Abstract
Looking at the hand can reduce the perception of pain and the magnitude of the event-related potentials (ERPs) elicited by nociceptive stimuli delivered onto the hand, whereas it can increase that of tactile ERPs. These differences could be related to the use of different experimental designs. Importantly, most studies on the effects of vision on pain have relied on a mirror to create the illusion that the reflected hand is a direct view of the stimulated hand. Here, we compared the effects of direct vs. mirror vision of the hand vs. an object on the perception and ERPs elicited by non-nociceptive and nociceptive stimuli. We did not observe any significant effect of vision on the perceived intensity. Vision of the hand reduced the nociceptive N240, and enhanced the non-nociceptive P200. Our results confirm that vision of the body differentially affects nociceptive and non-nociceptive processing, but question the robustness of visual analgesia.
Keywords: Vision, nociception, touch, event-related potentials, perception
1. Introduction
Experimental research on pain has highlighted the importance of the interactions between attention, multisensory integration and the perception of pain (Haggard, Iannetti, & Longo, 2013; Legrain, Iannetti, Plaghki, & Mouraux, 2011; Legrain, et al., 2012; Trojan, Diers, Valenzuela-Moguilllansky, & Torta, 2014). However, the actual mechanisms underlying these interactions are still unclear, and much remains to be done to translate these findings into applicable clinical tools. It has been recently suggested that vision of the body exerts a differential effect on the responses to non-nociceptive and nociceptive somatosensory input. Studies have shown that vision of the hand onto which tactile stimuli are applied improves discrimination performance (Kennett, Taylor-Clarke, & Haggard, 2001) and increases the magnitude of the elicited somatosensory event-related potentials (ERPs) (Taylor-Clarke, Kennett, & Haggard, 2002). This effect has been interpreted as resulting from a crossmodal effect of spatial attention: directing visual attention towards the stimulated hand would enhance the processing of somatosensory input originating from that hand (Taylor-Clarke, Kennett, & Haggard, 2002). Contrasting with these results, it was recently suggested that looking at the hand onto which nociceptive somatosensory stimuli are applied reduces the intensity of the elicited pain percept (Longo, Betti, Aglioti, & Haggard, 2009; Longo, Iannetti, Mancini, Driver, & Haggard, 2012; Mancini, Longo, Canzoneri, Vallar, & Haggard, 2013), as well as the magnitude of the elicited nociceptive ERPs (Longo, Betti, Aglioti, & Haggard, 2009), but see also (Hofle, Hauck, Engel, & Senkowski, 2012; Hofle, Pomper, Hauck, Engel, & Senkowski, 2013).
The notion that looking at the body part onto which somatosensory stimuli are applied induces differential effects on nociceptive and non-nociceptive somatosensory processing is highly interesting, as it suggests the existence of complex multisensory interactions between touch, pain and the other senses (De Paepe, Crombez, Spence, & Legrain, 2014; Favril, Mouraux, Sambo, & Legrain, 2014). However, the reported differences could result from many factors, including differences in experimental design, methods and outcome measures; as these studies did not compare directly the effects on the perception and brain responses to non-nociceptive and nociceptive inputs using the same experimental design. Most importantly, various means have been used to avoid the confounding effect of viewing the stimulator when viewing the stimulated body part. For example, most studies on the effect of viewing the hand on the responses to nociceptive stimuli applied to that hand have used a mirror aligned with the sagittal plane of the participant to create the illusion that the hand reflected in the mirror is a direct view of the stimulated hand (Longo, Betti, Aglioti, & Haggard, 2009; Longo, Iannetti, Mancini, Driver, & Haggard, 2012; Mancini, Longo, Canzoneri, Vallar, & Haggard, 2013). Small inconsistencies in the illusion could create a conflict between somatosensory, proprioceptive and visual inputs. Therefore, the observed reduction of the responses to nociceptive stimuli when viewing the hand could be due, at least in part, to the mirror illusion rather than to the actual viewing of the hand.
The aim of the present study was to compare the effects of vision of the stimulated body part on the intensity of perception and ERPs elicited by nociceptive and non-nociceptive somatosensory stimuli using strictly identical experimental designs within participants. We used a setup that allowed hiding the nociceptive stimulator from the view of the participant. This allowed assessing the specific effect of direct vs. mirror vision of the hand on the elicited responses. Based on previous findings suggesting that vision influences differentially nociception and touch, we hypothesized that direct vision of the stimulated hand would decrease the responses elicited by nociceptive stimulation and increase the responses elicited by non-nociceptive stimulation.
2. Materials and Methods
2.1. Participants
Sixteen subjects took part in the experiment (7 women, 9 men, mean age 26.2± 2.8, 1 left handed). Participants were recruited among students and staff of the university and were naïve to the aims of the study. Informed written consent was obtained before the beginning of the study, which was approved by the Ethics Committee of the Université catholique de Louvain.
