Abstract
Background and objectives
Donor dopamine improves initial graft function after kidney transplantation due to antioxidant properties. We investigated if a 4 µg/kg per minute continuous dopamine infusion administered after brain-death confirmation affects long-term graft survival and examined the exposure-response relationship with treatment duration.
Design, setting, participants, & measurements
Five-year follow-up of 487 renal transplant patients from 60 European centers who had participated in the randomized, multicenter trial of dopamine donor pretreatment between 2004 and 2007 (ClinicalTrials.gov identifier: NCT00115115).
Results
Follow-up was complete in 99.2%. Graft survival was 72.6% versus 68.7% (P=0.34), and 83.3% versus 80.4% (P=0.42) after death-censoring in treatment and control arms according to trial assignment. Although infusion times varied substantially in the treatment arm (range 0–32.2 hours), duration of the dopamine infusion and all-cause graft failure exhibited an exposure-response relationship (hazard ratio, 0.96; 95% confidence interval [95% CI], 0.92 to 1.00, per hour). Cumulative frequency curves of graft survival and exposure time of the dopamine infusion indicated a maximum response rate at 7.10 hours (95% CI, 6.99 to 7.21), which almost coincided with the optimum infusion time for improvement of early graft function (7.05 hours; 95% CI, 6.92 to 7.18). Taking infusion time of 7.1 hours as threshold in subsequent graft survival analyses indicated a relevant benefit: Overall, 81.5% versus 68.5%; P=0.03; and 90.3% versus 80.2%; P=0.04 after death-censoring.
Conclusions
We failed to show a significant graft survival advantage on intention-to-treat. Dopamine infusion time was very short in a considerable number of donors assigned to treatment. Our finding of a significant, nonlinear exposure-response relationship disclosed a threshold value of the dopamine infusion time that may improve long-term kidney graft survival.
Keywords: cadaver organ transplantation, ischemia-reperfusion, renal protection, renal transplantation, outcomes, dopamine, brain-dead donor, donor pretreatment, arm, brain, brain death, confidence intervals, death, dopamine, follow-up studies, graft survival, humans, intention to treat analysis, kidney transplantation, survival analysis, tissue donors, transplants
Introduction
Optimized care of the deceased brain-dead donor (DBD) in the intensive care unit (ICU) increases organ yield and improves transplantation success (1–3). Current recommendations refer to the judicious use of inotropic agents and vasopressors to maintain donor hemodynamic stability, and medications such as levothyroxine and steroids (3,4). However, these recommendations are not scientifically determined due to sparse clinical data from controlled trials on graft outcome. Very few studies have prospectively investigated donor interventions that aim to improve graft function among recipients (5,6). In 2009, we presented the results of a randomized controlled trial, which confirmed that dopamine donor pretreatment improved graft function immediately after kidney transplantation (7). Rather than stabilizing donor hemodynamics, protection depends on dopamine’s reducing activity and diffusion into cells (8,9). Post hoc analysis of cardiac allografts from multiorgan donors enrolled in the trial also indicated a survival benefit for recipients of dopamine-treated hearts after 1 and 3 years. The survival advantage appeared early after transplantation as improvements in initial graft function (10). The mechanism of action may be different in renal transplantation because, unlike heart transplantation, dialysis can routinely bridge the renal allograft with early dysfunction. Here, we report the 5-year follow-up of the renal trial. We also sought to examine if an exposure-response relationship exists between infusion time and long-term graft survival, because primary data analysis of the randomized dopamine trial had suggested positive correlations between treatment duration, dialysis independency, and recovery of kidney function (7).
Materials and Methods
Study Design, Patients, and Measurements
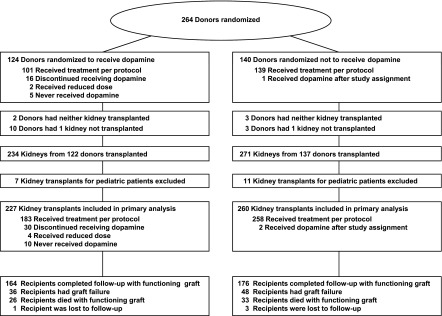
This study was embedded in the database of the randomized dopamine trial designed as a controlled donor intervention trial in adult kidney transplantation (ClinicalTrials.gov identifier: NCT00115115). Rationale, design, and execution of the dopamine trial have been described elsewhere (7). Briefly, between 2004 and 2007, 264 DBDs were randomized to receive a standardized 4 µg/kg per minute dopamine infusion after ascertainment of brain-death until crossclamping before the start of cold perfusion (Figure 1). The window of possible donor intervention, from brain-death confirmation to surgical removal of the kidneys, depended on organizational issues at the various procurement sites, but was very similar in both trial arms due to randomization. Eligible DBDs had serum-creatinine levels <1.3 mg/dl on admission and were stable without vasopressor support, except norepinephrine administered at <0.4 µg/kg per minute. Kidney allocation to recipients was centrally directed by Eurotransplant according to waiting time and prospective HLA-antigen match. The primary trial endpoint was requirement of more than a single post-transplant dialysis session. The reasoning for selecting requirement more than one dialysis session as primary endpoint rather than the standard definition of delayed graft function (DGF)—any dialysis post-transplant—is provided in the Supplemental Material (Supplemental Figure 1 and Supplemental Table 1). The trial was powered to detect a 12% difference in the primary endpoint at a significance level <0.05. The trial protocol was approved by the Independent Ethics Committee of the Medical Faculty of Mannheim, Germany and by the Kidney Advisory Committee of the Eurotransplant International Foundation, Leiden, The Netherlands.
Figure 1.
Study flow diagram.
