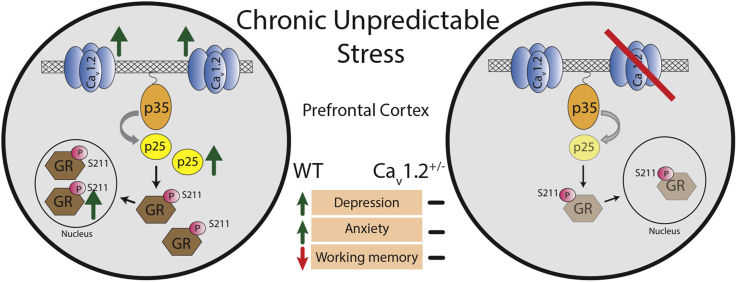
Abstract
Chronic stress is known to precipitate and exacerbate neuropsychiatric symptoms, and exposure to stress is particularly pathological in individuals with certain genetic predispositions. Recent genome wide association studies have identified single nucleotide polymorphisms (SNPs) in the gene CACNA1C, which codes for the Cav1.2 subunit of the L-type calcium channel (LTCC), as a common risk variant for multiple neuropsychiatric conditions. Cav1.2 channels mediate experience-dependent changes in gene expression and long-term synaptic plasticity through activation of downstream calcium signaling pathways. Previous studies have found an association between stress and altered Cav1.2 expression in the brain, however the contribution of Cav1.2 channels to chronic stress-induced behaviors, and the precise Cav1.2 signaling mechanisms activated are currently unknown. Here we report that chronic stress leads to a delayed increase in Cav1.2 expression selectively within the prefrontal cortex (PFC), but not in other stress-sensitive brain regions such as the hippocampus or amygdala. Further, we demonstrate that while Cav1.2 heterozygous (Cav1.2+/−) mice show chronic stress-induced depressive-like behavior, anxiety-like behavior, and deficits in working memory 1–2 days following stress, they are resilient to the effects of chronic stress when tested 5–7 days later. Lastly, molecular studies find a delayed upregulation of the p25/Cdk5-glucocorticoid receptor (GR) pathway in the PFC when examined 8 days post-stress that is absent in Cav1.2+/− mice. Our findings reveal a novel Cav1.2-mediated molecular mechanism associated with the persistent behavioral effects of chronic stress and provide new insight into potential Cav1.2 channel mechanisms that may contribute to CACNA1C-linked neuropsychiatric phenotypes.
Graphical abstract
1. Introduction
Deficits in the structure and connectivity of the prefrontal cortex (PFC) are a key feature of a multitude of neuropsychiatric conditions (Negrón-Oyarzo et al., 2016). As a highly connected structure, the PFC acts as a key coordinator of various limbic brain regions. Thus, dysfunction within the PFC can dramatically impact emotional regulation, attention, and cognition. Chronic stress, a known risk factor for neuropsychiatric disorders, induces profound impairments within the PFC, including dendritic atrophy (Liston et al., 2006, Izquierdo et al., 2006), changes in gene expression (Erburu et al., 2015), and impairments in long-term potentiation (Quan et al., 2011). In rodent models, exposure to prolonged, chronic stress leads to long-lasting changes in behavior (Elizalde et al., 2008, Matuszewich et al., 2007, Gourley and Taylor, 2009), making it an optimal model of stress-relevant neuropsychiatric disorders (Willner, 2016, Nestler and Hyman, 2010).
Current evidence supports the diathesis-stress model of mental illness, which postulates that genetic predispositions alter stress reactivity, leading to the manifestation of mental disorders. While exposure to stressful life events often precedes the onset of psychiatric symptoms (Fowles, 1992, Hammen and Gitlin, 1997, Kessler, 1997), the development of neuropsychiatric disorders is likely regulated by an interaction between these stressors and genetic vulnerability (Lazary et al., 2016, Daskalakis and Binder, 2015, Lovallo et al., 2015).
Several genetic studies have identified the gene CACNA1C, which encodes the Cav1.2 subunit of L-type calcium channels (LTCCs), as a common risk gene for multiple neuropsychiatric disorders (Heyes et al., 2015, Bhat et al., 2012, Casamassima et al., 2010), including bipolar disorder (Ferreira et al., 2008, Ament et al., 2015), schizophrenia (Nyegaard et al., 2010, Green et al., 2010), and major depressive disorder (Green et al., 2010, Rao et al., 2016). Nevertheless, how CACNA1C influences such a variety of neuropsychiatric conditions is not currently understood.
Cav1.2 channels are highly expressed throughout the forebrain (Hell et al., 1993) and in vitro studies have identified that disease-associated increases in Ca2+ influx via Cav1.2 channels can alter gene expression patterns (Paşca et al., 2011, Tian et al., 2014) and contribute to activity dependent dendritic retraction (Krey et al., 2013), which has also been observed following chronic stress (Liston et al., 2006, Izquierdo et al., 2006, Erburu et al., 2015). In support of this, CACNA1C risk allele carriers show reduced PFC activity during working memory tasks, correlating with impaired performance (Bigos et al., 2010), and altered PFC structure (Wang et al., 2011) and connectivity (Wang et al., 2011, Paulus et al., 2014). In animal models, Cav1.2 regulates PFC-dependent behaviors such as depressive-like behavior (Dao et al., 2010), anxiety-like behavior (Lee et al., 2012), and fear learning (Jeon et al., 2010, Bader et al., 2011). A previous study has reported that chronic stress increases Cav1.2 mRNA in the hippocampus and amygdala (Maigaard et al., 2012), stress-sensitive brain regions, but the effect of chronic stress on Cav1.2 levels in the PFC is unknown. Additionally, a direct link between Cav1.2 and the effects of chronic stress on behavior has yet to be explored, and the downstream molecular pathways within stress-sensitive regions including the PFC have not been characterized.
One potential Cav1.2 mechanism for regulating the stress response is via glucocorticoid receptors (GRs). GRs mediate effects of chronic stress on behavior (Barik et al., 2013, Chmielarz et al., 2013), and site-specific phosphorylation of GRs induces translocation to the nucleus, where GRs bind DNA and regulate gene expression (Galliher-Beckley and Cidlowski, 2009). Pharmacological blockade of LTCCs attenuates GR-mediated transcription following corticosterone treatment in vitro (Basta-Kaim et al., 2002), but the mechanisms by which LTCCs mediate this process, and if a similar mechanism exists in vivo, are unknown.
One mechanism for regulation of the GR pathway is Ca2+-activated cleavage of the protein p35 to p25. Through activation of cyclin-dependent kinase 5 (Cdk5), a kinase linked to stress responses (Plattner et al., 2015, Papadopoulou et al., 2015), p25 increases phosphorylation of GRs at serine 211 (Kino et al., 2007), a site specifically linked to altered downstream gene expression (Wang et al., 2007, Rei et al., 2015). p25 is upregulated in the PFC following chronic stress (Adzic et al., 2009) and induces memory deficits through regulation of GR-mediated transcription in the hippocampus (Rei et al., 2015). However, if Cav1.2 channels act as the calcium source for chronic stress-induced activation of the p25/Cdk5-GR pathway has yet to be explored.
Here we report that chronic unpredictable stress (CUS) leads to a delayed increase in expression of Cav1.2 protein within the PFC, but not within the hippocampus or amygdala, and that Cav1.2 heterozygous mice are resilient to the persistent effects of chronic stress on PFC-dependent behavioral tasks. Additionally, we find that CUS activates the p25/Cdk5-GR pathway specifically within the PFC in a delayed, Cav1.2-dependent manner, providing a potential mechanism by which Cav1.2 may regulate stress-susceptibility. Characterizing Cav1.2 as a mediator of chronic stress may help explain the pleiotropic effects of CACNA1C mutations on neuropsychiatric disorders.
2. Materials and methods
2.1. Animals
Male cacna1c heterozygous mice (Moosmang et al., 2005) and wild-type littermates were 8–12 weeks old at the start of the experiments. Full brain Cav1.2 knockout mice were generated by crossing homozygous cacna1c floxed mice (Moosmang et al., 2005) with mice expressing Cre recombinase under the control of the nestin promoter (Jackson Laboratory; B6.Cg-Tg (Nes-cre)1Kln/J). Animals were group housed, maintained on a 12hr light/dark cycle, and had food and water available ad libitum. All animal procedures were conducted in accordance with the rules of Weill Cornell Medicine Institutional Animal Care and Use Committee following the National Institutes of Health guide for the care and use of Laboratory animals. Efforts were put forth to minimize animal suffering and to reduce the number of animals utilized for experiments.
2.2. Chronic unpredictable stress protocol
A modified version of chronic unpredictable stress (CUS) protocol was used (Mineur et al., 2006). Mice were exposed to three variable stressors daily for 28 days. Stressors included: Strobe light (2 h), restraint stress (1 h), bedding deprivation (3 h), tilted cage (3 h), cold stress (30 min), social isolation (2 h), footshock (5, 0.7 mA shocks), water stress (1 h), wet bedding (2 h), lights on overnight (12 h), and wet bedding overnight (12 h). The daily and weekly order of stressors was randomized in order to ensure that the stress remained unpredictable. Behavior was tested at two time points in separate cohorts of mice. The first cohort began testing 24 h following the last CUS session (early) and the second began testing 5 days following the last CUS session (late). A no-stress control group was used for all experiments. These control animals were handled daily for the 28-day period and returned to their home cages.
2.3. Immunoblotting
2.3.1. Tissue collection
Mice were decapitated 24 h following behavioral testing. Brains were rapidly dissected and sectioned into 1.0 mm coronal sections using a metal mouse brain slicer matrix (Zivic instruments) placed on dry ice to maintain freezing conditions. Bilateral tissue punches of the prefrontal cortex, dorsal hippocampus, ventral hippocampus and amygdala were obtained on a glass surface placed on dry ice to maintain frozen sections. Tissue was placed immediately on dry ice and stored at −80 °C until processed.
