Abstract
Background
There are very few effective, scientifically validated treatments with known mechanisms of action for treatment of hair loss in both men and women. Fibroblast growth factor 5 (FGF5) is an important factor in the irreversible transition from anagen to catagen, and inhibition of FGF5 prolongs anagen phase and reduces hair loss.
Objective
We aimed to screen botanically derived molecules for FGF5 inhibitory activity in vitro and assess efficacy in a clinical setting.
Methods
We screened for FGF5 inhibitory efficacy via a novel 2-step in vitro pipeline consisting of an engineered FGF5 responsive cell line, followed by an activated dermal papillae (DP) cell method. Efficacy in a clinical setting was assessed in a randomized, single-blind, placebo-controlled trial against early- to mid-stage pattern hair loss in men and women.
Results
We observed FGF5 inhibitory activity for a number of compounds from the monoterpenoid family, many showing greater inhibitory efficacy than our previously reported crude plant extracts. Evaluation of a lead candidate in a clinical study over 112 days showed a significant improvement in anagen:telogen (AT) ratio (p = 0.002), reduced hair fall (p = 0.007) and improved visual grading (p = 0.004). Scientifically matched photography on a subgroup of randomly chosen participants highlighted significant improvement in hair density, with increases evident in all tested participants compared to baseline.
Conclusion
Isolates from the monoterpenoid family displayed efficacy in FGF5 inhibition in vitro. A topical formulation containing a leading isolate significantly improved AT ratio, reduced hair fall and increased apparent hair density in the tested population of men and women.
Keywords: hair growth, hair treatment, in vitro testing, hair loss, anagen, FGF5
Introduction
Pattern hair loss (PHL) affects a significant number of both men (MPHL) and women (FPHL). Approximately 50% of men will present with PHL by the age of 50, increasing to >70% of men by the age of 80.1 The incidence of PHL in women is somewhat lower, occurring in 3–13% of women under 40 years, increasing to 32–54% in women over 70 years.2 The psychosocial impact of PHL can be profound in many individuals, resulting in loss of self-confidence, pre-occupation and depression. The impact is less profound in men and many retain a high level of personality function,3 but in contrast, PHL in women has been identified as substantially more distressing and is associated with negative body image and a pattern of less adaptive functioning.4
The pathology of hair loss is complex, and there remain significant gaps in understanding, particularly for FPHL. In normal hair growth, the hair follicles cycle through distinct phases.5 Growth, known as anagen, where the hair shaft is actively generated and extends, can last as long as 6 years on the human scalp before entering a period of rest. In the rest period, the follicle regresses (catagen), which is followed by a period of quiescence (telogen) and release of the hair shaft (exogen). The signals that induce the transition between the different phases of the hair cycle are not fully understood; however, complex signaling cycles between the root sheath and the dermal papillae (DP), involving a number of protein families including the fibroblast growth factors (FGFs), bone morphogenic proteins, sonic hedgehog and Wnt signaling, are thought to be crucial.
In normal hair cycling, ~90% of follicles are in anagen phase, 1% in catagen phase and 9% in telogen phase at any given time,6 with the proportion of follicles in anagen phase declining with age.7 In PHL, there appears to be a dysfunction and imbalance in hair cycling, resulting in a reduced length of anagen phase, increased proportion of hair in catagen/telogen phase and a shift toward the production of fine, short hairs (~<2 cm), with a lack of pigment known as vellus.6 Ultimately, this leads to follicle miniaturization and hair follicle dormancy, which in the most extreme cases can progress to total hair loss.
The mechanisms that lead to the imbalance in the phases of the hair cycle and follicle miniaturization are not fully characterized, and there are significant gaps in the knowledge of mechanisms of PHL. In men, androgens are involved in many cases, and this has led to the umbrella term of androgenetic alopecia being generally applied to PHL. However, the underlying causes are poorly understood, particularly in women, and PHL may actually have a number of etiologies, a scenario that is perhaps reflected in the modest response rates to many current treatments (“Discussion” section). Nevertheless, it is known that in many men dihydrotestosterone (DHT), synthesized from testosterone by 5α reductases, is a major player (reviewed in Kaufman8). The intracellular signaling cascade after androgen receptor binding by DHT is poorly understood, but receptor binding leads to increased production of cytokines, such as transforming growth factor β1 (TGFβ1), which promote telogen and DP cell senescence. In women, there is less evidence regarding the involvement of androgens. The higher prevalence of FPHL in post-menopausal women has led to the association of hormonal changes in the etiology, but such changes may also be attributed to life-course reduction in hair density that only becomes visibly apparent later in life.9 Largely, the mechanisms underlying FPHL remain unknown.
There are only 2 Food and Drug Administration (FDA)-approved treatments for hair loss, finasteride and minoxidil, alone or in combination. Finasteride, a 5α reductase inhibitor that blocks the conversion of testosterone to DHT,10 has proven useful in the treatment of MPHL (reviewed in Mella et al11). However, finasteride is associated with a number of side effects, including sexual dysfunction that in some cases persists after ceasing therapy.12 Despite a small number of studies showing efficacy, the use of finasteride in women is controversial, and only off-label, uncontrolled studies and anecdotal evidence have reported positive results.13 Finasteride treatment in pre-menopausal women is also problematic, requiring concomitant contraceptive treatment due to the teratogenic nature of the compound.14
Minoxidil was initially developed for the treatment of hypertension. Hypertrichosis was noted as a side effect of treatment,15 and the product was repurposed as a hair loss therapy. Topical minoxidil is the only known treatment that is effective in both men and women; however, the mechanism of action is unknown.16 Minoxidil is generally well tolerated but is associated with a number of side effects, including an initial increase in hair shedding and exacerbation of hair loss after withdrawal from treatment.17
FGF5 was identified as a determinant of hair length through seminal studies of FGF5 knock-out mice that displayed the angora long-haired phenotype and were observed to have an abnormally extended anagen phase.18 Importantly, in a recent work by Higgins et al,19 FGF5 was identified as a crucial regulator of hair length in humans, with mutations in FGF5 underlying familial trichomegaly. Individuals with this mutation have longer hair and a greater proportion of hair follicles in anagen phase compared with ethnically matched controls.
Studies in rodents have found that FGF5 messenger RNA (mRNA) is upregulated during late anagen phase,20 DP cells that express the receptor for FGF5 (FGFR1)21 are responsive to FGF5 in vitro22 and FGF5 inhibits DP cell activation.23 Such findings demonstrate that FGF5 signaling to DP cells through FGFR1 is a critical regulatory process. Furthermore, FGF5 accumulates in the outer root sheath and in small macrophage-like cells surrounding the follicle, and the expression at these sites is increased at late anagen phase.18,19,24 Alternate mRNA splicing of FGF5 results in the translation of long (FGF5) and short (FGF5S) isoforms, which are known to act in concert. FGF5S acts as an antagonist to the catagen-inducing effect of FGF5,25 and in this vein there is potential for exogenous inhibitors of FGF5 to promote hair growth, as demonstrated by Ito et al.26
The evidence that FGF5 inhibits hair growth and is involved in the transition from anagen to catagen led us, in our previous work,27 to screen botanical extracts for FGF5 inhibitory activity. We identified Sanguisorba officinalis root extract (SO extract) as an effective FGF5 inhibitor and demonstrated that SO extract enhanced the multiplication of outer root sheath cells in vitro. Further, in vivo analyses indicated that while SO extract did not enhance the onset of growth, it significantly reduced the number of telogen-phase follicles and increased hair length. We subsequently tested a topical solution containing SO extract in a cohort of human participants vs. a placebo group in a double-blind study over a 4-month period. We observed significantly reduced number of shed hairs (~70% less shed hairs) and decreased number of soft hairs (vellus), while hair growth rate and anagen:telogen (AT) ratio were increased. Subjective assessments of effectiveness indicated that the extract was rated as somewhat effective, effective or very effective for 74% of the treated group vs. 25% of the control. We have since marketed a product containing SO extract under the brand name évolis®.
We describe here the results of a secondary screening program of FGF5 inhibitory activity in plant extracts and, importantly, single-molecule isolates of botanical origin. As with our previous work, we utilized an FGF5 responsive cell line, but with the addition of a secondary in vitro screening system using a DP cell culture method. The 2-stage in vitro testing has supplanted any reliance on animal testing. In our screening program of numerous botanical isolates, we identified a number of compounds from the monoterpenoid family as having FGF5 inhibitory activity. Monoterpenes are widely distributed in the plant kingdom and constitute the major volatile components of essential oils from plants, including citrus28 and eucalyptus species.29,30 Monoterpenes are C10 compounds that may be cyclic or acyclic with the generalized cyclic structure illustrated in Figure 1. Numerous monoterpenoid compounds of botanical origin are known to have pharmacological properties, including anti-inflammatory and anti-microbial activities, growth modulation and anti-tumor actions.29,31
Figure 1.
