Abstract
Bleeding because of impaired platelet function is a major side effect of the Bruton's tyrosine kinase (BTK) inhibitor ibrutinib. We quantitatively assessed ristocetin-induced platelet aggregation (RIPA) in 64 patients with chronic lymphocytic leukemia (CLL) under ibrutinib at 287 time points. Eighty-seven bleeding episodes in 39 patients were registered (85 Common Toxicity Criteria (CTC) grade 1 or 2, 2 CTC grade 3) during a median observation period of 10.9 months. At times of bleeding, RIPA values were significantly lower (14 vs 28 U; P<0.0001). RIPA was impaired in patients receiving concomitant antiplatelet therapy or anticoagulation (14 vs 25 U, P=0.005). A gradual decline of median RIPA values was observed with increasing bleeding severity. Importantly, no CTC grade 2 or 3 bleeding were observed with RIPA values of >36 U. Sequential monitoring indicated a decrease of RIPA values from a median of 17 to 9 U within 2 weeks after initiation of treatment as well as an increase above the critical threshold of 36 U within 7 days when ibrutinib was paused. Low RIPA values were similar during treatment with another BTK inhibitor, CC292. Quantitative assessment of platelet function is a practical tool to monitor bleeding tendency under BTK-inhibitor therapy.
Introduction
Targeting Bruton's tyrosine kinase (BTK) with the small-molecule ibrutinib has significantly improved progression-free and overall survival of patients with chronic lymphocytic leukemia (CLL) and mantle cell lymphoma and is currently investigated in other B-cell lymphomas.1, 2, 3, 4, 5 The drug is now widely used in clinical routine. Its mechanism of action is based on the inhibition of BTK as part of the B-cell receptor signaling cascade.6, 7, 8, 9, 10 BTK is also expressed in other hematopoietic lineages, including platelets.11, 12, 13 Inhibition of this kinase may therefore result in relevant hematologic side effects. Indeed, bleeding episodes were observed in 44% of patients in the CLL registration trial1 and in up to 61% of patients after a longer observation period.14
Bleeding is usually mild (Common Toxicity Criteria (CTC) grades 1–2, corresponding to spontaneous bruising or petechiae), but more severe bleeding is observed in 8% of patients.14 CTC grade 3 or 4 bleeding occurs in ∼5% of patients after trauma.1, 4, 15
BTK is a TEC family kinase and acts as a signaling molecule in von Willebrand factor (vWF)- and collagen-induced platelet activation.12 The corresponding receptors glycoprotein VI (GPVI, collagen receptor) and the glycoprotein Ib-IX-V complex (GPIb, vWF receptor) have previously been shown to be affected by BTK inhibition: in vitro binding of platelets from ibrutinib-treated patients to vWF matrix was significantly impaired16 as well as platelet response to collagen using light transmission aggregometry.11
Of note, vWF antigen and activity in plasma remain within normal range.17 The dependency of GPIb-IX-V signaling on BTK has also been shown in vivo using a BTK-deficient mouse model.13 However, there is a possibility that TEC, another member of the TEC kinase family, compensates for the loss of BTK in platelets as it is indicated by the partially preserved response to collagen in human X-linked agammaglobulinemia patients.18 Nevertheless, the cysteine residue that is required for the covalent binding of ibrutinib to an adenosine triphosphate-binding site can also be found in TEC.12 Taken together, there is strong evidence that pharmacologic inhibition of BTK and other TEC kinases by ibrutinib results in impaired platelet function.
Proper management of bleeding complications is important because they may affect the length and intensity of ibrutinib treatment. In addition, up to 50% of CLL patients with cardiovascular comorbidities are under concomitant treatment with anticoagulants or platelet function inhibitors.19, 20
We used an easy to perform method for quantitative assessment of vWF/ristocetin-induced platelet aggregation (RIPA) in ibrutinib-treated CLL patients in order to (1) correlate the degree of impairment of platelet function with bleeding tendency and (2) monitor platelet aggregation during drug therapy and before interventions.
Subjects and methods
Subjects
This observational study included 64 CLL patients from 4 different centers in 3 countries (Cambridge, Munich, Salzburg and Vienna). Patients received ibrutinib at a target dose of 420 mg daily and were either included in the RESONATE study (28 patients), in the ibrutinib named patient program (12 patients) or received ibrutinib in routine clinical use according to the licensed indication in Europe (24 patients). Of the patients, 18% were previously untreated but had a 17p deletion or TP53 mutation, whereas 82% had relapsed or refractory CLL with or without 17p/TP53 aberrations. The study was approved by the ethics committee of the Medical University of Vienna (ethics committee Nr 1631/2012 and 11/2005) and informed consent was obtained. Eleven patients had multiple measurements before start and during ibrutinib therapy and 53 patients were investigated under stable ibrutinib intake.
