Abstract
Background and objectives
Prior work has suggested a higher risk of hypertension in kidney stone formers but lacked disease validation and adjustment for potential confounders. Certain types of stone formers may also be at higher risk of hypertension.
Design, setting, participants, & measurements
In our study, incident symptomatic stone formers in Olmsted County from 2000 to 2011 were manually validated by chart review and age and sex matched to Olmsted County controls. We followed up patients through November 20, 2015. Hypertension was also validated by manual chart review, and the risk of hypertension in stone formers compared with controls was assessed both univariately and after adjusting for comorbidities. The risk of hypertension among different subtypes of stone formers was also evaluated.
Results
Among 3023 coded stone formers from 2000 to 2011, a total of 1515 were validated and matched to 1515 controls (mean age was 45 years old, and 56% were men). After excluding those with baseline hypertension (20% of stone formers and 18% of controls), 154 stone formers and 110 controls developed hypertension. Median follow-up time was 7.8 years in stone formers and 9.6 years in controls. Stone formers were found to have a higher risk of hypertension compared with controls (hazard ratio, 1.50; 95% confidence interval, 1.18 to 1.92), even after adjusting for age, sex, body mass index, serum creatinine, CKD, diabetes, gout, coronary artery disease, dyslipidemia, tobacco use, and alcohol abuse (hazard ratio, 1.58; 95% confidence interval, 1.12 to 2.21). Results were similar after excluding patients who were ever on a thiazide diuretic (hazard ratio, 1.65; 95% confidence interval, 1.16 to 2.38). Stone composition, radiographic stone burden, number of subsequent stone events, and stone removal surgeries were not associated with hypertension (P>0.05 for all).
Conclusions
The risk of hypertension was higher after the first symptomatic kidney stone event. However, kidney stone severity, type, and treatment did not associate with hypertension.
Keywords: kidney stones; Thiazide diuretics; chronic kidney disease; Epidemiology; Alcoholism; Body Mass Index; Comorbidity; coronary artery disease; creatinine; diabetes mellitus; Dyslipidemias; Follow-Up Studies; Gout; Humans; hypertension; Kidney Calculi; Male; Renal Insufficiency, Chronic; Tobacco Use; Sodium Chloride Symporter Inhibitors
Introduction
Over the last three decades, the incidence of kidney stones has sharply increased to become a significant health problem (1,2). Other than the risk of painful recurrence, kidney stone disease is a risk factor for CKD (3), cardiovascular disease (4,5), and bone fracture (6). Several studies have also suggested an increased risk of hypertension with kidney stones (7–14). Many of these studies lacked validation of stone formers and hypertension, had a cross–sectional study design, lacked adequate adjustment for comorbidities, or did not account for thiazide diuretics used to treat both kidney stone disease and hypertension.
Even if kidney stones are a risk factor for hypertension, it is unclear whether this is true for all types of stone formers, because certain types of stones have been associated with different comorbidities. For example, uric acid stones may be more strongly associated with diabetes, obesity, and metabolic syndrome (15,16). Whether stone composition is itself predictive of hypertension is not known. The severity of kidney stone disease can also be quite variable between different patients, but whether disease severity associates with risk of hypertension is also not known.
To address these questions, we performed a population–based cohort study with manual chart validation. We assessed whether incident symptomatic stone formers were at higher risk of developing hypertension compared with controls. We also assessed whether stone composition and stone disease severity in particular were predictive of hypertension among stone formers.
Materials and Methods
Study Population
After institutional review board approval (with a waiver of specific informed consent for a retrospective chart review), newly coded stone formers in Olmsted County from 2000 to 2011 were identified by International Classification of Diseases, Ninth Revision (ICD-9) codes 592 (calculus of kidney and ureter), 594 (calculus of lower urinary tract), and 274.11 (uric acid nephrolithiasis) and manually validated through chart review by trained nurse and physician abstractors to identify incident symptomatic stone formers. Patients were considered to have a symptomatic kidney stone if they had renal colic or atypical abdominal pain plus a stone obstructing the ureter on imaging or a documented voided stone. Patients were excluded if they had prior kidney stone episodes, only asymptomatic (incidental) kidney stones on imaging, only bladder stones, or a suspected symptomatic stone not confirmed with imaging or by a documented voided stone. Controls randomly sampled from the Olmsted County population were matched 1:1 to validated symptomatic stone formers by age ±1 year and sex. The index date for stone formers and their matched controls was the date of the first (incident) stone event in the stone former.