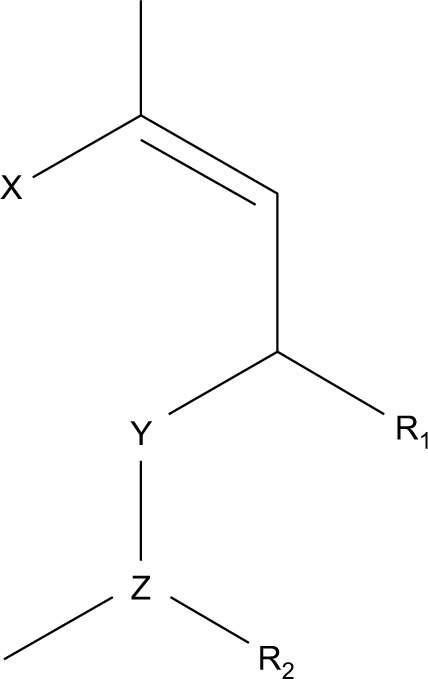
The generalized structure of the monoterpenoid family.
Notes: In those compounds which we tested for significant FGF5 inhibitory activity: R1 is hydrogen, hydroxyl or oxygen; R2 is absent or hydrogen or hydroxyl; X is CH3 or CH2OH or X is CH2CH2 or CHOHCH2 and X and Y together form a single bond within a 6-membered ring; Y is CH2 when X is CH3 or CH2OH or Y is CH or COH when X is CH2CH2 or CHOHCH2 and Z is a saturated or unsaturated C2–C5 alkyl or alkyl ester.
Abbreviation: FGF, fibroblast growth factor.
Our secondary objective was to test the lead extract identified in the FGF5 inhibitory screening in a single-blind, randomized, placebo-controlled trial. We assessed the ability of the new topical formulation of product to improve the hair growth profiles of men and women with early-stage PHL. Participants were monitored over 16 weeks (112 days), with periodic measurements of AT ratio, hair fall and expert visual grading. We also performed scientifically matched photography and image analysis for a small subset of participants.
Materials and methods
In vitro study
Tested compounds
We screened a series of botanical extracts for FGF5 inhibitory activity, including the FGF5 inhibitory SO extract (Maruzen Pharmaceuticals, Onomichi, Japan) described in our previous work,27 Camellia japonica seed extract (NOF Corporation, Tokyo, Japan) and crude eucalyptus essential oil (EPT extract) (Essentially Australia, Byron Bay, Australia). We also screened a number of isolates from the monoterpene family (Figure 1) including 3-carene, l-carveol, 1,8-cineole, β-citronellol, DL-rose oxide, geraniol, geranyl acetate, linalool, linalyl acetate, L-menthol, nerol, piperitone, α-terpineol, (−)terpinen-4-ol, (+)terpinen-4-ol and (±)terpinen-4-ol. The monoterpenoid isolates were sourced from Tokyo Chemical Industries (Tokyo, Japan) except for 3-carene, l-carveol and dl-rose oxide, which were kindly provided by Nippon Terpene Chemicals, Inc. (Kobe, Japan). A selection of the above will hereafter be referred to as MTP3–MTP6 without disclosure of their identities for reasons of commercial protection. The test compounds were diluted with 100% ethanol as working stocks and further diluted as necessary.
FGF5 inhibition assay
A genetically engineered BaF3 cell line with cell surface expression of human FGF receptor 1,27 the receptor for FGF5, was cultured in 96-well microplates (Corning, NY, USA; no special coating) to log phase. Cells were maintained in RPMI1640 (Wako Chemical, Osaka, Japan) supplemented with 10% fetal bovine serum (FBS) (Biological Industries, Cromwell, CT, USA) and 100 U/mL G418 (Calbiochem, San Diego, CA, USA). Dose–response activity for a number of test conditions was measured for 6 compound concentrations and performed in triplicate. Compound concentrations (in mg/mL) were MTP3–MTP6 (0, 0.0123, 0.0370, 0.111, 0.333, 1); EPT extract (0, 0.0123, 0.0370, 0.111, 0.333, 1) and SO extract (0, 0.0021, 0.0063, 0.019, 0.056, 0.169). To each well containing a different concentration of compound, 2.5 × 104 cells were seeded and either: Blank vehicle media; FGF5 (100 ng/mL) (Oriental Yeast, Tokyo, Japan); or interleukin (IL)-3 (2 ng/mL) (Preprotech, Rocky Hill NJ, USA), was added to the media. Cells were incubated for 3 days at 37°C in 5% CO2. The number of viable cells was quantified using Cell Counting Kit-8 (Dojindo, Kumamoto, Japan), based on the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) reaction. Absorbance (450 nm) measurements for triplicate experiments were averaged and adjusted for background absorbance, and the % cells for each test condition were calculated by comparison to wells with the compound concentration of 0 (negative control). Dose-dependence curves were plotted, and inhibitory concentration 50 (IC50) values for IL-3 (a measure of nonspecific inhibition) and FGF5 were calculated manually for each compound. From these 2 IC50 values, a relative measure of “FGF5 inhibitory efficacy” was calculated as the ratio of the IC50 of the compound against FGF5-stimulated cells to IC50 of the compound against IL-3-stimulated cells (IC50 for IL-3/IC50 for FGF5).
DP ALP assay (anagen recovery)
We have established a DP cell culture system in our laboratory. DP cells express the receptor for FGF5:FGFR1.21 Alkaline phosphatase (ALP) has been shown to be a reliable indicator of the anagen period.32 FGF5 protein causes suppression of ALP enzymatic activity in Wnt-activated DP cells (in-house data; Supplementary materials and Figure S1). By measuring the recovery of ALP activity in cultured DP cells in the presence of a test compound and FGF5, we can measure the FGF5 inhibitory effect of that compound.
DP cells (Advangen, Kashiwa, Japan) were washed and suspended in Dulbecco’s Modified Eagle’s Medium (DMEM; Wako Chemical) supplemented with 10% FBS (Biological Industries) and 100 U/mL penicillin and streptomycin (Wako Chemical). Dose–response activity to various compounds was measured in triplicate against cells in culture medium either supplemented with GSK3 inhibitor IX (500 ng/mL) (Calbiochem) alone or against GSK3 inhibitor IX (500 ng/mL), plus FGF5 (10 ng/mL) (Oriental Yeast). Compound concentrations (in mg/mL) were MTP3–MTP6 (0, 0.0185, 0.0556, 0.167, 0.5, 1.5); EPT extract (0, 0.062, 0.0185, 0.0556, 0.167, 0.5) and SO extract (0, 0.0001, 0.00031, 0.00094, 0.00282, 0.00845). Cells were incubated for 3 days at 37°C in 5% CO2, and the cell number was counted via the MTT reaction as described earlier. ALP enzymatic activity was subsequently measured via the following protocol: 27.5 µL of 1% NP-40/water (Wako Chemical), 7.5 µL of 0.5 M Tris–HCl (pH 8.8) (Wako Chemical) plus 10 mM MgCl2 (Kanto Chemical, Tokyo, Japan) and 15 µL of 1.0 mM nicotinamide adenine dinucleotide phosphate (Enzo Life Science, Farmingdale, NY, USA) were added to each assay well and incubated at room temperature for 30 minutes. A total of 7.5 µL of 0.5 M sodium phosphate (pH 8.8) (Wako Chemical) was added to each well alongside 70 µL of 2.5 mg/mL 2-(4-iodophenyl)-3-(4-nitrophenyl)-5-phenyl-2H-tetrazolium (INT) (Dojindo) dissolved in 12% EtOH (Wako Chemical), 15 µL of 0.08 mg/mL diaphorase (NADH) (Asahi Kasei Pharma, Tokyo, Japan) dissolved in water and 7.5 µL of 1.0 mg/mL alcohol dehydrogenase (Oriental Yeast) dissolved in 50% glycerin (Wako Chemical). Wells were colorized for 5 minutes at 37°C under 5% CO2 and absorbance measured at 490 nm in a microplate reader. Cell numbers and % cells for each condition were calculated as earlier. Background subtracted absorbance values were indicative of ALP levels, from which a corrected value of ALP/cell was calculated. To determine the relative recovery of ALP compared to control cells, we first calculated the change from baseline (relative amount of ALP/cell with respect to that seen at the compound concentration of 0), and a recovery factor was calculated by a comparison of the change from baseline for the treated samples, as calculated earlier, to that of the control experiment at each specific concentration.
Clinical study
All clinical efficacy experiments and safety testing were performed by an independent, FDA-registered, GCP/GLP-compliant and ISO 9001-certified laboratory (AMA Laboratories Inc., New City, NY, USA) following standard operating procedures. All testing and data recording were performed on site. AMA Laboratories Inc. performed the enrollment and allocation to treatment groups. The study protocol was reviewed and approved by the institutional review board of AMA Laboratories Inc. according to the US Code of Federal Regulations Title 21, Part 56.
