Abstract
Background and objectives
Residual kidney function can be assessed by simply measuring urine volume, calculating GFR using 24-hour urine collection, or estimating GFR using the proposed equation (eGFR). We aimed to investigate the relative prognostic value of these residual kidney function parameters in patients on dialysis.
Design, setting, participants, & measurements
Using the database from a nationwide prospective cohort study, we compared differential implications of the residual kidney function indices in 1946 patients on dialysis at 36 dialysis centers in Korea between August 1, 2008 and December 31, 2014. Residual GFR calculated using 24-hour urine collection was determined by an average of renal urea and creatinine clearance on the basis of 24-hour urine collection. eGFR-urea, creatinine and eGFR β2-microglobulin were calculated from the equations using serum urea and creatinine and β2-microglobulin, respectively. The primary outcome was all-cause death.
Results
During a mean follow-up of 42 months, 385 (19.8%) patients died. In multivariable Cox analyses, residual urine volume (hazard ratio, 0.96 per 0.1-L/d higher volume; 95% confidence interval, 0.94 to 0.98) and GFR calculated using 24-hour urine collection (hazard ratio, 0.98; 95% confidence interval, 0.95 to 0.99) were independently associated with all-cause mortality. In 1640 patients who had eGFR β2-microglobulin data, eGFR β2-microglobulin (hazard ratio, 0.98; 95% confidence interval, 0.96 to 0.99) was also significantly associated with all-cause mortality as well as residual urine volume (hazard ratio, 0.96 per 0.1-L/d higher volume; 95% confidence interval, 0.94 to 0.98) and GFR calculated using 24-hour urine collection (hazard ratio, 0.97; 95% confidence interval, 0.95 to 0.99). When each residual kidney function index was added to the base model, only urine volume improved the predictability for all-cause mortality (net reclassification index =0.11, P=0.01; integrated discrimination improvement =0.01, P=0.01).
Conclusions
Higher residual urine volume was significantly associated with a lower risk of death and exhibited a stronger association with mortality than GFR calculated using 24-hour urine collection and eGFR-urea, creatinine. These results suggest that determining residual urine volume may be beneficial to predict patient survival in patients on dialysis.
Keywords: dialysis, end-stage renal disease, glomerular filtration rate, mortality, residual kidney function, urine volume
Introduction
Residual kidney function (RKF) is a crucial predictor of clinical outcomes in patients with ESRD treated with dialysis therapy (1–10). There have been several attempts to improve patient outcomes by increasing urea or creatinine clearance derived from dialysis (2,4,9,11,12). However, previous observational studies showed that RKF was independently associated with survival and that RKF including renal creatinine (3), urea (8,9), or average of creatinine and urea clearance (5,7) was more important than urea or creatinine clearance delivered by dialysis. Reanalysis of the Canada-USA (CANUSA) Study showed that RKF significantly predicted mortality, whereas peritoneal creatinine clearance did not in patients on peritoneal dialysis (PD) (5). In patients on hemodialysis (HD), the Netherlands Cooperative Study on the Adequacy of Dialysis (NECOSAD-2) also indicated that renal Kt/Vurea was significantly associated with mortality and that the benefit of higher dialysis Kt/Vurea was not found in patients with RKF (8).
RKF contributes to solute and volume clearance. Furthermore, RKF increases renal salt and water excretion, mitigating the adverse effect of chronic volume overload (13,14). Greater clearance of middle molecule, including β2-microglobulin (B2M), and protein-bound uremic toxins, including p-cresol sulfate and indoxyl sulfate (15–19), better phosphate control (20,21), nutritional benefit (22,23), and better anemia control (10,21) are also thought to be closely associated with beneficial effect of RKF.
In clinical practice, clinicians often confront the inconvenient methodologic issue of RKF measurement. RKF can be determined by GFR derived from the renal clearance of urea and creatinine or the average of urea and creatinine using 24-hour urine collection (GFR-24U) or residual urine volume (UV) (24,25). Without urine collection, equations for eGFR using serum filtration markers have been proposed in patients on dialysis (26).
However, so far, there have been few studies to explore the relative discriminative value of RKF indices in predicting prognosis in both patients on HD and patients on PD. A recent study showed that overhydration measured by bioelectric impedance analyses was significantly associated with UV but was not associated with GFR-24U in patients on PD (27). On the basis of this result, we hypothesized that UV may be more closely associated with prognosis than other indices because of its relevant association with volume status. To address this issue, we compared prognostic powers of residual UV, GFR-24U, and eGFR for mortality in patients on dialysis from a nationwide prospective observational cohort.
Materials and Methods
Study Design and Participants
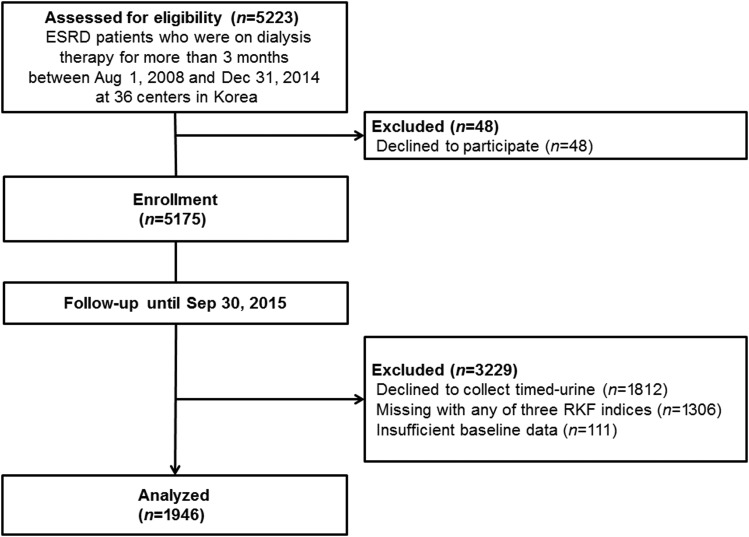
All patients who received HD or PD for >3 months between August 1, 2008 and December 31, 2014 at 36 centers of the Clinical Research Center for End-Stage Renal Disease (CRC for ESRD) in Korea were initially screened for this study. This study is a part of a nationwide multicenter prospective cohort study of patients with ESRD that aimed to improve clinical outcomes and develop efficient treatment guidelines in Korea (clinicaltrial.gov NCT00931970). To test our hypothesis, 3118 patients who declined to perform 24-hour urine collection or had missing values for any of the three RKF indices (residual UV, GFR-24U, and eGFR calculated from serum urea and creatinine [eGFR-urea,creat]) and 111 participants who had insufficient baseline data were excluded from the initially enrolled 5175 patients on dialysis. A total of 1946 patients on dialysis were included in the final analysis (Figure 1). This study was carried out in accordance with the Declaration of Helsinki. The study protocol was approved by the institutional review board at each participating center, and all patients provided their written informed consent to participate in the study.
