Abstract
Neurofibromatosis Type 1 (NF1) is a monogenetic autosomal-dominant disorder with a broad spectrum of clinical symptoms and is commonly associated with cognitive deficits. Patients with NF1 frequently exhibit cognitive impairments like attention problems, working memory deficits and dysfunctional inhibitory control. The latter is also relevant for the resolution of cognitive conflicts. However, it is unclear how conflict monitoring processes are modulated in NF1. To examine this question in more detail, we used a system neurophysiological approach combining high-density ERP recordings with source localisation analyses in juvenile patients with NF1 and controls during a flanker task. Behaviourally, patients with NF1 perform significantly slower than controls. Specifically on trials with incompatible flanker-target pairings, however, the patients with NF1 made significantly fewer errors than healthy controls. Yet, importantly, this overall successful conflict resolution was reached via two different routes in the two groups. The healthy controls seem to arrive at a successful conflict monitoring performance through a developing conflict recognition via the N2 accompanied by a selectively enhanced N450 activation in the case of perceived flanker-target conflicts. The presumed dopamine deficiency in the patients with NF1 seems to result in a reduced ability to process conflicts via the N2. However, NF1 patients show an increased N450 irrespective of cognitive conflict. Activation differences in the orbitofrontal cortex (BA11) and anterior cingulate cortex (BA24) underlie these modulations. Taken together, juvenile patients with NF1 and juvenile healthy controls seem to accomplish conflict monitoring via two different cognitive neurophysiological pathways.
Keywords: Conflict processing, Neurofibromatosis type 1, EEG, Source localisation, Cognitive control
Highlights
-
•
NF1 is often associated with deficits in attention, working memory and inhibition.
-
•
Conflict processing on the flanker task is intact, but slow in patients with NF1.
-
•
Different mechanisms lead to successful conflict processing in NF1 and controls.
-
•
The N2 in high-conflict conditions is reduced in NF1 compared to healthy controls.
-
•
The N450 is generally enhanced in NF1, possibly representing compensatory processes.
1. Introduction
Neurofibromatosis Type 1 (NF1) is an autosomal dominant monogenetic genetic disorder (MIM #162200) occurring in about 1 out of 3000 children (Lammert et al., 2005) that is caused by mutations in the tumor suppressor gene neurofibromin 1 (17q11.2, MIM*613113). Aside from the chronic and often progressive medical and neurological symptoms (Kayl and Moore, 2000) and an increased susceptibility to the development of benign and malignant tumors, cognitive deficits, often resembling those characteristic for patients with Attention Deficit/Hyperactivity Disorder (ADHD), occur in 30–70% of all NF1 cases (Kayl and Moore, 2000, Mautner et al., 2015, North et al., 1997, Pride et al., 2012, van der Voet et al., 2016). Generally, these deficits may occur in the form of intellectual impairments, learning disabilities and emotional as well as psychosocial problems (Kayl and Moore, 2000). The precise cognitive profiles are very heterogeneous and negatively impact quality of life, academic achievement and social functioning (Huijbregts and de Sonneville, 2011). Concerning the underlying pathomechanisms, NF1 has been suggested to be associated with deficits in dopaminergic signalling (Anastasaki et al., 2015, Brown et al., 2010, Brown et al., 2011, Diggs-Andrews et al., 2013, Diggs-Andrews and Gutmann, 2013, van der Voet et al., 2016, Wozniak et al., 2013).
