Abstract
The use of theory-driven models to develop and evaluate family-based intervention programs has a long history in psychology. Some of the first evidence-based parenting programs to address child problem behavior, developed in the 1970s, were grounded in causal models derived from longitudinal developmental research. The same translational strategies can also be applied to designing programs that leverage emerging scientific knowledge about the effects of early adverse experiences on neurobiological systems to reduce risk and promote well-being. By specifying not only behavioral targets but also affected underlying neural systems, interventions can become more precise and efficient. This chapter describes the development of a program of research focusing on an intervention for young children in foster care. The intervention emerged from social learning theory research and employs a translational neuroscience approach. The conceptual model guiding the research, which incorporates behavioral domains as well as stress-regulatory neural systems, is described. Finally, future directions for translational neuroscience in family-based intervention research are considered.
Considerable effort has been applied in recent decades to the development and empirical evaluation of family-focused interventions designed to mitigate the effects of environmental adversity (e.g., poverty, maltreatment, and neighborhood violence). Although the research has produced definitive “proof of concept” that some of the effects of adversity are reversible, this work has had a limited impact to date on the health of the population (Shonkoff & Fisher, 2013). Restricted progress in this area may be attributed in part to the slow uptake of evidence-based interventions in community settings. However, even if rates of uptake were increased, it is unclear what the impact might be, because—in general—only modest effect sizes from intervention trials have been reported in the literature. Moreover, challenges are often encountered in scaling interventions with issues, such as low rates of participation, difficulties with program 3-delity and sustainability, and high costs of implementation. Put simply, additional work is needed to produce impactful interventions, and strategies for scaling, that lead to detectable improvements in well-being among those exposed to adversity at the population level.
In this chapter, it is argued that one way in which progress will be made in this area is by emphasizing so-called “experimental medicine” approaches to intervention development and evaluation (Pine & Leibenluft, 2015). By definition, experimental medicine approaches pinpoint specific targets of interventions. They also use intervention trials as experimental tests of theory in which comparisons of intervention and control groups on changes in intervention targets and outcomes—and the associations between those changes—yield empirical evidence about the validity of a hypothesized conceptual model (Insel et al., 2013). Methodological advances in developmental neuroscience now permit noninvasive measurement of many brain and biological systems that are known to be affected by early adversity, including stress hormone systems; brain systems that are involved in attention, detection of threat, motivation, and reward; and immune and inflammatory systems (Chiang, Taylor, & Bower, 2015; Shonkoff et al., 2012; Tyrka, Burgers, Philip, Price, & Carpenter, 2013). This work has stimulated interest in applying experimental medicine approaches to the development and evaluation of translational neuroscience interventions that can help explicate underlying neural mechanisms of developmental psychopathology, and in doing so, determine whether targeting such mechanisms through family-based interventions leads to reductions in childhood symptomatology and psychopathology (Fisher & Berkman, 2015).
In the following sections, the opportunities and challenges facing the family-intervention field are considered, with respect to using translational neuroscience to address the effects of adversity. The discussion begins with a description of the manner in which experimental methods were first applied to the family intervention field by Gerald Patterson and colleagues as they developed coercion theory (Patterson, 1982). Consideration is given to the way in which Patterson et al.’s work, which included causal modeling of longitudinal data, led to the articulation of a conceptual model that, in turn, produced a set of evidence-based intervention strategies for treating disruptive behavior in childhood. A description is provided of the early randomized clinical trials that were employed, both to evaluate these intervention strategies and to experimentally test coercion theory. A brief synopsis is presented of how this led to subsequent iterations of theory and practice, including the developmentally downward extension of a coercion theory-based treatment from delinquent adolescents to preschool-age foster children. It was the intervention for foster preschoolers that created the opportunity for one of the first “translational neuroscience” research programs, which incorporated hypothesized neurobiological mechanisms and behavioral variables. Results are presented of studies conducted within this research program. The chapter concludes with a consideration of future directions for research in this area.
The Social Learning Model as an Application of an Experimental Approach to Family-Based Interventions
The concept of applying experimental approaches to family-based interventions has been described in the literature for many decades. Specifically, in the 1960s, some researchers began to find fault with the correlational approaches to empirical investigation that had long been in the mainstream of developmental psychology. A pioneering researcher, Gerald Patterson, moved beyond correlational research in the study of families (Patterson, Reid, & Eddy, 2002). Beginning in the late 1960s, Patterson and colleagues at the Oregon Social Learning Center set out to study families in their natural environments. Rather than rely solely on questionnaires and interviews to measure phenomena of interest, researchers used direct observation methods and microsocial coding to quantify parent-child interactions in naturalistic settings. In addition, families were studied longitudinally at frequent intervals to determine how processes observed at one time-point related to subsequent outcomes, both positive and negative, across childhood and adolescence.
