Abstract
Background and objectives
For many women pregnancy is the first contact with health services, thus providing an opportunity to identify renal disease. This study compares causes and long-term renal outcomes of biopsy-proven renal disease identified during pregnancy or within 1 year postpartum, with nonpregnant women.
Design, setting, participants, & measurements
Native renal biopsies (1997–2012), in women of childbearing age (16 to <50 years), from 21 hospitals were studied. The pregnancy-related diagnosis group included those women with abnormal urinalysis/raised creatinine identified during pregnancy or within 1 year postpartum. Pregnancy-related and control biopsies were matched for age and ethnicity (black versus nonblack).
Results
One hundred and seventy-three pregnancy-related biopsies (19 antenatal, 154 postpregnancy) were identified and matched with 1000 controls. FSGS was more common in pregnancy-related biopsies (32.4%) than controls (9.7%) (P<0.001) but there were no differences in Columbia classification. Women with a pregnancy-related diagnosis were younger (32.1 versus 34.2 years; P=0.004) and more likely to be black (26.0% versus 13.3%; P<0.001) than controls, although there were no differences in ethnicities in women with FSGS. The pregnancy-related group (excluding antenatal biopsies) was more likely to have a decline in Chronic Kidney Disease Epidemiology Collaboration eGFR in the follow-up period than the control group (odds ratio, 1.67; 95% confidence interval, 1.03 to 2.71; P=0.04), and this decline appeared to be more rapid (−1.33 versus −0.56 ml/min per 1.73 m2 per year, respectively; P=0.045). However, there were no differences between groups in those who required RRT or who died.
Conclusions
Pregnancy is an opportunity to detect kidney disease. FSGS is more common in women who have been pregnant than in controls, and disease identified in pregnancy or within 1 year postpartum is more likely to show a subsequent decline in renal function. Further work is required to determine whether pregnancy initiates, exacerbates, or reveals renal disease.
Keywords: pregnancy; renal biopsy; preeclampsia; focal segmental glomerulosclerosis; biopsy; creatinine; female; follow-up studies; glomerulosclerosis, focal segmental; humans; kidney; odds ratio; postpartum period; pregnancy; renal insufficiency, chronic; urinalysis; urinary tract physiological phenomena
Introduction
CKD is estimated to affect up to 6% of women of childbearing age in high-income countries (1). Frequently, antenatal visits are the first time women are assessed by health care, thus providing an opportunity to identify CKD. However, few studies have investigated the etiology of renal disease identified during or after pregnancy, and to our knowledge none have compared with the spectrum of disease in nonpregnant women. Improved understanding of renal pathologies identified during pregnancy will inform decision-making about the necessity of biopsy during or after pregnancy.
Pregnancy is a “stress test” for the kidney and may lead to progression of preexisting disease. It is possible that less severe disease may be revealed during pregnancy but there is limited study of natural history of renal disease identified during or after pregnancy to guide long-term prognosis and management of young women with newly-diagnosed CKD.
Our aims were to define the causes and long-term outcomes of renal disease identified during pregnancy or within 1 year postpartum, and compare these with nonpregnant women of childbearing age.
Materials and Methods
All renal biopsy reports for women of childbearing age (16 to <50 years) from five renal units serving 21 referring hospitals (1997–2012) were reviewed. The clinical details of the reason for biopsy were assessed, and pathology records interrogated. Women who had biopsies for abnormal urinalysis/raised serum creatinine (SCr) identified during pregnancy (regardless of the timing of the subsequent biopsy), and all women who had biopsies within 1 year postpartum were included in the pregnancy-related diagnosis group. Repeat and inadequate biopsies were excluded. Protein-to-creatinine ratio was recorded where available, and quantification in grams per 24 hours was converted to an estimated protein-to-creatinine ratio by multiplying by 100. Albumin-to-creatinine ratio (ACR) quantifications were excluded from analyses as they could not be reliably converted to an estimated protein-to-creatinine ratio.
Rate of change of eGFR per year was calculated using the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation (2). Women of mixed race that included black heritage were categorized as black. Women who were on RRT during follow-up were considered to have eGFR of 10 ml/min per 1.73 m2 for the purpose of calculating change in renal function over time. In view of the expected physiologic fall in SCr during pregnancy, for the purpose of assessing eGFR and rate of change in eGFR, comparison was made between controls and postpregnancy patients only, i.e., women who had biopsies during pregnancy were not included in this analysis because of inaccuracies of GFR estimates in pregnancy (3,4).
Each biopsy was assigned a primary diagnosis, and additional concurrent diagnoses recorded. If FSGS was present but deemed to be secondary to an alternative intrinsic renal pathology, patients were categorized according to the primary diagnosis and not FSGS. All pregnancy-related biopsies and all indeterminate cases (including in the control group) were re-reviewed by two histopathologists independently with consensus agreement for diagnosis. All FSGS biopsies (pregnancy-related and controls) were formally classified according to Columbia classification (5). For the remaining controls, the biopsy diagnosis in the original report was used.