Patients were seen regularly (at least monthly) in their corresponding centers. Bleeding tendency was recorded at each time point by standardized questions including bruising, petechiae and epistaxis, and a physical examination was performed. Clinical information included clinical stage, genetics, lines of therapy and concomitant treatment, particularly therapy with antiplatelet agents or anticoagulants. Patient characteristics are shown in Table 1. Severity of bleeding was scored according to the CTC grading scale (version 4.03: 14 June 2010) used in the RESONATE protocol.
Table 1. Patient characteristics.
| Characteristic | Ibrutinib patients (n=64) |
|---|---|
| Median age, years (range) | 71 (40–92) |
| ⩾70 Years | 56.3% |
| ⩾75 Years | 29.7% |
| Male, % | 64.1% |
| Median weight, kg (range) | 76 (44–114) |
| Male | 80 (62–114) |
| Female | 58 (44–84) |
| Median number of prior therapies (range) | 2 (0–6) |
| 0 | 18% |
| 1 | 27.9% |
| 2 | 32.8% |
| ⩾3 | 21.3% |
| Del17p or TP53 mutation, % | 47.6% |
| Median platelet count, G/l (range) | 114.5 (28–307) |
| Median observation period, months (range) | 10.9 (0.5–35.3) |
| Patients with at least one bleeding event | 39/64 (60.9%) |
| CTC grade 1 or 2 | 37 |
| CTC grade 3 | 2 |
| CTC grade 4 or more severe | None |
| Total number of bleeding events during follow-up | 87 |
| CTC grade 1 or 2 | 85/87 (97.7%) |
| CTC grade 3 | 2/87 (2.3%) |
| CTC grade 4 or more severe | None |
| Total number of patients in whom ibrutinib was paused or discontinued because of; | 11/64 (17.2%) |
| Progression of disease/death | 2/64 (3.1%) |
| Adverse event | 6/64 (9.4%) |
| Planned surgery | 3/64 (4.7%) |
| Total number of patients still responding and on ibrutinib after a median of 10.9 months (0.5–35.3) | 60/64 (93.8%) |
Abbreviation: CTC, Common Toxicity Criteria.
Controls included 13 CLL patients currently not on treatment, 11 patients receiving other treatments for CLL (4 with immunochemotherapy (rituximab plus fludarabine/cyclophosphamide or bendamustin) and 7 with phosphatidylinositol-3-kinase inhibitors (idelalisib, duvelisib)) as well as 1 patient with X-linked agammaglobulinemia and proven BTK protein deficiency.
Platelet function assessments
Fresh blood specimens (5 ml) for analysis of platelet function were drawn into hirudine containing tubes (Vacuette, Kremsmuenster, Austria) and analyzed within 3 h in a Multiplate Analyzer (Roche, Vienna, Austria) according to the manufacturer's recommendations. This whole blood platelet aggregometry measures the increase in electric resistance when platelets adhere and aggregate on electrodes. The in-house normal ranges of the performed aggregation tests were 44–176 U for RIPA and 22–110 U for collagen-induced platelet aggregation in healthy volunteers.21 The measured area under the curve allows quantification of platelet function. RIPA and collagen-induced platelet aggregation were performed for this study. Importantly, test results are dependent on platelet function and platelet count22, 23 and the assessment has to be performed on fresh whole blood locally.
Reproducibility
Variability of the RIPA test was evaluated in Vienna in five healthy donors and six patients under ibrutinib. A total of 4–10 measurements were performed from the same time point and on consecutive days in healthy donors. In total, median values for healthy donors ranged from 81.9 to 133.0 U with an intraindividual coefficient of variation of 7.6 to 9.1%. Median values for the 6 patients were 4.1 to 37.5 U with a coefficient of variation from 11.2 to 15.6% in 5 patients. In one patient with very low values the results varied from 1 to 8 U (coefficient of variation 49.4%), but still stayed in the same range.
In addition, vWF antigen and activity were assessed by standard methods as described previously.24
Statistical methods
Statistical analysis was performed using JMP Statistics (SAS Corporation, Cary, NC, USA) software. Statistical methods comprised χ2 analysis or Fisher's exact test to compare baseline characteristics, and t-test or analysis of variance where applicable for RIPA analysis. Correlations were determined by Pearson's r. The P-values of <0.05 (two sided) were considered statistically significant.
Results
Patient characteristics and bleeding events
The study cohort consisted of a typical CLL cohort treated with ibrutinib with a median age of 71 (range: 40–92) years, 2 (range: 0–6) prior treatment lines and aberrations of TP53 (17p deletion and/or TP53 mutation) in 47.6%. The median observation period under ibrutinib was 10.9 months. Importantly, 28.1% were treated with concomitant antiplatelet (acetyl salicylic acid, clopidogrel, 18.8%) or anticoagulation therapy (coumarin, heparin, novel direct oral anticoagulants, 10.9%) or both (in 1 case).
