Environmental conditions are associated with different aspects of circadian rhythmicity in wild barley.
Abstract
In plants, the circadian system controls a plethora of processes, many with agronomic importance, such as photosynthesis, photoprotection, stomatal opening, and photoperiodic development, as well as molecular processes, such as gene expression. It has been suggested that modifying circadian rhythms may be a means to manipulate crops to develop improved plants for agriculture. However, there is very little information on how the clock influences the performance of crop plants. We used a noninvasive, high-throughput technique, based on prompt chlorophyll fluorescence, to measure circadian rhythms and demonstrated that the technique works in a range of plants. Using fluorescence, we analyzed circadian rhythms in populations of wild barley (Hordeum vulgare ssp. spontaneum) from widely different ecogeographical locations in the Southern Levant part of the Fertile Crescent, an area with a high proportion of the total genetic variation of wild barley. Our results show that there is variability for circadian traits in the wild barley lines. We observed that circadian period lengths were correlated with temperature and aspect at the sites of origin of the plants, while the amplitudes of the rhythms were correlated with soil composition. Thus, different environmental parameters may exert selection on circadian rhythms.
Endogenous circadian (∼24-h) systems appear to be a common feature of all eukaryotic and a number of prokaryotic organisms. Circadian systems coordinate metabolism, enable organisms to anticipate predictable daily changes in the environment, and have a role in photoperiod measurement to regulate seasonality. Conceptually, a circadian system can be divided into three parts: the oscillator, input pathways, and output pathways. Oscillators are composed of interlocking positive/negative feedback loops of pacemaker elements and determine the period, phase, and amplitude of the output rhythms. The oscillators can be entrained by signals from the environment such as temperature and light changes. At the same time, built into the system is a strong temperature compensation capability that allows the oscillator to function with a period that is close to 24 h under a wide range of ambient temperatures. Output pathways regulate numerous physiological and molecular processes, including, in plants, chlorophyll biosynthesis, starch metabolism, hypocotyl growth, leaf movements, scent production, stomatal opening, photoperiodic flowering, and the expression of around 30% of the genome (Harmer et al., 2000; Nagel and Kay, 2012).
Consistent with its wide-ranging importance in the life of a plant, there are a number of reports showing that a robust, environment-matching circadian system improves growth and may be adaptive (Green et al., 2002; Dodd et al., 2005; Ni et al., 2009; Yerushalmi et al., 2011). A commonly used definition of an adaptation is a phenotypic variant that results in the highest fitness in a given environment, with fitness defined as the contribution of a genotype to future generations (Futuyma, 1998). A trait, such as the amplitude of a circadian rhythm, that improves fitness under a particular environmental condition will be selected for. This selection can manifest itself as increased numbers of individuals with that particular trait in subsequent generations. Thus, if correlations are observed between different environmental conditions and certain traits, this strongly suggests that these traits are adaptive. Such correlations can potentially tell us which attributes of circadian rhythms are most important for growth and reproductive success in different environmental conditions.
Studies on Arabidopsis (Arabidopsis thaliana) accessions collected from a broad geographic range have examined correlations between circadian period length and latitude, daylength during the growing season, and altitude at the geographic site of origin. The results of such studies suggest that correlations may be complex; Edwards et al. (2005) showed that longer periods correlated with lower altitudes but not with latitude, while Michael et al. (2003) showed that longer periods correlated with longer daylength and higher latitude but not with altitude. Accessions also show differences in their ability to compensate for changes in ambient temperatures, with most, but not all, accessions showing a faster circadian clock at high temperatures (Kusakina et al., 2014). These results suggest that properties of circadian rhythms may respond to selection forces of different conditions. However, the interpretation of these accession-dependent circadian rhythm differences is complicated by the wide range of potential environmental differences, including daylength, often coupled with a lack of precise data about the geographic sites of origin of all the accessions (Edwards et al., 2005).
Originating in the Fertile Crescent area of the Near East, wild barley (Hordeum vulgare ssp. spontaneum) is one of the world’s oldest cultivated crops (Mayer et al., 2012). It is still the world’s fourth most-produced cereal, heavily grown in northern Europe, America, and Asia, primarily for animal feed for the meat and dairy industries and also for beer (Blake et al., 2011). Barley is increasingly being recognized as a potentially valuable high-fiber component of human diets (Collins et al., 2010). Wild barley is the direct progenitor of cultivated, elite, and landrace barley (Hordeum vulgare ssp. vulgare), and the subspecies readily cross. The richest genetic variation is found in wild barley (Jakob et al., 2014), which typically grows in areas with harsh environmental conditions (e.g. low water, poor soils, and heat stress). Thus, not only is wild barley a valuable natural resource, it is also a potentially useful tool for studying the adaptive significance of circadian rhythms.
A significant challenge for plant circadian researchers is developing high-throughput techniques to analyze rhythms; one approach is to use chlorophyll fluorescence. Light energy absorbed by chlorophyll molecules in plants results in singlet-state excited molecules that can return to the ground state by one of several pathways: photosynthesis (photochemical quenching), dissipation as heat (nonphotochemical quenching), or reemission as light (fluorescence; Croce and van Amerongen, 2014). The three methods of energy dissipation are correlated; increasing the efficiency of one will decrease the yield of the other two (Maxwell and Johnson, 2000; Muller et al., 2001). At room temperature, most fluorescence is from PSII and can be divided into prompt chlorophyll fluorescence (F; within ∼2 ns [Kalaji et al., 2012]), originating in chlorophyll molecules that have been excited directly by light or fast exciton energy transfer from other chlorophyll molecules, and delayed chlorophyll fluorescence (DF; seconds time scale). In the dark when there is no forward electron transfer, electrons trapped at the secondary electron acceptor, quinone QB, site of PSII will flow back to the manganese cluster in an energetically uphill back electron transfer process. DF is the result of the regeneration of the excited state of the primary donor of PSII, P680, during this process (Keren et al., 1997). DF is at least 2 orders of magnitude less intense than F but, due to its uphill energetics, is emitted for a long time after the disappearance of F. DF has been used successfully to measure circadian rhythms (Kusakina et al., 2015; Gould et al., 2009; Gawronski et al., 2014), and recently, one of the parameters of F was shown to be under circadian control (Litthauer et al., 2015).
The circadian system affects numerous physiological and molecular processes of ecological and agricultural importance. Here, we start to explore the potential of the circadian system for optimizing plant adaptation and responsiveness to challenging environments. We show how F may be used as a high-throughput tool to measure circadian rhythms in a range of different plant species and use it to screen wild barley populations from the Fertile Crescent. We suggest how different aspects of circadian rhythmicity may be adaptive to optimize growth in diverse environmental conditions.
RESULTS
Circadian Rhythms of F
Our first aim was to develop a high-throughput platform to measure circadian rhythms in soil-grown plants. F analysis is a powerful technique that can be used to measure the efficiency of PSII photochemistry and, by taking a range of different measurements, gauge nonphotochemical quenching and plant vitality. Recently, Litthauer et al. (2015) showed that one aspect of F (F′q/F′m; Supplemental Table S1) is under circadian control in blue light in Arabidopsis. We explored whether other aspects of F are under circadian control and determined whether F also can reliably be used as a marker for circadian rhythms in white (blue + red) light.