Statistical Analyses
Control women were matched against pregnant women by age (at last birthday) and ethnic group (black versus nonblack) and unmatched controls were dropped. In order to maintain power, all controls matched for age and ethnicity were included. Proportions were compared using two-sided chi-squared test with Yate correction, and the nonparametric Mann–Whitney U test was used for non-normally distributed continuous variables. Logistic regression analysis with a dummy variable for matching group was used to adjust for any effect of age, ethnicity, or their interaction on diagnosis and renal outcomes. Spearman rank correlation was used to explore the relationship between non-normally distributed continuous variables. Statistical analyses were performed using Stata version 14.1 (StataCorp, College Station, TX) and GraphPad Prism (version 7.0).
Results
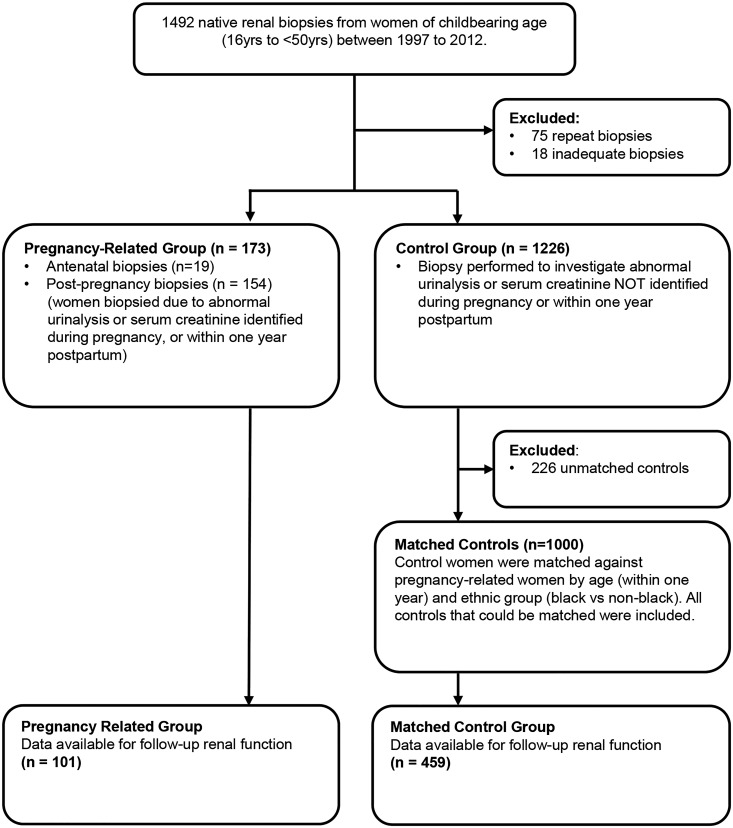
One thousand three hundred and ninety-nine biopsies were identified in women aged between 16 and 50 years, including 173 pregnancy-related biopsies (19 antenatal, 154 postpregnancy) and 1226 control biopsies, between 1997 and 2012. One thousand control women were matched for age and ethnicity, and included all women with equal ages and ethnicities as the pregnancy-related group i.e., women were not excluded if there was more than one match per pregnancy-related case. Figure 1 shows how the matched cohorts were assembled, and the baseline demographics are shown in Table 1. The median age of women with a pregnancy-related diagnosis was significantly lower than controls, and there was a higher proportion of women of black or black British ethnicity in the pregnancy-related group compared with controls. SCr at the time of biopsy was significantly lower in the pregnancy-related group. There was no difference in urinary protein-to-creatinine ratio between women with biopsies performed postpregnancy and controls, but protein-to-creatinine ratio in women with biopsies performed antenatally was significantly higher than those performed after delivery (P=0.02), and compared with controls (P=0.02).
Figure 1.
Flow diagram of the identification and assembly of matched cohorts.
Table 1.
Demographics of women of childbearing age—all diagnoses
| Variables | Controls | Pregnancy-Related (Antenatal and Postpregnancy) | P Value | Antenatal | Postpregnancy | P Value |
|---|---|---|---|---|---|---|
| N= | 1000 | 173 | 19 | 154 | ||
| Median age, yr (IQR) | 34.2 (28.0–41.0) | 32.1 (27.8–36.5) | 0.004a | 30.9 (25.5–36.0) | 32.2 (28.4–36.9) | 0.25b |
| Ethnicity, N (%) | ||||||
| White | 324 (32.4) | 53 (30.6) | 0.65a | 8 (42.1) | 45 (29.2) | 0.29b |
| Mixed | 12 (1.2) | 2 (1.2) | 0.96a | 0 (0.0) | 2 (1.3) | >0.99b |
| Asian/Asian British | 141 (14.1) | 18 (10.4) | 0.19a | 1 (5.3) | 17 (11.0) | 0.70b |
| Black/Black British | 133 (13.3) | 45 (26.0) | <0.001a | 7 (36.8) | 38 (24.7) | 0.27b |
| Other groups | 97 (9.7) | 14 (8.1) | 0.50a | 2 (10.5) | 12 (7.8) | 0.65b |
| Not stated | 293 (29.3) | 41 (23.7) | 0.13a | 1 (5.3) | 40 (26.0) | 0.05b |
| N= | 825 | 152 | 16 | 136 | ||
| Median SCr at time of biopsy, mg/dl (IQR) | 1.11 (0.77–2.06) | 0.90 (0.74–1.51) | 0.79 (0.63–1.33) | 0.90 (0.74–1.58) | 0.18b–0.002c | |
| Median CKD-EPI GFR at time of biopsy, ml/min per 1.73 m2 (IQR) | 65.9 (30.8–102.6) | 85.7 (47.6–114.1) | 108.2 (60.4–130.2) | 84.1 (46.8–112.3) | <0.001c | |
| N= | 609 | 131 | 14 | 117 | ||
| Median urine protein-to-creatinine at time of biopsy, mg/mmol (IQR) | 240.0 (100.0–576.0) | 237.0 (125.0–580.0) | 536.5 (280.3–824.5) | 200.0 (121.0–503.5) | 0.02b–0.80c |
IQR, interquartile range; SCr, serum creatinine; CKD-EPI, Chronic Kidney Disease Epidemiology Collaboration.