Test material and safety testing
We proceeded to test the best performing single-molecule isolate, which in this manuscript is designated as MTP3. There were 3 treatment arms. Placebo treatment of évolis tonic base with no active ingredients and 2 treatment formulations: treatment group 1 (évolis tonic formulation with 0.095% MTP3) and treatment group 2 (évolis tonic formulation with 0.5% MTP3). The tonic base consisted of the following ingredients (%w/v) in purified water: ethanol (60%) (Mandrila Group, Sydney, Australia), 1,3-butylene glycol (3%) (OXEA Corporation, Oberhausen, Germany), panthenyl ethyl ether (0.3%) (Rita Corporation, Crystal Lake, IL, USA), glycyrrhentinic acid (0.1%) (Maruzen Pharmaceuticals), citric acid, (0.025%) (LabChem Inc, Zelienople, PA, USA), and sodium citrate (0.024%) (LabChem Inc). Preservative efficacy and safety testing were performed, and results are described in the Supplementary materials.
Clinical study participant recruitment and selection
Male and female participants were recruited at the study site via advertisements and were inducted after written informed consent in accordance with US Code of Federal Regulations Title 21, Part 50. Hair loss criteria for participants were patterned baldness at Hamilton–Norwood scale 2–4 for men or Ludwig scale I-2 to II-2 for women (Figure 2), but not confounded by other specific hair disorders such as alopecia areata, cyclic alopecia, loose anagen syndrome, acute anagen or telogen effluvium and trichotillomania, confirmed by examination by a trained clinical evaluator.
Figure 2.
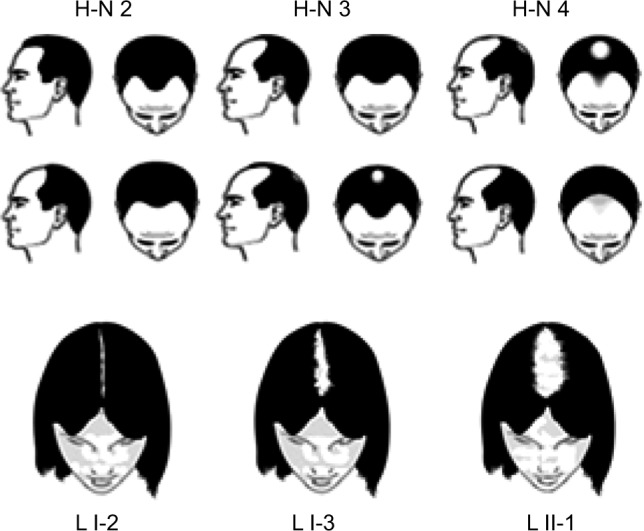
The Hamilton–Norwood (H-N) and Ludwig (L) scale ranges used in the study for men and women, respectively.
Participants were included if they were in general good health, non-obese (body mass index [BMI] 19–26 kg/m2 by standard calculation) and free of any health problems, including neurological, dermatological or systemic disorder. All participants agreed to use the same shampoo and maintain the same hair style, length and color during the entire study and refrain from cutting the scalp hair shorter than 1 inch (~2.5 cm) in length.
Participants were excluded if they displayed any of the confounding hair loss conditions described earlier. They were also excluded if they had been diagnosed with chronic skin allergies, generalized allergies, scalp inflammation or skin conditions or were being treated for any specific condition and/or taking any medication that may interfere with the study design. Additionally, individuals who were involved in any other clinical research studies, those who had previously experienced irritation or sensitivity to topical products, those receiving any hair loss treatments at the time of or in the last 6 weeks prior to enrollment and those suffering from hypothyroidism or receiving a thyroid hormone treatment in the last 6 weeks prior to enrollment were excluded. Females who were pregnant, lactating, had been pregnant, given birth within the 6-month period immediately preceding study commencement or planning pregnancy within 6 months were also excluded. Finally, participants with a history of any form of skin cancer, melanoma, lupus, psoriasis, connective tissue disease, diabetes or any disease that would increase the risk associated with study participation were additionally excluded from the study group.
Study design and treatment protocol
The study was conducted according to a single-blind placebo-controlled protocol, in which study participants were blind to treatment, with randomized complete block design of 3 age- and gender-matched groups with a 1:1:1 allocation ratio (placebo, 0.095% MTP3, 0.5% MTP3). The product for each treatment arm was supplied in bottles that were coded and de-identified as to their contents. All participants were trained in the correct application of the product to the target areas of the scalp and were directed to use the formulation twice a day, morning and evening, on dry or damp hair for the 16-week study period. Participants were instructed that if routine washing or conditioning was performed that the formulation was to be applied after such activities, but before any styling. Compliance was assessed via phone contact at weeks 1 and 4 and at clinic/facility visits at week 8 and 16.
AT ratio analysis
Hair shafts were excised from the treated region of the scalp using a standardized technique33 at days 0 (baseline), 56 and 112, with a minimum of 10 hair shafts required for analysis. Shafts were categorized as anagen (growth phase) or telogen (falling phase),34 and ratios of anagen to telogen counts (AT) were calculated according to Van Scott.35
Hair fall analysis
Hair fall was measured using gravimetric determination via the Maibach36 hair pull technique at days 0 (baseline), 56 and 112. A trained technician combed each participant’s hair 20 times using a standardized technique, capturing and weighing the “fall-off”.
Visual evaluation
A trained expert clinical evaluator assessed global hair release and recovery condition for hair volume at baseline and days 56 and 112 using a modified visual analog scale with an 11-point grading scale from 0 representing poor to 10 indicating excellent.
High-resolution scientifically matched photography
A small subset of 3 females and 3 males from treatment group 1 was chosen, at baseline, to participate in the photogrammetric investigation at days 0 (baseline), 56 and 112. High-resolution, digitally certified un-retouched photographs were taken of the top center of the scalp, with the front of the camera lens standardized for each participant, perpendicular to the basic plane, centered within the line of intersection of coronal and mid-sagittal planes of the head. Photographic procedures and settings were standardized with fixed camera background, angles, settings, lighting, participant positioning, color bars and white balance. Photographs were evaluated using PhotoGrammetrix® Image analysis (AMA Laboratories Inc.), quantifying hair density, release and recovery.
Statistical analysis
All data were examined via descriptive statistics to ensure assumptions of standard statistical analysis were met. If necessary and where appropriate, data were log2 transformed. Parametric and non-parametric tests were applied as appropriate to the data. Differences within treatment groups over successive time points were assessed through one-way repeated measures analysis of variance (ANOVA) or the Friedman test with post hoc pairwise comparisons via Tukey’s pairwise comparisons or paired Wilcoxon rank sign tests, respectively. Comparisons between test groups were performed via one-way ANOVA or Kruskal–Wallis with post hoc testing via Tukey’s pairwise comparisons and Mann–Whitney tests, respectively. Paired t-tests were also performed as appropriate.
Results
FGF5 inhibitory assays
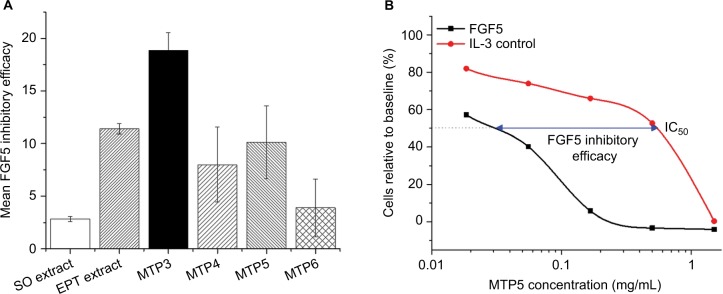
We tested the FGF5 inhibitory efficacy of monoterpenoids, in parallel to EPT extract, Camelia seed extract and SO extract using 2 cell-based assays of FGF5 activity. We first assessed the ability of test compounds to inhibit FGF5-induced proliferation of the engineered FGF receptor 1-expressing BaF3 cell line.27 The BaF3 cell line used in the study is a cell line engineered to express FGFR1c, originally used to study the splice variant of FGF5 known as FGF5s.26 The BaF3 cell lineage is IL-3 dependent and responds to the presence of IL-3 by proliferating. The cell line is commonly used in signaling research as it is readily transformed.37 The transfection of the BaF3 cell line with FGFR1c also makes it responsive to FGF5, proliferating in its presence. The use of IL-3 in this assay is thus a control that only indicates test compound non-specific inhibition of cell culture growth. When this is compared to specific inhibition of cell growth via blocking interaction of FGF5 with its receptor expressed on the engineered BaF3 cells, we can identify true FGF5-inhibiting compounds. The inhibitory efficacy was determined by the ratio of the IC50 of the test compound in the presence of FGF5 against the IC50 of the test compound in the presence of IL-3. The former indicating FGF5-dependent inhibition, and the latter being representative of non-specific inhibition of cell growth. Large differences in the specific and non-specific inhibition of cell growth by the test compounds were indicative of high efficacy of FGF5 inhibition, in contrast to non-specific effects on cell viability, which tended to occur at high concentrations. An example of a curve from an individual experiment is shown for illustrative purposes in Figure 3B. Analysis of these data showed that MTP3 had significant inhibitory efficacy: 1.6-fold higher than crude EPT extract (p = 0.065) and close to 7-fold more efficacy than SO extract (p = 0.0024), while having 2.4-fold, 1.9-fold and 4.8-fold higher mean efficacy measures than MTP4 (p = 0.013), MTP5 (p = 0.036) and MTP6 (p = 0.0018), respectively (Figure 3A). Data from samples that only had low activity are not shown.