To assess long-term graft survival, we sent a standard questionnaire to the 60 transplantation centers that had collaborated in the dopamine trial. We used Eurotransplant code numbers to ensure participant anonymity and requested information on graft function/failure as well as reports on recipient deaths until 5 years after transplantation. In six individual cases, when a recipient was no longer under surveillance of the transplantation center, the family physician was contacted.
At first we evaluated the outcomes according to trial group assignment. Separate survival analyses were performed with and without death-censoring. Hereafter, we investigated in-depth the dose-response relationship of the primary and secondary trial endpoints—requirement of more than one post-transplant dialysis session and all-cause graft failure 5 years after transplantation—with infusion time, which varied substantially in the treatment arm for the following reasons. First, the dopamine infusion was administered in the window after confirming brain-death and before crossclamping; there was no predefined infusion duration, because the trial protocol precluded any study-driven delay of the donation procedure. Second, the infusion was administered within predefined safety thresholds and was stopped if heart rate was >120 beats/min or BP was >160/90 mmHg to avoid possible donor destabilization. Therefore, dopamine infusion times ranged between 0 and 32.2 hours in the treatment arm (Figure 1).
To illustrate the influence of exposure, we created cumulative percentage frequency curves of the primary and secondary efficacy endpoints—freedom from multiple dialyses requirement and 5-year graft survival—and dopamine infusion time. We then used the infusion time with the maximum response rate as threshold in separate graft survival analyses on study medication.
Statistical Analyses
Statistical analyses were performed using the trial database after incorporating the 5-year data. We generated Kaplan–Meier survival curves and primarily assessed graft survival according to trial group assignment. Log-rank tests were applied to compare survival between groups.
For evaluation of the dose-effect with infusion time we used logistic regression to analyze the primary trial endpoint (more than one post-transplant dialysis session), and applied Cox regression to assess graft survival until 5 years. We utilized duration of the dopamine infusion as continuous explanatory variable with full adjustment for the time interval between brain-death confirmation and organ procurement. The latter was the defining factor of treatment duration. To control for other possible confounding influences, we included age and sex of donors and recipients, donor BP, urine production, multiorgan versus kidney-only donor, cause of brain-death, time interval from admission on ICU to brain-death confirmation, cold ischemic time (CIT), recipient weight, recipient diabetes, time spent on waiting list, HLA-antigen match, panel reactivity, history of a previous transplant, and immunosuppressive treatment at transplantation (induction therapy, tacrolimus versus cyclosporine) as covariates in the respective statistical models. Maximum likelihood estimates are displayed by odds ratios or hazard ratios (HR) with 95% confidence intervals (95% CI). Regression analyses are on the basis of the entire trial cohort including the controls.
We generated cumulative percentage frequency curves of freedom from multiple dialyses requirement and of 5-year graft survival by infusion time. Infusion times at the inflection point were estimated separately for either of the efficacy endpoints. Besides a graphical derivation, we modeled the cumulative frequency functions according to the Hill equation (11). The Hill equation is widely applied for curve-fitting purposes and enables accurate determination of the slopes of the tangents at the inflection point (maximum rate of response) in sigmoid-shaped time course relationships by means of a noniterative algebraic method (12,13). Numeric values of the infusion time at the respective inflection points of the cumulative frequency curves are presented with a 95% CI. In a secondary analysis, we used the infusion time with the maximum increase in 5-year graft survival to define a binary factor for multiple regression analysis, besides donor and recipient characteristics as specified above. In addition, we computed adjusted HRs according to rising thresholds of exposure to empirically derive the effective infusion time from the full survival model. To quantitate organ quality, we calculated the kidney donor profile index (KDPI) (14).
We evaluated quantitative data by using ANOVA for means of values, and the Kruskal–Wallis test for medians, as appropriate for data distribution. Qualitative data were compared with Fisher exact tests. A P value <0.05 was considered significant. Statistical analyses were performed with SAS release 9.3 (SAS Institute Inc., Cary, NC).
Results
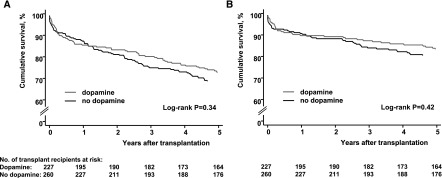
Characteristics of donors and recipients at baseline are presented in Table 1. Five-year survival data were complete in 483 of 487 patients (99.2%). Four patients were lost to follow-up, after 5 months, 28 months, and two patients after 31 months. These were censored on their last-seen date. During the 5-year follow-up, 143 graft failure events were reported (Figure 1). Fifty-nine failures were caused by death with functioning graft (41.3%). According to trial assignment, overall graft survival was 72.6% and 68.7% in the treatment and control groups, respectively (log-rank P=0.34). After death-censoring, the survival estimates were 83.3% and 80.4%, log-rank P=0.42 (Figure 2).
Table 1.