2.3.2. Tissue processing
Tissue was homogenized in 100 μl sucrose buffer (0.3M sucrose/0.01 mM HEPES) containing protease and phosphatase inhibitors, as previously published by Knackstedt et al. (2010). Homogenates were spun at 1000 × g for 10 min at 4 °C. Supernatant (cytoplasmic and membrane S1 fraction) was saved as the total protein lysate fraction and the pellet (nuclear P1 fraction) was washed in 50 μl sucrose buffer and spun at 1000 × g for 10 min at 4 °C. The pellet was sonicated in 1% SDS buffer, including protease and phosphatase inhibitors, and was spun at max speed for 15 min at 4° C. The supernatant was saved as the nuclear fraction. Protein concentration was determined using a BCA protein assay kit (Thermo Fischer Scientific).
2.3.3. Western blot analysis
20-30μg of protein was run on a 10% SDS gel using electrophoresis (constant 200 V, 50 min) and proteins were transferred to a PDVF membrane (constant 0.3 mA, 3 h). Transfer efficiency was confirmed using Ponceau S staining. Membranes were probed with primary antibodies against Cav1.2 (1:500, Alomone, ACC-003, RRID:AB_2039771), phospho-glucocorticoid receptor serine 211 (1:500, cell signaling #4161, RRID:AB_2155797), glucocorticoid receptor (1:1,000, cell signaling #3660, RRID:AB_11179215), p35/p25 (1:500, cell signaling #2680s, RRID:AB_1078214), CaMKII pan (cell signal, #3362S, RRID:AB_10692639), histone H3 (di Methyl K9) (Abcam, ab1220, RRID:AB_449854), Gapdh (1:20,000, Abcam, ab22555, RRID:AB_447153) and a peroxidase-labeled anti-rabbit secondary antibody (1:5,000, Vector Laboratories), peroxidase-labeled anti-goat secondary antibody (1:5,000, Vector Laboratories) or peroxidase-labeled anti-mouse secondary antibody (1:5,000, Vector Laboratories). Proteins were quantified using ImageJ analysis software (NIH), and protein quantities were normalized to Gapdh as a loading control.
2.4. Behavioral testing
2.4.1. Tail-suspension test
The tail-suspension test was used to measure depressive-like behavior (Kabir et al., 2017). A 17 cm long piece of adhesive tape (0.75” wide, Fisherbrand) was placed on the tail of each mouse and was used to suspend the mouse 30 cm above the ground. A hollow climbstopper (4 cm length x 1.5 cm diameter, 1.3 g) was placed over the tail to prevent mice from climbing. Mice were suspended for 6 min. Behavior was recorded using a camera placed in front of the apparatus and time immobile was measured by the experimenter. Immobility was defined as any time that there was a lack of body movement except of swinging caused by the momentum of the last movement. The apparatus and climbstopper were cleaned with 70% ethanol between trials.
2.4.2. Elevated plus maze
The elevated plus maze was used to measure anxiety-like behavior. The apparatus contained four arms (30.5 cm length), two of which were enclosed by walls (15.2 cm in height) and two of which were open. Each mouse was placed in the center of the apparatus and allowed to freely explore for 5 min. Behavior was recorded using a camera placed above the apparatus, and time spent in the open arms was recorded using AnyMaze software (Stoelting). The apparatus was cleaned with 70% ethanol between trials.
2.4.3. Spontaneous alternation
The spontaneous alternation task was used to measure working memory as previously published in Sierksma et al. (2013). The apparatus contained three enclosed arms (33 cm × 7.6 cm x 38.1 cm) spaced 120° apart. Each mouse was placed into the center of the apparatus and allowed to freely explore for 5 min. Behavior was recorded using a camera placed above the apparatus, and the subject's path was recorded by the experimenter. An alternation was defined as entry into all three arms without repeating entry into any of the arms. Percentage of spontaneous alternations was calculated as total number of alternations divided by the total number of possible alternations multiplied by 100. The apparatus was cleaned with 70% ethanol between trials.
2.5. Corticosterone ELISA
Following decapitation, trunk blood was collected in 1.5 mL microfuge tubes, incubated at room temperature for 60 min, and spun at 1200 × g for 15 min. Supernatant was collected and stored at −80° C until analyzed. Serum corticosterone levels were measured using a corticosterone high sensitivity EIA (Immunodiagnostic Systems, AC-15F1). Samples were tested in duplicate and concentration was determined as described by the manufacturer.
2.6. Statistical analyses
Normality was tested using the Shapiro-Wilk test and equal variance was confirmed using the F-test of equality of variances. Data were then analyzed using an unpaired t-test or two-way ANOVA followed by bonferroni post-hoc analysis. For data that did not show equal variances, Welch's correction was used, as specified in the text. Data were considered to be statistically significant if p < 0.05. All statistical analyses were completed using Graphpad Prism v6.0.
3. Results
3.1. Chronic unpredictable stress leads to a delayed increase in Cav1.2 protein expression within the prefrontal cortex
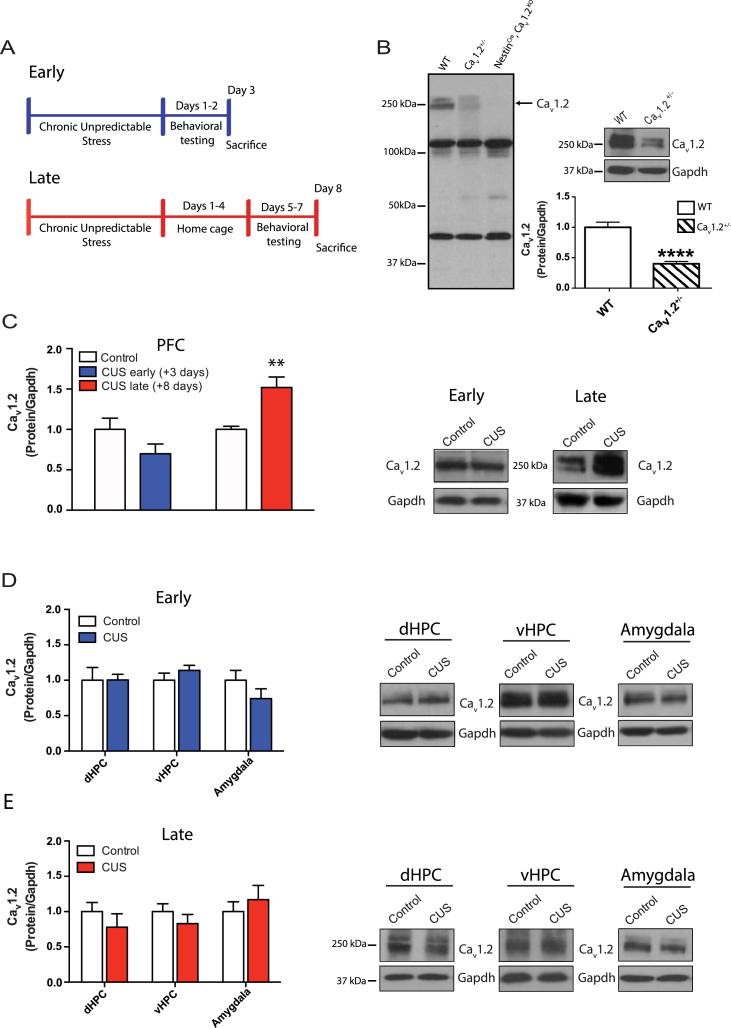
Western blot analysis was used to quantify protein expression of Cav1.2 in the prefrontal cortex (PFC) of WT mice 3 days (early) and 8 days (late) following chronic unpredictable stress (CUS) (Fig. 1A). We first validated the anti-Cav1.2 antibody (Alomone, ACC-003) using PFC protein lysate from full brain Cav1.2 knockout (NestinCre, Cav1.2KO) and Cav1.2 heterozygous (Cav1.2+/−) mice. Two bands surrounding the predicted 250 kDa full-length protein were present in the wildtype (WT) and absent in NestinCre, Cav1.2KO mice (Fig. 1B), indicating that these bands are specific Cav1.2 variants. The doublet appeared fainter in Cav1.2+/− mice, and when quantified (both bands combined), revealed significantly lower levels of Cav1.2 protein in the PFC of Cav1.2+/− mice compared to WT littermates (Fig. 1B; t(9) = 6.88, p < 0.0001, unpaired t-test).
Fig. 1.
Western blot analysis of Cav1.2 expression in the prefrontal cortex following chronic unpredictable stress. A) Schematics of the chronic unpredictable stress paradigms, indicating the early and late time points chosen. B) Left, Validation of the Cav1.2 antibody using a full brain knockout (NestinCre, Cav1.2KO) and Cav1.2+/− mice indicates that the two bands surrounding 250 kDa are specific Cav1.2 variants. Right, When quantified, these two bands are significantly reduced in Cav1.2+/− PFC (****p < 0.0001; WT, n = 5; Cav1.2+/−, n = 6). C) Chronic unpredictable stress (CUS) did not affect Cav1.2 protein expression in the PFC when examined at the early (3 days post-stress) time point, but increased Cav1.2 protein expression in the PFC when examined at the late (8 days post-stress) time point (**p < 0.01; control, n = 6; CUS, n = 7). Western blot images contain representative samples of each group taken from the same blot. D) CUS does not impact Cav1.2 protein levels in the dHPC (control, n = 7; CUS, n = 6), vHPC (control, n = 7; CUS, n = 7), or amygdala (control, n = 7; CUS, n = 7) when examined at the early time point. E) CUS does not impact Cav1.2 protein levels in the dHPC (control, n = 6; CUS, n = 5), vHPC (control, n = 6; CUS, n = 6) or amygdala (control, n = 6; CUS, n = 5) when examined at the late time point. Data are represented as mean ± SEM.
CUS had no effect on Cav1.2 protein expression in the PFC when examined 3 days post-stress, but significantly increased Cav1.2 protein expression when tested after 8 days (Fig. 1C; t(7.2) = 3.76, p = 0.007, unpaired t-test with Welch's correction). This CUS-induced increase in Cav1.2 was specific to the PFC, as there was no effect of CUS on Cav1.2 levels in other stress-sensitive regions, including the dorsal hippocampus (dHPC), ventral hippocampus (vHPC), and amygdala at the early (Fig. 1D) and late time points (Fig. 1E).