2.2. Nociceptive and non-nociceptive somatosensory stimuli
Nociceptive and non-nociceptive somatosensory stimuli were delivered to the right hand. The nociceptive stimulus consisted of a 20 ms pulse of radiant heat generated by an infrared CO2 laser stimulator (wavelength 10.6 μm; Université catholique de Louvain). Beam surface area at target site was 40 mm2. The target of the laser beam was adjusted using a computer-controlled high-speed 2-axis galvanometer (LSST-10.6-32-3808; Sintec Optronics, Singapore), hidden from the view of the participant. In a preliminary session, the stimulation target was visualized using a coaxial He–Ne laser beam such as to delimit the area on the hand dorsum onto which the stimuli could be applied. Then, the power of the laser was adjusted such that the stimuli elicited a clear pinprick sensation, detected with reaction times compatible with the conduction velocity of Aδ- fibers (<650 ms). The mean energy density of the stimulus was 15.5 ±3.5 mJ/mm2. This intensity is clearly above the energy required to activate heat-sensitive Aδ nociceptors and evoke a pinprick sensation (Mouraux, Guerit, & Plaghki, 2003). The non-nociceptive stimulus consisted in a non-painful 0.5 ms constant-current square wave electrical pulses (DS7 stimulator, Digitimer Ltd, UK) delivered to the right median nerve at the level of the wrist using a pair of square adhesive electrodes (5x5 mm) separated by approximately 20 mm. The intensity of the stimulation was set at twice the absolute detection threshold, established using the method of limits. The mean intensity was 2.27 ±0.65 mA.
2.3. Experimental procedure
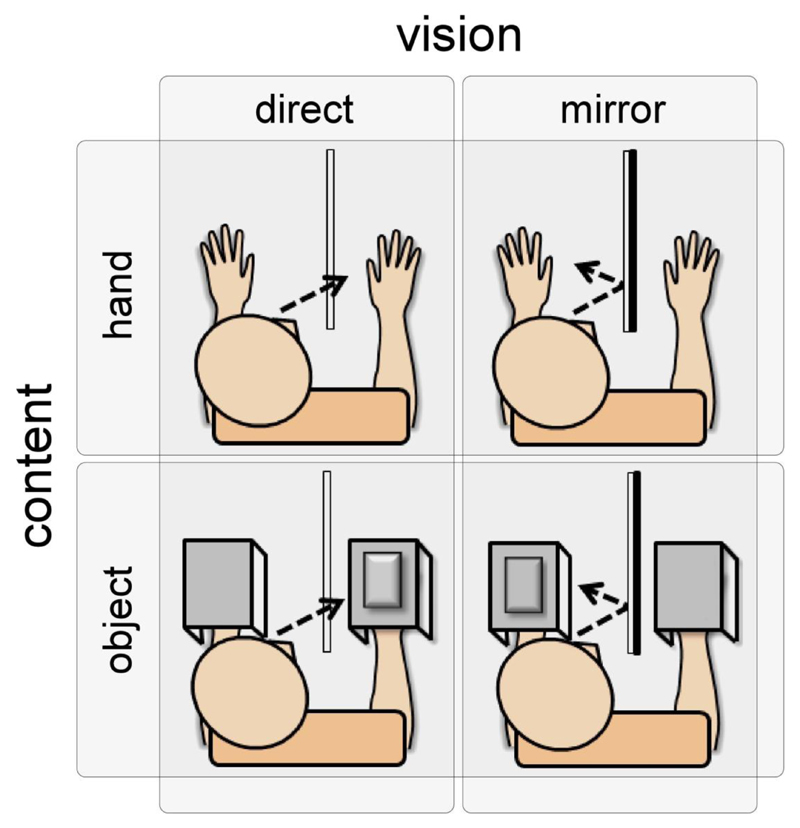
During the experiment, the participant was comfortably seated in front of a table onto which he/she placed his/her hands. The experiment included four experimental conditions aiming at teasing out the respective effects of direct vs. mirror vision of the hand vs. an object on the perception and ERPs elicited by nociceptive vs. non-nociceptive somatosensory stimuli (Figure 1).
Figure 1.
Experimental setup. In separate blocks, nociceptive laser stimuli and non-nociceptive electrical stimuli were delivered to the right hand while participants (1) looked directly at the stimulated hand, (2) looked directly at an object positioned on top of the stimulated hand, (3) looked at the reflection of the contralateral hand through a mirror and (4) looked at the reflection of an object positioned on top of their contralateral hand through a mirror. Both the nociceptive and the non-nociceptive stimuli were hidden from the participant.
The conditions were counterbalanced across participants. In the first experimental condition (direct vision of the hand), a transparent glass allowing direct vision of the right hand was placed on the body midline of the participant. In the second experimental condition (mirror vision of the hand) the glass was replaced by a mirror. Participants looked at the reflection of their left hand in the mirror, creating the illusion of looking at the right hand. In the third experimental condition (direct vision of the object), both hands were covered by a 31x22x7 cm box surmounted by an object, a black 22x15x2 cm book. Participants looked directly at the object placed on top of the right hand. In the fourth experimental condition (mirror vision of the object), the book was positioned on top of the box covering the left hand. Participants looked at the object reflected in the mirror, creating the illusion that both hands were covered by a box and object. The right hand was also covered by a box, in order to keep constant any peripheral sensation of the box touching the skin. An opening at the back of the box allowed the application of laser stimuli without any concomitant vision of the hand.
For each of the four experimental conditions, nociceptive and non-nociceptive stimuli were delivered in separate blocks, resulting in a total of eight blocks. The modality of the first block (nociceptive or non-nociceptive) was balanced across participants. In each block, 20 stimuli were delivered. The inter stimulus interval ranged between 5 and 8 seconds. After each nociceptive stimulus, the target of the laser beam was displaced to one of the predefined positions using the computer-controlled galvanometer, in order to avoid skin overheating and nociceptor fatigue or sensitization. The minimum distance between two consecutive stimuli was 20 mm. Importantly, the He-Ne laser was switched off such that participants could not see the target of the laser when directly viewing the stimulated hand. White noise was played for the whole duration of the experiment to mask any noise produced by the galvanometer.