Previous research has shown attentional problems (Pride et al., 2012) and deficits in working memory (Plasschaert et al., 2016) as well as inhibition (Diggs-Andrews and Gutmann, 2013, Huijbregts et al., 2010, Koini et al., 2016, Plasschaert et al., 2016, Ribeiro et al., 2015, Rowbotham et al., 2009). Crucially, inhibitory control has been shown to significantly influence the resolution of response conflicts (Klein et al., 2014, Ocklenburg et al., 2011, Verleger et al., 2009). This has received support by several human (Tandonnet et al., 2011, Taylor et al., 2007) and monkey studies (Cisek and Kalaska, 2005). Since inhibitory control processes have been shown to be altered in NF1 (Ribeiro et al., 2015), it is possible that related processes of conflict monitoring are also dysfunctional in NF1. To examine to what extent conflict monitoring processes are modulated in juvenile patients with NF1 compared to healthy controls, we compared performance on a simplified version of the flanker task. To examine the neurophysiological underpinnings, we combined high-density EEG recordings with source localisation methods to delineate the neurophysiological alterations associated with changes on the behavioural level. The advantage of electrophysiological (EEG) techniques and event-related potentials (ERPs) in particular is that different cognitive-neurophysiological subprocesses involved in information processing (and response inhibition) can be isolated on the basis of their temporal occurrence.
Conflict monitoring per se is crucially reflected by two main event-related potentials. On the one hand, the fronto-central N2 has been associated with the detection of conflicts on the stimulus- and on the response-level. Also, it is related to the adjustment of cognitive control to the perceived conflict, which mainly is associated with activation of the anterior cingulate cortex (ACC) (Falkenstein et al., 1999, Falkenstein et al., 2002, Folstein and Van Petten, 2008, Larson et al., 2014, van Veen and Carter, 2002). Neurobiologically, the N2 component has been strongly associated with dopaminergic transmission (Beste et al., 2016, Seer et al., 2016, Zhang et al., 2016). On the other hand, the N450 has been shown to be specifically sensitive to stimulus (and less so to response) conflicts. Similar to the N2, the N450 also reflects conflict-related activation of the ACC (Szűcs and Soltész, 2012). In contrast to the N2, the neurobiological underpinnings of the N450 are still rather unclear, although some dopaminergic involvement has been suggested (Larson et al., 2015, Li et al., 2013). Overall, the N2 and the N450 are important conflict-related components to be examined in patients with NF1, as specifically the functional connectivity of the ACC has been shown to be deficient in this patient group and this deficit has been explicitly linked to functional outcomes (Loitfelder et al., 2015).
Aside from conflict processing per se, basic sensory perception and especially perceptual categorisation of the conflict stimuli are important contributors to successful conflict processing (Herrmann and Knight, 2001). Also, basic attentional selection processes are crucial in this context. The occipito-parietal P1 and N1 components following the flanker as well as the target stimuli are well-established neurophysiological correlates of these early sensory and attentional processes (Herrmann and Knight, 2001) and thus also need to be examined within the current study. In addition, response selection processes (as represented by the central P3) will be examined as well, as they could be affected by the monitoring and resolution of conflicts (Beste et al., 2011, Mückschel et al., 2016, Stock et al., 2016).
2. Materials and methods
2.1. Samples
All subjects and their parents or legal guardians provided informed written consent according to the Declaration of Helsinki and the study was approved by the local ethics committee of the Medical Faculty of the TU Dresden. 14 patients with NF1 were included in the study (10 female, 2 brothers, age 13.4 ± 2.5 years). The NF1 diagnoses were based on the clinical criteria by the National Institutes of Health (NIH) Consensus Development Conference on Neurofibromatosis (NIH, 1988). 22 children were included in the control group (10 female, 12.7 ± 2.5 years). None of them were taking medication and none had a psychiatric diagnosis as confirmed by clinical interview. Age (t(34) = − 0.74; p = 0.47) and sex (χ2 = 2.3; p = 0.13) did not differ between the groups.
