Abstract
Striae distensae are a frequent skin condition associated with pregnancy, weight change or lack of skin elasticity. The aim of this research was to obtain a topical product containing herbal active ingredients with documented antioxidant and anti-inflammatory activity (Punica granatum seed oil and Croton lechleri resin extract) and demonstrate its positive effect on prevention and treatment of striae distensae. First, the cream base formulation was optimized through experimental design. Secondly, the cream containing the two active ingredients was investigated in an interventional nonrandomized clinical trial. The clinical outcome was assessed through biophysical parameters and ultrasonographic evaluation. The state of the skin was evaluated by biophysical measurements and ultrasonography at the beginning of the study and after 3 and 6 weeks. The experimental design was successfully used to set the best ranges for the technological and formulation factors to obtain a cosmetic formulation with optimal characteristics. The study of clinical efficacy on the optimal formulation revealed an increase in the dermis thickness, hydration and elasticity values in both groups after 6 weeks of cream application. The new oil-in-water cream containing P. granatum seed oil and C. lechleri resin extract can be helpful in the prevention or improving of skin changes associated with striae.
Keywords: stretch marks, ultrasonography, texture analysis, design of experiments, oil-in-water emulsion
Introduction
Striae distensae (SD) represent dermal lesions with multifactorial physiopathology, but their occurrence mechanisms have not been fully elucidated until now. Although rarely inducing major medical problems, striae represent an esthetic concern for those affected.1 SD appear as linear bands with an erythematous to violaceus color (striae rubrae) and gradually become hypopigmented atrophic lines (striae albae). Striae albae represent the stabilized form of striae, characterized by the absence of erythema and the decrease of melanization.2 Rarely, in people with darker skin, striae appear darker because of increased melanization as striae cerulae (blue) or striae nigrae (black).1–4 The color of SD is linked to the stage of their evolution and to the influence of melanocyte mechanobiology.5 Also, in case of striae nigrae and striae cerulae, the releasing of cytokines, such as the stem cell factor and hepatocyte growth factor, may induce epidermal hyperpigmentation.2
The anatomical appearance sites include abdomen and breasts areas in case of striae gravidarum, thighs and lumbosacral regions in adolescent males and abdomen, breasts and thighs in adolescent females.1,6,7 Numerous risk factors, such as topical or systemic use of corticosteroids, pregnancy, adolescence, weight variation and tissue expansion, Cushing, and Marfan syndrome, have been associated with the presence of striae.3,8–10 The genetic predisposition, family history, race and skin type also represent etiological risk factors.6 Among all forms of SD, striae gravidarum appear with a high prevalence, estimated at up to 90% of pregnant women. The most important factors involved in pathogenesis of striae gravidarum are the mechanical stress of the skin, hormonal variations and pre-existing dermal pathology.3,8,11,12
The physiopathogenetic mechanism of SD involves changes similar with scar formation process.1,7,10 In an initial stage, inflammation and edema of the dermis develop, followed by the deposition of dermal collagen along the lines of mechanical stress and the flattering of rete pegs.1,9 Another hypothesis suggests that the formation of SD is related to the reorganization and diminution of dermal elastic fibers network, rather than collagen transformation.13 Excessive mechanical stretching of the skin determines the rupture of the elastic fibers while the fibroblasts are unable to repair the components of the extracellular matrix.14 The quality of fibroblasts is also altered in striae, with a reduction in fibronectin, and type I and type III procollagen expression in fibroblasts from SD.8 The atrophic aspect of SD is determined by the decrease of the amounts of fibrillin surrounding the dermoepidermal junction and the elastin in the papillary dermis, together with the realignment of elastin and fibrillin fibers in deep dermis.1,11
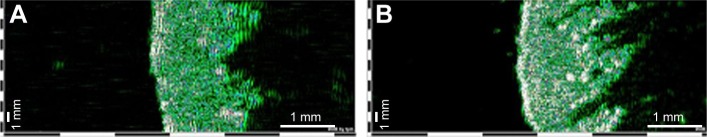
In recent years, ultrasonography emerged as an efficient method to monitor the evolution of skin characteristics after topical products application. Ultrasonographic evaluation of SD reveals a heteroechoic hyperechoic area with the decrease of dermis thickness and an increased heterogeneity and density, showing a morphologic similarity with atrophic scars (Figure 1).
Figure 1.
Ultrasonographic aspect of healthy skin (A) and skin with stretchmarks (B). 22 MHz transducer, B-scan; images from the same individual (Dub®cutis, Lüneburg, Germany). Striae distensae are delineated as low-echogenic bands inside the dermis surrounded by high-echogenic areas.
In order to improve SD aspect, multiple therapeutic options exist, but no standard treatment was yet found to cure striae albae with the pathology and associated symptoms.4,11 For this purpose, numerous topical treatments including tretinoin, glycolic acid, trichloroacetic acids, silicone gel and cosmetic preparations were evaluated,7,15–19 together with nonpharmacologic interventions, such as microdermabrasion, laser techniques and surgical methods.20–23 Vitamin C and fruity acids showed positive effects on striae rubrae, together with highly moisturizing preparations. Among the herbal actives, creams with Centella asiatica extract, as trofolastin cream, were the most efficient supporting treatment, even in striae gravidarum.
Topical preparations used in the prevention and treatment of the early-stage SD are expected to induce the following processes: increase fibroblastic activity, collagen and fibronectin production, improve blood perfusion and anti-inflammatory properties, and stimulate cell proliferation.4,19 At a global level, these effects correlate to the skin texture, meaning improved skin hydration and increased elasticity and dermis thickness.
Punica granatum seed oil represents a rich source of conjugated fatty acids (punicic acid), flavonoids and anthocyanidins (delphinidin, cyanidin and pelargonidin) with antioxidant and anti-inflammatory activity and conventionally used for the antiaging effect.24 According to previous research of P. granatum seeds exhibits DPPH free radicals scavenging, the antioxidant activity being three times greater than green tea extract.25,26 Also, on a human skin cells study, Aslam et al reported that P. granatum seed oil stimulates keratinocyte proliferation and induces a mild thickening of the epidermis without the loss of ordered differentiation.27 In complementary medicine, the effect of P. granatum extract in diabetes mellitus and irritable bowel syndrome was investigated.28,29
Croton lechleri (Dragon’s blood) presents antioxidant, anti-inflammatory and healing properties due mainly to the principal constituents of the resin: proanthocyanidins; taspin, an alkaloid; catechin; epigallocatechin; and epicatechin. Regarding the healing process, previous studies have showed that C. lechleri extract stimulates the proliferation and migration of fibroblasts and also the production of collagen and the epithelial regeneration. There are numerous studies concerning the multitude of medicinal properties of C. lechleri extract, such as wound healing, cicatrizing, antibacterial, antiviral, anti-inflammatory and antioxidant activity.30
The cosmetic cream represents a complex formulation, where the effect is not only due to the active ingredients but also to emollient ingredients which can improve SD. Emollient ingredients (Mangifera indica seed butter and Butyrospermum parkii butter) were incorporated in the formulation for the beneficial effect on improving skin elasticity. As shown in other previous histological studies, elastic fibers represent the primary target in the pathological process of SD. In the incipient phase, mast cell degranulation and macrophage activation lead to release of elastases, followed by alteration of collagen, elastin and fibrillin.31
So far, many cosmetic formulations containing herbal extracts were developed for antistretchmark effect, but the proofs supporting their clinical efficacy are limited.19
The aim of this research was to obtain a cosmetic cream containing herbal active ingredients with documented antioxidant and anti-inflammatory activity: P. granatum seed oil and C. lechleri resin extract. First, the cream base formulation was optimized according to a design of experiments (DoE) regarding its viscosity, texture and stability. In a second stage, the two active ingredients were added to the optimal cream base and the efficacy of the final product was investigated in an interventional nonrandomized study. The clinical outcome was assessed through biophysical parameters and ultrasonographic evaluation.
