Comparative analysis of tobacco EXO70 isoforms reveals their distinct functional properties in tip growth and subcellular localization to several compartments, including specific plasma membrane domains.
Abstract
The vesicle-tethering complex exocyst is one of the crucial cell polarity regulators. The EXO70 subunit is required for the targeting of the complex and is represented by many isoforms in angiosperm plant cells. This diversity could be partly responsible for the establishment and maintenance of membrane domains with different composition. To address this hypothesis, we employed the growing pollen tube, a well-established cell polarity model system, and performed large-scale expression, localization, and functional analysis of tobacco (Nicotiana tabacum) EXO70 isoforms. Various isoforms localized to different regions of the pollen tube plasma membrane, apical vesicle-rich inverted cone region, nucleus, and cytoplasm. The overexpression of major pollen-expressed EXO70 isoforms resulted in growth arrest and characteristic phenotypic deviations of tip swelling and apical invaginations. NtEXO70A1a and NtEXO70B1 occupied two distinct and mutually exclusive plasma membrane domains. Both isoforms partly colocalized with the exocyst subunit NtSEC3a at the plasma membrane, possibly forming different exocyst complex subpopulations. NtEXO70A1a localized to the small area previously characterized as the site of exocytosis in the tobacco pollen tube, while NtEXO70B1 surprisingly colocalized with the zone of clathrin-mediated endocytosis. Both NtEXO70A1a and NtEXO70B1 colocalized to different degrees with markers for the anionic signaling phospholipids phosphatidylinositol 4,5-bisphosphate and phosphatidic acid. In contrast, members of the EXO70 C class, which are specifically expressed in tip-growing cells, exhibited exocytosis-related functional effects in pollen tubes despite the absence of apparent plasma membrane localization. Taken together, our data support the existence of multiple membrane-trafficking domains regulated by different EXO70-containing exocyst complexes within a single cell.
The pollen tube is a structure indispensable for angiosperm sexual reproduction. The tube germinates from a pollen grain after it lands on the stigma of a pistil within a flower. During its growth through the pistil, the pollen tube needs to overcome, depending on the plant species, millimeters to centimeters of transmitting tract distance (Lora et al., 2016) so that sperm cells can be brought to the embryo sac. Overcoming such a long distance is enabled by a special type of polar growth called tip growth, the local delivery of cell materials to a focused growth site, forming an elongated cellular structure. Because of such extreme polar growth, pollen tubes are used as an important model system of cell polarity, and many fundamental cell polarity regulators like ROP GTPases and plant phospholipid-modifying enzymes were first characterized in pollen tubes and later appeared to play an analogous function in other cell types (Qin and Dong, 2015).
Plant cells have several polar membrane domains with different composition (Žárský et al., 2009; Łangowski et al., 2010, 2016), and additional polarized structures arise during the response to pathogens and symbionts (Dörmann et al., 2014). One of the key components regulating cell polarity is the exocyst complex. It is a heterooctameric protein complex (TerBush et al., 1996) that is shared across eukaryotes (Vaškovičová et al., 2013; Martin-Urdiroz et al., 2016) and that tethers secretory vesicles to the plasma membrane (PM) and regulates their subsequent fusion. In animal and yeast model systems, many molecular interactions involved in exocyst function have already been discovered: EXO70 and SEC3 drive exocyst to the target site by interaction with phosphatidylinositol 4,5-bisphosphate (PIP2) and small GTPases of the Rho family (He et al., 2007; Liu et al., 2007; Wu et al., 2010; Pleskot et al., 2015), both budding yeast (Saccharomyces cerevisiae) Sec15p (France et al., 2006) and Exo70p interact with the cell polarity determinant Bem1p (Liu and Novick, 2014), SEC15 interacts with secretory vesicle-associated Rab GTPases (Wu et al., 2005) and promotes myosin motor release after vesicle fusion with the PM (Donovan and Bretscher, 2015), and budding yeast Sec6p binds SNARE proteins and promotes SNARE complex assembly (Dubuke et al., 2015) and also binds the SNARE interactor Sec1p (Morgera et al., 2012). Besides facilitating canonical exocytosis, exocyst in opisthokonts plays a role in other processes such as fission yeast (Schizosaccharomyces pombe) cytokinesis (Wang et al., 2002, 2016), midbody scission (Chen et al., 2006), and the formation of tunneling nanotubes (Hase et al., 2009), and the animal exocyst subcomplex also contributes to the initiation of autophagy (Bodemann et al., 2011).
We previously discovered the exocyst complex in plants (Eliáš et al., 2003) and have unraveled some of its functions, like pectin deposition in seed coats (Kulich et al., 2010), recycling of auxin transporters (Drdová et al., 2013), cell plate initiation and maturation during cytokinesis (Fendrych et al., 2010), tip growth (Cole et al., 2005; Bloch et al., 2016), and defense against pathogen infection (Pečenková et al., 2011). One of the most striking findings was the presence of many paralogs encoding the EXO70 subunit in plant genomes (Eliáš et al., 2003; Cvrčková et al., 2012). Besides the trivial explanation that different EXO70 paralogs could play equivalent roles in different cell types, it was hypothesized that various EXO70 isoforms would coexist within a single cell type and regulate exocytosis to different PM domains (Žárský et al., 2009; Žárský and Potocký, 2010). Besides different lateral mobility and regulated endocytosis, targeted exocytosis was postulated as a key mechanism responsible for the presence of polar membrane domains with different composition. Thus, different activated cortical domains, docking platforms for exocytotic vesicles, could drive the secretion to different parts of the PM by employing different EXO70 isoforms and connected intracellular secretory machinery (diverse recycling endosomes, Rab GTPase, and SNARE protein isoforms, etc.; Žárský et al., 2009).
While exocyst acts as a complex in budding yeast (TerBush et al., 1996; Heider et al., 2016) and plant cells (Hála et al., 2008) and its subunits should be studied in the context of the whole complex, it also makes sense to analyze plant EXO70 proteins separately and infer valuable results with biological relevance. First, the EXO70 subunit is involved in the targeting of the whole complex. Localization of tagged EXO70 alone should reflect the localization where it can drive the rest of the complex. EXO70 proteins are subject to regulated proteolytic degradation (Samuel et al., 2009; Stegmann et al., 2012; Žárský et al., 2013), and exchange of different EXO70 isoform levels probably acts as a fast switch of secretion to polar domains preferred by different EXO70s. Second, some EXO70s may act as part of the exocyst subcomplex (Kulich et al., 2013) or could have functions completely independent of the rest of the exocyst, as was shown for animal EXO70 (Zuo et al., 2006; Dellago et al., 2011; Liu et al., 2012; Zhao et al., 2013). Due to the large number of predicted EXO70 protein interactors, EXO70 isoforms possibly serve as an important component of cell polarity-directing machines (Žárský et al., 2009). Systematic study of the subcellular localization of different EXO70 isoforms is thus of utmost importance.
Due to the large number of plant EXO70 paralogs, distinct intracellular localization of EXO70 protein isoforms to different membrane domains or other compartments was mapped only in some instances. Arabidopsis (Arabidopsis thaliana) AtEXO70B1 was shown to play a role in autophagy and localizes to internal compartments starting at the endoplasmic reticulum and directed to the Golgi-independent vacuolar pathway (Kulich et al., 2013). EXO70I was recently demonstrated to be important for the formation of the periarbuscular membrane subdomain during arbuscular mycorrhizal symbiosis in Medicago trunculata (Zhang et al., 2015). For most EXO70 paralogs, the available studies are based on genetic approaches combined with cytological analyses. This way, AtEXO70H4 was shown to be indispensable for correct trichome cell wall thickening and callose deposition (Kulich et al., 2015). The results of gene-silencing experiments suggest that the EXO70F-like subunits are essential in resistance to fungal penetration in barley (Hordeum vulgare; Ostertag et al., 2013). To our knowledge, no study simultaneously showing the localization of more EXO70 isoforms in a single cell has been published so far.
In the context of plant cell polarity research, the extremely polarized growing pollen tube represents a great model system for large-scale analyses due to easy transiently tagged protein expression and the availability of vast transcriptomic and proteomic data sets. Indeed, some PM domains are larger and better separated from each other in this cell type (Sekereš et al., 2015). Zones of active endocytosis (Derksen et al., 1995; Moscatelli et al., 2007) and exocytosis (Bove et al., 2008; Idilli et al., 2013; Luo et al., 2016) are very well separated. A previous study attempting to systematically examine the localization of exocyst components in tobacco (Nicotiana tabacum) BY-2 cells (Chong et al., 2010) resulted in the aggregation of tagged proteins into clusters caused by overexpression in many cases. Contrary to Chong et al. (2010), we also decided to study autologous tobacco proteins instead of Arabidopsis homologs, so that conditions would be as native as possible.
The aim of our study was to perform a systematic analysis of the localization and functional properties of EXO70 isoforms in growing tobacco pollen tubes. We demonstrate a distinct localization for several EXO70 isoforms and show that selected pollen-expressed isoforms induce specific morphological phenotypes upon overexpression. We provide evidence that isoforms NtEXO70A1a and NtEXO70B1 localize to distinct, mutually exclusive domains of the pollen tube PM and display different degrees of colocalization with markers for the anionic signaling phospholipids PIP2 and phosphatidic acid (PA). Both isoforms colocalize with the exocyst subunit NtSEC3a at the PM and are possibly part of distinct subpopulations of exocyst complex particles.
RESULTS
All Major Angiosperm EXO70 Clades Are Present in Solanaceae Genomes
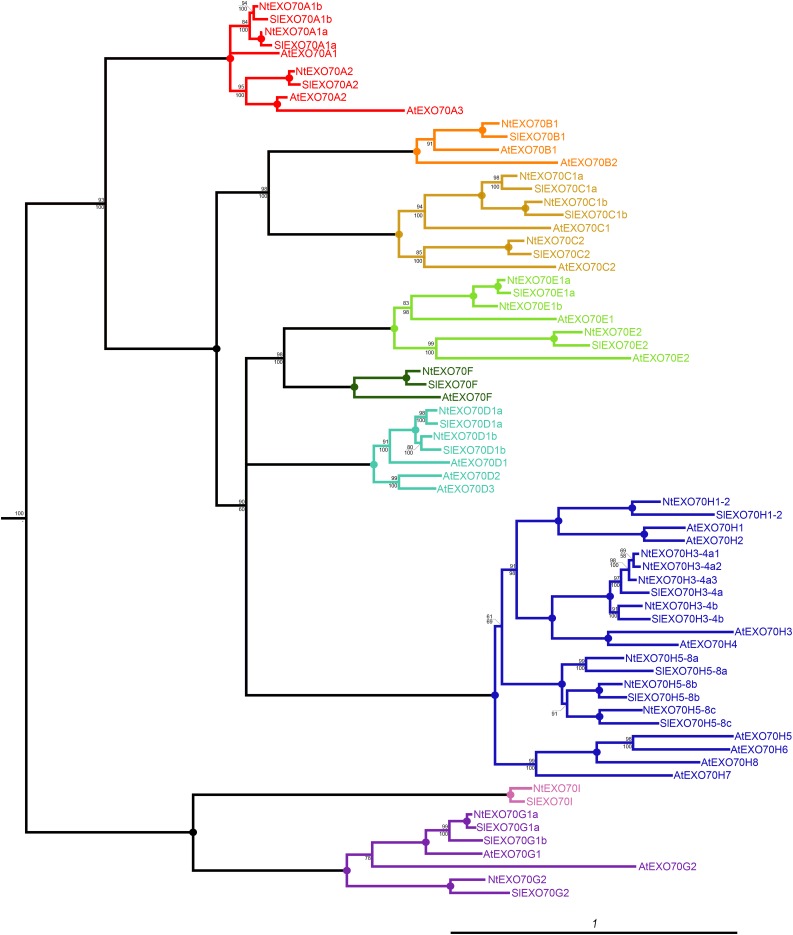
Plant EXO70s constitute an expanded gene family divided into nine clades that are present in most angiosperm groups (Cvrčková et al., 2012). We initiated a detailed study of tobacco EXO70 proteins by bioinformatic analysis that confirmed the presence of all major EXO70 clades in the Solanaceae, including tobacco (Fig. 1). It should be noted that tobacco is amphidiploid with two nearly identical copies of most genes in its genome (one from each parental species). For the sake of clarity, we further present data from only the one diploid gene set. An exhaustive BLAST analysis of the tobacco genome draft, together with searches in the genomes of the tobacco parental species Nicotiana sylvestris and Nicotiana tomentosiformis and the closely related tomato (Solanum lycopersicum), uncovered 24 EXO70 genes in a diploid tobacco gene set. In congruence with previous observations from other plant genomes (Synek et al., 2006; Cvrčková et al., 2012), most EXO70 genes outside clades A and I are intronless. While tobacco EXO70 genes can be easily sorted into the same clades as Arabidopsis EXO70 genes, one-to-one orthology relations cannot be established in most cases (Fig. 1). During the evolution from the last common ancestor of the Solanaceae and Brassicaceae, some genes have undergone duplications only in Solanaceae (e.g. ancestral EXO70C1 into NtEXO70C1a and NtEXO70C1b) while others have duplicated only in the lineage leading to Brassicaceae (e.g. ancestral EXO70A2 into AtEXO70A2 and AtEXO70A3). However, in the case of some isoforms (AtEXO70C2/NtEXO70C2, AtEXO70E2/NtEXO70E2, and AtEXO70F/NtEXO70F), clear orthology relationships can be inferred.
