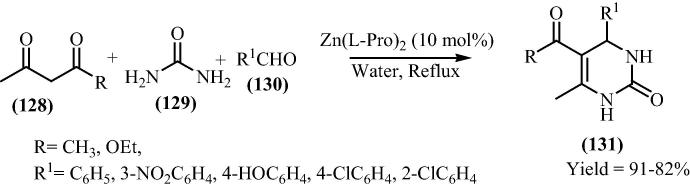Graphical abstract
Keywords: Bis[(l)prolinate-N,O]Zn; Amino-acid complex; Zinc; Asymmetric catalyst; Lewis acid; Organometallic chemistry
Abstract
Under the green chemistry perspective, bis[(l)prolinate-N,O]Zn (also called zinc–proline or Zn[(l)-pro]2) has proven its competence as a promising alternative in a plethora of applications such as catalyst or promoter. Owing to its biodegradable and non-toxic nature of bis[(l)prolinate-N,O]Zn, it is being actively investigated as a water soluble green catalyst for synthetic chemistry. Bis[(l)prolinate-N,O]Zn are readily utilized under mild conditions and have high selectivity and reactivity with broad range of substrate acceptance to make it better reaction medium for a wide variety of organic transformations. This Review summarizes the till date literature on its synthesis, characterization, and its catalytic role in various organic reactions.
Introduction
The recent past scientific and technological advances have provided a great insight regarding the catalytic properties and mechanism of metal-amino acid complexes. Metal salts with chiral amino acid have been used as promising materials for biological as well as chemical advancement as they tend to exhibit the advantage of the metal salts and the asymmetrical organic amino acids [1], [2]. α–Amino acids could act as chelating ligands and form five member ring because they have two types of coordination atoms [3], [4], [5], [6], [7] due to the presence of proton acceptor amino group (NH2) and the donor carboxylic acid group (COOH) in them.
Zinc catches eyes of several researchers due to several reasons, as it can show various coordination geometries, is abundant in nature, is redox-inactive [8], and forms stable complexes with nitrogen. Zinc is an essential micronutrient, which is involved in various biological processes such as transcription, cell signaling catalysis, hormone synthesis, and structural integrity of cell membrane [9], [10]. From the biological point of view, more than 300 zinc metallo-enzymes covering all six classes of enzymes have been discovered [11], [12]. In most cases, the zinc ion is an essential cofactor for the observed biological function of these metalloenzymes. By the virtue of biological activity, thousands of synthetic zinc complexes have been formed either to mimic natural structure or to completely diverge from the natural platform [13], [14], [15], [16], [17], [18]. Moreover zinc is present in active site of class II aldolases (an enzyme) witnessing the bis[(l)prolinate-N,O]Zn as a valid candidate for aldolase mimics.
Deprotonated amino acid coordination chemistry is dominated by the formation of the nitrogen and oxygen chelating motif producing the geometrically (and energetically) favoured five membered metallocyclic compounds [19].
Stability of the zinc complexes varies with different amino acids [20], [21], [22], [23]. Metal ion-ligand affinity increases as the polarizability of the donor atom is increased (O < N < S) [24]. So there is an increase in selectivity for the amino acid having (N, S) linkage followed by (N, O). It has been shown that cysteine and its derivatives are more selective for metal ion-ligand binding as compared to other amino acid having (N, O) linkage [25]. The cumulative energy required for the acid dissociation of carboxylic acid to carboxylate ion and ammonium ion to secondary amine for proline with Zinc (II) is lower than other amino acid which has primary amine group and acid group. In secondary amine, there is more inductive effect which makes it more labile for acid dissociation constant [26], [27].
Complex synthesis
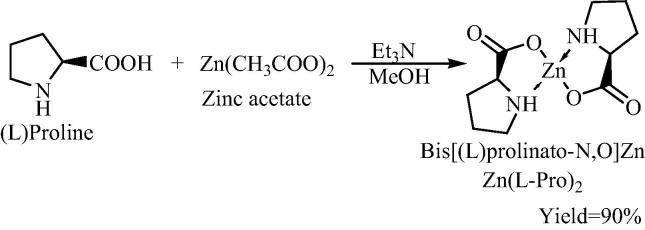
Originally Darbre and Machuquiero have synthesized this bis[(l)prolinate-N,O]Zn complex. They have synthesized bis[(l)prolinate-N,O]Zn complex by adding small quantity of Et3N as base to the proline in methanol followed by zinc acetate (double ratio of amino acid) (Scheme 1). After stirring a white precipitate was obtained which could be separated from reaction medium by simple filtration with good yield [28].
Scheme 1.
Structure and characterization of the catalyst
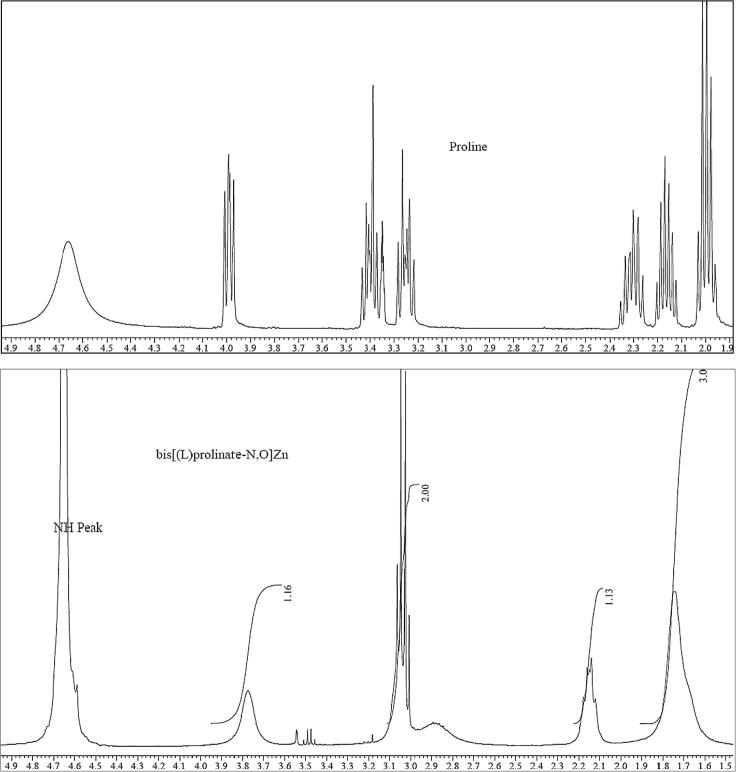
1H NMR analysis
In the comparison of 1H NMR of proline and bis[(l)prolinate-N,O]Zn complex in Fig.1, 1H NMR of the bis[(l)prolinate-N,O]Zn showed that there is proton shielding of protons of proline and the splitting pattern resolved in the presence of Zinc metal ion. Shielding is more in C(2), which indicate the formation of carboxylate ion; moreover, there is a noticeable shielding in C(5) as compared to proline, which further confirms the synthesis of bis[(l)prolinate-N,O]Zn [28].
Fig. 1.
1H NMR of proline and bis[(l)prolinate-N,O]Zn.
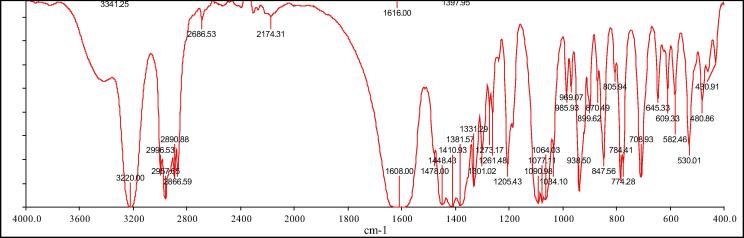
FTIR analysis
In IR spectra of bis[(l)prolinate-N,O]Zn complex shown in Fig. 2, the shift observed confirms the formation of the target compound in comparison with l-proline. There was decrease in broad band at 3422 cm−1 for OH stretching of COOH. The NH stretching band at 3220 cm−1 was very prominent while twisting was observed at 1205 cm−1. The COO− vibration peak appeared comes at 1410 cm−1 along with the carbonyl peak of carboxylic group at 1608 cm−1 while the in-plane deformation at 774 cm−1, scissoring at 703 cm−1 and rocking vibrational peak o at 530 cm−1 were also observed. The CH2 stretching, wagging, and rocking were observed at 2800–3216, 1330–1300, and 938–847 cm−1 respectively. The C—N stretching was observed in between 1330 and 1450 cm−1 while the C—N stretches due to absorption were noticed at 1077 and 1064 cm−1 [29].
Fig. 2.
FTIR of bis[(l)prolinate-N,O]Zn.
Single crystal X-ray diffraction
Structure of bis[(l)prolinate-N,O]Zn complex was first shown by Chew H-N, and he described trans complex [Zn(C6H7NO2)2] in Fig. 3 [30], as a spiral structure formed along the 21 direction with atoms O4 (2−x, y−1/2, −z), Zn, N(2), C(7) and C(6) constituting a repeating unit. The Zn atom is pentacoordinate, the fifth coordination site being occupied by the symmetry related atom O(4 i) [symmetry code: (i) 2−x, y−i ∼, −z] of a neighboring proline molecule so that an infinite polymeric chain is generated. The polymer shows a helical structure along the 2∼ direction. The zinc coordination here is unique, as most zinc-amino acid complexes are hexacoordinate. The Zn atom has trigonal bipyramidal geometry with O(4 i), N(1) and N (2) while O(1) and O(3) occupying the axial position and the pyrrolidine rings are transformed from planner to 3-dimension shape. The distance Zn—O and Zn—N and all the bond lengths of the proline unit were comparable and normal for metal-coordinated amino acids [31], [32], [33], [34]. The angle between O(3)—Zn(1)—O(1) is nearly linear with value of 173.8 (1)°.
Fig. 3.
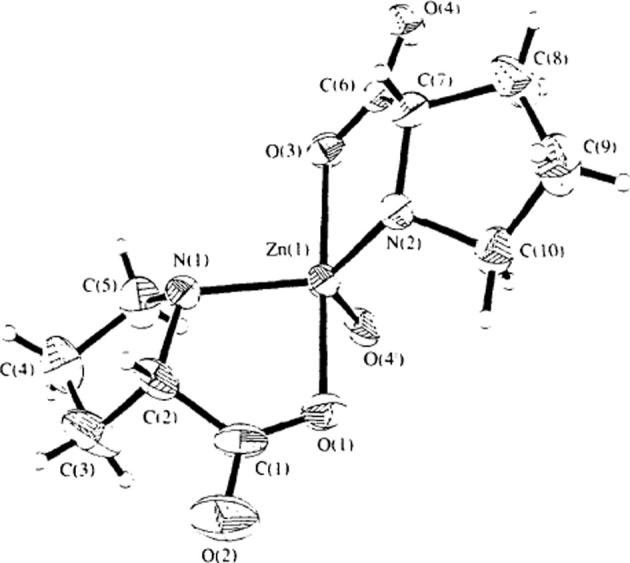
Single crystal X-ray diffraction of bis[(l)prolinateo-N,O]Zn.
Powder X-ray diffraction
Kidwai and his coworkers group have shown for the first time X-ray diffraction of the complex in the range 2θ = 0–100 as shown in Fig. 4. The characteristic peak obtained from powder XRD of bis[(l)prolinate-N,O]Zn of specific d value has showed that the complex is orthorhombic in structure since it is in agreement with data card 47-1919JCDPS [35], [36].
Fig. 4.
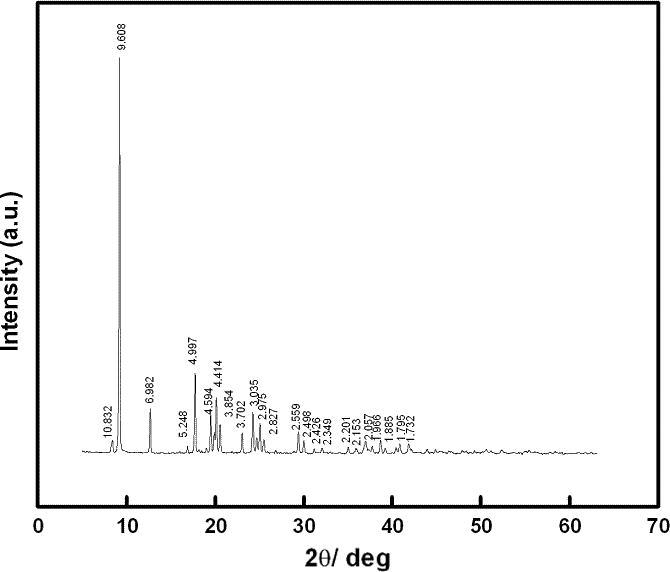
Powder XRD of bis[(l)prolinate-N,O]Zn.
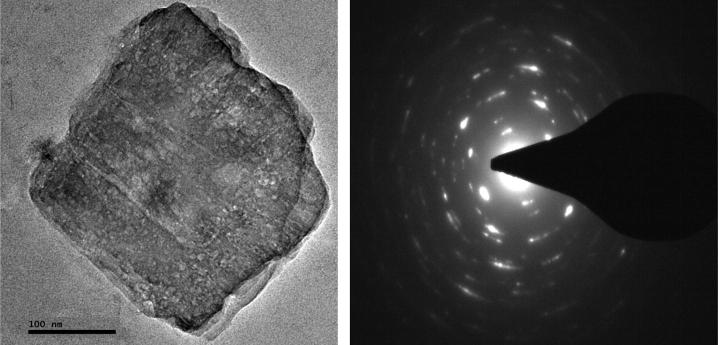
TEM image
For crystal assessment of bis[(l)prolinate-N,O]Zn, TEM technique was used. Kidwai and his co-workers (2011) had acquired various images of complex on carbon coated grid and confirmed the crystalline in nature of the complex as depicted in Fig. 5 [37].
Fig. 5.
TEM images of fresh bis[(l)prolinate-N,O]Zn.
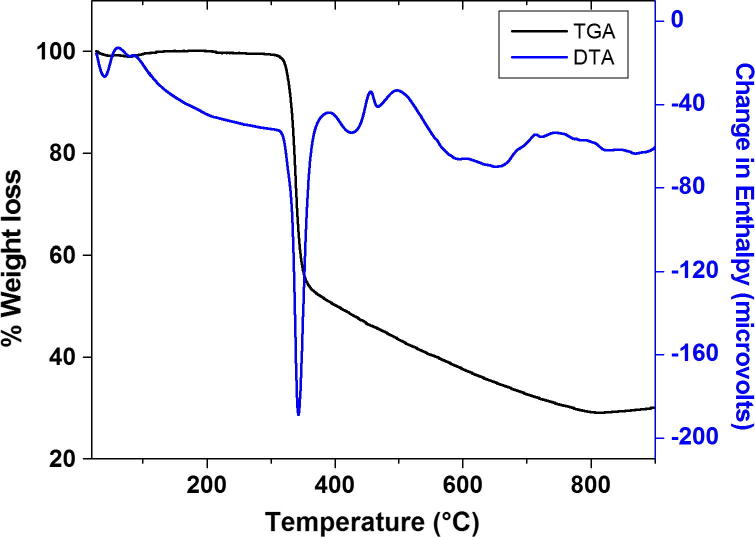
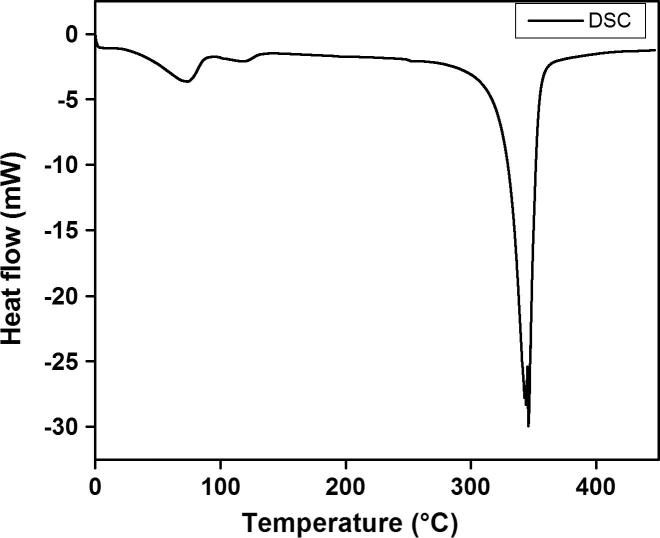
Thermal analysis
The thermal stability of bis[(l)prolinate-N,O]Zn complex was evaluated by TG/DTA and DSC experiments as described by kidwai and research group in Fig. 6, Fig. 7 [38]. Briefly the complex was heated at the rate of 10 K min−1 in N2 atmosphere. A blunt endothermic peak due to the release of adhered water molecules was observed at 100.62 °C in the DTA curve. The purity of crystal was further confirmed by the sharpness of endothermic peak at 342.81 °C in the DTA curve which matches the melting point of bis[(l)prolinate-N,O]Zn. TGA curve showed the detailed decomposition of the complex (Fig. 6). Differential scanning calorimetry (DSC) study was carried in the inert atmosphere from the temperature range 20–500 °C with a heating rate of 10 K min−1. Bis[(l)prolinate-N,O]Zn undergone through an irreversible endothermic transition at its melting point 342.81 °C. Henceforth it was confirmed that the material is stable up to its melting point making it suitable for various applications, where the complex is utilized at high temperatures.