Comparison between controls and pregnancy-related (antenatal and postpregnancy).
Comparison between antenatal and postpregnancy.
Comparison between controls and postpregnancy.
Histologic Diagnoses
FSGS was the primary diagnosis in 32.4% (56 of 173) of pregnancy-related biopsies compared with 9.7% (97 of 1000) of controls (P<0.001). Lupus nephritis (LN) was found more commonly in controls (23.5% [235 of 1000] versus 13.9% [24 of 173]; P=0.001) (Table 2).
Table 2.
Renal biopsy diagnoses in women of childbearing age comparing disease identified in pregnancy with controls
| Variables | Controls | Pregnancy-Related (Antenatal and Postpregnancy) | P Valuea | Adjusted for Age & Ethnicitya | Antenatal | Postpregnancy | P Valueb | |
|---|---|---|---|---|---|---|---|---|
| OR (95% CI) | P Value | |||||||
| N= | 1000 | 173 | 19 | 154 | ||||
| FSGS, N (%) | 97 (9.7) | 56 (32.4) | <0.001 | 4.42 (3.00 to 6.55) | <0.001 | 6 (31.6) | 104 (32.5) | >0.99 |
| Lupus, N (%) | 235 (23.5) | 24 (13.9) | 0.01 | 0.44 (0.28 to 0.70) | 0.001 | 5 (26.3) | 19 (12.3) | 0.15 |
| IgA, N (%) | 147 (14.7) | 25 (14.5) | 0.93 | 1.10 (0.69 to 1.76) | 0.69 | 1 (5.3) | 24 (15.6) | 0.32 |
| Interstitial nephritis, N (%) | 62 (6.2) | 8 (4.6) | 0.42 | 0.74 (0.35 to 1.59) | 0.45 | 0 (0.0) | 8 (5.2) | 0.60 |
| Membranous, N (%) | 53 (5.3) | 9 (5.2) | 0.96 | 1.01 (0.48 to 2.10) | 0.99 | 2 (10.5) | 7 (4.5) | 0.26 |
| Minimal change, N (%) | 50 (5.0) | 5 (2.9) | 0.23 | 0.59 (0.23 to 1.51) | 0.27 | 0 (0.0) | 5 (3.2) | >0.99 |
| Thin membrane, N (%) | 30 (3.0) | 9 (5.2) | 0.14 | 2.11 (0.97 to 4.60) | 0.06 | 0 (0.0) | 9 (5.8) | 0.60 |
| DM, N (%) | 35 (3.5) | 1 (0.6) | 0.04 | 0.18 (0.25 to 1.42) | 0.10 | 0 (0.0) | 1 (0.7) | >0.99 |
| Crescentic, N (%) | 14 (1.4) | 0 (0.0) | 0.12 | — | — | 0 (0.0) | 0 (0.0) | >0.99 |
| FSGS (HIV), N (%) | 6 (0.6) | 1 (0.6) | 0.97 | 0.51 (0.06 to 4.37) | 0.54 | 0 (0.0) | 1 (0.6) | >0.99 |
| Other, N (%) | 271 (27.1) | 35 (20.2) | 0.06 | 0.73 (0.50 to 1.08) | 0.12 | 5 (26.3) | 30 (19.5) | 0.54 |
OR, odds ratio; 95% CI, 95% confidence interval; DM, Diabetes mellitus; —, unable to calculate.
Comparison between controls and pregnancy-related (antenatal and postpregnancy).
Comparison between antenatal and postpregnancy.
Women with pregnancy-related FSGS were younger than controls but there were no significant differences in ethnicity or Colombia classification between groups. Creatinine at time of biopsy was significantly lower in the pregnancy-related group. (Table 3). There were no disagreements between histopathologists in diagnoses or Columbia classifications.
Table 3.
