Identification of putative regulatory genes in the ambient temperature flowering time pathway of tulip highlights several orthologs in Arabidopsis flowering control.
Abstract
The vegetative-to-reproductive phase change in tulip (Tulipa gesneriana) is promoted by increasing temperatures during spring. The warm winters of recent years interfere with this process and are calling for new adapted cultivars. A better understanding of the underlying molecular mechanisms would be of help, but unlike the model plant Arabidopsis (Arabidopsis thaliana), very little is known about floral induction in tulip. To shed light on the gene regulatory network controlling flowering in tulip, RNA sequencing was performed on meristem-enriched tissue collected under two contrasting temperature conditions, low and high. The start of reproductive development correlated with rounding of the shoot apical meristem and induction of TGSQA expression, a tulip gene with a high similarity to Arabidopsis APETALA1. Gene Ontology enrichment analysis of differentially expressed genes showed the overrepresentation of genes potentially involved in floral induction, bulb maturation, and dormancy establishment. Expression analysis revealed that TERMINAL FLOWER1 (TgTFL1) and SUPPRESSOR OF OVEREXPRESSION OF CONSTANS1-like1 (TgSOC1-like1) might be repressors, whereas TgSOC1-like2 likely is an activator, of flowering. Subsequently, the flowering time-associated expression of eight potential flowering time genes was confirmed in three tulip cultivars grown in the field. Additionally, heterologous functional analyses in Arabidopsis resulted in flowering time phenotypes in line with TgTFL1 being a floral repressor and TgSOC1-like2 being a floral activator in tulip. Taken together, we have shown that long before morphological changes occur in the shoot apical meristem, the expression of floral repressors in tulip is suppressed by increased ambient temperatures, leading either directly or indirectly to the activation of potential flowering activators shortly before the commencement of the phase change.
The monocotyledonous species tulip (Tulipa gesneriana) originates from Central Asia and grows in mountain-rich areas with a temperate climate (Kamenetsky and Okubo, 2012; Christenhusz et al., 2013). Most cultivated tulips are produced in The Netherlands, which has a temperate maritime climate, fairly resembling the climate of the tulip’s region of origin (Compton et al., 2007). The growth cycle of cultivated tulips starts in autumn, when the bulbs are planted in the field. At that moment, all organs, such as the stem, leaves, and flower, are already present inside the bulb. A subsequent period of prolonged cold is required for fast stem elongation as well as for internal preparation of the flower to bloom in spring (Lambrechts et al., 1994; Rietveld et al., 2000). After this cold winter period, the stem elongates, the leaves stretch and unfold, and blooming occurs around April or May, depending on the cultivar. The mother bulb is completely consumed after blooming, and the main daughter bulb, also known as axillary bud A, replaces the mother bulb (Botschantzeva, 1982). Increasing ambient temperatures in spring are assumed to induce the vegetative-to-reproductive phase change (floral induction) at the shoot apical meristem (SAM) in the daughter bulb, leading to the development of the floral organs and the induction of dormancy (Steward et al., 1971; Gilford and Rees, 1973; De Hertogh and Le Nard, 1993). Once the flower is completely developed inside the bulb, the life cycle starts again.
The morphology of the SAM during floral induction was well characterized by Beijer (1952); however, until now, the molecular regulation of floral induction has not been thoroughly investigated. In contrast, this process has been studied extensively in the model dicotyledonous species Arabidopsis (Arabidopsis thaliana). In Arabidopsis, floral induction can be triggered by long days after a period of prolonged cold (vernalization response), which leads to the down-regulation of the flowering repressor FLOWERING LOCUS C (FLC) gene. This repression of FLC facilitates flowering by making the SAM sensitive to flower-inducing cues such as ambient temperature and long days (Choi et al., 2011). When the days are getting longer, the photoperiod pathway is induced, leading to the activation of FLOWERING LOCUS T (FT) by the zinc finger transcriptional regulator CONSTANS. The perception of changes in the photoperiod is located in the leaves, but floral induction occurs at the SAM. In this respect, FT acts as a florigen. The FT protein is transported via the phloem to the SAM, where it interacts with the basic leucine zipper (bZIP) transcription factor FLOWERING LOCUS D (FD). The interaction between FT and FD results in the activation of the floral integrator SUPPRESSOR OF OVEREXPRESSION OF CONSTANS1 (SOC1), SQUAMOSA-BINDING PROTEIN-LIKE (SPL) genes, and finally the flower meristem identity genes APETALA1 (AP1), LEAFY (LFY), and FRUITFULL (Huijser and Schmid, 2011; Andres and Coupland, 2012).
In the absence of the photoperiod pathway in Arabidopsis, the phytohormone GA plays a major role in the regulation of flowering. GA is known to promote the expression of SOC1 and LFY, dependent or independent of the DELLA-mediated pathway, leading to the activation of the so-called A, B, and C class genes (Lee and Lee, 2010). Together with GA, it is believed that other endogenous (e.g. other hormones and carbohydrates) and external (e.g. nutrients and ambient temperature) signals also play a role in the floral induction (Mutasa-Göttgens and Hedden, 2009; Galvão et al., 2015). The molecular regulation of the phase change by ambient temperature has been studied to a lesser extent in comparison with the vernalization and photoperiod pathways (for review, see Verhage et al., 2014; McClung et al., 2016). Examples of genes that have been associated with ambient temperature-mediated flowering in Arabidopsis are FLOWERING LOCUS M, SHORT VEGETATIVE PHASE, EARLY FLOWERING3, TERMINAL FLOWER1 (TFL1), and PHYTOCHROME-INTERACTING FACTOR4 (Balasubramanian and Weigel, 2006; Strasser et al., 2009; Kumar et al., 2012; Lee et al., 2013; Pose et al., 2013; Box et al., 2015; Fernández et al., 2016).
Even though it has been shown that flowering time genes in Arabidopsis are regulated by changes in temperature, the change in daylength (photoperiod) is the key seasonal cue to trigger the reproduction process (Park et al., 1999; Jeong and Clark, 2005; Osnato et al., 2012). In contrast, for tulip, it is assumed that a high ambient temperature is the most important seasonal signal to trigger the floral induction (Khodorova and Boitel-Conti, 2013). In addition, Arabidopsis is a dicot while tulip is a monocot, and this large evolutionary distance may raise the question: how much of the flowering time network has been conserved (i.e. how much knowledge can be transferred from Arabidopsis to tulip)?
In the monocotyledonous model species rice (Oryza sativa), the homologous gene of FT (HEADING DATE3a) also acts as an activator of flowering, but under short-day conditions (Komiya et al., 2008). This, and many other examples, reveal that there is at least some similarity between dicots and monocots in the molecular mechanisms underlying flowering time control (Blümel et al., 2015). To date, only a few flowering time genes have been identified and characterized in ornamental geophytes, such as tulip. A study by Noy-Porat and colleagues (2013), focusing on daffodil (Narcissus tazetta), identified two potential flowering time genes with similarity to FT and LFY of Arabidopsis, respectively. In daffodil, NtFT was shown to be induced by high temperatures at the end of the growth period, correlating with the moment of flowering induction (Noy-Porat et al., 2013). Next to these single-gene approaches, Villacorta-Martin et al. (2015) were, to our knowledge, the first authors to publish a genome-wide study focusing on the vernalization response and flowering in the ornamental geophyte lily (Lilium longiflorum) by transcriptome profiling.
In this study, a genome-wide approach was undertaken to elucidate the molecular mechanism underlying the floral induction and the integration of temperature responses in tulip. In The Netherlands, the warm winters and high temperatures during spring in recent years interfered with the floral induction process and induced an early transition from vegetative to reproductive development, resulting in dehydration of the flower (floral bud blasting) or low-quality tulip flowers (van Dam and van Haaster, 2013). This problem calls for the development of new cultivars that are adapted to this climate change; hence, detailed molecular knowledge of the process is required. An experimental setup was designed with contrary environments, low and high temperature, to identify genes induced by high temperature and their possible role in floral induction. RNA sequencing (RNA-seq) was performed to identify differentially expressed genes in SAM-enriched tissues collected at the different temperatures. Subsequently, both a bottom-up and a top-down approach were followed to identify potential flowering time genes in tulip. For the bottom-up approach, a clustering analysis was performed to obtain an overall picture of the transcriptional changes that correlate with flowering induction, followed by a Gene Ontology (GO) enrichment analysis. For the top-down approach, a direct search based on high similarity with known flowering time genes was performed. From the identified potential flowering time genes, eight were further characterized, and their correlation with the flowering time response was validated in different tulip cultivars. Additionally, heterologous functional analysis of a small number of potential tulip key flowering time regulators was performed in Arabidopsis to confirm their proposed role in the control of this important phase transition.