Much of Patterson’s work focused on how antisocial and disruptive behavior develops and is sustained in children. At the time, it was well understood that intra-individual processes, such as a difficult child temperament; familial factors, such as parental substance use and maternal depression; and sociocultural factors, such as poverty, discrimination, and neighborhood violence, were known to contribute to the development of child problems. However, a seminal finding from the Patterson et al. research was that the above variables are most adequately conceptualized as distal, or indirect, influencers of subsequent child outcomes (Capaldi, DeGarmo, Patterson, & Forgatch, 2002). Parenting processes, in contrast, proved consistently to be the most proximal predictors of child antisocial behavior. In particular, families that (a) used harsh and inconsistent discipline strategies and (b) had low levels of warmth and support were found to be most likely to include children with disruptive behavior problems (Capaldi et al., 2002). The previously noted distal variables were inarguably also important, but their contribution had to do with the extent to which they disrupted effective parenting processes. Notably, when families with children who exhibited disruptive behavior were observed longitudinally, negative interactions between parents and children were seen to escalate over time, because parents and children resorted to increasingly coercive strategies for terminating negative and hostile interactions (Patterson, Reid, & Dishion, 1992). These escalating interactions seemed to train negative behavior in children, which, in turn, led to their difficulties adjusting to the academic and social demands of the school environment. Subsequently, during development, these family interactions were predictive of myriad negative outcomes, including increased probability of school failure, rejection by prosocial peers, substance abuse, and delinquent behavior (Patterson, DeBaryshe, & Ramsey, 1989). One of the most noteworthy aspects of Patterson and colleagues’ early work on coercion within families is the extent to which the core family processes that were identified as primary causes of the development of disruptive behavior had the potential to be malleable. That is, the possibility existed to intervene on and potentially change core predictors of child disruptive behavior: harsh and inconsistent parenting practices combined with low levels of support for positive behavior.
To investigate whether the development of disruptive child behavior could be effectively altered through changes in parenting, Patterson and colleagues developed a set of intervention strategies that collectively came to be known as Parent Management Training Oregon (PMTO; Patterson, Chamberlain, & Reid, 1982). In the years following the development of PMTO, a number of independent randomized clinical trials found that these strategies had an effective impact on the relevant domains of parenting (Kazdin, 1997). Moreover, changes in parenting were associated with decreases in problem behavior and increases in prosocial behavior (Kazdin, 1997). As such, these evaluation trials not only provided evidence for these parenting programs as efficacious approaches to promoting child well-being, but also corroborated evidence for the validity of the coercion model.
Extension of the Coercion Model to Incorporate Neurobiological Domains
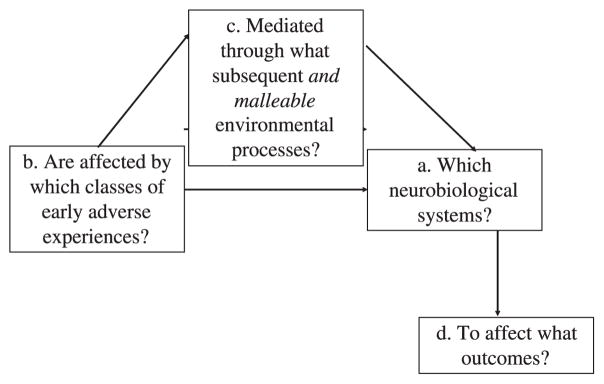
The iterative cycles that led to the development of PMTO are an example of how an experimental approach may be applied to family intervention research. Although the incorporation of a translational neuroscience focus into this work may not be completely intuitive, it is actually fairly straightforward. To accomplish it, however, the focus must broaden from one that is only on behavioral targets and outcomes to one that includes underlying neural processes that may mediate the relationship between targets and outcomes. As shown in Figure 7.1, this strategy leads to consideration of (a) specific neurobiological systems hypothesized to be affected by (b) specific classes of early adverse experiences, as mediated through (c) potentially modifiable family-based processes to affect (d) specific outcomes (Fisher, Stoolmiller, Gunnar, & Burraston, 2007). Intervention trials in this context may specify causal processes arising from early adversity that can be modified through parenting to influence underlying child neural systems that represent an intermediate, mechanistic step in improving behavioral outcomes.
Figure 7.1.
