Supplemental Digital Content is available in the text
Keywords: ambulatory blood pressure, bremelanotide, female sexual dysfunction, melanocortin agonists
Abstract
Background:
Melanocortin receptor agonists that bind to the melanocortin receptor 4 may cause increases in blood pressure (BP). Bremelanotide is an on-demand, subcutaneous melanocortin-receptor agonist that binds to the melanocortin receptor 4 and is being developed for the treatment of female sexual dysfunction.
Methods:
We studied the effects of bremelanotide administration on ambulatory BP and heart rate (HR), in a randomized, double-blind, placebo-controlled, and parallel-arm trial of three doses of bremelanotide (0.75, 1.25, and 1.75 mg) in 397 premenopausal women with female sexual dysfunction with normotension or controlled hypertension. Pharmacokinetic exposure was assessed in conjunction with ambulatory BP measurements.
Results:
Increases in ambulatory SBP relative to placebo of 2.4 and 3.0 mmHg (1.25 mg; P values: 0.029 and 0.076) and 3.1 and 3.2 mmHg (1.75 mg; P values: 0.006 and 0.027), respectively, occurred following two doses, separated by 24 h at the 0 to 4-h postdose interval; peak increases typically lasted less than 15 min. Similar increases in the DBP were observed. Increases in BP were accompanied by reductions in HR during the 0–4-h interval for the 1.75-mg dose (−4.6 to −4.7 bpm; P < 0.001). Twenty-six participants discontinued after randomization due to prespecified increases in BP but the proportions were similar among the four treatment groups.
Conclusion:
These data show that ambulatory monitoring was a useful methodology to detect small, transient increases in ambulatory BP accompanied by reductions in HR following bremelanotide. Results of this trial led to appropriate in-clinic BP monitoring during the larger clinical development trials of this agent for female sexual dysfunction.
INTRODUCTION
Bremelanotide is a novel cyclic heptapeptide melanocortin analog that acts as a melanocortin-receptor ligand and agonist with highest affinity for the type-4 receptor, which has the potential for downstream modulation of brain pathways involved in sexual response [1–3]. The drug was initially developed for intranasal administration; however, this route was associated with a wide variability in bioavailability [4,5], potentially exposing some patients to levels above those needed for efficacy, and a heightened risk of autonomic or other adverse effects including elevations in blood pressure (BP), while leaving other patients with inadequate exposure. Hence, a subcutaneous formulation was developed that might provide a tighter relation between dose and exposure, facilitating the identification of dosing schedules with clinical efficacy and improved safety profiles.
For drugs with off-target effects associated with increases in BP, out-of-office monitoring has been shown to be effective in ascertaining the extent and duration of pharmacodynamic effects during both phases 2 and 3 clinical development [6,7]. Hence, we conducted a large, multicenter, randomized, double-blind, placebo-controlled, and parallel-group trial of three different doses of bremelanotide administered subcutaneously on an at-home, as-needed basis for up to 12 weeks by premenopausal women with sexual dysfunction syndromes. The study was developed to characterize the incidence and time course of BP changes by using ambulatory monitoring and to determine the pharmacodynamics of bremelanotide following subcutaneous dosing.
METHODS
Study design
The study consisted of a 4-week, no-treatment screening period; a single in-clinic dose of single-blind placebo administration; a 4-week, single-blind placebo, outpatient self-treatment (baseline) period; a 2-week period during which randomized study participants received two single in-clinic doses of double-blind placebo/treatment spaced approximately 1 week apart; and a 12-week, double-blind, outpatient, self-treatment period (Fig. S1 in the online data supplement). The double-blind randomization, by an interactive voice/web response system, assigned study participants in a 1 : 1 : 1 : 1 ratio, with stratification by sexual dysfunction diagnosis, to placebo or bremelanotide 0.75, 1.25, or 1.75 mg provided in prefilled syringes for subcutaneous injection into the anterior thigh or abdomen approximately 45 min prior to anticipated sexual activity (not exceeding one dose per day or 16 doses during a 4-week period). During this latter 12-week study phase, participants were assessed every 4 weeks. The trial was registered with ClinicalTrials.gov (identifier: NCT01382719).
Study participants
All participants were premenopausal, nonpregnant women aged at least 21 years with hypoactive sexual desire disorder, female sexual arousal disorder, or a combination of these disorders with at least 6-month duration, as diagnosed by a qualified clinician using a diagnostic screening guide and validated instruments including the Female Sexual Distress Scale–Desire/Arousal/Orgasm [8] and the Female Sexual Function Index [9] questionnaires. Exclusion criteria included any unstable medical condition including any recent cardiac or cerebrovascular disorder, and uncontrolled treated or untreated hypertension, defined as a repeated observation of a SBP at least 140 mmHg or DBP at least 90 mmHg during the screening period, or change in any antihypertensive therapy in the preceding 6 weeks prior to study enrollment suggesting a lack of stable baseline BP values.
The trial was conducted in accordance with Good Clinical Practice requirements, as described in the current revision of International Conference on Harmonization of Technical Requirements of Pharmaceuticals for Human Use guidelines and the Declaration of Helsinki. Before any study procedures occurred, a written informed consent was obtained from each study participant.
