Abstract
Hyperalgesic priming, a model of pain chronification in the rat, is mediated by ryanodine receptor-dependent calcium release. Although ryanodine induces priming in both sexes, females are 5 orders of magnitude more sensitive, by an estrogen receptor α (EsRα)-dependent mechanism. An inositol 1,4,5-triphosphate (IP3) receptor inhibitor prevented the induction of priming by ryanodine. For IP3 induced priming, females were also more sensitive. IP3-induced priming was prevented by pretreatment with inhibitors of the sarcoendoplasmic reticulum calcium ATPase and ryanodine receptor. Antisense to EsRα prevented the induction of priming by low-dose IP3 in females. The induction of priming by an EsRα agonist was ryanodine receptor-dependent and prevented by the IP3 antagonist. Thus, an EsRα-dependent bidirectional interaction between endoplasmic reticulum IP3 and ryanodine receptor-mediated calcium signaling is present in the induction of hyperalgesic priming, in females. In cultured male DRG neurons, IP3 (100 μm) potentiated depolarization-induced transients produced by extracellular application of high-potassium solution (20 mm, K20), in nociceptors incubated with β-estradiol. This potentiation of depolarization-induced calcium transients was blocked by the IP3 antagonist, and not observed in the absence of IP3. IP3 potentiation was also blocked by ryanodine receptor antagonist. The application of ryanodine (2 nm), instead of IP3, also potentiated K20-induced calcium transients in the presence of β-estradiol, in an IP3 receptor-dependent manner. Our results point to an EsRα-dependent, reciprocal interaction between IP3 and ryanodine receptors that contributes to sex differences in hyperalgesic priming.
SIGNIFICANCE STATEMENT The present study demonstrates a mechanism that plays a role in the marked sexual dimorphism observed in a model of the transition to chronic pain, hyperalgesic priming. This mechanism involves a reciprocal interaction between the endoplasmic reticulum receptors, IP3 and ryanodine, in the induction of priming, regulated by estrogen receptor α in the nociceptor of female rats. The presence of this signaling pathway modulating the susceptibility of nociceptors to develop plasticity may contribute to our understanding of sex differences observed clinically in chronic pain syndromes.
Keywords: endoplasmic reticulum, hyperalgesia, hyperalgesic priming, IP3 receptor, nociceptor, ryanodine receptor
Introduction
Chronic pain is a substantial health problem (Aronoff, 2016; Jackson et al., 2016; Maixner et al., 2016; Turk et al., 2016), reflecting the current limitations in its management. A growing body of clinical and preclinical evidence indicates chronic pain as a disease state, a consequence of ongoing neuronal dysfunction (Walk and Poliak-Tunis, 2016). Animal models of persistent inflammation or nerve damage have shown a prominent role of peripheral sensory neurons in the long-term painful sensitization (Parada et al., 2005; Khasar et al., 2008; Costigan et al., 2009; Young et al., 2012; Ferrari et al., 2013c; Bali and Kuner, 2014), supporting the view that acute painful conditions can transition to persistent neuronal sensitization or exacerbated pain to stimulation, even well after acute tissue insults resolve (Bérubé et al., 2016; Peng et al., 2016; Walk and Poliak-Tunis, 2016).
We developed a model in the rat to investigate the peripheral mechanisms involved in the transition from acute to chronic pain, hyperalgesic priming, in which a permanently increased response of the nociceptor to proalgesic mediators develops after a prior inflammatory episode (Aley et al., 2000; Reichling and Levine, 2009). The induction of priming has been shown to be dependent on the activation of protein kinase Cε (PKCε) followed by ryanodine receptor-triggered release of calcium from the endoplasmic reticulum (ER) and persistent protein translation at the peripheral and central terminals of nociceptors (Aley et al., 2000; Parada et al., 2003a; Reichling and Levine, 2009; Ferrari et al., 2013a, b, 2016). Of note, a marked, estrogen receptor-dependent, sexual dimorphism is observed in this model, and activation of PKCε, directly or receptor-mediated, does not induce priming in female rats (Joseph et al., 2003). However, direct stimulation of second messengers downstream of PKCε, such as the ryanodine receptor, present at the ER (Sutko et al., 1985; Sorrentino and Volpe, 1993; Fill and Copello, 2002), induces priming in both males and females (Ferrari et al., 2013b, 2016).
Recently, we have shown that the sexual dimorphism observed in priming is not only limited to a negative regulation of PKCε by estrogen in female rats (Joseph et al., 2003), but also an interaction between estrogen receptor α (EsRα) and ryanodine receptor in females. Paradoxically, in this case, such an interaction markedly increases the sensitivity of female nociceptors to be primed, representing a dual role of estrogen in priming (Ferrari et al., 2016), preventing its induction through cell surface receptors and PKCε activation, but facilitating its triggering by activation of ryanodine receptors. This sensitizing effect of estrogen toward the ryanodine receptor in females has also been observed in in vitro experiments showing potentiation of the response to ryanodine application in cultured female, but not male, DRG neurons in the presence of β-estradiol or the EsRα agonist 1,3,5-tris(4-hydroxyphenyl)-4-propyl-1H-pyrazole (PPT), confirming a relationship between EsR and the calcium receptors at the ER in neuroplasticity, as suggested by previous studies (Fricke et al., 2007; Rybalchenko et al., 2009).
The activation of ryanodine receptors releases calcium from the ER (Sutko et al., 1985; Fill and Copello, 2002) and the consequent induction of calcium waves (Stutzmann and Mattson, 2011; Adasme et al., 2015; Futagi and Kitano, 2015; Evans et al., 2016) have been associated with some forms of neuroplasticity (Chen et al., 2015; Futagi and Kitano, 2015). Also, an interaction between ryanodine receptors and inositol 1,4,5-triphosphate (IP3) receptors (Smith et al., 2009; Taylor and Tovey, 2010), whose activation also releases calcium from the ER (Berridge and Taylor, 1988; Putney and Bird, 1993; Mak and Foskett, 2015; Taylor and Konieczny, 2016), has been shown to play a role in neuronal plasticity (Barbara, 2002; Raymond and Redman, 2006; Nagarkatti et al., 2008; Gruol et al., 2010; Silveira et al., 2015). In this study, we evaluated whether the IP3 receptor also plays a role in the nociceptor plasticity observed in this preclinical model of chronic pain.
Materials and Methods
Animals.
All experiments were performed on male and female adult Sprague Dawley rats (220–400 g; Charles River Laboratories). Rats were housed three per cage, under a 12 h light/dark cycle, in a temperature- and humidity-controlled animal care facility at the University of California, San Francisco. Food and water were available ad libitum. Nociceptive testing was done between 10:00 A.M. and 5:00 P.M. The experimental protocols were approved by the Institutional Animal Care and Use Committee at the University of California at San Francisco and adhered to the National Institutes of Health Guide for the care and use of laboratory animals. Effort was made to minimize the number of animals used and their suffering.
Testing mechanical nociceptive threshold.
Mechanical nociceptive threshold was quantified using an Ugo Basile Analgesymeter (Randall-Selitto paw-withdrawal test, Stoelting), which applies a linearly increasing mechanical force to the dorsum of the rat's hindpaw, as previously described (Randall and Selitto, 1957; Taiwo et al., 1989). Rats were placed in cylindrical acrylic restrainers designed to provide adequate comfort and ventilation, allow extension of the hind leg, and minimize restraint stress. To adapt rats to the testing procedure, they were acclimatized to the restrainer for 1 h before starting each study and for 30 min before experimental manipulations. The nociceptive threshold was defined as the force, in grams, at which the rat withdrew its paw. Baseline paw-pressure nociceptive threshold was defined as the mean of the three readings taken before the test agents were injected. Each paw was treated as an independent measure (Ferrari et al., 2015) and each experiment performed on a separate group of rats.
Drugs and reagents.
The following compounds were used in this study: the direct-acting hyperalgesic agent prostaglandin E2 (PGE2); the activator of IP3 receptors d-myo-inositol 1,4,5-tris-phosphate trisodium salt (IP3); xestospongin C, an IP3 receptor inhibitor; the ryanodine receptor modulator ryanodine; dantrolene sodium salt, a ryanodine receptor inhibitor; thapsigargin, a SERCA (Sarco-Endoplasmic Reticulum Calcium ATPase) inhibitor (Kijima et al., 1991; Lytton et al., 1991); β-estradiol-water-soluble (cyclodextrin-encapsulated 17β-estradiol), an EsR agonist; and PPT, an EsRα-specific agonist, all from Sigma-Aldrich; Griffonia simplicifolia isolectin B4 (IB4) conjugated to AlexaFluor-488 dye (Invitrogen); and fura-2 AM, a membrane-permeable form of the fluorescent calcium indicator fura-2 (Calbiochem). Selection of drug doses was based on our previous studies (Alessandri-Haber et al., 2009; Ferrari et al., 2013b, 2014, 2016; Hendrich et al., 2013). The required drug concentrations were achieved by dilutions in 0.9% NaCl (for in vivo experiments) or in external perfusion solution (for in vitro experiments).
Solutions of β-estradiol, dissolved in 0.9% NaCl, were freshly prepared. Stock solutions of PGE2 in absolute ethanol (1 μg/μl) were diluted in 0.9% NaCl (1:50, Cfinal = 0.02 μg/μl) immediately before injection. The ethanol concentration of the final PGE2 solution was ∼2% and the injection volume 5 μl. Ryanodine was also first prepared as a stock solution, in absolute ethanol, and then diluted with 0.9% NaCl to the required concentration/dose. Aliquots of IP3, dissolved in distilled water, were further diluted with 0.9% NaCl to the required concentrations, depending on the dose needed. Dantrolene was dissolved in DMSO at the time of the experiments and further diluted in 0.9% NaCl containing 10% DMSO; stock solutions of PPT, thapsigargin, xestospongin C, and fura-2 AM (1 mm) were prepared in 100% DMSO, and diluted in 0.9% NaCl containing 10% DMSO at the time of the experiments; IB4 was prepared as a stock solution (1 μg/μl), in calcium- and magnesium-free PBS (Invitrogen). Importantly, in vivo control experiments have previously shown that the final concentration of ethanol (2%), used to prepare the solutions of PGE2 or ryanodine, had no effect on the mechanical threshold per se; DMSO, used to dissolve dantrolene, PPT, thapsigargin, xestospongin C, and fura-2 AM, did not produce any effect on the mechanical nociceptor threshold (Ferrari et al., 2016).
In the behavioral experiments, drugs were administered intradermally at a marked site on the dorsum of the hindpaw via a beveled 30-gauge hypodermic needle that was attached to a Hamilton microsyringe by polyethylene (PE-10) tubing. The administration of IP3, ryanodine, dantrolene, xestospongin C, or thapsigargin, as well as their respective vehicles, was preceded by hypotonic shock to facilitate cell permeability to these agents (2 μl of distilled water, separated by an air bubble, to avoid mixing in the same syringe), to facilitate getting compounds into the nerve terminal (Borle and Snowdowne, 1982; Burch and Axelrod, 1987).
Induction of hyperalgesic priming.
The procedure to induce hyperalgesic priming was based on protocols previously described (Aley et al., 2000; Bogen et al., 2012; Ferrari et al., 2015, 2016). The inducer of priming in this study was IP3, ryanodine, or PPT (Ferrari et al., 2016), injected intradermally on the dorsum of the hindpaw. The presence of priming was confirmed by the marked prolongation of the hyperalgesia induced by injection of PGE2 (100 ng), at the same site, 1 week later. Importantly, at this point in time, the mechanical nociceptive thresholds were not different from preinducer baseline (see Data analysis). The mechanical hyperalgesia induced by injection of PGE2 in the previously untreated naive control paw lasts no more than 2 h (Aley and Levine, 1999). The prolongation of PGE2-induced hyperalgesia to >4 h is used as a marker for the presence of hyperalgesic priming (Aley et al., 2000; Parada et al., 2003b; Reichling and Levine, 2009; Ferrari et al., 2014). To investigate the mechanisms in the ER that play a role in the induction of hyperalgesic priming, pharmacological agents were injected before the injection of the inducers.
Oligodeoxynucleotide (ODN) antisense (AS) to EsRα mRNA.
To investigate the role of EsRα, shown previously to be involved in the regulation of the sensitivity of the nociceptor to be primed (Ferrari et al., 2016), in the induction of hyperalgesic priming by IP3, AS ODN against EsRα mRNA was administered to female rats. The sequence for the EsRα, 5′-CAT-GGT-CAT-GGT-CAG-3′ AS ODN (Invitrogen) was directed against unique regions of the rat EsRα (GeneBank accession number NM_012689.1), and has been previously shown to attenuate cellular levels of this receptor (Liang et al., 2002; Edinger and Frye, 2007). The mismatch (MM) ODN sequence, 5′-ATC-GTG-GAT-CGT-GAC-3′, was a scrambled AS ODN sequence that has the same base pairs and GC ratio, with the order randomized, and little or no homology to any mRNA sequence posted at GenBank.