Baseline characteristics according to trial group assignment
| Variable | Donors Assigned to Treatment | Donors Assigned to Controls | P Value | |
|---|---|---|---|---|
| Dopamine Not Given Or Prematurely Terminated | Dopamine Administered per Protocol | |||
| N | n=21 | n=103 | n=140 | |
| Mean age (SD), yr | 51.2 (11.3) | 50.6 (15.2) | 50.8 (14.8) | 0.99 |
| Women, n (%) | 11 (52.4) | 50 (48.5) | 76 (54.3) | 0.70 |
| Mean serum-creatinine (SD), mg/dla | 0.72 (0.19) | 0.86 (0.31) | 0.84 (0.26) | 0.12 |
| Mean systolic BP (SD), mmHga | 131 (19) | 133 (19) | 128 (17) | 0.13 |
| Mean diastolic BP (SD), mmHga | 75 (13) | 71 (11) | 71 (11) | 0.26 |
| Mean urine production last 24 h (SD), ml | 4880 (2880) | 4900 (2410) | 4760 (2460) | 0.90 |
| Mean urine production last h (SD), ml | 205 (122) | 204 (141) | 179 (107) | 0.26 |
| Mean Kidney Donor Profile Index (SD), % | 51 (23) | 54 (28) | 53 (26) | 0.91 |
| Median time from admission on ICU to brain death confirmation (IQR), d | 3 (1–4) | 3 (1–5) | 3 (2–8) | 0.18 |
| Median time from brain death confirmation to start of cold perfusion (IQR), h | 9.3 (7.9–10.5) | 9.8 (7.8–11.9) | 9.0 (6.8–11.9) | 0.32 |
| Median time from brain death confirmation to initiation of the dopamine infusion (IQR), h | 2.6 (1.7–4.2)b | 3.0 (0.8–5.6) | n.e. | 0.53 |
| Median duration of the dopamine infusion (IQR), h | 0.8 (0.7–2.0) | 6.2 (4.9–8.3) | 0 | — |
| Median time from cessation of dopamine to initiation of perfusion (IQR), h | 3.8 (2.1–6.2)b | 0 | n.e. | — |
| Recipients and transplant characteristics | ||||
| N | n=40 | n=187 | n=260 | |
| Mean age (SD), yr | 49.8 (11.9) | 53.4 (12.8) | 52.0 (12.4) | 0.18 |
| Women, n (%) | 15 (37.5) | 79 (42.3) | 96 (36.9) | 0.54 |
| Mean weight (SD), kg | 74.8 (14.4) | 75.0 (15.3) | 73.0 (12.9) | 0.30 |
| Diabetes, n (%) | 6 (15.0) | 30 (16.0) | 36 (13.9) | 0.81 |
| Previous transplant, n (%) | 3 (7.5) | 24 (12.8) | 47 (18.1) | 0.12 |
| Mean cold ischemic time (SD), h | 14.7 (5.3) | 13.4 (5.5) | 14.2 (5.2) | 0.19 |
| Mean antigen mismatches A, B, and DR (SD), n | 2.8 (1.7) | 2.8 (1.6) | 2.8 (1.6) | 0.95 |
| Panel reactive antibody >5%, n (%) | 3 (7.5) | 21 (11.2) | 34 (13.1) | 0.64 |
The intervention group is subdivided in donors and transplants treated per protocol and those prematurely withdrawn from the infusion for side effects or not given dopamine despite randomization to treatment. ICU, intensive care unit; IQR, interquartile range; n.e., not estimable; —, not applicable.
Last assessment before organ procurement.
Not estimable in five donors not given dopamine.
Figure 2.
Kaplan–Meier estimates of kidney graft survival until 5 years after transplantation according to trial group assignment failed to show a significant benefit of dopamine. (A) Overall graft survival. (B) Death-censored graft survival.
Regression analysis of the primary trial endpoint (more than one post-transplant dialysis session) resulted in a significant odds ratio of 0.92 (95% CI, 0.87 to 0.98) when infusion time measured in hours was used as continuous explanatory variable. By comparison, the statistical association of graft failure and dopamine infusion time was weaker but approached borderline significance (HR, 0.96; 95% CI, 0.92 to 1.00, per hour).
The association of treatment duration with the trial outcomes remained similar in the multivariable analysis after addition of various potentially confounding variables to the statistical regression models (Tables 2 and 3). Multiple regression disclosed in part different influence factors that were associated with the primary and secondary trial endpoint, respectively. Although donor age negatively affected initial graft function and 5-year graft survival, the discriminating powers of CIT and recipient weight were lost in the long-term. BP and urine production, which were marginally enhanced in the treatment arm presumably due to dopaminergic receptor stimulation (7), had no measurable effect on the outcomes (Tables 2–4). Although the period from brain-death diagnosis to organ procurement limited the window of possible donor intervention, this was not a significant variable of graft survival (Table 3). Notably, it was very similar in the control group (Table 1). Repeating the analyses per protocol confirmed these principal findings (Supplemental Tables 2 and 3).
Table 2.
Logistic regression of multiple dialyses requirement (>1 dialysis session) during first week after transplantation
| Variable | Single-Variable Analysis | Multivariable Analysis | ||||
|---|---|---|---|---|---|---|
| OR | 95% CI | P Value | OR | 95% CI | P Value | |
| Dopamine infusion time, h | 0.92 | 0.87 to 0.98 | 0.004 | 0.91 | 0.86 to 0.97 | 0.003 |
| Donor characteristics | ||||||
