Abstract
Vascular peroxidase 1 (VPO1) is a member of the peroxidase family which aggravates oxidative stress by producing hypochlorous acid (HOCl). Our previous study demonstrated that VPO1 plays a critical role in endothelial dysfunction through dimethylarginine dimethylaminohydrolase2 (DDAH2)/asymmetric Dimethylarginine (ADMA) pathway. Hereby we describe the regulatory role of VPO1 on endothelial nitric oxide synthase (eNOS) expression and activity in human umbilical vein endothelial cells (HUVECs). In HUVECs AngiotensinII (100 nM) treatment reduced Nitric Oxide (NO) production, decreased eNOS expression and activity, which were reversed by VPO1 siRNA. Knockdown of VPO1 also attenuated ADMA production and eNOS uncoupling while enhancing phosphorylated ser1177 eNOS expression level. Furthermore, HOCl stimulation was shown to directly induce ADMA production and eNOS uncoupling, decrease phosphorylated ser1177 eNOS expression. It also significantly suppressed eNOS expression and activity together with NO production. Therefore, VPO1 plays a vital role in regulating eNOS expression and activity via hydrogen peroxide (H2O2)-VPO1-HOCl pathway.
Abbreviation: DDAH2, dimethylarginine dimethylaminohydrolase2; H2O2, hydrogen peroxide; ADMA, asymmetric Dimethylarginine; HOCl, hypochlorous acid; eNOS, endothelial nitric oxide synthase; PRMT1, Protein arginine methyltransferase1; ROS, reactive oxygen species; NO, nitric oxide
Keywords: Vascular peroxidase 1, Endothelial nitric oxide synthase, Nitric oxide, Asymmetricdimethylarginine, Angiotensin II, Oxidative stress
Highlights
-
•
Angiotensin II decreased eNOS expression and activity in HUVECs.
-
•
VPO1 plays an important role in regulating eNOS expression and activity in HUVECs.
-
•
VPO1 regulates eNOS expression and activity through VPO1/H2O2/HOCl pathway.
1. Introduction
Vascular peroxidase (VPO1) is a heme-containing peroxidase that is primarily found in the cardiovascular system [1]. As a member of peroxidase family, VPO1 aggravates oxidative stress utilizing hydrogen peroxide (H2O2) and producing hypochlorous acid (HOCl) [2]. Recent study suggested that VPO1 decreases eNOS expression by increasing Asymmetric dimethylarginine (ADMA) level [3]. VPO1 also was found to decrease dimethylarginine dimethylaminohydrolase2 (DDAH2) expression and activity in HUVECs, which contributes to endothelial dysfunction [3].
ADMA is the L-arginine analogue that inhibits eNOS expression [4]. In the murine model of diabetic nephropathy with angiotensin II (Ang II) infusion, it was shown to have higher level of reactive oxygen species (ROS) and ADMA, as well as decreased eNOS expression [5]. This suggests that oxidative stress induced by Ang II correlates with ADMA production and eNOS expression. Protein arginine methyltransferase1 (PRMT1) is the predominant enzyme catalyzing the formation of ADMA. However, the exact relationship between ROS and PRMT1 regulation has not been fully determined.
eNOS has been shown to be a source of superoxide. Under pathological conditions, the homodimer of eNOS could be uncoupled, which result in dissociation of eNOS dimmers into monomer. The uncoupling prompts eNOS to produce superoxide instead of NO [6], which may induce oxidative stress. It has been demonstrated that HOCl treatment induced eNOS uncoupling in endothelial cells and with recombinant eNOS protein [7]. In addition, phosphorylation on serine (Ser1177) and theonine (Thr495) residues [8] have been discussed extensively to regulate eNOS. The phosphorylation of Ser1177 is involved in multiple signaling pathways in the cardiovascular conditions [9].
Overall, we hypothesize that under oxidative stress, eNOS expression and activity are regulated through a VPO1 mediated signaling pathway.
2. Material and methods
2.1. Cell culture and treatment
Human umbilical vein endothelial cells (HUVECs) were obtained from ATCC. HUVECs were cultured in Dulbecco's modified Eagle's medium (DMEM, 1 g/L glucose, 10 mmol/L sodium pyruvate) supplemented with 10% fetal bovine serum (FBS) and 1% streptomycin/penicillin at 37 °C in 5% CO2. HUVECs from passage 3 to passage 10 were used.
HUVECs were incubated with DMEM containing AngⅡ (100 nmmol/l) for 24 h which was shown to be the optimal treatment for VPO1 expression by our previous work. The protein and mRNA expression of VPO1, PRMT-1, eNOS, the levels of ADMA in the supernatant, the ratio of eNOS dimer and monomer, the concentration of H2O2, HOCl, cGMP in the cell lysate were determined. In addition, the cells were also exposed to Hank's buffered saline solution (HBSS) with 100 µmol/L HOCl at 37 °C for 2 h. HOCl was removed by extensive wash with PBS, the cells were then cultured in DMEM for another 24 h [10].
