Abstract
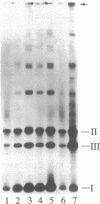
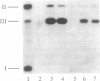
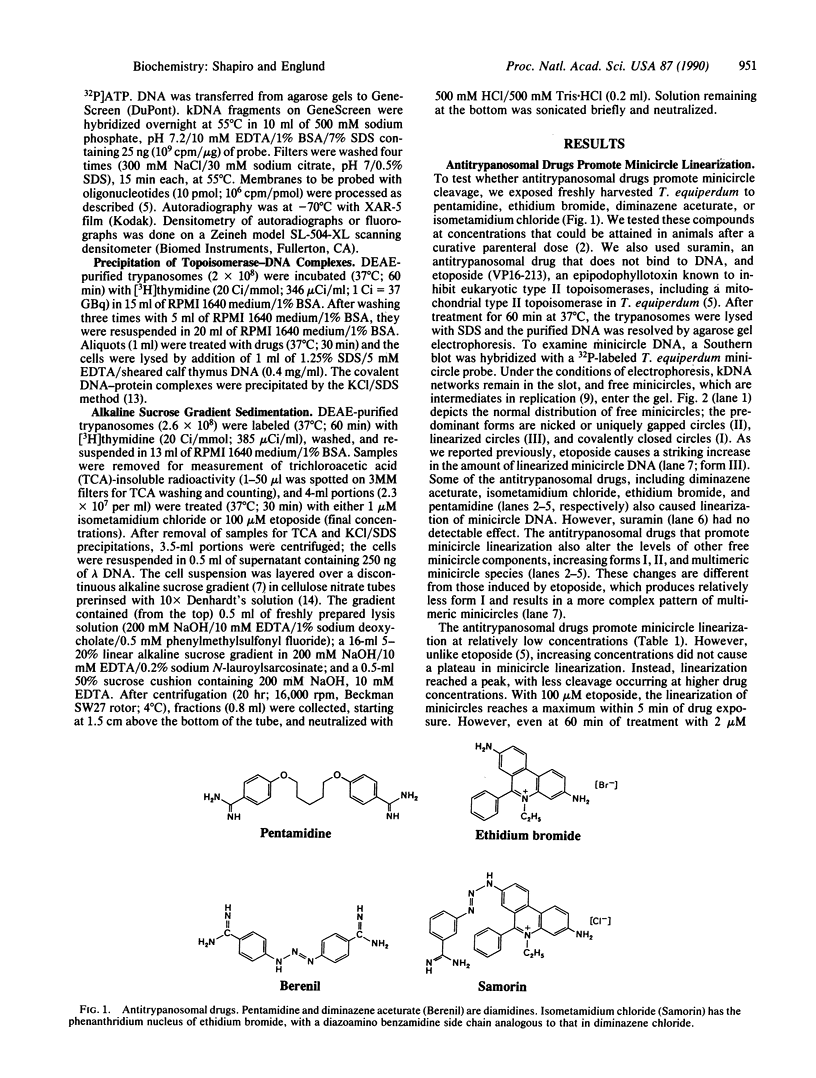
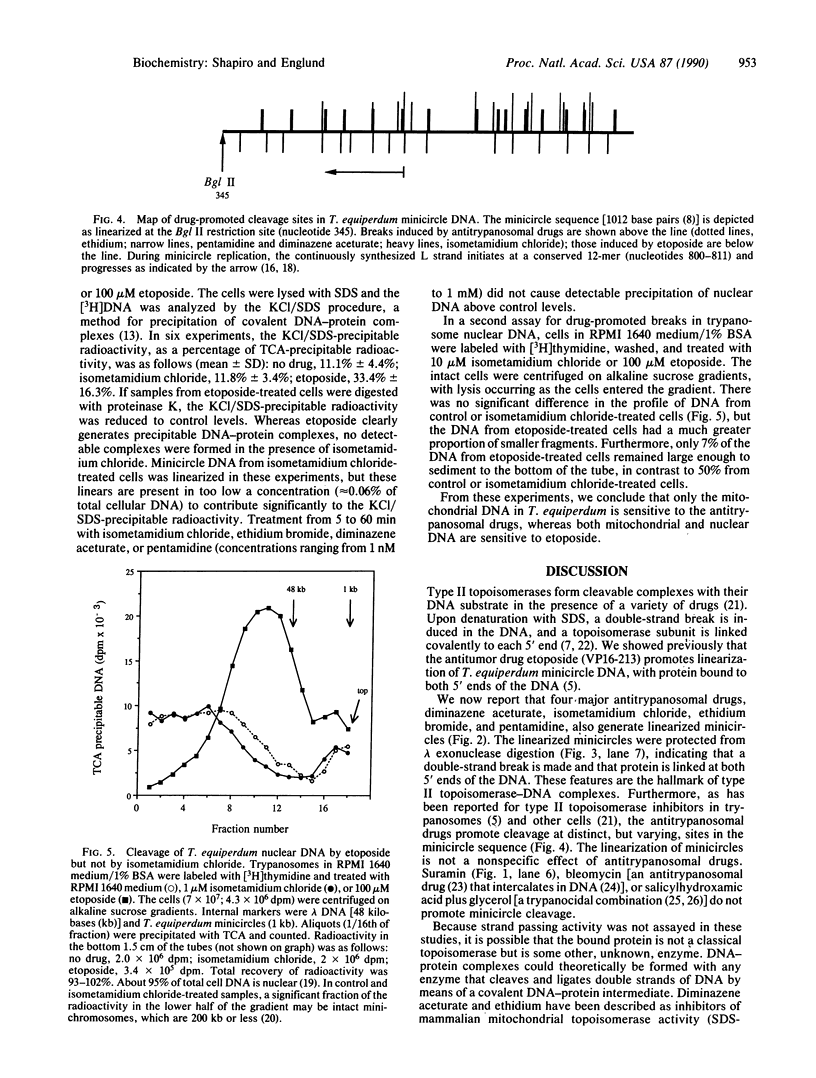
Pentamidine, diminazene aceturate (Berenil), isometamidium chloride (Samorin), and ethidium bromide, which are important antitrypanosomal drugs, promote linearization of Trypanosoma equiperdum minicircle DNA (the principal component of kinetoplast DNA, the mitochondrial DNA in these parasites). This effect occurs at therapeutically relevant concentrations. The linearized minicircles are protease sensitive and are not digested by lambda exonuclease (a 5' to 3' exonuclease), indicating that the break is double stranded and that protein is bound to both 5' ends of the molecule. The cleavage sites map to discrete positions in the minicircle sequence, and the cleavage pattern varies with different drugs. These findings are characteristic for type II topoisomerase inhibitors, and they mimic the effects of the antitumor drug etoposide (VP16-213, a semisynthetic podophyllotoxin analog) on T. equiperdum minicircles. However, the antitrypanosomal drugs differ dramatically from etoposide in that they do not promote detectable formation of nuclear DNA-protein complexes or of strand breaks in nuclear DNA. Selective inhibition of a mitochondrial type II topoisomerase may explain why these antitrypanosomal drugs preferentially disrupt mitochondrial DNA structure and generate dyskinetoplastic trypanosomes (which lack mitochondrial DNA).
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bangs J. D., Hereld D., Krakow J. L., Hart G. W., Englund P. T. Rapid processing of the carboxyl terminus of a trypanosome variant surface glycoprotein. Proc Natl Acad Sci U S A. 1985 May;82(10):3207–3211. doi: 10.1073/pnas.82.10.3207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrois M., Riou G., Galibert F. Complete nucleotide sequence of minicircle kinetoplast DNA from Trypanosoma equiperdum. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3323–3327. doi: 10.1073/pnas.78.6.3323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borst P., van der Ploeg M., van Hoek J. F., Tas J., James J. On the DNA content and ploidy of trypanosomes. Mol Biochem Parasitol. 1982 Jul;6(1):13–23. doi: 10.1016/0166-6851(82)90049-4. [DOI] [PubMed] [Google Scholar]
- Chen G. L., Yang L., Rowe T. C., Halligan B. D., Tewey K. M., Liu L. F. Nonintercalative antitumor drugs interfere with the breakage-reunion reaction of mammalian DNA topoisomerase II. J Biol Chem. 1984 Nov 10;259(21):13560–13566. [PubMed] [Google Scholar]
