Abstract
Background and objectives
Patients with chronic kidney disease (CKD) are often volume expanded and hypertensive. Few controlled studies have assessed the effects of a sodium-restricted diet (SRD) in CKD.
Design, setting, participants, & measurements
We conducted a randomized crossover trial to evaluate the effect of SRD (target <2 g sodium per day) versus usual diet on hydration status (by bioelectrical impedance spectroscopy) and blood pressure (BP) between May of 2009 and May of 2013. A total of 58 adults with stage 3–4 CKD were enrolled from two academic sites: University of Michigan (n=37) and University of North Carolina at Chapel Hill (n=21); 60% were men, 43% were diabetic, 93% were hypertensive, and mean age was 61 years. Participants followed SRD or usual diet for 4 weeks, followed by a 2-week washout period and a 4-week crossover phase. During the SRD, dieticians provided counseling every 2 weeks, using motivational interviewing techniques.
Results
Whole-body extracellular volume and calf intracellular volume decreased by 1.02 L (95% confidence interval [95% CI], −1.48 to −0.56; P<0.001) and −0.06 L (95% CI, −0.12 to −0.01; P=0.02), respectively, implying decreased fluid content on the SRD compared with usual diet. Significant reductions in urinary sodium (−57.3 mEq/24 h; 95% CI, −81.8 to −32.9), weight (−2.3 kg; 95% CI, −3.2 to −1.5), and 24-hour systolic BP (−10.8 mmHg; 95% CI, −17.0 to −4.6) were also observed (all P<0.01). Albumin-to-creatinine ratio did not change significantly and mean serum creatinine increased slightly (0.1 mg/dl; 95% CI, −0.01 to 0.2; P=0.06). No period or carryover effects were observed. Results were similar when analyzed from phase 1 only before crossover, although P values were modestly larger because of the loss of power.
Conclusions
In this randomized crossover trial, implementation of SRD in patients with CKD stage 3–4 resulted in clinically and statistically significant improvement in BP and hydration status. This simple dietary intervention merits a larger trial in CKD to evaluate effects on major clinical outcomes.
Keywords: salt sensitivity, crossover design, ambulatory blood pressure monitoring, bioelectrical impedance, motivational interviewing
Introduction
CKD is characterized by impaired volume homeostasis and frequently associated with hypertension (1). Hypertension is considered both a cause and consequence of CKD, and patients with CKD may have a “salt-sensitive” blood pressure (BP) phenotype (1–6). Many patients with stage 3–5 CKD have expanded extracellular volume (ECV), which may contribute to the excess cardiovascular risk associated with high sodium intake (7–10). Although prior studies have documented the efficacy of dietary sodium restriction in reducing BP in the general hypertensive population (2,11), few have explored this in the CKD population. Data from animal models and observational human studies suggest that dietary sodium restriction may slow the progression of intrinsic renal disease and its surrogate markers, such as albuminuria (3,12). On the basis of these observations, the Kidney Disease Outcome Quality Initiative clinical practice guidelines on hypertension and antihypertensive agents in CKD recommend restricting dietary sodium intake to <2.3 g/d (13). More recently, the 2012 Kidney Disease Improving Global Outcomes guidelines for management of BP in patients with CKD recommended lowering sodium intake to <2 g/d (14).
Challenges to implementing this strategy include the high sodium content of processed and restaurant foods, as well as patient-specific barriers related to knowledge, skills, and attitudes regarding sodium-restricted diets (SRD). Difficulties in assessing the impact of SRD include accurate tracking of intake and insensitivity of physical examination to detect mild to moderate volume expansion (15). As a result of these challenges and a lack of substantial clinical trial data, formal SRDs remain an underutilized approach in clinical practice (6,16,17).
We performed a randomized crossover trial of an SRD versus usual diet in patients with stage 3–4 CKD, postulating that SRD would reduce ECV, BP, and albuminuria. The study was conducted at two United States academic centers: the University of Michigan (UM; Ann Arbor, MI) and the University of North Carolina at Chapel Hill (UNC; Chapel Hill, NC).
Materials and Methods
Study Participants
Adults with eGFR 15–60 ml/min per 1.73 m2 (Modification of Diet in Renal Disease equation), without history of acute illness or hospitalization within the past 2 months, were eligible for participation. Exclusion criteria included sitting systolic BP (SBP) <100 mmHg, baseline 24-hour urinary sodium excretion ≤2.3 g/d, pregnancy or lactation, CKD related to salt-wasting diseases, history of persistent nonadherence, atrial fibrillation (to avoid inaccuracies in ambulatory BP [ABP] recording) or metal foreign objects in the body, including coronary stents or metal sutures, or limb amputation (to avoid potential interference with bioelectrical impedance measurements). The institutional review boards at both participating sites approved the study protocol, and all study participants gave written informed consent. This study adhered to the principles of the Declaration of Helsinki and is registered on Clinicaltrials.gov (identifier: NCT00974636).
Design/Intervention
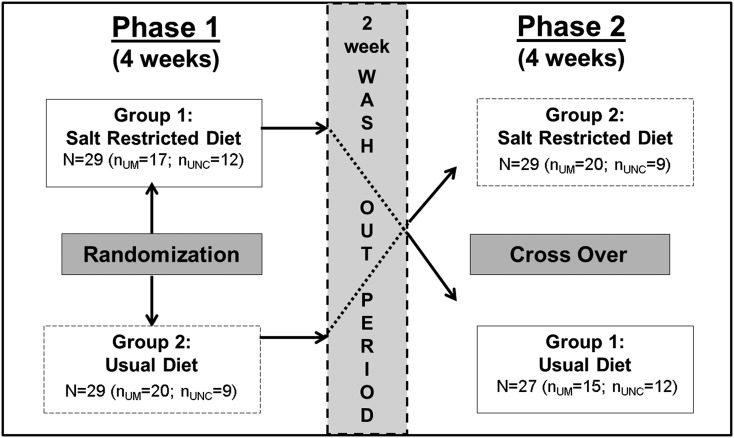
The study was a randomized, two-period crossover trial of SRD versus usual diet conducted between May of 2009 and May of 2013. Patients were randomized to consume <2 g sodium per day or usual diet for 4 weeks, followed by a 2-week washout period and a second 4-week crossover phase (Figure 1). Randomization was performed using sealed envelopes at each site. Study investigators were blinded to assigned treatment, which was only known to study dieticians and coordinators. The 2 g dietary sodium level was based on recommendations by the Seventh Joint National Committee (18) and the 2007 European Society of Hypertension guidelines for hypertensive individuals (19).