2.2. RNA interference and cell transfection
The small interference RNA (siRNA) including negative controls were synthesized and purchased from RiboBio Co Ltd (Guangzhou, China). HUVECs were seeded in six-well plates at a density of 5×105 per well and cultured in 10% FBS DMEM for 12–24 h until 40–50% confluent. The cells were transfected with VPO1 siRNA or negative control siRNA at a final concentration of 50 nM using the ribo FECT™ CP Transfection Kit (RiboBio Co Ltd, Guangzhou, China), following manufacturer's protocol. 24 h after transfection, cells were washed with PBS and treated with AngⅡ(100 nM) for 24 h.
2.3. Western blot analysis
The cells were lysed with RIPA(Beyotime, China) containing 1 mM phenylmethanesulfonyl fluoride (PMSF) on ice for 30 min to 1h. Cell lysate was sonicated at 4 °C and centrifuged at 12000 rpm for 10 min. Cell lysate containing 50–60 μg protein that was solubilized in 5×loading buffer (Beyotime, China) and resolved by 10% SDS-PAGE gels and transferred onto 0.22 µm polyvinylidene difuoride membranes (PVDF, Millipore). Specifically, eNOS dimers and monomers were assayed by low-temperature SDS-PAGE as described previously [11]. Briefly, after blocking the residual protein sites on the membranes with 5% nonfat dry milk, the membrane was incubated with anti-VPO1 (from Dr. Guangjie Cheng, University of Alabama at Birmingham), anti-PRMT-1 (Sigma-Aldrich, USA), eNOS, eNOS ser 1177 (BD transduction laboratories), 3-chlorotyrosine antibody (Cell Science), anti-GAPDH (Sigma-Aldrich, USA) overnight at 4 °C. And then the membrane was washed by TBST for 10 min 3 times and further incubated with a second horseradish peroxidase-conjugated antibody against with mouse or rabbit IgG for 1 h at room temperature. Lastly, the membrane was visualized using SuperSignal West Pico Chemiluminent Substrates (PIERCE) with a gel documentation system (Bio-Rad).
2.4. RNA isolation, reverse transcription, real-time PCR analysis
Total RNA isolation from HUVECs was carried out by trizol (Invitrogen, USA) at a constant temperature of 4 °C.The RNA was quantified by ultraviolet absorbance spectrophotometry. cDNA was synthesized from RNA (500 ng) using the PrimeScript™ RT Reagent Kit (TakaRa, Japan) according to the manufacture's instruction. Real-time PCR was perfomed by ABI 7500 Real-time PCR system (Foster City, CA, USA). Results were analyzed as the ratio of VPO1 to Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) gene expression.
2.5. ADMA concentration
ADMA concentration was detected by using High Performance Liquid Chromatography (HPLC) as described previously [12].
2.6. Hydrogen peroxide assay
Changes in intracellular H2O2 levels were measured by the hydrogen peroxide assay kit (Beyotime, China) according to the manufacturer's instruction. In this kit, ferric ions (Fe3+) that oxidated by H2O2 can form a purple complex, xylenol orange (3,3′-bis[N,N-di(carboxymethyl)-aminomethyl]-o-cresolsulfonephthalein, disodium salt and xylenol orange), which can be detected at a wavelength of 560 nm [13].
2.7. Hypochlorous acid assessment
HOCl can be combined with tyrosine to produce 3-chlorotyrosine (3-Cl-Tyr) [14]. Western blot and immunohistochemistry were used for detecting 3-Cl-Tyr expression. Cells were seeded to six-well glass glide. The slides of cells fixed by 4% paraformaldehyde for 20 min, permeabilized by 0.1% Triton×100 for 10 min, blotted by 1% BSA for 1 h, and then incubated anti-3-Cl-Tyr (Hycult Biotech, The Netherlands) overnight 4 °C. Secondary antibody was incubated for 1 h at room temperature. Lastly, 3,3′-diaminobenzidinc was used as color developing reagent. The results were determined by the amount of granular brown substance in the cytoplasm.
2.8. Assay of cGMP
The concentration of cGMP was determined using ELISA from R&D system.
2.9. eNOS activity
Cellular NOS activity was measured by the conversion of L-arginine to NO using a nitric-oxide synthase assay kit (Beyotime Institute of Biotechnology). After treated AngⅡ (100 nM) for 24 h, the culture supernatant was removed and 100 uL NOS assay buffer (1×) with or without eNOS inhibitor (L-NAME) were added to each well. Then 100 uL of NOS assay reaction solution(50%NOS assay buffer, 38.8% MilliQ water, 5% L-Arginine solution, 5% 0.1 mM NADPH, 0.2% DAF-FMDA) was added to each well and incubated for 2 h at 37 ℃. Cells were collected and washed twice by PBS. Fluorescence was measured by flow cytometry. eNOS activity was calculated by the decrease in NOS activity when applying eNOS inhibitor to HUVECs.