Figure 1.
Phylogenetic relationship of EXO70 proteins from Arabidopsis (At), tobacco (Nt), and tomato (Sl). The tree represents the protein maximum likelihood phylogeny, where numbers at nodes correspond to the approximate likelihood ratio test with SH (Shimodaira-Hasegawa)-like support from maximum likelihood (top) and posterior probabilities from Bayesian analysis (bottom). Circles represent 100% support by both methods, and branches were collapsed if the inferred topology was not supported by both methods. The tree was rooted using human EXO70 as an outgroup. The scale bar indicates the rate of substitutions per site.
Multiple EXO70 Isoforms Are Expressed in Germinating Pollen
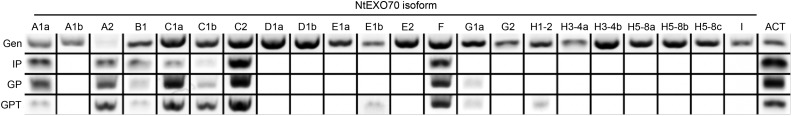
After compiling the repertoire of tobacco EXO70 genes, we investigated EXO70 isoforms that are expressed in tobacco pollen. We analyzed the transcription of tobacco EXO70 genes by reverse transcription (RT)-PCR amplification of EXO70 fragments from cDNA mixtures prepared from imbibed pollen, germinating pollen, and growing pollen tubes. Due to the high similarity of the gene-coding regions in the T and S genomes of amphidiploid tobacco, primer pairs were designed so that they would discriminate between particular isoforms indicated in Figure 1 but would not discriminate between copies from parental species. Furthermore, three members of the NtEXO70H3-4a subclade (Fig. 1) were not discriminated either, because of their extreme similarity at the nucleic acid level. RT-PCR analysis has shown that 10 EXO70s are transcribed in at least one stage of pollen germination or pollen tube growth (Fig. 2). Since the correlation between the transcription level and the protein abundance is often weak, we further investigated EXO70 expression in tobacco pollen at the protein level. Total cytoplasmic protein extract was separated by SDS protein electrophoresis, and proteins of around 70 kD were analyzed by mass spectrometry. Although this approach was not sensitive enough to detect all pollen isoforms, it uncovered the most abundant EXO70s. Table I summarizes the results of four independent mass spectrometry reads from two different biological replicates and shows that EXO70 clades A and C represent dominant subfamilies in growing pollen tubes.
Figure 2.
Several EXO70 isoforms are expressed in tobacco pollen and growing pollen tubes. Semiquantitative RT-PCR analysis is shown for the EXO70 family in tobacco imbibed pollen (IP), germinating pollen (GP), and growing pollen tube (GPT), with genomic DNA (Gen) used for the control amplification. Actin (ACT) was amplified as a control from the same premix solution. The image shown was assembled from two independent experiments for each tissue. Combinations that did not result in the presence of an active band are represented by blank spaces.
Table I. Incidence of EXO70 isoform detection in the proteomic expression analysis.
| Isoform | Total No. of Peptides |
|---|---|
| NtEXO70A2 | 14 |
| NtEXO70C1a | 25 |
| NtEXO70C1b | 5 |
| NtEXO70C2 | 7 |
Several EXO70 Isoforms Localize to Distinct Compartments in Growing Tobacco Pollen Tubes
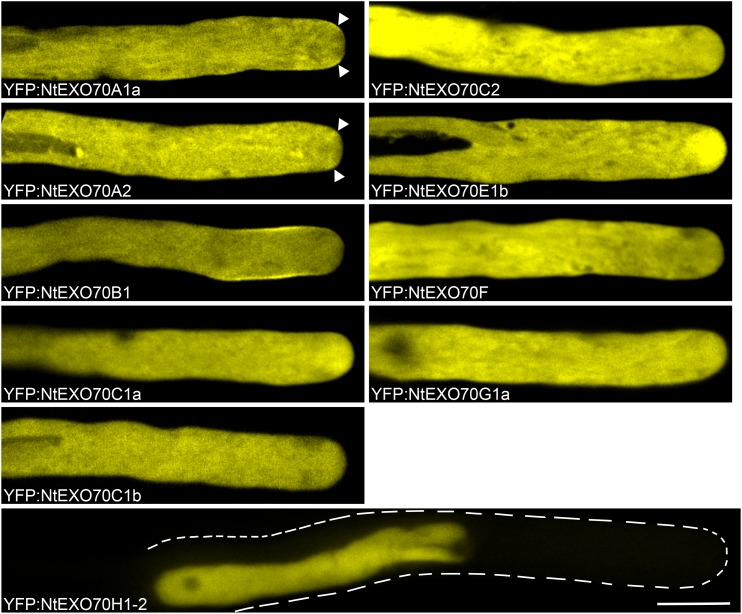
In order to get a first insight into the putative functions of pollen EXO70s, we fused 20 different tobacco EXO70 isoforms to the C terminus of yellow fluorescent protein (YFP) under the control of the LAT52 promoter (nucleotide sequences of our clones are provided in Supplemental File S1) and performed detailed subcellular localization analyses by spinning disk confocal microscopy. Since the transient expression of constructs results in variable levels of expression among different pollen tubes, we focused on pollen tubes that exhibited low levels of expression of the transgenic constructs (as assessed by fluorescence intensity readout) and normal growth rate (∼3–5 μm min−1). Our observations are summarized in Figure 3 for pollen-expressed isoforms and in Supplemental Figure S1 for isoforms not natively expressed in pollen. In systems where binding properties of EXO70 to membrane were well characterized, EXO70 was demonstrated to be a peripheral membrane protein (He et al., 2007). Thus, it is not surprising that, besides distinct subcellular patterns of different isoform localization, most tagged EXO70 isoforms also exhibit strong cytoplasmic fluorescence. These observations provided several noteworthy conclusions.
Figure 3.
Localization of natively pollen-expressed EXO70 isoforms in growing tobacco pollen tubes. Selected YFP-tagged tobacco EXO70 isoforms were transiently expressed in tobacco pollen tubes, and their subcellular localization was examined by spinning disk confocal microscopy. Growing pollen tubes with low expression levels of the transgene are shown. The images shown are representative for 20 or more transformed pollen tubes observed in at least two independent experiments for NtEXO70F, NtEXO70G1a, and NtEXO70H1-2 and for 30 or more transformed pollen tubes in three or more independent experiments for the rest of the isoforms. Arrowheads mark small areas of membrane localization for NtEXO70A1a and concentration of signal in the corresponding area for NtEXO70A2. In the case of NtEXO70H1-2, the contour of the pollen tube is marked because of negligent cytoplasmic signal due to nuclear accumulation of the signal. Bar = 10 µm.
(1) NtEXO70A1a localized to the small but very distinct PM region adjacent to the pollen tube tip (Fig. 3; Supplemental Movie S1). In contrast, NtEXO70A2 was concentrated in an area near the pollen tube tip but did not localize to the PM in most pollen tubes observed. Only occasionally did NtEXO70A2 display PM localization comparable to NtEXO70A1a (Fig. 3). Surprisingly, NtEXO70A1b, the closest homolog of NtEXO70A1a (sharing 90% identical residues with NtEXO70A1a; Supplemental Fig. S2), was localized in growing pollen tubes evenly throughout the cytoplasm and only rarely in the manner observed for NtEXO70A2. To our knowledge, PM localization of NtEXO70A1b in growing pollen tubes has never been observed. (2) We observed a very distinct localization pattern for YFP:NtEXO70B1, which localizes to a broader subapical PM region but is excluded from the area near the pollen tube tip (Fig. 3; Supplemental Movie S2). (3) Rather surprisingly, all members of the pollen-enriched C class localized exclusively in the cytoplasm. (4) We further discovered that NtEXO70E1b localizes to the inverted-cone region at the pollen tube tip, the zone enriched with secretory and recycling vesicles (Derksen et al., 1995). (5) NtEXO70E2 localized to mobile spots in the cytoplasm (Supplemental Fig. S1; Supplemental Movie S3). Because NtEXO70E2 is not expressed in pollen and aggregation to puncta is a common overexpression artifact, the localization pattern of NtEXO70E2 in the growing pollen tube is probably an artifact. (6) While tagged NtEXO70G1a localizes only to the cytoplasm upon very low levels of expression, in pollen tubes with slightly increased expression and slightly decreased growth rate, NtEXO70G1a decorates the subapical membrane and fibrillar structures, likely actin fibers (Supplemental Fig. S3; Supplemental Movie S4). (7) Unexpectedly, fluorescently tagged NtEXO70H1-2 and NtEXO70H5-8b localized predominantly to the nucleus. Since other tagged EXO70 isoforms do not exhibit nuclear fluorescence despite frequent strong cytoplasmic signals and the nuclear signal is stronger than the cytoplasmic signal, we can conclude that targeting to the nucleus is an active, isoform-specific process and not the result of a passive entry or degradation of the YFP-fused protein. In the case of NtEXO70H1-2, the tendency of nuclear import is so strong that almost no cytoplasmic signal can be detected.
Because the transient expression of fluorescently tagged proteins in growing tobacco pollen tubes does not allow a direct way to prove the functionality of the tagged proteins by rescue of the mutant phenotype (as is possible in the case for Arabidopsis mutants stably transformed with tagged proteins of interest), we decided to further verify that the subcellular localization of the N-terminally tagged NtEXO70 isoforms reflects the behavior of the native proteins by examining the subcellular localization of C-terminally YFP-tagged NtEXO70B1. NtEXO70B1:YFP displayed a nearly identical subcellular distribution to YFP:EXO70B1 (Supplemental Fig. S4A).
In summary, we observed a distinct localization pattern for several tobacco EXO70 isoforms. Interestingly, membrane binding was observed only for natively expressed pollen isoforms, and clear differences could be seen even between closely related EXO70 paralogs (e.g. NtEXO70A1a versus NtEXO70A1b and NtEXO70E1a versus NtEXO70E1b; Fig; 3; Supplemental Fig. S1). Most other nonpollen EXO70 isoforms also showed cytoplasm-only localization in pollen tubes (Supplemental Fig. S1). This strongly suggests that the nonoverlapping localizations observed for pollen NtEXO70 isoforms A1a, B1, and E1b reflect their functional significance.
