DOI] [PubMed] [Google Scholar]
- 305.Thompson LF, Ruedi JM, Glass A, Low MG, Lucas AH. Antibodies to 5’-nucleotidase (CD73), a glycosyl-phosphatidylinositol-anchored protein, cause human peripheral blood T cells to proliferate. J. Immunol. 1989;143:1815–1821. [PubMed] [Google Scholar]
- 306.Loertscher R, Lavery P. The role of glycosyl phosphatidyl inositol (GPI)-anchored cell surface proteins in T-cell activation. Transpl. Immunol. 2002;9:93–96. doi: 10.1016/s0966-3274(02)00013-8. [DOI] [PubMed] [Google Scholar]
- 307.MacKenzie WM, Hoskin DW, Blay J. Adenosine inhibits the adhesion of anti-CD3-activated killer lymphocytes to adenocarcinoma cells through an A3 receptor. Cancer Res. 1994;54:3521–3526. [PubMed] [Google Scholar]
- 308.Ohta A, Sitkovsky M. Role of G-protein-coupled adenosine receptors in downregulation of inflammation and protection from tissue damage. Nature. 2001;414:916–920. doi: 10.1038/414916a. [DOI] [PubMed] [Google Scholar]
- 309.Hay CM, Sult E, Huang Q, Mulgrew K, Fuhrmann SR, McGlinchey KA, Hammond SA, et al. Targeting CD73 in the tumor microenvironment with MEDI9447. OncoImmunology. 2016;5:e1208875. doi: 10.1080/2162402X.2016.1208875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 310.Forte G, Sorrentino R, Montinaro A, et al. Inhibition of CD73 Improves B Cell-Mediated Anti-Tumor Immunity in a Mouse Model of Melanoma. J. Immunol. 2012;189:2226–2233. doi: 10.4049/jimmunol.1200744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 311.Zhi X, Wang Y, Zhou X, et al. RNAi-mediated CD73 suppression induces apoptosis and cell-cycle arrest in human breast cancer cells. Cancer Sci. 2010;101:2561–2569. doi: 10.1111/j.1349-7006.2010.01733.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 312.Zhou X, Zhi X, Zhou P, et al. Effects of ecto-5’-nucleotidase on human breast cancer cell growth in vitro and in vivo. Oncol. Rep. 2007;17:1341–1346. [PubMed] [Google Scholar]
- 313.Rust S, Guillard S, Sachsenmeier K, et al. Combining phenotypic and proteomic approaches to identify membrane targets in a “triple negative” breast cancer cell type. Mol. Cancer. 2013;12:11. doi: 10.1186/1476-4598-12-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 314.Terp MG, Olesen KA, Arnspang EC, Lund RR, Lagerholm BC, Ditzel HJ, Leth-Larsen R. Anti-Human CD73 Monoclonal Antibody Inhibits Metastasis Formation in Human Breast Cancer by Inducing Clustering and Internalization of CD73 Expressed on the Surface of Cancer Cells. J. Immunol. 2013;191:4165–4173. doi: 10.4049/jimmunol.1301274. [DOI] [PubMed] [Google Scholar]
- 315.Mittal D, Sinha D, Barkauskas D, et al. Adenosine 2B Receptor Expression on Cancer Cells Promotes Metastasis. Cancer Res. 2016;76:4372–4382. doi: 10.1158/0008-5472.CAN-16-0544. [DOI] [PubMed] [Google Scholar]
- 316.Ntantie E, Gonyo P, Lorimer EL, et al. An Adenosine-Mediated Signaling Pathway Suppresses Prenylation of the GTPase Rap1B and Promotes Cell Scattering. Sci. Signal. 2013;6:ra39. doi: 10.1126/scisignal.2003374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 317.Desmet CJ, Gallenne T, Prieur A, et al. Identification of a pharmacologically tractable Fra-1/ADORA2B axis promoting breast cancer metastasis. Proc. Natl. Acad. Sci. 2013;110:5139–5144. doi: 10.1073/pnas.1222085110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 318.Allard B, Turcotte M, Spring K, Pommey S, Royal I, Stagg J. Anti-CD73 therapy impairs tumor angiogenesis. Int. J. Cancer J. Int. Cancer. 2014;134:1466–1473. doi: 10.1002/ijc.28456. [DOI] [PubMed] [Google Scholar]
- 319.Wang L, Tang S, Wang Y, et al. Ecto-5′-nucleotidase (CD73) promotes tumor angiogenesis. Clin. Exp. Metastasis. 2013;30:671–680. doi: 10.1007/s10585-013-9571-z. [DOI] [PubMed] [Google Scholar]
- 320.R S, Ac S. Dual, enzymatic and non-enzymatic, function of ecto-5’-nucleotidase (eN, CD73) in migration and invasion of A375 melanoma cells. Acta Biochim. Pol. 2011;59:647–652. [PubMed] [Google Scholar]
- 321.Sadej R, Inai K, Rajfur Z, et al. Tenascin C interacts with Ecto-5′-nucleotidase (eN) and regulates adenosine generation in cancer cells. Biochim. Biophys. Acta BBA - Mol. Basis Dis. 2008;1782:35–40. doi: 10.1016/j.bbadis.2007.11.001. [DOI] [PubMed] [Google Scholar]