Figure 1.
The experimental design is a randomized two-treatment two-period crossover design. Number of patients in each arm at each site during phase 1 (NUM=37, NUNC=21) and phase 2 (NUM=35, NUNC=21). At University of Michigan (UM), two patients withdrew in phase 1 because of illness unrelated to the intervention, and another withdrew because of competing priorities in phase 2 (n=34 completed). UNC, University of North Carolina at Chapel Hill.
At screening visit, baseline visit, week 2, and week 4 of each phase, patients received in-person dietary counseling by a registered study dietitian with training in motivational interviewing techniques (MIT) (20). Counseling time and depth of topic discussion was individualized. Basic informational materials were standardized and patients were provided source web links. Additional details regarding the intervention using MIT are presented according to the Template for Intervention Description and Replication framework checklist in Supplemental Table 1. Study participants were advised to maintain isocaloric diets and stable intake of total and saturated fat, potassium intake of 2–3 g/d, and phosphorus intake of ≤1 g/d during both study phases. They were instructed to maintain their usual levels of alcohol, caffeine, and nicotine, and continue their usual level of physical activity. Throughout the SRD phase, MIT was implemented with the goal of lessening the subject’s resistance to behavior change and empowering lower sodium choices. Three-day food diaries were collected at baseline and week 2 of each phase to assist dieticians with compliance monitoring and tailored counseling. Participants received phone calls at weeks 1 and 3 of each phase to maintain motivation, assess dietary compliance, and offer counseling.
Measurements and Outcomes
Comorbidities and medications were recorded at baseline, with changes in medications collected throughout the study. Clinical and laboratory measurements were made at baseline, week 2, and week 4 in each phase. Physical exams were conducted at baseline and week 4 during each phase.
The primary outcome was change in hydration status (intracellular volume [ICV], ECV, and total body water) as measured by bioelectrical impedance spectroscopy (BIS). Secondary outcomes included change in body weight, ABP, and albuminuria. Adherence to SRD was assessed by 24-hour urinary sodium excretion. Safety outcomes included development of hypotension defined by SBP<100 mmHg and/or antihypertensive medications reduced or discontinued because of dizziness, falls, or syncope.
BIS.
BIS measurements were carried out using a modified spectrum device and a switch box (Xitron 4200; Xitron Technologies, San Diego, CA) and included both segmental (arm, trunk, leg, and calf) and whole-body measurements. Study personnel were trained by experienced clinical researchers from the Renal Research Institute, New York, NY. Whole-body and calf ECV and ICV were calculated using the obtained BIS measures and Xitron software (21). Calf-normalized resistivity was calculated using 5 kHz calf resistivity and calf circumference (22).
BP Measurements.
Brachial BP and heart rate (three measurements at 2-minute intervals) were obtained at each visit in both supine and standing positions. 24-hour ABP was obtained at baseline and week 4 of each phase. Participants were provided an ABP monitor (SunTech Oscar 2; SunTech Medical, Morrisville, NC) and an appropriately sized arm cuff for the nondominant arm; accuracy was confirmed against manually obtained BP before leaving the clinic. ABP values were recorded every 30 minutes during daytime hours and every 60 minutes from 12:00 a.m. to 6:00 a.m. ABP recordings with fewer than ten daytime and/or five night time recordings were excluded from analysis (23).
Statistical Analyses
Tests for the fixed effects of treatment, period, and sequence were performed using linear mixed models with a random intercept for each subject. This method allowed inclusion of participants with missing data for one phase, which occurred for between two and nine persons, depending on the outcome. Equality of variance in the two groups and normality of residual errors were checked; tests for carryover effects were made. Sensitivity analyses included models restricted to participants with complete data and models including only phase 1 data. Skewed variables were natural log transformed and a P value <0.05 was considered significant. All analyses were conducted using SAS, version 9.3 (SAS Institute Inc., Cary, NC).
Results
Patients
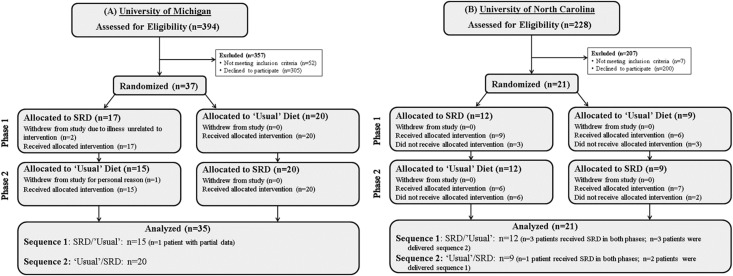
Figure 2 displays the Consolidated Standards of Reporting Trials flow diagram for each site. Of the 21 patients enrolled at UNC, six were given treatment in the reverse sequence from the randomized assignment for unknown reasons. In addition, four patients who were initially randomized to the SRD in phase 1 had poor compliance and, in violation of the protocol, were encouraged to continue the SRD in phase 2. Despite these protocol deviations, an intention-to-treat analysis was conducted and results are presented overall and separately for each site. Figure 1 depicts the study design and patient randomization at each site (N=58). Patient characteristics at enrollment are displayed in Table 1, by study site and treatment group (Supplemental Table 2).
Figure 2.