| Age, yr | 1.02 | 1.01 to 1.04 | 0.001 | 1.04 | 1.02 to 1.06 | <0.001 |
| Women | 1.20 | 0.81 to 1.76 | 0.37 | 1.24 | 0.77 to 1.97 | 0.37 |
| Systolic BP, mmHg | 1.00 | 0.99 to 1.01 | 0.94 | 1.00 | 0.98 to 1.01 | 0.54 |
| Diastolic BP, mmHg | 1.01 | 0.99 to 1.02 | 0.43 | 1.00 | 0.98 to 1.03 | 0.70 |
| Urine production last h, ml | 1.00 | 0.999 to 1.002 | 0.22 | 1.00 | 0.999 to 1.003 | 0.29 |
| Cause of brain death | ||||||
| Head trauma, yes | 0.85 | 0.53 to 1.36 | 0.50 | 1.59 | 0.87 to 2.90 | 0.14 |
| Cerebral stroke, yes | 1.24 | 0.65 to 2.35 | 0.51 | 1.32 | 0.63 to 2.80 | 0.46 |
| Cerebral ischemia/cerebral edema, yes | 1.55 | 0.81 to 2.96 | 0.18 | 2.23 | 1.05 to 4.76 | 0.04 |
| Time from admission on ICU to brain-death confirmation, d | 1.00 | 0.96 to 1.04 | 0.95 | 1.01 | 0.96 to 1.06 | 0.74 |
| Time from brain-death confirmation to organ procurement, h | 0.99 | 0.95 to 1.03 | 0.61 | 1.05 | 1.00 to 1.11 | 0.04 |
| Multiorgan versus kidney-only donor | 0.63 | 0.41 to 0.99 | 0.04 | 0.73 | 0.43 to 1.23 | 0.23 |
| Cold ischemic time, h | 1.07 | 1.03 to 1.11 | 0.001 | 1.07 | 1.02 to 1.12 | 0.002 |
| Recipient characteristics | ||||||
| Age, yr | 1.01 | 0.99 to 1.02 | 0.51 | 0.99 | 0.97 to 1.01 | 0.34 |
| Women | 0.73 | 0.48 to 1.09 | 0.12 | 1.03 | 0.63 to 1.67 | 0.92 |
| Weight, kg | 1.02 | 1.01 to 1.04 | 0.001 | 1.02 | 1.01 to 1.04 | 0.01 |
| Diabetes, yes | 0.73 | 0.41 to 1.30 | 0.28 | 1.13 | 0.56 to 2.29 | 0.73 |
| Time spent on waiting list, yr | 1.02 | 0.95 to 1.10 | 0.58 | 1.02 | 0.94 to 1.11 | 0.64 |
| Antigen mismatches A, B, and DR, n | 0.99 | 0.88 to 1.11 | 0.83 | 0.94 | 0.81 to 1.09 | 0.39 |
| Panel reactive antibody >5%, yes | 1.60 | 0.91 to 2.81 | 0.10 | 2.03 | 0.99 to 4.15 | 0.06 |
| Previous transplant, yes | 1.29 | 0.77 to 2.18 | 0.34 | 1.23 | 0.64 to 2.36 | 0.54 |
| Immunosuppressive treatmenta | ||||||
| Induction therapy, yes | 1.31 | 0.89 to 1.93 | 0.17 | 1.22 | 0.79 to 1.90 | 0.37 |
| Tacrolimus versus cyclosporine | 0.88 | 0.60 to 1.31 | 0.54 | 0.96 | 0.60 to 1.55 | 0.88 |
Analyses cover the entire trial cohort including control patients. OR, odds ratio; 95% CI, 95% confidence interval; ICU, intensive care unit.
At time of transplantation.
Table 3.
Cox regression of all-cause graft failure until 5 years after transplantation
| Variable | Single-Variable Analysis | Multivariable Analysis | ||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P Value | HR | 95% CI | P Value | |
| Dopamine infusion time, h | 0.96 | 0.92 to 1.00 | 0.05 | 0.95 | 0.90 to 0.99 | 0.03 |
| Donor characteristics | ||||||
| Age, yr | 1.03 | 1.02 to 1.05 | <0.001 | 1.02 | 1.01 to 1.04 | 0.01 |
| Women | 1.05 | 0.76 to 1.47 | 0.75 | 1.00 | 0.69 to 1.46 | 0.99 |
| Systolic BP, mmHg | 1.01 | 0.99 to 1.02 | 0.14 | 1.00 | 0.99 to 1.01 | 0.81 |
| Diastolic BP, mmHg | 1.00 | 0.98 to 1.02 | 0.75 | 1.00 | 0.98 to 1.02 | 0.98 |
| Urine production last h, ml | 1.00 | 0.998 to 1.001 | 0.55 | 1.00 | 0.998 to 1.001 | 0.86 |
| Cause of brain death | ||||||
| Head trauma, yes | 0.88 | 0.58 to 1.32 | 0.53 | 1.17 | 0.73 to 1.90 | 0.52 |
| Cerebral stroke, yes | 1.40 | 0.84 to 2.32 | 0.20 | 0.86 | 0.47 to 1.56 | 0.62 |
| Cerebral ischemia/cerebral edema, yes | 0.41 | 0.18 to 0.92 | 0.03 | 0.42 | 0.18 to 0.99 | 0.05 |
| Time from admission on ICU to brain-death confirmation, d | 1.00 | 0.96 to 1.04 | 0.95 | 1.01 | 0.97 to 1.04 | 0.73 |
| Time from brain-death confirmation to organ procurement, h | 0.97 | 0.94 to 1.01 | 0.17 | 1.00 | 0.96 to 1.05 | 0.92 |
| Multiorgan versus kidney-only donor | 0.78 | 0.54 to 1.14 | 0.20 | 1.00 | 0.66 to 1.54 | 0.99 |
| Cold ischemic time, h | 0.99 | 0.96 to 1.02 | 0.44 | 1.00 | 0.97 to 1.04 | 0.86 |
| Recipient characteristics | ||||||
| Age, yr | 1.04 | 1.03 to 1.06 | <0.001 | 1.03 | 1.01 to 1.05 | 0.001 |
| Women | 1.11 | 0.80 to 1.55 | 0.62 | 1.37 | 0.92 to 2.02 | 0.12 |
| Weight, kg | 1.01 | 0.99 to 1.02 | 0.26 | 1.01 | 0.99 to 1.03 | 0.12 |
| Diabetes, yes | 1.14 | 0.73 to 1.78 | 0.56 | 1.88 | 1.12 to 3.16 | 0.02 |
| Time spent on waiting list, yr | 0.98 | 0.92 to 1.04 | 0.49 | 1.00 | 0.94 to 1.07 | 0.92 |
| Antigen mismatches A, B, and DR, n | 1.13 | 1.02 to 1.25 | 0.02 | 1.05 | 0.93 to 1.18 | 0.45 |
| Panel reactive antibody >5%, yes | 1.23 | 0.76 to 1.99 | 0.41 | 1.17 | 0.67 to 2.04 | 0.58 |
| Previous transplant, yes | 1.33 | 0.87 to 2.02 | 0.19 | 2.07 | 1.26 to 3.39 | 0.004 |
| Immunosuppressive treatmenta | ||||||
| Induction therapy, yes | 1.01 | 0.72 to 1.40 | 0.97 | 0.84 | 0.59 to 1.20 | 0.33 |
| Tacrolimus versus cyclosporine | 0.78 | 0.56 to 1.09 | 0.15 | 0.79 | 0.54 to 1.16 | 0.23 |
Analyses cover the entire trial cohort including control patients. HR, hazard ratio; 95% CI, 95% confidence interval; ICU, intensive care unit.