Validation of Hypertension
Hypertension was identified in the medical record by electronic searches for the term hypertension or the presence of ICD-9 codes for hypertension (401.0 [malignant essential hypertension], 401.1 [benign essential hypertension], and 401.9 [unspecified essential hypertension]). The diagnosis of hypertension and date of onset were validated by manual chart review of all patients who were coded for hypertension or had the term hypertension in their medical record. Criteria for hypertension included (1) a documented diagnosis of hypertension, (2) a BP>140/90 mmHg on at least two occasions, and (3) medical intervention for hypertension (lifestyle modification and/or BP-lowering agents) initiated.
Comorbidities and Clinical and Laboratory Characteristics
Prevalent comorbidities at the time of the index date were identified from ICD-9 codes (Supplemental Table 1), including diabetes mellitus, gout, dyslipidemia, coronary artery disease, CKD, tobacco use, and alcohol abuse. Height and weight (to calculate body mass index [BMI]) and serum creatinine were electronically extracted from medical records.
Kidney Stone Disease Characteristics
The kidney stone disease at the first stone event was characterized. Stone composition was defined by the mutually exclusive categories of unknown, mostly calcium oxalate, mostly hydroxyapatite, any uric acid, or any struvite as previously described (17). The radiographic stone burden of the incident stone event was classified by largest stone diameter (<4, 4–7, or ≥8 mm), number of stones (zero, one, two, or more than two), any upper tract dilation (hydronephrosis), any renal pelvic or lower pole stone, and the type of stone surgery (none, shockwave lithotripsy, stenting alone, ureteroscopy, or laparoscopic/open lithotomy). The dates of any subsequent stone event and stone surgery were also identified. Thiazide or thiazide–like diuretic use was the only stone prevention medication used frequently enough to meaningfully study.
Statistical Analyses
Prevalent hypertension was defined as clinical hypertension before the index date or within 90 days after the index date to account for the increased clinical care in stone formers around the time of the stone event. The incidence of hypertension was estimated using the Kaplan–Meier method after excluding patients with prevalent hypertension. Patients who did not develop hypertension were censored at their last clinic visit or on November 30, 2015, whichever came first. The risk of hypertension in stone formers compared with controls was assessed using Cox proportional hazards models both with and without adjustments for age, sex, BMI, baseline serum creatinine, and prevalent comorbidities (present before the index date +90 days). Clinical CKD (by ICD-9 code) and serum creatinine were analyzed as time-dependent covariates in additional models. The proportional hazards assumption was checked using scaled Schoenfeld residuals. Because thiazide diuretics are used to treat both kidney stones and hypertension, additional analyses were performed after excluding patients who were on thiazide diuretics either at baseline or during follow-up.
Separate Cox models among stone formers were fit to assess whether stone disease characteristics (number of stone events, radiographic stone burden, stone composition, and surgical intervention) were predictive of hypertension with adjustment for age, sex, and BMI. All tests were two sided, with α-level =0.05. All analyses used JMP v10, SAS v9.4 software (SAS Institute, Cary, NC), or R 3.3.1.
Results
Baseline Characteristics
Figure 1 diagrams the sampling framework. A total of 3023 newly coded stone formers in Olmsted County between 2000 and 2011 were identified. A total of 1515 were validated as having a first symptomatic kidney stone event and matched to 1515 controls on the basis of age and sex (index date was the date of the stone event for both stone formers and controls). The average number of clinic visits before the index date was similar between stone formers and controls (mean of 48 versus 45; P=0.20) overall, although higher in stone formers than controls during the 1 year before the index date (mean of eight versus six; P<0.001). As a consequence of the matching, stone formers and controls had the same mean age (45 years old) and sex distribution (56% men). There were 23 stone formers and 22 control patients whose date of onset of hypertension could not be determined and who were considered to have prevalent hypertension. The overall prevalence of hypertension between stone formers and controls was similar (20% versus 18%; P=0.46), and also, it did not differ after adjusting for age, sex, and BMI (odds ratio, 0.90; 95% confidence interval [95% CI], 0.72 to 1.12; P=0.34).