Materials and methods
Materials
Abil® EM 180 (cetyl PEG/PPG-10/1 dimethicone; Evonik Goldschmidt, Essen, Germany), glyceryl stearate (Elemental, Oradea, Romania) and Olliva (cetearyl olivate/sorbitan olivate; Elemental) were chosen as lipophilic surfactants, and Simulgel INS100 (hydroxyethyl acrylate/sodium acryloyldimethyl taurate copolymer and isohexadecane and Polysorbate 60; Seppic, Paris, France) was chosen as the co-surfactant. The cetylstearyl alcohol was supplied by Vitamar (Bucharest, Romania); B. parkii butter (shea butter), M. indica seed butter (mango butter), glycerin, and Olliva were supplied by Elemental. The other materials used were the following: P. granatum seed oil (Elemental), C. lechleri resin extract (Aroma-Zone, Paris, France), caprylic/capric triglycerides (Croda, Snaith, UK), Sepigel 305 (polyacrylamide and C13-14 isoparaffin and laureth-7; Seppic), Euxyl PE 9010® (phenoxyethanol and ethylhexylglycerin; Schülke & Mayr, Norderstedt, Germany), and Xiameter® PMX-0246 (cyclohexasiloxane and cyclopentasiloxane; Dow Corning, Midland, MI, USA).
Methods
Experimental design for base formulation
The experimental work was conducted by a D-Optimal DoE with six factors and three levels (Modde 11.0 software; Umetrics, Umea, Sweden). The studied factors were the type of emulsifier (X1) with three variables: E1 (Abil EM 180), E2 (glyceryl stearate) or E3 (Olliva); the amount of oily phase (g; X2); the stirring rate (X3); the stirring time for the emulsification step (X4); the ratio of surfactant (%; X5); and the presence of 2% Simulgel INS100 as co-surfactant (X6). The software generated 18 experimental trials and 3 center points, presented in Table 1.
Table 1.
Experimental design matrix
| Exp | X1 | X2 (g) | X3 (rpm) | X4 (min) | X5 (%) | X6 |
|---|---|---|---|---|---|---|
| N1 | E1 | 19 | 500 | 5 | 1.50 | Yes |
| N2 | E1 | 28.5 | 1,000 | 10 | 2 | Yes |
| N3 | E1 | 38 | 1,500 | 15 | 3 | Yes |
| N4 | E2 | 19 | 500 | 10 | 2 | Yes |
| N5 | E2 | 28.5 | 1,000 | 15 | 2.50 | Yes |
| N6 | E2 | 38 | 1,500 | 5 | 1 | Yes |
| N7 | E3 | 19 | 1,000 | 5 | 3 | Yes |
| N8 | E3 | 28.5 | 1,500 | 10 | 2 | Yes |
| N9 | E3 | 38 | 500 | 15 | 2.5 | Yes |
| N10 | E1 | 19 | 1,500 | 15 | 2 | No |
| N11 | E1 | 28.5 | 500 | 5 | 2.5 | No |
| N12 | E1 | 38 | 1,000 | 10 | 1.5 | No |
| N13 | E2 | 19 | 1,000 | 5 | 1 | No |
| N14 | E2 | 28.5 | 1,500 | 5 | 1.5 | No |
| N15 | E2 | 38 | 500 | 10 | 2.5 | No |
| N16 | E3 | 19 | 1,500 | 10 | 3 | No |
| N17 | E3 | 28.5 | 500 | 15 | 2 | No |
| N18 | E3 | 38 | 1,000 | 10 | 2.5 | No |
| N19 | E1 | 28.5 | 1,000 | 10 | 2 | Yes |
| N20 | E1 | 28.5 | 1,000 | 10 | 2 | Yes |
| N21 | E1 | 28.5 | 1,000 | 10 | 2 | Yes |
Abbreviations: Exp, experiment; E1, surfactant Abil EM 180; E2, surfactant glyceryl stearate; E3, surfactant Olliva; X1, emulsifier type; X2, amount of oily phase; X3, stirring rate; X4, time of stirring; X5, ratio of the surfactant; X6, the use of a co-surfactant (yes/no).
The dependent variables were firmness (Y1), consistency (Y2), adhesive force (Y3), adhesiveness (Y4), stringiness (Y5), stringiness length (Y6), spreadability (Y7), viscosity (Y8), and the physical stability of the creams (Y9).
Quality-of-fit assessment, coefficient calculation and statistic parameters evaluation were performed with Modde 11.0 software (Umetrics). The data fitting was done using partial least-squares method. To determine the influence of each factor on the properties of the emulsions, the results were evaluated by means of statistical analysis using analysis of variance (ANOVA) test.
Based on the experimental design results, an optimal formulation was generated using the same software, by applying a set of constraints for each of the responses. The model validity was assessed by comparing the obtained results with the ones predicted by the program.
Cream preparation
The cream was prepared by heating separately the aqueous phase (Sepigel 305, Euxyl PE 9010, glycerol and distilled water) and the oily phase (cetylstearyl alcohol, B. parkii butter, M. indica seed butter, caprylic/capric triglycerides and Xiameter PMX-0246) containing the surfactants (E1, E2, or E3 in the proportions indicated in Table 1). The aqueous phase was added in the oily phase under continuous stirring (DLS Stirrer; Velp Scientifica, Usmate, Italy) at controlled temperature (50°C±2°C). The active ingredients were added in the oily and aqueous phase, depending on their solubility.
Characterization of creams
Viscosity (Y8)
Viscosity measurements were performed at 50 rpm using a rotational rheometer DV-III Ultra (Brookfield Engineering Laboratories, Middleborough, MA, USA), equipped with cylindrical spindle 64. The measurements were carried out in triplicate at 22°C±2°C, and mean viscosities ± standard deviation were reported.