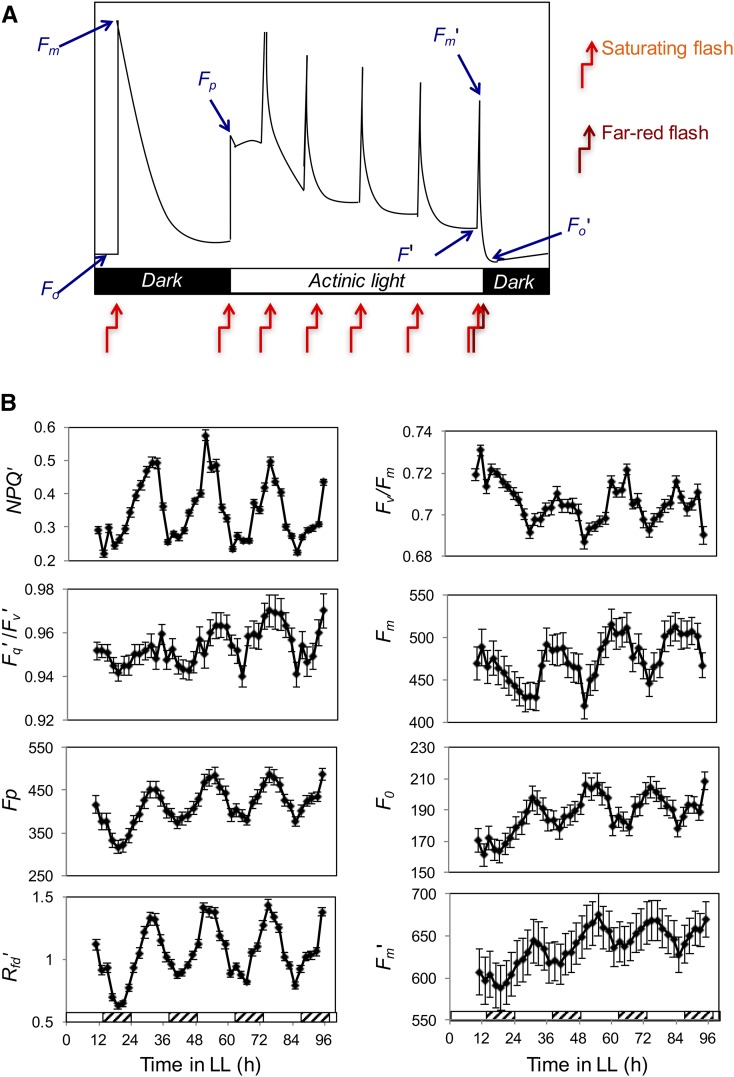
Using an Open FluorCam system (see “Materials and Methods”), we assessed a number of F parameters in Arabidopsis during the transition from dark adaptation to light. Our protocol (Fig. 1A) was as follows. Plants were entrained in 14 h of light/10 h of dark (LD) at 22°C for 4 weeks before being transferred to continuous white light (LL). At 2-h intervals, the plants were given a brief period of dark adaptation and chlorophyll fluorescence was measured. This measurement, Fo, is the minimum fluorescence that occurs when the reaction centers of PSII are completely open and the primary quinone acceptor is fully oxidized. Following a brief saturating pulse of white light to transiently reduce the primary quinone acceptor and plastoquinone pool, Fm was measured. The saturating light pulse is too brief to initiate nonphotochemical quenching, so Fm is the maximum possible fluorescence in the absence of heat dissipation. The plants were then subjected to a dark period that allowed reoxidation of the primary quinone acceptor and plastoquinone pool and then adapted to light during a period of actinic (suitable for photosynthesis) blue light, which caused the plastoquinone pool to become reduced and resulted in another peak of fluorescence, Fp. After an extended period in actinic light, PSI starts to transfer electrons, the plastoquinone pool becomes reoxidized, and fluorescence decreases to a steady state, F′. At the same time, nonphotochemical quenching is activated; this could be seen when additional brief saturating pulses of light were superimposed on the actinic light regime. Fm′ is the maximum fluorescence in the light-adapted state and is lower than Fm measured in the dark. Finally, the actinic light was switched off, the primary quinone acceptor and plastoquinone pool were reoxidized by a far-red light flash, and Fo′ was measured. From these measurements, a number of parameters of fluorescence can be calculated (Table I). A comparison of the terminology for F parameters used in this article and by other researchers is given in Supplemental Table S1. Almost all the measured and calculated F parameters showed rhythmicity (Fig. 1B). Fo, Fp, Fm′, Fq′/Fv′, Rfd′, and NPQ peak during the subjective day, while Fm and Fv/Fm peak at night. These results show that many aspects of F are rhythmic in Arabidopsis.
Figure 1.
F parameters are rhythmic in Arabidopsis. A, F parameters measured during dark adaptation and subsequent light adaptation. B, Arabidopsis plants were entrained to LD for 4 weeks before being transferred to LL for 4 d. At 2-h intervals, the plants were given a brief period of dark adaptation (as described in “Materials and Methods”) and chlorophyll fluorescence was measured using the FluorCam. Fq′/Fv′, Rfd′, NPQ, and Fv/Fm were calculated from the measurements described in Table I.
Table I. Chlorophyll fluorescence parameters.
| Symbol | Formula | Description |
|---|---|---|
| Fo | Measured | Minimum F when the primary electron-accepting plastoquinone of PSII is oxidized and there is no nonphotochemical quenching |
| Fm | Measured | Maximum F when the primary electron-accepting plastoquinone of PSII is reduced and there is no nonphotochemical quenching |
| Fv | Fm − Fo | F during the transition from the dark state with open reaction centers to the light state with closed reaction centers |
| Fp | Measured | Peak of F after transfer to actinic light when there is photochemical and nonphotochemical quenching |
| F′ | Measured | Steady-state F after adaptation to actinic light |
| Fm′ | Measured | Maximum F after adaptation to actinic light when there is nonphotochemical quenching |
| Fo′ | Measured | Steady-state minimum F immediately after transfer to dark following adaptation to actinic light |
| Fq′ | Fm′ − F′ | Photochemical quenching of fluorescence by open PSII centers |
| Fq′/Fv′ | (Fm′ − F′)/(Fm′ − Fo′) | Fraction of open PSII reaction centers |
| Rfd′ | (Fp − F′)/F′ | Empiric parameter for plant vitality |
| Fv/Fm | Fv/Fm | Maximum PSII quantum yield after dark adaptation |
| NPQ | (Fm − Fm′)/Fm′ | Steady-state nonphotochemical quenching in actinic light |
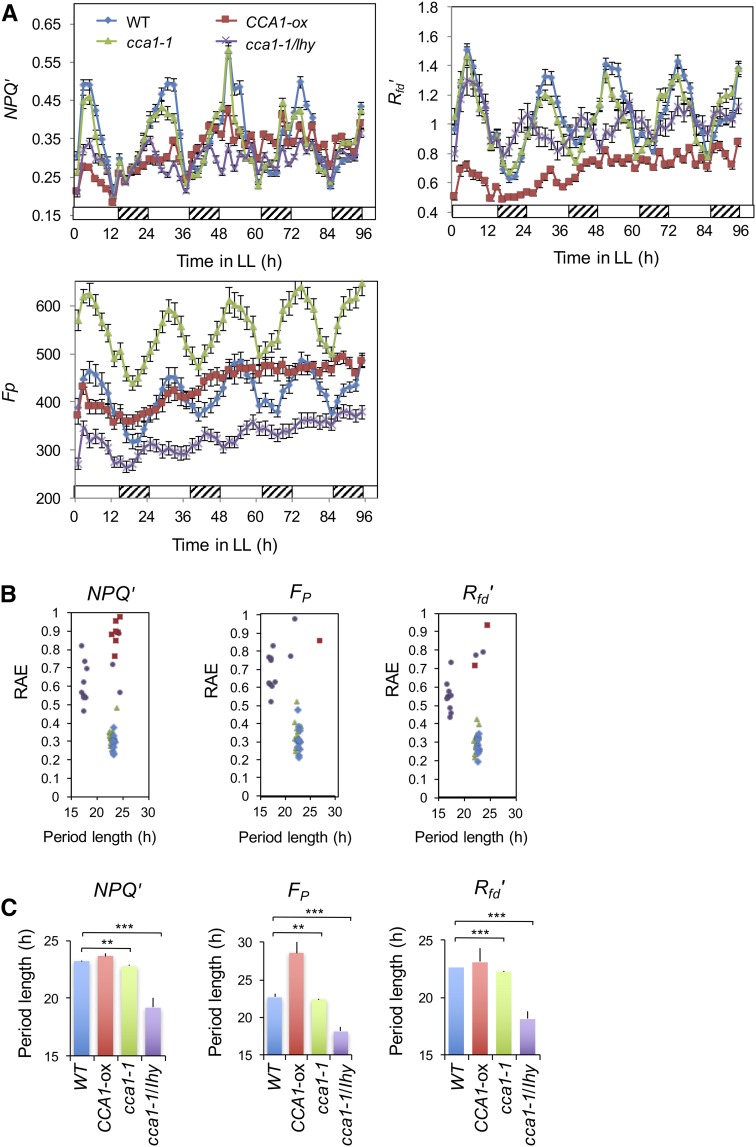
To confirm that the F oscillations we observed were regulated by the circadian system in Arabidopsis, we used mutants; misexpression of circadian oscillator genes affects circadian rhythms. Depending on the gene and the levels of expression, plants show longer or shorter periods or become arrhythmic (Hsu and Harmer, 2014). We tested whether F rhythms were affected in plants that misexpressed the key oscillator genes CIRCADIAN CLOCK ASSOCIATED1 (CCA1) and the closely related LATE ELONGATED HYPOCOTYL (LHY). Figure 2 shows the results for NPQ, Fp, and Rfd′ for wild-type, CCA1 null (cca1-1), CCA1 LHY double null (cca1-1 lhy), and CCA1 overexpresser (CCA1-ox) plants. All three F parameters oscillated robustly in both wild-type and cca1-1 plants (Fig. 2A). Consistent with previously published results (Green and Tobin, 1999; Mizoguchi et al., 2002; Lu et al., 2009), cca1-1 plants had significantly shorter periods of F (NPQ, 22.81 ± 0.13 h; Fp, 22.38 ± 0.1 h; Rfd′, 22.27 ± 0.08 h) than wild-type plants (NPQ, 23.22 ± 0.03 h; Fp, 22.72 ± 0.04 h; Rfd′, 22.65 ± 0.04 h). cca1-1 lhy plants showed much shorter periods (NPQ, 19.21 ± 0.86 h; Fp, 18.18 ± 0.54 h; Rfd′, 18.18 ± 0.54 h) than either cca1-1 or wild-type plants with a higher relative amplitude error (RAE; Fig. 2B). RAE is used to assess individual rhythm robustness; values close to 0 indicate robust cycling and values at or near 1 indicate a rhythm with an error value as large as the amplitude itself (not statistically significant; Plautz et al., 1997). CCA1-ox plants were largely arrhythmic; a few CCA1-ox individuals showed very weak rhythmicity, especially of NPQ, but none of the CCA1-ox plants had an RAE of less than 0.75, which is well above the threshold for being considered a significant circadian rhythm (Fig. 2, B and C). Taken together, our results confirm that F rhythms are regulated by the circadian system in Arabidopsis.