Extension of the experimental medicine approach for family-based interventions to translational neuroscience research
To illustrate how translational neuroscience can be used in experimental medicine approaches to family-based interventions to address the effects of adversity, it is helpful to return to the work of Patterson and colleagues after the development and validation of PMTO. In the 1980s, one of Patterson’s protégés, Patricia Chamberlain, developed a more intensive family-based approach called Treatment Foster Care Oregon (TFCO1; Eddy & Chamberlain, 2000) to treat child and adolescent conduct disorders. Although TFCO focused on the same parenting practices as did PMTO, it was implemented in a foster home setting in which these parenting practices were emphasized in the absence of overlearned coercive interactions among family members. Once a youth responded to the parenting practices in the foster home and the birth parents had learned to use similar parenting strategies, the youth was transitioned back home to parents who had received PMTO-type training. The TFCO program has been evaluated in several randomized clinical trials and found to be an extremely efficacious approach to reducing problem behavior and preventing subsequent delinquency in very troubled youths (Leve et al., 2012).
In the 1990s, the TFCO intervention was adapted downward developmentally to meet the specific needs of preschool-age children in the foster care system (Fisher, Ellis, & Chamberlain, 1999). As with the TFCO program for older children, the program for preschoolers (TFCO-P2) supported foster parents intensively to engage in efficacious parenting. In addition, to facilitate preschoolers’ social skills development and successful entry into kindergarten, the program included a playgroup component. This helped children gain experience and practice in a typical classroom setting and engage in activities that required self-regulation and early literacy skills. In addition, TFCO-P provided support to birth and adoptive families after the child transitioned out of the foster home and into a permanent placement.
As work unfolded using the TFCO-P model, clinicians noted that many children initially showed very limited treatment response and continued to behave as if they were still in a threatening or neglectful environment. After a period of time that could last as long as several months, however, many of the children who had shown limited response at first subsequently underwent positive behavior change. Even more noteworthy were anecdotal observations that progress also seemed to occur in terms of developmental functioning (e.g., letter and color recognition, gross, and fine motor skills). The observed changes were relatively rapid in nature compared to normative development, spanning weeks or sometimes even days. This led the group to speculate that ongoing dysregulation in a stress regulatory neural system might be initially preventing neurocognitive and behavioral development from proceeding in spite of the child being in a more responsive and enriched environment. An ensuing collaborative effort by intervention researchers and developmental neuroscientists (Gunnar, Fisher, & the Early Experience, Stress, and Prevention Network, 2006) led to the identification of the hypothalamic-pituitary-adrenal (HPA) axis as a candidate mechanism that might underlie these phenomena. The HPA axis is a neuroendocrine system that helps the body return to homeostatic balance following the experience of stress. Activation of the HPA results in a hormonal cascade, which in turn leads to bodily changes such as metabolism of stored energy and activation of the immune system (space considerations prevent further discussion of how the physiological development of the HPA axis may be disrupted by adverse early experiences; see Fisher, Gunnar, Dozier, Bruce, & Pears, 2006, for details). When the TFCO-P program for preschoolers was being developed, evidence already existed for the elevation in activity of the HPA axis among many clinical populations and those exposed to acute life and occupational stress (Heim & Nemeroff, 2001; Melamed et al., 1999). Moreover, some researchers were also finding alterations in the HPA among children reared in institutional settings (i.e., orphanages) in developing countries (Carlson & Earls, 1997).
Notably, however, rather than showing elevated levels of cortisol (the end product of the hormonal HPA cascade), these children exhibited diminished levels of HPA axis activity3 (Gunnar & Vazquez, 2001). Remarkably, this initial research involving foster children not only showed patterns of cortisol activity similar to those of the institutionally reared children, but documented that such patterns of cortisol activity were most common among individuals in foster care with histories of significant neglect (Bruce, Fisher, Pears, & Levine, 2009). This finding was of considerable importance for two reasons: First, neglect was the distinguishing characteristic of the institutional orphanages in which children’s diminished HPA activity had also been observed; second, contrary to popular conceptions that child physical and sexual abuse are the most common reasons children are remanded to foster care, neglect is in fact the primary reason that children are placed in foster homes.
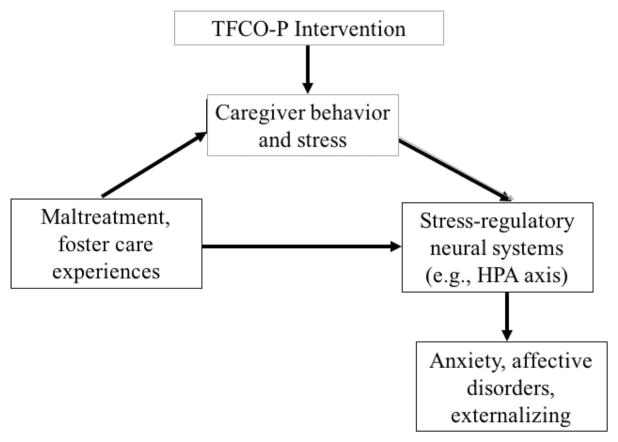
TFCO-P researchers developed a conceptual model for the randomized trial of the program that was an extension of the basic coercion theory model, but also incorporated neurobiological functioning as an underlying mechanism associated with later outcomes (Fisher et al., 2007). As shown in Figure 7.2, in this model, alterations in HPA axis functioning arising from maltreatment (in particular, neglect) are hypothesized to be mediated through parenting practices that the child experiences after being placed in foster care. By targeting those parenting practices through the TFCO-P intervention, it was hypothesized that outcomes such as anxiety and affective disorders, school success, and externalizing behavior would be affected as a result of changes in, and in particular, increased regulation of, the HPA axis.
