Abstract
Vasculogenesis is the process of de novo blood vessel formation observed primarily during embryonic development. Emerging evidence suggest that post-natal mesenchymal stem cells are capable of recapitulating vasculogenesis when these cells are engaged in tissue regeneration. However, the mechanisms underlining the vasculogenic differentiation of mesenchymal stem cells remain unclear. Here, we used stem cells from human permanent teeth (DPSC) or deciduous teeth (SHED) as models of post-natal primary human mesenchymal stem cells to understand mechanisms regulating their vasculogenic fate. GFP-tagged mesenchymal stem cells seeded in human tooth slice/scaffolds and transplanted into immunodeficient mice differentiate into human blood vessels that anastomize with the mouse vasculature. In vitro, VEGF induced the vasculogenic differentiation of DPSC and SHED via potent activation of Wnt/β-catenin signaling. Further, activation of Wnt signaling is sufficient to induce the vasculogenic differentiation of post-natal mesenchymal stem cells, while Wnt inhibition blocked this process. Notably, β-catenin-silenced DPSC no longer differentiate into endothelial cells in vitro, and showed impaired vasculogenesis in vivo. Collectively, these data demonstrate that VEGF signaling through the canonical Wnt/β-catenin pathway defines the vasculogenic fate of post-natal mesenchymal stem cells.
Keywords: Vasculogenesis, Angiogenesis, Dental pulp stem cells, Multipotency, Self-renewal, Tissue engineering
Graphical Abstract
Schematic representation of the role of the Wnt/β-catenin signaling axis on the determination of the vasculogenic fate of post-natal mesenchymal stem cells. It has been previously demonstrated that dentin-derived BMPs induce the odontoblastic differentiation of dental pulp stem cells [39]. This manuscript shows that VEGF signaling through the canonical Wnt/β-catenin pathway regulates the differentiation of post-natal mesenchymal stem cells into vascular endothelial cells.
Introduction
Mesenchymal stem cells are characterized by multipotency and self-renewal, and play critical roles in embryonic development and tissue regeneration. Several populations of tooth-related mesenchymal stem cells have been characterized over the last 15 years, as for example the dental pulp stem cells (DPSC) from permanent teeth [1], the stem cells from human exfoliated deciduous teeth (SHED) [2], and the stem cells from the apical papilla (SCAP) [3, 4]. These mesenchymal stem cells display multipotency being able to differentiate into osteo/odontogenic, adipogenic, and neurogenic lineages [5]. Interestingly, stromal stem cells from human dental pulps can differentiate into both, osteoblasts and endotheliocytes [6]. At about the same time, the Nakashima laboratory demonstrated that a sub-fraction of side population (SP) cells that are negative for CD31 and CD146 are capable of stimulating blood vessel formation via secretion of pro-angiogenic factors such as vascular endothelial growth factor (VEGF)-A [7]. Evidence from our laboratory suggested that dental pulp stem cells have the ability to differentiate into vascular endothelial cells [8, 9]. However, mechanisms underlying the determination of dental stem cell fate are poorly understood. Understanding mechanisms regulating the mesenchymal stem cell fate will help determining the role of these cells during tooth development, and likely unveil biological modifiers that can be exploited when using these cells therapeutically in tissue regeneration purposes.
Wnt signaling plays important roles in the regulation of cell proliferation and polarity, apoptosis, branching morphogenesis, inductive processes, and differentiation of stem cells [10–13]. It has been shown that Wnt signaling promotes hematoendothelial cell development from human embryonic stem cells [14]. Wnt signaling controls the specification of definitive and primitive hematopoiesis from human pluripotent stem cells (hPSCs), and the specification of definitive progenitor cells (KDR+CD235-) [15]. Wnt-β-catenin signaling mediates endothelial cell differentiation from human pluripotent stem cells (hPSCs) via a β-catenin-dependent process [16]. In the central nervous system (CNS), canonical Wnt signaling regulates organ-specific assembly and differentiation of the vasculature [12]. Notably, loss- and gain-of-function experiments of members of the Wnt signaling pathway caused marked alterations of vascular development and endothelial cell specification [17]. Collectively, these studies demonstrate a prominent role for the Wnt-β-catenin signaling pathway in angiogenic differentiation during development. However, the role of this pathway in the regulation of vasculogenic differentiation of post-natal mesenchymal stem cells remains poorly understood.
VEGF is a 46-kDa homodimeric protein that specifically binds to two receptor tyrosine kinases (RTK), namely the fms-like tyrosine kinase Flt-1 (VEGFR1) and KDR (VEGFR2) [18–22]. VEGF binds to VEGFR1 and VEGFR2 with high affinity, i.e. Kd=16 pM and Kd=760 pM respectively [23]. While VEGFR1 expression can be found rather ubiquitously, the expression of VEGFR2 is more limited to vascular endothelial cells [24]. We have shown that VEGF-dependent tumor angiogenesis requires an inverse and reciprocal regulation of VEGFR1 and VEGFR2 [25]. We have also shown that quiescent dental pulp stem cells strongly express VEGFR1 but no VEGFR2, and that long-term VEGF treatment modifies this balance by inducing VEGFR2 expression [8]. Notably, gene-silencing experiments revealed that VEGFR1 signaling is required for VEGF-induced endothelial differentiation of dental pulp stem cells. We postulate that VEGFR1 enables VEGF signaling in mesenchymal stem cells, and that these cells begin expressing VEGFR2 while differentiating into vascular endothelial cells.
Here, we performed a series of studies that explored downstream mechanisms triggered by VEGF that regulate the vasculogenic fate of dental pulp stem cells. Collectively, our data demonstrate that Wnt/β-catenin signaling regulates the vasculogenic differentiation of dental pulp stem cells. These studies unveiled a series of signaling events that explain how post-natal human mesenchymal stem cells can differentiate into endothelial cells that form blood vessels.
Materials and Methods
Cell culture
Dental pulp stem cells (DPSC) [1] and stem cells from human exfoliated deciduous teeth (SHED) [2] (kindly provided by Songtao Shi) were cultured in α-Minimum Essential Medium (MEM; Invitrogen, Carlsbad, CA, USA) supplemented with 5–15% fetal bovine serum (FBS, Invitrogen) and 1% penicillin/streptomycin (Invitrogen) at 37°C and 5% CO2. Human bone marrow stem cells (HBMSC) kindly provided by Russell S. Taichman (University of Michigan) and University of Michigan Squamous Cell Carcinoma (UM-SCC)-1 cells (obtained from the Tissue Biorepository, University of Michigan Head and Neck SPORE) were cultured in Dulbecco’s Modified Eagle’s Medium (DMEM; Invitrogen) supplemented with 10% FBS, 100 U/ml penicillin, and 100 µg/ml streptomycin. Pooled human dermal microvascular endothelial cells (HDMEC; Lonza, Walkersville, MD, USA) were cultured in endothelial growth medium-2 for microvascular cells (EGM2-MV; Lonza). Cells were serum-starved overnight, and treated with 0–50 ng/ml rhVEGF165 (R&D Systems, Minneapolis, MN, USA) or 0–50 ng/ml rhWnt1 (Cell Sciences, Canton, MA, USA) for indicated time points. When indicated, cells were cultured with α-MEM supplemented with 5% FBS and 0–10 µM JW67 or 0–10 µM CHIR99021 (Tocris Bioscience, Minneapolis, MN, USA).