Figure 2.
F rhythms are affected in plants that misexpress key oscillator genes. Wild-type (WT), cca1-1, cca1-1 lhy, and CCA1-ox plants were grown in LD for 4 weeks and then transferred to LL, and F measurements were taken every 2 h for 4 d. Fp, Rfd′, and NPQ were measured and calculated. The graphs were plotted with the se (n = 12). **, P < 0.01 and ***, P < 0.001 (Student’s two-tailed t test). The experiment was carried out twice with similar results.
F Can Be Used to Measure Circadian Rhythms in a Range of Plants
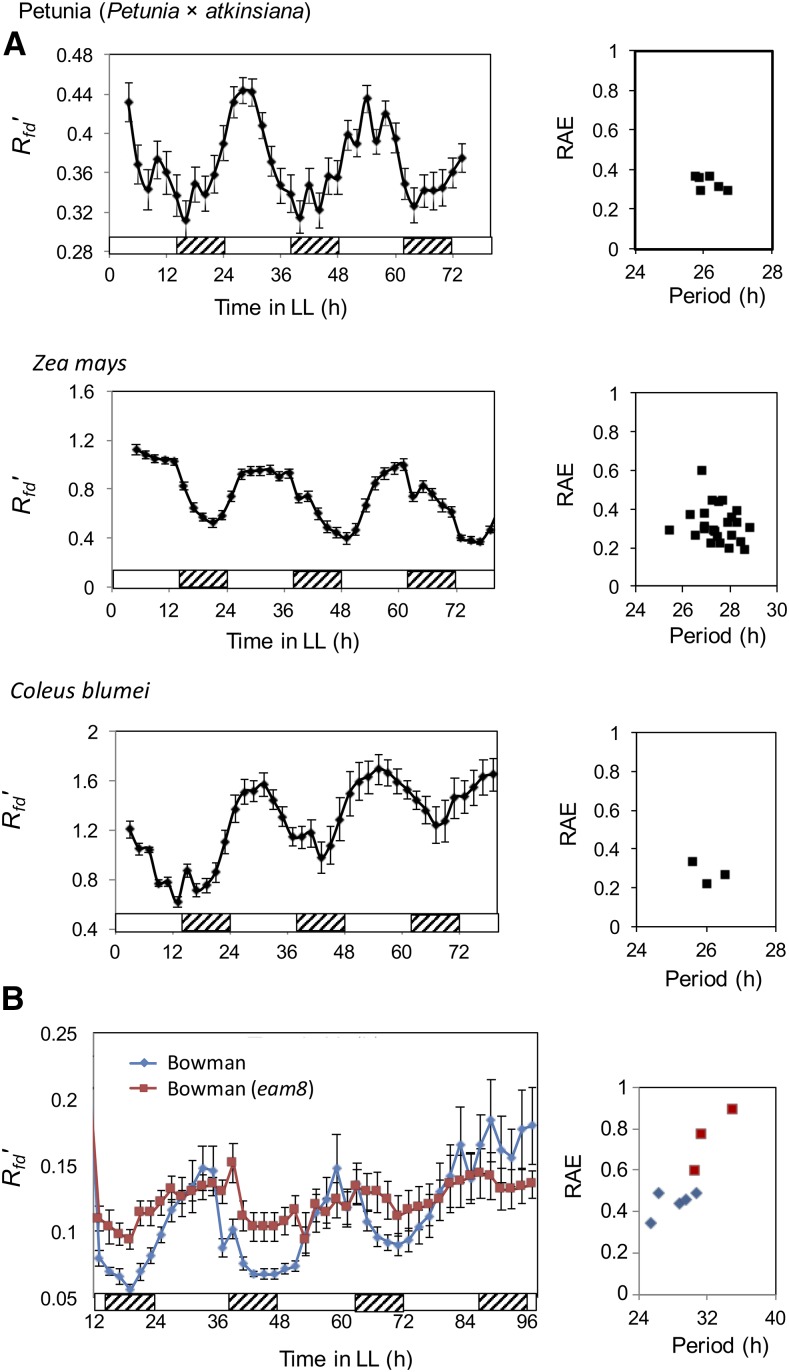
To determine whether F parameters may be used as markers for measuring circadian rhythmicity in other plants, we surveyed the C3 angiosperms petunia (Petunia × atkinsiana) and Coleus blumei and the C4 angiosperm maize (Zea mays). All the plants tested showed robust circadian rhythms of F parameters, including Rfd′ with low RAEs (Fig. 3).
Figure 3.
F parameters are rhythmic in a range of plants. A, Petunia, , and C. blumei. B, Wild barley cv Bowman and cv Bowman(eam8). Plants were grown in LD for 4 weeks and then transferred to LL, and F measurements were taken every 2 h. Left graphs show circadian rhythms, and right graphs show RAE. The graphs were plotted with the se.
We also examined two barley cultivars, cv Bowman, a spring barley line, and a derived introgression line, cv Bowman(eam8), carrying a mutation at the clock gene EARLY FLOWERING3 (HvELF3; Faure et al., 2012). Figure 3B and Supplemental Figure S1 show that cv Bowman plants exhibited clear rhythms of Rfd′ with peaks in the middle of the subjective day, but rhythms in cv Bowman(eam8) were significantly dampened (Fig. 3B), a result that corresponds well with the reduced amplitude of HvCCA1 expression demonstrated previously in cv Bowman(eam8) (Faure et al., 2012). Thus, F measurements appear to be robust markers for analyzing circadian variation in intact living plants from a wide range of species.
Circadian Variation in Wild Barley Accessions
Using F measurements as a tool, we addressed the main aim of this article: examining whether and how circadian rhythms correlate with the environment. For this study, we chose to focus on barley; as we note in the introduction, barley is an important crop and wild barley shows rich genetic variation. We used the Barley 1K (B1K) collection of wild barley accessions (Hübner et al., 2009, 2013) collected from a narrow range of latitudes, between 29°N and 33°N, in the southwestern part of the Fertile Crescent, an area with a high proportion of the total genetic variation of wild barley (Nevo, 1998; Jakob et al., 2014). It is one of the few collections of wild barley that comes directly from the wild without long-term storage, and the germplasm has been single seed descent propagated. The plants in the B1K collection are from different ecological environments but almost identical daylength conditions (Hübner et al., 2009), and there is detailed passport data for the sites of origin of each accession that include elevation, midday temperatures in January (MDT1) and August (MDT8), average annual rainfall (MAR), aspect, slope, soil organic matter (OM), soil bulk density (Db), and soil water content (WC).