This diagram shows the patient flow through each phase of the randomized crossover trial. The study was conducted at the University of Michigan (A) and the University of North Carolina (B), following the guidelines of the Consolidated Standards of Reporting Trials. Of the 58 patients randomized, 29 (50%) were allocated to sodium-restricted diet (SRD) and 29 to usual diet in phase 1. The target sample size of 60 was on the basis of having 80% power to detect an effect size of 0.74 using a two-sample t test with 0.05, two-sided significance level.
Table 1.
Baseline descriptive statistics by study site and treatment group (n=58)
| Variable | University of Michigan | University of North Carolina at Chapel Hill | ||
|---|---|---|---|---|
| Group 1, n=17 | Group 2, n=20 | Group 1, n=12 | Group 2, n=9 | |
| Phase 1 diet | Salt restricted | Usual | Salt restricted | Usual |
| Demographics/anthropometrics | ||||
| Age, yr | 56.2±14.0 | 64.0±11.4 | 56.9±14.7 | 67.0±8.6 |
| Men | 47.1% (8) | 75.0% (15) | 58.3% (7) | 55.6% (5) |
| Black | 11.8% (2) | 15.0% (3) | 66.7% (8) | 44.4% (4) |
| Body mass index, kg/m2 | 33.7±6.6 | 31.8±4.8 | 29.8±5.1 | 33.1±6.8 |
| CKD stage | ||||
| 3a, eGFR≥45 ml/min per 1.73 m2 | 29.4% (5) | 40.0% (8) | 8.3% (1) | 33.3% (3) |
| 3b, eGFR<45 to ≤30 ml/min per 1.73 m2 | 35.3% (6) | 25.0% (5) | 50.0% (6) | 66.7% (6) |
| 4, eGFR<30 to ≤15 ml/min per 1.73 m2 | 35.3% (6) | 35.0% (7) | 41.7% (5) | 0.0% (0) |
| Cause of CKD | ||||
| Diabetes | 17.6% (3) | 40.0% (8) | 8.3% (1) | 11.1% (1) |
| Hypertension | 35.3% (6) | 45.0% (9) | 25.0% (3) | 33.3% (3) |
| Other | 29.4% (5) | 25.0% (5) | 50.0% (6) | 33.3% (3) |
| Unknown | 11.8% (2) | 10.0% (2) | 33.3% (4) | 0.0% (0) |
| Comorbidities | ||||
| Diabetes | 41.2% (7) | 45.0% (9) | 33.3% (4) | 55.6% (5) |
| Hypertension | 94.1% (16) | 95.0% (25) | 91.7% (11) | 88.9% (8) |
| Cardiovascular disease | 23.5% (4) | 20.0% (4) | 16.7% (2) | 44.4% (4) |
| Cancer | 17.6% (3) | 40.0% (8) | 0.0% (0) | 22.2% (2) |
| Gastrointestinal disorders | 11.8% (2) | 0.0% (0) | 33.3% (4) | 44.4% (4) |
| Hyperlipidemia | 35.3% (6) | 50.0% (10) | 41.7% (5) | 77.8% (7) |
| Average systolic BP (SBP) | ||||
| In-center standing SBP, mm/Hg | 128.4±17.1 | 137.9±17.0 | 129.8±18.8 | 125.1±11.5 |
| In-center supine SBP, mm/Hg | 129.9±14.6 | 139.5±19.1 | 130.8±19.8 | 126.1±7.6 |
| 24-hour ambulatory SBP, mm/Hg | 142.7±21.8 | 142.3±13.0 | 133.4±13.8 | 128.6±12.3 |
| 24-hour ambulatory heart rate, bpm | 73.3±12.1 | 68.8±8.8 | 72.7±9.1 | 72.9±10.2 |
| Volume statusa | ||||
| Edema (yes/no) | 29.4% (5) | 50.0% (10) | 16.7% (2) | 33.3% (3) |
| Clinically euvolemic (yes/no) | 88.2% (15) | 65.0% (13) | 83.3% (10) | 87.5% (7) |
| Clinically hypervolemic (yes/no) | 11.8% (2) | 35.0% (7) | 8.3% (1) | 12.5% (1) |
| Laboratory measurements (serum) | ||||
| Creatinine, mg/dl | 2.0±0.8 | 1.8±0.6 | 2.1±0.5 | 1.6±0.3 |
| eGFR, ml/min per 1.73 m2 | 36.4±11.9 | 40.5±13.6 | 34.5±9.3 | 43.4±8.2 |
| Sodium, mEq/L | 139.2±3.8 | 140.2±2.4 | 140.7±3.3 | 141.3±2.6 |
| Potassium, mEq/L | 4.5±0.6 | 4.5±0.6 | 4.7±0.7 | 4.5±0.5 |
| Carbon dioxide, mEq/L | 27.2±2.6 | 28.0±2.6 | 24.4±3.3 | 27.4±3.4 |
| Glucose, mg/dlb | 108 (90, 137) | 105.0 (88.5, 145.5) | 101.5 (80.5, 127) | 149 (107, 202.8) |
| Laboratory measurements (urine) | ||||
| Albumin excretion, mg/24 hb | 69.2 (17.3, 442.1) | 116.5 (12.8, 720.5) | 143.5 (44.4, 200.8) | 42.8 (32.5, 102.3) |
| Potassium, mmol/24 h | 60.6±24.9 | 59.2±23.4 | 69.0±37.5 | 73.7±25.5 |
| Creatinine, g/24 h | 1.3±0.4 | 1.5±0.3 | 1.7±0.6 | 1.5±0.4 |
| Sodium, mEq/24 h | 169.9±39.9 | 167.3±48.1 | 180.8±51.0 | 167.6±55.9 |
| Albumin-to-creatinine ratio, mg/gb | 41.1 (12.0, 290.1) | 107.7 (14.2, 447.8) | 80.1 (13.9, 805.7) | 18.5 (11.1, 70.8) |
Continuous variables are reported as mean±SD for normally distributed variables and as median (25th, 75th percentiles) for skewed variables. Categorical variables are reported as % (n).
Volume status was evaluated by physical at initial evaluation. Patients were deemed clinically euvolemic if there was no jugular venous pressure elevation, pulmonary rales, and peripheral and/or sacral edema.