Figure 1.
Among 5175 initially enrolled patients with ESRD on dialysis therapy, 1946 patients were finally analyzed. CRC for ESRD, Clinical Research Center for End-Stage Renal Disease; RKF, residual kidney function.
Data Collection
Demographic, clinical, and laboratory data were retrieved from the CRC for ESRD electronic database. Demographics, including age, sex, dialysis modality, dialysis vintage before study enrollment, and medication, were recorded at enrollment in the study. The modified Charlson comorbidity index (CCI) was calculated (28). The biochemical data were collected at study entry and every 6 months thereafter. High-sensitivity C-reactive protein (hs-CRP) concentrations were measured by a latex-enhanced immunonephelometric method using a BNII Analyzer (Dade Behring, Newark, DE). The B2M level was determined by chemiluminescent immunoassay (DiaSorin, Saluggia, Italy), and N-terminal pro–B-type natriuretic peptide level was determined by the Elecsys proBNP Electrochemiluminescence Immunoassay (Roche Diagnostics, Indianapolis, IN).
Assessment of RKF
RKF was ascertained once by residual UV, GFR-24U, and eGFR at enrollment in the study. If patients agreed to urine collection, patients were educated on collecting all voided urine at home by research coordinators. During urine collection, patients were asked to record voiding time and UV. Research coordinators interviewed patients and reviewed the records to assess the completion of urine collection. In patients on HD, residual urine was collected during a 44-hour period of the interdialytic interval. In patients on PD, a 24-hour timed urine collection was performed, regardless of timing. UV during the interdialytic period was divided by its duration and expressed as UV per day (liters per day). Anuria was defined as UV of <0.1 L/d. GFR-24U was calculated as the mean of 24-hour urinary urea and creatinine clearances and normalized to the standard body surface area of 1.73 m2 (milliliters per minute per 1.73 m2) (25). To calculate the urea and creatinine clearances, creatinine and urea concentrations were measured from plasma at the start and the end of urine collection, respectively. The average urea and creatinine concentrations at the start and the end of urine collections were used for calculation of urinary urea and creatinine clearances (GFR-24U). In patients on PD, blood sampling timing differed between dialysis modalities; sampling was taken anytime during daytime, generally in the morning for continuous ambulatory PD and the middle of the noncycling daytime period for automated PD, when urea concentration represents the average value of blood urea for a day. eGFR was calculated from the serum urea and creatinine concentrations using the equation proposed by Shafi et al. (26): eGFR-urea,creat (milliliters per minute per 1.73 m2) =2.4×BUN0.984× creatinine−1.868. In this study, 1640 patients had data for B2M concentrations, and eGFR was determined by the equation using B2M concentrations proposed by Shafi et al. (26): eGFR B2M (milliliters per minute per 1.73 m2) =2852×B2M−2.417×1.592 if a man. For calculation of eGFRs, we used predialysis urea, creatinine, and B2M concentrations, which were obtained at the study entry as baseline biochemical variables.
Follow-Up
Patients were followed until September 30, 2015. All death events were extracted from the CRC for ESRD database and collated with the medical record at each participating center and the Korea National Statistics database.
Statistical Analyses
Statistical analysis was performed using SPSS for Windows version 20.0 (IBM Corp., Armonk, NY), SAS version 9.2 (SAS Institute, Cary, NC), and R (R Foundation for Statistical Computing, Vienna, Austria; www.r-project.org). Continuous variables were expressed as the mean±SD or the median (interquartile range [IQR]), and categorical variables were expressed as a number (percentage). Baseline characteristics were compared between survivors and nonsurvivors using a t test, Mann–Whitney U test, or chi-squared test. The association between UV and baseline characteristics was tested by uni- and multivariate linear regression analyses. Cox regression analyses were performed to identify the independent prognostic value of each RKF index. To test our hypothesis that UV may be a better predictor of mortality than the other RKF indices, we assessed the additional effect of each RKF index on a base model: model 1, base model + GFR-24U; model 2, base model + eGFR-urea,creat; and model 3, base model + UV. In 1640 patients who had available B2M data, model 2 was constructed with eGFR B2M instead of eGFR-urea,creat. A base model was constructed including age, sex, dialysis modality, dialysis vintage, modified CCI, smoking status, and serum albumin concentrations, all of which were significant risk factors for mortality in univariate analyses. Then, the net reclassification index (NRI) and the integrated discrimination improvement (IDI) were calculated to ascertain which RKF indices improved the discriminatory ability when added to the base model. Furthermore, Harrell C index and differences in c statistics were also calculated using bootstrapping with 1000 replicates. For sensitivity analysis, subgroup analyses were performed in patients with UV≥0.1 or <0.1 L/d, patients on HD, patients on PD, and patients with dialysis vintage <2 or ≥2 years. Although there was no interaction between each RKF index and dialysis modality (P value for interaction; GFR-24U: P=0.74; eGFR-urea,creat: P=0.11; UV: P=0.44), stratified analyses were performed due to clinical relevance. P<0.05 was considered statistically significant.
Results
Baseline Characteristics
Baseline characteristics of the study participants are shown in Table 1. The mean age was 56.1±13.5 years old, and 840 (43.2%) patients were women. The median values of GFR-24U and eGFR-urea,creat were 2.0 (IQR, 0.1–5.4) and 2.5 (IQR, 1.7–3.9) ml/min per 1.73 m2, respectively. The median value of UV was 0.50 (IQR, 0.04–1.10) L/d, and 639 (32.8%) patients were anuric. During a mean follow-up duration of 42 months, 385 (19.8%) patients died. Compared with non-survivors, the mean age, dialysis vintage, modified CCI, proportion with diabetes, proportion of smokers, and hs-CRP, B2M, and N-terminal pro–B-type natriuretic peptide concentrations were significantly lower in survivors. Serum albumin concentrations of survivors were significantly higher than those of non-survivors. GFR-24U and UV were significantly higher, but eGFR-urea,creat was lower in the survivor group. Meanwhile, there were no significant differences in baseline characteristics between patients with and without 24-hour collection data, except the cause of primary kidney disease and body mass index (Supplemental Table 1).
