Abstract
Purpose
Hepatitis C virus (HCV) infection is associated with increased systemic oxidative stress, which leads to cardiovascular events, diabetes, and chronic kidney disease. Similarly, cataract is also associated with increased oxidative stress. The association between HCV infection and increased risk of cataract remains unclear.
Methods
A total of 11,652 HCV-infected patients and 46,608 age- and sex-matched non-HCV infected patients were identified during 2003–2011. All patient data were tracked until a diagnosis of cataract, death, or the end of 2011. Cumulative incidences and hazard ratios (HRs) were calculated.
Results
The mean follow-up durations were 5.29 and 5.86 years for the HCV and non-HCV cohorts, respectively. The overall incidence density rate for cataract was 1.36 times higher in the HCV cohort than in the non-HCV cohort (1.86 and 1.37 per 100 person-y, respectively). After adjusting for age, sex, comorbidities of diabetes, hypertension, hyperlipidemia, asthma, chronic obstructive pulmonary disease, coronary artery disease, and anxiety, patients with HCV infection had an increased risk of cataract compared with those without HCV infection [adjusted HR = 1.23, 95% confidence interval (CI) = 1.14–1.32]. HCV-infected patients receiving interferon–ribavirin therapy had a 1.83 times higher (95% CI = 1.40–2.38) risk of cataract than non-HCV infected patients did.
Conclusion
HCV infection, even without the complication of cirrhosis, is associated with an increased risk of cataract, and this risk is higher in HCV-infected patients undergoing interferon–ribavirin therapy.
Introduction
Cataract, which has an estimated prevalence of 33% in the general population, is the second leading cause of visual impairment and vision loss worldwide [1]. Cataract is associated with increasing incidences of falls and traffic accidents and impair self-care ability and quality of life [2]. Increasing associated medical expenditures makes cataract being a major public health concern [3]. Aging is the major etiology of cataract. Meanwhile, cataract can also be hereditary or result from other eye conditions, trauma, previous eye surgery, long-term steroid use, and several medical conditions such as diabetes [4–6]. The pathogenesis of cataract is complex, and includes photochemical generation of reactive oxygen species, oxidative stress [7], DNA damage, polyol formation [8], osmotic stress, as well as protein misfolding and aggregation [9]. Several experimental animal studies have shown that topical antioxidants such as caffeine, ascorbate, and vitamin Effectively prevent cataract formation and that oxidative stress play a central role in cataract pathogenesis [10 11].
Hepatitis C virus (HCV) infection has an estimated global prevalence of 2.2% and is also a major health concern worldwide [12]. HCV leads to liver cirrhosis, hepatic decompensation, and hepatocellular carcinoma. HCV infection has also various extrahepatic presentations, including renal dysfunction, cardiovascular events, and metabolic syndromes; these extrahepatic presentations are believed to be related with HCV-induced systemic oxidative stress and hyperinsulinemia [13].
Although cataract is also a manifestation of oxidative stress and has long-term effects involving visual impairment, the association between cataract risk and HCV infection has seldom been investigated in detail. Yoshida et al have reported that patients with age-related cataract had significantly higher seropositivity for HCV than an age-matched general population [14]. In this study, we investigated this association by using the National Health Insurance (NHI) database of Taiwan, which is a nationwide longitudinal database with medical claims of 23 million people. Additionally, this study examined whether HCV infection is a risk factor for cataract.
Methods
Data source
In Taiwan, National Health Insurance (NHI) is a single-payer program that has been launched since 1995, covering 98% of the population [15]. National Health Insurance handled a research database (NHIRD), which encompassed patient demographics, diagnosis of disease, mediations, and procedures performed in the hospital and in outpatient claims. Several datasets were extracted from the complete NHIRD for researches purposes. This population-based retrospective cohort study was conducted by analyzing one of the extracted set of NHIRD, the Longitudinal Health Insurance Database 2000 (LHID2000) of the NHI program. The details of the NHI program and LHID2000 have been well-addressed previously [16, 17].
Ethics statement
The NHIRD encrypts patient personal information to protect privacy and provides researchers with anonymous identification numbers associated with relevant claims information, including sex, date of birth, medical services received, and prescriptions. Therefore, patient consent is not required to access the NHIRD. This study was approved to fulfill the condition for exemption by the Institutional Review Board (IRB) of China Medical University (CMUH104-REC2-115-CR1). The IRB also specifically waived the consent requirement.
Sample
We conducted a retrospective cohort of HCV infected patients from the one million patients in representative LHID 2000 sample dataset. The HCV cohort contained adult patients newly diagnosed with HCV infection (ICD-9-CM codes 070.41, 070.44, 070.51, 070.54, and V02.62) from January 1, 2000, to December 31, 2010; the date of first diagnosis was set as the index date. We excluded patients with a history of hepatitis B virus infections (ICD-9-CM codes 070.20, 070.22, 070.30, 070.32, and V02.61) and cataract (ICD-9-CM code 366) in medical records before index date. The comparison cohort was comprised of non-HCV infection patients. To control for the confounding effects of age (every 5-year span), sex, and index year, we constructed a matched variable containing the age at index data and sex for each HCV infection patient from the comparison cohort. All participants were followed from the index date until the date of cataract diagnosis, withdrawal from the NHI program, death, or the end of 2011 (December 31, 2011).