Texture analysis (Y1–Y7)
CT3 Texture Analyzer (Brookfield Engineering Laboratories) was used for texture profile determination, following the methods listed in Table 2 for each of the following parameters: firmness, consistency, adhesive force, adhesiveness, stringiness, stringiness length and spreadability. Compression test type was used with TA-BT-KIT fixture, having a specified distance as target (Table 2). Both the test speed and the post-test speed were 2 mm/s. The results were recorded using Texture ProCT Software 1.5 (Brookfield Engineering Laboratories). For each formulation, three measurements were carried out, and the mean ± standard deviation was reported.
Table 2.
Texture analyzer settings and test methods used for the calculated parameters
| Texture parameter | Probe | Target value (mm) | Trigger load (g) |
|---|---|---|---|
| Firmness (g) | TA-DEC | 25 | 10 |
| Consistency (mJ) | TA-DEC | 25 | 10 |
| Adhesive force (g) | TA-SF | 15 | 2 |
| Adhesiveness (mJ) | TA-SF | 15 | 2 |
| Spreadability (g) | TA-SF | 15 | 2 |
| Stringiness work done (mJ) | TA-10 | 25 | 10 |
| Stringiness length (mm) | TA-10 | 25 | 10 |
Physical stability evaluation (Y9)
The physical stability of the creams was evaluated after storage under standardized test conditions at 25°C±2°C for 60 days. The stability of the disperse system was assessed by visual observation using a 5-point scale where: 1 = stable homogeneous emulsion system and 5 = system where breakdown processes such as creaming or coalescence occurred.
In vivo cream efficacy evaluation
Study design
Subjects
An interventional, nonrandomized study, approved by the Research Ethics Committee of the Iuliu Haţieganu University of Medicine and Pharmacy (clinical trial registration number: 244; date of approval: June 9, 2016) was carried out on 20 healthy female volunteers aged between 21 and 48 years, divided into two subgroups: 10 volunteers with striae albae at the hip level and 10 volunteers with no stretchmarks on the test area. The in vivo study aimed to observe the clinical changes at the epidermis and dermis level both in case of already defined striae and in case of no striae on the area of interest.
Before enrolling in the study, every subject was informed about the purpose of the study and signed an informed consent. The cosmetic cream was applied once daily, on the hip area, for a period of 6 weeks. During the study, each volunteer used the same hygiene product and no other cosmetic cream was applied on the tested areas.
The instrumental measurements (skin hydration level, skin elasticity and thickness of the dermis) were performed at the beginning of the study (T0), after 3 weeks (T1) and at the end of the study – after 6 weeks (T2). The volunteers were preconditioned in the test room for at least 15 minutes before the measurements.
Ultrasound scanning
The ultrasonographic evaluation was performed using Dub®cutis skinscanner system (Taberna pro medicum, Lüneburg, Germany), a high-resolution ultrasound scanner using a 22-MHz transducer that allows visualizing structures up to 6–7 mm in depth, including epidermis, dermis and partially subcutaneous fatty tissue.
Biophysical parameter measurements
Skin hydration values and elasticity measurements were recorded using Multi Probe Adapter (MPA Systems) (Courage + Khazaka Electronic GmbH, Köln, Germany) equipped with the probes Corneometer® CM 825 (stratum corneum hydration) and Cutometer® dual MPA 580 (viscoelasticity of the skin).
Results and discussion
Experimental design
Table 3 presents the matrix of the responses analyzed in the experimental plan. The validity of the experimental design was checked, and the results obtained after data fitting showed that the results fit the models. Partial least-squares method was applied to fit the experimental results to the model, and the statistical parameters R2, Q2 and model validity were calculated (Table 4).
Table 3.
Matrix of responses
| Firmness (g) (Y1) | Consistency (mJ) (Y2) | Adhesive force (g) (Y3) | Adhesiveness (mJ) (Y4) | Stringiness work done (Y5) | Stringiness length (Y6) | Spreadability (g) (Y7) | Viscosity (cP) (Y8) | Stability (Y9) | |
|---|---|---|---|---|---|---|---|---|---|
| N1 | 1,045.30±36.70 | 212.26±7.77 | 191.80±32.20 | 8.72±0.56 | 0.66±0.10 | 3.72±0.21 | 313.80±42.70 | 102,800±1,744 | 2.00 |
| N2 | 1,361.00±23.50 | 281.97±9.20 | 207.70±65.30 | 9.30±0.86 | 2.17±0.22 | 9.88±2.08 | 355.30±89.20 | 133,200±6,548 | 3.00 |
| N3 | 1,233.00±118.60 | 250.53±18.28 | 284.80±40.10 | 8.32±1.32 | 1.18±0.03 | 8.33±0.42 | 494.20±83.40 | 80,600±2,163 | 2.00 |
| N4 | 1,941.20±157.40 | 367.57±24.01 | 179.00±25.90 | 9.91±1.45 | 4.54±0.24 | 18.03±2.02 | 331.50±39.50 | 68,933±3,172 | 1.00 |
| N5 | 2,477.80±58.40 | 425.51±11.37 | 142.20±27.30 | 3.01±0.51 | 6.97±1.23 | 16.48±2.54 | 551.70±122.30 | 67,867±4,601 | 3.00 |
| N6 | 2,468.50±27.50 | 476.99±35.68 | 138.70±7.80 | 2.98±0.40 | 6.76±1.29 | 13.31±3.30 | 498.50±47.30 | 47,867±6,839 | 4.00 |
| N7 | 1,453.00±80.20 | 290.32±13.53 | 135.00±12.70 | 6.29±1.90 | 4.55±0.43 | 23.76±1.33 | 282.30±8.60 | 47,067±4,549 | 2.00 |
| N8 | 2,093.30±83.00 | 433.09±14.95 | 112.80±27.20 | 2.62±0.34 | 8.23±1.50 | 20.37±2.71 | 411.80±68.40 | 57,333±1,155 | 3.00 |