Table 1.
Baseline characteristics of study participants at the time of study enrollment
| Variable | All, n=1946 | Survivors, n=1561 | Nonsurvivors, n=385 | P Value |
|---|---|---|---|---|
| Age, yr | 56.1±13.5 | 53.8±13.1 | 65.1±11.0 | <0.001 |
| Women | 840 (43.2%) | 698 (44.7%) | 143 (37.1%) | 0.01 |
| Hemodialysis | 1254 (64.4%) | 981 (62.8%) | 273 (70.9%) | 0.02 |
| Dialysis vintage, mo (IQR) | 9 (3–39) | 7 (3–36) | 19 (3–48) | <0.001 |
| Patients on incident dialysis | 826 (42.4%) | 721 (46.2%) | 105 (27.3%) | <0.001 |
| Primary renal disease | <0.001 | |||
| Diabetic nephropathy | 920 (47.3%) | 677 (43.4%) | 243 (63.1%) | |
| Hypertensive | 365 (18.8%) | 295 (18.9%) | 70 (18.2%) | |
| GN | 349 (17.9%) | 318 (20.4%) | 31 (8.1%) | |
| Polycystic kidney disease | 60 (3.1%) | 53 (3.4%) | 7 (1.8%) | |
| Modified CCI | 5.2±2.4 | 4.7±2.2 | 6.9±2.4 | <0.001 |
| Diabetes mellitus | 996 (51.2%) | 738 (47.3%) | 258 (67.0%) | <0.001 |
| CVDa | 709 (36.4%) | 492 (31.5%) | 217 (56.4%) | <0.001 |
| Smoker | 766 (39.4%) | 595 (38.1%) | 171 (44.4%) | 0.03 |
| SBP, mmHg | 139.2±21.0 | 139.2±20.9 | 139.3±21.4 | 0.90 |
| Body mass index, kg/m2 | 22.6±3.3 | 22.6±3.3 | 22.4±3.2 | 0.27 |
| Hemoglobin, g/dl | 9.9±1.7 | 9.9±1.7 | 10.1±1.6 | 0.03 |
| BUN, mg/dl | 71.7±31.1 | 73.7±32.2 | 63.3±24.9 | <0.001 |
| Creatinine, mg/dl | 9.3±3.5 | 9.6±3.7 | 8.2±2.7 | <0.001 |
| Albumin, g/dl | 3.7±0.6 | 3.7±0.5 | 3.6±0.6 | <0.001 |
| Glucose, mg/dl | 136.9±72.1 | 131.5±63.1 | 154.9±81.1 | <0.001 |
| Calcium, mg/dl | 8.4±1.2 | 8.4±1.2 | 8.4±0.8 | 0.002 |
| Phosphorus, mg/dl | 5.3±1.9 | 5.3±1.9 | 4.6±1.5 | <0.001 |
| hs-CRP (IQR), mg/dl | 0.19 (0.04–0.74) | 0.16 (0.04–0.62) | 0.33 (0.08–1.06) | <0.001 |
| B2M (IQR), mg/Lb | 20.5 (12.1–29.0) | 20.0 (11.3–28.6) | 22.5 (14.4–30.6) | <0.001 |
| NT-proBNP (IQR), pg/mlc | 3296 (1715–30,597) | 6901 (1539–29,938) | 16,787 (2580–38,268) | <0.001 |
| Medications | ||||
| RAS blockers | 1118 (57.5%) | 899 (57.6%) | 219 (56.9%) | 0.82 |
| β-Blockers | 906 (46.6%) | 703 (45.0%) | 203 (52.7%) | 0.01 |
| Calcium channel blockers | 987 (50.7%) | 806 (51.6%) | 181 (47.0%) | 0.11 |
| Diuretics | 958 (49.2%) | 782 (50.1%) | 176 (45.7%) | 0.13 |
| Dialysis urea clearance | ||||
| Kt/Vurea (HD)d | 1.4±0.3 | 1.4±0.4 | 1.4±0.3 | 0.19 |
| Weekly peritoneal Kt/Vurea (PD)e | 1.4±0.5 | 1.4±0.5 | 1.4±0.6 | 0.27 |
| GFR-24U (IQR), ml/min per 1.73 m2 | 2.0 (0.1–5.4) | 2.3 (0.1–5.6) | 0.8 (0.1–4.6) | <0.001 |
| eGFR-urea,creat (IQR), ml/min per 1.73 m2 | 2.5 (1.7–3.9) | 2.4 (1.6–3.8) | 2.7 (1.8–4.2) | 0.003 |
| eGFR B2M (IQR), ml/min per 1.73 m2b | 2.3 (1.0–7.7) | 2.5 (1.1–8.1) | 1.9 (0.9–5.5) | 0.001 |
| UV (IQR), L/d | 0.50 (0.04–1.10) | 0.60 (0.04–1.10) | 0.20 (0.03–0.81) | <0.001 |
Data are expressed as the mean±SD, median (IQR), or number of patients (percentage). IQR, interquartile range; CCI, Charlson comorbidity index; CVD, cardiovascular disease; SBP, systolic BP; hs-CRP, high-sensitivity C-reactive protein; B2M, β2-microglobulin; NT-proBNP, N-terminal pro–B-type natriuretic peptide; RAS, renin-angiotensin system; HD, hemodialysis; PD, peritoneal dialysis; GFR-24U, GFR by 24-hour urine collection; eGFR-urea,creat, estimated GFR calculated from the serum urea and creatinine concentrations; UV, urine volume.
CVD is a composite of coronary artery disease, peripheral artery disease, cerebrovascular accidents, and congestive heart failure.
B2M concentrations were available for 1640 patients.
NT-proBNP concentrations were available for 721 patients.
Kt/Vurea was measured in 1254 patients on HD.
Weekly peritoneal Kt/Vurea was measured in 692 patients on PD.
Association between UV and Clinical Characteristics
Multivariate linear regression analysis revealed that PD, dialysis vintage, body mass index, and log hs-CRP were independently associated with UV. In 1640 patients who had B2M value, B2M showed a significant negative association with UV (Supplemental Table 2).