Figure 7.2.
The conceptual model guiding TFCO-P
A large-scale randomized clinical trial was conducted to evaluate this conceptual model during the course of a 10-year period (Fisher et al., 2006). The study design had three arms: foster children randomly assigned to receive TFCO-P, those assigned to services as usual, and low-income community children who had no history of maltreatment or child welfare involvement. The total sample of 177 was equally distributed across groups (regular foster care [RFC] = 60; TFCO-P = 57; community comparison [CC] = 60).
Many positive effects of TFCO-P were observed. For example, the intervention was found to be associated with increased stability of foster care placements and conversely with fewer placement disruptions than for RFC children (Fisher, Stoolmiller, Mannering, Takahashi, & Chamberlain, 2011). It was also associated with an increased likelihood that when a child was placed in a permanent adoptive family or reunified with birth parents following foster care, that placement would remain intact over time (Fisher, Burraston, & Pears, 2005). One of the most important findings from prior research showed that among children in foster care, disrupted placements are very common and, germane to the TFCO-P study, that one of the primary causes of disruptions is high rates of problem behavior exhibited by the child. In contrast, the TFCO-P randomized trial showed that children who received the intervention while in foster care were buffered against the likelihood that their foster placement would disrupt as a result of their problem behavior. Specifically, RFC children had a threshold of six problem behaviors per day (as reported by foster parents), and their risk for disrupted placement increased substantially with each additional problem behavior. However, in the intervention condition, children showed no such threshold effect; that is, TFCO-P children with more than six problem behaviors per day remained at the same low level of risk for disruption as did foster children in both groups who had six or fewer problem behaviors per day (Fisher et al., 2011).
The TFCO-P intervention also had a positive impact on attachment-related behaviors. Children in the intervention condition were more likely to seek out a caregiver when their distress levels began to approach those observed in the CC sample. In contrast, RFC children showed a decreased likelihood over time that they would approach the caregiver when distressed (Fisher & Kim, 2007).
Results related to children’s cortisol levels were also obtained. Specifically, results showed that among children in the intervention condition, morning cortisol levels were more likely to remain at levels comparable to those of CC children in the study, whereas RFC children showed dramatically decreased morning cortisol levels over time (similar to the type of neuroendocrine dysregulation observed among severely neglected children in other studies; Fisher et al., 2007).
The research also focused on variables hypothesized to be associated with children’s changes in cortisol levels. One set of analyses on this topic found that among RFC children, foster parents reported high levels of stress managing children’s problem behavior at baseline, and this continued throughout the course of the foster placement. By contrast, stress levels among the foster parents in the intervention condition dropped immediately after baseline and remained low when they were managing children’s problem behavior. Notably, in the RFC condition caregiver stress levels were directly and temporally associated with children’s morning cortisol levels. That is, high foster parent stress on one day was associated with diminished child cortisol levels the next morning. On the other hand, there was no association between foster parent stress and cortisol level the next day for children in the intervention group (Fisher & Stoolmiller, 2008).
In a second analysis of the data, researchers found that among RFC children, changes in placement from one foster home to another or from foster to permanent placement was associated with diminished and atypical morning cortisol levels. In contrast, among children who received the intervention, cortisol levels remained stable during placement changes. This may be because efforts were made to prepare children for transitions in the intervention condition. Moreover, because of TFCO-P’s emphasis on consistent parenting strategies for all child caregivers, the environment may have remained more similar before and after placement change for intervention condition children (Fisher, Van Ryzin, & Gunnar, 2011).