Western Blots
Cells were lysed in 1% Nonidet P-40 (NP-40) lysis buffer (50 mM Tris-HCL, PH 7.4, 10% glycerol, 200 mM NaCl and 2 mM MgCl2) containing protease inhibitors. Protein lysates were loaded onto 8–15% SDS-PAGE. Membranes were blocked with 5% non fat milk in 1X TBS containing 0.3% Tween-20, then incubated overnight at 4°C with the following primary antibodies: rabbit anti-human EGFR, rabbit anti-human VEGFR1, VEGFR2, E-cadherin, CD31, mouse anti-human VE-cadherin, mouse anti-human β-catenin, rabbit anti-human Frizzled-3, Frizzled-4 (Fzd-3, Fzd-4), mouse anti-human β-actin conjugated with HRP (Santa Cruz Biotechnology, Santa Cruz, CA, USA); rabbit anti human LRP-5, LRP-6, rabbit anti-human Frizzled-5, Frizzled-6 (Fzd-5, Fzd-6), rabbit anti-human P-GSK-3β, GSK-3β, rabbit anti-human Bmi-1, rabbit anti-human Axin2, rabbit anti-human Tie-2, rabbit anti-human Wnt1 (Invitrogen), mouse anti-GAPDH (Chemicon, Billerca, MA, USA). Affinity-purified secondary antibodies conjugated with horseradish peroxidase (Jackson Laboratories, West Grove, PA, USA) were used and immunoreactive proteins were visualized by SuperSignal West Pico chemiluminescent substrate (Thermo Scientific, Rockford, IL, USA).
In vitro sprouting assay
5×104 DPSC cells/well were seeded in 12-well plates coated with growth factor reduced Matrigel (BD Biosciences, Bedford, MA, USA). HDMEC cells were used as positive control for capillary tube formation. The cells were cultured with EGM2-MV medium supplemented with 50 ng/ml rhVEGF165 in presence of 0–5 µM JW67 (Tocris Bioscience).
Reverse transcriptase PCR
Total RNA was prepared in Trizol (Invitrogen) according to manufacturer’s instructions. cDNA was synthesized with SuperScript II Reverse Transcriptase (RT) (Invitrogen) and PCR was performed with Platinum Taq DNA Polymerase (Invitrogen). The primers used in this study were, as follows: VEGFR-1, sense 5’-ACT CCC TTG AAC ACG AGA GTT C-3’, antisense 5’-GAT TTC TCA GTC GCA GGT AAC C-3’; VEGFR-2 sense 5’-GCT GTC TCA GTG ACA AAC CCA T-3’, antisense 5’-CTC CCA CAT GGA TTG GCA GAG G-3’; CD31 sense 5’-TAC TCA GTC ATG GCC ATG GT −3’, antisense 5’-TTG GCC TTG GCT TTC CTC AG −3’; VE-cadherin sense 5’-CCT GGT ATA ACC TGA CTG TG-3’, antisense 5’-TGT GAT GGT GAG GAT GCA GA-3’; Tie-2 sense 5’-TAC ACC TGC CTC ATG CTC AG-3’, antisense 5’-GCA GAG ACA TCC TTG GAA GC-3’; DSPP sense 5’-TCA CAA GGG AGA AGG GAA TG-3’, antisense 5’-TGC CAT TTG CTG TGA TGT TT-3’; DMP-1 sense 5’-CAG GAG CAC AGG AAA AGG AG-3’, antisense 5’-CTG GTG GTA TCT TGG GCA CT-3’; GAPDH sense 5’-GAC CCC TTC ATT GAC CTC AAC T-3’, antisense 5’-CAC CAC CTT CTT GAT GTC ATC-3’. RT-PCR products were verified by electrophoresis in agarose gel.
Immunohistochemistry and immunofluorescence
4 µm-thick sections were deparaffinized and rehydrated. Antigen retrieval was performed, and sections were incubated overnight at 4°C with the following primary antibodies: rabbit anti-human CD31 (Bethyl Laboratories, Montgomery, TX, USA), rabbit anti-mouse CD31 (Abcam), rabbit anti-factor VIII related antigen/Von Willebrand Factor Ab-1 (Thermo Scientific), rabbit anti-GFP (Abcam) or mouse anti-GFP (Santa Cruz), mouse anti-SMA-α (Millipore). The EnVision™+ system (Dako, Troy, MI, USA) and 3,3-diamino benzidine (Dako) were utilized for visualization (IHC). Alexa Flour 488 goat anti-rabbit, goat anti-mouse IgG (green) (Life Technologies) and Alexa Flour 594 goat–anti mouse, goat anti-rabbit IgG (red) (Life Technologies) were used as secondary antibody to detect blood vessels labelled with anti-human-CD31, anti-GFP or anti-SMA-α primary antibody, respectively. Isotype-matched non-specific IgG was used as negative control.
β-catenin silencing in DPSC
HEK293T cells were transiently co-transfected with the lentiviral packaging vectors psPAX2, pMD2G (Vector Core, University of Michigan) and shRNA-β-catenin or scramble sequence control (shRNA-C) (Addgene, Cambridge, MA, USA) by the calcium phosphate method. DPSC cells were infected with supernatants containing lentivirus and selected with 1 µg/ml of puromycin (Sigma-Aldrich, St. Louis, MO) for at least 1 week. Knock-down of β-catenin was verified by western blot.
Tooth slice/scaffolds for stem cell transplantation
Extracted non-carious human third molars were collected in the Department of Oral Surgery (University of Michigan) under an approved Institutional Review Board protocol. The pulp tissue was thoroughly removed, 1.2 mm thick tooth slices were prepared and poly-L-lactic acid (PLLA) (Boehringer Ingelheim, Ingelheim, Germany) scaffolds were casted within the pulp chamber [26]. 1×106 DPSC stably transduced with GFP, shRNA-β-catenin, or shRNA-scrambled sequence vector control were resuspended in a 1:1 mix of growth factor reduced Matrigel (BD Biosciences) and EGM2-MV medium (Lonza), seeded in tooth slice/scaffolds (n=8), and transplanted into the subcutaneous space of immunodeficient mice (CB.17.SCID; Taconic, Germantown, NY, USA), as we described [26]. After 1–4 weeks, mice were euthanized, tooth slice/scaffolds were removed, fixed with 10% buffered formalin phosphate, decalcified with Decalcifier II (Leica Biosystems, Buffalo Grove, IL, USA) and prepared for immunohistochemistry or immunofluorescence.
Statistical Analysis
Data was evaluated by t-test or one-way ANOVA followed by appropriate post-hoc tests using the SigmaStat 2.0 software (SPSS, Chicago, IL, USA). Statistical significance was determined at p<0.05.