| N9 | 2,615.00±156.60 | 530.71±42.27 | 103.70±13.70 | 2.35±0.63 | 8.06±0.13 | 21.2±1.21 | 359.20±12.90 | 35,800±2,307 | 3.00 |
| N10 | 1,033.50±30.04 | 170.71±12.73 | 229.00±42.50 | 6.65±0.81 | 0.55±0.08 | 3.04±0.51 | 334.50±61.70 | 10,7067±5,001 | 1.00 |
| N11 | 993.50±42.30 | 200.06±14.08 | 212.80±60.30 | 7.62±0.11 | 1.57±0.37 | 7.22±0.85 | 334.80±86.50 | 74,533±2,626 | 2.00 |
| N12 | 1,183.50±54.70 | 243.66±16.58 | 179.80±34.20 | 8.79±0.73 | 1.79±0.34 | 5.53±0.23 | 336.80±51.60 | 86,600±1,249 | 2.00 |
| N13 | 2,230.70±205.30 | 419.03±46.58 | 160.50±12.80 | 9.80±0.74 | 1.76±0.29 | 6.59±0.75 | 241.80±21.50 | 125,333±7,943 | 1.00 |
| N14 | 1,741.30±73.90 | 308.65±6.71 | 159.30±33.20 | 8.74±1.16 | 0.87±0.18 | 2.47±0.35 | 282.50±44.20 | 75,467±3,029 | 2.00 |
| N15 | 2,128.70±135.40 | 404.51±28.40 | 168.80±64.80 | 5.31±2.91 | 4.56±0.63 | 10.04±1.17 | 377.80±27.50 | 35,200±6,437 | 3.00 |
| N16 | 1,715.80±241.30 | 312.25±35.92 | 82.50±9.20 | 5.12±0.42 | 2.66±3.50 | 0.23±0.06 | 191.30±14.80 | 67,867±3,802 | 1.00 |
| N17 | 2,362.50±117.70 | 477.92±27.15 | 95.00±9.70 | 5.31±0.54 | 4.49±0.28 | 17.8±2.76 | 247.50±22.30 | 79,933±8,556 | 1.00 |
| N18 | 2,229.70±26.40 | 379.78±44.92 | 87.00±8.20 | 2.33±0.26 | 6.68±0.43 | 21.2±1.20 | 245.30±16.60 | 41,200±4,970 | 3.00 |
| N19 | 1,025.00±58.70 | 203.00±11.04 | 242.70±38.50 | 7.30±0.72 | 0.75±0.06 | 8.1±1.14 | 391.50±54.40 | 105,333±3,802 | 2.50 |
| N20 | 1,258.00±15.60 | 259.72±23.43 | 192.70±22.30 | 8.41±0.24 | 2.93±0.14 | 0.14±0.02 | 320.30±31.40 | 126,233±4,306 | 3.00 |
| N21 | 1,408.00±17.05 | 301.00±14.72 | 244.70±37.30 | 9.05±1.11 | 1.68±0.09 | 8.87±0.37 | 409.00±58.80 | 119,267±2,386 | 3.50 |
Notes: Y1, firmness; Y2, consistency; Y3, adhesive force; Y4, adhesiveness; Y5, stringiness; Y6, stringiness length; Y7, spreadability; Y8, viscosity; Y9, physical stability (mean ± SD).
Abbreviation: Exp, experiment.
Table 4.
Statistical parameters – ANOVA test
| Response | SS | df | MS (variance) | F | P-value | Lack of fit | R2 | Q2 | Model validity |
|---|---|---|---|---|---|---|---|---|---|
| Y1 | 6.79E+07 | 21 | 3.23E+06 | 24.2954 | 0 | 0.298 | 0.859 | 0.756 | 0.697 |
| Y2 | 2.52E+06 | 21 | 119,854 | 17.2079 | 0 | 0.408 | 0.811 | 0.688 | 0.775 |
| Y3 | 663,238 | 21 | 31,582.7 | 19.6317 | 0 | 0.67 | 0.867 | 0.731 | 0.900 |
| Y4 | 1,033.86 | 21 | 49.2316 | 8.99197 | 0.001 | 0.178 | 0.613 | 0.404 | 0.569 |
| Y5 | 389.273 | 21 | 18.5368 | 16.441 | 0 | 0.287 | 0.804 | 0.673 | 0.687 |
| Y6 | 3,537.78 | 21 | 168.466 | 6.97795 | 0.001 | 0.523 | 0.699 | 0.352 | 0.838 |
| Y7 | 2.71E+06 | 21 | 129,028 | 9.21479 | 0 | 0.278 | 0.697 | 0.489 | 0.680 |
| Y8 | 1.54E+11 | 21 | 7.34E+009 | 8.71337 | 0 | 0.266 | 0.789 | 0.552 | 0.668 |
| Y9 | 126.5 | 21 | 6.02381 | 14.838 | 0 | 0.352 | 0.724 | 0.58 | 0.739 |
Abbreviations: ANOVA, analysis of variance; SS, sum of squares; df, degrees of freedom; MS, mean of square; F, Fischer’s ratio; R2, regression coefficient; Q2, predictive power of the model.
ANOVA was performed to evaluate model significance (Table 4). All models were significant since P-value was <0.05 and lack of fit was not significant (P>0.05).
R2 is a measure of how well the regression model fits the experimental data. The closer the correlation values are to 1, the better is the developed model and its prediction. Our results showed a good fit for each of the responses, with values over 0.6, which indicates high model significance. The validity parameter shows if the appropriate type of model was chosen; in our case, the validity was good for all responses. Reproducibility, which reflects experimental variability, attained high values, between 0.782 and 0.971.
Influence of independent variables on the texture analysis parameters
Texture analysis involves the compression of a sample using a certain probe, at a constant predetermined rate. The product resistance is recorded as load values versus distance or time. Each compression cycle results in a graphical representation of the load (g) as a function of time (s); all the texture describing parameters can be calculated directly from the texture profile plot, using appropriate mathematical equations.
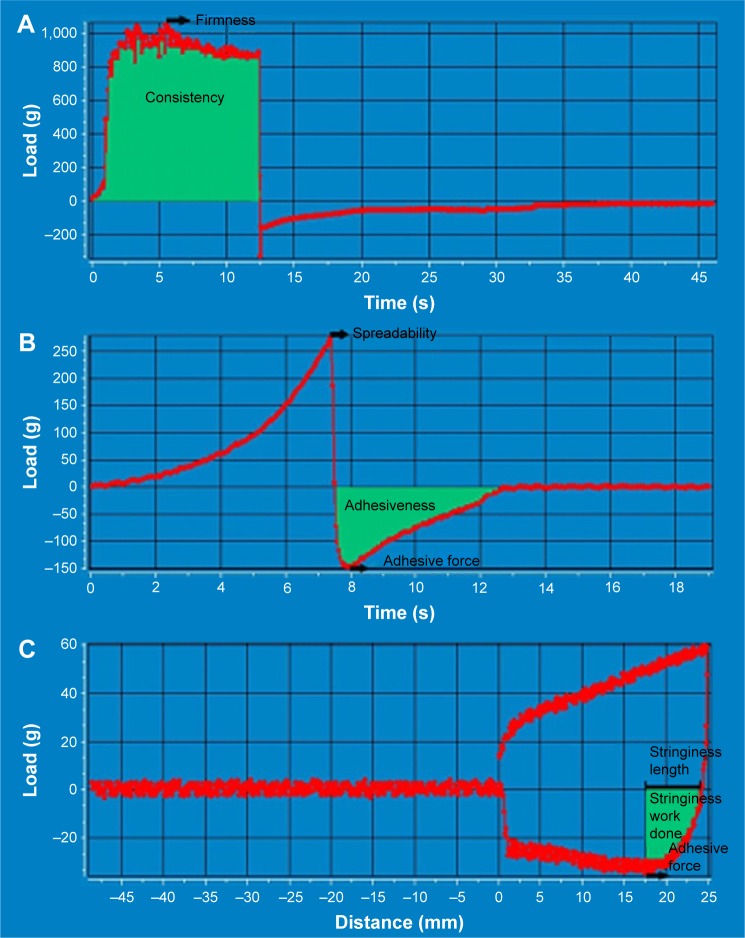
The maximum force on the graph measures firmness (Y1), whereas the area under the positive part of the graph indicates the consistency (Y2) of the sample (Figure 2A). Spreadability of the samples was (Y7) recorded while the cone probe was forced down into the conical sample holder filled with the cream. The firmness of the sample measured as the maximum force on the graph indicates the spreadability of the sample (Figure 2B). The negative part of the plot is generated while the probe returns to the starting point and the maximum negative force of the compression cycle is considered as adhesive force (Y3), while the area under the negative part leads to the adhesiveness value (Y4). Stringiness (Y5) of the sample is calculated as the area under the load versus distance curve, measured from where cycle one reaches zero load, to the adhesive force. Stringiness length (Y6) represents the distance from the zero load to the adhesive force (Figure 2C).