Before use, ODNs were reconstituted in nuclease-free 0.9% NaCl, and then administered intrathecally at a dose of 2 μg/μl in a volume of 20 μl, for 3 consecutive days, starting 3 d before the injection of IP3, and then continued for 3 additional days, at which time the evaluation for the presence of priming was performed by intradermal administration of PGE2 on the dorsum of the hindpaw. As described previously (Alessandri-Haber et al., 2009), rats were anesthetized with isoflurane (2.5% in O2), and the ODN injected using a microsyringe (10 μl) with a 30-gauge needle, inserted into the subarachnoid space, between the L4 and L5 vertebrae.
Preparation of cultures of DRG neurons.
Primary cultures of rat DRG sensory neurons were obtained from adult males and prepared as described previously (Hendrich et al., 2013; Ferrari et al., 2016). In brief, under isoflurane anesthesia, rats were decapitated, the dorsum of the vertebral column was then opened, and the L4 and L5 DRGs rapidly removed, chilled in HBSS on ice, and desheathed. Ganglia were treated with 0.125% collagenase P (Worthington Biochemical) in HBSS for 90 min at 37°C, and then treated with 0.25% trypsin (Worthington Biochemical) in calcium- and magnesium-free PBS (Invitrogen) for 10 min, followed by 3 times washout and trituration in Neurobasal-A medium (Invitrogen) to produce a single-cell suspension. The suspension was centrifuged at 1000 RPM for 3 min and resuspended in Neurobasal-A medium supplemented with 50 ng/ml nerve growth factor, 100 U/ml penicillin/streptomycin, and B-27 (Invitrogen). In some experimental series, the medium was additionally supplemented with β-estradiol (100 nm) for activation of estrogen receptors. Cells were then plated on coverslips and incubated at 37°C in 5% CO2 for at least 24 h before use in experiments.
In vitro recordings.
Cultured DRG neurons were used for in vitro experiments between 24 and 96 h after dissociation and plating. While small-, medium-, and large-sized neurons were routinely observed in the same preparation, this study was focused only on cells with a cell body diameter <30 μm (small DRG neurons, predominantly representing the C-type nociceptor subpopulation). After mounting to a recording chamber, the culture medium was replaced with Tyrode's solution containing 140 mm NaCl, 4 mm KCl, 2 mm MgCl2, 2 mm CaCl2, 10 mm glucose, 10 mm HEPES, and adjusted to pH 7.4 with NaOH. Tyrode's solution was used in the in vitro experiments as external perfusion solution, and all fluorescent dyes, stimulating and modulating drugs were applied diluted in this solution. Depolarization-induced calcium transients were produced by application of K20 solution, containing 20 mm KCl, 124 mm NaCl, 2 mm MgCl2, 2 mm CaCl2, 10 mm glucose, 10 mm HEPES, and adjusted to pH 7.4 with NaOH. The volume of the recording chamber was 150 μl. The perfusion system was gravity-driven at a flow rate of 1–2 ml/min. All experiments were performed at room temperature of 20°C-23°C.
Calcium imaging.
The bright-field imaging system consisted of an inverted microscope (Eclipse TE-200, Nikon) with epifluorescence attachment, using a mercury lamp for excitation. Illumination was controlled by a Lambda 10–2 filter wheel controller and Lambda SC Smart Shutter controller (Sutter Instruments); an Andor Clara Interline CCD camera (Andor Technology) was used for high-resolution digital image acquisition. MetaFluor software (Molecular Devices) provided computer interface and controlled the whole system as well as being used for image processing. A Plan Fluor objective (20×UV, NA 0.50; Nikon) was used for both fluorescent and transmitted light imaging with phase contrast. Calcium imaging was performed using the fluorescent calcium indicator fura-2 AM as previously described (Hendrich et al., 2013; Ferrari et al., 2016). Briefly, neurons were loaded with 5 μm fura-2 AM by incubation for 20 min directly in the recording chamber. Then cells were perfused with Tyrode's solution for 10 min before the beginning of the recording to allow for complete deesterification of the fura-2 AM. Measurement of the concentration of free calcium ions ([Ca2+]i) was performed by ratiometric imaging. Fluorescence was excited at 340 and 380 nm for 2–10 ms each, and the emitted light was long filtered at 520 nm using a standard Fura-2 filter set (Chroma Technology). Using MetaFluor software (Molecular Devices), corresponding pairs of digital images were acquired every 1–10 s (depending on the rate of the examined process, to minimize UV exposure and excitotoxicity); the fluorescence ratio (F340/F380) was calculated on a pixel-by-pixel basis with background correction and averaged for the region of interest defined for each neuron. The fluorescence ratio was used to characterize [Ca2+]i without recalculation into concentration. The amplitude of response was measured as the difference between fluorescence ratios at the peak and the base of the responses.
Histochemistry.
Cells were incubated in Tyrode's solution supplemented with 10 μg/ml IB4 conjugated to AlexaFluor-488 dye (Invitrogen) for 10–12 min, in the dark. After washout, fluorescent images were captured during the first 15 min of each experiment (before prolonged calcium imaging) using a standard GFP filter set (Chroma Technology). Cells demonstrating bright fluorescence and a halo around the neurons plasma were recognized as IB4-positive (IB4+), whereas those having intensity <20% of maximum for selected field of view were considered as IB4-negative (IB4−), and, thus, not used in our measurements.
Data analysis.
All behavioral data are presented as mean ± SEM of N independent observations. Statistical comparisons were made using GraphPad Prism 5.0 statistical software (GraphPad Software). A p value <0.05 was considered statistically significant. In the behavioral experiments, the dependent variable was change in mechanical paw-withdrawal threshold, expressed as percentage change from baseline. To evaluate the role of SERCA, IP3 receptors or ryanodine receptors in the induction of priming by ryanodine (see Fig. 1), IP3 (see Fig. 2b), or PPT (see Fig. 3b) inhibitors were injected in one paw while the contralateral paw received vehicle (control). Thus, the rats did not receive the same treatment in both paws. Importantly, the use of contralateral paws as control is supported by previous demonstrations that paws from the same animal can be considered as independent, in regard to the treatments performed on the dorsum of the hindpaw, in a volume of 5 μl, in the doses used in our experiments and previous work from our group (Ferrari et al., 2013a, 2014, 2015). In the experiments shown in Figure 3a, in which the ODN AS or MM was injected intrathecally to the spinal cord, only the left paws were used (6 rats per group). No significant difference in mechanical nociceptive thresholds was observed before the injection of priming stimuli (ryanodine, IP3, or PPT) and immediately before injection of PGE2 (average mechanical nociceptive threshold before priming stimuli: 121.6 ± 0.7 g; average mechanical nociceptive threshold before PGE2 injection: 121.0 ± 0.8 g; N = 168 paws; paired Student's t test, t(167) = 1.053, p = 0.2937). As specified in the figure legends, Student's t test or two-way repeated-measures ANOVA, followed by Bonferroni post hoc test, was performed to compare the magnitude of the hyperalgesia induced by PGE2 injection in the different groups, or to compare the effect produced by different treatments on the prolongation of the PGE2-induced hyperalgesia (evaluated 4 h after injection) with the control groups. Of note, in Figure 2a, the mechanical nociceptive thresholds at the fourth hour after PGE2 injection were compared with the respective baseline thresholds before the injection of PGE2, to evaluate whether the mechanical hyperalgesia was present at that time point.
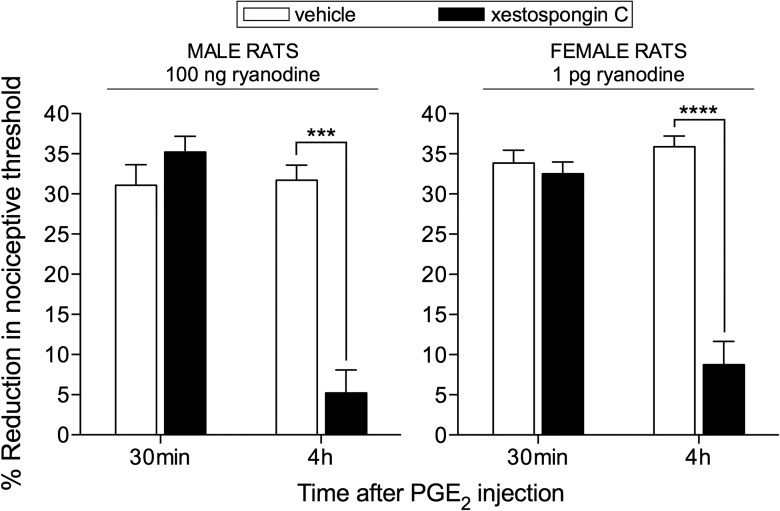
Figure 1.
Ryanodine-induced priming is IP3 receptor dependent. The IP3 receptor antagonist xestospongin C (0.2 μg, black bars) was injected intradermally on the dorsum of the hindpaw of male (left) and female (right) rats; control groups (white bars) received vehicle. After 10 min, the doses of ryanodine previously shown to induce priming (100 ng in males and 1 pg in females) were injected at the same site. One week later, evaluation for the presence of priming was performed by intradermal injection of PGE2 (100 ng) at the same site as ryanodine. Two-way repeated-measures ANOVA, followed by Bonferroni post hoc test, showed a significant attenuation of the hyperalgesia induced by PGE2 at the fourth hour, in groups that had been pretreated with xestospongin C, compared with the control groups, pretreated with vehicle (males: F(1,5) = 130.6; ***p < 0.0001; females: F(1,5) = 89.10; ****p = 0.0002, when the hyperalgesia in the vehicle- and xestospongin C-treated groups is compared at the fourth hour), demonstrating that the induction of priming by ryanodine is dependent on the activation of IP3 receptors (N = 6 paws per group).
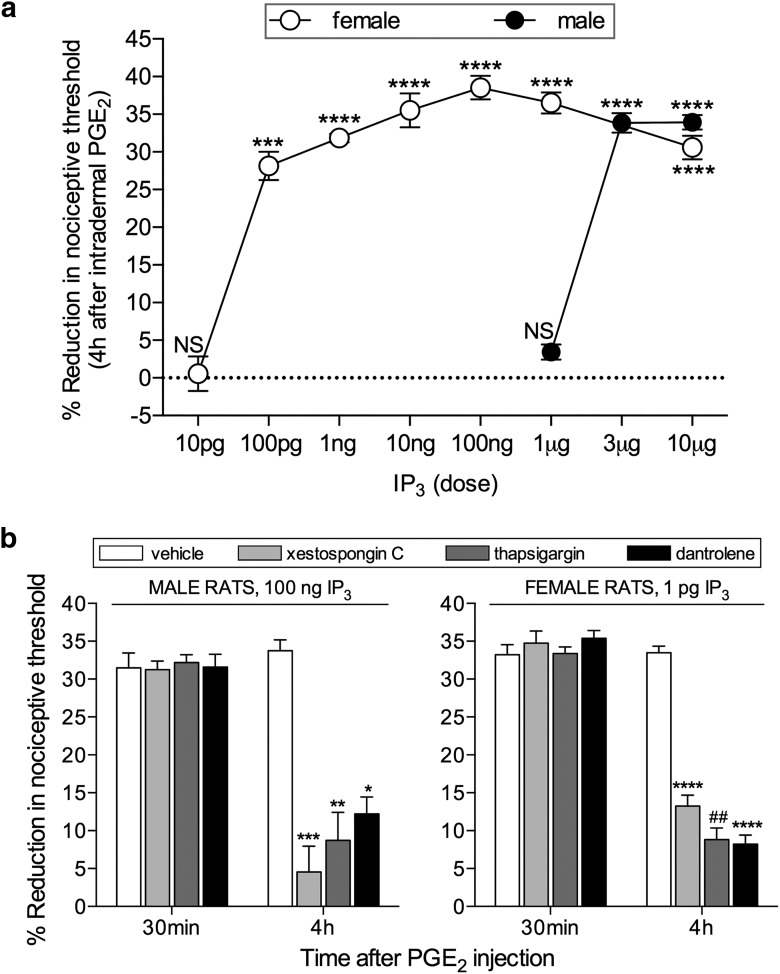
Figure 2.