Results
Vasculogenic differentiation of dental pulp stem cells
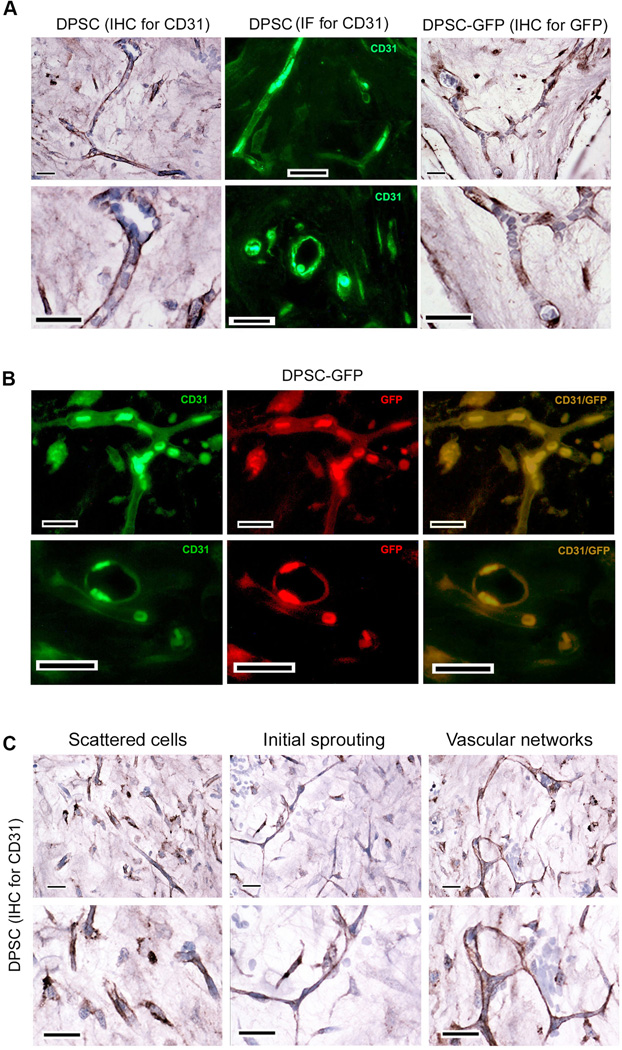
To begin to understand mechanisms regulating the vasculogenic differentiation of dental pulp stem cells (DPSC), cells stably transduced with green fluorescence protein (DPSC-GFP) were seeded in tooth slice/scaffolds [26] and transplanted into the subcutaneous space in the dorsum of immunodeficient mice. Several complementary methods were used to confirm that, indeed, the blood vessels (i.e. blood-carrying vessels) observed within the scaffolds were lined with human DPSC cells that were transplanted and that differentiated into vascular endothelial cells. Human DPSC-derived vessels were identified by immunohistochemistry (IHC) and immunofluorescence (IF) with anti-human specific CD31 antibody in tooth slice/scaffolds seeded with DPSC cells (Fig. 1). In addition, we also performed IHC for GFP and direct fluorescence identification in tooth slice/scaffolds seeded with DPSC-GFP cells (Fig. 1). IHC showed human blood vessels containing mouse blood that were lined with cells that were positively stained for human CD31 and for GFP (Fig. 1A). Co-immunofluorescence for human CD31 and GFP demonstrated that the endothelial cells forming blood vessels are DPSC cells that underwent endothelial differentiation (Fig. 1B). We also found that these blood vessels were positively stained for GFP and human CD31 but negative for mouse CD31 (Supporting Information Fig. S1A). These data provide further evidence that the blood vessels are indeed lined by human endothelial cells derived from the vasculogenic differentiation of the human DPSC cells. Interestingly, we observed areas where most human CD31-positive cells were scattered (Fig. 1C). We interpret these findings as areas with predominance of primitive vascular structures at the onset of vasculogenesis. In addition, we observed cells that were positive for GFP, but negative for both human and mouse CD31 (Supporting Information Fig. S1B). We interpret these findings as DPSC-GFP cells that remained in an undifferentiated state and/or divided into daughter stem cells via self-renewal. CD31-positive cells aligned into long sprouts that eventually formed branches and vascular networks that anastomized with the mouse vasculature, as demonstrated by the presence of mouse blood cells in the lumen (Fig. 1C). This series of cellular events closely resembles the process of vasculogenesis, i.e. de novo blood vessel formation [27]. Interestingly, areas of anastomosis between human and mouse blood vessels were depicted by the presence of human-CD31 positive endothelial cells and human-CD31 negative cells (mouse endothelial cells) side-by-side in the walls of chimeric blood vessels (Supporting Information Fig. S1C).
Figure 1.
Dental pulp stem cells (DPSC) differentiate into blood vessels in vivo. 1×106 DPSC or DPSC-GFP (stably transduced with GFP) cells were seeded in tooth slice/scaffolds and transplanted in the subcutaneous space of the dorsum of SCID mice (n=8). Five weeks later, tooth slice/scaffolds were retrieved and processed for imaging by immunohistochemistry (IHC) or immunofluorescence (IF). (A): Blood vessels originated from the human DPSC were detected by IHC for GFP (brown color) in tooth slice/scaffolds seeded with DPSC-GFP. Alternatively, we performed IHC or IF for CD31 in tooth slice/scaffolds seeded with DPSC cells. (B): DPSC-derived human blood vessels were detected by double staining with anti-human CD31 (green) and GFP (red) in tooth slice/scaffolds seeded with DPSC-GFP cells. (C): Stages of DPSC-mediated vasculogenesis as shown by IHC for anti-human CD31. Left panel: Scattered CD31-positive cells. Middle panel: Elongated CD31-positive cells connecting with each other. Right panel: Connected branches forming complex vascular networks. Scale bars: 25 µm.
To begin to understand the process of vessel maturation upon vasculogenic differentiation of dental pulp stem cells, tooth slice/scaffolds containing DPSC were transplanted into mice and retrieved after 1–4 weeks. We observed that, 1 week after transplantation, most of the cells were scattered throughout the tissue. After 2 weeks, a few blood vessels were formed. They were positively stained for human CD31 with a few of them also stained for smooth muscle actin (SMA)-α, a marker for smooth muscle cells/pericytes [28, 29]. After 3–4 weeks, the majority of the blood vessels were clearly positive for human CD31 and for SMA-α. Collectively, these data indicates the maturation of DPSC-derived blood vessels, as defined by progressive investment of these vessels with smooth muscle cells/pericytes (Fig. 2).
Figure 2.
Process of DPSC-derived vessel maturation, as determined by progressive investment with smooth muscle actin (SMA)-positive smooth muscle cells/pericytes. 1×106 DPSC cells were seeded in tooth slice/scaffolds and transplanted in the subcutaneous space of the dorsum of SCID mice. Tooth slice/scaffolds were collected after 1–4 weeks (n=4), retrieved and processed for immunofluorescence. DPSC-derived human blood vessels were detected in the pulp chamber with anti-human CD31 (green) and anti-SMA antibody was used to detect smooth muscle cells/pericytes (red) lining the walls of these newly formed blood vessels. Green arrow points to a human CD31-positive cell and red arrow points to a SMA-positive smooth muscle cell. Scale bars: 25 ***m.