3.2. Cav1.2 channels mediate the persistent effects of chronic unpredictable stress on depressive-like behavior, anxiety-like behavior, and working memory
To test the role of Cav1.2 in chronic stress-induced behavioral deficits, Cav1.2+/− and WT littermates were exposed to CUS, followed by behavioral testing for depressive-like behavior using the tail suspension test (TST), anxiety-like behavior using the elevated plus maze (EPM), and working memory capacity using a non-reward based spontaneous alternation task beginning either 24 h (early) or 5 days (late) post-stress. Heterozygous mice were utilized as homozygous knockout of Cav1.2 is embryonic lethal (Seisenberger et al., 2000). The non-reward based spontaneous alternation task was chosen in place of a standard reinforced alternation task, as it has been used previously as a test of working memory (Wietrzych et al., 2005, Galeano et al., 2014, Sierksma et al., 2013), and because chronic unpredictable stress is known to alter reward (Papp et al., 1992, Haile et al., 2001). The non-reward based spontaneous alternation task, therefore, eliminates this potential confound when attempting to isolate working memory.
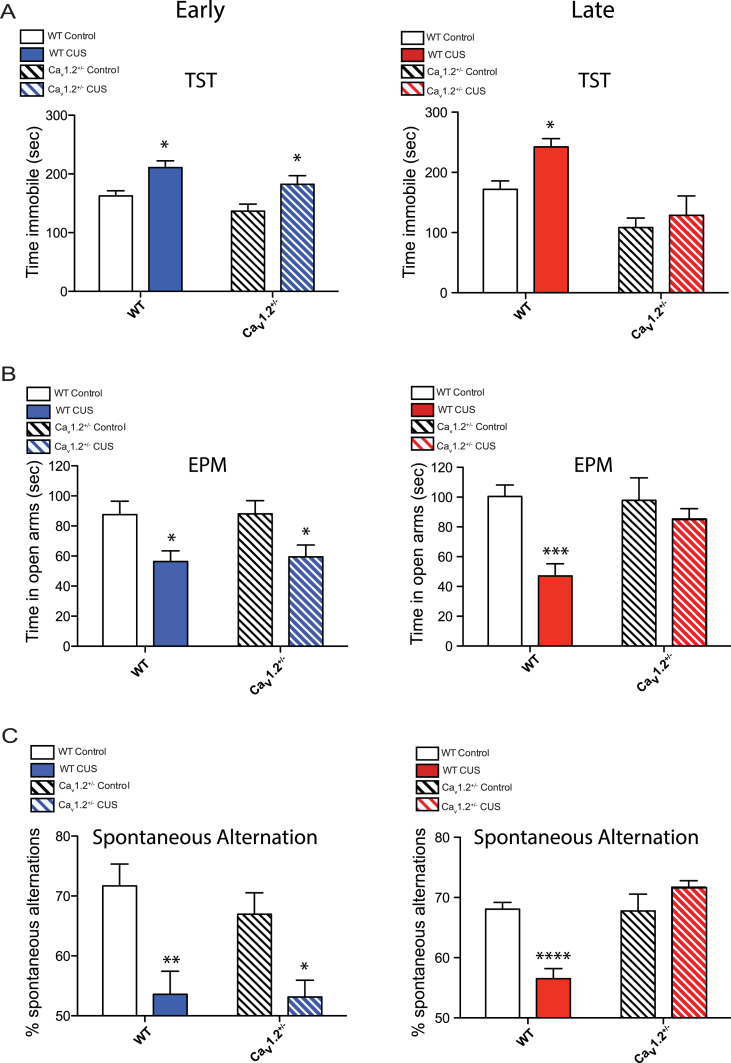
In the TST, CUS significantly increased time spent immobile in both WT and Cav1.2+/− mice when tested 1–2 days post-stress (Fig. 2A; main effect of CUS: F(1,24) = 14.70, p = 0.0008), indicating a depressive-like phenotype that is not dependent upon Cav1.2 channels at this time point. WT mice continued to show this depressive-like behavior when tested 5–7 days post-stress, while Cav1.2+/− mice did not (Fig. 2A; main effect of CUS: F(1,19) = 5.38, p = 0.032; main effect of genotype: F(1,19) = 20.49, p = 0.0002), indicating that Cav1.2 mediates the persistent effects of CUS on depressive-like behaviors.
Fig. 2.
Behavior of Cav1.2 heterozygous (Cav1.2+/−) and wildtype littermates (WT) following exposure to chronic unpredictable stress (CUS). A) Left, CUS increased depressive-like behavior in the tail suspension test (TST) in both WT and Cav1.2+/− mice when tested at the early (1–2 days post-stress) time point (*p < 0.05, WT control vs CUS, bonferroni post-hoc; *p < 0.05, Cav1.2+/− control vs CUS, bonferroni post-hoc; WT control, n = 6; WT CUS, n = 7; Cav1.2+/− control, n = 8; Cav1.2+/− CUS, n = 7). Right, when tested at the late (5–7 days post-stress) time piont, CUS increased depressive-like behavior in WT mice but not in Cav1.2+/− mice (*p < 0.05, WT control vs CUS, bonferroni post-hoc; p > 0.05, Cav1.2+/− control vs CUS, bonferroni post-hoc; WT control, n = 5; WT CUS, n = 8; Cav1.2+/− control, n = 5; Cav1.2+/− CUS, n = 5). B) Left, when tested at the early time point, CUS increased anxiety-like behavior in the elevated plus maze (EPM) in both WT and Cav1.2+/− mice (*p < 0.05, WT control vs CUS, bonferroni post-hoc; *p < 0.05, Cav1.2+/− control vs CUS, bonferroni post-hoc; WT control, n = 7; WT CUS, n = 7; Cav1.2+/− control, n = 8; Cav1.2+/− CUS, n = 7). Right, when tested at the late time point, CUS increased anxiety-like behavior in WT but not in Cav1.2+/− mice (***p < 0.001, WT control vs CUS, bonferroni post-hoc; p = 0.60, Cav1.2+/− control vs CUS, bonferroni post-hoc; WT control, n = 12; WT CUS, n = 14; Cav1.2+/− control, n = 10; Cav1.2+/− CUS, n = 13). C) Left, CUS induced working memory deficits in the spontaneous alternation task in WT and Cav1.2+/− mice when tested at the early time point (**p < 0.01, WT control vs CUS, bonferroni post-hoc; *p < 0.05, Cav1.2+/− control vs CUS, bonferroni post-hoc; WT control, n = 7; WT CUS, n = 7; Cav1.2+/− control, n = 8; Cav1.2+/− CUS, n = 7). Right, when tested at the late time point, however, CUS decreased spontaneous alternations in WT but not in Cav1.2+/− mice (****p < 0.0001 WT control vs CUS, bonferroni post-hoc; p = 0.22, Cav1.2+/− control vs CUS, bonferroni post-hoc; WT control, n = 12; WT CUS, n = 14; Cav1.2+/− control, n = 10; Cav1.2+/− CUS, n = 13). Data are represented as mean ± SEM.
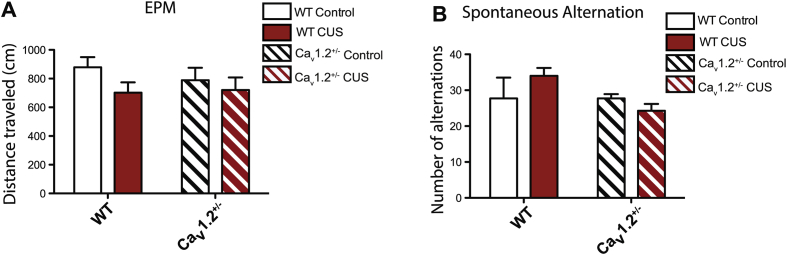
Exposure to CUS significantly decreased time spent in the open arms of the EPM in both WT and Cav1.2+/− mice when tested 1–2 days post-stress (Fig. 2B; main effect of CUS: F(1,25) = 12.98, p = 0.001), indicating a Cav1.2-independent anxiety-like phenotype. However, when tested 5–7 days later, WT mice continued to demonstrate increased anxiety-like behavior while Cav1.2+/− mice did not (Fig. 2B; interaction (genotype x CUS): F(1,45) = 4.74, p = 0.035, two-way ANOVA). No effect of genotype or CUS was observed on distance traveled during the EPM test (Supp. Fig. 1A). These results demonstrate that Cav1.2 is necessary for persistent CUS-induced anxiety-like behavior.
Additionally, in the spontaneous alternation test, CUS significantly decreased the percentage of spontaneous alternations in both WT and Cav1.2+/− mice when tested 1–2 days post-stress (Fig. 2C; main effect of CUS: F(1,25) = 20.62, p = 0.0001), indicating a Cav1.2-independent working memory deficit. When tested 5–7 days later, however, WT but not Cav1.2+/− mice continued to show a decrease in percentage of spontaneous alternations (Fig. 2C; interaction (genotype x CUS): F(1,45) = 20.77, p < 0.0001, two-way ANOVA). No effect of genotype or CUS was observed on total number of alternations during the test, indicating no difference in exploratory behavior (Supp. Fig. 1B). These results demonstrate a role of Cav1.2 in mediating the persistent effects of chronic stress on working memory.