Adequacy of the Evidence for Translational Neuroscience and Practice, and Implications for the Field
The results of the TFCO-P randomized clinical trial can be interpreted in several ways. At one level, they provide evidence that the use of parenting techniques known to be efficacious in shaping children’s behavior has the potential to dramatically alter the life course trajectories of young foster children, even after significant adversity. The breadth of positive, observed effects of the intervention is particularly noteworthy and includes improved likelihood of successful foster care and permanency of placements and improved psychosocial adjustment. In addition, the randomized trial provided some of the first evidence that by intervening in the context of the family, it is possible to improve the functioning of neurobiological systems known to be affected by exposure to early adversity. This set of findings is especially meaningful when considering that an economic analysis conducted during this trial found that during a 12-month period, the associated costs of the intervention were less than the cost of placing children in conventional foster care (Lynch, Dickerson, Saldana, & Fisher, 2014).
Although these results provide strong justification for TFCO-P as an efficacious program, the intervention did not fare as well from an experimental medicine perspective. Figure 7.3 is a synopsis of the results of the study in terms of validating the conceptual model shown in Figure 7.2. The thickness of arrows in Figure 7.3 is intended to depict an estimate of the strength of observed associations. As shown, although the intervention did have an impact on parenting stress, changes in parenting behaviors specified in the model were not readily observed. Similarly, although behavioral adjustment (specifically, secure attachment behaviors) improved in the intervention condition, and salivary cortisol measures also seemed to indicate a positive effect of the intervention, the path from the intervention behavioral changes did not appear to be mediated through underlying stress neurobiology or caregiving. Indeed, the one mediational process in the model that appeared to be robust was the connection between the stress caregivers reported while managing children’s problem behavior and children’s cortisol levels the next morning.
Figure 7.3.
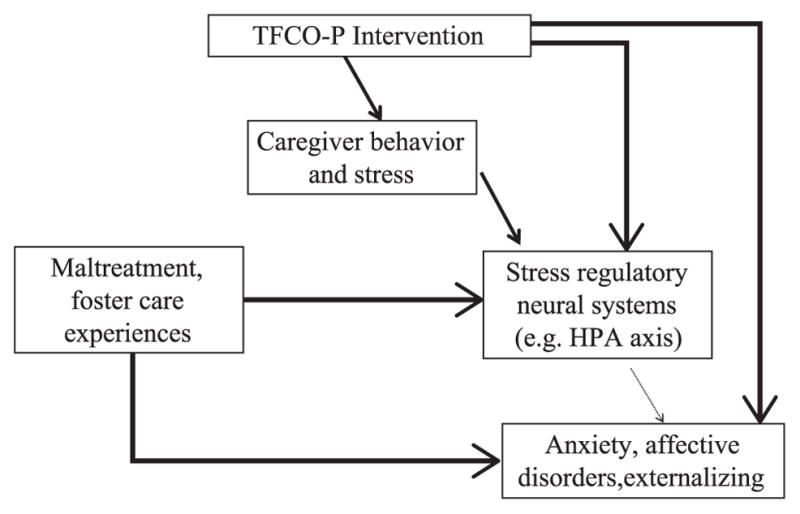
Evidence for the TFCO-P Conceptual Model (thicker arrows denote stronger evidence of associations
These results present something of a conundrum, albeit a not-uncommon one in the family-based intervention field. Although it may be sufficient to document the positive effects of an intervention on meaningful outcomes from a policy perspective, from a scientific perspective, the results of the TFCO-P randomized trial provide less understanding about how the intervention works. Equally important, by focusing on the main effects of the intervention, variations in response on outcomes of interest are easily overlooked.
How then might the field progress beyond this point? To advance the science in this area, three domains require attention: First, greater effort must be applied to examining intervention targets. There are two subdomains in this area: (1) in many cases, interventions must show sufficient specificity with respect to their targets, and (2) even when intervention targets are clearly specified, more evidence must confirm that the intervention effectively engages those targets before large-scale evaluation is conducted. Second, evidence of the mediating role of specific underlying neurobiological systems’ connection between targets and behavioral or psychosocial outcomes must be obtained before large-scale intervention research is conducted. In the case of the present research, although the HPA axis was effectively influenced by the intervention, the mediating role between parenting, HPA axis function, and child outcomes remains considerably less clear. Third, more effort must be devoted at the outset and throughout the design of intervention research to understanding the question of moderators that can be used to predict individual variability in response to intervention. The overall effectiveness of interventions may be increased when it is understood who is most likely to benefit and what systematic processes or factors might underlie variations in that response. In this way subsequent and supplemental interventions can be developed and evaluated.
Translational neuroscience has the potential to play a role in future research in this area. For example, there is evidence that some neural systems represent common pathways between adversity and negative outcomes (Heim, Plotsky, & Nemeroff, 2004). The aforementioned HPA axis is one such system. In addition, a scientific knowledge base exists with regard to specific neural regions and circuits associated with the wide range of common phenotypes underlying developmental psychopathology. For example, individuals who have experienced high levels of early adversity typically show alterations in the development of executive functioning (Pechtel & Pizzagalli, 2011). These alterations may manifest in poor performance on behavioral tests and real-world behavior, such as impulsivity and poor self-control; the alterations may also be measured via underlying neural circuitry.
