Abstract
A series of studies were carried out in Farber disease (OMIM #228000) cells and mice to evaluate the feasibility of enzyme replacement therapy (ERT) for this disorder. Media from Chinese hamster ovary (CHO) cells overexpressing human recombinant acid ceramidase (rhAC) was used to treat fibroblasts from a Farber disease patient, leading to significantly reduced ceramide. We also found that chondrocytes from Farber disease mice had a markedly abnormal chondrogenic phenotype, and this was corrected by rhAC as well. Acute dosing of rhAC in Farber mice confirmed the enzyme's bioactivity in vivo, and showed that it could be safely administered at doses up to 50 mg/kg. These studies also revealed little or no re-accumulation of ceramide in tissues for at least 7 days after enzyme administration. Once weekly administration of rhAC moderately improved survival of the mice, which could be enhanced by starting enzyme administration at an earlier age (3 days vs. 3 weeks). Repeat administration of the enzyme also led to normalization of spleen size, significantly reduced plasma levels of monocyte chemoattractant protein 1 (MCP-1), reduced infiltration of macrophages into liver and spleen, and significantly reduced ceramide and sphingosine in tissues. Overall, we conclude that ERT should be further developed for this debilitating and life-threatening disorder.
Keywords: Enzyme replacement, Ceramide, Arthritis, Mouse model
1. Introduction
Farber disease (OMIM #228000) was first described in 1952 in a 14-month-old infant with granulomatous lesions on multiple joints and evidence of lipid storage [1]. Over the ensuing decade other similar cases were described, all of whom had lesions and often exhibited a characteristic “hoarse” cry or voice due to their presence on the larynx. The involvement of other organ systems in some of these patients, including the lung, liver, spleen and central nervous system (CNS), also was noted [2].
In 1972, Sugita and colleagues [3] demonstrated that post-mortem tissues and cells from several Farber disease patients exhibited acid ceramidase (AC) deficiency. Acid ceramidase (E.C. #3.5.1.23) is a lipid hydrolase first identified in 1963 [4]. The enzyme was shown to have a pH optimum of ~ 5, suggesting that it was a component of the lysosomal system, although at physiologic pH it also could carry out a “reverse” reaction in which ceramides were synthesized using fatty acids and sphingosine as substrates [for review see [5]].
The first substantial purification of AC was in 1995 from human urine [6]. The purified urinary enzyme was found to be an ~ 50 kDa polypeptide that could be reduced into 13 kDa and 40 kDa polypeptides (α and β-subunits, respectively). Deglycosylation studies revealed the presence of 5 or 6 N-glycosylation sites on the β-subunit. The availability of the highly purified urinary enzyme led to the isolation of the cDNA and gene encoding AC (ASAH1) [7], [8], the identification of the first mutations causing Farber disease [7], and the production and purification of recombinant human AC (rhAC) from Chinese hamster ovary (CHO) cells [9].
Characterization of rhAC purified from CHO cell media showed that it was composed of three polypeptides associated by disulfide bonds; a precursor polypeptide and the α and β-subunits. The precursor polypeptide was inactive until an internal cleavage at cysteine residue 142 resulted in the subunit formation. Further studies showed that AC was a self-cleaving enzyme and underwent “auto-activation” [10]. Farber disease cells internalized rhAC, leading to the degradation of the accumulating ceramides. The recombinant enzyme also was able to carry out the reverse reaction, although the physiological significance of this reaction and the factors that govern the balance between the degradative and synthetic functions remain unknown [11].
The availability of the human AC cDNA also led to the isolation of the murine gene (asah1) and the construction of the first AC deficient animal models [12], [13]. These studies revealed that a complete knockout of asah1 led to embryonic death by the 4-cell stage, demonstrating the essential role of this enzyme in early development [14], and leading to the use of rhAC to enhance embryo production by in vitro fertilization [15]. Subsequent studies led to the construction of a tamoxifin-inducible conditional KO mouse, and provided the first in vivo evidence that AC was required for oocyte maturation [16].
In 2013 a “knock-in” mouse was produced that introduced a mutation originally described in a severely affected Farber disease patient (P362R) into the conserved residue (P361) of mouse AC [17]. Mice homozygous for this mutation exhibited growth retardation by 3 weeks, progressive weight loss, and death between 7 and 13 weeks. Spleens were firm, pale and significantly enlarged, as were the thymuses and lymph nodes. Ovaries were small with a low number of follicles. Several blood abnormalities also were documented, and macrophage infiltration into many organs was evident. This was correlated with the elevation of macrophage chemoattractant protein-1 (MCP-1) and several other cytokines in the plasma, as well as the massive accumulation of ceramides in tissues. These animals represent the first viable model to study the pathophysiology and treatment of Farber disease.
In 2012, Zhou and colleagues showed that mutations in the ASAH1 gene also were responsible for a rare form of spinal muscular atrophy with myoclonic epilepsy (SMA-PME) [18]. Cells from patients with SMA-PME have reduced AC activity, and knock-down of the activity in zebrafish resulted in a loss of motor neuron axonal branching in the spinal cord. Patients with SMA-PME do not exhibit joint lesions or nodules as in Farber disease, although as a recent publication illustrates [19], there is likely a continuum of phenotypes due to AC deficiency.
To date, treatment for Farber disease patients has been symptomatic and principally aimed at reducing pain. Hematopoietic stem cell transplantation (HSCT) has been undertaken in several patients [e.g., [20], [21]], and overall the outcome on the non-neurological phenotype has been positive provided that the transplant procedure itself was successful. Such transplanted patients exhibited significant reduction in pain, increased mobility and joint range of motion, and in most cases shrinking and complete resolution of the subcutaneous nodules. However, successful transplantation requires histocompatible donor cells, and exposes patients to invasive and potentially dangerous immunosuppressant regimes. One alternative to HSCT is gene therapy in which autologous donor cells are transduced with a vector expressing the therapeutic protein, obviating the need for histocompatible donors. Gene therapy has been evaluated in the Farber disease knock-in mice, and resulted in reduction of tissue ceramides and macrophage infiltration [17].
Another approach is enzyme replacement therapy (ERT) in which the recombinant protein is introduced into patients by intravenous infusions [for reviews see [22], [23]. ERTs are currently available for 7 lysosomal storage disorders (LSDs), and under evaluation for several others. Overall, the ERTs have proven to be safe and effective, although the recombinant enzymes cannot cross the blood-brain barrier, and therefore have little or no effect in the CNS. Direct (e.g., intrathecal) CNS administration of enzyme has been evaluated to overcome this limitation, and new therapies that utilize fusion proteins containing CNS targeting moieties are under development [e.g., [24]].
The current study was designed to use the Farber disease knock-in mouse model to evaluate the potential of ERT in this disorder. We hypothesize that a ceramide-driven inflammatory response is responsible for the infiltration of lipid-filled macrophages into tissues of Farber mice and patients, particularly at cartilage sites, and that reduction of ceramide by rhAC treatment will reverse and/or prevent this. Although the knock-in Farber mice do not develop visible granuloma-like nodules as in patients, they do exhibit massive ceramide storage and infiltration of macrophages in most tissues, as well as elevations of plasma MCP-1 and other cytokines, making them a suitable model in which to evaluate therapy.
2. Materials and methods
2.1. Production & characterization of rhAC
A human AC cDNA (derived from NM_177924.3; ASAH1 variant 1) was introduced into the Selexis SURE CHO-M cell line™ (Selexis SA, Switzerland), and clones overexpressing AC activity were selected. One overexpressing clone (MST-cp07-cp47) was further expanded and grown in a bioreactor system (GE Healthcare Life Sciences Inc.). After filtration, rhAC was purified from the media by sequential ion-exchange and size-fractionation chromatography. Prior to its use in animals, the in vitro physical and biochemical characteristics (e.g., pH optimum, isoelectric point, molecular weight, subunit association) were compared to the previously described CHO-derived rhAC [9] by established methods.
2.2. Farber mouse colony
A colony of asah1P361R/P361R mutant mice (i.e., Farber disease mice) were maintained on a mixed genetic background (W4/129Sv/CD1) by breeding heterozygous mating pairs as previously described [17]. Genotyping was carried out by analysis of toe clip DNA at weaning (3 weeks) unless otherwise noted. All experiments were performed at the Icahn School of Medicine under a protocol (#98-0089) approved by the Institutional Animal Care and Use Committee. Wild-type mice were used as controls and derived from within the colony. Animals were fed ad libitum food and water, and euthanasia was performed by ketamine/xylazine injections followed by cervical dislocation according to NIH guidelines.