Figure 3.
In vitro FGF5 inhibitory efficacy from the engineered BaF3 cell line assay.
Notes: (A) Significant differences in inhibitory efficacy were observed, ANOVA p = 0.016. Post hoc pairwise comparisons identified specific differences: MTP3 vs. SO extract p = 0.002; MTP3 vs. MTP4 p = 0.013; MTP3 vs. MTP5 p = 0.036 and MTP3 vs MTP6 p = 0.002. Borderline significant results were also observed between MTP3 vs. EPT extract p = 0.065; EPT extract vs. SO extract p = 0.060 and EPT extract vs. MTP6 p = 0.065. The unit of measurement in the y axis is a ratio reflecting the fold difference between non-specific IC50 and specific FGF5 inhibitory IC50 (or IC50 for IL-3/IC50 for FGF5). This ratio is referred to as inhibitory efficacy. (B) Example inhibitory efficacy curve for 1 experiment using MTP5 showing inhibition of FGF5-stimulated cell growth at lower compound concentrations compared to controls using IL-3-dependent proliferation.
Abbreviations: FGF, fibroblast growth factor; ANOVA, analysis of variance; EPT, eucalyptus essential oil; SO, Sanguisorba officinalis; IL, interleukin.
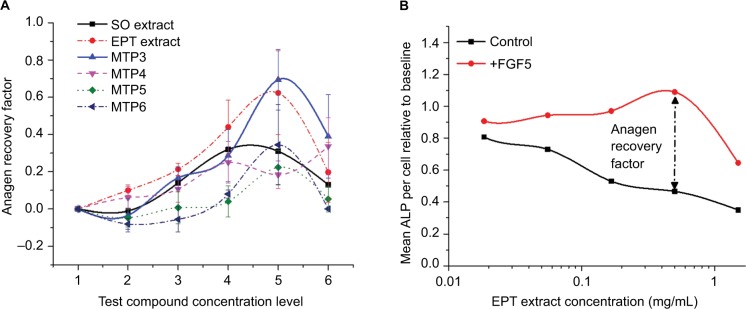
We also tested the FGF5 inhibitory activity of MTP3 in vitro with our DP cell-based assay, using ALP activity as an indicator of the anagen period. We use this method as an anagen/catagen transition model with exogenous FGF5 interacting with FGFR1 on the DP cells, inducing catagen and correspondingly reducing ALP activity. FGF5-inhibiting compounds, when added to the cell culture alongside exogenous FGF5, should stimulate the recovery of anagen and hence increase ALP activity in a dose-dependent manner. An anagen recovery factor was calculated by comparing the maximum increase of ALP activity over baseline for FGF5-inhibited cells to the ALP activity over baseline for the test compound alone at that specific concentration. An example from 1 experiment is displayed in Figure 4B. We observed that MTP3 had the strongest ability to recover anagen phase (Figure 4A), with an average recovery factor double that of SO extract (0.69 compared with 0.32) and improved performance compared to the other monoterpene isolates. Such results were indicative of effective FGF5 inhibitory activity and highlighted MTP3 as a strong candidate for testing in a clinical setting.
Figure 4.
Recovery of anagen phase as indicated by ALP activity measured after treatment of DP cells with increasing concentrations of test compounds in the presence of FGF5.
Notes: (A) MTP3 showed the highest recovery of anagen phase with respect to baseline and was more effective than EPT extract and much more effective than SO extract and the other MTP isolates. (B) Example of a dose–response curve from the ALP anagen recovery assay using mean values from each EPT extract experiment.
Abbreviations: ALP, alkaline phosphatase; DP, dermal papillae; FGF, fibroblast growth factor; EPT, eucalyptus essential oil; SO, Sanguisorba officinalis.
Clinical cohort characteristics
We recruited 32 individuals to our single-blind placebo-controlled study, the majority White Caucasian. Individuals were de-identified and randomized into 3 equal groups matched by age and gender (Table 1). When we compared baseline measurements of hair loss scale scores, visual grading, hair fall and AT between the cohorts in a post hoc manner, we also found a good balance between the tested parameters except for hair fall. In the hair fall category, we observed the 0.095% MTP3 test group as having significantly higher hair fall at baseline vs. the placebo group (p = 0.008) and vs. the 0.5% MTP3 group (p = 0.02). We also noted some outliers in hair fall, particularly in females at baseline. All participants completed the study, and no adverse events were reported.
Table 1.
Demographics of the study cohort
| Placebo | 0.095% MTP3 |
0.5% MTP3 |
p-value | |
|---|---|---|---|---|
| Number of participants | 10 | 11 | 11 | NA |
| Age (years) | 41.8±7.6 | 48±6.5 | 43±7.3 | n.d. |
| Gender (F:M) | 3:7 | 3:8 | 4:7 | n.d. |
| Ludwig scale score for females (median) | I-2 | I-3 | I-2, I-3 | n.d. |
| Hamilton–Norwood scale score for males | 3.0±1.0 | 2.9±0.6 | 3.0±1.0 | n.d. |
| Baseline visual grading score | 4.5±2.0 | 4.0±1.2 | 4.3±1.2 | n.d. |
| Baseline hair fall (mg) | 28.2±144.2 | 46.3±54.4 | 10±5.1 | 0.008 |
| Baseline anagen (%) | 51±24.7 | 41±16.0 | 36±9.7 | n.d. |
Notes: All values are mean ± SD unless otherwise noted. p = results of statistical tests to monitor differences in cohorts.
Abbreviations: NA, not applicable; F, female; M, male; n.d., no difference between cohorts; SD, standard deviation.
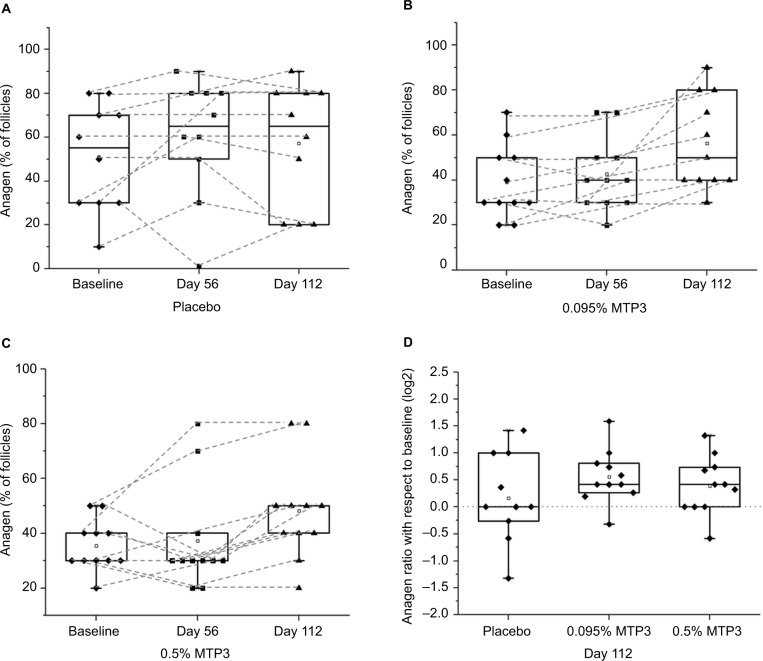
AT ratio comparisons
AT ratio of trial participants at baseline and after treatment was determined through inspection of hair follicles using a standard method.31 In the study cohort, most of the individuals had low baseline anagen ratios, with the majority below 50% anagen (0.5 AT ratio; Figure 5A–C, baseline). There was also non-significant imbalance between the treated groups and the placebo, and the latter group had a higher baseline variance. Nevertheless, when the responses of each of the groups to treatment were compared to the group baseline, significant increases in anagen ratios for both 0.095% MTP3 (p = 0.002; Figure 5B) and 0.5% MTP3 (p = 0.03; Figure 5C) were evident at day 112. No differences were found in the placebo group (Figure 5A). The mean improvement in AT ratio (95% confidence interval [95% CI]) and median improvement for each group at day 112 were as follows: 0.095% MTP3, 0.173 (0.075–0.27), 0.10; 0.5% MTP3, 0.127 (0.040–0.215), 0.10; and placebo, 0.060 (−0.074 to 0.194), 0.
Figure 5.
Follicles in anagen phase from baseline to day 112.