Comorbidity and medication
All confounding variables were defined according to the diagnosis before the index date in HCV and non-HCV patients. Cirrhosis was defined as three outpatient claims with ICD-9-CM codes 571.2, 571.5, and 571.6. Diabetes was defined as defined as three outpatient claims with ICD-9-CM code 250. The following confounding variables for cataract all defined as three outpatients claims with the corresponding ICD-9 code: hypertension (ICD-9-CM codes 401–405), hyperlipidemia (ICD-9-CM code 272), asthma (ICD-9-CM code 493), chronic obstructive pulmonary disease (COPD; ICD-9-CM codes 491, 492, and 496), coronary artery disease (ICD-9-CM codes 410–414), alcohol-related illness (ICD-9-CM codes 291, 303, 305, 571.0, 571.1, 571.2, 571.3, 790.3, A215, and V11.3), and anxiety (ICD-9-CM code 300.00).
Individuals in the HCV group would visit clinics more regularly than those in the non-HCV group, thus HCV patients may be more likely to be diagnosed with cataract at an earlier stage. To alleviate such surveillance bias, frequency of clinical visits was added as confounding variable.
Regimen of HCV infection, including ribavirin, interferon alpha, and interferon alpha-ribavirin combination therapy were classified for evaluating the effects of the HCV therapy on risk of cataract.
Statistical analysis
Data on the HCV and non-HCV cohorts involving age, follow-up duration, number and proportion of age group, sex, and comorbidity are presented as means and deviations (SDs). We applied a t test for age and follow-up duration and the chi-square test for age group, sex, and comorbidity. The Kaplan–Meier curve analysis was performed to reveal the cumulative incidence of cataract, and the log-rank test was applied to identify the differences between the HCV and non-HCV cohorts. The incidence density rate of cataract was estimated as the number of cataract events identified during the follow-up duration divided by the total follow-up person-years. Univariate and multivariate Cox proportion hazard regression models were used to examine the effect of HCV infection on cataract risk through hazard ratios (HRs) with their respective 95% confidence intervals (CIs). The multivariable models included all statistically significant risk factors identified in the univariable Cox model. SAS 9.4 software (SAS Institute, Cary, NC, USA) was used to analyze all data. All statistical analyses were performed with a two-tailed significance level of 0.05.
Results
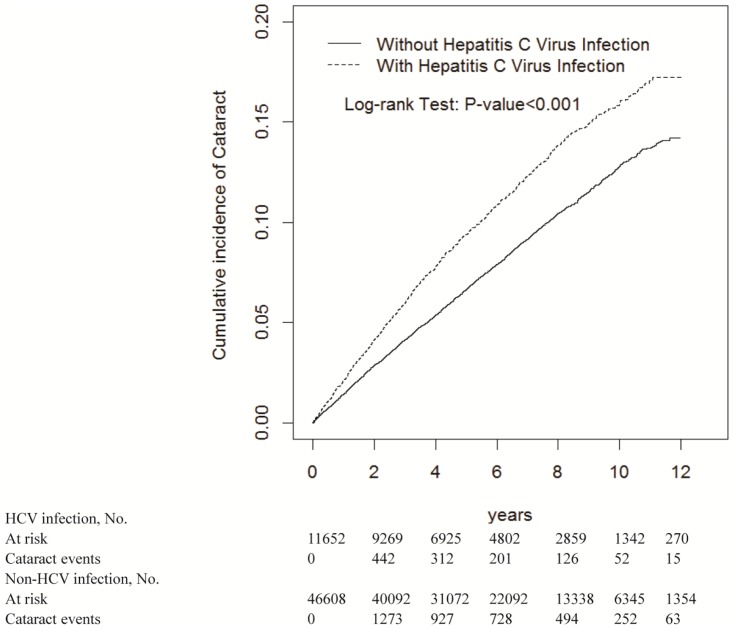
We have enrolled a total of 11,652 HCV patients and 46,608 non-HCV patients. The mean follow-up duration was 5.29 and 5.86 years for the HCV and non-HCV cohorts, respectively. Similar characteristics were recognized in both cohorts which 55.0% were men and 48.3% of participants were aged ≤49 years. The mean ages of the HCV and non-HCV cohorts were 50.5±14.3 and 50.0±14.6 years, respectively. The prevalence of cirrhosis, hypertension, diabetes, hyperlipidemia, asthma, chronic obstructive pulmonary disease, coronary artery disease, alcohol-related illness, and anxiety were significantly in HCV patients, compared with non-HCV patients (p <0.001) (Table 1). Kaplan–Meier curve analysis revealed that the HCV cohort exhibited a higher cumulative cataract incidence than the non-HCV cohort did (log-rank test, p <0.001; Fig 1).
Table 1. Demographic characteristics and comorbidities in cohorts with and without HCV infection.
| HCV infection | |||
|---|---|---|---|
| No | Yes | ||
| Variable | N = 46608 | N = 11652 | p-value |
| Age, year | 0.99 | ||
| ≤ 49 | 22532(48.3) | 5633(48.3) | |
| 50–64 | 16420(35.2) | 4105(35.2) | |
| 65+ | 7656(16.4) | 1914(16.4) | |
| Mean(SD)† | 50.0(14.6) | 50.5(14.3) | 0.01 |
| Average number of clinic visits/per year, Mean(SD) | 13.5(12.8) | 21.0(16.4) | <0.001 |
| Sex | 0.99 | ||
| Female | 20992(45.0) | 5248(45.0) | |
| Male | 25616(55.0) | 6404(55.0) | |
| Comorbidity | |||
| Cirrhosis | 167(0.36) | 1391(11.9) | <0.001 |
| Diabetes | 2904(6.23) | 1523(13.1) | <0.001 |
| Hypertension | 11561(24.8) | 3892(33.4) | <0.001 |
| Hyperlipidemia | 7175(15.4) | 2575(22.1) | <0.001 |
| Asthma | 2252(4.83) | 856(7.35) | <0.001 |
| COPD | 3372(7.23) | 1394(12.0) | <0.001 |
| Coronary artery disease | 4822(10.4) | 1857(15.9) | <0.001 |
| Alcohol-related illness | 1322(2.84) | 1312(11.3) | <0.001 |
| Anxiety | 2269(4.87) | 1158(9.94) | <0.001 |
Chi-Square Test;
†: T-Test
Fig 1. Cummulative incidence comparison of cataract for patients with (dashed line) or without (solid line) HCV infection.