Safety assessments
At each clinic visit following the screening visit, all study participants were queried about adverse events. Safety assessments also included physical examination, vital signs, 12-lead ECG [at screening; at the second in-clinic double-blind study-drug dosing (approximately 2-h postdose, the expected time of bremelanotide maximal plasma concentration (Cmax)); and at end of study], and clinical laboratory tests.
Blood pressure monitoring
The BP was monitored manually for 2 h following each of the three in-clinic study visits (the first was a single-blind placebo dose, whereas the second and third visits were double-blind placebo/study drug). Before each dose was administered in the clinic, study participants were fitted with an ambulatory BP monitor (ABPM) that was initiated to measure the BP at 15-min intervals (Spacelabs 90207 ABP Monitor; Spacelabs Healthcare, Issaquah, Washington, USA). The ABPM data were searched both manually and programmatically (by standardized, computerized methods, CoreLab Partners, Inc.; Rockville, Maryland, USA) for SBP values at least 150 mmHg or DBP values at least 95 mmHg and for postdosing SBP changes from baseline at least 30 mmHg or DBP changes at least 15 mmHg. All reports with these flagged values were reviewed by safety personnel, including an independent clinical hypertension expert blinded to treatment assignment (W.B.W.), who also reviewed related physical/mental activities, heart rate (HR), and nonstudy-drug medications. If the clinic or ambulatory BPs met measurement criteria based on the absolute prespecified levels, change from baseline in BP and duration of the increase from baseline (Table S1 in the online data supplement), the patient was withdrawn from the trial.
Pharmacokinetic assessments
At the two in-clinic, double-blind, study-drug dosing visits, blood draws were performed predose and at 0.5, 1.0, and 2.0-h postdose for pharmacokinetic analyses including determination of the Cmax of bremelanotide. Plasma concentrations were analyzed using a validated liquid chromatography coupled with tandem mass spectrometry method. Noncompartmental analysis of the plasma concentration versus time data with validated pharmacokinetic software was used to calculate Cmax, time of maximal concentration (Tmax), area under the time–concentration curve (AUC)(0–4), and AUC(0–2)[10].
Statistical analyses
Multiple BP safety endpoints were assessed in an exploratory manner in the trial. The key endpoint was the difference in mean change in ambulatory BP from baseline in the active treatment group versus placebo after the initial double-blind dose according to the following time intervals: 0–4, more than 4–8, more than 8–24, and 0–24 h postdose. These same ambulatory BP data were also evaluated after the second double-blind dose that was administered in the study clinic. Other endpoints included changes from baseline in the HR and systolic pressure–HR product, the duration of SBP shifts in 15-min intervals using thresholds of changes of 10 and 15 mmHg and absolute values of 150 and 160 mmHg. Pharmacokinetic analyses included calculation of the mean plasma Cmax (ng/ml) and range as well as the coefficient of variation of the Cmax after the first and second in-clinic doses of the three dose levels of bremelanotide. The relationship between the maximal observed SBP and DBP and BP changes, HR and HR change, and the plasma Cmax of bremelanotide (using data from all dose levels) were individually examined by simple linear regression analysis with Cmax as the independent variable and BP and HR (individually) as the dependent variables. Finally, the incidence of adverse events was tabulated in all participants who received at least one dose of double-blind study drug (safety population).
Sample-size calculation
The sample size of this trial was based on the efficacy of bremelanotide with an assumption that 100 study participants per treatment group would have 80% power to detect a clinically important difference in sexually satisfying experiences at the 0.05 significance level between the placebo group and the 1.25/1.75 mg groups pooled. There were no assumptions made regarding cardiovascular safety utilized in the clinical efficacy power calculations. Post-hoc computations showed that 100 patients per treatment group provided power to detect a difference between treatment groups of 3 mmHg in DBP and SBP as follows: 85.4% power for a common SD of 7 mmHg (a conservative assumption for DBP) and 75.1% power for a common SD of 8 mmHg (a reasonable assumption for SBP).
RESULTS
Characteristics of the study participants at baseline
Of 1142 potential participants screened at 67 US and Canadian clinical sites, 612 were enrolled and 397 were randomized into one of the four dose groups. Among the 397 randomized participants, 327 used double-blind study drug at home and were assessed at 4 double-blind weeks (modified intent-to-treat population), and 287 completed the study. Disposition of the participants, including reasons for nonrandomization into the study, is shown in Fig. S2 in the online data supplement. Baseline characteristics of the double-blind study-drug recipients were similar among the treatment groups (shown in Table 1), as were baseline BP values. Average clinic SBP ranged from 113.4 to 114.7 mmHg and DBP from 73.7 to 75.3 mmHg.
TABLE 1.