3.3. Cav1.2 mediates the delayed production of p25 and phosphorylation of glucocorticoid receptors in the PFC resulting from chronic unpredictable stress
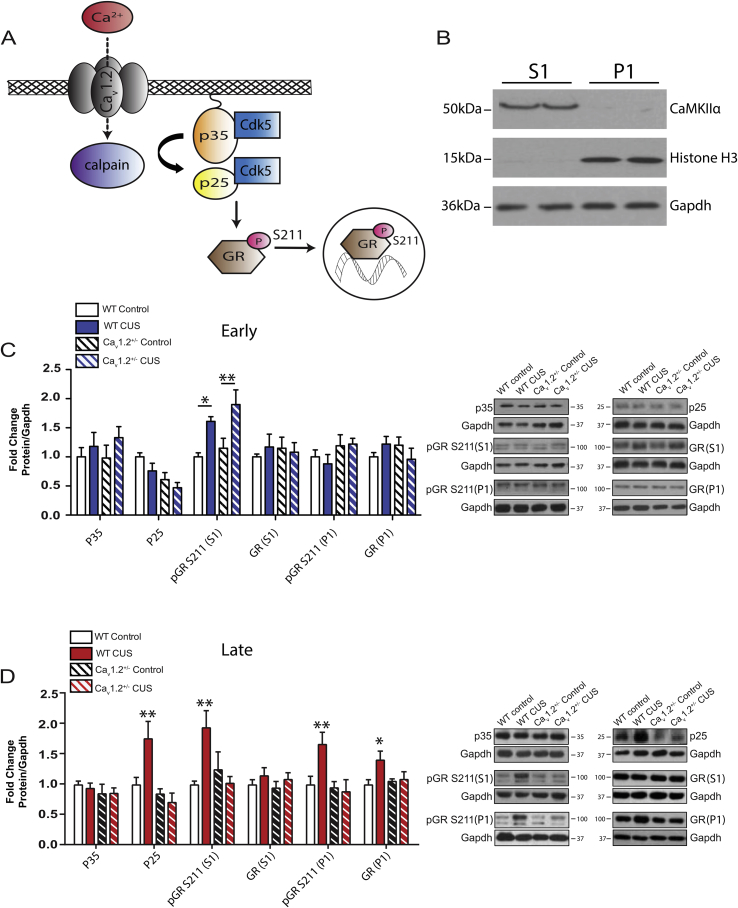
Calcium-mediated cleavage of p35 to p25 has been associated with the effects of chronic stress on behavior (Rei et al., 2015) (Fig. 3A). To determine a potential role of Cav1.2 in mediating this process, WT and Cav1.2+/− mice were exposed to CUS, and western blot analysis was performed on PFC S1 (cytoplasmic and membrane) and P1 (nuclear) fractions.
Fig. 3.
Western blot analysis of the p25/Cdk5-GR pathway in the PFC following exposure to chronic unpredictable stress (CUS) in WT and Cav1.2+/− mice. A) Working model of Cav1.2 activation of the p25/Cdk5-GR pathway. B) Representative western blots with S1 (cytoplasmic and membrane) and P1 (nuclear) fractions demonstrating purity of fractions using the S1 and P1 fraction-specific proteins CaMKIIα and Histone H3, respectively. C) In the PFC, CUS increased expression of phosphorylated glucocorticoid receptors at S211 (pGR S211) in the S1 fraction in both WT and Cav1.2+/− mice (*p < 0.05, WT control vs CUS, bonferroni post-hoc; **p < 0.01, Cav1.2+/− control vs CUS, bonferroni post-hoc) when examined at the early (3 days post-stress) time point. At this time point, CUS had no effect on expression of p35, p25, GR in the S1 fraction, pGR S211 in the P1 fraction or GR in the P1 fraction (WT control, n = 5–7; WT CUS, n = 7; Cav1.2+/− control, n = 7; Cav1.2+/− CUS, n = 6–7). D) When examined at the late (8 days post-stress) time point, in the PFC of WT but not Cav1.2+/− mice, CUS increased expression of p25 (WT control vs CUS, **p < 0.01, bonferroni post-hoc; Cav1.2+/− control vs CUS, p > 0.99, bonferroni post-hoc; WT control, n = 11; WT CUS, n = 10; Cav1.2+/− control, n = 9; Cav1.2+/− CUS, n = 11), phosphorylation of glucocorticoid receptors at S211 (pGR S211) in both the S1 fraction (WT control vs CUS, **p < 0.01, bonferroni post-hoc; Cav1.2+/− control vs CUS, p = 0.70, bonferroni post-hoc; WT control, n = 6; WT CUS, n = 7; Cav1.2+/− control, n = 7; Cav1.2+/− CUS, n = 7) and in the P1 fraction (**p < 0.01 WT control vs CUS, bonferroni post-hoc; p = 0.96 Cav1.2+/− control vs CUS, bonferroni post-hoc; WT control, n = 6; WT CUS, n = 8; Cav1.2+/− control, n = 6; Cav1.2+/− CUS, n = 5), as well as P1 levels of GR (*p < 0.05 WT control vs CUS, bonferroni post-hoc; p = 0.85 Cav1.2+/− control vs CUS, bonferroni post-hoc; WT control, n = 6; WT CUS, n = 8; Cav1.2+/− control, n = 6; Cav1.2+/− CUS, n = 6). CUS had no effect on S1 expression of p35 or GR in either WT or Cav1.2+/− mice (WT control, n = 6–8; WT CUS, n = 7; Cav1.2+/− control, n = 6–7; Cav1.2+/− CUS, n = 7). Western blot images contain representative samples of each group taken from the same blot. Data are represented as mean ± SEM.
CUS had no effect on p35 or p25 protein levels in the S1 fraction of the PFC in either WT or Cav1.2+/− mice when examined 3 days post-stress (Fig. 3C). However, when examined 8 days post-stress, WT mice exposed to CUS had increased levels of p25 in protein lysates from the PFC while Cav1.2+/− mice did not (Fig. 3D; interaction (genotype x CUS): F(1,37) = 7.47, p = 0.009, two-way ANOVA). Neither genotype showed changes in p35 protein levels in the PFC at this time point, suggesting that Cav1.2 mediates CUS-induced cleavage of p35 to p25. Downstream of p25, we next examined the effect of CUS and the role of Cav1.2 channels therein on phosphorylation of the glucocorticoid receptor (GR) at the p25-activated Cdk5 site (S211) (Fig. 3A) in S1 and P1 fractions, as phosphorylation of GR induces translocation to the nucleus. S1 and P1 fractions were first validated for purity; CaMKIIα was detected in S1 fractions but not in P1 fractions (Fig. 3B). Similarly, nuclear protein histone H3 was detected in P1 fractions while absent in the S1 fractions.
Exposure to CUS increased levels of S1 pGR S211 in both WT and Cav1.2+/− mice when examined 3 days post-stress (Fig. 3C; main effect of CUS: F(1,24) = 18.01, p = 0.0003), while 8 days post-stress, WT mice continued to show an increase in pGR S211 in the PFC but Cav1.2+/− mice did not (Fig. 3D; interaction (genotype x CUS): F(1,23) = 7.90, p = 0.01, two-way ANOVA). No change in total S1 GR levels following CUS was observed in WT or Cav1.2+/− mice at either time point.
We next examined levels of GR and pGR S211 in P1 fractions from the PFC. CUS had no effect on nuclear GR or pGR S211 levels in either WT or Cav1.2+/− mice 3 days post-stress (Fig. 3C). When examined 8 days post-stress, though, WT mice exposed to CUS showed an increase in protein levels of nuclear GR (Fig. 3D; interaction (genotype x CUS): F(1,22) = 4.88, p = 0.04, two-way ANOVA) as well as nuclear pGR S211 (Fig. 3D; interaction (genotype x CUS): F(1,21) = 5.41, p = 0.03, two-way ANOVA) that was not observed in Cav1.2+/− mice exposed to CUS. Taken together, these findings indicate that while CUS induces a Cav1.2-independent phosphorylation of S1 GR early on after CUS in the PFC, Cav1.2 is necessary for activation of the p25/Cdk5 pathway at the later time point and may be responsible for the maintenance of GR phosphorylation and nuclear translocation of GR.
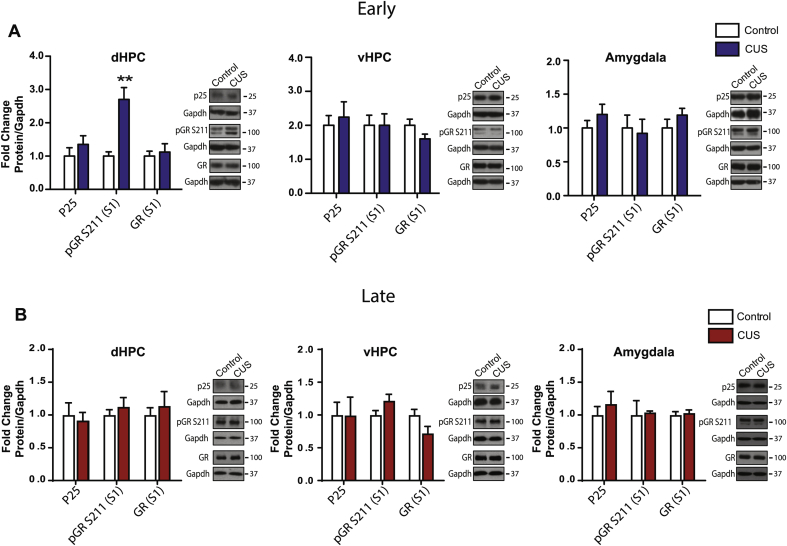
We additionally looked at activation of the p25/cdk5-GR pathway in the S1 fraction of other stress-sensitive brain regions including the dHPC, vHPC and amygdala of WT mice. Three days post-stress, CUS increased pGR S211 in the dHPC (Supp. Fig. 2A; t(7) = 4.44, p = 0.003), but had no effect on p25 or GR levels. Additionally, in the vHPC and amygdala, CUS had no effect on levels of p25, GR or pGR S211 at this early time point (Supp. Fig. 2A). When examined 8 days post-stress, CUS had no effect on p25, GR or pGR S211 in the dHPC, vHPC, or amygdala (Supp. Fig. 2B).
3.4. Cav1.2 is not necessary for stress-induced regulation of corticosterone
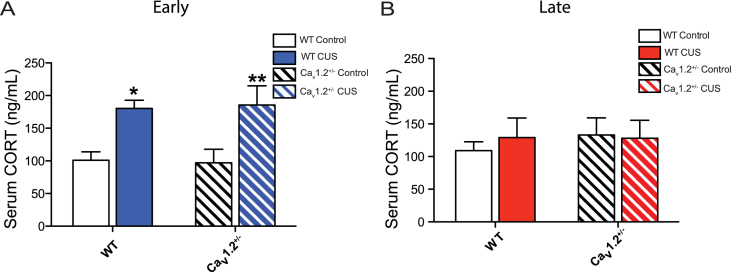
To determine if Cav1.2 might mediate the effects of chronic stress through regulation of circulating corticosterone (CORT), Cav1.2+/− and WT mice were exposed to CUS and serum was collected. When examined 3 days post-stress, CUS increased serum CORT in both WT and Cav1.2+/− mice (Fig. 4A; main effect of CUS: F(1,24) = 18.37, p = 0.0003, two-way ANOVA). When examined 8 days after CUS, serum CORT had normalized to baseline levels in both WT and Cav1.2+/− mice (Fig. 4B).