At least one bleeding event was recorded in 39 of the 64 patients (60.9% Table 1). Bleeding was mild in most patients (CTC grade 1 or 2) and only two CTC grade 3 bleedings were observed. The total number of bleeding episodes was 87, again predominantly grade 1 or 2. None of the patients had a grade 4 or 5 bleeding event. Of note, 60 of the 64 patients (93.8%) were still responding and on ibrutinib after 10.9 months. Reasons for discontinuation or pause are shown in Table 1.
Bleeding tendency, platelet number and anticoagulation
The occurrence of bleeding events under ibrutinib was significantly associated with lower platelet counts (116.5 G/l, range: 38–303 vs 137 G/l, range: 51–328; P=0.0012; Supplementary Figure 1). Patients receiving antiplatelet therapy or anticoagulation (13/18, 72%) were more frequently affected than patients without concomitant treatment (26/46; 57%, P=0.25). Patients with anticoagulation had the highest frequency of bleeding (6 of 7 patients; Table 2). However, this was not statistically significant.
Table 2. Characteristics of bleeding and nonbleeding patients.
| Characteristic | Bleeding | No bleeding | P-value |
|---|---|---|---|
| Total number (n=64) | 39/64 (60.9%) | 25/64 (39.1%) | NA |
| Median body weight, kg (range) | 74 (48–108) | 69.5 (44–114) | 0.49 |
| Male, % | 64.1% | 64% | 0.99 |
| Antiplatelet or anticoagulant Tx | 13/39 (33.3%) | 5/25 (20%) | 0.25 |
| Antiplatelet therapy | 8/39 (20.5%) | 4/25 (16%) | 0.65 |
| Oral AC/heparin | 6/39 (15.4%) | 1/25 (4%) | 0.15 |
| Median platelet count, G/l (range) | 116.5 (38–303) | 137 (51–328) | 0.0012 |
| Median hemoglobin, mg/dl (range) | 12.6 (9.2–16.7) | 12.1 (8.3–17.1) | 0.16 |
| Median WBC, G/l (range) | 21.8 (4.3–347.8) | 16.0 (2.2–397) | 0.41 |
Abbreviations: AC, anticoagulant; NA, not available; Tx, treatment; WBC, white blood cell count. Bold value indicates significant P-value.
Impairment of platelet aggregation during ibrutinib treatment
In total, 287 quantitative assessments were performed by RIPA (median: 3 per patient). CLL patients without current treatment (median 57 U, range: 8–111) or during immunochemotherapy (median 68.5 U, range: 27–94) had RIPA values close to normal, whereas patients under ibrutinib had considerably impaired RIPA values, although with a wide range (median 19.5 U, range: 0–199; Supplementary Figure 2).
Bleeding tendency and platelet function
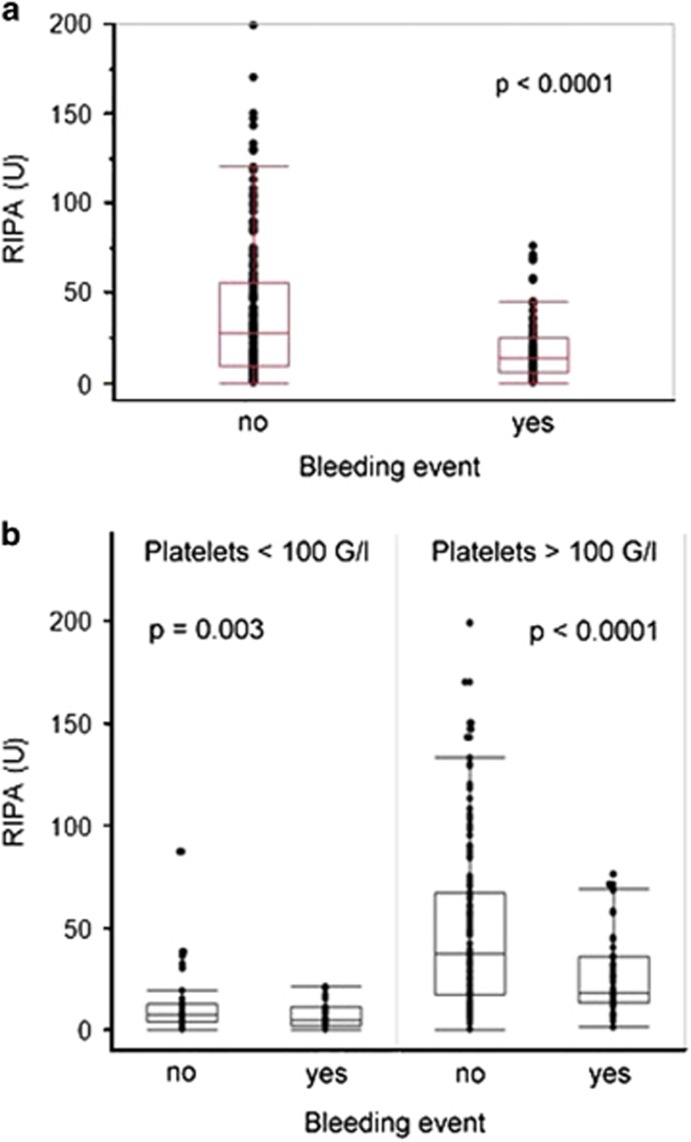
The major goal of this study was to establish an association between bleeding and platelet function during ibrutinib treatment. The median RIPA measured at times of bleeding events under ibrutinib was significantly impaired (median 14 U, range: 0–76) compared with time points when no bleeding was observed (median 28 U, range: 0–199; P<0.0001; Figure 1a). Collagen-induced platelet aggregation testing confirmed the findings obtained by RIPA measurements. Platelet function showed significantly lower values at time points when bleeding occurred (P=0.0015; Supplementary Figure 3). We confirmed that RIPA values with this assay correlate with platelet numbers (P<0.0001; Supplementary Figure 4).23 However, low RIPA values were associated with bleeding regardless of platelet count (Figure 1b).