DPSC organize into sprout-like structures in Matrigel
In attempt to establish an in vitro model that would be more amenable to mechanistic studies, we performed a series of experiments to address whether DPSC can form sprout-like structures in Matrigel. DPSC cells were seeded in growth factor-reduced Matrigel-coated plates and cultured with vasculogenic differentiation medium, i.e. endothelial cell growth medium (EGM2-MV, Lonza) supplemented with rhVEGF165. Human dermal microvascular endothelial cells (HDMEC) were used as positive control for tube formation. After 1 day, HDMEC cells formed capillary sprouts-like structures when cultured vasculogenic differentiation medium, but not when cultured in serum-free, growth factor-free endothelial basal medium (EBM). Interestingly, DPSC cells cultured for 1 day in vasculogenic differentiation medium did not form sprout-like structures (Supporting Information Fig. S2A). We then performed a time course experiment for 11 days with HDMEC and DPSC cells seeded in Matrigel and cultured with vasculogenic medium or control EBM medium (Supporting Information Fig. S2B). Since HDMEC are fully differentiated cells, sprout-like structures were readily visible within 24 hours and persisted for 11 days albeit a gradual decrease in the density of sprouts being observed over time. With DPSC cells, the overall trends are very different. At day 1, individual DPSC cells cultured with vasculogenic differentiation medium were spread throughout the 3-D matrix. By day 3, small branches of sprouting cells began to be observed. Longer branches and vascular networks were observed around day 8 and were readily visible throughout the Matrigel by day 11 (Supporting Information Fig. S2B). In contrast, DPSC cells cultured in serum-free, growth factor-free EBM medium did not form sprout-like structures in any time period of this experiment (Supporting Information Fig. S2B). Collectively, these data demonstrate that undifferentiated DPSC cells require culture medium supplemented with vasculogenic factors and several days to differentiate into endothelial cells and form sprout-like structures in vitro, while the fully differentiated endothelial cells can form sprouts within 24 hours.
VEGF activates the canonical Wnt/β-catenin signaling in dental pulp stem cells
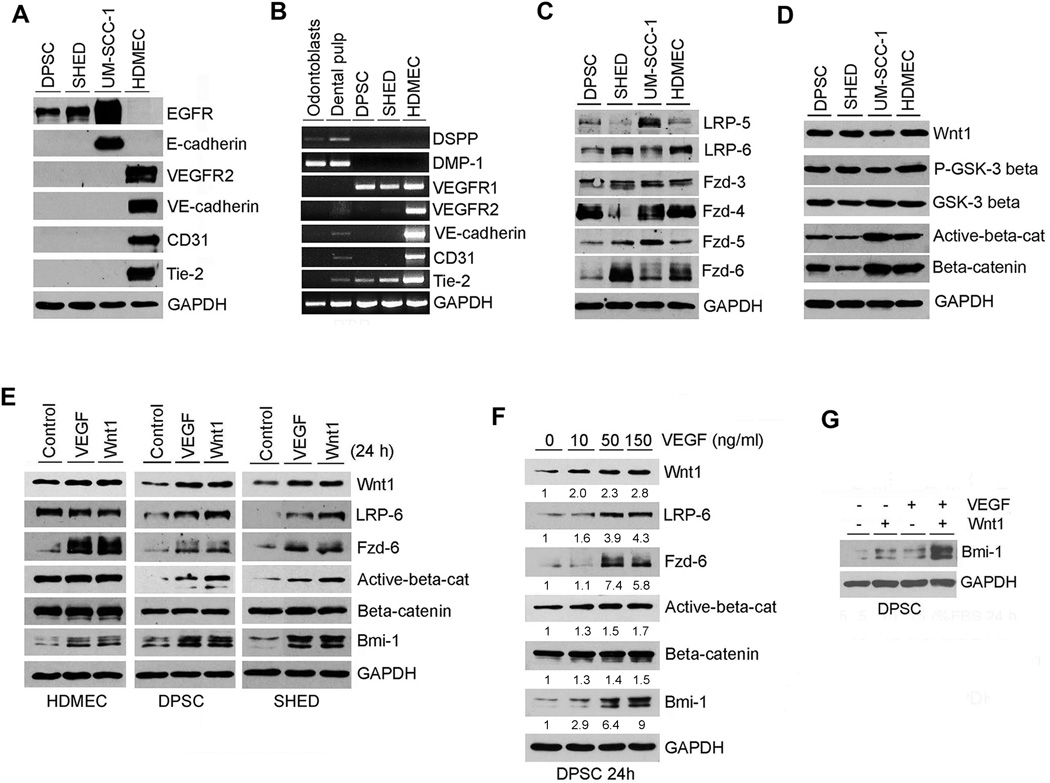
To understand signaling pathways involved in the regulation of endothelial differentiation of dental pulp stem cells, we determined the baseline expression levels of endothelial markers (Fig. 3A, 3B) and Wnt pathway-related proteins (Fig. 3C, 3D) in DPSC and SHED cells. Endothelial cells (HDMEC) were used as positive controls. Human head and neck squamous cell carcinoma cells (UM-SCC-1) were used as negative controls for endothelial cell markers, and positive control for epithelial cell markers. Unstimulated DPSC and SHED cells did not express the endothelial cell markers VEGFR2, Tie-2, VE-cadherin and CD31 (Fig. 3A). DPSC and SHED did not express the epithelial-specific marker E-cadherin, but showed baseline expression of EGFR (Fig. 3A). We also evaluated the transcriptional expression level of odontoblastic/osteoblastic and endothelial cell markers by RT-PCR (Fig. 3B). As expected, DPSC and SHED did not express odontoblastic/osteoblastic markers DSPP and DMP-1. In contrast, they strongly expressed VEGFR1, confirming our previous reports [8, 9]. Aligned with the Western blots (Fig. 3A), unstimulated DPSC and SHED did not express the endothelial markers VEGFR2, VE-cadherin and CD31 (Fig. 3B). Interestingly, DPSC and SHED expressed Tie-2 at the RNA level (Fig. 3B) but not at the protein level (Fig. 3A), suggesting some level of post-transcriptional regulation for this gene in these cells. Unstimulated DPSC and SHED expressed variable levels of key Wnt signaling-related proteins, i.e. LRP-5, LRP-6, Fzd-3, Fzd-4, Fzd-5, Fzd-6, Wnt1, P-GSK-3β, GSK-3β, active β-catenin and β-catenin (Fig. 3C, 3D). Collectively, these data demonstrate that DPSC and SHED express VEGFR1 and key components of the Wnt signaling pathway and therefore are capable of responding to signaling initiated by VEGF and Wnt.
Figure 3.