Median (25th, 75th percentiles) reported for skewed variables.
Adherence to SRD
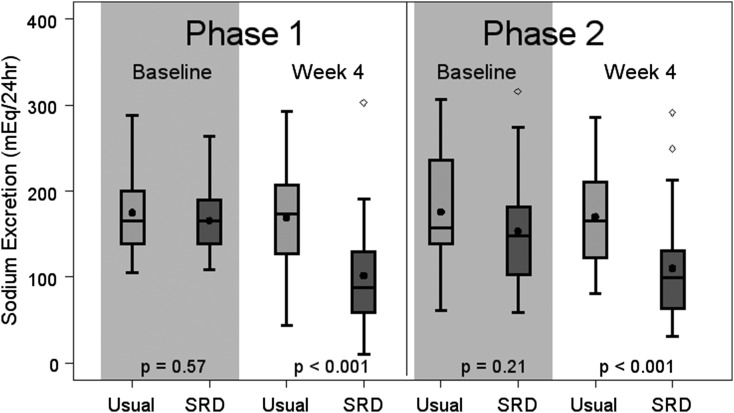
Compared with the usual diet phase, the mean reduction in urinary sodium excretion was 57.3 mEq/24 h (95% confidence interval [95% CI], −81.8 to −32.9; P<0.001; Supplemental Table 3). In 79% of participants, dietary sodium was reduced during the SRD phase; 65% of patients reduced their intake by >20%. Baseline 24-hour sodium excretion did not significantly differ in either phase (Figure 3), suggesting that the washout period was effective, further supported by the absence of significant carryover effects in this crossover trial. Notably, urinary sodium reduction during the SRD phase varied by study site (Supplemental Table 3); at UM, the mean reduction was 77.9 mEq/24 h (95% CI, −108.5 to −7.3; P<0.001) while at UNC, the mean reduction was 22.4 mEq/24 h (95% CI, −63.9 to 19.1; P=0.27).
Figure 3.
Sodium excretion at baseline is similar between treatment groups in both phases, but at Week 4 is lower in the sodium-restricted diet (SRD) than in usual diet in both phases (n=58). The length of the box defines the interquartile range (IQR). Medians are represented by horizontal lines and means by black dots. Outliers (values more than 1.5 times the IQR from either end of the box) are represented by circles. P value <0.05 indicates difference in 24-hour sodium excretion between treatment groups.
Primary Outcome: Change in Hydration Status
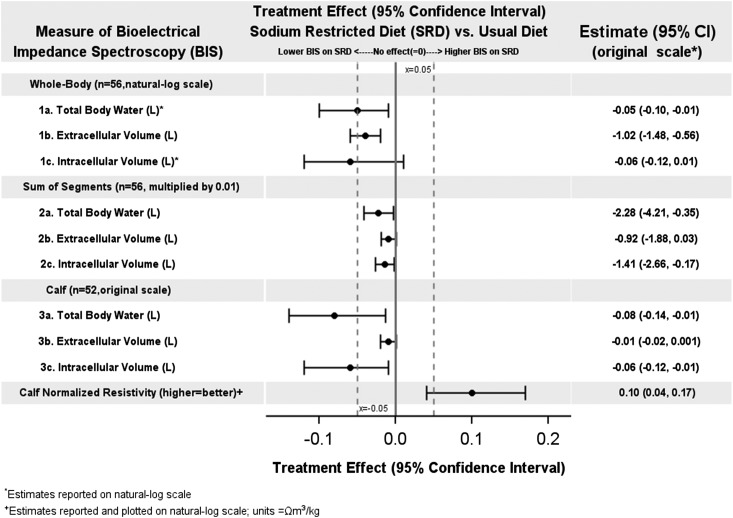
Table 2 shows BIS measurements for all patients at enrollment for each study site and treatment group. BIS measurements for all patients in each randomization group at the beginning and end of each phase are reported in Supplemental Table 2. Figure 4 displays results for whole-body, sum of segments, and calf measures of BIS for the combined group. Results for all measures of BIS for both the combined study and each site are reported in Supplemental Table 3. On average, during the SRD phase participants experienced a mean 1.02 L (95% CI, −1.48 to −0.56; P<0.001) reduction in whole-body ECV and 0.06 L (95% CI, −0.12 to −0.01; P=0.02) reduction in calf ICV. At UM, the SRD was associated with a mean 1.48 L reduction in whole-body ECV (95% CI, −2.10 to −0.85; P<0.001), a 0.08 L reduction in calf ICV (95% CI, −0.15 to −0.01; P=0.04), and a 0.08 L reduction in whole-body total body water (95% CI, −0.15 to −0.01; P=0.02). At UNC, significant changes in whole-body and calf measures of BIS were not observed during SRD.
Table 2.