RESULTS
Morphological Characterization of Floral Induction and Early Flower Development under High-Temperature Conditions
Tulips bloom in spring, and during development toward blooming the resources in the mother bulb are completely consumed. The mother bulb is replaced by a small number of daughter bulbs including one main daughter bulb (known as axillary bud A), which is competent to flower (Botschantzeva, 1982). The SAM within this daughter bulb is present in the middle of the bulb on top of the basal plate and is surrounded by fleshy scales that function as storage organs and provide energy for growth (Fig. 1A; Van der Toorn et al., 2000). The vegetative-to-reproductive phase transition occurs in the main daughter bulb shortly after blooming of the mother bulb and is supposed to be induced by high temperatures during spring (Khodorova and Boitel-Conti, 2013). To prove whether temperature is indeed the primary trigger for the floral induction and to investigate the process of floral induction at the morphological level, tulip bulbs of cv Dynasty were lifted from the field at the end of spring. The bulbs were transferred to controlled-climate cells with long-day conditions to match with field conditions; they were separated into two groups and exposed to low (8°C–9°C) or high (18°C) ambient temperature conditions. The temperature courses during the growth season in the field and in the climate cells were monitored (Supplemental Fig. S1, A and B). Figure 1B shows the morphological changes of the SAM that were observed in the main daughter bulb at 8°C to 9°C in comparison with 18°C. Based on the morphological changes of the SAM, Beijer (1952) divided flower induction and development into seven stages (Supplemental Fig. S2). In order to confirm that floral meristem identity is indeed established around stage II, the expression of a putative floral meristem identity gene was investigated. In Arabidopsis, AP1 has been identified as a floral meristem identity gene, and this gene is not expressed during the vegetative stage of development but specifies floral meristems from the earliest moment onward (Irish and Sussex, 1990; Sundström et al., 2006). In tulip, two genes belonging to the SQUAMOSA subfamily were identified previously in viridiflora tulips and named TGSQA and TGSQB (Hirai et al., 2010). We isolated these two genes that show high similarity with Arabidopsis AP1 and the AP1-like MADS box gene OsMADS28 of rice (Supplemental Fig. S3; Yamaguchi and Hirano, 2006) from the tulip cv Dynasty and adapted the names TGSQA and TGSQB. The expression of TGSQA was investigated in both low- and high-temperature conditions (Fig. 1C). This analysis shows that floral meristem identity is indeed established just after the moment that the SAM enlarges and transforms into a dome-like structure, which is approximately 6 weeks after the start of the high-temperature treatment. This confirms the staging as proposed by Beijer (1952); therefore, the same classification is used in this study.
Figure 1.
Morphology of the vegetative-to-reproductive phase change at the SAM and transcriptional changes over time. A, Morphology of the SAM inside the bulb and its surrounding tissues in spring prior to the temperature experiment. Note that the SAM is still vegetative and that one leaf primordium has developed. Bar = 1 mm. B, Morphological changes at the SAM inside the main daughter bulbs of cv Dynasty during low- and high-temperature conditions. In the first 5 weeks of both temperature conditions, only one leaf primordium developed (green) and the SAM remained flat (yellow). After 6 weeks at 18°C, the SAM got a dome-like appearance, which is the first known morphological change upon making the switch from vegetative to reproductive development. Shortly after this, the floral meristem (FM; orange) gives rise to the development of the different floral organs (tepals, cyan; stamens, violet; carpel, red). Note that the SAM of bulbs in the low-temperature condition (8°C–9°C) remains vegetative and flat for the complete period of 8 weeks. The different tissues have been artificially colored in the right image of each group. Bars = 1 mm. C, Expression pattern of TGSQA at low-temperature (8°C–9°C) and high-temperature (18°C) conditions. D, MDS plot revealing global transcriptional changes over time. The bulbs from the low-temperature (8°C–9°C) condition remain in a relatively stable transcriptional state, whereas the bulbs from the high-temperature (18°C) condition show significant transcriptional changes over time associated with the switch from the vegetative to the reproductive phase. C, Cold; W, warm; the number indicates the week after the start of the experiment. FC, Fold change.
During the first 5 weeks, under both temperature conditions, the SAM of the main daughter bulbs was morphologically in stage I and displayed a similar appearance, with one leaf primordium developed and the SAM remaining flat (Fig. 1B). The first morphological differences between the 8°C to 9°C and 18°C treatments were observed from 6 weeks onward. The main daughter bulbs at 8°C to 9°C continued to develop the first leaf primordium and the SAM remained flat, while at 18°C, the SAM started rounding and forming a dome-like structure (stage II). At 7 weeks, the first floral organ primordium appeared (stage P1) and two additional leaf primordia began to develop. More defined tepal, stamen, and carpel structures were observed after 8 weeks at 18°C (stage A2+). In contrast, bulbs at 8°C to 9°C developed one leaf primordium only and the SAM remained vegetative, even after 8 weeks of low-temperature treatment. Above the soil, the mother plants remained green at the low-temperature condition (Supplemental Fig. S1C), whereas the mother plants at the high-temperature condition senesced completely, resembling the phenotype under normal field conditions (Supplemental Fig. S1D).
Transcriptome Analysis during the Floral Induction: A Top-Down Approach
To obtain a better understanding of floral induction in tulip, transcriptional changes were investigated. RNA-seq was performed on RNA collected from SAM-enriched daughter bulb material collected from week 0 (1 d before transfer) up to 7 weeks after the transfer to the low- or high-temperature environment. Transcripts were reconstructed de novo using Trinity (Haas et al., 2013). A total of 346,016 transcripts were reconstructed, representing 244,383 Trinity genes (Supplemental Table S1). This large number of putative genes is not unusual when using de novo assembly in the absence of a reference genome. In addition, no filtering was used after the transcriptome assembly to prevent the loss of sequence information of lowly expressed transcripts (Moreno-Pachon et al., 2016). The multi-dimensional scaling (MDS) plot in Figure 1D shows global transcriptional changes over time in the SAM at the two different temperature regimes. In the low-temperature condition, very few morphological changes are occurring in the SAM inside the bulb (Fig. 1B), which is accompanied by only a few transcriptional changes. In contrast, gene activity in bulbs in the high-temperature condition is changing substantially over time. The samples taken from week 2.5 until week 6 cluster together, while samples from 7 weeks after the transfer form a separate cluster. This clustering reveals that high temperatures have an immediate effect and that floral induction, and likely other high-temperature-induced processes, are affected directly from the start of the temperature treatment (week 2.5). Subsequently, based on global transcriptional changes, the bulbs remain in this stage for several weeks, followed by a second change in global expression at week 7. This later burst of differential expression coincides perfectly with the morphological changes of the SAM (Fig. 1B) and the induction of flowering, as confirmed by the increase of TGSQA transcript abundance (Fig. 1C).
For further identification of putative flowering time-controlling genes and to gain insight into the global transcriptional changes, an initial top-down approach was followed (Leeggangers et al., 2013). The top-down approach consisted of an untargeted analysis using GO enrichment and a clustering analysis. In the GO enrichment analysis, genes differentially expressed upon high-temperature treatment were selected to get an indication of the biological processes affected by this treatment. For this purpose, transcript abundance at each interval was compared with the situation at the moment just before the transfer to controlled environmental conditions (week 0). Figure 2 displays a selection of GO terms that were found to be overrepresented in the significantly up- and down-regulated genes at high ambient temperature but not in low-temperature conditions at the same time point. A more complete overview of overrepresented GO terms can be found in Supplemental Figure S4. As expected, the panel of up-regulated genes contains GO terms related to the flowering process, such as the regulation of flower development and vegetative-to-reproductive phase transition of the meristem. These GO terms corroborate the morphological changes (Fig. 1B). Besides these directly flowering-related GO terms, several others, such as sugar-mediated signaling pathway, cell cycle, response to temperature stimulus, and RNA splicing, are connected with the vegetative-to-reproductive phase transition. One example of sugar-mediated signaling involved in the flowering process is trehalose-6-phosphate signaling in Arabidopsis, for which the gene TREHALOSE-6-PHOSPHATE SYNTHASE1 is required for the timing of the initiation of flowering (Wahl et al., 2013). Also, the process of RNA splicing has been shown to play a role in ambient temperature-mediated flowering time control in Arabidopsis (Verhage et al., 2014; Capovilla et al., 2015).
Figure 2.
Overview of the GO enrichment analysis in the transcriptome data of the floral induction in tulip. Output GO enrichment analysis was performed by comparing each week with week 0. At left in red, the GO terms listed are specifically overrepresented in the up-regulated genes upon high temperatures. At right in blue, GO terms specifically overrepresented in the down-regulated genes are shown.
In addition to direct flowering-related GO terms, other GO terms related to plant metabolism are overrepresented in both groups of up- and down-regulated genes. One example is carbohydrate biosynthetic process, which is found to be overrepresented in the up-regulated genes, while carbohydrate metabolic process is overrepresented in the down-regulated genes. The up-regulated genes are mostly present in secondary metabolite biosynthesis and glycan biosynthesis/metabolism, while the down-regulated genes are mostly present in lipid metabolism (fatty acid biosynthesis) and metabolism of other amino acids. In this respect, it is good to realize that, at the same moment that a decision is made to flower or not to flower, parts of the mother bulb (e.g. leaves, stem, and scales) are senescing and the daughter bulbs mature and become dormant (De Hertogh and Le Nard, 1993). Therefore, it is possible that the overrepresentation of these metabolism-specific terms is not, or not only, related to floral induction but also to these physiological changes.
The GO terms overrepresented in the down-regulated genes in the high-temperature condition are mostly related to metabolic processes such as amine metabolism and alcohol metabolism, but also hormone related, such as brassinosteroid biosynthesis and jasmonic acid metabolism. Thus, the GO enrichment analysis revealed that, among the up-regulated differentially expressed genes, flowering-related GO terms are present together with GO terms related to bulb maturation and the induction of dormancy.