Demographics of women of childbearing age with FSGS
| Variables | Controls | Pregnancy-Related (Antenatal and Postpregnancy) | P Value | Antenatal | Postpregnancy | P Value |
|---|---|---|---|---|---|---|
| N= | 97 | 56 | 6 | 50 | ||
| Median age, yr (IQR) | 35.7 (30.3–41.9) | 33.4 (27.3–39.1) | 0.05a | 29.5 (22.5–37.1) | 33.7 (28.7–39.2) | 0.26b |
| Ethnicity, N (%) | ||||||
| White | 29 (29.9) | 18 (32.1) | 0.77a | 3 (50.0) | 15 (30.0) | 0.38b |
| Mixed | 1 (1.0) | 1 (1.8) | 0.69a | 0 (0.0) | 1 (2.0) | >0.99b |
| Asian/AsianBritish | 10 (10.3) | 4 (7.1) | 0.51a | 0 (0.0) | 4 (8.0) | >0.99b |
| Black/Black British | 22 (22.7) | 18 (32.1) | 0.20a | 3 (50.0) | 15 (30.0) | 0.37b |
| Other groups | 9 (9.3) | 2 (3.8) | 0.19a | 0 (0.0) | 2 (4.0) | >0.99b |
| Not stated | 26 (26.8) | 13 (23.2) | 0.62a | 0 (0.0) | 13 (26.0) | 0.32b |
| Columbia classification, N (%) | ||||||
| Cellular | 3 (3.1) | 3 (5.4) | 0.49a | 1 (16.7) | 2 (4.0) | 0.29b |
| Collapsing | 7 (7.2) | 1 (1.8) | 0.15a | 0 (0.0) | 1 (2.0) | >0.99b |
| NOS | 67 (69.1) | 38 (67.9) | 0.88a | 5 (83.3) | 33 (66.0) | 0.65b |
| Perihilar | 8 (8.2) | 8 (14.3) | 0.24a | 0 (0.0) | 8 (16.0) | 0.58b |
| Tip | 8 (8.2) | 6 (10.7) | 0.61a | 0 (0.0) | 6 (12.0) | >0.99b |
| Not classified | 4 (4.1) | 0 (0.0) | 0.12a | 0 (0.0) | 0 (0.0) | >0.99b |
| N= | 79 | 49 | 5 | 44 | ||
| Median SCr at time of biopsy mg/dl (IQR) | 1.07 (0.84–1.75) | 0.92 (0.72–1.32) | 0.92 (0.64–1.48) | 0.93 (0.75–1.29) | 0.85b–0.04c | |
| Median CKD-EPI GFR at time of biopsy ml/min per 1.73m2 (IQR) | 71.6 (41.2–92.6) | 86.0 (58.3–112.0) | 102.1 (49.5–135.7) | 85.4 (58.4–110.2) | 0.02c | |
| N= | 72 | 43 | 5 | 38 | ||
| Median urine protein-to-creatinine at time of biopsy mg/mmol (IQR) | 340.0 (165.0–629.5) | 237.0 (160.0–600.0) | 600.0 (250.0–708.0) | 200.0 (157.5–495.3) | 0.17b–0.27c |
IQR, interquartile range; SCr, serum creatinine; CKD-EPI, Chronic Kidney Disease Epidemiology Collaboration.
Comparison between controls and pregnancy-related (antenatal and postpregnancy).
Comparison between antenatal and postpregnancy.
Comparison between controls and postpregnancy.
Renal Outcomes
The date of delivery was available in 67.5% of biopsies performed after delivery. The median time from delivery to biopsy was 199 days (interquartile range, 92–312), including nine biopsies performed within 6 weeks. Follow-up data on renal function were available for 58.4% (101 of 173) of the pregnancy-related group and 45.9% (459 of 1000) of the control group (Table 3). There was no difference in the median follow-up time between pregnancy-related and control groups. Women with a pregnancy-related diagnosis (excluding those diagnosed antenatally), were more likely to have an overall decline in eGFR in the follow-up period than matched controls despite adjustment for remaining differences in age and ethnicity between groups (odds ratio, 1.67; 95% confidence interval, 1.03 to 2.71; P=0.04). This decline also appeared to be more rapid in the pregnancy-related group (−1.33 versus −0.56 ml/min per 1.73 m2 per year, respectively; P=0.045). There was no correlation between age and rate of decline in eGFR (Spearman rho=0.03; n=546; P=0.53). There were no significant differences between the proportion of women requiring RRT between pregnancy-related cases and controls, or deaths during the follow-up period (Table 4).
Table 4.
Follow-up of women of childbearing age—all diagnoses
| Variables | Controls | Pregnancy-Related (Antenatal and Postpregnancy) | P Value | Antenatal | Postpregnancy | P Value |
|---|---|---|---|---|---|---|
| N = | 459 | 101 | 14 | 87 | ||
| Median follow-up time months (IQR) | 44.3 (20.1–77.2) | 42.8 (17.4–70.9) | 0.48a | 40.8 (24.2–75.6) | 43.3 (17.0–70.8) | >0.99b |
| Median rate of change in CKD-EPI GFR ml/min per 1.73 m2 per yr (IQR) | −0.56 (−4.26–3.22) | −2.43 (−8.16–0.18) | −7.36 (−15.44–−4.35) | −1.33 (−6.97–0.94) | 0.002b–0.045c | |
| Died, % (N) | 3.4 (34 of 1000) | 2.3 (4 of 173) | 0.46a | 10.5 (2 of 19) | 1.3 (2 of 154) | 0.06b |
| RRT, % (N) | 13.0 (130 of 1000) | 12.7 (22 of 173) | 0.92a | 15.8 (3 of 19) | 12.3 (19 of 154) | 0.72b |
| Time to reach RRT, m (IQR) | 18.55 (5.37–45.79) | 17.8 (7.3–46.5) | 0.93a | 17.8 (11.3–89.5) | 19.8 (4.5–44.7) | 0.53b |
| Age at RRT, yr (IQR) | 37.9 (30.4–44.1) | 34.0 (27.7–40.8) | 0.27a | 34.2 (24.9–40.3) | 33.2 (27.7–42.2) | 0.74b |
IQR, interquartile range; CKD-EPI, Chronic Kidney Disease Epidemiology Collaboration.