Independent Prognostic Value of RKF Indices for Mortality
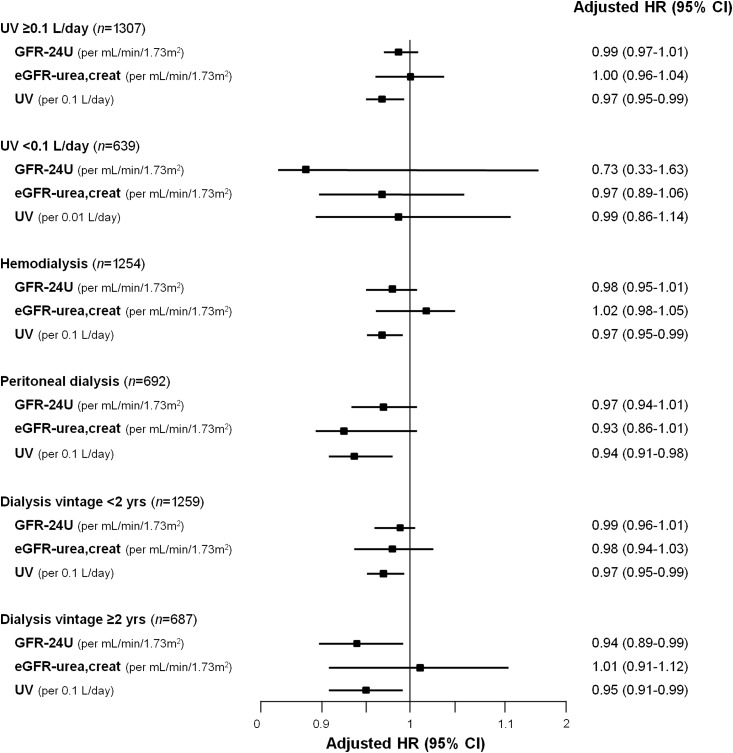
To determine the independent prognostic value of RKF indices for mortality, Cox regression analyses were performed (Table 2). Patients were censored at the time of loss to follow-up, kidney transplantation, or recovery of renal function. When each RKF index was added to the base model, GFR-24U (per milliliters per minute per 1.73 m2 increase; hazard ratio [HR], 0.98; 95% confidence interval [95% CI], 0.95 to 0.99) and higher UV (per 0.1-L/d higher volume; HR, 0.96; 95% CI, 0.94 to 0.98) were independently associated with lower risk of mortality. However, eGFR-urea,creat did not show a significant association with mortality in a multivariate analysis (model 2 in Table 2). In 1640 patients who had available B2M data, GFR-24U (per milliliters per minute per 1.73 m2 increase; HR, 0.97; 95% CI, 0.95 to 0.99), eGFR B2M (per milliliters per minute per 1.73 m2 increase; HR, 0.98; 95% CI, 0.96 to 0.99), and UV (per 0.1-L/d higher volume; HR, 0.96; 95% CI, 0.94 to 0.98) were significantly associated with all-cause mortality (Table 3). For sensitivity analysis, we further tested prognostic values of RKF indices in several subgroups (Figure 2). In 1307 patients who had residual UV ≥0.1 L/d, UV showed the highest association with mortality among the three RKF indices (per 0.1-L/d higher volume; HR, 0.97; 95% CI, 0.95 to 0.99). In the remaining 639 patients with residual UV <0.1 L/d, UV did not show a significant association with mortality as well as the other RKF indices. In both patients on HD and patients on PD, UV remained the only independent risk factor for death (per 0.1-L/d higher volume; HR, 0.97; 95% CI, 0.95 to 0.99 in HD and HR, 0.94; 95% CI, 0.91 to 0.98 in PD). Moreover, UV was also significantly associated with all-cause mortality in 1259 patients with dialysis vintage <2 years (per 0.1-L/d higher volume; HR, 0.97; 95% CI, 0.95 to 0.99).
Table 2.
Multivariable Cox regression analysis of GFR by 24-hour urine collection, eGFR-urea,creat, and urine volume for all-cause mortality (n=1946)
| Variable | HR (95% CI) | |||
|---|---|---|---|---|
| Base modela | Model 1b | Model 2c | Model 3d | |
| Age, yr | 1.04 (1.03 to 1.06) | 1.04 (1.03 to 1.06) | 1.04 (1.03 to 1.06) | 1.04 (1.03 to 1.06) |
| Women (versus men) | 0.90 (0.69 to 1.17) | 0.88 (0.68 to 1.14) | 0.91 (0.70 to 1.19) | 0.89 (0.69 to 1.16) |
| PD (versus HD) | 1.15 (0.90 to 1.45) | 1.27 (0.98 to 1.63) | 1.16 (0.91 to 1.47) | 1.15 (0.91 to 1.46) |
| Dialysis vintage, mo | 1.00 (1.00 to 1.01) | 1.00 (1.00 to 1.00) | 1.00 (1.00 to 1.06) | 1.00 (1.00 to 1.01) |
| Modified CCI (per 1) | 1.21 (1.15 to 1.27) | 1.21 (1.16 to 1.28) | 1.21 (1.15 to 1.28) | 1.21 (1.15 to 1.28) |
| Smoker (versus nonsmoker) | 1.09 (0.84 to 1.42) | 1.08 (0.83 to 1.40) | 1.10 (0.85 to 1.42) | 1.09 (0.84 to 1.41) |
| Albumin, per mg/dL | 0.61 (0.51 to 0.74) | 0.60 (0.49 to 0.72) | 0.61 (0.50 to 0.73) | 0.61 (0.50 to 0.74) |
| GFR-24U, per ml/min per 1.73 m2 | — | 0.98 (0.95 to 0.99) | — | — |
| eGFR-urea,creat, per ml/min per 1.73 m2 | — | — | 0.99 (0.95 to 1.03) | — |
| UV, per 0.1 L/d | — | — | — | 0.96 (0.94 to 0.98) |
HR, hazard ratio; 95% CI, 95% confidence interval; PD, peritoneal dialysis; HD, hemodialysis; CCI, Charlson comorbidity index; GFR-24U, GFR by 24-hour urine collection; eGFR-urea,creat, estimated GFR calculated from the serum urea and creatinine concentrations; UV, urine volume.
Base model: adjusted for age, sex, dialysis modality, dialysis vintage, modified CCI, smoking status, and serum albumin concentrations.
Model 1: Base model and GFR-24U.
Model 2: Base model and eGFR-urea,creat.
Model 3: Base model and UV.