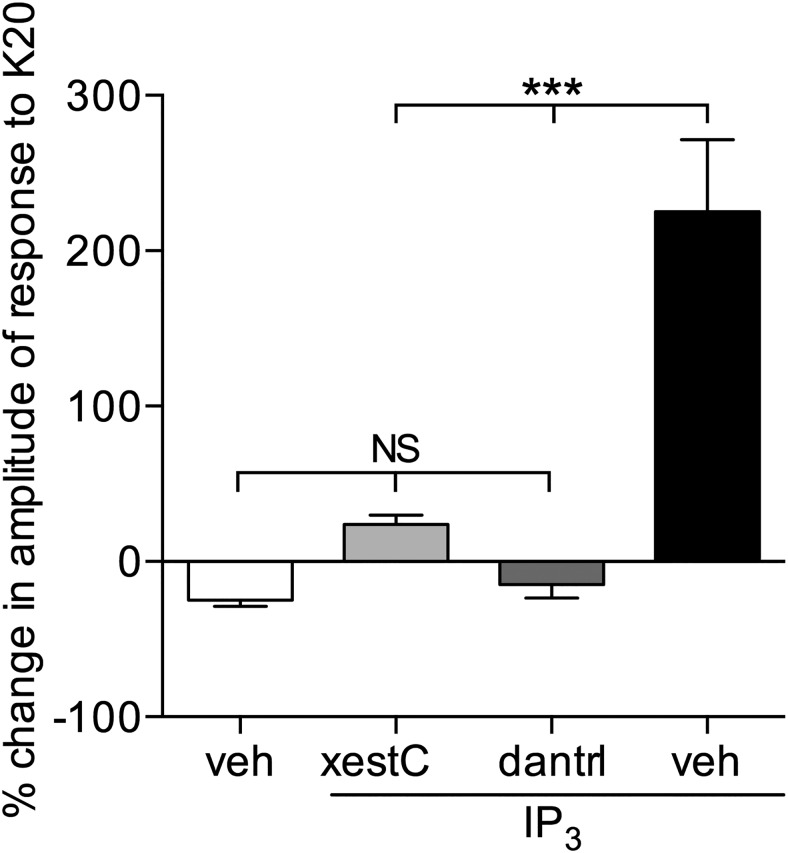
Induction of hyperalgesic priming by IP3 in male and female rats. a, Different doses of IP3 were injected on the dorsum of the hindpaw in different groups of female (open circles represent 10 pg; 100 pg; 1 ng; 10 ng; 100 ng; 1 and 10 μg) and male (filled circles represent 1 μg; 3 μg; 10 μg) rats. No change in the mechanical nociceptive threshold was observed after the injection of IP3 (data not shown). PGE2 (100 ng) was injected at the same site, 1 week later, and the mechanical hyperalgesia evaluated by the Randall-Sellitto paw-withdrawal test. This figure shows the mechanical hyperalgesia at the fourth hour after the injection of PGE2; the presence of hyperalgesia at that time point was used to confirm the induction of priming by the previous injection of IP3. In the groups of female rats that had received a dose of 100 pg and higher, and in the group of male rats previously treated with 3 or 10 μg, but not with 1 μg, the hyperalgesia induced by PGE2 was still present at the fourth hour (female rats: 10 pg, t(5) = 1.627, p = 0.1647 (not significant, NS); 100 pg, t(5) = 8.788, p = 0.0003 (***); 1 ng, t(5) = 27.81; 10 ng, t(5) = 12.59; 100 pg, t(5) = 21.24; 1 μg, t(5) = 15.53; 10 μg, t(5) = 13.94, all p < 0.0001 (****); male rats: 1 μg, t(5) = 0.4385, p = 0.6793 (NS); 3 μg, t(5) = 16.54; 10 μg, t(5) = 23.27, both p < 0.0001 (****), when the mechanical nociceptive thresholds before and 4 h after the injection of PGE2, for each group, are compared, paired Student's t test). These results support the suggestion that nociceptors in females are significantly more sensitive to induction of priming by IP3 because a dose much lower was required. b, Groups of male (left) and female (right) rats received an injection of vehicle (white bars), the IP3 receptor inhibitor xestospongin C (0.2 μg, light gray bars), the SERCA inhibitor thapsigargin (1 μg, dark gray bars), or the ryanodine receptor inhibitor dantrolene (1 μg, black bars) on the dorsum of the hindpaw. Ten minutes later, the smallest doses of IP3 that induced priming (a; 3 μg in male; 100 pg in female) were injected at the same site. No significant change in mechanical nociceptive threshold was observed after injection of IP3 (data not shown). One week later, the presence of priming was determined by the evaluation of the prolonged mechanical hyperalgesia induced by intradermal injection of PGE2 (100 ng), at the same site as IP3. Two-way repeated-measures ANOVA, followed by Bonferroni post hoc test, showed that, while the hyperalgesia induced by PGE2 in control groups (vehicle-treated) was still present 4 h after injection, and not different from the 30 min time point, in the groups pretreated with xestospongin C, thapsigargin, or dantrolene, its magnitude was significantly smaller at the 4 h time point (males, xestospongin C group: F(1,5) = 81.30, ***p = 0.0003; thapsigargin group: F(1,5) = 31.11, **p = 0.0026; dantrolene group: F(1,5) = 44.44, *p = 0.0011; females: xestospongin C group: F(1,5) = 230.5, ****p < 0.0001; thapsigargin group: F(1,5) = 58.63, ##p = 0.0006; dantrolene group: F(1,5) = 16.48, ****p < 0.0001, when the hyperalgesia in the vehicle- and inhibitor-treated groups is compared at the fourth hour), indicating that the induction of priming by IP3 is dependent on the activation of IP3 and ryanodine receptors (N = 6 paws all groups).
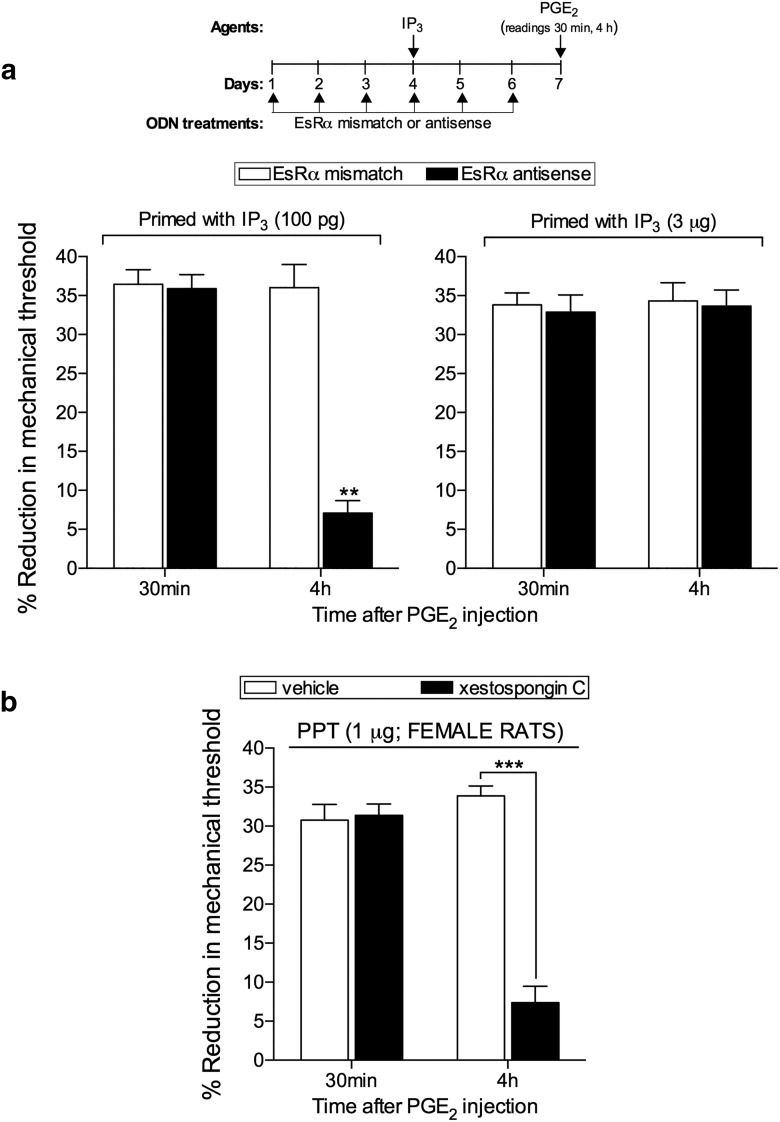
Figure 3.
EsRα regulates the induction of hyperalgesic priming by IP3 in females. a, Female rats were treated with ODN AS (black bars) or MM (white bars) for EsRα mRNA, for 6 consecutive days. IP3 (100 pg, left; 3 μg, right) was injected on the dorsum of the left hindpaws on the fourth day of ODN treatment. On the seventh day, PGE2 (100 ng) was injected at the same site as IP3, and the mechanical nociceptive threshold evaluated, 30 min and 4 h later. No significant difference was observed in the mechanical nociceptive thresholds before the injections of IP3 and immediately before injection of PGE2 (data not shown). PGE2-induced hyperalgesia was still present 4 h after injection in all groups, except in the group that received the low dose of IP3 (100 pg) treated with ODN AS (F(1,10) = 31.89; **p = 0.0002, when the low-dose groups treated with ODNs are compared; F(1,10) = 0.1479; p = 0.7086, not significant, when the high-dose groups treated with ODNs are compared; two-way repeated-measures ANOVA followed by Bonferroni post hoc test). These results support the suggestion that EsRα regulates the ability of a low dose of IP3 to induce priming in the female rat. b, Female rats received an intradermal injection of vehicle (white bars) or the IP3 receptor inhibitor xestospongin C (0.2 μg, black bars) on the dorsum of the hindpaw. Ten minutes later, the specific EsRα agonist PPT (1 μg) was injected at the same site. After 1 week, testing for the presence of hyperalgesic priming was performed by injecting PGE2 (100 ng). Mechanical hyperalgesia was observed in both groups when evaluated 30 min after PGE2 injection. However, at the fourth hour, the magnitude of the PGE2-induced hyperalgesia was significantly smaller in the group previously treated with xestospongin C (F(1,5) = 69.81, ***p = 0.0004; when both groups are compared at the fourth hour; two-way repeated-measures ANOVA followed by Bonferroni post hoc test), indicating that the inhibition of IP3 receptors prevented the induction of priming by PPT (N = 6 paws all groups).
Calcium imaging results are presented as change in amplitude of the responses to drug application, calculated for each cell as the percentage of the amplitude of its initial (premodulated) response. Differences between means of two groups were analyzed using two-tailed unpaired Student's t test for unequal variances (with Welch correction), whereas one-way ANOVA followed by Tukey's post hoc analysis was used in case of multiple comparisons.
Results
Induction of priming by ryanodine is dependent on the IP3 receptor
While the activation of receptors that signal through PKCε produces priming only in male rats (Joseph et al., 2003), triggering signals downstream of this kinase, for example, by activating ryanodine receptors, induces priming in both sexes (Ferrari et al., 2013b, 2016). However, due to an interaction between EsRα and ryanodine receptors, females need a much smaller dose of ryanodine to be primed than males (Ferrari et al., 2016). The activation of ryanodine receptors triggers the release of calcium from the ER (Sutko et al., 1985; Fill and Copello, 2002), thus implicating a role of this organelle in the induction of priming (Ferrari et al., 2013b, 2016). Because the release of calcium from the ER has been shown to activate local calcium-activated receptors (Taylor and Konieczny, 2016), such as the IP3 receptor (Smith et al., 2009; Taylor and Tovey, 2010; Taylor and Konieczny, 2016), we investigated whether the induction of priming by ryanodine is dependent on the activation of the IP3 receptor. Male and female rats received an intradermal injection of xestospongin C (0.2 μg), an IP3 receptor inhibitor, on the dorsum of the hindpaw, 10 min before the injection of ryanodine [100 ng in males and 1 pg in females, previously shown to induce priming (Ferrari et al., 2016)], at the same site. One week later, when we evaluated for the presence of priming by injecting PGE2 (Aley et al., 2000; Ferrari et al., 2014), mechanical hyperalgesia was observed 30 min after injection in both the control- and the xestospongin C-treated groups (Fig. 1). However, compared at the fourth hour, the magnitude of hyperalgesia in the xestospongin C-treated group was significantly smaller. Because the prolongation of PGE2-induced hyperalgesia is the main indicator of the presence of hyperalgesic priming in the nociceptor (Aley et al., 2000; Ferrari et al., 2014), this result indicates that the induction of hyperalgesic priming by ryanodine is dependent on the activation of IP3 receptors (Fig. 1).
IP3 induces priming in male and female rats
Because the induction of priming by ryanodine is IP3 receptor-dependent, we tested whether the injection of IP3 itself induces priming. When the dose of IP3 was varied between 10 pg and 10 μg, we found that the minimum dose able to induce priming, expressed as prolongation of the PGE2-induced hyperalgesia observed at the fourth hour after injection (Aley et al., 2000; Ferrari et al., 2014), was 3 μg in males and 100 pg in females (Fig. 2a).
Induction of priming by IP3 is dependent on IP3 receptor, SERCA, and ryanodine receptor
To confirm that IP3 induces priming in male and female rats by acting at the ER IP3 receptor, we evaluated the effect of the IP3 receptor inhibitor xestospongin C, or thapsigargin, an inhibitor of SERCA, the calcium pump located in the ER (Kijima et al., 1991; Lytton et al., 1991), on the induction of priming by IP3. The inhibitors were injected 10 min before IP3, at the same site. When PGE2 was injected at the same site 1 week later, it induced mechanical hyperalgesia that was still present at the fourth hour after injection, only in the control group, whereas in the groups that had been previously treated with xestospongin C (0.2 μg) or thapsigargin (1 μg), it was significantly attenuated at that time point (Fig. 2b).
Because interaction between IP3 and ryanodine receptors has been shown to play a role in some models of neuroplasticity (Rose and Konnerth, 2001; Verkhratsky, 2002; Lohmann and Wong, 2005; Bardo et al., 2006), we next investigated whether the induction of priming by IP3 is also dependent on the activation of ryanodine receptors. The injection of the ryanodine receptor inhibitor dantrolene (1 μg), 10 min before the injection of IP3, at the same site, prevented the development of priming, measured as the prolongation of the hyperalgesia induced by PGE2 (Fig. 2b).