NtEXO70A1a and NtEXO70B1 Occupy Mutually Exclusive Parts of the PM in Growing Tobacco Pollen Tubes, and Both Overlap with Exocyst Subunit NtSEC3a PM Localization
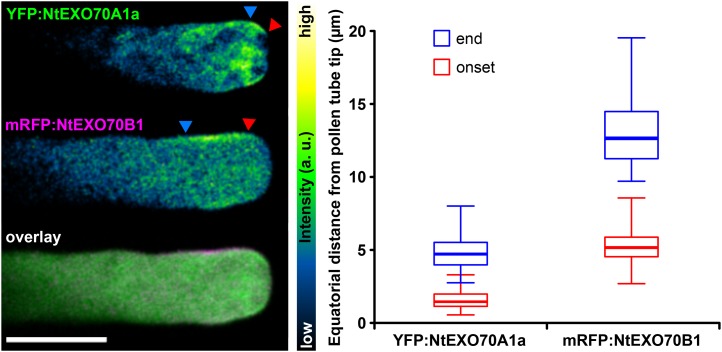
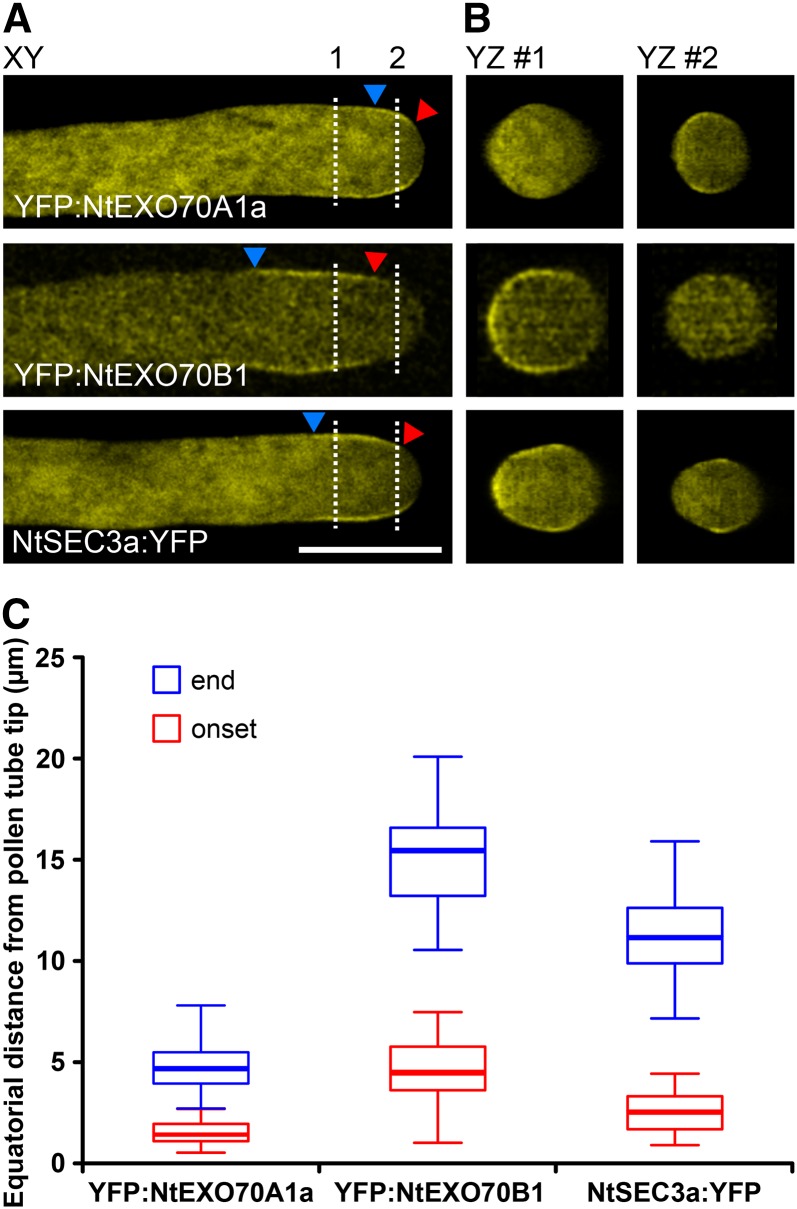
The localization of tagged NtEXO70A1a and NtEXO70B1 (Fig. 3) strongly suggested that the proteins inhabit mutually exclusive PM domains in the growing tobacco pollen tube. To analyze this in more detail, we transiently cotransformed tobacco pollen with YFP-tagged NtEXO70A1a and monomeric red fluorescent protein (mRFP)-tagged NtEXO70B1. In growing pollen tubes with low levels of transgene expression, besides their cytoplasmic signal, the tagged EXO70 proteins indeed localized to distinct membrane zones (Fig. 4). We repeatedly observed a similar pattern of localization and quantified the distance of onset and end of EXO70 localization from the pollen tube apex (Fig. 4). Please note that, although onset and end of localization were variable among the pollen tube population, NtEXO70A1a and NtEXO70B1 did not overlap in the vast majority of pollen tubes. End of NtEXO70A1a and onset of NtEXO70B1 localization were separated by less than 1 μm (n = 49). Recently published work (Bloch et al., 2016) characterized the localization and mechanism of membrane binding of the exocyst subunit SEC3 to a broader region near the tobacco pollen tube tip. In order to elucidate the relation of NtEXO70A1a and NtEXO70B1 to other components of the exocyst complex, we compared the distribution of the membrane signals of YFP:NtEXO70A1a and YFP:NtEXO70B1 with the exocyst subunit NtSEC3a:YFP. Interestingly, typical NtSEC3a:YFP localization partially overlaps with both YFP:NtEXO70A1a and YFP:NtEXO70B1 localization (Fig. 5A). We also investigated the distribution of the membrane signal of YFP:NtEXO70A1a, YFP:NtEXO70B1, and NtSEC3a:YFP along the annular surface of growing tobacco pollen tubes using optical sectioning. As documented by orthogonal views, these experiments also showed the partial overlap of NtSEC3a with both NtEXO70A1a and NtEXO70B1 and indicated that, in pollen tubes, the fluorescence signal of exocyst subunits at the PM seems to be mostly continuous (Fig. 5B). The observed relation between NtEXO70A1a, NtEXO70B1, and NtSEC3a also was corroborated by measuring equatorial distances of onset and end of YFP:NtEXO70A1a, YFP:NtEXO70B1, and NtSEC3a:YFP membrane localization from the pollen tube apex (Fig. 5C). This was further supported by a colocalization analysis of tobacco pollen tubes expressing mRFP:NtEXO70B1 and NtSEC3a:YFP (Supplemental Fig. S4B). The membrane signal of mRFP:NtEXO70B1 and NtSEC3a:YFP largely overlapped, but the signal of NtSEC3a:YFP clearly reached farther to the tip, probably to the zone of NtEXO70A1a membrane localization.
Figure 4.
Mutually exclusive localization of NtEXO70A1a and NtEXO70B1 in growing tobacco pollen tubes. YFP:NtEXO70A1a and mRFP:NtEXO70B1 were transiently coexpressed in tobacco pollen tubes, and their subcellular localization was examined by spinning disk confocal microscopy with representative results shown (left). Images of individual channels are represented using a color intensity code in order to display local enrichment of the YFP/mRFP signal. In the overlay, YFP is represented by green, mRFP by magenta, and white indicates the overlapping signal. Red and blue arrowheads mark the onset and end of the particular membrane signal, measured in middle optical sections as the equatorial distance from the pollen tube apex (right; n = 25). a.u., Arbitrary unit. Bar = 10 µm.
Figure 5.
Membrane NtSEC3a localization overlaps with both NtEXO70A1a and NtEXO70B1 localization. YFP:NtEXO70A1a, YFP:NtEXO70B1, and NtSEC3a:YFP were expressed individually in tobacco pollen tubes, and their subcellular localization was examined by optical sectioning using spinning disk confocal microscopy. The main image shows a single optical section at the x-y plane (A), and dashed lines indicate where the stack was sectioned to show the y-z planes (B). Red and blue arrowheads mark the onset and end of the particular membrane signal, measured as the equatorial distance from the pollen tube apex (C; n ≥ 15). Bar = 10 µm.
The Zone of Active Clathrin-Mediated Endocytosis Maximum Is Distinct from the NtEXO70A1a PM Localization and Largely Overlaps with NtEXO70B1 Membrane Localization
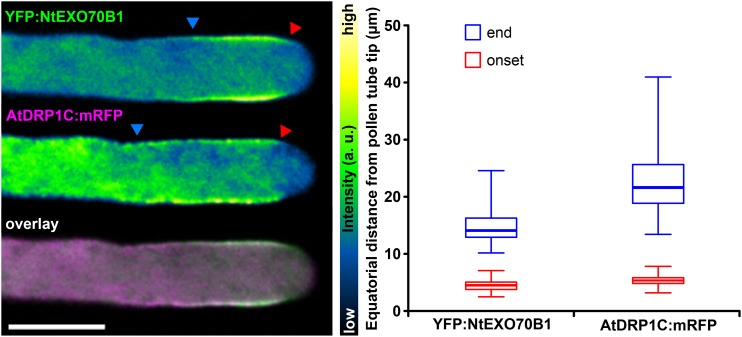
The unexpected localization of NtEXO70B1 in the pollen tube subapex far from textbook sites of secretion prompted us to further analyze its distribution. We noted that the localization of NtEXO70B1 resembled the previously described maximum of clathrin-mediated endocytosis (Derksen et al., 1995; Moscatelli et al., 2007). To confirm this, we used an mRFP-tagged dynamin-related protein from Arabidopsis (AtDRP1C; Konopka and Bednarek, 2008) as a marker of clathrin-mediated endocytosis and visualized it together with YFP-tagged NtEXO70B1. Similar patterns of localization were observed repeatedly, and distances of membrane localization onset and end from the apex were quantified (Fig. 6). In healthy growing pollen tubes with low levels of transgene expression, areas of NtEXO70B1 and AtDRP1C membrane localization largely overlapped. The onset of membrane localization was almost identical for both proteins, and the membrane localization of AtDRP1C was more extended. On the other hand, colocalization of YFP:NtEXO70A1a and AtDRP1C:mRFP demonstrated that the observed membrane localization of the two constructs is mutually exclusive (Supplemental Fig. S5).
Figure 6.
Overlap of NtEXO70B1 localization and the zone of clathrin-mediated endocytosis maximum marked by AtDRP1C. YFP:NtEXO70B1 and AtDRP1C:mRFP were transiently coexpressed in tobacco pollen tubes, and their subcellular localization was examined by spinning disk confocal microscopy with representative results shown (left). Images of individual channels are represented using a color intensity code in order to display local enrichment of the YFP/mRFP signal. In the overlay, YFP is represented by green, mRFP by magenta, and white indicates the overlapping signal. Red and blue arrowheads mark the onset and end of the particular membrane signal, measured as the equatorial distance from the pollen tube apex (right; n = 50). a.u., Arbitrary unit. Bar = 10 µm.
NtEXO70A1a and NtEXO70B1 Exhibit Different Degrees of Overlap with Genetically Encoded Markers for Anionic Signaling Phospholipids
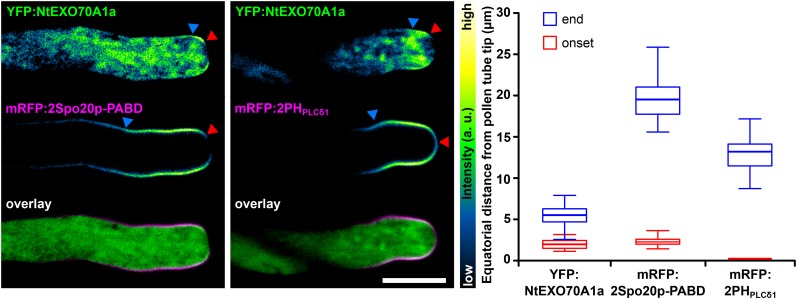
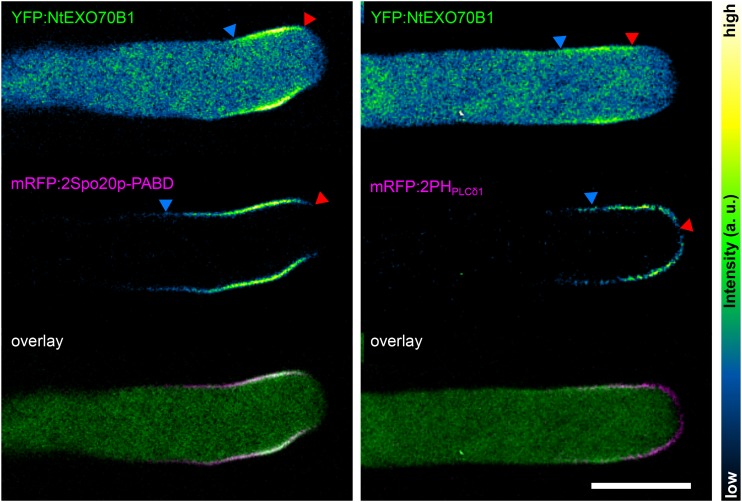
Budding yeast (He et al., 2007) and animal (Liu et al., 2007) EXO70 were demonstrated to directly bind PIP2 at the PM. Recently, the affinity of the SEC3 exocyst subunit toward PIP2 and its biological relevance, previously known from opisthokonts (Zhang et al., 2008), were confirmed in plants (Bloch et al., 2016). Amino acid residues identified as responsible for opisthokont EXO70 lipid binding are conserved to different degrees among plant EXO70 isoforms (Žárský et al., 2009). Thus, it is probable that the local lipid composition of the PM is responsible for the differential binding of different EXO70s. In the light of this hypothesis, we transiently cotransformed tobacco pollen with YFP-tagged EXO70s and mRFP-tagged genetically encoded lipid markers for PA and PIP2. In growing pollen tubes with low levels of transgene expression, NtEXO70A1a (Fig. 7) and NtEXO70B1 (Fig. 8) displayed different degrees of overlap with PA (mRFP:2Spo20p-PABD) and PIP2 (mRFP:2PHPLCδ1) markers. Membrane YFP:NtEXO70A1a was fully included within the range of mRFP:2PHPLCδ1 signal in every pollen tube observed. Interestingly, the very tip covered by mRFP:2PHPLCδ1 but devoid of mRFP:2Spo20p-PABD was devoid of YFP:NtEXO70A1a as well (Fig. 7). This was confirmed by the quantification of YFP:NtEXO70A1a and mRFP:2Spo20p-PABD membrane signal onset distances from the pollen tube tip, which appeared to be the same (Fig. 7). Note that, although the onset and end of localization were variable among pollen tube population, the YFP:NtEXO70A1a signal never reached closer to the very tip than mRFP:2Spo20p-PABD in individual pollen tubes. Although with current tools we cannot equivocally say whether the YFP:EXO70A1a signal membrane localization matches the maximum of mRFP:2PHPLCδ1 signal, it is clear that the mRFP:2PHPLCδ1 signal always completely covers the area of PM YFP:EXO70A1a localization. The YFP:NtEXO70B1 membrane signal matched the area of mRFP:2Spo20p-PABD signal maximum and also largely overlapped with mRFP:2PHPLCδ1 (Fig. 8).