We tested 18 B1K lines representing 11 of the accession sites with the most geographic diversity to determine whether environmental conditions at their sites of origin affect circadian rhythms. The period, RAE, amplitude, and phase of NPQ, Fv/Fm, and Rfd′ were calculated for each line. Our results show that the lines had a range of circadian periods, RAEs, phases, and amplitudes (Table II; Fig. 4). For example, Rfd′ periods varied from 23.3 to 28.1 h, while NPQ periods varied between 24 and 27.1 h. Fv/Fm showed less period length variation (26.6–24.2 h) and was antiphasic to Rfd′, but NPQ was still robustly rhythmic. Supplemental Figure S2 shows that period lengths for NPQ, Fv/Fm, and Rfd′ were correlated with Pearson coefficient correlations (r) of NPQ versus Rfd′ (0.65), NPQ versus Fv/Fm (0.3), and Fv/Fm versus Rfd′ (0.29). The lower correlation between Fv/Fm and the other parameters may reflect the reduced period length variation. However, our results suggest that all three F parameters are regulated by the same core oscillator, although it is possible that this regulation may be via different output pathways or differentially affected by noncircadian constraints on photosynthesis.
Table II. Circadian period, phase, and amplitude for the B1K lines.
| Line |
NPQ |
Fv/Fm |
Rfd′ |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Period | RAE | Amplitude | Phase | Period | RAE | Amplitude | Phase | Period | RAE | Amplitude | Phase | |
| h | h | h | ||||||||||
| B1K-02-05 | 24.6 ± 0.5 | 0.41 | 0.061 ± 0.005 | −6.9 ± 1.4 | 26.1 ± 0.3 | 0.35 | 0.021 ± 0.002 | 7.2 ± 1.4 | 25.5 ± 0.4 | 0.47 | 0.064 ± 0.004 | −2.3 ± 2.6 |
| B1K-02-13 | 24.0 ± 0.2 | 0.36 | 0.073 ± 0.005 | −2.9 ± 2.2 | 24.2 ± 0.3 | 0.29 | 0.022 ± 0.001 | 5.1 ± 0.8 | 25.5 ± 0.4 | 0.41 | 0.074 ± 0.004 | −7.9 ± 0.4 |
| B1K-03-04 | 25.0 ± 0.4 | 0.43 | 0.060 ± 0.005 | −1.8 ± 1.2 | 26.6 ± 0.4 | 0.30 | 0.021 ± 0.001 | 6.2 ± 2.0 | 25.9 ± 0.3 | 0.35 | 0.113 ± 0.008 | −3.7 ± 0.6 |
| B1K-03-09 | 24.5 ± 0.5 | 0.60 | 0.038 ± 0.004 | −1.9 ± 1.9 | 25.4 ± 0.5 | 0.43 | 0.014 ± 0.001 | 3.7 ± 1.9 | 25.7 ± 0.2 | 0.39 | 0.083 ± 0.005 | −4.3 ± 0.5 |
| B1K-05-07 | 24.1 ± 0.9 | 0.38 | 0.044 ± 0.005 | −4.6 ± 2.0 | 26.1 ± 0.2 | 0.31 | 0.017 ± 0.002 | 8.3 ± 1.8 | 25.6 ± 0.8 | 0.42 | 0.053 ± 0.004 | −6.3 ± 2.3 |
| B1K-08-01 | 25.2 ± 0.3 | 0.42 | 0.046 ± 0.004 | −9.2 ± 0.4 | 26.6 ± 0.3 | 0.34 | 0.019 ± 0.001 | 6.3 ± 0.3 | 26.0 ± 0.2 | 0.28 | 0.117 ± 0.007 | −8.1 ± 0.3 |
| B1K-08-10 | 25.2 ± 0.2 | 0.27 | 0.082 ± 0.005 | −6.2 ± 0.9 | 25.8 ± 0.1 | 0.24 | 0.030 ± 0.001 | 6.0 ± 1.5 | 26.9 ± 0.5 | 0.40 | 0.055 ± 0.003 | −4.6 ± 0.5 |
| B1K-12-03 | 25.8 ± 0.5 | 0.41 | 0.056 ± 0.006 | −7.6 ± 0.6 | 25.6 ± 0.2 | 0.27 | 0.021 ± 0.002 | 7.2 ± 0.5 | 26.8 ± 0.3 | 0.34 | 0.10 ± 0.01 | −5.2 ± 0.7 |
| B1K-12-17 | 24.3 ± 0.3 | 0.46 | 0.044 ± 0.003 | −0.4 ± 2.6 | 26.5 ± 0.5 | 0.43 | 0.022 ± 0.002 | 5.5 ± 1.9 | 25.9 ± 0.2 | 0.35 | 0.071 ± 0.007 | −9.1 ± 0.7 |
| B1K-26-08 | 25.3 ± 0.6 | 0.47 | 0.050 ± 0.003 | −6.2 ± 1.2 | 26.2 ± 0.5 | 0.43 | 0.018 ± 0.001 | 4.5 ± 1.9 | 26.6 ± 0.4 | 0.41 | 0.065 ± 0.005 | −4.5 ± 0.2 |
| B1K-26-16 | 26.1 ± 0.6 | 0.53 | 0.040 ± 0.002 | −8.7 ± 1.4 | 26.4 ± 0.4 | 0.43 | 0.014 ± 0.001 | 5.1 ± 1.5 | 26.8 ± 0.4 | 0.56 | 0.051 ± 0.003 | −8.4 ± 0.5 |
| B1K-29-02 | 24.6 ± 0.2 | 0.39 | 0.058 ± 0.005 | −5.0 ± 0.9 | 25.6 ± 0.1 | 0.30 | 0.015 ± 0.001 | 8.2 ± 0.1 | 24.8 ± 0.2 | 0.29 | 0.090 ± 0.005 | −5.1 ± 0.3 |
| B1K-29-13 | 27.0 ± 0.3 | 0.46 | 0.025 ± 0.001 | −9.0 ± 1.6 | 25.8 ± 0.5 | 0.60 | 0.009 ± 0.001 | 5.5 ± 1.3 | 23.3 ± 1.0 | 0.54 | 0.033 ± 0.003 | −2.3 ± 0.8 |
| B1K-33-03 | 26.1 ± 0.3 | 0.36 | 0.070 ± 0.006 | −7.1 ± 1.1 | 25.9 ± 0.2 | 0.35 | 0.018 ± 0.001 | 7.7 ± 0.9 | 26.5 ± 0.3 | 0.32 | 0.10 ± 0.01 | −6.9 ± 0.3 |
| B1K-33-09 | 24.6 ± 0.4 | 0.45 | 0.060 ± 0.007 | −6.4 ± 0.7 | 25.8 ± 0.3 | 0.38 | 0.016 ± 0.001 | 6.8 ± 1.7 | 23.9 ± 0.4 | 0.35 | 0.079 ± 0.005 | −6.9 ± 0.9 |
| B1K-39-02 | 25.9 ± 0.4 | 0.42 | 0.050 ± 0.005 | −8.5 ± 0.8 | 25.7 ± 0.2 | 0.35 | 0.019 ± 0.001 | 5.3 ± 1.2 | 26.1 ± 0.2 | 0.28 | 0.096 ± 0.007 | −5.8 ± 0.3 |
| B1K-42-16 | 27.1 ± 0.1 | 0.55 | 0.028 ± 0.003 | −10.2 ± 1.5 | –a | –a | 0.014 ± 0.001 | −7.7 ± 1.4 | 28.0 ± 0.3 | 0.35 | 0.083 ± 0.007 | −5.1 ± 0.5 |
| B1K-50-14 | 26.4 ± 0.3 | 0.49 | 0.027 ± 0.002 | −1.3 ± 2.6 | 25.8 ± 0.6 | 0.52 | 0.014 ± 0.002 | −4.6 ± 0.7 | 28.1 ± 0.5 | 0.54 | 0.038 ± 0.005 | −4.2 ± 0.2 |
Data missing.