3. Results
3.1. Angiotensin II enhances VPO1 expression, decreases eNOS expression and activity as well as NO production
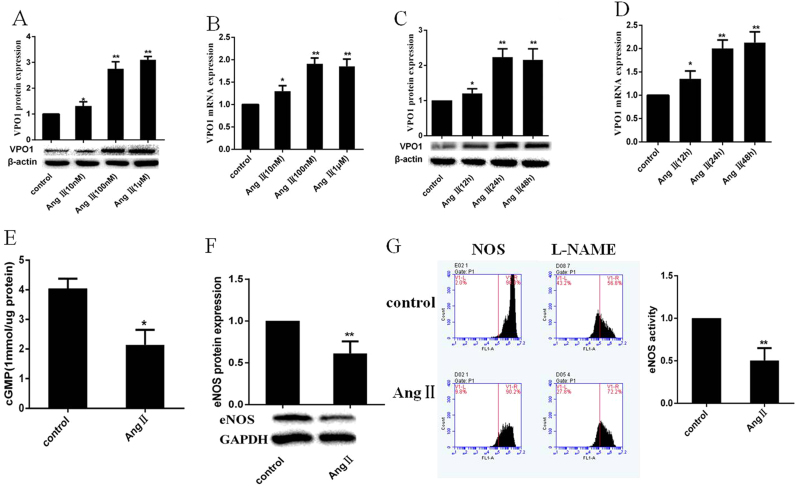
As described by our groups previously, AngⅡ induced VPO1 protein and mRNA expression in a concentration and time-dependent manner in VASMCs [10]. In this study, AngⅡ also up-regulated VPO1 protein and mRNA expression in the same manner in HUVECs. The induction effect peaked at 24 h of treatment with 100 nM AngⅡ (Fig. 1A–D). Also, AngⅡ (100 nM) significantly decreased eNOS expression and activity as well as cGMP level (Fig. 1E–G).
Fig. 1.
Effects of AngⅡ on VPO1 and eNOS in HUVECs. (A–D): AngⅡ treatment up-regulated protein and mRNA of expression of VPO1 in a time and dose dependent manner (**P<0.01) vs control group. (E) AngⅡ (100 nM) treatment inhibited cGMP production. (*P<0.05) vs control group. (F–G) incubation of HUVECs with Ang (100 nM) for 24 h decreased eNOS expression and activity. (**P<0.01) vs control group. (n=3 per group).
3.2. VPO1 regulates eNOS expression through PRMT1/ADMA pathway
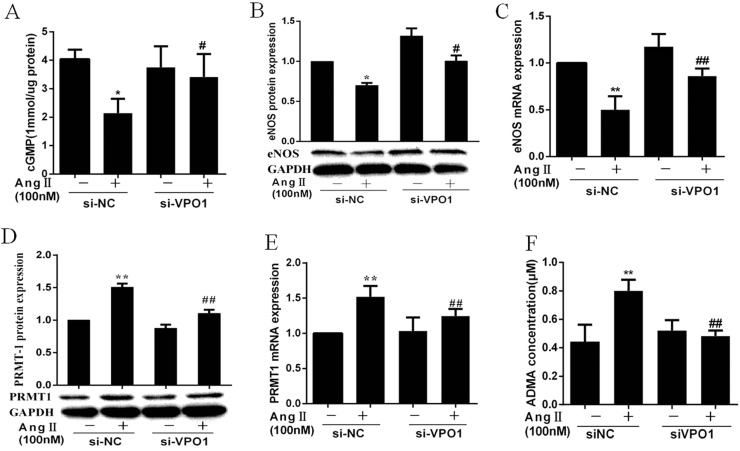
siRNA transfection was used to knockdown gene and protein expression of VPO1 in HUVECs. VPO1 expression (both protein and mRNA) was significantly inhibited compared with the negative control (Supplementary Figs. A–B). we treated HUVECs with AngⅡ (100 nM) for 24 h after siRNA transfection. The decrease in cGMP concentration induced by AngⅡ was reversed by VPO1siRNA transfection (Fig. 2A). VPO1 siRNA also alleviated the inhibiting effect of AngⅡ on eNOS gene and protein expression (Fig. 2B–C). ADMA is a competitive inhibitor of eNOS and synthesized by protein arginine methyltransferase1 (PRMT1) [15]. As shown in Fig. 2D–F, AngⅡup-regulated PRMT1 protein and mRNA expression and ADMA concentration, which were inhibited by VPO1 siRNA.
Fig. 2.
VPO1 regulates eNOS expression through PRMT1/ADMA pathway. HUVECs were treated with AngⅡ for 24 h after transfected with siVPO1. (A) VPO1 gene knockdown reversed AngⅡ induced decrease of cGMP generation. (B–C) the eNOS protein and mRNA expression. (D–E) the PRMT1 protein and mRNA expression. (F) AMDA was measured by HPLC(n=3 per group) (**P<0.01) vs control group. (##P<0.01) vs AngⅡ group, (n=3 per group).