Figure 7.
Colocalization of NtEXO70A1a with lipid markers in growing tobacco pollen tubes. NtEXO70A1a was transiently coexpressed together with the PA marker mRFP:2Spo20p-PABD (left) and the PIP2 marker mRFP:2PHPLCδ1 (middle), and the subcellular localization of the constructs was examined by spinning disk confocal microscopy. Images of individual channels are represented using a color intensity code in order to display local enrichment of the YFP/mRFP signal. In the overlays, YFP is represented by green, mRFP by magenta, and white indicates the overlapping signal. Red and blue arrowheads mark the onset and end of the particular membrane signal, measured as the equatorial distance from the pollen tube apex (right; n ≥ 13). The onset value for mRFP:2PHPLCδ1 was set to zero because it always covers the very tip of the pollen tube PM. Bar = 10 µm.
Figure 8.
Colocalization of NtEXO70B1 with lipid markers in growing tobacco pollen tubes. NtEXO70B1 was transiently coexpressed together with the PA marker mRFP:2Spo20p-PABD (left) and the PIP2 marker mRFP:2PHPLCδ1 (right), and the subcellular localization of the constructs was examined by spinning disk confocal microscopy with representative results shown. Images of individual channels are represented using a color intensity code in order to display local enrichment of the YFP/mRFP signal. In the overlays, YFP is represented by green, mRFP by magenta, and white indicates the overlapping signal. Red and blue arrowheads mark the onset and end of the particular membrane signal. a.u., Arbitrary unit. Bar = 10 µm.
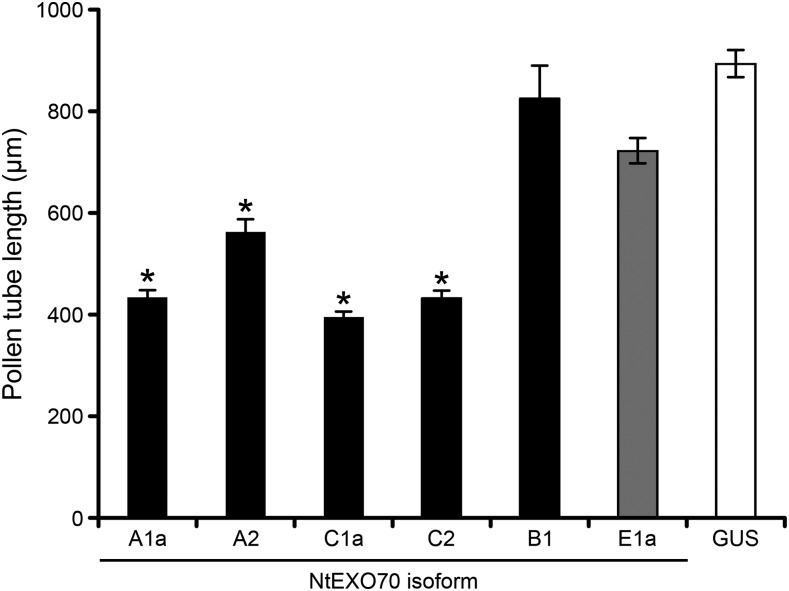
Major Pollen EXO70 Isoforms Inhibit Pollen Tube Growth upon Overexpression
Overexpression of the protein of interest in the tobacco pollen tube is often used to infer a protein’s function (Ischebeck et al., 2008). To test the physiological role of most prevalent tobacco pollen EXO70 isoforms, we analyzed the effect of their overexpression on pollen tube growth. From the A clade of EXO70 proteins, the most expressed isoform (NtEXO70A2) and the isoform with the most distinct subcellular localization (NtEXO70A1a) were selected for functional characterization. From the C clade, we chose the two most expressed isoforms at the protein level: NtEXO70C1a and NtEXO70C2. When overexpressed, all four of these isoforms significantly impaired pollen tube growth. The transformed tubes grew to about half of the length of the control pollen tubes overexpressing YFP:GUS (Fig. 9). To rule out a possible nonspecific effect caused by the overexpression of membrane trafficking-related protein, we also analyzed the overexpression effect of YFP:NtEXO70E1a, a selected isoform that is neither expressed in pollen nor exhibits specific subcellular localization (Fig. 2; Supplemental Fig. S1). Pollen tubes overexpressing YFP:NtEXO70E1a elongated almost identically to the tubes overexpressing YFP:GUS, which strongly indicates that the ability of a particular EXO70 isoform to inhibit pollen growth upon overexpression correlates with its physiological role in pollen growth. We did not observe any effect of NtEXO70B1 overexpression (Fig. 9), probably because YFP-tagged NtEXO70B1 (regardless of the YFP position) loses its distinct membrane localization and forms artificial aggregates in cytoplasm once the level of expression reaches a certain threshold (Supplemental Fig. S4A). Thus, in the case of NtEXO70B1, overexpression cannot be used to assess the physiological functionality of the protein.
Figure 9.
Overexpression of the major pollen EXO70 class members inhibits pollen tube growth. Tobacco pollen tubes transiently expressing high levels (5 µg of DNA) of selected tagged EXO70 isoforms and control (YFP-tagged GUS) were imaged 8 h after biolistic transformation by epifluorescence microscopy, and pollen tube lengths were determined. At least 100 cells from at least two independent transformations were measured for each construct. Asterisks indicate significant differences (P < 0.001) from the corresponding controls (GUS and NtEXO70E1a) according to ANOVA followed by posthoc multiple mean comparison test with Tukey contrasts using the multcomp R package. Data represent means of 100 or more pollen tubes ± se.
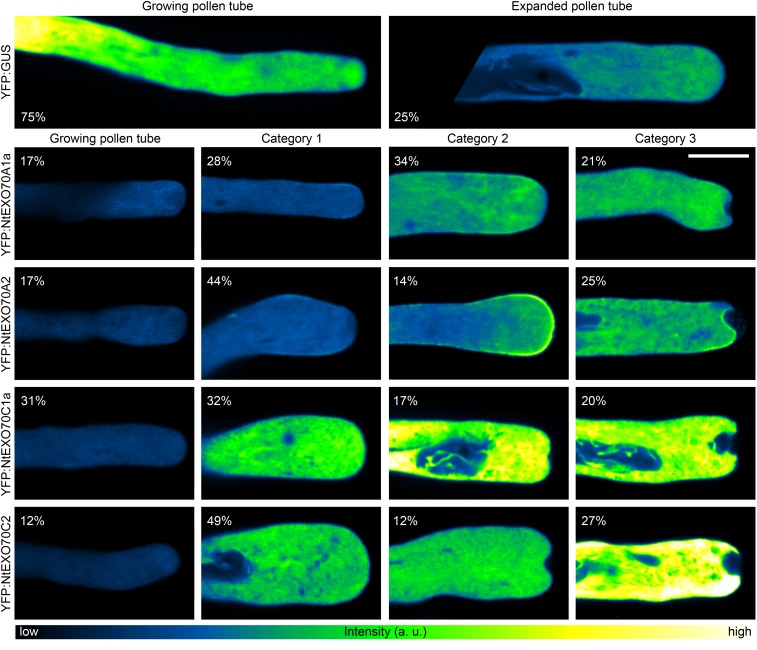
Overexpression of Major Pollen EXO70 Isoforms in Growing Pollen Tubes Results into Distinct Morphological Phenotypes
After demonstrating the functional significance of selected pollen isoforms in pollen tube growth, we further aimed to infer more about the role of these proteins from phenotypic changes in pollen tubes overexpressing particular constructs. Such an approach was demonstrated repeatedly as a successful method to get insight into the functions of proteins of interest in pollen tube polar growth (Ischebeck et al., 2008, 2011; Stenzel et al., 2012). Thus, we carefully investigated pollen tube morphology upon overexpression of selected YFP-tagged EXO70 isoforms that strongly inhibited pollen tube growth, namely NtEXO70A1a, NtEXO70A2, NtEXO70C1a, and NtEXO70C2. Despite the high inherent variability in the population of transformed pollen tubes, several distinct phenotypic categories induced by the overexpression of YFP:EXO70s could be assigned by careful examination of a large data set of transformed pollen tubes (Fig. 10). We compared these effects with mild alterations inevitably caused by high overexpression of a control construct encoding YFP:GUS. The overview of different phenotypic category occurrence is summarized in Figure 10. Generally, mild overexpression of pollen EXO70s (as assessed by fluorescence level; Supplemental Fig. S6) induced the expansion of the pollen tube apical region. Even though the extended tip phenotype also was observed occasionally in the control set overexpressing YFP:GUS, the incidence of the phenotype and the severity of tip expansion were significantly lower than in the population of EXO70-overexpressing pollen tubes. Strong overexpression of EXO70 isoforms from both the A and C classes resulted in the formation of apical invaginations (Fig. 10). Note that the observed phenotypic categories of pollen tubes overexpressing particular isoforms do not necessarily represent a succession order (e.g. pollen tubes overexpressing NtEXO70A2 with the morphology classified as category 2 would not necessarily change morphology to category 3 given enough time to develop).
Figure 10.
Effects of major pollen EXO70 isoform overexpression on the phenotypes of tobacco pollen tubes. Tobacco pollen tubes transiently expressing high levels of selected tagged EXO70 isoforms were imaged 8 h after biolistic transformation by spinning disk confocal microscopy. For each EXO70 isoform, pollen tubes were classified into four phenotypic categories (with the percentage of occurrence shown for each image). Control pollen tubes expressing YFP:GUS could be classified only into two categories. Images are displayed using a color intensity code with the same upper limit set for all images (see “Materials and Methods”), and the representative images thus reflect differences in signal intensity between the individual pollen tubes. The data are based on 33 or more transformed pollen tubes observed for each construct in three independent experiments. a.u., Arbitrary unit. Bar = 10 µm.
During a systematic localization study of EXO70 isoforms in growing pollen tubes with low transgene expression, cells with high levels of YFP-tagged EXO70 isoforms (as assessed by level of fluorescence; Supplemental Fig. S6) also were often observed, because of the variable nature of transgene expression in the transiently transformed pollen population. A similar trend to that reported for the overexpression of NtEXO70C1a and NtEXO70C2 (Fig. 10) also was observed in pollen tubes overexpressing NtEXO70C1b. Also, YFP:NtEXO70A1b was seen occasionally to be recruited to the tip PM upon overexpression but did so far less frequently than YFP:NtEXO70A1a or YFP:NtEXO70A2. However, the apical invagination phenotype was never observed outside A- and C-class EXO70 proteins, and strong membrane recruitment upon EXO70 overexpression was never observed outside the A class. Furthermore, the morphology of cells overexpressing nonpollen YFP:EXO70 isoforms was comparable to that of cells overexpressing YFP:GUS. It is noteworthy that, while the overexpression of A-class EXO70 isoforms resulted in increased membrane recruitment of the tagged proteins to the pollen tube membrane tip and induced invaginations, C-class EXO70 isoforms never localized to the PM, even upon strong overexpression (Fig. 10). This indicated completely different molecular mechanisms of A- and C-class EXO70 action in pollen tube tip growth. In order to further test this hypothesis, we attempted to recruit NtEXO70C2 to the PM by increasing the levels of a minor acidic phospholipid in situ. We chose PIP2 as a candidate phospholipid because it was demonstrated previously to recruit EXO70 to the PM in budding yeast and mammalian cells (He et al., 2007; Liu et al., 2007) and the enzymes catalyzing the conversion of phosphatidylinositol 4-phosphate into PIP2 are well characterized in plant cells, including tobacco pollen tubes (Ischebeck et al., 2008, 2013). As the positive control, we used NtSEC3a as a bona fide peripheral membrane protein binding PIP2. Plant SEC3 was demonstrated recently to directly bind PIP2 in plant cells, including growing tobacco pollen tubes, with the molecular mechanism of PIP2 well characterized and supported by both in vitro and in vivo experiments (Bloch et al., 2016). We transformed the pollen tubes with a high amount of the cyan fluorescent protein-tagged phosphatidylinositol 4-phosphate 5-kinase 5 (PIP5K5:CFP, Ischebeck et al., 2008) in order to rapidly increase the PIP2 level in situ and low amount of NtSEC3a or NtEXO70C2 so that exocyst subunits would not perturb cellular morphology by themselves and only serve as a readout of their membrane-binding properties. The morphological aberrations of the pollen tubes observed (Supplemental Fig. S7) are thus the sole result of PIP5K5 overexpression, as reported previously (Ischebeck et al., 2008). In the case of NtSEC3a, we repeatedly observed strong recruitment of the protein to the PM upon increased levels of PIP2 with increased PM-cytoplasm signal ratio (Supplemental Fig. S7). On the other hand, NtEXO70C2 was never recruited to the PM after increasing PIP2 level, which confirms the notion that C-class EXO70 isoforms do not bind the PM.
