Abstract
Objectives
In October 2012, the Haitian Ministry of Health endorsed the “Option B+” strategy to eliminate mother-to-child transmission of HIV and achieve HIV epidemic control. The objective of this paper is to assess and identify risk factors for attrition from the national ART program among Option B+ patients in the 12 months after ART initiation.
Design
This retrospective cohort study included patients newly initiating ART from October 2012-August 2013 at 68 ART sites covering 45% of all newly enrolled ART patients in all regions of Haiti.
Methods
With data from electronic medical records, we carried out descriptive analysis of sociodemographic, clinical, and pregnancy-related correlates of ART attrition, and used a modified Poisson regression approach to estimate relative risks in a multivariable model.
Results
There were 2,166 Option B+ patients who initiated ART, of whom 1,023 were not retained by 12 months (47.2%). One quarter (25.3%) dropped out within 3 months of ART initiation. Protective factors included older age, more advanced HIV disease progression, and any adherence counseling prior to ART initiation, while risk factors included starting ART late in gestation, starting ART within 7 days of HIV testing, and using an atypical ART regimen.
Discussion
Our study demonstrates early ART attrition among Option B+ patients and contributes evidence on the characteristics of women who are most at risk of attrition in Haiti. Our findings highlight the importance of targeted strategies to support retention among Option B+ patients.
Introduction
In October 2012, the Haitian Ministry of Health endorsed the “Option B+” strategy to accelerate coverage of prevention of mother-to-child transmission of HIV (PMTCT) services toward the dual goals of PMTCT and achieving overall HIV epidemic control by 2030 [1,2] Under this strategy, pregnant and breastfeeding women living with HIV are routinely enrolled on lifelong HIV antiretroviral therapy (ART) regardless of immunologic or clinical status, thereby decreasing delays and barriers to ART enrollment [3,4]
HIV is among the top five causes of death and disability globally [5], and the high prevalence of HIV among adults of childbearing age results in approximately 240,000 perinatal HIV infections annually[1]. Haiti accounts for the largest number of prevalent and incident HIV infections in the Caribbean region [1], with prevalence among adult women of 2.7% [6]. In 2013, use of ARVs for PMTCT during childbirth was estimated at 87% among HIV-positive women [7]. Despite this high coverage, it is estimated that 5.8–7.7% of pregnancies still result in perinatal HIV infections, for a total of approximately 325–430 new pediatric HIV cases per year [8,9] High retention on ART under Option B+ is critical to preserving the health of pregnant and postpartum women, achieving elimination of mother-to-child HIV transmission (eMTCT), preventing transmission of HIV to sero-discordant partners, and preventing development of antiretroviral drug resistance.
Early evidence from Option B+ programs globally shows rapid expansion in PMTCT coverage [3,10,11,12,13] but with a concerning pattern of elevated attrition among those newly enrolling on ART [10,14] A retrospective cohort studies in Haiti by our research team, the parent study to the present analysis, also demonstrated this pattern[15]. Among 17,059 newly initiating ART patients, which adjusted for individual-level demographic and health characteristics, we found up to a 59% greater risk of attrition among women enrolled on ART under Option B+ compared to non-pregnant women. In the present study, we report on ART attrition outcomes after ART start following adoption of the Option B+ policy in Haiti, using the subset of the cohort with sufficient follow up time to observe 12 month outcomes. The aims of the present study are: 1) to describe ART attrition among Option B+ patients in Haiti; and 2) to examine risk factors for ART attrition among Option B+ patients, with a specific focus on the timing of HIV diagnosis, enrollment in care, and ART initiation relative to pregnancy. We also compare outcomes in Haiti with outcomes reported from other settings. The study provides an important contribution in the context of building a public health agenda, towards eMTCT and “test and treat” goals [16,17]
Methods
Data source
The study used longitudinal electronic medical record (EMR) data from the iSanté data system, a networked system used in more than 100 health facilities where HIV care and treatment services are provided. The iSanté system has been described elsewhere [18,19]. Facility-level iSanté data are securely replicated to a consolidated server housed within the Haitian Ministry of Health (MSPP) in Port-au-Prince on a daily basis, or as Internet connectivity permits, and the study used de-identified data from this iSanté consolidated server. Records from health facilities with out-of-date data, defined as having less than 90% of patient visit forms saved to the iSanté consolidated server within 90 days of the patient’s visit, were excluded from the analysis. Each site had a study close date based on last replication of data to the consolidated server on or before November 24, 2014. The study cohort was drawn from 68 out of 131 national ART sites, located in 9 out of 10 administrative Departments in Haiti, and covered approximately 45% of all newly-enrolled ART patients in Haiti.
Study setting and population
Haiti’s PMTCT program has steadily evolved since the early 2000s. Starting in March 2009, for women not already enrolled on ART for their own health, the PMTCT program adopted Option B, with triple ART therapy after 28 weeks gestation through one week after cessation of breastfeeding, regardless of CD4 count. Those who met the CD4-based ART eligibility threshold of <350 cells/mm3 were advised to continue ART. Starting in June 2011, Option B was recommended after 14 weeks gestation [20]. The adoption of Option B+ in 2012 reflected MSPP’s consistent embrace of new strategies to reach national eMTCT goals. Over this period, PMTCT services extended from 113 sites in 2008 to 137 sites in 2013 [8], and ART use in PMTCT increased from 27% in 2008 to 87% in 2014 [7]. By 2014, 99% of the ARV regimens used for PMTCT were multidrug regimens suitable for life-long therapy. Early infant diagnosis services, first piloted in 2009 in Haiti, reached 3,600 HIV exposed infants in 2014, with a positivity rate of 5.3% [21].