At time of transplantation.
Table 4.
Multiple Cox regression of all-cause graft failure taking infusion time ≥7.1 h as cut-off value
| Variable | HR | 95% Confidence Interval | P Value |
|---|---|---|---|
| Dopamine infusion time ≥7.1 h | 0.52 | 0.29 to 0.94 | 0.03 |
| Donor age, yr | 1.02 | 1.00 to 1.04 | 0.01 |
| Female donor | 1.03 | 0.71 to 1.50 | 0.88 |
| Donor systolic BP, mmHg | 1.00 | 0.99 to 1.01 | 0.93 |
| Donor diastolic BP, mmHg | 1.00 | 0.98 to 1.02 | 0.99 |
| Donor urine production last h, ml | 1.00 | 0.998 to 1.001 | 0.79 |
| Head trauma, yes | 1.20 | 0.74 to 1.94 | 0.46 |
| Cerebral stroke, yes | 0.89 | 0.49 to 1.61 | 0.69 |
| Cerebral ischemia/cerebral edema, yes | 0.42 | 0.18 to 1.00 | 0.05 |
| Time from admission on ICU to brain-death confirmation, d | 1.01 | 0.97 to 1.05 | 0.65 |
| Time from brain-death confirmation to organ procurement, h | 1.00 | 0.96 to 1.05 | 0.86 |
| Multiorgan versus kidney-only donor, yes | 1.02 | 0.67 to 1.55 | 0.93 |
| Cold ischemic time, h | 1.01 | 0.97 to 1.04 | 0.72 |
| Recipient age, yr | 1.03 | 1.01 to 1.05 | 0.001 |
| Female recipient | 1.34 | 0.91 to 1.99 | 0.14 |
| Recipient weight, kg | 1.01 | 0.99 to 1.02 | 0.14 |
| Recipient diabetes, yes | 1.86 | 1.10 to 3.12 | 0.02 |
| Time spent on waiting list, yr | 1.00 | 0.94 to 1.07 | 0.95 |
| Antigen mismatches A, B, and DR, n | 1.05 | 0.93 to 1.18 | 0.45 |
| Panel reactive antibody >5%, yes | 1.22 | 0.70 to 2.14 | 0.48 |
| Repeat transplant, yes | 2.10 | 1.28 to 3.46 | 0.003 |
| Induction therapy, yes | 0.84 | 0.59 to 1.19 | 0.33 |
| Tacrolimus versus cyclosporinea | 0.78 | 0.53 to 1.14 | 0.20 |
Analyses cover the entire trial cohort including control patients. HR, hazard ratio; ICU, intensive care unit.
At time of transplantation.
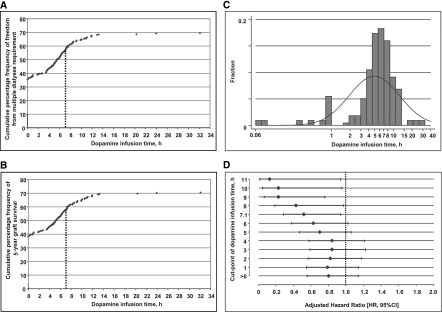
Cumulative percentage frequency curves produced a sigmoid-shaped relationship with both efficacy endpoints, freedom from multiple dialyses requirement post-transplant, and 5-year graft survival (Figure 3, A and B). Although the frequency distribution of the dopamine infusion times appeared left-skewed with a median of 5.8 hours (Figure 3C), the maximum increase in efficacy occurred at 7.05 hours (95% CI, 6.92 to 7.18) for avoidance of multiple dialyses sessions, and at 7.10 hours (95% CI, 6.99 to 7.21) for improvement of graft survival (Figure 3, A and B), a value lying on the 67th percentile in the treatment arm. Accordingly, around two thirds of donors assigned to dopamine were exposed to suboptimal treatment duration.
Figure 3.
Frequency graphs of the trial efficacy endpoints and dopamine infusion time displayed an effective treatment duration of around 7.1 hours which was achieved only in one third of donors assigned to dopamine. Cumulative percentage frequency of (A) freedom from multiple dialyses requirement immediately after transplantation, and (B) survival with functioning graft 5 years after transplantation, by dopamine infusion time. Vertical dashed lines denote the infusion time at the inflection point. (C) Frequency distribution of dopamine infusion time. (D) Adjusted hazard ratios (HR) of all-cause graft failure 5 years after transplantation and related 95% confidence intervals (95% CI) according to rising thresholds of exposure to the continuous dopamine infusion.
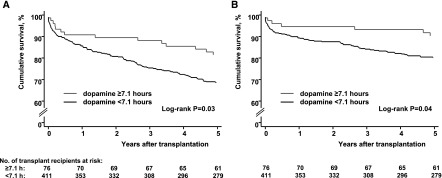
Taking infusion time of 7.1 hours as cut-point for comparison of graft survival indicated a relevant benefit: Overall 81.5% versus 68.5%, log-rank P=0.03 and 90.3% versus 80.2%, log-rank P=0.04 in the death-censored evaluation (Figure 4). The survival benefit was reassured by multiple Cox regression (Table 4). Because KDPI was marginally lower in the subgroup of kidneys treated for longer than 7.1 hours (46% versus 54%, P=0.02), we reanalyzed the data with KDPI as explanatory variable. The principal findings persisted (Supplemental Table 4). Also, the threshold of around 7 hours for improvement of graft survival was confirmed by empirical derivation from the adjusted survival model (Figure 3D). Serum-creatinine values in patients with functioning grafts are presented in Supplemental Tables 5 and 6.