Fig. 6.
TGA/DTA graph of bis[(l)prolinate-N,O]Zn.
Fig. 7.
DSC graph of bis[(l)prolinate-N,O]Zn.
Solubilities of bis[(l)prolinate-N,O]Zn
Bis[(l)prolinate-N,O]Zn is highly soluble in water and insoluble in organic solvent due to its ionic nature. The N, O and Zn atoms form H-bond with water molecules and make it hydrated which is not possible in organic solvent. The recyclability of complex depends upon its solubility in the reaction medium. Majority of the reactions with complex are performed in aqueous medium and extracted with organic solvent (Ethyl acetate, ether, chloroform or DCM) from the aqueous layer and reused for further reaction [29], [36], [37]. In aqueous medium the reactivity of metal complexes is restricted because water molecules can participate as substrate for metal bonding. Criterion for water stable Lewis acids (improbable to hydrolysis) has been investigated based on the relationship between the catalyst activity with two parameters viz water exchange rate constant and hydrolysis constant [26]. Zinc complexes are found to be appropriate for various organic reactions in aqueous medium.
Bis[(l)prolinate-N,O]Zn distribution in biological system
Although metal ions and complexing agents occur ubiquitously in biological tissues and fluids, few studies have been done for the distribution of the metal ions among the competing ligands in such systems [39], [40]. First time equilibria of complex were understood in Bjerrum's book “Metal Ammine Formation in Aqueous Solution” that was published in Denmark in 1941 [42]. It has been confirmed that the equilibrium between a complex forming agent and an ion is usually thermodynamically reversible and occurs instantaneously without significant energy of activation. So equilibria can be written in mass-action equations. Furthermore, Bjerrum has established that complex formation is occurred in stepwise course. Quantitative studies by Albert (1950) for the avidity of l-proline for Zn(II) ion have been reported [41]. It was found that pKa value for l-proline is 10.68 and stability constant of the bis[(l)prolinate-N,O]Zn complex is 10.2, implying that l-proline has the greatest avidity for Zn(II) ion and forms a stable complex with it.
The computed distribution of Zn(II) ion among seventeen amino acids present in human blood plasma had been studied and approximately 50% of the Zn(II) is coordinated to cysteine and histidine (as their stability constant is highest among all amino acids), but considerable amino acid complex formation occurs with most of the other amino acids [43].
Recently, metal ions have been used in metallization of biomacromolecules [44]. These processes rely upon the specific metal ion amino acid interaction, which allow an efficient metal deposition and attachment to biological systems. The molecular mechanism of the metallization process was studied by means of chemical quantum calculations of metal ion- amino acid interaction [45]. An interesting feature of the zinc(II) ion is its ability to adopt a tetrahedral, a trigonal bipyramidal, or an octahedral geometry depending on the ligands bonded to the ion. On the other hand the Zn2+ aqua ion, as well as Zn2+ complexed to two N donors, is six-coordinated [46], [47]. Zinc(II) ion coordinated by at least three N or S donor forms either tetrahedral or trigonal bipyramidal complexes [48]. A theoretical study of Zn(II) interaction with l-proline was carried out using density functional theory method with Becke’s three parameter, hybrid exchange functional and the Lee-Yang-Parr correlation functional (B3LYP). A moderately high affinity (−13.4 kJ mol−1) was predicted for the proline residue complexing a zinc ion via the nitrogen atom of the five membered ring [49].
In plant, there is increase in concentration of proline to get rid of heavy metals which are toxic in nature. To check the importance of proline in plant reactions to heavy metal stress, Sharma et al. have studied the effect of proline on Zn-induced inhibition of glucose-6-phosphate dehydrogenase and nitrate reductase in vitro. Proline appeared to protect both enzymes against Zinc. There were no indications of any significant role for proline-water or proline-protein interactions. The significance of these findings with regard to heavy metal-induced proline accumulation in vivo has been discussed [50]. A synergistic immunological adjuvant formulation having bis[(l)prolinate-N,O]Zn complex as synergist has been patented which showed the pharmaceutical properties associated with the complex [51].
Bis[(l)prolinate-N,O]Zn in organic synthesis as catalyst
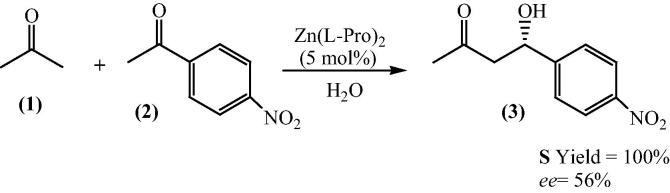
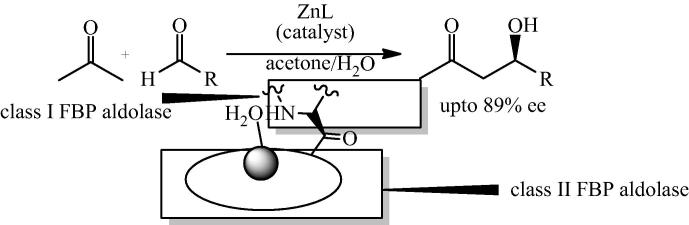
Bis[(l)prolinate-N,O]Zn has received immense attention over the last eight years which provided intriguing opportunities in organic synthesis because of its ability to act as Lewis acid and ease of preparation. The following section illustrates various synthetic approaches exploiting bis[(l)prolinate-N,O]Zn as a catalyst. In most cases, water had been used as a part of the reaction media. Henceforth, in each synthetic approach, examples related to the use of this organometallic complex in biphasic systems, water saturated organic solvents and even water as a sole reaction media have been described. This section examines the growing opportunities and applications of bis[(l)prolinate-N,O]Zn catalyzed reactions. Originally Darbre et al. (2003) have shown bis[(l)prolinate-N,O]Zn as a selective catalyst for the direct aldol reaction in aqueous media. They have investigated that 5 mol% of the Zn complexes of lysine, arginine and proline are catalysts for the aldol addition of acetone (1) and p-nitrobenzaldehyde (2) in aqueous medium, giving considerable yields and enantiomeric excess up to 56% at room temperature (Scheme 2) [28].
Scheme 2.
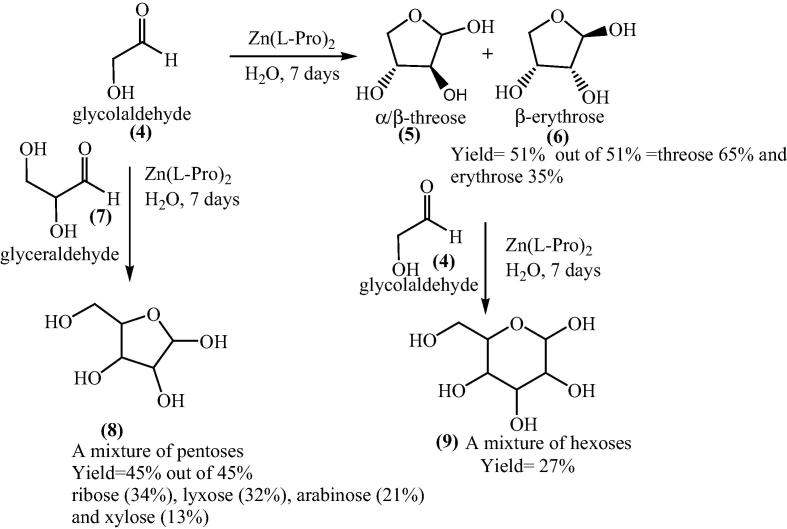
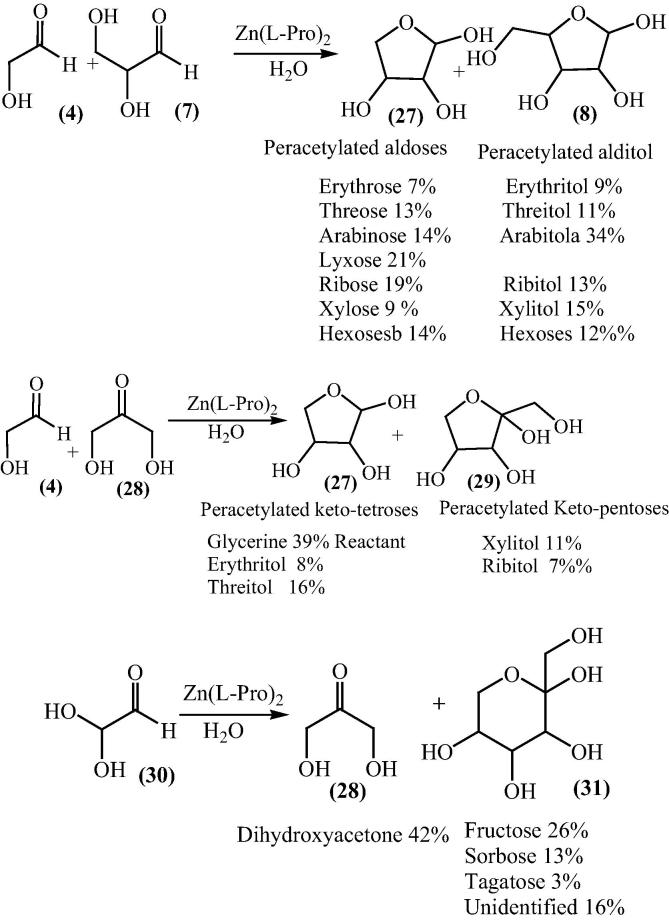
The catalytic ability of other with 5 mol% Zn-(l)-amino acid complexes had been studied in water-acetone medium. The complexes were prepared and isolated as described for Zn-proline [52], [53], [54], [55], [56], [57]. In the absence of zinc, product (3) was obtained in 74% yield and 6% ee with the R-1 enantiomer in excess. The higher ee values were observed with different amino acids requiring chiral Lewis acid as catalyst. Moreover in 2004, Darbre and Reymond et al. together explored the bis[(l)prolinate-N,O]Zn complex catalyzed pathway for the formation of sugars [58]. Bis[(l)prolinate-N,O]Zn complex catalyzed the aldolization of unprotected glycolaldehyde (4) in water to give tetroses (5,6) in 51 % yield which further aldolization gave hexoses (9) with 10% enantiomeric excess of the D-isomer (Scheme 3). A mixture of pentoses (8) was produced by the reaction of glycolaldehyde with glyceraldehyde (7) in the presence of bis[(l)prolinate-N,O]Zn complex in water.
Scheme 3.
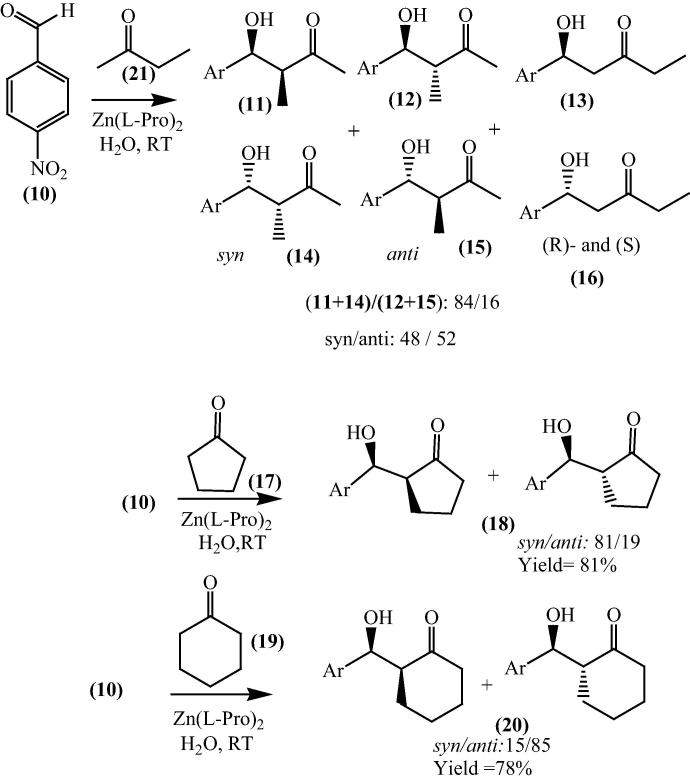
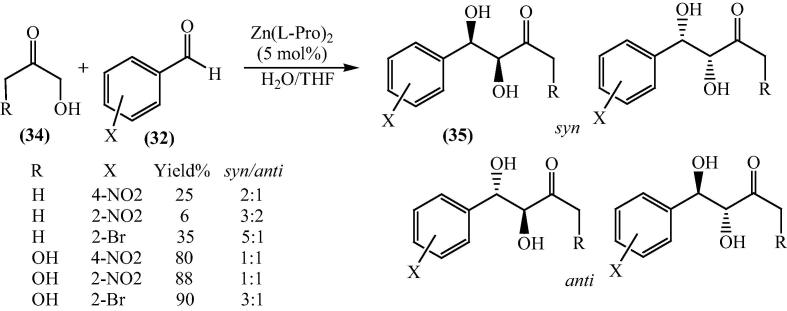
The aldol reaction of 4-nitrobenzaldehyde catalyzed with three different ketones (2-butanone, cyclopentanone and cyclohexanone) in three different combinations with aqueous media, has been studied to explore selectivity of environmentally benign reaction. The combination included conditions are bis[(l)prolinate-N,O]Zn complex, NaHCO3/bis[(l)prolinate-N,O]Zn complex and l-proline/bis[(l)prolinate-N,O]Zn complex. For the synthesis of β-hydroxy ketones NaHCO3 was surprisingly found to be a proficient catalyst, showing high-quality diastereo- and regioselectivity within 9 h. Cyclopentanone (17) were mainly found to give syn diastereoisomers while cyclohexanone (19) produced anti isomers being the major product which was an exceptional result (Scheme 4) [59].
Scheme 4.
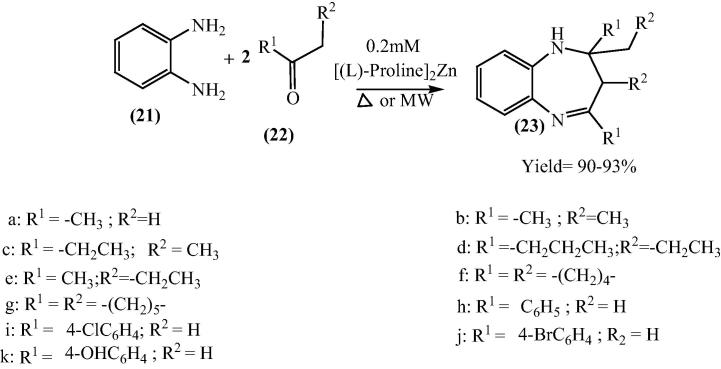
Sivamurugan and his research group have performed the reaction of o-phenylene diamine (21) and α-hydrogen carbonyl (22) with 0.2 mmol of bis[(l)prolinate-N,O]Zn as catalyst to produce 1,5-benzodiazepine derivatives a one pot reaction under solvent-free conditions [60]. The effectiveness of the catalyst has been checked by microwave irradiation technique as well as conventional method. 1,5-Benzodiazepine (23) was obtained in moderate to good yield (90–93%) in all the reactions within a shorter reaction time (2–3 mins) under microwave irradiation while in conventional the yield (80–88%) was lower and had in longer reaction time (2 h). The catalyst was recycled up to five times with marginal loss of its catalytic reactivity (Scheme 5).