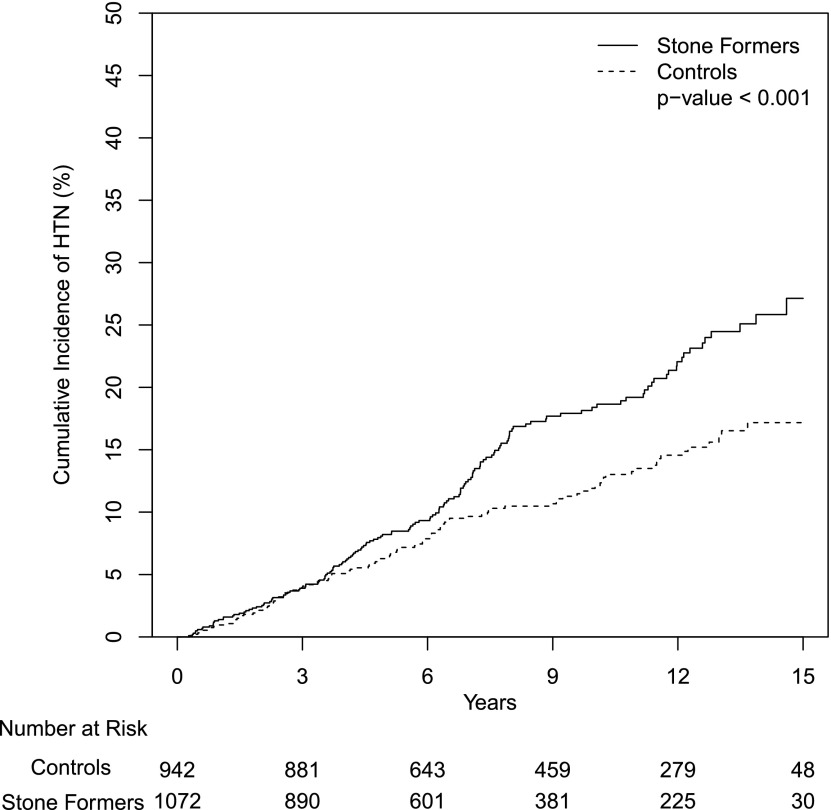
Figure 1.
Study design. HTN, hypertension.
After excluding patients with prevalent hypertension (n=531), patients lacking follow-up clinic visits (n=191), and patients lacking height or weight to calculate BMI (n=249), there were 1072 stone formers and 942 controls who were followed for incident hypertension. As shown in Table 1, stone formers had a higher BMI, serum creatinine level, and clinical CKD rate compared with controls at baseline.
Table 1.
Baseline characteristics of the stone former and control cohorts
| Baseline Characteristics | Stone Formers, n=1072 | Controls, n=942 | P Value |
|---|---|---|---|
| Age, yr | 41 (±14) | 42 (±14) | 0.08 |
| Men | 570 (53) | 496 (53) | 0.82 |
| Body mass index, kg/m2 | 29.2 (±6.8) | 28.1 (±7) | <0.001 |
| Thiazide use | 6 (0.6) | 3 (0.3) | 0.41 |
| Serum creatinine, mg/dl | 1.02 (±0.3) | 0.98 (±0.3) | <0.01 |
| CKD | 112 (10) | 12 (1) | <0.001 |
| Diabetes mellitus | 55 (5) | 40 (4) | 0.35 |
| Dyslipidemia | 96 (9) | 95 (10) | 0.39 |
| Gout | 1 (0.1) | 4 (0.4) | 0.13 |
| Coronary artery disease | 2 (0.2) | 4 (0.4) | 0.33 |
| Tobacco use | 46 (4) | 36 (4) | 0.59 |
| Alcohol abuse | 13 (1.2) | 15 (1.6) | 0.47 |
Values are presented as n (%) or mean (±SD).