Figure 1.
RIPA measurements in CLL patients during stable ibrutinib therapy. (a) RIPA measurements were significantly lower at the time when bleeding-related adverse events occurred (median 14 vs 28 U, P<0.0001). (b) When patients were grouped by platelet count, RIPA results remained impaired at the time of bleeding-related adverse events (P=0.003 and P<0.0001, respectively).
Effect of concomitant anticoagulation
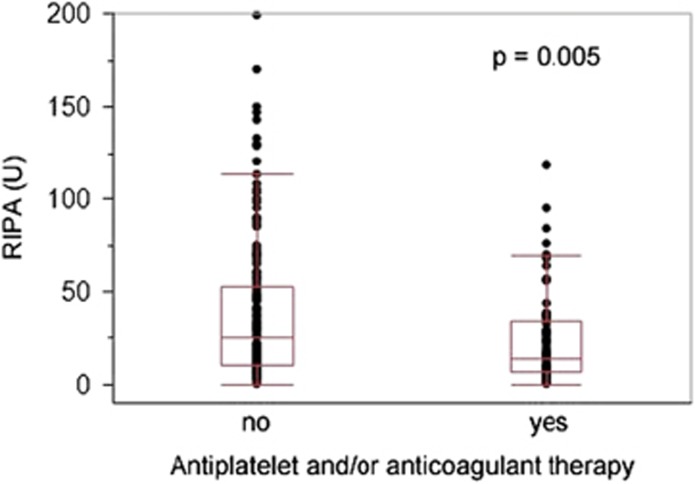
RIPA was significantly impaired in patients receiving concomitant antiplatelet therapy or anticoagulation (14 vs 25 U, P=0.005; Figure 2). This effect was seen regardless of platelet count, but more prominent when platelets were >100 G/l (Supplementary Figure 5). There was no difference between patients receiving antiplatelet or anticoagulant therapy (data not shown).
Figure 2.
RIPA in ibrutinib-treated CLL patients with concomitant antiplatelet and/or anticoagulant medication. Ibrutinib-treated CLL patients under concomitant antiplatelet and/or anticoagulant therapy showed reduced RIPA values (median 14 U, range: 0–118 U) when compared with patients without concomitant medication (median 25 U, range: 0–199 U; P=0.005).
Bleeding severity and platelet function
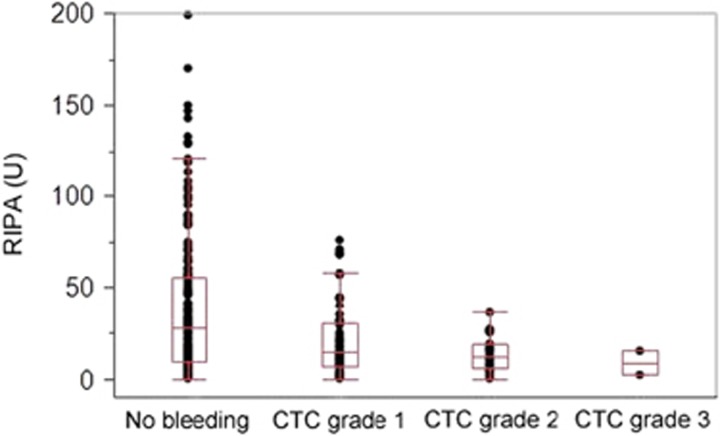
We next analyzed platelet function in relation to the severity of the event expressed by CTC grade: a steady decline of median RIPA values was observed from time points without bleeding (28 U) to CTC grade 1 (14.5 U), grade 2 (12 U) and grade 3 (8.5 U) events (Figure 3). Importantly, no CTC grade 2 or 3 bleeding was observed with RIPA values of >36 U. These data indicate that impairment of platelet function is associated with severity of bleeding and that significant bleeding does not occur beyond a defined threshold.
Figure 3.
Relationship of RIPA with severity of bleeding. Results of RIPA measurements showed a median value of 28 U (range: 0–199 U) when no bleeding-related adverse event was reported, and decreased gradually from CTC grade 1 (median 14.5 U, range: 0–76 U) to CTC grade 2 (median 12 U, range: 0–36 U) and CTC grade 3 events (median 8.5 U, range: 2–15 U) dependent on adverse event severity.