Baseline bioelectrical impedance spectroscopy (BIS) measurements by study site and treatment group (n=58)
| University of Michigan | University of North Carolina at Chapel Hill | |||
|---|---|---|---|---|
| Measurement of BIS | Group 1, n=17 | Group 2, n=20 | Group 1, n=12 | Group 2, n=9 |
| Phase 1 diet | Salt restricted | Usual | Salt restricted | Usual |
| Calf BIS | ||||
| Total body water (TBW), L | 0.65±0.17 | 0.72±0.20 | 0.68±0.17 | 0.62±0.18 |
| Extracellular volume (ECV), L | 0.18±0.04 | 0.20±0.08 | 0.17±0.03 | 0.16±0.03 |
| Intracellular volume (ICV), L | 0.47±0.15 | 0.52±0.15 | 0.51±0.15 | 0.46±0.19 |
| ECV/ICV | 0.41±0.11 | 0.40±0.11 | 0.35±0.10 | 0.43±0.25 |
| ECV/TBW | 0.29±0.05 | 0.28±0.05 | 0.26±0.05 | 0.28±0.12 |
| ECV/weight, 100×L/kg | 0.19±0.04 | 0.20±0.05 | 0.19±0.03 | 0.18±0.05 |
| Calf standardized resistivity, Ω·m3/kg | 0.14±0.04 | 0.14±0.04 | 0.17±0.03 | 0.14±0.06 |
| Sum of segment BIS | ||||
| TBW, L | 49.1±12.0 | 51.5±8.9 | 45.5±10.41 | 40.9±7.3 |
| ECV, L | 21.7±5.7 | 23.6±5.0 | 21.4±4.6 | 20.9±3.2 |
| ICV, L | 27.4±6.7 | 28.0±4.6 | 24.2±6.2 | 20.0±4.6 |
| ECV/ICV | 0.80±0.10 | 0.85±0.12 | 0.90±0.13 | 1.23±0.58 |
| ECV/TBW | 0.44±0.03 | 0.46±0.04 | 0.47±0.04 | 0.51±0.04 |
| ECV/weight, L/kg | 0.23±0.04 | 0.24±0.03 | 0.24±0.03 | 0.23±0.03 |
| Whole-body BIS | ||||
| TBW, L | 48.7±15.4 | 47.9±9.3 | 45.1±10.5 | 39.4±9.2 |
| ECV, L | 20.2±5.6 | 21.3±4.4 | 19.3±4.2 | 18.1±3.6 |
| ICV, L | 28.5±10.8 | 26.6±5.4 | 25.9±6.5 | 21.3±5.9 |
| ECV/ICV | 0.74±0.12 | 0.81±0.10 | 0.75±0.07 | 0.87±0.16 |
| ECV/TBW | 0.42±0.04 | 0.45±0.03 | 0.43±0.02 | 0.46±0.04 |
| ECV/weight, L/kg | 0.21±0.03 | 0.22±0.02 | 0.22±0.03 | 0.20±0.03 |
Mean±SDs are reported.
Figure 4.
Treatment effects (means and 95% confidence intervals [95% CIs]) for selected bioelectrical impedance spectroscopy (BIS) measurements were more favorable for those on the sodium-restricted versus usual diet. Values are plotted on different scales; results are reported on original scale. Results for all BIS measurements may be found in Supplemental Table 3.
Secondary Outcomes
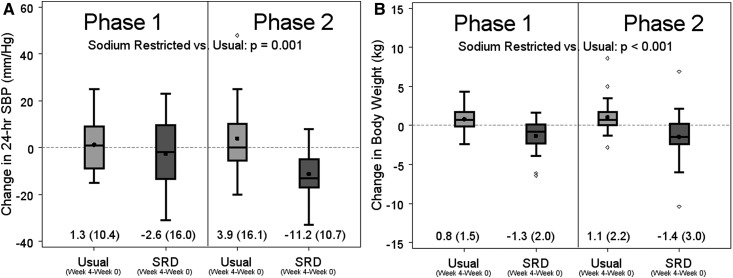
Supplemental Table 3 presents results for all secondary outcomes overall and by study site. Compared with usual diet, participants in the SRD phase lost an average of 2.3 kg (95% CI, −3.2 to −1.5; P<0.001) and experienced a mean decrease in 24-hour systolic ABP of 10.8 mmHg (95% CI, −17.0 to −4.6; P=0.001; Figure 5). During the SRD phase, significant reductions in body weight (3.4 kg; 95% CI, −4.5 to −2.3; P<0.001) and 24-hour systolic ABP (17.9 mmHg; 95% CI, −26.8 to −9.0; P<0.001) were observed at UM, but not at UNC.
Figure 5.
Compared to baseline in each phase, the sodium-restricted diet (SRD) group had significantly lower 24-hour systolic blood pressure (SBP) and significantly lower body weight than the usual diet group. Box plots of (A) change in 24-hour systolic BP (SBP), and (B) change in body weight in the combined study. The length of the box defines the interquartile range (IQR). Medians are represented by horizontal lines and means by black dots. Outliers (values more than 1.5 times the IQR from either end of the box) are represented by circles. Mean (SD) displayed.
At enrollment, 48 patients were taking at least one antihypertensive medication, with 77% taking two or more and 48% taking three or more. During the study, 12 patients discontinued 27 medications; eight patients discontinued 15 medications during the SRD phase compared with six patients (and 12 medications) during the usual diet phase. The majority (75%) of the changes during the usual diet phase occurred during phase 2, after the SRD phase.
For both primary and secondary outcomes, results did not change when the analyses were restricted to participants with complete data, or when baseline weight was included in the model (data not shown). When the analyses were conducted using data before crossover only (i.e., phase 1 only), effect sizes were similar although P values were modestly larger because of the loss of power (Supplemental Table 4).
Safety Outcomes
In two patients, clinic SBP was <100 mmHg while following usual diet. No patients received medical treatment for symptomatic hypotension, falls, or syncope. On average, serum creatinine (SCR) rose 0.1 mg/dl (P=0.06) corresponding to a nonsignificant drop in eGFR (P=0.33) during the SRD phase. At UM, average SCR rose by 0.3 mg/dl (P<0.01) and eGFR decreased by 3.3 ml/min per 1.73 m2 (P=0.02). At UNC however, SCR decreased significantly by 0.1 mg/dl (P=0.04) along with a marginal increase in eGFR (P=0.05).
Discussion
Optimal BP control remains a cornerstone of CKD management. In this randomized crossover trial, we have demonstrated the feasibility and efficacy of dietary sodium restriction using motivational interviewing without invoking preprepared meals, in effecting meaningful improvements in both hydration status and BP among patients with CKD. The intervention was well tolerated and no clinically significant adverse outcomes were observed.
In the SRD arm, the average body weight loss was 2.3 kg, whereas the reduction in ECV was estimated to be a liter (equivalent to 1.0 kg). In the short term, reductions in weight and ECV are most likely because of water loss. However, as a result of repeated encounters with study dieticians, participants may have more generally modified their dietary habits (e.g., reducing calorie or fat intake, increasing fruits and vegetables) and/or increased physical activity during the course of the trial. We speculate that these factors could be responsible for the discrepancy between weight loss and estimated ECV reduction and may have additionally contributed to the ABP reduction in the SRD arm.