Baseline characteristics of the study participants
| Variable | Placebo group, n = 97 | Bremelanotide groups | ||
| 0.75 mg, n = 100 | 1.25 mg, n = 99 | 1.75 mg, n = 98 | ||
| Age (years) | ||||
| Mean (SD) | 37.0 (7.7) | 37.6 (7.8) | 35.7 (7.2) | 37.0 (7.6) |
| Median (range) | 39.0 (22–53) | 38.0 (22–53) | 37.0 (21–52) | 37.0 (21–50) |
| Race, n (%) | ||||
| White | 75 (77%) | 71 (71%) | 65 (66%) | 70 (71%) |
| Black | 19 (20%) | 25 (25%) | 32 (32%) | 23 (23%) |
| Other | 3 (3%) | 4 (4%) | 2 (2%) | 5 (5%) |
| Weight at screening (lbs) | ||||
| Mean (SD) | 164.4 (42.1) | 168.2 (37.9) | 174.0 (43.2) | 179.2 (45.9)a |
| Median (range) | 155.6 (99–286) | 161.0 (98–289) | 168.5 (95–323) | 167.8 (107–308)a |
| BMI at screening (kg/m2) | ||||
| Mean (SD) | 27.7 (6.2) | 28.5 (6.6) | 29.2 (7.1) | 29.9 (7.2)a |
| Median (range) | 26.6 (18.6–45.9) | 27.5 (17.6–52.4) | 27.3 (17.6–53.2) | 28.7 (18.0–49.3)a |
| BP (mmHg) | ||||
| SBP | 113.6 (12.0) | 113.4 (10.0) | 114.1 (10.5) | 114.7 (9.7) |
| DBP | 73.7 (9.0) | 74.2 (7.9) | 75.3 (7.8) | 74.9 (7.8) |
| Heart rate (bpm) | 70.1 (9.1) | 70.9 (9.3) | 69.8 (9.5) | 71.5 (8.4) |
| FSD diagnosis, n (%) | ||||
| FSAD | 4 (4%) | 3 (3%) | 3 (3%) | 2 (2%) |
| HSDD | 24 (25%) | 20 (20%) | 24 (24%) | 24 (24%) |
| Mixed | 69 (71%) | 77 (77%) | 72 (73%) | 72 (73%) |
BP, blood pressure; FSAD, female sexual arousal disorder; FSD, female sexual dysfunction; HSDD, hypoactive sexual desire disorder.
an = 97.
Protocol-directed, blood pressure-related withdrawals
Throughout the study, no study participant had in-clinic BP results that met any of the predefined criteria for BP-related withdrawal (Table S1 in the online data supplement). Based on ambulatory BP findings prior to randomization, 40 participants (8.2%) met the criteria for withdrawal after receiving a single-blind dose of placebo. Based on ambulatory BP findings after randomization, 27 participants (7% of the 394 women who were randomized and dosed) either met specific BP criteria (n = 24), created clinical concern (n = 2), or had a technically unacceptable ambulatory BP recording (n = 1) and were withdrawn from further study participation – this included six (6% of 97) after a placebo dose, four (4% of 100) after a 0.75-mg bremelanotide dose, nine (9% of 99) after a 1.25-mg dose, and seven (7% of 98) after a 1.75-mg dose.
Ambulatory blood pressure and heart rate changes following in-clinic injections
Mean ambulatory BP and HR changes from baseline obtained at 15-min intervals for up to 12 h after double-blind study-drug dosing at the two visits are shown in Fig. 1. The placebo-subtracted mean ambulatory BP changes from baseline from the single-blind in-clinic placebo dose to the first and second double-blind in-clinic bremelanotide doses are shown in Table 2. Bremelanotide was associated with small increases in the SBP and DBP, with a maximum mean systolic change of +3.1 mmHg in the 1.75-mg group at 0–4 h after the first double-blind dose. The 0–4-h changes in SBP were statistically significant for the two highest doses. Changes from baseline in SBP in the 1.25 and 1.75-mg doses were NS following 4–8 h after the initial dose but were for the second dose of 1.25 mg only (Table 2). Similar findings were observed for the ambulatory DBP. Statistically significant reductions in placebo-subtracted mean HR were confined to the first 8 h and consisted of decreases that were similar in all treatment groups (range of means, −4.8 to −6.6 bpm). Changes from baseline in the placebo-subtracted systolic pressure–HR product were lower in all three treatment groups. There were no inverse relationships between the changes in ambulatory HR and changes in SBP seen for bremelanotide or placebo (data not shown).
FIGURE 1.