VEGF activates the canonical Wnt/β-catenin signaling in dental pulp stem cells. (A): Western blots for EGFR, E-cadherin, VEGFR-2, Tie-2, VE-cadherin and CD31 from whole cell lysates of DPSC, SHED, UM-SCC-1 and HDMEC. (B): RT-PCR for odontogenic/osteoblastic markers (DSPP, DMP-1), and for endothelial cell markers (VEGFR2, Tie-2, VE-cadherin, CD-31). Total RNA was prepared from odontoblasts retrieved from freshly extracted sound human 3rd molars, whole human pulp tissue, or from DPSC, SHED, HDMEC cells. (C): Western blot for Wnt pathway receptors LRP-5, LRP-6, Fzd-3, Fzd-4, Fzd-5 and Fzd-6 in DPSC and SHED, using as controls UM-SCC-1 and HDMEC. (D): Western blot for Wnt1, P-GSK-3β, GSK-3β, active-β-catenin and β-catenin. (E): Western blots for LRP-6, Fzd-6, active-β-catenin, β-catenin, Wnt1 and Bmi-1 in DPSC, SHED or HDMEC starved overnight and treated with 50 ng/ml rhVEGF165 or 50 ng/ml rhWnt1 for 24 hours. (F): Western blot for LRP-6, Fzd-6, active-β-catenin, β-catenin, Wnt1 and Bmi-1 in DPSC starved overnight and treated with 0–150 ng/ml rhVEGF165 for 24 hours. Numbers depict the band density normalized against untreated controls and GAPDH. (G): Western blot for Bmi-1 in DPSC starved overnight and treated with 50 ng/ml rhVEGF165 and/or 50 ng/ml rhWnt1 for 24 hours.
Next, we evaluated the effect of VEGF- and Wnt1-initiated signaling in dental pulp stem cells, using endothelial cells (HDMEC) as controls. Cells were starved overnight, then treated with 50 ng/ml rhVEGF165, 50 ng/ml Wnt1, or left untreated as negative controls (Fig. 3E). Both VEGF and Wnt1 induced expression of the Wnt receptors LRP-6 and Fzd-6, and induced activation of β-catenin in DPSC and SHED (Fig. 3E). In contrast, VEGF and Wnt1 induced expression of Fzd-6 in endothelial cells, but did not induce β-catenin activation. The possible reason for this difference in response might be the high endogenous level of active-β-catenin in HDMEC. Also, VEGF induced expression of Wnt1 in DPSC and SHED, but not in the fully differentiated endothelial cells (Fig. 3E). To confirm these observations, we exposed DPSC to increasing concentrations of VEGF and observed that it upregulated Wnt1, LRP-6, Fzd-6, and activated β-catenin in a dose-dependent manner (Fig. 3F). To understand which receptor is mediating VEGF-induced activation of the Wnt signaling pathway, we used neutralizing anti-VEGFR1 or anti-VEGFR2 antibodies (Supporting Information Fig. S3). These experiments revealed that VEGFR1 is the primary receptor mediating these responses. Blockade of VEGFR1 signaling inhibited VEGF-induced expression of Wnt1, LRP-6, Fzd-6, and active β-catenin, while VEGFR2 blockade had no measurable effect. Surprisingly, we observed that both VEGF and Wnt1 induce expression of the self-renewal marker Bmi-1 in short-term experiments (Fig. 3E, 3F), particularly when used together to treat DPSC cells (Fig. 3G). We will explore these unexpected observations further in long-term experiments shown in Figure 4.
Figure 4.
VEGF and Wnt mediate endothelial differentiation of dental pulp stem cells. (A): Western blots for VEGFR1, VEGFR2 and VE-cadherin from DPSC cells treated with 50 ng/ml rhVEGF165 or 50 ng/ml rhWnt1 for 5–15 days. (B, C): Western blots for VEGFR2 and Tie-2 from DPSC treated with 50 ng/ml rhWnt1 for 7–21 days (B) or with 50 ng/ml VEGF and/or 50 ng/ml rhWnt1 for 14 days (C). (D): Western blots for VEGFR2 and CD31 from SHED treated with 50 ng/ml VEGF and/or 50 ng/ml rhWnt1 for 14 days. Numbers depict the band density normalized against untreated controls and GAPDH. (E, F): Western blots for the self renewal marker Bmi-1 from DPSC (E) or SHED (F) treated with 50 ng/ml VEGF or 50 ng/ml rhWnt1 for the indicated time points.
VEGF and Wnt mediate endothelial differentiation of dental pulp stem cells
To understand mechanisms of vasculogenic differentiation of dental pulp stem cells, we have exposed DPSC and SHED to 50 ng/ml VEGF and/or 50 ng/ml Wnt1 for several days and evaluated expression of endothelial cell differentiation markers (e.g. VEGFR2, VE-Cadherin, Tie-2 and/or CD31) by Western blot (Fig. 4). Both, VEGF and Wnt1 induced expression of endothelial cell markers in DPSC, particularly after 7–10 days of treatment (Fig. 4A, 4B), While individually VEGF or Wnt1 are sufficient to induce expression of markers of endothelial differentiation in DPSC (Fig. 4C) and SHED (Fig. 4D), when cells are exposed to VEGF and Wnt1 together we observed a further increase in the expression levels of endothelial markers in both cell types. To verify the specificity of the effect of VEGF and Wnt on the endothelial differentiation of dental pulp stem cells, we repeated these experiments with another mesenchymal stem cell, i.e. human bone marrow stem cells (HBMSC). We observed that VEGF and Wnt1 induced expression of the endothelial markers (VEGFR2, VE-cadherin, Tie-2, CD31) in HBMSC (Supporting Information Fig. S4). This effect was potentiated by the treatment with VEGF and Wnt1 together. Interestingly, as DPSC and SHED acquired the endothelial phenotype characterized by expression of differentiation markers, these cells gradually lost self-renewal as demonstrated by a reduction in the expression of the self-renewal marker Bmi-1 (Fig. 4E, 4F). Collectively, these data indicates that both VEGF and Wnt1 are capable of inducing endothelial differentiation of dental pulp stem cells.
GSK-3β inhibition is sufficient to induce vasculogenic differentiation of dental pulp stem cells
To evaluate the effect of Wnt signaling on the endothelial differentiation in dental pulp stem cells, we used the GSK-3β inhibitor CHIR99021 [30] to upregulate signaling through the canonical Wnt signaling pathway. As expected, CHIR99021 downregulated P-GSK-3β in a time-dependent and a dose-dependent manner (Fig. 5A). P-GSK-3β downregulation with CHIR99021 was accompanied by the expected activation of β-catenin (Fig. 5B). Interestingly, we also observed a time-dependent and dose-dependent increase in expression of Frizzled-6 and its co-receptor LDL-receptor-related protein (LRP)-6. Surprisingly, enforced activation of β-catenin with CHIR99021 for 14 days was sufficient to induce vasculogenic differentiation of DPSC and SHED cells, as shown by upregulated expression of VEGFR2, VE-Cadherin, CD31 and Tie-2 (Fig. 5C). To confirm these unexpected observations, we used the Wnt pathway inhibitor JW67 [31] to perform reverse experiments and verify the responses obtained with CHIR99021. As expected, JW67 induced a time-dependent and dose-dependent phosphorylation of P-GSK-3β and upregulation of AXIS inhibition protein (Axin)-2 (Fig. 5D), which forms a complex with β-catenin leading to its degradation. Inhibition of the canonical pathway with JW67 resulted in inhibition of vasculogenic differentiation of DPSC and SHED cells, as measured by decreased VEGFR2 and Tie-2 expression (Fig. 5E). A capillary sprouting assay with DPSC cells seeded in Matrigel-coated plates confirmed that inhibition of Wnt signaling with JW67 is sufficient to inhibit the vasculogenic potential of DPSC cells (Fig. 5F, 5G), without significant toxicity to the cells (data not shown).