In all of our study sites, PMTCT and ART services were located within the same health facility. In general, Option B+ patients initiated ART through the maternal health service. The national guidelines specify that transition of care to the ART service after cessation of breastfeeding, although in some facilities transition occurred earlier. Most health facilities operate a single ART pharmacy serving both PMTCT and other ART patients. The study used data on patients newly enrolling on ART from October 2012, when the Option B+ policy was considered to be operationalized in Haiti, through August 2013. Patients with at least 365 days of follow up time possible as of the health facility-specific study close date were included in the analysis. The iSanté system includes a de-duplication algorithm based on patient names and demographic details; in the case of duplicates, the record with the first ART use was retained. Patients with missing age or sex were excluded from the analysis (0.4% of records).
Measurements
The main outcome of interest was attrition from the ART program due to death or loss-to-follow-up in the 12 months after ART initiation, under definitions used by the MSPP and the President’s Emergency Plan for AIDS Relief (PEPFAR). The MSPP definition considers patients to be cases of attrition if they made no visits for ART pick up within the 275–365 day period after ART initiation. The PEPFAR definition considers patients to be cases of attrition if: 1) after their last visit they did not have enough ARVs to last through the end of the 12th month of treatment, or 2) if they died, stopped, or were lost to follow up. Both definitions count patients who drop out of care for a time after ART initiation but who return to care by 12 months as retained patients. We also considered the outcome of never returning to care after the ART start visit and not returning to care in the first 90 days post-partum. This final outcome recognizes that, among Option B+ patients, attrition as a function of time could be more closely related to timing of delivery than to timing since ART start. Known patient transfers to other health facilities, representing either patients which were coded as transfers by health workers at the index site or patients which were detected as transfers based upon the iSanté de-duplication algorithm, were excluded from the attrition analyses (n = 13, <1% patients).
The methodology for identifying Option B+ patients has been described elsewhere [15]. Briefly, the iSanté data system contains information on pregnancy and labor and delivery, but not on breastfeeding status, so patients who enrolled on ART during pregnancy or up to 12 weeks post-partum were considered as Option B+ patients. Among patients with evidence of pregnancy but with no data on last menstrual period, gestational age, or an estimated or observed delivery date, pregnancy dates were imputed, based upon the assumption that each pregnancy-related visit fell at the mid-point of a typical pregnancy of 40-weeks duration. Very few labor and delivery events are recorded within iSanté, reflecting the fact that only about one-third of deliveries take place within a health facility in Haiti [6], as well as generally low use of the iSanté EMR within labor and delivery services.
Measurement of patient demographic and clinical covariates was consistent with our earlier work [15]. Weight measures falling after the first trimester of pregnancy were not used to calculate baseline body mass index (BMI), since pregnancy-related weight gain could distort a patient’s BMI as a marker of nutritional status and health. We evaluated timing of HIV testing relative to pregnancy, timing of enrollment in HIV care and treatment relative to pregnancy, and gestational age at the time of ART initiation.
Data analysis
We used descriptive statistical analysis to characterize the patient population, by their demographic and clinical covariates. To compare the distribution of covariates across by attrition status, we used the Chi-squared test of proportions for categorical covariates, and the Kruskal-Wallis non-parametric test for numeric covariates. We examined the timing of last visit, and analyzed interruptions in care among those who were retained at 12 months. We carried out bivariate analyses to identify whether attrition levels varied by pregnancy-related covariates (gestational age at ART initiation and timing of HIV testing relative to pregnancy). We limited these analyses to patients with more precise pregnancy start and end dates available based upon last menstrual period, gestational age, or estimated or observed delivery date. Unless otherwise specified, we used the MSPP-defined attrition outcome in our analyses.
Finally, to explore factors associated with attrition among Option B+ patients, we carried out adjusted analysis for the attrition outcome based upon the MSPP definition of retention. For all bivariate and adjusted models, we used a modified Poisson regression approach to estimate relative risks (RRs). We used generalized estimating equations (GEE) models with a log link, a Poisson family, exchangeable correlation structure, and robust variance to address correlation of data by health facility [22,23]. In the adjusted model approximately 10% of cases were complete in all covariates. We used multiple imputation by chained equations to impute missing covariate data for the GEE models. We specified 90 imputations, following the guideline that the number of imputations be equal to the proportion of incomplete cases [24]. Multiple imputation is a reasonable method when it is assumed that patterns in missingness can be explained by the observed covariates. Stata 13.1 (Stata Corp, College Station, TX) was used for all analyses.
Ethical review
The study received scientific and ethical review and approval from the US Centers for Disease Control and Prevention and the Haiti National Committee on Bioethics, and was exempted from human subjects review by University of Washington.
Results
There were 3,533 previously ART-naive Option B+ patients enrolled on ART from October 2012 through August 2014 in the cohort of the parent study. Of these, 2,166 women had at least 12 months of time under study following ART initiation and were included in the present analysis (Table 1). Only 968 patients (44.7%) had precise pregnancy start or end dates available, and only 850 patients (39.2%) had known dates for pregnancy and HIV testing. There were also high levels of missing data in several covariates of interest, particularly household size and presence of another known HIV+ person within the household, baseline body mass index, WHO disease stage, and hemoglobin level.
Table 1. Characteristics of Option B+ patients (Categorical variables).