2.3. Cell culture analysis
Dr. Thierry Levade (Toulouse, France) kindly provided a previously characterized [25] EBV-transformed fibroblast cell line from a Farber disease patient. Cells were grown in RPMI culture media (Sigma-Aldrich) containing 10% heat inactivated fetal bovine serum (FBS) (v/v), 1% penicillin/streptomycin (v/v) and 1% l-glutamine (v/v). To evaluate rhAC secreted into the MST-cp07-cp47 media, conditioned media (CM) was collected from the CHO cell clone grown in shaker flasks for 48 h. The transformed Farber disease fibroblasts were grown to ~ 80% confluency and the standard RPMI medium was exchanged for media containing RPMI diluted with CM such that the final concentration of rhAC was 10 μg/ml. Cells were then grown for an additional 24 h, after which they were trypsinized and harvested with a rubber policeman. The cell pellets were washed 3 × with PBS, and lipid assays were performed on cell lysates as described below.
Chondrocytes were isolated from the articular surfaces of mouse menisci. The tissue isolates were collected in fresh DMEM (Thermo Fisher) containing 10% FBS (v/v), 1% penicillin/streptomycin (v/v), 1% l-glutamine (v/v) and 0.1% fungizone (v/v), minced with scissors, and then transferred to DMEM containing 1 mg/ml protease and rotated at 37 °C for 2 h. They were then incubated in DMEM containing 1 mg/ml collagenase type II and rotated at 37 °C overnight. The remaining tissue was strained three times through 40 μm nylon mesh filters to remove debris. The cell suspension was centrifuged (3000 × g for 5 min) and cells were plated at a density of 10,000 cells/cm2 and cultured in complete DMEM. Media was changed every 3 days. For rhAC supplementation experiments, cells were grown with (12.5 μg/ml) or without rhAC in the medium. rhAC was only added on day 1, when the cells were first plated. Subsequent media changes did not include rhAC.
2.4. rhAC preparation & enzyme administration
Purified rhAC obtained from the media of the MST-cp07-cp47 CHO cell clone was maintained at a concentration of 10 mg/ml in sterile PBS and stored at − 20 °C. It was subjected to only one thaw cycle prior to use. Enzyme administration into Farber mice was by intraperitoneal (i.p.) injection unless otherwise noted. The amount of enzyme administered to mice was based on the desired dose and the weight of the animals (in μg/g). If necessary, the enzyme was diluted in sterile PBS prior to administration. Control Farber disease mice were injected with PBS alone.
2.5. Quantification of total tissue ceramides & sphingosine
Lipid extracts were prepared from tissue homogenates or cell lysates by the classic Folch method [26] using chloroform/methanol (2:1). The lipid extract was then dried under nitrogen gas and re-dissolved in a 2% Igepal solution. For ceramide determination, a ceramide hydrolysis buffer (0.2 M citric/phosphate buffer, pH 4.5 containing 0.3 M NaCl and 0.2 mg/ml of rhAC) was mixed with the total lipid extract solution (1:1, v/v) and incubated at 37 °C for 60 min. For our standard reaction, 2 μl each of lipid extract and ceramide hydrolysis buffer was used. This mixture was then incubated for an additional 10 min at 50 °C with 56 μl of a fluorogenic reaction buffer (25 mM sodium borate buffer, pH 9) containing 1.25 mM sodium cyanide and 1.25 mM naphthalene-2,3-dicarboxyaldehyde (NDA) to derivatize the ceramide hydrolysis product, sphingosine.
The mixture was then centrifuged (13,000 × g/10 min) and 5 μl of the supernatant was analyzed using an Acquity H-Class UPLC system (Waters) equipped with a Waters Acquity UPLC BEH RP18 column (2.0 × 50 mm, 1.7 μm). The mobile phase composition for the gradient system was 0.1% ammonium hydroxide for mobile phase A, and 100% acetonitrile for mobile phase B. The gradient program was 0–0.01 min 36-4% A, 64–96% B, 0.01–0.3 min 4–36% A, 96–64% B, 0.3–1 min 36% A, 64% B at a flow rate of 1 ml/min. The fluorescent (NDA) sphingosine was monitored at excitation and emission wavelengths of 252 and 483 nm, respectively. Quantification of the sphingosine peak was calculated using the Waters Empower software according to a standard curve derived from commercial (Invitrogen) NDA sphingosine.
For quantification of sphingosine the same procedure was used except that the ceramide hydrolysis step was excluded. In this way endogenous sphingosine present in the lipid extract could be derivatized directly with NDA.
2.6. AC activity assay
Samples (tissue homogenates or cell lysates) were incubated at 37 °C (1:1, v/v) with substrate buffer (0.2 mM NBD-C12 ceramide, 0.2 M citrate/phosphate buffer, pH 4.5, 0.3 M NaCl, 10% FBS, and 0.2% Igepal) for 30 min. NBD-C12 ceramide was purchased from Cayman Chemical. The reaction was stopped by ethanol (10 ×) and centrifuged (13,000 × g/10 min), and the supernatant (5 μl) was analyzed using the Acquity H-Class UPLC system (Waters). Separation of the undegraded NBD-C12 ceramide substrate and NBD-C12 fatty acid reaction product was achieved using a Waters Acquity UPLC BEH C18 column (2.0 × 30 mm, 1.7 μm). The mobile phase composition for the gradient system was 13 mM ammonium acetate buffer (pH 7.2) for mobile phase A and 100% acetonitrile for mobile phase B. The gradient program was 0–0.1 min 68–0% A, 32–100% B, 0.1–0.4 min 0–68% A, 100–32% B, 0.4–0.8 min 68% A, 32% B at a flow rate of 1.2 ml/min. The fluorescent product (NBD-C12 fatty acid) was monitored at excitation and emission wavelengths of 435 nm and 525 nm, respectively. Quantification of the product peak was calculated using the Waters Empower software according to a standard curve derived from commercial NBD-C12 fatty acid (Avanti).
2.7. MCP-1 Elisa
Immediately following euthanasia, blood was collected from mice by heart puncture and plasma was frozen at − 20 °C. MCP-1 levels were determined by ELISA using a commercial kit (#MJE00, R & D Systems) according to a protocol supplied by the manufacturer.
2.8. RT-qPCR analysis
After 7 or 14 days of expansion, chondrocytes with and without rhAC supplementation were harvested from the culture flasks (~ 1 × 106 cells/pellet). RNA was extracted using the qiaShredder and RNeasy Mini Kit (Qiagen, Limburg, Netherlands) and quantified using the Nanodrop 1000 (Thermo Scientific, Walthman, MA). Complementary DNA was synthesized using the same amount of RNA from each group using the high capacity cDNA reverse transcription kit (Life Technologies, Grand Island, NY) and a Bio—Rad S1000 thermal cycler (Bio—Rad, Hercules, CA). RT-qPCR was completed using the fast universal PCR Master Mix and primers (Life Technologies, Grand Island, NY) specific for collagen II (Rn01637087_m1), aggrecan (Rn00573424_m1), Sox-9 (Rn01751069_mH), and GAPDH (Rn01775763_g1 as a housekeeping gene), and run on a 7900HT qPCR machine (Life Technologies, Grand Island, NY). The ΔΔct method was used to analyze the data and the results were presented as relative quantity (RQ) fold increase.
2.9. Histopathology
Following euthanasia, the tissues were harvested from mice and fixed in 10% formalin for 24 h, and then stored in ethanol until ready for analysis. For H&E staining they were paraffin embedded and sectioned (5 μl) with a microtome.
2.10. Statistics
Comparisons between two groups were performed with a Student's t-test. In cases where more than two groups were compared to each other, a one-way analysis of variance (ANOVA) was used, followed by a Tukey's HSD test.
3. Results
3.1. In vitro assessment of rhAC
Fig. 1.
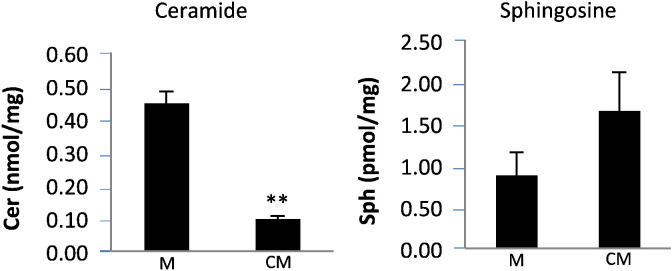
Effect of rhAC on ceramide and sphingosine levels in Farber disease cells. Serum-free conditioned media (CM) was obtained from a CHO cell line overexpressing rhAC and added to an SV40-transformed skin fibroblast line derived from a Farber disease patient (see Materials and Methods). Standard RPMI media (M) lacking rhAC was used as a control. After media exchange, the Farber cells were grown for 24 h, harvested, washed 3 × with PBS, and the amounts of total ceramide (Cer) and sphingosine (Sph) were quantified and expressed per milligram (mg) total cell protein. **indicates p value < 0.01 comparing cells grown with CM to cells grown in M.