2.2. Task
We used a modified Flanker task to examine response inhibition processes in the two groups (Bluschke et al., 2016, Chmielewski et al., 2014). Participants were required to press one of two buttons in response to a central target stimulus. If this central arrow pointed to the right/left, participants were required to also press the button on the right/left. 200 ms before target presentation, two distractor stimuli (flanker arrows) were presented above and below the location at which the target arrow would then appear. These flanker arrows could either point into the same (compatible condition, 67% of trials) or into the opposite direction (incompatible condition, 33% of trials) as the subsequent target stimulus. Participants were instructed to ignore the flanker arrows and to respond according to the direction of the target arrow as fast as possible. Additional time pressure was created through an auditory warning stimulus (1000 Hz, 60 db sound pressure level) presented 1200 ms after the response if it had not occurred within 450 ms after target presentation. Targets were presented for 300 ms, with the flankers being switched off simultaneously. The intertrial-interval was pseudo-randomised between 1400 and 1800 ms. The experiment was comprised of four blocks of 120 trials. To examine the effects of the predictability of trial order on performance, trials in two out of the four blocks (predictable blocks) were presented in a repetitive order (30 compatible, 20 alternating compatible-incompatible, 20 incompatible, 20 alternating incompatible-compatible, 30 compatible). In the other two (unpredictable) blocks, trials were presented in a pseudo-randomised order. In order to avoid occurrences of certain trial sequences (i.e. a succession of three equal trials and/or five trials with the same direction of the target arrow), a sequence of 16 compatible and 8 incompatible trials was compiled and repeatedly tested in Matlab until recurrent sequences could be excluded, thus making trial order unpredictable. Such sequences of 24 trials were used five times within each of the two unpredictable blocks.
2.3. EEG recording and analyses
We recorded the EEG from 60 Ag/AgCl electrodes at a sampling rate of 500 Hz with impedances being kept below 5 kΩ (reference electrode at Fpz, ground electrode at θ = 58, ф = 78). Off-line data pre-processing included a down-sampling of the data to 256 Hz, the application of a band-pass filter (0.5–20 Hz, slope of 48 db/oct) and the removal of technical as well as periodic artifacts (pulse artifacts, horizontal and vertical eye movements). A raw data inspection as well as an independent component analysis was applied to detect and remove/correct such artifacts. Subsequently, the EEG was segmented to the onset of the target stimuli. Only trials with correct responses were included in further analyses. Each trial was segmented to the period from 2000 ms before to 2000 ms after target onset. An automated artifact rejection procedure was applied to exclude any remaining trials containing artifacts (rejection criteria: amplitude criterion (maximal amplitude: 200 μV, minimal amplitude: − 200 μV), maximal value difference (200 μV in a 200 ms interval) and low activity (below 0.5 μV in a 100 ms period)). A current source density (CSD) transformation was used to allow a reference-free evaluation of the EEG data which helps to find the electrodes showing the strongest effects (Nunez and Pilgreen, 1991). Before averaging the segments for each condition (compatible and incompatible trials in the predictable and unpredictable blocks), a baseline correction was applied from − 200 ms to 0 ms. Based on scalp topography, event-related potentials (ERPs) were quantified in the following time windows and over the following electrodes: The N2 component was measured over FCz at 250–290 ms (both groups). The N450 component was quantified over electrodes Fz and FCz in the time window of 450–500 ms after target onset. The P1Flanker was measured over electrodes P7, P8, P9 and P10 in both groups during the time window of − 100 to − 80 ms before target onset. The N1 Flanker was quantified over the same electrodes in the time window of − 30 to − 10 ms (controls)/− 10–10 ms (NF1). For the P1Target, amplitudes in both groups were measured over the same four electrodes 115–135 ms after the target. N1Target was quantified at the same locations at 220–260 ms for the control group and at 200–220 ms for the patients with NF1. The P3 was quantified at Pz. Based on the different temporal course apparent in the control group, time windows were differentially quantified for the compatible (285–325 ms) and the incompatible (340–380 ms) trials. No such distinction was necessary in the patients with NF1 (285–325 ms). This choice of electrodes and time windows was validated using a statistical procedure described in Mückschel et al. (2014).