- 322.Geoghegan JC, Diedrich G, Lu X, et al. Inhibition of CD73 AMP hydrolysis by a therapeutic antibody with a dual, non-competitive mechanism of action. mAbs. 2016;8:454–467. doi: 10.1080/19420862.2016.1143182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 323.Barnhart BC, Sega E, Yamniuk A, et al. Abstract 1476: A therapeutic antibody that inhibits CD73 activity by dual mechanisms. Cancer Res. 2016;76:1476–1476. [Google Scholar]
- 324.Paoli MG, Augier S, Blemont MR, et al. Abstract 2344: Discovery and characterization of new original blocking antibodies targeting the CD73 immune checkpoint for cancer immunotherapy. Cancer Res. 2016;76:2344–2344. [Google Scholar]
- 325.Beavis PA, Milenkovski N, Henderson MA, et al. Adenosine Receptor 2A Blockade Increases the Efficacy of Anti–PD-1 through Enhanced Antitumor T-cell Responses. Cancer Immunol. Res. 2015;3:506–517. doi: 10.1158/2326-6066.CIR-14-0211. [DOI] [PubMed] [Google Scholar]
- 326.Waickman AT, Alme A, Senaldi L, Zarek PE, Horton M, Powell JD. Enhancement of tumor immunotherapy by deletion of the A2A adenosine receptor. Cancer Immunol. Immunother. 2011;61:917–926. doi: 10.1007/s00262-011-1155-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 327.Cekic C, Linden J. Adenosine A2A Receptors Intrinsically Regulate CD8+ T Cells in the Tumor Microenvironment. Cancer Res. 2014;74:7239–7249. doi: 10.1158/0008-5472.CAN-13-3581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 328.Cekic C, Sag D, Day Y-J, Linden J. Extracellular adenosine regulates naive T cell development and peripheral maintenance. J. Exp. Med. 2013;210:2693–2706. doi: 10.1084/jem.20130249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 329.Ryzhov S, Novitskiy SV, Zaynagetdinov R, et al. Host A2B Adenosine Receptors Promote Carcinoma Growth. Neoplasia N. Y. N. 2008;10:987–995. doi: 10.1593/neo.08478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 330.Allard B, Turcotte M, Stagg J. Targeting CD73 and downstream adenosine receptor signaling in triple-negative breast cancer. Expert Opin. Ther. Targets. 2014;18:863–881. doi: 10.1517/14728222.2014.915315. [DOI] [PubMed] [Google Scholar]
- 331.Leone RD, Lo Y-C, Powell JD. A2aR antagonists: Next generation checkpoint blockade for cancer immunotherapy. Comput. Struct. Biotechnol. J. 2015;13:265–272. doi: 10.1016/j.csbj.2015.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 332.Allard B, Pommey S, Smyth MJ, Stagg J. Targeting CD73 Enhances the Antitumor Activity of Anti-PD-1 and Anti-CTLA-4 mAbs. Clin. Cancer Res. 2013;19:5626–5635. doi: 10.1158/1078-0432.CCR-13-0545. [DOI] [PubMed] [Google Scholar]
- 333.Iannone R, Miele L, Maiolino P, Pinto A, Morello S. Adenosine limits the therapeutic effectiveness of anti-CTLA4 mAb in a mouse melanoma model. Am. J. Cancer Res. 2014;4:172–181. [PMC free article] [PubMed] [Google Scholar]
- 334.Verbrugge I, Hagekyriakou J, Sharp LL, et al. Radiotherapy Increases the Permissiveness of Established Mammary Tumors to Rejection by Immunomodulatory Antibodies. Cancer Res. 2012;72:3163–3174. doi: 10.1158/0008-5472.CAN-12-0210. [DOI] [PubMed] [Google Scholar]
- 335.Loi S, Pommey S, Haibe-Kains B, Beavis PA, Darcy PK, Smyth MJ, Stagg J. CD73 promotes anthracycline resistance and poor prognosis in triple negative breast cancer. Proc. Natl. Acad. Sci. 2013;110:11091–11096. doi: 10.1073/pnas.1222251110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 336.Wennerberg E, Kawashima N, Demaria S. Adenosine regulates radiation therapy-induced anti-tumor immunity. J. Immunother. Cancer. 2015;3:378. [Google Scholar]
- 337.Wirsdörfer F, Leve S de, Cappuccini F, et al. Extracellular Adenosine Production by ecto-5’-Nucleotidase (CD73) Enhances Radiation-Induced Lung Fibrosis. Cancer Res. 2016;76:3045–3056. doi: 10.1158/0008-5472.CAN-15-2310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 338.Young A, Ngiow SF, Barkauskas DS, et al. Co-inhibition of CD73 and A2AR Adenosine Signaling Improves Anti-tumor Immune Responses. Cancer Cell. 2016;30:391–403. doi: 10.1016/j.ccell.2016.06.025. [DOI] [PubMed] [Google Scholar]
- 339.Di Virgilio F, Boeynaems JM, Robson SC. Extracellular nucleotides as negative modulators of immunity. Curr Opin Pharmacol. 2009;9:507–513. doi: 10.1016/j.coph.2009.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]