The intervention proved safe, although a slight increase in SCR was observed. This effect was more pronounced at the site where SRD adherence was greater. With decreases in ECV and BP, the slight rise in SCR could relate to small reductions in intraglomerular pressure, analogous to the slight rise in SCR after angiotensin-converting enzyme inhibitor initiation.
There have been relatively few controlled studies examining the role of SRD in CKD. In a nonrandomized study of 43 Chinese patients with IgA nephropathy, SRD<100 mmol/d for 7 days resulted in mean SBP reduction of 11.1 mmHg compared with 5.0 mmHg reduction in controls (P=0.02), a change that correlated with decreases in urinary sodium excretion (24). No ABP or fluid status measurements were performed. A randomized controlled trial of SRD in 56 South Asian patients reported significant decreases in urinary sodium excretion and reduction in SBP by 8 mmHg compared with controls (P<0.001) (25). The interventions in this study (including cooking classes) were culturally tailored to a population with high dietary sodium intake. A randomized crossover trial of SRD in 20 Australian patients, demonstrated a mean SBP reduction of 10 mmHg compared with controls, comparable to our findings (6). Methodological similarities with our trial include use of BIS and 24-hour ABP measurements. However, their study included a shorter (2-week) intervention period, provision of food as part of the study protocol, and use of salt tablets to create a high sodium intake group on a background of previously attained low sodium consumption. In contrast, by utilizing the regular diet of our study participants as a control and focusing solely on relatively frequent MIT-based dietary counseling, our findings extend the results of the above studies by demonstrating the feasibility and benefits of sodium restriction.
Two previous studies have suggested that SRD can potentiate the effects of the renin-angiotensin-aldosterone system blockade and decrease proteinuria as well as BP (26,27). We did not observe a statistically significant decrease in albuminuria after SRD. Notably, our population had remarkably lower baseline proteinuria compared with the patients in those studies (where mean protein excretion was 3.7 and 3.8 g/d respectively) (26,27). However, a decrease in proteinuria was also seen in two other SRD studies with more comparable baseline degrees of proteinuria (6,24). In contrast to our study, one study enrolled only patients with IgA nephropathy and the other reported low usage of renin-angiotensin-aldosterone system blockade. Further study is needed to clarify potential benefits of SRD in reducing proteinuria.
Hypertension in CKD is often resistant to multiple medications (28), and typically associated with ECV expansion (29). BP lowering is a major therapeutic goal, as uncontrolled ABP triples long-term risk of ESRD and major cardiovascular events (30). The beneficial effects may go beyond reduction in BP, as SRD can reduce arterial stiffness and left ventricular diastolic dysfunction (9,10). Emerging data indicate that venous congestion can cause endothelial oxidative stress and release of inflammatory mediators, which may contribute to cardiovascular risk (31). Our study demonstrating reductions in both ABP and ECV supports SRD as an attractive method to reduce cardio-renal risk in CKD. Further, we report on the use of calf-normalized resistivity. This somewhat novel approach to measuring segmental bioimpedance has been used as an aid to achieving postdialysis target weight in the hemodialysis population, but requires further validation before generalized use in the CKD population (32).
An important aspect of any lifestyle modification intervention is long-term sustainability. The Dietary Approaches to Stop Hypertension–sodium study demonstrated that lowering dietary sodium results in decreases in BP in patients without CKD, (33). In that trial, participants were provided all food to ensure adherence to prescribed sodium intake, raising questions of generalizability. Indeed, subsequent studies without food provision have noted varying degrees of adherence to prescribed SRD (34). At the UM site, we found that using an individualized approach and MIT provided by trained dieticians resulted in excellent compliance with SRD. This was accomplished through ongoing coaching and reinforcement but without any food provision or direct observation. In general, participants reported that sodium restriction became easier over the 4-week intervention phase, and no patients withdrew from the study for discomfort related to following the dietary advice. Conversely, urinary sodium excretion did not decrease in the UNC cohort, and changes in BP and whole-body BIS measures were modest and inconsistent compared with the UM cohort. These results illustrate the potential challenges with the approach and the importance of SRD adherence in patients with CKD. Additionally, the fact that sodium excretion after SRD returned to prestudy levels during the washout period for most patients suggests that ongoing support may be necessary.
Our study has important strengths, including the crossover design, the use of objective measurements for hydration status and BP, and application of interventions in a more real-world, albeit clinical trial setting. Some limitations are also notable, specifically, violation in randomization procedure at one site, which both diminished power for overall statistical comparisons and may have been the cause of different effect magnitudes in site-specific analyses. In particular, the benefits of SRD with respect to hydration status (1.48 L reduction in ECV), weight (3.4 kg loss), and systolic ABP reduction (17.9 mmHg) are more compelling when the randomized single-center results were examined (UM; n=35). We were not able to collect complete 24 hours of ABP readings at all the time points stipulated in all participants; however, our results were consistent in both the overall pool with ABP readings and in the subset with full 24-hour ABP measurements. We have not tested the specific effects of changes (if any) of dietary components other than sodium (e.g., calorie, macro or micronutrients) on BP or fluid/hydration status. The authors were somewhat surprised by the absence of proteinuria reduction during SRD. We speculate that this may be because a majority of patients were receiving renin-angiotensin-aldosterone system inhibitors along with other antihypertensives, and their baseline proteinuria was modest. A larger or longer trial may be necessary to further investigate this somewhat unexpected finding.
In summary, by encouraging sodium restriction through MIT-based dietary counseling, we observed significant reductions in ECV and ABP in patients with stage 3–4 CKD. We did not utilize preprepared low sodium meals. Our findings build on the results of recent trials of SRD by extending generalizability and feasibility beyond constrained clinical trial protocols. Sodium restriction represents a significant opportunity to improve outcomes of morbidity, mortality, and quality of life among patients with CKD. Larger trials are warranted with this approach, using BIS measurements as recommended for cardiorenal syndromes (35), and with long-term measures of CKD progression. These measures would include effects on eGFR, proteinuria, cardiovascular structure and function, and most importantly, the potential for reduction in mortality or hospitalization (36).