DISCUSSION
Previous studies clearly demonstrated the involvement of exocyst in plant cell polarity regulation, including polar tip growth (Cole et al., 2005; Synek et al., 2006; Hála et al., 2008). The significance of EXO70 family diversity in angiosperms (Eliáš et al., 2003; Cvrčková et al., 2012) has become one of the key topics in understanding plant cell polarity regulation (Žárský et al., 2009). However, most studies carried out to date were limited to Arabidopsis and none involved expression, localization, and functional study of EXO70 family diversity within a single cell. Here, we attempted to overcome these shortcomings by systematic investigation of tobacco EXO70 isoforms in tobacco pollen tubes, a cell type particularly favorable for analyses of genes regulating cell morphogenesis and membrane traffic.
First, we performed a phylogenetic analysis of the EXO70 family in Solanaceae and studied it in the context of previous findings (Eliáš et al., 2003; Cvrčková et al., 2012). All nine angiosperm EXO70 clades are present in Solanaceae genomes, with 22 members in the diploid tomato genome and 24 members in the diploid tobacco genome. As summarized in Figure 1, in some cases, clear orthologs between Arabidopsis and Solanaceae isoforms can be detected. In other instances, the EXO70 genes have undergone a few independent duplications in lineages leading to Brassicaceae and Solanaceae. Most striking examples of independent duplications were detected for the H clade. Noteworthy, the rate of substitution detected between EXO70 genes from tobacco and its parental species (N. sylvestris and N. tomentosiformis) was very low for A-clade EXO70s and highest for H-clade EXO70s (data not shown). This underpins the relatively high evolutionary rate of the H-clade EXO70s and offers insight for a future study of EXO70 family evolution in plants.
Furthermore, we analyzed the expression of EXO70 family members in tobacco pollen at the transcript level in three different stages of pollen germination and by a proteomic approach. In total, transcripts of 10 genes from seven clades were detected (Fig. 2). Proteomic analysis identified the presence of NtEXO70A2 and all three members of the EXO70 C clade. Compared with known EXO70 family expression analyses of Arabidopsis pollen, the following picture can be drawn. At the transcript level, AtEXO70A2, AtEXO70C1, AtEXO70C2, AtEXO70F, AtEXO70G2, AtEXO70H3, and AtEXO70H5 can be detected in Arabidopsis pollen (Winter et al., 2007; Loraine et al., 2013; L. Synek, unpublished data), with C-clade EXO70s having the highest level of expression. Pollen expression of AtEXO70A2, AtEXO70C1, and AtEXO70C2 was further confirmed at the protein level (Grobei et al., 2009), with the prevalence of C-clade isoforms in the proteome, followed by AtEXO70A2. Together with our data obtained in tobacco, these results suggest that A- and C-clade EXO70s are the major angiosperm pollen isoforms. The importance of NtEXO70B1 (see below) for pollen growth in angiosperms outside the Solanaceae remains to be confirmed. Both tobacco and Arabidopsis express H-class EXO70s in pollen, although the particular isoforms differ, probably due to the rapid evolution of H-class EXO70s. Lower expression of certain isoforms suggests that they play marginal or regulatory roles.
We then investigated the subcellular localization of 20 EXO70 isoforms in growing tobacco pollen tubes by spinning disk confocal microscopy. NtEXO70A1a and NtEXO70B1 localized to distinct PM domains, as discussed below.
NtEXO70E1b localized to the inverted cone region, the structure where secretory/recycling vesicles accumulate (Hepler and Winship, 2015). NtEXO70E1b might play a regulatory role in earlier steps of the secretory pathway. However, biological interpretations of NtEXO70E1b localization to the inverted cone should be considered with caution due to the low native expression of NtEXO70E1b in tobacco pollen.
Interestingly, NtEXO70G1a decorated fibrillar structures resembling actin and the subapical membrane. This is similar to the subcellular localization of NET2A, a member of a newly discovered actin-interactor family, in Arabidopsis pollen (Deeks et al., 2012). NET superfamily proteins mediate the interaction of actin and various membranes in plant cells. In opisthokonts, exocyst interacts directly with both actin and the microtubule cytoskeleton (Synek et al., 2014), animal EXO70 was shown previously to interact directly with the Arp2/3 complex and regulate actin polymerization (Zuo et al., 2006; Liu et al., 2012), and EXO70 directly binds membrane in budding yeast and mammalian cells (He et al., 2007; Liu et al., 2007). Thus, NtEXO70G1a (or G-class EXO70s generally) might regulate part of the actin cytoskeleton and its interaction with the PM. The functions of G-clade EXO70s remain largely unexplored, and future studies are required to confirm our conclusions regarding NtEXO70G1a.
We further observed the accumulation of NtEXO70E2 in distinct bodies with little cytoplasmic localization. It is probable that NtEXO70E2 has the property of forming artificial structures when overexpressed. The expression of AtEXO70E2 is largely restricted to a few tissues like stomata (eFP browser), and the function of AtEXO70E2 is still not known. Since NtEXO70E2 is not natively expressed in pollen, the localization of tagged NtEXO70E2 is probably an artifact, and its native biological function remains to be established.
Surprisingly, two members of the rapidly evolving H class, NtEXO70H1-2 and NtEXO70H5-8b, exhibit strong enrichment in the nucleus with respect to the cytoplasm (NtEXO70H1-2 signal is almost absent from the cytoplasm). Since NtEXO70H1-2 is present in tobacco pollen, we believe that the nuclear localization of the tagged NtEXO70H1-2 reflects the native behavior of the protein. Furthermore, interactors of closely related Arabidopsis AtEXO70H1 identified by high-throughput screen include transcription factors (Žárský et al., 2013), and animal EXO70 was shown previously to shuttle into the nucleus and interact with spliceosomal components (Dellago et al., 2011). It is not clear whether ancestral eukaryotic EXO70 played a role in the nucleus among other processes or whether such a function was independently secondarily acquired for animal EXO70 and selected plant EXO70s. Our findings epitomize the previous notion that plant EXO70 proteins should be investigated not only in the light of the exocyst complex but also with the possibility of autonomous exocyst-independent function (Žárský et al., 2013).
One of the key aims of this study was to use the polar tip-growing pollen tube as a universal model system to categorize the subcellular localization of the whole spectrum of the EXO70 family. Surprisingly, tagged versions of almost all isoforms not natively expressed in pollen localized only to the cytoplasm. Even in the case of closely related isoforms like NtEXO70E1a and NtEXO70E1b, the pollen-expressed isoform displayed distinct localization to the inverted cone while the isoform not expressed in pollen did not. Moreover, we observed very different localization for three closely related isoforms of the A clade. Even upon overexpression, the nonpollen NtEXO70A1b localized to the PM only rarely, while pollen isoforms from the A clade displayed a significant tendency to bind PM near the pollen tube tip (Figs. 3 and 10). Thus, coincident interactions with cell-specific protein partners are probably indispensable for the proper targeting of EXO70 isoforms. Small GTPases are important for budding yeast and animal EXO70 targeting (Wu et al., 2008; Pleskot et al., 2015). Plant EXO70s have been shown to interact with ROH1 (Kulich et al., 2010), and NOI family proteins might play a role in EXO70 membrane targeting (Afzal et al., 2013; Sabol et al, 2017). Given our observations, it is paramount to study EXO70s in cell types where they are natively expressed.
NtEXO70A1a, NtEXO70A2, NtEXO70C1a, and EXO70C2 significantly inhibited pollen tube growth upon overexpression, in contrast to YFP:GUS and NtEXO70E1a, a nonpollen isoform, used as controls. This is in agreement with the notion that EXO70 isoforms not natively expressed in pollen do not have functional properties there, probably due to the lack of tissue-specific protein interactors. We continued with more detailed analysis focused on morphological phenotypes of pollen tubes overexpressing major pollen EXO70s. Such an approach has been applied previously to elucidate the functions of proteins of interest in pollen tubes (Ischebeck et al., 2008, 2010; Stenzel et al., 2012). All four analyzed EXO70 isoforms induced distinct changes in pollen tube tips (Fig. 10). In each case, strong overexpressors displayed apical membrane invaginations. Such invaginations were reported previously as a result of the overexpression of phospholipid-modifying enzymes and are generally interpreted as a hallmark of disrupted cell polarity (Ischebeck et al., 2008; Stenzel et al., 2012). Previously, this phenotype was interpreted as a consequence of excessive endocytosis (Zhao et al., 2010), but it is often difficult to distinguish between an imbalance in exocytosis and endocytosis in plant cells without very detailed studies (Sekereš et al., 2015).
In striking contrast to YFP-tagged A-class EXO70s, C-class EXO70s never localized to the PM, even under conditions of strong overexpression or upon an increased intracellular PIP2 level induced by the overexpression of PIP5K5. This suggests mutually exclusive roles of the two EXO70 families. Pollen tube tip swelling was described previously as a consequence of Rac-type GTPase overexpression (Klahre et al., 2006) and has been attributed to changes in the actin cytoskeleton (Fu et al., 2001). Although tip swelling of the C-class EXO70 overexpressors is much less pronounced than in the case of Rac-type GTPase overexpression, C-class EXO70s might regulate actin properties, similar to animal EXO70 (Zuo et al., 2006; Liu et al., 2012). Another possible interpretation could be that EXO70 C-class isoforms play the negative regulatory function of exocyst membrane binding. The proteins are overly similar to A-class EXO70s but lack the ability to bind membrane. In Arabidopsis, C-class EXO70s are expressed specifically in tip-growing cells: root hairs and pollen. Tip-growing cells grow relatively fast and need to focus exocytosis to a small area near the growing tip. It is possible that exocyst is bound by C-class EXO70 isoforms not capable of targeting the complex and secretory vesicle to the membrane in most of the cell body. In the tip, where A-class EXO70s bind to the PM, possibly due to local lipid and protein interactors, the secretory vesicle would be allowed to fuse with the PM and deliver the cargo. Exchange of the EXO70 isoform of exocyst was already proposed as an important cellular reaction to changing conditions (Žárský et al., 2013), but it also could act as a spatial regulatory event after exocyst relocalization within the cell. This model also is supported by the fact that AtEXO70C1 and AtEXO70C2 are expressed in a nonstoichiometric manner with respect to the exocyst subunits in Arabidopsis (eFP browser), and C-class EXO70s are prevalent over A-class EXO70s in the pollen proteome (Grobei et al., 2009; this study). Thus, an inhibitory C class without membrane-binding properties is prevalent in cytoplasm and could prevent ectopic vesicle fusion before vesicles reach the tip where A-class EXO70s reside. Therefore, overexpression of C-class EXO70s would cause an imbalance in exocytosis regulation, resulting in the described phenotypes.