3.3. The effect of VPO1 on eNOS activity in AngⅡ treated HUVECs
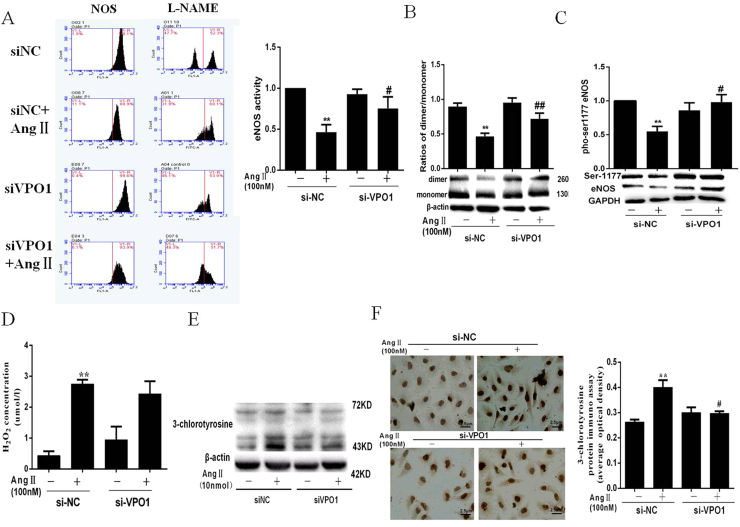
As shown in Fig. 3A, VPO1 siRNA successfully attenuated the decreased eNOS activity which induced by AngⅡ. eNOS activity can be regulated by several mechanisms. Studies have shown the dimeric structure is crucial to eNOS activity [16]. We assessed the ratio of eNOS dimer and monomer after transfection with VPO1 siRNA. The results showed that VPO1 siRNA reversed the eNOS uncoupling induced by AngⅡ (Fig. 3B). In addition, eNOS phosphorylation is a critical way of regulating its activity [17], [18]. We subsequently examine the ser1177 phosphorylation of eNOS. As depicted in Fig. 3C, AngⅡimpaired ser1177 phosphorylation of eNOS, which reversed by VPO1 siRNA.
Fig. 3.
The effect of VPO1 on eNOS activity in AngⅡ treated HUVECs. (A) eNOS activity was measured by Flow CytoMetry. VPO1-siRNA attenuated the decreased eNOS activity induced by AngⅡ. (B) The eNOS dimer and monomer were measured by low-temperature SDS-PAGE. (C) The level of p-eNOS. (D) The level of intracellular H2O2. (E–F) the 3-chlorotyrosine expression assessed by western blot and immunohistochemistry. (**P<0.01) vs control group. (##P<0.01) vs AngⅡgroup. (n=3 per group).
3.4. Influence of VPO1 on H2O2 and HOCl production
Our previous study has been demonstrated that VPO1 is responsible for the production of HOCl in the cardiovascular diseases [1]. As shown in Fig. 3D–F, AngⅡincubation significantly increased the levels of H2O2 and HOCl. Also, VPO1-siRNA evidently inhibited the HOCl generation compared with the controls (Fig. 3E–F). However, in the AngⅡ treated state, VPO1-siRNA increased H2O2 production without statistical significance (Fig. 3D).
3.5. HOCl directly decreases eNOS expression and activity in HUVECs
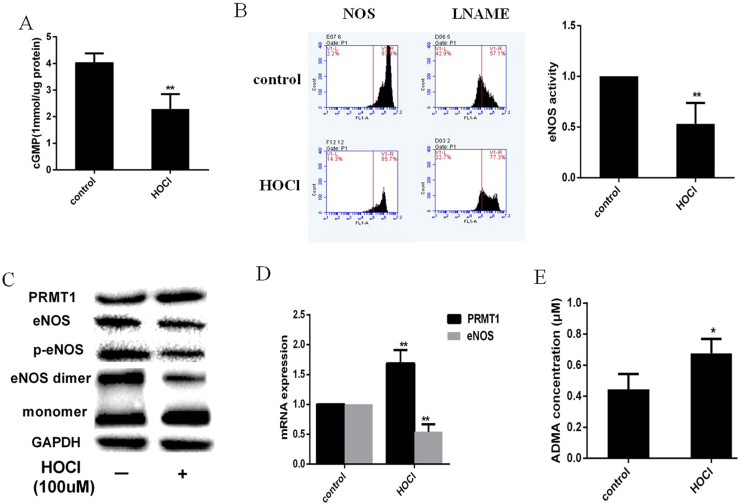
It has been demonstrated that VPO1-generated HOCl aggravates vascular oxidative stress that induced vascular smooth cell proliferation and endothelial dysfunction [19]. HUVECs were treated with HOCl (100 µmol/L) for 2 h and replaced with normal medium for 24 h. As shown in Fig. 4A–D, HOCl significantly decreased eNOS expression and activity, cGMP production. Moreover, PRMT-1 expression and ADMA production were increased while the dimer and phosphorylation of eNOS were decreased compared with the controls (Fig. 4C–E).
Fig. 4.