Notes: No difference was observed in the placebo group (A); however, differences were observed in between baseline and day 112 for both 0.095% MTP3 (p = 0.002) (B) and 0.5% MTP3 (p = 0.03) (C), with a number of study participants recovering to levels close to 80% anagen for the treatment groups. (D) Compared to baseline (indicated with dotted line at log2 zero), the majority of participants on both treatment arms showed improvement in anagen ratio at day 112. The placebo group had no trend in either direction.
The majority of individuals in the treatment groups showed improvement in anagen ratio at day 112 (Figure 5D), with the effect most apparent in the 0.095% MTP3 group with >40% of participants showing improvement in anagen by at least 20 percentage points. While the effect was less consistent in the 0.5% MTP3 group, >35% of participants had an increase in anagen ratio by at least 20 percentage points. We did not find a significant difference in change from baseline of treated groups compared to placebo (Figure 5D), which was likely influenced by the high baseline variance in the placebo group. As stated earlier, there was a strong trend for improvement in the treated groups, but in the placebo group, there was no clear trend, with some participants decreasing in anagen ratio and some increasing, resulting in a median change of 0. Median changes with respect to baseline for treated groups were positive.
Although gender-based analysis was not a primary outcome of the study, we also separated the data into male and female participants. However, the small sample size in this pilot study, particularly in the female cohort, meant that statistical analyses suffered from issues with power. To improve this, we combined the treatment arms (0.095% and 0.5%) into a single treated group prior to gender-based analyses. The results of all gender-based analyses, including both combined and also separated treatment arms, are presented in Figure S2. For the AT ratio, we saw significant difference from baseline in both male (p = 0.007) and female (p = 0.003), with the effect most apparent at day 112 (p = 0.02 and p = 0.016 for female and male, respectively) (Figure S2A). No differences were seen in the placebo group for either gender (Figure S2B). Effect comparisons with respect to baseline between groups (treated vs. placebo) suffered from low sample numbers and high variance in the female placebo group (Figure S2C), but in the male subgroup, we observed a borderline significant difference compared to placebo (p = 0.08), which was not seen in the whole cohort analysis.
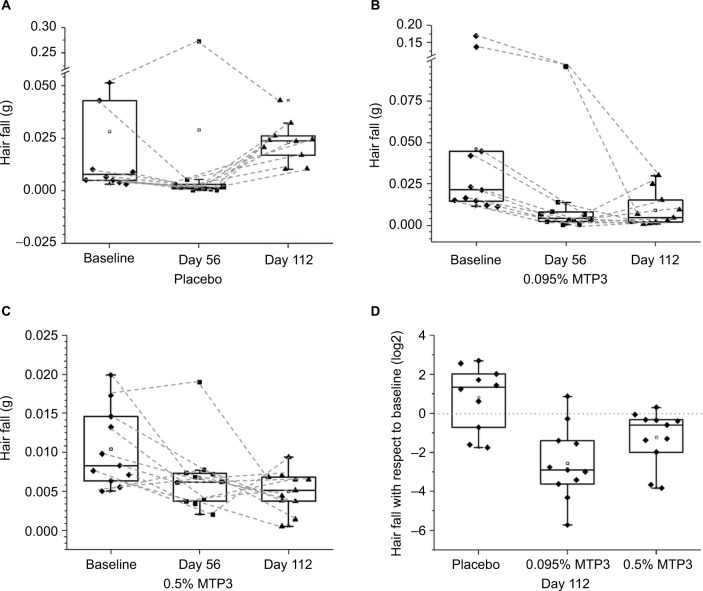
Hair fall analysis
We assessed hair fall by collecting hair using the Maibach36 pull technique. We observed no significant differences in the placebo group compared to baseline at either time point (Figure 6A), but we did see a trend for decreased hair fall at day 56 (p = 0.08). Contrastingly, significant decreases in hair fall were observed compared to baseline for both MTP3 formulations (Figure 6B and C) at both day 56 (0.095% MTP3, p = 0.004; 0.5% MTP3, p = 0.007) and day 112 (0.095% MTP3, p = 0.029; 0.5% MTP3, p = 0.013). The mean and median % improvements from baseline for each group at day 112 were as follows: 0.095% MTP3, 80.21% and 78.4%; 0.5% MTP3, 51.9% and 38.4% and placebo, 17.37% and −204%.
Figure 6.
Hair fall comparisons within treatment groups and between treatment groups.
Notes: (A) No difference in hair fall was observed in the placebo group, although there was a trend for decreased hair fall at day 56 (p = 0.08). (B) In the 0.095% MTP3 treatment group, significant reductions in hair fall were observed between baseline and day 56 (p = 0.004) and between baseline and day 112 (p = 0.007). (C) In the 0.5% MTP3 treatment group, significant reductions in hair fall were observed between baseline and day 56 (p = 0.029) and between baseline and day 112 (p = 0.013). (D) Compared to placebo, significant differences in hair fall relative to baseline at day 112 were observed for 0.095% and 0.5% MTP3 formulations (p = 0.002 and 0.019, respectively), with both showing decreased hair fall. The line at 0 indicates no change relative to baseline on the log2 scale.
Greater than 90% of participants in the MTP3 treatment arms showed improvement in hair fall over baseline at day 112, in contrast 70% of the placebo group showed increased hair fall at day 112. Analyzing hair fall after treatment with respect to baseline enabled comparison between treatment arms (Figure 6D) that highlighted significant differences between the performance of both treatment arms against the placebo at day 112 (0.095% MTP3, p = 0.002; 0.5% MTP3, p = 0.019).
As with the AT ratio earlier, the small sample size in this pilot study, particularly in the female cohort, meant that statistical analyses of the male and female sub-cohorts suffered from issues with power. Therefore, we combined the treatment arms (0.095% and 0.5%) into a single group prior to analysis. For hair fall (Figure S3A), there was a significant difference from baseline in both male (p = 0.002) and female (p = 0.012), with the effect seen at both time points, but most apparent at day 112 for females (p = 0.02) and day 56 for males (p = 0.001). No differences were evident in the female placebo group, and in the male placebo group, there was a significant increase in hair fall at day 112 (p = 0.02) (Figure S3B). Effect comparisons with respect to baseline between groups (treated vs. placebo) suffered from low sample numbers and low power (Figure S3C), but in the male subgroup, a significant difference was apparent in both treatment arms compared to placebo: 0.095% p = 0.001 and 0.5% p = 0.006. All treated females improved in hair fall compared to baseline and to a larger magnitude on average than placebo.
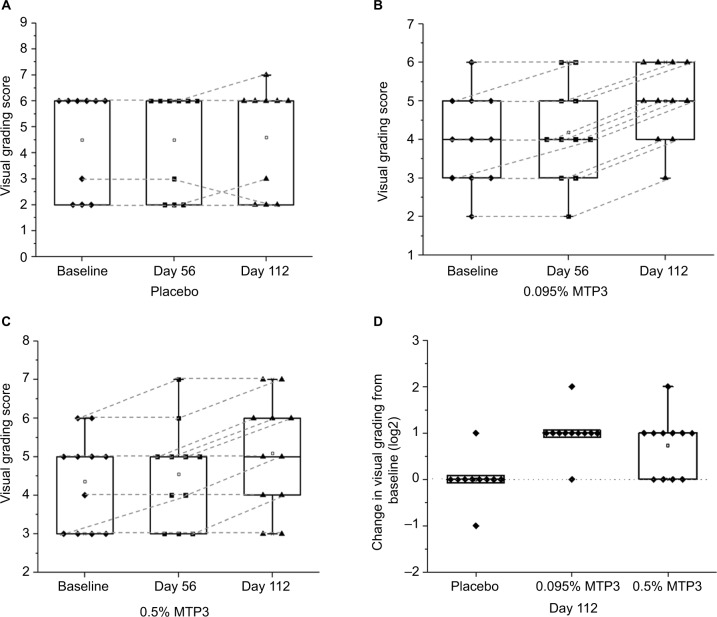
Hair volume via global release and recovery
As an additional measure of hair quality and appearance, we performed expert evaluation of hair volume via global hair release and recovery grading, scored via an 11-point intensity grading scale from 0 = poor and 10 = excellent. Over the course of the study, we observed no significant difference in the placebo group (Figure 7A), with only 2 of the 10 participants showing a slight increase in visual grading, while 1 participant had a slight decrease. Significant improvements in visual grading were observed for both MTP3 formulations compared to baseline at day 112, with all participants in the 0.095% MTP3 group (Figure 7B) increasing in visual grading (p = 0.002), some by 2 grading points. Similarly, 7 (out of 11) participants in the 0.5% MTP3 group (Figure 7C) showed an improvement over baseline at day 112 (p = 0.020), and all participants in this group remained stable in hair volume, with none showing any decrease in hair volume. The mean improvement in grading on the 10-point scale (95% CI) and median improvement for each group at day 112 were as follows: 0.095% MTP3, 1 (0.76–1.25), 1; 0.5% MTP3, 0.73 (0.37–1), 1; placebo, 0.1 (−0.22 to 0.42), 0.