Comparison between controls and pregnancy-related (antenatal and postpregnancy).
Comparison between antenatal and postpregnancy.
Comparison between controls and postpregnancy.
There were no significant differences in rate of change in eGFR in women with FSGS between groups, or requirement for RRT, or death (Table 5).
Table 5.
Follow-up of women of childbearing age with FSGS
| Variables | Controls | Pregnancy-Related (Antenatal and Postpregnancy) | P Value | Antenatal | Postpregnancy | P Value |
|---|---|---|---|---|---|---|
| N= | 49 | 31 | 4 | 27 | ||
| Median follow-up time, m (IQR) | 45.9 (26.3–92.6) | 48.0 (30.6–75.9) | 0.83a | 41.9 (37.0–78.3) | 50.3 (20.0–75.9) | >0.99b |
| Median rate of change in CKD-EPI GFR ml/min per 1.73 m2 per yr (IQR) | −1.98 (−5.98–0.26) | −2.66 (−8.97–0.00) | −8.48 (−12.85–−6.89) | −1.62 (−7.55–0.21) | 0.06b–0.91c | |
| Died, % (N) | 3.1 (3 of 97) | 1.8 (1 of 56) | 0.63a | 16.7 (1 of 6) | 0.0 (0 of 50) | 0.11b |
| RRT, % (N) | 15.5 (15 of 97) | 7.1 (4 of 56) | 0.13a | 16.7 (1 of 6) | 6.0 (3 of 50) | 0.37b |
| Time to reach RRT, m (IQR) | 46.0 (12.99–68.13) | 64.7 (50.3–89.5) | 0.17a | 89.5 | 57.5 (50.3–64.7) | — |
| Age at RRT, yr (IQR) | 39.3 (33.4–46.6) | 40.3 (32.1–40.3) | 0.77a | 40.3 | 36.2 (32.1–40.3) | — |
IQR, interquartile range; –, numbers too small to calculate.
Comparison between controls and pregnancy-related (antenatal and postpregnancy).
Comparison between antenatal and postpregnancy.
Comparison between controls and postpregnancy.
Comparison between Antenatal and Postpartum Biopsies
Median SCr was similar, and urinary protein-to-creatinine ratio higher, at the time of biopsy in women who had biopsies antenatally compared with those who had biopsies postpregnancy (Table 1). There were no differences in biopsy diagnoses between groups (Table 2) and no difference in those that died or required RRT (Table 3).
Discussion
Main Findings
This study demonstrates the wide range of glomerular diseases identified by renal biopsy during or within 1 year of pregnancy, supporting the role of renal biopsy for confirmation of diagnosis in this patient group. FSGS was more frequently reported in pregnancy-related biopsies than in controls, but no differences in Colombia classification were observed. Conversely, LN was less commonly identified during or after pregnancy than in controls. Women with a pregnancy-related diagnosis had lower SCr concentrations at time of biopsy than controls, thus pregnancy may provide an opportunity to identify CKD at earlier stages. Women with biopsies after pregnancy also had a more rapid decline of eGFR during follow-up than controls, despite comparable severity of proteinuria, highlighting the importance of detection and diagnosis of renal disease revealed by pregnancy. Nearly one in forty women (2.3%) with a pregnancy-related renal biopsy died and one in eight (12.7%) required RRT during the follow-up period. This emphasizes the severity of a diagnosis of glomerular disease in young women, and the substantial implications it has for the individual and her new family.
Strengths and Weaknesses
To our knowledge, this is the largest study of pregnancy and postpregnancy biopsies with secondary histologic classification, and the only study to include controls with matching for age and ethnicity. Data were from 21 referring centers hence unlikely to be confounded by center-specific subjective decision-making about indications for biopsy. However, the study is unable to address the long-term renal outcomes of other renal diseases identified within 1 year of pregnancy that do not require biopsy for diagnosis e.g., reflux nephropathy or cystic kidney disease, thus these data relate only to glomerular disease. One of the limitations of our study was the absence of detailed pregnancy data (including parity and diagnosis of preeclampsia) hence it was not possible to establish the relationship between pregnancy outcomes and renal biopsy lesions. Furthermore, because of the large number of centers included it was not possible to confirm that all control women had not had a recent pregnancy which was not reported on the biopsy request form. We acknowledge also that follow-up data on renal function was available for approximately half of the pregnant and control groups.
FSGS
FSGS was found in nearly a third of pregnancy-related biopsies, with no differences in Columbia classification of FSGS between pregnancy-related and control groups. FSGS was also the most common diagnosis in postpartum biopsies by Day et al. (6), identified in a comparable proportion of pregnancies (28%), but Columbia classification of FSGS was not reported. FSGS lesions (7,8) and FSGS-like lesions (9) have been described in some biopsy series of women with preeclampsia, with correlation between severity of lesions and clinical findings (10).