Source localisation was conducted using sLORETA (standardized low resolution brain electromagnetic tomography; (Pascual-Marqui, 2002)), providing a single solution to the inverse problem (Marco-Pallarés et al., 2005, Pascual-Marqui, 2002, Sekihara et al., 2005). For sLORETA, the intracerebral volume is partitioned into 6239 voxels at 5 mm spatial resolution. Then, the standardized current density at each voxel is calculated in a realistic head model (Fuchs et al., 2002) based on the MNI152 template (Mazziotta et al., 2001). It has been mathematically proven that sLORETA provides reliable results without a localisation bias (Sekihara et al., 2005). Moreover, there is evidence from EEG/fMRI and neuronavigated EEG/TMS studies underlining the validity of the sources estimated using sLORETA (Dippel and Beste, 2015, Sekihara et al., 2005). In this study, the voxel-based sLORETA images were compared across groups (NF1 vs. controls) using the sLORETA-built-in voxel-wise randomization tests with 2000 permutations, based on statistical nonparametric mapping (SnPM). Voxels with significant differences (p < 0.01, corrected for multiple comparisons) between contrasted conditions were located in the MNI-brain www.unizh.ch/keyinst/NewLORETA/sLORETA/sLORETA.htm.
2.4. Statistics
Data was analysed using repeated-measures/univariate ANOVAs and t-tests. Group (controls vs. patients with NF1) was used as the between-subjects factor. Compatibility (compatible vs. incompatible) (not applicable to P1Flanker and N1Flanker) as well as Predictability (predictable vs. unpredictable) were used as the within-subject factors when analysing both behavioural and neurophysiological data. If necessary in the neurophysiological analysis, Electrode was used as an additional within-subjects factor, but is only reported when a relevant interaction with Group is observed. Greenhouse-Geisser correction was applied for all ANOVAs. Post-hoc tests were Bonferroni-corrected when necessary. All variables included in the analysis were normally distributed, as indicated by Kolmogorov-Smirnov tests (all z < 1.05; p > 0.2).
3. Results
3.1. Behavioural data
3.1.1. Reaction times (RTs)
Concerning the RTs, we found a significant main effect of Compatibility (F(1,32) = 206.4; p < 0.001; ηp2 = 0.87). Reaction times were generally faster in compatible (345 ± 12 ms) than in incompatible trials (432 ± 13 ms). In addition, we found a significant main effect of Group (F(1,32) = 13.6; p = 0.001; ηp2 = 0.30), with healthy controls generally reacting faster (345 ± 15 ms) than patients with NF1 (432 ± 19 ms). The interaction of Compatibility*Group was not significant (F(1,32) = 0.2; p = 0.66; ηp2 = 0.006).
3.1.2. Accuracy
Concerning error rates, we also found significant main effects of Compatibility (F(1,32) = 103.7; p < 0.001; ηp2 = 0.76) and Group (F(1,32) = 10.0; p = 0.003; ηp2 = 0.24). Overall, incompatible trials (37.6 ± 2.3%) resulted in more erroneous responses than compatible trials (10.9 ± 1.9%). Healthy controls committed more errors (29.5 ± 2.0%) than patients with NF1 (19.1 ± 2.6%). Most importantly, the interaction of Compatibility*Group (F(1,32) = 6.1; p = 0.02; ηp2 = 0.16) was significant. Analysing this further, we found that the two groups differed in their error rates on incompatible trials (F(1,32) = 14.0; p = 0.001; ηp2 = 0.31) (controls: 46.1 ± 2.8%; NF1: 29.2 ± 3.5%), but not on compatible trials (F(1,32) = 1.02; p = 0.32; ηp2 = 0.03) (controls: 12.9 ± 2.4%; NF1: 8.9 ± 3.1%). The main effect of Compatibility was significant both within the healthy controls (F(1,20) = 86.3; p < 0.001; ηp2 = 0.81) and within the patients with NF1 (F(1,12) = 37.3; p < 0.001; ηp2 = 0.76).