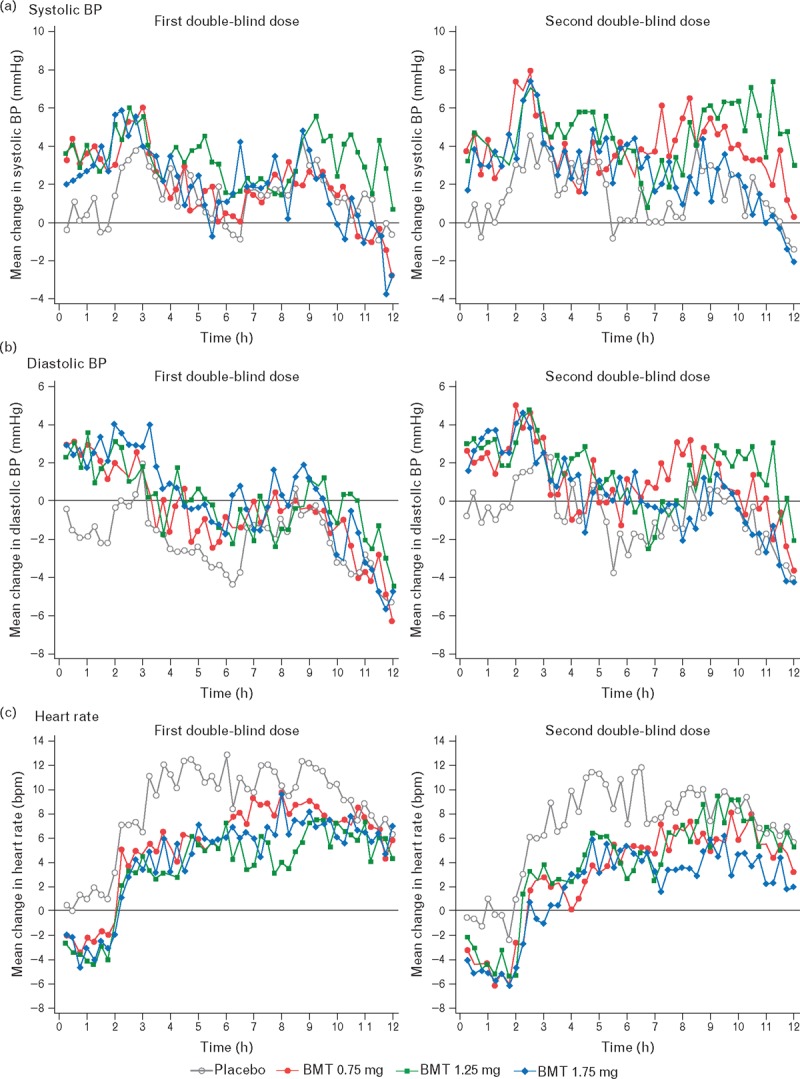
Acute changes from baseline in blood pressure (panels a and b) and heart rate (panel c) using ambulatory monitoring postdosing of bremelanotide. BMT, bremelanotide.
TABLE 2.
Placebo-subtracted ambulatory monitoring changes from single-blind in-clinic placebo dose to double-blind in-clinic bremelanotide doses
| Variable | Bremelanotide dose groups (mg) | ||
| 0.75 | 1.25 | 1.75 | |
| First double-blind dose | |||
| n (bremelanotide, placebo) | 100, 95 | 97, 95 | 98, 95 |
| SBP (mmHg), hours postdose | |||
| 0–4 | +1.8 | +2.4* | +3.1* |
| 4–8 | +0.9 | +1.4 | +2.1 |
| 8–24 | +0.9 | +0.7 | +0.9 |
| 0–24 | +1.1 | +1.1 | +1.6 |
| DBP (mmHg), hours postdose | |||
| 0–4 | +1.5 | +3.0* | +3.2* |
| 4–8 | +1.3 | +2.2* | +2.3* |
| 8–24 | +1.0 | +1.4* | +1.4* |
| 0–24 | +1.1* | +1.9* | +1.9* |
| Heart rate (bpm), hours postdose | |||
| 0–4 | −5.2* | −5.2* | −4.6* |
| 4–8 | −6.2* | −6.1* | −6.6* |
| 8–24 | −0.4 | −1.5 | −0.8 |
| 0–24 | −2.2* | −2.9* | −2.2* |
| Heart rate × systolic pressure product, hours postdose | |||
| 0–4 | −492.8* | −436.4* | −305.9 |
| 4–8 | −676.5* | −621.0* | −608.1* |
| 8–24 | +5.2 | −127.4 | −23.7 |
| 0–24 | −187.7 | −265.9 | −139.1 |
| Second double-blind dose | |||
| n (bremelanotide, placebo) | 90, 92 | 86, 92 | 88, 92 |
| SBP (mmHg), hours postdose | |||
| 0–4 | +1.1 | +2.1* | +2.5* |
| 4–8 | +1.6 | +1.3* | +2.2 |
| 8–24 | +1.6 | +1.5* | +0.6 |
| 0–24 | +1.5 | +1.6* | +1.3 |
| DBP (mmHg), hours postdose | |||
| 0–4 | +0.6 | +2.2* | +2.6* |
| 4–8 | +1.7 | +0.9 | +2.2* |
| 8–24 | +1.3* | +1.7* | +1.4 |
| 0–24 | +1.3* | +1.7* | +1.8* |
| Heart rate (bpm), hours postdose | |||
| 0–4 | −4.8* | −6.1* | −4.7* |
| 4–8 | −5.5* | −6.5* | −6.6* |
| 8–24 | +0.1 | −0.7 | −0.5 |
| 0–24 | −1.6 | −2.6* | −2.2* |
| Heart rate × systolic pressure product, hours postdose | |||
| 0–4 | −491.9* | −583.3* | −375.4* |
| 4–8 | −503.3* | −669.7* | −624.5* |
| 8–24 | +114.9 | +4.2 | −31.3 |
| 0–24 | −82.3 | −206.5 | −184.1 |
BP, blood pressure.