| Option B+ | Attritionǂ | p-valueǁ | |||
|---|---|---|---|---|---|
| n | % | n | % | ||
| Total | 2,166 | 100.0% | 1,023 | 47.2% | |
| Marital status | |||||
| Married/concubinage | 1,425 | 65.8% | 683 | 66.8% | 0.67 |
| Widow/divorce | 90 | 4.2% | 38 | 3.7% | |
| Single | 236 | 10.9% | 107 | 10.5% | |
| Missing/Unknown | 415 | 19.2% | 195 | 19.1% | |
| Location of residence | |||||
| Different commune | 647 | 29.9% | 303 | 29.6% | 0.70 |
| Same commune | 1,470 | 67.9% | 694 | 67.8% | |
| Missing | 49 | 2.3% | 26 | 2.5% | |
| HIV+ household member | |||||
| No | 818 | 37.8% | 363 | 35.5% | <0.001 |
| Yes | 106 | 4.9% | 35 | 3.4% | |
| Missing | 1,242 | 57.3% | 625 | 61.1% | |
| Household size | |||||
| 1–3 members | 698 | 32.2% | 314 | 30.7% | <0.001 |
| >3 members | 226 | 10.4% | 84 | 8.2% | |
| Missing | 1,242 | 57.3% | 625 | 61.1% | |
| WHO stage | |||||
| I | 941 | 43.4% | 468 | 45.7% | <0.001 |
| II | 517 | 23.9% | 231 | 22.6% | |
| III | 369 | 17.0% | 145 | 14.2% | |
| IV | 100 | 4.6% | 44 | 4.3% | |
| Missing | 239 | 11.0% | 135 | 13.2% | |
| Period of ART start | |||||
| Oct12-Mar13 | 893 | 41.2% | 401 | 39.2% | 0.13 |
| Apr13-Sep13 | 1,086 | 50.1% | 536 | 52.4% | |
| Oct13-Mar14 | 187 | 8.6% | 86 | 8.4% | |
| Starting ART regimen | |||||
| AZT-3TC-EFV | 201 | 9.3% | 82 | 8.0% | 0.11 |
| AZT-3TC-NVP | 155 | 7.2% | 80 | 7.8% | |
| TDF-3TC-EFV | 1,752 | 80.9% | 829 | 81.0% | |
| All Other | 58 | 2.7% | 32 | 3.1% | |
| Supportive services at ART start | |||||
| Treatment supporter named | 274 | 12.7% | 102 | 10.0% | <0.001 |
| Counseling before ART start | 273 | 12.6% | 77 | 7.5% | <0.001 |
| Treatment or prophylaxis for opportunistic infections at ART start | |||||
| TB prophylaxis or treatment | 542 | 25.0% | 269 | 26.3% | 0.20 |
| Cotrimoxizole prophylaxis | 1,441 | 66.5% | 646 | 63.1% | <0.01 |
| Pregnancy | |||||
| Dates of pregnancy known* | 968 | 44.7% | 439 | 42.9% | 0.12 |
| Dates of HIV test and pregnancy known | 850 | 39.2% | 389 | 38.0% | 0.27 |
| HIV test after start of pregnancy** | 687 | 80.8% | 350 | 51.0% | <0.001 |
| Registered for HIV care after preg start*** | 792 | 81.8% | 398 | 50.3% | <0.001 |
ǂAttrition by MSPP definition.
ǁp-value for Chi-2 test statistic comparing attrition and non-attrition cases.
*Pregnancy dates were considered to be known when data were available on last menstrual period date, gestational age, estimated delivery date, or actual delivery date.
**Among cases with dates of HIV test and pregnancy known.
***Among cases with dates of pregnancy known.
AZT = Zidovudine; 3TC = Lamivudine; NVP = Nevirapine; TDF = Tenofovir; EFV = Efavirenz.
Option B+ patients had a median age of 28.1 years, and were likely to be married or in a stable partnership (65.8%), live in the same commune as the health facility (67.9%), be asymptomatic (43.4%), and be prescribed the first-line regimen of Tenofovir + Lamivudine + Efavirenz (TDF-3TC-EFV) (80.9%) (Table 1). Many were started on Cotrimoxizole prophylaxis at ART start (66.5%), but few had ART adherence counseling documented (12.6%) or a treatment supporter named at ART start (12.7%). Among those with known HIV testing dates, the median time from HIV testing to ART start was 5 days (interquartile range or IQR: 0–12 days). Among those with known pregnancy and/or HIV testing dates, HIV testing typically occurred at 15.5 weeks gestation (IQR: 7.0–23.9 weeks) while ART start typically occurred at 19.1 weeks gestation (IQR: 13.0–26.7 weeks) (Table 2). Only 18.2% of women registered for HIV care before their pregnancy (Table 1).
Table 2. Characteristics of Option B+ patients (Continuous variables).
| Option B+ | p-valueǁ | ||||
| n | % missing | median | IQR | ||
| Age at ART start (years) | 2166 | 0% | 28.1 | (23.9, 33.1) | <0.001 |
| Body mass index | 285 | 87% | 22.5 | (20.5, 25.9) | 0.10 |
| Hemoglobin | 1055 | 51% | 10.7 | (9.8, 11.7) | <0.01 |
| Time: HIV test to registration (days) | 1614 | 25% | 0 | (0, 1) | 0.03 |
| Time: registration to ART start (days) | 2166 | 0% | 1 | (0, 62) | <0.001 |
| Time: HIV test to ART start (days) | 1614 | 25% | 5 | (0, 120) | <0.001 |
| Gestational age at HIV test (weeks) | 850 | 61% | 15.5 | (7.0, 23.9) | <0.001 |
| Gestational age at ART start (weeks) | 968 | 55% | 19.1 | (13.0, 26.7) | <0.001 |
| Attritionǂ | p-valueǁ | ||||
| n | % missing | median | IQR | ||
| Age at ART start (years) | 1023 | 0% | 27.0 | (22.8, 31.9) | <0.001 |
| Body mass index | 54 | 95% | 21.6 | (20.1, 24.2) | 0.10 |
| Hemoglobin | 428 | 58% | 10.6 | (9.6, 11.6) | <0.01 |
| Time: HIV test to registration (days) | 758 | 26% | 0 | (0, 1) | 0.03 |
| Time: registration to ART start (days) | 1023 | 0% | 0 | (0, 18) | <0.001 |
| Time: HIV test to ART start (days) | 758 | 26% | 2 | (0, 34) | <0.001 |
| Gestational age at HIV test (weeks) | 389 | 62% | 17.7 | (11.0, 25.1) | <0.001 |
| Gestational age at ART start (weeks) | 439 | 57% | 21.0 | (14.1, 28.7) | <0.001 |
ǂ Attrition by MSPP definition
ǁ Kruskal—Wallis equality of populations rank test (with ties) comparing variable values between attrition and non-attrition cases.