Figure 2.
Texture analysis plot showing calculations for (A) firmness and consistency, (B) spreadability, adhesive force and adhesiveness and (C) stringiness and stringiness length.
Revised models (Table 5) were developed to evaluate the effects of independent variables for each response, based on the regression coefficient values and signs (“+” for positive influence and “−” for negative influence).
Table 5.
The revised quantitative factor effects of the responses
| Factor | Firmness (g) (Y1) | Consistency (mJ) (Y2) | Adhesive force (g) (Y3) | Adhesiveness (mJ) (Y4) | Stringiness work done (mJ) (Y5) | Stringiness length (Y6) | Spreadability (g) (Y7) | Viscosity (cP) (Y8) | Stability (Y9) |
|---|---|---|---|---|---|---|---|---|---|
| Constant | 1,804.71 | 346.75a | 159.69a | 6.26a | 3.72a | 11.22a | 339.53 | 763.12a | 2.22a |
| X1 (E1) | −633.51 | −110.87a | 58.64a | 1.89a | −2.56a | −6.13a | 8.87 | 276.47a | – |
| X1 (E2) | 359.99 | 53.62a | −1.61 | 0.36 | 0.51 | −0.07 | 41.09 | −62.01 | – |
| X1 (E3) | 273.51 | 57.25a | −57.03a | −2.25a | 2.05 | 6.20a | −49.97 | −214.45a | – |
| X2 | 203.24 | 42.83a | – | −1.36 a | 1.19a | 2.02 | 51.38 | −159.83a | 0.75a |
| X3 | – | – | – | – | – | −2.52 | – | 32.49 | – |
| X4 | – | – | 7.55 | – | – | – | – | 89.72 | −0.33a |
| X5 | −115.16 | −31.64a | 12.29 | – | – | – | – | −105.61a | – |
| X6 (yes) | – | – | 6.95 | – | 0.95a | 2.98a | 51.50 | – | 0.44a |
| X6 (no) | – | – | −6.95 | – | −0.95a | −2.98a | −51.50 | – | −0.44a |
Note:
Statistically significant effects (P<0.05).
Abbreviations: E1, surfactant Abil EM 180; E2, surfactant glyceryl stearate; E3, surfactant Olliva; X1, emulsifier type; X2, amount of oily phase; X3, stirring rate; X4, time of stirring; X5, ratio of the surfactant; X6, the use of a co-surfactant.
The firmness of the creams ranged between 993.50±42.30 and 2,615.00±156.60 g, whereas the consistency ranged between 170.71±12.73 and 530.71±42.27 mJ. Y1 and Y2 were mainly influenced by the type of emulsifying agent (X1) and the oily phase amount (X2). The use of E1, a liquid emulsifier, had a negative influence on the firmness and consistency values, whereas E2 and E3 (solid waxy emulsifiers) increased Y1 and Y2 values. The formulations with a high ratio of shea butter and mango butter in the oily phase also increased the load profile due to the solid emollients added.
Previous studies confirmed that between texture analysis and sensory characteristics exist direct correlations, and the sensory profile of a cosmetic product could be predicted through instrumental assessments.32 Adhesive properties define the sensory attributes of the cosmetic cream and influence consumers’ acceptance, while the spreadability also influences the efficacy of the cosmetic product. In our study, adhesive force (Y3), adhesiveness (Y4) and spreadability (Y7) were significantly influenced by the type of emulsifying agent (E1 enhanced the adhesive properties, whereas E3 led to less sticky formulations). Spreadability was favored by a higher emulsifying agent content, especially E2 and also by adding of the co-surfactant.
Stringiness (Y5 and Y6) of the creams is correlated with filament-stretching properties and influences the easy pick-up of the cosmetic product from the container. In the current study, stringiness was influenced by E3, the co-surfactant and a high level of lipophilic phase. The viscosity (Y8) varied between and was mainly influenced by emulsifying agent type and ratio. E1 determined higher viscosity values, whereas E3 determined lower ones.
A high percentage of lipophilic phases and the presence of the co-surfactant improved the stability (Y9) of the emulsions, but apparently, long processing times led to less stable products. Stirring time usually increases emulsions stability, up to an optimal value that when exceeded can produce system creaming.
Cream optimization
The sensation produced after the topical application of a cosmetic product is an important factor for the consumer preference. Consumer acceptability and the efficacy of topical products involve optimal mechanical properties (firmness), appropriate adhesiveness and a good spreadability.33
To achieve an optimal formulation regarding the stability and the sensorial characteristics, an optimization process was carried out. The conditions selected were the following: the spreadability and the stability were maximized, the firmness and the adhesiveness were minimized and the consistency was maintained at a medium value. The experimental conditions and the qualitative and quantitative parameters for the optimal formulation are shown in Table 6.
Table 6.
Experimental conditions for the optimal formulation
| Factor | Optimal level |
|---|---|
| X1 | E3 |
| X2 | −0.5190 |
| X3 | 0.9983 |
| X4 | 0.9880 |
| X5 | −0.9974 |
| X6 | No |
| Ingredients | Amount (%) |
| Cetylstearyl alcohol | 2.48 |
| Mangifera indica seed butter | 6.20 |
| Butyrospermum parkii butter | 6.20 |
| Caprylic/capric triglycerides | 6.20 |
| Xiameter® PMX-0246 | 2.48 |
| Olliva | 2.00 |
| Sepigel 305 | 1.50 |
| Euxyl PE 9010® | 1.00 |
| Glycerol | 5.00 |
| Distilled water | to 100 |
Abbreviations: E3, surfactant Olliva; X1, emulsifier type; X2, amount of oily phase; X3, stirring rate; X4, time of stirring; X5, ratio of the surfactant; X6, the use of a co-surfactant.
For the optimal formulation, the experimental data revealed a good correlation between the predicted model and the experimental values, thus indicating the validity of the proposed model (data not shown).