Risk of Hypertension in Stone Formers Compared with Controls
The median follow-up time was 7.8 years for stone formers and 9.6 years for controls. The cumulative incidence of hypertension over a maximum follow-up of 15 years was 27% among stone formers and 17% among controls (hazard ratio [HR], 1.50; 95% CI, 1.22 to 1.97; P<0.001) (Figure 2, Table 2). The Cox proportional hazards assumption was found to be satisfied. Between 4 and 15 years of follow-up, when medical care was less likely to be related to the stone event, the number of clinic visits was similar between stone formers and controls (mean of 25 versus 24; P=0.62). The risk of hypertension among stone formers remained higher after adjusting for age, sex, BMI, serum creatinine (time dependent), clinically diagnosed CKD (time dependent), diabetes mellitus, gout, dyslipidemia, coronary artery disease, tobacco use, and alcohol abuse (Table 2). There were 125 (11%) stone formers and 75 (7%) control patients who were on thiazide diuretics either at baseline or during follow-up; the higher risk of hypertension in stone formers remained significant after excluding this subset of patients (HR, 1.65; 95% CI, 1.16 to 2.38; P<0.01). There were 188 stone formers who had symptomatic stone recurrence during follow-up (32 of whom subsequently developed hypertension). In analyses with additional censoring at stone recurrence, the risk of hypertension in stone formers remained significant (HR, 1.42; 95% CI, 1.10 to 1.84; P<0.01).
Figure 2.
Higher risk of hypertension in stone formers. The unadjusted cumulative incidence of hypertension (HTN) among incident symptomatic stone formers compared with age- and sex-matched controls.
Table 2.
Risk of hypertension among stone formers compared with controls
| Adjusting Factors | Hazard Ratio (95% Confidence Interval) | P Value |
|---|---|---|
| None | 1.50 (1.18 to 1.92) | 0.001 |
| Age and sex | 1.53 (1.20 to 1.96) | <0.001 |
| Age, sex, and BMI | 1.49 (1.17 to 1.90) | 0.001 |
| Age, sex, BMI, baseline serum creatinine, diabetes mellitus, gout, coronary artery disease, dyslipidemia, tobacco use, and alcohol abuse | 1.44 (1.13 to 1.84) | 0.004 |
| Age, sex BMI, CKD,a and serum creatininea | 1.56 (1.12 to 2.18) | <0.01 |
| Age, sex, BMI, diabetes mellitus, gout, coronary artery disease, dyslipidemia, tobacco use, alcohol abuse, CKD,a and serum creatininea | 1.58 (1.12 to 2.21) | <0.01 |
| Age, sex, and BMI (excluding those who ever used thiazide diuretics) | 1.65 (1.16 to 2.38) | <0.01 |
| Age, sex, BMI, diabetes mellitus, gout, coronary artery disease, dyslipidemia, tobacco use, alcohol abuse, CKD,a and serum creatininea (excluding those who ever used thiazide diuretics) | 1.71 (1.07 to 2.74) | 0.03 |
BMI, body mass index.
Serum creatinine and clinically diagnosed CKD were modeled as a time-dependent covariates; 95% of stone formers and 91% of controls had at least one serum creatinine level.
Stone-Specific Risk Factors for Hypertension
Subgroup analysis among the 1072 stone formers was performed to identify any stone disease–specific risk factors for hypertension. During follow-up, 83% had no recurrent stone events, 11% had one recurrent event, 6% had two or more recurrent events during follow-up, and 38% had one or more stone surgeries (Supplemental Table 2). A subset of 518 (48%) had a stone composition analysis of their first stone. Overall, the number of stone events, number of stone surgeries, type of stone surgery, stone composition, and radiographic stone burden were not predictive of hypertension (Table 3).
Table 3.