HOCl directly decreases eNOS expression and activity in HUVECs. HUVECs were incubated with HOCl (100 µmol/L) for 2 h, and then cultured with DMEM for 24 h. (A) cGMP production measured by ELISA. (B) eNOS activity. (C)PRMT1, total eNOS, eNOS dimer and monomer were analyzed by western blot. (D) The eNOS and PRMT1 mRNA expression. (E) ADMA production. n=3 per group (** P<0.01, * P<0.05) vs control group. (n=3 per group).
4. Discussion
The data presented above indicates that VPO1 plays a critical role in regulating eNOS expression and activity. VPO1 expression was up-regulated in AngⅡ-treated HUVECs. Knockdown of VPO1 was sufficient to attenuate the AngⅡ induced decrease in eNOS expression and activity. Moreover, the effect of VPO1 on eNOS was mainly achieved by HOCl formation. This experiment implied that VPO1 may be a key regulator on eNOS, which is a potential therapeutic target in cardiovascular diseases.
Vascular peroxidase 1 (VPO1) is a newly identified family member of peroxidases expressed in cardiovascular system. VPO1-mediated oxidative stress plays an essential role in endothelial cell apoptosis, smooth muscle cell proliferation, caridiac hypertrophy and vascular calcification [10], [20], [21], [22]. Recently our laboratory demonstrated that activation of NOX/VPO1/HOCl pathway played an important role in endothelial dysfunction via DDAH/ADMA pathway in SHR and HUVECs treated with AngⅡ [3].
NO is one of the factors secreted by endothelium to maintain the vascular tone. These factors modulate the relaxatory behavior of vascular smooth muscle, platelet aggregation and inflammation [23]. NO synthesized enzymatically from L-arginine by eNOS [24], [25] is the most important signaling molecule that modulates physiological endothelium function. Previous studies have shown that increased oxidative stress is often associated with decreased eNOS expression and activity [23], [26], [27]. This is consistent with our data that eNOS expression and activity were decreased in AngⅡ treated HUVECs.
ADMA is the most important upstream regulator for eNOS expression [28]. It is potentially an indicator for cardiovascular diseases, as decrease in NO production leads to adverse vascular effects including dysregulation of blood pressure, loss of antithrombotic activity, disorder in homeostasis and fibrinolysis, and inhibition of platelet aggregation [29], [30]. Protein arginine methyltransferase1 (PRMT1) is the predominant enzyme catalyzing the formation of ADMA by transferring methyl groups from S-adenosyl-methionine to guanidino groups of arginine residues in a variety of proteins; DDAH metabolize ADMA to produce citrulline [31]. Our group has demonstrated that VPO1 was correlated with ADMA metabolism through regulating DDAH expression and activity [12]. In this study, we found that AngⅡ-induced PRMT1 expression was also inhibited by VPO1 siRNA, which significantly suppressed ADMA production and enhanced eNOS expression. These results implicated that VPO1 is a key enzyme regulating ADMA generation and eNOS expression.
The activity of eNOS is subjected to a discreet and complexed mechanism of regulation. The active form of eNOS enzyme consists of two identical subunits that form a head to tail homodimer [27]. Intriguingly, it has been demonstrated that the eNOS could be a source of superoxidase when uncoupled [32]. Recent studies in vivo and in vitro demonstrated increased eNOS uncoupling in many cardiovascular disorders including hypertension [33], ischemia reperfusion [34] and diabetes [35]. In this study,we observed that the VPO1 siRNA attenuated AngⅡ-induced eNOS uncoupling. In addition, protein phosphorylation is a posttranslational modification and a key mediator of eNOS activity. The primary sites where eNOS gets phosphorylated are serine residues and, to a lesser extent, on tyrosine (Tyr) and threonine residues [36]. Studies showed that Resveratrol restored endothelial function by preserving eNOS phosphorylation (Ser1177). In our study, we observed the knockdown of VPO1 expression significantly increased serine1177 phosphorylation of eNOS. These data suggest that VPO1 may act as a novel regulator of eNOS activity via structure change and phoshorylation.
The predominant role of VPO1 entails utilization of H2O2 to yield HOCl, which aggravates oxidative stress in the pathological conditions [19]. In this study, we demonstrated that AngⅡ increased H2O2 and HOCl production in HUVECs. Interestingly, VPO1 expression inhibition had no effect on H2O2 production, but significantly decreased the level of HOCl. HOCl was shown to inactivate eNOS by inducing eNOS uncoupling and inhibiting eNOS phosphorylation [37], [38]. To further test the hypothesis that VPO1 regulates eNOS via HOCl formation, HUVECs were incubated with HOCl for an hour. HOCl is the strong oxidant substance that participates in redox reaction [37]. We found that HOCl treatment decreased eNOS expression and activity by inducing ADMA production, eNOS uncoupling and inhibiting p-eNOS. These results indicate that HOCl-derived from VPO1 directly induced a decreased eNOS expression and activity which inhibited NO generation.