*P ≤ 0.05.
Outlier analyses
There were five clinically important shifts in SBP (>10 or >15 mmHg lasting ≥30 min) lasting more than 30 min (three on bremelanotide and two on placebo). Most of these events – 91% after placebo dosing and 88% after bremelanotide dosing – lasted 15 min or less. Of the five observed elevations of SBP values more than 170 mmHg for more than 15 min, two were in 0.75-mg recipients, one in a 1.25-mg recipient, two in 1.75-mg recipients, and none in placebo recipients (Table 3). No patient in any bremelanotide group exhibited an SBP more than 180 mmHg for more than 15 min or an increase at least 15 mmHg for more than 30 min.
TABLE 3.
Maximal SBP elevations by the duration and number of events following bremelanotide and placebo
| Treatment arms | SBP > 150 mmHg | SBP > 160 mmHg | SBP > 170 mmHg | SBP > 180 mmHg | ||||||||||||
| Number of events by event duration (min) | ||||||||||||||||
| <15 | >15 | >30 | >45 | <15 | >15 | >30 | >45 | <15 | >15 | >30 | >45 | <15 | >15 | >30 | >45 | |
| Placebo | 11 | 2 | 0 | 0 | 7 | 0 | 0 | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 0.75 mg | 22 | 1 | 0 | 0 | 3 | 0 | 0 | 0 | 2 | 1 | 0 | 0 | 1 | 0 | 0 | 0 |
| 1.25 mg | 14 | 2 | 2 | 1 | 6 | 0 | 0 | 0 | 2 | 0 | 0 | 0 | 1 | 0 | 0 | 0 |
| 1.75 mg | 17 | 0 | 1 | 0 | 12 | 1 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 |
Pharmacokinetics
The bremelanotide plasma Cmax findings among all recipients of an in-clinic bremelanotide dose are shown in Table 4. Following both in-clinic doses, the pharmacokinetic results indicate dose proportionality. There was no relationship observed between the maximal change in the SBP and the Cmax following the initial in-clinic dose nor the maximal change in the HR and the Cmax (Fig. 2).
TABLE 4.
Bremelanotide pharmacology results following subcutaneous dosing of premenopausal women
| Variable | Subcutaneous dose (mg) | ||
| 0.75 | 1.25 | 1.75 | |
| First in-clinic dose | |||
| n | 95 | 97 | 92 |
| Cmax, mean (ng/ml) | 37.5 | 60.0 | 77.2 |
| Coefficient of variation (%) | 26.6 | 30.6 | 25.2 |
| Cmax range (ng/ml) | 16.5–59.7 | 28.9–126.0 | 14.7–115.0 |
| Median Tmax (h) | 0.50 | 0.50 | 0.58 |
| (min, max) | (0.43, 1.17) | (0.00, 2.00) | (0.50, 2.08) |
| Second in-clinic dose | |||
| n | 89 | 84 | 88 |
| Cmax, mean (ng/ml) | 37.3 | 60.1 | 77.7 |
| Coefficient of variation (%) | 29.3 | 34.6 | 27.0 |
| Cmax range (ng/ml) | 0–77.5 | 0–150.0 | 0–127.0 |
| Median Tmax (h) | 0.52 | 0.53 | 0.55 |
| (min, max) | (0.0, 1.08) | (0.0, 2.00) | (0.50, 2.08) |
Cmax, maximal concentration; Tmax, time of maximal concentration.
FIGURE 2.
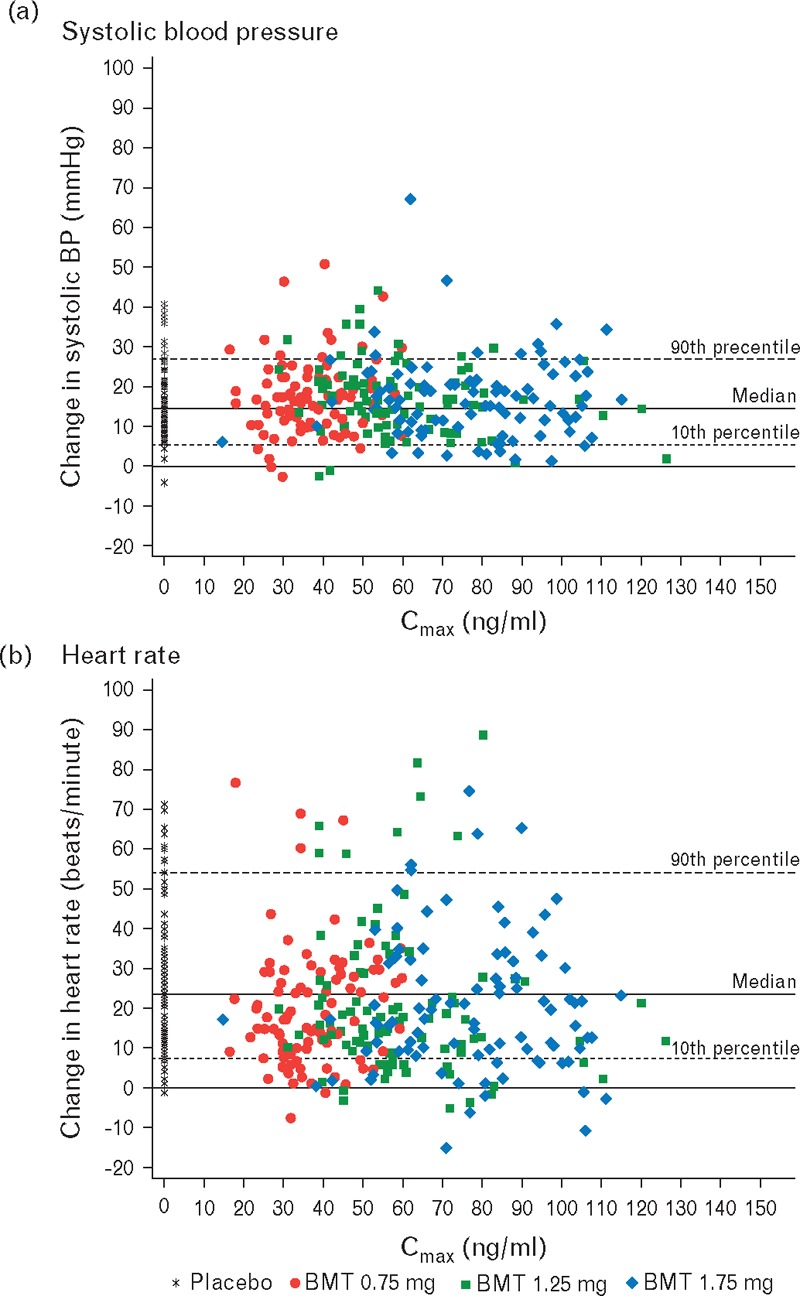
Relations among the maximal concentrations of three doses of bremelanotide and changes in systolic BP (a) and changes in heart rate (b) after the first double-blind dose (controlled by placebo).
Adverse events overall