Figure 7.
Visual grading scores within groups over the treatment course and between groups at day 112.
Notes: (A) No differences were observed in the placebo group over time. (B) In the 0.095% MTP3 treatment group, significant differences were observed between day 112 vs. baseline (p = 0.002) and day 112 and day 56 (p = 0.003). (C) In the 0.5% MTP3 treatment group, significant differences were observed between day 112 vs. baseline (p = 0.016) and day 122 and day 56 (p = 0.020). (D) The 0.095% MTP3 performed significantly better compared to baseline than placebo (p = 0.0005). The 0.5% MTP3 group also performed better than placebo at day 112 (p = 0.012). The line at y axis = 0 indicates no change from baseline.
When the data were analyzed in terms of absolute change from baseline for comparison between treatment arms (Figure 7D), a significant difference between the groups was identified. The 0.095% MTP3 group performed significantly better than placebo (p = 0.0005), and the 0.5% MTP3 group also significantly improved compared to placebo (p = 0.012). No difference was found between treatment arms.
As with the AT ratio and hair fall analysis earlier, the small sample size in this pilot study, particularly in the female cohort, meant that statistical analyses of the male and female sub-cohorts suffered from issues with power; therefore, the treatment arms (0.095% and 0.5%) were combined into a single group prior to gender-based analysis. For visual grading (Figure S4A), we saw significant differences from baseline in both male (p = 0.002) and female (p = 0.038), with the effect most apparent at day 112 for females (p = 0.026) and only evident at day 112 for males (p = 0.005). No differences were seen in the placebo groups (Figure S4B). Effect comparisons with respect to baseline between groups (treated vs. placebo) suffered from low sample numbers and low power (Figure S4C), but in the male subgroup, there was a significant difference compared to placebo (p = 0.03). All but one treated female improved in grading compared to the baseline group in which only 1 of 3 placebo females did, and we saw a trend for improvement in females compared to placebo p = 0.075.
High-resolution scientifically matched photography
We performed quantitative analysis of hair volume using high-resolution scientifically matched photography, carried out in a controlled environment in an independent laboratory (AMA Laboratories Inc.). Photographs were converted to a format that enabled quantitation of hair density using PhotoGrammetrix. Due to the small sample size and limitations of this method, these data are presented as supporting information only (Figure S5). Due to large differences in baseline, we analyzed the increase in hair density as a function of baseline (Figure 8). Despite the limitations of this method, we observed that all participants had an increase in the density of hair at day 56, and 5 of 6 participants continued to show an increase in hair density up to day 112. A significant increase in hair density with respect to baseline at day 112 compared to day 56 (p = 0.03; Figure 8) was apparent, highlighting the continued improvement in hair density for this group of participants applying the topical formulation with 0.095% MTP3.
Figure 8.
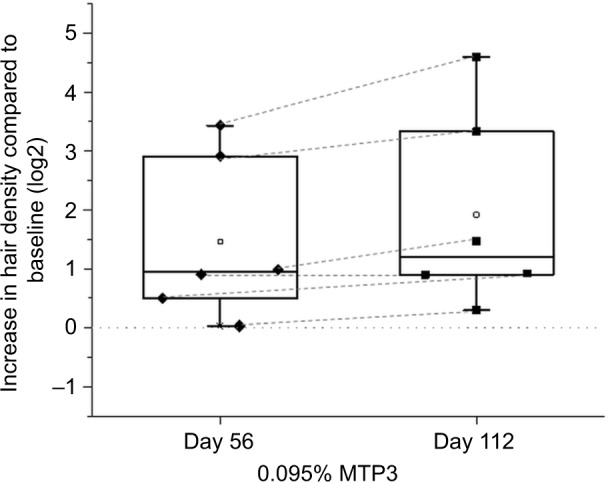
Scientifically matched photography compared to baseline.
Notes: There was a significant increase in digital grading between day 56 and 112 (p = 0.030), and both time points displayed increased hair density from baseline for all participants for whom this assessment was performed. Line at 0 is indicative of “no change” in the log2 scale.
Discussion
MTP3 is a potent inhibitor of FGF5
We previously observed FGF5 inhibitory activity in botanical extracts and demonstrated efficacy of a topical formulation containing SO extract in a randomized, double-blind, placebo-controlled trial in humans.27 Following the success of those studies, we developed a novel 2-step in vitro screening approach for testing activity of not only crude plant extracts, as we previously described, but also individual compounds, such as the monoterpenoids analyzed in this study. The in vitro screening process removes the need for animal testing in pre-clinical programs, and additionally, the analysis of single molecules rather than crude isolates is a significant progression in the precision of our approach. We observed potent FGF5 inhibitory activity for a number of the botanically derived monoterpenoid molecules using both in vitro assays. Exposure of the engineered cell line that proliferates in the presence of FGF5 to a number of monoterpenoid compounds revealed significant inhibition of cell growth, with inhibitory efficacy scores for the isolate MTP3 up to 7 times greater than those for the previously published FGF5 inhibitor SO extract.27 Further evidence of FGF5 inhibition was provided by a second assay, where activated DP cells in culture recovered anagen activity (measured via ALP activity), compared to control cells and in a dose-responsive manner, when the monoterpenoids were added to cells alongside FGF5. The recovery response seen for MTP3 was at least double that seen for SO extract and approximately double or triple that of the other monoterpene isolates. Such recovery of anagen in the presence of FGF5, which should initiate the termination of the anagen phase, further highlighted the ability of MTP3 to block FGF5 activity.
Clinical study highlighted improved outcomes in individuals with PHL
As the assays earlier indicated that MTP3 was an excellent inhibitor of FGF5, we developed a number of formulations containing MTP3 (0.095% and 0.5%), for clinical testing as a topical treatment. We recruited 32 individuals for a randomized, single-blind, placebo-controlled trial with 3 treatment arms: placebo of tonic formulation base, tonic with 0.095% MTP3 and tonic with 0.5% MTP3. Participants were treated for 112 days and results compared to baseline at day 56 (8 weeks) and day 112 (16 weeks). We observed significant improvements for all tested parameters, particularly after 112 days. Participants receiving placebo did not show improvement in any parameter.
In adults who are not experiencing hair loss, 80–90% of hair follicles should be in anagen,6 while those experiencing hair loss tend to present with low anagen ratios. FGF5 is important in the transition of hair follicles out of anagen in humans,19 and therefore, blocking its activity should result in the lengthening of anagen for individual follicles,27 resulting in an apparent increase over time in the ratio of hair follicles in the anagen phase to those in telogen phase. As mutations in FGF5 have similar effects in both men and women,19 and as the shortening of anagen phase is common to both male and female hair loss, we would expect antagonism of FGF5 to be effective in both men and women. For those individuals treating their scalps with both the MTP3 formulations, we observed significant increases in the proportion of hair roots in anagen, with many participants improving by up to 20 percentage points (improvement in AT ratio of 0.2), and some treated individuals recovering to ~80% anagen. There were no significant differences at any time point in the placebo-treated group. Such observations are indicative of FGF5 inhibitory effects resulting in less hair follicles prematurely entering telogen. Although it was not a primary objective of this study, statistical analysis after separation of the cohorts into male and female participants, despite low power due to small sample size and high variance in the female placebo group, identified highly significant differences from baseline in both females and males, highlighting the utility of FGF5 inhibition in the treatment of both male and female hair loss.
We did note some non-significant differences in the baseline anagen measurements between groups and a larger spread of values in the placebo, particularly in females, likely due to the small sample size, which consequently may have influenced the statistical analyses and led us to approach the interpretation of these data carefully. We also noted that several participants had very low anagen measurements at some sampling time points, likely due to sampling errors and the relatively small number of hairs that are excised from the scalp for this measurement. However, when the overall trends in the data compared to baseline are analyzed, the consistent mean and median increase in anagen for most participants in both treatment groups and for both genders over the course of the study, in contrast to placebo where the median change was 0, is more reflective of a response to treatment, than sampling-based inconsistencies, normal hair cycling or natural variation around a baseline. The placebo group showed no clear pattern over the course of the study.
Hair fall is also a useful indicator of hair cycling, with increased cycling of follicles out of anagen and into catagen/telogen resulting in a higher number of shed hairs. Application of topical formulations containing the FGF5 inhibitory compound MTP3 resulted in significant decreases in shed hair overall and in both males and females. We did note a trend in all cohorts for decreased hair fall at day 56. However, this was only statistically significant for the MTP3-treated groups. Such a trend in hair fall for all groups at day 56 could represent a normal variation in hair cycling or effects through variation in sampling technique. Importantly, statistically significant differences in hair fall were only observed in the MTP3-treated groups, and similarly, the trend for continued reduction in hair loss compared to baseline over the course of the study was only observed in the MTP3 treatment arms. Some imbalance in the baseline hair fall for all 3 cohorts was apparent, with the 0.5% MTP3 group showing higher hair fall at day 0. As with the AT data, the slight imbalances in allocation are likely due to the relatively small sample sizes used in this study. Such imbalance had no effect on the intra-group comparisons, but when comparing magnitude of change with respect to baseline, such imbalance may result in underestimation of biological responses, that is, if starting with a low hair fall baseline, such as in the 0.05% MTP3 group. Nevertheless, we observed a significant reduction compared to baseline for both treatment arms after 112 days of treatment with >90% of participants showing improvement, with significant differences from baseline evident in both males and females. In contrast, 70% of the placebo group had increased hair fall measured at day 112.