Table 3.
Multivariable Cox regression analysis of GFR by 24-hour urine collection, eGFR β2-microglobulin, and urine volume for all-cause mortality (n=1640)
| Variable | HR (95% CI) | |||
|---|---|---|---|---|
| Base modela | Model 1b | Model 2c | Model 3d | |
| Age, yr | 1.05 (1.04 to 1.06) | 1.05 (1.04 to 1.06) | 1.05 (1.04 to 1.06) | 1.05 (1.04 to 1.06) |
| Women (versus men) | 0.88 (0.66 to 1.18) | 0.86 (0.64 to 1.15) | 0.85 (0.63 to 1.13) | 0.84 (0.63 to 1.13) |
| PD (versus HD) | 1.21 (0.94 to 1.56) | 1.35 (1.93 to 1.76) | 1.36 (1.04 to 1.78) | 1.44 (1.11 to 1.89) |
| Dialysis vintage, mo | 1.00 (1.00 to 1.01) | 1.00 (1.00 to 1.01) | 1.00 (1.00 to 1.01) | 1.00 (1.00 to 1.00) |
| Modified CCI (per 1) | 1.20 (1.14 to 1.27) | 1.21 (1.14 to 1.28) | 1.21 (1.14 to 1.28) | 1.20 (1.13 to 1.26) |
| Smoker (versus nonsmoker) | 1.08 (0.81 to 1.45) | 1.06 (0.80 to 1.42) | 1.08 (0.81 to 1.44) | 1.05 (0.79 to 1.40) |
| Albumin, per mg/dL | 0.65 (0.53 to 0.80) | 0.63 (0.51 to 0.78) | 0.63 (0.51 to 0.78) | 0.62 (0.50 to 0.76) |
| GFR-24U, per ml/min per 1.73 m2 | 0.97 (0.95 to 0.99) | — | — | |
| eGFR B2M, per ml/min per 1.73 m2 | 0.98 (0.96 to 0.99) | — | ||
| UV, per 0.1 L/d | 0.96 (0.94 to 0.98) | |||
These analyses were performed in 1640 patients who had available B2M data. HR, hazard ratio; 95% CI, 95% confidence interval; PD, peritoneal dialysis; HD, hemodialysis; CCI, Charlson comorbidity index; GFR-24U, GFR by 24-hour urine collection; B2M, β2-microglobulin; UV, urine volume.
Base model: adjusted for age, sex, dialysis modality, dialysis vintage, modified CCI, smoking status, and serum albumin concentrations.
Model 1: Base model and GFR-24U.
Model 2: Base model and eGFR B2M.
Model 3: Base model and UV.
Figure 2.
Among three residual kidney function (RKF) indices, only residual urine volume (UV) indicated an independent prognostic value in patients with UV≥0.1 or <0.1 L/d, patients on hemodialysis, patients on peritoneal dialysis, and patients with dialysis vintage <2 or ≥2 years. Adjusted hazard ratios (HRs) were calculated after adjustment for age, sex, dialysis modality, dialysis vintage, modified Charlson comorbidity index, smoking status, and serum albumin concentrations. In subgroup analysis regarding hemodialysis and peritoneal dialysis, dialysis modality was not adjusted. Similarly, in subgroup analysis regarding patients with dialysis duration <2 and ≥2 years, dialysis vintage was not adjusted. 95% CI, 95% confidence interval; eGFR-urea,creat, estimated GFR calculated from the serum and creatinine concentrations; GFR-24U, GFR by 24-hour urine collection.
Comparison of Predictive Value of Each RKF Index for Mortality
To clarify the additive predictive power of RKF indices for mortality, we calculated the NRI and the IDI (Table 4). When UV was added to the base model, it showed a significant improvement to predict all-cause mortality compared with the base model (NRI=0.11, P=0.01; IDI=0.01, P=0.01). However, adding GFR-24U, eGFR-urea,creat, or eGFR B2M to the base model did not improve the discriminative ability of each model for all-cause mortality. In addition, the c statistics of the base model with UV significantly increased compared with the base model (mean difference in c statistics =0.01; 95% CI, 0.001 to 0.01) (Table 4).
Table 4.
Predictability of four residual kidney function index models for all-cause mortality using net reclassification index, integrated discrimination improvement, and c statistic
| Models | NRI (SEM; 95% CI) | P Value | IDI (SEM; 95% CI) | P Value | c Statistics (95% CI) | Mean of Differences in c Statistics (95% CI)a |
|---|---|---|---|---|---|---|
| Base modelb | Reference | Reference | 0.75 (0.73 to 0.78) | |||
| Base model and GFR-24U | 0.14 (−0.05 to 0.25) | 0.09 | 0.002 (−0.001 to 0.01) | 0.12 | 0.76 (0.73 to 0.78) | 0.002 (−0.004 to 0.01) |
| Base model and eGFR-urea,creat | 0.19 (−0.24 to 0.27) | 0.47 | 0.001 (−0.001 to 0.01) | 0.44 | 0.76 (0.73 to 0.78) | 0.001 (−0.001 to 0.004) |
| Base modelc and eGFR B2M | 0.23 (−0.16 to 0.34) | 0.13 | 0.01 (−0.001 to 0.01) | 0.11 | 0.76 (0.74 to 0.79) | 0.01 (0.003 to 0.03) |
| Base model and UV | 0.11 (0.01 to 0.21) | 0.01 | 0.01 (0.001 to 0.02) | 0.01 | 0.76 (0.73 to 0.78) | 0.01 (0.001 to 0.01) |
NRI, net reclassification index; 95% CI, 95% confidence interval; IDI, integrated discrimination improvement; GFR-24U, GFR by 24-hour urine collection; eGFR-urea,creat, estimated GFR calculated from the serum urea and creatinine concentrations; B2M, β2-microglobulin; UV, urine volume.
Differences in c statistics were calculated using bootstrapping with 1000 replicates.
Base model included age, sex, dialysis modality, dialysis vintage, modified Charlson comorbidity index, smoking status, and serum albumin concentrations.
B2M data were available in 1640 patients. Comparison between base model and base model and eGFR B2M was performed in 1640 patients.
Discussion
This study evaluated the relative prognostic values of RKF indices on all-cause mortality in a nationwide prospective cohort of patients with ESRD. In this study, both residual UV and GFR-24U were independently associated with mortality risk in patients on dialysis. However, residual UV was the only indicator to improve discriminative power for mortality, suggesting that determining residual UV may be useful and additive to predict prognosis in patients on dialysis compared with the other RKF indices.