However, the mechanism of how HOCl regulates PRMT-1 and eNOS uncoupling remains unexplained. Recently, a novel redox-based PRMT regulatory mechanism was reported. It is revealed that two cysteine residues (C101 and C208) which can influence PRMT1 activity can be regulated by the redox environment [39]. Additionally, S-glutathionylation of eNOS is a new and important mechanism providing redox regulation of cellular signaling [40]. It has been demonstrated that HOCl contributed to the formation of s-glutathionylation [41]. These are interesting directions in which further investigation is necessary in uncovering the mechanism behind.
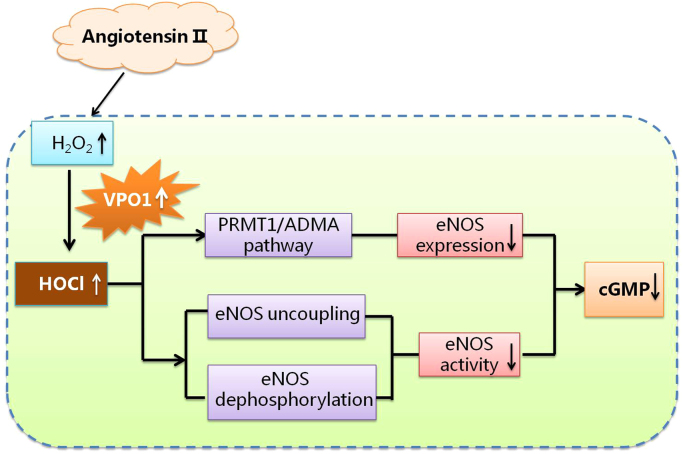
In conclusion, our work suggested that VPO1 inhibited eNOS expression by decreasing ADMA production. It also decreases eNOS activity by structural change and de-phosphorylation. The proposed pathway of VPO1 regulation of eNOS is summarized in Fig. 5. Overall, VPO1 is a pivotal regulator of eNOS.
Fig. 5.
The proposed pathway of VPO1 regulation of eNOS. AngⅡ up-regulates H2O2 production and VPO1 expression. VPO1-derived HOCl decreases eNOS expression through PRMT1/ADMA pathway and down-regulates eNOS activity via inducing eNOS uncoupling and eNOS ser1177 dephosphorylation.
Sources of funding
This work was supported by grants from the National Youth Science Foundation of China (No. 81102440 to S.R.Z.) and the National Natural Science Fund of China (No. 81170261 to Z.G.G.). And this work was also supported by National Basic Research Program of China (973 Program) (No. 2014CB542402), grants from Natural Science Fund of Hunan Province (2016JJ2159) and grant from the Postdoctor Fund of China (No. 2013M542143).
Acknowledgments
The International Postdoctoral Exchange Fellowship Program for Ruizheng Shi.
Footnotes
Supplementary data associated with this article can be found in the online version at doi:10.1016/j.redox.2017.02.022.
Contributor Information
Guogang Zhang, Email: xyzgg2006@sina.com.
Ruizheng Shi, Email: xyshiruizheng@csu.edu.cn.
Appendix A. Supplementary material
Supplementary material
.
References
- 1.Cheng G., Salerno J.C., Cao Z. Identification and characterization of VPO1, a new animal heme-containing peroxidase. Free Radic. Biol. Med. 2008;45(12):1682–1694. doi: 10.1016/j.freeradbiomed.2008.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cheng G., Salerno J.C., Cao Z. Identification and characterization of VPO1, a new animal heme-containing peroxidase. Free Radic. Biol. Med. 2008;45(12):1682–1694. doi: 10.1016/j.freeradbiomed.2008.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Peng H., Chen L., Huang X. Vascular peroxidase 1 up regulation by angiotensin II attenuates nitric oxide production through increasing asymmetrical dimethylarginine in HUVECs. J. Am. Soc. Hypertens. 2016;10(9):741–751. doi: 10.1016/j.jash.2016.06.036. [DOI] [PubMed] [Google Scholar]
- 4.Aldamiz-Echevarria L., Andrade F. Asymmetric dimethylarginine, endothelial dysfunction and renal disease. Int. J. Mol.. Sci. 2012;13(9):11288–11311. doi: 10.3390/ijms130911288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Onozato M.L., Tojo A., Leiper J. Expression of NG,NG-dimethylarginine dimethylaminohydrolase and protein arginine N-methyltransferase isoforms in diabetic rat kidney: effects of angiotensin II receptor blockers. Diabetes. 2008;57(1):172–180. doi: 10.2337/db06-1772. [DOI] [PubMed] [Google Scholar]
- 6.Rochette L., Lorin J., Zeller M. Nitric oxide synthase inhibition and oxidative stress in cardiovascular diseases: possible therapeutic targets? Pharm. Ther. 2013;140(3):239–257. doi: 10.1016/j.pharmthera.2013.07.004. [DOI] [PubMed] [Google Scholar]
- 7.Xu J., Xie Z., Reece R. Uncoupling of endothelial nitric oxidase synthase by hypochlorous acid: role of NAD(P)H oxidase-derived superoxide and peroxynitrite. Arterioscler. Thromb. Vasc. Biol. 2006;26(12):2688–2695. doi: 10.1161/01.ATV.0000249394.94588.82. [DOI] [PubMed] [Google Scholar]