Monitoring of platelet function during ibrutinib therapy
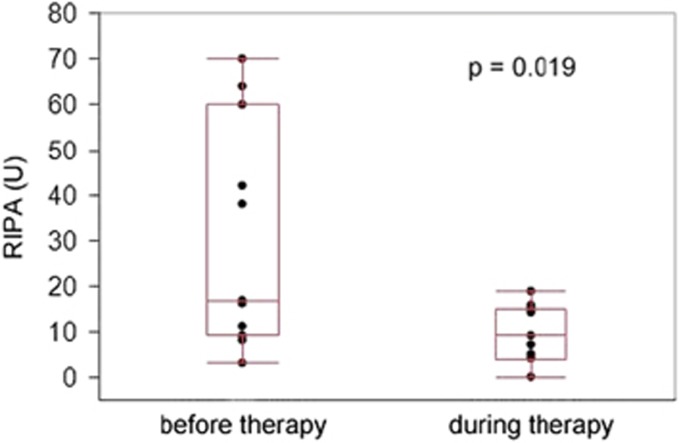
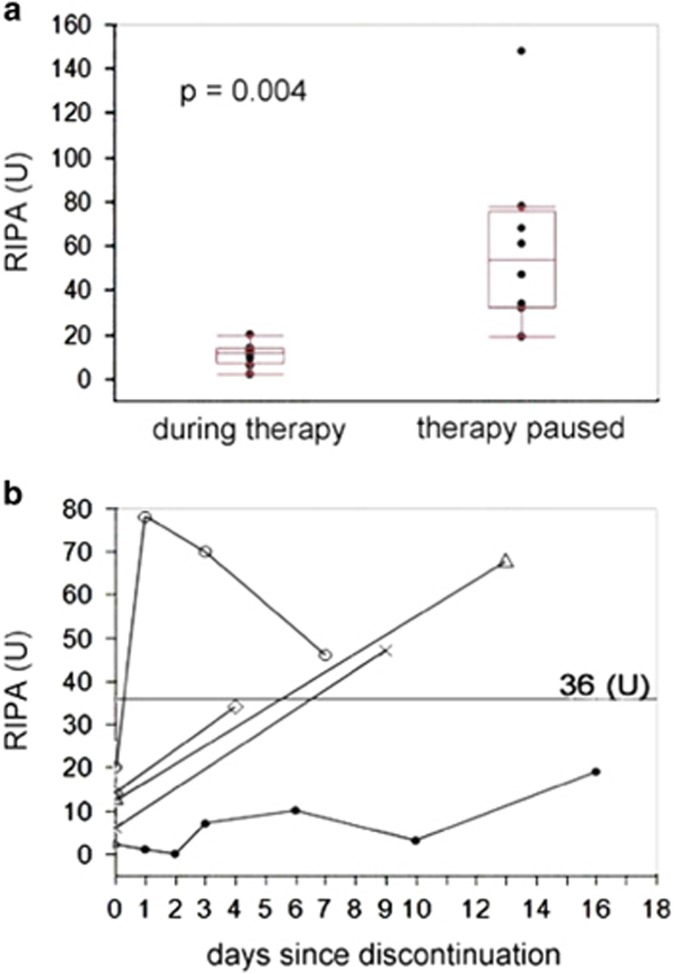
Consecutive samples before and after start of ibrutinib were available in 11 patients. A decline of platelet aggregation from 17 to 9 U was observed when therapy was initiated (P=0.019; Figure 4). The median interval between measurements was 13 days. When ibrutinib was paused or stopped (9 instances) the median RIPA values increased from 12 to 54 U (P=0.004; median interval 18 days; Figure 5a). Short-term RIPA kinetics were available in 5 patients (Figure 5b): some variability of platelet function recovery was observed with 4 of 5 patients crossing the imaginary threshold of 36 U within 7 days, whereas recovery was slow in 1 patient. These data indicate that quantitative assessment of RIPA may be used to monitor platelet function, for example, in the context of planned surgical interventions.
Figure 4.
Dynamics of RIPA after initiation of ibrutinib therapy in CLL patients. Consecutive measurements were available in 11 CLL patients and showed a marked decrease (median 17 U, range: 3–70 U vs median 9 U, range: 0–19 U, P=0.019) after a median period of 13 days of ibrutinib therapy.
Figure 5.
Dynamics of RIPA measurements after pause/discontinuation of ibrutinib therapy in CLL patients. (a) Platelet function partly recovered (median 12 vs 54 U, P=0.004) after pause/discontinuation of ibrutinib therapy. The median time between measurements was 18 days in 9 patients. (b) In five patients, RIPA was measured in shorter intervals after discontinuation. The threshold of 36 U was reached within 7 days by 4/5 patients.