RKF is an important determinant for clinical outcomes, independent of elevated urea and creatinine clearance derived from dialysis therapy (3,5,7–9). Previous observational studies clearly showed that RKF was independently associated with patient survival (1–10). In the reanalysis of the CANUSA Study, preserved RKF was independently associated with lower death risk (5). In contrast, peritoneal creatinine clearance had no association with mortality, suggesting that RKF is more important than peritoneal clearance (5). In HD, the NECOSAD-2 showed that survival advantage of increasing the dialysis dose was strongly dependent on the presence of renal Kt/Vurea (29). Furthermore, aside from renal solute clearance, RKF has been known to be valuable in volume control, middle molecule clearance, and nutritional aspects (13–23). On the basis of these findings, preservation of adequate RKF has emerged as a pivotal strategy in the treatment of patients on dialysis, because loss of RKF cannot be superseded by increasing dialysis dose.
However, although several treatment guidelines recommend regular monitoring of RKF in patients on dialysis, measurement of RKF is cumbersome in clinical practice (24,30). RKF was calculated from GFR-24U in most guidelines (31). Therefore, complete and accurate urine collection is required, and patients should bring the collection bag to the laboratory. Concern for reliability of timed urine collection and technical issues regarding additional blood and urine sampling for urea and creatinine concentrations has made physicians hesitant to order RKF measurement in clinical practice. In contrast, determining residual UV without calculation of urea and creatinine clearance is simple and is not confined to urea and creatinine clearance. So far, there is little evidence on which RKF index is more closely associated with clinical outcomes than other RKF indices. The CANUSA Study first suggested that UV was more important than GFR in predicting adverse outcomes in patients on PD (5). In agreement with those findings, we showed that both GFR-24U and residual UV were independently associated with mortality risk. Of note, only UV added to the base model improved discriminative power for predicting mortality compared with the other RKF indices. The independent association of residual UV with patient survival still held true for nonanuric patients, patients on HD, and patients on PD, irrespective of dialysis modality. From these findings, we speculated that simple measurement of residual UV might have greater clinical advantage and implication than GFR-based indices in the management of patients on dialysis. Using the residual UV index can alleviate the burden on patients and health care providers of collecting 24-hour urine specimens and calculating clearance.
There are several possible explanations for why residual UV was more closely associated with adverse outcomes compared with the other RKF indices. One potential explanation is that UV has a stronger association with volume status. Several studies also revealed that extracellular volume expansion was associated with UV in patients on dialysis (27,32). Recently, overhydration measured by the Body Composition Monitor significantly correlated with UV but did not correlate with GFR-24U in patients on PD (27), supporting our observation. In patients on HD, UV was the largest in patients with low interdialytic weight gain (interdialytic weight gain/dry weight <1.0%), despite comparable GFR-24U (33). However, unfortunately, objective assessments of volume status, such as relative plasma volume monitoring, bioelectrical impedance analyses, or echocardiography, were not performed, making it difficult to affirm a stronger relationship between volume overload and UV in this study. Another possible explanation for stronger association between residual UV and mortality is that UV might reflect actual GFR better than GFR-24U. However, this issue cannot be verified in this study. It is worth investigating in a future study.
Patients with preserved RKF were found to have higher clearance of middle molecules, such as B2M, and protein-bound uremic toxins, including p-cresol sulfate and indoxyl sulfate, all of which are associated with a higher risk of death (15–19). In this study, UV showed a significant inverse association with serum B2M concentrations in accordance with previous studies (15,16,18,19). However, because this is the result from cross-sectional evaluation in which B2M clearance was not measured, we cannot assure causal effect of UV on middle molecule clearance. Moreover, RKF has a significant association with better phosphate control (20,21), nutritional benefit (22,23), and anemia control (10,21). In this study, the proportion of patients requiring phosphate binders or erythropoiesis stimulating agents was significantly higher in patients with UV<0.5 L/d (data not shown), suggesting favorable effect of residual UV on phosphorous control and anemia.
Our study has several limitations. First, RKF indices were measured once at baseline and were not serially monitored. It would be interesting to examine the changes in RKF indices during the study period and analyze how those changes affect clinical outcomes. Second, by virtue of its observational study design, any information regarding interventions to preserve RKF was not included. Interestingly, we recently reported that the use of icodextrin PD solution significantly preserved UV but not residual GFR better than conventional solution (34). Thus, the effect of RKF preservation on patient survival as measured by each RKF index might be worth investigating. Third, although measuring UV is simpler than calculating solute clearance, timed urine collection may still be inconvenient to patients and dialysis unit practitioners. In this study, >30% of initially enrolled patients declined a timed urine collection. From a clinical viewpoint, measuring UV by cup or volumetric container can be easily done at home. In fact, Shafi et al. (10) defined the presence of RKF by using the self-described ability to produce at least 1 cup (250 ml) per day and found that preserved RKF was independently associated with lower mortality in HD. Fourth, although there was no significant difference in the baseline characteristics between patients with and without 24-hour urine collection, 1946 patients may not be representative of the initially enrolled population, leading to potential selection bias. Fifth, new filtration markers, such as cystatin C and β-trace protein, were not available in our cohort. Recent studies proposed an equation using these markers without urine collection (26,35). Furthermore, precision and accuracy of β-trace protein, B2M, and cystatin C equations were significantly greater than for the urea- or creatinine-based equation (26). However, additional relatively expensive cost limits measurement of these markers in routine practice of dialysis units; β-trace protein assay is available only for research purposes in Korea. On the contrary, determining UV does not require additional cost. Although eGFR B2M was significantly associated with all-cause mortality, there was no significant improvement of predictability when eGFR B2M was added to the base model. Therefore, we suggest that measuring UV has advantage with respect to cost-effectiveness. Moreover, calculating GFR by the equation using the serum filtration markers, such as urea, creatinine, and B2M, may lead to imprecise estimation of kidney function, because these solutes are not in steady state during interdialytic period. Thus, equations using new filtration markers should be validated through additional studies. Notwithstanding these limitations, our study is the first investigation to explore the relative prognostic value of various RKF indices in a nationally distributed group of both patients on HD and patients on PD.