- 8.Zhang H., Zhang J., Ungvari Z. Resveratrol improves endothelial function: role of TNF{alpha} and vascular oxidative stress. Arterioscler. Thromb. Vasc. Biol. 2009;29(8):1164–1171. doi: 10.1161/ATVBAHA.109.187146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Signorello M.G., Segantin A., Passalacqua M. Homocysteine decreases platelet NO level via protein kinase C activation. Nitric Oxide. 2009;20(2):104–113. doi: 10.1016/j.niox.2008.11.005. [DOI] [PubMed] [Google Scholar]
- 10.Shi R., Hu C., Yuan Q. Involvement of vascular peroxidase 1 in angiotensin II-induced vascular smooth muscle cell proliferation. Cardiovasc Res. 2011;91(1):27–36. doi: 10.1093/cvr/cvr042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zou M.H., Shi C., Cohen R.A. Oxidation of the zinc-thiolate complex and uncoupling of endothelial nitric oxide synthase by peroxynitrite. J. Clin. Invest. 2002;109(6):817–826. doi: 10.1172/JCI14442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Peng H., Chen L., Huang X. Vascular peroxidase 1 up regulation by angiotensin II attenuates nitric oxide production through increasing asymmetrical dimethylarginine in HUVECs. J. Am. Soc. Hypertens. 2016;10(9):741–751. doi: 10.1016/j.jash.2016.06.036. [DOI] [PubMed] [Google Scholar]
- 13.Deiana L., Carru C., Pes G. Spectrophotometric measurement of hydroperoxides at increased sensitivity by oxidation of Fe2+ in the presence of xylenol orange. Free Radic. Res. 1999;31(3):237–244. doi: 10.1080/10715769900300801. [DOI] [PubMed] [Google Scholar]
- 14.Knutson C.G., Mangerich A., Zeng Y. Chemical and cytokine features of innate immunity characterize serum and tissue profiles in inflammatory bowel disease. Proc. Natl. Acad. Sci. USA. 2013;110(26):E2332–E2341. doi: 10.1073/pnas.1222669110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Alpoim P.N., Sousa L.P., Mota A.P. Asymmetric dimethylarginine (ADMA) in cardiovascular and renal disease. Clin. Chim. Acta. 2015;440:36–39. doi: 10.1016/j.cca.2014.11.002. [DOI] [PubMed] [Google Scholar]
- 16.Roe N.D., Ren J. Nitric oxide synthase uncoupling: a therapeutic target in cardiovascular diseases. Vasc. Pharm. 2012;57(5–6):168–172. doi: 10.1016/j.vph.2012.02.004. [DOI] [PubMed] [Google Scholar]
- 17.Corson M.A., James N.L., Latta S.E. Phosphorylation of endothelial nitric oxide synthase in response to fluid shear stress. Circ. Res. 1996;79(5):984–991. doi: 10.1161/01.res.79.5.984. [DOI] [PubMed] [Google Scholar]
- 18.Dimmeler S., Fleming I., Fisslthaler B. Activation of nitric oxide synthase in endothelial cells by Akt-dependent phosphorylation. Nature. 1999;399(6736):601–605. doi: 10.1038/21224. [DOI] [PubMed] [Google Scholar]
- 19.Li H., Cao Z., Zhang G. Vascular peroxidase 1 catalyzes the formation of hypohalous acids: characterization of its substrate specificity and enzymatic properties. Free Radic. Biol. Med. 2012;53(10):1954–1959. doi: 10.1016/j.freeradbiomed.2012.08.597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tang Y., Xu Q., Peng H. The role of vascular peroxidase 1 in ox-LDL-induced vascular smooth muscle cell calcification. Atherosclerosis. 2015;243(2):357–363. doi: 10.1016/j.atherosclerosis.2015.08.047. [DOI] [PubMed] [Google Scholar]
- 21.Yang W., Liu Z., Xu Q. Involvement of vascular peroxidase 1 in angiotensin II-induced hypertrophy of H9c2 cells. J. Am. Soc. Hypertens. 2016 doi: 10.1016/j.jash.2016.08.002. [DOI] [PubMed] [Google Scholar]
- 22.Bai Y.P., Hu C.P., Yuan Q. Role of VPO1, a newly identified heme-containing peroxidase, in ox-LDL induced endothelial cell apoptosis. Free Radic. Biol. Med. 2011;51(8):1492–1500. doi: 10.1016/j.freeradbiomed.2011.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Erqou S., Kip K.E., Mulukutla S.R. Endothelial dysfunction and racial disparities in mortality and adverse cardiovascular disease outcomes. Clin. Cardiol. 2016;39(6):338–344. doi: 10.1002/clc.22534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Forstermann U. Oxidative stress in vascular disease: causes, defense mechanisms and potential therapies. Nat. Clin. Pr. Cardiovasc. Med. 2008;5(6):338–349. doi: 10.1038/ncpcardio1211. [DOI] [PubMed] [Google Scholar]