Figure 4.
Kaplan–Meier estimates of kidney graft survival until 5 years after transplantation according to dopamine infusion time ≥7.1 hours indicated a relevant benefit of dopamine. (A) Overall graft survival. (B) Death-censored graft survival.
To exclude major selection bias, we carefully analyzed clinical characteristics in the subgroup of donors who required early termination of the trial intervention or remained untreated despite randomization to dopamine. These donors were neither different from controls nor from donors receiving dopamine until crossclamping (Table 1). Furthermore, kidneys with premature withdrawal from the dopamine infusion did not result in inferior graft survival compared with organs from the control group (73.3% versus 68.8%, log-rank P=0.57).
Discussion
One of our principal findings is that we did not observe a statistically significant survival advantage between the groups according to trial assignment. Hence, a recommendation for the routine use of dopamine in DBDs on the basis of the long-term results on intention-to-treat is not well supported. A previous large 1993 cohort study of 2407 DBD kidney grafts on the basis of the Eurotransplant registry reported a survival advantage of donor pretreatment with adrenergic agents of approximately 6% after 4 years (15). Although the randomized dopamine trial was underpowered to detect a survival advantage of this magnitude, the 1993 cohort study was recently confirmed in a nationwide multicenter survey from Germany which assessed deceased donor parameters on graft survival in 4411 kidney transplants between 2006 and 2008 (16).
Because of protocol restrictions (7), the treatment group of our trial was rather heterogeneous concerning dopamine infusion times. A number of kidneys were treated shortly, which likely diluted the effectiveness of the trial intervention and reduced the discriminatory power to detect a survival benefit on intention-to-treat. This view is supported by the observed significant association between 5-year graft survival and dopamine infusion time (Table 3). Our finding of a nonlinear exposure-response relationship owns biologic plausibility and consistency: Biologic plausibility, because dopamine’s mode of action relies on its antioxidant properties (8,17,18). Effectiveness, however, requires dopamine’s diffusion into cells before the kidney graft gets exposed to cold preservation. This is under steady-state conditions of the continuous infusion, where rapid enzymatic degradation occurs by monoamine oxidase and catecholamine-O-methyltransferase in the bloodstream (19), a time-dependent process.
Consistency is documented in two respects. The cumulative frequency functions of both prevention of the primary trial endpoint and graft survival exhibited very similar curve characteristics with almost equivalent values of infusion time at the inflection point (Figure 3, A and B); the statistical associations remained robust when various influencing factors known to affect the transplantation outcomes were considered for multiple regression analyses. (Tables 2 and 3). Particularly, the varying time interval until organ procurement, which limited treatment duration after brain-death confirmation, was no relevant confounding variable. These findings largely exclude that the statistical associations between infusion time and graft outcomes were caused by factors other than dopamine itself.
DGF represents the final common pathway of multiple injurious events during the transplantation process (20). Various donor and recipient risks have been evaluated prospectively for improved management of DGF after kidney transplantation. CIT, donor age, recipient body mass index, and induction therapy showed high predictive values in a French multicenter cohort study (21). A large-scale study from the Scientific Registry of Transplant Recipients confirmed that DGF negatively affects long-term graft and—to a lesser extent—recipient survival (22). Novel insights at the molecular level reveal that DGF is not only related to the complex interplay of deleterious events but also to repair mechanisms (23). It is therefore conceivable that interventions that ameliorate cold ischemia–reperfusion injuries do not directly induce improved long-term graft survival. Although CIT causally associates with DGF (24), it was no determinant of long-term graft failure in this study (Tables 2–4). This is in line with registry data indicating that a CIT up to 18 hours is not detrimental to the renal allograft (25); the majority of our patients were transplanted with a CIT<18 hours (7). Multiple regression analysis found no association of donor BP and urine production with any trial endpoint. Hence, in accordance with previous reports (7,26), our data do not support that dopamine’s protective properties are mediated by transient changes in donor renal hemodynamics.
Adverse side effects occurred in 12.9% of donors assigned to treatment which resulted in premature termination of the dopamine infusion. Assuming a longer treatment duration was not protective, but just a surrogate for hemodynamic stability, would have distorted the outcomes in the same direction as the study has. However, that scenario is extremely unlikely, as pointed out in the original trial report: Adverse side effects referring to tachycardia and hypertension were transient and fully reversible after termination of the dopamine infusion. Not a single donor was further compromised when dopamine was stopped according to the predefined termination criteria (7). Nonetheless, we carefully reanalyzed the subgroup of donors withdrawn from the dopamine infusion or not having received dopamine despite randomization to treatment (Figure 1), and compared them with donors treated per protocol, either in the intervention or control group. Our data do not support that these donors were of poorer quality (Table 1). In addition, they were not different regarding the length of stay on ICU, and the time interval between brain-death ascertainment and crossclamping. The latter largely excludes that donors not treated per protocol were unstable, necessitating urgent referral to the operation theater. Also, premature termination of the dopamine infusion did not alter graft survival compared with kidneys from untreated controls (log rank P=0.57).