Treatment-emergent adverse events reported during the entire 12 weeks, double-blind study-drug treatment are shown in Table S2 of the online data supplement. At all bremelanotide dosages, the most common adverse events were nausea, flushing, and headache, with no marked dosage dependence. There was one adverse event classified as serious: in the 1.75-mg group, a 41-year-old obese woman was admitted to a hospital with a diagnosis of anxiety-related noncardiac chest pain. Two cardiovascular adverse events – palpitation and flushing accompanied by normal ECG findings in a 0.75-mg user and asymptomatic sinus bradycardia among otherwise normal ECG findings in a 1.75-mg user – were classified as possibly study-drug related.
DISCUSSION
In premenopausal women with sexual dysfunction syndromes, bremelanotide administered subcutaneously was followed by small and transient increases in BP. For the highest dose, 1.75 mg, the mean maximum increases for SBP and DBP were 3.1 and 3.2 mmHg, respectively, and the SBP elevations were generally confined to 4 h following drug administration. There was a small number of participants in whom the proportion of increases in SBP to values more than 150 mmHg were higher after bremelanotide doses than placebo, but there were no sustained elevations (>15 min) exceeding 180 mmHg. One patient (of 99 patients) on the 1.75-mg dose had an elevation of SBP of 170 mmHg lasting 30 min. In total, approximately 7% of participants met predefined BP criteria and were withdrawn from the study. Of note, these participants were evenly distributed among the randomized treatment assignments, including placebo.
The BP changes associated with subcutaneous bremelanotide were accompanied by mean HR reductions of 4.6–6.6 bpm, which persisted for up to 8 h following administration. Although it is unclear whether this represents a baroreceptor reflex, a central adaptive process, or a combination of peripheral and central mechanisms, the reduction in HR led to a decline in the rate-pressure product, a clinical surrogate for myocardial oxygen consumption [11].
The three doses of bremelanotide demonstrated dose-proportional increases in concentrations following subcutaneous dosing of premenopausal women. Pharmacokinetic analyses performed in conjunction with 24-h monitoring of the BP showed no relation between pharmacologic exposure and the maximally observed changes in the SBP or HR. Because bremelanotide is a central nervous system agonist that allows the drug to remain on the target tissue/cells, it can have pharmacodynamics effects after the systemic concentrations are not measurable. In an earlier study of high doses of subcutaneous bremelanotide in men with erectile dysfunction [5], dose-dependent increases in plasma concentrations of bremelanotide were observed with a Tmax of 0.5–1 h and mean half-life ranging from 1.9 to 2.7 h. The same study also showed transient increases in clinic SBP of 4–5 mmHg that were similar to the present study for single subcutaneously administered doses of 4–6 mg.