Platelet function with higher doses of ibrutinib and other BTK inhibitors
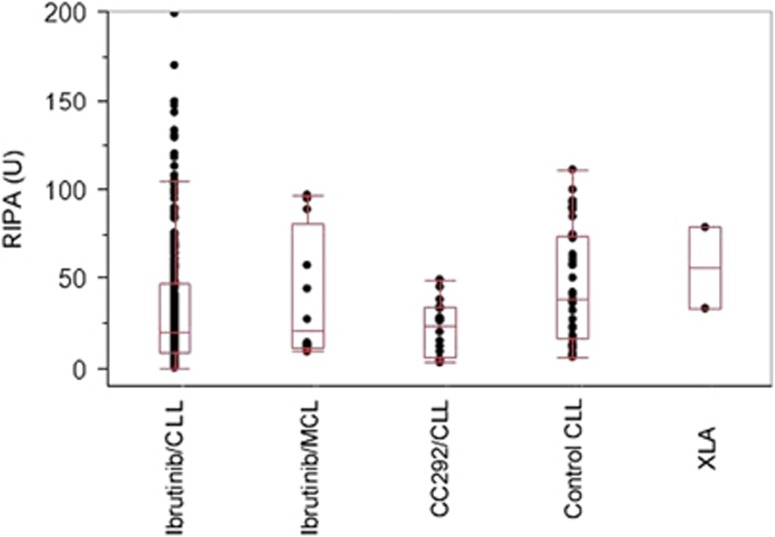
We also assessed RIPA in patients receiving higher doses of ibrutinib (560 mg daily) in mantle cell lymphoma patients (n=7). RIPA values were comparable to those in CLL patients (Figure 6). Ibrutinib also inhibits other kinases including TEC kinases that are also involved in the regulation of platelet function. Interestingly, patients under treatment with a different BTK inhibitor, CC292 (n=6), showed almost identical RIPA values (19.5 vs 23 U). Two different measurements in a patient with a hereditary BTK deficiency (X-linked agammaglobulinemia) showed less impairment of platelet function.
Figure 6.
Comparison of platelet function in ibrutinib-treated CLL (n=64), ibrutinib-treated mantle cell lymphoma (MCL; n=7), CC292-treated CLL (n=6), CLL control (n=13) and X-linked agammaglobulinemia (XLA; n=1) patients. Median RIPA values: ibrutinib/CLL (19.5 U, range: 0–199 U), ibrutinib/MCL (20.5 U, range: 9–97 U), CC292/CLL (23 U, range: 3–49 U), Control CLL (37 U, range: 6–94 U) and XLA (56 U, range 33–79 U).
Discussion
Ibrutinib has become an integral part of the CLL treatment algorithm. The drug is well tolerated, but bleeding is a frequent side effect.14 Here we show that quantitative assessment of RIPA is a practical tool to monitor and manage bleeding tendency, and may thereby increase the safety of ibrutinib.
The theoretical basis for the impairment of platelet function has been well described by others13, 16, 18 (Supplementary Figure 6). BTK is an important component of the signaling cascades downstream of the GPIb and GPVI platelet receptors.13, 18 Therefore, ristocetin- as well as collagen-induced platelet aggregation are impaired and could both be used as diagnostic tools.16 Unfortunately, assessment of platelet function by light transmission aggregometry has turned out to be tedious, variable and operator dependent. Here we used an easy to perform whole blood aggregometry method that is frequently used in clinical routine. We chose the RIPA assay for our investigations because of its slightly better discriminatory power in preliminary experiments. However, similar results were obtained with collagen-induced platelet aggregation (Supplementary Figure 3). There are some uncertainties as to the contribution of BTK in comparison with other Tec kinases in impairing platelet function as ibrutinib inhibits several other kinases. However, the data obtained with a different BTK inhibitor, CC292, argue for a strong involvement of BTK in platelet function. It remains to be established whether this will translate into clinical bleeding tendency when other selective BTK inhibitors enter the clinic.25, 26
The major clinical finding of this study is that low RIPA values are strongly associated with bleeding tendency. This is supported by the following observations: (1) a decline in platelet aggregation was observed when ibrutinib is initiated; (2) platelet function was significantly impaired in CLL patients under stable ibrutinib therapy compared with CLL patients under immunochemotherapy or without current intervention; and (3) at times when bleeding events occurred, RIPA values were significantly lower (14 vs 28 U). This also is in line with previous observations made by other groups.11, 16, 17 Bleeding under ibrutinib is usually mild, but can be severe in 5 to 8% of patients.14, 16 A practical clinical implication of our study is that RIPA values decrease with the degree of bleeding. Thus, it seems possible to define a threshold (36 U in this study) above which no major bleeding events (CTC grade ⩾3) will occur. This will allow monitoring of bleeding tendency with RIPA tests before elective surgery or in cases of emergency, where platelet transfusions may be indicated.16 We note that the recommended washout period of 7 days before surgical interventions correlated well with the increase in RIPA above the threshold level of 36 U in most patients. However, this may be different in some patients in whom monitoring will add important information for clinical decisions.