Our study has limitations. First, it was beyond the scope of the randomized dopamine trial to prospectively explore the well-known recipient risks of graft failure in the long-term, such as trough levels and changes of immunosuppressive therapy, rejections beyond the first month post-transplant, prescription of antihypertensive drugs, etc. We restricted the covariates (risk factors) of graft failure to those that were primarily assessed according to the original trial protocol. It is deemed unlikely that the aforesaid additional covariates have confounded our finding of an exposure-response relationship. Usually a confounding variable requires any (at least putative) association with exposure (in the donor population) from which the cases (survivors with functioning graft) derive. This is clearly not the case. Second, according to the eligibility criteria, our findings solely relate to stable donors with normal renal function. Third, a shortcoming of the randomized dopamine trial is that the runtime of the infusion was not predefined. On the other hand, dopamine’s minimum effective duration was not yet known during the planning and execution of the trial. Fourth, although infusion times were to be documented (7), estimations of the optimal treatment duration were not intended. Hence, comparing survival according to the cut-point of 7.1 hours (Figure 4) complies with a post hoc analysis and requires confirmation in another prospective evaluation.
Nonetheless, despite missing statistical significance in the primary analysis on intention-to-treat, our data at least suggest that a graft survival advantage emerges when the continuous dopamine infusion is administered long enough before organ procurement. We feel that this implies considerable clinical relevance, offering the potential to improve the outcomes after renal transplantation without major effort and—more importantly—without side-effects among recipients. Moreover, the administration of low-dose dopamine (4 µg/kg per minute) is a safe procedure in the DBD, as long as the termination criteria of possible hemodynamic destabilization are respected. Given confirmed efficacy for improvement of early graft function and given that controlled clinical data on long-term results will not be available in the near future, it appears reasonable on the basis of the present findings to target an infusion time of around 7 hours for improving the ultimate prognosis of the kidney graft. From an ethical point of view this approach appears justifiable, because it would most likely be in accordance with the presumed decision and interest of the deceased willing to donate her/his organs with the best possible result for the recipient.
In summary, this study did not show a significant 5-year graft survival advantage according to trial assignment on intention-to-treat, most likely because duration of the continuous dopamine infusion was too short in a number of donors in the treatment arm. Our observation of a sigmoid-shaped exposure-response relationship suggests a threshold above which dopamine may improve long-term graft survival. Further studies on this issue are warranted.
Disclosures
None.
Supplementary Material
Acknowledgments
We would like to thank all 60 collaborating transplantation centers within Eurotransplant for their invaluable contributions to this study, and Dr. I. Tieken from Eurotransplant International Foundation for administrative support.
The study was an investigator-driven clinical trial conducted by the University Medical Center of Mannheim, Germany. It was partially supported by a medical school grant from Novartis Pharmaceuticals released in November 2002, before the study started recruiting eligible donors.
Footnotes
Published onlineahead of print. Publication dateavailable at www.cjasn.org.
See related editorial, “Optimizing Graft Survival by Pretreatment of the Donor,” on pages 388–390.
This article contains supplemental material online at http://cjasn.asnjournals.org/lookup/suppl/doi:10.2215/CJN.07600716/-/DCSupplemental.
References
- 1.Rosendale JD, Kauffman HM, McBride MA, Chabalewski FL, Zaroff JG, Garrity ER, Delmonico FL, Rosengard BR: Aggressive pharmacologic donor management results in more transplanted organs. Transplantation 75: 482–487, 2003 [DOI] [PubMed] [Google Scholar]
- 2.Novitzky D, Cooper DK, Rosendale JD, Kauffman HM: Hormonal therapy of the brain-dead organ donor: Experimental and clinical studies. Transplantation 82: 1396–1401, 2006 [DOI] [PubMed] [Google Scholar]
- 3.Malinoski DJ, Patel MS, Ahmed O, Daly MC, Mooney S, Graybill CO, Foster CE, Salim A; United Network for Organ Sharing (UNOS) Region 5 Donor Management Goals (DMG) Workgroup : The impact of meeting donor management goals on the development of delayed graft function in kidney transplant recipients. Am J Transplant 13: 993–1000, 2013 [DOI] [PubMed] [Google Scholar]
- 4.Wood KE, Becker BN, McCartney JG, D’Alessandro AM, Coursin DB: Care of the potential organ donor. N Engl J Med 351: 2730–2739, 2004 [DOI] [PubMed] [Google Scholar]
- 5.Kainz A, Wilflingseder J, Mitterbauer C, Haller M, Burghuber C, Perco P, Langer RM, Heinze G, Oberbauer R: Steroid pretreatment of organ donors to prevent postischemic renal allograft failure: A randomized, controlled trial. Ann Intern Med 153: 222–230, 2010 [DOI] [PubMed] [Google Scholar]
- 6.Niemann CU, Feiner J, Swain S, Bunting S, Friedman M, Crutchfield M, Broglio K, Hirose R, Roberts JP, Malinoski D: Therapeutic hypothermia in deceased organ donors and kidney-graft function. N Engl J Med 373: 405–414, 2015 [DOI] [PubMed] [Google Scholar]