3.2. Acute dosing of Farber disease mice with rhAC
Initial studies in the Farber mice evaluated single administration of purified rhAC into ~ 9-week-old animals at 4 different doses (0.1, 1, 10 and 50 mg/kg). At this age, affected mice exhibit massive ceramide storage in tissues [17]. Intraperitoneal (i.p.) injections were used rather than tail vein due to the extremely small size of the mice and relatively large volume (~ 75 μl) required for the high dose (50 mg/kg) injection.
A similar pharmacokinetic pattern was observed in spleens, except that the peak AC activity was slightly delayed compared to the liver (peak AC activity at 24 vs. 12 h). We also evaluated the plasma half-life of AC activity in Farber disease mice following single tail vein injection, and found that the T1/2 was ~ 36 min (Supplemental Fig. 2).
Based on the above dosing studies, we concluded that rhAC was bioactive in vivo (reduced ceramide and produced sphingosine), and that single i.p. injections into Farber disease mice could be well tolerated at doses up to 50 mg/kg. We also hypothesized that repeat dosing of at least once per week should be sufficient to maintain low ceramides and achieve therapeutic effects due to the relatively slow rate of re-accumulation after enzyme administration.
3.3. Repeat dosing of Farber disease mice with rhAC
Next, groups of Farber disease mice were injected i.p. with three doses of rhAC (1, 3 and 10 mg/kg), once per week beginning at ~ 3 weeks of age. Mice were maintained on enzyme treatment until they required euthanasia according to IACUC protocol (loss of > 10% body weight within 1 week). Thus, the primary endpoint for this study was survival.
Fig. 5.
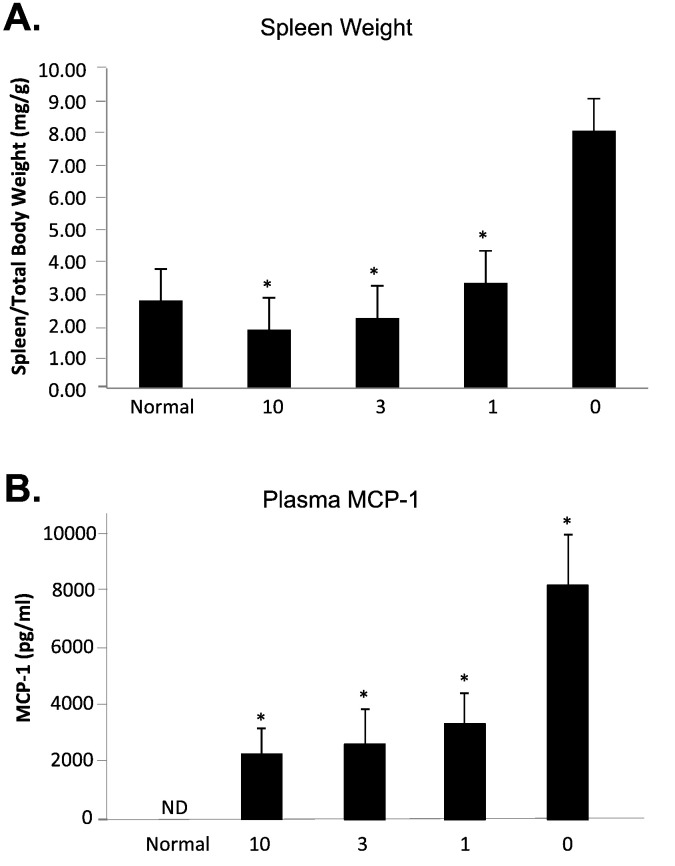
Spleen weight and plasma MCP-1 levels in Farber disease mice receiving repeat injections of rhAC. Approximately 3-week-old Farber disease mice received once weekly i.p. injections of purified rhAC at doses of 1 mg/kg, 3 mg/kg and 10 mg/kg (n = 4-9/dose). Mice were euthanized when they lost > 10% body weight within a one week period. Spleen weight (A) was expressed per total body weight. MCP-1 (B) was measured in plasma using ELISA kits (see Materials and Methods). “0” indicates Farber mice receiving once weekly injections of PBS. The spleen weight and MCP-1 levels in age-matched, wild-type mice (normal) also is shown. *indicates p value < 0.05 comparing mice treated with rhAC to mice treated with PBS.
Fig. 7.
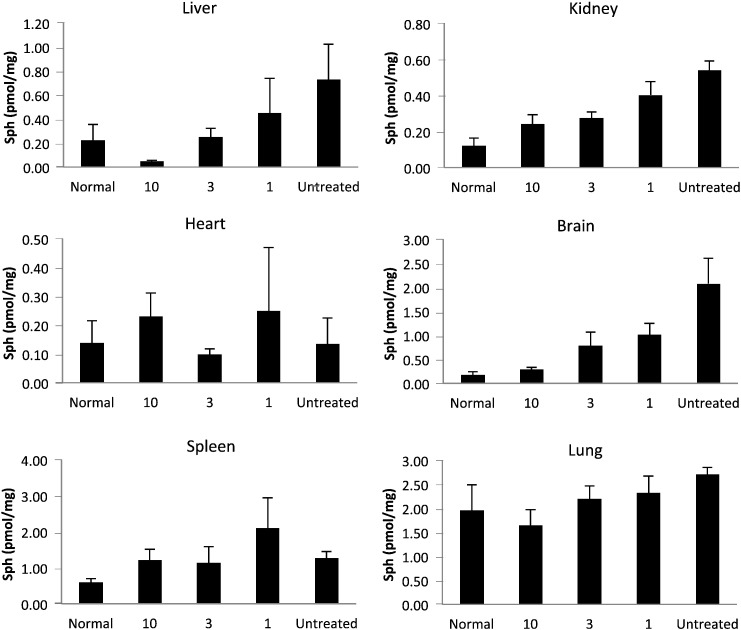
Sphingosine in tissues of Farber disease mice receiving repeat injections of rhAC. Approximately 3-week-old Farber disease mice received once weekly i.p. injections of purified rhAC at doses of 1 mg/kg, 3 mg/kg and 10 mg/kg (n = 4–10/dose). Mice were euthanized when they lost > 10% body weight within a one week period. Total sphingosine (Sph) levels were determined in tissue extracts as described in the Materials and Methods. “0” indicates Farber disease mice receiving once weekly injections of PBS. *indicates p value < 0.05 comparing mice treated with rhAC to mice treated with PBS. **indicates p value < 0.01.
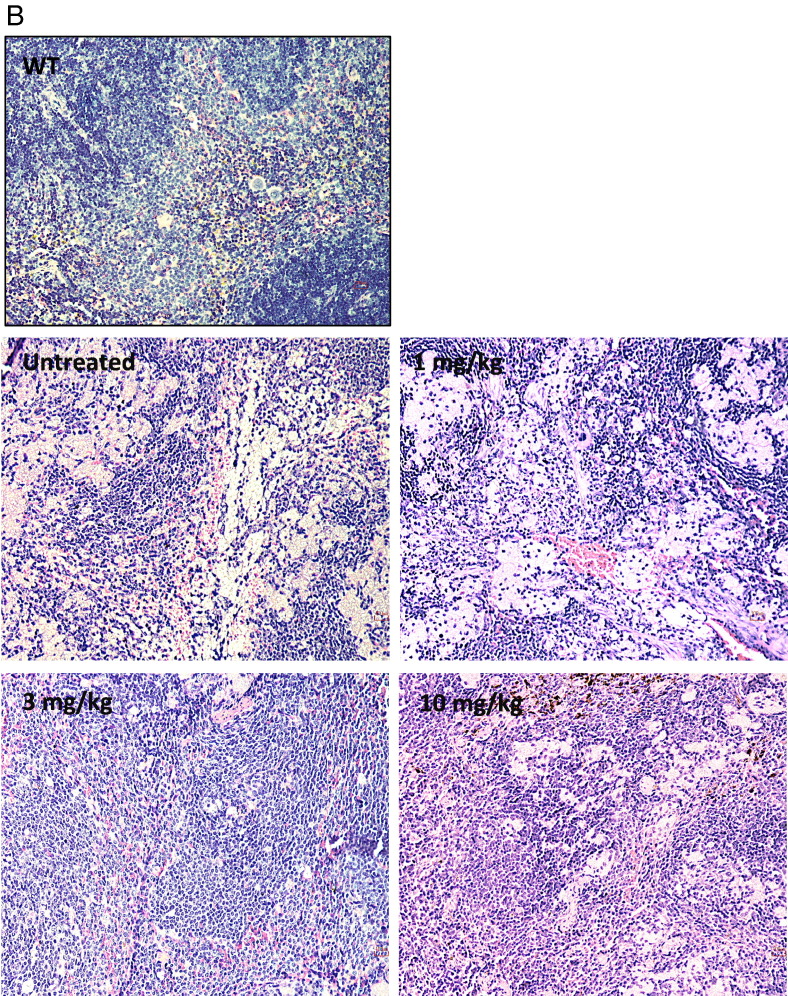
Fig. 8.