An oil-in-water cream containing Olliva (2%) as emulsifying agent and 23.56% oily phase was obtained using following process parameters: stirring rate − 1,500 rpm and stirring time −15 min. The active ingredients P. granatum seed oil (4%) and C. lechleri resin extract (3%) were incorporated in the optimal formulation, and the potential effect on preventing or improving SD was investigated.
In vivo efficacy study
In vivo efficacy study was carried out on 20 volunteers to observe the skin characteristics comparatively, before and after the treatment. Preclinical studies on animals were also considered, but there is no animal model for SD and this skin condition cannot be induced experimentally. Considering the mechanism of action of the ingredients from the cosmetic formulation, hydration and elasticity level of the skin and thickness of the dermis have been assessed to evaluate the clinical changes.
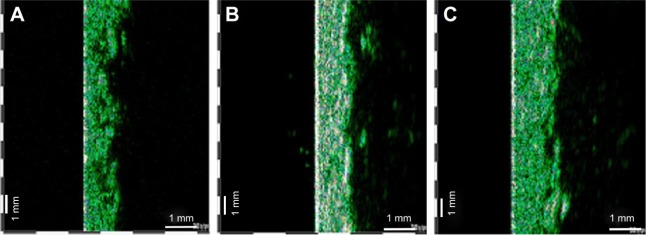
Figure 3 shows comparative ultrasonographic images of the skin at baseline (A), after 3 weeks (B) and after 6 weeks of cream application (C). Statistical analysis highlighted a significant increase in dermis thickness at the end of the study (Figure 4) in both subgroups. Ultrasound investigation of the skin revealed new echogenic areas instead of SD and also an improved acoustic homogeneity of the dermis.
Figure 3.
Ultrasonographic aspect of SD before (A), after 3 weeks (B) and after 6 weeks (C) of cream application- 22 MHz transducer, B-scan. Abbreviation: SD, striae distensae.
Figure 4.
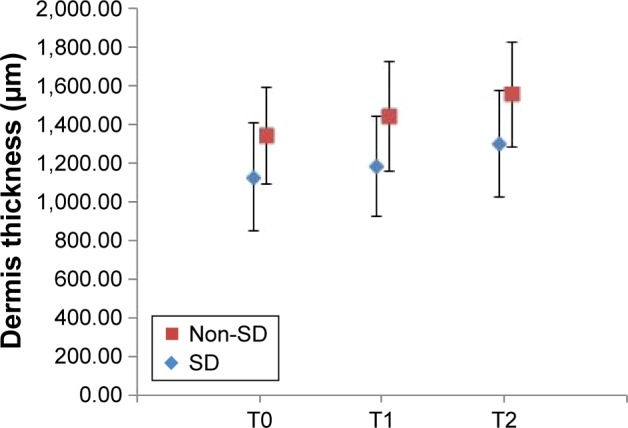
Relative changes in dermis thickness values during the study (mean ± standard deviation).
Notes: T0 – baseline determination, T1 – after 3 weeks, T2 – after 6 weeks of cream application; non-SD – subgroup without striae (N=10, P=0.0000486709), SD – subgroup with striae (N=10, P=0.0000455191).
Abbreviation: SD, striae distensae.
Statistical analysis also demonstrated positive results on hydration and elasticity levels of the skin in both subgroups. Mean values and standard deviation are represented in Figures 5 and 6.
Figure 5.
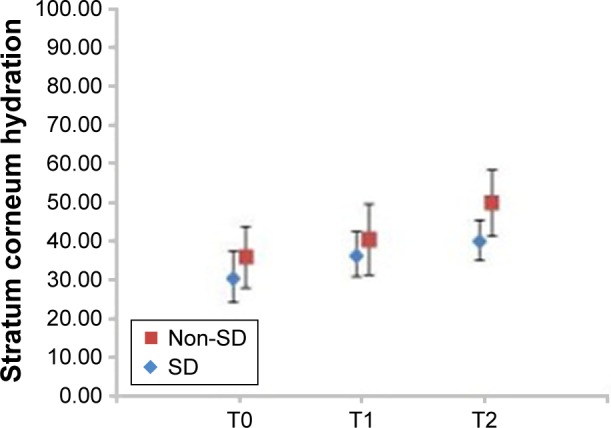
Relative changes of stratum corneum hydration level during the study (mean ± standard deviation).
Notes: T0 – baseline determination, T1 – after 3 weeks, T2 – after 6 weeks of cream application; non-SD – subgroup without striae (N=10, P=0.000109062), SD – subgroup with striae (N=10, P=0.0000332375).
Abbreviation: SD, striae distensae.
Figure 6.
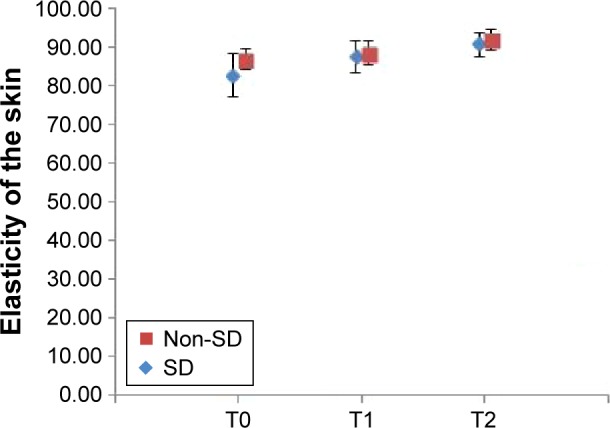
Relative changes in the elasticity of the skin during the study (mean ± standard deviation).
Notes: T0 – baseline determination, T1 – after 3 weeks, T2 – after 6 weeks of cream application; non-SD – subgroup without striae (N=10, P=0.0017748), SD – subgroup with striae (N=10, P=0.000067221).
Abbreviation: SD, striae distensae.
In vivo clinical study revealed significant skin changes of the dermis thickness, hydration and elasticity of stratum corneum. For the SD subgroup, the increase in dermis thickness was 14.85%, the hydration increased 30.32% and the elasticity increased 9.75% from baseline. The subjective self-assessment of the volunteers indicated that, after 6 weeks of cream application, striae become less defined and less depressed.
In case of the subgroup with no SD, the increase in dermis thickness was 15.86%, whereas hydration and elasticity increased from baseline, 38.40% and 5.86%, respectively. The volunteers also noticed an improved appearance of the skin. The results obtained in both subgroups suggest that it is possible to minimize or prevent the skin alterations which appear in SD.