Additional evidence of improved hair retention and density was provided by visual grading. Despite being carried out by an identity-blinded independent trained expert evaluator, such assessment remains a subjective measure of hair thickness. However, the striking results seen in this evaluation provide compelling evidence for the efficacy of the topical formulation with MTP3 in blocking FGF5 and reducing hair loss in both males and females. All participants in the 0.095% MTP3 treatment group improved in hair grading over the course of the study, 7 of 11 participants in the 0.5% MTP3 arm increased their visual grading scores and 6 of 7 females overall improved their grading scores. The placebo group had only 2 of 9 participants improve in score. Such findings are supported by our sub-cohort study using scientifically matched serial photography. Quantification of the photography showed improvements in hair density of all measured individuals, and the majority of these continued to increase over the 112 days of the trial. The global photographic method used was somewhat influenced by hair combing, particularly in the male subjects, but this is a feature that could also be influenced by changes in hair density. As such, this method is a limitation of our study, and the data are provided only as supporting information. In larger clinical trials of our FGF5-inhibiting compounds, we plan on assessing hair growth using more sophisticated techniques such as phototrichography.
The earlier findings demonstrate that participants treated with MTP3, the newly identified FGF5-inhibiting monoterpenoid compound, performed significantly better for all measures of hair recovery. We observed improvements in the AT ratio indicating increased hair follicles in the anagen (growth) phase, decreased shedding of hair and improved volume and appearance in the MTP3-treated groups. It appeared that the 0.095% MTP3-treated group performed better than the 0.5% MTP3 group for all measures, and as such, this concentration of MTP3 should be taken forward into larger studies to further confirm efficacy and also to monitor improvements over longer time periods. Performing additional large studies will address the limitation of this small study in terms of sample size and statistical power and will help us further address the efficacy of our FGF5-inhibiting compounds in both genders.
As there are no standardized methods for assessing response of individuals with PHL to treatment, it remains difficult to objectively compare the results of our efficacy study to other hair loss treatments. However, cursory comparisons of our study to those of other botanical extracts do highlight the efficacy of the FGF5-inhibiting active MTP3. For example, treatment with rice bran extract resulted in only modest changes in hair density (10% increase), with significant improvements only observed in males.38 Likewise, methyl vanillate treatment resulted in a 6% increase in hair count and 12% increase in hair mass index.39 The same is true for comparisons of this study against our previous efficacy trial of SO extract, where participants were also from a different ethnic background (Japanese). Nevertheless, an improvement in shed hair after MTP3 treatment was evident compared to our previous trial of FGF5-inhibiting SO extract: 80% reduction in hair fall at day 112 for the MTP3-treated groups vs. 68% at 4 months.27
There is a paucity of published information regarding the efficacy of minoxidil, with many studies reporting subjective measures rather than quantitative data.40,41 Although significant improvements over placebo are generally reported in men, many of the improvements are modest, with increases in hair growth of ~20% on average (reviewed in Gupta),42 and reported for only limited proportion of the study group, for example, in 52% of enrolled participants.43 Similar results were observed for women with mean hair count increases of ~23%.44 It will be interesting to directly compare demonstrated FGF5 inhibitory products such as the monoterpenoid MTP3 against the FDA-approved treatments in our preparation for larger trials in men and women. One consistently reported feature of minoxidil treatment is an initial increase in hair shedding as follicles transition to anagen phase and existing telogen hair shafts enter exogen.45 Such initial shedding can impact on patients’ compliance and continued use of hair loss products containing minoxidil. As FGF5 inhibition acts to extend the anagen phase, and therefore gradually increases the number of anagen follicles on the head over time, we would not expect any shedding to occur, and this is supported in our data where reductions in hair fall and improvements in density were noted after 6 weeks.
Our FGF5 inhibitor screening process has identified novel potent inhibitors of FGF5 activity, with many members of the monoterpenoid family showing inhibitory activity. Our current novel 2-step in vitro screening approach has removed the need for animal testing and has allowed us to rapidly probe for inhibitory extracts enabling the identification of individual inhibitory molecules. We have translated this in vitro data into treatments with significant clinical effects. With such promising results, we aim to further characterize compounds acting as FGF5 inhibitors and their biochemical effects on hair growth, in order to improve outcomes in individuals affected by pattern hair loss.
Conclusion
We identified isolates from the monoterpenoid family as potent inhibitors of FGF5 activity using a well-defined novel 2-step in vitro protocol. Such FGF5 inhibition was reflected in a clinical setting by increases in AT ratios, reduced hair fall, improved hair density and improved appearance in both males and females affected by thinning hair.
Acknowledgments
The authors would like to thank Associate Professor Graham Robertson for his comments during the preparation of this manuscript. The authors would also like to thank the peer review panel for their helpful comments and their suggestions for improving the manuscript.
Footnotes
Disclosure
Maria Halasz and Koichiro Koike are shareholders in the parent company Cellmid. The authors report no other conflicts of interest in this work.
References
- 1.Hamilton JB. Male pattern hair loss in man: types and incidence. Ann N Y Acad Sci. 1951;53(3):708–728. doi: 10.1111/j.1749-6632.1951.tb31971.x. [DOI] [PubMed] [Google Scholar]
- 2.Birch MP, Messenger JF, Messenger AG. Hair density, hair diameter and the prevalence of female pattern hair loss. Br J Dermatol. 2001;144(2):297–304. doi: 10.1046/j.1365-2133.2001.04018.x. [DOI] [PubMed] [Google Scholar]
- 3.Cash TF. The psychological effects of androgenetic alopecia in men. J Am Acad Dermatol. 1992;26(6):926–931. doi: 10.1016/0190-9622(92)70134-2. [DOI] [PubMed] [Google Scholar]
- 4.Cash TF, Price VH, Savin RC. Psychological effects of androgenetic alopecia on women: comparisons with balding men and with female control subjects. J Am Acad Dermatol. 1993;29(4):568–575. doi: 10.1016/0190-9622(93)70223-g. [DOI] [PubMed] [Google Scholar]
- 5.Stenn KS. Molecular insights into the hair follicle and its pathology: a review of recent developments. Int J Dermatol. 2003;42(1):40–43. doi: 10.1046/j.1365-4362.2003.01726.x. [DOI] [PubMed] [Google Scholar]
- 6.Whiting DA. Male pattern hair loss: current understanding. Int J Dermatol. 1998;37(8):561–566. doi: 10.1046/j.1365-4362.1998.00542.x. [DOI] [PubMed] [Google Scholar]
- 7.Courtois M, Loussouarn G, Hourseau C, Grollier J. Ageing and hair cycles. Br J Dermatol. 1995;132(1):86–93. doi: 10.1111/j.1365-2133.1995.tb08630.x. [DOI] [PubMed] [Google Scholar]
- 8.Kaufman KD. Androgens and alopecia. Mol Cell Endocrinol. 2002;198(1–2):89–95. doi: 10.1016/s0303-7207(02)00372-6. [DOI] [PubMed] [Google Scholar]
- 9.Messenger AG. Hair through the female life cycle. Br J Dermatol. 2011;165(Suppl):2–6. doi: 10.1111/j.1365-2133.2011.10628.x. [DOI] [PubMed] [Google Scholar]