Risk of hypertension with specific stone disease characteristics
| Variables | No. of Stone Formers Who Developed Hypertension (%) | No. of Stone Formers Who Did Not Develop Hypertension (%) | HR (95% CI)a |
|---|---|---|---|
| Time-dependent variables | |||
| No. of kidney stone events | 1.18 (0.96 to 1.44) | ||
| No. of urologic interventions | 1.10 (0.88 to 1.39) | ||
| Urologic intervention | |||
| Total | 154 | 918 | |
| No urologic intervention | 95 (62) | 652 (66) | 1.00 (Reference) |
| Any urologic interventions | 59 (38) | 318 (35) | 1.09 (0.78 to 1.52) |
| Extracorporeal shockwave lithotripsy | 11 (7) | 40 (4) | 1.58 (0.81 to 2.79) |
| Ureteroscopy | 37 (24) | 218 (24) | 1.06 (0.71 to 1.52) |
| Open or laparoscopic lithotomy | 3 (2) | 23 (3) | 0.78 (0.19 to 2.06) |
| Stenting alone (cystoscopy) | 8 (5) | 39 (4) | 0.84 (0.37 to 1.61) |
| Stone composition | |||
| Total | 154 | 918 | |
| Known stone composition | 67 (44) | 451 (46) | 1.00 (Reference) |
| Unknown stone composition | 87 (56) | 531 (54) | 1.10 (0.81 to 1.53) |
| Calcium oxalate stone | 55 (82) | 309 (69) | 1.00 (Reference) |
| Calcium phosphate stone | 10 (15) | 128 (28) | 0.95 (0.43 to 1.88) |
| Uric acid stone | 2 (3) | 10 (2) | 0.83 (0.13 to 2.73) |
| Struvite stone | 0 (0) | 3 (0.7) | NAb |
| Others | 0 (0) | 1 (0.3) | NAb |
| Radiographic stone burden | |||
| Total | 144 | 844 | |
| Upper urinary tract dilation | 96 (67) | 600 (71) | 0.85 (0.60 to 1.22) |
| Pelvic or lower pole stone | 28 (19) | 145 (17) | 1.21 (0.78 to 1.80) |
| Focal renal scarring or cortical thinning | 1 (1) | 14 (2) | 0.38 (0.02 to 1.72) |
| Stone size, mm | 0.97 (0.75 to 1.23) | ||
| <4 | 78 (54) | 433 (51) | |
| 4 to <8 | 51 (35) | 319 (38) | |
| ≥8 | 15 (10) | 92 (11) | |
| No. of stones | 1.10 (0.89 to 1.36) | ||
| 0 | 7 (5) | 56 (7) | |
| 1 | 20 (14) | 112 (13) | |
| 2 | 69 (48) | 402 (48) | |
| >2 | 48 (33) | 274 (32) |
HR, hazard ratio; 95% CI, 95% confidence interval; NA, not applicable.
Adjusted for age, sex, and body mass index.
Too few events in the model.
Discussion
In this population–based cohort study, the incidence of hypertension was significantly higher after a first kidney stone event, whereas preexisting hypertension did not differ between stone formers and controls. The higher risk was independent of factors known to have an effect on the occurrence of hypertension, including age, sex, BMI, coronary artery disease, dyslipidemia, diagnosis of CKD, serum creatinine, alcohol abuse, and tobacco use. Stone-specific characteristics were not associated with a higher incidence of hypertension, including severity of stone disease, stone composition, and surgical treatments.
These findings are consistent with prior studies that identified a higher risk of hypertension among stone formers (12–14). We did not find that a prior history of hypertension increased the risk of kidney stone disease, whereas prior studies are conflicted on this point. A prospective study among Italian factory workers found a higher risk of kidney stones in hypertensive patients compared with controls (8). Another study found a higher risk of kidney stones in hypertensive patients seen in hypertension clinic compared with healthy volunteers (7). Inadequate validation of kidney stones and hypertension, small sample size, or selection bias among the studied participants may explain the associations reported in these prior studies. Consistent with our findings, a large prospective population–based cohort study found a higher risk of developing hypertension in stone formers compared with controls but did not find an increased risk of developing kidney stones in persons with hypertension compared with those without (12,13).
The mechanism by which kidney stones lead to hypertension is not clear. Interestingly, we found that stone composition and severity of stone disease were not predictive of the risk of hypertension. This suggests that these factors are not causal for hypertension. Urine chemistries could potentially mediate the risk of hypertension, particularly urine sodium or calcium, but were not available for analysis in this study. We did not find surgical management of kidney stones to be predictive of the risk of hypertension. Some but not all studies have linked shock wave lithotripsy to a higher risk of hypertension (18–22). In particular, a recent population–based study found a 40% higher risk of hypertension with shock wave lithotripsy (HR, 1.40; 95% CI, 1.19 to 1.66) (22). Similarly, we found a 58% higher risk of hypertension with shock wave lithotripsy that was not statistically significant, possibly because of our smaller sample size. However, the risk of hypertension among stone formers in our cohort remained statistically significant, even after excluding those who underwent shock wave lithotripsy (HR, 1.44; 95% CI, 1.13 to 1.85 adjusted for age, sex, and BMI).