2.4. Behavioral measures
Participants were asked to provide a verbal rating of the intensity of the perception elicited by each stimulus using a numerical rating scale (NRS) ranging from 0 (no sensation) to 10 (most intense sensation). Ratings were collected approximately 3 seconds after each stimulus.
At the end of the experiment, participants had to respond to a Likert questionnaire assessing how much they felt they were looking directly at the hand rather than a mirror image of their hand, and how much they felt they were looking at their left or right hand. Ratings ranged from -3 (strongly disagree) to +3 (strongly agree) for the first question and from -100 (strongly left) to+100 (strongly right) for the second question (Longo, Betti, Aglioti, & Haggard, 2009).
2.5. Electrophysiological measures
The EEG was recorded at a 1 kHz sampling rate using a 64-channel amplifier and digitizer (ASA-LAB EEG system; Advanced Neuro Technologies, The Netherlands). Scalp signals were acquired with an average reference, using 64 shielded electrodes, positioned according to the 10-10 system (Waveguard; Advanced Neuro Technologies, The Netherlands). The ground electrode was positioned at FCz. A pair of bipolar electrodes placed at the upper-left and lower-right sides of the right eye were used to record eye-blinks and ocular movement.
Analysis of the EEG data was carried out using Letswave 5 (http://nocions.webnode.com/letswave). The continuous EEG recordings were band-pass filtered using 0.5-30 Hz Butterworth zero phase filter, segmented in 1.5 s epochs extending from -0.5 to +1 s relative to stimulus onset. EOG artifacts were subtracted using a validated method based on independent component analysis (ICA; (Jung, et al., 2000). In all datasets, ICs related to eye movements had a large EOG channel contribution and a frontal scalp distribution. Baseline correction was performed using the -0.5 to 0 s pre-stimulus interval. Epochs exceeding ± 100 µV were excluded. For each participant, eight separate average waveforms were computed for each experimental condition (direct vs. mirror vision of the hand vs. object) and type of stimulus (nociceptive vs. non-nociceptive).
2.6. Statistical analysis
Our primary aim was to assess the differential effects of direct vs. mirror vision of the hand vs. a neutral object on the responses elicited by nociceptive and non-nociceptive somatosensory delivered to the hand.
For this purpose, we compared the intensity of perception using a three-way repeated measures ANOVA with the factors ‘modality’ (nociceptive vs. non nociceptive), ‘vision’ (direct vs. mirror vision) and ‘content’ (viewing the hand vs. viewing the object). The Greenhouse-Geisser correction was used to correct for violations of sphericity. When appropriate, tests of within-subjects contrasts were used. With these analyses, a significant interaction between the factors modality and content would be indicative of a differential effect of viewing the hands on the perception of nociceptive and non-nociceptive stimuli.
To assess the effects of vision and content on the magnitude of the nociceptive and non-nociceptive ERPs, we performed a two-way repeated-measures ANOVA with the factors ‘vision’ (direct vs. mirror vision) and ‘content’ (viewing the hand vs. viewing the object) on each time point of the ERP waveforms. This yielded, for each modality, the time courses of the main effect of vision, the main effect of content and the interaction between the two factors. A cluster-based permutation testing was then used to assess the time intervals during which the ERP waveforms showed significant differences. Cluster-based thresholding is an approach commonly used in neuroimaging. The technique assumes that true neural activity will tend to generate signal changes over contiguous time points (Forman, et al., 1995; Maris & Oostenveld, 2007). First, the raw F-statistic waveforms were thresholded at p <0.05 to identify the clusters of contiguous points showing a significant effect. An estimate of the magnitude of each cluster was then obtained by computing the sum of the F values constituting each cluster. Second, permutation testing (1000 random permutations of the conditions within all subjects) was used to assess the distribution of cluster magnitudes in the permuted data. This was then used to define, for each within-subject effect, a cluster magnitude threshold, set at Z>2 standard deviations from the mean. Applying this cluster threshold yielded waveforms highlighting the time intervals where the ERP waveforms showed a significant effect of vision, a significant effect of content and/or a significant interaction between the two factors.
2.7. Control experiment
Because our behavioral results did not replicate the modulation effect of viewing the hands reported in previous studies, we conducted a control experiment in eight additional subjects (6 women, 2 men, mean age 27.5± 3.6, all right handed) to examine whether this could be due (1) to the fact that participants were not explicitly asked to look passively for 60 seconds at their hand or the object before each block (Longo, Betti, Aglioti, & Haggard, 2009) and/or (2) to the use of a numerical rating scale extending from 0 to 10 instead of from 0 to 100 (Longo, Betti, Aglioti, & Haggard, 2009; Longo, Iannetti, Mancini, Driver, & Haggard, 2012). For this purpose, in the control experiment, participants were asked to passively look at the hand or object for 60 s before the beginning of each block (as in Longo et al., 2009). Furthermore, participants were asked to provide a rating of stimulus intensity as well as a rating of unpleasantness using a 0-100 scale. Finally, (3) we increased the duration of the laser stimulus from 20 ms to 40 ms such as to elicit a more consistent and long-lasting pain percept. Indeed, because of their difference in wavelength, the CO2 laser used in the present study only heats the most superficial layers of the skin whereas the Nd:YAP laser used in other studies penetrates more deeply, thereby heating a greater volume of skin tissue for a longer time. The mean energy density of the stimulus was 20.3 ±7.3 mJ/mm2. All other experimental factors were identical to the main experiment except for the fact that the EEG was not recorded.