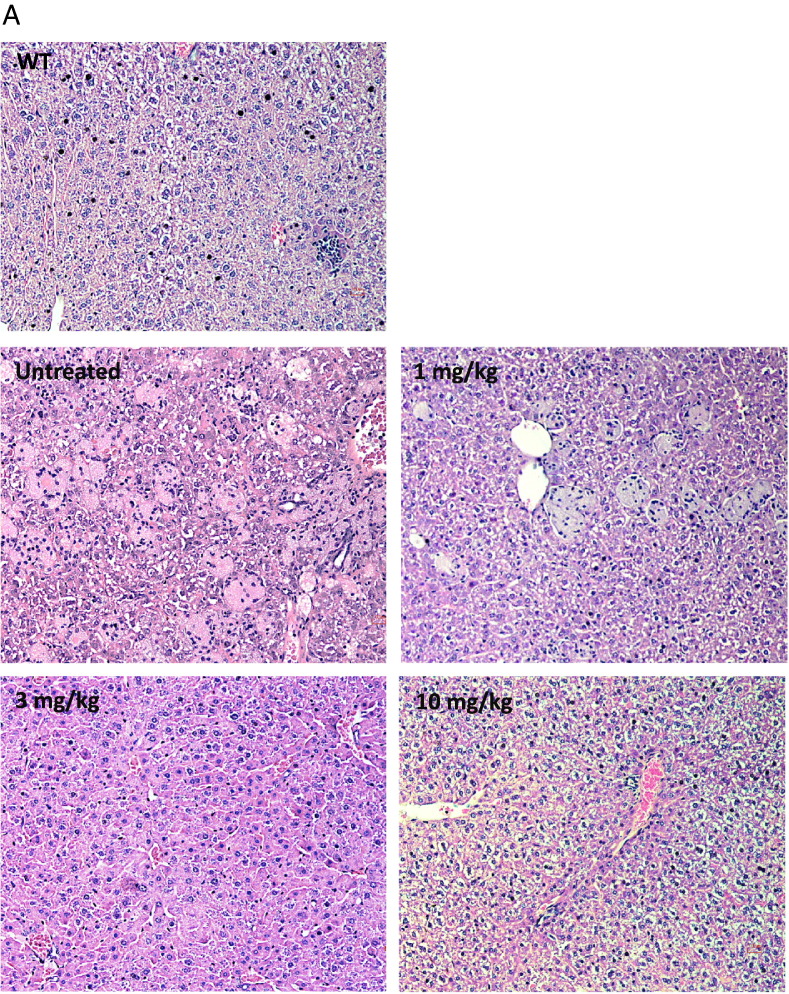
Histology in liver and spleen sections from Farber disease mice receiving repeat injections of rhAC. Approximately 3-week-old Farber disease mice received once weekly i.p. injections of purified rhAC at doses of 1 mg/kg, 3 mg/kg and 10 mg/kg (n = 4–10/dose). Mice were euthanized when they lost > 10% body weight within a one week period. Livers (A) and spleens (B) were fixed in formalin, sectioned and subjected to H&E staining. “0” indicates Farber disease mice receiving once weekly injections of PBS. WT indicates age-matched wild-type mice. Representative sections are shown from mice in each dose group. Magnification = 20 ×.
Overall, the above findings demonstrated that ERT in Farber disease mice had a significant impact on several important endpoints, including tissue ceramides and sphingosine, macrophage infiltration, spleen size, and plasma MCP-1 levels. Surprisingly, there also was some evidence that rhAC might influence the brain (e.g., reduction of accumulating sphingosine).
To further explore these findings, we next carried out a study in which Farber disease mice were treated with rhAC at a dose of 10 mg/kg (once weekly, i.p.), starting at ~ 3 days of age, immediately after genotyping. The rationale underlying this study was that early treatment might provide additional benefit in the brain, as in other mouse LSD studies [28].
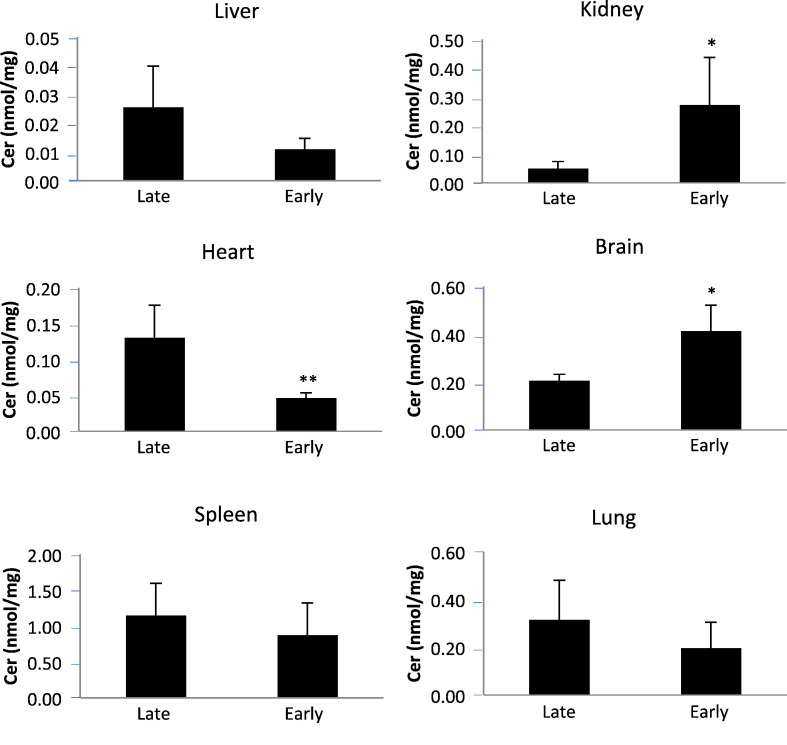
Fig. 9.
Ceramide levels in tissues of Farber disease mice receiving repeat injections of rhAC starting at two different ages. Farber disease mice received once weekly i.p. injections of purified rhAC (10 mg/kg) starting at 3 days (early) or 3 weeks (late) of age (n = 7–11/age). Mice were euthanized when they lost > 10% body weight within a one week period. Total ceramide (Cer) levels were determined in tissue extracts as described in the Materials and Methods. *indicates p value < 0.05 comparing mice treated early to late. **indicates p value < 0.01.
3.4. In vitro assessment of rhAC treatment on Farber disease mouse chondrocytes
Fig. 10.
Effect of rhAC on chondrocytes from Farber disease mice. Primary chondrocytes were obtained from the humeri of 4-6 week-old Farber disease mice. Cells from 5 to 10 mice were pooled to establish cultures, and grown in standard media (−) or media containing purified rhAC (12.5 μg/ml) for 7 days or 14 days. rhAC was added once at the time of cell plating. qPCR was then used to assess the expression of three chondrogenic marker genes: collagen 2, aggrecan and Sox-9. The dotted lines indicate the expression of these genes in chondrocytes grown from healthy mice in standard media. The results of two separate experiments (Exp 1 and 2) are shown.
4. Discussion
The goal of these experiments was to use the mouse model of Farber disease to establish proof-of-concept for future ERT studies in Farber disease patients. The Farber disease mouse is a knock-in of a severe human Farber disease mutation (P362R), and correspondingly the mice exhibit a severe disease that results in death between ~ 7–13 weeks of age [17]. Affected mice are exceptionally small and exhibit failure to thrive and a rapid decline beginning at ~ 4–5 weeks of age. As in patients, they accumulate ceramides in most tissues and exhibit macrophage infiltration. They also have high levels of several inflammatory biomarkers, of which MCP-1 is the most significant and consistent. Unlike patients, Farber disease mice do not develop visible nodules at cartilage sites, although there is histological evidence of macrophage infiltration in cartilage and synovial tissues. The fact that they do not develop the lipogranulomatous nodules characteristic of human disease might be due to their markedly shortened lifespan.