Even if there are a large number of marketed topical products, no standard treatment exists for the management of SD. An increase in the elasticity of connective tissue represents one of the main goals of the antistriae formulations. Other topical formulations for SD present different mechanisms of action, which include the increasing collagen production, anti-inflammatory effects as well as rehydration of the skin.4
Although numerous studies showed multiple advantages of some topical products recommended for SD, there is limited evidence that topical therapy can thoroughly eradicate the lesions. Moreover, only a few studies are supported by high-quality controlled trials to demonstrate antistriae efficacy.24 In one of those trials, a double-blind controlled study, Ud-Din et al reported an improving SD albae and SD rubrae after 6 weeks of topical application of a silicone gel. They evaluated the clinical efficacy with noninvasive devices, histologically, and the preliminary findings revealed a decrease in the vascularity, hemoglobin, pliability, collagen and an increase in the pigmentation.7
In another study, Bleve et al carried out a clinical trial to monitor SD maturation and to evaluate their changes after the topical treatment with a marketed commercial product. The evaluation of topical treatment efficacy was performed using ultrasound scanning and Primos system, and the results showed that after 2 months of treatment only striae rubra were treated successfully.1
In all those cases, the topical products were applied by local massage on SD and it is difficult to establish the influence of the massage on the improvement of striae. Timur Taşhan and Kafkasli evaluated the application of almond oil with or without massage therapy and demonstrated that almond oil applied through a 15-minute massage reduced the development of striae gravidarum, whereas the use of almond oil without massage had no effect.34
As well, various vegetal oils have been evaluated for their beneficial effect in SD, the most commonly investigated being olive oil. The results of the study noticed no significant difference in development or severity of SD between treatment and control groups.35–37
The results of the present study are in accordance with those presented in similar research.35–37 Although the topical application of the tested cosmetic cream improved skin parameters (dermis thickness, hydration and elasticity), the repairing process is incomplete since the changes in SD are irreversible. Therefore, the most appropriate approach in SD management is prophylaxis.
To our knowledge, this is the first study reported in the literature which evaluates the antistriae effect of a formulation containing P. granatum seed oil and C. lechleri resin extract. The results are supported by an interventional nonrandomized clinical study, where the changes were evaluated both in volunteers with SD or on healthy skin. In addition, the use of DoE provides a new approach to the cosmetic formulation which overcomes the deficiencies of traditional formulation methods to obtain a cosmetic product with desired characteristics using a minimal number of experimental runs.
Conclusion
An emollient oil-in-water cream containing P. granatum seed oil and C. lechleri resin extract was formulated. The experimental design was successfully used to set the best ranges for the technological and formulation factors to obtain a cosmetic formulation with optimal characteristics. The study of clinical efficacy on the optimal formulation revealed an increase in the dermis thickness, hydration and elasticity values, suggesting that it can be helpful in prevention or improving skin changes associated with striae.
Acknowledgments
The authors gratefully acknowledge Sanorama Company, Cluj-Napoca, Romania, for allowing the use of the ultrasound equipment and Farmec Company, Cluj-Napoca, Romania, for the substances supplied. This research was financially supported by the University of Medicine and Pharmacy, Cluj-Napoca, Romania, Grant 1495/7/28.01.2014.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Bleve M, Capra P, Pavanetto F, Perugini P. Ultrasound and 3D skin imaging: methods to evaluate efficacy of striae distensae treatment. Dermatol Res Prat. 2012;2012:673706. doi: 10.1155/2012/673706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hermanns JF, Piérard GE. High-resolution epiluminescence colorimetry of striae distensae. J Eur Acad Dermatol Venereol. 2006;20(3):282–287. doi: 10.1111/j.1468-3083.2006.01426.x. [DOI] [PubMed] [Google Scholar]
- 3.Cordeiro RC, Zecchin KG, de Moraes AM. Expression of estrogen, androgen, and glucocorticoid receptors in recent striae distensae. Int J Dermatol. 2010;49(1):30–32. doi: 10.1111/j.1365-4632.2008.04005.x. [DOI] [PubMed] [Google Scholar]
- 4.Brennan M, Young G, Devane D. Topical preparations for preventing stretch marks in pregnancy. Cochrane Database Syst Rev. 2012;11:CD000066. doi: 10.1002/14651858.CD000066.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pierard-Franchimont C, Hermanns JF, Hermanns-Le T, Piérard GE. Striae distensae in darker skin types: the influence of melanocyte mechanobiology. J Cosmet Dermatol. 2005;4(3):174–178. doi: 10.1111/j.1473-2165.2005.00306.x. [DOI] [PubMed] [Google Scholar]
- 6.Al-Himdani, Ud-Din S, Gilmore S, Bayat A. Striae distensae: a comprehensive review and evidence-based evaluation of prophylaxis and treatment. Br J Dermatol. 2014;170(3):527–547. doi: 10.1111/bjd.12681. [DOI] [PubMed] [Google Scholar]
- 7.Ud-Din S, McAnelly SL, Bowring A, et al. A double-blind controlled clinical trial assessing the effect of topical gels on striae distensae (stretch marks): a non-invasive imaging, morphological and immunohistochemical study. Arch Dermatol Res. 2013;305(7):603–617. doi: 10.1007/s00403-013-1336-7. [DOI] [PubMed] [Google Scholar]
- 8.Gilmore SJ, Vaughan BL, Jr, Madzvamuse A, Maini PK. A mechanochemical model of striae distensae. Math Biosci. 2012;240(2):141–147. doi: 10.1016/j.mbs.2012.06.007. [DOI] [PubMed] [Google Scholar]
- 9.Hengge UR, Ruzicka T, Schwartz RA, Cork MJ. Adverse effects of topical glucocorticosteroids. J Am Acad Dermatol. 2006;54(1):1–15. doi: 10.1016/j.jaad.2005.01.010. [DOI] [PubMed] [Google Scholar]
- 10.Yosipovitch G, DeVore A, Dawn A. Obesity and the skin: skin physiology and skin manifestations of obesity. J Am Acad Dermatol. 2007;56(6):901–916. doi: 10.1016/j.jaad.2006.12.004. [DOI] [PubMed] [Google Scholar]