3. Results
3.1. Behavioral results
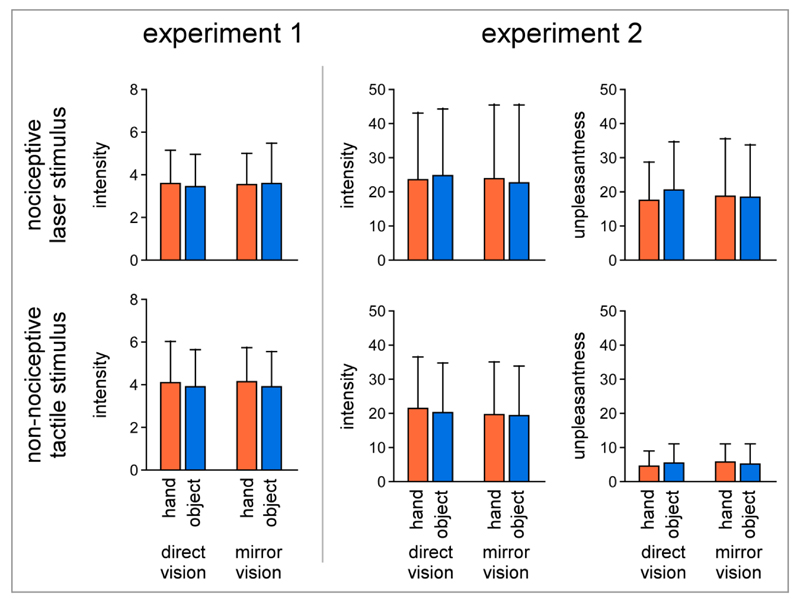
The three-way repeated-measures ANOVA performed on the intensity of perception did not show significant main effects of ‘modality’, ‘vision’ or ‘content’ on the perceived intensity of nociceptive and non-nociceptive stimuli, both in the main experiment and in the control experiment. Most importantly, there were no significant interaction effects (see Figure 2, Table 1 and Table 2).
Figure 2.
Behavioral results. In both experiments, there was no significant effect of vision on the ratings of intensity of the percept elicited by nociceptive and non-nociceptive stimuli. In experiment 2, there was also no effect of vision on the perceived unpleasantness of the stimuli.
Table 1.
Statistics of the ratings of intensity of perception in experiment 1. The repeated-measures ANOVA did not reveal any significant effect of the factors ‘vision’ and ‘content’ on the ratings of intensity obtained for both modalities on a scale ranging from 0 to 10.
| F value | p-value | Partial eta square | |
|---|---|---|---|
| Modality | 0.690 | 0.434 | 0.090 |
| Content | 0.005 | 0.944 | 0.001 |
| Vision | 0.461 | 0.519 | 0.062 |
| Modality x Content | 0.365 | 0.565 | 0.050 |
| Modality x Vision | 0.000 | 0.991 | 0.000 |
| Content x Vision | 0.135 | 0.724 | 0.019 |
| Modality x Content x Vision | 0.556 | 0.480 | 0.074 |
Table 2.
Statistics of the ratings of intensity of perception in experiment 2. Such as for the first experiment, the repeated-measures ANOVA did not reveal any significant effect of the factors ‘vision’ and ‘content’ on the perceived intensity of the somatosensory nociceptive and non-nociceptive stimuli. Ratings of intensity were obtained, in this experiment, on a scale ranging from 0 to 100.
| F value | p-value | Partial eta square | |
|---|---|---|---|
| Modality | 3.985 | 0.064 | 0.210 |
| Content | 0.811 | 0.382 | 0.051 |
| Vision | 0.003 | 0.955 | 0.000 |
| Modality x Content | 0.145 | 0.708 | 0.010 |
| Modality x Vision | 0.238 | 0.633 | 0.016 |
| Content x Vision | 0.145 | 0.708 | 0.010 |
| Modality x Content x Vision | 0.783 | 0.390 | 0.050 |
The three-way repeated-measures ANOVA performed on the ratings of unpleasantness in Experiment 2 showed a significant effect of ‘modality’ F(1,7)=10.560 p=0.014 η2 = 0.601, but no significant main effect of ‘vision’ or ‘content’, and no significant interaction between the factors (see Figure 2 and Table 3).
Table 3.
Statistics of the ratings of unpleasantness experiment 2. the repeated-measures ANOVA did not reveal any significant effect of the factors ‘vision’ and ‘content’ on the perceived unpleasantness of the somatosensory nociceptive and non-nociceptive stimuli. Ratings were obtained on a 0-100 scale.