- 7.Schnuelle P, Gottmann U, Hoeger S, Boesebeck D, Lauchart W, Weiss C, Fischereder M, Jauch KW, Heemann U, Zeier M, Hugo C, Pisarski P, Krämer BK, Lopau K, Rahmel A, Benck U, Birck R, Yard BA: Effects of donor pretreatment with dopamine on graft function after kidney transplantation: A randomized controlled trial. JAMA 302: 1067–1075, 2009 [DOI] [PubMed] [Google Scholar]
- 8.Brinkkoetter PT, Song H, Lösel R, Schnetzke U, Gottmann U, Feng Y, Hanusch C, Beck GC, Schnuelle P, Wehling M, van der Woude FJ, Yard BA: Hypothermic injury: The mitochondrial calcium, ATP and ROS love-hate triangle out of balance. Cell Physiol Biochem 22: 195–204, 2008 [DOI] [PubMed] [Google Scholar]
- 9.Lösel RM, Schnetzke U, Brinkkoetter PT, Song H, Beck G, Schnuelle P, Höger S, Wehling M, Yard BA. N-octanoyl dopamine, a non-hemodyanic dopamine derivative, for cell protection during hypothermic organ preservation. PLoS One 5(3): e9713, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Benck U, Hoeger S, Brinkkoetter PT, Gottmann U, Doenmez D, Boesebeck D, Lauchart W, Gummert J, Karck M, Lehmkuhl HB, Bittner HB, Zuckermann A, Wagner F, Schulz U, Koch A, Bigdeli AK, Bara C, Hirt S, Berchtold-Herz M, Brose S, Herold U, Boehm J, Welp H, Strecker T, Doesch A, Birck R, Krämer BK, Yard BA, Schnuelle P: Effects of donor pre-treatment with dopamine on survival after heart transplantation: A cohort study of heart transplant recipients nested in a randomized controlled multicenter trial. J Am Coll Cardiol 58: 1768–1777, 2011 [DOI] [PubMed] [Google Scholar]
- 11.Hill AV: The possible effects of the aggregation of the molecules of haemoglobin on its dissociation curves. J Physiol 40: iv–vii, 1910 [Google Scholar]
- 12.Goutelle S, Maurin M, Rougier F, Barbaut X, Bourguignon L, Ducher M, Maire P: The Hill equation: A review of its capabilities in pharmacological modelling. Fundam Clin Pharmacol 22: 633–648, 2008 [DOI] [PubMed] [Google Scholar]
- 13.Bounias M: Algebraic potential of the Hill equation as an alternative tool for plotting dose (or time)/effects relationships in toxicology: A theoretical study. Fundam Clin Pharmacol 3: 1–9, 1989 [DOI] [PubMed] [Google Scholar]
- 14.U.S. Department of Health & Human Services: KDPI open source calculator, 2016. Available at: https://optn.transplant.hrsa.gov/resources/allocation-calculators/kdpi-calculator/. Accessed September 24, 2016
- 15.Schnuelle P, Berger S, de Boer J, Persijn G, van der Woude FJ: Effects of catecholamine application to brain-dead donors on graft survival in solid organ transplantation. Transplantation 72: 455–463, 2001 [DOI] [PubMed] [Google Scholar]
- 16.Fischer-Fröhlich CL, Kutschmann M, Feindt J, Schmidtmann I, Kirste G, Frühauf NR, Wirges U, Rahmel A, Schleicher C: Influence of deceased donor and pretransplant recipient parameters on early overall kidney graft-survival in Germany. J Transplant 2015: 307230, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yard B, Beck G, Schnuelle P, Braun C, Schaub M, Bechtler M, Göttmann U, Xiao Y, Breedijk A, Wandschneider S, Lösel R, Sponer G, Wehling M, van der Woude FJ: Prevention of cold-preservation injury of cultured endothelial cells by catecholamines and related compounds. Am J Transplant 4: 22–30, 2004 [DOI] [PubMed] [Google Scholar]
- 18.Brinkkoetter PT, Beck GC, Gottmann U, Loesel R, Schnetzke U, Rudic B, Hanusch C, Rafat N, Liu Z, Weiss C, Leuvinik HG, Ploeg R, Braun C, Schnuelle P, van der Woude FJ, Yard BA: Hypothermia-induced loss of endothelial barrier function is restored after dopamine pretreatment: Role of p42/p44 activation. Transplantation 82: 534–542, 2006 [DOI] [PubMed] [Google Scholar]
- 19.Yan M, Webster LT Jr, Blumer JL: Kinetic interactions of dopamine and dobutamine with human catechol-O-methyltransferase and monoamine oxidase in vitro. J Pharmacol Exp Ther 301: 315–321, 2002 [DOI] [PubMed] [Google Scholar]
- 20.Cavaillé-Coll M, Bala S, Velidedeoglu E, Hernandez A, Archdeacon P, Gonzalez G, Neuland C, Meyer J, Albrecht R: Summary of FDA workshop on ischemia reperfusion injury in kidney transplantation. Am J Transplant 13: 1134–1148, 2013 [DOI] [PubMed] [Google Scholar]
- 21.Chapal M, Le Borgne F, Legendre C, Kreis H, Mourad G, Garrigue V, Morelon E, Buron F, Rostaing L, Kamar N, Kessler M, Ladrière M, Soulillou JP, Launay K, Daguin P, Offredo L, Giral M, Foucher Y: A useful scoring system for the prediction and management of delayed graft function following kidney transplantation from cadaveric donors. Kidney Int 86: 1130–1139, 2014 [DOI] [PubMed] [Google Scholar]
- 22.Butala NM, Reese PP, Doshi MD, Parikh CR: Is delayed graft function causally associated with long-term outcomes after kidney transplantation? Instrumental variable analysis. Transplantation 95: 1008–1014, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schröppel B, Legendre C: Delayed kidney graft function: From mechanism to translation. Kidney Int 86: 251–258, 2014 [DOI] [PubMed] [Google Scholar]
- 24.Kayler LK, Srinivas TR, Schold JD: Influence of CIT-induced DGF on kidney transplant outcomes. Am J Transplant 11: 2657–2664, 2011 [DOI] [PubMed] [Google Scholar]
- 25.Opelz G, Döhler B: Multicenter analysis of kidney preservation. Transplantation 83: 247–253, 2007 [DOI] [PubMed] [Google Scholar]
- 26.Schnuelle P, Yard BA, Braun C, Dominguez-Fernandez E, Schaub M, Birck R, Sturm J, Post S, van der Woude FJ: Impact of donor dopamine on immediate graft function after kidney transplantation. Am J Transplant 4: 419–426, 2004 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.