EsRα regulates the induction of priming by low-dose IP3 in female rats
We have previously reported that the sexual dimorphism in hyperalgesic priming induced by ryanodine is EsRα-dependent (Ferrari et al., 2016). Because the induction of priming by IP3 is dependent on the ryanodine receptor (Fig. 2b), we next investigated whether the induction of priming by the low dose of IP3 in female rats is also EsRα-dependent. Intrathecal ODN AS for EsRα mRNA prevented the induction of priming by low-dose (100 pg), but not by high-dose (3 μg, the smallest dose able to induce priming in male rats) IP3, in female rats (Fig. 3a). This result supports the hypothesis that EsRα plays a role as a modulator of the response to IP3, similar to ryanodine (Ferrari et al., 2016), likely amplifying it and allowing a very small dose of IP3 to induce priming. This observation is in line with our previous study (Ferrari et al., 2016), indicating the presence of an EsRα-IP3/ryanodine receptor-dependent calcium release pathway in the development of hyperalgesic priming in females. Still, the mechanism by which circulating estrogen interacts with the IP3 or ryanodine receptors to regulate the sensitivity to induce priming remains to be established.
EsRα agonist-induced priming is IP3 receptor-dependent
We previously demonstrated that the injection of the selective EsRα agonist PPT induces priming in female rats, in a ryanodine receptor-dependent manner (Ferrari et al., 2016). Because estrogen can signal to activate ryanodine and IP3 receptors (Evinger and Levin, 2005; Fricke et al., 2007; Muchekehu and Harvey, 2008; Rybalchenko et al., 2009; Szatkowski et al., 2010; Reuquén et al., 2015; Tabatadze et al., 2015), and the priming induced by ryanodine is dependent on IP3 receptors (Fig. 1), we investigated the role of IP3 receptors in the induction of priming by PPT. The administration of xestospongin C (0.2 μg), 10 min before the injection of PPT (1 μg), on the dorsum of the hindpaw of female rats prevented the induction of priming, tested 1 week later by injecting PGE2 at the same site (Fig. 3b). Thus, EsRα, which regulates the activation of ryanodine receptors in female rats, leading to priming (Ferrari et al., 2016), also interacts with IP3 receptors.
In the presence of β-estradiol, IP3 potentiates depolarization-induced calcium transients
To elucidate the intracellular mechanism by which IP3 receptors contribute to priming, a series of in vitro experiments was conducted in cultured male DRG neurons. These experiments only considered responses from small DRG neurons (with soma diameter <30 μm), as they predominantly represent the C-type nociceptive subpopulation of primary sensory neurons (Harper and Lawson, 1985; Gold et al., 1996). Additionally, because the population of nociceptors that develop hyperalgesic priming is IB4+ (Joseph and Levine, 2010), all observations were performed on IB4+ nociceptors, which constituted ∼75% of the small neurons examined (351 of 466), in agreement with the proportion reported by other studies (Hendrich et al., 2012; Khomula et al., 2013; Ferrari et al., 2016).
Using fluorescent calcium imaging with fura-2, we tested whether direct extracellular application of IP3 produces changes in cytosolic free calcium ion concentration ([Ca2+]i) (i.e., calcium transients). Initially, no significant change in [Ca2+]i was observed during a 10 min application of IP3, in a concentration as high as 400 μm (as shown in Fig. 4a, gray horizontal bar), confirming that the extracellular application of IP3, by itself, does not induce calcium transients.
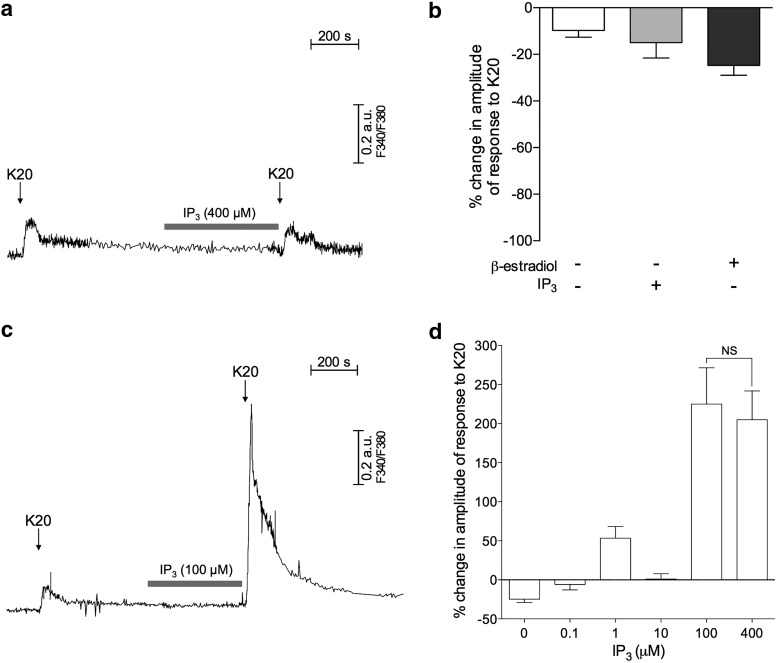
Figure 4.
IP3 in the presence of β-estradiol potentiates depolarization-induced calcium transients. a, Representative recording of calcium transients in an IB4+ small DRG neuron incubated without β-estradiol. Calcium transients were induced by two identical depolarizing stimuli (arrows indicate short applications of K20, 20 mm), before and after exposure to IP3 (gray horizontal bar represents 400 μm), which was applied for 10 min when [Ca2+]i returned to baseline after the first application of K20. No changes in the response to K20, even after the application of IP3, and no changes in baseline in response to application of IP3 by itself, are observed. b, Pooled relative changes in the amplitudes of the second response to K20, as percentage of the first response in the following: neurons incubated without β-estradiol submitted to application of vehicle between the applications of K20 (as control, white bar, N = 32), neurons incubated without β-estradiol submitted to the application of IP3 alone (as described in a, gray bar, N = 34), and neurons incubated with β-estradiol (100 nm) submitted to application of vehicle between applications of K20 (black bar, N = 28). Negative values indicate lack of potentiation of the response to K20 application in all cases. The absence of a significant difference among the groups (F(2,91) = 2.22, p = 0.12; one-way ANOVA) suggests that neither the exposure to IP3 alone nor the incubation with β-estradiol by itself significantly affects the responses to K20 compared with control conditions (no IP3/no β-estradiol). c, Recordings of calcium transients in IB4+ small DRG neurons, incubated in the presence of β-estradiol (100 nm). IP3 was applied as described in a. Although no response was produced by IP3 itself, a significant potentiation of the response to K20 occurred after exposure to IP3 (second transient). d, Pooled relative changes in the amplitudes of the second response to K20, as percentage of the first response, in IB4+ small DRG neurons incubated in the presence of β-estradiol (100 nm), after exposure to different concentrations of IP3: 0 (N = 28, the same as the black bar in b); 0.1 μm (N = 4); 1 μm (N = 23); 10 μm (N = 13); 100 μm (N = 13, same recording shown in c); and 400 μm (N = 21). Maximum potentiation was observed for 100 μm of IP3, with no significant difference between the magnitudes of potentiation produced by 100 and 400 μm (t(25) = 0.34, p = 0.73; two-tailed unpaired Student's t test with Welch's correction). Therefore, all further experiments were performed with 100 μm of IP3. a, c, Horizontal scale bars: 200 s; vertical bars, 0.2 arbitrary units (a.u.) of the fluorescence ratio F340/F380. b, + and −, located under the bars, indicate presence and absence of the corresponding agent, respectively.
We have previously shown that β-estradiol potentiated the facilitating effect of ryanodine on calcium release from endoplasmic reticulum in IB4+ DRG neurons (Ferrari et al., 2016). This effect of β-estradiol was suggested as responsible for the increased sensitivity of female rats to be primed by ryanodine, compared with male rats, observed in vivo (Ferrari et al., 2016). Thus, considering that: (1) the presence of estrogen sensitizes the ryanodine receptor; (2) this sensitization is observed as a higher sensitivity of female rats to be primed by ryanodine; (3) similarly, females are more sensitive to be primed by IP3 (Fig. 2a); and (4) activation of either ryanodine or IP3 receptors trigger calcium release from the ER (Berridge and Taylor, 1988; Putney and Bird, 1993; Mak and Foskett, 2015; Taylor and Konieczny, 2016), we next determined whether β-estradiol would have an effect on the IP3 application in cultures of DRG neurons. We used as a test stimulus two identical short applications of a solution containing high concentration of K+ (K20, Fig. 4a), known to activate influx of calcium via voltage-gated calcium channels in the plasma membrane, as well as release calcium from ER stores (Usachev et al., 1993; Kostyuk and Verkhratsky, 1994; Shmigol et al., 1995a; Kostyuk et al., 2000; Solovyova et al., 2002b; Kruglikov et al., 2004; Shutov et al., 2006), producing calcium transients. As shown in Figure 4a, b, the calcium transient produced by the second application of K20 was not increased (no potentiation), compared with the first one (used as a reference) regardless of the presence of IP3 (400 μm) or its vehicle applied for 10 min between the applications of K20. In sequence, the same protocol was applied to cultured DRG neurons incubated for 24–72 h in the presence of β-estradiol (100 nm, used as saturating concentration) (Fig. 4c). In this case, we observed increased (“potentiated”) response to K20, after exposure to IP3 (Fig. 4c). Of note, the result of a concentration dependence analysis for this potentiating effect of IP3 showing maximum effect produced by the concentration of 100 μm (Fig. 4d) is consistent with our in vivo finding that IP3 doses ≤1 μg did not induce hyperalgesic priming in male rats (Fig. 2a), as these doses (diluted in 5 μl of the injection solution) corresponded to the IP3 concentrations ≤400 μm that were tested. Importantly, the observed potentiation could not be attributed to an effect of β-estradiol itself because there was no potentiation in cultures incubated with β-estradiol when IP3 was not coapplied (Fig. 4b).
Potentiation of depolarization-induced calcium transients by IP3 is ryanodine- as well as IP3 receptor-dependent
To confirm that extracellularly applied IP3 penetrates into the cytosol to activate intracellular IP3 receptors, we evaluated whether the potentiation of the calcium transients produced by K20 was mediated by IP3 receptors. The IP3 receptor antagonist xestospongin C (1 μm) was applied after the first application of K20 for 10 min and remained in the bath along with the application of IP3 (100 μm). We observed that the potentiation of calcium transients was significantly attenuated compared with the control group, treated with vehicle and IP3 (Fig. 5). Importantly, even though some reports suggest that high concentrations of xestospongin C can antagonize SERCA (Castonguay and Robitaille, 2002; Solovyova et al., 2002a), an involvement of SERCA in our observations is unlikely. Because inhibiting SERCA would deplete ER calcium stores (Shmigol et al., 1995a; Solovyova et al., 2002b) and significantly decrease any response to K20, the fact that we still observed calcium transients produced by the second application of K20, with a small potentiation in the presence of xestospongin C (Fig. 5, light gray bar), suggests that: (1) calcium transients mediated by ryanodine receptors were not affected, and (2) probably not all IP3 receptors were blocked because we intentionally used a very low concentration of xestospongin C.
Figure 5.
Potentiation of depolarization-induced calcium transients by IP3 is dependent on both IP3 and ryanodine receptors. Pooled relative changes in the amplitudes of the second response to K20, as percentage of the first response, after exposure to IP3 (100 μm) in IB4+ small DRG neurons, incubated in the presence of β-estradiol (100 nm), shows lack of potentiation in the neurons preincubated with the selective IP3 receptor antagonist xestospongin C (xestC, 1 μm, light gray bar; N = 25), or the selective ryanodine receptor antagonist dantrolene (dantrl, 1 μm, dark gray bar; N = 13). The “vehicle alone” group (white bar) and the “vehicle + IP3” group (black bar) are the “0 μm” and “100 μm” groups, respectively, from Figure 4d and are shown here for comparison. Both antagonists significantly attenuated the potentiation of the response to the second application of K20 compared with the control group (black bar) (F(3,75) = 39, ***p < 0.0001; one-way ANOVA followed by Tukey's multiple comparison test). No significant difference was observed compared with the “vehicle alone” group (white bar).
It has been previously demonstrated that neuroplasticity mediated by ryanodine receptors can contribute to depolarization-induced calcium transients in DRG neurons (Usachev et al., 1993; Kostyuk and Verkhratsky, 1994; Shmigol et al., 1995a, b). Our result showing that xestospongin C attenuated the potentiation of K20-induced calcium transients by IP3 strongly suggests a contribution of sensitized IP3 receptors to the plasticity in the nociceptor, observed also in hyperalgesic priming, in addition to ryanodine receptors. Nevertheless, it remains unclear whether the contribution of IP3 receptors in the effect of K20 was just additive and independent on the activation of ryanodine receptors, or the IP3 receptors operated in synergy with ryanodine receptors. To address this question, we added the ryanodine receptor antagonist dantrolene (1 μm) to the bathing solution 30 min before the first application of K20 (and consequently before the administration of IP3) to cultures incubated with β-estradiol (Fig. 5, dark gray bar). This protocol eliminated the contribution of ryanodine receptors to the K20-induced calcium transients, showing only the effect of the sensitization of IP3 receptors. After preincubation of the cultured DRG neurons with dantrolene, the increase in the magnitude of calcium transients produced by 100 μm IP3, which produced maximum effect (Fig. 4d), was not observed, suggesting that, to induce potentiation of the calcium transients produced by calcium release from ER in small IB4+ DRG neurons cultured with β-estradiol, a cooperation between IP3 and ryanodine receptors is needed. It is also possible that the release of calcium, specifically via ryanodine receptors, activates IP3 receptors sensitized by IP3, or that there is a reciprocal interaction between IP3 and ryanodine receptors in this phenomenon.