As a second top-down approach, a coexpression clustering analysis of all transcription factors was performed to focus specifically on regulatory genes for which the expression correlates with high-temperature-induced floral induction in tulip and corresponding morphological changes. Of the clusters with an expression pattern that can be related to floral induction, three selected clusters contain at least one gene showing high sequence similarity with an Arabidopsis flowering time regulator (Fig. 3; Supplemental Fig. S5). In cluster 17, a transcript showing high similarity with the floral repressor AP2 (Jofuku et al., 1994) is present that shows a steep drop in expression after week 4 (Fig. 3A). This is approximately 2 weeks before the SAM obtains its characteristic dome-like structure. In addition to AP2, also a transcript showing high similarity with the floral repressor ALB3 (Wang and Wang, 2009) is present in this cluster. Other putative transcription factor genes in this cluster are ATIPS2, ARF22, and JAZ1. Although these genes are not associated directly with flowering in Arabidopsis, their expression patterns suggest a relation with the repression of flowering. Further detailed analyses are needed to explore possible roles of these regulatory genes in the flowering time response. Cluster 37 contains a transcript showing high similarity with the Arabidopsis ATC gene, another repressor of flowering (Huang et al., 2012). Its expression decreased gradually until week 5 (Fig. 3B). The fact that both clusters 17 and 37 contain putative flowering repressors suggests that the block on flowering is removed around week 4 after high-temperature induction. Other transcripts present in cluster 37 have been related in Arabidopsis to trichrome branching and seed coat development (MYB5; Li et al., 2009), cell wall biosynthesis (GAUT15; Persson et al., 2007), and lignin biosynthesis (PRR2; Nakatsubo et al., 2008). Finally, in cluster 238, two transcripts showing high similarity with known flowering time functions in Arabidopsis were present (Fig. 3C). The first is the flowering time gene FLK, which acts as a repressor of FLC in Arabidopsis (Mockler et al., 2004). The second gene is FT, which acts in Arabidopsis as a floral integrator (Yamaguchi et al., 2005). The expression of these putative flowering time genes increased steadily from week 0 onward until they reached a plateau of maximum expression around week 4. This interesting cluster also contains the genes TBP2 and EDA35. It is attractive to attach a potential function as a flowering inducer to these genes, but it is good to realize that, at the same moment during development, other biological processes are active to which these genes might be related. Hence, we cannot exclude that their correlation with morphological flowering is coincidental.
Figure 3.
Three selected clusters from the cluster analysis of all tulip transcripts that have high similarity with known transcription factors in Arabidopsis. The clusters represent transcripts of the high-temperature condition. On the x axis, the different time points are plotted, and on the y axis, the z score is shown (normalized cpm). A, Expression of the genes in cluster 17 remains stable until week 4, after which their expression decreases. The cluster includes AP2, INOSITOL 3-PHOSPHATE SYNTHASE2 (ATIPS2), AUXIN RESPONSE FACTOR22 (ARF22), JASMONATE-ZIM-DOMAIN PROTEIN1 (JAZ1), and ALBINO3 (ALB3). B, Expression of the genes in cluster 37 decreases slowly over time. This cluster includes ARABIDOPSIS CENTRORADIALIS (ATC), MYB5, GALACTURONOSYLTRANSFERASE15 (GAUT15), and PINORESINOL REDUCTASE2 (PRR2). C, Expression of the genes in cluster 238 increasing from week 0 onward and reaching a plateau around week 4. This cluster includes FLOWERING LOCUS K (FLK), FT, TATA BOX-BINDING PROTEIN2 (TBP2), and EMBRYO SAC DEVELOPMENT ARREST35 (EDA35). Transcripts with a high similarity (BLAST cutoff value of 1e-05) to a known flowering time gene in Arabidopsis are marked with red stars.
Identification and Characterization of Putative Flowering Time Genes: A Bottom-Up Approach
The top-down approach provided first insights into the flowering time gene regulatory network and pointed toward genes potentially involved in a variety of high-temperature-induced biological processes, including floral induction. However, it also revealed limitations of the identification of key regulatory genes of a single defined process based solely on expression association. Therefore, a bottom-up approach was followed as well, guided by the wealth of knowledge on the molecular network of flowering time control in Arabidopsis. In this model species, over 170 genes have been identified and described that are known to play a role in flowering time control (Fornara et al., 2010).
For 57 Arabidopsis flowering time genes, one or more sequences with high similarity could be identified in tulip (Supplemental Fig. S6). Based on the observed expression patterns in tulip during high-temperature-induced flowering, eight genes were selected for further detailed studies of their proposed functioning in flowering induction. To confirm their expression pattern, as well as the overall quality of our RNA-seq assembly and differential gene expression analysis, the expression patterns of these selected genes were confirmed by quantitative reverse transcription PCR (Fig. 4). Among these eight genes is a gene with high similarity to the floral repressor TFL1; therefore, it was designated TgTFL1 (Supplemental Fig. S7A). In the high-temperature condition, the expression of this gene decreased instantly after the start of the treatment, while under the low-temperature treatment, transcript abundance decreased gradually but slowly over the whole period of 8 weeks (Fig. 4A). A similar pattern was observed for the gene belonging to the TM3 subfamily TgSOC1-like1 (TgSOC1L1; Fig. 4B, Supplemental Fig. S7B). Based on these expression patterns, both genes seem to act as repressors of flowering. For TgTFL1, this is expected, based on Arabidopsis data (Hanano and Goto, 2011), but for TgSOC1L1, this is a surprising observation, taking into account the function of the floral integrator AtSOC1 (Lee and Lee, 2010). However, besides this TgSOC1L1 gene, another member of the TM3 subfamily clade was identified and named TgSOC1-like2 (TgSOC1L2; Supplemental Fig. S7B). Expression of TgSOC1L2 increased between weeks 4 and 6 and decreased again between weeks 6 and 7 in the high-temperature condition (Fig. 4C). AtSOC1 showed a similar increase in expression toward the vegetative-to-reproductive phase change, after which its expression diminished during further flower and floral organ development (Lee and Lee, 2010). Another potential floral integrator that could be identified is homologous to Arabidopsis FT, designated TgFT-like (Supplemental Fig. S7B). The abundance of this TgFT-like transcript also increased from week 4 onward, but instead of a decrease in expression, like TgSOC1L2, its expression was induced throughout the whole measured period (Fig. 4D). In the low-temperature condition, both genes were not expressed; hence, for both genes, a positive correlation with floral induction was observed, providing evidence that these genes might act as activators of flowering in tulip.
Figure 4.
Expression analysis by quantitative reverse transcription-PCR of eight putative tulip flowering time genes in the SAM region of the main daughter bulb during 8 weeks of high- or low-temperature treatment. A, Expression of TgTFL1. B, Expression of TgSOC1L1. C, Expression of TgSOC1L2. D, Expression of TgFT-like. E, Expression of TgSEP1. F, Expression of TGSQB. G, Expression of TgSPL1. H, Expression of TgSPL2.
Morphological data and the expression of the AP1-like gene TGSQA in tulip (Fig. 1C) suggested that flower development starts during week 6, and the expression of the putative floral organ identity gene SEPALLATA1 (SEP1) and TGSQB correlates with this (Fig. 4, E and F; Supplemental Fig. S6). Furthermore, these genes were not expressed in the low-temperature condition in which the SAM remains vegetative. TGSQA has a high similarity to AP1 from Arabidopsis and the snapdragon (Antirrhinum majus) SQUAMOSA gene (Supplemental Fig. S3). SQUAMOSA genes are regulated by SQUAMOSA PROMOTOR-BINDING PROTEIN box genes (Preston and Hileman, 2010), of which two were identified in the tulip transcriptome, designated TgSPL1 and TgSPL2. Both genes have different expression patterns (Fig. 4, G and H). TgSPL2 might act as a floral repressor, because its expression decreased from weeks 0 to 4 under high-temperature conditions but increased under the low-temperature condition. In contrast, TgSPL1 was induced specifically by high temperature, and this increase coincided with the up-regulation of TGSQA, making it a putative candidate for this regulatory function upstream of TGSQA and suggesting a conservation of this regulatory link between Arabidopsis and tulip.
Genetic Diversity as a Tool to Confirm the Role of Putative Tulip Flowering Time Genes
To date, no efficient tools are available to transform Tulipa spp. (Kanno et al., 2007). Therefore, genetic diversity was used as a tool to obtain additional confirmation of the proposed role of a selection of genes in the flowering time response. Second, the potential tulip flowering time regulators were identified under controlled temperature conditions in climate cells; therefore, the experiment was repeated with the original cultivar (cv Dynasty) and selected additional cultivars under their natural conditions in the field. Unfortunately, no detailed information is available about the moment of floral induction in different tulip genetic backgrounds. However, the moment of blooming in spring has been reported for a large number of tulip cultivars, and we hypothesized that there is a direct correlation between the timing of blooming and the moment of the vegetative-to-reproductive phase change inside the main daughter bulb. Initially, six tulip cultivars were selected with variable blooming times in spring (Supplemental Fig. S8A). After blooming of the mother bulb, bulbs of all cultivars were lifted at the same time. From 1 month before lifting until 8 weeks after lifting, the morphological changes related to the floral induction were monitored (Supplemental Fig. S8B). Surprisingly, earliness in the floral induction appeared not to be correlated with early blooming in spring. One of the latest-blooming cultivars (cv Strong Gold) of our selection was shown to be one of the first making the developmental switch from the vegetative to the reproductive phase. However, it is important to note that cv Strong Gold is known to be a temperature-sensitive cultivar. The differences in the timing of the phase change appeared to be limited to approximately 1 to 2 weeks only, and all cultivars reached stage P1 (first whorl of tepals formed) almost at the same time (Supplemental Fig. S8B). After reaching this stage, the floral buds developed at a similar speed. Based on these observations, the most diversified cultivars in the moment of the floral induction were selected for molecular analysis (cv Strong Gold, early; cv Purple Prince, mid; cv Dynasty, late; Fig. 5A). From these three cultivars, cv Purple Prince and cv Strong Gold have one parent in common (cv Yokohama).