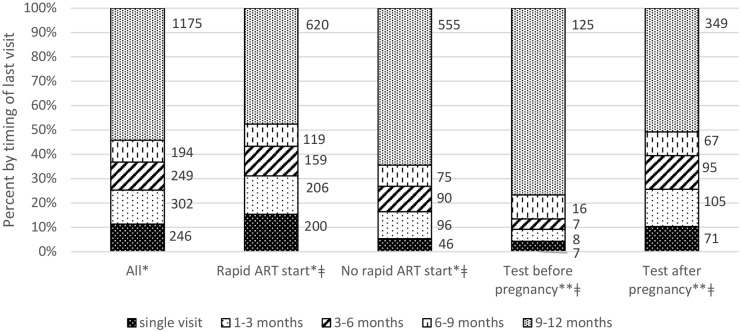
At 12 months after ART start, 1,023 (47.2%) Option B+ patients experienced attrition according to the MSPP definition, while 1,255 (57.9%) experienced attrition according to the PEPFAR definition. Many women dropped out soon after their ART start, with 246 patients (11.4%) never returning after their initial ART visit and 302 patients (13.9%) dropping out within 3 months of ART initiation (Fig 1). Even among the 1,143 patients who were retained on ART at 12 months (MSPP definition), a large proportion experienced at least one instance of being 30 or more days late for an ART pick up visit during the first year on ART (59.1%) (results not shown).
Fig 1. Timing of last visit in 12 months after ART start.
*Among 2,166 Option B+ clients. **Among 850 Option B+ clients with known pregnancy and HIV testing dates. Rapid ART start = ART start within 7 days of registration in HIV care; No rapid ART start = ART start >7 days after registration in HIV care. ǂp<0.001 for Chi2 test of equality of proportions between Rapid ART start vs. No rapid ART start and between HIV test before vs. after start of pregnancy.
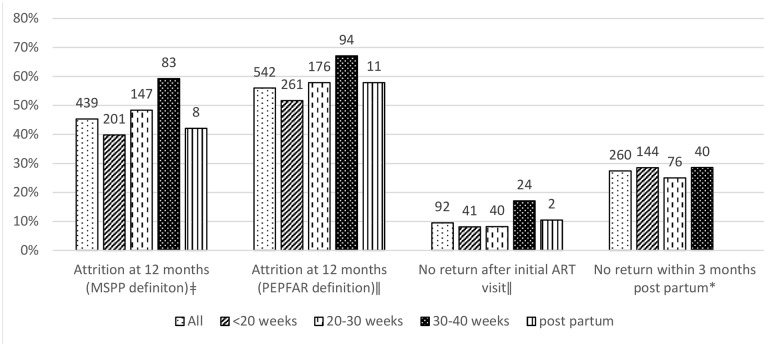
Those who started ART within 7 days of enrolling in HIV care (“rapid ART start”) were at heightened risk for 12 month attrition (RR = 1.43; 95% confidence interval or CI: 1.24–1.64, p<0.001) (results not shown). They were also at risk for early attrition; specifically, 15.3% of patients with a rapid ART start never returned after their initial ART visit, while 5.3% of patients with more time before starting ART never returned (RR = 2.70; 95% CI: 2.00–3.66, p<0.001) (Fig 1). Timing of HIV testing relative to pregnancy and gestational age at ART start were also important. Among the 850 women with known pregnancy and HIV testing dates, the 687 women (80.8%) who first enrolled in HIV care after the start of pregnancy had a 2-fold risk of attrition compared to those who were diagnosed with HIV before pregnancy (RR = 2.06, 95% CI: 1.60–2.65, p<0.001) (Fig 1). Among the 968 women with known pregnancy dates, those who initiated ART in the last 10 weeks of pregnancy were at higher risk of ART attrition, compared to those who initiated in the first 20 weeks of gestation (RR = 1.52, 95% CI: 1.30–1.7; p<0.01) (Fig 2).
Fig 2. ART attrition indicators by gestational age at ART start.
**Among 968 Option B+ patients with available data on pregnancy dates. ǂ p<0.001 ǁ p = 0.01 * p = 0.54 for Chi2 test of equality of proportions across gestational age groups.
In the adjusted analyses, significant protective factors with respect to ART attrition were older age (RR = 0.89, 95% CI: 0.86–0.93 for each 5-year increase in age, p<0.0001), having more advanced HIV disease progression by clinical staging or baseline CD4 (RR = 0.88, 95% CI: 0.77–1.00 for stage III/IV vs. I/II, p<0.05), and having any counseling on ART adherence prior to ART initiation (RR = 0.74, 95% CI: 0.62–0.88, p<0.01) (Table 3). Significant risk factors were starting ART at 30–40 weeks gestation (RR = 1.20, 95% CI: 1.02–1.42, p = 0.03), starting ART within 7 days of HIV testing (RR = 1.19, 95% CI: 1.05–1.34, p<0.01), or having an atypical ART regimen prescribed (RR = 1.35, 95% CI: 1.08–1.69, p<0.01) (Table 3).
Table 3. Risk factors for ART attrition among Option B+ patients*.