Subclinical renal insults from kidney stone disease may also contribute to the risk of hypertension. Our previous studies have shown that kidney stone disease is associated with an increased risk of CKD and ESRD (3,23). However, kidney stones remained a significant risk factor for hypertension, even after adjustment for serum creatinine and clinically diagnosed CKD. Calcium oxalate crystals are toxic to the tubular epithelial cells and lead to the production of reactive oxygen species as well as potentially, development of oxidative stress and inflammation (24,25). Major chronic inflammation and renal injury markers are detectable in stone formers’ urine (26,27), and more renal parenchymal thinning was observed in stone formers (28). Hypertension also correlates with papillary calcifications (29). In theory, the end result of this subtle tubulointerstitial injury may be the development of hypertension without manifestations of overt CKD.
Because 97% of stone compositions were common calcium (calcium oxalate or calcium phosphate), the higher risk of hypertension detected is most relevant to calcium stone formers. Alterations in calcium handling may also have an important role in the pathogenesis of both nephrolithiasis and hypertension. Hypercalciuria is a significant risk factor for kidney stones (30), and increased urinary calcium excretion has been observed in hypertensive patients in some (7,31,32) but not all (33) studies. Aggregation of hypertension in families of patients with hypercalciuria has been described (34) as well as increased urinary calcium among offspring of hypertensive parents (35).
Strengths of this study include the population-based design and careful validation of symptomatic kidney stones and hypertension. There were also potential limitations in this study. First, the study population was predominately white, which may limit generalizability to other race groups. Second, first–time stone formers in the community have limited clinical evaluations, and thus, we lacked sufficient urine chemistry and dietary data for meaningful analysis. Third, stone formers may have been followed more closely for hypertension compared with controls. However, there were not more clinic visits in stone formers compared with controls beyond 4 years of follow-up.
Our findings support the notion that kidney stones are a risk factor for hypertension but not vice versa. Factors beyond the stones themselves may play a role in the mechanism underlying this higher risk. There may be some shared genetic or environmental factors that manifest earlier as kidney stone disease and later as hypertension. Additional studies are needed to investigate the role of specific metabolic or genetic abnormalities in explaining a higher incidence of hypertension in stone formers.
Disclosures
None.
Supplementary Material
Acknowledgments
This project was supported by National Institute of Diabetes and Digestive and Kidney Diseases Mayo Clinic O’Brien Urology Research Center grants DK100227 and DK83007 and Center for Translational Science Activities grant UL1 TR000135 from the National Center for Advancing Translational Sciences, a component of the National Institutes of Health (NIH), and made possible by Rochester Epidemiology Project AG034676 from the NIH, US Public Health Service.
Preliminary results of this work were presented at the Scientific Meeting of the American Society of Hypertension held May 13–17, 2016 in New York, New York.
The funding sources had no role in the study design, conduct, or reporting. Its contents are solely the responsibility of the authors and do not necessarily represent the official view of NIH.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
This article contains supplemental material online at http://cjasn.asnjournals.org/lookup/suppl/doi:10.2215/CJN.06600616/-/DCSupplemental.