The rhAC used for these studies was purified from the media of overexpressing CHO cells. The cell line expressed the same cDNA as in our previous work [9], except that it was prepared and maintained under “good manufacturing practice” (GMP) conditions. In addition, the purification protocol was modified from our previous work to accommodate scale-up and eventual clinical use. As shown in supplemental Fig. 1, this rhAC exhibited the same molecular weight and subunit association as our previous rhAC. To confirm the bioactivity of this enzyme, conditioned media from the overexpressing CHO cells was used to grow SV40-transformed Farber disease skin fibroblasts. This co-mixing experiment resulted in significant ceramide reduction and sphingosine elevation compared to cells grown in standard tissue culture media.
Acute injection studies using this rhAC in the Farber disease mice further revealed the bioactivity of this enzyme, resulting in a reduction of tissue ceramides and production of sphingosine. Several important findings were observed from these acute dosing studies: a) doses as low as 0.1 mg/kg had biological effect, b) doses up to 50 mg/kg were well tolerated and exhibited no toxic effect, c) there was no advantage of the 50 mg/kg dose compared to 10 mg/kg, d) sphingosine elevation exhibited a more linear and dose responsive pattern than ceramide reduction, and e) even in 9-week-old animals with considerable disease, reversal of ceramide storage was evident. It was also notable that after a single injection of rhAC ceramide levels remained reduced for up to 1 week, indicating a relatively slow re-accumulation rate.
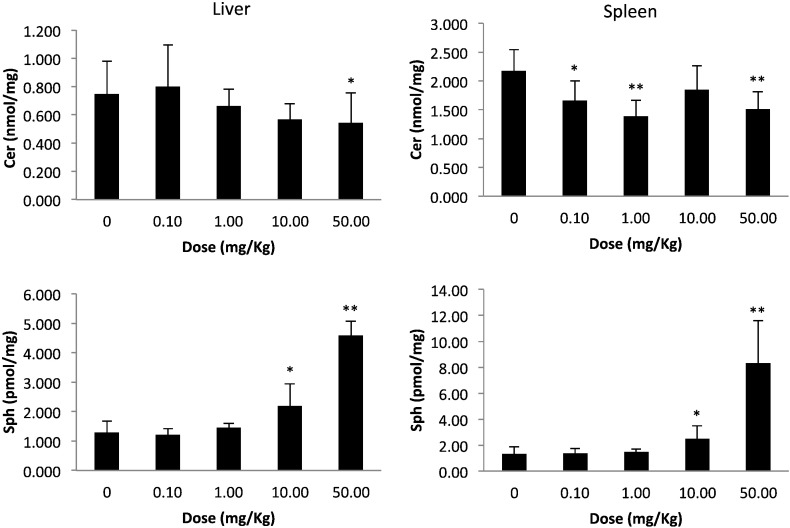
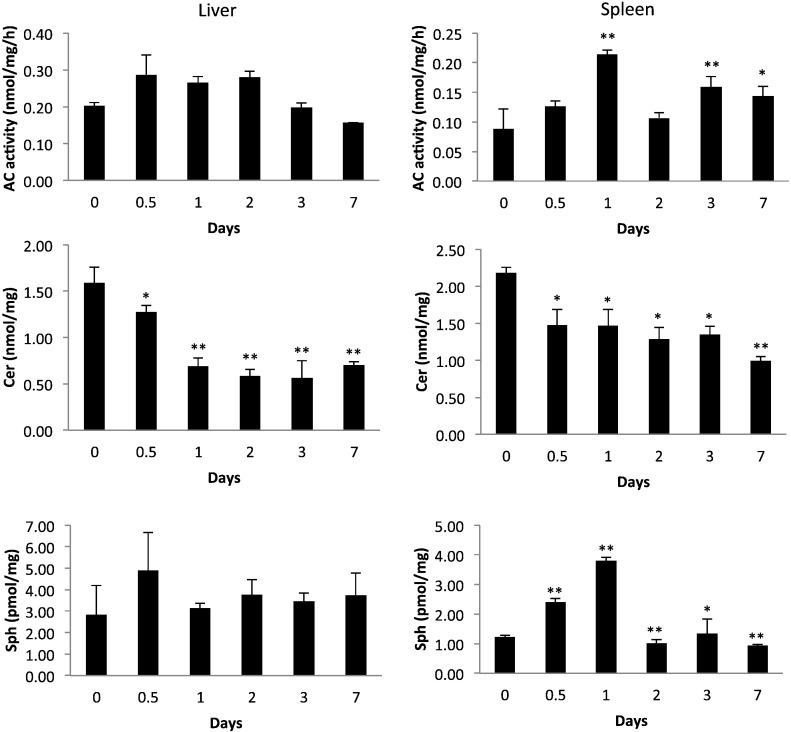
Several interesting points arise from these observations. At the outset we predicted that we might observe some toxicity to high dose rhAC administration in Farber disease mice, in part due to the production of sphingosine, a highly toxic and bioactive sphingolipid, particularly in the liver where the majority of the rhAC is delivered. Such a high dose toxicity has been observed in acid sphingomyelinase deficient (Niemann-Pick disease) mice, and was attributed to the production of ceramide, which is subsequently converted to sphingosine [29]. We did not observe this in the Farber disease mice, perhaps due to the relatively low levels of sphingosine produced and the transient nature of the elevation. In fact, although nanomole levels of sphingosine should have been produced in tissues from ceramide hydrolysis by rhAC, only picomole levels were detected (see Fig. 2, Fig. 3). This indicated that the majority of the sphingosine produced by rhAC was either rapidly degraded or re-metabolized. In the future it will be of interest to examine the baseline levels of enzymes involved in sphingosine metabolism in Farber disease mice and patients, including sphingosine kinases and sphingosine lysase, to see if overexpression of these enzymes could be responsible for this observation. The current data also warrant a more detailed analysis to identify additional sphingolipid metabolites that might be produced by rhAC treatment and could represent pharmacodynamics markers for future clinical trials. Also, the fact that acute dosing studies in the Farber disease mice revealed a relatively slow re-accumulation of ceramide after enzyme administration suggests that this ERT can likely be administered to Farber disease patients in the clinic every other week, or perhaps less frequently. It is also of interest that following rhAC administration we observed expression of AC activity in the liver for up to 48 h (Fig. 3), which could provide a sustained pool of enzyme for delivery to other tissues.
Fig. 2.
Ceramide and sphingosine levels in the livers and spleens of Farber disease mice after a single injection of rhAC. Groups of ~ 9-week-old Farber disease mice received single i.p. injections of purified rhAC at the indicated doses (n = 3/dose). After 24 h the mice were euthanized and the amounts of total ceramide (Cer) and sphingosine (Sph) were quantified and expressed per milligram total protein in tissue extracts. “0” indicates age-matched Farber mice that were injected with PBS. *indicates p value < 0.05 comparing mice injected with rhAC to mice injected with PBS; **indicates p value < 0.01.
Fig. 3.
Time-course of ceramide, sphingosine and AC activity in the livers and spleens of Farber disease mice after a single injection of rhAC. Approximately 9-week-old Farber disease mice received single i.p. injections of purified rhAC at a dose of 10 mg/kg. At the indicated times post-injection, mice were euthanized (n = 3 per time point), and the amounts of total ceramide (Cer), sphingosine (Sph), and AC activity were quantified and expressed per milligram protein in tissue extracts. FD indicates untreated, age-matched Farber disease mice injected with PBS. *indicates p value < 0.05 comparing mice injected with rhAC to mice injected with PBS. **indicates p value < 0.01.
We next evaluated repeat administration of the enzyme in the Farber disease mice. Treatment was started at ~ 3 weeks (immediately after weaning) since even at this young age affected mice exhibit pathology. We considered this design clinically relevant since Farber disease patients commonly go through a period of delayed diagnosis, and are therefore likely to exhibit established disease before treatment is initiated. Due to their very small size and difficulty injecting the tail vein, we chose to use i.p. injections for more consistent dosing. However, in the clinic it is likely that rhAC will be administered by i.v. infusion. Work in other LSD animal models has shown that following i.p. injections in mice, recombinant enzymes exhibit a delayed but sustained release into the blood, after which they follow a similar pharmacology to tail vein injections [30].