Fig. 4.
Analysis of serum corticosterone concentration following acute and chronic stress. A) Chronic unpredictable stress (CUS) increased serum corticosterone (CORT) levels in both WT and Cav1.2+/− mice when examined at the early (3 days post-stress) time point (*p < 0.05, WT control vs CUS, bonferroni post-hoc; **p < 0.01, Cav1.2+/− control vs CUS, bonferroni post-hoc; WT control, n = 7; WT CUS, n = 7; Cav1.2+/− control, n = 8; Cav1.2+/− CUS, n-6). B) When examined at the late (8 days post-stress) time point, (CUS had no effect on serum CORT levels in either WT or Cav1.2+/− mice (WT control, n = 9; WT CUS, n = 14; Cav1.2+/− control, n = 11; Cav1.2+/− CUS, n = 13). Data are represented as mean ± SEM.
4. Discussion
Our data presented here demonstrate that chronic stress leads to a delayed increase in Cav1.2 protein levels within the prefrontal cortex (PFC), and that this increase is associated with deficits in behavioral tasks as well as activation of stress-induced downstream molecular signaling within the PFC. Using heterozygous Cav1.2 mice, we show that Cav1.2 is necessary for the persistent effects of chronic stress on depressive-like behavior, anxiety-like behavior and working memory deficits, as well as delayed activation of the p25/Cdk5-glucocorticoid receptor (GR) pathway within the PFC. Given the association of CACNA1C with various neuropsychiatric disorders, as well as the known role of stress in precipitating psychiatric symptoms, these data give new insight into how changes in CACNA1C may impact risk for mental illness.
We show that exposure to chronic unpredictable stress (CUS) increases Cav1.2 protein expression within the PFC when examined 8 days post-stress but not 3 days post-stress. This delayed increase is consistent with a previous report that following acute stress, Cav1.2 expression shows a delayed increase in the hippocampus several days post-stress (Yang et al., 2012). This increase in Cav1.2 following CUS was specific to the PFC, as we saw no changes in expression in other stress-sensitive regions such as the dorsal hippocampus (dHPC), ventral hippocampus (vHPC) or amygdala. These data contradict a previous report by Maigaard et al. (2012), reporting an increase in Cav1.2 mRNA expression in both the HPC and amygdala following chronic restraint stress in rats. In addition to potential protocol and species differences, an increase in mRNA does not always translate to an increase in protein expression. Thus, Cav1.2 mRNA may be increased by exposure to chronic stress, but protein expression may not be altered. It is also plausible that despite the lack of an increase in Cav1.2 protein, Cav1.2 channels are involved in CUS-induced changes in these brain regions via alterations in Cav1.2 channel dynamics and physiology. Future experiments will address these aspects of Cav1.2 channel function following stress.
As expected, we found that exposure to CUS increased depressive-like behavior in the tail suspension test (TST), increased anxiety-like behaviors in the elevated plus maze (EPM), and reduced working memory in the spontaneous alternation task. These results replicate previous findings regarding the behavioral effects of similar chronic stress protocols (Mineur et al., 2006, Liston et al., 2006, Monteiro et al., 2015, Rei et al., 2015). In wildtype mice, these behavioral alterations persisted 5–7 days post-stress, consistent with previous reports that chronic stress leads to long-lasting changes in behavior (Elizalde et al., 2008, Matuszewich et al., 2007, Gourley and Taylor, 2009). However, we found that Cav1.2 was necessary for the persistent effects of CUS on behavior in the TST, EPM, and spontaneous alternation task, as Cav1.2 heterozygous mice show CUS-induced deficits in these tasks 1–2 days post-stress but not when tested 5–7 days post-stress. To the best of our knowledge, this is the first demonstration of the role of Cav1.2 channels in chronic stress-induced behavioral phenotypes. These findings are consistent, though, with previous studies, which showed that pharmacological inhibition of LTCCs could rescue behavioral deficits induced by acute stress (Kumar et al., 2012).
The TST, EPM, and spontaneous alternation task all recruit the PFC (Delatour and Gisquet-Verrier, 1996, Jinks and McGregor, 1997, Laroche et al., 2000, Shah and Treit, 2003, Adhikari et al., 2010, Divac et al., 2013). Our behavioral data, along with the molecular data showing an increase in Cav1.2 and its downstream stress-induced intracellular mediators in the PFC but not in the HPC or amygdala (discussed below), suggest that Cav1.2 in the PFC contributes to CUS-induced behavioral and molecular changes. It is important to consider, though, that while the TST, EPM and spontaneous alternation tasks recruit the PFC, these behavioral tests rely on specific sub-regions of the PFC as well as circuitry between numerous brain regions, including the HPC and amygdala. For example, optogenetic stimulation of the medial PFC can attenuate the effects of stress on depressive-like behaviors in the TST, and this stimulation is correlated with increased synchrony between the amygdala, ventral tegmental area, and nucleus accumbens (Kumar et al., 2012). Additionally, projections from the medial PFC to the dorsal raphe nucleus have been shown to attenuate depressive-like behavior in the forced swim test (Warden et al., 2012). Furthermore, optogenetic stimulation of the ventromedial PFC projections to the basomedial amygdala inhibit chronic CORT-induced anxiety-like behavior (Adhikari et al., 2015). In the spontaneous alternation task, optogenetic inhibition of the projection from the CA1 region of the ventromedial HPC to the infralimbic and prelimbic regions of the PFC was shown to attenuate encoding of spatial working memory (Spellman et al., 2015). While these findings indicate a critical role of the PFC in mediating these behavioral tasks, future studies will need to explore the effects of chronic stress on Cav1.2 expression in PFC sub-regions as well as the subsequent effects on circuitry. Although Cav1.2 levels are unchanged within the HPC and amygdala following CUS, these channels, both independently and through connections with the PFC, may still play a role during stress and could impact behavioral phenotypes. It will be vital in future studies to address the effect of loss of Cav1.2 on the intrinsic electrophysiological properties of PFC neurons and downstream target brain regions and the effect of stress therein.
Consistent with previous studies (Adzic et al., 2009, Rei et al., 2015), we found that exposure to CUS increased production of p25, phosphorylation of the glucocorticoid receptor (GR) at serine 211, a cyclin-dependent kinase 5 (Cdk5)-phosphorylation site, and nuclear translocation of GR within the PFC. Adding to this previously characterized pathway, we demonstrate that Cav1.2 is necessary for production of p25 and activation of GR signaling following chronic stress. However, in contrast to these previous reports, we found that activation of this pathway occurred only 8 days post-stress, not 3 days following chronic stress. Additionally, we did not find an increase in activation of the p25/cdk5-GR pathway in the HPC following CUS at either time point, inconsistent with the previous finding by Rei et al. (2015) that examined changes up to 90 min post-stress. This previous report utilized the Swiss Webster mouse strain, which is highly sensitive to the effects of stress. Indeed, these mice only required eight days of footshock stress to induce behavioral deficits, while chronic stress paradigms in C57BL/6 mice typically require 21–28 days. It is possible, therefore, that these mice exhibit molecular changes following chronic stress that are not observed in the C57BL/6 mouse strain. We did, however, find an increase in phosphorylation of GR at S211 in both the PFC and HPC at the early time point following chronic stress. This increase may be due, in part, to increased circulating corticosterone (discussed below).
Cleavage of p35 to p25 is induced by calpains (Kusakawa et al., 2000), proteases that are activated by calcium, but no connection between Cav1.2 and calpains has been previously explored in the brain. Here we demonstrate that Cav1.2 is necessary for production of p25 following chronic stress without affecting p35 levels, suggesting a role of Cav1.2 in mediating calpain activity. This hypothesis is supported by the recent finding that the LTCC subunit Cav1.4 increases calpain activity in photoreceptors (Schön et al., 2016). Future studies will address the role of calpains in the Cav1.2-mediated increase in p25 following chronic stress.
p25 activates and dysregulates Cdk5 by sequestering it from the plasma membrane to the cytoplasm and nucleus and by prolonging its activation (Patrick et al., 1999). Cdk5, which is crucial for regulating brain development, synaptic plasticity and learning and memory, has been highly studied in the context of neurodegenerative diseases (Su and Tsai, 2011), but has more recently been implicated in mediating the stress response (Papadopoulou et al., 2015, Plattner et al., 2015). While we did not directly test Cdk5 activity, an increase in p25 along with increased phosphorylation of GR at the Cdk5 phosphorylation site (S211), suggests an increase in the activity of this kinase. This suggests that Cav1.2 may be important for stress-induced activation of Cdk5. As Cdk5 levels are increased in the postmortem dorsolateral PFC of depressed patients (Papadopoulou et al., 2015), Cav1.2-activated p25/Cdk5 signaling may contribute to the neuropathology underlying chronic stress-induced neuropsychiatric disorders, particularly in patients harboring CACNA1C risk SNPs.
Previous studies using pharmacology have identified a key role of LTCCs in mediating GR-induced transcription following corticosterone exposure in cell culture (Basta-Kaim et al., 2002). Our data indicate that a similar mechanism may exist in vivo. Additionally, we identify p25 production as a mechanism by which Cav1.2 may regulate GR phosphorylation and nuclear trafficking. The fact that we only see activation of this pathway 8 days post-stress, when Cav1.2 is elevated, and not 3 days post-stress, when no increase in Cav1.2 is observed, supports the hypothesis that an increase in Cav1.2 in the PFC following chronic stress mediates the activation of the p25/Cdk5-GR pathway. Given that GRs mediate stress-induced transcription of genes that can alter synaptic transmission and synaptic structure (Polman et al., 2012, Ota et al., 2014, Papadopoulou et al., 2015), Cav1.2's recruitment of GR-activated genes may have implications for synaptic architecture and neural connectivity following exposure to chronic stress. For example, the p25/cdk5/GR pathway regulates transcription of the histone deacetylase 2 (Hdac2) gene, which in turn negatively regulates expression of genes related to synaptic function, neuronal plasticity and learning and memory, including Gria1, Gria2, Grin2a, Grin2b, Bdnf, and Syp (Graff et al., 2012, Rei et al., 2015). GR additionally enhances transcription of the Redd1 gene, which is known to mediate chronic stress-induced behavioral and synaptic changes within the PFC (Ota et al., 2014). Future studies will determine whether Cav1.2-mediated GR phosphorylation and nuclear translocation leads to alterations in downstream gene expression, and will look to identify specific Cav1.2/GR-mediated transcriptional profiles and their contribution to persistent behavioral, molecular, and synaptic changes induced by chronic stress.
