Abstract
Focal segmental glomerulosclerosis (FSGS) is a leading cause of kidney disease worldwide. The presumed etiology of primary FSGS is a plasma factor with responsiveness to immunosuppressive therapy and a risk of recurrence after kidney transplant–important disease characteristics. In contrast, adaptive FSGS is associated with excessive nephron workload due to increased body size, reduced nephron capacity, or single glomerular hyperfiltration associated with certain diseases. Additional etiologies are now recognized as drivers of FSGS: high-penetrance genetic FSGS due to mutations in one of nearly 40 genes, virus-associated FSGS, and medication-associated FSGS. Emerging data support the identification of a sixth category: APOL1 risk allele–associated FSGS in individuals with sub-Saharan ancestry. The classification of a particular patient with FSGS relies on integration of findings from clinical history, laboratory testing, kidney biopsy, and in some patients, genetic testing. The kidney biopsy can be helpful, with clues provided by features on light microscopy (e.g., glomerular size, histologic variant of FSGS, microcystic tubular changes, and tubular hypertrophy), immunofluorescence (e.g., to rule out other primary glomerulopathies), and electron microscopy (e.g., extent of podocyte foot process effacement, podocyte microvillous transformation, and tubuloreticular inclusions). A complete assessment of renal histology is important for establishing the parenchymal setting of segmental glomerulosclerosis, distinguishing FSGS associated with one of many other glomerular diseases from the clinical-pathologic syndrome of FSGS. Genetic testing is beneficial in particular clinical settings. Identifying the etiology of FSGS guides selection of therapy and provides prognostic insight. Much progress has been made in our understanding of FSGS, but important outstanding issues remain, including the identity of the plasma factor believed to be responsible for primary FSGS, the value of routine implementation of genetic testing, and the identification of more effective and less toxic therapeutic interventions for FSGS.
Keywords: nephrotic syndrome, immunosuppression, Alleles, Biopsy, Body Size, Fluorescent Antibody Technique, Genetic Testing, Segmental glomerulosclerosis, Glomerulosclerosis, Focal Segmental, Humans, hypertrophy, kidney, Kidney Diseases, Kidney Glomerulus, kidney transplantation, Microscopy, Electron, Mutation, Penetrance, Podocytes, Workload
Introduction
Focal segmental glomerulosclerosis (FSGS) is the leading glomerular cause of ESRD in the United States. FSGS refers to a histologic pattern that is a characteristic of perhaps six distinct underlying etiologies sharing a common theme of podocyte injury and depletion. The diagnosis and evaluation of FSGS rely on integration of the clinical history (family history, birth history, peak weight and body mass, and medication use), clinical laboratory findings (serum albumin, urine protein, and viral serologies), and renal histopathology. Proteinuria may be in the nephrotic or subnephrotic range. Critical is the elimination of other systemic diseases or primary renal diseases that may result in a similar presentation. Many reviews cover various aspects of FSGS and include comprehensive reviews (1–4), disease mechanisms (5–8), pediatric disease (9), immunologic aspects (10), treatment in children (11), and treatment in adults (12–14). We focus here on a practical approach to FSGS assessment on clinical and histopathologic grounds in the context of our current understanding of disease mechanisms and genetics.
Epidemiology and Global Burden
The prevalence of FSGS, relative to other glomerular disease diagnoses, seems to be increasing worldwide, and it is a major contributor to ESRD. However, the absolute incidence and prevalence of FSGS are difficult to ascertain given the large global variations in the indications, accessibility, and pathology support for kidney biopsy. McGrogan et al. (15) reviewed published literature from around the world and reported that annual incidence rates ranged from 0.2 to 1.8/100,000 population per year. Australia, with a liberal biopsy policy, had among the highest incidence of FSGS (15,16). A population-based study in the southwestern United States examined 2501 adult kidney biopsies performed between the years 2000 and 2011 (17). Over the 12 years studied, FSGS was the most common diagnosis (39% of biopsies), with an increasing incidence rate (from 1.6 to 5.3 patients per million). Although the average incidence rate was 2.7 patients per million, there was a significant racial/ethnic predilection. FSGS incidence rates are generally higher in men, being approximately 1.5-fold higher than in women.
In 2004, Kitiyakara et al. (18) noted a two-decade-long trend of increasing ESRD attributed to FSGS in the United States. Incident rates, expressed as patients per million, were 6.8 in blacks, 3.7 in Hispanics, and 1.9 in whites. The rise in FSGS prevalence has been observed in other populations as well. In Nigeria, the leading cause of nephrotic syndrome has shifted from quartan malaria (ca. 1960s) to membranoproliferative GN (ca. 1980s) to FSGS (present) (19). The factors responsible for the increasing incidence and prevalence of FSGS are largely unknown. Some of the increase is likely attributable to improved recognition (particularly where indications for kidney biopsy are broadening and the procedure is more available). There may well be an absolute rise in incidence of adaptive FSGS compounded by obesity and chronic inflammation, but epidemiologic data are lacking.
Among the primary nephrotic diseases, FSGS is most likely to progress to ESRD. Within FSGS categories, emerging data indicate that an association with APOL1 defines a group most likely to progress to ESRD. Histologic variants portend outcome with variable rates of progression (collapsing variant greater than not otherwise specified [NOS] greater than tip lesion).
Classification
Classification of FSGS is multifaceted and includes pathophysiologic, histologic, and genetic considerations. D’Agati et al. (20) initially proposed that FSGS be divided into primary (idiopathic) and secondary forms. The latter might be considered to include familial/genetic forms, virus-associated forms, drug-induced forms, and forms mediated by adaptive structural-functional responses (i.e., in the setting of congenital or acquired reduction of renal mass/nephron complement). Clinical response and prognosis may relate to the histologic variant, most notably the glucocorticioid responsiveness of the tip lesion and the aggressive, unrelenting nature of the collapsing variants (21,22). It is with this in mind that the variants are included in standard pathology reports. More recently, efforts to identify genetic drivers of FSGS in at-risk populations have gained momentum, with the most recent addition including the APOL1 genetic variant as a major association with FSGS in individuals of sub-Saharan African descent with FSGS (23).
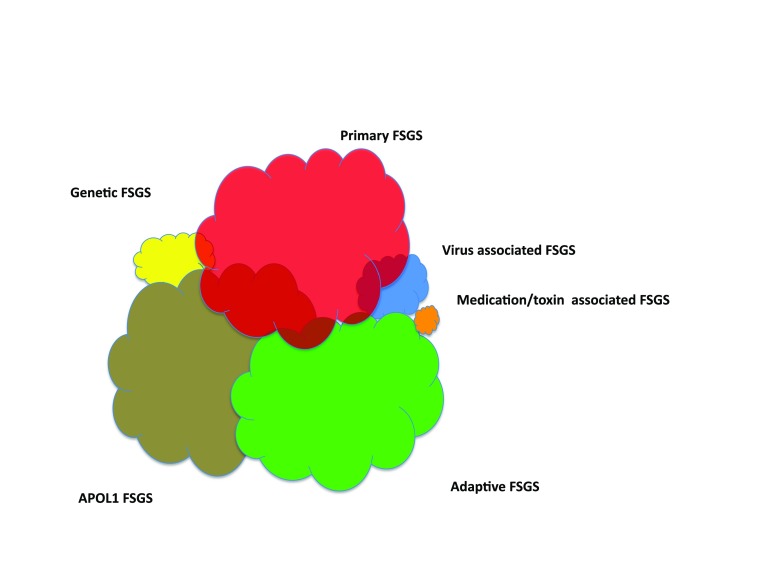
Putting together the genetic susceptibility, pathophysiologic drivers, clinical history, and response to therapy (summarized in Table 1), we believe that it is useful to cluster FSGS into six clinical forms (Figure 1, Table 2). These include two common forms (primary FSGS and adaptive FSGS) and three less common forms (high-penetrance genetic FSGS, viral-mediated FSGS, and medication-associated FSGS) (24). Evidence is mounting to consider another form—APOL1-associated FSGS (discussed below). With regard to primary FSGS, some would prefer the term idiopathic FSGS; both are defined as a disease that arises spontaneously or is of unknown cause, and we would view these terms as interchangeable.
Table 1.
Data relevant in evaluating a patient with the histologic diagnosis of FSGS
| Relevant Clinical History | Laboratory Data | Renal Biopsy Findings |
|---|---|---|
| Family history of kidney disease | Serum albumin before therapy | FSGS histologic variant |
| Birth weight, gestational age at birth, congenital cyanotic heart disease | Urine protein-to-creatinine ratio | Glomerular size (glomerulomegaly) |
| Sickle cell disease | Change in urine protein-to-creatinine ratio after maximal renin-angiotensin-aldosterone therapy and dietary sodium restriction | Electron microscopy: extent of foot process effacement; podocyte mircrovillus transformation |
| History consistent with reflux nephropathy or reduced renal mass | Change in urine protein-to-creatinine ratio after immunosuppressive therapy | Electron microscopy: tubuloreticular inclusions in glomerular endothelial cells (IFN effect) |
| Peak and present body mass index: obesity, extreme muscular development | ||
| Viral infection: HIV, cytomegalovirus | ||
| Medication, past or present: IFN, lithium, bisphosphonate, androgen abuse, chronic use of nephrotoxic drugs |
Modified from Kopp (24), with permission.
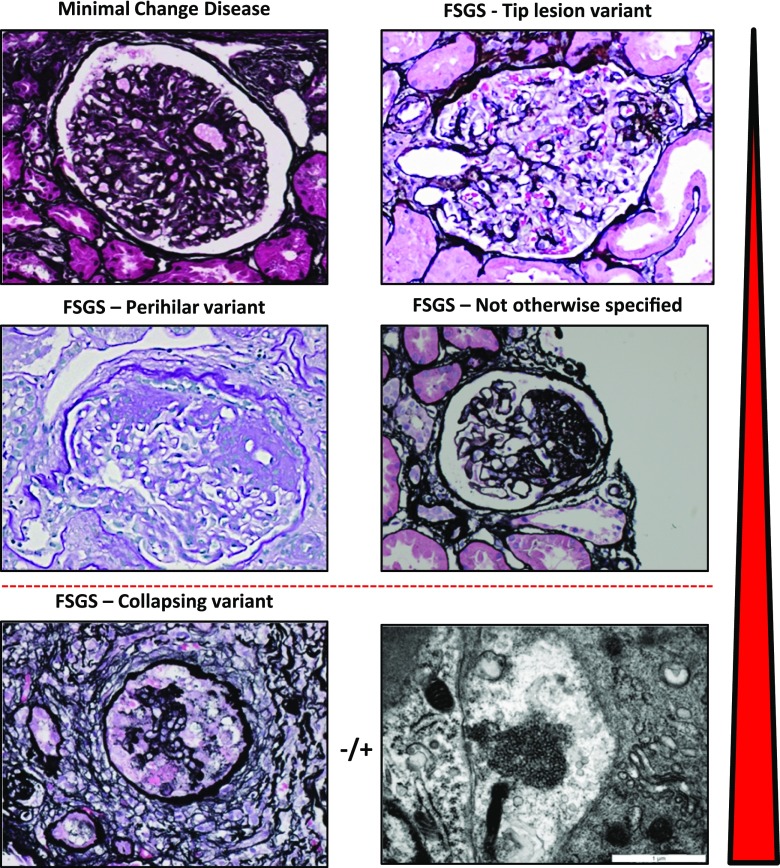
Figure 1.