Most importantly, we demonstrated the localization of NtEXO70A1a and NtEXO70B1 to two distinct, mutually exclusive domains of the pollen tube PM (Fig. 4). The small area of NtEXO70A1a localization corresponds to the previously described area of exocytosis maximum in the pollen tube (Bove et al., 2008; Idilli et al., 2013; Luo et al., 2016). This localization is in accordance with previous characterizations of Arabidopsis EXO70A1 as the isoform generally responsible for exocytosis in many plant tissues (Synek et al., 2006; Hála et al., 2008; Samuel et al., 2009; Kulich et al., 2010; Drdová et al., 2013; Fendrych et al., 2013; Zhang et al., 2016; Vukašinović et al., 2017). In accordance with the published observations, NtEXO70A1a localizes both to the cytoplasm and to the specific area of the PM. The enrichment of PM signal with respect to the cytoplasmic signal in growing pollen tubes was less pronounced compared with tissues like Arabidopsis rhizodermis and stigmatic papillae (Samuel et al., 2009; Zhang et al., 2016). Generally, the ratio of membrane to the cytoplasmic population of EXO70A1 differs among various cell types. For example, previous observations regarding the AtEXO70A1 distribution in Arabidopsis root tissues demonstrated a strong enrichment of GFP:AtEXO70A1 at the outer lateral PM of rhizodermal cells but significantly weaker apolar membrane enrichment of GFP:AtEXO70A1 in root cortical cells (Zhang et al., 2016). The overall abundance of the cytoplasmic NtEXO70A1a population in growing tobacco pollen tubes might be caused by the fact that the membrane domain of NtEXO70A1a localization and cellular secretion is very small and focused. Thus, a high concentration of the peripheral membrane protein targeting the secretion could facilitate its dynamic recruitment to this focused area.
According to previous findings, the role of EXO70B1 in plants is more complex. It is important for autophagy and autophagy-derived transport to the vacuole (Kulich et al., 2013) but also was shown to localize to the PM during light-induced stomatal opening when ectopically expressed in fava bean (Vicia faba) protoplasts (Hong et al., 2016). EXO70B1 also was suggested to be involved in unconventional secretion in specific circumstances like defense against pathogen attack (Žárský et al., 2013; Kulich and Žárský, 2014). Although it is an extremely useful model system for examining the membrane-binding properties of peripheral membrane proteins in plant cells, the tobacco pollen tube is still limited by the impossibility of directly testing the functionality of fluorescently tagged proteins by rescuing the phenotype in a mutant background. Thus, we verified our observations of N-terminal YFP:NtEXO70 fusion proteins by examining the subcellular localization of the C-terminally YFP-tagged NtEXO70B1, which confirmed the previous observation of the corresponding N-terminally tagged protein (Supplemental Fig. S4A). Moreover, AtEXO70B1 was demonstrated previously to rescue the mutant phenotype in Arabidopsis (Kulich et al., 2013). We thus conclude that the observed localization patterns of tagged EXO70 isoforms are biologically relevant.
There are several possible hypotheses explaining the localization of NtEXO70B1 to the subapical region of the pollen tube posterior to the zone of NtEXO70A1a localization. First, NtEXO70B1 might drive an unconventional secretory pathway delivering a specific cargo, alternative to the bulk secretion driven by NtEXO70A1a. This would be in accordance with the general model involving exocytosis to different membrane domains by different EXO70 isoforms (Žárský et al., 2009). Indeed, both NtEXO70A1a and NtEXO70B1 overlap with NtSEC3a at the PM (Fig. 5; Supplemental Fig. S4B). This supports the idea that both EXO70s can act as part of a complex together with other exocyst subunits. One possible mechanism would involve the dynamic replacement of NtEXO70A1a by NtEXO70B1 after cargo delivery. Alternatively, there can be two subpopulations of NtSEC3a-containing exocyst particles: one binding NtEXO70A1a and the other binding NtEXO70B1. This would also explain why the previously described membrane signal of NtSEC3a (Bloch et al., 2016) seems rather broad in comparison with the narrow area of bulk exocytosis in the tobacco pollen tube (Bove et al., 2008; Idilli et al., 2013; Luo et al., 2016). Second, NtEXO70B1 also could play a role in pollen tube endocytosis. Large-scale analysis previously suggested exocyst to be an important linking hub between exocytosis and endocytosis in budding yeast cells (Jose et al., 2015). After delivering secretory cargo to the PM, exocyst or its subcomplex could orchestrate subsequent endocytic recycling. NtEXO70B1 localizes to the area where most of the clathrin-mediated endocytic recycling occurs (Derksen et al., 1995; Moscatelli et al., 2007). This is further supported by colocalization studies with a clathrin endocytosis marker, AtDRP1C, that largely overlaps with NtEXO70B1, and the subapical onset of the membrane localization is identical for both proteins. Importantly, the zone of clathrin endocytosis visualized by DRP1C does not overlap with the region decorated by NtEXO70A1a (Supplemental Fig. S5). Third, subapical NtEXO70B1 could play an exocyst-independent role in pollen tube membrane traffic by assisting membrane remodeling similar to animal Exo70p (Zhao et al., 2013).
Notably, the distinct localization of NtEXO70A1a and NtEXO70B1 also may reflect their different phospholipid-binding properties. As proposed previously (Potocký et al., 2014), the near-subapical overlap of PA and PIP2 could define a distinct domain responsible for the recruitment of certain effectors. Since onsets of NtEXO70A1a and PA marker localization are identical and membrane NtEXO70A1a signal also overlaps with PIP2 localization (Fig. 7), it is possible that the membrane recruitment of NtEXO70A1a requires the simultaneous detection of both PA and PIP2. Conversely, NtEXO70B1 localization is largely excluded from the PIP2-decorated apex and corresponds to the area of the pollen tube strongly decorated by the PA marker. Thus, it is probable that the local phospholipid composition of the PM fine-tunes the membrane affinity toward selected EXO70 isoforms. Protein interactors also are likely to be involved, because A-class EXO70 isoforms display different affinity toward the target PM domain, even though they share amino acid residues predicted to bind minor acidic phospholipids (Žárský et al., 2009).
We conclude that the diversity of EXO70 isoforms in plant cells can play a role in targeting different exocyst complexes or subcomplexes to specific PM domains, but EXO70 isoforms also can be involved in processes not related to membrane trafficking, possibly acting outside the exocyst complex. C-class EXO70 proteins, specifically expressed in tip-growing cells, do not directly bind membrane and probably have a regulatory function. Our results also strongly indicate that EXO70 isoforms need to be studied in cell types in which they are normally expressed. Two of the studied isoforms, NtEXO70A1a and NtEXO70B1, occupy mutually exclusive areas of the pollen tube PM. They exhibit different localization patterns toward the endocytosis marker and lipid probes, and both overlap with the exocyst subunit NtSEC3 at the PM. Since specific interactions with membrane lipids may be important for the membrane recruitment of these isoforms, functional and pharmacological studies will be used in the future to elucidate causal relationships between the distribution of different minor acidic phospholipids and the recruitment of particular EXO70 isoforms.
MATERIALS AND METHODS
Sequence and Phylogenetic Analyses
Tobacco (Nicotiana tabacum) and tomato (Solanum lycopersicum) EXO70 sequences were identified by BLAST searches against corresponding genome databases at the Sol Genomics Network database (https://solgenomics.net) with multiple members of the AtEXO70 family as input query sequences. In most cases, default search parameters were used with occasional modifications (word size 2, scoring matrix BLOSUM45). Multiple alignments were constructed with MAFFT algorithms in E-INS-i mode (Katoh and Toh, 2008) and manually adjusted. Conserved sequence blocks were concatenated, giving alignment with 70 sequences and 588 positions. The maximum likelihood method using the PhyML program (Guindon and Gascuel, 2003) was employed for phylogeny inference with the LG matrix, γ-corrected for among-site rate variation with four rate site categories plus a category for invariable sites, with all parameters estimated from the data. Bayesian tree searches were performed using MrBayes 3.1 (Ronquist and Huelsenbeck, 2003) with a WAG amino acid model, where the analysis was performed in four runs with six chains and 1,000,000 generations, and trees were sampled every 100 generations. All four runs asymptotically approached the same stationarity after the first 200,000 generations, which were omitted from the final analysis.
RT-PCR Expression Analysis
RNA from freshly hydrated pollen, germinating pollen (hydrated and incubated in liquid medium [Klahre et al., 2006] for 40 min), and growing pollen tubes (hydrated and incubated in liquid medium for 3 h) of tobacco was isolated using the RNeasy Kit (Qiagen). Residual DNA was removed with the Turbo DNA-free Kit (Thermo Fisher), and the RNA was transcribed to cDNA using the Transcriptor High Fidelity cDNA Synthesis Kit (Roche). Primers for specific detection of EXO70 paralogs (Supplemental Table S1) were used for subsequent PCR analysis, and tobacco actin was used as the control gene as described by Bosch et al. (2005).
Proteomic Expression Analysis
Pollen tubes were cultivated in liquid medium (Klahre et al., 2006) for 2 h at 150 rpm. They were subsequently vacuum filtered, resuspended in lysis buffer (Potocký et al., 2012), and lysed by sonication. Lysed tubes were centrifuged three times (8,000g, 10 min, 4°C) to get the crude extract. Crude extract protein samples were separated on 10% SDS-PAGE gels, and the band corresponding to 70 kD was cut. Alternatively, the peripheral membrane protein fraction was prepared according to McLoughlin et al. (2013), proteins were separated by 10% SDS-PAGE, and the lane was cut into three bands. After in-gel digestion with trypsin, eluted peptides were identified using UHPLC Dionex Ultimate3000 RSLC nano (Dionex) connected with the mass spectrometer ESI-Q-TOF Maxis Impact (Bruker). Measurements were carried out in positive ion mode with precursor ion selection in the range of 400 to 1,400 mass-to-charge ratio; up to 10 precursor ions were selected for fragmentation from each mass spectrometry spectrum. Peak lists were extracted from raw data by Data Analysis version 4.1 (Bruker Daltonics) and uploaded to the data management system Proteinscape (Bruker Daltonics). For protein identification, the Mascot server (version 2.4.1; Matrix Science) was used with a custom-made database containing tobacco variety K326 and SwissProt proteins.
Molecular Cloning
Most EXO70 coding sequences are intronless and were amplified from tobacco genomic DNA (prepared using Plant DNAzol reagent; Thermo Fisher). EXO70s from the A clade were amplified from a mix of tobacco leaf and pollen cDNA (for preparation, see “RT-PCR Expression Analysis”). AtDRP1C was cloned from Arabidopsis (Arabidopsis thaliana) cDNA. Primers used for molecular cloning together with specific restriction sites are listed in Supplemental Table S2. For N-terminal YFP and mRFP fusions, EXO70 isoforms were cloned into pWEN240 (LAT52:YFP-GA5-MCS:NOS) and pHD222 (LAT52:MCS-GA5-mRFP:NOS) vectors, respectively (Klahre et al., 2006). For C-terminal mRFP fusion of AtDRP1C, we used the pHD223 vector (LAT52:mRFP-GA5-MCS:NOS; Klahre et al., 2006).
Pollen Transformation and Microscopic Analysis
Expression vectors were transferred into tobacco pollen grains germinating on solid culture medium by particle bombardment using a helium-driven particle-delivery system (PDS-1000/He; Bio-Rad) as described previously (Kost et al., 1998). Particles were coated with 1 μg of DNA for subcellular protein localization studies and with 5 μg of DNA for overexpression studies. When two constructs were coexpressed, particles were coated with 1 μg of each DNA plasmid (unless explicitly stated). For observation of subcellular protein localization, 6- to 8-h-old pollen tubes were observed. For overexpression analyses, 8- to 10-h-old pollen tubes were used. For measurement of pollen tube growth inhibition, we used an upright wide-field microscope (Zeiss Axioimager, HPX120V excitation, with camera Axiocam 506 mono) with Achromat 5× dry (numerical aperture = 0.16) objective. For the observation of subcellular protein localization and detailed studies of overexpression-induced phenotypes, we used a spinning disk confocal microscope (Yokogawa CSU-X1 on Nikon Ti-E platform, laser box Agilent MLC400, with sCMOS camera Andor Zyla) with Lense Plan Apochromat 60× WI (numerical aperture = 1.2) objective and 488- and 561-nm laser lines.
Quantitative Image Analysis
For measurements of the equatorial distance of the onset and end of membrane signal, we subtracted background signal and then manually measured the distances from the tip of pollen tubes along the pollen tube membrane (using segmented line and length measurements in ImageJ software). Data are presented as box plots where the horizontal line in the box represents the median, the top and bottom lines of the box represent the 75th and 25th percentiles, and the top-most and bottom-most lines represent extreme values. For evaluation of the effect of EXO70 isoform overexpression on pollen tube morphology and presentation of pollen tubes with typical morphological phenotypes, we applied the following criteria on all images (obtained with the same acquisition setup): the background signal was subtracted, the maximum value of the intensity scale was set to 2,500 (an arbitrary value we empirically found to cover the whole intensity spectrum of signal even for very strong representative overexpressors), and a color intensity code was used to present the processed images. For the quantitative evaluation of YFP-tagged protein overexpression, we measured maximum intensity values of individual images.