In conclusion, higher residual UV was significantly associated with a lower risk of death and showed stronger association with mortality compared with the other RKF indices in patients with ESRD, regardless of dialysis modality. These results suggest that determining residual UV may be beneficial to predict patient survival in patients on dialysis.
Disclosures
None.
Supplementary Material
Acknowledgments
The authors extend sincere appreciation to all participants and investigators of the cohort study (Clinical Research Center for End-Stage Renal Disease) in Korea.
This work was supported by the Brain Korea PLUS 21 Project for Medical Science, Yonsei University; National Research Foundation of Korea grant 2011-0030711 funded by the Korean Government (Ministry of Education, Science, and Technology); and grant HC15C1129 of the Korea Healthcare Technology R&D Project through the Korean Heath Industry Development Institute funded by the Ministry of Health & Welfare, Republic of Korea.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
See related editorial, “Preservation of Residual Kidney Function and Urine Volume in Patients on Dialysis,” on pages 377–379.
This article contains supplemental material online at http://cjasn.asnjournals.org/lookup/suppl/doi:10.2215/CJN.05520516/-/DCSupplemental.
References
- 1.Maiorca R, Brunori G, Zubani R, Cancarini GC, Manili L, Camerini C, Movilli E, Pola A, d’Avolio G, Gelatti U: Predictive value of dialysis adequacy and nutritional indices for mortality and morbidity in CAPD and HD patients. A longitudinal study. Nephrol Dial Transplant 10: 2295–2305, 1995 [DOI] [PubMed] [Google Scholar]
- 2.Canada-USA (CANUSA) Peritoneal Dialysis Study Group : Adequacy of dialysis and nutrition in continuous peritoneal dialysis: Association with clinical outcomes. Canada-USA (CANUSA) Peritoneal Dialysis Study Group. J Am Soc Nephrol 7: 198–207, 1996 [DOI] [PubMed] [Google Scholar]
- 3.Diaz-Buxo JA, Lowrie EG, Lew NL, Zhang SM, Zhu X, Lazarus JM: Associates of mortality among peritoneal dialysis patients with special reference to peritoneal transport rates and solute clearance. Am J Kidney Dis 33: 523–534, 1999 [DOI] [PubMed] [Google Scholar]
- 4.Szeto CC, Wong TY, Leung CB, Wang AY, Law MC, Lui SF, Li PK: Importance of dialysis adequacy in mortality and morbidity of chinese CAPD patients. Kidney Int 58: 400–407, 2000 [DOI] [PubMed] [Google Scholar]
- 5.Bargman JM, Thorpe KE, Churchill DN; CANUSA Peritoneal Dialysis Study Group : Relative contribution of residual renal function and peritoneal clearance to adequacy of dialysis: A reanalysis of the CANUSA study. J Am Soc Nephrol 12: 2158–2162, 2001 [DOI] [PubMed] [Google Scholar]
- 6.Shemin D, Bostom AG, Laliberty P, Dworkin LD: Residual renal function and mortality risk in hemodialysis patients. Am J Kidney Dis 38: 85–90, 2001 [DOI] [PubMed] [Google Scholar]
- 7.Termorshuizen F, Korevaar JC, Dekker FW, van Manen JG, Boeschoten EW, Krediet RT; NECOSAD Study Group : The relative importance of residual renal function compared with peritoneal clearance for patient survival and quality of life: An analysis of the Netherlands Cooperative Study on the Adequacy of Dialysis (NECOSAD )-2. Am J Kidney Dis 41: 1293–1302, 2003 [DOI] [PubMed] [Google Scholar]
- 8.Termorshuizen F, Dekker FW, van Manen JG, Korevaar JC, Boeschoten EW, Krediet RT; NECOSAD Study Group : Relative contribution of residual renal function and different measures of adequacy to survival in hemodialysis patients: An analysis of the Netherlands Cooperative Study on the Adequacy of Dialysis (NECOSAD)-2. J Am Soc Nephrol 15: 1061–1070, 2004 [DOI] [PubMed] [Google Scholar]
- 9.Vilar E, Wellsted D, Chandna SM, Greenwood RN, Farrington K: Residual renal function improves outcome in incremental haemodialysis despite reduced dialysis dose. Nephrol Dial Transplant 24: 2502–2510, 2009 [DOI] [PubMed] [Google Scholar]
- 10.Shafi T, Jaar BG, Plantinga LC, Fink NE, Sadler JH, Parekh RS, Powe NR, Coresh J: Association of residual urine output with mortality, quality of life, and inflammation in incident hemodialysis patients: The Choices for Healthy Outcomes in Caring for End-Stage Renal Disease (CHOICE) Study. Am J Kidney Dis 56: 348–358, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Eknoyan G, Beck GJ, Cheung AK, Daugirdas JT, Greene T, Kusek JW, Allon M, Bailey J, Delmez JA, Depner TA, Dwyer JT, Levey AS, Levin NW, Milford E, Ornt DB, Rocco MV, Schulman G, Schwab SJ, Teehan BP, Toto R; Hemodialysis (HEMO) Study Group : Effect of dialysis dose and membrane flux in maintenance hemodialysis. N Engl J Med 347: 2010–2019, 2002 [DOI] [PubMed] [Google Scholar]
- 12.Paniagua R, Amato D, Vonesh E, Correa-Rotter R, Ramos A, Moran J, Mujais S; Mexican Nephrology Collaborative Study Group : Effects of increased peritoneal clearances on mortality rates in peritoneal dialysis: ADEMEX, a prospective, randomized, controlled trial. J Am Soc Nephrol 13: 1307–1320, 2002 [DOI] [PubMed] [Google Scholar]
- 13.Bragg-Gresham JL, Fissell RB, Mason NA, Bailie GR, Gillespie BW, Wizemann V, Cruz JM, Akiba T, Kurokawa K, Ramirez S, Young EW: Diuretic use, residual renal function, and mortality among hemodialysis patients in the Dialysis Outcomes and Practice Pattern Study (DOPPS). Am J Kidney Dis 49: 426–431, 2007 [DOI] [PubMed] [Google Scholar]
- 14.Konings CJ, Kooman JP, Schonck M, Struijk DG, Gladziwa U, Hoorntje SJ, van der Wall Bake AW, van der Sande FM, Leunissen KM: Fluid status in CAPD patients is related to peritoneal transport and residual renal function: Evidence from a longitudinal study. Nephrol Dial Transplant 18: 797–803, 2003 [DOI] [PubMed] [Google Scholar]