Scheme 5.
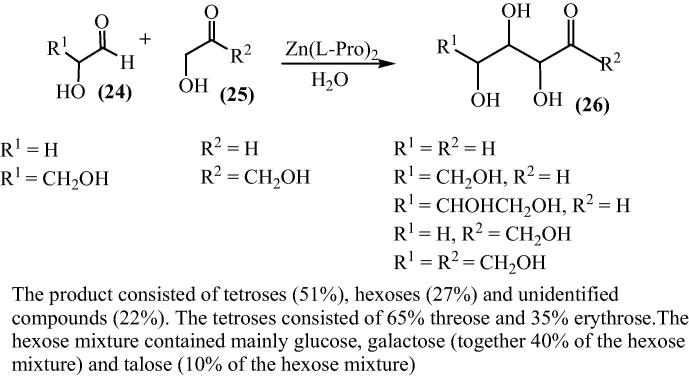
To explore the wide applicability of bis[(l)prolinate-N,O]Zn, the aldolization of different hydroxyl aldehydes and ketones was studied by Darbre group using the complex [61]. Glycolaldehyde (4) gave mainly tetroses whereas in the cross-aldolization of glycolaldehyde and rac glyceraldehydes (7), pentoses accounted for 60% of the sugars formed with 20% of ribose. They suggested that generally, unprotected α-hydroxy aldehydes and ketones could undergo aldol additions in the presence of bis[(l)prolinate-N,O]Zn as catalyst in water. Depending on the starting aldehyde, the products formed may include tetroses, pentonse, hexoses, keto-pentoses, keto-hexoses with smaller yields of higher sugars. For the simplicity of analysis, the sugars were also reduced to polyols using NaBH4 (Schemes 6 and 7) [62].
Scheme 6.
Scheme 7.
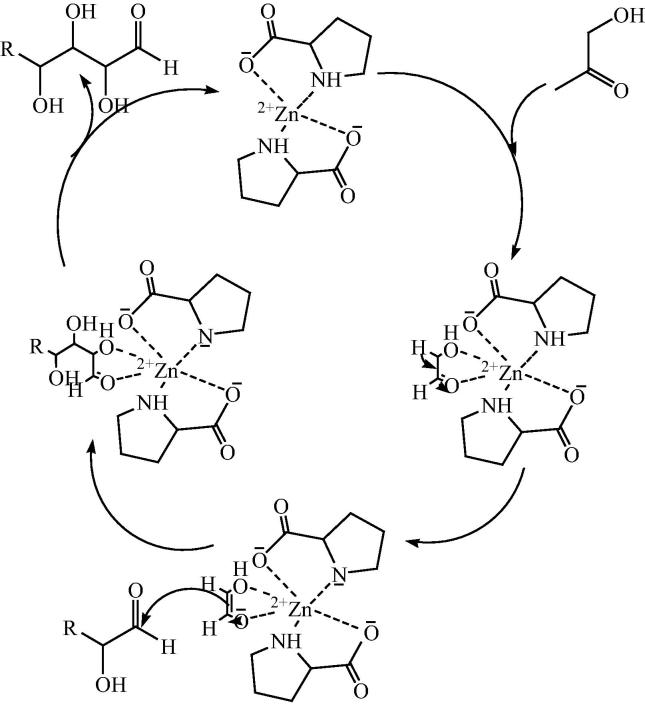
An appropriate mechanism was proposed by darbre for bis[(l)prolinate-N,O]Zn to catalyze the aldol reaction shown in Fig. 8. The chelating enolate formation took place by bonding of glycolaldehyde (4) to the zinc. This is similar step which occurs in class II aldolase enzyme having zinc (II) in active site as cofactor. The electron deficient carbonyl reacted with the enolate which does not require to coordinating with zinc.
Fig. 8.
Plausible mechanism for the bis[(l)prolinate-N,O]Zn catalyzed the formation of ribose and other pentoses.
The main difficulty to use pentoses as probable prebiotic reagents was the lack of stabilities in earlier days. Previously, the self condensation of formaldehyde in basic medium was used to synthesize pentoses to yield less than 1% of riboses [63]. So the investigations were carried out to escalate the amount and stability of pentoses. The results showed that synthesis of pentoses should be done using Lewis acid and maximum stability of products could be achieved at room temperature in aqueous.
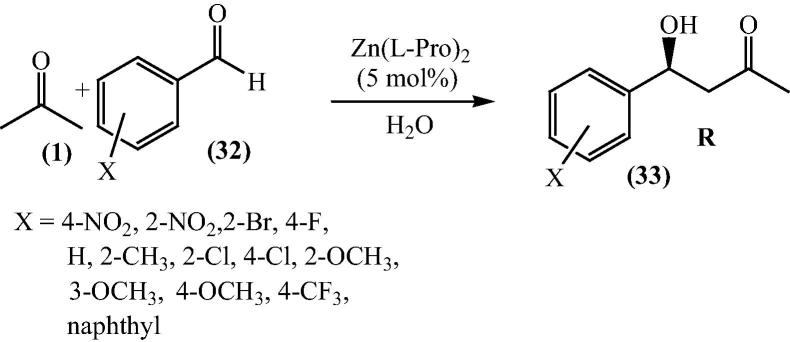
In another publication by Lopez et al. [64], bis[(l)prolinate-N,O]Zn complex was depicted to catalyze the very famous aldol reaction of acetone (1) and broad range of aromatic aldehydes (32) in aqueous media, and even less reactive aromatic aldehydes such as methoxybenzaldehyde gave good yields. The reaction was also comprehensive to hydroxyacetone and dihydroxyacetone as donors (Scheme 8, Scheme 9).
Scheme 8.
Scheme 9.
Heterocyclic aldehydes with acetone were also established to be appropriate substrate for the aldol reaction. Variation in molar concentration acetone was also done and good to better yields were achieved with even cyclopentanone. Moreover e 2-butanone and cyclohexanone underwent aldol reaction with 4-nitrobenzaldehyde. They also extended bis[(l)prolinate-N,O]Zn complex catalyzed reaction with Hydroxyacetone and Dihydroxyacetone. Encouraging results were obtained with ketones too. They postulated a mechanism linking a formation zinc-assisted enamine, where zinc complexation stabilized the enamine intermediate [65]. Coordination to zinc stabilized the enamine in aqueous, possibility of the condensation with the aldehyde shown in Fig. 9.
Fig. 9.
Proposed intermediates for the zinc-supported enamine mechanism of the bis[(l)prolinate-N,O]Zn complex-catalyzed aldol reaction.
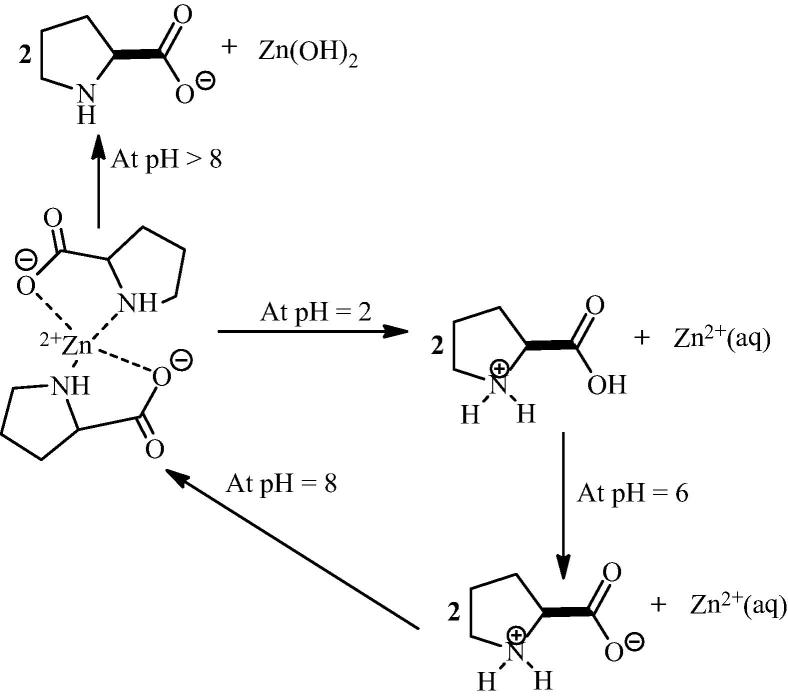
In 2006, Kofoed et al. have explored the dual mechanism of bis[(l)prolinate-N,O]Zn complex catalyzed aldol reactions in water. They found that the aldol condensation of aldehydes with acetone in water medium under numerous catalyst e.g. proline, bis[(l)prolinate-N,O]Zn complex, (S)-(+)-1-(2-pyrrolidinomethyl)pyrrolidine and (2S,4R)-4-hydroxyproline progressed via an enamine mechanism, while the aldol reaction of dihydroxyacetone catalyzed by bis[(l)prolinate-N,O]Zn complex and by organic bases such as N-methylmorpholine occured under rate-limiting deprotonation of the α-carbon and formation of an enolate intermediate [66]. Bis[(l)prolinate-N,O]Zn complex appeared to be a particularly efficient catalyst for both enamine and enolate type catalyses. Addition of a base to bis[(l)prolinate-N,O]Zn complex induced precipitation of Zn(OH)2 above pH 9, implying that the conjugate base [(OH)((l)prolinate-N,O)2]Zn was not available as a general base for deprotonating dihydroxyacetone, while the pH curve showed that proline could easily disintegrate from zinc upon protonation from pH 8 to pH 6 (Scheme 10).
Scheme 10.
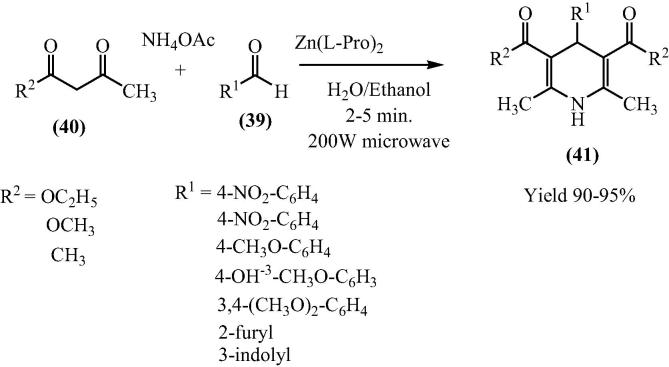
Bis[(l)prolinate-N,O]Zn complex was shown to be an capable catalyst for the Hantzsch synthetic route for the synthesis of 1,4-Dihydropyridine (DHP) (41) derivatives using a broad variety of aromatic aldehydes (39) and dicarbonyl compounds (40) in aqueous medium under microwave irradiation. The Bis[(l)prolinate-N,O]Zn exhibited greater catalytic activity even with low MW power (≈200 W) and gave excellent yield (90–98%) in short reaction times (<5 min) [67] (Scheme 11).
Scheme 11.
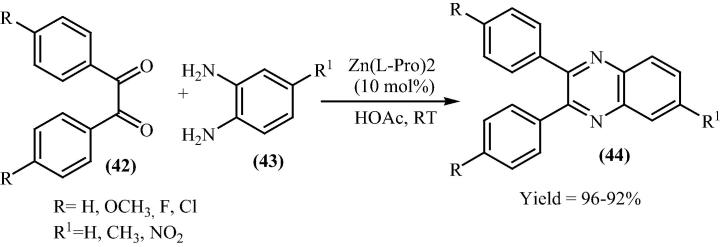
Quinoxaline derivatives show broad spectrum of biological activities. They have been used in dyes [68], [69], pharmaceuticals [70], [71] and building blocks for the synthesis of organic semiconductors [72]. An ecofriendly straightforward, proficient method for the preparation of quinoxalines (44) by the condensation of 1,2-diamines (43) with various 1,2-diketones (42) using bis[(l)prolinate-N,O]Zn as a catalyst has been reported by Heravi et al. in 2007 [73]. In his reaction acetic acid was used as a solvent which was unable to precede the reaction (Scheme 12).
Scheme 12.
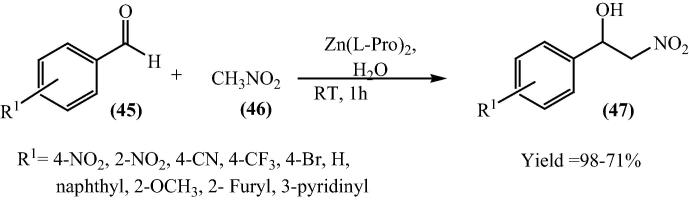
Direct nitroaldol reaction by bis[(l)prolinate-N,O]Zn complex was performed in 2007 by Reddy et al. [74]. The Henry reaction or nitroaldol is one of the influential Carbon-Carbon bond formation reactions in organic chemistry to produce important functionalized skeletons such as α-hydroxy carboxylic acids and 1,2-amino alcohols [75], [76]. The standard nitroaldol reaction is carried out in the presence of inorganic (alkali metal hydroxides, calcium hydroxide, alkoxides, aluminum ethoxides, carbonates, bicarbonates) or organic base (primary, secondary, and tertiary amines) in an organic solvent [77]. To conquer some of the inconveniences associated, the selective, homogeneous and reusable catalysts are highly recommended. Hence, bis[(l)prolinate-N,O]Zn complex was used as a catalyst for this reaction (Scheme 13).
Scheme 13.
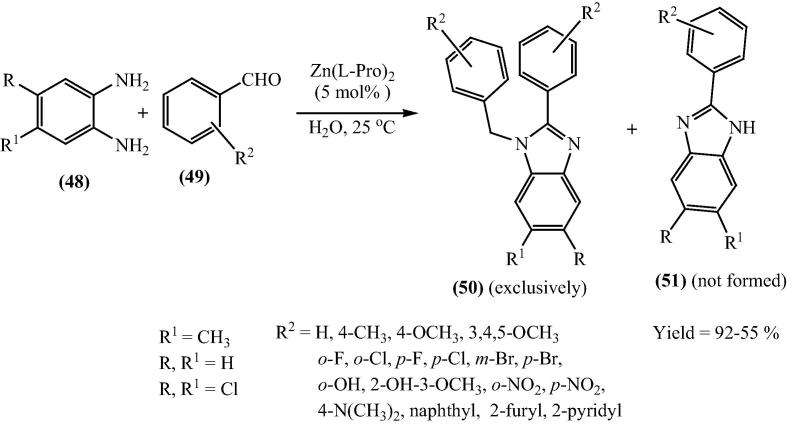
Bis[(l)prolinate-N,O]Zn complex also acted as a water-soluble and recyclable Lewis acid catalyst for the selective synthesis of 1,2-disubstituted benzimidazoles via the reaction of substituted o-phenylenediamines (48) and aldehydes (49) in moderate to excellent isolated yields (42–92%) using water as solvent at ambient temperature [78]. Under the optimized reaction conditions, in all cases the yields were high and 1,2-disubstituted product (50) was formed selectively rather than 2-substituted product (51). This selectivity could be useful in synthesizing a mini library of biologically relevant 1,2-disubstituted benzimidazoles in moderate to excellent yields (Scheme 14).
Scheme 14.