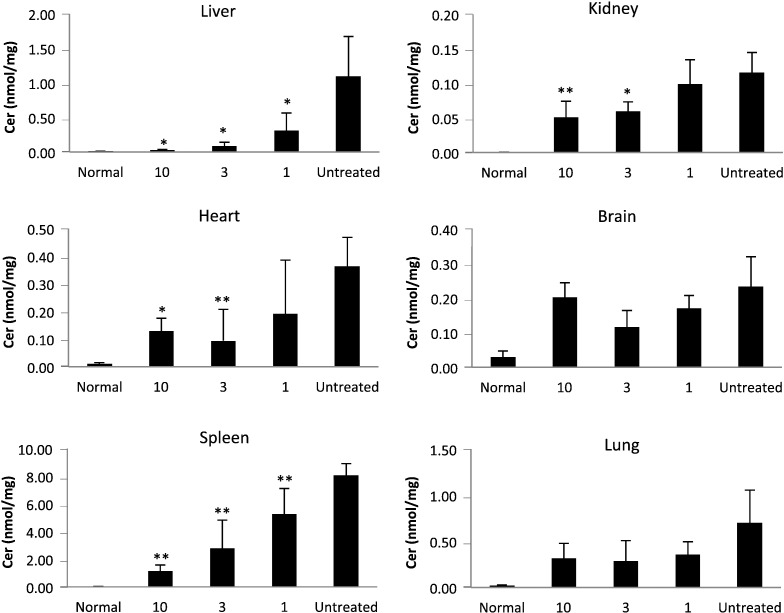
The liver is the major site of enzyme uptake for both i.v. and i.p. injections. Correspondingly, in our studies liver exhibited the most consistent response to rhAC administration. Liver ceramides, which are massively elevated in the Farber disease mice, were reduced to normal (Fig. 6). The lowest dose evaluated (1 mg/kg) exhibited a > 80% reduction in liver ceramides. Spleen, heart, kidney and lung each exhibited significant ceramide reductions as well. Surprisingly, even brain exhibited some ceramide reduction at the 1 mg/kg and 3 mg/kg doses.
Fig. 6.
Ceramide in tissues of Farber disease mice receiving repeat injections of rhAC. Approximately 3-week-old Farber disease mice received once weekly i.p. injections of purified rhAC at doses of 1 mg/kg, 3 mg/kg and 10 mg/kg (n = 4–10/dose). Mice were euthanized when they lost > 10% body weight within a one week period. Total ceramide (Cer) levels were determined in tissue extracts as described in the Materials and Methods. “0” indicates Farber disease mice receiving once weekly injections of PBS. *indicates p value < 0.05 comparing mice treated with rhAC to mice treated with PBS. **indicates p value < 0.01.
It is also notable that three tissues in the Farber disease mice (brain, liver and kidney) exhibited significant sphingosine storage, and that rhAC treatment resulted in depletion from each of these tissues. The origin of sphingosine storage in Farber disease remains unknown, but could be due to progressive degradation of accumulating ceramides that distribute from lysosomes to other cellular compartments where they are exposed to other ceramidases.
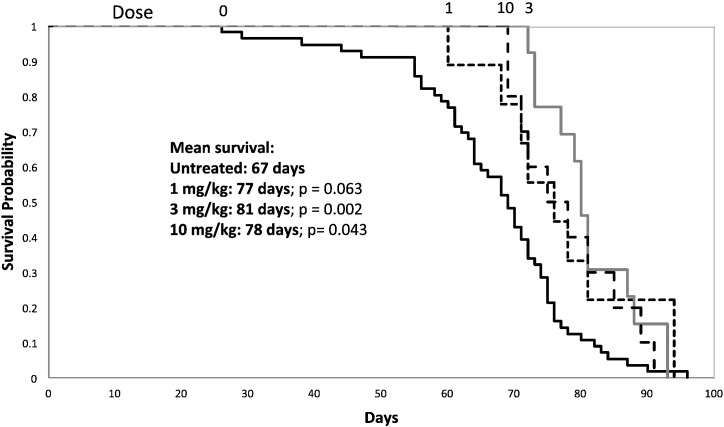
It is important to recognize that the primary endpoint in these repeat administration experiments was survival, and that we therefore treated the Farber disease mice until they exhibited a weight loss that required euthanasia according to our IACUC protocol. Thus, not all animals received the same number of injections, even if they received the same dose. This could contribute to variability of the data within and between dose groups. Overall, we did observe a modest survival benefit to rhAC administration (on average ~ 10-days), although there was no clear dose response (Fig. 4).
Fig. 4.
Survival of Farber disease mice receiving repeat injections of rhAC. Approximately 3-week-old Farber disease mice received once weekly i.p. injections of purified rhAC at doses of 1 mg/kg, 3 mg/kg and 10 mg/kg (n = 9-56/dose). Mice were euthanized when they lost > 10% body weight within a one week period. Kaplan-Meir plots were used to indicate survival probability. “0” indicates Farber disease mice receiving once weekly injections of PBS.
Of interest, when we started rhAC administration (10 mg/kg) at an earlier age (~ 3 days vs. 3 weeks), the survival benefit was significantly extended (supplemental Fig. 4). Studies in other LSD mouse models have shown that such early enzyme administration may facilitate delivery to the brain due to the delayed closing of the blood-brain barrier [28], and we hypothesize that this might be the case here. Although the cause of death has not been established in the Farber disease mice, there is substantial brain pathology in this model that could be contributing to the early death [31]. However, ceramide was not substantially reduced in the brain by early treatment, and in fact was even elevated (as in the kidney). The mechanism underlying this finding remains unknown, but it did not seem to reduce the survival benefit resulting from early rhAC therapy.
In the future these dose-responsive lipid changes should be further evaluated. For example, the question of why some tissues demonstrated higher ceramide levels at the higher doses or when enzyme administration was started at a very early age requires further attention, as does its relationship to the enhanced survival. That said, the data presented here clearly show elevation of tissue ceramides and sphingosine in the Farber disease mice, and that repeat administration of rhAC led to significant reductions of these lipids in most tissues.
We also followed spleen weight in the treated mice, and noted a very significant reduction in response to rhAC administration. Even at the 1 mg/kg dose the spleen size was reduced to near normal. We similarly determined the levels of MCP-1 in the plasma of the treated mice. MCP-1 is a macrophage chemokine that is significantly elevated in the blood of Farber disease mice and patients [17], [27], and we observed a significant reduction in the mice receiving rhAC administration. In animals receiving 1 mg/kg rhAC, MCP-1 was reduced ~ 50%, although no clear dose response was observed in the 3 and 10 mg/kg groups and the levels never reached those of normal animals. Consistent with the reduction in MCP-1, we also observed reduced macrophage infiltration into Farber disease mouse liver and spleen following rhAC administration.
Thus, from the repeat administration studies we conclude that rhAC a) can be safely administered to Farber disease mice, b) is bioactive and results in significant ceramide and sphingosine changes in tissues, c) results in a small survival advantage in a model of severe Farber disease that can be improved by early administration, d) normalizes spleen size, e) significantly reduces plasma MCP-1, and f) reduces macrophage infiltration into tissues.
Lastly, although this Farber disease mouse model does not develop visible nodules at cartilage sites, we did isolate primary chondrocytes and show for the first time that there was very low expression of several genes essential for chondrodifferentiation (aggrecan, collagen 2, sox-9). Addition of rhAC to the culture media could correct this phenotype, further indicating the bioactivity of the enzyme and the fact that it can be taken up by this important cell type. We and others have shown that there is an important and unique relationship between sphingolipids and cartilage integrity [32], [33], and in the future it will be interesting to examine this further in the context of Farber disease.
Overall, we conclude that these proof-of-concept studies support the further development of ERT for this disorder. rhAC administration resulted in significant correction of the tissue lipid profiles and also significantly reduced at least one important inflammatory cytokine (MCP-1), as well as the infiltration of inflammatory cells into tissues. Spleen weight also was normalized and there was a modest effect on lifespan. Additional studies are required to further delineate the dose responsive changes in this model, particularly the changes in individual ceramides and changes in additional plasma biomarkers. Although the potential effect of ERT on the lipogranulomatous nodules in skin and cartilage of Farber disease patients could not be evaluated in this model, given the clear effect of the enzyme on reducing the inflammatory profile and macrophage infiltration in other tissues it is expected that they will be reduced significantly in patients receiving ERT since they are primarily composed of such macrophages. This supposition is further supported by the resolution of the macrophage-filled subcutaneous and laryngeal nodules in Farber disease patients after HSCT [21].