Research that is examining young foster children’s response to corrective feedback is an example of how translational neuroscience has the potential to lead to new insights and family-based interventions. In a pilot study based on a subsample of children from the TFCO-P randomized trial, preliminary evidence revealed the impact of intervention on an electrophysiological measure of brain activity. Specifically, it was observed that RFC children showed very limited response on an event-related potential (ERP) measure obtained during a computer task in which children received corrective feedback after having made a mistake. The task, a color version of the flanker, required children to inhibit a prepotent response. In contrast, intervention condition children showed a typical increase in electrical activity in the prefrontal region following corrective feedback (Bruce, McDermott, Fisher, & Fox, 2009). The specific ERP measure, called feedback-related negativity, has also been shown to be diminished in other clinical populations, including those with anxiety and depression.
These results are important because they provide clarity about a particular area of neurobehavioral deficiency in young foster children that has potential to affect their functioning in school and social settings, but that also appears to be malleable in the context of an intervention. By using new knowledge about narrowly focused domains of behavioral functioning and their underlying circuitry, interventions have the potential to become more precise and efficient and more effective. In the long run, this may address some of the criticisms of the family-based prevention field and allow for more readily scalable and effective interventions.
Clearly, more work in this area is needed, and has great potential to occur as understanding of the neurobiological effects of adversity and its relationship to subsequent outcomes continues to grow. In addition to evidence that early adversity causes alterations in stress hormone and executive functioning systems, there is evidence that it affects systems involved in monitoring threats (Tottenham & Sheridan, 2009) and in reward and motivation (Dillon et al., 2009). There is also evidence of early adversity effects on the connectivity among these different neural systems (Pollak, 2005), and it is likely that networks, rather than individual regions or even circuits, will ultimately provide the most effective underlying targets for interventions.
On the positive side, research continues to provide an increasingly acute understanding of the connection between classes of early adversity and alterations in underlying systems (Champagne, 2010). Studies are also examining how familial and extrafamilial variables, as well as intraindividual variables, protect individuals from adversity (McCrory, De Brito, & Viding, 2010). These models, if tied to intervention work, have the potential to move the field forward and make significant breakthroughs in improving the scientific knowledge base and the well-being of the most vulnerable individuals in our society.
Footnotes
The approach developed by Chamberlain and colleagues was originally (and in many prior publications) referred to as Multidimensional Treatment Foster Care, or MTFC, and was renamed and retrademarked as Treatment Foster Care Oregon (TFCO) in 2015.
TFCO-P was originally called Multidimensional Treatment Foster Care for Preschoolers, or MTFC-P, and was renamed and retrademarked as Treatment Foster Care Oregon for Preschoolers (TFCO-P) in 2015.
It is important to denote the two distinct measures of HPA axis function that are described in the literature. Researchers assess both the reactivity (i.e., activation and recovery) of the system in response to social stressors in the laboratory and the diurnal activity of the system via measurement of cortisol levels from awakening through bedtime. Most of the research on institutionally reared children, postinstitutional adoptees, and foster children has for practical and ethical reasons employed diurnal HPA axis activity measures.