Ryanodine receptor-mediated potentiation of depolarization-induced calcium transients is dependent on IP3 receptors and enhanced in the presence of β-estradiol
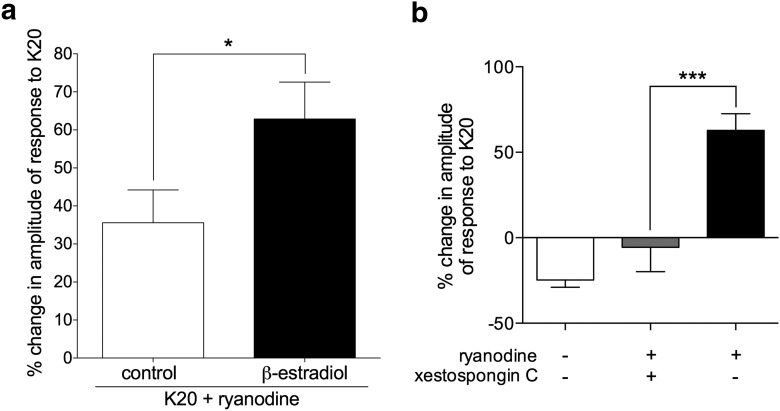
Our above-described in vivo experiments strongly suggest a contribution of IP3 receptors to the induction of hyperalgesic priming by ryanodine (Fig. 1). Therefore, we next investigated whether ryanodine could, by itself, stimulate or modulate the stimulation of calcium release (by depolarization) from the ER, thus contributing to depolarization-induced calcium transients, and how IP3 receptors could contribute to that effect of ryanodine. Recently, we have demonstrated that ryanodine, in very low concentration (2 nm), produces a significant, estrogen receptor-dependent, facilitating effect on the release of calcium from the ER in small IB4+ female DRG neurons cultured in the presence of β-estradiol (Ferrari et al., 2016). When ryanodine (2 nm), instead of IP3, was applied, for 10 min, in the same protocol as used for testing the IP3-induced potentiation (Fig. 4c), no visible response or change in baseline was observed, in the presence or absence of β-estradiol. However, there was a significant potentiation of the K20-induced calcium transients (Fig. 6a). Of note, although this effect was observed in cultures incubated with and without β-estradiol in the medium, the magnitude of the potentiation was significantly higher in the cultures that contained β-estradiol.
Figure 6.
Potentiation of depolarization-induced calcium transients by ryanodine is dependent on IP3 receptors and enhanced in the presence of β-estradiol. a, Pooled relative changes in the amplitude of the second response to K20 after exposure to ryanodine (2 nm) in IB4+ small DRG neurons, incubated without (control, white bar, N = 36) or in the presence of β-estradiol (100 nm, black bar, N = 28), reveal significant potentiation in both groups (positive values significantly >0: control group, t(35) = 4.1, p = 0.0002; β-estradiol group, t(27) = 6.5, p < 0.0001). Importantly, potentiation was significantly higher in the group incubated with β-estradiol (t(58) = 2.1, *p = 0.04) when both groups are compared (two-tailed unpaired Student's t test with Welch's correction). b, Pooled relative changes in the amplitude of the second response to K20 after exposure to ryanodine (2 nm) in IB4+ small DRG neurons, incubated with β-estradiol (100 nm), show lack of potentiation in neurons preincubated with the selective IP3 receptor antagonist xestospongin C (1 μm, gray bar; N = 8). Black bar is the same as the “β-estradiol” bar in a. White bar (no ryanodine) is the same as “β-estradiol alone” from Figure 4b, shown here for comparison. Xestospongin C significantly inhibited the ryanodine-induced potentiation of calcium transients compared with the control group (black bar) (F(2,61) = 35, ***p = 0.0004; one-way ANOVA followed by Tukey's multiple comparison test).
Again, similar to the ability of the ryanodine receptor antagonist to abolish the potentiation induced by IP3, the potentiation of the depolarization-induced calcium transients produced by ryanodine was blocked by the IP3 receptor antagonist xestospongin C (1 μm), applied 30 min before testing the effect of ryanodine (Fig. 6b). This result shows that the enhancement of the stimulated calcium release from the ER in the presence of ryanodine depends on IP3 receptors.
Discussion
Several studies have demonstrated the involvement of calcium channels in neuroplasticity, including ones present in the ER (Raymond and Redman, 2006; Fernández de Sevilla et al., 2008; Adasme et al., 2011; Segal and Korkotian, 2016). Changes in calcium dynamics, leading to synaptic plasticity (Nakata and Nakamura, 2007; Futagi and Kitano, 2015; Segal and Korkotian, 2016), and/or changes in neuronal response to stimulation (Kárai et al., 2004; Rigaud et al., 2009) have been shown to play a role in processes associated with the development of memory [such as long-term potentiation (Raymond and Redman, 2006; Baker et al., 2013; Del Prete et al., 2014; Paula-Lima et al., 2014; Ashhad et al., 2015)]. Plasticity due to the activation of calcium-dependent mechanisms, leading to the amplification of responses in neuronal cells that detect noxious stimulation, has also been suggested by studies in models of chronic pain (Bauer et al., 2009; Xia et al., 2014; D'Arco et al., 2015). We recently demonstrated participation of ryanodine receptors, whose activation releases calcium from the ER, in the induction of hyperalgesic priming (Ferrari et al., 2016), a “model of pain memory” in nociceptors (Reichling and Levine, 2009; Bogen et al., 2012). In that study, we showed that ryanodine receptors, in addition to being fundamental for the induction of priming, play an important role in sex differences in susceptibility (Ferrari et al., 2016). In female rats, this type of priming, namely, Type I (Araldi et al., 2015), can only be induced by activating targets downstream of PKCε, such as ryanodine receptors (Joseph et al., 2003; Ferrari et al., 2013b, 2016). Interestingly, we found that an interaction between the estrogen and the ryanodine receptor increases the susceptibility of females to be primed, such that a significantly smaller stimulus, which releases calcium from the ER (injection of ryanodine), is able to produce priming (Ferrari et al., 2016). This unexpected observation represents a dual role of estrogen in females, preventing induction of priming by the activation of PKCε, while at the same time facilitating its induction by a significantly smaller dose of ryanodine. Together, these results establish an important role of ER and estrogen in the regulation of priming, which could contribute to mechanisms involved in the sexual dimorphism observed in chronic pain.
In this study, we evaluated a possible mechanism downstream the activation of ryanodine receptors. Within the ER, most calcium signaling is mediated by both the ryanodine and the IP3 receptors (Taylor and Konieczny, 2016). Both are stimulated by calcium, generating signals that propagate as calcium released by one receptor triggers the activity of nearby receptors (Smith et al., 2009). Thus, we investigated whether IP3 receptors participate in the induction of priming by ryanodine. The pretreatment with the IP3 receptor inhibitor xestospongin C prevented the induction of priming by ryanodine, indicating that functional IP3 receptors are required for ryanodine to induce priming, probably for amplification of the calcium signal initially produced by activation of ryanodine receptors (Reber et al., 1993; Gordienko and Bolton, 2002; Valdés et al., 2007; Hirose et al., 2008; Hund et al., 2008). This additionally released calcium could in turn stimulate further release of calcium via ryanodine receptors, thus producing positive feedback between these two calcium-activated channels via calcium signaling and, finally, releasing enough calcium to produce neuroplasticity. A similar dependence of ryanodine-induced calcium release on functional IP3 receptors has been shown in mouse pancreatic acinar cells (Ashby et al., 2003), in which coordinated release from ryanodine and IP3 receptors has been suggested to underlie the increased sensitivity to initiation of calcium signals.
As the IP3 receptor participates in the induction of priming by ryanodine, we next investigated whether its direct activation could induce priming. As mentioned above, the induction of priming by ryanodine, in addition to being IP3-dependent, is sexually dimorphic (Ferrari et al., 2016). Thus, we tested different doses of IP3 in both sexes, to evaluate whether there were also sex differences in the induction of priming by IP3. We found that the profile of IP3 doses able to induce priming in males and females was similar to the doses of ryanodine. Moreover, considering that the release of calcium by activation of ryanodine receptors activates IP3 receptors, and vice versa (Berridge et al., 2000; Smith et al., 2009; Taylor and Tovey, 2010; Taylor and Konieczny, 2016), we tested whether IP3-induced priming was also dependent on the subsequent activation of ryanodine receptors. Pretreatment with a ryanodine receptor inhibitor prevented the induction of priming by IP3, suggesting that the release of calcium, and probably its amplification, by activation of ryanodine or IP3 receptors, can induce neuroplasticity. Indeed, the injection of a calcium ionophore (Babcock et al., 1976), or a solution containing high concentration of potassium (150 mm KCl), which produces depolarization, did not induce priming (data not shown), indicating that a short increase in calcium concentration is not sufficient to trigger neuroplasticity, but a more prolonged, sustained calcium release, from the ER, as produced by interaction between the ryanodine and the IP3 receptors, does activate the priming pathway.
The mechanism that regulates sexual dimorphism in priming induced by ryanodine and IP3 was also similar, as the knockdown of EsRα prevented the induction of priming by a low-dose, but not high-dose, IP3. This suggests the importance of the interaction between estrogen and ER calcium-related receptors, previously reported as the sensitizing effect of estrogen for the IP3 receptor, as well as the ryanodine receptor (Morales et al., 2005; Zhao et al., 2005; Micevych and Sinchak, 2008; Rybalchenko et al., 2009; Andruska et al., 2015; Tabatadze et al., 2015), contributing to pain chronification in females. In this sense, a role of PKA has been shown in the regulation of ryanodine receptors by estrogen, in processes such as calcium handling (Kravtsov et al., 2007) and calcium-induced calcium release (CICR)-dependent neuroplasticity in hippocampal neurons (Lin et al., 2007). A specific role of EsRα in the sensitization of IP3 receptors in the hippocampus, through metabotropic glutamate receptor 1, stimulation of phospholipase C, and generation of IP3, by estrogen has also been demonstrated (Tabatadze et al., 2015). However, our current experiments do not allow to determine whether the abovementioned mechanisms participate in our model.
The plasticity induced by IP3 observed in the behavior experiments was also observed in in vitro experiments performed in IB4+, male DRG neurons. Even at high doses, extracellular application of IP3 to the cultures did not induce detectable calcium transients, in agreement with the lack of changes in the mechanical nociceptive threshold after intradermal injection of IP3 (data not shown). Although the absence of response to IP3 in our cultures could be caused by a low membrane permeability to IP3 ions, a small amount of IP3 ions still could penetrate into the cytosol, particularly by pinocytosis (Tischner, 1977; Okada and Rechsteiner, 1982; Park et al., 1988), and sensitize the receptors, increasing their sensitivity to cytosolic calcium. This could result in activation of IP3 receptors during the increase in calcium concentration and, in particular, enhance their contribution to CICR, usually attributed to activation of ryanodine receptors in DRG neurons (Kostyuk and Verkhratsky, 1994; Shmigol et al., 1995b; Kostyuk et al., 2000). Interestingly, however, the application of IP3 to cultures that had been incubated in the presence of β-estradiol produced a significant potentiation of calcium transients induced by depolarization that was inhibited by antagonists of IP3 and ryanodine receptors. Because neither IP3 nor β-estradiol, by themselves, induced potentiation of calcium transients, it is possible that an interaction between β-estradiol and the IP3 receptor is involved in this potentiated response to a depolarizing stimulus (K20, in this case). Our experiments also confirm a crosstalk between IP3 and ryanodine receptors that, due to the interaction with β-estradiol, triggers a mechanism that increases the release of calcium from the endoplasmic reticulum, which can result in plastic changes in nociceptor function. These results are in line with the induction of priming by IP3 observed in the in vivo experiments, and also with previous reports showing potentiation of calcium transients produced by application of ryanodine, only in presence of β-estradiol, to DRG neurons in cultures (Ferrari et al., 2016). The data also support the idea of an interaction of this sex hormone and the ryanodine/IP3 receptors, increasing the release of calcium from the ER, and demonstrating that a sustained, as opposed to brief release of calcium from the ER, is necessary to produce priming.
Our results demonstrate a bidirectional interaction between IP3 and ryanodine receptors, resulting in facilitation of calcium release from the ER in small DRG neurons, which could be a part of the molecular mechanism involved in the induction of hyperalgesic priming. The current findings, integrating results from our previous report (Ferrari et al., 2016), and proposed mechanisms involved, are summarized in Figure 7, emphasizing the paradigm of “shared amplification system” formed by the ER receptors. The strong dependence of this process on the presence of β-estradiol could be the basis for the marked sexual dimorphism observed in the ability of IP3 and ryanodine to induce priming.