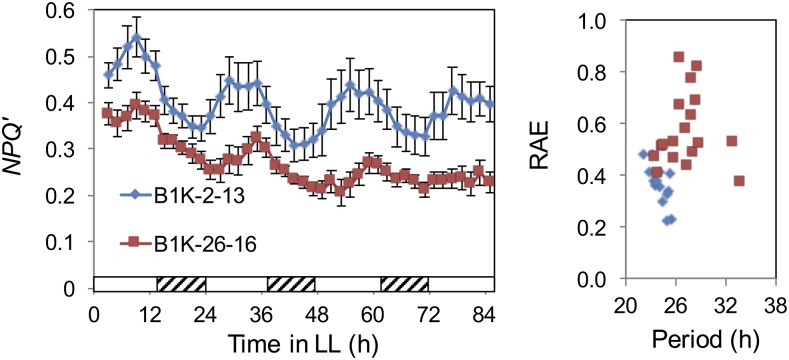
Figure 4.
Circadian rhythms are accession dependent. NPQ oscillations are shown in two B1K lines from different geographic locations. B1K-02-13 is from Yeruham at 535 m above sea level, and B1K-26-16 is from Ein Gev at 158 m below sea level. The left graph shows circadian rhythms, and the right graph shows RAE. The NPQ graph was plotted with the se.
Overall, the differences in circadian attributes strongly suggest that there is allelic variability underlying circadian trait variation in the naturally evolved wild barley populations.
Do Environmental Conditions Correspond with Different Aspects of Circadian Rhythmicity?
To start to understand how the physical environment may act as a selection force for circadian rhythms, we asked whether and how the variation in the three key circadian attributes (phase, amplitude, and period) in the B1K wild barley lines was correlated with geographic and climate variables at the sites of origin of the plants (Hübner et al., 2009). Our results (Figs. 2 and 3) show that F is regulated by the circadian system. However, it is possible that there are other factors affecting F patterns, such as variation in photosynthesis between the lines; therefore, to minimize, as much as possible, noncircadian effects on the circadian readout, we averaged the period and amplitude data for the NPQ, Fv/Fm, and Rfd′ parameters (Supplemental Table S1). However, since circadian phase was parameter dependent and could not be averaged, we used the Rfd′ phase. Environmental data consisted of nine variables (elevation, MDT1, MDT8, MAR, aspect, slope, OM, Db, and WC); aspect was replaced by its north-south and east-west components. Each of the circadian attributes (average period, average amplitude, and Rfd′ phase) was subjected to full-subset linear regression modeling. The models were ranked and weighted according to the Akaike Information Criterion, corrected for small sample sizes (AICc).
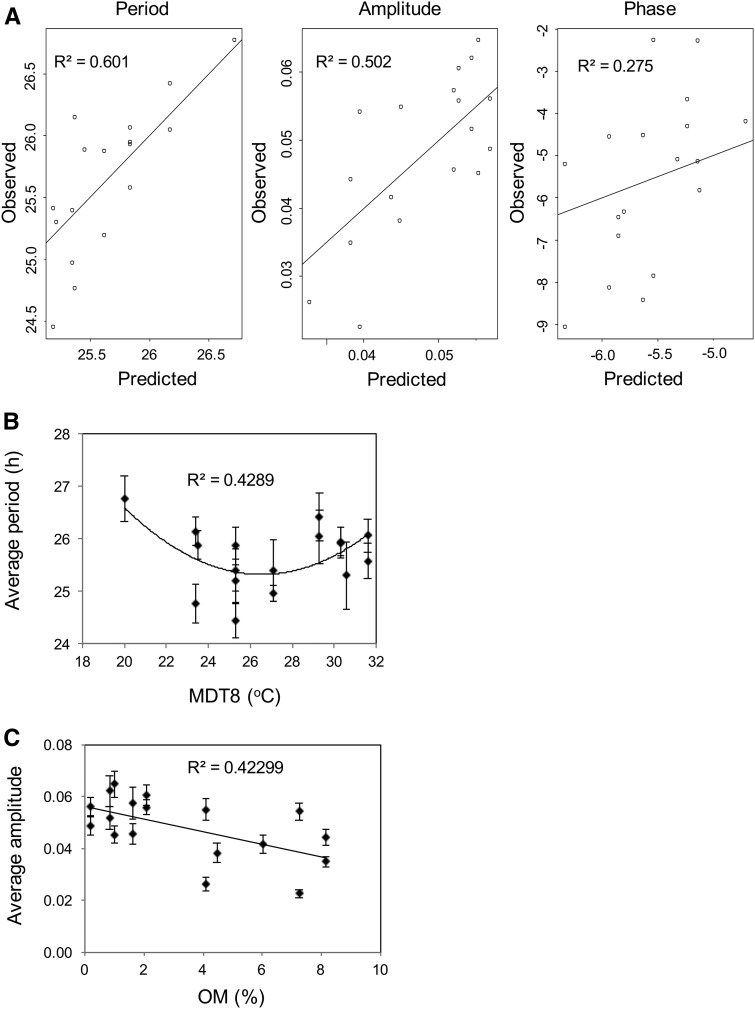
The resulting averaged models provided a good fit for average period (r2 = 0.60, P < 0.001) and average amplitude (r2 = 0.50, P < 0.001) but less so for Rfd′ phase (r2 = 0.27, P = 0.025; Fig. 5A). Table III shows that the average period length of circadian rhythms in the B1K wild barley lines was correlated with the east-west aspect and MDT8. Intriguingly, the relationship between the average period and MDT8 was nonlinear; both lower and higher MDT8 values correlated with longer period rhythms (Table III; Fig. 5B). Circadian amplitude, by contrast, correlated primarily with OM and, to a lesser extent, with MAR (Table III). Higher amplitude rhythms were associated with a lower soil organic matter content (Fig. 5C) and rainfall. Overall, our results demonstrate a correlation between circadian parameters of chlorophyll fluorescence and environmental variables that may be the result of selection.
Figure 5.
The averaged models provide a good fit for period and amplitude but not phase. A, The fit between the observed circadian attributes and those predicted based on the averaged models. Solid lines depict equality. B, Average circadian period correlates with MDT8. 0.019 Prob(F) by one-way ANOVA. C, Average amplitude correlates with OM. 0.004 Prob(F) by one-way ANOVA. For B and C, the graphs were plotted with the se. The r2 values were calculated using OriginLab with errors as weights.
Table III. Multimodel averaged coefficients (with se) for the regression of clock attributes on environmental factors.
Importance is the sum of the AIC weights of the models that include the specified variable. The number of models including each variable, per attribute, is provided in parentheses. Square brackets show the total number of models with AICc < 2.
| Parameter | Variable | Estimate | se | Importance |
|---|---|---|---|---|
| Average period [3] | Intercept | 25.189 | 0.323 | |
| Eness^2 | 0.368 | 0.259 | 0.75 (2) | |
| mdt8^2 | 0.174 | 0.147 | 0.70 (2) | |
| OM | 0.075 | 0.140 | 0.25 (1) | |
| wc^2 | −0.069 | 0.129 | 0.25 (1) | |
| Average amplitude [4] | Intercept | 0.050 | 0.003 | |
| OM | −0.007 | 0.002 | 1.00 (4) | |
| mar^2 | −0.002 | 0.002 | 0.60 (2) | |
| mdt8^2 | −0.001 | 0.002 | 0.19 (1) | |
| wc^2 | 0.000 | 0.001 | 0.16 (1) | |
| Rfd′ phase [7] | Intercept | −5.420 | 0.639 | |
| MAR | 0.225 | 0.443 | 0.28 (2) | |
| mdt8^2 | −0.393 | 0.806 | 0.27 (2) | |
| mar^2 | 0.191 | 0.454 | 0.18 (1) | |
| MDT8 | −0.075 | 0.260 | 0.13 (1) | |
| wc^2 | 0.045 | 0.195 | 0.10 (1) | |
| WC | 0.041 | 0.194 | 0.09 (1) |
DISCUSSION
Litthauer et al. (2015) recently reported that Fq′/Fv′ is under circadian control in Arabidopsis in blue light, raising the exciting possibility of exploiting F as a noninvasive, high-throughput marker for circadian rhythms. Here, we have expanded on these findings and shown that the circadian system controls many other parameters of F in a range of plant species in white light. It is important to note that our protocol for F measurements was optimized for analyzing circadian rhythms and may not accurately indicate the photosynthetic capacity of the plant. Thus, for example, the 5 min of dark adaptation we used for Arabidopsis is probably not sufficient to ensure that the photosynthetic apparatus is genuinely dark adapted, but it was the length of dark adaptation that resulted in the best circadian rhythms. However, overall, measuring F is a valuable technique for understanding circadian rhythms in close-to-natural conditions or for examining the effects of stress on circadian rhythms.