- 15.Bammens B, Evenepoel P, Verbeke K, Vanrenterghem Y: Removal of middle molecules and protein-bound solutes by peritoneal dialysis and relation with uremic symptoms. Kidney Int 64: 2238–2243, 2003 [DOI] [PubMed] [Google Scholar]
- 16.Pham NM, Recht NS, Hostetter TH, Meyer TW: Removal of the protein-bound solutes indican and p-cresol sulfate by peritoneal dialysis. Clin J Am Soc Nephrol 3: 85–90, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Roumelioti ME, Nolin T, Unruh ML, Argyropoulos C: Revisiting the middle molecule hypothesis of uremic toxicity: A systematic review of beta 2 microglobulin population kinetics and large scale modeling of hemodialysis trials in silico. PLoS One 11: e0153157, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fry AC, Singh DK, Chandna SM, Farrington K: Relative importance of residual renal function and convection in determining beta-2-microglobulin levels in high-flux haemodialysis and on-line haemodiafiltration. Blood Purif 25: 295–302, 2007 [DOI] [PubMed] [Google Scholar]
- 19.Marquez IO, Tambra S, Luo FY, Li Y, Plummer NS, Hostetter TH, Meyer TW: Contribution of residual function to removal of protein-bound solutes in hemodialysis. Clin J Am Soc Nephrol 6: 290–296, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang AY, Woo J, Wang M, Sea MM, Sanderson JE, Lui SF, Li PK: Important differentiation of factors that predict outcome in peritoneal dialysis patients with different degrees of residual renal function. Nephrol Dial Transplant 20: 396–403, 2005 [DOI] [PubMed] [Google Scholar]
- 21.Penne EL, van der Weerd NC, Grooteman MP, Mazairac AH, van den Dorpel MA, Nubé MJ, Bots ML, Lévesque R, ter Wee PM, Blankestijn PJ; CONTRAST investigators : Role of residual renal function in phosphate control and anemia management in chronic hemodialysis patients. Clin J Am Soc Nephrol 6: 281–289, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang AY, Sea MM, Ip R, Law MC, Chow KM, Lui SF, Li PK, Woo J: Independent effects of residual renal function and dialysis adequacy on actual dietary protein, calorie, and other nutrient intake in patients on continuous ambulatory peritoneal dialysis. J Am Soc Nephrol 12: 2450–2457, 2001 [DOI] [PubMed] [Google Scholar]
- 23.Szeto CC, Lai KN, Wong TY, Law MC, Leung CB, Yu AW, Li PK: Independent effects of residual renal function and dialysis adequacy on nutritional status and patient outcome in continuous ambulatory peritoneal dialysis. Am J Kidney Dis 34: 1056–1064, 1999 [DOI] [PubMed] [Google Scholar]
- 24.European Best Practice Guidelines Expert Group on Hemodialysis, European Renal Association : Section I. Measurement of renal function, when to refer and when to start dialysis. Nephrol Dial Transplant 17[Suppl 7]: 7–15, 2002 [DOI] [PubMed] [Google Scholar]
- 25.Du Bois D, Du Bois EF: A formula to estimate the approximate surface area if height and weight be known. 1916. Nutrition 5: 303–311, 1989 [PubMed] [Google Scholar]
- 26.Shafi T, Michels WM, Levey AS, Inker LA, Dekker FW, Krediet RT, Hoekstra T, Schwartz GJ, Eckfeldt JH, Coresh J: Estimating residual kidney function in dialysis patients without urine collection. Kidney Int 89: 1099–1110, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jung ES, Sung JY, Han SY, Kim AJ, Ro H, Jung JY, Lee HH, Chung W, Chang JH: Residual urinary volume is a predictor of overhydration in patients on peritoneal dialysis. Tohoku J Exp Med 233: 295–300, 2014 [DOI] [PubMed] [Google Scholar]
- 28.Hemmelgarn BR, Manns BJ, Quan H, Ghali WA: Adapting the Charlson Comorbidity Index for use in patients with ESRD. Am J Kidney Dis 42: 125–132, 2003 [DOI] [PubMed] [Google Scholar]
- 29.Nolph KD, Moore HL, Prowant B, Meyer M, Twardowski ZJ, Khanna R, Ponferrada L, Keshaviah P: Cross sectional assessment of weekly urea and creatinine clearances and indices of nutrition in continuous ambulatory peritoneal dialysis patients. Perit Dial Int 13: 178–183, 1993 [PubMed] [Google Scholar]
- 30.Peritoneal Dialysis Adequacy Work Group : Clinical practice guidelines for peritoneal dialysis adequacy. Am J Kidney Dis 48[Suppl 1]: S98–S129, 2006 [DOI] [PubMed] [Google Scholar]
- 31.Tattersall J, Martin-Malo A, Pedrini L, Basci A, Canaud B, Fouque D, Haage P, Konner K, Kooman J, Pizzarelli F, Tordoir J, Vennegoor M, Wanner C, ter Wee P, Vanholder R: EBPG guideline on dialysis strategies. Nephrol Dial Transplant 22[Suppl 2]: ii5–ii21, 2007 [DOI] [PubMed] [Google Scholar]
- 32.Davenport A, Sayed RH, Fan S: Is extracellular volume expansion of peritoneal dialysis patients associated with greater urine output? Blood Purif 32: 226–231, 2011 [DOI] [PubMed] [Google Scholar]
- 33.Lee MJ, Doh FM, Kim CH, Koo HM, Oh HJ, Park JT, Han SH, Yoo TH, Kim YL, Kim YS, Yang CW, Kim NH, Kang SW: Interdialytic weight gain and cardiovascular outcome in incident hemodialysis patients. Am J Nephrol 39: 427–435, 2014 [DOI] [PubMed] [Google Scholar]
- 34.Chang TI, Ryu DR, Yoo TH, Kim HJ, Kang EW, Kim H, Chang JH, Kim DK, Moon SJ, Yoon SY, Han SH; Yonsei Associate Network CHronic Kidney Disease Trial (YACHT) investigators : Effect of icodextrin solution on the preservation of residual renal function in peritoneal dialysis patients: A randomized controlled study. Medicine (Baltimore) 95: e2991, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hoek FJ, Korevaar JC, Dekker FW, Boeschoten EW, Krediet RT: Estimation of residual glomerular filtration rate in dialysis patients from the plasma cystatin C level. Nephrol Dial Transplant 22: 1633–1638, 2007 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.