Figure 5.
Morphological and molecular analysis of the vegetative-to-reproductive phase change in three tulip cultivars. A, Morphological analysis of the changes at the SAM in cv Purple Prince, cv Dynasty, and cv Strong Gold. I, Vegetative; II, reproductive; P1, first whorl of tepals; P2, second whorl of tepals; A2, second whorl of stamens; A2+, beginning of carpel development. FM, Floral meristem. The first visual observation of the transition from vegetative to reproductive development is marked with an asterisk. Bars = 1 mm. B, Expression of TgTFL1. C, Expression of TgSOC1L1. D, Expression of TgSOC1L2. E, Expression of TgFT-like. F, Expression of TGSQA. G, Expression of TgSEP1. H, Expression of TgSPL1. I, Expression of TgSPL2.
Expression of the eight selected putative flowering time genes was monitored in the three cultivars (Fig. 5, B–I). The expression of TgTFL1 decreased first in cv Strong Gold, starting from 4 weeks before lifting, followed 1 to 2 weeks later in cv Purple Prince and cv Dynasty (Fig. 5B). In the case of TgSOC1L1, the expression in all three cultivars decreased in a similar manner (Fig. 5C). The same was observed for the putative floral inducer TgSOC1L2, the expression of which increased from 1 week before lifting (week −1) in all three analyzed cultivars and, after reaching a high steady-state level, started to decrease slowly after the transition to reproductive development (Fig. 5D). Also, TgFT-like expression increased 1 week before lifting (week −1) in all cultivars (Fig. 5E). The expression of TgSEP1 and TgSPL2 was similar for all cultivars and increased from 3 weeks after lifting onward (Fig. 5, G and I). For the TGSQA and TgSPL1 genes, a slightly earlier induction was observed in cv Strong Gold in comparison with cv Dynasty, which is in line with the earlier floral induction in this cultivar (Fig. 5, F and H).
In conclusion, all selected genes showed similar behavior in expression pattern in this field experiment performed in 2015 to the previous controlled-climate chamber experiment in 2013. The observed expression patterns and levels were in line with the supposed functions of the analyzed genes in flowering time control and, as such, provided additional evidence for their proposed roles in this biological process. Whereas for some of the genes no differences in expression could be observed at the exact moment of repression or induction in the three cultivars, TgTFL1, TGSQUA, and TgSPL1 showed expression changes tightly linked to the small differences in the timing of the phase switch from vegetative to reproductive development.
Heterologous Expression of Tulip Flowering Time Genes in Arabidopsis
To further investigate the functions of two selected potential tulip flowering time regulators, heterologous overexpression studies were performed in Arabidopsis. Transgenic Arabidopsis lines in which the tulip genes TgSOC1L2 and TgTFL1 were placed under the control of the constitutive cauliflower mosaic virus 35S promoter (Odell et al., 1985) were generated and phenotyped for flowering time. Overexpression of TgSOC1L2 resulted in a weak early-flowering phenotype (Fig. 6, A and E–G), while overexpression of TgTFL1 resulted in a severe late-flowering phenotype (Fig. 6, B and H–J). In addition to the late-flowering phenotype upon overexpressing TgTFL1, floral organ morphological changes were observed that are similar to those observed when ectopically expressing AtTFL1 (Fig. 6, C and D; Shannon and Meeks-Wagner, 1991), confirming that TgTFL1 is similar in function and behavior to AtTFL1.
Figure 6.
Phenotypic and molecular analyses of Arabidopsis overexpressing different potential tulip flowering time genes. A, 35S:TgSOC1L2. B, 35S:TgTFL1. C, Wild-type flower. D, 35S:TgTFL1 flower. E, Number of days to flowering for 35S:TgSOC1L2. F, Leaf number of 35S:TgSOC1 when the inflorescence reaches a length of 1 cm. G, Expression of TgSOC1L2 in the overexpression line TgSOC1L2-2 in comparison with Columbia-0 (Col-0). H, Number of days to flowering for 35S:TgTFL1. I, Leaf number of 35S:TgTFL1 when the inflorescence reaches a length of 1 cm. J, Expression of TgTFL1 in the overexpression line TgTFL1-1 in comparison with Columbia-0. * represents <0.05 significance and ** represents <0.01 significance.
Protein Interaction Partners of Potential Tulip Flowering Time Regulators
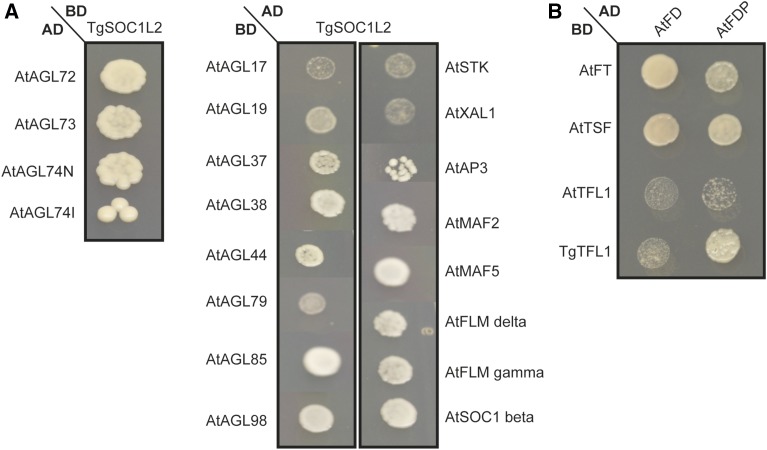
For both AtSOC1L2 and AtTFL1, protein-protein interaction studies have been reported (de Folter et al., 2005; van Dijk et al., 2010; Hanano and Goto, 2011) that provide information about their biological and molecular functions. In total, 25 protein-protein interactions between AtSOC1 and other MADS domain transcription factor proteins were reported in the study of de Folter and colleagues (2005). To test whether the tulip homolog TgSOC1L2 is able to interact with the same set of MADS domain proteins as AtSOC1, the protein-protein interaction between TgSOC1L2 and the collection of Arabidopsis MADS domain proteins was studied. Yeast two-hybrid analyses revealed that TgSOC1L2 is able to interact with 18 Arabidopsis MADS domain proteins (Fig. 7A), of which, remarkably, only four are in common with AtSOC1 (AGAMOUS-LIKE12 [AGL12]/XAANTAL1, AGL17, AGL19, and AGL44/ARABIDOPSIS NITRATE REGULATED1). This difference in protein-protein interaction pattern between AtSOC1 and TgSOC1L2 might explain the weak early-flowering phenotype upon overexpressing TgSOC1L2 in Arabidopsis (Fig. 6). The lack of interaction with the classical ABC-class proteins also could explain why no flower phenotypes appeared upon ectopic expression of TgSOC1L2, in contrast to what has been found when ectopically expressing AtSOC1 in flowers (Borner et al., 2000). In AtSOC1, certain interaction motifs have been characterized and found to be required for protein-protein interactions (van Dijk et al., 2010). When aligning the TgSOC1L2 and AtSOC1 protein sequences, mutations are present at almost all these motifs supposed to be important for protein-protein interactions, except for motif 2 (Supplemental Fig. S9). This supports the difference observed in the protein-protein interactions of TgSOC1L2. Overexpression of TgTFL1 in Arabidopsis gave a similar phenotype to overexpression of AtTFL1 (Ratcliffe et al., 1998). It is known that, in Arabidopsis, both AtFT and AtTFL1 can interact with the bZIP transcription factor FD (Hanano and Goto, 2011). To test whether TgTFL1 is able to interact with AtFD, a yeast two-hybrid assay was performed. TgTFL1 showed interaction with AtFD and AtFDP (Fig. 7B), suggesting that, similar to AtTFL1, TgTFL1 can interfere with the FT/FD-dependent transcriptional activation of flowering time (Hanano and Goto, 2011).
Figure 7.
Yeast two-hybrid assay of potential tulip flowering time regulators. A, Protein-protein interactions between TgSOC1L2 and Arabidopsis MADS domain proteins (synthetic drop-out medium – Leu, Trp, and His + 1 mm 3-aminotriazole). B, Protein-protein interaction of TgTFL1 and Arabidopsis FD and FDP proteins (synthetic drop-out medium – Leu, Trp, and His + 1 mm 3-aminotriazole). AtFT, AtTSF, and AtTFL1 were added to the assay as positive controls. AD, Activation domain; BD, binding domain.
DISCUSSION
In this study, a deep-sequencing RNA-seq approach was followed to shed light on the transcriptional changes occurring prior to and during the switch from vegetative to reproductive development in the bulbous plant species tulip. A broad range of in silico analyses and confirmation of observed expression patterns by quantitative reverse transcription-PCR provided strong evidence for a set of tulip genes to represent regulators of flowering. For two of the identified genes, their supposed roles as repressor and activator of flowering, respectively, could be confirmed by heterologous functional analyses in Arabidopsis. We showed that high ambient temperatures are inducing flowering in tulip and that transcriptional changes associated with the flowering time response occur already 4 to 5 weeks before the first flowering-related morphological changes of the SAM become visible.