Recently, off-target increases in BP have attracted both regulatory and scientific interest, focusing attention on how carefully these increases are determined during drug development and their potential impact on the benefit versus risk of the therapy under investigation [6]. As there is evidence that out-of-office BP predicts cardiovascular outcomes better than in-clinic monitoring from prospective cohort studies [12,13], ABPM has become more widely used in assessing the BP effects of drugs. Furthermore, phase 2 is an opportune time to evaluate drug-induced off-target BP increases because these studies typically involve patients with the disease or disorder of interest, have more intensive monitoring of vital signs compared with phase 3, and use a wider dose spectrum [6]. Many classes of drugs – from antidepressants to NSAIDs to oncologic therapies – can increase BP via distinct mechanisms ranging from activation of the sympathetic nervous system, salt and water retention to inhibition of vascular endothelial growth factor [14]. For example, a number of prior studies using ABPM with the NSAIDs demonstrated the ability to provide more precise information on the pressor effects of these agents outside of the clinical research environment along or when combined with antihypertensive therapies [15–17]. These earlier trials have led the way for utilizing out-of-office BP monitoring that has affected regulatory advice in drug development as it relates to BP changes [6,14].
The data from this large phase 2 study with bremelanotide were important for future development of the drug for at-home, as-needed subcutaneous self-injection by premenopausal women with female sexual dysfunction. The BP and HR effects of bremelanotide at doses 1.75 mg or less were intensively studied using 24-h monitoring measurements that captured both the time course of the BP and HR changes as well as allowed for assessment of outliers compared with placebo. The results showed small and transient increases in BP accompanied by reductions in the HR. The results on repeated dosing were similar to the initial dose administration with very few dropouts between the 2 test days. Importantly, the results of this large phase 2 study in the target population were helpful to avoid this level of intensive vital signs monitoring during phase 3 development. As the BP effects of the drug have been well characterized in this trial, the phase 3 program can obtain BP data in the clinic using standardized measurements. Strength of the data comes from the subcutaneous delivery of bremelanotide that improves the predictability of exposure compared with the intranasal delivery of this drug [4], with potential benefits in treatment safety, tolerability, and consistency of effectiveness. An important limitation is the exclusion of women with uncontrolled hypertensive values at baseline whether treated or not with antihypertensive therapy. More information may be derived from the larger phase 3 development program as it relates to the effects of bremelanotide on BP in women with less well controlled hypertension.
In conclusion, off-target BP elevations occur with several classes of medications for noncardiovascular indications. Bremelanotide is a subcutaneously administered melanocortin receptor agonist that is being developed for female sexual dysfunction. Prior research with an intranasal formulation of this drug suggested that it could elevate BP in both men and women but the absorption of the drug was less predictable than subcutaneous administration. During a large phase 2 program with subcutaneous administration of bremelanotide, we studied the effects of its administration (three separate doses) on two separate days in a randomized, placebo-controlled study using ambulatory BP measurement that could characterize the pharmacodynamics effects of this drug in the patients at home environment. We found a transient rise in the BP accompanied by a reduction in the HR following bremelanotide. The levels of BP were not related to drug exposure and persistent moderate or severe hypertension did not occur on any dose of bremelanotide. Hence, these data supported a level of safety of this drug that allowed for its further development with standard clinical trial assessment of vital signs and demonstrated the utility of ABPM in the assessment of a novel compound for a noncardiovascular indication.
ACKNOWLEDGEMENTS
Final high-resolution figures for this manuscript were produced by The Curry Rockefeller Group, Tarrytown, New York, USA. This study was supported by Palatin Technologies, Cranbury, New Jersey, USA.
Conflicts of interest
W.B.W. was the medical monitor for this study in 2011–2012 and received compensation for that activity from Palatin Technologies, Inc.; R.J. and J.L. are full-time employees of Palatin Technologies, Inc.; and M.G.M. reports no conflicts of interest.
Supplementary Material
Supplementary Material
Reviewers’ Summary Evaluations
Reviewer 1
Strengths: This is large phase II study describing the BP rising effect of different subcutaneous doses of a melanocortin receptor agonist in women with sexual dysfunction.
Limitations: This rise in blood pressure is likely due to a rise in systemic vascular resistance, but data explaining this rise in vascular resistance are not provided.
Reviewer 2
The strength of this study is showing the drug effect in women when bremelanotide is delivered subcutaneously, with potential benefits and tolerability; and the evaluation of transient elevation of blood pressure, in the patient's living environment (home), using ambulatory blood pressure monitoring.
The study excludes women with uncontrolled hypertension whether treated or not; that is a limitation of the study. No evaluation has been done for possible differences in BP changes in women with treated and controlled hypertension in comparison with non hypertensive women.
Anyhow, the methodology applied in this study allows a better evaluation of bremelanotide than in other studies. The same type of BP evaluation method described here seems good enough to be applied to other drugs with incidental changes in BP values, allowing a better evaluation and quantification of the magnitude and duration of BP and HR changes induced by drugs under evaluation.