Given the caveat that our F measuring protocol was optimized for measuring circadian rhythms, the various F parameters that can be measured and calculated may still indicate how the circadian system affects the status quo of plants and regulates photosynthesis. NPQ is an indirect measurement of a plant’s capacity for nonphotochemical quenching (heat dissipation) of excess energy from light absorption. NPQ is highest during the middle of the subjective day (Figs. 1B and 4), when the adverse effects of high light intensity are most likely to be experienced by plants (Ishida et al., 2014). Although the mechanism for the circadian regulation of NPQ in barley is unclear, the principal features of NPQ appear to be conserved in all plant species and involve the xanthophyll cycle (Demmig-Adams et al., 2012). Recently, altering the levels of genes regulating NPQ was shown to have dramatic effects on plant productivity (Kromdijk et al., 2016). In Arabidopsis, violaxanthin deepoxidase, a key regulator of the xanthophyll cycle, is under circadian control (Edwards et al., 2006; Covington et al., 2008), and it is possible that the circadian system regulates NPQ at the level of gene expression. Rfd′ reflects the potential photosynthetic activity of the leaf and can be used as a vitality index for plants (Lichtenthaler et al., 1986; Lichtenthaler and Miehe, 1997). Rfd′ is calculated from Fv and F′ and, like the other F measurements, is affected by chlorophyll pigment levels. Both chlorophyll and chlorophyll b have been shown to be under circadian control in soybean (Pan et al., 2015), suggesting that this may be a potential mechanism for the circadian rhythms of Rfd′ and Fv/Fm that we observed. In the future, it will be interesting to carry out further experiments to understand the mechanism(s) by which the circadian system controls F parameters.
Using F as a marker, we have shown that there are significant differences between B1K lines from different sites. It is possible that there is also variation between lines from the same sites; thus, while lines including B1K8 and B1K26 have similar period rhythms, others, such as B1K33, are different. In the Arabidopsis relative Boechera stricta, there can be as much variation within a population as between populations from different sites (Salmela et al., 2016), and in the future, it will be interesting to analyze additional lines from each site to compare intersite- and intrasite-specific variation.
We have demonstrated correlations between rhythm parameters and environmental variables. In the wild barley accessions, there were significant correlations between period length and temperature and aspect (Table III; Fig. 5B) at the site of origins of the lines. These correlations may reflect the critical role of the circadian system in regulating photoperiodic development, especially reproductive development. An early switch from vegetative to reproductive development might be an escape mechanism to allow flowering before local conditions become too hot. Our observation that the correlation between period length and MDT8 was not linear in the B1K lines suggests that the relationship may be complex. In general, the region has hot, dry summers and cooler, wet winters. In areas with low MDT8, the winters are much colder and longer, and in areas with high MDT8, the very hot summer conditions start early. The longer period at both high and low MDT8 might reflect a constricted growing season at both extremes. Consistent with the idea that temperature at the site of origin may be a selection force on photoperiodic flowering, strong correlations between flowering time and MDT1 have been reported for the B1K lines (Hübner et al., 2013). Although in our experiments we measured circadian rhythms in constant light and in natural conditions plants grow under daily light/dark (diel) conditions, it is possible that the differences we observed in period length may affect the expression of photoperiod components in diel conditions. For example, the evening-expressed HvELF3 represses the floral promoters GIBBERELLIN20 OXIDASE2 and FLOWERING LOCUS T (Faure et al., 2012; Boden et al., 2014); thus, plants with long period rhythms and delayed HvELF3 expression may show accelerated flowering. However, under diel conditions, the regulation of photoperiod components can be complex, since rhythms are modified by light signals (de Montaigu et al., 2015).
The correlation between period and temperature and aspect also may be related to nonreproductive causes; links have been suggested between circadian period and the environment and growth rate. B. stricta plants growing at higher elevations have shorter period rhythms that are associated with more rapid growth, a trait that the authors suggest may beneficial at sites with shorter growing seasons (Salmela et al., 2016). Studies also have tested the correlations between period length and growth under different temperatures; in both Brassica rapa and Arabidopsis accessions, circadian period was shorter at higher temperatures (Lou et al., 2011; Kusakina et al., 2014). Intriguingly, Arabidopsis accessions with circadian systems that were more poorly buffered against temperature showed greater increases in growth at higher temperatures, indicating that having a flexible oscillator may be advantageous (Kusakina et al., 2014). It has been suggested that a faster clock at high temperatures may enable plants to shift temperature-sensitive activities to earlier in the day. However, the nonlinear correlation between period and temperature suggests that, at least for the plants we analyzed from the B1K collection, the relationship is more complex; period length may be determined by an intricate balance between the requirements for growth, metabolism, and development.
By contrast with circadian period, our results suggest that circadian amplitude correlated with soil conditions, specifically the percentage of organic matter (Table III; Fig. 5C). Although at this stage we cannot rule out the possibility that the amplitude differences may be affected by variations in photosynthetic capacity between the lines, we suggest that it is possible that plants with high amplitude rhythms grow better and are selected for under challenging environmental conditions. We have reported previously that, in Arabidopsis, when plants are grown under stressful high-density conditions, the next generation has higher amplitude rhythms, suggesting that the stressful conditions may select for amplitude (Yerushalmi et al., 2011). Supporting the idea that the selective force of the environmental conditions may impact different aspects of barley growth and development, Db at the site of origin is strongly correlated with plant size but not with flowering time in the B1K wild barley lines (Hübner et al., 2013).
Our results raise the possibility that circadian amplitude may be a target for optimizing crop plant growth and yield. Consistent with this idea, Ni et al. (2009) have shown that increased productivity arising from hybrid vigor may be at least partially caused by an increase in the circadian amplitude of key regulatory genes. However, seemingly paradoxically, several studies have associated the loss of circadian amplitude with domestication and the spread of crops, such as in tomato (Solanum lycopersicum; Muller et al., 2016) and some barley cultivars (Faure et al., 2012), and mutations in the rice (Oryza sativa) GIGANTEA gene cause a reduction in the amplitude of gene expression in day/night conditions without adverse effects on photosynthesis and growth (Izawa et al., 2011). Clearly, plants with lower amplitude rhythms can thrive. We suggest, though, that while such plants may perform well under the optimal or close-to optimal conditions of modern agriculture, having a higher amplitude rhythm may still confer an advantage when conditions are less than ideal.
CONCLUSION
The world is facing pressure to produce more food for an increasing population with less suitable agricultural land and under conditions of changing climate. Although the circadian system regulates numerous physiological and molecular processes of agricultural and ecological importance, remarkably little is known about how the circadian clock affects plant-environment interactions in major crop plants (Bendix et al., 2015). Our results suggest how the circadian system may be an important target for optimizing plant growth and yield under potentially stressful conditions.
MATERIALS AND METHODS
Plant Materials and Growth
For the Arabidopsis (Arabidopsis thaliana) experiments, wild-type, CCA1-ox, and cca1-1 lhy (SALK_031092) plants in the Columbia ecotype were used (Wang and Tobin, 1998; Yakir et al., 2009). For the barley (Hordeum vulgare ssp. spontaneum) experiments, the spring barley cv Bowman and an introgression line, cv Bowman(eam8), provided by Maria Von Korff (Department of Plant Developmental Biology, Max Planck Institute for Plant Breeding Research), and lines from the B1K collection (Hübner et al., 2009) were used. All experiments were carried out on plants growing on commercial potting soil. Seeds were cold treated at 4°C for 4 d (Arabidopsis) or 14 d (barley) to optimize germination. Unless stated otherwise, all plants were germinated and grown in LD. The barley plants were grown under short-day conditions (8 h of light/16 h of dark) at 150 µE m−2 s−1 light intensity and 22°C until germination was complete (appearance of the first leaf). Other plants were obtained as young specimens grown on soil from a local nursery. Philips fluorescent lights TLD 18W/29 and TLD 18W/33CW were used as the light sources for plant growth and entrainment.