- 11.Nussbaum R, Bendetto AV. Cosmetic aspects of pregnancy. Clin Dermatol. 2006;25:133–141. doi: 10.1016/j.clindermatol.2005.10.007. [DOI] [PubMed] [Google Scholar]
- 12.Kasielska-Trojan A, Sobczak M, Antoszewski B. Risk factors of striae gravidarum. Int J Cosmet Sci. 2015;37(2):236–240. doi: 10.1111/ics.12188. [DOI] [PubMed] [Google Scholar]
- 13.Watson RE, Parry EJ, Humphries JD, et al. Fibrillin microfibrils are reduced in skin exhibiting striae distensae. Br J Dermatol. 1998;138(6):931–937. doi: 10.1046/j.1365-2133.1998.02257.x. [DOI] [PubMed] [Google Scholar]
- 14.Mitts TF, Jimenez F, Hinek A. Skin biopsy analysis reveals predisposition to stretch mark formation. Aesthet Surg J. 2005;25(6):593–600. doi: 10.1016/j.asj.2005.09.004. [DOI] [PubMed] [Google Scholar]
- 15.Rangel O, Arias I, García E, Lopez-Padilla S. Topical tretinoin 0.1% for pregnancy-related abdominal striae: an open-label, multicenter, prospective study. Adv Ther. 2001;18(4):181–186. doi: 10.1007/BF02850112. [DOI] [PubMed] [Google Scholar]
- 16.Adatto MA, Deprez P. Striae treated by a novel combination treatment–sand abrasion and a patent mixture containing 15% trichloracetic acid followed by 6–24 hrs of a patent cream under plastic occlusion. J Cosmet Dermatol. 2003;2(2):61–67. doi: 10.1111/j.1473-2130.2004.00023.x. [DOI] [PubMed] [Google Scholar]
- 17.Mazzarello V, Farace F, Ena P, et al. A superficial texture analysis of 70% glycolic acid topical therapy and striae distensae. Plast Reconstr Surg. 2012;129(3):589e–590e. doi: 10.1097/PRS.0b013e3182419c40. [DOI] [PubMed] [Google Scholar]
- 18.Summers B, Lategan M. The effect of a topically-applied cosmetic oil formulation on striae distensae. SA Fam Pract. 2009;51:332–336. [Google Scholar]
- 19.Ud-Din S, McGeorge D, Bayat A. Topical management of striae distensae (stretch marks): prevention and therapy of striae rubrae and albae. J Eur Acad Dermatol Venereol. 2016;30(2):211–222. doi: 10.1111/jdv.13223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Karimipour DJ, Kang S, Johnson TM, et al. Microdermabrasion: a molecular analysis following a single treatment. J Am Acad Dermatol. 2005;52(2):215–223. doi: 10.1016/j.jaad.2004.10.008. [DOI] [PubMed] [Google Scholar]
- 21.Suh DH, Chang KY, Son HC, Ryu JH, Lee SJ, Song KY. Radiofrequency and 585-nm pulsed dye laser treatment of striae distensae: a report of 37 Asian patients. Dermatol Surg. 2007;33(1):29–34. doi: 10.1111/j.1524-4725.2007.33004.x. [DOI] [PubMed] [Google Scholar]
- 22.Trelles MA, Levy JL, Ghersetich I. Effects achieved on stretch marks by a nonfractional broadband infrared light system treatment. Aesthetic Plast Surg. 2008;32(3):523–530. doi: 10.1007/s00266-008-9115-0. [DOI] [PubMed] [Google Scholar]
- 23.Al-Dhalimi MA, Abo Nasyria AA. A comparative study of the effectiveness of intense pulsed light wavelengths (650 nm vs 590 nm) in the treatment of striae distensae. J Cosmet Laser Ther. 2013;15(3):120–125. doi: 10.3109/14764172.2012.748200. [DOI] [PubMed] [Google Scholar]
- 24.Jadoon S, Karim S, Bin Asad MH, et al. Anti-aging potential of phytoextract loaded-pharmaceutical creams for human skin cell longetivity. Oxid Med Cell Longev. 2015;2015:709628. doi: 10.1155/2015/709628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Singh RP, Chidambara Murthy KN, Jayaprakasha GK. Studies on the antioxidant activity of pomegranate (Punica granatum) peel and seed extracts using in vitro models. J Agric Food Chem. 2002;50(1):81–86. doi: 10.1021/jf010865b. [DOI] [PubMed] [Google Scholar]
- 26.Rahimi HR, Arastoo M, Ostad SN. A comprehensive review of Punica granatum (Pomegranate) properties in toxicological, pharmacological, cellular and molecular biology researches. Iran J Pharm Res. 2012;11(2):385–400. [PMC free article] [PubMed] [Google Scholar]
- 27.Aslam MN, Lansky EP, Varani J. Pomegranate as a cosmeceutical source: pomegranate fractions promote proliferation and procollagen synthesis and inhibit matrix metalloproteinase-1 production in human skin cells. J Ethnopharmacol. 2006;103(3):311–318. doi: 10.1016/j.jep.2005.07.027. [DOI] [PubMed] [Google Scholar]
- 28.Lauche R, Kumar S, Hallmann J, et al. Efficacy and safety of ayurvedic herbs in diarrhoea-predominant irritable bowel syndrome: a randomised controlled crossover trial. Complement Ther Med. 2016;26:171–177. doi: 10.1016/j.ctim.2016.04.002. [DOI] [PubMed] [Google Scholar]
- 29.Ceylan S, Azal O, Taşlipinar A, Türker T, Açikel CH, Gulec M. Complementary and alternative medicine use among Turkish diabetes patients. Complement Ther Med. 2009;17(2):78. doi: 10.1016/j.ctim.2008.07.003. [DOI] [PubMed] [Google Scholar]
- 30.Namjoyan F, Kiashi F, Moosavi ZB, Saffari F, Makhmalzadeh BS. Efficacy of Dragon’s blood cream on wound healing: a randomized, double-blind, placebo-controlled clinical trial. J Tradit Complement Med. 2015;6(1):37–40. doi: 10.1016/j.jtcme.2014.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sheu HM, Yu HS, Chang CH. Mast cell degranulation and elastolysis in the early stage of striae distensae. J Cutan Pathol. 1991;18:410–416. doi: 10.1111/j.1600-0560.1991.tb01376.x. [DOI] [PubMed] [Google Scholar]
- 32.Gilbert L, Savary G, Grisel M, Picard C. Predicting sensory texture properties of cosmetic emulsions by physical measurements. Chemometr Intell Lab Syst. 2013;124:21–31. [Google Scholar]
- 33.Estanqueiro M, Amaral MH, Sousa Lobo JM. Comparison between sensory and instrumental characterization of topical formulations: impact of thickening agents. Int J Cosmet Sci. 2016;38(4):389–398. doi: 10.1111/ics.12302. [DOI] [PubMed] [Google Scholar]
- 34.Timur Taşhan S, Kafkasli A. The effect of bitter almond oil and massaging on striae gravidarum in primiparaous women. J Clin Nurs. 2012;21(11–12):1570–1576. doi: 10.1111/j.1365-2702.2012.04087.x. [DOI] [PubMed] [Google Scholar]
- 35.Soltanipour F, Delaram M, Taavoni S, Haghani H. The effect of olive oil and the Saj® cream in prevention of striae gravidarum: a randomized controlled clinical trial. Complement Ther Med. 2014;22(2):220–225. doi: 10.1016/j.ctim.2013.11.011. [DOI] [PubMed] [Google Scholar]
- 36.Soltanipoor F, Delaram M, Taavoni S, Haghani H. The effect of olive oil on prevention of striae gravidarum: a randomized controlled clinical trial. Complement Ther Med. 2012;20(5):263–266. doi: 10.1016/j.ctim.2012.05.001. [DOI] [PubMed] [Google Scholar]
- 37.Taavoni S, Soltanipour F, Haghani H, Ansarian H, Kheirkhah M. Effects of olive oil on striae gravidarum in the second trimester of pregnancy. Complement Ther Clin Pract. 2011;17(3):167–169. doi: 10.1016/j.ctcp.2010.10.003. [DOI] [PubMed] [Google Scholar]