| Adjusted Relative Risk | 95% Confidence Interval | p-value | |
|---|---|---|---|
| Marital status | |||
| Non-married vs. married | 0.97 | (0.83, 1.12) | 0.67 |
| Age | |||
| Each 5-year increase | 0.89 | (0.86, 0.93) | <0.0001 |
| Household characteristics | |||
| Live in same commune as health facility | 0.94 | (0.80, 1.10) | 0.42 |
| 4+ members vs. 1–3 members | 0.98 | (0.82, 1.17) | 0.81 |
| Known HIV+ household member | 0.80 | (0.60, 1.06) | 0.13 |
| Timing of ART start | |||
| Gestational age 30–40 weeks at ART start | 1.20 | (1.02, 1.42) | 0.03 |
| ART start within 7 days of HIV test | 1.19 | (1.05, 1.34) | <0.01 |
| Period of ART initiation (reference = Oct12-Mar13) | |||
| Apr13-Sep13 | 1.06 | (0.95, 1.19) | 0.26 |
| Oct13-Mar14 | 0.97 | (0.81, 1.18) | 0.79 |
| ART starting regimen (reference = TDF+3TC+EFV) | |||
| AZT-3TC-NVP | 1.06 | (0.83, 1.36) | 0.62 |
| AZT-3TC-EFV | 0.88 | (0.72, 1.07) | 0.21 |
| All other | 1.35 | (1.08, 1.69) | <0.01 |
| Clinical status at ART start | |||
| WHO stage III/IV vs. I/II | 0.88 | (0.77, 1.00) | <0.05 |
| BMI (each 1 unit increase) | 0.94 | (0.94, 1.01) | 0.23 |
| Moderate or severe anemia | 1.08 | (0.91, 1.28) | 0.35 |
| Supportive services at ART start | |||
| Treatment buddy named | 0.86 | (0.70, 1.06) | 0.15 |
| Pre-ART counseling provided | 0.74 | (0.62, 0.88) | <0.01 |
| Treatment or prophylaxis for opportunistic infections at ART start | |||
| Tuberculosis-related therapy | 1.03 | (0.87, 1.22) | 0.74 |
| Cotrimoxazole therapy | 0.94 | (0.82, 1.07) | 0.37 |
* Attrition by MSPP definition.
Discussion
In the context of Haiti’s eMTCT agenda and the MSPP’s adoption in June 2016 of the universal “test and treat” approach to HIV care and treatment, our findings provide important evidence about sub-optimal retention on ART among women who initiate ART during or after pregnancy under Option B+. Haiti’s national PMTCT program has made important gains in recent years. Still, closing the “last mile” to achieve eMTCT will require improved retention on ART during pregnancy and post-partum periods and beyond.
Our study raises notable concerns about the high level of early ART attrition among Option B+ patients throughout Haiti. It demonstrates the profile of women at highest risk of attrition from the Option B+ program: younger women, women newly diagnosed with HIV during pregnancy or post-partum, women who initiated ART soon after diagnosis, and women who initiated ART late in pregnancy. Women who immediately move into the ART enrollment process upon learning their HIV diagnosis face the challenge of accepting their diagnosis and assimilating information about HIV treatment at the very time they are expected to start taking ART medications. Those who tested days or weeks before ART initiation would have had a greater chance to accept their diagnosis or disclose their HIV status to others, leading to lower risk of attrition. Our finding that having ART adherence counseling prior to ART initiation was protective suggests that a greater level of counseling and support at the time of enrollment is helpful; however, this finding deserves cautious interpretation. It is possible that ART adherence counseling was given in some sites, but without capturing data on this within the iSanté data system. We were unable to distinguish between the service not being offered and the data not being collected.
Our evidence on a national scale corroborates results from a study of ART retention outcomes at a single Departmental hospital in Les Cayes in Southern Haiti, during the era of the Option B approach from 2009–12 [20]. That study found elevated loss to follow up among those with HIV diagnosis during vs. before pregnancy and with ART initiation during the third trimester of pregnancy, although their results were not statistically significant in adjusted analyses. A recent mixed methods study on the PMTCT care cascade, sponsored by the MSPP and the Pan American Health Organization, investigated reasons women dropped out of PMTCT programs. The study focused on 10 health facilities in the 4 Departments of Haiti located along the border with the Dominican Republic, and was able to locate and interview 56/179 PMTCT patients (31%) who were lost to follow up from those facilities [25]. Key themes explaining loss to follow up were: lack of money to cover transport to the clinic and time away from economic activities, denial of HIV status (including a belief that a negative PCR result for the infant indicated a negative status in the mother), stigma and lack of disclosure to partners and family members, migration, ART side effects, and dissatisfaction with clinic services based upon wait time, reception, and concerns that health workers would fail to protect confidentiality.
Haiti’s experience with retention on ART following adoption of Option B+ echoes early results from other countries, as described in both quantitative and qualitative studies. In Malawi, at the start of the Option B+ program in 2011–12, 23.2% of Option B+ patients were not retained on ART after 12 months [10,26], with wide variation in outcomes across facilities [27]. In a rural area of Northern Mozambique, attrition at 12 months was reported among 64.9% of Option B+ patients [28]. Younger age [26,27,29] and rapid ART initiation following HIV diagnosis [28,30] have each been demonstrated as risk factors for attrition under Option B+ in other settings. Reasons for lost to follow up in Malawi based upon qualitative research [31,32] appear similar to reasons reported in Haiti [20,25].
In the early days of Haiti’s ART program, when only the sickest patients met ART eligibility criteria, a treatment buddy (or “accompagnateur”) model, involving directly observed therapy by community health workers, was the main model used in Haiti to reinforce ART adherence and retention in care [33]. As the ART program expanded nationally in Haiti, this model was adapted to a volunteer-based model where patients could name a friend or family member as a treatment buddy who could accompany the patient to clinic visits, collect medications on behalf of the patient, and otherwise support them. This voluntary treatment buddy model has its limitations in the context of ART use by patients who are not obviously sick, and retention support models have evolved in Haiti over time in recognition of the multi-level challenges to ART retention. Recently, the MSPP and its technical assistance partners have undertaken several promising initiatives to increase ART retention, including moves toward multi-month prescriptions and “rapid pathway” services for stable ART patients, community-based ART distribution, and active tracing of patients within 1–2 weeks of missed visits [34]. Several strategies for improving ART retention among pregnant women have shown promise in other countries, including promotion of couples testing [35,36], clinic- or community-based peer support [35,37,38,39], case management using community health workers [40], clinic level systems analysis and workflow re-design and other quality improvement approaches [35,41,42,43]. Targeted retention support initiatives for pregnant and post-partum women, especially younger women, need to be systematically tested in Haiti to reveal which innovations are most effective and cost-effective to optimize Option B+.