We observed an association of platelet number with bleeding tendency confirming previous observations (Supplementary Figure 1), indicating that bleeding events were more frequent during the initial treatment phase of CLL patients, when platelets are still low.14 In addition, there was a strong correlation between RIPA and platelet number in the assay used for this study, as reported during the evaluation of the test (Supplementary Figure 4).23 However, the association of low RIPA values and bleeding under ibrutinib was still significant regardless of platelet counts (Figure 1b). In addition, the median platelet values were quite high with a median of 116.5 G/l in bleeding patients.
Increased bleeding tendency with concomitant antiplatelet or anticoagulation therapy has been reported and has led to the recommendation that warfarin should not be used together with ibrutinib.10 There was a trend for, but no significant association of, concomitant antiplatelet or anticoagulation treatment with bleeding in our study. This is probably because of low patient numbers. However, in our study low RIPA values were associated with both antiplatelet and anticoagulation therapy indicating a higher propensity for bleeding.
There are some limitations to this study. The RIPA impedance test has to be performed on site within a limited time frame with fresh samples. Frozen or shipped samples cannot be used. On the other hand, the analyzer used is now widely available in larger hospitals. In addition, similar and reliable results could be obtained in 4 different centers in 3 countries (median RIPA values were 26 U (range: 0–199; Vienna), 14 U (range: 1–148; Cambridge), 16 U (range: 2–76; Munich) and 25 U (range: 3–68; Salzburg)). Measurements under stable ibrutinib intake on consecutive visits showed little variability (Supplementary Figure 7). There was no significant statistical difference between centers. The test has a reasonably low intraindividual variation (coefficient of variation <10% in healthy donors and <16% in patients under ibrutinib) confirming its clinical reliability. The threshold for major bleeding events is currently only based on 64 CLL patients and 287 measurements. A more detailed and formal evaluation such as for other CLL diagnostic tests will be required for robust generalized diagnostics.27, 28 Reporting of bleeding events is dependent on thorough clinical evaluation of patients at any visit. We have therefore developed a questionnaire that can be used in the future. Last but not the least, further confirmation in larger studies is still needed.
It is important to note that 93.8% of our CLL patients were still responding to ibrutinib after 10.9 months and many will remain on the drug for much longer. Monitoring of bleeding tendency with platelet function tests may therefore contribute to enhance safety, adherence and the successful long-term use of ibrutinib.
Acknowledgments
Expert assistance by Michaela Bronhagl and Bernadette Hilgarth is gratefully acknowledged. This study was in part supported by Grant SFB54-P04 (to BJ) from the Austrian Science Funds (FWF).
Footnotes
Supplementary Information accompanies this paper on the Leukemia website (http://www.nature.com/leu)
This work has been presented in part at the 57th Annual Meeting of the American Society of Hematology in Orlando 2015.
MB, PS, AE, C-MW and UJ received honoraria from Janssen. LK, CD, CS, WT, TM, SS, EP, CE, MH, AH, MR, IP, RT, GH, PQ and BJ declare no conflict of interest.
Supplementary Material
References
- Byrd JC, Furman RR, Coutre SE, Flinn IW, Burger JA, Blum KA et al. Targeting BTK with ibrutinib in relapsed chronic lymphocytic leukemia. N Engl J Med 2013; 369: 32–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrd JC, Brown JR, O'Brien S, Barrientos JC, Kay NE, Reddy NM et al. Ibrutinib versus of atumumab in previously treated chronic lymphoid leukemia. N Engl J Med 2014; 371: 213–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson WH, Young RM, Schmitz R, Yang Y, Pittaluga S, Wright G et al. Targeting B cell receptor signaling with ibrutinib in diffuse large B cell lymphoma. Nat Med 2015; 21: 922–926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang ML, Rule S, Martin P, Goy A, Auer R, Kahl BS et al. Targeting BTK with ibrutinib in relapsed or refractory mantle-cell lymphoma. N Engl J Med 2013; 369: 507–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang ML, Blum KA, Martin P, Goy A, Auer R, Kahl BS et al. Long-term follow-up of MCL patients treated with single-agent ibrutinib: updated safety and efficacy results. Blood 2015; 126: 739–745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aalipour A, Advani RH. Bruton tyrosine kinase inhibitors: a promising novel targeted treatment for B cell lymphomas. Br J Haematol 2013; 163: 436–443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiestner A. Emerging role of kinase-targeted strategies in chronic lymphocytic leukemia. Blood 2012; 120: 4684–4691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiestner A. Targeting B-Cell receptor signaling for anticancer therapy: the Bruton's tyrosine kinase inhibitor ibrutinib induces impressive responses in B-cell malignancies. J Clin Oncol 2013; 31: 128–130. [DOI] [PubMed] [Google Scholar]