There were 1,148 cataract events among 11,752 HCV patients and 3,738 cataract events among 46,608 non-HCV patients. The incidence of cataract was 1.86% (1,148/61,686) in HCV patients. The overall incidence density rate of cataract in the HCV cohort was 1.36 times higher in the non-HCV cohort (1.86 vs. 1.37 per 100 person-y; Table 2). Women had a higher risk of cataract than men (adjusted HR (aHR) = 1.22, 95% CI = 1.15–1.29). After adjusting for age, sex, and comorbidities of diabetes, hypertension, hyperlipidemia, asthma, COPD, coronary artery disease, and anxiety, patients in the HCV cohort were associated with an increased risk of cataract compared with those in the non-HCV cohort [adjusted HR (aHR) = 1.23, 95% CI = 1.14–1.32]. Compared with patients aged ≤49 years, the risk of cataract was 9.23 times higher in those aged 50–64 years (95% CI = 8.29–10.3) and 15.2 times higher in those aged ≥65 years (95% CI = 13.5–17.0). In HCV infection patients, comorbidities independently associated with cataract were diabetes (aHR = 1.41, 95% CI = 1.30–1.52), hypertension (aHR = 1.13, 95% CI = 1.06–1.21), hyperlipidemia (aHR = 1.25, 95% CI = 1.17–1.34), and COPD (aHR = 1.12, 95% CI = 1.03–1.22).
Table 2. The incidence and hazard ratio for cataract and cataract-associated risk factor.
| Variable | Event | PY | Rate# | Crude HR(95% CI) | Adjusted HR† (95% CI) |
|---|---|---|---|---|---|
| HCV infection | |||||
| No | 3738 | 273224 | 1.37 | 1.00 | 1.00 |
| Yes | 1148 | 61686 | 1.86 | 1.36(1.27, 1.45)*** | 1.23(1.14, 1.32)*** |
| Age, year | |||||
| ≤ 49 | 396 | 181705 | 0.22 | 1.00 | 1.00 |
| 50–64 | 2686 | 11684 | 2.41 | 11.1(10.0, 12.4)*** | 9.20(8.27, 10.3)*** |
| 65+ | 1804 | 41520 | 4.34 | 20.2(18.1, 22.6)*** | 14.8(13.2, 16.6)*** |
| Sex | |||||
| Female | 2653 | 153042 | 1.73 | 1.41(1.34, 1.50)*** | 1.22(1.15, 1.29)*** |
| Male | 2233 | 181868 | 1.23 | 1.00 | 1.00 |
| Comorbidity | |||||
| Cirrhosis | |||||
| No | 4728 | 329724 | 1.43 | 1.00 | 1.00 |
| Yes | 158 | 5186 | 3.05 | 2.09(1.79, 2.45)*** | 1.09(0.92, 1.29) |
| Diabetes | |||||
| No | 4079 | 315418 | 1.29 | 1.00 | 1.00 |
| Yes | 807 | 19472 | 4.14 | 3.18(2.94, 3.43)*** | 1.41(1.30, 1.52)*** |
| Hypertension | |||||
| No | 2458 | 258134 | 0.95 | 1.00 | 1.00 |
| Yes | 2428 | 76776 | 3.16 | 3.31(3.13, 3.51)*** | 1.13(1.06, 1.21)*** |
| Hyperlipidemia | |||||
| No | 3341 | 285523 | 1.17 | 1.00 | 1.00 |
| Yes | 1545 | 49386 | 3.13 | 2.66(2.51, 2.83)*** | 1.25(1.17, 1.34)*** |
| Asthma | |||||
| No | 4445 | 320217 | 1.39 | 1.00 | 1.00 |
| Yes | 441 | 14693 | 3.00 | 2.14(1.94, 2.37)*** | 1.01(0.91, 1.13) |
| COPD | |||||
| No | 4125 | 312943 | 1.32 | 1.00 | 1.00 |
| Yes | 761 | 21967 | 3.46 | 2.61(2.41, 2.82)*** | 1.12(1.03, 1.22)** |
| Coronary artery disease | |||||
| No | 3670 | 302987 | 1.21 | 1.00 | 1.00 |
| Yes | 1216 | 31923 | 3.81 | 3.13(2.93, 3.34)*** | 0.97(0.87, 1.08) |
| Alcohol-related illness | |||||
| No | 4486 | 319089 | 1.41 | 1.00 | 1.00 |
| Yes | 400 | 15821 | 2.53 | 0.86(0.73, 1.02) | - |
| Anxiety | |||||
| No | 4743 | 323774 | 1.46 | 1.00 | 1.00 |
| Yes | 143 | 11136 | 1.28 | 1.78(1.60, 1.97)*** | 0.97(0.87, 1.08) |
Rate#, incidence rate, per 100 person-years; Crude HR, relative hazard ratio; Adjusted HR†: multivariable analysis including age, average number of clinic visits/per year, and comorbidities of diabetes, hypertension, hyperlipidemia, asthma, COPD, coronary artery disease, and anxiety;
** p < 0.01,
*** p<0.001
The incidence of cataract increased with age in both cohorts (Table 3). HCV patients had significant higher risk of cataract across all age categories including aged ≤49 years (aHR = 1.47, 95% CI = 1.18–1.84), 50–64 years (aHR = 1.30, 95% CI = 1.18–1.42), and ≧65 years(aHR = 1.20, 95% CI = 1.06–1.36). The stratification analysis of sex showed that HCV cohort had increasing risks of cataract for both women (aHR = 1.37, 95% CI = 1.24–1.50) and men (aHR = 1.24, 95% CI = 1.11–1.37). The stratification analysis of comorbidities showed that the risk of cataract was aHR = 1.26 (95% CI = 1.16–1.35) for HCV cohort with comorbidities and aHR = 1.45 (95% CI = 1.25–1.69) for HCV cohorts without comorbidities. The interaction analysis of HCV infection and confounding variables showed that significant interaction between HCV infection and diabetes (P for interaction < 0.001), hyperlipidemia (P for interaction = 0.003), and alcohol related disease (P for interaction = 0.003).