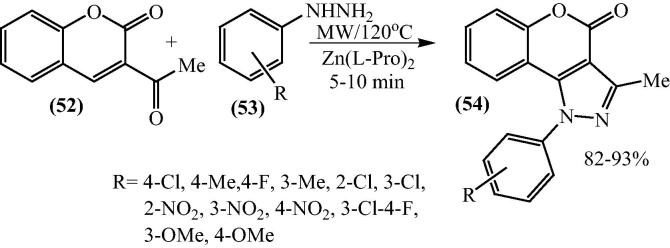
Shah and co-worker have revealed [79] that the bis[(l)prolinato-N,O]Zn, a Lewis acid catalyst under microwave irradiation could afford 3-methyl-1-substituted-phenyl-1H-chromeno[4,3-c]pyrazol-4-ones (54) by cyclization of hydrazones of 3-acetyl-4-hydroxycoumarin. The range of yields of various products was obtained to be 82–93%. In the absence of the catalyst, no reaction occurred. There was no remarkable increase in the yields of product at high temperatures and at high microwave power (Scheme 15).
Scheme 15.
Itoh et al. have utilized the concept that that stereospecific aldol reactions are catalyzed by aldolase enzymes in a reversible manner. Aldolases enzymes are subdivided into two classes aldolase I (on catalyzing stereospecific aldol reaction through the enamine intermediates) and aldolase II (in which Zn2+ enolates of substrates react with acceptor aldehydes) [80] in Fig. 10. Mechanistic studies suggested that the amino acid part and the Zn2+ ion of the catalyst function in a cooperative manner to generate Zn2+ - enolates to give aldol adducts (Fig. 11).
Fig. 10.
Ligands that can bind to Zn2+ to form mimic of aldolase enzymes that catalyze stereospecific aldol reactions in a reversible manner.
Fig. 11.
Initially hypothesized scheme for aldol reactions catalyzed by the amino acid part and the Zn2+ ion of the catalyst function in a cooperative manner to generate Zn2+ - enolates.
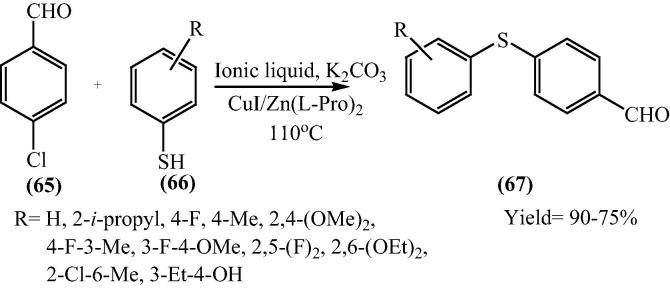
Transition metal ion catalyzed coupling reaction is one of the most significant reactions to form a carbon–heteroatom bond. Out of carbon-heteroatom, the C—S bond formation has received much attraction due to its occurrence in many molecules that are of pharmaceutical used in drugs, building block material interests and biologically active. In 2009, Sheng-Rong et al. reported a palladium-free and mild synthetic procedure for the cross-coupling reaction of thiols (66) and aryl chlorides (65) with bis[(l)prolinate-N,O]Zn with K2CO3 as inorganic base, in ionic liquid 1-butyl-3-methylimidazolium tetrafluoroborate ([bmim]BF4) (Scheme 16) [81].
Scheme 16.
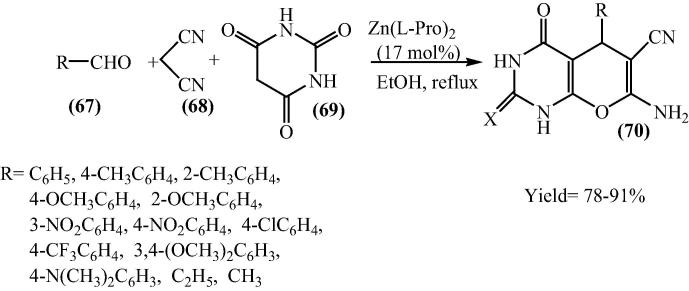
Multicomponent reactions (MCRs) are one pot processes in which three or more reactants come together in a single reaction vessel to form a product containing substantial elements of all the reactants [82]. Pyrano[2,3-d]pyrimidines derivatives have acquired much attraction during the last decade owing to their broad spectrum of biological activities. Uracil derivatives have shown antibacterial, antitumor, antihypertensive, bronchiodilator, vasodilator, cardiotonic, hepatoprotective and antiallergic activities. Some of them also demonstrate herbicidal, analgesic, antifungal and antimalarial properties [83], [84], [85], [86], [87], [88], [89], [90], [91]. Influenced by this attractive importance of pyrano[2,3-d]pyrimidine derivatives, Heravi et al. in 2010 have synthesized these compounds using bis[(l)prolinate-N,O]Zn complex as a catalyst [92] (Scheme 17).
Scheme 17.
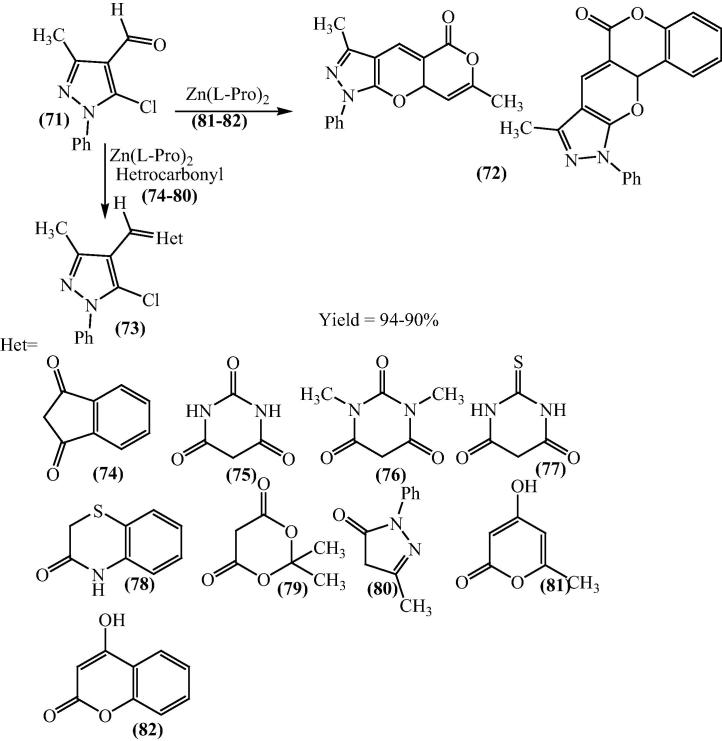
Siddiqui and her research groups have further explored the activity of bis[(l)prolinate-N,O]Zn as a Lewis acid catalyst in Knoevenagel condensation. The Knoevenagel condensation products from 5-chloro-3-methyl-1-phenylpyrazole-4-carboxaldehyde (71) with different cyclic active methylene compounds, using water soluble and recyclable bis[(l)prolinate-N,O]Zn both are under solvent-free conditions and using water as a reaction medium in good yields [93], [94] (Scheme 18).
Scheme 18.
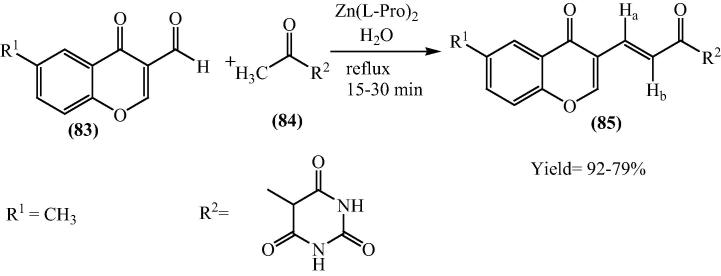
Naturally origin as well as synthetic chromone derivatives forms an important constituent of pharmacophores of a variety of biologically active molecules having significant medicinal applications [95], [96], [97], [98], [99], [100], [101], [102]. On the other side, chalcones family correspond to flavanoid class and have shown a remarkable range of biological activities [103]. Bis[(l)prolinate-N,O]Zn catalyzed the preparation of a library of chromonyl chalcones (85) from different cyclic active methyl groups (84) and 3-formylchromones (83) [104]. The yield of substituted chromonyl chalcones was found to 79–92% in 15–30 mins (Scheme 19).
Scheme 19.
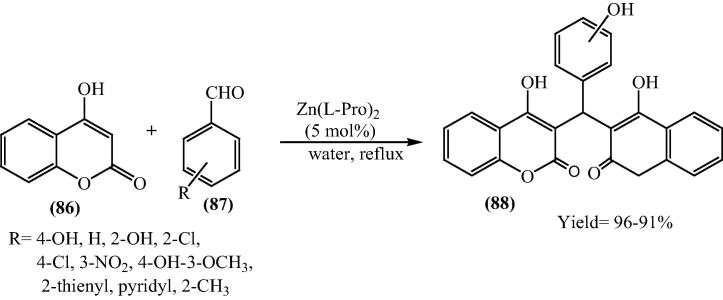
From the point of view of green chemistry to use green solvent, water is the best option for reaction solvent to proceed and there is no requirement to mention properties of water [105], [106]. Since water has specific properties [107], [108], [109], [110], [111], [112], [113], [114] a disadvantage comes with the insolubility of organic compounds [115], [116], [117]. A novel, greener approach was adopted for the synthesis [118] of dicoumarols (88) using bis[(l)prolinate-N,O]Zn as a mild, non-toxic, Lewis acid catalyst in water employing 4-hydroxycoumarin (86) and aromatic/heteroaromatic aldehydes (87) (Scheme 20).
Scheme 20.
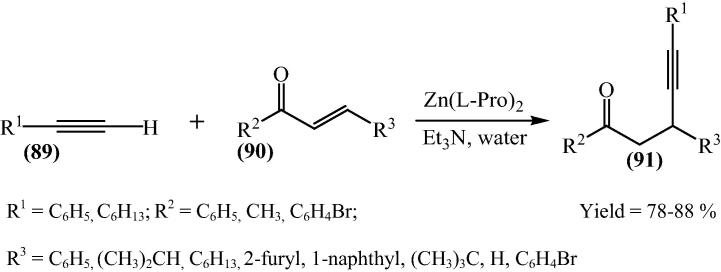
Kidwai and his research group [37] have done more progress toward the catalytic evaluation of bis[(l)prolinato-N,O]Z, they synthesized χ, δ-Acetylenic ketones (91) from phenylacetylene (89) and benzalacetophenone (90) using bis[(l)prolinato-N,O]Zn as a catalyst and Et3N as an additive (Scheme 21) and the yield of product was up to 85%.
Scheme 21.
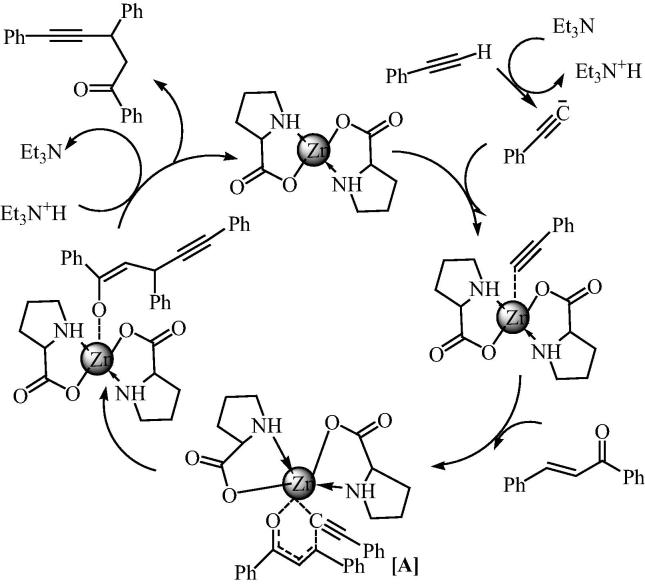
It was also indicated that only bis[(l)prolinate-N,O]Zn was capable of acting as an efficient catalyst for the synthesis of γ, δ-acetylenic ketones. The reason is only in bis[(l)prolinato-N,O]Zn, and amino acid contains secondary amine which ultimately enhances the catalytic efficiency of bis[(l)prolinato-N,O]Zn. The use of zinc reagent to affect the 1,4 - addition of an alkynyl group to an α,β-unsaturated ketone could be explained by the tendency with which zinc binds to alkyne ligand. It suggests that highly water soluble catalyst could coordinate with the alkyne easily and could transform it into product via intermediate. A plausible pathway involves the intramolecular delivery of an alkynyl group through a six membered transition state [A], which has been shown in the mechanism in Fig. 12. This on further rearrangement gives desirable product. Mechanism illustrates that there is no scope for the formation of side product. Furthermore, it is also shown that Et3N was added in fractional amount to deprotonate the terminal alkyne and could be recovered back in the aqueous layer, when product was extracted.
Fig. 12.
Plausible mechanism for the bis[(l)prolinate-N,O]Zn catalyzed 1,4-addition of terminal alkyne to conjugated enone.
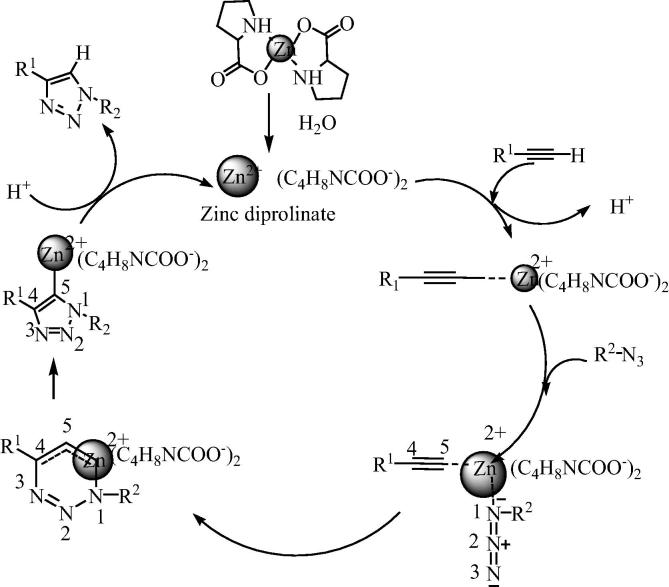
To explore more from bis[(l)prolinate-N,O]Zn as a catalyst to devise greener chemical transformations, kidwai and Jain [38] have further used bis[(l)prolinate-N,O]Zn as a catalyst for the preparation of triazoles by the reaction of alkyne, azide and benzyl halides in water as a solvent (Scheme 22).
Scheme 22.
A probable mechanism for this reaction is shown in Fig. 13. Since the reaction is carried out in water, hence in bis[(l)prolinate-N,O]Zn complex, metal ion would exist in the form of hydrated cation and the corresponding amino acid in the form of anion. The bis[(l)prolinate-N,O]Zn complex would be abstract proton from alkyne and make it acetylide [119], [120]. With the bonding of azide with acetylide, the reaction would take the conduit frequently permitted for this transformation to form a triazolide intermediate, which then ultimately forms the target triazole and the recycling of the catalyst.
Fig. 13.
Proposed mechanism for the preparation of triazoles by the reaction of alkyne, azide and benzyl halides using bis[(l)prolinate-N,O]Zn as a catalyst.