References
- 1.Sakhaee K, Maalouf NM, Sinnott B: Clinical review. Kidney stones 2012: Pathogenesis, diagnosis, and management. J Clin Endocrinol Metab 97: 1847–1860, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Scales CD Jr., Smith AC, Hanley JM, Saigal CS; Urologic Diseases in America Project : Prevalence of kidney stones in the United States. Eur Urol 62: 160–165, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rule AD, Bergstralh EJ, Melton LJ 3rd , Li X, Weaver AL, Lieske JC: Kidney stones and the risk for chronic kidney disease. Clin J Am Soc Nephrol 4: 804–811, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cheungpasitporn W, Thongprayoon C, Mao MA, O’Corragain OA, Edmonds PJ, Erickson SB: The risk of coronary heart disease in patients with kidney stones: A systematic review and meta-analysis. N Am J Med Sci 6: 580–585, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rule AD, Roger VL, Melton LJ 3rd , Bergstralh EJ, Li X, Peyser PA, Krambeck AE, Lieske JC: Kidney stones associate with increased risk for myocardial infarction. J Am Soc Nephrol 21: 1641–1644, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Taylor EN, Feskanich D, Paik JM, Curhan GC: Nephrolithiasis and risk of incident bone fracture. J Urol 195: 1482–1486, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Borghi L, Meschi T, Guerra A, Briganti A, Schianchi T, Allegri F, Novarini A: Essential arterial hypertension and stone disease. Kidney Int 55: 2397–2406, 1999 [DOI] [PubMed] [Google Scholar]
- 8.Cappuccio FP, Siani A, Barba G, Mellone MC, Russo L, Farinaro E, Trevisan M, Mancini M, Strazzullo P: A prospective study of hypertension and the incidence of kidney stones in men. J Hypertens 17: 1017–1022, 1999 [DOI] [PubMed] [Google Scholar]
- 9.Cappuccio FP, Strazzullo P, Mancini M: Kidney stones and hypertension: Population based study of an independent clinical association. BMJ 300: 1234–1236, 1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cirillo M, Laurenzi M: Elevated blood pressure and positive history of kidney stones: Results from a population-based study. J Hypertens Suppl 6: S485–S486, 1988 [DOI] [PubMed] [Google Scholar]
- 11.Gillen DL, Coe FL, Worcester EM: Nephrolithiasis and increased blood pressure among females with high body mass index. Am J Kidney Dis 46: 263–269, 2005 [DOI] [PubMed] [Google Scholar]
- 12.Madore F, Stampfer MJ, Rimm EB, Curhan GC: Nephrolithiasis and risk of hypertension. Am J Hypertens 11: 46–53, 1998 [DOI] [PubMed] [Google Scholar]
- 13.Madore F, Stampfer MJ, Willett WC, Speizer FE, Curhan GC: Nephrolithiasis and risk of hypertension in women. Am J Kidney Dis 32: 802–807, 1998 [DOI] [PubMed] [Google Scholar]
- 14.Strazzullo P, Barba G, Vuotto P, Farinaro E, Siani A, Nunziata V, Galletti F, Mancini M, Cappuccio FP: Past history of nephrolithiasis and incidence of hypertension in men: A reappraisal based on the results of the Olivetti Prospective Heart Study. Nephrol Dial Transplant 16: 2232–2235, 2001 [DOI] [PubMed] [Google Scholar]
- 15.Cho ST, Jung SI, Myung SC, Kim TH: Correlation of metabolic syndrome with urinary stone composition. Int J Urol 20: 208–213, 2013 [DOI] [PubMed] [Google Scholar]
- 16.Daudon M, Traxer O, Conort P, Lacour B, Jungers P: Type 2 diabetes increases the risk for uric acid stones. J Am Soc Nephrol 17: 2026–2033, 2006 [DOI] [PubMed] [Google Scholar]
- 17.Singh P, Enders FT, Vaughan LE, Bergstralh EJ, Knoedler JJ, Krambeck AE, Lieske JC, Rule AD: Stone composition among first-time symptomatic kidney stone formers in the community. Mayo Clin Proc 90: 1356–1365, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Barbosa PV, Makhlouf AA, Thorner D, Ugarte R, Monga M: Shock wave lithotripsy associated with greater prevalence of hypertension. Urology 78: 22–25, 2011 [DOI] [PubMed] [Google Scholar]