| F value | p-value | Partial eta square | |
|---|---|---|---|
| Modality | 10.560 | 0.014 | 0.601 |
| Content | 1.423 | 0.272 | 0.169 |
| Vision | 0.007 | 0.935 | 0.001 |
| Modality x Content | 1.063 | 0.337 | 0.132 |
| Modality x Vision | 0.663 | 0.442 | 0.086 |
| Content x Vision | 1.974 | 0.203 | 0.220 |
| Modality x Content x Vision | 0.715 | 0.426 | 0.093 |
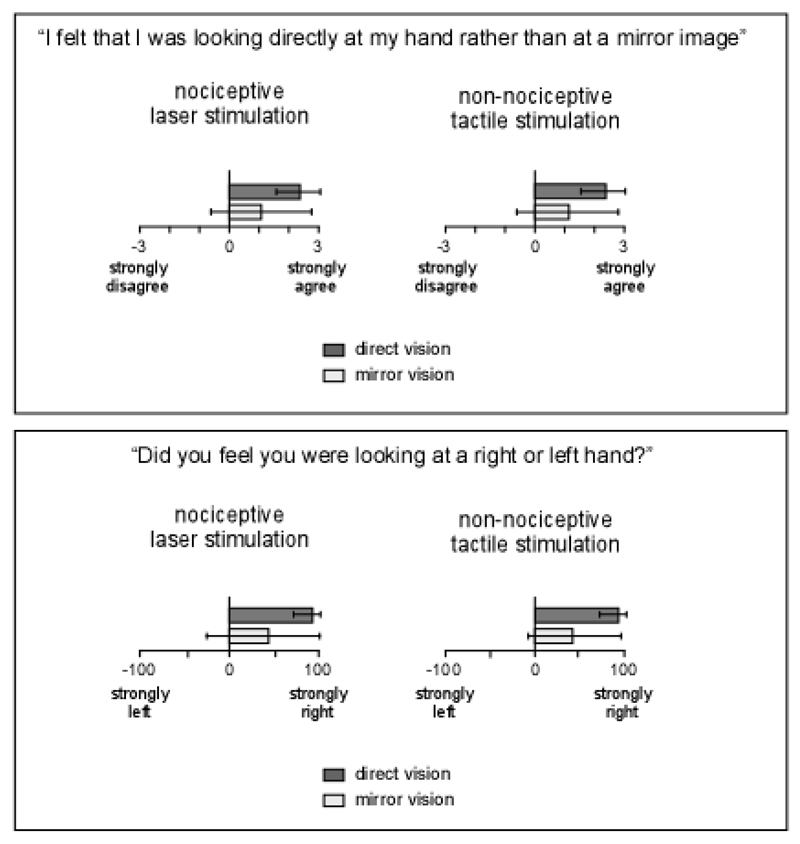
Results of the Likert questionnaire showed that, on average, when participants looked directly at their hand through the glass window, they felt more strongly that they were looking directly at their hand (laser p=0.016, electrical p=0.025), and felt more strongly that the hand they were looking at was a right hand (laser p=0.014, electrical p=0.003) (Figure 3). This suggests that looking at the hand through the mirror did not equal looking at the hand through the glass.
Figure 3.
Participants’ report data from experiment 1. Bars indicate SD
3.2. Electrophysiological results
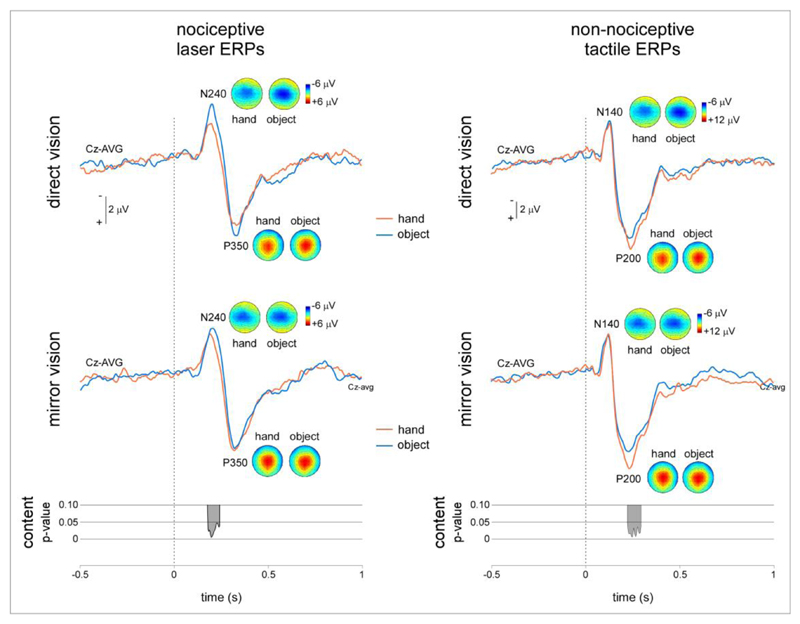
In each experimental condition and each participant, nociceptive and non-nociceptive somatosensory stimuli elicited a consistent ERP maximal at the scalp vertex (Figure 3). Following nociceptive stimulation, this consisted in a negative peak (N240: 213 ±31 ms) followed by a positive peak (P350: 320 ±33 ms). Both peaks were maximal at the scalp vertex. Following non-nociceptive stimulation, this consisted in a negative peak (N140: 127 ±17 ms) followed by a positive peak (P200: 233 ±13 ms). Both peaks were also maximal at the scalp vertex, but the negative peak extended towards the central and temporal electrodes contralateral to the stimulated hand.
The results of the point-by-point two-way repeated measures ANOVA with cluster-based thresholding are shown in Figure 4.
Figure 4.
Nociceptive and non-nociceptive ERPs. The magnitude of the nociceptive N240 was, on average, greater during vision of the object as compared to vision of the hand. However, this difference was not significant. The magnitude of the non-nociceptive P200 was greater when during vision of the hand as compared to vision of the object. This difference was significant.