Circadian F Analysis
F measurements were done with a customized Open FluorCam system and a growth chamber supplied by Photon System Instruments. The growth chamber does not control humidity or CO2 concentration. The FluorCam system uses the pulse-amplitude-modulated mode technique to measure the Kautsky effect (Nedbal et al., 2000). The barley plants that had been grown in short days were transferred to LD at 22°C for 5 d of entrainment. All plants were acclimated in the FluorCam chamber for at least 1 d of LD before switching to LL (200 µE m−2 s−1, 50% red [615 nm] and 50% blue [450 nm]). At 2-h intervals, barley plants were given 15 min of dark adaptation (all other species were given 5 min of dark adaptation), and then the sequence of F was imaged. We used four to six plants for each line and visually selected three areas on each plant for imaging. The selected areas were checked to ensure that they remained fully focused on the plant during the course of the experiment. For the F measurements, blue (450 nm) actinic light (200 µE m−2 s−1) was used to drive photochemistry. F emission was induced by saturating flashes of 2,250 µE m−2 s−1 from cool-white 5700K light-emitting diodes. The far-red light flashes were provided by 735-nm light-emitting diodes. Fluorescence images were captured by a 512- × 512-pixel CCD camera. Results were analyzed using the FluorCam7 software package from the manufacturer. Data were imported into the Biological Rhythms Analysis Software System (available from http://www.amillar.org), and the period, RAE, phase, and amplitude of the rhythms were analyzed with the FFT-NLLS suite of programs, as described previously (Plautz et al., 1997).
Data Analysis
Environmental data consisted of nine variables (Table III). Aspect, being a circular variable, was replaced by its north-south and east-west components, calculated by taking the cosine and sine of the angle, respectively. All environmental variables were standardized prior to subsequent analyses. To resolve issues of multicollinearity, variables were removed sequentially until the variance inflation factor (VIF) of all remaining variables fell below 3 (Zuur et al., 2010). Variables in order of removal were north-south, elevation, slope, MDT1, and Db.
Each of the three clock attributes (average period, average amplitude, and Rfd′ phase) was subjected to full-subset regression modeling. To allow for nonlinearities, the global model included a second polynomial term for each of the variables but no interactions. The models were ranked and weighted according to their AICc scores. Averaged (with shrinkage) coefficients and relative variable importance were calculated for all models with AICc < 2 using the MuMin package (Barton, 2015). The quality of the averaged model was evaluated by correlating the observed and expected responses (clock attributes). R was used for all statistical analyses (R Core Team, 2014).
Supplemental Data
The following supplemental materials are available.
Supplemental Figure S1. Rfd′ is rhythmic in cv Bowman barley.
Supplemental Figure S2. Correlations between the three F parameters used in the B1K screen.
Supplemental Table S1. Comparison of the terminology for chlorophyll fluorescence parameters.
Supplementary Material
Acknowledgments
We thank N. Keren for advice on photosynthesis and fluorescence, Dr. M. Von Korff for the cultivated barley lines, and M. Shtaya, M. Haddad, S. Davis, and the technical and engineering staff at Photon System Instruments for advice and help in setting up the FluorCam as a system for measuring circadian rhythms.
Glossary
- F
prompt chlorophyll fluorescence
- DF
delayed chlorophyll fluorescence
- LD
14 h of light/10 h of dark
- LL
continuous white light
- RAE
relative amplitude error
- MDT1
midday temperature in January
- MDT8
midday temperature in August
- MAR
average annual rainfall
- OM
soil organic matter
- Db
soil bulk density
- WC
soil water content
Footnotes
This work was supported by the Deutsche Forschungsgemeinschaft and the Israel Science Foundation (grant no. ISF 649/12).
Articles can be viewed without a subscription.
References
- Barton K. (2015) MuMIn - Model selection and model averaging based on information criteria (AICc and alike). R package version 1.15.1.https://CRAN.R-project.org/package=MuMIn/
- Bendix C, Marshall CM, Harmon FG (2015) Circadian clock genes universally control key agricultural traits. Mol Plant 8: 1135–1152 [DOI] [PubMed] [Google Scholar]
- Blake T, Blake V, Bowman J, Abdel-Haleem H (2011) Barley feed uses and quality improvement. In SE Ullrich, ed, Barley: Production, Improvement and Uses. Blackwell Publishing; Wiley-Blackwell, New Jersey, pp. 522–531 [Google Scholar]
- Boden SA, Weiss D, Ross JJ, Davies NW, Trevaskis B, Chandler PM, Swain SM (2014) EARLY FLOWERING3 regulates flowering in spring barley by mediating gibberellin production and FLOWERING LOCUS T expression. Plant Cell 26: 1557–1569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins HM, Burton RA, Topping DL, Liao ML, Bacic A, Fincher GB (2010) Variability in fine structures of noncellulosic cell wall polysaccharides from cereal grains: potential importance in human health and nutrition. Cereal Chem 87: 272–282 [Google Scholar]
- Covington MF, Maloof JN, Straume M, Kay SA, Harmer SL (2008) Global transcriptome analysis reveals circadian regulation of key pathways in plant growth and development. Genome Biol 9: R130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croce R, van Amerongen H (2014) Natural strategies for photosynthetic light harvesting. Nat Chem Biol 10: 492–501 [DOI] [PubMed] [Google Scholar]
- Demmig-Adams B, Cohu CM, Muller O, Adams WW III (2012) Modulation of photosynthetic energy conversion efficiency in nature: from seconds to seasons. Photosynth Res 113: 75–88 [DOI] [PubMed] [Google Scholar]
- de Montaigu A, Giakountis A, Rubin M, Tóth R, Cremer F, Sokolova V, Porri A, Reymond M, Weinig C, Coupland G (2015) Natural diversity in daily rhythms of gene expression contributes to phenotypic variation. Proc Natl Acad Sci USA 112: 905–910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodd AN, Salathia N, Hall A, Kévei E, Tóth R, Nagy F, Hibberd JM, Millar AJ, Webb AA (2005) Plant circadian clocks increase photosynthesis, growth, survival, and competitive advantage. Science 309: 630–633 [DOI] [PubMed] [Google Scholar]
- Edwards KD, Anderson PE, Hall A, Salathia NS, Locke JC, Lynn JR, Straume M, Smith JQ, Millar AJ (2006) FLOWERING LOCUS C mediates natural variation in the high-temperature response of the Arabidopsis circadian clock. Plant Cell 18: 639–650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards KD, Lynn JR, Gyula P, Nagy F, Millar AJ (2005) Natural allelic variation in the temperature-compensation mechanisms of the Arabidopsis thaliana circadian clock. Genetics 170: 387–400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faure S, Turner AS, Gruszka D, Christodoulou V, Davis SJ, von Korff M, Laurie DA (2012) Mutation at the circadian clock gene EARLY MATURITY 8 adapts domesticated barley (Hordeum vulgare) to short growing seasons. Proc Natl Acad Sci USA 109: 8328–8333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Futuyma DJ. (1998) Evolutionary Biology, Ed 3 Sinauer Associates, Sunderland, MA [Google Scholar]
- Gawronski P, Ariyadasa R, Himmelbach A, Poursarebani N, Kilian B, Stein N, Steuernagel B, Hensel G, Kumlehn J, Sehgal SK, Gill BS, Gould P, Hall A, Schnurbusch T (2014) A distorted circadian clock causes early flowering and temperature-dependent variation in spike development in the Eps-3Am mutant of einkorn wheat. Genetics 196: 1253–1261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould PD, Diaz P, Hogben C, Kusakina J, Salem R, Hartwell J, Hall A (2009) Delayed fluorescence as a universal tool for the measurement of circadian rhythms in higher plants. Plant J 58: 893–901 [DOI] [PubMed] [Google Scholar]