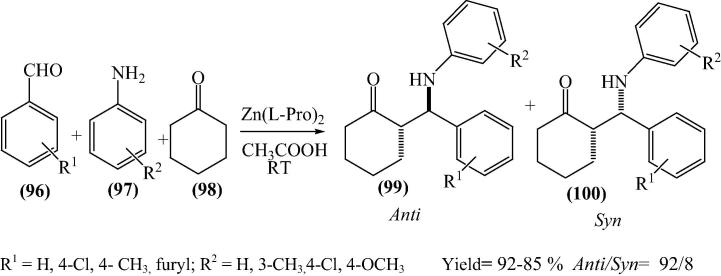
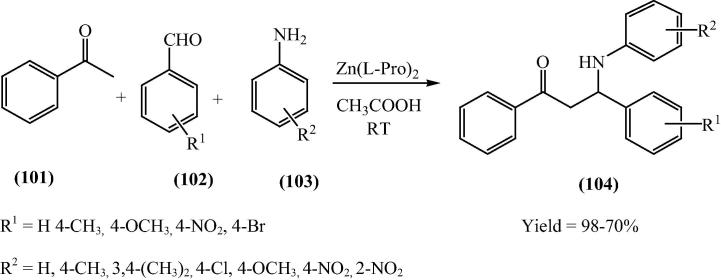
β-Amino carbonyl compounds are found to be attractive targets for various chemical syntheses as they are widely used in biologically active molecules as well as are important reactants for various pharmaceuticals [121]. Kidwai and his research group [29] have reported bis[(l)prolinate-N,O]Zn catalyzed three-component stereoselective Mannich reaction for the synthesis of β-amino carbonyl compounds in aqueous medium (Scheme 23, Scheme 24). One of the most significant rewards of this reaction is the purity of the products. All the products were of very high purity and do not need any additional purification application such as recrystallization or column chromatography.
Scheme 23.
Scheme 24.
Products formed showed excellent anti selectivity. The anti 105 and syn 106 isomers were identified by the coupling constants (J) of the vicinal protons adjacent to C O and NH in their 1H NMR spectra (Fig. 14).
Fig. 14.
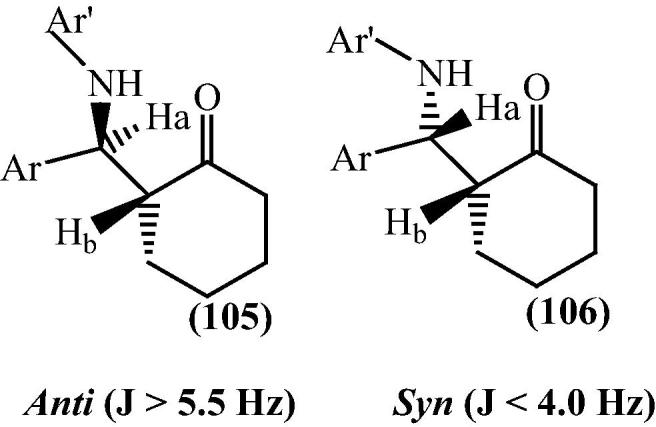
Identification of anti and syn isomers by 1H NMR spectroscopy.
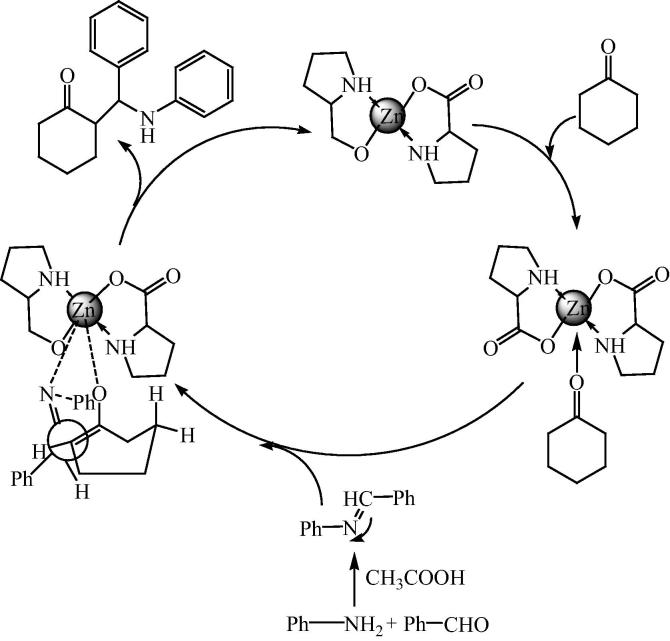
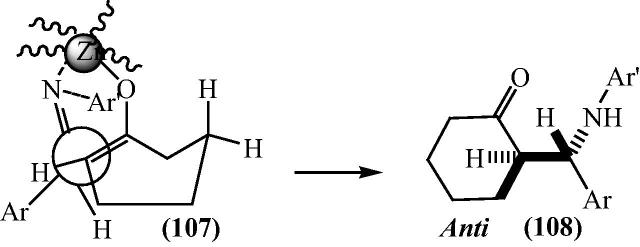
A plausible transition state was possible in which bonding of imine and enol with zinc produces cyclohexanone (Fig. 15). Transition state (107) gives less steric repulsion between the methylene groups of cyclohexanone and aryl group on the carbon atom and more space for the aryl groups of the aldimine, which is the most stable transition state, produces the anti-isomer shown in Fig. 16.
Fig. 15.
Plausible mechanism for the bis[(l)prolinato-N,O]Zn catalyzed reaction for β-aminocarbonyl compounds.
Fig. 16.
Possible transition state leading to anti product.
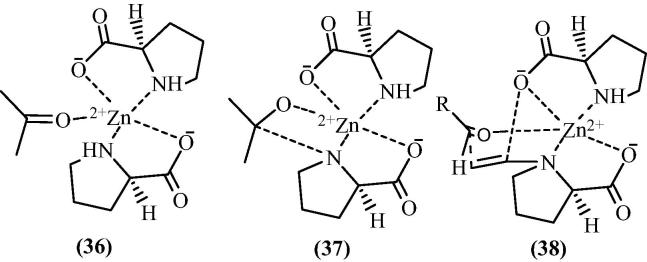
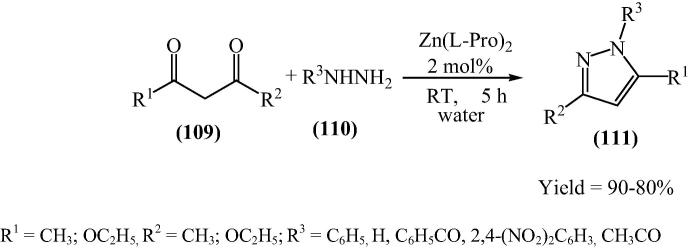
In continuation of further studies on developing economically viable and environmentally benign methodologies for organic reactions and to reveal the efficient utility of transition metals and their derivatives, Kidwai and his research group [36] have reported for the first time bis[(l)prolinate-N,O]Zn catalyzed an efficient synthesis of pyrazoles by the reaction of 1,3 diketone and phenyl hydrazine or hydrazine or hydrazides and 1,3 diketone and o-phenylenediamine in pure water (Scheme 25, Scheme 26). However phenyl hydrazine can give pyrazole in the absence of catalyst in a less amount. But less reactive hydrazines and hydrazides take an evident advantage of the use of this catalyst. Generally 2, 4-dinitrophenylhydrazine with acetylacetone afforded enamine types of compound (A) and ethylacetoacetate with hydrazines afforded pyrazolones [122] (B). But by using this catalyst, reaction led to the formation of pyrazole only (Fig. 17). It is remarkable to point out that in the presence of Zn(OAc)2 and in the absence of l-proline, the reaction did not occur. Even l-proline alone was not able to give any desirable product.
Scheme 25.
Scheme 26.
Fig. 17.
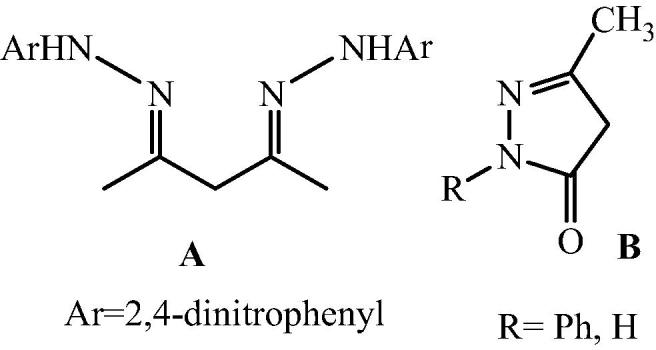
Enamine(A), Pyrazolone(B).
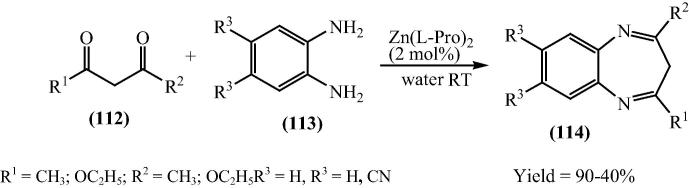
Kidwai and Jain [123] have also extended their work and described a convenient and a resourceful process for the synthesis of xanthenediones with greater stereoselective manner. The prominent features of this protocol are rapid synthesis, simple experimental procedure, mild reaction conditions, manageable work-up, environmental friendliness by avoiding the use of volatile organic compounds as reaction media, reusability of the catalyst, improved yields, and cleaner reaction profile, which make it an efficient, economic and ecofriendly process (Scheme 27).
Scheme 27.
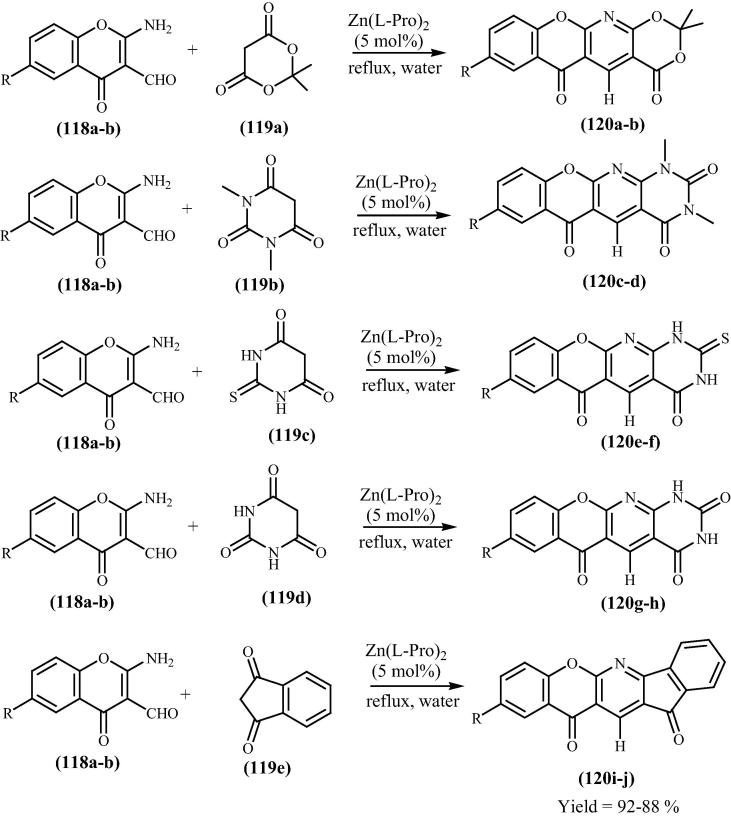
Friedlander condensation produces heteroannulated pyridines by the condensation–cyclodehydration reaction of reactive active methylene group and an aromatic 2-aminoaldehyde or ketone in acid or base medium. In this context, Siddiqui [124] has reported the preparation of novel benzopyrano [2,3-b] pyridine derivatives 120(a–j) in aqueous media via Friedlander condensation using 2-amino-3-formyl chromone 118(a–b) and cyclic active methylene compounds 119(a-e) (Scheme 28). It was excellent reports on the preparation of benzopyranopyridine derivatives using bis[(l)prolinate-N,O]Zn as Lewis acid catalyst.
Scheme 28.
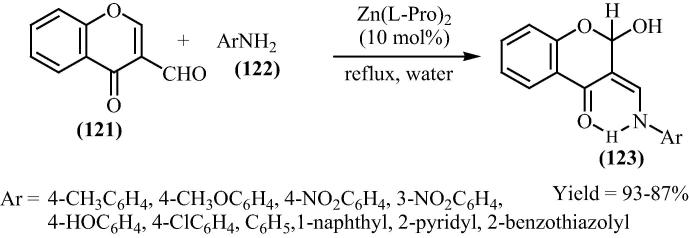
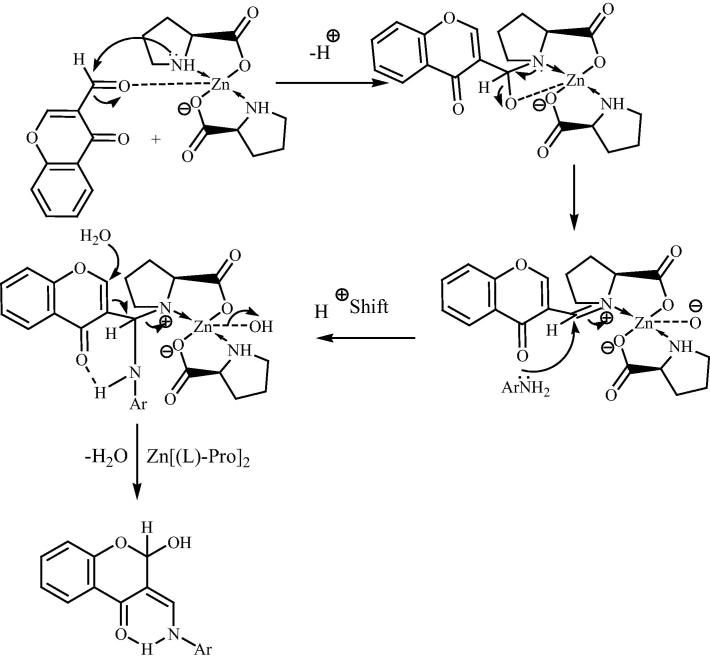
In continuation of efforts in developing selective, efficient, mild and ecofriendly synthetic methodologies for the preparation of biologically relevant heterocyclic derivatives, Siddiqui and Farooq [125] have reported a simple and convenient method for the synthesis of 4-chromanone derivatives (123) by the reaction of 3-formylchromone (121) with different primary aromatic and heteroaromatic amines (122) using bis[(l)prolinate-N,O]Zn complex as a water-tolerant Lewis acid catalyst in water. The plausible mechanism for the synthesis of (123) in the presence of bis[(l)prolinate-N,O]Zn has been shown in Scheme 29. Zn is accomplished of binding with the carbonyl oxygen raising the reactivity of parent carbonyl group in 121 which led to formation of imine with proline takes place in Fig. 18. This is followed by nucleophilic attack of amines (122) to the imine to form hydrogen bonded adduct. Finally, water as nucleophile attacks on electrophilic C-2 center with the expulsion of bis[(l)prolinate-N,O]Zn gives the preferred 2-hydroxy chromanones (123).
Scheme 29.
Fig. 18.
Plausible mechanism for the bis[(l)prolinato-N,O]Zn catalyzed reaction for 4-chromanone derivative compounds.
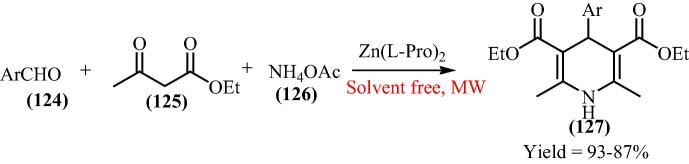
To further explore bis[(l)prolinate-N,O]Zn as heterogeneous Lewis catalyst in microwave, the Pourshamsian and his research group [126] have described the preparation of 1,4-dihydropyridines (127) by condensation of ethyl acetoacetate (125), ammonium acetate (126), and aldehydes (124) under the influence of microwave irradiation in solvent free conditions (Scheme 30). They reported that the reaction offers several advantages, such as the absence of any volatile and hazardous organic solvent, high yields and simple procedure with an easy work-up. Moreover, the catalyst can be easily recycled and reused at least three times without appreciable loss of its catalytic activity.
Scheme 30.
Siddiqui [127] has further investigated the utilities of bis[(l)prolinate-N,O]Zn as heterogeneous Lewis catalyst for the preparation of 3,4-dihydropyrimidin-2(1H)-one derivatives (136a–j) by the condensation of 1,3 dicarbonyl (128), urea (129) and aldehyde (130) in Scheme 31. Again they have shown bis[(l)prolinate-N,O]Zn as an suitable catalyst for this transformation.
Scheme 31.