Flowering Induction Cooccurs with Bulb Maturation and the Initiation of Dormancy
Simultaneously with flower initiation in the SAM, the daughter bulbs mature and are prepared for a period of dormancy (De Hertogh and Le Nard, 1993). In line with these developmental and physiological conditions, we identified an overrepresentation of GO terms such as dormancy process, seed maturation, and response to abscisic acid (ABA) in the GO analysis of the differentially expressed genes. The phytohormone ABA is often associated with the establishment and maintenance of seed dormancy (McCarty, 1995). Seeds are prevented from precocious germination by the presence of ABA, the osmotic environment, and, possibly, by limiting the availability of energy and nutrients (Garciarrubio et al., 1997; Bewley et al., 2013). In the transcriptome data of tulip, something similar is observed, as many genes annotated with metabolite-associated GO terms, such as amine metabolic process and carbohydrate metabolism, are down-regulated in the meristem-enriched tissue collected from tulip. Down-regulation of metabolism likely is associated here with the preparation or establishment of dormancy, very similar to what is observed in seeds. Thus, based on the transcriptome, maturation and preparation for dormancy in tulips resembles the process of maturation and dormancy induction in seeds. In addition, several studies have shown that ABA can either inhibit or promote flowering, depending on the species (Wang et al., 2002, 2013; Frankowski et al., 2014). In Arabidopsis, the bZIP transcription factor ABSCISIC ACID-INSENSITIVE MUTANT5 is involved in the repression of the floral transition by up-regulation of the vernalization-responsive gene FLC (Wang et al., 2013). Also, in Pharbitis nil (Japanese morning glory), ABA has been shown to have an inhibitory effect on flowering, likely through the modulation of ethylene biosynthesis (Frankowski et al., 2014). In contrast to Arabidopsis and P. nil, in which ABA inhibits flowering, ABA promotes flowering in Litchi chinensis (lychee nut). In this species, the application of exogenous ABA promoted flowering, and this was impaired by the expression of LcAP1, the homolog of Arabidopsis AP1 (Cui et al., 2013). Thus, ABA has been associated with several biological processes, ranging from metabolic arrest, dormancy initiation, and tissue maturation to the control of flowering time. Obviously, more research is required to pinpoint the exact function of ABA during floral bud initiation in tulip.
Functioning of a Tulip TFL1 Gene as a Potential Flowering Repressor
We have identified TgTFL1 as a potential inhibitor of flowering in tulip. Down-regulation of its expression appears to be temperature dependent and is initiated 4 to 5 weeks prior to the switch to reproductive development. In the dicot Arabidopsis, TFL1 also acts as a flowering repressor and has been proposed to function in the ambient temperature pathway as a hub between the photoperiodic and ambient temperature flowering time signaling (Strasser et al., 2009). Also, in strawberry (Fragaria vesca), the AtTFL1 homolog FvTFL1 integrates photoperiod and temperature signals in order to repress flowering (Rantanen et al., 2015). Different from Arabidopsis and strawberry, tulip is a day-neutral plant; therefore, photoperiod is not supposed to play a role in the regulation of flowering time (Kamenetsky and Okubo, 2012). However, surprisingly, GO terms related to photoperiod, such as photoperiodism and response to light stimulus, were found to be overrepresented in the genes up-regulated by high flowering-inducing ambient temperatures, and overall, their expression patterns correlated perfectly with the genes belonging to the GO category vegetative-to-reproductive phase transition of meristem. A bulb is an underground plant structure, but when these specific genes are induced, the plants still have green leaves aboveground that may translate a light or photoperiodic signal to the SAM in the daughter bulbs. It will be of great interest to investigate whether the observed expression differences of photoperiodic genes plays a role in the induction of flowering in tulip and whether the function of TFL1 as an integrator of the photoperiod and ambient temperature pathways is conserved in monocots and in bulbous plant species such as tulip. The life cycles of tulip and the short-day plant strawberry have a lot in common. Under short-day and low-temperature conditions in autumn, strawberry makes the transition of the vegetative to reproductive phase change. Then, after the winter period, the flowers emerge and blooming occurs in spring (Koskela et al., 2012). FvTFL1 is a strong regulator controlling the seasonal flowering of strawberry (Rantanen et al., 2014). Not only in strawberry, but likely also in other perennials, including tulip, TFL1-like genes play an important role in the timing of flowering and the duration of blooming.
TgTFL1 is of interest not only in relation to flowering time control but also to the function of AtTFL1 in maintaining inflorescence meristem identity and, as such, is essential for indeterminate inflorescence development and the production of multiple flowers. A mutation in the Arabidopsis TFL1 gene transforms the indeterminate inflorescence into a terminal floral meristem (Shannon and Meeks-Wagner, 1991), which is similar to tulip reproductive stage morphology. The difference between these two species most likely can be explained by the fact that, in contrast with Arabidopsis, TgTFL1 expression remains low throughout flower development following its reduction toward the phase switch. Nonetheless, when overexpressing TgTFL1 in Arabidopsis, not only is flowering delayed but also petals of most flowers are absent, suggesting that TgTFL1 in Arabidopsis is able to repress AtAP1. This shows that the sequences of TgTFL1 and AtTFL1 are sufficiently similar and conserved to maintain this function in the repression of AtAP1 and that terminal flower formation in tulip is most likely not due to a mutation in the TgTFL1 protein but to a mutation of the TgTFL1 expression pattern.
Existence of TgSOC1-Like Genes with Possible Antagonistic and Novel Functions in Flowering
Two SOC1-like genes were identified in tulip that, surprisingly, appeared to respond in an opposite manner to the temperature treatments. The gene that we designated TgSOC1L1 has an expression pattern typical for a flowering repressor, whereas TgSOC1L2 resembles the expression of AtSOC1 and that of a flowering inducer. In line with this observation, overexpression of TgSOC1L2 in Arabidopsis caused a weak but significant early-flowering phenotype. In this respect, it should be realized that the tulip’s life cycle is different from the life cycle of Arabidopsis (Anderson, 2006; Sofo, 2016). This difference is not only in duration but also when the transition to the reproductive phase is made and blooming occurs. In Arabidopsis, flowering commences directly upon the switch from vegetative to reproductive development, whereas tulip flower buds become dormant after their initiation and still require a period of prolonged cold in order to bloom in spring (Lambrechts et al., 1994). We found that TgSOC1L2 has a dimerization pattern different from AtSOC1 in the heterologous yeast two-hybrid screen. One of the striking differences is that TgSOC1L2 interacts with members of the FLC/MADS AFFECTING FLOWERING (MAF) clade, which are known for their function in vernalization and ambient temperature flowering time responses. Therefore, it seems that TgSOC1L2 evolved different functions in comparison with AtSOC1 but seems to be a key regulator of the ambient temperature-controlled flowering time in tulip.
CONCLUSION
This study has confirmed that high temperature is an important trigger of the vegetative-to-reproductive phase transition in tulip. A large number of potential flowering time regulators have been identified that partially appear to be conserved when compared with the dicot Arabidopsis. Our results are summarized in a putative model of the molecular regulation of the floral induction in tulip (Fig. 8). We have identified a large number of potential novel flowering time regulators that might be bulbous plants or tulip specific. The initiation of flower development, maturation of the bulb, and establishment of dormancy all take place at the same moment. Therefore, it is of great interest to study the interactions between these processes in more detail and to resolve the complexity of events occurring in daughter bulbs, when from the outside nothing seems to be happening and the bulbs are establishing summer dormancy.
Figure 8.
Proposed model of the vegetative-to-reproductive phase change in tulip. During spring, high temperature induces the floral induction in tulip by first repressing TgTFL1 and TgSOC1L1. After this suppression, the floral activators TgSOC1L2 and TgFT-like are induced, leading to direct or indirect activation of floral meristem and organ identity genes (TGSQA, TGSQB, and TgSEP1).
MATERIALS AND METHODS
Plant Material and Growth Conditions
Tulip (Tulipa gesneriana ‘Dynasty’; size 10/11) bulbs were planted in crates in the field in early November 2012 and transferred to two different controlled temperature conditions, 8°C to 9°C and 19°C, at the beginning of June 2013 after decapitation of the flower. The bulbs were planted in crates to prevent damage to the roots by transfer to the temperature-controlled climate cells. The bulbs were maintained in the climate cells at the two indicated temperature regimes with a 16-h photoperiod, with 100 µmol s−1 m−2 light, for 9 weeks. A mix of meristem-enriched tissues (square cutting of 0.5 × 0.5 cm including the meristem and leaf primordia; Fig. 1A), derived from five individual tulip bulbs, was dissected with a scalpel and pooled together to form one biological replicate, and this was repeated three times, once every week in the afternoon (Central European Time, 1–3 pm). Each mix of meristem-enriched tissue was homogenized by the use of liquid nitrogen, mortar, and pestle and stored at −80°C until use. In addition to artificially stimulating or preventing the floral induction by controlled temperature conditions, six cultivars (cv Northgo, cv Purple Prince, cv Dynasty, cv Ile de France, cv Strong Gold, and cv Yellow Flight) were planted directly in the field at the end of October 2014 and harvested in June 2015. After harvest, the bulbs were placed at 25°C for 10 d to dry. After these 10 d, the bulbs were stored at 17°C to 20°C in the dark. Samples of meristem-enriched tissue were collected during the cycle as described above and stored at −80°C until use.