Analysis of the nociceptive ERP waveforms obtained at electrode Cz showed a sustained significant main effect of the factor ‘content’ during the time window corresponding to the latency of the N240 wave (one cluster: 212-276 ms after stimulus onset). There was no main effect of the factor ‘vision’ and no interaction between the two factors. Both during direct vision and during mirror vision, the amplitude of the N240 wave was reduced when participants viewed their hand as compared to when they viewed the object (Figure 4). Analysis of the non-nociceptive ERP waveforms obtained at electrode Cz showed a sustained significant main effect of the factor ‘content’ during the time window corresponding to the latency of the P200 wave (one cluster: 248-320 ms after stimulus onset). There was no main effect of the factor ‘vision’ and no interaction between the two factors. Both during direct vision and mirror vision, the amplitude of the P200 wave was increased when participants viewed their hand as compared to when they viewed the object (Figure 4).
4. Discussion
The aim of the present experiment was to examine whether vision of a body part exerts a differential effect on the perception and cortical processing of non-nociceptive and nociceptive somatosensory inputs applied to that body part. This has been suggested by the results of previous studies examining the effect of vision on either touch or pain (Kennett, Taylor-Clarke, & Haggard, 2001; Longo, Betti, Aglioti, & Haggard, 2009; Longo, Iannetti, Mancini, Driver, & Haggard, 2012; Mancini, Longo, Canzoneri, Vallar, & Haggard, 2013; Taylor-Clarke, Kennett, & Haggard, 2002). However, because no study had so far included nociceptive and non-nociceptive stimuli in the same experimental design, conclusions about this differential effect of vision remained speculative. For this purpose, we compared, within subjects, and using a strictly identical experimental setup, the respective effects of direct vs. mirror vision of the hand vs. a neutral object on the perception and ERPs elicited by nociceptive vs. non-nociceptive somatosensory stimuli delivered to the hand.
The first main result of our study is that we did not replicate the “analgesic effect” of viewing the hand on nociception and pain that has been reported in several previous studies (Longo, Betti, Aglioti, & Haggard, 2009; Longo, Iannetti, Mancini, Driver, & Haggard, 2012; Mancini, Longo, Canzoneri, Vallar, & Haggard, 2013). Indeed, both in the main experiment and in the control experiment, the intensity of the percept elicited by laser stimuli was not reduced by direct or mirror vision of the hand as compared to direct or mirror vision of a neutral object. There was also no effect of vision on the ratings of unpleasantness. Taken together, these negative findings question the robustness of the previously reported effect of “visual analgesia” (Longo, Betti, Aglioti, & Haggard, 2009; Longo, Iannetti, Mancini, Driver, & Haggard, 2012; Mancini, Longo, Canzoneri, Vallar, & Haggard, 2013). At present, we can only speculate on the reasons why viewing the hand clearly did not modulate pain perception in both experiments of the present study.
A non-trivial difference between the present study and previous studies concerns the position of the hands in the different conditions. In previous studies (e.g. (Longo, Betti, Aglioti, & Haggard, 2009)), participants had both hands on the table during mirror vision of the hand but only one hand on the table and the other hand on their lap during mirror vision of the object. In the present experiment, participants always kept both hands on the table to ensure that observed differences were not due to a difference in hand position rather than a difference in vision (when viewing the object, the hand remained on the table and was covered with a box onto which the object was placed). This could be important, as it has been shown that differences in the relative position of the hands can modify the processing of stimuli applied to the hands (Shore, Gray, Spry, & Spence, 2005). It should be noted that Mancini et al. (Mancini, Longo, Canzoneri, Vallar, & Haggard, 2013) did find that viewing the hand induces a significant reduction of the perception elicited by painful tonic heat even though participants kept both hands on the table in all conditions. However, when participants viewed their hand in that study, they also viewed the stimulation thermode attached to the hand. In contrast, the stimulator was never visible in the present experiment. Interestingly, Mancini et al. (Mancini, Longo, Canzoneri, Vallar, & Haggard, 2013) also found that vision of the hand reduced the perception of noxious heat stimuli but not the perception of innocuous warmth stimuli, as compared to the vision of an neutral object. Whether the lack of effect in the present study could have been related to the use of lower nociceptive stimulation intensities and/or shorter stimulus durations should thus be considered. However, this seems unlikely as stimuli were qualified as clearly pricking in both experiments of the present study. Furthermore, no effect was observed in the control experiment in which the duration of the stimulus was increased such as to elicit a more consistent and long-lasting pain percept. Finally, the finding that no effect on perception was observed both when participants rated intensity using a 0-10 scale and a 0-100 scale indicates that the lack of effect was not due to a difference in numerical rating scales.
The effect of viewing the stimulated body part on the perceived intensity of non-nociceptive somatosensory stimuli has not been studied as extensively. Previous studies have reported that non-informative vision of the hand improves the spatial discrimination of tactile stimuli delivered to the hand (Kennett, Taylor-Clarke, & Haggard, 2001). However, no systematic investigation was carried out about how vision may affect perceived intensity. In the present study, vision of the hand did not exert any effect on the intensity and unpleasantness ratings of the non-nociceptive somatosensory stimuli.
Contrasting with the lack of effect on intensity of perception, we found that both during mirror vision and direct vision, the magnitude of the N240 component elicited by nociceptive stimuli was significantly reduced when participants looked at their hand as compared to when they looked at a neutral object (Figure 4). No effect was observed at the latency of the later P350 component. This result is compatible with previous findings reporting that looking at the hand is associated with a significant reduction of the magnitude of the ERPs delivered to the watched hand. Most interestingly, different effects were observed when analyzing the non-nociceptive ERP waveforms. Indeed, both during mirror vision and direct vision, the magnitude of the P200 component elicited by non-nociceptive stimuli was significantly increased when participants looked at their hand as compared to when they looked at the object (Figure 4). No effect was observed at the latency of the earlier N140 wave.