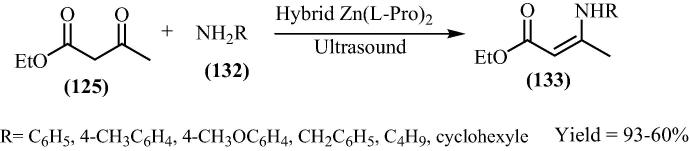
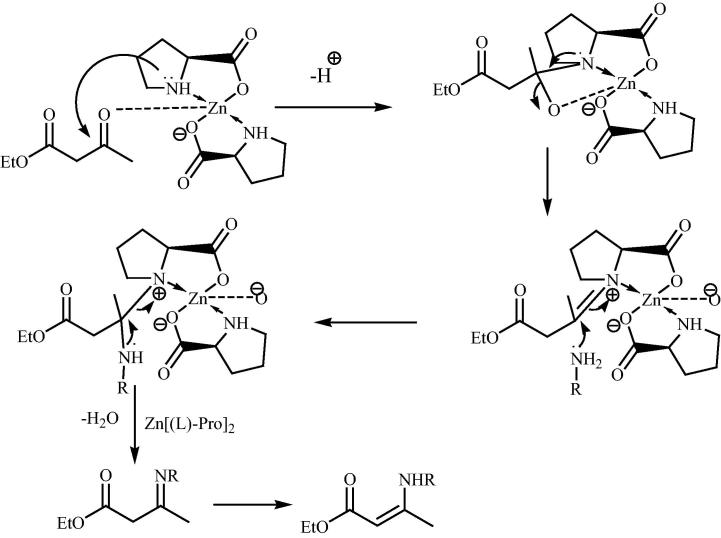
Recently hybrid materials have seized attention from scientific community mainly as heterogenic catalysts in organic reactions on a large scale succeeding in some organic compounds with high yields. One of the most important classes of hybrid materials used for this purpose involves the complex of Zn and amino acids. Winck and his research group [128] have introduced bis[(l)prolinate-N,O]Zn and Zn[Gly]2 hybrid materials for the synthesis of several β-enaminones via solvent free protocol under the influence of ultrasound (Scheme 32). β-enaminones are known for their flexible reactivity, as nucleophiles and electrophiles. In mechanistic point of view there is a nucleophilic attack of the nitrogen from the catalyst on the ketone carbonyl which has methyl group (Fig. 19). This attack produced the iminium ion which was attacked by the amine. The obtained N,N acetal produced the corresponding imine which finally rearranged itself resulting in the β-enaminone while there is no nucleophilic attack on the ester carbonyl group.
Scheme 32.
Fig. 19.
Plausible mechanism for the bis[(l)prolinato-N,O]Zn catalyzed reaction for β-enaminone compounds.
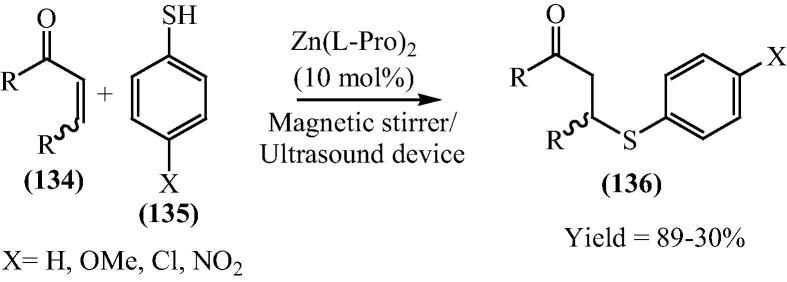
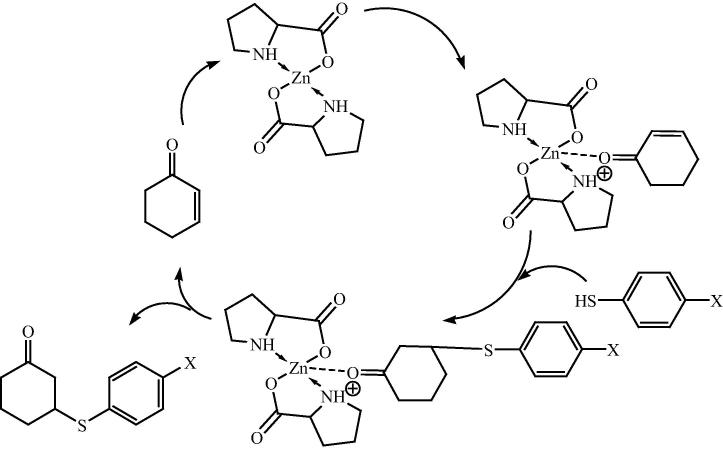
Recently Darbem [129] and his research group further extent catalytic property of bis[(l)prolinate-N,O]Zn as a heterogeneous catalyst in a thio-Michael reaction using the ultrasound method (Scheme 33). The 80% yield is obtained in 1 h for the thio-Michel adduct when 10 mol% of the bis[(l)prolinato-N,O]Zn was employed at the same time the Ultrasound device used. It is worth noting that when using a chiral hybrid catalyst, bis[(l)prolinato-N,O]Zn, a dextro thio-Michael adduct was obtained. This result is important and contrary to the results from porcine pancreatic lipase [130]. However, the use of the ultrasound device did not result in a substantial increase in the yields for thio-Michael adducts. The reaction using isophorone did not produce the thio-Michael adduct, and to the best of their knowledge, they attributed this effect to the presence of dimethyl groups bonded to carbon 5, which made it impossible for a nucleophilic attack to occur in the transition state following the reaction between bis[(l)prolinate-N,O]Zn and isophorone. This fact was confirmed by the result of 3-methyl-cyclohexen-2-one, which also shows a hindered effect but in the C3 position. For this compound, yields were lower when compared to the other Michael acceptor. Taking into account all results, they presented a mechanism involving the thiophenol and cyclohexen-2-one (Fig. 20).
Scheme 33.
Fig. 20.
Plausible mechanism for the bis[(l)prolinate-N,O]Zn catalyzed reaction for thio-Michael reaction.
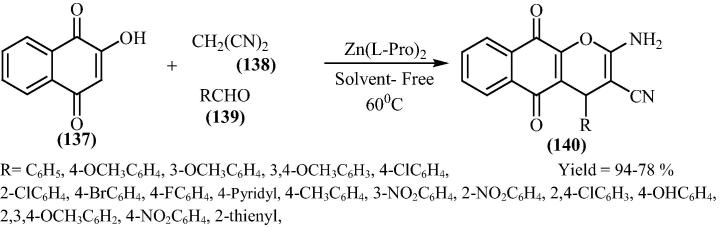
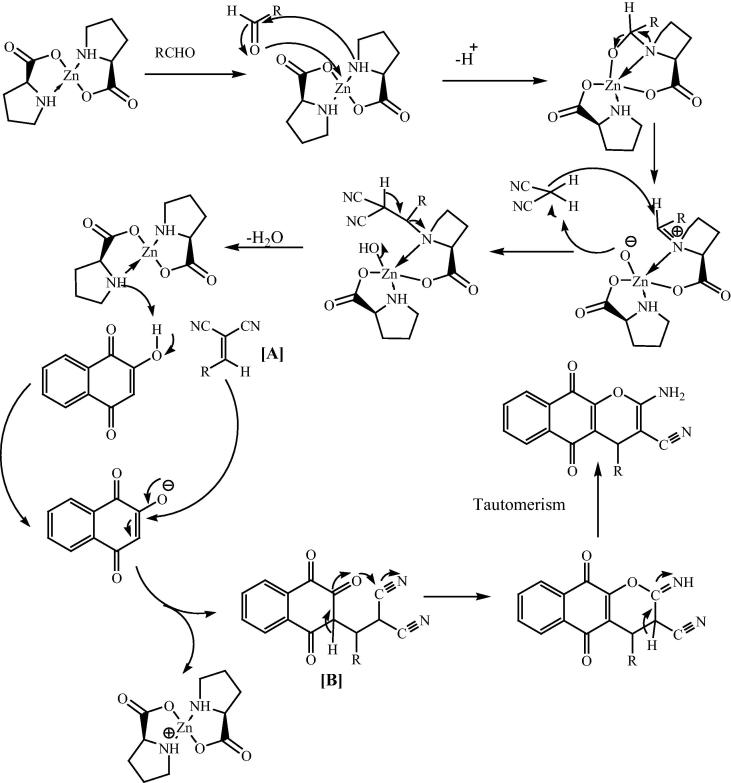
Maleki and his research group [131] have reported a simple, clean and environmentally friendly process for the synthesis of 2-amino-4H-benzo[g]chromene derivatives by reaction of various aldehydes, malononitrile and 2-hydroxy-1,4-naphthaquinone in the presence of 20 mol% of bis[(l)prolinate-N,O]Zn under solvent-free conditions at 60 °C (Scheme 34). A plausible mechanism of the reaction is shown in Fig. 21 exhibits that bis[(l)prolinate-N,O]Zn complex facilitates cyanoolefin formation and synthesis of 2-amino-4H-benzo[g]chromenes. The reaction occurs via initial formation cyanoolefin [A] from condensation of aldehydes and malononitrile, which reacts with 2-hydroxynaphthalene-1,4-dione to give intermediate [B] which subsequently underwent cyclization to afford the desired products. There was no effect observed on the reaction time and the yield of the corresponding products when electron-donating groups and electron-withdrawing groups on benzaldehydes are used. On further examination it was found that aliphatic aldehydes such as butanal instead of benzaldehydes in the reaction, showed no desired products after 1 h. In addition to the aromatic aldehydes, the reaction also precedes smoothly using heterocyclic aldehydes in high yield.
Scheme 34.
Fig. 21.
Plausible mechanism for the bis[(l)prolinate-N,O]Zn catalyzed 2-amino-4H-benzo[g]chromene by reaction of aldehydes, malononitrile and 2-hydroxy-1,4-naphthaquinone.
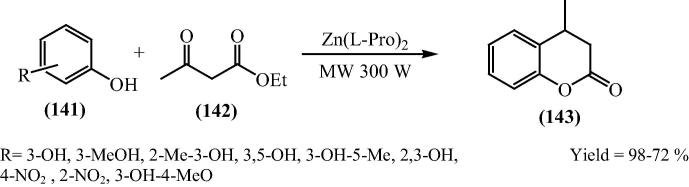
The author Chavan and his research group [132] have reported the Pechmann condensation reaction of phenols and β-ketoesters employing bis[(l)prolinate-N,O]Zn complex as a simple, efficient, eco-friendly, organometallic catalyst under solvent free condition. They carried out a series of substituted phenols with ethylacetoacetate to obtain corresponding coumarin derivatives in a very good yield (72–98%) (Scheme 35). The catalyst is reusable up to five cycles with marginal loss of its catalytic activity.
Scheme 35.
Conclusions and future perspectives
This review demonstrates the synthesis, characteristics and catalysis of bis[(l)prolinato-N,O]Zn. This study shows the organic synthetic applications of bis[(l)prolinate-N,O]Zn in water provide alternative, environmentally friendly methods that can be easily prepared and stored as stable solids in non-inert conditions and can be used to substitute a host of traditional Lewis acid applied along with VOCs (Volatile organic compounds) [133], [134], [135], [136], [137]. The increased application of bis[(l)prolinate-N,O]Zn in organic reactions will definitely develop in the future as our thinking of this complex and new complexes are revealed and brought to market. Concerning the catalysts, the tendency toward reusable solids will accelerate in the near future. It is anticipated that the reaction conditions under which bis[(l)prolinate-N,O]Zn performs will be broadened and this will open further research opportunities. Given societies demand for green chemistry solutions and the creativity opportunities surrounding this unique amino acid-complex, it is believed that the next decade will give one of the most productive and hopeful sections in the long history of metal-complexes. In future, there are chance that formation of complex alike bis[(l)prolinate-N,O]Zn where zinc metal ion can be substituted with other transition metal dications i.e. Fe2+, Cu2+, Mn2+, Mg2+ or proline with other α-amino acid in which amine is secondary which will create a series of green and economic metal complexes.
Conflict of Interest
The authors have declared no conflict of interest.
Compliance with Ethics Requirements
This article does not contain any studies with human or animal subjects.
Acknowledgments
All the authors are grateful to the University of Delhi, Delhi, India, for the financial support.
Biographies

Roona Poddar is an Assistant Professor of Chemistry and teaches post graduate student in Department of Chemistry, University of Delhi, Delhi, India. She has a Master Degree in Chemistry from Indian Institute of Technology (IIT) Delhi, and a Ph.D. in Chemistry from the University of Delhi (DU). She has worked as Post Doctorate Fellow for three years before joining as faculty in Department of Chemistry, University of Delhi, Delhi, India She has published numerous research papers in peer reviewed journals.

Arti Jain obtained her PhD (Organic Chemistry) from University of Delhi, India, in 2013. She is currently an Assistant Professor in Department of Chemistry, Daulat Ram College, University of Delhi, India. Her research area is based on the exploration of newer environmental benign protocol for various traditional reactions, use of agricultural waste material to apply cradle to cradle approach etc. She has 4 years of teaching experience to the undergraduate students. She is still doing research in the college.

M. Kidwai is working as Professor at the Department of Chemistry, Delhi University, delhi, India. He has 30 years of teaching experience at the university level. Currently he has 260 papers in the Journal of National and International repute and supervised 40 Ph.D. students and 31 M. Phil students. Pioneer in the field of Green Chemistry, who has started first research work in this field in India in 1990. From Asia among 5 members is inclusive of himself in the international Advisory board make Globally figure in the exclusive field of Green chemistry.
Footnotes
Peer review under responsibility of Cairo University.