Figure 7.
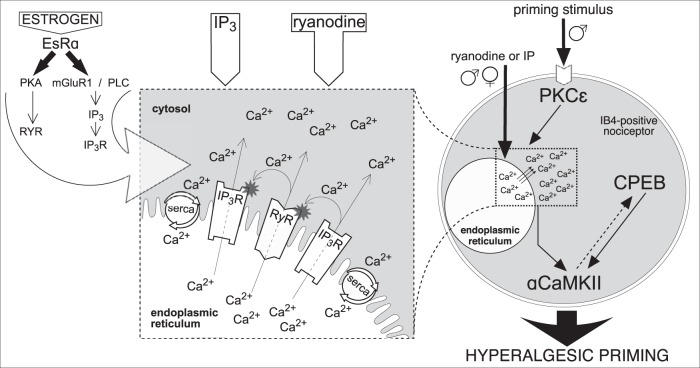
Schematic diagram of proposed mechanism of reciprocal interaction between IP3 and ryanodine receptors in the induction of hyperalgesic priming. Activation of PKCε (in males, only) by the priming stimulus in the peripheral terminal of an IB4+ nociceptor (right side of figure) triggers a cascade of events that ultimately produces hyperalgesic priming (Aley et al., 2000; Joseph et al., 2003; Joseph and Levine, 2010; Ferrari et al., 2013b), which includes calcium release from ER and requires both ryanodine (RyR) and IP3 (IP3R) receptors, activation of αCaMKII, and cytoplasmic polyadenylation element binding protein (CPEB) (Ferrari et al., 2013b). Direct activation of RyR and IP3R also produces priming, in males and females. We hypothesize that release of calcium ions (Ca2+) from ER stores, causing a local increase in [Ca2+]i (dashed and magnified boxes, middle) (Ehrlich et al., 1994; MacKrill, 1999, 2012) is a key factor required for the induction of priming. The suggested interaction between RyR and IP3R, by Ca2+ released through these ionotropic receptors, is shown in the magnified box. Once outside the ER, Ca2+ can activate nearby RyR and IP3R, increasing [Ca2+]i even more, via CICR, to reach a level high enough to trigger mechanisms, such as calmodulin and αCaMKII (Shakiryanova et al., 2007, 2011; Wong et al., 2009), leading to induction of priming (Ferrari et al., 2013b). The ER calcium pump, SERCA, transports the Ca2+ released into the cytosol, back to stores, to refill them, restricting Ca2+ signals spatially and temporally, a “loop” mechanism that has been associated with the development of neuroplasticity (Pal et al., 2001; Scullin and Partridge, 2010; Kato et al., 2012; Komin et al., 2015; Yasuda and Mukai, 2015). Additional Ca2+ buffering in the cytosol limits the fast diffusion of Ca2+, supporting its local accumulation. Thus, the whole system of RyR and IP3R can be triggered by administration of IP3 or ryanodine. When the concentration of agonists reaches a certain level, the effective doses able to induce priming (Ferrari et al., 2016) (Fig. 2a), the local cascade of propagating CICR becomes self-supported. We believe this “small” (in the scale of the whole cell), but long-lasting, change in Ca2+ homeostasis is a crucial factor for the development of hyperalgesic priming. In contrast, when the Ca2+ signal produced is strong but of short duration, for example, that produced by calcium ionophore, due to the bell-shaped sensitivity of RyR and IP3R for [Ca2+]i, the production of the self-supported long-lasting local [Ca2+]i increase is prevented, and hyperalgesic priming does not develop. Although the direct interaction between the receptors is important to generate amplification, we consider Ca2+ a key mediator of the reported reciprocal modulation of RyR and IP3R, and the amount of Ca2+ initially released is the most important factor in the mechanism, regardless of which receptor is stimulated. This constitutes our paradigm of “the common (shared) amplifier,” postulating the amplification of Ca2+ signals produced by either input (RyR or IP3R), which explains the similarity in the difference between males and females in the sensitivity to be primed by either IP3 or ryanodine. Although this amplification system is present in both males and females, it is less potent in males (a 100,000-fold difference). In regard of the higher sensitivity of females to be primed by ryanodine or IP3, the action of estrogen on EsRα (left) potentiates the amplification system, likely by sensitizing the ER receptors, as suggested by previous reports: modulation of RyR by estrogen-PKA signaling (Lin et al., 2007) or potentiation of IP3R by estrogen through metabotropic glutamate receptor 1, phospholipase C, and generation of IP3 (Tabatadze et al., 2015) (left, large arrow). This latter pathway could also result in tonic sensitization of IP3R in females, due to an increased basal IP3 levels. As shown in Figures 4 and 6, tonic activation of EsR in cultured male nociceptors, by incubation in the presence of β-estradiol, also potentiates their sensitivity to both ryanodine and IP3. This finding suggests that the sensitizing machinery of EsR is also present in males, but probably not active enough due to a lower level (or lack) of estrogen. Nevertheless, there is still a possibility that the EsRα-dependent sensitization in females (as well as in males, when activated) is indirect, due to the suppression of some blocking pathway active in males and females when EsRα is not stimulated. Although little is known about endogenous competitive antagonists shared by RyR and IP3R, according to our “shared amplification system paradigm,” suppression of either RyR and IP3R by their antagonists, including endogenous (Lenzen and Rustenbeck, 1991; Uehara et al., 1996; Vervliet et al., 2015), could indeed reduce the sensitivity of the whole system (as shown in Figs. 1, 2b). Whether this mechanism is involved in the observed difference between males and females in our study remains to be a determined.
Footnotes
This work was supported by National Institutes of Health Grant NS084545.
The authors declare no competing financial interests.
References
- Adasme T, Haeger P, Paula-Lima AC, Espinoza I, Casas-Alarcón MM, Carrasco MA, Hidalgo C (2011) Involvement of ryanodine receptors in neurotrophin-induced hippocampal synaptic plasticity and spatial memory formation. Proc Natl Acad Sci U S A 108:3029–3034. 10.1073/pnas.1013580108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adasme T, Paula-Lima A, Hidalgo C (2015) Inhibitory ryanodine prevents ryanodine receptor-mediated Ca2+ release without affecting endoplasmic reticulum Ca2+ content in primary hippocampal neurons. Biochem Biophys Res Commun 458:57–62. 10.1016/j.bbrc.2015.01.065 [DOI] [PubMed] [Google Scholar]
- Alessandri-Haber N, Dina OA, Chen X, Levine JD (2009) TRPC1 and TRPC6 channels cooperate with TRPV4 to mediate mechanical hyperalgesia and nociceptor sensitization. J Neurosci 29:6217–6228. 10.1523/JNEUROSCI.0893-09.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aley KO, Levine JD (1999) Role of protein kinase A in the maintenance of inflammatory pain. J Neurosci 19:2181–2186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aley KO, Messing RO, Mochly-Rosen D, Levine JD (2000) Chronic hypersensitivity for inflammatory nociceptor sensitization mediated by the epsilon isozyme of protein kinase C. J Neurosci 20:4680–4685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andruska ND, Zheng X, Yang X, Mao C, Cherian MM, Mahapatra L, Helferich WG, Shapiro DJ (2015) Estrogen receptor α inhibitor activates the unfolded protein response, blocks protein synthesis, and induces tumor regression. Proc Natl Acad Sci U S A 112:4737–4742. 10.1073/pnas.1403685112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araldi D, Ferrari LF, Levine JD (2015) Repeated mu-opioid exposure induces a novel form of the hyperalgesic priming model for transition to chronic pain. J Neurosci 35:12502–12517. 10.1523/JNEUROSCI.1673-15.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aronoff GM. (2016) What do we know about the pathophysiology of chronic pain? Implications for treatment considerations. Med Clin North Am 100:31–42. 10.1016/j.mcna.2015.08.004 [DOI] [PubMed] [Google Scholar]
- Ashby MC, Petersen OH, Tepikin AV (2003) Spatial characterisation of ryanodine-induced calcium release in mouse pancreatic acinar cells. Biochem J 369:441–445. 10.1042/bj20021039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashhad S, Johnston D, Narayanan R (2015) Activation of InsP3 receptors is sufficient for inducing graded intrinsic plasticity in rat hippocampal pyramidal neurons. J Neurophysiol 113:2002–2013. 10.1152/jn.00833.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babcock DF, First NL, Lardy HA (1976) Action of ionophore A23187 at the cellular level: separation of effects at the plasma and mitochondrial membranes. J Biol Chem 251:3881–3886. [PubMed] [Google Scholar]
- Baker KD, Edwards TM, Rickard NS (2013) The role of intracellular calcium stores in synaptic plasticity and memory consolidation. Neurosci Biobehav Rev 37:1211–1239. 10.1016/j.neubiorev.2013.04.011 [DOI] [PubMed] [Google Scholar]
- Bali KK, Kuner R (2014) Noncoding RNAs: key molecules in understanding and treating pain. Trends Mol Med 20:437–448. 10.1016/j.molmed.2014.05.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbara JG. (2002) IP3-dependent calcium-induced calcium release mediates bidirectional calcium waves in neurones: functional implications for synaptic plasticity. Biochim Biophys Acta 1600:12–18. 10.1016/S1570-9639(02)00439-9 [DOI] [PubMed] [Google Scholar]
- Bardo S, Cavazzini MG, Emptage N (2006) The role of the endoplasmic reticulum Ca2+ store in the plasticity of central neurons. Trends Pharmacol Sci 27:78–84. 10.1016/j.tips.2005.12.008 [DOI] [PubMed] [Google Scholar]
- Bauer CS, Nieto-Rostro M, Rahman W, Tran-Van-Minh A, Ferron L, Douglas L, Kadurin I, Sri Ranjan Y, Fernandez-Alacid L, Millar NS, Dickenson AH, Lujan R, Dolphin AC (2009) The increased trafficking of the calcium channel subunit α2δ-1 to presynaptic terminals in neuropathic pain is inhibited by the α2δ ligand pregabalin. J Neurosci 29:4076–4088. 10.1523/JNEUROSCI.0356-09.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge MJ, Taylor CW (1988) Inositol trisphosphate and calcium signaling. Cold Spring Harb Symp Quant Biol 53:927–933. 10.1101/SQB.1988.053.01.107 [DOI] [PubMed] [Google Scholar]
- Berridge MJ, Lipp P, Bootman MD (2000) The versatility and universality of calcium signalling. Nat Rev Mol Cell Biol 1:11–21. 10.1038/35036035 [DOI] [PubMed] [Google Scholar]
- Bérubé M, Choinière M, Laflamme YG, Gélinas C (2016) Acute to chronic pain transition in extremity trauma: a narrative review for future preventive interventions (part 1). Int J Orthop Trauma Nurs 23:47–59. 10.1016/j.ijotn.2016.04.002 [DOI] [PubMed] [Google Scholar]
- Bogen O, Alessandri-Haber N, Chu C, Gear RW, Levine JD (2012) Generation of a pain memory in the primary afferent nociceptor triggered by PKCε activation of CPEB. J Neurosci 32:2018–2026. 10.1523/JNEUROSCI.5138-11.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borle AB, Snowdowne KW (1982) Measurement of intracellular free calcium in monkey kidney cells with aequorin. Science 217:252–254. 10.1126/science.6806904 [DOI] [PubMed] [Google Scholar]
- Burch RM, Axelrod J (1987) Dissociation of bradykinin-induced prostaglandin formation from phosphatidylinositol turnover in Swiss 3T3 fibroblasts: evidence for G protein regulation of phospholipase A2. Proc Natl Acad Sci U S A 84:6374–6378. 10.1073/pnas.84.18.6374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castonguay A, Robitaille R (2002) Xestospongin C is a potent inhibitor of SERCA at a vertebrate synapse. Cell Calcium 32:39–47. 10.1016/S0143-4160(02)00093-3 [DOI] [PubMed] [Google Scholar]
- Chen M, Van Hook MJ, Thoreson WB (2015) Ca2+ diffusion through endoplasmic reticulum supports elevated intraterminal Ca2+ levels needed to sustain synaptic release from rods in darkness. J Neurosci 35:11364–11373. 10.1523/JNEUROSCI.0754-15.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costigan M, Scholz J, Woolf CJ (2009) Neuropathic pain: a maladaptive response of the nervous system to damage. Annu Rev Neurosci 32:1–32. 10.1146/annurev.neuro.051508.135531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Arco M, Margas W, Cassidy JS, Dolphin AC (2015) The upregulation of α2δ-1 subunit modulates activity-dependent Ca2+ signals in sensory neurons. J Neurosci 35:5891–5903. 10.1523/JNEUROSCI.3997-14.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Prete D, Checler F, Chami M (2014) Ryanodine receptors: physiological function and deregulation in Alzheimer disease. Mol Neurodegener 9:21. 10.1186/1750-1326-9-21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edinger KL, Frye CA (2007) Androgens' effects to enhance learning may be mediated in part through actions at estrogen receptor-beta in the hippocampus. Neurobiol Learn Mem 87:78–85. 10.1016/j.nlm.2006.07.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrlich BE, Kaftan E, Bezprozvannaya S, Bezprozvanny I (1994) The pharmacology of intracellular Ca(2+)-release channels. Trends Pharmacol Sci 15:145–149. 10.1016/0165-6147(94)90074-4 [DOI] [PubMed] [Google Scholar]