- Clarkson A. B., Jr, Brohn F. H. Trypanosomiasis: an approach to chemotherapy by the inhibition of carbohydrate catabolism. Science. 1976 Oct 8;194(4261):204–206. doi: 10.1126/science.986688. [DOI] [PubMed] [Google Scholar]
- Delain E., Brack C., Riou G., Festy B. Ultrastructural alterations of Trypanosoma cruzi kinetoplast induced by the interaction of a trypanocidal drug (hydroxystilbamidine) with the kinetoplast DNA. J Ultrastruct Res. 1971 Oct;37(1):200–218. doi: 10.1016/s0022-5320(71)80051-5. [DOI] [PubMed] [Google Scholar]
- Fairfield F. R., Bauer W. R., Simpson M. V. Mitochondria contain a distinct DNA topoisomerase. J Biol Chem. 1979 Oct 10;254(19):9352–9354. [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Festy B., Sturm J., Daune M. Interaction between hydroxystilbamidine and DNA. I. Binding isotherms and thermodynamics of the association. Biochim Biophys Acta. 1975 Sep 12;407(1):24–42. [PubMed] [Google Scholar]
- Liu L. F., Rowe T. C., Yang L., Tewey K. M., Chen G. L. Cleavage of DNA by mammalian DNA topoisomerase II. J Biol Chem. 1983 Dec 25;258(24):15365–15370. [PubMed] [Google Scholar]
- Macadam R. F., Williamson J. Lesions in the fine structure of Trypanosoma rhodesiense specifically associated with drug treatment. Trans R Soc Trop Med Hyg. 1969;63(4):421–422. [PubMed] [Google Scholar]
- Maxwell A., Gellert M. Mechanistic aspects of DNA topoisomerases. Adv Protein Chem. 1986;38:69–107. doi: 10.1016/s0065-3233(08)60526-4. [DOI] [PubMed] [Google Scholar]
- Nathan H. C., Bacchi C. J., Sakai T. T., Rescigno D., Stumpf D., Hutner S. H. Bleomycin-induced life prolongation of mice infected with Trypanosoma brucei brucei EATRO 110. Trans R Soc Trop Med Hyg. 1981;75(3):394–398. doi: 10.1016/0035-9203(81)90101-2. [DOI] [PubMed] [Google Scholar]
- Ntambi J. M., Englund P. T. A gap at a unique location in newly replicated kinetoplast DNA minicircles from Trypanosoma equiperdum. J Biol Chem. 1985 May 10;260(9):5574–5579. [PubMed] [Google Scholar]
- Ntambi J. M., Shapiro T. A., Ryan K. A., Englund P. T. Ribonucleotides associated with a gap in newly replicated kinetoplast DNA minicircles from Trypanosoma equiperdum. J Biol Chem. 1986 Sep 5;261(25):11890–11895. [PubMed] [Google Scholar]
- Opperdoes F. R., Borst P., Fonck K. The potential use of inhibitors of glycerol-3-phosphate oxidase for chemotherapy of African trypanosomiasis. FEBS Lett. 1976 Feb 15;62(2):169–172. doi: 10.1016/0014-5793(76)80045-2. [DOI] [PubMed] [Google Scholar]