Strengths of our study were the large number of patients and facilities covered by the EMR data source and the ability to investigate the relationship between timing of pregnancy, HIV testing, enrollment in care, and ART start based upon patient-level EMR data. A limitation was the lack of precise pregnancy start and end dates and HIV testing dates among many patients, meaning our results on timing of ART start during pregnancy may not reflect the broader population of Option B+ patients. Another limitation was that reasons for ART discontinuation at any given health facility were only documented in 26% of non-retained patients, so transfers of care to other health facilities could have been under-reported. However, our previous analyses suggest that “silent transfer” explains <10% of cases of attrition [15]. Our findings on rapidly starting ART as a risk factor for ART attrition may reflect selection bias in that those who return to initiate ART days or weeks after HIV diagnosis may be inherently more likely to remain in care. Finally, while the study raises an interesting hypothesis about the helpful role of ART adherence counseling prior to ART start among Option B+ patients, this finding deserves cautious interpretation. Further investigation of the variation in ART enrollment processes and quality of patient education and counseling at ART initiation across health facilities under Option B+ would be helpful, and interventions to strengthen ART adherence counseling at the time of ART initiation would need to be tested via an experimental or quasi-experimental study design.
Conclusion
Our study reports on the retention outcomes of a large cohort of newly-enrolled ART patients from 68 health facilities in Haiti following the adoption of the Option B+ policy in October 2012. Women of younger age, who tested for HIV after the start of pregnancy, who enrolled on ART late in pregnancy, who had little time between HIV diagnosis and ART initiation, and who had no pre-ART counseling were at particular risk of attrition. This highlights the importance of finding feasible strategies to support retention in care among these women, in order to fulfill the eMTCT and “test and treat” agendas in Haiti.
Acknowledgments
NP led the study design, analysis, interpretation, and manuscript writing. JWD, KF, RGP, and DL conceived the study and facilitated data acquisition. KY and MM assisted in data analysis. All authors contributed to interpretation of results and provided substantial intellectual contributions to the final manuscript.
The iSanté data system depends upon the dedicated efforts of the many individuals who care for patients and who work to assure data quality, including health care workers, disease reporting officers, regional strategic information officers, and PEPFAR implementing partners. Without their efforts, this analysis would not have been possible. The authors would like to acknowledge Lydia Liu and Dana Duncan (formerly with the US Centers for Disease Control and Prevention) for thoughtful scientific input on the analysis, Steven Wagner (International Training and Education Center for Health/I-TECH) for assistance in preparation of data extracts, and Gillian O’Bryan and Joanna Diallo (International Training and Education Center for Health/I-TECH) for editorial assistance.
Meetings at which data was presented
8th International AIDS Society Conference on HIV Pathogenesis, Treatment and Prevention (IAS 2015), Vancouver Convention Centre, 19–22 July 2015, Vancouver, Canada.
Data Availability
The data for the study were obtained from the iSanté data system with permission of the Haitian Ministry of Health and Population (MSPP), a third party. Analysts who wish to use the data would need to seek permission from the MSPP in Haiti. The Haitian Ministry of Health and Population does not have a formal Data Access Committee for iSanté data as of this writing. To obtain permission to use iSanté data, analysts interested in obtaining the data are advised to contact the following individuals to make a data request: 1. Dr. Paul Adrian, Director, MSPP Division of Epidemiology, Laboratory and Research (DELR): padrien@yahoo.fr; padrien_2004@gmail.com. 2. Mr. Lavoisier Lamothe, MSPP Unit of Planning and Management (UGP): lavoisier.lamothe@ugp.ht. Analysts would need to obtain local ethical approval for data use prior to accessing the data, from the Haitian National Bioethics Committee of the MSPP. The contact for this body is Dr. Gerald Lerebours: gerald_lerebours@hotmail.com.
Funding Statement
This research has been supported by the President’s Emergency Plan for AIDS Relief (PEPFAR) through the Health Resources and Services Administration, under award number U91HA0680, and the US Centers for Disease Control and Prevention (https://www.cdc.gov/), under award number NU2GGH001130-04-00, to the International Training and Education Center for Health (I-TECH) at the University of Washington. Research reported in this publication was also supported by NIAID, NCI, NIMH, NIDA, NICHD, NHLBI, NIA, NIGMS, NIDDK of the National Institutes of Health (https://www.nih.gov/) under award number AI027757 to the University of Washington Center for AIDS Research (CFAR). The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention, the National Institutes of Health, or the Health Resources and Services Administration.
References
- 1.UNAIDS (2014) The Gap Report. http://www.unaids.org/en/resources/documents/2014/20140716_UNAIDS_gap_report.
- 2.UNAIDS (2014) 90-90-90: An ambitious treatment target to help end the AIDS epidemic. http://www.unaids.org/en/resources/documents/2014/90-90-90.