- Evans AM, Fameli N, Ogunbayo OA, Duan J, Navarro-Dorado J (2016) From contraction to gene expression: nanojunctions of the sarco/endoplasmic reticulum deliver site- and function-specific calcium signals. Sci China Life Sci 59:749–763. 10.1007/s11427-016-5071-0 [DOI] [PubMed] [Google Scholar]
- Evinger AJ 3rd, Levin ER (2005) Requirements for estrogen receptor alpha membrane localization and function. Steroids 70:361–363. 10.1016/j.steroids.2005.02.015 [DOI] [PubMed] [Google Scholar]
- Fernández de Sevilla D, Núñez A, Borde M, Malinow R, Buño W (2008) Cholinergic-mediated IP3-receptor activation induces long-lasting synaptic enhancement in CA1 pyramidal neurons. J Neurosci 28:1469–1478. 10.1523/JNEUROSCI.2723-07.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrari LF, Bogen O, Chu C, Levine JD (2013a) Peripheral administration of translation inhibitors reverses increased hyperalgesia in a model of chronic pain in the rat. J Pain 14:731–738. 10.1016/j.jpain.2013.01.779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrari LF, Bogen O, Levine JD (2013b) Role of nociceptor αCaMKII in transition from acute to chronic pain (hyperalgesic priming) in male and female rats. J Neurosci 33:11002–11011. 10.1523/JNEUROSCI.1785-13.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrari LF, Levine E, Levine JD (2013c) Role of a novel nociceptor autocrine mechanism in chronic pain. Eur J Neurosci 37:1705–1713. 10.1111/ejn.12145 [DOI] [PubMed] [Google Scholar]
- Ferrari LF, Bogen O, Levine JD (2014) Second messengers mediating the expression of neuroplasticity in a model of chronic pain in the rat. J Pain 15:312–320. 10.1016/j.jpain.2013.12.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrari LF, Bogen O, Reichling DB, Levine JD (2015) Accounting for the delay in the transition from acute to chronic pain: axonal and nuclear mechanisms. J Neurosci 35:495–507. 10.1523/JNEUROSCI.5147-13.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrari LF, Khomula EV, Araldi D, Levine JD (2016) Marked sexual dimorphism in the role of the ryanodine receptor in a model of pain chronification in the rat. Sci Rep 6:31221. 10.1038/srep31221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fill M, Copello JA (2002) Ryanodine receptor calcium release channels. Physiol Rev 82:893–922. 10.1152/physrev.00013.2002 [DOI] [PubMed] [Google Scholar]
- Fricke O, Kow LM, Bogun M, Pfaff DW (2007) Estrogen evokes a rapid effect on intracellular calcium in neurons characterized by calcium oscillations in the arcuate nucleus. Endocrine 31:279–288. 10.1007/s12020-007-0034-7 [DOI] [PubMed] [Google Scholar]
- Futagi D, Kitano K (2015) Ryanodine-receptor-driven intracellular calcium dynamics underlying spatial association of synaptic plasticity. J Comput Neurosci 39:329–347. 10.1007/s10827-015-0579-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold MS, Dastmalchi S, Levine JD (1996) Co-expression of nociceptor properties in dorsal root ganglion neurons from the adult rat in vitro. Neuroscience 71:265–275. 10.1016/0306-4522(95)00433-5 [DOI] [PubMed] [Google Scholar]
- Gordienko DV, Bolton TB (2002) Crosstalk between ryanodine receptors and IP(3) receptors as a factor shaping spontaneous Ca(2+)-release events in rabbit portal vein myocytes. J Physiol 542:743–762. 10.1113/jphysiol.2001.015966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruol DL, Netzeband JG, Nelson TE (2010) Somatic Ca2+ signaling in cerebellar Purkinje neurons. J Neurosci Res 88:275–289. 10.1002/jnr.22204 [DOI] [PubMed] [Google Scholar]
- Harper AA, Lawson SN (1985) Conduction velocity is related to morphological cell type in rat dorsal root ganglion neurones. J Physiol 359:31–46. 10.1113/jphysiol.1985.sp015573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendrich J, Alvarez P, Joseph EK, Ferrari LF, Chen X, Levine JD (2012) In vivo and in vitro comparison of female and male nociceptors. J Pain 13:1224–1231. 10.1016/j.jpain.2012.09.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendrich J, Alvarez P, Joseph EK, Chen X, Bogen O, Levine JD (2013) Electrophysiological correlates of hyperalgesic priming in vitro and in vivo. Pain 154:2207–2215. 10.1016/j.pain.2013.07.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirose M, Stuyvers B, Dun W, Ter Keurs H, Boyden PA (2008) Wide long lasting perinuclear Ca2+ release events generated by an interaction between ryanodine and IP3 receptors in canine Purkinje cells. J Mol Cell Cardiol 45:176–184. 10.1016/j.yjmcc.2008.05.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hund TJ, Ziman AP, Lederer WJ, Mohler PJ (2008) The cardiac IP3 receptor: uncovering the role of “the other” calcium-release channel. J Mol Cell Cardiol 45:159–161. 10.1016/j.yjmcc.2008.06.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson T, Thomas S, Stabile V, Shotwell M, Han X, McQueen K (2016) A systematic review and meta-analysis of the global burden of chronic pain without clear etiology in low- and middle-income countries: trends in heterogeneous data and a proposal for new assessment methods. Anesth Analg 123:739–748. 10.1213/ANE.0000000000001389 [DOI] [PubMed] [Google Scholar]
- Joseph EK, Levine JD (2010) Hyperalgesic priming is restricted to isolectin B4-positive nociceptors. Neuroscience 169:431–435. 10.1016/j.neuroscience.2010.04.082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joseph EK, Parada CA, Levine JD (2003) Hyperalgesic priming in the rat demonstrates marked sexual dimorphism. Pain 105:143–150. 10.1016/S0304-3959(03)00175-1 [DOI] [PubMed] [Google Scholar]
- Kárai LJ, Russell JT, Iadarola MJ, Oláh Z (2004) Vanilloid receptor 1 regulates multiple calcium compartments and contributes to Ca2+-induced Ca2+ release in sensory neurons. J Biol Chem 279:16377–16387. 10.1074/jbc.M310891200 [DOI] [PubMed] [Google Scholar]
- Kato HK, Kassai H, Watabe AM, Aiba A, Manabe T (2012) Functional coupling of the metabotropic glutamate receptor, InsP3 receptor and L-type Ca2+ channel in mouse CA1 pyramidal cells. J Physiol 590:3019–3034. 10.1113/jphysiol.2012.232942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khasar SG, Burkham J, Dina OA, Brown AS, Bogen O, Alessandri-Haber N, Green PG, Reichling DB, Levine JD (2008) Stress induces a switch of intracellular signaling in sensory neurons in a model of generalized pain. J Neurosci 28:5721–5730. 10.1523/JNEUROSCI.0256-08.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khomula EV, Viatchenko-Karpinski VY, Borisyuk AL, Duzhyy DE, Belan PV, Voitenko NV (2013) Specific functioning of Cav3.2 T-type calcium and TRPV1 channels under different types of STZ-diabetic neuropathy. Biochim Biophys Acta 1832:636–649. 10.1016/j.bbadis.2013.01.017 [DOI] [PubMed] [Google Scholar]
- Kijima Y, Ogunbunmi E, Fleischer S (1991) Drug action of thapsigargin on the Ca2+ pump protein of sarcoplasmic reticulum. J Biol Chem 266:22912–22918. [PubMed] [Google Scholar]
- Komin N, Moein M, Ellisman MH, Skupin A (2015) Multiscale modeling indicates that temperature dependent [Ca2+]i spiking in astrocytes is quantitatively consistent with modulated SERCA activity. Neural Plast 2015:683490. 10.1155/2015/683490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kostyuk P, Verkhratsky A (1994) Calcium stores in neurons and glia. Neuroscience 63:381–404. 10.1016/0306-4522(94)90537-1 [DOI] [PubMed] [Google Scholar]
- Kostyuk PG, Shmigol' AV, Voitenko NV, Svichar NV, Kostyuk EP (2000) The endoplasmic reticulum and mitochondria as elements of the mechanism of intracellular signaling in the nerve cell. Neurosci Behav Physiol 30:15–18. 10.1007/BF02461387 [DOI] [PubMed] [Google Scholar]
- Kravtsov GM, Kam KW, Liu J, Wu S, Wong TM (2007) Altered Ca(2+) handling by ryanodine receptor and Na(+)-Ca(2+) exchange in the heart from ovariectomized rats: role of protein kinase A. Am J Physiol Cell Physiol 292:C1625–C1635. 10.1152/ajpcell.00368.2006 [DOI] [PubMed] [Google Scholar]
- Kruglikov I, Gryshchenko O, Shutov L, Kostyuk E, Kostyuk P, Voitenko N (2004) Diabetes-induced abnormalities in ER calcium mobilization in primary and secondary nociceptive neurons. Pflugers Arch 448:395–401. 10.1007/s00424-004-1263-8 [DOI] [PubMed] [Google Scholar]
- Lenzen S, Rustenbeck I (1991) Effects of IP3, spermine, and Mg2+ on regulation of Ca2+ transport by endoplasmic reticulum and mitochondria in permeabilized pancreatic islets. Diabetes 40:323–326. 10.2337/diab.40.3.323 [DOI] [PubMed] [Google Scholar]
- Liang YQ, Akishita M, Kim S, Ako J, Hashimoto M, Iijima K, Ohike Y, Watanabe T, Sudoh N, Toba K, Yoshizumi M, Ouchi Y (2002) Estrogen receptor beta is involved in the anorectic action of estrogen. Int J Obes Relat Metab Disord 26:1103–1109. 10.1038/sj.ijo.0802054 [DOI] [PubMed] [Google Scholar]
- Lin F, Xin Y, Wang J, Ma L, Liu J, Liu C, Long L, Wang F, Jin Y, Zhou J, Chen J (2007) Puerarin facilitates Ca(2+)-induced Ca(2+) release triggered by KCl-depolarization in primary cultured rat hippocampal neurons. Eur J Pharmacol 570:43–49. 10.1016/j.ejphar.2007.05.023 [DOI] [PubMed] [Google Scholar]
- Lohmann C, Wong RO (2005) Regulation of dendritic growth and plasticity by local and global calcium dynamics. Cell Calcium 37:403–409. 10.1016/j.ceca.2005.01.008 [DOI] [PubMed] [Google Scholar]
- Lytton J, Westlin M, Hanley MR (1991) Thapsigargin inhibits the sarcoplasmic or endoplasmic reticulum Ca-ATPase family of calcium pumps. J Biol Chem 266:17067–17071. [PubMed] [Google Scholar]
- MacKrill JJ. (1999) Protein-protein interactions in intracellular Ca2+-release channel function. Biochem J 337:345–361. 10.1042/bj3370345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacKrill JJ. (2012) Ryanodine receptor calcium release channels: an evolutionary perspective. Adv Exp Med Biol 740:159–182. 10.1007/978-94-007-2888-2_7 [DOI] [PubMed] [Google Scholar]
- Maixner W, Fillingim RB, Williams DA, Smith SB, Slade GD (2016) Overlapping chronic pain conditions: implications for diagnosis and classification. J Pain 17:T93–T107. 10.1016/j.jpain.2016.06.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mak DO, Foskett JK (2015) Inositol 1,4,5-trisphosphate receptors in the endoplasmic reticulum: a single-channel point of view. Cell Calcium 58:67–78. 10.1016/j.ceca.2014.12.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Micevych P, Sinchak K (2008) Estradiol regulation of progesterone synthesis in the brain. Mol Cell Endocrinol 290:44–50. 10.1016/j.mce.2008.04.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morales A, Díaz M, Guelmes P, Marín R, Alonso R (2005) Rapid modulatory effect of estradiol on acetylcholine-induced Ca2+ signal is mediated through cyclic-GMP cascade in LHRH-releasing GT1–7 cells. Eur J Neurosci 22:2207–2215. 10.1111/j.1460-9568.2005.04432.x [DOI] [PubMed] [Google Scholar]
- Muchekehu RW, Harvey BJ (2008) 17beta-estradiol rapidly mobilizes intracellular calcium from ryanodine-receptor-gated stores via a PKC-PKA-Erk-dependent pathway in the human eccrine sweat gland cell line NCL-SG3. Cell Calcium 44:276–288. 10.1016/j.ceca.2007.12.001 [DOI] [PubMed] [Google Scholar]
- Nagarkatti N, Deshpande LS, DeLorenzo RJ (2008) Levetiracetam inhibits both ryanodine and IP3 receptor activated calcium induced calcium release in hippocampal neurons in culture. Neurosci Lett 436:289–293. 10.1016/j.neulet.2008.02.076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakata H, Nakamura S (2007) Brain-derived neurotrophic factor regulates AMPA receptor trafficking to post-synaptic densities via IP3R and TRPC calcium signaling. FEBS Lett 581:2047–2054. 10.1016/j.febslet.2007.04.041 [DOI] [PubMed] [Google Scholar]