Histopathology of minimal change disease and focal segmental glomerulosclerosis. Minimal change disease shows patent glomeruli in the absence of tubulointersitial scarring (silver stain, ×40). The tip lesion represents a focal adhesion of the glomerular tuft to Bowman’s capsule near the proximal tubule takeoff (silver stain, ×400). The most common forms of FSGS seen in adaptive FSGS and across all etiologies of FSGS are the perihilar variant (periodic acid–Schiff stain, ×40) and not otherwise specified pattern (silver stain, ×400). The most distinctive variant is the collapsing variant (collapsing glomerulopathy; silver stain, ×40). A specific instance of collapsing variant can be appreciated in the setting of endothelial tubuloreticular inclusions seen on ultrastructural analysis. These may be observed in high IFN states, including viral infection and exogenous IFN. The red arrowhead indicates the relative response to therapy and propensity of progression of these various forms, with minimal change disease and tip lesion being most responsive and least progressive and collapsing glomerulopathy being most therapy resistant and rapidly progressing.
Table 2.
Characteristic clinical and pathology features of the six forms of FSGS
| Characteristic Features | Primary FSGS | Adaptive FSGS | APOL1 FSGS | Genetic FSGS | Infection/Inflammation Associated | Medication-Associated FSGS |
|---|---|---|---|---|---|---|
| Mechanism of Podocyte Injury | Circulating factor, possibly a cytokine | Mismatch between metabolic load and glomerular capacity | APOL1 variant–initiated inflammation | High-penetrance genetic variants (Mendelian or mitochondrial inheritance) | Postulated role of IFN and possible other cytokines | Presumed direct effect on podocytes |
| History | Acute onset of edema | Reduced renal mass: low birth weight, oligomeganephronia, ureteral reflux, morbid obesity; increased single-nephron GFR: cyanotic congenital heart disease, sickle cell anemia | Family history, may be unremarkable | Family history, may be unremarkable with recessive inheritance genes | HIV, CMV, possible: parvovirus B19, Still disease, natural killer cell leukemia | Bisphosphonate, lithium |
| Laboratory tests | Many have high-grade proteinuria and nephrotic syndrome | Any level of proteinuria, serum albumin may be normal | Any level of proteinuria | Any level of proteinuria | Any level of proteinuria | Any level of proteinuria |
| Renal pathology | Widespread foot process effacement | Large glomeruli, perihilar sclerosis variant most typical, partial foot process effacement | May resemble primary or adaptive forms | Variable | Variable | Variable |
| Treatment and response | May respond to IST | Responds well to RAAS antagonism, often with >50% proteinuria reduction | May respond to therapies used for primary and adaptive forms | High-penetrance genetic mutations: usually does not respond to IST | Treat the virus | Stop the medication |
| Recurrence after renal transplant | Possible | Unlikely | Possible | Unlikely | Possible if infection/inflammation persists | Unlikely |
CMV, cytomegalovirus; IST, immunosuppressive therapy; RAAS, renin-angiotensin-aldosterone system; CG, collaspsing glomerulopathy. Modified from Kopp (24), with permission.
Histopathology
Historically, degenerative glomerular lesions of FSGS were those seen in the progression of minimal change disease (MCD; formerly lipoid nephrosis), and it was subsequently noted that these patients had an accelerated clinical course (25,26). In contrast to MCD, FSGS glomeruli show segmental solidification of the glomerular tuft. Tubulointerstitial scarring indicative of glomerular disease may also be observed. It is not uncommon that a biopsy with minimal glomerular changes may have tubulointerstitial damage, suggesting that FSGS might have been present on unsampled glomeruli.
The distinction between MCD and FSGS is critical, because both present with proteinuria, podocyte injury, and minimal immune deposits. For the biopsy to exclude FSGS as a cause of nephrotic syndrome and assess the degree of cortical involvement, adequate renal cortical sampling is required. The involvement of glomeruli in focal glomerular processes follows a binomial distribution, and thus, for focal disease, more glomeruli are necessary to observe an affected glomerulus (27,28). There is also zonal proclivity for FSGS, such that juxtamedullary/inner cortex glomeruli are the first affected progressing to involve the outer cortex at a later stage of disease (24). Fogo et al. (29) further show that the focality of sclerotic lesions is greater in children than in adults, suggesting a need for more comprehensive sampling. Differential staining patterns for synaptopodin (30) and dystroglycan (31) may distinguish steroid-sensitive MCD versus steroid-resistant MCD (likely unsampled FSGS) and FSGS.
A simulation study has highlighted two issues with respect to diagnostic yield of renal biopsies in FSGS (28). (1) If glomerular scars are uniformly distributed in a biopsy with 10–30 glomeruli, the diagnostic accuracy for the detection of at least one scarred glomerulus will be 80% when at least 10% of juxtamedullary glomeruli or 20% of other cortical glomeruli are scarred. (2) With an average of 20 glomeruli with 20%–60% glomerular sclerosis, the predicted error rate is ±50% for extent of glomerular involvement. This suggests caution when making treatment recommendations on the basis of the extent of involved glomeruli (Supplemental Figure 1).
The Columbia classification of FSGS recognized five morphologic patterns, all of which involve obliteration of the capillary lumens and for the most part, have good reproducibility across independent observers (20,32) (Figure 2). Two distinctive forms are the tip lesion and the collapsing variant. Tip lesions affect the portion of the glomerular tuft juxtaposed to the tubular pole. Abnormalities include adhesion to Bowman’s capsule at the tip, hypercellularity, presence of foam cells, and/or sclerosis. By contrast, the collapsing variant shows segmental or global mesangial consolidation and loss of endocapillary patency in association with extracapillary epithelial hypertrophy and/or proliferation. Microcystic tubular dilation is frequently present. The perihilar and NOS variants are determined by whether the segmental sclerosis/segmental obliteration of capillary loops with matrix increase (with or without hyalinosis) involves the segment near the hilum or the specific segment cannot be determined, respectively. The cellular lesion is perhaps the most difficult lesion to identify reproducibly and shows segmental endocapillary hypercellularity occluding lumens with or without foam cells and karyorrhexis (32). Some biopsies will show multiple morphologic lesions (33,34), and the Columbia classification suggests a hierarchy for diagnosis, such that the presence of the higher-ranking lesion determines the clinical course: collapsing variant, tip lesion, cellular variant, perihilar variant, and lastly, NOS (reviewed in Stokes and D’Agati [35]). Including the pattern of FSGS has standardized the reporting of FSGS lesions and provides prognostic information. Nonetheless, this classification system was designed to rely solely on pathologic criteria and does not integrate these findings with clinical and genetic information.
Figure 2.
The six forms of FSGS. These syndromes include three forms that are most common, including primary FSGS, adaptive FSGS, and APOL1 FSGS. These forms are probably of approximately equal prevalence in the United States adult population. Three forms are less common, including genetic FSGS (by which is meant high-penetrance genetic causes), medication-associated FSGS, and viral FSGS. The approximate relative distribution of these variants in the United States population at present is shown by the size each cloud, although firm data on prevalence are lacking. The absolute frequency of these FSGS forms is influenced by race/ethnicity (particularly in the frequency of APOL1 risk variants), age (children are less likely to have adaptive FSGS), and the frequency of exposures to toxins (e.g., heroin) and medications and having viruses (e.g., HIV).
Complete renal pathologic evaluation includes immunofluorescence analysis and electron microscopy, which help to exclude focal and segmental glomerular scarring as an injury pattern that can be seen as an element of any chronic progressive renal diseases, including lupus nephritis, IgA nephropathy, and diabetic nephropathy (36). As such, to avoid confusion, it is probably best to avoid using the term FSGS in this setting. Low or moderate amounts of IgM are frequently present in the mesangium of patients with FSGS. Thurman and colleagues (37) have proposed that IgM may bind neoepitopes in the mesangium that are exposed after nonimmune injury, resulting in complement deposition and glomerular injury.
Ultrastructural examination adds to the assessment in three ways. First, it can exclude the presence of immune complexes and abnormal deposits, such as amyloid. Second, it can exclude basement membrane abnormalities seen in genetic disorders of collagen; the appearance of COL4 mutations among the list of genes associated with FSGS suggests that the alterations in basement membrane at the ultrastructural level may be subtle or undetectable. Third, it can provide an estimate of the severity of podocyte injury (fractional foot process effacement) and injury pattern (microvillus transformation). Mean foot process width is greater in primary FSGS compared with adaptive FSGS and may serve as a helpful, although subtle, clue (38).
The use of all three renal pathology modalities (light microscopy, electron microscopy, and staining for Ig and complement) is critical in making the distinction between MCD and FSGS. Extensive podocyte injury at the ultrastructural level with adequate numbers of normal-appearing glomeruli and nonscarred tubulointerstitium by light microscopy suggests MCD. Nevertheless, individuals with a diagnosis of MCD who are resistant to glucocorticoid therapy or manifest deterioration in renal function may have FSGS that was not sampled on the first biopsy and is shown on a subsequent renal biopsy.
Mechanisms of Disease
FSGS is a diverse syndrome that arises after podocyte injury from diverse causes: some known and others unknown (39). The sources of podocyte injury are varied (circulating factors [primary FSGS], genetic abnormalities, viral infection, and medication), although the effect on podocytes is similar. For the most part, the interplay among these drivers is unclear and likely complex. For instance, adaptive FSGS involves both podocyte stress (a mismatch between glomerular load and glomerular capacity) and a genetic susceptibility. Wiggins and colleagues (40) have elegantly shown this in a rat model that combines genetic susceptibility, renal mass reduction (uninephrectomy), and obesity (increased glomerular load).
Importantly, podocyte injury from any of the forms of FSGS (or from other glomerular diseases) will initiate a similar process, resulting in the pathologic features of adaptive FSGS.
It is thought that there is progressive loss of injured podocytes into the urinary space. Podocyte depletion arising from an inability to replicate (although nuclear division may occur, at least in animals) and results in podocyte catastrophe (41). To balance this deficit, podocytes compensate by hypertrophy to cover more of the glomerular capillary surface. In a recent insightful and provocative review, Kriz and Lemley (42) proposed that shear stress forces on podocytes are a critical factor driving podocyte injury. In adaptive FSGS, glomerular hypertrophy occurs early in the disease process; in other forms of FSGS and other glomerular diseases, glomerular hypertrophy occurs with progressive nephron loss, leading to increased pressures and flows in the remaining patent glomeruli.
The following sections address pathologic mechanisms, therapy, and treatment for each of the FSGS syndromes. The typical onset ages and approximate relative incidence rates are shown schematically in Supplemental Figure 1.
Primary FSGS
Primary FSGS is a distinct entity, and paradoxically, it is best defined, at this time, as what it is not (i.e., not one of the other forms of FSGS). As a practical matter, this means assessing the likelihood of other forms. This includes ruling out adaptive FSGS (by medical history, peak or current body weight, renal biopsy features, serum albumin, or proteinuria response to renin-angiotensin-aldosterone system [RAAS] antagonism), genetic FSGS (via genetic test panels on the basis of family history and age of onset), viral FSGS (by appropriate virologic testing), and medication-associated FSGS (by medication history). Emerging data support that APOL1-associated FSGS may make up yet another form of primary FSGS.
The mechanism of podocyte injury in at least some patients with primary FSGS likely involves a circulating factor, possibly a cytokine that makes particular subjects susceptible. The best evidence for a circulating factor comes from the experience with recurrent FSGS immediately (on a scale of hours to several weeks) after kidney transplant. The cytokine or cytokines responsible for recurrent FSGS after kidney transplant remain to be defined. In an unusual and striking case that supports the cytokine hypothesis, a kidney was transplanted into a recipient with FSGS; proteinuria developed, and the transplanted kidney showed podocyte foot process effacement. Subsequently, the kidney was removed and transplanted into a patient with ESRD due to diabetes, and in the new host, the kidney functioned well without proteinuria (43). Current candidates for the recurrent FSGS factor, reviewed recently (44), include cardiotrophin-like cytokine factor 1 (45), apoA1b (an isoform of ApoA1) (46), anti-CD40 antibody (47), and serum urine–type plasminogen activator receptor (suPAR) (48). The role of suPAR remains controversial (49). suPAR levels were reported in FSGS and other primary glomerular diseases (50). In primary and adaptive FSGS (51), elevated levels may (52) or may not (53) predict glucocorticoid sensitivity, and a role for different forms of suPAR has been suggested as reviewed recently (54). Recurrent FSGS plasma affects the cytoskeleton of cultured podocytes, including promoting cell mobility by phosphorylating vasodilator-stimulated phosphoprotein (55) and disassembling focal adhesion complexes (56). The lack of suitable animal models has hindered progress in this area.