References
- Bruce J, Fisher PA, Pears KC, Levine S. Morning cortisol levels in preschool-aged foster children: Differential effects of maltreatment type. Developmental Psychobiology. 2009;51(1):14–23. doi: 10.1002/dev.20333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruce J, McDermott JM, Fisher PA, Fox NA. Using behavioral and electrophysiological measures to assess the effects of a preventive intervention: A preliminary study with preschool-aged foster children. Prevention Science. 2009;10(2):129–140. doi: 10.1007/s11121-008-0115-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capaldi D, DeGarmo D, Patterson GR, Forgatch M. Contextual risk across the early life span and association with antisocial behavior. In: Reid JB, Patterson GR, Snyder J, editors. Antisocial behavior in children and adolescents: A developmental analysis and model for intervention. Washington, DC: American Psychological Association; 2002. pp. 123–145. [DOI] [Google Scholar]
- Carlson M, Earls F. Psychological and neuroendocrinological sequelae of early social deprivation in institutionalized children in Romania. Annals of the New York Academy of Sciences. 1997;807(1):419–428. doi: 10.1111/j.1749-6632.1997.tb51936.x. [DOI] [PubMed] [Google Scholar]
- Champagne FA. Early adversity and developmental outcomes interaction between genetics, epigenetics, and social experiences across the life span. Perspectives on Psychological Science. 2010;5(5):564–574. doi: 10.1177/1745691610383494. [DOI] [PubMed] [Google Scholar]
- Chiang JJ, Taylor SE, Bower JE. Early adversity, neural development, and inflammation. Developmental Psychobiology. 2015;57(8):887–907. doi: 10.1002/dev.21329. [DOI] [PubMed] [Google Scholar]
- Dillon DG, Holmes AJ, Birk JL, Brooks N, Lyons-Ruth K, Pizzagalli DA. Childhood adversity is associated with left basal ganglia dysfunction during reward anticipation in adulthood. Biological Psychiatry. 2009;66(3):206–213. doi: 10.1016/j.biopsych.2009.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eddy JM, Chamberlain P. Family management and deviant peer association as mediators of the impact of treatment condition on youth antisocial behavior. Journal of Consulting and Clinical Psychology. 2000;68(5):857–863. doi: 10.1037//0022-006X.68.5.857. [DOI] [PubMed] [Google Scholar]
- Fisher PA, Berkman ET. Designing interventions informed by scientific knowledge about effects of early adversity: A translational neuroscience agenda for next-generation addictions research. Current Addiction Reports. 2015 doi: 10.1007/s40429-015-0071-x. Advance online publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher PA, Burraston B, Pears K. The early intervention foster care program: Permanent placement outcomes from a randomized trial. Child Maltreatment. 2005;10(1):61–71. doi: 10.1177/1077559504271561. [DOI] [PubMed] [Google Scholar]
- Fisher PA, Ellis BH, Chamberlain P. Early intervention foster care: A model for preventing risk in young children who have been maltreated. Children’s Services: Social Policy, Research, and Practice. 1999;2(3):159–182. doi: 10.1207/s15326918cs0203_3. [DOI] [Google Scholar]
- Fisher PA, Gunnar MR, Dozier M, Bruce J, Pears KC. Effects of therapeutic interventions for foster children on behavioral problems, caregiver attachment, and stress regulatory neural systems. Annals of the New York Academy of Sciences. 2006;1094(1):215–225. doi: 10.1196/annals.1376.023. [DOI] [PubMed] [Google Scholar]
- Fisher PA, Kim HK. Intervention effects on foster preschoolers’ attachment-related behaviors from a randomized trial. Prevention Science. 2007;8(2):161–170. doi: 10.1007/s11121-007-0066-5. http://dx.doi.org/10.1007/s11121-007-0066-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher PA, Stoolmiller M. Intervention effects on foster parent stress: Associations with child cortisol levels. Development and Psychopathology. 2008;20(03):1003–1021. doi: 10.1017/S0954579408000473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher PA, Stoolmiller M, Gunnar MR, Burraston BO. Effects of a therapeutic intervention for foster preschoolers on diurnal cortisol activity. Psychoneuroendocrinology. 2007;32(8):892–905. doi: 10.1016/j.psyneuen.2007.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher PA, Stoolmiller M, Mannering AM, Takahashi A, Chamberlain P. Foster placement disruptions associated with problem behavior: Mitigating a threshold effect. Journal of Consulting and Clinical Psychology. 2011;79(4):481–487. doi: 10.1037/a0024313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher PA, Van Ryzin MJ, Gunnar MR. Mitigating HPA axis dysregulation associated with placement changes in foster care. Psychoneuroendocrinology. 2011;36(4):531–539. doi: 10.1016/j.psyneuen.2010.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunnar MR, Fisher PA the Early Experience, Stress and Prevention Network. Bringing basic research on early experience and stress neurobiology to bear on preventive intervention research on neglected and maltreated children. Development and Psychopathology. 2006;18:651–677. doi: 10.1017/S0954579406060330. [DOI] [PubMed] [Google Scholar]