Table 3. Incidence of cataract by age, sex and comorbidity and Cox model measured hazards ratio for patients with HCV infection compared those without HCV infection.
| HCV infection | ||||||||
|---|---|---|---|---|---|---|---|---|
| No | Yes | |||||||
| Variables | Event | PY | Rate# | Event | PY | Rate# | Crude HR (95% CI) | Adjusted HR† (95% CI) |
| Age, years | ||||||||
| ≤ 49 | 266 | 146547 | 0.18 | 130 | 35159 | 0.37 | 2.05(1.66, 2.53)*** | 1.47(1.18, 1.84)*** |
| 50–64 | 2026 | 91782 | 2.21 | 660 | 19902 | 3.32 | 1.52(1.39, 1.66)*** | 1.30(1.18, 1.42)*** |
| 65+ | 1446 | 34896 | 4.14 | 358 | 6624 | 5.40 | 1.28(1.14, 1.44)*** | 1.20(1.06, 1.36)** |
| P for interaction | 0.12 | |||||||
| Sex | ||||||||
| Female | 2007 | 124459 | 1.61 | 646 | 28583 | 2.26 | 1.40(1.28, 1.53)*** | 1.37(1.24, 1.50)*** |
| Male | 1731 | 148765 | 1.16 | 502 | 33103 | 1.52 | 1.30(1.18, 1.44)*** | 1.24(1.11, 1.37)*** |
| P for interaction | 0.28 | |||||||
| Comorbidity‡ | ||||||||
| No | 1235 | 178228 | 0.69 | 195 | 27008 | 0.72 | 1.04(0.90, 1.21) | 1.45(1.25, 1.69)*** |
| Yes | 2503 | 94996 | 2.63 | 953 | 34678 | 2.75 | 1.04(0.97, 1.12) | 1.26(1.16, 1.35)*** |
| P for interaction | 0.99 | |||||||
| Cirrhosis | ||||||||
| No | 3719 | 272567 | 1.36 | 1009 | 57157 | 1.77 | 1.29(1.21, 1.39)*** | 1.32(1.23, 1.42)*** |
| Yes | 19 | 657 | 2.89 | 139 | 4529 | 3.07 | 1.03(0.64, 1.66) | 1.02(0.63, 1.66) |
| P for interaction | 0.41 | |||||||
| Diabetes | ||||||||
| No | 3189 | 260099 | 1.23 | 890 | 55319 | 1.61 | 1.31(1.22, 1.41)*** | 1.35(1.25, 1.45)*** |
| Yes | 549 | 13125 | 4.18 | 258 | 6367 | 4.05 | 0.97(0.84, 1.12) | 1.09(0.94, 1.27) |
| P for interaction | <0.001 | |||||||
| Hypertension | ||||||||
| No | 1935 | 214096 | 0.90 | 523 | 44038 | 1.19 | 1.32(1.20, 1.45)*** | 1.46(1.32, 1.62)*** |
| Yes | 1803 | 59128 | 3.05 | 625 | 17648 | 3.54 | 1.16(1.06, 1.27)** | 1.20(1.09, 1.32)*** |
| P for interaction | 0.07 | |||||||
| Hyperlipidemia | ||||||||
| No | 2622 | 236880 | 1.11 | 719 | 48643 | 1.48 | 1.33(1.23, 1.45)*** | 1.31(1.20, 1.43)*** |
| Yes | 1116 | 36344 | 3.07 | 429 | 13043 | 3.29 | 1.07(0.96, 1.20) | 1.24(1.11, 1.39)*** |
| P for interaction | 0.002 | |||||||
| Asthma | ||||||||
| No | 3429 | 262296 | 1.31 | 1016 | 57921 | 1.75 | 1.34(1.25, 1.44)*** | 1.31(1.22, 1.42)*** |
| Yes | 309 | 10928 | 2.83 | 132 | 3765 | 3.51 | 1.23(1.01, 1.51)* | 1.28(1.03, 1.58)* |
| P for interaction | 0.47 | |||||||
| COPD | ||||||||
| No | 3213 | 257267 | 1.25 | 912 | 55676 | 1.64 | 1.31(1.22, 1.41)*** | 1.31(1.21, 1.42)*** |
| Yes | 525 | 15957 | 3.29 | 236 | 6010 | 3.93 | 1.19(1.02, 1.39)* | 1.26(1.07, 1.47)** |
| P for interaction | 0.28 | |||||||
| Coronary artery disease | ||||||||
| No | 2871 | 249646 | 1.15 | 799 | 53341 | 1.50 | 1.30(1.20, 1.41)*** | 1.34(1.23, 1.46)*** |
| Yes | 867 | 23578 | 3.68 | 349 | 8345 | 4.18 | 1.13(1.00, 1.28) | 1.20(1.05, 1.37)** |
| P for interaction | 0.07 | |||||||
| Alcohol-related illness | ||||||||
| No | 3660 | 267617 | 1.37 | 1083 | 56157 | 1.93 | 1.41(1.32, 1.51)*** | 1.35(1.25, 1.45)*** |
| Yes | 78 | 5607 | 1.39 | 65 | 5529 | 1.18 | 0.84(0.61, 1.17) | 0.98(0.69, 1.40) |
| P for interaction | 0.003 | |||||||
| Anxiety | ||||||||
| No | 3488 | 262698 | 1.33 | 998 | 56392 | 1.77 | 1.33(1.24, 1.43)*** | 1.29(1.19, 1.39)*** |