References
- 1.Christiansons W.D., Lipscomb W.N. Carboxypeptidase A. Acc Chem Res. 1992;22:62–69. [Google Scholar]
- 2.Caroline M.L., Kandasamy A., Mohan R., Vasudevan S. Growth and characterization of dichlorobis l-prolineZn(II): a semi organic nonlinear optical single crystal. J Crystal Growth. 2009;311:1161–1165. [Google Scholar]
- 3.Aoki S., Kimura E. Zinc-nucleic acid interaction. Chem Rev. 2004;104:769–787. doi: 10.1021/cr020617u. [DOI] [PubMed] [Google Scholar]
- 4.Yukawa Y., Inomata Y., Takeuchi T., Ouchi M.A.S. The structure of dichloro(4-hydroxy-l-proline)cadmium (II) Bull Chem Soc Jpn. 1982;55:3135–3137. [Google Scholar]
- 5.Yukawa Y., Inomata Y., Takeuchi T. Structure and properties of dichloro(4-hydroxy-l-proline)cadmium (II) hydrate. Bull Chem Soc Jpn. 1983;56:2125–2128. [Google Scholar]
- 6.Yukawa Y., Yasukawa N., Inomata Y., Takeuchi T. The structure of dichlorobis (l-proline)zinc (II) Bull Chem Soc Jpn. 1985;58:1591–1592. [Google Scholar]
- 7.Itoh S., Yukawa Y., Inomata Y., Takeuchi T. Structure of aqauchloro(4-hydroxy-l-prolinato)copper (II) Bull Chem Soc Jpn. 1987;60:899–902. [Google Scholar]
- 8.Jiang P., Guo Z. Fluorescent detection of zinc in biological systems: recent development on the design of chemosensors and biosensors. Coordin Chem Rev. 2004;248:205–229. [Google Scholar]
- 9.Prasad A.S. Clinical, biochemical and pharmacological role of zinc. Annu Rev Pharmatoxicol. 1979;20:393–426. doi: 10.1146/annurev.pa.19.040179.002141. [DOI] [PubMed] [Google Scholar]
- 10.Vallee B.L., Falchuk K.H. The biochemical basis of zinc physiology. Physiol Rev. 1993;73:79–118. doi: 10.1152/physrev.1993.73.1.79. [DOI] [PubMed] [Google Scholar]
- 11.Vallee B.L., Auld D.S. Active-site zinc ligands and activated H2O of zinc enzymes. Proc Natl Acad Sci USA. 1990;87:220–224. doi: 10.1073/pnas.87.1.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vallee B.L., Auld D.S. Cocatalytic zinc motifs in enzyme catalysis. Proc Natl Acad Sci USA. 1993;90:2715–2718. doi: 10.1073/pnas.90.7.2715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ruf M., Burth R., Weis K., Vahrenkamp H. Coordination of cysteine and histidine derivatives to the pyrazolylborate-zinc unit. Chem Ber. 1996;129:1251–1257. [Google Scholar]
- 14.Brand U., Rombach M., Seebacher J., Vahrenkamp H. Functional modeling of cobalamine-independent methionine synthase with pyrazolylborate-zinc-thiolate complexes. Inorg Chem. 2001;40:6151–6157. doi: 10.1021/ic0105112. [DOI] [PubMed] [Google Scholar]
- 15.Orchard Katherine L., Harris J.E., White A.J.P., Shaffer M.S.P., Williams C.K. Solvent dependence of the structure of ethylzinc acetate and its application in CO2/epoxide copolymerization. Organometallics. 2011;301:223–229. [Google Scholar]
- 16.Daka P., Xu Z., Alexa A., Wang H. Primary amine-metal Lewis acid bifunctional catalysts based on a simple bidentate ligand: direct asymmetric aldol reaction. Chem Commun. 2011;47:224–226. doi: 10.1039/c0cc00917b. [DOI] [PubMed] [Google Scholar]
- 17.Parkin G. Synthetic analogues relevant to the structure and function of zinc enzymes. Chem Rev. 2004;104:699–767. doi: 10.1021/cr0206263. [DOI] [PubMed] [Google Scholar]
- 18.Paradowska J., Pasternak M., Gut B., Gryzło B., Mlynarski J. Direct asymmetric aldol reactions inspired by two types of natural aldolases: water-compatible organocatalysts and ZnII complexes. J Org Chem. 2012;77:173–187. doi: 10.1021/jo201584w. [DOI] [PubMed] [Google Scholar]
- 19.Laurie S.H. In: Wilkinson G., Gillard R.D., McCleverty J.A., editors. vol. 2. Pergamon; Oxford: 1987. p. 739. (Comprehensive coordination chemistry). [Google Scholar]
- 20.Irving H.M., Pettit L.D. The stabilities of metal complexes of some C-substituted derivatives of glycine. J Chem Soc. 1963:1546–1553. [Google Scholar]
- 21.Sigel H., Prijs B. Stability and structure of binary and ternary complexes of α-lipoate and lipoate derivatives with Mn2+, Cu2+, and Zn2+ in solution. Arch Biochem Biophys. 1978;187:208–214. doi: 10.1016/0003-9861(78)90025-5. [DOI] [PubMed] [Google Scholar]
- 22.Perrin D.D. The stability of iron complexes. Part III. A comparison of 1: 1 ferric and ferrous amino-acid complexes. J Chem Soc. 1959:290–296. [Google Scholar]
- 23.Perrin D.D. The stability of complexes of ferric ion and amino-acids. J Chem Soc. 1958:3125–3128. [Google Scholar]
- 24.Irving H.M., Williams R.J.P. 637. The solubility of transition-metal complexes. J Chem Soc. 1953:3192–3210. [Google Scholar]
- 25.Williams R.J.P. The biochemistry of zinc. Polyhedron. 1987;6:61–69. [Google Scholar]
- 26.Gilliard R.D., Irving H.M., Parkins R.M., Payne N.C., Pettit L.D. The isomers of complexes of α-amino-acids with copper(II) J Chem Soc (A) 1966:1159–1164. [Google Scholar]
- 27.Kobayashi S., Nagayama S., Busujima T. Lewis acid catalysts stable in water. Correlation between catalytic activity in water and hydrolysis constants and exchange rate constants for substitution of inner-sphere water ligands. J Am Chem Soc. 1998;120:8287–8288. [Google Scholar]
- 28.Darbre T., Machuquiero M. Zn-Proline catalyzed direct aldol reaction in aqueous media. Chem Commun. 2003:1090–1091. [PubMed] [Google Scholar]
- 29.Kidwai M., Jain A., Poddar R., Bhardwaj S. Bis[(l)prolinato-N, O]Zn in acetic acid–water: a novel catalytic system for the synthesis of β-amino carbonyls via Mannich reaction at room temperature. Appl Organometal Chem. 2011;25:335. [Google Scholar]
- 30.Ng C.-H., Fun H.-K., Teo S.-B., Teoh S.-G., Chinnakali K. Bis(l-prolinato-N, O)zinc(II) Acta Crystallogr Sec C. 1995;51:244–245. [Google Scholar]
- 31.Alcock N.W., Berry A., Moore P. Structure of a complex of 1,4,8,11-Tetraazacyclotetradecane (cyclam) with zinc (II) Acta Cryst. 1992;C48:16–19. [Google Scholar]
- 32.Shearer N.A., Shearer H.M.M., Spencer C.B. Tetrameric methylzinc acetoximate, Zn(CH3)(C3H6NO)4. Acta Cryst. 1984;C40:613–616. [Google Scholar]
- 33.Freeman H.C. Crystal structures of metal-peptide complexes. Adv Protein Chem. 1976;22:257–424. doi: 10.1016/s0065-3233(08)60043-1. [DOI] [PubMed] [Google Scholar]
- 34.Kratochvil B., Ondracek J., Novotny J. Structure of a Zinc(II) complex with a non-symmetrical tetradentate Schiff base. Acta Cryst. 1991;C47:2207–2209. [Google Scholar]
- 35.Lonibala R., Rao T. X-ray diffraction study of Zn(II) complexes of 2–Acetyl–pyridine (N–Benzoyl)glycylhydrazone. Crys Res Technol. 1991;26:77. [Google Scholar]
- 36.Kidwai M., Jain A., Poddar R. Zn[(l)proline]2 in water: a new easily accessible and recyclable catalytic system for the synthesis of pyrazoles. J Organomet Chem. 2011;696:1939. [Google Scholar]
- 37.Kidwai M., Jain A., Bhardwaj S. 1,4-addition of terminal alkynes to conjugated enones in water using green catalyst Bis[(l)prolinato-N, O]Zn—An environmentally benign protocol. Catal Lett. 2011;141:183–185. [Google Scholar]
- 38.Kidwai M., Jain A. Regioselective synthesis of 1,4-disubstituted triazoles using bis[(l)prolinato-N, O]Zn complex as an efficient catalyst in water as a sole solvent. Appl Organometal Chem. 2011;25:620. [Google Scholar]
- 39.Orgel LE. In: Crook EM, editor. Metals, enzyme activity, p-14. Cambridge University Press; 1958.
- 40.Neumann P.Z., S-Kortsak A. The state of copper in human serum: evidence for an amino acid-bound fraction. J Clin Invest. 1967;46:646–658. doi: 10.1172/JCI105566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Albert A. Quantitative studies of the avidity of naturally occurring substances for trace. Met Biochem J. 1950;47:531–538. doi: 10.1042/bj0470531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bjerrum J. P. Haase and Son; Copenhagen: 1941. Metal ammine formation in aqueous solution. [Google Scholar]
- 43.Hallman P.S., Perrin D.D., Watt A.E. The computed distribution of copper(II) and zinc(II) ions among seventeen amino acids present in human blood plasma. Biochem J. 1971;121:549–555. doi: 10.1042/bj1210549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Braun E., Eichen Y., Sivan U., Ben-Yoseph G. DNA-templated assembly and electrode attachment of a conducting silver wire. Nature. 1998;391:775–778. doi: 10.1038/35826. [DOI] [PubMed] [Google Scholar]
- 45.Trzaskowski B., Deymier P.A., Adamowicz L. Metallization of nanobiostructures: a theoretical study of copper nanowires growth in microtubules. J Mater Chem. 2006;16:4649–4656. [Google Scholar]
- 46.Munoz-Paez A., Pappalardo R.R., Marcos E.S. Determination of the second hydration shell of Cr3+ and Zn2+ in aqueous solutions by extended X-ray absorption fine structure. J Am Chem Soc. 1995;117:11710–11720. [Google Scholar]
- 47.Slocik JM, Knecht MR, Wright DW. In: King RB. editor. Encyclopedia of inorganic chemistry, 2nd ed. New York: John Wiley and Sons; 2005. p. 371.
- 48.Zhang C., Janiak C.J. Six-coordinated zinc complexes: [Zn(H2O)4(phen)] (NO3)2·H2O and [ZnNO3(H2O)(bipy)(Him)]NO3 (phen = 1,10-phenanthroline, bipy = 2,2′-bipyridine, and Him = imidazole) Chem Crystallogr. 2001;31:29. [Google Scholar]
- 49.Trzaskowski B., Adamowicz L., Deymier P.A. A theoretical study of zinc(II) interactions with amino acid models and peptide fragments. J Biol Inorg Chem. 2008;13:133–137. doi: 10.1007/s00775-007-0306-y. [DOI] [PubMed] [Google Scholar]
- 50.Sharma S.S., Schat H., Vooijs R. In vitro alleviation of heavy metal-induced enzyme inhibition by proline. Phytochemistry. 1998;49:1531–1535. doi: 10.1016/s0031-9422(98)00282-9. [DOI] [PubMed] [Google Scholar]
- 51.Grobhofer N. An adjuvant formulation based on N-acetylglucosaminyl-N-acetylmuramyl -l-alanyl-d-isoglutamine with dimethyldioctadecylammonium chloride and zinc-l-proline complex as synergists. Immuno Lett. 1995;44:19. doi: 10.1016/0165-2478(94)00188-w. [DOI] [PubMed] [Google Scholar]
- 52.Rombach M., Gelinsky M., Vahrenkamp H. Coordination modes of aminoacids to zinc. Inorg Chem Acta. 2002;334:25–33. [Google Scholar]
- 53.Gockel P., Vahrenkamp H., Zuberbühler A.D. Zine complexes of cysteine, histidine, and derivatives thereof: potentiometric determination of their compositions and stabilities. Helv Chim Acta. 1993;76:511. [Google Scholar]
- 54.Gockel P., Vogler R., Vahrenkamp H. Solution behavior and zinc complexation of dipeptides made up solely from histidine and cysteine. Chem Ber. 1996;129:887–895. [Google Scholar]
- 55.Förster M., Vahrenkamp H. Zinc complexes of histidine-containing Di- and tripeptides. Chem Ber. 1995;128:541–550. [Google Scholar]
- 56.Kistenmacher T.J. A refinement of the structure of bis-(l-histidinato)zinc(II) dihydrate. Acta Crystallogr B. 1972;28:1302–1304. [Google Scholar]
- 57.Helm Dvan der, Nicholas A.F., Fisher C.G. The crystal structure of bis(l-serinato)zinc. Acta Crystallogr. 1970;B26:1172–1173. [Google Scholar]
- 58.Kofoed J., Machuqueiro M., Reymond J.-L., Darbre T. Zinc–proline catalyzed pathway for the formation of sugars. Chem Commun. 2004:1540–1541. doi: 10.1039/b404465g. [DOI] [PubMed] [Google Scholar]
- 59.Wu Y.-S., Shao W.-Y., Zheng C.-Q., Huang Z.-L., Cai J., Deng Q.-Y. Studies on direct stereoselective aldol reactions in aqueous media. Helv Chim Acta. 2004;87:1377–1384. [Google Scholar]
- 60.Sivamurugan V., Deepa K., Palanichamy M., Murugesan V. [(l)Proline]2Zn catalysed synthesis of 1,5-benzodiazepine derivatives under solvent-free condition. Synth Commun. 2004;34:3833–3846. [Google Scholar]
- 61.Kofoed J., Reymond J.-L., Darbre T. Prebiotic carbohydrate synthesis: zinc–proline catalyzes direct aqueous aldol reactions of α-hydroxy aldehydes and ketones. Org Biomol Chem. 2005;10:1850–1855. doi: 10.1039/b501512j. [DOI] [PubMed] [Google Scholar]
- 62.Sawardeker J.S., Sloneker J.H., Jeanes A. Quantitative determination of monosaccharides as their alditol acetates by gas liquid chromatography. Anal Chem. 1965;37:1602–1604. [Google Scholar]
- 63.Weber A.L. Prebiotic sugar synthesis: hexose and hydroxy acid synthesis from glyceraldehyde catalyzed by iron(III) hydroxide oxide. J Mol Evol. 1992;35:1–6. doi: 10.1007/BF00160255. [DOI] [PubMed] [Google Scholar]
- 64.Lopez R.F., Kofoed J., Machuqueiro M., Darbre T. A selective direct aldol reaction in aqueous media catalyzed by zinc-proline. Eur J Org Chem. 2005:5268–5276. [Google Scholar]
- 65.Dickerson T.J., Lovell T., Meijer M.M., Noodleman L., Janda K.D. Nornicotine aqueous aldol reactions: synthetic and theoretical investigations into the origins of catalysis. J Org Chem. 2004;69:6603–6609. doi: 10.1021/jo048894j. [DOI] [PubMed] [Google Scholar]
- 66.Kofoed J., Darbre T., Reymond J.-L. Dual mechanism of zinc-proline catalyzed aldol reactions in water. Chem Commun. 2006:1482–1484. doi: 10.1039/b600703a. [DOI] [PubMed] [Google Scholar]
- 67.Sivamurugan V., Vinu A., Palanichamy M., Murugesan V. Rapid and cleaner synthesis of 1,4-dihydropyridines in aqueous medium. Heteroatom Chem. 2006;17:267–271. [Google Scholar]
- 68.Brock ED, Lewis DM, Yousaf TI, Harper HH. Procter & Gamble Company, Reactive dye compounds USA WO; 1999. 9951688.