Chronic exposure to CORT is known to impact behavior in rodents (Kalynchuk et al., 2004, Mishima et al., 2015). Our data indicate that Cav1.2 does not impact stress-induced CORT release; both wildtype and Cav1.2 heterozygous mice showed stress-induced increases in CORT when examined 3 days post-stress, and this increase in CORT normalized by 8 days post-stress in both genotypes. While there were no differences in CORT levels between WT and Cav1.2+/− mice, suggesting that altered activation of the HPA axis does not explain the resiliency of Cav1.2+/− mice to CUS-induced behavioral and molecular changes, it is highly plausible that activation of the HPA axis and increase in CORT release at the early time point following CUS induces increase in Cav1.2 and the downstream p25/Cdk5-GR pathway observed at the time point examined in this study.
5. Conclusion
Our data characterize Cav1.2 channels as key regulators of susceptibility to the persistent effects of chronic stress. We find that exposure to chronic stress increases Cav1.2 protein expression in the PFC, a brain region which shows deficits in neuropsychiatric patients, including CACNA1C SNP carriers. Additionally, we show that Cav1.2 is necessary for the long-lasting effects of chronic stress on depressive-like behavior, anxiety-like behavior and working memory deficits symptoms common to the neuropsychiatric disorders for which CACNA1C mutations confer risk. Lastly, we identify activation of the p25/Cdk5-GR pathway in the PFC as a downstream mediator of the delayed activation of Cav1.2 signaling following chronic stress. These findings shed light on how CACNA1C mutations may impact neuropsychiatric risk and add to our understanding of how genetic predisposition and environment interact to impact mental health.
Conflicts of interest
Authors report no conflict of interest.
Funding sources
This work was supported by funding from The Hartwell Foundation (A.M.R) and the Weill Cornell Autism Research Program (A.M.R).
Acknowledgments
We would like to thank Baila Hall for scientific guidance and educational discussions and Rosa Chen for technical assistance.
Footnotes
Supplementary data related to this article can be found at http://dx.doi.org/10.1016/j.ynstr.2017.02.004.
Appendix A. Supplementary data
The following are the supplementary data related to this article:
Bavley et al._Supp Figure 1-05.
Behavioral analyses of Cav1.2 heterozygous (Cav1.2+/−) and wildtype littermates (WT) following exposure to chronic unpredictable stress (CUS). A) No effect of genotype or CUS was observed on distance traveled in the elevated plus maze (EPM) (genotype: F(1,45) = 0.20, p = 0.66; CUS: F(1,45) = 2.36, p = 0.13; WT control, n = 12; WT CUS, n = 14; Cav1.2+/− control, n = 10; Cav1.2+/− CUS, n = 13). B) No effect of genotype or CUS was observed on total number of alternations in the spontaneous alternation task (genotype: F(1,24) = 2.08, p = 0.16; CUS: F(1,24) = 0.19, p = 0.67; WT control, n = 7; WT CUS, n = 8; Cav1.2+/− control, n = 7; Cav1.2+/− CUS, n = 7). Data are represented as mean ± SEM.
Bavley et al._Supp Figure 2-06.
Western blot analysis of the p25/ Cdk5-GR pathway in the dHPC, vHPC, and amygdala following exposure to chronic unpredictable stress (CUS) in WT and Cav1.2+/− mice. A) CUS increased phosphorylation of the glucocorticoid receptor (GR) at S211 in the dHPC when examined at the early (3 days post-stress) time point (t(7) = 4.44, **p = 0.003) but had no effect on p25 or GR expression (control, n = 7; CUS, n = 7). In the vHPC and amygdala, CUS had no effect on protein levels of p25, pGR S211 or GR when examined at the early time point (control, n = 7; CUS, n = 7). B) CUS had no effect on protein levels of p25, pGR S211 or GR in the dHPC, vHPC or amygdala when examined at late (8 days post-stress) time point (control, n = 6; CUS, n = 5–7). Data are represented as mean ± SEM.
References
- Adhikari A., Topiwala M.A., Gordon J.A. Synchronized activity between the ventral hippocampus and the medial prefrontal cortex during anxiety. Neuron. 2010;65:257–269. doi: 10.1016/j.neuron.2009.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adhikari A., Lerner T.N., Finkelstein J., Pak S., Jennings J.H., Davidson T.J., Ferenczi E., Gunaydin L.A., Mirzabekov J.J., Ye L., Kim S.Y., Lei A., Deisseroth K. Basomedial amygdala mediates top-down control of anxiety and fear. Nature. 2015;527:179–185. doi: 10.1038/nature15698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adzic M., Djordjevic J., Djordjevic A., Niciforovic A., Demonacos C., Radojcic M., Krstic-Demonacos M. Acute or chronic stress induce cell compartment-specific phosphorylation of glucocorticoid receptor and alter its transcriptional activity in Wistar rat brain. J. Endocrinol. 2009;202:87–97. doi: 10.1677/JOE-08-0509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ament S.A., Szelinger S., Glusman G., Ashworth J., Hou L., Akula N., Shekhtman T., Badner J.A., Brunkow M.E., Mauldin D.E., Stittrich A.B., Rouleau K., Detera-Wadleigh S.D., Nurnberger J.I., Jr., Edenberg H.J., Gershon E.S., Schork N., Bipolar Genome Study, Price ND. Gelinas R., Hood L., Craig D., McMahon F.J., Kelsoe J.R., Roach J.C. Rare variants in neuronal excitability genes influence risk for bipolar disorder. Proc. Natl. Acad. Sci. 2015;112:3576–3581. doi: 10.1073/pnas.1424958112. (U.S.A) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bader P.L., Faizi M., Kim L.H., Owen S.F., Tadross M.R., Alfa R.W., Bett G.C., Tsien R.W., Rasmusson R.L., Shamloo M. Mouse model of Timothy syndrome recapitulates triad of autistic traits. Proc. Natl. Acad. Sci. 2011;108:15432–15437. doi: 10.1073/pnas.1112667108. (U.S.A.) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barik J., Marti F., Morel C., Fernandez S.P., Lanteri C., Godeheu G., Tassin J.P., Mombereau C., Faure P., Tronche F. Chronic stress triggers social aversion via glucocorticoid receptor in dopaminoceptive neurons. Science. 2013;339:332–335. doi: 10.1126/science.1226767. [DOI] [PubMed] [Google Scholar]
- Basta-Kaim A., Budziszewska B., Jaworska-Feil L., Tetich M., Leśkiewicz M., Kubera M., Lasoń W. Chlorpromazine inhibits the glucocorticoid receptor-mediated gene transcription in a calcium-dependent manner. Neuropharmacology. 2002;43:1035–1043. doi: 10.1016/s0028-3908(02)00149-1. [DOI] [PubMed] [Google Scholar]
- Bhat S., Dao D.T., Terrillion C.E., Arad M., Smith R.J., Soldatov N.M., Gould T.D. CACNA1C (Cav1.2) in the pathophysiology of psychiatric disease. Prog. Neurobiol. 2012;99:1–14. doi: 10.1016/j.pneurobio.2012.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bigos K.L., Mattay V.S., Callicott J.H., Straub R.E., Vakkalanka R., Kolachana B., Hyde T.M., Lipska B.K., Kleinman J.E., Weinberger D.R. Genetic variation in CACNA1C affects brain circuitries related to mental illness. Archives General Psychiatry. 2010;67:939–945. doi: 10.1001/archgenpsychiatry.2010.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casamassima F., Huang J., Fava M., Sachs G.S., Smoller J.W., Cassano G.B., Lattanzi L., Fagerness J., Stange J.P., Perlis R.H. Phenotypic effects of a bipolar liability gene among individuals with major depressive disorder. Am. J. Med. Genet. Part B Neuropsychiatr. Genet. 2010;153B:303–309. doi: 10.1002/ajmg.b.30962. [DOI] [PubMed] [Google Scholar]
- Chmielarz P., Kuśmierczyk J., Parlato R., Schütz G., Nalepa I., Kreiner G. Inactivation of glucocorticoid receptor in noradrenergic system influences anxiety- and depressive-like behavior in mice. PLoS One. 2013;8:e72632. doi: 10.1371/journal.pone.0072632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dao D.T., Mahon P.B., Cai X., Kovacsics C.E., Blackwell R.A., Arad M., Shi J., Zandi P.P., O'Donnell P., Knowles J.A., Weissman M.M., Cryell W., Scheftner W.A., Lawson W.B., Levinson D.F., Thompson S.M., Potash J.B., Gould T.D. Mood disorder susceptibility gene CACNA1C modifies mood-related behaviors in mice and interacts with sex to influence behavior in mice and diagnosis in humans. Biol. Psychiatry. 2010;68:801–810. doi: 10.1016/j.biopsych.2010.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daskalakis N.P., Binder E.B. Schizophrenia in the spectrum of gene-stress interactions: the FKBP5 example. Schizophr. Bull. 2015;41:323–329. doi: 10.1093/schbul/sbu189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delatour B., Gisquet-Verrier P. Prelimbic cortex specific lesions disrupt delayed-variable response tasks in the rat. Behav. Neurosci. 1996;110:1282–1298. doi: 10.1037//0735-7044.110.6.1282. [DOI] [PubMed] [Google Scholar]
- Divac I., Wikmark R.G.E., Gade A. Spontaneous alternation in rats with lesions in the frontal lobes: an extension of the frontal lobe syndrome. Physiol. Psychol. 2013;3:39–42. [Google Scholar]
- Erburu M., Cajaleon L., Guruceaga E., Venzala E., Muñoz-Cobo I., Beltrán E., Puerta E., Tordera R.M. Chronic mild stress and imipramine treatment elicit opposite changes in behavior and in gene expression in the mouse prefrontal cortex. Pharmacol. Biochem. Behav. 2015;135:227–236. doi: 10.1016/j.pbb.2015.06.001. [DOI] [PubMed] [Google Scholar]