It is quite likely that other patients with what is now diagnosed as primary FSGS will be reassigned to alternative etiologies, including new genes, environmental factors, and/or microorganisms.
Primary FSGS has several prototypical characteristics. It is probably the most common form in adolescents and young adults, although it may occur at any age. It is commonly associated with nephrotic-range proteinuria (sometimes massive), reduced plasma albumin levels, and hyperlipidemia. Histologically, it may manifest as the tip variant, collapsing variant, or NOS variant.
Current therapy for primary FSGS is on the basis of immunosuppressive agents; it is now apparent that a number of these, including glucocorticoids and calcineurin inhibitors, directly modulate the podocyte phenotype (57). Recently, it has been shown that the effects of glucocorticoids may be mediated by Krüppel factor 15, a zinc finger transcription factor (57,58). In a recent retrospective case series involving 476 subjects, the use of glucocorticoids and/or cyclosporin was associated with improved outcomes compared to no immunosuppression, with a hazard ratio of 0.49 (95% CI, 0.28 to 0.86) for ESRD whereas the use of cyclosporine with or without glucocorticoids was not associated with benefit HR 0.42 (95% CI, 0.15 to 1.18) (59). Despite the limitations of a retrospective study design, this provides new evidence to support therapy.
Recurrent FSGS remains a vexing clinical problem. The histologic variant in the native kidney does not predict recurrence, although it is notable that only one of 77 initial kidney biopsies from subjects who subsequently had recurrent FSGS showed the perihilar variant (60). Therapy with plasma exchange may induce a remission, typically temporary. Canaud et al. (61) reported favorable results from a small series of adult subjects (n=10) with a regimen that included glucocorticoids and cyclosporin initially administered intravenously.
Adaptive FSGS
Adaptive FSGS, which has also been called, perhaps more accurately, postadaptive FSGS (62), arises after a period of nephron-level glomerular hyperfiltration and glomerular hypertension after pathophysiology as identified by Brenner and Mackenzie (63). Total GFR may be elevated, normal, or decreased at the outset.
Conditions that are associated with an increase in total kidney GFR include congenital cyanotic heart disease (64), sickle cell anemia (65), obesity (66), androgen abuse (67), sleep apnea (68), and high-protein diet. The duration of single-nephron glomerular hyperfiltration is typically measured in decades before progressive glomerulosclerosis eventually reduces total GFR. Conditions associated with reduced renal mass include prematurity and/or small for gestation age (69), renal anomalies, reflux nephropathy, and AKI. Any chronic glomerular or tubular disease may reduce the total nephron function and result in adaptive FSGS that is superimposed on the primary disorder.
Adaptive FSGS arises from the processes described above involving increased single-nephron GFR (often with intraglomerular hypertension), leading to progressive cycles of glomerular hypertrophy; podocyte hypertrophy, stress, and depletion; and synechia formation and excess extracellular matrix deposition within the glomerulus as described so well by Kriz and Lemley (70).
Renal biopsy features that support the diagnosis of adaptive FSGS include large glomeruli, a preponderance of perihilar scars among glomeruli showing sclerotic changes, and only partial foot process effacement. Clinical features include a normal serum albumin, which is unusual in primary FSGS. A complete response to RAAS antagonism, particularly when combined with sodium restriction and a rise or normalization of serum albumin, supports the diagnosis of adaptive FSGS, although it does not exclude other forms of FSGS.
Genetic FSGS
Genetic FSGS takes two forms. First, some patients are associated with variants in susceptibility genes (i.e., some individuals with a particular variant will develop FSGS, and other individuals will not). By far, the most common example of this is the newly identified association with APOL1 as discussed below. Other genetic risk loci include PDSS1 (71) and numerous others (72,73). More are likely to be discovered in the near future. Second, other patients are associated with high-penetrance mutations that manifest either Mendelian inheritance (for nuclear genes) or maternal inheritance (for genes encoded by mitochondrial DNA). The number of genes associated with FSGS rises every year, in large part because of the dissemination of whole-exome sequencing. To date, at least 38 genes have been identified as shown in Table 3 . (For simplicity, genes such as CFH and C3, which represent distinct syndromes that could be mistaken for FSGS when ultrastructure examination is not performed, have not been listed in Table 3. Other genes that have provisionally been associated with FSGS but lack the robust data needed to firmly establish causality are not shown [e.g., ACLS4, ALG1, NEIL1, PMM2, PODLX, SYNPO, and ZEB1]).
Table 3.
Genetic FSGS: Genetic variants with syndromes with Mendelian and mitochondrial inheritance
| Cell Matrix | Slit Diaphragm Complex | Cytoskeleton and Related | Mitochondria Function | DNA Repair, Transcription, Nuclear Transport | Cell Signaling | Lysosome | Cilia |
|---|---|---|---|---|---|---|---|
| Nonsyndromic | |||||||
| COL4A3 | NPHS (nephrin) | ACTN4 | INF2 | WT 1 (Denys–Drash, Frasier) | PLCE1 | TTC21B | |
| COL4A4 | NPHS2 (podocin) | INF2 | NUP95 | TRPC6 | |||
| COL4A5 | CD2AP | AHRGP24 | NUP203 | ||||
| PTPRO (GLEPP1) | AHRGDIA | XP05 (exportin 5) | |||||
| MYO1E | NXF5 (nuclear export factor 5) | ||||||
| PAX2 | |||||||
| Syndromic | |||||||
| ITGB4 (epidermolysiss bullosa) | MYH9 (Esptein, Fechtner) | INF2 (Charcot–Marie–Tooth) | WT1 (Denys–Drash, Frasier) | KANK4 | SCARB2 (action myoclonus) | ||
| LAMB2 (Pearson) | MT-TL1, MT-TL2 tRNA leucine (MELAS)a | LMX1B (Nail-patella) | |||||
| MT-TY, tRNA tyrosine (MELAS)a | |||||||
| COQ2 | SMARCAL1 (Schimke immune-osseous dysplasia) | ||||||
| COQ6 | NXF5 | ||||||
| PDSS2 (Leigh) | EYA1 (Branchio-oto-renal) | ||||||
| ADCK | WDR73 (Galloway–Mowat, nephrocerebellar syndrome) | ||||||
| LMNA (partial lipodystrophy) | |||||||
| 5 | 5 | 5 | 7 | 11 | 3 | 1 | 1 |
Genetic FSGS can be categorized as susceptibility genes (not shown; e.g., APOL1) and genes with Mendelian and mitochondrial inheritance (38 genes shown above); genes for which casual evidence is not yet entirely convincing or associations are with unbiopsied nephrotic syndrome are omitted. These gene products are located in various podocyte cell compartments, although some proteins are present in more than one location (the number of genes in each compartment are tabulated in the last row). Nonsyndromic genes are those in which mutations are associated with manifestations only in the kidney; syndromic genes are those in which mutations are also associated with extrarenal manifestations, in some cases with variable penetrance (some individuals with certain mutations may have a purely renal phenotype). For some diseases, eponyms or syndromic names are provided in parentheses. Most genes affecting mitochondrial function are encoded by nuclear genes. MELAS, mitochondrial encephalopathy, lactic acidosis and stroke-like episodes.
The two MELAS genes are encoded by mitochondrial DNA and show mitochondrial inheritance.
Some genes are associated with a syndrome that includes extrarenal manifestations, and this can provide a clinical clue that a patient might have a mutation in a particular gene. Some genes are associated with characteristic changes in basement membrane morphology (e.g., LXMB1 and COL4A3–5) or mitochondrial morphology (e.g., the genes associated with mitochondrial encephalopathy, lactic acidosis, stroke-like episode syndrome) that may provide such clues. Other genes are associated with therapeutic response. (Table 5 does not include genes associated with steroid-sensitive nephrotic syndrome, for which renal biopsies have not been done. Some genetic variants shown in Table 5 are associated with glucocorticoid sensitivity; e.g., PTPRO [74]).
Table 5.
Treatment recommendations for adults with FSGS
| Setting | Therapy | Comment |
|---|---|---|
| Nephrotic forms of primary FSGS,a APOL1 FSGS, certain steroid-sensitive genetic forms of FSGS | Prednisone, initially daily or alternate daysa | Alternate for patients at high risk for steroid complications: calcineurin inhibitorsa |
| Steroid-resistant FSGS with nephrotic syndromea | Calcineurin inhibitora (cyclosporin and possibly, tacrolimus) | |
| Refractory FSGS with nephrotic syndromea | Mycophenolate mofetil plus high-dose dexamethasonea | |
| All forms of FSGS with subnephrotic proteinuria | ACE inhibitor and angiotensin receptor blocker; dietary sodium restriction | Thiazide diuretic may potentiate the antiproteinuric of RAAS antagonism |
Therapy doses are in ref. 46. Cyclosporin has been shown effective in randomized, controlled trials, whereas tacrolimus has not. In the nosology presented here, these recommendations would apply, when nephrotic syndrome is present, to primary FSGS, APOL1 FSGS, and certain rare forms of genetic FSGS that may be steroid sensitive. ACE, angiotensin-converting enzyme; RAAS, renin-angiotensin-aldosterone system.
Recommendations from the Kidney Disease Improving Outcomes Global initiatives for idiopathic FSGS with nephrotic syndrome are extended here to other forms of FSGS as shown.
Should patients with FSGS undergo genetic testing? The answer to this question remains unclear. With regard to pediatric patients, Hildebrandt and coworkers (75) have suggested that every family with a child who has FSGS deserves to be offered an opportunity to have a genetic diagnosis (RE). Benefits of this approach may include a guide to appropriate therapy (e.g., avoidance of glucocorticoids except for in genetic forms that may be responsive and coenzyme Q10 therapy for particular mitochondrial mutations), prognosis (typical native kidney outcomes and likelihood of transplant recurrence), and family issues (identification of disease in other family members and prenatal testing). To be sure, caution is warranted, in that systematic studies of the response of genetic forms of FSGS to various therapies have not been carried out. High-penetrance genetic causes are more likely to be identified in childhood nephrotic syndrome (approximately 60%) than in that in older children and adolescents (approximately 5%), with lower rates in adults (75,76). Genetic testing in adults with FSGS is indicated where there is a family history, because some genes have mutations associated with autosomal dominant mutations (IFN2 and ATCN4).
What is the most appropriate and cost-effective way to carry out genetic testing? When a family has not had gene testing previously that identified a specific gene, the most effective approach is to use panels that focus on early-onset FSGS (infant and early childhood) or adult-onset FSGS. Genetic test resources around the world are available at the Genetic Test Registry, National Center for Biotechnology Information, National Institutes of Health (http://www.ncbi.nlm.nih.gov/gtr).
A more comprehensive approach is a whole-exome scan, which provides DNA sequence for the approximately 180,000 exons that make up the approximately 22,000 human genes; this may, in the near future, replace the use of selected gene panels. Genetic testing may identify known mutations that prior experience has shown are associated with FSGS, but it may also identify protein-altering mutations (e.g., missense mutations that change an amino acid or introduce a stop codon) of unknown significance. With comprehensive genetic exploration, there is also the possibility of unexpected identification of genetic variants that have significance for the individual and family. An advisory group from the American College of Human Genetics has provided a recommended list of 56 genes for which variants that are likely to be disease associated should be reported to the individual or family; this list will likely expand over time (77). Therapy for genetic FSGS is generally conservative and on the basis of RAAS antagonism. Calcineurin inhibitors may have an effect in a minority of patients (78). As noted above, coenzyme Q10 may benefit individuals with mutations in certain mitochondrial defects.