| Yes | 250 | 10527 | 2.37 | 150 | 5294 | 2.83 | 1.19(0.97, 1.46) | 1.45(1.18, 1.80)*** |
| P for interaction | 0.32 | |||||||
Rate#, incidence rate, per 100 person-years; Crude HR, relative hazard ratio; Adjusted HR†: multivariable analysis including age, average number of clinic visits/per year, and comorbidities of diabetes, hypertension, hyperlipidemia, asthma, COPD, coronary artery disease, and anxiety;
Comorbidity‡: Only to have one of comorbidities (including cirrhosis, diabetes, hypertension, hyperlipidemia, asthma, COPD, coronary artery disease, alcohol-related illness and anxiety) classified as the comorbidity group
* p < 0.05,
** p<0.01,
*** p<0.001
Table 4 presents the results of the analysis of the effects of different therapy regimens of HCV infection on the risk of cataract, with the non-HCV cohort as comparison. The onset of cataract from the initiation of HCV treatment was 3.08(SD = 2.66). Compared with non-HCV cohort, associated with risk of cataract was no treatment (aHR = 1.29, 95% CI = 1.20–1.39), interferon-alpha only (aHR = 1.29, 95% CI = 1.20–1.39), and interferon-ribavirin combination (aHR = 1.83, 95% CI = 1.40–2.38). Subgroup analysis showed that HCV patients without cirrhosis receiving interferon-ribavirin combination had significantly higher risk of cataract (aHR = 1.70, 95% CI = 1.28–2.25), compared with non-HCV cohort.
Table 4. Incidence, and hazard ratio of cataract between patients with HCV infection with and without treatment.
| Variables | N | Event | PY | Rate# | Crude HR(95% CI) | Adjusted HR† (95% CI) |
|---|---|---|---|---|---|---|
| Without HCV infection | 46608 | 3738 | 273224 | 1.37 | 1(Reference) | 1(Reference) |
| HCV infection without treatment | 11198 | 1092 | 58918 | 1.85 | 1.35(1.26, 1.45)** | 1.29(1.20, 1.39)*** |
| HCV infection with interferon only | 27 | 6 | 127 | 4.47 | 3.44(1.55, 7.66)*** | 1.29(1.20, 1.39)*** |
| HCV infection with Ribavirin only | 10 | 0 | 76 | 0.00 | - | - |
| HCV infection with interferon-Ribavirin combination | 454 | 56 | 2768 | 2.02 | 1.49(1.14, 1.93)** | 1.83(1.40, 2.38)*** |
| Without Cirrhosis | ||||||
| Without HCV infection | 46441 | 3719 | 272567 | 1.36 | 1(Reference) | 1(Reference) |
| HCV infection without treatment | 9835 | 960 | 54509 | 1.76 | 1.29(1.20, 1.38)*** | 1.30(1.21, 1.40)*** |
| HCV infection with interferon-Ribavirin combination | 426 | 49 | 2648 | 1.85 | 1.36(1.03, 1.81)* | 1.70(1.28, 2.25)*** |
Rate#, incidence rate, per 100 person-years; Crude HR, relative hazard ratio; Adjusted HR†: multivariable analysis including age, average number of clinic visits/per year, and comorbidities of diabetes, hypertension, hyperlipidemia, asthma, COPD, coronary artery disease, and anxiety;
* p < 0.05,
** p<0.01,
*** p<0.001
Table 5 showed that synergistic effects of HCV with diabetes, hyperlipidemia, and alcohol-related illness on the risk of cataract. Diabetic HCV patients had increasing risk of cataract (aHR = 1.69, 95% CI = 1.48–1.93), compared with non-HCV and non-diabetic patients. HCV patients with accompanying hyperlipidemia had 1.67-fold higher risk of cataract, compared with non-HCV and non-hyperlipidemia patients.
Table 5. Cox method estimated hazard ratios of cataract associated HCV infection and comorbidity.