- Elizalde N., Gil-Bea F.J., Ramírez M.J., Aisa B., Lasheras B., Del Rio J., Tordera R.M. Long-lasting behavioral effects and recognition memory deficit induced by chronic mild stress in mice: effect of antidepressant treatment. Psychopharmacol. Berl. 2008;199:1–14. doi: 10.1007/s00213-007-1035-1. [DOI] [PubMed] [Google Scholar]
- Ferreira M.A. Collaborative genome-wide association analysis supports a role for ANK3 and CACNA1C in bipolar disorder. Nat. Genet. 2008;40:1056–1058. doi: 10.1038/ng.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fowles D.C. Schizophrenia: diathesis-stress revisited. Annu. Rev. Psychol. 1992;43:303–336. doi: 10.1146/annurev.ps.43.020192.001511. [DOI] [PubMed] [Google Scholar]
- Galeano P., Martino Adami P.V., Do Carmo S., Blanco E., Rotondaro C., Capani F., Castaño E.M., Cuello A.C., Morelli L. Longitudinal analysis of the behavioral phenotype in a novel transgenic rat model of early stages of Alzheimer's disease. Front. Behav. Neurosci. 2014;8:321. doi: 10.3389/fnbeh.2014.00321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galliher-Beckley A., Cidlowski J.A. Emerging roles of glucocorticoid receptor phosphorylation in modulating glucocorticoid hormone action in health and disease. IUBMB Life. 2009;61:979–986. doi: 10.1002/iub.245. [DOI] [PubMed] [Google Scholar]
- Gourley S., Taylor J.R. Recapitulation and reversal of a persistent depression-like syndrome in rodents. Curr. Protoc. Neurosci. 2009 doi: 10.1002/0471142301.ns0932s49. (Chapter 9): Unit 9.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graff J., Rei D., Guan J.S., Wang W.Y., Seo J., Hennig K.M., Nieland T.J., Fass D.M., Kao P.F., Kahn M., Su S.C., Samiei A., Joseph N., Haggarty S.J., Delalle I., Tsai L.H. An epigenetic blockade of cognitive functions in the neurodegenerating brain. Nature. 2012;483:222–226. doi: 10.1038/nature10849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green E.K., Grozeva D., Jones I., Jones L., Kirov G., Caesar S., Gordon-Smith K., Fraser C., Forty L., Russell E., Hamshere M.L., Moskvina V., Nikolov I., Farmer A., McGuffin P., Wellcome Trust Case Control Consortium. Holmans P.A., Owen M.J., O'Donovan M.C., Craddock N. The bipolar disorder risk allele at CACNA1C also confers risk of recurrent major depression and of schizophrenia. Mol. Psychiatry. 2010;15:1016–1022. doi: 10.1038/mp.2009.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haile C.N., GrandPre T., Kosten T.A. Chronic unpredictable stress, but not chronic predictable stress, enhances the sensitivity to the behavioral effects of cocaine in rats. Psychopharmacology. 2001;154:213–220. doi: 10.1007/s002130000650. [DOI] [PubMed] [Google Scholar]
- Hammen C., Gitlin M. Stress reactivity in bipolar patients and its relation to prior history of disorder. Am. J. Psychiatry. 1997;154:856–857. doi: 10.1176/ajp.154.6.856. [DOI] [PubMed] [Google Scholar]
- Hell J.W., Westenbroek R.E., Warner C., Ahlijanian M.K., Prystay W., Gilbert M.M., Snutch T.P., Catterall W.T. Identification and differential subcellular localization of the neuronal class C and class D L-type calcium channel alpha 1 subunits. J. Cell Biol. 1993;123:949–962. doi: 10.1083/jcb.123.4.949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heyes S., Pratt W.S., Rees E., Dahimene S., Ferron L., Owen M.J., Dolphin A.C. Genetic disruption of voltage-gated calcium channels in psychiatric and neurological disorders. Prog. Neurobiol. 2015;134:36–54. doi: 10.1016/j.pneurobio.2015.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izquierdo A., Wellman C.L., Holmes A. Brief uncontrollable stress causes dendritic retraction in infralimbic cortex and resistance to fear extinction in mice. J. Neurosci. 2006;26:5733–5738. doi: 10.1523/JNEUROSCI.0474-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeon D., Kim S., Chetana M., Jo D., Ruley H.E., Lin S.Y., Rabah D., Kinet J.P., Sin H.S. Observational fear learning involves affective pain system and Cav1.2 Ca2+ channels in ACC. Nat. Neurosci. 2010;13:482–488. doi: 10.1038/nn.2504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jinks A., McGregor I. Modulation of anxiety-related behaviours following lesions of the prelimbic or infralimbic cortex in the rat. Brain Res. 1997;772:181–190. doi: 10.1016/s0006-8993(97)00810-x. [DOI] [PubMed] [Google Scholar]
- Kabir Z.D., Lee A.S., Burgdorf C.E., Fischer D.K., Rajadhyaksha A.M., Mok E., Rizzo B., Rice R.C., Singh K., Ota K.T., Gerhard D.M., Schierberl K.C., Glass M.J., Duman R., Rajadhyaksha A.M. Cacna1c in the prefrontal cortex regulates depression-related behaviors via REDD1. Neuropsychopharmacology. 2017 doi: 10.1038/npp.2016.271. ([Epub ahead of print]) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalynchuk L.E., Gregus A., Boudreau D., Perrot-Sinal T.S. Corticosterone increases depression-like behavior, with some effects on predator odor-induced defensive behavior, in male and female rats. Behav. Neurosci. 2004;118:1365–1377. doi: 10.1037/0735-7044.118.6.1365. [DOI] [PubMed] [Google Scholar]
- Kessler R. The effect of stressful life events on depression. Annu. Rev. Psychol. 1997;48:191–214. doi: 10.1146/annurev.psych.48.1.191. [DOI] [PubMed] [Google Scholar]
- Kino T., Ichijo T., Amin N.D., Kesavapany S., Wang Y., Kim N., Rao S., Player A., Zheng Y.L., Garabedian M.J., Kawasaki E., Pant H.C., Chrousos G.P. Cyclin-dependent kinase 5 differentially regulates the transcriptional activity of the glucocorticoid receptor through phosphorylation: clinical implications for the nervous system response to glucocorticoids and stress. Mol. Endocrinol. 2007;21:1552–1568. doi: 10.1210/me.2006-0345. [DOI] [PubMed] [Google Scholar]
- Knackstedt L., Moussawi K., Lalumiere R., Schwendt M., Klugmann M., Kalivas P. Extinction training after cocaine self-administration induces glutamatergic plasticity to inhibit cocaine-seeking. J. Neurosci. 2010;30:7984–7992. doi: 10.1523/JNEUROSCI.1244-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krey J.F., Paşca S.P., Shcheglovitov A., Yazawa M., Schwemberger R., Rasmusson R., Dolmetsch R.E. Timothy syndrome is associated with activity-dependent dendritic retraction in rodent and human neurons. Nat. Neurosci. 2013;16:201–209. doi: 10.1038/nn.3307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar N., Singh N., Jaggi A.S. Anti-stress effects of cilnidipine and nimodipine in immobilization subjected mice. Physiology Behav. 2012;105:1148–1155. doi: 10.1016/j.physbeh.2011.12.011. [DOI] [PubMed] [Google Scholar]
- Kusakawa G., Saito T., Onuki R., Ishiguro K., Kishimoto T., Hisanaga S. J. Biol. Chem. 2000;275:17166–17172. doi: 10.1074/jbc.M907757199. [DOI] [PubMed] [Google Scholar]
- Laroche S., Davis S., Jay T.M. Plasticity at hippocampal to prefrontal cortex synapses: dual roles in working memory and consolidation. Hippoacmpus. 2000;10:438–446. doi: 10.1002/1098-1063(2000)10:4<438::AID-HIPO10>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- Lazary J., Eszlari N., Juhasz G., Bagdy G. Genetically reduced FAAH activity may be a risk for the development of anxiety and depression in persons with repetitive childhood trauma. Eur. Neuropsychopharmacol. 2016;26:1020–1028. doi: 10.1016/j.euroneuro.2016.03.003. [DOI] [PubMed] [Google Scholar]
- Lee A.S., Ra S., Rajadhyaksha A., Britt J., De Jesus-Cortes H., Gonzales K.L., Lee A., Moosmang S., Hofmann F., Pieper A.A., Rajadhyaksha A.M. Forebrain elimination of cacna1c mediates anxiety-like behavior in mice. Mol. Psychiatry. 2012;17:1054–1055. doi: 10.1038/mp.2012.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liston C., Miller M.M., Goldwater D.S., Radley J.J., Rocher A.B., Hof P.R., Morrison J.H., McEwen B.S. Stress-induced alterations in prefrontal cortical dendritic morphology predict selective impairments in perceptual attentional set-shifting. J. Neurosci. 2006;26:7870–7874. doi: 10.1523/JNEUROSCI.1184-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovallo W.R., Enoch M.A., Acheson A., Cohoon A.J., Sorocco K.H., Hodgkinson C.A., Vincent A.S., Goldman D. Early-life adversity interacts with FKBP5 genotypes: altered working memory and cardiac stress reactivity in the Oklahoma family health patterns project. Neuropsychopharmacology. 2015;41:1724–1732. doi: 10.1038/npp.2015.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maigaard K., Hageman I., Jørgensen A., Jørgensen M., Wörtwein G. Electroconvulsive stimulations prevent chronic stress-induced increases in L-type calcium channel mRNAs in the hippocampus and basolateral amygdala. Neurosci. Lett. 2012;51:24–28. doi: 10.1016/j.neulet.2012.03.043. [DOI] [PubMed] [Google Scholar]
- Matuszewich L., Karney J.J., Carter S.R., Janasik S.P., O'Brien J.L., Friedman R.D. The delayed effects of chronic unpredictable stress on anxiety measures. Physiology Behav. 2007;90:674–681. doi: 10.1016/j.physbeh.2006.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mineur Y., Belzung C., Crusio W. Effects of unpredictable chronic mild stress on anxiety and depression-like behavior in mice. Behav. Brain Res. 2006;175:43–50. doi: 10.1016/j.bbr.2006.07.029. [DOI] [PubMed] [Google Scholar]