Virus-Associated FSGS
Among infections, viruses are predominantly implicated in causing FSGS. HIV-1 is strongly associated with FSGS, particularly the collapsing glomerulopathy variant, although other variants are also seen (79). The mechanisms likely involve direct infection of podocytes (80). A similar renal syndrome can be reproduced in transgenic mice bearing HIV-1 Nef (81) or Vpr (82) accessory proteins, suggesting possible mechanisms.
Interestingly, the effect of HIV on podocytes is strongest in individuals with two APOL1 risk alleles, with an odds ratio of 29 in the United States (83) and an odds ratio of 89 in South Africa (84). Most individuals with HIV-associated nephropathy (HIVAN) have one or two APOL1 risk alleles.
Other viruses that have been implicated in causing FSGS include cytomegalovirus, parvovirus B19, and Epstein-Barr virus, with the evidence perhaps stronger for cytomegalovirus compared with the others (reviewed in Chandra and Kopp [85]).
Certain parasites have also been associated with FSGS, presumably by stimulating innate immune pathways in ways that injure podocytes. These include Plasmodium (malaria) (86), Schistosoma mansoni (87), and filiariasis (88), although these seem to be rare events given the frequency of these infections.
Medication-Associated FSGS
There is a relatively short list of medications that cause FSGS. IFN-α, -β, or -γ therapy has been associated with the development of collapsing glomerulopathy in a case series of 11 subjects (89).
Several other medications have been associated with FSGS; a genetic predisposition is plausible but has not been shown. Bisphosphonates are associated with podocyte injury, including MCD, FSGS, and particularly, collapsing FSGS (collapsing glomerulopathy) as first reported by Markowitz et al. (89). As in HIVAN, the abundant cells in Bowman’s space are derived from the parietal epithelium (90). The mechanism by which bisphosphonates act remains obscure. Lithium therapy has been associated with MCD, which typically reverses within weeks after stopping the medication, and FSGS, which may also be reversible (91). The mechanism of podocyte injury, if that is the mechanism, remains to be determined. Sirolimus, particularly when the plasma levels are high, has been associated with FSGS (92) as well as exacerbating preexisting FSGS (93). Anthracycline medications, including doxorubicin (Adriamcyin) and daunomycin, have been associated with FSGS (94), and doxorubicin, a podocyte toxin, has been widely used to generate a mouse model of FSGS (95).
Emerging Pathologic Mechanisms: APOL1-Associated FSGS
The identification of genetic variants in APOL1 in patients with FSGS has been an important discovery for FSGS and related diseases. Unlike certain genes that have not shown firm causality to FSGS, data are mounting for the role of APOL1. Although confirmatory data are required, the strength and consistency of the genetic association, the exclusion of other genetic variants in this genomic region, and the demonstration that the expression of the renal risk variants (but not the common allele) causes FSGS in transgenic mice (96) are highly supportive of the role of APOL1 genetic variants as causal for FSGS.
APOL1-associated FSGS is a major form of FSGS in countries with individuals of sub-Saharan African descent. The effect is largely recessive, requiring two risk alleles, although in certain situations (e.g., HIV-positive South Africans), a single copy of a risk allele G1 has a significant association with HIVAN (84). In the United States, approximately 40% of ESRD attributed to FSGS occurs in blacks, and of this, 72% is associated with APOL1 genetic variants (83); thus, approximately one third of FSGS in the United States is associated with APOL1 variants.
APOL1-associated FSGS can present in various ways: it may present as a primary FSGS, with severe nephrotic syndrome, or as recurrence after kidney transplant, or it manifest as adaptive FSGS with preserved serum albumin and minimal edema. One setting in which the adaptive processes drive APOL1 FSGS is perhaps the report that APOL1 variants are associated with proteinuric sickle cell nephropathy (97), although a systematic study of kidney histology in APOL1-associated sickle nephropathy is still lacking. APOL1 high-risk alleles are strongly associated with collapsing glomerulopathy in several settings: (1) HIVAN, in which 72% have two APOL1 high-risk alleles (83), and (2) the use of exogenous IFN (98) and in lupus (99).
Emerging data support defining APOL1 FSGS as a separate category of FSGS. First, the APOL1 risk alleles represented confer susceptibility, but most subjects with two risk alleles will not develop kidney disease; thus, APOL1 differs from the high-penetrance genetic variants in most other forms of genetic FSGS. Second, APOL1 FSGS is by far the most common form of genetic FSGS in countries with substantial African descent populations. Third, the diagnosis of APOL1 FSGS may have implications for prognosis, with more rapid progression to ESRD, and it may have implications for the selection of living donors. Fourth, novel therapeutics may become available that target APOL1 variant–driven molecular pathways.
Therapeutic Approaches
Therapy should be individualized on the basis of the particular form of FSGS as well as factors particular to the patient, such as age and comorbidities. Therapy for primary FSGS and in rare instances, genetic FSGS, particularly when nephrotic proteinuria is present, involves the use of medications that are immunosuppressive but also have direct effects on podocytes. These therapies are summarized in Tables 4 and 5 and discussed below. A comprehensive analysis of approaches to steroid-sensitive and -resistant FSGS is available from the Kidney Diseases Improving Global Outcomes initiative, and the reader is referred to this initiative for a detailed and thoughtful discussion (100) as well as recent reviews (13). FSGS may be responsive to therapy with glucocorticoids, although relapses are typical. Randomized, controlled trials are few. Interestingly, functional polymorphisms in the glucocorticoid receptor encoded by NR3C1 have been associated with both relapse and frequent relapse (101). Cyclosporin plus low-dose prednisone was shown in a randomized, controlled trial to be superior to prednisone alone in preserving renal function assessed as creatinine clearance (102). Mycophenolate mofetil has not been tested as monotherapy for FSGS, but it has been tested in combination with glucocorticoids in three trials against active comparators that were either glucocorticoids or cyclosporin (reviewed in Senthil Nayagam et al. [103]). None of the trials showed significant differences between groups, and although sample sizes were small, particularly for noninferiority trials, these data suggest efficacy.
Table 4.
Treatment recommendations for children with FSGS
| Setting | Therapy | Comment |
|---|---|---|
| On presentation | Prednisone: initially daily and then alternate days | |
| Frequently relapsing or steroid dependent | Prednisone: initially daily and then alternate days at lowest dose to prevent relapse | |
| Same, with steroid-related adverse events | Alkylating agent (cyclophosphamide or chlorambucil), calcineurin inhibitor, levamisole, mycophenolate mofetil | No RCT comparing one agent with another; listed in alphabetical order |
| Steroid resistant | Calcineurin inhibitor | Also ACE inhibitor and angiotensin receptor blocker; sodium restriction |
| If no remission, mycophenolate mofetil, corticosteroids, or both |
Recommendations from the Kidney Disease Improving Outcomes Global initiatives. Doses and durations of therapy are provided in Lopez-Hellin et al. (46). In the nosology presented here, these recommendations would apply to nephrotic forms of primary FSGS, APOL1 FSGS, and certain rare forms of genetic FSGS. RCT, randomized, controlled trial; ACE, angiotensin-converting enzyme.
Several Cochrane reviews are available covering treatment of FSGS in adults (104) and children using not only glucocorticoids (105) but also, other therapies, including cyclophosphamide, azathioprine, levamisole, mizorbine, and rituximab (106,107). Many other agents have been tested in small trials or reported as case series, and they have been the subject of recent reviews (13,108–111). These agents include the following: adalimumab, an anti-TNF mAb (112); pirfenidone, an antifibrotic agent that suppresses TGF-β signaling (113); fresolimumab, an anti–TGF-β mAb (114); pulse steroids plus cyclophosphamide (115); and saquinivir (116). Achtar gel is unusual in that it was approved in the 1950s by the US Food and Drug Administration for nephrotic syndrome under criteria that were less stringent than required today. Small case series suggest limited efficacy of Acthar in some individuals with FSGS (117,118) as has been reviewed (119); a randomized, controlled trial is in progress. Galactose, proposed as therapy for recurrent FSGS, has been shown to lack efficacy in steroid-resistant primary FSGS in children (120). Sirolimus may accelerate progression of FSGS and should be avoided (93). Ongoing and recently completed phase 3 trials have shown some efficacy with sparsentan, an irbesartan-like molecule that also antagonizes endothelin receptor, and abatacept, a CD80 antagonist (121).
There is considerable incentive for the pharmaceutical and biotechnology industry to develop novel therapies for FSGS, a rare disease as defined by the US Food and Drug Administration that lacks therapies that are both safe and effective. Gipson and colleagues (122) have proposed a new paradigm for therapeutics development that involves a deep understanding of disease mechanisms in patient subsets, enriching study populations for subjects most likely to respond on the basis of those biologic insights, and adaptive trial designs to improve efficiency (122,123).
Medication-associated FSGS can generally be managed by stopping the offending medication, and virus-associated FSGS can be best addressed by initiating antiviral therapy with additional adjunctive therapies as indicated. Currently, therapy for APOL1 FSGS is directed toward addressing the other FSGS classes (e.g., primary, adaptive, virus associated, or medication associated) that may coexist with APOL1 FSGS. Future therapies for cell injury pathways initiated by APOL1 variant expression are likely to be developed.
The customary definition of response is similar to that of other nephrotic diseases and assumes baseline proteinuria in the nephrotic range (i.e., urine protein-to-creatinine ratio [uPCR] ≥2 g/g). First void urine is preferred, because that uPCR most closely correlates with the 24-hour uPCR. Complete remission (CR) is defined as uPCR<0.2 g/g, and partial remission (PR) is defined as having 50% reduction from baseline proteinuria and uPCR<2 g/g.
RAAS antagonism therapy is central to all progressive CKD, and it is the treatment that directly addresses the hemodynamic alterations in adaptive FSGS. RAAS antagonist therapy also has a role in genetic FSGS (evidence limited to case reports [124]) and APOL1 FSGS (no published data), particularly when adaptive hemodynamic mechanisms are likely at work (e.g., an individual who was born prematurely is now obese and has a normal serum albumin, despite substantial proteinuria), and may reduce proteinuria in primary FSGS adjunctive to immunosuppressive therapies. RAAS antagonist monotherapy was shown in a retrospective study to be superior to immunotherapy in a pediatric FSGS cohort in Philadelphia (with 55% black subjects) (125). As with all progressive CKD, attention to BP control, smoking, obesity, and serum bicarbonate and urate are indicated, and consideration should be given to statin therapy.
The effects of RAAS antagonist therapy are potentiated by dietary sodium restriction and low-dose thiazide diuretic (126). Although hydrochlorothiazide is the most widely used thiazide diuretic, an alternative is chlorthalidone, which has certain features to recommend its more widespread use: it has longer duration of action, it is more kaliuretic (particularly helpful when low GFR compromises potassium excretion), and it has been shown to improve cardiovascular outcomes, which other thiazides have not been shown to do.
The issue of combining an angiotensin-converting enzyme inhibitor (ACEi) and an angiotensin receptor blocker (ARB) is fraught with uncertainty. A meta-analysis of this combination therapy in IgA nephropathy suggested proteinuria reduction compared with single therapy, but only one study involved subjects with nephrotic-range proteinuria (127). The Ongoing Telmisartan Alone and in Combination with Ramipril Global End Point Trial Study showed that this combination is associated with adverse cardiovascular outcomes in an older population with a high prevalence of cardiovascular disease (128); similarly, the VA Diabetes in Nephropathy Study of diabetic nephropathy (median age of 64 years old) showed a trend toward benefit but was stopped early due to safety concerns (129). These studies may have limited relevance to younger patients with FSGS, particularly those with preserved GFR, who may tolerate this combination better. RAAS antagonism and particularly, the combination of ACEi and ARB will lower GFR by reducing efferent arteriolar vascular tone and thus, reducing intraglomerular capillary pressure, the driving force for glomerular filtration. Thus, a modest decrease in GFR may be tolerated, providing evidence that RAAS antagonism has been achieved. Nevertheless, there are no trials of suitable size to justify this approach from an efficacy or safety standpoint.