Unlike animal micropuncture studies (11) which report unchanged intraglomerular pressure during pregnancy, a recent systematic review, using synthesized estimations from formal assessment of renal plasma flow and GFR, described an increase in filtration fraction in healthy pregnancy (12), thus further exacerbation of hemodynamic changes could contribute to the development of FSGS in preeclampsia. However, the proportion of biopsies with the perihilar variant of FSGS, which is the typical pattern of adaptive FSGS in nonpregnant patients (13), was not greater in pregnancy-related cases than control groups in our study, although true discrepancies may not have been identified by the small numbers within classification subgroups.
More recently, podocyte loss has been proposed to lead to progressive renal injury in women with preeclampsia. Podocyturia is reported in women with preeclampsia, before, at time of diagnosis, and postpartum (14–16), and downregulation of podocyte-specific proteins (e.g., nephrin, synaptopodin, and GLEPP-1) is reported in the renal biopsies of women with preeclampsia (17,18). Similarly, podocyturia is observed in patients with FSGS (19) and with progression of other glomerular diseases (20). However, there is a regression of histologic findings of preeclampsia, including complete resolution of FSGS-like lesions after delivery in historic large biopsy series (10,21). Furthermore, detailed renal physiologic assessment of 57 women with preeclampsia observed that functional manifestations of glomerular endothelial injury were undetectable after 4 weeks (22), suggesting that immediate pathophysiologic changes secondary to preeclampsia may not be contributory to persistent renal abnormalities in the postpartum period, and that preexisting renal injury may be important. For example, one in five women with severe preeclampsia had underlying renal disease in a biopsy series of 86 women (23), and a wide range of renal pathologies were reported in a population study of renal biopsies performed in pregnancies complicated by preeclampsia (24). Moreover, Norwegian population studies have identified preeclampsia in a previous pregnancy to be associated with increased relative risk of having a future renal biopsy (24), and developing future ESRD (25), but the risk of progression to ESRD in women with renal biopsies remote from pregnancy is not augmented by a history of previous preeclampsia (26).
Other Histologic Diagnoses
Higher rates of LN were observed in the renal biopsies of the control group compared with women in the pregnancy-related group. A recent systematic review reported an estimated rate of 16.1% (95% confidence interval, 9.0% to 23.2%) LN flare during pregnancy (27). However, Day et al. (6) reported LN to be present in 35% and 8% of biopsies performed in pregnancy and postpartum, respectively. The lower incidence of LN diagnosed within 1 year of pregnancy in this study may reflect more women with SLE conceiving with quiescent disease or differences in local practice. For example, because of perceived risks of renal biopsy during pregnancy, some physicians may treat women who develop LN empirically without biopsy.
Progression of CKD
In this study, women with a pregnancy-related diagnosis had lower SCr at time of biopsy than controls, even after exclusion of antenatal biopsies and adjustment for age. Pregnancy is associated with a 50% increase in glomerular filtration thus is a stress test for the kidney (28), and may provide an opportunity to detect early disease. Our study also identified a more rapid decline in GFR in women with renal disease identified within 1 year of pregnancy, despite comparable levels of proteinuria, less severe disease at diagnosis, and adjustment for age and ethnicity. The National Institute of Clinical Health Excellence (NICE) recommend that the absolute risk of renal disease after hypertensive complications in pregnancy is low and no specific advice or follow-up is required (29), and there are no specific recommendations regarding follow-up of renal disease identified during pregnancy. NICE guidelines recommend that 24-hour urine collection remains the gold standard of analysis of proteinuria in pregnancy; however, spot urine protein-to-creatinine ratio is an acceptable alternative therefore both methods were included. There is insufficient evidence on the use of ACR in pregnancy (29,30) and therefore they were not included in the analysis. The CKD-EPI formula may underestimate GFR in pregnant women (2,31), hence patients with antenatal biopsies were not included in analyses of renal function. Nine of the postpartum group had biopsies within 6 weeks of delivery, and a persistent pregnancy-related elevation in eGFR may have influenced findings, although this effect is likely to be minimal. It is also possible that nephrology-led follow-up of only women with more severe disease diagnosed during or after pregnancy may confound analysis of progression of renal disease, although the inclusion of five renal centers in this study reduces the influence of individual center policy on selection of women who continued to have nephrologic follow-up. The rate of ESRD (12.7%) in women with a pregnancy-related diagnosis was not higher than controls, but appears to be lower than a smaller study of 53 women with proteinuria identified in pregnancy performed over two decades ago (21% progressed to ESRD) (32). In contrast, Day et al. reported much higher rates of women developing ESRD (30%) after women who had biopsies antenatally which may reflect differences in thresholds for biopsy or length of follow-up. The high rate of progression of renal disease identified during pregnancy suggests that earlier intervention and nephrology follow-up, even for those with less severe disease, is warranted.
Indications and Safety of Biopsy
Because of the large number of centers included we were unable to obtain detailed clinical information after the biopsy which is a limitation of this study. Thus, we are unable to compare risk of biopsy during or after pregnancy with controls. The decision to perform a renal biopsy during pregnancy is complex for both the clinician and mother. However, our data and others support the role of renal biopsy either during or after pregnancy as histologic confirmation is likely to lead to a change in management in the majority of women (33).