- Brown JR. Ibrutinib (PCI-32765), the first BTK (Bruton's tyrosine kinase) inhibitor in clinical trials. Curr Hematol Malig Rep 2013; 8: 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown JR. Ibrutinib in chronic lymphocytic leukemia and B cell malignancies. Leuk Lymphoma 2014; 55: 263–269. [DOI] [PubMed] [Google Scholar]
- Kamel S, Horton L, Ysebaert L, Levade M, Burbury K, Tan S et al. Ibrutinib inhibits collagen-mediated but not ADP-mediated platelet aggregation. Leukemia 2015; 29: 783–787. [DOI] [PubMed] [Google Scholar]
- Berglof A, Hamasy A, Meinke S, Palma M, Krstic A, Mansson R et al. Targets for ibrutinib beyond B cell malignancies. Scand J Immunol 2015; 82: 208–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Fitzgerald ME, Berndt MC, Jackson CW, Gartner TK. Bruton tyrosine kinase is essential for botrocetin/VWF-induced signaling and GPIb-dependent thrombus formation in vivo. Blood 2006; 108: 2596–2603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrd JC, Furman RR, Coutre SE, Burger JA, Blum KA, Coleman M et al. Three-year follow-up of treatment-naive and previously treated patients with CLL and SLL receiving single-agent ibrutinib. Blood 2015; 125: 2497–2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Advani RH, Buggy JJ, Sharman JP, Smith SM, Boyd TE, Grant B et al. Bruton tyrosine kinase inhibitor ibrutinib (PCI-32765) has significant activity in patients with relapsed/refractory B-cell malignancies. J Clin Oncol 2013; 31: 88–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levade M, David E, Garcia C, Laurent PA, Cadot S, Michallet AS et al. Ibrutinib treatment affects collagen and von Willebrand factor-dependent platelet functions. Blood 2014; 124: 3991–3995. [DOI] [PubMed] [Google Scholar]
- Wiestner A. The role of B-cell receptor inhibitors in the treatment of patients with chronic lymphocytic leukemia. Haematologica 2015; 100: 1495–1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atkinson BT, Ellmeier W, Watson SP. Tec regulates platelet activation by GPVI in the absence of Btk. Blood 2003; 102: 3592–3599. [DOI] [PubMed] [Google Scholar]
- Goede V, Bahlo J, Chataline V, Eichhorst B, Durig J, Stilgenbauer S et al. Evaluation of geriatric assessment in patients with chronic lymphocytic leukemia: Results of the CLL9 trial of the German CLL study group. Leuk Lymphoma 2016; 57: 789–796. [DOI] [PubMed] [Google Scholar]
- Jones JA, Hillmen P, Coutre S, Tam C, Furman RR, Barr PM et al. Pattern of use of anticoagulation and/or antiplatelet agents in patients with chronic lymphocytic leukemia (CLL) treated with single-agent ibrutinib therapy. Blood 2014; 124: 1990. [Google Scholar]
- Spiel AO, Bartko J, Schwameis M, Firbas C, Siller-Matula J, Schuetz M et al. Increased platelet aggregation and in vivo platelet activation after granulocyte colony-stimulating factor administration. A randomised controlled trial. Thromb Haemost 2011; 105: 655–662. [DOI] [PubMed] [Google Scholar]
- Femia EA, Scavone M, Lecchi A, Cattaneo M. Effect of platelet count on platelet aggregation measured with impedance aggregometry (Multiplate analyzer) and with light transmission aggregometry. J Thromb Haemost 2013; 11: 2193–2196. [DOI] [PubMed] [Google Scholar]
- Hanke AA, Roberg K, Monaca E, Sellmann T, Weber CF, Rahe-Mayer N et al. Impact of platelet count on results obtained from multiple electrode platelet aggregometry (Multiplate). Eur J Med Res 2010; 15: 214–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jilma-Stohlawetz P, Knobl P, Gilbert JC, Jilma B. The anti-von Willebrand factor aptamer ARC1779 increases von Willebrand factor levels and platelet counts in patients with type 2B von Willebrand disease. Thrombosis Haemost 2012; 108: 284–290. [DOI] [PubMed] [Google Scholar]
- Walter HS, Rule SA, Dyer MJ, Karlin L, Jones C, Cazin B et al. A phase 1 clinical trial of the selective BTK inhibitor ONO/GS-4059 in relapsed and refractory mature B-cell malignancies. Blood 2016; 127: 411–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrd JC, Harrington B, O'Brien S, Jones JA, Schuh A, Devereux S et al. Acalabrutinib (ACP-196) in relapsed chronic lymphocytic leukemia. N Engl J Med 2016; 374: 323–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rawstron AC, Fazi C, Agathangelidis A, Villamor N, Letestu R, Nomdedeu J et al. A complementary role of multiparameter flow cytometry and high-throughput sequencing for minimal residual disease detection in chronic lymphocytic leukemia: an European Research Initiative on CLL study. Leukemia 2015; 30: 929–936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pospisilova S, Sutton LA, Malcikova J, Tausch E, Rossi D, Montserrat E et al. Innovation in the prognostication of chronic lymphocytic leukemia: how far beyond TP53 gene analysis can we go? Haematologica 2016; 101: 263–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.