| Variables | Crude HR(95% CI) | Adjusted HR† (95% CI) | p-value# | |
|---|---|---|---|---|
| HCV infection | Diabetes | <0.001 | ||
| No | No | 1(Reference) | 1(Reference) | |
| No | Yes | 3.39(3.09, 3.71)*** | 1.44(1.15, 1.34)*** | |
| Yes | No | 1.31(1.22, 1.41)*** | 1.24(1.15, 1.34)*** | |
| Yes | Yes | 3.28(2.89, 3.72)*** | 1.69(1.48, 1.93)*** | |
| HCV infection | Hyperlipidemia | 0.002 | ||
| No | No | 1(Reference) | 1(Reference) | |
| No | Yes | 2.76(2.57, 2.96)*** | 1.39(1.29, 1.50)*** | |
| Yes | No | 1.33(1.23, 1.45)*** | 1.24(1.13, 1.35)*** | |
| Yes | Yes | 2.96(2.67, 3.28)*** | 1.67(1.50, 1.87)*** | |
| HCV infection | Alcohol-related illness | |||
| No | No | 1(Reference) | 1(Reference) | 0.003 |
| No | Yes | 1.00(0.80, 1.25) | 1.02(0.81, 1.28) | |
| Yes | No | 1.41(1.32, 1.51)*** | 1.24(1.15, 1.33)*** | |
| Yes | Yes | 0.85(0.66, 1.08) | 1.04(0.81, 1.330 | |
Crude HR, relative hazard ratio;
† Model was adjusted for age, average number of clinic visits/per year, and comorbidities of diabetes, hypertension, hyperlipidemia, asthma, COPD, coronary artery disease, and anxiety;
#p-value for interaction;
*** p < 0.001
Discussion
In this population-based cohort study, we observed that HCV infection, even in the absence of cirrhosis, was associated with an increased risk of cataract. HCV-infected patients receiving interferon–ribavirin therapy had a 1.83 times higher risk of cataract compared with those without HCV infection and not receiving interferon–ribavirin therapy. This suggested that all patients with HCV, regardless of receiving HCV treatment, should be closely monitored for subsequent cataract development.
Even after adjusting for major confounding variables such as age and diabetes, HCV infection remained independently associated with cataract. The effects of HCV on ocular manifestations have been seldom studied. We hypothesized that the pathogenic mechanism connecting HCV infection and cataract is oxidative stress. Evidence has increasingly indicated that HCV infection-induced oxidative stress is systemic and not limited to the liver [18–21]. HCV-infected patients have high insulin resistance and oxidative stress, which increases their risk of coronary artery disease [22], chronic kidney disease [23], glucose metabolism derangements, and diabetes [24]. Similarly, HCV may induce high oxidative stress within the lens, initiating a cascade of free radial formation, oxidized material accumulation, and ultimately, cataract development. Because we have adjusted our data for diabetes, a major risk factor for cataract and a recognized sequela of HCV infection, our results clearly demonstrated an independent association between HCV infection and cataract.
In this study, we observed a positive association between pegylated interferon and ribavirin therapy for HCV infection and risk of cataract. Our data showed that HCV patients receiving interferon alpha-only regimen had 1.29-fold higher risk of cataract, interferon alpha-ribavirin regimen had 1.83-fold higher risk of cataract, compared with non-HCV patients. Thus, the regimen combining Interferon alpha and ribavirin would have addictive and synergistic effects on the risk of developing cataract. Few case reports and studies with small sample sizes have reported similar findings [25, 26]. This current large-scale cohort study clearly demonstrated that pegylated interferon and ribavirin therapy for HCV infection is associated with a 1.83 times higher risk of cataract. Interferon-associated retinopathy has been described in the literature [27, 28], interferon alfa–ribavirin-associated cataract has seldom been mentioned. There are several possible explanations accounting for the association between HCV therapy and cataract. One supposed pathway would be direct effects of interferon/ribavirin treatment or to HCV itself. It has been reported that about 0.5% patients taking ribavirin have cataract, with the incidence being up to 55.36% during the initial 6 months of ribavirin use, and the most co-used medication in these cataract patients was interferon alfa-2a [29]. The sample size of our HCV patients receiving ribavirin only was too small to determine whether ribavirin would be direct related to the risk of cataract. Further prospective randomized control study would be needed to classify the association between ribavirin and cataract.
Our HCV cohort had significantly higher prevalence of comorbidities compared with non-HCV cohort. Our interaction analysis showed that HCV interacted with diabetes, hyperlipidemia, and alcohol-related illness on the increasing risk of cataract. HCV patients had diabetes had highest risk of cataract, among those with or without HCV or diabetes. Our data demonstrated that clinical relevance of HCV infection is not equal to those of the other confounding factors of high oxidative stress, including diabetes and aging.
The other possible explanations would be competing risk between HCV-related mortality and cataract. It would be reasonable to speculate that systemic oxidative stress would be positively associated with the degree of HCV infection. However, in our stratified analysis, there was no significant difference in developing cataract between cirrhosis and non-cirrhosis. Thus, we suppose that there are competing risk between cataract and HCV- related morbidity and mortality in those HCV decompensation patients. On the other hand, current guideline indicated therapy should be considered for all compensated treatment—naïve HCV patients [30]. Thus, possible bias resulting from competing risk would be minimized in those HCV patients receiving interferon–ribavirin therapy since those who receiving therapy all had compensated liver function. However, possible selection bias would exist since viral load of HCV, genotype of HCV, status of liver reserve, and mounted immune response to therapy, these possible predisposing factors of cataract, of whom receiving interferon/ribavirin would be different at baseline from those HCV patients not receiving interferon/ribavirin. Further large scale prospective study would be required to investigate the association between HCV treatment and cataract.
This study has several limitations. First, information regarding the cataract subtype and family history of each cataract patient was unavailable. Second, details regarding smoking habit, vitamin supplement use, ultraviolet exposure, and sun protection were not available from the ICD-9-CM codes. Third, because the NHIRD encrypts patient personal information to protect privacy, no records regarding HCV genotype, viral load of HCV, serological response to HCV treatment, and severity of cataract were available; therefore, a direct and substantial dose–response relationship between HCV infection and cataract could not be established. Nevertheless, we controlled for possible confounding variables such as frequency of clinical visits, diabetes, coronary artery disease, hypertension, hyperlipidemia, COPD, asthma, and alcohol-related illness; thereafter, HCV infection was found to be independently associated with risk of cataract. Finally, treatment outcome, i.e. response to HCV therapy was unavailable in NHIRD. However, lack of treatment outcome of HCV therapy would not have effects on our conclusion that HCV infection is associated with an increased risk of cataract especially undergoing interferon–ribavirin therapy.