Footnotes
Abbreviations: ABPM, ambulatory blood pressure monitoring; AUC, area under the concentration curve; Cmax, maximal concentration; CV, cardiovascular; FSAD, female sexual arousal disorder; FSFI, Female Sexual Function Index; HSDD, hypoactive sexual desire disorder; LC/MS, liquid chromatography coupled with tandem mass spectrometry; MCr4, melanocortin receptor 4; mITT, modified intent-to-treat; PK, pharmacokinetics; Tmax, time of maximal concentration
REFERENCES
- 1.Wikberg JE, Muceniece R, Mandrika I, Prusis P, Lindblom J, Post C, Skottner A. New aspects on the melanocortins and their receptors. Pharmacol Res 2000; 42:393–420. [DOI] [PubMed] [Google Scholar]
- 2.Pfaus JG, Shadiack A, Van Soest T, Tse M, Molinoff P. Selective facilitation of sexual solicitation in the female rat by a melanocortin receptor agonist. Proc Natl Acad Sci USA 2004; 101:10201–10204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pfaus J, Giuliano F, Gelez H. Bremelanotide: an overview of preclinical CNS effects on female sexual function. J Sex Med 2007; 4 suppl 4:269–279. [DOI] [PubMed] [Google Scholar]
- 4.Diamond LE, Earle DC, Rosen RC, Willett MS, Molinoff PB. Double-blind, placebo-controlled evaluation of the safety, pharmacokinetic properties and pharmacodynamic effects of intranasal PT-141, a melanocortin receptor agonist, in healthy males and patients with mild-to-moderate erectile dysfunction. Int J Impot Res 2004; 16:51–59. [DOI] [PubMed] [Google Scholar]
- 5.Rosen RC, Diamond LE, Earle DC, Shadiack AM, Molinoff PB. Evaluation of the safety, pharmacokinetics and pharmacodynamic effects of subcutaneously administered PT-141, a melanocortin receptor agonist, in healthy male subjects and in patients with an inadequate response to Viagra. Int J Impot Res 2004; 16:135–142. [DOI] [PubMed] [Google Scholar]
- 6.Sager P, Heilbraun J, Turner JR, Gintant G, Geiger MJ, Kowey PR, et al. Assessment of drug-induced increases in blood pressure during drug development: report from the Cardiac Safety Research Consortium. Am Heart J 2013; 165:477–488. [DOI] [PubMed] [Google Scholar]
- 7.White WB, Salzman P, Schwid SR. Parkinson's Rasagaline: Efficacy and Safety in the Treatment of Off (PRESTO) Parkinson Study Group Transtelephonic home blood pressure to assess the monoamine oxidase-B inhibitor rasagiline in Parkinson disease. Hypertension 2008; 52:587–593. [DOI] [PubMed] [Google Scholar]
- 8.Derogatis LR, Rosen R, Leiblum S, Burnett A, Heiman J. The Female Sexual Distress Scale (FSDS): initial validation of a standardized scale for assessment of sexually related personal distress in women. J Sex Marital Ther 2002; 28:317–330. [DOI] [PubMed] [Google Scholar]
- 9.Rosen R, Brown C, Heiman J, Leiblum S, Meston C, Shabsigh R, et al. The Female Sexual Function Index (FSFI): a multidimensional self-report instrument for the assessment of female sexual function. J Sex Marital Ther 2000; 26:191–208. [DOI] [PubMed] [Google Scholar]
- 10.Vengurlekar S. ABC Method 48354-MI-08, HPLC-MS/MS Assay Method for the Determination of Bremelanotide (BMT, PT-14) in Human Plasma, ABC Laboratories, Inc., 20 August 2012. [Google Scholar]
- 11.White WB, Krishnan S, Giacco S, Mallareddy M. Effects of metoprolol succinate extended release vs amlodipine besylate on the blood pressure, heart rate, and the rate-pressure product in patients with hypertension. J Am Soc Hypertens 2008; 2:378–384. [DOI] [PubMed] [Google Scholar]
- 12.Hansen TW, Kikuya M, Thijs L, Bjorklund-Bodegard K, Kuznetsova T, Ohkubo T, et al. IDACO Investigators Prognostic superiority of daytime ambulatory over conventional blood pressure in four populations: a meta-analysis of 7030 individuals. J Hypertens 2007; 25:1554–1564. [DOI] [PubMed] [Google Scholar]
- 13.Verdecchia P, Angeli F, Cavallini C. Ambulatory blood pressure for cardiovascular risk stratification. Circulation 2007; 115:2091–2093. [DOI] [PubMed] [Google Scholar]
- 14.O’Brien E, Turner JR. Assessing blood pressure response to noncardiovascular drugs: the beneficial role of ambulatory blood pressure monitoring. J Clin Hypertens (Greenwich) 2013; 15:55–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sudano I, Flammer AJ, Periat D, Enseleit F, Hermann M, Wolfrum M, et al. Acetaminophen increases blood pressure in patients with coronary artery disease. Circulation 2010; 122:1789–1796. [DOI] [PubMed] [Google Scholar]
- 16.White WB, Kent J, Taylor A, Verburg KM, Lefkowith JB, Whelton A. Effects of celecoxib on ambulatory blood pressure in hypertensive patients on ACE inhibitors. Hypertension 2002; 39:929–934. [DOI] [PubMed] [Google Scholar]
- 17.Sowers JR, White WB, Pitt B, Whelton A, Simon LS, Winer N, et al. CRESCENT Investigators The effects of cyclooxygenase-2 inhibitors and nonsteroidal anti-inflammatory therapy on 24-h blood pressure in patients with hypertension, osteoarthritis, and type 2 diabetes mellitus. Arch Intern Med 2005; 165:161–168. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