Arabidopsis (Arabidopsis thaliana) seeds were stratified for 2 to 3 d at 4°C and germinated, and a segregating plant population (30–50 plants) was grown under long-day conditions (16/8 h of light/dark) at 20°C on Rockwool blocks. Flowering time was scored by counting the number of rosette leaves at the moment the inflorescence reached a length of 1 cm.
Microscopic Imaging
Morphological changes of the SAM region inside the bulb during the vegetative and reproductive phases were monitored with a Carl Zeiss SV11 stereomicroscope (Zeiss), and photographs were taken with a Nikon digital sight DS-Fi1 camera (Nikon).
Total RNA Extraction and cDNA Synthesis
To extract the total RNA of meristem-enriched tissue, the Tripure protocol (Roche) was used according to the manufacturer’s instructions with the addition of 2% (w/v) polyvinylpyrrolidone and 2% (v/v) β-mercaptoethanol to the extraction buffer. Subsequently, a DNase treatment with RQ1 (Promega) was performed to remove DNA, followed by a phenol:chloroform (1:1) extraction and ethanol precipitation. A total amount of 500 ng was used for first-strand cDNA synthesis using Moloney murine leukemia virus reverse transcriptase (Thermo Scientific) following the protocol from the manufacturer and oligo(dT) primers. All reactions were performed in a MyCycler (Bio-Rad).
Strand-Specific RNA-seq
Total RNA of meristem-enriched tissues, collected between June and late July 2013, was used for RNA-seq. For the preparation of the RNA-seq cDNA library, the TruSeq stranded mRNA sample preparation kit (Illumina) was used according to the manufacturer’s instructions. The quality of the libraries was examined with the Bioanalyzer 2100 DNA 1000 chip (Agilent Technologies). The Illumina HiSeq2000 platform was used to obtain 100-bp paired-end reads.
RNA-seq Data Analysis
The quality of the reads obtained from RNA-seq was examined by FastQC (http://www.bioinformatics.babraham.ac.uk/projects/fastqc/). Trimming of the reads by Trimmomatic version 0.32 (Bolger et al., 2014) was used to improve their quality. After trimming, a de novo assembly was performed using Trinity version 2.0.6 (Haas et al., 2013). Trinity assembles short-read RNA-seq data into contigs, which are likely (parts of) transcripts (Trinity genes). Low-abundance transcripts can be assembled in two or more contigs if regions of the transcript are not covered by any read. Based on sequence similarity, contigs are grouped together with the assumption that they represent isoforms derived from the same genetic locus. Kallisto version 0.42.1 (Bray et al., 2016) was used to quantify gene expression. Differential gene expression analysis was done with the edgeR package version 3.10/5 (Robinson et al., 2010) using the estimated counts produced by Kallisto as input. Each transcript was annotated with the best Arabidopsis hit (BLAST cutoff value of 1e-05). For this, Arabidopsis was chosen because of its well-annotated genome and because an extensive amount of functional analysis has been performed in comparison with the monocot rice (Oryza sativa).
GO Enrichment Analysis
Based on the differentially expressed genes identified in the high-temperature condition, the Plant GeneSet Enrichment Analysis Toolkit was used for GO enrichment. From these sets of differentially expressed genes, the genes also showing differential expression in the cold environment were removed for each weekly interval. The hypergeometric statistical test method and the Yekutieli (false discovery rate under dependency) multitest adjustment method settings were used for the analysis. The significance level and the false discovery rate were set at 0.05.
Clustering Analysis
Clustering of the transcripts with a similar expression pattern was done with the R package hclust using Pearson correlation as the distance measure (https://stat.ethz.ch/R-manual/R-devel/library/stats/html/hclust.html). To include as many transcripts as possible, all transcripts were annotated with the name of the best Arabidopsis hit (cutoff value of 1e-05). The expression values were normalized per gene, and then the z scores per time point were plotted. If the z score for a gene at a time point is 1, this means that the expression value differs by 1 sd from the mean of the expression of this gene over all time points.
Identification of Potential Flowering Time Regulators
Protein sequences of AtSOC1, AtFT, AtSEP1, AtTFL1, and all SPLs were used for BLASTx (cutoff of 1e-05) to identify the tulip transcripts with the highest similarity. All matching sequences were aligned, including the Arabidopsis gene, and the hit with the highest sequence similarity (greater than 50%) was chosen for further characterization.
Phylogenetic Analysis
For the AP1-like genes TGSQA and TGSQB, a maximum likelihood tree was reconstructed with MEGA 5.0 (Tamura et al., 2011). The alignment was made with the default ClustalW settings in MEGA 5.0. For the construction of the maximum likelihood tree, the Whelan and Goldman model was used as the substitution model and 500 bootstraps were generated to test the reliability of the tree. In addition, the setting gaps/missing data treatment was changed to partial deletion with 95% as the site coverage cutoff. These same settings were used to generate the maximum likelihood tree of the FT/TFL1 protein sequences. Here, a cutoff of 70% for the bootstrap value was used to adjust the branches. The neighbor-joining tree of the MADS box proteins was constructed in a similar way but using the Dayhoff model.
Real-Time PCR for Expression Analysis
Real-time PCR was performed in a total volume of 20 µL containing 10 µL of iQ SYBR Green Supermix (Bio-Rad), 5 µL of each forward and reverse primer (0.05 µm; primer details are listed in Supplemental Table S2), and 5 µL of a 1:15 dilution of the cDNA reaction mixture as template. Reactions were performed on the CFX Connect real-time PCR detection system (Bio-Rad) with an initial 3-min denaturation at 95°C followed by 40 cycles of 95°C for 10 s and 60°C for 30 s for the amplification. Final steps used for elongation were 95°C for 1 min, 55°C for 10 s, and 95°C for 30 s with afterward a melt curve determination. Normalized expression levels were calculated by the ΔΔCt method (Livak and Schmittgen, 2001) with TgACT as the reference gene. Calculations were based on three technical replicates and two to three biological replicates.
Construction of Overexpression Lines in Arabidopsis
Two selected genes were amplified from cDNA by PCR with the primers TgTFL1 (forward, 5′-ATGGCAAGAGTGCTGGAGC-3′, and reverse, 5′-TCACTGCTCCCACTTAACAT-3′) and TgSOC1L2 (forward, 5′-ATGAAGAGGGGGAAGACACA-3′, and reverse, 5′-CCATCCAATATGCAAGTCCG-3′). The PCR fragments of the flowering time genes were cloned in the Gateway overexpression vector pGD625 (Immink et al., 2002), driving ectopic expression of the transgene from the cauliflower mosaic virus 35S promoter. All generated constructs were introduced into Agrobacterium tumefaciens AGL0 and transformed into Arabidopsis Columbia-0 plants using the floral dip method (Clough and Bent, 1998).
Yeast Two-Hybrid Assays
Yeast two-hybrid screens were performed according to de Folter and Immink (2011). All baits were tested for autoactivation capacity prior to the screening for potential protein-protein interactions. None of the tested baits showed autoactivation capacity. Saccharomyces cerevisiae, strains PJ69-4a and PJ69-4alpha were used in all two-hybrid analyses.
Accession Numbers
Sequences of the potential flowering time genes described in this study can be found in GenBank (http://www.ncbi.nlm.nih.gov) under the accession numbers BAJ09453.1 (TGSQA), BAJ09452.1 (TGSQB), KY464928 (TgFT-like), KY464929 (TgSOC1L1), KY464930 (TgSOC1L2), KY464931 (TgSEP1), KY464932 (TgTFL1), KY464933 (TgSPL1), and KY464934 (TgSPL2). The raw RNA-seq data have been submitted to the Sequence Read Archive depository and can be obtained from http://www.ncbi.nlm.nih.gov/bioproject/327809.
Supplemental Data
The following supplemental materials are available.
Supplemental Figure S1. Temperature conditions and tulip morphology after transfer from the field to the climate cells.
Supplemental Figure S2. Morphological changes of the SAM during the vegetative-to-reproductive phase change in tulip.
Supplemental Figure S3. Phylogenetic analysis of TGSQA and TGSQB.
Supplemental Figure S4. General overview of the GO enrichment analysis.
Supplemental Figure S5. Selection of clusters (high-temperature condition) with potential flowering time regulators.
Supplemental Figure S6. Expression patterns of a selection of 25 tulip genes that have a high similarity to known flowering time genes in Arabidopsis.
Supplemental Figure S7. Phylogenetic analysis.
Supplemental Figure S8. Morphological analysis of the six different tulip cultivars.
Supplemental Figure S9. Alignment of tulip TgSOC1L2 and Arabidopsis AtSOC1 protein sequences.
Supplemental Table S1. Statistical overview of the de novo assembly by Trinity.
Supplemental Table S2. Quantitative PCR primers for the expression analysis of tulip genes.
Supplementary Material
Acknowledgments
We thank Gebroeders Klaver, Maliepaard Bloembollen, and Van der Gulik Tulpen for the plant material; Maarten Holdinga and Alex Silfhout for planting the bulbs in the field and Juliette Silven for help with gene cloning; Bioscience of Wageningen Plant Research for allowing use of the Carl Zeiss SV11 stereomicroscope; and Mariana Silva Artur for suggestions on the construction of phylogenetic trees.