- Okada CY, Rechsteiner M (1982) Introduction of macromolecules into cultured mammalian cells by osmotic lysis of pinocytic vesicles. Cell 29:33–41. 10.1016/0092-8674(82)90087-3 [DOI] [PubMed] [Google Scholar]
- Pal S, Sun D, Limbrick D, Rafiq A, DeLorenzo RJ (2001) Epileptogenesis induces long-term alterations in intracellular calcium release and sequestration mechanisms in the hippocampal neuronal culture model of epilepsy. Cell Calcium 30:285–296. 10.1054/ceca.2001.0236 [DOI] [PubMed] [Google Scholar]
- Parada CA, Yeh JJ, Joseph EK, Levine JD (2003a) Tumor necrosis factor receptor type-1 in sensory neurons contributes to induction of chronic enhancement of inflammatory hyperalgesia in rat. Eur J Neurosci 17:1847–1852. 10.1046/j.1460-9568.2003.02626.x [DOI] [PubMed] [Google Scholar]
- Parada CA, Yeh JJ, Reichling DB, Levine JD (2003b) Transient attenuation of protein kinase Cε can terminate a chronic hyperalgesic state in the rat. Neuroscience 120:219–226. 10.1016/S0306-4522(03)00267-7 [DOI] [PubMed] [Google Scholar]
- Parada CA, Reichling DB, Levine JD (2005) Chronic hyperalgesic priming in the rat involves a novel interaction between cAMP and PKCε second messenger pathways. Pain 113:185–190. 10.1016/j.pain.2004.10.021 [DOI] [PubMed] [Google Scholar]
- Park RD, Sullivan PC, Storrie B (1988) Hypertonic sucrose inhibition of endocytic transport suggests multiple early endocytic compartments. J Cell Physiol 135:443–450. 10.1002/jcp.1041350311 [DOI] [PubMed] [Google Scholar]
- Paula-Lima AC, Adasme T, Hidalgo C (2014) Contribution of Ca2+ release channels to hippocampal synaptic plasticity and spatial memory: potential redox modulation. Antioxid Redox Signal 21:892–914. 10.1089/ars.2013.5796 [DOI] [PubMed] [Google Scholar]
- Peng J, Gu N, Zhou L, B Eyo U, Murugan M, Gan WB, Wu LJ (2016) Microglia and monocytes synergistically promote the transition from acute to chronic pain after nerve injury. Nat Commun 7:12029. 10.1038/ncomms12029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Putney JW Jr, Bird GS (1993) The inositol phosphate-calcium signaling system in nonexcitable cells. Endocr Rev 14:610–631. 10.1210/edrv-14-5-610 [DOI] [PubMed] [Google Scholar]
- Randall LO, Selitto JJ (1957) A method for measurement of analgesic activity on inflamed tissue. Arch Int Pharmacodyn Ther 111:409–419. [PubMed] [Google Scholar]
- Raymond CR, Redman SJ (2006) Spatial segregation of neuronal calcium signals encodes different forms of LTP in rat hippocampus. J Physiol 570:97–111. 10.1113/jphysiol.2005.098947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reber BF, Stucki JW, Reuter H (1993) Unidirectional interaction between two intracellular calcium stores in rat phaeochromocytoma (PC12) cells. J Physiol 468:711–727. 10.1113/jphysiol.1993.sp019796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichling DB, Levine JD (2009) Critical role of nociceptor plasticity in chronic pain. Trends Neurosci 32:611–618. 10.1016/j.tins.2009.07.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuquén P, Oróstica ML, Rojas I, Díaz P, Parada-Bustamante A, Orihuela PA (2015) Estradiol increases IP3 by a nongenomic mechanism in the smooth muscle cells from the rat oviduct. Reproduction 150:331–341. 10.1530/REP-15-0137 [DOI] [PubMed] [Google Scholar]
- Rigaud M, Gemes G, Weyker PD, Cruikshank JM, Kawano T, Wu HE, Hogan QH (2009) Axotomy depletes intracellular calcium stores in primary sensory neurons. Anesthesiology 111:381–392. 10.1097/ALN.0b013e3181ae6212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose CR, Konnerth A (2001) Stores not just for storage: intracellular calcium release and synaptic plasticity. Neuron 31:519–522. 10.1016/S0896-6273(01)00402-0 [DOI] [PubMed] [Google Scholar]
- Rybalchenko V, Grillo MA, Gastinger MJ, Rybalchenko N, Payne AJ, Koulen P (2009) The unliganded long isoform of estrogen receptor beta stimulates brain ryanodine receptor single channel activity alongside with cytosolic Ca2+. J Recept Signal Transduct Res 29:326–341. 10.3109/10799890903295168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scullin CS, Partridge LD (2010) Contributions of SERCA pump and ryanodine-sensitive stores to presynaptic residual Ca2+. Cell Calcium 47:326–338. 10.1016/j.ceca.2010.01.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segal M, Korkotian E (2016) Roles of calcium stores and store-operated channels in plasticity of dendritic spines. Neuroscientist 22:477–485. 10.1177/1073858415613277 [DOI] [PubMed] [Google Scholar]
- Shakiryanova D, Klose MK, Zhou Y, Gu T, Deitcher DL, Atwood HL, Hewes RS, Levitan ES (2007) Presynaptic ryanodine receptor-activated calmodulin kinase II increases vesicle mobility and potentiates neuropeptide release. J Neurosci 27:7799–7806. 10.1523/JNEUROSCI.1879-07.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shakiryanova D, Morimoto T, Zhou C, Chouhan AK, Sigrist SJ, Nose A, Macleod GT, Deitcher DL, Levitan ES (2011) Differential control of presynaptic CaMKII activation and translocation to active zones. J Neurosci 31:9093–9100. 10.1523/JNEUROSCI.0550-11.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shmigol A, Kostyuk P, Verkhratsky A (1995a) Dual action of thapsigargin on calcium mobilization in sensory neurons: inhibition of Ca2+ uptake by caffeine-sensitive pools and blockade of plasmalemmal Ca2+ channels. Neuroscience 65:1109–1118. 10.1016/0306-4522(94)00553-H [DOI] [PubMed] [Google Scholar]
- Shmigol A, Verkhratsky A, Isenberg G (1995b) Calcium-induced calcium release in rat sensory neurons. J Physiol 489:627–636. 10.1113/jphysiol.1995.sp021078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shutov L, Kruglikov I, Gryshchenko O, Khomula E, Viatchenko-Karpinski V, Belan P, Voitenko N (2006) The effect of nimodipine on calcium homeostasis and pain sensitivity in diabetic rats. Cell Mol Neurobiol 26:1541–1557. 10.1007/s10571-006-9107-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silveira PE, Lima RF, Guimarães JD, Molgó J, Naves LA, Kushmerick C (2015) Ryanodine and inositol triphosphate receptors modulate facilitation and tetanic depression at the frog neuromuscular junction. Muscle Nerve 52:623–630. 10.1002/mus.24571 [DOI] [PubMed] [Google Scholar]
- Smith IF, Wiltgen SM, Shuai J, Parker I (2009) Ca(2+) puffs originate from preestablished stable clusters of inositol trisphosphate receptors. Sci Signal 2:ra77. 10.1126/scisignal.2000466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solovyova N, Fernyhough P, Glazner G, Verkhratsky A (2002a) Xestospongin C empties the ER calcium store but does not inhibit InsP3-induced Ca2+ release in cultured dorsal root ganglia neurones. Cell Calcium 32:49–52. 10.1016/S0143-4160(02)00094-5 [DOI] [PubMed] [Google Scholar]
- Solovyova N, Veselovsky N, Toescu EC, Verkhratsky A (2002b) Ca(2+) dynamics in the lumen of the endoplasmic reticulum in sensory neurons: direct visualization of Ca(2+)-induced Ca(2+) release triggered by physiological Ca(2+) entry. EMBO J 21:622–630. 10.1093/emboj/21.4.622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorrentino V, Volpe P (1993) Ryanodine receptors: how many, where and why. Trends Pharmacol Sci 14:98–103. 10.1016/0165-6147(93)90072-R [DOI] [PubMed] [Google Scholar]
- Stutzmann GE, Mattson MP (2011) Endoplasmic reticulum Ca(2+) handling in excitable cells in health and disease. Pharmacol Rev 63:700–727. 10.1124/pr.110.003814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutko JL, Ito K, Kenyon JL (1985) Ryanodine: a modifier of sarcoplasmic reticulum calcium release in striated muscle. Fed Proc 44:2984–2988. [PubMed] [Google Scholar]
- Szatkowski C, Parys JB, Ouadid-Ahidouch H, Matifat F (2010) Inositol 1,4,5-trisphosphate-induced Ca2+ signalling is involved in estradiol-induced breast cancer epithelial cell growth. Mol Cancer 9:156. 10.1186/1476-4598-9-156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabatadze N, Huang G, May RM, Jain A, Woolley CS (2015) Sex differences in molecular signaling at inhibitory synapses in the hippocampus. J Neurosci 35:11252–11265. 10.1523/JNEUROSCI.1067-15.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taiwo YO, Coderre TJ, Levine JD (1989) The contribution of training to sensitivity in the nociceptive paw-withdrawal test. Brain Res 487:148–151. 10.1016/0006-8993(89)90950-5 [DOI] [PubMed] [Google Scholar]
- Taylor CW, Konieczny V (2016) IP3 receptors: take four IP3 to open. Sci Signal 9:1–4. 10.1126/scisignal.aaf6029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor CW, Tovey SC (2010) IP(3) receptors: toward understanding their activation. Cold Spring Harb Perspect Biol 2:1–22. 10.1101/cshperspect.a004010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tischner K. (1977) Uptake of horseradish peroxidase by sensory nerve fibres in vitro. J Anat 124:83–97. [PMC free article] [PubMed] [Google Scholar]
- Turk DC, Fillingim RB, Ohrbach R, Patel KV (2016) Assessment of psychosocial and functional impact of chronic pain. J Pain, 17:T21–T49. 10.1016/j.jpain.2016.02.006 [DOI] [PubMed] [Google Scholar]
- Uehara A, Fill M, Vélez P, Yasukochi M, Imanaga I (1996) Rectification of rabbit cardiac ryanodine receptor current by endogenous polyamines. Biophys J 71:769–777. 10.1016/S0006-3495(96)79276-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Usachev Y, Shmigol A, Pronchuk N, Kostyuk P, Verkhratsky A (1993) Caffeine-induced calcium release from internal stores in cultured rat sensory neurons. Neuroscience 57:845–859. 10.1016/0306-4522(93)90029-F [DOI] [PubMed] [Google Scholar]
- Valdés JA, Hidalgo J, Galaz JL, Puentes N, Silva M, Jaimovich E, Carrasco MA (2007) NF-κB activation by depolarization of skeletal muscle cells depends on ryanodine and IP3 receptor-mediated calcium signals. Am J Physiol Cell Physiol 292:C1960–C1970. 10.1152/ajpcell.00320.2006 [DOI] [PubMed] [Google Scholar]
- Verkhratsky A. (2002) The endoplasmic reticulum and neuronal calcium signalling. Cell Calcium 32:393–404. 10.1016/S0143416002001896 [DOI] [PubMed] [Google Scholar]
- Vervliet T, Parys JB, Bultynck G (2015) Bcl-2 and FKBP12 bind to IP3 and ryanodine receptors at overlapping sites: the complexity of protein-protein interactions for channel regulation. Biochem Soc Trans 43:396–404. 10.1042/BST20140298 [DOI] [PubMed] [Google Scholar]
- Walk D, Poliak-Tunis M (2016) Chronic pain management: an overview of taxonomy, conditions commonly encountered, and assessment. Med Clin North Am 100:1–16. 10.1016/j.mcna.2015.09.005 [DOI] [PubMed] [Google Scholar]
- Wong MY, Shakiryanova D, Levitan ES (2009) Presynaptic ryanodine receptor-CamKII signaling is required for activity-dependent capture of transiting vesicles. J Mol Neurosci 37:146–150. 10.1007/s12031-008-9080-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia J, Pan R, Gao X, Meucci O, Hu H (2014) Native store-operated calcium channels are functionally expressed in mouse spinal cord dorsal horn neurons and regulate resting calcium homeostasis. J Physiol 592:3443–3461. 10.1113/jphysiol.2014.275065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasuda H, Mukai H (2015) Turning off of GluN2B subunits and turning on of CICR in hippocampal LTD induction after developmental GluN2 subunit switch. Hippocampus 25:1274–1284. 10.1002/hipo.22435 [DOI] [PubMed] [Google Scholar]
- Young EE, Lariviere WR, Belfer I (2012) Genetic basis of pain variability: recent advances. J Med Genet 49:1–9. 10.1136/jmedgenet-2011-100386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao X, MacBride MM, Peterson BR, Pfaff DW, Vasudevan N (2005) Calcium flux in neuroblastoma cells is a coupling mechanism between non-genomic and genomic modes of estrogens. Neuroendocrinology 81:174–182. 10.1159/000087000 [DOI] [PubMed] [Google Scholar]