- 19.Elves AW, Tilling K, Menezes P, Wills M, Rao PN, Feneley RC: Early observations of the effect of extracorporeal shockwave lithotripsy on blood pressure: A prospective randomized control clinical trial. BJU Int 85: 611–615, 2000 [DOI] [PubMed] [Google Scholar]
- 20.Krambeck AE, Gettman MT, Rohlinger AL, Lohse CM, Patterson DE, Segura JW: Diabetes mellitus and hypertension associated with shock wave lithotripsy of renal and proximal ureteral stones at 19 years of followup. J Urol 175: 1742–1747, 2006 [DOI] [PubMed] [Google Scholar]
- 21.Sato Y, Tanda H, Kato S, Ohnishi S, Nakajima H, Nanbu A, Nitta T, Koroku M, Akagashi K, Hanzawa T: Shock wave lithotripsy for renal stones is not associated with hypertension and diabetes mellitus. Urology 71: 586–591, 2008 [DOI] [PubMed] [Google Scholar]
- 22.Denburg MR, Jemielita TO, Tasian GE, Haynes K, Mucksavage P, Shults J, Copelovitch L: Assessing the risk of incident hypertension and chronic kidney disease after exposure to shock wave lithotripsy and ureteroscopy. Kidney Int 89: 185–192, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.El-Zoghby ZM, Lieske JC, Foley RN, Bergstralh EJ, Li X, Melton LJ 3rd , Krambeck AE, Rule AD: Urolithiasis and the risk of ESRD. Clin J Am Soc Nephrol 7: 1409–1415, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Khan SR: Hyperoxaluria-induced oxidative stress and antioxidants for renal protection. Urol Res 33: 349–357, 2005 [DOI] [PubMed] [Google Scholar]
- 25.Knoll T, Steidler A, Trojan L, Sagi S, Schaaf A, Yard B, Michel MS, Alken P: The influence of oxalate on renal epithelial and interstitial cells. Urol Res 32: 304–309, 2004 [DOI] [PubMed] [Google Scholar]
- 26.Baggio B, Gambaro G, Ossi E, Favaro S, Borsatti A: Increased urinary excretion of renal enzymes in idiopathic calcium oxalate nephrolithiasis. J Urol 129: 1161–1162, 1983 [DOI] [PubMed] [Google Scholar]
- 27.Boonla C, Wunsuwan R, Tungsanga K, Tosukhowong P: Urinary 8-hydroxydeoxyguanosine is elevated in patients with nephrolithiasis. Urol Res 35: 185–191, 2007 [DOI] [PubMed] [Google Scholar]
- 28.Lorenz EC, Lieske JC, Vrtiska TJ, Krambeck AE, Li X, Bergstralh EJ, Melton LJ 3rd , Rule AD: Clinical characteristics of potential kidney donors with asymptomatic kidney stones. Nephrol Dial Transplant 26: 2695–2700, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stoller ML, Low RK, Shami GS, McCormick VD, Kerschmann RL: High resolution radiography of cadaveric kidneys: Unraveling the mystery of Randall’s plaque formation. J Urol 156: 1263–1266, 1996 [DOI] [PubMed] [Google Scholar]
- 30.Pak CY: Medical management of nephrolithiasis in Dallas: Update 1987. J Urol 140: 461–467, 1988 [DOI] [PubMed] [Google Scholar]
- 31.Young EW, Morris CD, McCarron DA: Urinary calcium excretion in essential hypertension. J Lab Clin Med 120: 624–632, 1992 [PubMed] [Google Scholar]
- 32.Eisner BH, Porten SP, Bechis SK, Stoller ML: Hypertension is associated with increased urinary calcium excretion in patients with nephrolithiasis. J Urol 183: 576–579, 2010 [DOI] [PubMed] [Google Scholar]
- 33.Taylor EN, Mount DB, Forman JP, Curhan GC: Association of prevalent hypertension with 24-hour urinary excretion of calcium, citrate, and other factors. Am J Kidney Dis 47: 780–789, 2006 [DOI] [PubMed] [Google Scholar]
- 34.Mente A, Honey RJ, McLaughlin JM, Bull SB, Logan AG: High urinary calcium excretion and genetic susceptibility to hypertension and kidney stone disease. J Am Soc Nephrol 17: 2567–2575, 2006 [DOI] [PubMed] [Google Scholar]
- 35.van Hooft IM, Grobbee, Frölich M, Pols HA, Hofman A: Alterations in calcium metabolism in young people at risk for primary hypertension. The Dutch Hypertension and Offspring Study. Hypertension 21: 267–272, 1993 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.