3.2. Neurophysiological data
3.2.1. Conflict detection processes (N2 and N450)
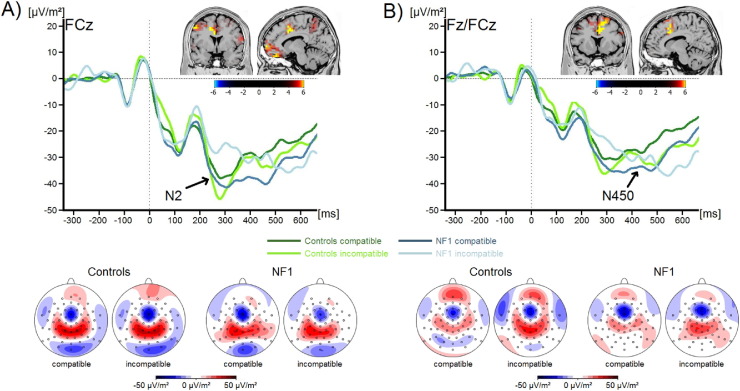
The N2 and N450 components are shown in Fig. 1.
Fig. 1.
Event-related potentials (ERPs) (current source density) and topographic maps showing the (A) N2 and (B) N450 component during compatible and incompatible flanker trials for both groups. The time windows represented in the scalp topographies are stated in the methods section. Positive values are given in red, negative values are given in blue. Time point zero denotes the occurrence of the target stimulus. Negative values are plotted downwards. Inlays display sLORETA analyses. Plots show the difference between compatible and incompatible trials compared between the two groups. Colours denote t-values corrected using randomization tests.
Concerning the N2 amplitude, we found a significant interaction of Compatibility*Group (F(1,34) = 8.2; p = 0.007; ηp2 = 0.19). Further analyses revealed a significant main effect of Compatibility within the group of patients with NF1 (F(1, 13) = 5.8; p = 0.03; ηp2 = 0.31). Amplitudes were generally more negative in compatible (− 38.9 ± 8.4 μV/m2) compared to incompatible trials (− 27.7 ± 7.6 μV/m2). The distinction between compatible (− 35.5 ± 4.6 μV/m2) and incompatible trials (− 42.5 ± 6.3 μV/m2) was not significant in the control group (F(1,21) = 2.9; p = 0.11; ηp2 = 0.12). None of the other main effects or interactions were significant (all F < 3.7; all p > 0.07; all ηp2 < 0.09). The sLORETA analysis showed that the differences in N2 amplitudes between the compatibility conditions and the two groups were due to activation differences in the ACC (BA24) and the orbitofrontal cortex (BA11).
Examining the N450 component, we found a significant interaction of Compatibility*Group (F(1,34) = 5.1; p = 0.03; ηp2 = 0.13). Within the control group, the main effect of Compatibility was significant (F(1, 34) = 8.8; p = 0.007; ηp2 = 0.13), while this was not the case in the patients with NF1 (F(1, 34) = 0.5; p = 0.48; ηp2 = 0.04). While the N450 measured in control participants showed a significant distinction between compatible (− 23.9 ± 3.6 μV/m2) and incompatible trials (− 31.3 ± 5.0 μV/m2), this was not the case in the group of patients with NF1 (compatible: − 34.3 ± 6.5 μV/m2; incompatible: − 31.0 ± 9.1 μV/m2). Concerning the N450, no further main effects or interactions were significant (all F < 2.9; all p > 0.09; all ηp2 < 0.08). Here also, the sLORETA analysis revealed for the observed differences to originate from the ACC (BA24).