The notion that vision of the body exerts apparently different effects on the processing of nociceptive and non-nociceptive somatosensory inputs relies mainly on the finding that vision of the stimulated body part can decrease the magnitude of the nociceptive N240-P360 (Longo, Betti, Aglioti, & Haggard, 2009) whereas it can increase the magnitude of the non-nociceptive N80 (Taylor-Clarke, Kennett, & Haggard, 2002). However, in the same experiment, Taylor-Clarke et al. (Taylor-Clarke, Kennett, & Haggard, 2002) found that the N140 was systematically reduced when viewing the hand. Comparison of the effect of vision on the magnitude of nociceptive ERPs to the effect of vision on the magnitude of the non-nociceptive N140 could be more appropriate considering that the nociceptive N240 and the non-nociceptive N140 are thought to reflect functionally-similar processes (Legrain, Guerit, Bruyer, & Plaghki, 2002; Mouraux & Iannetti, 2009; Sambo, Gillmeister, & Forster, 2009a; Van der Lubbe, Buitenweg, Boschker, Gerdes, & Jongsma, 2012) originating at least partly from the same brain areas (Garcia-Larrea, Frot, & Valeriani, 2003; Garcia-Larrea, Lukaszewicz, & Mauguiere, 1995). Such a comparison would lead to an entirely different conclusion: that vision of the body decreases the responses to both nociceptive and non-nociceptive somatosensory inputs. Indeed
The enhancement of the non-nociceptive P200 observed while viewing the hand in the present study appeared to be also present in the results of Taylor-Clarke et al. (Taylor-Clarke, Kennett, & Haggard, 2002). This effect could be mediated by a modulation of activity in multimodal areas (Forster & Gillmeister, 2011; Gillmeister & Forster, 2010; Sambo, Gillmeister, & Forster, 2009b).
In conclusion, our results support the notion that vision of the body can affect differently the processing of nociceptive and non-nociceptive stimuli. Indeed, vision of the hand decreased the magnitude of the N240 elicited by nociceptive stimuli whereas it increased the magnitude of the P200 elicited by non-nociceptive stimuli. Most importantly, the lack of interaction between the factors ‘content’ and ‘mirror’ indicate that this effect was independent of whether the participants looked directly at the stimulated hand or whether they looked at a mirror image of the contralateral hand. The differential modulation of nociceptive and non-nociceptive ERPs by vision indicates that they reflect, at least in part, functionally distinct cortical processes. However, the lack of any concomitant change in the intensity of the percept elicited by nociceptive and non-nociceptive stimuli indicates that these changes in brain activity may be unrelated to the previously reported analgesic effect of viewing the hands.
Furthermore, the lack of effect of vision on the perception of nociceptive stimuli observed in both experiments of the present study raises questions on the contextual factors required for this multisensory interaction to appear, and leaves open the question about the processes underlying the modulation of nociceptive and non-nociceptive ERPs.
Supplementary Material
Acknowledgments
DMT is an AuL-Marie Curie Incoming Post-doc. VL is supported by the Fund for Scientific Research of the French-speaking Community of Belgium (F.R.S.-FNRS). AM received support from an ERC starting grant (“PROBING-PAIN”).
References
- De Paepe AL, Crombez G, Spence C, Legrain V. Mapping nociceptive stimuli in a peripersonal frame of reference: Evidence from a temporal order judgment task. Neuropsychologia. 2014;56:219–228. doi: 10.1016/j.neuropsychologia.2014.01.016. [DOI] [PubMed] [Google Scholar]
- Favril L, Mouraux A, Sambo CF, Legrain V. Shifting attention between the space of the body and external space: Electrophysiological correlates of visual-nociceptive crossmodal spatial attention. Psychophysiology. 2014 doi: 10.1111/psyp.12157. [DOI] [PubMed] [Google Scholar]
- Forman SD, Cohen JD, Fitzgerald M, Eddy WF, Mintun MA, Noll DC. Improved assessment of significant activation in functional magnetic resonance imaging (fMRI): use of a cluster-size threshold. Magn Reson Med. 1995;33:636–647. doi: 10.1002/mrm.1910330508. [DOI] [PubMed] [Google Scholar]
- Forster B, Gillmeister H. ERP investigation of transient attentional selection of single and multiple locations within touch. Psychophysiology. 2011;48:788–796. doi: 10.1111/j.1469-8986.2010.01147.x. [DOI] [PubMed] [Google Scholar]
- Garcia-Larrea L, Frot M, Valeriani M. Brain generators of laser-evoked potentials: from dipoles to functional significance. Neurophysiologie Clinique. 2003;33:279–292. doi: 10.1016/j.neucli.2003.10.008. [DOI] [PubMed] [Google Scholar]
- Garcia-Larrea L, Lukaszewicz AC, Mauguiere F. Somatosensory responses during selective spatial attention: The N120-to-N140 transition. Psychophysiology. 1995;32:526–537. doi: 10.1111/j.1469-8986.1995.tb01229.x. [DOI] [PubMed] [Google Scholar]