- Gunnar MR, Vazquez DM. Low cortisol and a flattening of expected daytime rhythm: Potential indices of risk in human development. Development and Psychopathology. 2001;13(3):515–538. doi: 10.1017/S0954579401003066. [DOI] [PubMed] [Google Scholar]
- Heim C, Nemeroff CB. The role of childhood trauma in the neurobiology of mood and anxiety disorders: Preclinical and clinical studies. Biological Psychiatry. 2001;49(12):1023–1039. doi: 10.1016/S0006-3223(01)01157-X. [DOI] [PubMed] [Google Scholar]
- Heim C, Plotsky PM, Nemeroff CB. Importance of studying the contributions of early adverse experience to neurobiological findings in depression. Neuropsychopharmacology. 2004;29(4):641–648. doi: 10.1038/sj.npp.1300397. [DOI] [PubMed] [Google Scholar]
- Insel TR, Voon V, Nye JS, Brown VJ, Altevogt BM, Bullmore ET, … Marston HM. Innovative solutions to novel drug development in mental health. Neuroscience & Biobehavioral Reviews. 2013;37(10):2438–2444. doi: 10.1016/j.neubiorev.2013.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kazdin AE. Parent management training: Evidence, outcomes, and issues. Journal of the American Academy of Child & Adolescent Psychiatry. 1997;36(10):1349–1356. doi: 10.1097/00004583-199710000-00016. [DOI] [PubMed] [Google Scholar]
- Leve LD, Harold GT, Chamberlain P, Landsverk JA, Fisher PA, Vostanis P. Practitioner review: Children in foster care–vulnerabilities and evidence-based interventions that promote resilience processes. Journal of Child Psychology and Psychiatry. 2012;53(12):1197–1211. doi: 10.1111/j.1469-7610.2012.02594.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch FL, Dickerson JF, Saldana L, Fisher PA. Incremental net benefit of early intervention for preschool-aged children with emotional and behavioral problems in foster care. Children and Youth Services Review. 2014;36:213–219. doi: 10.1016/j.childyouth.2013.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCrory E, De Brito SA, Viding E. Research review: The neurobiology and genetics of maltreatment and adversity. Journal of Child Psychology and Psychiatry. 2010;51(10):1079–1095. doi: 10.1111/j.1469-7610.2010.02271.x. [DOI] [PubMed] [Google Scholar]
- Melamed S, Ugarten U, Shirom A, Kahana L, Lerman Y, Froom P. Chronic burnout, somatic arousal and elevated salivary cortisol levels. Journal of Psychosomatic Research. 1999;46(6):591–598. doi: 10.1016/S0022-3999(99)00007-0. [DOI] [PubMed] [Google Scholar]
- Patterson GR. Coercive family process. Vol. 3. Eugene, OR: Castalia; 1982. [Google Scholar]
- Patterson GR, Chamberlain P, Reid JB. A comparative evaluation of a parent-training program. Behavior Therapy. 1982;13(5):638–650. doi: 10.1016/S0005-7894(82)80021-X. [DOI] [PubMed] [Google Scholar]
- Patterson GR, DeBaryshe BD, Ramsey E. A developmental perspective on antisocial behavior. American Psychologist. 1989;44(2):329–335. doi: 10.1037/0003-066X.44.2.329. [DOI] [PubMed] [Google Scholar]
- Patterson GR, Reid JB, Dishion TJ. Antisocial boys: A social interactional approach. Eugene, OR: Castalia; 1992. [Google Scholar]
- Patterson GR, Reid JB, Eddy JM. A brief history of the Oregon model. In: Reid JB, Patterson GR, Snyder J, editors. Antisocial behavior in children and adolescents: A developmental analysis and model for intervention. Washington, DC: American Psychological Association; 2002. pp. 3–20. [DOI] [Google Scholar]
- Pechtel P, Pizzagalli DA. Effects of early life stress on cognitive and affective function: An integrated review of human literature. Psychopharmacology. 2011;214(1):55–70. doi: 10.1007/s00213-010-2009-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pine DS, Leibenluft E. Biomarkers with a mechanistic focus. JAMA Psychiatry. 2015;72(7):633–634. doi: 10.1001/jamapsychiatry.2015.0498. [DOI] [PubMed] [Google Scholar]
- Pollak SD. Early adversity and mechanisms of plasticity: Integrating affective neuroscience with developmental approaches to psychopathology. Development and Psychopathology. 2005;17(3):735–752. doi: 10.1017/S0954579405050352. [DOI] [PubMed] [Google Scholar]
- Shonkoff JP, Fisher PA. Rethinking evidence-based practice and two-generation programs to create the future of early childhood policy. Development and Psychopathology. 2013;25(4pt2):1635–1653. doi: 10.1017/S0954579413000813. doi:S0954579413000813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shonkoff JP, Garner AS, Siegel BS, Dobbins MI, Earls MF, McGuinn L, … Wood DL. The lifelong effects of early childhood adversity and toxic stress. Pediatrics. 2012;129(1):e232–e246. doi: 10.1542/peds.2011-2663. [DOI] [PubMed] [Google Scholar]
- Tottenham N, Sheridan MA. A review of adversity, the amygdala and the hippocampus: A consideration of developmental timing. Frontiers in Human Neuroscience. 2009;3:68. doi: 10.3389/neuro.09.068.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyrka AR, Burgers DE, Philip NS, Price LH, Carpenter LL. The neurobiological correlates of childhood adversity and implications for treatment. Acta Psychiatrica Scandinavica. 2013;128(6):434–447. doi: 10.1111/acps.12143. [DOI] [PMC free article] [PubMed] [Google Scholar]