3.2.2. Further ERPs influencing conflict detection performance
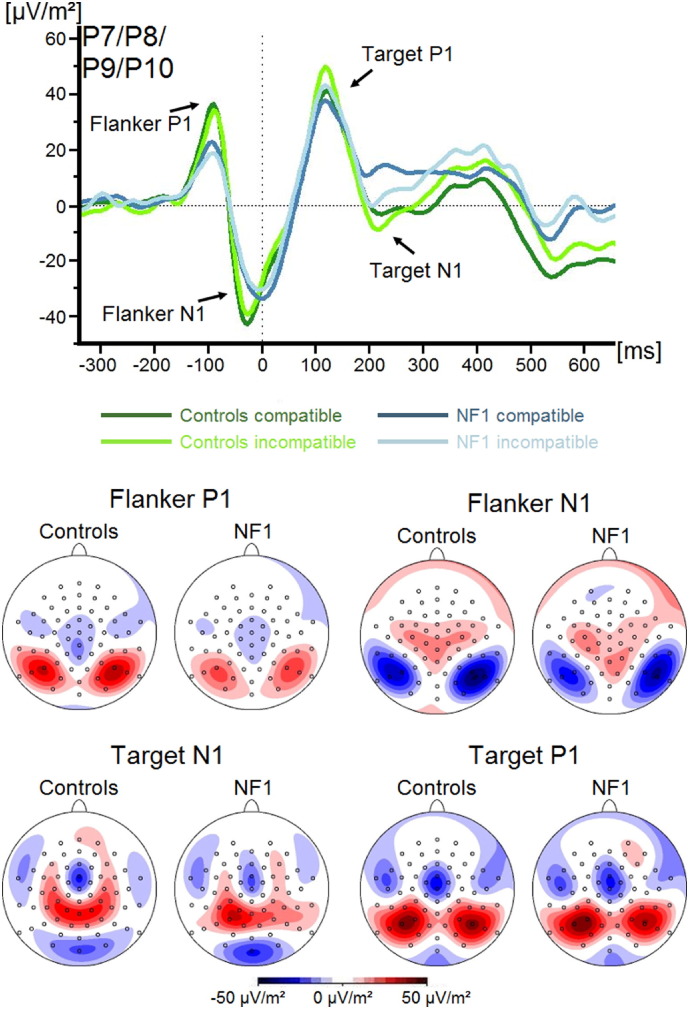
P1 and N1 ERP components for flanker and target stimuli are shown in Fig. 2.
Fig. 2.
Event-related potentials (ERPs) (current source density) and topographic maps showing the flanker P1, flanker N1, target P1 and target N1 components during compatible and incompatible flanker trials for both groups. The time windows represented in the scalp topographies are stated in the methods section. Positive values are given in red, negative values are given in blue. Time point zero denotes the occurrence of the target stimulus. Negative values are plotted downwards.
Regarding the amplitudes of the P1Flanker and N1Flanker (representing the perceptual categorisation and attentional selection of the Flanker stimuli, respectively), analyses revealed no significant main effects of any of the factors and no significant interactions between them (all F < 2.45; all p > 0.08; all ηp2 = 0.19).
Examining the perceptual categorisation of the target via the P1Target component, we found a significant main effect of Compatibility (F(1,34) = 22.4; p < 0.001; ηp2 = 0.39). Amplitudes of the P1Target were significantly more positive in incompatible (44.5 ± 6.3 μV/m2) compared to compatible trials (37.7 ± 5.8 μV/m2). All other main effects and interactions, including those containing the factor Group, were not significant (all F < 1.9; all p > 0.15; all ηp2 < 0.15).
Concerning the N1Target component, which indicates attentional selection processes related to the target stimulus, we found a significant main effect of Compatibility (F(1,34) = 8.0; p = 0.008; ηp2 = 0.19). The N1Target was more negative in incompatible (− 3.2 ± 5.5 μV/m2) than in compatible (4.2 ± 4.6 μV/m2) trials. All other main effects and interactions, including those containing the factor Group, were not significant (all F < 2.9; all p > 0.09; all ηp2 < 0.08).