Glossary
- SAM
shoot apical meristem
- RNA-seq
RNA sequencing
- GO
Gene Ontology
- ABA
abscisic acid
Footnotes
This work was supported by the Technological Top Institute Green Genetics, the Koninklijke Algemeene Vereeniging voor Bloembollencultuur, and the Dutch Ministry of Economic Affairs.
References
- Anderson N. (2006) Flower Breeding and Genetics: Issues, Challenges and Opportunities for the 21st Century. Springer, The Netherlands [Google Scholar]
- Andres F, Coupland G (2012) The genetic basis of flowering responses to seasonal cues. Nat Rev Genet 13: 627–639 [DOI] [PubMed] [Google Scholar]
- Balasubramanian S, Weigel D (2006) Temperature induced flowering in Arabidopsis thaliana. Plant Signal Behav 1: 227–228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beijer JJ. (1952) De ontwikkelingsstadia van tulp. Publication of the Laboratory of bulb research Lisse, The Netherlands: 92: 1–7 [Google Scholar]
- Bewley JD, Bradford KJ, Hilhorst HWM, Nonogaki H (2013) Seeds: Physiology of Development, Germination and Dormancy, Ed 3 Springer, New York [Google Scholar]
- Blümel M, Dally N, Jung C (2015) Flowering time regulation in crops: what did we learn from Arabidopsis? Curr Opin Biotechnol 32: 121–129 [DOI] [PubMed] [Google Scholar]
- Bolger AM, Lohse M, Usadel B (2014) Trimmomatic: a flexible trimmer for Illumina sequence data. Bioinformatics 30: 2114–2120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borner R, Kampmann G, Chandler J, Gleissner R, Wisman E, Apel K, Melzer S (2000) A MADS domain gene involved in the transition to flowering in Arabidopsis. Plant J 24: 591–599 [DOI] [PubMed] [Google Scholar]
- Botschantzeva Z. (1982) Tulips: Taxonomy, Morphology, Cytology, Phytogeography and Physiology. Balkema, Rotterdam, The Netherlands [Google Scholar]
- Box MS, Huang BE, Domijan M, Jaeger KE, Khattak AK, Yoo SJ, Sedivy EL, Jones DM, Hearn TJ, Webb AA, et al. (2015) ELF3 controls thermoresponsive growth in Arabidopsis. Curr Biol 25: 194–199 [DOI] [PubMed] [Google Scholar]
- Bray NL, Pimentel H, Melsted P, Pachter L (2016) Near-optimal probabilistic RNA-seq quantification. Nat Biotechnol 34: 525–527 [DOI] [PubMed] [Google Scholar]
- Capovilla G, Pajoro A, Immink RGH, Schmid M (2015) Role of alternative pre-mRNA splicing in temperature signaling. Curr Opin Plant Biol 27: 97–103 [DOI] [PubMed] [Google Scholar]
- Choi K, Kim J, Hwang HJ, Kim S, Park C, Kim SY, Lee I (2011) The FRIGIDA complex activates transcription of FLC, a strong flowering repressor in Arabidopsis, by recruiting chromatin modification factors. Plant Cell 23: 289–303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christenhusz MJM, Govaerts R, David JC, Hall T, Borland K, Roberts PS, Tuomisto A, Buerki S, Chase MW, Fay MF (2013) Tiptoe through the tulips: cultural history, molecular phylogenetics and classification of Tulipa (Liliaceae). Bot J Linn Soc 172: 280–328 [Google Scholar]
- Clough SJ, Bent AF (1998) Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J 16: 735–743 [DOI] [PubMed] [Google Scholar]
- Compton TJ, Rijkenberg MJA, Drent J, Piersma T (2007) Thermal tolerance ranges and climate variability: a comparison between bivalves from differing climates. J Exp Mar Biol Ecol 352: 200–211 [Google Scholar]
- Cui Z, Zhou B, Zhang Z, Hu Z (2013) Abscisic acid promotes flowering and enhances LcAP1 expression in Litchi chinensis Sonn. S Afr J Bot 88: 76–79 [Google Scholar]
- de Folter S, Immink RGH (2011) Yeast protein-protein interaction assays and screens. In Yuan L, Perry ES, eds, Plant Transcription Factors: Methods and Protocols. Humana Press, Totowa, NJ, pp 145–165 [DOI] [PubMed] [Google Scholar]
- de Folter S, Immink RGH, Kieffer M, Parenicová L, Henz SR, Weigel D, Busscher M, Kooiker M, Colombo L, Kater MM, et al. (2005) Comprehensive interaction map of the Arabidopsis MADS box transcription factors. Plant Cell 17: 1424–1433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Hertogh A, Le Nard M (1993) The Physiology of Flower Bulbs: A Comprehensive Treatise on the Physiology and Utilization of Ornamental Flowering Bulbous and Tuberous Plants. Elsevier, New York [Google Scholar]
- Fernández V, Takahashi Y, Le Gourrierec J, Coupland G (2016) Photoperiodic and thermosensory pathways interact through CONSTANS to promote flowering at high temperature under short days. Plant J 86: 426–440 [DOI] [PubMed] [Google Scholar]
- Fornara F, de Montaigu A, Coupland G (2010) SnapShot: control of flowering in Arabidopsis. Cell 141: 550.e1–550.e2 [DOI] [PubMed] [Google Scholar]
- Frankowski K, Wilmowicz E, Kućko A, Kęsy J, Świeżawska B, Kopcewicz J (2014) Ethylene, auxin, and abscisic acid interactions in the control of photoperiodic flower induction in Pharbitis nil. Biol Plant 58: 305–310 [Google Scholar]
- Galvão VC, Collani S, Horrer D, Schmid M (2015) Gibberellic acid signaling is required for ambient temperature-mediated induction of flowering in Arabidopsis thaliana. Plant J 84: 949–962 [DOI] [PubMed] [Google Scholar]
- Garciarrubio A, Legaria JP, Covarrubias AA (1997) Abscisic acid inhibits germination of mature Arabidopsis seeds by limiting the availability of energy and nutrients. Planta 203: 182–187 [DOI] [PubMed] [Google Scholar]
- Gilford JM, Rees AR (1973) Growth of the tulip shoot. Sci Hortic (Amsterdam) 1: 143–156 [Google Scholar]
- Haas BJ, Papanicolaou A, Yassour M, Grabherr M, Blood PD, Bowden J, Couger MB, Eccles D, Li B, Lieber M, et al. (2013) De novo transcript sequence reconstruction from RNA-seq using the Trinity platform for reference generation and analysis. Nat Protoc 8: 1494–1512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanano S, Goto K (2011) Arabidopsis TERMINAL FLOWER1 is involved in the regulation of flowering time and inflorescence development through transcriptional repression. Plant Cell 23: 3172–3184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirai M, Ochiai T, Kanno A (2010) The expression of two DEFICIENS-like genes was reduced in the sepaloid tepals of viridiflora tulips. Breed Sci 60: 110–120 [Google Scholar]
- Huang NC, Jane WN, Chen J, Yu TS (2012) Arabidopsis thaliana CENTRORADIALIS homologue (ATC) acts systemically to inhibit floral initiation in Arabidopsis. Plant J 72: 175–184 [DOI] [PubMed] [Google Scholar]
- Huijser P, Schmid M (2011) The control of developmental phase transitions in plants. Development 138: 4117–4129 [DOI] [PubMed] [Google Scholar]
- Immink RGH, Gadella TWJ, Ferrario S, Busscher M, Angenent GC (2002) Analysis of MADS-box protein-protein interactions in living plant cells. Proc Natl Acad Sci USA 99: 2416–2421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irish VF, Sussex IM (1990) Function of the apetala-1 gene during Arabidopsis floral development. Plant Cell 2: 741–753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeong S, Clark SE (2005) Photoperiod regulates flower meristem development in Arabidopsis thaliana. Genetics 169: 907–915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jofuku KD, den Boer BG, Van Montagu M, Okamuro JK (1994) Control of Arabidopsis flower and seed development by the homeotic gene APETALA2. Plant Cell 6: 1211–1225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamenetsky R, Okubo H (2012) Ornamental Geophytes: From Basic Science to Sustainable Production. CRC Press, Boca Raton, FL [Google Scholar]
- Kanno A, Nakada M, Akita Y, Hirai M (2007) Class B gene expression and the modified ABC model in nongrass monocots. Sci World J 7: 268–279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khodorova NV, Boitel-Conti M (2013) The role of temperature in the growth and flowering of geophytes. Plants (Basel) 2: 699–711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komiya R, Ikegami A, Tamaki S, Yokoi S, Shimamoto K (2008) Hd3a and RFT1 are essential for flowering in rice. Development 135: 767–774 [DOI] [PubMed] [Google Scholar]
- Koskela EA, Mouhu K, Albani MC, Kurokura T, Rantanen M, Sargent DJ, Battey NH, Coupland G, Elomaa P, Hytönen T (2012) Mutation in TERMINAL FLOWER1 reverses the photoperiodic requirement for flowering in the wild strawberry Fragaria vesca. Plant Physiol 159: 1043–1054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar SV, Lucyshyn D, Jaeger KE, Alos E, Alvey E, Harberd NP, Wigge PA (2012) Transcription factor PIF4 controls the thermosensory activation of flowering. Nature 484: 242–245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambrechts H, Rook F, Kolloffel C (1994) Carbohydrate status of tulip bulbs during cold-induced flower stalk elongation and flowering. Plant Physiol 104: 515–520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J, Lee I (2010) Regulation and function of SOC1, a flowering pathway integrator. J Exp Bot 61: 2247–2254 [DOI] [PubMed] [Google Scholar]