- 69.Gazit A., App H., McMahon G., Chen J., Levitzki A., Bohmer F.D. Tyrphostins. 5. Potent inhibitors of platelet-derived growth factor receptor tyrosine kinase: structure-activity relationships in quinoxalines, quinolines, and indole tyrphostins. J Med Chem. 1996;39:2170–2177. doi: 10.1021/jm950727b. [DOI] [PubMed] [Google Scholar]
- 70.Sehlstedt U., Aich P., Bergman J., Vallberg H., Norden B., Graslund A. Interactions of the antiviral quinoxaline derivative 9-OH-B220 2,3-dimethyl-6-(dimethylaminoethyl)-9-hydroxy-6H-indolo-[2,3-b]quinoxaline with Duplex and Triplex Forms of Synthetic DNA and RNA. J Mol Biol. 1998;278:31–56. doi: 10.1006/jmbi.1998.1670. [DOI] [PubMed] [Google Scholar]
- 71.Dailey S., Feast J.W., Peace R.J., Till I.C., Sage S., Wood E.L. Synthesis and device characterisation of side-chain polymer electron transport materials for organic semiconductor applications. J Mater Chem. 2001;11:2238–2243. [Google Scholar]
- 72.O’Brien D., Weaver M.S., Lidzey D.G., Bradley D.D.C. Use of poly(phenylquinoxaline) as an electron transport material in polymer light-emitting diodes. Appl Phys Lett. 1996;9:881–883. [Google Scholar]
- 73.Heravi M.M., Tehrani M.H., Bakhtiari K., Oskooie H.A. Zn[(l)proline]2: a powerful catalyst for the very fast synthesis of quinoxaline derivatives at room temperature. Catal Commun. 2007;8:1341–1344. [Google Scholar]
- 74.Reddy K.R., Rajasekhar C.V., Krishna G.G. Zinc-proline complex: an efficient, reusable catalyst for direct nitroaldol reaction in aqueous media. Synth Commun. 2007;37:1971–1976. [Google Scholar]
- 75.Luzzio F.A. The Henry reaction: recent examples. Tetrahedron. 2001;57:915–945. [Google Scholar]
- 76.Ono N. Wiley-VCH; New York: 2001. The nitro group in organic synthesis. [Google Scholar]
- 77.Rosini G. In: Trost B.M., Fleming I., Heathcock C.H., editors. vol. 2. Pergamon; Oxford, New York: 1991. p. 321. (Comprehensive organic synthesis). [Google Scholar]
- 78.Ravi V., Ramu E., Vijay K., Rao A.S. Zn-proline catalyzed selective synthesis of 1,2-disubstituted benzimidazoles in water. Chem Pharm Bull. 2007;55:1254–1257. doi: 10.1248/cpb.55.1254. [DOI] [PubMed] [Google Scholar]
- 79.Manvar A., Bochiya P., Virsodia V., Khunt R., Shah A. Microwave-assisted and Zn[l-proline]2 catalyzed tandem cyclization under solvent free conditions: rapid synthesis of chromeno[4,3-c]pyrazol-4-ones. J Mol Catal A: Chem. 2007;275:148–152. [Google Scholar]
- 80.Itoh S., Kitamura M., Yamada Y., Aoki S. Chiral catalysts dually functionalized with amino acid and Zn2+ complex components for enantioselective direct aldol reactions inspired by natural aldolases: design, synthesis, complexation properties, catalytic activities, and mechanistic study. Chem Eur J. 2009;15:10570–10584. doi: 10.1002/chem.200900733. [DOI] [PubMed] [Google Scholar]
- 81.Sheng-Rong G., Yan-Qin Y., Li-Jin W. Zn(Pro)2-promoted CuI-catalyzed synthesis of diphenyl sulfide derivations in ionic liquid. J Sulf Chem. 2009;30:10–16. [Google Scholar]
- 82.Dömling A. Recent developments in isocyanide based multicomponent reactions in applied chemistry. Chem Rev. 2006;106:17–89. doi: 10.1021/cr0505728. [DOI] [PubMed] [Google Scholar]
- 83.Fenn D., editor. The pyrimidines. Wiley; New York: 1994. [Google Scholar]
- 84.Heber D., Heers C., Ravens U. Positive inotropic activity of 5-amino-6-cyano-1,3-dimethyl-1,2,3,4-tetrahydropyrido[2,3-d]pyrim idine-2,4-dione in cardiac muscle from guinea-pig and man. Part 6: compounds with positive inotropic activity. Pharmazie. 1993;48:537. [PubMed] [Google Scholar]
- 85.Griva E.M., Lee S., Siyal C.W., Duch D.S., Nichol C.A. Synthesis and antitumor activity of 2,4-diamino-6-(2,5-dimethoxybenzyl)-5-methylpyrido[2,3-d]pyrimidine. J Med Chem. 1980;23:327–329. doi: 10.1021/jm00177a025. [DOI] [PubMed] [Google Scholar]
- 86.Ghorab M.M., Hassan A.Y. Synthesis and antibacterial properties of new dithienyl containing pyran, pyrano[2,3-b] pyridine, pyrano[2,3-d]pyrimidine and pyridine derivatives. Phosphorus Sulfur Silicon Relat Elem. 1998;141:251–261. [Google Scholar]
- 87.Furuya S, Ohtaki T. Pyrido[2,3-d]pyrimidines and their use as endothelin antagonists. Eur Patent; 1994. 608565.
- 88.Coates WJ. Preparation of pyrimidopyrimidine derivatives useful as bronchodilators, vasodilators, antiallergic agents, and phosphodiesterase inhibitors. Eur Patent; 1990. 351058.
- 89.Kitamura N, Onishi A. Pyrimidopyrimidinedione derivatives and their use antiallergic agent. Eur Patent; 1984. 163599.
- 90.Davoll J., Clarke J., Elslager E.F. Folate antagonists. 4. Antimalarial and antimetabolite effects of 2,4-diamino-6- [(benzyl)amino] pyrido [2,3-d]-pyrimidine. J Med Chem. 1972;15:837–839. doi: 10.1021/jm00278a009. [DOI] [PubMed] [Google Scholar]
- 91.Levitt G. Herbicidal sulfonamides. US Patent; 1982. 4339267.
- 92.Heravia M.M., Ghodsa A., Bakhtiaria K., Derikvanda F. Zn[(l)proline]2: an efficient catalyst for the synthesis of biologically active pyrano[2,3-d]pyrimidine derivatives. Synth Commun. 2010;40:1927–1931. [Google Scholar]
- 93.Siddiqui Z.N., Musthafa T.N.M., Praveen S., Farooq F. Zn(Proline)2-catalyzed Knoevenagel condensation under solvent free/aqueous conditions and biological evaluation of products. Med Chem Res. 2011;20:1438–1445. [Google Scholar]
- 94.Siddiqui Z.N., Asad M. Folate antagonists. 4. Antimalarial and antimetabolite effects of 2,4-diamino-6-[(benzyl)amino]pyrido [2,3-d] -pyrimidine. Indian J Chem. 2009;48B:1609–1613. doi: 10.1021/jm00278a009. [DOI] [PubMed] [Google Scholar]
- 95.Foroumadi A., Kermani A.S., Emami S., Dehghan G., Sorkhi M., Arabsorkhi F., et al. Synthesis and antioxidant properties of substituted 3-benzylidene-7-alkoxychroman-4-ones. Bioorg Med Chem Lett. 2007;17:6764–6769. doi: 10.1016/j.bmcl.2007.10.034. [DOI] [PubMed] [Google Scholar]
- 96.Dewick PM. In: Harborne JB, editor. The flavonoids: advances in research since 1986. New York: Chapmann & Hall; 1994. p. 38.
- 97.Valenti P., Bisi A., Rampa A., Belluti F., Gobbi S., Zampiron A., et al. Synthesis and biological activity of some rigid analogues of flavone-8-acetic acid. Bioorg Med Chem. 2000;8:239–246. doi: 10.1016/s0968-0896(99)00282-5. [DOI] [PubMed] [Google Scholar]
- 98.Boumendjel A., Bois F., Beney C., Mariotte A.M., Conseil G., Di Petro A. B-ring substituted 5,7-dihydroxyflavonols with high-affinity binding to P-glycoprotein responsible for cell multidrug resistance. Bioorg Med Chem Lett. 2001;11:75–77. doi: 10.1016/s0960-894x(00)00595-3. [DOI] [PubMed] [Google Scholar]
- 99.Larget R., Lockhart B., Renard P., Largeron M. A CONVENIENT EXTENSION OF THE Wessely-Moser rearrangement for the synthesis of substituted alkylaminoflavones as neuroprotective agents in vitro. Bioorg Med Chem Lett. 2000;10:835–838. doi: 10.1016/s0960-894x(00)00110-4. [DOI] [PubMed] [Google Scholar]
- 100.Groweiss A., Cardellins J.H., Boyd M.R. HIV-inhibitory prenylated xanthones and flavones from maclura tinctoria. J Nat Prod. 2000;63:1537–1539. doi: 10.1021/np000175m. [DOI] [PubMed] [Google Scholar]
- 101.Gross A., Borcherding D.R., Friedrich D., Sabol J.S. A stereocontrolled approach to substituted piperidones and piperidines: flavopiridol D-ring analogs. Tetrahedron Lett. 2001;42:1631–1633. [Google Scholar]
- 102.Prakash O., Kumar R., Parkash V. Synthesis and antifungal activity of some new 3-hydroxy-2-(1-phenyl-3-aryl-4-pyrazolyl)chromones. Eur J Med Chem. 2008;43:435–440. doi: 10.1016/j.ejmech.2007.04.004. [DOI] [PubMed] [Google Scholar]
- 103.Nowakowska Z. A review of anti-infective and anti-inflammatory chalcones. Eur J Med Chem. 2007;42:125–137. doi: 10.1016/j.ejmech.2006.09.019. [DOI] [PubMed] [Google Scholar]
- 104.Siddiqui Z.N., Musthafa T.N.M. An efficient and novel synthesis of chromonyl chalcones using recyclable Zn(l-proline)2 catalyst in water. Tetrahedron Lett. 2011;52:4008–4013. [Google Scholar]
- 105.Anastas P.T., Warner J. Oxford Univ. Press; Oxford: 1998. Green chemistry theory, practice. [Google Scholar]
- 106.Clark J.H. Green chemistry: challenges and opportunities. Green Chem. 1999;1:1–8. [Google Scholar]
- 107.Wang H., Deng S.X., Shen Z.R., Wang J.G., Ding D.T., Chen T.H. Facile preparation of Pd/organoclay catalysts with high performance in solvent-free aerobic selective oxidation of benzyl alcohol. Green Chem. 2009;11:1499–1502. [Google Scholar]
- 108.Shirakawa S., Kobayashi S. Carboxylic acid catalyzed three-component aza-friedel-crafts reactions in water for the synthesis of 3-substituted indoles. Org Lett. 2006;8:4939–4942. doi: 10.1021/ol062031q. [DOI] [PubMed] [Google Scholar]
- 109.Zhuang W., Jorgensen K.A. Friedel-Crafts reactions in water of carbonyl compounds with heteroaromatic compounds. Chem Commun. 2002:1336–1337. [Google Scholar]
- 110.Boersma A.J., Feringa B.L., Roelfes G. Enantioselective friedel-crafts reactions in water using a DNA based catalyst. Angew Chem Int Ed. 2009;48:3346–3348. doi: 10.1002/anie.200900371. [DOI] [PubMed] [Google Scholar]
- 111.Manabe K., Aoyama N., Kobayashi S. Friedel-Crafts-type conjugate addition of Indoles using a Lewis acid-surfactant-combined catalyst in water. Adv Synth Catal. 2001;343:171–173. [Google Scholar]
- 112.Kobayashi S., Manabe K. Development of novel Lewis acid catalysts for selective organic reactions in aqueous media. Acc Chem Res. 2002;35:209–217. doi: 10.1021/ar000145a. [DOI] [PubMed] [Google Scholar]
- 113.Azizi N., Arynasab F., Saidi M.R. Efficient Friedel-Crafts alkylation of indoles and pyrrole with enones and nitroalkene in water. Org Biomol Chem. 2006;4:4275–4277. doi: 10.1039/b610263h. [DOI] [PubMed] [Google Scholar]
- 114.Breslow R. Hydrophobic effects on simple organic reactions in water. Acc Chem Res. 1991;24:159–164. [Google Scholar]
- 115.Venkatraman S., Li C.-J. Rhodium catalyzed conjugated addition of unsaturated carbonyl compounds by triphenylbismuth in aqueous media and under an air atmosphere. Tetrahedron Lett. 2001;42:781–784. [Google Scholar]
- 116.Lubineau A., Auge J. vol. 2. Springer; Berlin: 1999. p. 1. (Topic in current chemistry). [Google Scholar]
- 117.Pirrung M.C., Sarma K.D. Multicomponent reactions are accelerated in water. J Am Chem Soc. 2004;126:444–445. doi: 10.1021/ja038583a. [DOI] [PubMed] [Google Scholar]
- 118.Siddiqui Z.N., Farooq F. Zn(Proline)2: a novel catalyst for the synthesis of dicoumarols. Catal Sci Technol. 2011;1:810–817. [Google Scholar]
- 119.Gonzalez S.D., Nolan S.P. [(NHC)2Cu]X complexes as efficient catalysts for azide-alkyne click chemistry at low catalyst loadings. Angew Chem Int Ed. 2008;47:8881–8888. doi: 10.1002/anie.200803289. [DOI] [PubMed] [Google Scholar]
- 120.Namitharan K., Kumarraja M., Pitchumani K. CuII–Hydrotalcite as an efficient heterogeneous catalyst for Huisgen [3+2] cycloaddition. Chem Eur J. 2009;15:2755–2758. doi: 10.1002/chem.200802384. [DOI] [PubMed] [Google Scholar]
- 121.Muller R., Goesmann H., Waldmann H. N,N-phthaloylamino acids as chiral auxiliaries in asymmetric Mannich type reactions. Angew Chem Int Ed. 1999;38:184–187. [Google Scholar]
- 122.Wang Z.-X., Qin H.-L. Solventless syntheses of pyrazole derivatives. Green Chem. 2004;6:90–92. [Google Scholar]
- 123.Kidwai M., Jain A. Zn[(l)Proline]2: an eligible candidate for the synthesis of xanthenediones in water. Appl Organometal Chem. 2012;26:528–535. [Google Scholar]
- 124.Siddiqui Z.N. One pot synthesis of new benzopyranopyridines via Friedlander condensation. Tetrahedron Lett. 2012;53:4974–4978. [Google Scholar]
- 125.Siddiqui Z.N., Farooq F. A practical one pot synthesis of novel 2-hydroxy-4-chromanone derivatives from 3-formylchromone. J Chem Sci. 2012;124:1097–1105. [Google Scholar]
- 126.Pourshamsian N.M.K., Zoghi R., Mahjoob S. Zn[(l)-proline], as a recyclable and green catalyst for efficient and one-pot three-component synthesis of 1,4-dihydro- pyridines under solvent-free and microwave irradiation conditions. Oriental J Chem. 2012;28:103–107. [Google Scholar]
- 127.Siddiqui Z.N. Bis[(l)prolinato-N, O]Zn–water: a green catalytic system for the synthesis of 3,4-dihydropyrimidin-2(1H)-ones via the Biginelli reaction. CR Chimie. 2013;16:183–188. [Google Scholar]
- 128.Winck C.R., Darbem M.P., Gomes R.S., Rinaldi A.W. Domingues NLC. Zn[aminoacid]2 hybrid materials applied as heterogeneous catalysts in the synthesis of β-enaminones. Tetrahedron Lett. 2014;55:4123–4125. [Google Scholar]
- 129.Darbem M.P., Oliveira A.R., Winck C.R., Rinaldi A.W., Domingues N.L.C. Hybrid material from Zn[aminoacid]2 applied in the thio-Michael synthesis. Tetrahedron Lett. 2014;55:5179–5181. [Google Scholar]
- 130.Rizzo P.V.S., Boarin L.A., Freitas I.O.M., Gomes R.S., Beatriz A., Rinaldi A.W., et al. The study of biocatalyzed thio-Michael reaction: a greener and multi-gram protocol. Tetrahedron Lett. 2014;55:430–434. [Google Scholar]
- 131.Maleki B., Babaee S., Tayebee R. Zn(l-proline)2 as a powerful and reusable organometallic catalyst for the very fast synthesis of 2-amino-4H-benzo[g]chromene derivatives under solvent-free conditions. Appl Organometal Chem. 2015;29:408–411. [Google Scholar]
- 132.Chavan O.S., Jadhav S.A., Shioorkar M.G., Chavan S.B., Baseer M.A., Shinde D.B. Microwave assisted solvent free synthesis of Coumarins using Zn[(l)-proline]2 catalyst Rasayan. J Chem. 2015;8:194–197. [Google Scholar]
- 133.Kidwai M., Chauhan R., Bhatnagar S. Nafion-H®: a versatile catalyst for organic synthesis. Curr Org Chem. 2015;19:72–98. [Google Scholar]
- 134.Kidwai M., Poddar R., Diwaniyan S., Kuhad R.C. Laccase from basidiomycetous fungus catalyzes the synthesis of substituted 5-deaza-10-oxaflavins via a domino reaction. Adv Synth Catal. 2009;351:589–595. [Google Scholar]
- 135.Kidwai M., Poddar R., Bhardwaj S., Singh S., Luthra P.M. Aqua mediated synthesis of 2-amino-6-benzothiazol-2-ylsulfanyl-chromenes and its in vitro study, explanation of the structure-activity relationships (SARs) as antibacterial agent. Eur J Med Chem. 2010;45:5031–5038. doi: 10.1016/j.ejmech.2010.08.010. [DOI] [PubMed] [Google Scholar]
- 136.Kidwai M., Poddar R., Mothsra P. Beilstein N-acylation of ethanolamine using lipase: a chemoselective catalyst. J Org Chem. 2009;5(10) doi: 10.3762/bjoc.5.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Dhanda R., Kidwai M. Magnetically separable CuFe2O4/reduced graphene oxide nanocomposites: as a highly active catalyst for solvent free oxidative coupling of amines to imines. RSC Adv. 2016;6:53430–53437. [Google Scholar]