- Gillmeister H, Forster B. Vision enhances selective attention to body-related information. Neurosci Lett. 2010;483:184–188. doi: 10.1016/j.neulet.2010.08.004. [DOI] [PubMed] [Google Scholar]
- Haggard P, Iannetti GD, Longo MR. Spatial sensory organization and body representation in pain perception. Curr Biol. 2013;23:R164–176. doi: 10.1016/j.cub.2013.01.047. [DOI] [PubMed] [Google Scholar]
- Hofle M, Hauck M, Engel AK, Senkowski D. Viewing a needle pricking a hand that you perceive as yours enhances unpleasantness of pain. Pain. 2012;153:1074–1081. doi: 10.1016/j.pain.2012.02.010. [DOI] [PubMed] [Google Scholar]
- Hofle M, Pomper U, Hauck M, Engel AK, Senkowski D. Spectral signatures of viewing a needle approaching one's body when anticipating pain. Eur J Neurosci. 2013 doi: 10.1111/ejn.12304. [DOI] [PubMed] [Google Scholar]
- Jung TP, Makeig S, Humphries C, Lee TW, McKeown MJ, Iragui V, Sejnowski TJ. Removing electroencephalographic artifacts by blind source separation. Psychophysiology. 2000;37:163–178. [PubMed] [Google Scholar]
- Kennett S, Taylor-Clarke M, Haggard P. Noninformative vision improves the spatial resolution of touch in humans. Curr Biol. 2001;11:1188–1191. doi: 10.1016/s0960-9822(01)00327-x. [DOI] [PubMed] [Google Scholar]
- Legrain V, Guerit JM, Bruyer R, Plaghki L. Attentional modulation of the nociceptive processing into the human brain: selective spatial attention, probability of stimulus occurrence, and target detection effects on laser evoked potentials. Pain. 2002;99:21–39. doi: 10.1016/s0304-3959(02)00051-9. [DOI] [PubMed] [Google Scholar]
- Legrain V, Iannetti GD, Plaghki L, Mouraux A. The pain matrix reloaded: a salience detection system for the body. Prog Neurobiol. 2011;93:111–124. doi: 10.1016/j.pneurobio.2010.10.005. [DOI] [PubMed] [Google Scholar]
- Legrain V, Mancini F, Sambo CF, Torta DM, Ronga I, Valentini E. Cognitive aspects of nociception and pain: bridging neurophysiology with cognitive psychology. Neurophysiol Clin. 2012;42:325–336. doi: 10.1016/j.neucli.2012.06.003. [DOI] [PubMed] [Google Scholar]
- Longo MR, Betti V, Aglioti SM, Haggard P. Visually induced analgesia: seeing the body reduces pain. J Neurosci. 2009;29:12125–12130. doi: 10.1523/JNEUROSCI.3072-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longo MR, Iannetti GD, Mancini F, Driver J, Haggard P. Linking pain and the body: neural correlates of visually induced analgesia. J Neurosci. 2012;32:2601–2607. doi: 10.1523/JNEUROSCI.4031-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mancini F, Longo MR, Canzoneri E, Vallar G, Haggard P. Changes in cortical oscillations linked to multisensory modulation of nociception. Eur J Neurosci. 2013;37:768–776. doi: 10.1111/ejn.12080. [DOI] [PubMed] [Google Scholar]
- Maris E, Oostenveld R. Nonparametric statistical testing of EEG- and MEG-data. J Neurosci Methods. 2007;164:177–190. doi: 10.1016/j.jneumeth.2007.03.024. [DOI] [PubMed] [Google Scholar]
- Mouraux A, Guerit JM, Plaghki L. Non-phase locked electroencephalogram (EEG) responses to CO2 laser skin stimulations may reflect central interactions between A partial partial differential- and C-fibre afferent volleys. Clin Neurophysiol. 2003;114:710–722. doi: 10.1016/s1388-2457(03)00027-0. [DOI] [PubMed] [Google Scholar]
- Mouraux A, Iannetti GD. Nociceptive laser-evoked brain potentials do not reflect nociceptive-specific neural activity. J Neurophysiol. 2009;101:3258–3269. doi: 10.1152/jn.91181.2008. [DOI] [PubMed] [Google Scholar]
- Sambo CF, Gillmeister H, Forster B. Viewing the body modulates neural mechanisms underlying sustained spatial attention in touch. European Journal of Neuroscience. 2009a;30:143–150. doi: 10.1111/j.1460-9568.2009.06791.x. [DOI] [PubMed] [Google Scholar]
- Sambo CF, Gillmeister H, Forster B. Viewing the body modulates neural mechanisms underlying sustained spatial attention in touch. Eur J Neurosci. 2009b;30:143–150. doi: 10.1111/j.1460-9568.2009.06791.x. [DOI] [PubMed] [Google Scholar]
- Shore DI, Gray K, Spry E, Spence C. Spatial modulation of tactile temporal-order judgments. Perception. 2005;34:1251–1262. doi: 10.1068/p3313. [DOI] [PubMed] [Google Scholar]
- Taylor-Clarke M, Kennett S, Haggard P. Vision modulates somatosensory cortical processing. Curr Biol. 2002;12:233–236. doi: 10.1016/s0960-9822(01)00681-9. [DOI] [PubMed] [Google Scholar]
- Trojan J, Diers M, Valenzuela-Moguilllansky C, Torta DM. Body, space and pain. Front Hum Neurosci. 2014 doi: 10.3389/fnhum.2014.00369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van der Lubbe RH, Buitenweg JR, Boschker M, Gerdes B, Jongsma ML. The influence of transient spatial attention on the processing of intracutaneous electrical stimuli examined with ERPs. Clin Neurophysiol. 2012;123:947–959. doi: 10.1016/j.clinph.2011.08.034. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.