In conclusion, the current nationwide cohort study revealed that HCV-infected patients have an increased risk of cataract. Furthermore, HCV-infected patients receiving interferon–ribavirin therapy have a 1.83 times higher prevalence of cataract than those without HCV infection. Considering the surgical curability of cataract and serious HCV infection-related morbidity, we do not discourage the use of anti-HCV therapy for HCV-infected patients. Instead, we recommend routine screening of these HCV patients for ocular problems, especially those received interferon alpha–ribavirin therapy.
Supporting information
(DOC)
Abbreviations
- CI
confidence interval
- HCV
Hepatitis C virus
- HR
hazard ratio
- LHID2000
Longitudinal Health Insurance Database 2000
- SD
standard deviation
Data Availability
All data and related metadata were deposited in an appropriate public repository in the National Health Research Institutes (NHRI, http://english.nhri.org.tw/NHRI_WEB/nhriw001Action.do). The data on the study population that were obtained from the NHIRD (http://nhird.nhri.org.tw/en/index.html) are maintained in the NHIRD (http://nhird.nhri.org.tw/). The NHRI is a nonprofit foundation established by the government. Only citizens of the Republic of China (Taiwan) who fulfill the requirements of conducting research projects are eligible to apply for the NHIRD. The use of NHIRD is limited to research purposes only. Applicants must follow the Computer-Processed Personal Data Protection Law (http://www.winklerpartners.com/?p=987) and related regulations of National Health Insurance Administration and NHRI, and an agreement must be signed by the applicant and his/her supervisor upon application submission. All applications are reviewed for approval of data release. We also confirm that the data used in our study are third party data that are not owned by and have not been collected by the authors. In addition, these authors do not have special access privileges to these data.
Funding Statement
This study is supported in part by Taiwan Ministry of Health and Welfare Clinical Trial and Research Center of Excellence (MOHW105-TDU-B-212-133019), China Medical University Hospital, Academia Sinica Taiwan Biobank Stroke Biosignature Project (BM10501010037), NRPB Stroke Clinical Trial Consortium (MOST105-2325-B-039-003), Tseng-Lien Lin Foundation, Taichung, Taiwan, Taiwan Brain Disease Foundation, Taipei, Taiwan, and Katsuzo and Kiyo Aoshima Memorial Funds, Japan. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. No additional external funding received for this study.
References
- 1.Pascolini D, Mariotti SP. Global estimates of visual impairment: 2010. Br J Ophthalmol. 2012; 96:614–8. 10.1136/bjophthalmol-2011-300539 [DOI] [PubMed] [Google Scholar]
- 2.Chi MJ, Lee CY, Wu SC. The prevalence of chronic conditions and medical expenditures of the elderly by chronic condition indicator (CCI). Arch Gerontol Geriatr. 2011;52:284–9. [DOI] [PubMed] [Google Scholar]
- 3.Grimes CE, Henry JA, Maraka J, Mkandawire NC, Cotton M. Cost-effectiveness of surgery in low-and middle-income countries: a systematic review. World J Surg. 2014;38:252–63. 10.1007/s00268-013-2243-y [DOI] [PubMed] [Google Scholar]
- 4.Shiels A, Hejtmancik J. Genetics of human cataract. Clin Genet. 2013;84:120–27 10.1111/cge.12182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vashist P, Talwar B, Gogoi M, Maraini G, Camparini M, Ravindran RD, et al. Prevalence of cataract in an older population in India: the India study of age-related eye disease. Ophthalmology 2011;118:272–78. e2 10.1016/j.ophtha.2010.05.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Thorne JE, Woreta FA, Dunn JP, Jabs DA. Risk of cataract development among children with juvenile idiopathic arthritis-related uveitis treated with topical corticosteroids. Ophthalmology 2010;117:1436–41 10.1016/j.ophtha.2009.12.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Varma SD, Chand D, Sharma YR, Kuck JF Jr, Richards RD. Oxidative stress on lens and cataract formation: role of light and oxygen. Curr Eye Res. 1984;3:35–57. [DOI] [PubMed] [Google Scholar]
- 8.Pollreisz A, Schmidt-Erfurth U. Diabetic cataract—pathogenesis, epidemiology and treatment. J Ophthalmol. 2010;2010:608751 10.1155/2010/608751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Moreau KL, King JA. Protein misfolding and aggregation in cataract disease and prospects for prevention. Trends Mol Med. 2012;18:273–82. 10.1016/j.molmed.2012.03.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Williams DL. Oxidation, antioxidants and cataract formation: a literature review. Vet Ophthalmol. 2006;9:292–98 10.1111/j.1463-5224.2006.00498.x [DOI] [PubMed] [Google Scholar]
- 11.Babizhayev MA, Deyev AI, Yermakova VN, Semiletov YA, Davydova NG, Kurysheva NI, et al. N-Acetylcarnosine, a natural histidine-containing dipeptide, as a potent ophthalmic drug in treatment of human cataracts. Peptides 2001;22:979–94 [DOI] [PubMed] [Google Scholar]
- 12.Alter MJ. Epidemiology of hepatitis C virus infection. World J Gastroenterol. 2007;13:2436–41 10.3748/wjg.v13.i17.2436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lonardo A, Adinolfi LE, Loria P, Carulli N, Ruggiero G, Day CP. Steatosis and hepatitis C virus: mechanisms and significance for hepatic and extrahepatic disease. Gastroenterology 2004;126:586–97 [DOI] [PubMed] [Google Scholar]