An alternate approach to RAAS antagonism is to combine an ACEi or ARB with an aldosterone antagonist (130). This approach may have less effect on reducing glomerular capillary pressure, but aldosterone antagonists are potent antifibrotic agents. Recent data have shown that aldosterone is positioned upstream of multiple profibrotic pathways and that angiotensin 2 is only one of many stimuli to aldosterone production (131). This combination confers a risk for hyperkalemia, particularly with reduced GFR; this may be controlled with dietary restriction and diuretics, although close monitoring may be required.
Glucocorticoids have some efficacy in primary FSGS, although they have not been tested against placebo in a randomized, controlled trial. Response rates, defined as CR plus PR, tend to be higher in children (often approximately 50%) compared with adults. For APOL1 FSGS, response rates to glucocorticoids are probably similar to those seen in primary FSGS, although the data are limited (83). The mechanism by which glucocorticoids affect podocytes are poorly understood; these agents are known to have direct effects on cultured podocytes (132). Recently, it has been suggested that these agents can expand the population of myeloid-derived suppressor cells that downregulate T cell function (133). Reliable and validated biomarkers of steroid responsiveness would be valuable. There are few data on which to base a determination of the appropriate dose and duration of glucocorticoid therapy. Biomarkers for responsiveness to glucocorticoid therapy would be valuable to reduce or avoid the need to expose patients to drugs to which they will not respond. Preliminary studies that await validation in larger cohorts include the ratio of podocin mRNA to synaptopodin mRNA, particularly when the diagnostic uncertainty with regard to MCD versus FSGS exists (134). Immunohistochemical detection of reduced expression of synaptopodin and receptor-type tyrosine-protein phosphatase O (PTPRO or GLEPP1) may be useful in identifying steroid-resistant patients (135). Conversely, reduced eGFR at presentation does not predict nonresponsiveness to glucorticoids (136).
Calcineurin inhibitors, including cyclosporin and tacrolimus, are mainstays of FSGS therapy for both steroid-sensitive individuals who cannot tolerate continued steroid therapy and steroid-resistant FSGS. Cyclosporin was shown superior to placebo in the North American collaborative trial of steroid-resistant FSGS, with the primary outcome of CR or PR occurring in 70% with cyclosporin and 4% with placebo (102). Similarly, an open label study of cyclosporin in steroid-resistant nephrotic syndrome in China showed a 75% response rate (137). The FSGS Clinical Trial, which enrolled mostly children, showed that response rates are similar for cyclosporin and mycophenolate mofetil in blacks with and without high-risk APOL1 genotypes, although group sizes were small (138). Although patients may relapse when calcineurin inhibitors are discontinued, many subjects remain in remission.
A number of novel therapies have been explored in small trials. Some of these therapies have some promise, including pirfenidone (113) and saquinivir (116). Other therapies that have been shown to be ineffective or even harmful include sirolimus (93), galactose (139), and adalimumab (mAb directed against TNF) (139). Other immunosuppressive agents that have been used include azathioprine and mizoribine, although limited data for their success are available.
Recently, there has been a surge of interest in developing new therapies for FSGS and other primary glomerular diseases, all of which are defined as rare by the US Food and Drug Administration, and therefore, new therapies have certain commercial benefits. This is an exciting time for researchers of FSGS, and this is a time that patients with FSGS and their families have reason for cautious optimism. Ongoing or recently completed trials within the United States include a phase 1 study of N-acetyl mannosamine (metabolic precursor of sialic acid) and phase 2/3 studies of sparsentan (a modified form of irbesartan that also antagonizes endothelin-1), abatacept (a fusion protein that targets CD80), Acthar (pituitary extract), fresolimumab (mAb directed against TGF-β), isotretinion (retinoic acid derivative), and losmapimod (an mitogen-activated protein kinase inhibitor). Trials in other countries are studying dapagliflozin (Canada), lipoprotein removal (Japan), and mesenchymal stem cell therapy (Iran). Given the diverse forms of FSGS and the multiple molecular pathways involved in the pathogenesis of each form, it is critical that these trials and future trials characterize subjects with sufficient depth to determine the characteristics of responders and nonresponders.
FSGS comprises a complex set of syndromes, most with multiple causes, and hence, FSGS represents a diagnostic challenge that requires close and thoughtful collaboration between patient, family, nephrologist, pathologist, and geneticist to gather the necessary data. Information from clinical history, laboratory testing, renal biopsy, and in some patients, genetic testing can be used to identify which syndrome is present, guide therapy, and provide prognostic information. Many forms of FSGS tend to progress to ESRD, but new therapies are being tested that may improve the prognosis.
Disclosures
None.
Supplementary Material
Acknowledgments
The authors gratefully acknowledge the editorial assistance of Dr. Carla Nester, who improved the organization and clarity of this manuscript.
This work was supported by the Intramural Research Program, National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health and the Departments of Pathology of the Children’s National Medical Center and Johns Hopkins Medical Institutions.
Footnotes
This article contains supplemental material online at http://cjasn.asnjournals.org/lookup/suppl/doi:10.2215/CJN.05960616/-/DCSupplemental.
Published online ahead of print. Publication date available at www.cjasn.org.
References
- 1.Chen YM, Liapis H: Focal segmental glomerulosclerosis: Molecular genetics and targeted therapies. BMC Nephrol 16: 101, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.D’Agati VD, Kaskel FJ, Falk RJ: Focal segmental glomerulosclerosis. N Engl J Med 365: 2398–2411, 2011 [DOI] [PubMed] [Google Scholar]
- 3.Gbadegesin R, Lavin P, Foreman J, Winn M: Pathogenesis and therapy of focal segmental glomerulosclerosis: An update. Pediatr Nephrol 26: 1001–1015, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sethi S, Glassock RJ, Fervenza FC: Focal segmental glomerulosclerosis: Towards a better understanding for the practicing nephrologist. Nephrol Dial Transplant 30: 375–384, 2015 [DOI] [PubMed] [Google Scholar]
- 5.D’Agati VD: Pathobiology of focal segmental glomerulosclerosis: New developments. Curr Opin Nephrol Hypertens 21: 243–250, 2012 [DOI] [PubMed] [Google Scholar]
- 6.Fogo AB: Causes and pathogenesis of focal segmental glomerulosclerosis. Nat Rev Nephrol 11: 76–87, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mondini A, Messa P, Rastaldi MP: The sclerosing glomerulus in mice and man: Novel insights. Curr Opin Nephrol Hypertens 23: 239–244, 2014 [DOI] [PubMed] [Google Scholar]
- 8.Schell C, Huber TB: New players in the pathogenesis of focal segmental glomerulosclerosis. Nephrol Dial Transplant 27: 3406–3412, 2012 [DOI] [PubMed] [Google Scholar]
- 9.Kiffel J, Rahimzada Y, Trachtman H: Focal segmental glomerulosclerosis and chronic kidney disease in pediatric patients. Adv Chronic Kidney Dis 18: 332–338, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kronbichler A, Leierer J, Oh J, Meijers B, Shin JI: Immunologic changes implicated in the pathogenesis of focal segmental glomerulosclerosis. BioMed Res Int 2016: 2150451, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sethna CB, Gipson DS: Treatment of FSGS in children. Adv Chronic Kidney Dis 21: 194–199, 2014 [DOI] [PubMed] [Google Scholar]
- 12.Coppo R: Different targets for treating focal segmental glomerular sclerosis. Contrib Nephrol 181: 84–90, 2013 [DOI] [PubMed] [Google Scholar]
- 13.Korbet SM: Treatment of primary FSGS in adults. J Am Soc Nephrol 23: 1769–1776, 2012 [DOI] [PubMed] [Google Scholar]
- 14.Meyrier A: Focal and segmental glomerulosclerosis: Multiple pathways are involved. Semin Nephrol 31: 326–332, 2011 [DOI] [PubMed] [Google Scholar]
- 15.McGrogan A, Franssen CF, de Vries CS: The incidence of primary glomerulonephritis worldwide: A systematic review of the literature. Nephrol Dial Transplant 26: 414–430, 2011 [DOI] [PubMed] [Google Scholar]
- 16.Briganti EM, Dowling J, Finlay M, Hill PA, Jones CL, Kincaid-Smith PS, Sinclair R, McNeil JJ, Atkins RC: The incidence of biopsy-proven glomerulonephritis in Australia. Nephrol Dial Transplant 16: 1364–1367, 2001 [DOI] [PubMed] [Google Scholar]
- 17.Sim JJ, Batech M, Hever A, Harrison TN, Avelar T, Kanter MH, Jacobsen SJ: Distribution of biopsy-proven presumed primary glomerulonephropathies in 2000-2011 among a racially and ethnically diverse US population. Am J Kidney Dis 68: 533–544, 2016 [DOI] [PubMed] [Google Scholar]
- 18.Kitiyakara C, Eggers P, Kopp JB: Twenty-one-year trend in ESRD due to focal segmental glomerulosclerosis in the United States. Am J Kidney Dis 44: 815–825, 2004 [PubMed] [Google Scholar]
- 19.Asinobi AO, Ademola AD, Okolo CA, Yaria JO: Trends in the histopathology of childhood nephrotic syndrome in Ibadan Nigeria: Preponderance of idiopathic focal segmental glomerulosclerosis. BMC Nephrol 16: 213, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.D’Agati VD, Fogo AB, Bruijn JA, Jennette JC: Pathologic classification of focal segmental glomerulosclerosis: A working proposal. Am J Kidney Dis 43: 368–382, 2004 [DOI] [PubMed] [Google Scholar]
- 21.Silverstein DM, Craver R: Presenting features and short-term outcome according to pathologic variant in childhood primary focal segmental glomerulosclerosis. Clin J Am Soc Nephrol 2: 700–707, 2007 [DOI] [PubMed] [Google Scholar]
- 22.Thomas DB, Franceschini N, Hogan SL, Ten Holder S, Jennette CE, Falk RJ, Jennette JC: Clinical and pathologic characteristics of focal segmental glomerulosclerosis pathologic variants. Kidney Int 69: 920–926, 2006 [DOI] [PubMed] [Google Scholar]