- 10.Drake L, Hordinsky M, Fiedler V, et al. The effects of finasteride on scalp skin and serum androgen levels in men with androgenetic alopecia. J Am Acad Dermatol. 1999;41(4):550–554. [PubMed] [Google Scholar]
- 11.Mella JM, Perret MC, Manzotti M, Catalano H, Guyatt G. Efficacy and safety of finasteride therapy for androgenetic alopecia: a systematic review. Arch Dermatol. 2010;146(10):1141–1150. doi: 10.1001/archdermatol.2010.256. [DOI] [PubMed] [Google Scholar]
- 12.Irwig MS, Kolukula S. Persistent sexual side effects of finasteride for male pattern hair loss. J Sex Med. 2011;8(6):1747–1753. doi: 10.1111/j.1743-6109.2011.02255.x. [DOI] [PubMed] [Google Scholar]
- 13.Stout SM, Stumpf JL. Finasteride treatment of hair loss in women. Ann Pharmacother. 2010;44(6):1090–1097. doi: 10.1345/aph.1M591. [DOI] [PubMed] [Google Scholar]
- 14.Clark RL, Antonello JM, Grossman SJ, et al. External genitalia abnormalities in male rats exposed in utero to finasteride, a 5 alpha-reductase inhibitor. Teratology. 1990;42(1):91–100. doi: 10.1002/tera.1420420111. [DOI] [PubMed] [Google Scholar]
- 15.Mehta PK, Mamdani B, Shansky RM, Mahurkar S, Dunea G. Severe hypertension. Treatment with minoxidil. JAMA. 1975;233(3):249–252. [PubMed] [Google Scholar]
- 16.Messenger AG, Rundergren J. Minoxidil: mechanism of action on hair growth. Br J Dermatol. 2004;150(2):186–194. doi: 10.1111/j.1365-2133.2004.05785.x. [DOI] [PubMed] [Google Scholar]
- 17.Kulick MI. Topical minoxidil: its use in treatment of male pattern baldness. Ann Plast Surg. 1988;21(3):273–275. [PubMed] [Google Scholar]
- 18.Hébert JM, Rosenquist T, Götz J, Martin G. FGF5 as a regulator of the hair growth cycle: evidence from targeted and spontaneous mutations. Cell. 1994;78(6):1017–1025. doi: 10.1016/0092-8674(94)90276-3. [DOI] [PubMed] [Google Scholar]
- 19.Higgins C, Petukhova L, Harel S, et al. FGF5 is a crucial regulator of hair length in humans. Proc Natl Acad Sci U S A. 2014;111(29):3–8. doi: 10.1073/pnas.1402862111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Petho-Schramm A, Muller H-J, Paus R. FGF5 and the murine hair cycle. Arch Dermatol Res. 1996;288(5–6):264–266. doi: 10.1007/BF02530098. [DOI] [PubMed] [Google Scholar]
- 21.He X, Chao Y, Zhou G, Chen Y. Fibroblast growth factor 5-short (FGF5s) inhibits the activity of FGF5 in primary and secondary hair follicle dermal papilla cells of cashmere goats. Gene. 2016;2(2 pt 2):393–398. doi: 10.1016/j.gene.2015.09.034. [DOI] [PubMed] [Google Scholar]
- 22.Rosenquist TA, Martin GR. Fibroblast growth factor signaling in the hair growth cycle: expression of the fibroblast growth factor receptor and ligand genes in the murine hair follicle. Dev Dyn. 1996;205(4):379–386. doi: 10.1002/(SICI)1097-0177(199604)205:4<379::AID-AJA2>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 23.Ota Y, Saitoh Y, Suzuki S, Ozawa K, Kawano M, Imamura T. Fibroblast growth factor 5 inhibits hair growth by blocking dermal papilla cell activation. Biochem Biophys Res Commun. 2002;290(1):169–176. doi: 10.1006/bbrc.2001.6140. [DOI] [PubMed] [Google Scholar]
- 24.Suzuki S, Kato T, Takimoto H, et al. Localization of rat FGF-5 protein in skin macrophage-like cells and FGF-5S protein in hair follicle: possible involvement of two. Growth. 1998;111(6):963–972. doi: 10.1046/j.1523-1747.1998.00427.x. [DOI] [PubMed] [Google Scholar]
- 25.Suzuki S, Ota Y, Ozawa K, Imamura T. Dual-mode regulation of hair growth cycle by two Fgf-5 gene products. J Invest Dermatol. 2000;114(3):456–463. doi: 10.1046/j.1523-1747.2000.00912.x. [DOI] [PubMed] [Google Scholar]
- 26.Ito C, Saitoh Y, Fujita Y, et al. Decapeptide with fibroblast growth factor (FGF)-5 partial sequence inhibits hair growth suppressing activity of FGF-5. J Cell Physiol. 2003;197(2):272–283. doi: 10.1002/jcp.10369. [DOI] [PubMed] [Google Scholar]
- 27.Maeda T, Yamamoto T, Isikawa Y, et al. Sanguisorba officinalis root extract has FGF-5 inhibitory activity and reduces hair loss by causing prolongation of the anagen period. Nishinihon J Dermatol. 2007;69(1):81–86. [Google Scholar]
- 28.Mustafa NM. Citrus essential oils: current and prospective uses in the food industry. Recent Pat Food Nutr Agric. 2015;7(2):115–127. doi: 10.2174/2212798407666150831144239. [DOI] [PubMed] [Google Scholar]
- 29.Barbosa LA, Filomeno CA, Teixeira RR. Chemical variability and biological activities of eucalyptus spp. Essential Oils. Molecules. 2016;21(12):1–33. doi: 10.3390/molecules21121671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Soliman FM, Fathy MM, Salama MM, Saber FR. Chemical composition and bioactivity of the volatile oil from leaves and stems of Eucalyptus cinerea. Pharm Biol. 2014;52(10):1272–1277. doi: 10.3109/13880209.2014.889177. [DOI] [PubMed] [Google Scholar]
- 31.Koziol A, Stryjewska A, Librowski T, et al. An overview of the pharmacological properties and potential applications of natural monoterpenes. Mini Rev Med Chem. 2014;14(14):1156–1168. doi: 10.2174/1389557514666141127145820. [DOI] [PubMed] [Google Scholar]
- 32.Iida M, Ihara S, Matsuzaki T. Hair cycle-dependent changes of alkaline phosphatase activity in the mesenchyme and epithelium in mouse vibrissal follicles. Dev Growth Differ. 2007;49(3):185–195. doi: 10.1111/j.1440-169X.2007.00907.x. [DOI] [PubMed] [Google Scholar]
- 33.Crounse RG. The First Hair Symposium. New York: Medcom Press; 1973. The hair pluck: a diagnostic tool; pp. 147–156. [Google Scholar]
- 34.Peereboom-Wynia JDR. Comparative studies of the diameters of hairshafts in anagen and in telogen phases in male adults without alopecia and in male adults with androgenetic alopecia. In: Orfana E, Montagna W, Stuttgen G, editors. Hair Research. Heidelberg: Springer-Verlag; 1981. pp. 294–302. [Google Scholar]
- 35.Van Scott EJ. Responses of hair roots to chemical and physical influences. In: Montagna W, Ellis RA, editors. Biology of Hair Growth. New York: Academic Press; 1958. pp. 441–449. [Google Scholar]
- 36.Maibach HI. The First Human Hair Symposium. New York: Medcom Press; 1973. Hair growth measurements in man; pp. 399–406. [Google Scholar]
- 37.Warmuth M, Kim S, Gu X, Xia G, Adrián F. Ba/F3 cells and their use in kinase drug discovery. Curr Opin Oncol. 2007;19(1):55–60. doi: 10.1097/CCO.0b013e328011a25f. [DOI] [PubMed] [Google Scholar]
- 38.Choi J, Park JB, Moon W, et al. Safety and efficacy of rice bran supercritical CO2 extract for hair growth in androgenic alopecia: a 16-week double-blind randomized controlled trial. Biol Pharm Bull. 2015;38(12):1856–1863. doi: 10.1248/bpb.b15-00387. [DOI] [PubMed] [Google Scholar]
- 39.Tosti A, Zaiac MN, Canazza A, et al. Topical application of the Wnt/beta-catenin activator methyl vanillate increases hair count and hair mass index in women with androgenetic alopecia. J Cosmet Dermatol. 2016;15(4):469–474. doi: 10.1111/jocd.12225. [DOI] [PubMed] [Google Scholar]
- 40.McMichael A, Pham H, von Grote E, Meckfessel M. Efficacy and safety of minoxidil 2% solution in combination with a botanical hair solution in women with female pattern hair loss/androgenic alopecia. J Drugs Dermatol. 2016;15(4):398–404. [PubMed] [Google Scholar]
- 41.Keaney TC, Pham H, von Grote E, Meckfessel M. Efficacy and safety of minoxidil 5% foam in combination with a botanical hair solution in men with androgenic alopecia. J Drugs Dermatol. 2016;15(4):406–412. [PubMed] [Google Scholar]
- 42.Gupta AK, Charrette A. Topical minoxidil: systematic review and meta-analysis of its efficacy in androgenetic alopecia. Skinmed. 2015;13(3):185–189. [PubMed] [Google Scholar]
- 43.Arca E, Acikgoz G, Tastan HB, Kose O, Kurumlu Z. An open, randomized, comparative study of oral finasteride and 5% topical minoxidil in male androgenetic alopecia. Dermatology. 2004;209(2):117–125. doi: 10.1159/000079595. [DOI] [PubMed] [Google Scholar]
- 44.Gupta AK, Foley KA. 5% minoxidil: treatment for female pattern hair loss. Skin Therapy Lett. 2014;19(6):5–7. [PubMed] [Google Scholar]
- 45.Levy LL, Emer JJ. Female pattern alopecia: current perspectives. Int J Womens Health. 2013;5(1):541–556. doi: 10.2147/IJWH.S49337. [DOI] [PMC free article] [PubMed] [Google Scholar]