Accession Numbers
Sequence data from this article can be found in the GenBank/EMBL data libraries under accession numbers NtExo70A1a, KY499241; NtEXO70A1b, KY499242; NtEXO70A2, KY499243; NtEXO70B1, KY499244; NtExo70C1a, KY499245; NtEXO70C1b, KY499246; NtEXO70C2, KY499247; NtEXO70D1a, KY499248; NtExo70D1b, KY499249; NtEXO70E1a, KY499250; NtEXO70E1b, KY499251; NtEXO70E2, KY499252; NtEXO70F, KY499253; NtEXO70G1a, KY499254; NtEXO70G2, KY499255; NtEXO70H1-2, KY499256; NtEX070H3-4b, KY499257; NtEXO70H5-8a, KY499258; NtEXO70H5-8b, KY499259; NtEXO70H5-8c, KY499260.
Supplemental Data
The following supplemental materials are available.
Supplemental Figure S1. Localization of nonpollen EXO70 isoforms in growing tobacco pollen tubes.
Supplemental Figure S2. Comparison of the NtEXO70A1a and NtEXO70A1b amino acid sequences.
Supplemental Figure S3. Localization of YFP:NtEXO70G1a in growing tobacco pollen tubes with different expression levels.
Supplemental Figure S4. Localization of YFP:NtEXO70B1 and NtEXO70B1:YFP in growing tobacco pollen tubes with different expression levels and colocalization of NtEXO70B1 with NtSEC3a.
Supplemental Figure S5. Mutually exclusive localization of NtEXO70A1a and AtDRP1C in growing tobacco pollen tubes.
Supplemental Figure S6. Fluorescence signal intensity distribution in pollen tubes overexpressing major pollen N-terminally YFP-tagged EXO70 isoforms or the YFP:GUS control.
Supplemental Figure S7. Overexpression of PIP5K5 results in increased NtSEC3a recruitment to the PM in pollen tubes but does not recruit NtEXO70C2 to the PM.
Supplemental Table S1. Primers used for RT-PCR expression analysis of the EXO70 family.
Supplemental Table S2. Primers used for molecular cloning.
Supplemental Movie S1. Localization of YFP:NtEXO70A1a in a growing tobacco pollen tube.
Supplemental Movie S2. Localization of YFP:NtEXO70B1 in a growing tobacco pollen tube.
Supplemental Movie S3. Localization of YFP:NtEXO70E2 in a growing tobacco pollen tube.
Supplemental Movie S4. Localization of YFP:NtEXO70G1a in a growing tobacco pollen tube.
Supplemental File S1. Nucleotide sequences of EXO70 clones used in this study.
Supplementary Material
Acknowledgments
We thank Dr. Ingo Heilmann and colleagues for providing the plasmid encoding PIP5K5:CFP and Dr. Roman Pleskot for careful and critical reading of the article, including useful comments.
Glossary
- PM
plasma membrane
- PA
phosphatidic acid
- RT
reverse transcription
Footnotes
This work was supported by the Czech Science Foundation (grant nos. GA13-19073S and GA15-24711S), the Grant Agency of Charles University (grant no. 394815), the Ministry of Education, Youth, and Sport of the Czech Republic (grant no. NPUI LO1417 to V.Ž. and Specific University Research grant no. 20/2016 to J.Š.), and the Operational Programme Prague – Competitiveness (grant no. CZ.2.16/3.1.00/21519 to the Institute of Experimental Botany).
Articles can be viewed without a subscription.
References
- Afzal AJ, Kim JH, Mackey D (2013) The role of NOI-domain containing proteins in plant immune signaling. BMC Genomics 14: 327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloch D, Pleskot R, Pejchar P, Potocký M, Trpkošová P, Cwiklik L, Vukašinović N, Sternberg H, Yalovsky S, Žárský V (2016) Exocyst SEC3 and phosphoinositides define sites of exocytosis in pollen tube initiation and growth. Plant Physiol 172: 980–1002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodemann BO, Orvedahl A, Cheng T, Ram RR, Ou YH, Formstecher E, Maiti M, Hazelett CC, Wauson EM, Balakireva M, et al. (2011) RalB and the exocyst mediate the cellular starvation response by direct activation of autophagosome assembly. Cell 144: 253–267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosch M, Cheung AY, Hepler PK (2005) Pectin methylesterase, a regulator of pollen tube growth. Plant Physiol 138: 1334–1346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bove J, Vaillancourt B, Kroeger J, Hepler PK, Wiseman PW, Geitmann A (2008) Magnitude and direction of vesicle dynamics in growing pollen tubes using spatiotemporal image correlation spectroscopy and fluorescence recovery after photobleaching. Plant Physiol 147: 1646–1658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen XW, Inoue M, Hsu SC, Saltiel AR (2006) RalA-exocyst-dependent recycling endosome trafficking is required for the completion of cytokinesis. J Biol Chem 281: 38609–38616 [DOI] [PubMed] [Google Scholar]
- Chong YT, Gidda SK, Sanford C, Parkinson J, Mullen RT, Goring DR (2010) Characterization of the Arabidopsis thaliana exocyst complex gene families by phylogenetic, expression profiling, and subcellular localization studies. New Phytol 185: 401–419 [DOI] [PubMed] [Google Scholar]
- Cole RA, Synek L, Žárský V, Fowler JE (2005) SEC8, a subunit of the putative Arabidopsis exocyst complex, facilitates pollen germination and competitive pollen tube growth. Plant Physiol 138: 2005–2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cvrčková F, Grunt M, Bezvoda R, Hála M, Kulich I, Rawat A, Žárský V (2012) Evolution of the land plant exocyst complexes. Front Plant Sci 3: 159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deeks MJ, Calcutt JR, Ingle EK, Hawkins TJ, Chapman S, Richardson AC, Mentlak DA, Dixon MR, Cartwright F, Smertenko AP, et al. (2012) A superfamily of actin-binding proteins at the actin-membrane nexus of higher plants. Curr Biol 22: 1595–1600 [DOI] [PubMed] [Google Scholar]
- Dellago H, Löscher M, Ajuh P, Ryder U, Kaisermayer C, Grillari-Voglauer R, Fortschegger K, Gross S, Gstraunthaler A, Borth N, et al. (2011) Exo70, a subunit of the exocyst complex, interacts with SNEV(hPrp19/hPso4) and is involved in pre-mRNA splicing. Biochem J 438: 81–91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derksen J, Rutten T, Lichtscheidl IK, de Win AHN, Pierson ES, Rongen G (1995) Quantitative analysis of the distribution of organelles in tobacco pollen tubes: implications for exocytosis and endocytosis. Protoplasma 3: 267–276 [Google Scholar]
- Donovan KW, Bretscher A (2015) Tracking individual secretory vesicles during exocytosis reveals an ordered and regulated process. J Cell Biol 210: 181–189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dörmann P, Kim H, Ott T, Schulze-Lefert P, Trujillo M, Wewer V, Hückelhoven R (2014) Cell-autonomous defense, re-organization and trafficking of membranes in plant-microbe interactions. New Phytol 204: 815–822 [DOI] [PubMed] [Google Scholar]
- Drdová EJ, Synek L, Pečenková T, Hála M, Kulich I, Fowler JE, Murphy AS, Žárský V (2013) The exocyst complex contributes to PIN auxin efflux carrier recycling and polar auxin transport in Arabidopsis. Plant J 73: 709–719 [DOI] [PubMed] [Google Scholar]
- Dubuke ML, Maniatis S, Shaffer SA, Munson M (2015) The exocyst subunit Sec6 interacts with assembled exocytic SNARE complexes. J Biol Chem 290: 28245–28256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eliáš M, Drdová E, Žiak D, Bavlnka B, Hála M, Cvrčková F, Soukupová H, Žárský V (2003) The exocyst complex in plants. Cell Biol Int 27: 199–201 [DOI] [PubMed] [Google Scholar]
- Fendrych M, Synek L, Pečenková T, Drdová EJ, Sekereš J, de Rycke R, Nowack MK, Žársky V (2013) Visualization of the exocyst complex dynamics at the plasma membrane of Arabidopsis thaliana. Mol Biol Cell 24: 510–520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fendrych M, Synek L, Pečenková T, Toupalová H, Cole R, Drdová E, Nebesárová J, Sedinová M, Hála M, Fowler JE, et al. (2010) The Arabidopsis exocyst complex is involved in cytokinesis and cell plate maturation. Plant Cell 22: 3053–3065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- France YE, Boyd C, Coleman J, Novick PJ (2006) The polarity-establishment component Bem1p interacts with the exocyst complex through the Sec15p subunit. J Cell Sci 119: 876–888 [DOI] [PubMed] [Google Scholar]
- Fu Y, Wu G, Yang Z (2001) Rop GTPase-dependent dynamics of tip-localized F-actin controls tip growth in pollen tubes. J Cell Biol 152: 1019–1032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grobei MA, Qeli E, Brunner E, Rehrauer H, Zhang R, Roschitzki B, Basler K, Ahrens CH, Grossniklaus U (2009) Deterministic protein inference for shotgun proteomics data provides new insights into Arabidopsis pollen development and function. Genome Res 19: 1786–1800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guindon S, Gascuel O (2003) A simple, fast, and accurate algorithm to estimate large phylogenies by maximum likelihood. Syst Biol 52: 696–704 [DOI] [PubMed] [Google Scholar]
- Hála M, Cole R, Synek L, Drdová E, Pečenková T, Nordheim A, Lamkemeyer T, Madlung J, Hochholdinger F, Fowler JE, et al. (2008) An exocyst complex functions in plant cell growth in Arabidopsis and tobacco. Plant Cell 20: 1330–1345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hase K, Kimura S, Takatsu H, Ohmae M, Kawano S, Kitamura H, Ito M, Watarai H, Hazelett CC, Yeaman C, et al. (2009) M-Sec promotes membrane nanotube formation by interacting with Ral and the exocyst complex. Nat Cell Biol 11: 1427–1432 [DOI] [PubMed] [Google Scholar]
- He B, Xi F, Zhang X, Zhang J, Guo W (2007) Exo70 interacts with phospholipids and mediates the targeting of the exocyst to the plasma membrane. EMBO J 26: 4053–4065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heider MR, Gu M, Duffy CM, Mirza AM, Marcotte LL, Walls AC, Farrall N, Hakhverdyan Z, Field MC, Rout MP, et al. (2016) Subunit connectivity, assembly determinants and architecture of the yeast exocyst complex. Nat Struct Mol Biol 23: 59–66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hepler PK, Winship LJ (2015) The pollen tube clear zone: clues to the mechanism of polarized growth. J Integr Plant Biol 57: 79–92 [DOI] [PubMed] [Google Scholar]
- Hong D, Jeon BW, Kim SY, Hwang JU, Lee Y (2016) The ROP2-RIC7 pathway negatively regulates light-induced stomatal opening by inhibiting exocyst subunit Exo70B1 in Arabidopsis. New Phytol 209: 624–635 [DOI] [PubMed] [Google Scholar]
- Idilli AI, Morandini P, Onelli E, Rodighiero S, Caccianiga M, Moscatelli A (2013) Microtubule depolymerization affects endocytosis and exocytosis in the tip and influences endosome movement in tobacco pollen tubes. Mol Plant 6: 1109–1130 [DOI] [PubMed] [Google Scholar]
- Ischebeck T, Stenzel I, Heilmann I (2008) Type B phosphatidylinositol-4-phosphate 5-kinases mediate Arabidopsis and Nicotiana tabacum pollen tube growth by regulating apical pectin secretion. Plant Cell 20: 3312–3330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ischebeck T, Stenzel I, Hempel F, Jin X, Mosblech A, Heilmann I (2011) Phosphatidylinositol-4,5-bisphosphate influences Nt-Rac5-mediated cell expansion in pollen tubes of Nicotiana tabacum. Plant J 65: 453–468 [DOI] [PubMed] [Google Scholar]
- Ischebeck T, Vu LH, Jin X, Stenzel I, Löfke C, Heilmann I (2010) Functional cooperativity of enzymes of phosphoinositide conversion according to synergistic effects on pectin secretion in tobacco pollen tubes. Mol Plant 3: 870–881 [DOI] [PubMed] [Google Scholar]
- Ischebeck T, Werner S, Krishnamoorthy P, Lerche J, Meijón M, Stenzel I, Löfke C, Wiessner T, Im YJ, Perera IY, et al. (2013) Phosphatidylinositol 4,5-bisphosphate influences PIN polarization by controlling clathrin-mediated membrane trafficking in Arabidopsis. Plant Cell 25: 4894–4911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jose M, Tollis S, Nair D, Mitteau R, Velours C, Massoni-Laporte A, Royou A, Sibarita JB, McCusker D (2015) A quantitative imaging-based screen reveals the exocyst as a network hub connecting endocytosis and exocytosis. Mol Biol Cell 26: 2519–2534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katoh K, Toh H (2008) Recent developments in the MAFFT multiple sequence alignment program. Brief Bioinform 9: 286–298 [DOI] [PubMed] [Google Scholar]