Conflict of interest statement
E.H.S. is a consultant for Roivant Sciences Inc., a company developing ERT for Farber disease and other indications, and owns equity in Enzyvant Sciences Ltd., a wholly owned subsidiary of Roivant. He is also a co-founder, equity holder, and Chief Scientific Officer of Plexcera Therapeutics, who provided funding for this work, and an inventor on patents licensed by the Icahn School of Medicine to Roivant Sciences and Plexcera Therapeutics. J.A.M. and the University Health Network, Toronto, have licensed the Farber disease mice to Roivant Sciences for the development of ERT. A.S. is an employee of Enzyvant Sciences Ltd.
Transparency document
Transparency document
Acknowledgements and grant funding
This research was supported by grants from the National Institutes of Health (R01DK54801) and Plexcera Therapeutics. The authors wish to thank Professor Thierry Levade (Cancer Research Institute of Toulouse, France) for providing the transformed Farber disease cell line.
Footnotes
The Transparency document associated with this article can be found, in online version.
Supplementary data to this article can be found online at http://dx.doi.org/10.1016/j.bbacli.2017.02.001.
Appendix A. Supplementary data
Supplemental Fig. 1. Representative SDS-PAGE analysis of rhAC. rhAC was purified from the media of an overexpressing CHO cell line and subjected to SDS-PAGE under non-reducing (NR) and reducing (R) conditions. Staining was with SimpleBlue®. Molecular weights (in kDa) according to the migration of commercially-supplied molecular weight standards are shown to the left. Bands corresponding to rhAC precursor, alpha, and beta subunits also are indicated. Supplemental Fig. 2. Plasma clearance of rhAC activity after tail vein injection. rhAC (10 mg/kg) was injected into the tail vein of 4–6 week old Farber disease mice. At various times post-injection mice were euthanized (n = 3/time point) and AC activity in plasma was determined. Supplemental Fig. 3. Weight of Farber disease mice receiving repeat injections of rhAC. Approximately 3-week-old Farber disease mice received once weekly i.p. injections of purified rhAC at doses of 1, 3 and 10 mg/kg (n = 9–56/dose). Mice were euthanized when they lost > 10% body weight within a one week period. “0” indicates Farber disease mice receiving once weekly injections of PBS. The average weight of surviving mice at the indicated ages is graphed. Supplemental Fig. 4. Survival of Farber disease mice receiving repeat injections of rhAC starting at two different ages. Farber disease mice received once weekly i.p. injections of purified rhAC (10 mg/kg) starting at 3 days (early) or 3 weeks (late) of age (n = 7–10/age). Mice were euthanized when they lost > 10% body weight within a one week period. Kaplan-Meir plots were used to indicate survival probability. “0” indicates Farber disease mice receiving once weekly injection of PBS. Supplemental Fig. 5. Sphingosine levels in tissues of Farber disease mice receiving repeat injections of rhAC starting at two different ages. Farber disease mice received once weekly i.p. injections of purified rhAC (10 mg/kg) starting at 3 days (early) or 3 weeks (late) of age (n = 7–11/age). Mice were euthanized when they lost > 10% body weight within a one week period. Sphingosine (Sph) levels were determined in tissue extracts as described in the Materials and Methods. *indicates p value < 0.05 comparing mice treated early to late. **indicates p value < 0.01.
References
- 1.Farber S. A lipid metabolic disorder – Disseminated “lipogranulomatosis” – A syndrome with similarity to, and important difference from, Niemann-pick and hand-Schuller-Christian disease. Am. J. Dis. Child. 1952;84:499. [PubMed] [Google Scholar]
- 2.Moser H.W., Chen W.W. Metabolic Basis of Inherited Disease. Vol. 40. 1983. Ceramidase Deficiency: Farber's Lipogranulomatosis; pp. 820–830. [Google Scholar]
- 3.Sugita M., Dulaney J.T., Moser H.W. Ceramidase deficiency in Farber's disease (lipogranulomatosis) Science. 1972;178:1100–1102. doi: 10.1126/science.178.4065.1100. [DOI] [PubMed] [Google Scholar]
- 4.Gatt S. Enzymic hydrolysis and synthesis of ceramides. J. Biol. Chem. 1963;238:3131–3133. [PubMed] [Google Scholar]
- 5.Schuchman E.H. Acid ceramidase and the treatment of ceramide diseases. The expanding role of enzyme replacement therapy. Biochim. Biophys. Acta. 2016;1862:1459–1471. doi: 10.1016/j.bbadis.2016.05.001. [DOI] [PubMed] [Google Scholar]
- 6.Bernardo K.R., Hurwitz R., Zenk T., Desnick R.J., Ferlinz K., Schuchman E.H., Sandhoff K. Purification, characterization, and biosynthesis of human acid ceramidase. J. Biol. Chem. 1995;270:11098–11102. doi: 10.1074/jbc.270.19.11098. [DOI] [PubMed] [Google Scholar]
- 7.Koch J., Gartner S., Li C.M., Quintern L.E., Bernardo K., Levran O., Schnabel D., Desnick R.J., Schuchman E.H., Sandhoff K. Molecular cloning and characterization of a full-length complementary DNA encoding human acid ceramidase. Identification of the first molecular lesion causing Farber disease. J. Biol. Chem. 1996;271:33110–33115. doi: 10.1074/jbc.271.51.33110. [DOI] [PubMed] [Google Scholar]
- 8.Li C.M., Park J.H., He X., Levy B., Chen F., Arai K., Adler A.D., Disteche C.M., Koch J., Sandhoff K., Schuchman E.H. The human acid ceramidase gene (asah): Structure, chromosomal location, mutation analysis, and expression. Genomics. 1999;62:223–231. doi: 10.1006/geno.1999.5940. [DOI] [PubMed] [Google Scholar]
- 9.He X., Okino N., Dhami R., Dagan A., Gatt S., Schulze H., Sandhoff K., Schuchman E.H. Purification and characterization of recombinant, human acid ceramidase. Catalytic reactions and interactions with acid sphingomyelinase. J. Biol. Chem. 2003;278:32978–32986. doi: 10.1074/jbc.M301936200. [DOI] [PubMed] [Google Scholar]
- 10.Shtraizent N., Eliyahu E., Park J.H., He X., Shalgi R., Schuchman E.H. Autoproteolytic cleavage and activation of human acid ceramidase. J. Biol. Chem. 2008;283:11253–11259. doi: 10.1074/jbc.M709166200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Okino N., He X., Gatt S., Sandhoff K., Ito M., Schuchman E.H. The reverse activity of human acid ceramidase. J. Biol. Chem. 2003;278:29948–29953. doi: 10.1074/jbc.M303310200. [DOI] [PubMed] [Google Scholar]
- 12.Li C.M., Hong S.B., Kopal G., He X., Linke T., Hou W.S., Koch J., Gatt S., Sandhoff K., Schuchman E.H. Cloning and characterization of the full-length cDNA and genomic sequences encoding murine acid ceramidase. Genomics. 1998;50:267–274. doi: 10.1006/geno.1998.5334. [DOI] [PubMed] [Google Scholar]
- 13.Li C.M., Park J.H., Simonaro C.M., He X., Gordon R.E., Friedman A.H., Ehleiter D., Paris F., Manova K., Hepbildikler S., Fuks Z., Sandhoff K., Kolesnick R., Schuchman E.H. Insertional mutagenesis of the mouse acid ceramidase gene leads to early embryonic lethality in homozygotes and progressive lipid storage disease in heterozygotes. Genomics. 2002;79:218–224. doi: 10.1006/geno.2002.6686. [DOI] [PubMed] [Google Scholar]
- 14.Eliyahu E., Park J.H., Shtraizent N., He X., Schuchman E.H. Acid ceramidase is a novel factor required for early embryo survival. FASEB J. 2007;21:1403–1409. doi: 10.1096/fj.06-7016com. [DOI] [PubMed] [Google Scholar]