- 14.Yoshida K, Nakano H, Yoshitomi F, Oshika T. Prevalence of seropositivity for hepatitis C virus in cataract patients and the general population. J Cataract Refract Surg. 2002; 28:1789–92. [DOI] [PubMed] [Google Scholar]
- 15.Chen PC, Muo CH, Lee YT, Yu YH, Sung FC. Lung cancer and incidence of stroke: a population-based cohort study. Stroke. 2011;42:3034–9. 10.1161/STROKEAHA.111.615534 [DOI] [PubMed] [Google Scholar]
- 16.Lin SY, Lin CL, Chang YJ, Hsu WH, Lin CC, Wang IK, et al. Association Between Kidney Stones and Risk of Stroke: A Nationwide Population-Based Cohort Study. Medicine 2016;95:e2847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lin SY, Lin CL, Tseng CH, Chang YJ, Wang IK, Yeh HC, et al. Association Between Chronic Osteomyelitis and Risk of End-Stage Renal Disease: A Nationwide Population-Based Cohort Study. Medicine 2015;94:e1141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sumida Y, Nakashima T, Yoh T, Nakajima Y, Ishikawa H, Mitsuyoshi H, et al. Serum thioredoxin levels as an indicator of oxidative stress in patients with hepatitis C virus infection. J Hepatol. 2000;33:616–22. [DOI] [PubMed] [Google Scholar]
- 19.Levent G, Ali A, Ahmet A, Polat EC, Aytaç C, Ayşe E, et al. Oxidative stress and antioxidant defense in patients with chronic hepatitis C patients before and after pegylated interferon alfa-2b plus ribavirin therapy. J Transl Med. 2006;4:25 10.1186/1479-5876-4-25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gong G, Waris G, Tanveer R, Siddiqui A. Human hepatitis C virus NS5A protein alters intracellular calcium levels, induces oxidative stress, and activates STAT-3 and NF-κB. Proc Natl Acad Sci U S A. 2001;98:9599–604. 10.1073/pnas.171311298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.El-Zayadi A-R, Anis M. Hepatitis C virus induced insulin resistance impairs response to anti viral therapy. World J Gastroenterol. 2012;18:212–24. 10.3748/wjg.v18.i3.212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Roed T, Lebech AM, Kjaer A, Weis N. Hepatitis C virus infection and risk of coronary artery disease: a systematic review of the literature. Clin Physiol Funct Imaging. 2012;32:421–30. 10.1111/j.1475-097X.2012.01152.x [DOI] [PubMed] [Google Scholar]
- 23.Chen YC, Lin HY, Li CY, Lee MS, Su YC. A nationwide cohort study suggests that hepatitis C virus infection is associated with increased risk of chronic kidney disease. Kidney Int. 2014;85:1200–7. 10.1038/ki.2013.455 [DOI] [PubMed] [Google Scholar]
- 24.Negro F, Alaei M. Hepatitis C virus and type 2 diabetes. World J Gastroenterol. 2009;15:1537–47. 10.3748/wjg.15.1537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ku JY, Sharma A. Pegylated interferon and ribavirin therapy for hepatitis C causing cataract. Clin Exp Ophthalmol. 2009;37:743–5. 10.1111/j.1442-9071.2009.02125.x [DOI] [PubMed] [Google Scholar]
- 26.Farel C, Suzman DL, McLaughlin M, Campbell C, Koratich C, Masur H, et al. Serious ophthalmic pathology compromising vision in HCV/HIV co-infected patients treated with peginterferon alpha-2b and ribavirin. AIDS 2004;18:1805–09 [DOI] [PubMed] [Google Scholar]
- 27.Jain K, Lam WC, Waheeb S, Thai Q, Heathcote J. Retinopathy in chronic hepatitis C patients during interferon treatment with ribavirin. Br J Ophthalmol. 2001;85:1171–3. 10.1136/bjo.85.10.1171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Okuse C, Yotsuyanagi H, Nagase Y, Kobayashi Y, Yasuda K, Koike K, et al. Risk factors for retinopathy associated with interferon alpha-2b and ribavirin combination therapy in patients with chronic hepatitis C. World J Gastroenterol. 2006;12:3756–9. 10.3748/wjg.v12.i23.3756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Review: could Ribavirin cause Cataract? http://www.ehealthme.com/ds/ribavirin/cataract/ last modified June 2, 2016
- 30.Cosman F, de Beur SJ, LeBoff MS, Lewiecki EM, Tanner B, Randall S, et al. Clinician’s Guide to Prevention and Treatment of Osteoporosis. Osteoporos Int. 2014;25:2359–81. 10.1007/s00198-014-2794-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOC)
Data Availability Statement
All data and related metadata were deposited in an appropriate public repository in the National Health Research Institutes (NHRI, http://english.nhri.org.tw/NHRI_WEB/nhriw001Action.do). The data on the study population that were obtained from the NHIRD (http://nhird.nhri.org.tw/en/index.html) are maintained in the NHIRD (http://nhird.nhri.org.tw/). The NHRI is a nonprofit foundation established by the government. Only citizens of the Republic of China (Taiwan) who fulfill the requirements of conducting research projects are eligible to apply for the NHIRD. The use of NHIRD is limited to research purposes only. Applicants must follow the Computer-Processed Personal Data Protection Law (http://www.winklerpartners.com/?p=987) and related regulations of National Health Insurance Administration and NHRI, and an agreement must be signed by the applicant and his/her supervisor upon application submission. All applications are reviewed for approval of data release. We also confirm that the data used in our study are third party data that are not owned by and have not been collected by the authors. In addition, these authors do not have special access privileges to these data.