- Klahre U, Becker C, Schmitt AC, Kost B (2006) Nt-RhoGDI2 regulates Rac/Rop signaling and polar cell growth in tobacco pollen tubes. Plant J 46: 1018–1031 [DOI] [PubMed] [Google Scholar]
- Konopka CA, Bednarek SY (2008) Comparison of the dynamics and functional redundancy of the Arabidopsis dynamin-related isoforms DRP1A and DRP1C during plant development. Plant Physiol 147: 1590–1602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kost B, Spielhofer P, Chua NH (1998) A GFP-mouse talin fusion protein labels plant actin filaments in vivo and visualizes the actin cytoskeleton in growing pollen tubes. Plant J 16: 393–401 [DOI] [PubMed] [Google Scholar]
- Kulich I, Cole R, Drdová E, Cvrčková F, Soukup A, Fowler J, Žárský V (2010) Arabidopsis exocyst subunits SEC8 and EXO70A1 and exocyst interactor ROH1 are involved in the localized deposition of seed coat pectin. New Phytol 188: 615–625 [DOI] [PubMed] [Google Scholar]
- Kulich I, Pečenková T, Sekereš J, Smetana O, Fendrych M, Foissner I, Höftberger M, Žárský V (2013) Arabidopsis exocyst subcomplex containing subunit EXO70B1 is involved in autophagy-related transport to the vacuole. Traffic 14: 1155–1165 [DOI] [PubMed] [Google Scholar]
- Kulich I, Vojtíková Z, Glanc M, Ortmannová J, Rasmann S, Žárský V (2015) Cell wall maturation of Arabidopsis trichomes is dependent on exocyst subunit EXO70H4 and involves callose deposition. Plant Physiol 168: 120–131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulich I, Žárský V (2014) Autophagy-related direct membrane import from ER/cytoplasm into the vacuole or apoplast: a hidden gateway also for secondary metabolites and phytohormones? Int J Mol Sci 15: 7462–7474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Łangowski L, Růzicka K, Naramoto S, Kleine-Vehn J, Friml J (2010) Trafficking to the outer polar domain defines the root-soil interface. Curr Biol 20: 904–908 [DOI] [PubMed] [Google Scholar]
- Łangowski Ł, Wabnik K, Li H, Vanneste S, Naramoto S, Tanaka H, Friml J (2016) Cellular mechanisms for cargo delivery and polarity maintenance at different polar domains in plant cells. Cell Discov 2: 16018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu D, Novick P (2014) Bem1p contributes to secretory pathway polarization through a direct interaction with Exo70p. J Cell Biol 207: 59–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Zhao Y, Sun Y, He B, Yang C, Svitkina T, Goldman YE, Guo W (2012) Exo70 stimulates the Arp2/3 complex for lamellipodia formation and directional cell migration. Curr Biol 22: 1510–1515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Zuo X, Yue P, Guo W (2007) Phosphatidylinositol 4,5-bisphosphate mediates the targeting of the exocyst to the plasma membrane for exocytosis in mammalian cells. Mol Biol Cell 18: 4483–4492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lora J, Hormaza JI, Herrero M (2016) The diversity of the pollen tube pathway in plants: toward an increasing control by the sporophyte. Front Plant Sci 7: 107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loraine AE, McCormick S, Estrada A, Patel K, Qin P (2013) RNA-seq of Arabidopsis pollen uncovers novel transcription and alternative splicing. Plant Physiol 162: 1092–1109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo N, Yan A, Yang Z (2016) Measuring exocytosis rate using corrected fluorescence recovery after photoconversion. Traffic 17: 554–564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin-Urdiroz M, Deeks MJ, Horton CG, Dawe HR, Jourdain I (2016) The exocyst complex in health and disease. Front Cell Dev Biol 4: 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLoughlin F, Arisz SA, Dekker HL, Kramer G, de Koster CG, Haring MA, Munnik T, Testerink C (2013) Identification of novel candidate phosphatidic acid-binding proteins involved in the salt-stress response of Arabidopsis thaliana roots. Biochem J 450: 573–581 [DOI] [PubMed] [Google Scholar]
- Morgera F, Sallah MR, Dubuke ML, Gandhi P, Brewer DN, Carr CM, Munson M (2012) Regulation of exocytosis by the exocyst subunit Sec6 and the SM protein Sec1. Mol Biol Cell 23: 337–346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moscatelli A, Ciampolini F, Rodighiero S, Onelli E, Cresti M, Santo N, Idilli A (2007) Distinct endocytic pathways identified in tobacco pollen tubes using charged nanogold. J Cell Sci 120: 3804–3819 [DOI] [PubMed] [Google Scholar]
- Ostertag M, Stammler J, Douchkov D, Eichmann R, Hückelhoven R (2013) The conserved oligomeric Golgi complex is involved in penetration resistance of barley to the barley powdery mildew fungus. Mol Plant Pathol 14: 230–240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pečenková T, Hála M, Kulich I, Kocourková D, Drdová E, Fendrych M, Toupalová H, Žársky V (2011) The role for the exocyst complex subunits Exo70B2 and Exo70H1 in the plant-pathogen interaction. J Exp Bot 62: 2107–2116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pleskot R, Cwiklik L, Jungwirth P, Žárský V, Potocký M (2015) Membrane targeting of the yeast exocyst complex. Biochim Biophys Acta 1848: 1481–1489 [DOI] [PubMed] [Google Scholar]
- Potocký M, Pejchar P, Gutkowska M, Jiménez-Quesada MJ, Potocká A, Alché JdeD, Kost B, Žárský V (2012) NADPH oxidase activity in pollen tubes is affected by calcium ions, signaling phospholipids and Rac/Rop GTPases. J Plant Physiol 169: 1654–1663 [DOI] [PubMed] [Google Scholar]
- Potocký M, Pleskot R, Pejchar P, Vitale N, Kost B, Žárský V (2014) Live-cell imaging of phosphatidic acid dynamics in pollen tubes visualized by Spo20p-derived biosensor. New Phytol 203: 483–494 [DOI] [PubMed] [Google Scholar]
- Ronquist F, Huelsenbeck JP (2003) MrBayes 3: Bayesian phylogenetic inference under mixed models. Bioinformatics 19: 1572–1574 [DOI] [PubMed] [Google Scholar]
- Qin Y, Dong J (2015) Focusing on the focus: what else beyond the master switches for polar cell growth? Mol Plant 8: 582–594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabol P, Kulich I, Žárský V (2017) RIN4 recruits the exocyst subunit EXO70B1 to the plasma membrane. J Exp Bot doi/10.1093/jxb/erx007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuel MA, Chong YT, Haasen KE, Aldea-Brydges MG, Stone SL, Goring DR (2009) Cellular pathways regulating responses to compatible and self-incompatible pollen in Brassica and Arabidopsis stigmas intersect at Exo70A1, a putative component of the exocyst complex. Plant Cell 21: 2655–2671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sekereš J, Pleskot R, Pejchar P, Žárský V, Potocký M (2015) The song of lipids and proteins: dynamic lipid-protein interfaces in the regulation of plant cell polarity at different scales. J Exp Bot 66: 1587–1598 [DOI] [PubMed] [Google Scholar]
- Stegmann M, Anderson RG, Ichimura K, Pečenková T, Reuter P, Žárský V, McDowell JM, Shirasu K, Trujillo M (2012) The ubiquitin ligase PUB22 targets a subunit of the exocyst complex required for PAMP-triggered responses in Arabidopsis. Plant Cell 24: 4703–4716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stenzel I, Ischebeck T, Quint M, Heilmann I (2012) Variable regions of PI4P 5-kinases direct PtdIns(4,5)P2 toward alternative regulatory functions in tobacco pollen tubes. Front Plant Sci 2: 114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Synek L, Schlager N, Eliáš M, Quentin M, Hauser MT, Žárský V (2006) AtEXO70A1, a member of a family of putative exocyst subunits specifically expanded in land plants, is important for polar growth and plant development. Plant J 48: 54–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Synek L, Sekereš J, Žárský V (2014) The exocyst at the interface between cytoskeleton and membranes in eukaryotic cells. Front Plant Sci 4: 543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TerBush DR, Maurice T, Roth D, Novick P (1996) The exocyst is a multiprotein complex required for exocytosis in Saccharomyces cerevisiae. EMBO J 15: 6483–6494 [PMC free article] [PubMed] [Google Scholar]
- Vaškovičová K, Žárský V, Rösel D, Nikolič M, Buccione R, Cvrčková F, Brábek J (2013) Invasive cells in animals and plants: searching for LECA machineries in later eukaryotic life. Biol Direct 8: 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vukašinović N, Oda Y, Pejchar P, Synek L, Pečenková T, Rawat A, Sekereš J, Potocký M, Žárský V (2017) Microtubule-dependent targeting of the exocyst complex is necessary for xylem development in Arabidopsis. New Phytol 213: 1052–1067 [DOI] [PubMed] [Google Scholar]
- Wang H, Tang X, Liu J, Trautmann S, Balasundaram D, McCollum D, Balasubramanian MK (2002) The multiprotein exocyst complex is essential for cell separation in Schizosaccharomyces pombe. Mol Biol Cell 13: 515–529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang N, Lee IJ, Rask G, Wu JQ (2016) Roles of the TRAPP-II complex and the exocyst in membrane deposition during fission yeast cytokinesis. PLoS Biol 14: e1002437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winter D, Vinegar B, Nahal H, Ammar R, Wilson GV, Provart NJ (2007) An “Electronic Fluorescent Pictograph” browser for exploring and analyzing large-scale biological data sets. PLoS ONE 2: e718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu H, Rossi G, Brennwald P (2008) The ghost in the machine: small GTPases as spatial regulators of exocytosis. Trends Cell Biol 18: 397–404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu H, Turner C, Gardner J, Temple B, Brennwald P (2010) The Exo70 subunit of the exocyst is an effector for both Cdc42 and Rho3 function in polarized exocytosis. Mol Biol Cell 21: 430–442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu S, Mehta SQ, Pichaud F, Bellen HJ, Quiocho FA (2005) Sec15 interacts with Rab11 via a novel domain and affects Rab11 localization in vivo. Nat Struct Mol Biol 12: 879–885 [DOI] [PubMed] [Google Scholar]
- Žárský V, Cvrčková F, Potocký M, Hála M (2009) Exocytosis and cell polarity in plants: exocyst and recycling domains. New Phytol 183: 255–272 [DOI] [PubMed] [Google Scholar]
- Žárský V, Kulich I, Fendrych M, Pečenková T (2013) Exocyst complexes multiple functions in plant cells secretory pathways. Curr Opin Plant Biol 16: 726–733 [DOI] [PubMed] [Google Scholar]
- Žárský V, Potocký M (2010) Recycling domains in plant cell morphogenesis: small GTPase effectors, plasma membrane signalling and the exocyst. Biochem Soc Trans 38: 723–728 [DOI] [PubMed] [Google Scholar]
- Zhang C, Brown MQ, van de Ven W, Zhang ZM, Wu B, Young MC, Synek L, Borchardt D, Harrison R, Pan S, et al. (2016) Endosidin2 targets conserved exocyst complex subunit EXO70 to inhibit exocytosis. Proc Natl Acad Sci USA 113: E41–E50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Orlando K, He B, Xi F, Zhang J, Zajac A, Guo W (2008) Membrane association and functional regulation of Sec3 by phospholipids and Cdc42. J Cell Biol 180: 145–158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Pumplin N, Ivanov S, Harrison MJ (2015) EXO70I is required for development of a sub-domain of the periarbuscular membrane during arbuscular mycorrhizal symbiosis. Curr Biol 25: 2189–2195 [DOI] [PubMed] [Google Scholar]
- Zhao Y, Liu J, Yang C, Capraro BR, Baumgart T, Bradley RP, Ramakrishnan N, Xu X, Radhakrishnan R, Svitkina T, et al. (2013) Exo70 generates membrane curvature for morphogenesis and cell migration. Dev Cell 26: 266–278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y, Yan A, Feijó JA, Furutani M, Takenawa T, Hwang I, Fu Y, Yang Z (2010) Phosphoinositides regulate clathrin-dependent endocytosis at the tip of pollen tubes in Arabidopsis and tobacco. Plant Cell 22: 4031–4044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuo X, Zhang J, Zhang Y, Hsu SC, Zhou D, Guo W (2006) Exo70 interacts with the Arp2/3 complex and regulates cell migration. Nat Cell Biol 8: 1383–1388 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.