- 25.Forstermann U., Sessa W.C. Nitric oxide synthases: regulation and function. Eur. Heart J. 2012;33(7):829–837. doi: 10.1093/eurheartj/ehr304. (837a) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Park K.H., Park W.J. Endothelial dysfunction: clinical implications in cardiovascular disease and therapeutic approaches. J. Korean Med. Sci. 2015;30(9):1213–1225. doi: 10.3346/jkms.2015.30.9.1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jeremy J.Y., Yim A.P., Wan S. Oxidative stress, nitric oxide, and vascular disease. J. Card. Surg. 2002;17(4):324–327. doi: 10.1111/j.1540-8191.2001.tb01151.x. [DOI] [PubMed] [Google Scholar]
- 28.Wilcox C.S. Asymmetric dimethylarginine and reactive oxygen species: unwelcome twin visitors to the cardiovascular and kidney disease tables. Hypertension. 2012;59(2):375–381. doi: 10.1161/HYPERTENSIONAHA.111.187310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bermudez V., Bermudez F., Acosta G. Molecular mechanisms of endothelial dysfunction: from nitric oxide synthesis to ADMA inhibition. Am. J. Ther. 2008;15(4):326–333. doi: 10.1097/MJT.0b013e318160beda. [DOI] [PubMed] [Google Scholar]
- 30.Boger R.H. Asymmetric dimethylarginine (ADMA): a novel risk marker in cardiovascular medicine and beyond. Ann. Med. 2006;38(2):126–136. doi: 10.1080/07853890500472151. [DOI] [PubMed] [Google Scholar]
- 31.Sydow K., Schwedhelm E., Arakawa N. ADMA and oxidative stress are responsible for endothelial dysfunction in hyperhomocyst(e)inemia: effects of L-arginine and B vitamins. Cardiovasc Res. 2003;57(1):244–252. doi: 10.1016/s0008-6363(02)00617-x. [DOI] [PubMed] [Google Scholar]
- 32.Alderton W.K., Cooper C.E., Knowles R.G. Nitric oxide synthases: structure, function and inhibition. Biochem. J. 2001;357(Pt 3):593–615. doi: 10.1042/0264-6021:3570593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Antoniades C., Channon K.M., Stefanadis C. Letter by Antoniades et al. regarding article, "Uncoupled cardiac nitric oxide synthase mediates diastolic dysfunction". Circulation. 2010;122(21):e558. doi: 10.1161/CIRCULATIONAHA.110.952861. (e559) [DOI] [PubMed] [Google Scholar]
- 34.Moens A.L., Champion H.C., Claeys M.J. High-dose folic acid pretreatment blunts cardiac dysfunction during ischemia coupled to maintenance of high-energy phosphates and reduces postreperfusion injury. Circulation. 2008;117(14):1810–1819. doi: 10.1161/CIRCULATIONAHA.107.725481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Roe N.D., Thomas D.P., Ren J. Inhibition of NADPH oxidase alleviates experimental diabetes-induced myocardial contractile dysfunction. Diabetes Obes. Metab. 2011;13(5):465–473. doi: 10.1111/j.1463-1326.2011.01369.x. [DOI] [PubMed] [Google Scholar]
- 36.Kolluru G.K., Siamwala J.H., Chatterjee S. eNOS phosphorylation in health and disease. Biochimie. 2010;92(9):1186–1198. doi: 10.1016/j.biochi.2010.03.020. [DOI] [PubMed] [Google Scholar]
- 37.Xu J., Xie Z., Reece R. Uncoupling of endothelial nitric oxidase synthase by hypochlorous acid: role of NAD(P)H oxidase-derived superoxide and peroxynitrite. Arterioscler. Thromb. Vasc. Biol. 2006;26(12):2688–2695. doi: 10.1161/01.ATV.0000249394.94588.82. [DOI] [PubMed] [Google Scholar]
- 38.Pennathur S., Heinecke J.W. Oxidative stress and endothelial dysfunction in vascular disease. Curr. Diab Rep. 2007;7(4):257–264. doi: 10.1007/s11892-007-0041-3. [DOI] [PubMed] [Google Scholar]
- 39.Morales Y., Caceres T., May K. Biochemistry and regulation of the protein arginine methyltransferases (PRMTs) Arch. Biochem. Biophys. 2015;590:138–152. doi: 10.1016/j.abb.2015.11.030. [DOI] [PubMed] [Google Scholar]
- 40.Chen C.A., Wang T.Y., Varadharaj S. S-glutathionylation uncouples eNOS and regulates its cellular and vascular function. Nature. 2010;468(7327):1115–1118. doi: 10.1038/nature09599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Stacey M.M., Cuddihy S.L., Hampton M.B. Protein thiol oxidation and formation of S-glutathionylated cyclophilin A in cells exposed to chloramines and hypochlorous acid. Arch. Biochem. Biophys. 2012;527(1):45–54. doi: 10.1016/j.abb.2012.07.011. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material