- 23.Genovese G, Friedman DJ, Ross MD, Lecordier L, Uzureau P, Freedman BI, Bowden DW, Langefeld CD, Oleksyk TK, Uscinski Knob AL, Bernhardy AJ, Hicks PJ, Nelson GW, Vanhollebeke B, Winkler CA, Kopp JB, Pays E, Pollak MR: Association of trypanolytic ApoL1 variants with kidney disease in African Americans. Science 329: 841–845, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kopp JB: Focal segmental glomerulosclerosis and collapsing glomerulopathy syndromes. In: Essentials of Chronic Kidney Disease, edited by Fadem SZ, Hauppauge, Nova Science Publishers, 2015 [Google Scholar]
- 25.Fahr T: Pathologische anatomie des morbus brightii. In: Handbuch der Speziellen Pathologischen Anatomie und Histologie, edited by Henke F, Lubarsch O, Berlin, Springer, 1925 [Google Scholar]
- 26.Rich AR: A hitherto undescribed vulnerability of the juxtamedullary glomeruli in lipoid nephrosis. Bull Johns Hopkins Hosp 100: 173–186, 1957 [PubMed] [Google Scholar]
- 27.Corwin HL, Schwartz MM, Lewis EJ: The importance of sample size in the interpretation of the renal biopsy. Am J Nephrol 8: 85–89, 1988 [DOI] [PubMed] [Google Scholar]
- 28.Schachter AD: Computational simulation of renal biopsy accuracy in focal segmental glomerulosclerosis. Pediatr Nephrol 21: 953–957, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fogo A, Glick AD, Horn SL, Horn RG: Is focal segmental glomerulosclerosis really focal? Distribution of lesions in adults and children. Kidney Int 47: 1690–1696, 1995 [DOI] [PubMed] [Google Scholar]
- 30.Wagrowska-Danilewicz M, Danilewicz M: [Synaptopodin immunoexpression in steroid-responsive and steroid-resistant minimal change disease and focal segmental glomerulosclerosis]. Nefrologia 27: 710–715, 2007 [PubMed] [Google Scholar]
- 31.Giannico G, Yang H, Neilson EG, Fogo AB: Dystroglycan in the diagnosis of FSGS. Clin J Am Soc Nephrol 4: 1747–1753, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Meehan SM, Chang A, Gibson IW, Kim L, Kambham N, Laszik Z: A study of interobserver reproducibility of morphologic lesions of focal segmental glomerulosclerosis. Virchows Arch 462: 229–237, 2013 [DOI] [PubMed] [Google Scholar]
- 33.Han MH, Kim YJ: Practical application of columbia classification for focal segmental glomerulosclerosis. BioMed Res Int 2016: 9375753, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schwartz MM, Korbet SM: Primary focal segmental glomerulosclerosis: Pathology, histological variants, and pathogenesis. Am J Kidney Dis 22: 874–883, 1993 [DOI] [PubMed] [Google Scholar]
- 35.Stokes MB, D’Agati VD: Morphologic variants of focal segmental glomerulosclerosis and their significance. Adv Chronic Kidney Dis 21: 400–407, 2014 [DOI] [PubMed] [Google Scholar]
- 36.Zhong Y, Xu F, Li X, Chen H, Liang S, Zhu X, Liu Z, Zeng C: The evolution of morphological variants of focal segmental glomerulosclerosis: A repeat biopsy-based observation. Nephrol Dial Transplant 31: 87–95, 2016 [DOI] [PubMed] [Google Scholar]
- 37.Strassheim D, Renner B, Panzer S, Fuquay R, Kulik L, Ljubanović D, Holers VM, Thurman JM: IgM contributes to glomerular injury in FSGS. J Am Soc Nephrol 24: 393–406, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Deegens JK, Dijkman HB, Borm GF, Steenbergen EJ, van den Berg JG, Weening JJ, Wetzels JF: Podocyte foot process effacement as a diagnostic tool in focal segmental glomerulosclerosis. Kidney Int 74: 1568–1576, 2008 [DOI] [PubMed] [Google Scholar]
- 39.Wiggins RC: The spectrum of podocytopathies: A unifying view of glomerular diseases. Kidney Int 71: 1205–1214, 2007 [DOI] [PubMed] [Google Scholar]
- 40.Fukuda A, Chowdhury MA, Venkatareddy MP, Wang SQ, Nishizono R, Suzuki T, Wickman LT, Wiggins JE, Muchayi T, Fingar D, Shedden KA, Inoki K, Wiggins RC: Growth-dependent podocyte failure causes glomerulosclerosis. J Am Soc Nephrol 23: 1351–1363, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kriz W, Gretz N, Lemley KV: Progression of glomerular diseases: Is the podocyte the culprit? Kidney Int 54: 687–697, 1998 [DOI] [PubMed] [Google Scholar]
- 42.Kriz W, Lemley KV: A potential role for mechanical forces in the detachment of podocytes and the progression of CKD. J Am Soc Nephrol 26: 258–269, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gallon L, Leventhal J, Skaro A, Kanwar Y, Alvarado A: Resolution of recurrent focal segmental glomerulosclerosis after retransplantation. N Engl J Med 366: 1648–1649, 2012 [DOI] [PubMed] [Google Scholar]
- 44.Komatsu K, Frohlich ED, Ono H, Ono Y, Numabe A, Willis GW: Glomerular dynamics and morphology of aged spontaneously hypertensive rats. Effects of angiotensin-converting enzyme inhibition. Hypertension 25: 207–213, 1995 [DOI] [PubMed] [Google Scholar]
- 45.Savin VJ, Sharma M, Zhou J, Gennochi D, Fields T, Sharma R, McCarthy ET, Srivastava T, Domen J, Tormo A, Gauchat JF: Renal and hematological effects of CLCF-1, a B-Cell-stimulating cytokine of the IL-6 family. J Immunol Res 2015: 714964, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lopez-Hellin J, Cantarell C, Jimeno L, Sanchez-Fructuoso A, Puig-Gay N, Guirado L, Vilariño N, Gonzalez-Roncero FM, Mazuecos A, Lauzurica R, Burgos D, Plumed JS, Jacobs-Cacha C, Jimenez C, Fernandez A, Fernandez-Alvarez P, Torregrosa V, Nieto JL, Meseguer A, Alonso A; GREAT Study Group: A form of apolipoprotein a-I is found specifically in relapses of focal segmental glomerulosclerosis following transplantation. Am J Transplant 13: 493–500, 2013 [DOI] [PubMed] [Google Scholar]
- 47.Delville M, Sigdel TK, Wei C, Li J, Hsieh SC, Fornoni A, Burke GW, Bruneval P, Naesens M, Jackson A, Alachkar N, Canaud G, Legendre C, Anglicheau D, Reiser J, Sarwal MM: A circulating antibody panel for pretransplant prediction of FSGS recurrence after kidney transplantation. Sci Transl Med 6: 256ra136, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wei C, El Hindi S, Li J, Fornoni A, Goes N, Sageshima J, Maiguel D, Karumanchi SA, Yap HK, Saleem M, Zhang Q, Nikolic B, Chaudhuri A, Daftarian P, Salido E, Torres A, Salifu M, Sarwal MM, Schaefer F, Morath C, Schwenger V, Zeier M, Gupta V, Roth D, Rastaldi MP, Burke G, Ruiz P, Reiser J: Circulating urokinase receptor as a cause of focal segmental glomerulosclerosis. Nat Med 17: 952–960, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Maas RJ, Deegens JK, Wetzels JF: Serum suPAR in patients with FSGS: Trash or treasure? Pediatr Nephrol 28: 1041–1048, 2013 [DOI] [PubMed] [Google Scholar]
- 50.Spinale JM, Mariani LH, Kapoor S, Zhang J, Weyant R, Song PX, Wong HN, Troost JP, Gadegbeku CA, Gipson DS, Kretzler M, Nihalani D, Holzman LB; Nephrotic Syndrome Study Network: A reassessment of soluble urokinase-type plasminogen activator receptor in glomerular disease. Kidney Int 87: 564–574, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Huang J, Liu G, Zhang YM, Cui Z, Wang F, Liu XJ, Chu R, Chen Y, Zhao MH: Plasma soluble urokinase receptor levels are increased but do not distinguish primary from secondary focal segmental glomerulosclerosis. Kidney Int 84: 366–372, 2013 [DOI] [PubMed] [Google Scholar]
- 52.Peng Z, Mao J, Chen X, Cai F, Gu W, Fu H, Shen H, Wang J, Jin X, Zhu X, Liu A, Shu Q, Du L: Serum suPAR levels help differentiate steroid resistance from steroid-sensitive nephrotic syndrome in children. Pediatr Nephrol 30: 301–307, 2015 [DOI] [PubMed] [Google Scholar]
- 53.Maas RJ, Wetzels JF, Deegens JK: Serum-soluble urokinase receptor concentration in primary FSGS. Kidney Int 81: 1043–1044, 2012 [DOI] [PubMed] [Google Scholar]
- 54.Wada T, Nangaku M: A circulating permeability factor in focal segmental glomerulosclerosis: The hunt continues. Clin Kidney J 8: 708–715, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Harris JJ, McCarthy HJ, Ni L, Wherlock M, Kang H, Wetzels JF, Welsh GI, Saleem MA: Active proteases in nephrotic plasma lead to a podocin-dependent phosphorylation of VASP in podocytes via protease activated receptor-1. J Pathol 229: 660–671, 2013 [DOI] [PubMed] [Google Scholar]
- 56.Kachurina N, Chung CF, Benderoff E, Babayeva S, Bitzan M, Goodyer PR, Kitzler T, Matar D, Cybulsky AV, Alachkar N, Torban E: Novel unbiased assay for circulating podocyte-toxic factors associated with recurrent focal segmental glomerulosclerosis. Am J Physiol Renal Physiol 310: F1148–F1156, 2015 [DOI] [PubMed] [Google Scholar]
- 57.Mallipattu SK, He JC: The podocyte as a direct target for treatment of glomerular disease? Am J Physiol Renal Physiol 311: F46–F51, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mallipattu SK, Guo Y, Revelo MP, Roa-Pena L, Miller T, Ling J, Shankland SJ, Bialkowska AB, Ly V, Estrada C, Jain MK, Lu Y, Ma’ayan A, Mehrotra A, Yacoub R, Nord EP, Woroniecki RP, Yang VW, He JC: Kruppel-like factor 15 mediates glucocorticoid-induced restoration of podocyte differentiation markers. J Am Soc Nephrol 28: 166–184, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Laurin LP, Gasim AM, Poulton CJ, Hogan SL, Jennette JC, Falk RJ, Foster BJ, Nachman PH: Treatment with glucocorticoids or calcineurin inhibitors in primary FSGS. Clin J Am Soc Nephrol 11: 386–394, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Canaud G, Dion D, Zuber J, Gubler MC, Sberro R, Thervet E, Snanoudj R, Charbit M, Salomon R, Martinez F, Legendre C, Noel LH, Niaudet P: Recurrence of nephrotic syndrome after transplantation in a mixed population of children and adults: Course of glomerular lesions and value of the Columbia classification of histological variants of focal and segmental glomerulosclerosis (FSGS). Nephrol Dial Transplant 25: 1321–1328, 2010 [DOI] [PubMed] [Google Scholar]
- 61.Canaud G, Zuber J, Sberro R, Royale V, Anglicheau D, Snanoudj R, Gaha K, Thervet E, Lefrère F, Cavazzana-Calvo M, Noël LH, Méjean A, Legendre C, Martinez F: Intensive and prolonged treatment of focal and segmental glomerulosclerosis recurrence in adult kidney transplant recipients: A pilot study. Am J Transplant 9: 1081–1086, 2009 [DOI] [PubMed] [Google Scholar]
- 62.Barisoni L, Schnaper HW, Kopp JB: A proposed taxonomy for the podocytopathies: A reassessment of the primary nephrotic diseases. Clin J Am Soc Nephrol 2: 529–542, 2007 [DOI] [PubMed] [Google Scholar]
- 63.Brenner BM, Mackenzie HS: Nephron mass as a risk factor for progression of renal disease. Kidney Int Suppl 63: S124–S127, 1997 [PubMed] [Google Scholar]