- 3.Chimbwandira F, Makombe S, Midiani D, Mwansambo C, Njala J, et al. (2013) Impact of an Innovative Approach to Prevent Mother-to-Child Transmission of HIV—Malawi, July 2011–September 2012. Morbidity and Mortality Weekly Report 62: 148–151. [PMC free article] [PubMed] [Google Scholar]
- 4.WHO (2013) Consolidated Guidelines on the Use of Antiretroviral Drugs for Treating and Preventing HIV Infection: Recommendations for a Public Health Approach. Geneva: World Health Organization. [PubMed] [Google Scholar]
- 5.Ortblad KF, Lozano R, Murray CJL (2013) The burden of HIV: Insights from the Global Burden of Disease Study 2010. AIDS 27: 2003–2017. 10.1097/QAD.0b013e328362ba67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cayemittes M, Busangu MF, Bizimana JdD, Barrère B, Sévère B, et al. (2013) Enquête Mortalité, Morbidité et Utilisation des Services (EMMUS-V): Haiti 2012. Intitut Haitien d'Enfance and Measure DHS. [Google Scholar]
- 7.Domercant JW, Guillaume FD, Marston BJ, Lowrance DW (2015) Update on Progress in Selected Public Health Programs After the 2010 Earthquake and Cholera Epidemic—Haiti, 2014. Morbidity and Mortality Weekly Report 64: 137–140. [PMC free article] [PubMed] [Google Scholar]
- 8.MSPP/PNLS (2014) Declaration d'Engagement sur le VIH/SIDA. Port au Prince: Ministere de Sante Publique et de la Population. [Google Scholar]
- 9.UNICEF (2014) Elimination of Mother to Child Transmission of HIV (EMTCT) In: UNICEF, editor. UNICEF Data: Monitoring the Health of Children and Women. http://data.unicef.org/hiv-aids/emtct. [Google Scholar]
- 10.Tenthani L, Haas AD, Tweya H, Jahn A, van Oosterhout JJ, et al. (2014) Retention in care under universal antiretroviral therapy for HIV-infected pregnant and breastfeeding women (‘Option B+’) in Malawi. Aids 28: 589–598. 10.1097/QAD.0000000000000143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ebuy H, Yebyo H, Alemayehu M (2015) Level of adherence and predictors of adherence to the Option B+ PMTCT programme in Tigray, northern Ethiopia. International Journal of Infectious Diseases 33: 123–129. 10.1016/j.ijid.2014.12.026 [DOI] [PubMed] [Google Scholar]
- 12.Kieffer MP, Mattingly M, Giphart A, Ven Rvd, Chouraya C, et al. (2014) Lessons Learned From Early Implementation of Option B+: The Elizabeth Glaser Pediatric AIDS Foundation Experience in 11 African Countries. J Acquir Immune Defic Syndr 67: S188–S194. 10.1097/QAI.0000000000000372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Price AJ, Kayange M, Zaba B, Chimbwandira FM, Jahn A, et al. (2014) Uptake of prevention of mother-to-child-transmission using Option B+ in northern rural Malawi: a retrospective cohort study. Sexually Transmitted Infections 90: 309–314. 10.1136/sextrans-2013-051336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kim MH, Ahmed S, Hosseinipour MC, Giordano TP, Chiao EY, et al. (2015) The impact of Option B+ on the antenatal PMTCT cascade in Lilongwe, Malawi. JAIDS Journal of Acquired Immune Deficiency Syndromes 68: e77–e83. 10.1097/QAI.0000000000000517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Domercant J, Puttkammer N, Lu L, Francois K, Duncan D, et al. Attrition from antiretroviral treatment services among pregnant and non-pregnant patients following adoption of Option B+ in Haiti; 2015 19–22 July, 2015; Vancouver, BC. [DOI] [PMC free article] [PubMed]
- 16.Lundgren JD, Babiker AG, Gordin F, Group ISS (2015) Initiation of Antiretroviral Therapy in Early Asymptomatic HIV Infection. New England Journal of Medicine 373: 795–807. 10.1056/NEJMoa1506816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.WHO (2015) Guideline on When to Start Antiretroviral Therapy and Pre-Exposure Prophylaxis for HIV Geneva: World Health Organization. [PubMed] [Google Scholar]
- 18.Lober W, Quiles C, Wagner S, Cassagnol R, Lamothes R, et al. (2008) Three years experience with the implementation of a networked electronic medical record in Haiti. AMIA Annual Symposium Proceedings November 6: 434–438. [PMC free article] [PubMed] [Google Scholar]
- 19.Matheson AI, Baseman JG, Wagner SH, O'Malley GE, Puttkammer NH, et al. (2012) Implementation and expansion of an electronic medical record for HIV care and treatment in Haiti: an assessment of system use and the impact of large-scale disruptions. International Journal of Medical Informatics 81: 244–256. 10.1016/j.ijmedinf.2012.01.011 [DOI] [PubMed] [Google Scholar]
- 20.Dionne-Odom J, Massaro C, Jogerst KM, Li Z, Deschamps MM, et al. (2016) Retention in Care among HIV-Infected Pregnant Women in Haiti with PMTCT Option B. AIDS Research and Treatment 2016: 6284290 10.1155/2016/6284290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Desinor O, Jean S, Charles M, Buteau J, Segaren N, et al. (May 2011) Diagnostic précoce du VIH chez les nourrissons par l'utilisation du PCR. Bulletin Epidemiologique: Programme National de Lutte contre les IST/VIH/SIDA.