- 15.Eliyahu E., Shtraizent N., Martinuzzi K., Barritt J., He X., Wei H., Chaubal S., Copperman A.B., Schuchman E.H. Acid ceramidase improves the quality of oocytes and embryos and the outcome of in vitro fertilization. FASEB J. 2010;24:1229–1238. doi: 10.1096/fj.09-145508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Eliyahu E., Shtraizent N., Shalgi R., Schuchman E.H. Construction of conditional acid ceramidase knockout mice and in vivo effects on oocyte development and fertility. Cell. Physiol. Biochem. 2012;30:735–748. doi: 10.1159/000341453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Alayoubi A.M., Wang J.C., Au B.C., Carpentier S., Garcia V., Dworski S., El-Ghamrasni S., Kirouac K.N., Exertier M.J., Xiong Z.J., Prive G.G., Simonaro C.M., Casas J., Fabrias G., Schuchman E.H., Turner P.V., Hakem R., Levade T., Medin J.A. Systemic ceramide accumulation leads to severe and varied pathological consequences. EMBO Mol. Med. 2013;5:827–842. doi: 10.1002/emmm.201202301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhou J., Tawk M., Tiziano F.D., Veillet J., Bayes M., Nolent F., Garcia V., Servidei S., Bertini E., Castro-Giner F., Renda Y., Carpentier S., Andrieu-Abadie N., Gut I., Levade T., Topaloglu H., Melki J. Spinal muscular atrophy associated with progressive myoclonic epilepsy is caused by mutations in asah1. Am. J. Hum. Genet. 2012;91:5–14. doi: 10.1016/j.ajhg.2012.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Teoh H.L., Solyom A., Schuchman E.H., Mowat D., Roscioli T., Farrar M., Sampaio H. Polyarticular arthritis and spinal muscular atrophy in acid ceramidase deficiency. Pediatrics. 2016;E20161068 doi: 10.1542/peds.2016-1068. (Epub ahead of print) [DOI] [PubMed] [Google Scholar]
- 20.Ehlert K., Frosch M., Fehse N., Zander A., Roth J., Vormoor J. Farber disease: Clinical presentation, pathogenesis and a new approach to treatment. Pediatr. Rheumatol. Online J. 2007;5:15. doi: 10.1186/1546-0096-5-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Torcoletti M., Petaccia A., Pinto R.M., Hladnik U., Locatelli F., Agostoni C., Corona F. Farber disease in infancy resembling juvenile idiopathic arthritis: Identification of two new mutations and a good early response to allogeneic haematopoietic stem cell transplantation. Rheumatology. 2014;53:1533–1544. doi: 10.1093/rheumatology/keu010. [DOI] [PubMed] [Google Scholar]
- 22.Desnick R.J., Schuchman E.H. Enzyme replacement therapy for lysosomal storage diseases: Lessons from 20 years of experience and remaining challenges. Annu. Rev. Genomics Hum. Genet. 2012;13:307–335. doi: 10.1146/annurev-genom-090711-163739. [DOI] [PubMed] [Google Scholar]
- 23.Hollak C.E., Wijburg F.A. Treatment of lysosomal storage disorders: Successes and challenges. J. Inherit. Metab. Dis. 2014;37:587–598. doi: 10.1007/s10545-014-9718-3. [DOI] [PubMed] [Google Scholar]
- 24.Boado R.J., Lu J.Z., Hui E.K., Lin J., Pardridge W.M. Insulin receptor antibody-alpha-N-acetylglucosaminidase fusion protein penetrates the primate blood-brain barrier and reduces glycosaminoglycans in Sanfillippo type B fibroblasts. Mol. Pharm. 2016;13:1385–1392. doi: 10.1021/acs.molpharmaceut.6b00037. [DOI] [PubMed] [Google Scholar]
- 25.Chatelut M., Harzer K., Christomanou H., Feunteun J., Pieraggi M.T., Paton B.C., Kishimoto Y., O'Brien J.S., Basile J.P., Thiers J.C., Salvayre R., Levade T. Model SV40-transformed fibroblast lines for metabolic studies of human prosaposin and acid ceramidase deficiencies. Clin. Chim. Acta. 1997;262:61–76. doi: 10.1016/s0009-8981(97)06527-3. [DOI] [PubMed] [Google Scholar]
- 26.Folch Y., Lees M., Sloane G.H. A simple method for the isolation and purification of total lipids from animal tissues. J. Biol. Chem. 1957;226:497–509. [PubMed] [Google Scholar]
- 27.Dworski S., Lu P., Khan A., Maranda B., Mitchell J.J., Parini R., Di Rocco M., Hugle B., Yoshimitus M., Magnusson B., Makay B., Arsian N., Guelbert N., Ehlert K., Jarisch A., Gardner-Medwin J., Dagher R., Terreri M.T., Lorenco C.M., Barillas-Arias L., Tanpaiboon P., Solyom A., Norris J.S., He X., Schuchman E.H., Levade T., Medin J.A. Acid ceramidase deficiency is characterized by a unique cytokine and ceramide profile that is altered by therapy. Biochim. Biophys. Acta. 2016;1863:386–394. doi: 10.1016/j.bbadis.2016.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Volger C., Levy B., Galvin N.J., Thorpe C., Sands M.S., Barker J.E., Baty J., Birkenmeier E.H., Sly W.S. Enzyme replacement in murine mucopolysaccharidosis type VII: Neuronal and glial response to bet-glucuronidase requires early initiation of enzyme replacement therapy. Pediatr. Res. 1999;45:838–844. doi: 10.1203/00006450-199906000-00010. [DOI] [PubMed] [Google Scholar]
- 29.Murray J.M., Thompson A.M., Vitsky A., Hawes M., Chuang W.L., Pacheco J., Wilson S., McPherson J.M., Thurberg B.L., Karey K.P., Andrews L. Nonclinical safety assessment of recombinant human acid sphingomyelinase (rhASM) for the treatment of acid sphingomyelinase deficiency: The utility of animal models of disease in the toxicology evaluation of potential therapeutics. Mol. Genet. Metab. 2015;114:217–225. doi: 10.1016/j.ymgme.2014.07.005. [DOI] [PubMed] [Google Scholar]
- 30.Bae J.S., Jang K.H., Schuchman E.H., Jin H.K. Comparative effects of recombinant acid sphingomyelinase administration by different routes in Niemann-pick disease mice. Exp. Anim. 2004;53:417–421. doi: 10.1538/expanim.53.417. [DOI] [PubMed] [Google Scholar]
- 31.Unpublished, manuscript submitted.
- 32.Simonaro C.M., Sachot S., Ge Y., He X., DeAngelis V.A., Eliyahu E., Leong D.J., Sun H.B., Mason J.B., Haskins M.E., Richardson D.W., Schuchman E.H. Acid ceramidase maintains the chondrogenic phenotype of expanded primary chondrocytes and improves the chondrogenic differentiation of bone marrow-derived mesenchymal stem cells. PLoS One. 2013;8 doi: 10.1371/journal.pone.0062715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Frohbergh M.E., Guevara J.M., Greisamer R.P., Barbe M.F., He X., Simonaro C.M., Schuchman E.H. Acid ceramidase treatment enhances the outcome of autologous chondrocyte implantation in a rat osteochondral defect model. Osteoarthr. Cartil. 2016;24:752–762. doi: 10.1016/j.joca.2015.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Transparency document
Supplemental Fig. 1. Representative SDS-PAGE analysis of rhAC. rhAC was purified from the media of an overexpressing CHO cell line and subjected to SDS-PAGE under non-reducing (NR) and reducing (R) conditions. Staining was with SimpleBlue®. Molecular weights (in kDa) according to the migration of commercially-supplied molecular weight standards are shown to the left. Bands corresponding to rhAC precursor, alpha, and beta subunits also are indicated. Supplemental Fig. 2. Plasma clearance of rhAC activity after tail vein injection. rhAC (10 mg/kg) was injected into the tail vein of 4–6 week old Farber disease mice. At various times post-injection mice were euthanized (n = 3/time point) and AC activity in plasma was determined. Supplemental Fig. 3. Weight of Farber disease mice receiving repeat injections of rhAC. Approximately 3-week-old Farber disease mice received once weekly i.p. injections of purified rhAC at doses of 1, 3 and 10 mg/kg (n = 9–56/dose). Mice were euthanized when they lost > 10% body weight within a one week period. “0” indicates Farber disease mice receiving once weekly injections of PBS. The average weight of surviving mice at the indicated ages is graphed. Supplemental Fig. 4. Survival of Farber disease mice receiving repeat injections of rhAC starting at two different ages. Farber disease mice received once weekly i.p. injections of purified rhAC (10 mg/kg) starting at 3 days (early) or 3 weeks (late) of age (n = 7–10/age). Mice were euthanized when they lost > 10% body weight within a one week period. Kaplan-Meir plots were used to indicate survival probability. “0” indicates Farber disease mice receiving once weekly injection of PBS. Supplemental Fig. 5. Sphingosine levels in tissues of Farber disease mice receiving repeat injections of rhAC starting at two different ages. Farber disease mice received once weekly i.p. injections of purified rhAC (10 mg/kg) starting at 3 days (early) or 3 weeks (late) of age (n = 7–11/age). Mice were euthanized when they lost > 10% body weight within a one week period. Sphingosine (Sph) levels were determined in tissue extracts as described in the Materials and Methods. *indicates p value < 0.05 comparing mice treated early to late. **indicates p value < 0.01.