Disclosures
N.W.L. and P. Kotanko hold stock in Fresenius Medical Care, Waltham, Massachusetts.
Supplementary Material
Acknowledgments
We are grateful to all study coordinators and dieticians and participating patients.
This publication was funded by a grant from the Renal Research Institute and made possible by grant UL1TR000433 from the National Center for Research Resources (NCRR), a component of the National Institutes of Health (NIH), and NIH Roadmap for Medical Research. S.L.H. is supported by the National Heart, Lung, and Blood Institute (NHLBI) grant K23-109176.
This publication's contents are solely the responsibility of the authors and do not necessarily represent the official views of NCRR, NIH, or NHLBI.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
This article contains supplemental material online at http://cjasn.asnjournals.org/lookup/suppl/doi:10.2215/CJN.01120216/-/DCSupplemental.
References
- 1.Horowitz B, Miskulin D, Zager P: Epidemiology of hypertension in CKD. Adv Chronic Kidney Dis 22: 88–95, 2015 [DOI] [PubMed] [Google Scholar]
- 2.Cook NR, Cutler JA, Obarzanek E, Buring JE, Rexrode KM, Kumanyika SK, Appel LJ, Whelton PK: Long term effects of dietary sodium reduction on cardiovascular disease outcomes: Observational follow-up of the trials of hypertension prevention (TOHP). BMJ 334: 885–888, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Heerspink HL, Ritz E: Sodium chloride intake: Is lower always better? J Am Soc Nephrol 23: 1136–1139, 2012 [DOI] [PubMed] [Google Scholar]
- 4.Townsend RR, Taler SJ: Management of hypertension in chronic kidney disease. Nat Rev Nephrol 11: 555–563, 2015 [DOI] [PubMed] [Google Scholar]
- 5.Sachdeva A, Weder AB: Nocturnal sodium excretion, blood pressure dipping, and sodium sensitivity. Hypertension 48: 527–533, 2006 [DOI] [PubMed] [Google Scholar]
- 6.McMahon EJ, Bauer JD, Hawley CM, Isbel NM, Stowasser M, Johnson DW, Campbell KL: A randomized trial of dietary sodium restriction in CKD. J Am Soc Nephrol 24: 2096–2103, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Akdam H, Öğünç H, Alp A, Özbek Ö, Ömürlü IK, Yeniçerioğlu Y, Akar H: Assessment of volume status and arterial stiffness in chronic kidney disease. Ren Fail 36: 28–34, 2014 [DOI] [PubMed] [Google Scholar]
- 8.Hung SC, Kuo KL, Peng CH, Wu CH, Lien YC, Wang YC, Tarng DC: Volume overload correlates with cardiovascular risk factors in patients with chronic kidney disease. Kidney Int 85: 703–709, 2014 [DOI] [PubMed] [Google Scholar]
- 9.Hummel SL, Seymour EM, Brook RD, Sheth SS, Ghosh E, Zhu S, Weder AB, Kovács SJ, Kolias TJ: Low-sodium DASH diet improves diastolic function and ventricular-arterial coupling in hypertensive heart failure with preserved ejection fraction. Circ Heart Fail 6: 1165–1171, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Oberleithner H, Riethmüller C, Schillers H, MacGregor GA, de Wardener HE, Hausberg M: Plasma sodium stiffens vascular endothelium and reduces nitric oxide release. Proc Natl Acad Sci USA 104: 16281–16286, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.He FJ, Li J, Macgregor GA: Effect of longer-term modest salt reduction on blood pressure. Cochrane Database Syst Rev (4): CD004937, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rodríguez-Iturbe B, Vaziri ND, Herrera-Acosta J, Johnson RJ: Oxidative stress, renal infiltration of immune cells, and salt-sensitive hypertension: All for one and one for all. Am J Physiol Renal Physiol 286: F606–F616, 2004 [DOI] [PubMed] [Google Scholar]
- 13.Kidney Disease Outcomes Quality Initiative (K/DOQI) : K/DOQI clinical practice guidelines on hypertension and antihypertensive agents in chronic kidney disease. Am J Kidney Dis 43[Suppl 1]: S1–S290, 2004 [PubMed] [Google Scholar]
- 14.Wheeler DC, Becker GJ: Summary of KDIGO guideline. What do we really know about management of blood pressure in patients with chronic kidney disease? Kidney Int 83: 377–383, 2013 [DOI] [PubMed] [Google Scholar]
- 15.Fonseca C, Morais H, Mota T, Matias F, Costa C, Gouveia-Oliveira A, Ceia F, Investigators E: The diagnosis of heart failure in primary care: Value of symptoms and signs. Eur J Heart Fail 6: 795–800, 821-792, 2004 [DOI] [PubMed]
- 16.Agarwal R: Resistant hypertension and the neglected antihypertensive: Sodium restriction. Nephrol Dial Transplant 27: 4041–4045, 2012 [DOI] [PubMed] [Google Scholar]
- 17.Verbeke F, Lindley E, Van Bortel L, Vanholder R, London G, Cochat P, Wiecek A, Fouque D, Van Biesen W: A European Renal Best Practice (ERBP) position statement on the Kidney Disease: Improving Global Outcomes (KDIGO) clinical practice guideline for the management of blood pressure in non-dialysis-dependent chronic kidney disease: An endorsement with some caveats for real-life application. Nephrol Dial Transplant 29: 490–496, 2014 [DOI] [PubMed] [Google Scholar]
- 18.Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL Jr, Jones DW, Materson BJ, Oparil S, Wright JT Jr, Roccella EJ; Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. National Heart, Lung, and Blood Institute; National High Blood Pressure Education Program Coordinating Committee : Seventh report of the Joint National Committee on prevention, detection, evaluation, and treatment of high blood pressure. Hypertension 42: 1206–1252, 2003 [DOI] [PubMed] [Google Scholar]