Figure 5.
GSK-3β inhibition is sufficient to induce vasculogenic differentiation of dental pulp stem cells. (A): Western blots for Fzd-6, P-GSK-3β, and GSK-3β from DPSC treated with 10 µM CHIR99021 for up to 24 hours. Alternatively, we performed a dose-dependent experiment with DPSC cells treated with 0–10 µM CHIR99021 for 24 hours. Numbers depict the band density normalized against untreated controls and GAPDH. (B): Western blots for LRP-6, Fzd-6, active-β-catenin, β-catenin and Wnt1 from DPSC or SHED starved overnight, and treated with 0–10 µM CHIR99021 for 24 hours. (C): Western blots for endothelial differentiation markers (VEGFR2, VE-Cadherin, Tie-2 and CD31) from DPSC or SHED cells treated with 0–2.5 µM CHIR99021 for 14 days. (D): Western blots for Axin-2, P-GSK-3β, and GSK-3β from DPSC or SHED treated with 0–10 µM JW67 for 24 hours. (E): Western blots for vasculogenic differentiation markers (VEGFR2, Tie-2) from DPSC or SHED cells treated with 50 ng/ml rhWnt1 and/or 0–5 µM JW67 for 14 days. (F): Photomicrographs of DPSC cells seeded in 12-well plates (5×104 cells/well) coated with growth factor-reduced Matrigel and incubated with EGM2-MV supplemented with 50 ng/ml rhVEGF165 with or without 0–5 µM JW 67 for up to 11 days. Scale bars: 100 µm. (G): Graph depicting the number of sprouts formed by DPSC treated with 0, 2.5 or 5 µM JW67 in EGM2-MV medium supplemented with 50 ng/ml rhVEGF165. Three independent experiments using triplicate wells per experimental condition were performed to verify reproducibility of the data. * p<0.01, ** p<0.001.
β-catenin silencing is sufficient to inhibit vasculogenic differentiation of dental pulp stem cells
To verify the data obtained with chemical inhibitors, we used a genetic approach to inhibit the canonical Wnt signaling pathway by silencing β-catenin expression in DPSC cells with short hairpin-encoding lentiviral vectors. Transduced DPSC were incubated with 50 ng/ml Wnt1 or 50 ng/ml VEGF to confirm silencing of total β-catenin and inhibition of β-catenin phosphorylation (Fig. 6A, 6C). Two shRNA sequences were evaluated, but the sequence shRNA-β-catenin(1) proved to be the most efficient in silencing β-catenin expression (Fig. 6A). Silencing of β-catenin abrogated the vasculogenic differentiation of DPSC cells induced by Wnt1 (Fig. 6B) or VEGF (Fig. 6D). Sprouting assays with DPSC cells cultured in Matrigel confirmed these results, as β-catenin-silenced cells showed significantly less sprouting formation (Fig. 6E, 6F). Finally, the effect of β-catenin-silencing on the vasculogenic differentiation of DPSC cells was also evaluated in vivo with cells seeded in tooth slice/scaffolds [26] and transplanted in the subcutaneous space of immunodeficient mice (Fig. 7). We observed a significant decrease in microvessel density in scaffolds seeded with β-catenin-silenced DPSC cells when compared to scrambled sequence controls (Fig. 7A, 7B). Collectively, these data showed that the canonical Wnt signaling pathway is required for the vasculogenic differentiation of dental pulp stem cells.
Figure 6.
Knockdown of β-catenin inhibits angiogenic differentiation of DPSC in vitro(A–D): DPSC stably transduced with shRNA-scrambled or two different sequences (1 or 2) of shRNA-β-catenin were treated with 50 ng/ml rhWnt1 (A, B) or 50 ng/ml VEGF (C, D) for 14 days. Western blots were performed for active-β-catenin, β-catenin (A, C) or VEGFR2, Tie-2 (B, D). HDMEC cells were used as positive control for VEGFR2 and Tie-2. Numbers depict the band density normalized against untreated controls and GAPDH. (E): Photomicrographs of DPSC-shRNA-β-catenin (sequence 1 or 2) or control DPSC-shRNA-Scrambled cells seeded in 12-well plates (5×104 cells/well) coated with growth factor-reduced Matrigel and incubated with EGM2-MV supplemented with 50 ng/ml rhVEGF165 for up to 16 days. Scale bars: 100 µm. (F) Graph depicting the number of sprouts formed by DPSC-shRNA-β-catenin (sequence 1 or 2) or control DPSC-shRNA-Scrambled cells (E). Three independent experiments using triplicate wells per experimental condition were performed to verify reproducibility of the data. Asterisk indicates p<0.01.
Figure 7.
Knockdown of β-catenin inhibits angiogenic differentiation of DPSC in vivo(A): DPSC stably transduced with shRNA-β-catenin(1) or control shRNA-scrambled were seeded in tooth slice/scaffolds (n=8), and transplanted into the subcutaneous space of immunodeficient mice. Five weeks after transplantation, the tooth slice/scaffolds were retrieved from the mice, fixed, decalcified and prepared for IHC. Blood vessels (brown color) were detected with anti-factor VIII antibody. Scale bars: 50 µm. (B): Graph depicting the blood vessel density observed in the tissues generated with DPSC cells in (A). Asterisk indicates p<0.01.
Discussion
Vasculogenesis and angiogenesis are the processes by which new blood vessels are formed [27, 32, 33]. Vasculogenesis is defined as the differentiation of precursor cells into endothelial cells and de novo formation of a primitive vascular network, whereas angiogenesis is defined as the growth of new capillaries from pre-existing blood vessels [33]. Here, we showed that dental pulp stem cells can be induced to differentiate into vascular endothelial cells that formed de novo blood vessels via a process that closely resembles the vasculogenesis observed during early embryonic development. We also showed that the canonical Wnt-β-catenin signaling pathway plays a critical role regulating this process.
In the early 2000s, we demonstrated that human microvascular endothelial cells seeded in biodegradable scaffolds and transplanted into the subcutaneous space of immunodeficient mice were capable of organizing themselves into blood vessels [34]. At that time, we postulated that endothelial cells were “pre-programmed” to form tubular structures and find existing host vessels to anastomize and become blood-carrying vessels. Here, we went one step further and showed that human mesenchymal stem cells of dental origin can be induced to differentiate into vascular endothelial cells that are capable of de novo blood vessel formation. Individual stem cells that can be found spread throughout the scaffold eventually begin to proliferate and form initial lumen-less sprouts that further mature into blood-carrying vessels. These findings suggest that these post-natal mesenchymal stem cells have the potential to function as angioblast-like cells that can initiate the process of vasculogenesis in ways that resemble processes observed only early in embryonic development.