- Green RM, Tingay S, Wang ZY, Tobin EM (2002) Circadian rhythms confer a higher level of fitness to Arabidopsis plants. Plant Physiol 129: 576–584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green RM, Tobin EM (1999) Loss of the circadian clock-associated protein 1 in Arabidopsis results in altered clock-regulated gene expression. Proc Natl Acad Sci USA 96: 4176–4179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harmer SL, Hogenesch JB, Straume M, Chang HS, Han B, Zhu T, Wang X, Kreps JA, Kay SA (2000) Orchestrated transcription of key pathways in Arabidopsis by the circadian clock. Science 290: 2110–2113 [DOI] [PubMed] [Google Scholar]
- Hsu PY, Harmer SL (2014) Wheels within wheels: the plant circadian system. Trends Plant Sci 19: 240–249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hübner S, Bdolach E, Ein-Gedy S, Schmid KJ, Korol A, Fridman E (2013) Phenotypic landscapes: phenological patterns in wild and cultivated barley. J Evol Biol 26: 163–174 [DOI] [PubMed] [Google Scholar]
- Hübner S, Höffken M, Oren E, Haseneyer G, Stein N, Graner A, Schmid K, Fridman E (2009) Strong correlation of wild barley (Hordeum spontaneum) population structure with temperature and precipitation variation. Mol Ecol 18: 1523–1536 [DOI] [PubMed] [Google Scholar]
- Ishida S, Uebayashi N, Tazoe Y, Ikeuchi M, Homma K, Sato F, Endo T (2014) Diurnal and developmental changes in energy allocation of absorbed light at PSII in field-grown rice. Plant Cell Physiol 55: 171–182 [DOI] [PubMed] [Google Scholar]
- Izawa T, Mihara M, Suzuki Y, Gupta M, Itoh H, Nagano AJ, Motoyama R, Sawada Y, Yano M, Hirai MY, et al. (2011) Os-GIGANTEA confers robust diurnal rhythms on the global transcriptome of rice in the field. Plant Cell 23: 1741–1755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakob SS, Rödder D, Engler JO, Shaaf S, Ozkan H, Blattner FR, Kilian B (2014) Evolutionary history of wild barley (Hordeum vulgare subsp. spontaneum) analyzed using multilocus sequence data and paleodistribution modeling. Genome Biol Evol 6: 685–702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalaji MH, Carpentier R, Allakhverdiev SI, Bosa K (2012) Fluorescence parameters as early indicators of light stress in barley. J Photochem Photobiol B 112: 1–6 [DOI] [PubMed] [Google Scholar]
- Keren N, Berg A, van Kan PJ, Levanon H, Ohad I, (1997) Mechanism of photosystem II photoinactivation and D1 protein degradation at low light: the role of back electron flow. Proc Natl Acad Sci USA 94: 1579–1584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kromdijk J, Głowacka K, Leonelli L, Gabilly ST, Iwai M, Niyogi KK, Long SP (2016) Improving photosynthesis and crop productivity by accelerating recovery from photoprotection. Science 354: 857–861 [DOI] [PubMed] [Google Scholar]
- Kusakina J, Gould PD, Hall A (2014) A fast circadian clock at high temperatures is a conserved feature across Arabidopsis accessions and likely to be important for vegetative yield. Plant Cell Environ 37: 327–340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kusakina J, Rutterford Z, Cotter S, Martí MC, Laurie DA, Greenland AJ, Hall A, Webb AA (2015) Barley Hv CIRCADIAN CLOCK ASSOCIATED 1 and Hv PHOTOPERIOD H1 Are Circadian Regulators That Can Affect Circadian Rhythms in Arabidopsis. PLoS One 10:e0127449 [DOI] [PMC free article] [PubMed]
- Lichtenthaler HK, Buschmann C, Rinderle U, Schmuck G (1986) Application of chlorophyll fluorescence in ecophysiology. Radiat Environ Biophys 25: 297–308 [DOI] [PubMed] [Google Scholar]
- Lichtenthaler HK, Miehe JA (1997) Fluorescence imaging as a diagnostic tool for plant stress. Trends Plant Sci 2: 316–320 [Google Scholar]
- Litthauer S, Battle MW, Lawson T, Jones MA (2015) Phototropins maintain robust circadian oscillation of PSII operating efficiency under blue light. Plant J 83: 1034–1045 [DOI] [PubMed] [Google Scholar]
- Lou P, Xie Q, Xu X, Edwards CE, Brock MT, Weinig C, McClung CR (2011) Genetic architecture of the circadian clock and flowering time in Brassica rapa. Theor Appl Genet 123: 397–409 [DOI] [PubMed] [Google Scholar]
- Lu SX, Knowles SM, Andronis C, Ong MS, Tobin EM (2009) CIRCADIAN CLOCK ASSOCIATED1 and LATE ELONGATED HYPOCOTYL function synergistically in the circadian clock of Arabidopsis. Plant Physiol 150: 834–843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer KFX, Waugh R, Langridge P, Close TJ, Wise RP, Graner A, Matsumoto T, Sato K, Schulman A, Muehlbauer GJ, et al. (2012) A physical, genetic and functional sequence assembly of the barley genome. Nature 491: 711–716 [DOI] [PubMed] [Google Scholar]
- Maxwell K, Johnson GN (2000) Chlorophyll fluorescence—a practical guide. J Exp Bot 51: 659–668 [DOI] [PubMed] [Google Scholar]
- Michael TP, Salomé PA, Yu HJ, Spencer TR, Sharp EL, McPeek MA, Alonso JM, Ecker JR, McClung CR (2003) Enhanced fitness conferred by naturally occurring variation in the circadian clock. Science 302: 1049–1053 [DOI] [PubMed] [Google Scholar]
- Mizoguchi T, Wheatley K, Hanzawa Y, Wright L, Mizoguchi M, Song HR, Carré IA, Coupland G (2002) LHY and CCA1 are partially redundant genes required to maintain circadian rhythms in Arabidopsis. Dev Cell 2: 629–641 [DOI] [PubMed] [Google Scholar]
- Muller P, Li XP, Niyogi KK (2001) Non-photochemical quenching. A response to excess light energy. Plant Physiol 125: 1558–1566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller NA, Wijnen CL, Srinivasan A, Ryngajllo M, Ofner I, Lin T, Ranjan A, West D, Maloof JN, Sinha NR, et al. (2016) Domestication selected for deceleration of the circadian clock in cultivated tomato. Nat Genet 48: 89–93 [DOI] [PubMed] [Google Scholar]
- Nagel DH, Kay SA (2012) Complexity in the wiring and regulation of plant circadian networks. Curr Biol 22: R648–R657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nedbal L, Soukupová J, Kaftan D, Whitmarsh J, Trtílek M (2000) Kinetic imaging of chlorophyll fluorescence using modulated light. Photosynth Res 66: 3–12 [DOI] [PubMed] [Google Scholar]
- Nevo E. (1998) Genetic diversity in wild cereals: regional and local studies and their bearing on conservation ex situ and in situ. Genet Resour Crop Evol 45: 355–370 [Google Scholar]
- Ni Z, Kim ED, Ha M, Lackey E, Liu J, Zhang Y, Sun Q, Chen ZJ (2009) Altered circadian rhythms regulate growth vigour in hybrids and allopolyploids. Nature 457: 327–331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan WJ, Wang X, Deng YR, Li JH, Chen W, Chiang JY, Yang JB, Zheng L (2015) Nondestructive and intuitive determination of circadian chlorophyll rhythms in soybean leaves using multispectral imaging. Sci Rep 5: 11108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plautz JD, Straume M, Stanewsky R, Jamison CF, Brandes C, Dowse HB, Hall JC, Kay SA (1997) Quantitative analysis of Drosophila period gene transcription in living animals. J Biol Rhythms 12: 204–217 [DOI] [PubMed] [Google Scholar]
- R Core Team (2014) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria; http://www.R-project.org/ [Google Scholar]
- Salmela MJ, Greenham K, Lou P, McClung CR, Ewers BE, Weinig C (2016) Variation in circadian rhythms is maintained among and within populations in Boechera stricta. Plant Cell Environ 39: 1293–1303 [DOI] [PubMed] [Google Scholar]
- Wang ZY, Tobin EM (1998) Constitutive expression of the CIRCADIAN CLOCK ASSOCIATED 1 (CCA1) gene disrupts circadian rhythms and suppresses its own expression. Cell 93: 1207–1217 [DOI] [PubMed] [Google Scholar]
- Yakir E, Hilman D, Kron I, Hassidim M, Melamed-Book N, Green RM (2009) Posttranslational regulation of CIRCADIAN CLOCK ASSOCIATED1 in the circadian oscillator of Arabidopsis. Plant Physiol 150: 844–857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yerushalmi S, Yakir E, Green RM (2011) Circadian clocks and adaptation in Arabidopsis. Mol Ecol 20: 1155–1165 [DOI] [PubMed] [Google Scholar]
- Zuur AF, Ieno EN, Elphick CS (2010) A protocol for data exploration to avoid common statistical problems. Methods Ecol Evol 1: 3–14 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.