- Mishima Y., Shinoda Y., Sadakata T., Kojima M., Wakana S., Furuichi T. Lack of stress responses to long-term effects of corticosterone in Caps2 knockout mice. Sci. Rep. 2015;5 doi: 10.1038/srep08932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monteiro S., Roque S., de Sá-Calçada D., Sousa N., Correia-Neves M., Cerqueira J.J. An efficient chronic unpredictable stress protocol to induce stress-related responses in C57BL/6 mice. Front. Psychiatry. 2015;6:1–11. doi: 10.3389/fpsyt.2015.00006. article 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moosmang S., Haider N., Klugbauer N., Adelsberger H., Langwieser N., Müller J., Stiess M., Marais E., Schulla V., Lacinova L., Goebbels S., Nave K.A., Storm D.R., Hofmann F., Kleppisch T. Role of Hippocampal Cav1.2 Ca2+ channels in NMDA receptor-independent syanptic plasticity and spatial memory. J. Neurosci. 2005;25:9883–9892. doi: 10.1523/JNEUROSCI.1531-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Negrón-Oyarzo I., Aboitiz F., Fuentealba P. Impaired functional connectivity in the prefrontal cortex: a mechanism for chronic stress-induced neuropsychiatric disorders. Neural Plast. 2016;2016 doi: 10.1155/2016/7539065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nestler E.J., Hyman S.E. Animal models of neuropsychiatric disorders. Nat. Neurosci. 2010;13:1161–1169. doi: 10.1038/nn.2647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nyegaard M., Demontis D., Foldager L., Hedemand A., Flint T.J., Sørensen K.M., Andersen P.S., Nordentoft M., Werge T., Pedersen C.B., Hougaard D.M., Mortensen P.B., Mors O., Børglum A.D. CACNA1C (rs1006737) is associated with schizophrenia. Mol. Psychiatry. 2010;15:119–121. doi: 10.1038/mp.2009.69. [DOI] [PubMed] [Google Scholar]
- Ota K.T., Liu R.J., Voleti B., Maldonado-Aviles J.G., Duric V., Iwata M., Dutheil S., Duman C., Boikess S., Lewis D.A., Stockmeier C.A., Dileone R.J., Rex C., Aghajanian G.K., Duman R.S. REDD1 is essential for stress-induced synaptic loss and depressive behavior. Nat. Med. 2014;20:531–535. doi: 10.1038/nm.3513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papadopoulou A., Siamatras T., Delgado-Morales R., Amin N.D., Shukla V., Zheng Y.L., Pant H.C., Almeida O.F., Kino T. Acute and chronic stress differentially regulate cyclin-dependent kinase 5 in mouse brain: implications to glucocorticoid actions and major depression. Transl. Psychiatry. 2015;5:e578. doi: 10.1038/tp.2015.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papp M., Lappas S., Muscat R., Willner P. Attenuation of place preference conditioning but not place aversion conditioning by chronic mild stress. J. Psychopharmacol. 1992;6:352–356. doi: 10.1177/026988119200600302. [DOI] [PubMed] [Google Scholar]
- Paşca S.P., Portmann T., Voineagu I., Yazawa M., Shcheglovitov A., Paşca A.M., Cord B., Palmer T.D., Chikahisa S., Nishino S., Bernstein J.A., Hallmayer J., Geschwind D.H., Dolmetsch R.E. Using iPSC-derived neurons to uncover cellular phenotypes associated with Timothy syndrome. Nat. Med. 2011;17:1657–1662. doi: 10.1038/nm.2576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patrick G.N., Zukerberg L., Nikolic M., de la Monte S., Dikkes P., Tsai L.H. Conversion of p35 to p25 deregulates Cdk5 activity and promotes neurodegeneration. Nature. 1999;402:615–622. doi: 10.1038/45159. [DOI] [PubMed] [Google Scholar]
- Paulus F., Bedenbender J., Krach S., Pyka M., Krug A., Sommer J., Mette M., Nöthen M., Witt S., Rietschel M., Kircher T., Jansen A. Association of rs1006737 in CACNA1C with alterations in prefrontal activation and fronto-hippocampal connectivity. Hum. Brain Mapp. 2014;35:1190–1200. doi: 10.1002/hbm.22244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plattner F., Hayashi K., Hernández A., Benavides D., Tassin T.C., Tan C., Day J., Fina M.W., Yuen E.Y., Yan Z., Goldberg M.S., Nairn A.C., Greengard P., Nestler E.J., Taussig R., Nishi A., Houslay M.D., Bibb J.A. The role of ventral striatal cAMP signaling in stress-induced behaviors. Nat. Neurosci. 2015;18:1094–1100. doi: 10.1038/nn.4066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polman J.A., Welten J.E., Bosch D.S., de Jonge R.T., Balog J., van der Maarel S.M., de Kloet E.R., Datson N.A. A genome-wide signature of glucocorticoid receptor binding in neuronal PC12 cells. BMC Neurosci. 2012;13 doi: 10.1186/1471-2202-13-118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quan M., Zheng C., Zhang N., Han D., Tian Y., Zhang T., Yang Z. Impairments of behavior, information flow between thalamus and cortex, and prefrontal cortical synaptic plasticity in an animal model of depression. Brain Res. Bull. 2011;85:109–116. doi: 10.1016/j.brainresbull.2011.03.002. [DOI] [PubMed] [Google Scholar]
- Rao S., Yao Y., Zheng C., Ryan J., Mao C., Zhang F., Meyre D., Xu Q. Common variants in CACNA1C and MDD susceptibility: a comprehensive meta-analysis. Am. J. Med. Genet. Part B, Neuropsychiatr. Genet. 2016;171:896–903. doi: 10.1002/ajmg.b.32466. [DOI] [PubMed] [Google Scholar]
- Rei D., Mason X., Seo J., Gräff J., Rudenko A., Wang J., Rueda R., Siegert S., Cho S., Canter R., Mungenast A., Deisseroth K., Tsai L.H. Basolateral amygdala bidirectionally modulates stress-induced hippocampal learning and memory deficits through a p25/Cdk5-dependent pathway. Proc. Natl. Acad. Sci. 2015;112:7291–7296. doi: 10.1073/pnas.1415845112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schön C., Paquet-Durand F., Michalakis S. Cav1.4 L-type calcium channels contribute to calpain activation in degenerating photoreceptors of rd1 mice. PLoS One. 2016;11:e0156974. doi: 10.1371/journal.pone.0156974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seisenberger C., Specht V., Welling A., Platzer J., Pfeifer A., Kühbandner S., Striessnig J., Klugbauer N., Feil R., Hofmann F. Functional embryonic cardiomyocytes after disruption of the L-type alpha1C (Cav1.2) calcium channel gene in the mouse. J. Biol. Chem. 2000;275:39193–39199. doi: 10.1074/jbc.M006467200. [DOI] [PubMed] [Google Scholar]
- Shah A., Treit D. Excitotoxic lesions of the medial prefrontal cortex attenuate fear responses in the elevated-plus maze, social interaction and shock probe burying tests. Brain Res. 2003;969:183–194. doi: 10.1016/s0006-8993(03)02299-6. [DOI] [PubMed] [Google Scholar]
- Sierksma A.S., van den Hove D.L., Pfau F., Philippens M., Bruno O., Fedele E., Ricciarelli R., Steinbusch H.W., Vanmierlo T., Prickaerts J. Improvement of spatial memory function in APPswe/PS1dE9 mice after chronic inhibition of phosphodiesterase type 4D. Neuropharmacology. 2013;77:120–130. doi: 10.1016/j.neuropharm.2013.09.015. [DOI] [PubMed] [Google Scholar]
- Spellman T., Rigotti M., Ahmari S.E., Fusi S., Gogos J.A., Gordon J.A. Hippocampal-prefrontal input supports spatial encoding in working memory. Nature. 2015;522:309–314. doi: 10.1038/nature14445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su S.C., Tsai L.H. Cyclin-dependent kinases in brain development and disease. Annu. Rev. Cell Dev. Biol. 2011;27:465–491. doi: 10.1146/annurev-cellbio-092910-154023. [DOI] [PubMed] [Google Scholar]
- Tian Y., Voineagu I., Paşca S.P., Won H., Chandran V., Horvath S., Dolmetsch R.E., Geschwind D.H. Alteration in basal and depolarization induced transcriptional network in iPSC derived neurons from Timothy syndrome. Genome Med. 2014;6:75. doi: 10.1186/s13073-014-0075-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang F., McIntosh A.M., He Y., Gelernter J., Blumberg H. The association of genetic variation in CACNA1C with structure and function of a frontotemporal system. Bipolar Disord. 2011;13:696–700. doi: 10.1111/j.1399-5618.2011.00963.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z., Chen W., Kono E., Dang T., Garabedian M. Modulation of glucocorticoid receptor phosphorylation and transcriptional activity by a C-Terminal-Associated protein phosphatase. Mol. Endocrinol. 2007;21:625–634. doi: 10.1210/me.2005-0338. [DOI] [PubMed] [Google Scholar]
- Warden M.R., Selimbeyoglu A., Mirzabekov J.J., Lo M., Thompson K.R., Kim S.Y., Adhikari A., Tye K.M., Frank L.M., Deisseroth K. A prefrontal cortex-brainstem neuronal projection that controls response to behavioural challenge. Nature. 2012;492:428–432. doi: 10.1038/nature11617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wietrzych M., Meziane H., Sutter A., Ghyselinck N., Chapman P.F., Chambon P., Krężel1 W. Working memory deficits in retinoid X receptor γ-deficient mice. Learn. Mem. 2005;12:318–326. doi: 10.1101/lm.89805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willner P. The chronic mild stress (CMS) model of depression: history, evaluation and usage. Neurobiol. stress. 2016 doi: 10.1016/j.ynstr.2016.08.002. http://dx.doi.org/10.1016/j.ynstr.2016.08.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang C.H., Huang C.C., Hsu K.S. A critical role for protein tyrosine phosphatase nonreceptor type 5 in determining individual susceptibility to develop stress-related cognitive and morphological changes. J. Neurosci. 2012;32:7550–7562. doi: 10.1523/JNEUROSCI.5902-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]