VEGF is the major regulator of endothelial cell proliferation, migration, differentiation, and survival [22, 35, 36]. It induces differentiation of human embryonic stem cells (hESCs) into vascular endothelial cells that can be used to provide vascularization of tissue engineered implants [37]. VEGF functions via signaling through its major vascular receptors, VEGFR1 (expressed in several cell types) and VEGFR2 (expressed primarily in endothelial cells), to regulate vasculogenesis and angiogenesis [38]. We have shown that unstimulated mesenchymal stem cells from permanent teeth or deciduous teeth express constitutively VEGFR1 [8] providing a pathway through which VEGF can initiate signaling in these stem cells. Indeed, we have shown that VEGFR1-silenced SHED cells no longer differentiate into endothelial cells [8]. What we did not understand at that time is how the vasculogenic fate of dental pulp stem cells could be downregulated even in presence of VEGF. Such regulatory mechanism has to exist to explain how some stem cells seeded in tooth slice/scaffolds differentiate into endothelial cells while others differentiate into odontoblasts [8, 39] when transplanted into the relatively hypoxic subcutaneous space of immunodeficient mice where there is ample supply of VEGF. The observation that the canonical Wnt-β-catenin signaling pathway plays a major role in the regulation of vasculogenic differentiation of these mesenchymal stem cells provides a possible explanation for the different cell fates observed under similar environmental conditions.
Elegant work from the Wang laboratory demonstrated that Wnt-β-catenin signaling inhibits odontoblastic differentiation of dental pulp stem cells [40]. We have demonstrated previously that dental pulp stem cells are capable of differentiating into functional odontoblasts or vascular endothelial cells [8]. Here, we showed that activation of the canonical Wnt-β-catenin signaling is sufficient to induce vasculogenic differentiation of these cells. In contrast, blockade of this pathway with a small molecule inhibitor (JW67), or by β-catenin gene silencing, prevents the vasculogenic differentiation of dental pulp stem cells. Collectively, these studies demonstrate that dental pulp stem cells are driven towards the vasculogenic fate when the canonical Wnt-β-catenin signaling pathway is active. These data also suggest that, if Wnt-β-catenin signaling is blocked, the vasculogenic fate is inhibited, enabling these stem cells to follow other fates (e.g. odontoblastic, osteoblastic differentiation). Our laboratory has recently started studies that will further address the intriguing hypothesis that the Wnt-β-catenin pathway serves as a “switch” regulating the decision between the osteoblastic/odontoblastic fate and the vasculogenic fate of mesenchymal stem cells.
Conclusions
In conclusion, we have shown that post-natal mesenchymal stem cells can be induced to differentiate into blood vessels in vivo via a process that resembles embryonic vasculogenesis. The vasculogenic potential of these stem cells requires the function of the canonical Wnt-β-catenin signaling pathway. Indeed, we observed that direct activation of the canonical Wnt-β-catenin signaling pathway is sufficient to induce the vasculogenic differentiation of these mesenchymal stem cells. These findings may have implications to the understanding of mechanisms involved in the vascularization of the dental papilla during early tooth development. They may also accelerate the therapeutic use of dental pulp stem cells for tissue regeneration. Understanding the process of vasculogenic differentiation of mesenchymal stem cells may lead to mechanism-based strategies to effectively modulate cell fate to optimize tissue regeneration.
Supplementary Material
Acknowledgments
Funding: This work was funded by grant R01-DE21410 from the NIH/NIDCR (JEN)
Footnotes
Author contributions: ZZ conceived the work, performed the experiments, and drafted the manuscript; FN, MO, and CC played an important role in the in vivo experiments and revised the manuscript; SS provided the DPSC and SHED cells used here, helped with the interpretation of the data, and revised the manuscript; JEN conceived the work, helped in the interpretation of the data, and edited the manuscript.
Conflict of interest
The authors declare no conflict of interest.
References
- 1.Gronthos S, Mankani M, Brahim J, et al. Postnatal human dental pulp stem cells (DPSCs) in vitro and in vivo . Proc Natl Acad Sci USA. 2000;97:13625–13630. doi: 10.1073/pnas.240309797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Miura M, Gronthos S, Zhao M, et al. SHED: stem cells from human exfoliated deciduous teeth. Proc Natl Acad Sci USA. 2003;100:5807–5812. doi: 10.1073/pnas.0937635100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sonoyama W, Liu Y, Fang D, et al. Mesenchymal stem cell-mediated functional tooth regeneration in Swine. PLoS One. 2006;1:e79. doi: 10.1371/journal.pone.0000079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sonayama W, Liu Y, Yamaza T, et al. Characterization of the apical and its residing stem cells from human immature permanent teeth: a pilot study. J Endod. 2008;34:166–171. doi: 10.1016/j.joen.2007.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Huang GT, Gronthos S, Shi S. Mesenchymal stem cells derived from dental tissues vs. those from other sources: their biology and role in regenerative medicine. J Dent Res. 2009;88:792–806. doi: 10.1177/0022034509340867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.d’Aquino R, Graziano A, Sampaolesi M, et al. Human postnatal dental pulp cells co-differentiate into osteoblasts and endotheliocytes: a pivotal synergy leading to adult bone tissue formation. Cell Death Differ. 2007;14:1162–1171. doi: 10.1038/sj.cdd.4402121. [DOI] [PubMed] [Google Scholar]
- 7.Iohara K, Zheng L, Wake H, et al. A novel stem cell source for vasculogenesis in ischemia: subfraction of side population cells from dental pulp. Stem Cells. 2008;26:2408–2418. doi: 10.1634/stemcells.2008-0393. [DOI] [PubMed] [Google Scholar]
- 8.Sakai VT, Zhang Z, Dong Z, et al. SHED differentiate into functional odontoblasts and endothelium. J Dent Res. 2010;89:791–796. doi: 10.1177/0022034510368647. [DOI] [PubMed] [Google Scholar]
- 9.Bento LW, Zhang Z, Imai A, et al. Endothelial differentiation of SHED requires MEK1/ERK signaling. J Dent Res. 2013;92:51–57. doi: 10.1177/0022034512466263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wodarz A, Nusse R. Mechanisms of Wnt signaling in development. Annu Rev Cell Dev Biol. 1998;14:59–88. doi: 10.1146/annurev.cellbio.14.1.59. [DOI] [PubMed] [Google Scholar]