- 64.Morgan C, Al-Aklabi M, Garcia Guerra G: Chronic kidney disease in congenital heart disease patients: A narrative review of evidence. Can J Kidney Health Dis 2: 27, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Aygun B, Mortier NA, Smeltzer MP, Hankins JS, Ware RE: Glomerular hyperfiltration and albuminuria in children with sickle cell anemia. Pediatr Nephrol 26: 1285–1290, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wickman C, Kramer H: Obesity and kidney disease: Potential mechanisms. Semin Nephrol 33: 14–22, 2013 [DOI] [PubMed] [Google Scholar]
- 67.Herlitz LC, Markowitz GS, Farris AB, Schwimmer JA, Stokes MB, Kunis C, Colvin RB, D’Agati VD: Development of focal segmental glomerulosclerosis after anabolic steroid abuse. J Am Soc Nephrol 21: 163–172, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hanly PJ, Ahmed SB: Sleep apnea and the kidney: Is sleep apnea a risk factor for chronic kidney disease? Chest 146: 1114–1122, 2014 [DOI] [PubMed] [Google Scholar]
- 69.Ikezumi Y, Suzuki T, Karasawa T, Yamada T, Hasegawa H, Nishimura H, Uchiyama M: Low birthweight and premature birth are risk factors for podocytopenia and focal segmental glomerulosclerosis. Am J Nephrol 38: 149–157, 2013 [DOI] [PubMed] [Google Scholar]
- 70.Kriz W, Lemley KV: Mechanical challenges to the glomerular filtration barrier: Adaptations and pathway to sclerosis [published online ahead of print March 23, 2016]. Pediatr Nephrol doi:10.1007/s00467-016-3358-9 [DOI] [PubMed] [Google Scholar]
- 71.Gasser DL, Winkler CA, Peng M, An P, McKenzie LM, Kirk GD, Shi Y, Xie LX, Marbois BN, Clarke CF, Kopp JB: Focal segmental glomerulosclerosis is associated with a PDSS2 haplotype and, independently, with a decreased content of coenzyme Q10. Am J Physiol Renal Physiol 305: F1228–F1238, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Yu H, Artomov M, Brähler S, Stander MC, Shamsan G, Sampson MG, White JM, Kretzler M, Miner JH, Jain S, Winkler CA, Mitra RD, Kopp JB, Daly MJ, Shaw AS: A role for genetic susceptibility in sporadic focal segmental glomerulosclerosis. J Clin Invest 126: 1603, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Gast C, Pengelly RJ, Lyon M, Bunyan DJ, Seaby EG, Graham N, Venkat-Raman G, Ennis S: Collagen (COL4A) mutations are the most frequent mutations underlying adult focal segmental glomerulosclerosis. Nephrol Dial Transplant 31: 961–970, 2016 [DOI] [PubMed] [Google Scholar]
- 74.Ozaltin F, Ibsirlioglu T, Taskiran EZ, Baydar DE, Kaymaz F, Buyukcelik M, Kilic BD, Balat A, Iatropoulos P, Asan E, Akarsu NA, Schaefer F, Yilmaz E, Bakkaloglu A; PodoNet Consortium: Disruption of PTPRO causes childhood-onset nephrotic syndrome. Am J Hum Genet 89: 139–147, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Sadowski CE, Lovric S, Ashraf S, Pabst WL, Gee HY, Kohl S, Engelmann S, Vega-Warner V, Fang H, Halbritter J, Somers MJ, Tan W, Shril S, Fessi I, Lifton RP, Bockenhauer D, El-Desoky S, Kari JA, Zenker M, Kemper MJ, Mueller D, Fathy HM, Soliman NA, Hildebrandt F; SRNS Study Group: A single-gene cause in 29.5% of cases of steroid-resistant nephrotic syndrome. J Am Soc Nephrol 26: 1279–1289, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Trautmann A, Bodria M, Ozaltin F, Gheisari A, Melk A, Azocar M, Anarat A, Caliskan S, Emma F, Gellermann J, Oh J, Baskin E, Ksiazek J, Remuzzi G, Erdogan O, Akman S, Dusek J, Davitaia T, Özkaya O, Papachristou F, Firszt-Adamczyk A, Urasinski T, Testa S, Krmar RT, Hyla-Klekot L, Pasini A, Özcakar ZB, Sallay P, Cakar N, Galanti M, Terzic J, Aoun B, Caldas Afonso A, Szymanik-Grzelak H, Lipska BS, Schnaidt S, Schaefer F; PodoNet Consortium: Spectrum of steroid-resistant and congenital nephrotic syndrome in children: The PodoNet registry cohort. Clin J Am Soc Nephrol 10: 592–600, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Green RC, Berg JS, Grody WW, Kalia SS, Korf BR, Martin CL, McGuire AL, Nussbaum RL, O’Daniel JM, Ormond KE, Rehm HL, Watson MS, Williams MS, Biesecker LG; American College of Medical Genetics and Genomics: ACMG recommendations for reporting of incidental findings in clinical exome and genome sequencing. Genet Med 15: 565–574, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Büscher AK, Beck BB, Melk A, Hoefele J, Kranz B, Bamborschke D, Baig S, Lange-Sperandio B, Jungraithmayr T, Weber LT, Kemper MJ, Tönshoff B, Hoyer PF, Konrad M, Weber S; German Pediatric Nephrology Association (GPN): Rapid response to cyclosporin A and favorable renal outcome in nongenetic versus genetic steroid-resistant nephrotic syndrome. Clin J Am Soc Nephrol 11: 245–253, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Meehan SM, Kim L, Chang A: A spectrum of morphologic lesions of focal segmental glomerulosclerosis by Columbia criteria in human immunodeficiency virus infection. Virchows Arch 460: 429–435, 2012 [DOI] [PubMed] [Google Scholar]
- 80.Marras D, Bruggeman LA, Gao F, Tanji N, Mansukhani MM, Cara A, Ross MD, Gusella GL, Benson G, D’Agati VD, Hahn BH, Klotman ME, Klotman PE: Replication and compartmentalization of HIV-1 in kidney epithelium of patients with HIV-associated nephropathy. Nat Med 8: 522–526, 2002 [DOI] [PubMed] [Google Scholar]
- 81.Husain M, Gusella GL, Klotman ME, Gelman IH, Ross MD, Schwartz EJ, Cara A, Klotman PE: HIV-1 Nef induces proliferation and anchorage-independent growth in podocytes. J Am Soc Nephrol 13: 1806–1815, 2002 [DOI] [PubMed] [Google Scholar]
- 82.Hiramatsu N, Hiromura K, Shigehara T, Kuroiwa T, Ideura H, Sakurai N, Takeuchi S, Tomioka M, Ikeuchi H, Kaneko Y, Ueki K, Kopp JB, Nojima Y: Angiotensin II type 1 receptor blockade inhibits the development and progression of HIV-associated nephropathy in a mouse model. J Am Soc Nephrol 18: 515–527, 2007 [DOI] [PubMed] [Google Scholar]
- 83.Kopp JB, Nelson GW, Sampath K, Johnson RC, Genovese G, An P, Friedman D, Briggs W, Dart R, Korbet S, Mokrzycki MH, Kimmel PL, Limou S, Ahuja TS, Berns JS, Fryc J, Simon EE, Smith MC, Trachtman H, Michel DM, Schelling JR, Vlahov D, Pollak M, Winkler CA: APOL1 genetic variants in focal segmental glomerulosclerosis and HIV-associated nephropathy. J Am Soc Nephrol 22: 2129–2137, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kasembeli AN, Duarte R, Ramsay M, Mosiane P, Dickens C, Dix-Peek T, Limou S, Sezgin E, Nelson GW, Fogo AB, Goetsch S, Kopp JB, Winkler CA, Naicker S: APOL1 risk variants are strongly associated with HIV-associated nephropathy in black South Africans. J Am Soc Nephrol 26: 2882–2890, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Chandra P, Kopp JB: Viruses and collapsing glomerulopathy: A brief critical review. Clin Kidney J 6: 1–5, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Sehar N, Gobran E, Elsayegh S: Collapsing focal segmental glomerulosclerosis in a patient with acute malaria. Case Rep Med 2015: 420459, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Martinelli R, Pereira LJ, Brito E, Rocha H: Clinical course of focal segmental glomerulosclerosis associated with hepatosplenic schistosomiasis mansoni. Nephron 69: 131–134, 1995 [DOI] [PubMed] [Google Scholar]
- 88.Pakasa NM, Nseka NM, Nyimi LM: Secondary collapsing glomerulopathy associated with Loa loa filariasis. Am J Kidney Dis 30: 836–839, 1997 [DOI] [PubMed] [Google Scholar]
- 89.Markowitz GS, Appel GB, Fine PL, Fenves AZ, Loon NR, Jagannath S, Kuhn JA, Dratch AD, D’Agati VD: Collapsing focal segmental glomerulosclerosis following treatment with high-dose pamidronate. J Am Soc Nephrol 12: 1164–1172, 2001 [DOI] [PubMed] [Google Scholar]
- 90.Dijkman HB, Weening JJ, Smeets B, Verrijp KC, van Kuppevelt TH, Assmann KK, Steenbergen EJ, Wetzels JF: Proliferating cells in HIV and pamidronate-associated collapsing focal segmental glomerulosclerosis are parietal epithelial cells. Kidney Int 70: 338–344, 2006 [DOI] [PubMed] [Google Scholar]
- 91.Sakarcan A, Thomas DB, O’Reilly KP, Richards RW: Lithium-induced nephrotic syndrome in a young pediatric patient. Pediatr Nephrol 17: 290–292, 2002 [DOI] [PubMed] [Google Scholar]
- 92.Letavernier E, Bruneval P, Mandet C, Duong Van Huyen JP, Péraldi MN, Helal I, Noël LH, Legendre C: High sirolimus levels may induce focal segmental glomerulosclerosis de novo. Clin J Am Soc Nephrol 2: 326–333, 2007 [DOI] [PubMed] [Google Scholar]
- 93.Cho ME, Hurley JK, Kopp JB: Sirolimus therapy of focal segmental glomerulosclerosis is associated with nephrotoxicity. Am J Kidney Dis 49: 310–317, 2007 [DOI] [PubMed] [Google Scholar]
- 94.Mohamed N, Goldstein J, Schiff J, John R: Collapsing glomerulopathy following anthracycline therapy. Am J Kidney Dis 61: 778–781, 2013 [DOI] [PubMed] [Google Scholar]
- 95.Lee VW, Harris DC: Adriamycin nephropathy: A model of focal segmental glomerulosclerosis. Nephrology (Carlton) 16: 30–38, 2011 [DOI] [PubMed] [Google Scholar]
- 96.Park A, Gharavi A, Kopp J, Winkler C, Pullman JA, Miner J, Suztak K: Podocyte Specific Expression of Mutant APOL1 Induces Albuminuria and Global Sclerosis in Mice, San Diego, CA, American Society of Nephrology, 2015 [Google Scholar]
- 97.Ashley-Koch AE, Okocha EC, Garrett ME, Soldano K, De Castro LM, Jonassaint JC, Orringer EP, Eckman JR, Telen MJ: MYH9 and APOL1 are both associated with sickle cell disease nephropathy. Br J Haematol 155: 386–394, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Nichols B, Jog P, Lee JH, Blackler D, Wilmot M, D’Agati V, Markowitz G, Kopp JB, Alper SL, Pollak MR, Friedman DJ: Innate immunity pathways regulate the nephropathy gene Apolipoprotein L1. Kidney Int 87: 332–342, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Larsen CP, Beggs ML, Saeed M, Walker PD: Apolipoprotein L1 risk variants associate with systemic lupus erythematosus-associated collapsing glomerulopathy. J Am Soc Nephrol 24: 722–725, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.KDIGO Glomerulonephritis Work Group: KDIGO clinical practice guidelines for glomerulonephritis. Kidney Int 2[Suppl]: 139–274, 2012 [Google Scholar]
- 101.Teeninga N, Kist-van Holthe JE, van den Akker EL, Kersten MC, Boersma E, Krabbe HG, Knoers NV, van der Heijden AJ, Koper JW, Nauta J: Genetic and in vivo determinants of glucocorticoid sensitivity in relation to clinical outcome of childhood nephrotic syndrome. Kidney Int 85: 1444–1453, 2014 [DOI] [PubMed] [Google Scholar]
- 102.Cattran DC, Appel GB, Hebert LA, Hunsicker LG, Pohl MA, Hoy WE, Maxwell DR, Kunis CL; North America Nephrotic Syndrome Study Group: A randomized trial of cyclosporine in patients with steroid-resistant focal segmental glomerulosclerosis. Kidney Int 56: 2220–2226, 1999 [DOI] [PubMed] [Google Scholar]