- 22.Zou G (2004) A Modified Poisson Regression Approach to Prospective Studies with Binary Data. American Journal of Epidemiology 159: 702–706. [DOI] [PubMed] [Google Scholar]
- 23.Yelland LN, Salter AB, Ryan P (2011) Performance of the Modified Poisson Regression Approach for Estimating Relative Risks From Clustered Prospective Data. American Journal of Epidemiology 174: 984–992. 10.1093/aje/kwr183 [DOI] [PubMed] [Google Scholar]
- 24.White IR, Royston P, Wood AM (2011) Multiple imputation using chained equations: Issues and guidance for practice. Statistics in Medicine 30: 377–399. 10.1002/sim.4067 [DOI] [PubMed] [Google Scholar]
- 25.MSPP (2015) Etude operationnelle sur la deperdition des cas dans la cascade PTME en Haiti. Port-au-Prince, Haiti: Ministere de la Sante Publique et de la Population, Direction de la Sante Familiale. [Google Scholar]
- 26.Haas AD, Tenthani L, Msukwa MT, Tal K, Jahn A, et al. (2016) Retention in care during the first 3 years of antiretroviral therapy for women in Malawi's option B+ programme: an observational cohort study. The Lancet HIV 3: e175–e182. 10.1016/S2352-3018(16)00008-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.van Lettow M, Bedell R, Mayuni I, Mateyu G, Landes M, et al. (2014) Towards elimination of mother-to-child transmission of HIV: performance of different models of care for initiating lifelong antiretroviral therapy for pregnant women in Malawi (Option B+). Journal of the International AIDS Society 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Llenas-García J, Wikman-Jorgensen P, Hobbins M, Ehmer J, Mbofana F, et al. (2015) Retention in care of HIV-infected pregnant and lactating women starting Option B+ ART in rural Mozambique. International AIDS Society (IAS) Vancouver, Canada. [DOI] [PubMed] [Google Scholar]
- 29.Ford D, Muzambi M, Nkhata MJ, Abongomera G, Joseph S, et al. (2016) Implementation of antiretroviral therapy for life in pregnant/breastfeeding HIV+ women (Option B+) alongside rollout and changing guidelines for ART initiation in rural Zimbabwe: the Lablite Project experience. Journal of Acquired Immune Deficiency Syndromes Dec 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chan AK, Kanike E, Bedell R, Mayuni I, Manyera R, et al. (2016) Same day HIV diagnosis and antiretroviral therapy initiation affects retention in Option B+ prevention of mother-to-child transmission services at antenatal care in Zomba District, Malawi. Journal of the International AIDS Society 19: 20672 10.7448/IAS.19.1.20672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cataldo F, Chiwaula L, Nkhata M, Lettow Mv, Kasende F, et al. (2016) Exploring the experiences of women and health care workers in the context of PMTCT Option B Plus in Malawi. J Acquir Immune Defic Syndr. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kim MH, Zhou A, Mazenga A, Ahmed S, Markham C, et al. (2015) Why did I stop? Barriers and facilitators to uptake and retention in Option B+ HIV care in Lilongwe, Malawi. International AIDS Society (IAS) Vancouver, Canada. [Google Scholar]
- 33.Mukherjee JS, Ivers L, Leandre F, Farmer P, Behforouz H (2006) Antiretroviral Therapy in Resource-Poor Settings: Decreasing Barriers to Access and Promoting Adherence. Journal of Acquired Immune Deficiency Syndromes 43: S123–S126. 10.1097/01.qai.0000248348.25630.74 [DOI] [PubMed] [Google Scholar]
- 34.MSPP. ART Retention Forum led by the Haiti Ministry of Health and the Pan American Health Organization. In: Ministère de la Santé Publique et de la Population PNdLClSP, editor; 2016. September 29–30; Moulin sur Mer, Haiti. [Google Scholar]
- 35.Herce ME, Mtande T, Chimbwandira F, Mofolo I, Chingondole CK, et al. (2015) Supporting Option B+ scale up and strengthening the prevention of mother-to-child transmission cascade in central Malawi: results from a serial cross-sectional study. BMC Infectious Diseases 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rosenberg NE, Mtande TK, Saidi F, Stanley C, Jere E, et al. (2015) Recruiting male partners for couple HIV testing and counselling in Malawi's option B+ programme: an unblinded randomised controlled trial. The Lancet HIV 2: e483–e491. 10.1016/S2352-3018(15)00182-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rosenberg NE, Lettow Mv, Tweya H, Kapito-Tembo A, Bourdon CM, et al. (2014) Improving PMTCT Uptake and Retention Services Through Novel Approaches in Peer-Based Family-Supported Care in the Clinic and Community: A 3-Arm Cluster Randomized Trial (PURE Malawi). J Acquir Immune Defic Syndr 67: S114–S119. 10.1097/QAI.0000000000000319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schmitz K, Scheepers E, Okonji E, Kawooya V (2015) Retaining mother-baby-pairs in care and treatment: the mothers2mothers mentor mother model. International AIDS Society (IAS) Vancouver, Canada. [Google Scholar]
- 39.Sam-Agudu NA, Cornelius LJ, Okundaye JN, Adeyemi OA, Isah Haroun O, et al. (2014) The Impact of Mentor Mother Programs on PMTCT Service Uptake and Retention-in-Care at Primary Health Care Facilities in Nigeria: A Prospective Cohort Study (MoMent Nigeria). J Acquir Immune Defic Syndr 67: S132–S138. 10.1097/QAI.0000000000000331 [DOI] [PubMed] [Google Scholar]
- 40.Kim MH, Ahmed S, Buck WC, Preidis GA, Hosseinipour MC, et al. (2012) The Tingathe programme: a pilot intervention using community health workers to create a continuum of care in the prevention of mother to child transmission of HIV (PMTCT) cascade of services in Malawi. Journal of the International AIDS Society 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sherr K, Gimbel S, Rustagi A, Nduati R, Cuembelo F, et al. (2014) Systems analysis and improvement to optimize pMTCT (SAIA): a cluster randomized trial. Implementation Science 9: 55 10.1186/1748-5908-9-55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Moga T, Ncomanzi T, Mutede B, Tumbare E (2015) Taking lessons learned in the implementation of a clinical mentorship program to increase the confidence of nurses to initiate ART, in support of the roll out of Option B+ in Zimbabwe. International AIDS Society; Vancouver, Canada. [Google Scholar]
- 43.Mutede BR, Musarandega R, Tumbare E, Muponda J, Chinaka E, et al. (2015) Improving retention of young children and women on Option B+/ART at six months in Zimbabwe: A quality improvement approach. International AIDS Society (IAS) Vancouver, Canada. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data for the study were obtained from the iSanté data system with permission of the Haitian Ministry of Health and Population (MSPP), a third party. Analysts who wish to use the data would need to seek permission from the MSPP in Haiti. The Haitian Ministry of Health and Population does not have a formal Data Access Committee for iSanté data as of this writing. To obtain permission to use iSanté data, analysts interested in obtaining the data are advised to contact the following individuals to make a data request: 1. Dr. Paul Adrian, Director, MSPP Division of Epidemiology, Laboratory and Research (DELR): padrien@yahoo.fr; padrien_2004@gmail.com. 2. Mr. Lavoisier Lamothe, MSPP Unit of Planning and Management (UGP): lavoisier.lamothe@ugp.ht. Analysts would need to obtain local ethical approval for data use prior to accessing the data, from the Haitian National Bioethics Committee of the MSPP. The contact for this body is Dr. Gerald Lerebours: gerald_lerebours@hotmail.com.