- Lee JH, Ryu HS, Chung KS, Posé D, Kim S, Schmid M, Ahn JH (2013) Regulation of temperature-responsive flowering by MADS-box transcription factor repressors. Science 342: 628–632 [DOI] [PubMed] [Google Scholar]
- Leeggangers HACF, Moreno-Pachon N, Gude H, Immink RGH (2013) Transfer of knowledge about flowering and vegetative propagation from model species to bulbous plants. Int J Dev Biol 57: 611–620 [DOI] [PubMed] [Google Scholar]
- Li SF, Milliken ON, Pham H, Seyit R, Napoli R, Preston J, Koltunow AM, Parish RW (2009) The Arabidopsis MYB5 transcription factor regulates mucilage synthesis, seed coat development, and trichome morphogenesis. Plant Cell 21: 72–89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Δ Δ C(T)) method. Methods 25: 402–408 [DOI] [PubMed] [Google Scholar]
- McCarty DR. (1995) Genetic control and integration of maturation and germination pathways in seed development. Annu Rev Plant Physiol Plant Mol Biol 46: 71–93 [Google Scholar]
- McClung CR, Lou P, Hermand V, Kim JA (2016) The importance of ambient temperature to growth and the induction of flowering. Front Plant Sci 7: 1266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mockler TC, Yu X, Shalitin D, Parikh D, Michael TP, Liou J, Huang J, Smith Z, Alonso JM, Ecker JR, et al. (2004) Regulation of flowering time in Arabidopsis by K homology domain proteins. Proc Natl Acad Sci USA 101: 12759–12764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno-Pachon NM, Leeggangers HACF, Nijveen H, Severing E, Hilhorst H, Immink RGH (2016) Elucidating and mining the Tulipa and Lilium transcriptomes. Plant Mol Biol 92: 249–261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mutasa-Göttgens E, Hedden P (2009) Gibberellin as a factor in floral regulatory networks. J Exp Bot 60: 1979–1989 [DOI] [PubMed] [Google Scholar]
- Nakatsubo T, Mizutani M, Suzuki S, Hattori T, Umezawa T (2008) Characterization of Arabidopsis thaliana pinoresinol reductase, a new type of enzyme involved in lignan biosynthesis. J Biol Chem 283: 15550–15557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noy-Porat T, Cohen D, Mathew D, Eshel A, Kamenetsky R, Flaishman MA (2013) Turned on by heat: differential expression of FT and LFY-like genes in Narcissus tazetta during floral transition. J Exp Bot 64: 3273–3284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odell JT, Nagy F, Chua NH (1985) Identification of DNA sequences required for activity of the cauliflower mosaic virus 35S promoter. Nature 313: 810–812 [DOI] [PubMed] [Google Scholar]
- Osnato M, Castillejo C, Matías-Hernández L, Pelaz S (2012) TEMPRANILLO genes link photoperiod and gibberellin pathways to control flowering in Arabidopsis. Nat Commun 3: 808. [DOI] [PubMed] [Google Scholar]
- Park DH, Somers DE, Kim YS, Choy YH, Lim HK, Soh MS, Kim HJ, Kay SA, Nam HG (1999) Control of circadian rhythms and photoperiodic flowering by the Arabidopsis GIGANTEA gene. Science 285: 1579–1582 [DOI] [PubMed] [Google Scholar]
- Persson S, Caffall KH, Freshour G, Hilley MT, Bauer S, Poindexter P, Hahn MG, Mohnen D, Somerville C (2007) The Arabidopsis irregular xylem8 mutant is deficient in glucuronoxylan and homogalacturonan, which are essential for secondary cell wall integrity. Plant Cell 19: 237–255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pose D, Verhage L, Ott F, Yant L, Mathieu J, Angenent GC, Immink RGH, Schmid M (2013) Temperature-dependent regulation of flowering by antagonistic FLM variants. Nature 503: 414–417 [DOI] [PubMed] [Google Scholar]
- Preston JC, Hileman LC (2010) SQUAMOSA-PROMOTER BINDING PROTEIN 1 initiates flowering in Antirrhinum majus through the activation of meristem identity genes. Plant J 62: 704–712 [DOI] [PubMed] [Google Scholar]
- Rantanen M, Kurokura T, Jiang P, Mouhu K, Hytönen T (2015) Strawberry homologue of terminal flower1 integrates photoperiod and temperature signals to inhibit flowering. Plant J 82: 163–173 [DOI] [PubMed] [Google Scholar]
- Rantanen M, Kurokura T, Mouhu K, Pinho P, Tetri E, Halonen L, Palonen P, Elomaa P, Hytönen T (2014) Light quality regulates flowering in FvFT1/FvTFL1 dependent manner in the woodland strawberry Fragaria vesca. Front Plant Sci 5: 271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratcliffe OJ, Amaya I, Vincent CA, Rothstein S, Carpenter R, Coen ES, Bradley DJ (1998) A common mechanism controls the life cycle and architecture of plants. Development 125: 1609–1615 [DOI] [PubMed] [Google Scholar]
- Rietveld PL, Wilkinson C, Franssen HM, Balk PA, van der Plas LHW, Weisbeek PJ, Douwe de Boer A (2000) Low temperature sensing in tulip (Tulipa gesneriana L.) is mediated through an increased response to auxin. J Exp Bot 51: 587–594 [DOI] [PubMed] [Google Scholar]
- Robinson MD, McCarthy DJ, Smyth GK (2010) edgeR: a Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics 26: 139–140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shannon S, Meeks-Wagner DR (1991) A mutation in the Arabidopsis TFL1 gene affects inflorescence meristem development. Plant Cell 3: 877–892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sofo A. (2016) Arabidopsis thaliana: Cultivation, Life Cycle and Functional Genomics. Nova Science, New York [Google Scholar]
- Steward FC, Barber JT, Bleichert EF, Roca WM (1971) The behavior of shoot apices of Tulipa in relation to floral induction. Dev Biol 25: 310–335 [DOI] [PubMed] [Google Scholar]
- Strasser B, Alvarez MJ, Califano A, Cerdán PD (2009) A complementary role for ELF3 and TFL1 in the regulation of flowering time by ambient temperature. Plant J 58: 629–640 [DOI] [PubMed] [Google Scholar]
- Sundström JF, Nakayama N, Glimelius K, Irish VF (2006) Direct regulation of the floral homeotic APETALA1 gene by APETALA3 and PISTILLATA in Arabidopsis. Plant J 46: 593–600 [DOI] [PubMed] [Google Scholar]
- Tamura K, Peterson D, Peterson N, Stecher G, Nei M, Kumar S (2011) MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol Biol Evol 28: 2731–2739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Dam MFN, Haaster AJM (2013) Onderzoek naar de oorzaak van vroege bloemverdroging in tulpen. Praktijkonderzoek Plant en Omgeving BBF, Lisse, The Netherlands: pp 1–29 [Google Scholar]
- Van der Toorn A, Zemah H, Van As H, Bendel P, Kamenetsky R (2000) Developmental changes and water status in tulip bulbs during storage: visualization by NMR imaging. J Exp Bot 51: 1277–1287 [DOI] [PubMed] [Google Scholar]
- van Dijk ADJ, Morabito G, Fiers M, van Ham RCHJ, Angenent GC, Immink RGH (2010) Sequence motifs in MADS transcription factors responsible for specificity and diversification of protein-protein interaction. PLOS Comput Biol 6: e1001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verhage L, Angenent GC, Immink RGH (2014) Research on floral timing by ambient temperature comes into blossom. Trends Plant Sci 19: 583–591 [DOI] [PubMed] [Google Scholar]
- Villacorta-Martin C, Núñez de Cáceres González FF, de Haan J, Huijben K, Passarinho P, Lugassi-Ben Hamo M, Zaccai M (2015) Whole transcriptome profiling of the vernalization process in Lilium longiflorum (cultivar White Heaven) bulbs. BMC Genomics 16: 550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahl V, Ponnu J, Schlereth A, Arrivault S, Langenecker T, Franke A, Feil R, Lunn JE, Stitt M, Schmid M (2013) Regulation of flowering by trehalose-6-phosphate signaling in Arabidopsis thaliana. Science 339: 704–707 [DOI] [PubMed] [Google Scholar]
- Wang AX, Wang DY (2009) Regulation of the ALBINO3-mediated transition to flowering in Arabidopsis depends on the expression of CO and GA1. Biol Plant 53: 484–492 [Google Scholar]
- Wang WY, Chen WS, Chen WH, Hung LS, Chang PS (2002) Influence of abscisic acid on flowering in Phalaenopsis hybrida. Plant Physiol Biochem 40: 97–100 [Google Scholar]
- Wang Y, Li L, Ye T, Lu Y, Chen X, Wu Y (2013) The inhibitory effect of ABA on floral transition is mediated by ABI5 in Arabidopsis. J Exp Bot 64: 675–684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi A, Kobayashi Y, Goto K, Abe M, Araki T (2005) TWIN SISTER OF FT (TSF) acts as a floral pathway integrator redundantly with FT. Plant Cell Physiol 46: 1175–1189 [DOI] [PubMed] [Google Scholar]
- Yamaguchi T, Hirano HY (2006) Function and diversification of MADS-box genes in rice. Sci World J 6: 1923–1932 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.