The findings of this study support the use of renal biopsy as a diagnostic tool for the investigation of renal disease identified during or within 1 year of pregnancy. Pregnancy provides an opportunity for detection of disease and thus prevention of CKD progression and of future cardiovascular disease. FSGS is more commonly found in women who have been pregnant than in controls and, furthermore, women with a pregnancy-related diagnosis have a more rapid progression of disease. Further work is required to determine whether pregnancy initiates, exacerbates, or reveals renal disease.
Disclosures
None.
Acknowledgments
P.W., L.L., and K.B. were responsible for study design, data collection, statistical analysis, and manuscript submission. P.T.S. performed statistical analysis. L.M.W., I.L., M.H., C.S., and R.V. were responsible for data collection. H.T.C. and C.H. reviewed all renal biopsies and Columbia classified biopsies with FSGS.
We acknowledge support from Guy’s and St Thomas’ National Institute for Health Research Biomedical Research Centre for K.B.’s salary and from Imperial College National Institute for Health Research Biomedical Research Centre. P.T.S. is partly funded by Tommy’s (Registered charity no. 1060508) and Collaboration for Leadership in Applied Health Research and Care South London (National Institute for Health Research).
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
References
- 1.Mills KT, Xu Y, Zhang W, Bundy JD, Chen C-S, Kelly TN, Chen J, He J: A systematic analysis of worldwide population-based data on the global burden of chronic kidney disease in 2010. Kidney Int 88: 950–957, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF 3rd, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T, Coresh J; CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration) : A new equation to estimate glomerular filtration rate. Ann Intern Med 150: 604–612, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alper AB, Yi Y, Rahman M, Webber LS, Magee L, von Dadelszen P, Pridjian G, Aina-Mumuney A, Saade G, Morgan J, Nuwayhid B, Belfort M, Puschett J: Performance of estimated glomerular filtration rate prediction equations in preeclamptic patients. Am J Perinatol 28: 425–430, 2011 [DOI] [PubMed] [Google Scholar]
- 4.Smith MC, Moran P, Ward MK, Davison JM: Assessment of glomerular filtration rate during pregnancy using the MDRD formula. BJOG 115: 109–112, 2008 [DOI] [PubMed] [Google Scholar]
- 5.D’Agati VD, Fogo AB, Bruijn JA, Jennette JC: Pathologic classification of focal segmental glomerulosclerosis: A working proposal. Am J Kidney Dis 43: 368–382, 2004 [DOI] [PubMed] [Google Scholar]
- 6.Day C, Hewins P, Hildebrand S, Sheikh L, Taylor G, Kilby M, Lipkin G: The role of renal biopsy in women with kidney disease identified in pregnancy. Nephrol Dial Transplant 23: 201–206, 2008 [DOI] [PubMed] [Google Scholar]
- 7.Kida H, Takeda S, Yokoyama H, Tomosugi N, Abe T, Hattori N: Focal glomerular sclerosis in pre-eclampsia. Clin Nephrol 24: 221–227, 1985 [PubMed] [Google Scholar]
- 8.Nochy D, Hinglais N, Jacquot C, Gaudry C, Remy P, Bariety J: De novo focal glomerular sclerosis in preeclampsia. Clin Nephrol 25: 116–121, 1986 [PubMed] [Google Scholar]
- 9.Nagai Y, Arai H, Washizawa Y, Ger Y, Tanaka M, Maeda M, Kawamura S: FSGS-like lesions in pre-eclampsia. Clin Nephrol 36: 134–140, 1991 [PubMed] [Google Scholar]
- 10.Gärtner HV, Sammoun A, Wehrmann M, Grossmann T, Junghans R, Weihing C: Preeclamptic nephropathy -- an endothelial lesion. A morphological study with a review of the literature. Eur J Obstet Gynecol Reprod Biol 77: 11–27, 1998 [DOI] [PubMed] [Google Scholar]
- 11.Baylis C: The mechanism of the increase in glomerular filtration rate in the twelve-day pregnant rat. J Physiol 305: 405–414, 1980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Odutayo A, Hladunewich M: Obstetric nephrology: Renal hemodynamic and metabolic physiology in normal pregnancy. Clin J Am Soc Nephrol 7: 2073–2080, 2012 [DOI] [PubMed] [Google Scholar]