- 19.Mancia G, De Backer G, Dominiczak A, Cifkova R, Fagard R, Germano G, Grassi G, Heagerty AM, Kjeldsen SE, Laurent S, Narkiewicz K, Ruilope L, Rynkiewicz A, Schmieder RE, Boudier HA, Zanchetti A; ESH-ESC Task Force on the Management of Arterial Hypertension : 2007 ESH-ESC practice guidelines for the management of arterial hypertension: ESH-ESC task force on the management of arterial hypertension. J Hypertens 25: 1751–1762, 2007 [DOI] [PubMed] [Google Scholar]
- 20.Miller WR: RSE: Motivational Interviewing: Preparing People for Change, 2nd Ed., New York, Guilford Press, 2002 [Google Scholar]
- 21.De Lorenzo A, Andreoli A, Matthie J, Withers P: Predicting body cell mass with bioimpedance by using theoretical methods: A technological review. J Appl Physiol (1985) 82: 1542–1558, 1997 [DOI] [PubMed] [Google Scholar]
- 22.Zhu F, Kotanko P, Handelman GJ, Raimann JG, Liu L, Carter M, Kuhlmann MK, Seibert E, Leonard EF, Levin NW: Estimation of normal hydration in dialysis patients using whole body and calf bioimpedance analysis. Physiol Meas 32: 887–902, 2011 [DOI] [PubMed] [Google Scholar]
- 23.Kikuya M, Hansen TW, Thijs L, Björklund-Bodegård K, Kuznetsova T, Ohkubo T, Richart T, Torp-Pedersen C, Lind L, Ibsen H, Imai Y, Staessen JA; International Database on Ambulatory blood pressure monitoring in relation to Cardiovascular Outcomes Investigators : Diagnostic thresholds for ambulatory blood pressure monitoring based on 10-year cardiovascular risk. Circulation 115: 2145–2152, 2007 [DOI] [PubMed] [Google Scholar]
- 24.Yu W, Luying S, Haiyan W, Xiaomei L: Importance and benefits of dietary sodium restriction in the management of chronic kidney disease patients: Experience from a single Chinese center. Int Urol Nephrol 44: 549–556, 2012 [DOI] [PubMed] [Google Scholar]
- 25.de Brito-Ashurst I, Perry L, Sanders TA, Thomas JE, Dobbie H, Varagunam M, Yaqoob MM: The role of salt intake and salt sensitivity in the management of hypertension in South Asian people with chronic kidney disease: A randomised controlled trial. Heart 99: 1256–1260, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Esnault VL, Ekhlas A, Delcroix C, Moutel MG, Nguyen JM: Diuretic and enhanced sodium restriction results in improved antiproteinuric response to RAS blocking agents. J Am Soc Nephrol 16: 474–481, 2005 [DOI] [PubMed] [Google Scholar]
- 27.Vogt L, Waanders F, Boomsma F, de Zeeuw D, Navis G: Effects of dietary sodium and hydrochlorothiazide on the antiproteinuric efficacy of losartan. J Am Soc Nephrol 19: 999–1007, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tanner RM, Calhoun DA, Bell EK, Bowling CB, Gutiérrez OM, Irvin MR, Lackland DT, Oparil S, Warnock D, Muntner P: Prevalence of apparent treatment-resistant hypertension among individuals with CKD. Clin J Am Soc Nephrol 8: 1583–1590, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vasavada N, Agarwal R: Role of excess volume in the pathophysiology of hypertension in chronic kidney disease. Kidney Int 64: 1772–1779, 2003 [DOI] [PubMed] [Google Scholar]
- 30.Minutolo R, Gabbai FB, Agarwal R, Chiodini P, Borrelli S, Bellizzi V, Nappi F, Stanzione G, Conte G, De Nicola L: Assessment of achieved clinic and ambulatory blood pressure recordings and outcomes during treatment in hypertensive patients with CKD: A multicenter prospective cohort study. Am J Kidney Dis 64: 744–752, 2014 [DOI] [PubMed] [Google Scholar]
- 31.Colombo PC, Onat D, Harxhi A, Demmer RT, Hayashi Y, Jelic S, LeJemtel TH, Bucciarelli L, Kebschull M, Papapanou P, Uriel N, Schmidt AM, Sabbah HN, Jorde UP: Peripheral venous congestion causes inflammation, neurohormonal, and endothelial cell activation. Eur Heart J 35: 448–454, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhu F, Kuhlmann MK, Sarkar S, Kaitwatcharachai C, Khilnani R, Leonard EF, Greenwood R, Levin NW: Adjustment of dry weight in hemodialysis patients using intradialytic continuous multifrequency bioimpedance of the calf. Int J Artif Organs 27: 104–109, 2004 [DOI] [PubMed] [Google Scholar]
- 33.Sacks FM, Svetkey LP, Vollmer WM, Appel LJ, Bray GA, Harsha D, Obarzanek E, Conlin PR, Miller ER 3rd, Simons-Morton DG, Karanja N, Lin PH; DASH-Sodium Collaborative Research Group : Effects on blood pressure of reduced dietary sodium and the Dietary Approaches to Stop Hypertension (DASH) diet. N Engl J Med 344: 3–10, 2001 [DOI] [PubMed] [Google Scholar]
- 34.McMahon EJ, Campbell KL, Mudge DW, Bauer JD: Achieving salt restriction in chronic kidney disease. Int J Nephrol 2012: 720429, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ronco C, Kaushik M, Valle R, Aspromonte N, Peacock WF 4th: Diagnosis and management of fluid overload in heart failure and cardio-renal syndrome: The “5B” approach. Semin Nephrol 32: 129–141, 2012 [DOI] [PubMed] [Google Scholar]
- 36.McMahon EJ, Campbell KL, Bauer JD, Mudge DW: Altered dietary salt intake for people with chronic kidney disease. Cochrane Database Syst Rev (2): CD010070, 2015 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.