- Riou G. F., Belnat P., Benard J. Complete loss of kinetoplast DNA sequences induced by ethidium bromide or by acriflavine in Trypanosoma equiperdum. J Biol Chem. 1980 Jun 10;255(11):5141–5144. [PubMed] [Google Scholar]
- Riou G. F., Saucier J. M. Characterization of the molecular components in kinetoplast-mitochondrial DNA of Trypanosoma equiperdum. Comparative study of the dyskinetoplastic and wild strains. J Cell Biol. 1979 Jul;82(1):248–263. doi: 10.1083/jcb.82.1.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan K. A., Shapiro T. A., Rauch C. A., Englund P. T. Replication of kinetoplast DNA in trypanosomes. Annu Rev Microbiol. 1988;42:339–358. doi: 10.1146/annurev.mi.42.100188.002011. [DOI] [PubMed] [Google Scholar]
- Ryan K. A., Shapiro T. A., Rauch C. A., Griffith J. D., Englund P. T. A knotted free minicircle in kinetoplast DNA. Proc Natl Acad Sci U S A. 1988 Aug;85(16):5844–5848. doi: 10.1073/pnas.85.16.5844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro T. A., Klein V. A., Englund P. T. Drug-promoted cleavage of kinetoplast DNA minicircles. Evidence for type II topoisomerase activity in trypanosome mitochondria. J Biol Chem. 1989 Mar 5;264(7):4173–4178. [PubMed] [Google Scholar]
- Sundin O., Varshavsky A. Arrest of segregation leads to accumulation of highly intertwined catenated dimers: dissection of the final stages of SV40 DNA replication. Cell. 1981 Sep;25(3):659–669. doi: 10.1016/0092-8674(81)90173-2. [DOI] [PubMed] [Google Scholar]
- TRAGER W., RUDZINSKA M. A. THE RIBOFLAVIN REQUIREMENT AND THE EFFECTS OF ACRIFLAVIN ON THE FINE STRUCTURE OF THE KINETOPLAST OF LEISHMANIA TARENTOLAE. J Protozool. 1964 Feb;11:133–145. doi: 10.1111/j.1550-7408.1964.tb01734.x. [DOI] [PubMed] [Google Scholar]
- Tewey K. M., Chen G. L., Nelson E. M., Liu L. F. Intercalative antitumor drugs interfere with the breakage-reunion reaction of mammalian DNA topoisomerase II. J Biol Chem. 1984 Jul 25;259(14):9182–9187. [PubMed] [Google Scholar]
- Tewey K. M., Rowe T. C., Yang L., Halligan B. D., Liu L. F. Adriamycin-induced DNA damage mediated by mammalian DNA topoisomerase II. Science. 1984 Oct 26;226(4673):466–468. doi: 10.1126/science.6093249. [DOI] [PubMed] [Google Scholar]
- Van der Ploeg L. H., Cornelissen A. W., Barry J. D., Borst P. Chromosomes of kinetoplastida. EMBO J. 1984 Dec 20;3(13):3109–3115. doi: 10.1002/j.1460-2075.1984.tb02266.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waring M. J. Complex formation between ethidium bromide and nucleic acids. J Mol Biol. 1965 Aug;13(1):269–282. doi: 10.1016/s0022-2836(65)80096-1. [DOI] [PubMed] [Google Scholar]