- 11.Reya T, Duncan AW, Ailles L, et al. A role for Wnt signalling in self-renewal of haematopoietic stem cells. Nature. 2003;423:409–414. doi: 10.1038/nature01593. [DOI] [PubMed] [Google Scholar]
- 12.Stenman JM, Rajagopal J, Carroll TJ, et al. Canonical Wnt signaling regulates organ-specific assembly and differentiation of CNS vasculature. Science. 2008;322:1247–1250. doi: 10.1126/science.1164594. [DOI] [PubMed] [Google Scholar]
- 13.Willert K, Brown JD, Danenberg E, et al. Wnt proteins are lipid-modified and can act as stem cell growth factors. Nature. 2003;423:448–452. doi: 10.1038/nature01611. [DOI] [PubMed] [Google Scholar]
- 14.Woll PS, Morris JK, Painschab MS, et al. Wnt signaling promotes hematoendothelial cell development from human embryonic stem cells. Blood. 2008;111:122–131. doi: 10.1182/blood-2007-04-084186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sturgeon CM, Ditadi A, Awong G, et al. Wnt signaling controls the specification of definitive and primitive hematopoiesis from human pluripotent stem cells. Nat Biotechnol. 2014;32:554–561. doi: 10.1038/nbt.2915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lian X, Bao X, Al-Ahmad A, et al. Efficient differentiation of human pluripotent stem cells to endothelial progenitors via small-molecule activation of WNT signaling. Stem Cell Reports. 2014;3:804–816. doi: 10.1016/j.stemcr.2014.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dejana E. The role of Wnt signaling in physiological and pathological angiogenesis. Circ Res. 2010;107:943–952. doi: 10.1161/CIRCRESAHA.110.223750. [DOI] [PubMed] [Google Scholar]
- 18.Leung DW, Cachianes G, Kuang WJ, et al. Vascular endothelial growth factor is a secreted angiogenic mitogen. Science. 1989;246:1306–1309. doi: 10.1126/science.2479986. [DOI] [PubMed] [Google Scholar]
- 19.Keck PJ, Hauser SD, Krivi G, et al. Vascular permeability factor, an endothelial cell mitogen related to PDGF. Science. 1989;246:1309–1312. doi: 10.1126/science.2479987. [DOI] [PubMed] [Google Scholar]
- 20.de Vries C, Escobedo JA, Ueno H, et al. The fms-like tyrosine kinase, a receptor for vascular endothelial growth factor. Science. 1992;255:989–991. doi: 10.1126/science.1312256. [DOI] [PubMed] [Google Scholar]
- 21.Terman BI, Dougher-Vermazen M, Carrion ME, et al. Identification of the KDR tyrosine kinase as a receptor for vascular endothelial cell growth factor. Biochem Biophys Res Commun. 1992;187:1579–1586. doi: 10.1016/0006-291x(92)90483-2. [DOI] [PubMed] [Google Scholar]
- 22.Breier G, Albrecht U, Sterrer S, et al. Expression of vascular endothelial growth factor during embryonic angiogenesis and endothelial differentiation. Development. 1992;114:521–532. doi: 10.1242/dev.114.2.521. [DOI] [PubMed] [Google Scholar]
- 23.Waltenberger J, Claesson-Welsh L, Siegbahn A, et al. Different signal transduction properties of KDR and Flt1, two receptors for vascular endothelial growth factor. J Biol Chem. 1994;269:26988–26995. [PubMed] [Google Scholar]
- 24.Shibuya M. Differential roles of vascular endothelial growth factor receptor-1 and receptor-2 in angiogenesis. J Biochem Mol Biol. 2006;39:469–78. doi: 10.5483/bmbrep.2006.39.5.469. [DOI] [PubMed] [Google Scholar]
- 25.Zhang Z, Neiva KG, Lingen MW, et al. VEGF-dependent tumor angiogenesis requires inverse and reciprocal regulation of VEGFR1 and VEGFR2. Cell Death Differ. 2010;17:499–512. doi: 10.1038/cdd.2009.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sakai VT, Cordeiro MM, Dong Z, et al. Tooth slice/scaffold model of dental pulp tissue engineering. Adv Dent Res. 2011;23:325–332. doi: 10.1177/0022034511405325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Risau W, Flamme I. Vasculogenesis. Annu Rev Cell Dev Biol. 1995;11:73–91. doi: 10.1146/annurev.cb.11.110195.000445. [DOI] [PubMed] [Google Scholar]
- 28.Armulik A, Genové G, Betsholtz C. Pericytes: developmental, physiological, and pathological perspectives, problems, and promises. Dev Cell. 2011;21:193–215. doi: 10.1016/j.devcel.2011.07.001. [DOI] [PubMed] [Google Scholar]
- 29.Nehls V, Drenckhahn D. Heterogeneity of microvascular pericytes for smooth muscle type alpha-actin. J Cell Biol. 1991;113:147–154. doi: 10.1083/jcb.113.1.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ring DB, Johnson KW, Henriksen EJ, et al. Selective glycogen synthase kinase 3 inhibitors potentiate insulin activation of glucose transport and utilization in vitro and in vivo. Diabetes. 2003;52:588–595. doi: 10.2337/diabetes.52.3.588. [DOI] [PubMed] [Google Scholar]
- 31.Waaler J, Machon O, Von Kries JP, et al. Novel synthetic antagonists of canonical Wnt signaling inhibit colorectal cancer cell growth. Cancer Res. 2011;71:197–205. doi: 10.1158/0008-5472.CAN-10-1282. [DOI] [PubMed] [Google Scholar]
- 32.Carmeliet P. Mechanisms of angiogenesis and arteriogenesis. Nat Med. 2000;6:389–395. doi: 10.1038/74651. [DOI] [PubMed] [Google Scholar]
- 33.Risau W. Mechanisms of angiogenesis. Nature. 1997;386:671–674. doi: 10.1038/386671a0. [DOI] [PubMed] [Google Scholar]
- 34.Nör JE, Peters MC, Christensen JB, et al. Engineering and characterization of functional human microvessels in immunodeficient mice. Lab Invest. 2001;81:453–463. doi: 10.1038/labinvest.3780253. [DOI] [PubMed] [Google Scholar]
- 35.Ferrara N, Heinsohn H, Walder CE, et al. The regulation of blood vessel growth by vascular endothelial growth factor. Ann N Y Acad Sci. 1995;752:246–256. doi: 10.1111/j.1749-6632.1995.tb17435.x. [DOI] [PubMed] [Google Scholar]
- 36.Nör JE, Christensen J, Mooney DJ, et al. Vascular endothelial growth factor (VEGF)-mediated angiogenesis is associated with enhanced endothelial cell survival and induction of Bcl-2 expression. Am J Pathol. 1999;154:375–384. doi: 10.1016/S0002-9440(10)65284-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nourse MB, Halpin DE, Scatena M, et al. VEGF induces differentiation of functional endothelium from human embryonic stem cells implications for tissue engineering. Arterioscler Thromb Vasc Biol. 2010;30:80–89. doi: 10.1161/ATVBAHA.109.194233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Millauer B, Wizigmann-Voos S, Schnürch H, et al. High affinity VEGF binding and developmental expression suggest Flk-1 as major regulator of vasculogenesis and and angiogenesis. Cell. 1993;72:835–846. doi: 10.1016/0092-8674(93)90573-9. [DOI] [PubMed] [Google Scholar]
- 39.Casagrande L, Demarco FF, Zhang Z, et al. Dentin-derived BMP-2 and odontoblast differentiation. J Dent Res. 2010;89:603–608. doi: 10.1177/0022034510364487. [DOI] [PubMed] [Google Scholar]
- 40.Scheller EL, Chang J, Wang CY. Wnt/beta-catenin inhibits dental pulp stem cell differentiation. J Dent Res. 2008;87:126–130. doi: 10.1177/154405910808700206. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.