- 103.Senthil Nayagam L, Ganguli A, Rathi M, Kohli HS, Gupta KL, Joshi K, Sakhuja V, Jha V: Mycophenolate mofetil or standard therapy for membranous nephropathy and focal segmental glomerulosclerosis: A pilot study. Nephrol Dial Transplant 23: 1926–1930, 2008 [DOI] [PubMed] [Google Scholar]
- 104.Braun N, Schmutzler F, Lange C, Perna A, Remuzzi G, Risler T, Willis NS: Immunosuppressive treatment for focal segmental glomerulosclerosis in adults. Cochrane Database Syst Rev 3: CD003233, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Hahn D, Hodson EM, Willis NS, Craig JC: Corticosteroid therapy for nephrotic syndrome in children. Cochrane Database Syst Rev 3: CD001533, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Hodson EM, Knight JF, Willis NS, Craig JC: Corticosteroid therapy for nephrotic syndrome in children (Cochrane Review). In: The Cochrane Library, Oxford, UK, Oxford Update Software, 2003 [DOI] [PubMed] [Google Scholar]
- 107.Pravitsitthikul N, Willis NS, Hodson EM, Craig JC: Non-corticosteroid immunosuppressive medications for steroid-sensitive nephrotic syndrome in children. Cochrane Database Syst Rev 10: CD002290, 2013 [DOI] [PubMed] [Google Scholar]
- 108.Beer A, Mayer G, Kronbichler A: Treatment strategies of adult primary focal segmental glomerulosclerosis: A systematic review focusing on the last two decades. BioMed Res Int 2016: 4192578, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Hogan J, Radhakrishnan J: The treatment of idiopathic focal segmental glomerulosclerosis in adults. Adv Chronic Kidney Dis 21: 434–441, 2014 [DOI] [PubMed] [Google Scholar]
- 110.Meyrier A: An update on the treatment options for focal segmental glomerulosclerosis. Expert Opin Pharmacother 10: 615–628, 2009 [DOI] [PubMed] [Google Scholar]
- 111.Ponticelli C, Graziani G: Current and emerging treatments for idiopathic focal and segmental glomerulosclerosis in adults. Expert Rev Clin Immunol 9: 251–261, 2013 [DOI] [PubMed] [Google Scholar]
- 112.Joy MS, Gipson DS, Powell L, MacHardy J, Jennette JC, Vento S, Pan C, Savin V, Eddy A, Fogo AB, Kopp JB, Cattran D, Trachtman H: Phase 1 trial of adalimumab in focal segmental glomerulosclerosis (FSGS): II. Report of the FONT (Novel Therapies for Resistant FSGS) study group. Am J Kidney Dis 55: 50–60, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Cho ME, Smith DC, Branton MH, Penzak SR, Kopp JB: Pirfenidone slows renal function decline in patients with focal segmental glomerulosclerosis. Clin J Am Soc Nephrol 2: 906–913, 2007 [DOI] [PubMed] [Google Scholar]
- 114.Trachtman H, Fervenza FC, Gipson DS, Heering P, Jayne DR, Peters H, Rota S, Remuzzi G, Rump LC, Sellin LK, Heaton JP, Streisand JB, Hard ML, Ledbetter SR, Vincenti F: A phase 1, single-dose study of fresolimumab, an anti-TGF-β antibody, in treatment-resistant primary focal segmental glomerulosclerosis. Kidney Int 79: 1236–1243, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Hari P, Bagga A, Jindal N, Srivastava RN: Treatment of focal glomerulosclerosis with pulse steroids and oral cyclophosphamide. Pediatr Nephrol 16: 901–905, 2001 [DOI] [PubMed] [Google Scholar]
- 116.Coppo R, Camilla R, Porcellini MG, Peruzzi L, Gianoglio B, Amore A, Daprà V, Loiacono E, Fonsato V, Dal Canton A, Esposito C, Esposito P, Tovo PA: Saquinavir in steroid-dependent and -resistant nephrotic syndrome: A pilot study. Nephrol Dial Transplant 27: 1902–1910, 2012 [DOI] [PubMed] [Google Scholar]
- 117.Hogan J, Bomback AS, Mehta K, Canetta PA, Rao MK, Appel GB, Radhakrishnan J, Lafayette RA: Treatment of idiopathic FSGS with adrenocorticotropic hormone gel. Clin J Am Soc Nephrol 8: 2072–2081, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Madan A, Mijovic-Das S, Stankovic A, Teehan G, Milward AS, Khastgir A: Acthar gel in the treatment of nephrotic syndrome: A multicenter retrospective case series. BMC Nephrol 17: 37, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Lieberman KV, Pavlova-Wolf A: Adrenocorticotropic hormone therapy for the treatment of idiopathic nephrotic syndrome in children and young adults: A systematic review of early clinical studies with contemporary relevance [published online ahead of print April 16, 2016]. J Nephrol doi:10.1007/s40620-016-0308-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Sgambat K, Banks M, Moudgil A: Effect of galactose on glomerular permeability and proteinuria in steroid-resistant nephrotic syndrome. Pediatr Nephrol 28: 2131–2135, 2013 [DOI] [PubMed] [Google Scholar]
- 121.Yu CC, Fornoni A, Weins A, Hakroush S, Maiguel D, Sageshima J, Chen L, Ciancio G, Faridi MH, Behr D, Campbell KN, Chang JM, Chen HC, Oh J, Faul C, Arnaout MA, Fiorina P, Gupta V, Greka A, Burke GW 3rd, Mundel P: Abatacept in B7-1-positive proteinuric kidney disease. N Engl J Med 369: 2416–2423, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Spino C, Jahnke JS, Selewski DT, Massengill S, Troost J, Gipson DS: Changing the paradigm for the treatment and development of new therapies for FSGS. Front Pediatr 4: 25, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Mariani LH, Pendergraft WF 3rd, Kretzler M: Defining glomerular disease in mechanistic terms: Implementing an integrative biology approach in nephrology. Clin J Am Soc Nephrol 11: 2054–2060, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Copelovitch L, Guttenberg M, Pollak MR, Kaplan BS: Renin-angiotensin axis blockade reduces proteinuria in presymptomatic patients with familial FSGS. Pediatr Nephrol 22: 1779–1784, 2007 [DOI] [PubMed] [Google Scholar]
- 125.Fujisaki K, Tsuruya K, Nakano T, Taniguchi M, Higashi H, Katafuchi R, Kanai H, Nakayama M, Hirakata H, Kitazono T; Impact of Combined Losartan/Hydrochlorothiazide on Proteinuria in Patients with Chronic Kidney Disease and Hypertension (ILOHA) Study Investigators: Impact of combined losartan/hydrochlorothiazide on proteinuria in patients with chronic kidney disease and hypertension. Hypertens Res 37: 993–998, 2014 [DOI] [PubMed] [Google Scholar]
- 126.Kangovi S, Edwards M, Woloszynek S, Mitra N, Feldman H, Kaplan BS, Meyers KE: Renin-angiotensin-aldosterone system inhibitors in pediatric focal segmental glomerulosclerosis. Pediatr Nephrol 27: 813–819, 2012 [DOI] [PubMed] [Google Scholar]
- 127.Cheng J, Zhang X, Tian J, Li Q, Chen J: Combination therapy an ACE inhibitor and an angiotensin receptor blocker for IgA nephropathy: A meta-analysis. Int J Clin Pract 66: 917–923, 2012 [DOI] [PubMed] [Google Scholar]
- 128.Mann JF, Schmieder RE, McQueen M, Dyal L, Schumacher H, Pogue J, Wang X, Maggioni A, Budaj A, Chaithiraphan S, Dickstein K, Keltai M, Metsärinne K, Oto A, Parkhomenko A, Piegas LS, Svendsen TL, Teo KK, Yusuf S; ONTARGET investigators: Renal outcomes with telmisartan, ramipril, or both, in people at high vascular risk (the ONTARGET study): A multicentre, randomised, double-blind, controlled trial. Lancet 372: 547–553, 2008 [DOI] [PubMed] [Google Scholar]
- 129.Fried LF, Emanuele N, Zhang JH, Brophy M, Conner TA, Duckworth W, Leehey DJ, McCullough PA, O’Connor T, Palevsky PM, Reilly RF, Seliger SL, Warren SR, Watnick S, Peduzzi P, Guarino P; VA NEPHRON-D Investigators: Combined angiotensin inhibition for the treatment of diabetic nephropathy. N Engl J Med 369: 1892–1903, 2013 [DOI] [PubMed] [Google Scholar]
- 130.Ando K, Ohtsu H, Uchida S, Kaname S, Arakawa Y, Fujita T; EVALUATE Study Group: Anti-albuminuric effect of the aldosterone blocker eplerenone in non-diabetic hypertensive patients with albuminuria: A double-blind, randomised, placebo-controlled trial. Lancet Diabetes Endocrinol 2: 944–953, 2014 [DOI] [PubMed] [Google Scholar]
- 131.Shavit L, Lifschitz MD, Epstein M: Aldosterone blockade and the mineralocorticoid receptor in the management of chronic kidney disease: Current concepts and emerging treatment paradigms. Kidney Int 81: 955–968, 2012 [DOI] [PubMed] [Google Scholar]
- 132.Xing CY, Saleem MA, Coward RJ, Ni L, Witherden IR, Mathieson PW: Direct effects of dexamethasone on human podocytes. Kidney Int 70: 1038–1045, 2006 [DOI] [PubMed] [Google Scholar]
- 133.Li L, Zhang T, Diao W, Jin F, Shi L, Meng J, Liu H, Zhang J, Zeng CH, Zhang MC, Liang S, Liu Y, Zhang CY, Liu Z, Zen K: Role of myeloid-derived suppressor cells in glucocorticoid-mediated amelioration of FSGS. J Am Soc Nephrol 26: 2183–2197, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Schmid H, Henger A, Cohen CD, Frach K, Gröne HJ, Schlöndorff D, Kretzler M: Gene expression profiles of podocyte-associated molecules as diagnostic markers in acquired proteinuric diseases. J Am Soc Nephrol 14: 2958–2966, 2003 [DOI] [PubMed] [Google Scholar]
- 135.Hirakawa M, Tsuruya K, Yotsueda H, Tokumoto M, Ikeda H, Katafuchi R, Fujimi S, Hirakata H, Iida M: Expression of synaptopodin and GLEPP1 as markers of steroid responsiveness in primary focal segmental glomerulosclerosis. Life Sci 79: 757–763, 2006 [DOI] [PubMed] [Google Scholar]
- 136.Jafry N, Ahmed E, Mubarak M, Kazi J, Akhter F: Raised serum creatinine at presentation does not adversely affect steroid response in primary focal segmental glomerulosclerosis in adults. Nephrol Dial Transplant 27: 1101–1106, 2012 [DOI] [PubMed] [Google Scholar]
- 137.Fan L, Liu Q, Liao Y, Li Z, Ji Y, Yang Z, Chen J, Fu J, Zhang J, Kong Y, Fu P, Lou T, Liu Z, Yu X, Chen W: Tacrolimus is an alternative therapy option for the treatment of adult steroid-resistant nephrotic syndrome: A prospective, multicenter clinical trial. Int Urol Nephrol 45: 459–468, 2013 [DOI] [PubMed] [Google Scholar]
- 138.Kopp JB, Winkler CA, Zhao X, Radeva MK, Gassman JJ, D’Agati VD, Nast CC, Wei C, Reiser J, Guay-Woodford LM, Pollak MR, Hildebrandt F, Moxey-Mims M, Gipson DS, Trachtman H, Friedman AL, Kaskel FJ; FSGS-CT Study Consortium: Clinical features and histology of apolipoprotein L1-associated nephropathy in the FSGS clinical trial. J Am Soc Nephrol 26: 1443–1448, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Trachtman H, Vento S, Herreshoff E, Radeva M, Gassman J, Stein DT, Savin VJ, Sharma M, Reiser J, Wei C, Somers M, Srivastava T, Gipson DS: Efficacy of galactose and adalimumab in patients with resistant focal segmental glomerulosclerosis: Report of the font clinical trial group. BMC Nephrol 16: 111, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.