- 13.D’Agati VD, Alster JM, Jennette JC, Thomas DB, Pullman J, Savino DA, Cohen AH, Gipson DS, Gassman JJ, Radeva MK, Moxey-Mims MM, Friedman AL, Kaskel FJ, Trachtman H, Alpers CE, Fogo AB, Greene TH, Nast CC: Association of histologic variants in FSGS clinical trial with presenting features and outcomes. Clin J Am Soc Nephrol 8: 399–406, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Craici IM, Wagner SJ, Bailey KR, Fitz-Gibbon PD, Wood-Wentz CM, Turner ST, Hayman SR, White WM, Brost BC, Rose CH, Grande JP, Garovic VD: Podocyturia predates proteinuria and clinical features of preeclampsia: Longitudinal prospective study. Hypertension 61: 1289–1296, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.White WM, Garrett AT, Craici IM, Wagner SJ, Fitz-Gibbon PD, Butters KA, Brost BC, Rose CH, Grande JP, Garovic VD: Persistent urinary podocyte loss following preeclampsia may reflect subclinical renal injury. PLoS One 9: e92693, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Garovic VD, Craici IM, Wagner SJ, White WM, Brost BC, Rose CH, Grande JP, Barnidge DR: Mass spectrometry as a novel method for detection of podocyturia in pre-eclampsia. Nephrol Dial Transplant 28: 1555–1561, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Garovic VD, Wagner SJ, Petrovic LM, Gray CE, Hall P, Sugimoto H, Kalluri R, Grande JP: Glomerular expression of nephrin and synaptopodin, but not podocin, is decreased in kidney sections from women with preeclampsia. Nephrol Dial Transplant 22: 1136–1143, 2007 [DOI] [PubMed] [Google Scholar]
- 18.Zhao S, Gu X, Groome LJ, Wang Y: Decreased nephrin and GLEPP-1, but increased VEGF, Flt-1, and nitrotyrosine, expressions in kidney tissue sections from women with preeclampsia. Reprod Sci 16: 970–979, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hara M, Yanagihara T, Kihara I: Urinary podocytes in primary focal segmental glomerulosclerosis. Nephron 89: 342–347, 2001 [DOI] [PubMed] [Google Scholar]
- 20.Wickman L, Afshinnia F, Wang SQ, Yang Y, Wang F, Chowdhury M, Graham D, Hawkins J, Nishizono R, Tanzer M, Wiggins J, Escobar GA, Rovin B, Song P, Gipson D, Kershaw D, Wiggins RC: Urine podocyte mRNAs, proteinuria, and progression in human glomerular diseases. J Am Soc Nephrol 24: 2081–2095, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kincaid-Smith P: The renal lesion of preeclampsia revisited. Am J Kidney Dis 17: 144–148, 1991 [DOI] [PubMed] [Google Scholar]
- 22.Hladunewich MA, Myers BD, Derby GC, Blouch KL, Druzin ML, Deen WM, Naimark DM, Lafayette RA: Course of preeclamptic glomerular injury after delivery. Am J Physiol Renal Physiol 294: F614–F620, 2008 [DOI] [PubMed] [Google Scholar]
- 23.Murakami S, Saitoh M, Kubo T, Koyama T, Kobayashi M: Renal disease in women with severe preeclampsia or gestational proteinuria. Obstet Gynecol 96: 945–949, 2000 [DOI] [PubMed] [Google Scholar]
- 24.Vikse BE, Irgens LM, Bostad L, Iversen BM: Adverse perinatal outcome and later kidney biopsy in the mother. J Am Soc Nephrol 17: 837–845, 2006 [DOI] [PubMed] [Google Scholar]
- 25.Vikse BE, Irgens LM, Leivestad T, Skjaerven R, Iversen BM: Preeclampsia and the risk of end-stage renal disease. N Engl J Med 359: 800–809, 2008 [DOI] [PubMed] [Google Scholar]
- 26.Vikse BE, Hallan S, Bostad L, Leivestad T, Iversen BM: Previous preeclampsia and risk for progression of biopsy-verified kidney disease to end-stage renal disease. Nephrol Dial Transplant 25: 3289–3296, 2010 [DOI] [PubMed] [Google Scholar]
- 27.Smyth A, Oliveira GHM, Lahr BD, Bailey KR, Norby SM, Garovic VD: A systematic review and meta-analysis of pregnancy outcomes in patients with systemic lupus erythematosus and lupus nephritis. Clin J Am Soc Nephrol 5: 2060–2068, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cornelis T, Odutayo A, Keunen J, Hladunewich M: The kidney in normal pregnancy and preeclampsia. Semin Nephrol 31: 4–14, 2011 [DOI] [PubMed] [Google Scholar]
- 29.Hypertension in pregnancy: Diagnosis and management | Guidance and guidelines | NICE, 2016. Available at: http://www.nice.org.uk/guidance/CG107. Accessed July 28, 2016
- 30.Morris RK, Riley RD, Doug M, Deeks JJ, Kilby MD: Diagnostic accuracy of spot urinary protein and albumin to creatinine ratios for detection of significant proteinuria or adverse pregnancy outcome in patients with suspected pre-eclampsia: Systematic review and meta-analysis. BMJ 345: e4342, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pamella SO, Vega JA: Comparison of 24-hour urine to estimated renal function using CKD-EPI, MDRD4 and cockcroft-gault in specific patient subsets. J Pharm Care Heal Syst 2: 129, 2015 [Google Scholar]
- 32.Stettler RW, Cunningham FG: Natural history of chronic proteinuria complicating pregnancy. Am J Obstet Gynecol 167: 1219–1224, 1992 [DOI] [PubMed] [Google Scholar]
- 33.Piccoli GB, Daidola G, Attini R, Parisi S, Fassio F, Naretto C, Deagostini MC, Castelluccia N, Ferraresi M, Roccatello D, Todros T: Kidney biopsy in pregnancy: Evidence for counselling? A systematic narrative review. BJOG 120: 412–427, 2013 [DOI] [PubMed] [Google Scholar]