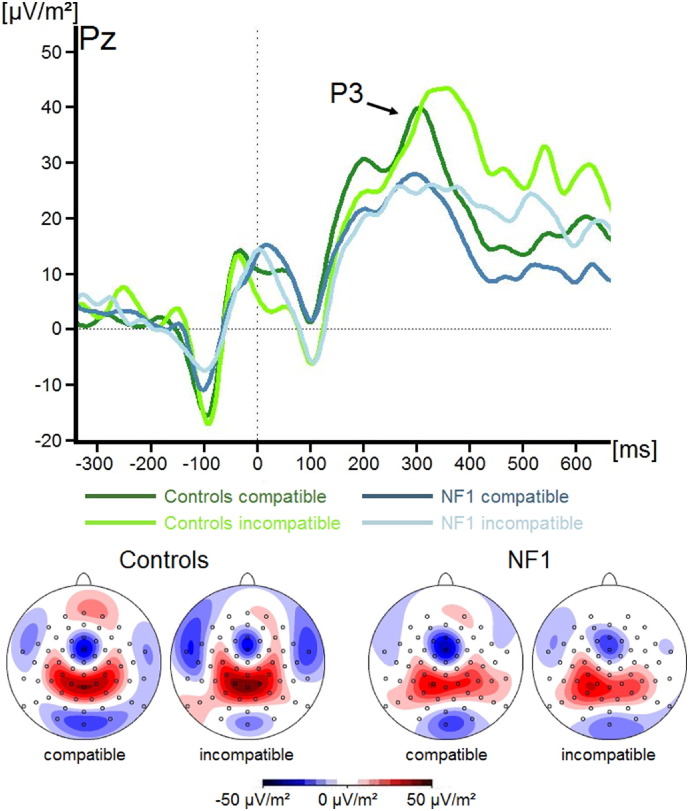
As response selection processes may also have a significant impact on conflict processing, we additionally examined the amplitude of the P3 (see Fig. 3). Here, we only found a trend level main effect of Group (F(1,34) = 3.6; p = 0.06; ηp2 = 0.10), with amplitudes being slightly reduced in patients with NF1 (26.2 ± 5.5 μV/m2) compared to those in the healthy control group (39.4 ± 4.4 μV/m2). No other main effects or interactions were significant (all F < 1.1; all p > 0.31; all ηp2 < 0.03).
Fig. 3.
Event-related potentials (ERPs) (current source density) and topographic maps showing the P3 component during compatible and incompatible flanker trials for both groups. The time windows represented in the scalp topographies are stated in the methods section. Positive values are given in red, negative values are given in blue. Time point zero denotes the occurrence of the target stimulus. Negative values are plotted downwards.
4. Discussion
The monogenetic disorder Neurofibromatosis Type 1 (NF1) is frequently associated with cognitive deficits often resembling those characteristic for attention deficit disorders. One of the barriers to successful therapeutic intervention is the wide spectrum of described cognitive dysfunction. Inhibitory control processes have been shown to be altered in NF1, but no closer look has yet been taken at associated conflict monitoring processes. Thus, in the current study, we examined conflict monitoring abilities in patients with NF1 compared to healthy controls using a flanker task. Patients with NF1 performed significantly slower than healthy controls but committed significantly fewer errors overall, especially on incompatible flanker trials. No such distinctions between compatible and incompatible trials were present concerning reaction times. Consequently, the reported results are not purely a result of a speed-accuracy trade-off.
Neurophysiologically, it became apparent that conflict processing seemingly takes place via two different mechanisms in the two groups. In the healthy control participants, the amplitude of the N2 did not vary significantly depending on flanker-target compatibility. When considering the mean values, it becomes clear that there was a trend towards more pronounced N2 amplitudes in incompatible trials. This lack of a significant N2-modulation (as well as the relatively poor performance specifically on the incompatible trials) can most likely be attributed to developmental effects concerning executive functions and the structure and function of the ACC in this group of young adolescents (Chmielewski et al., 2015, Ladouceur et al., 2007, Lamm et al., 2006). In line with this interpretation, a fully developed frontal N2 has only been found around 17 years of age (Oades et al., 1997). Concerning the N450 in the healthy controls, we found significantly larger amplitudes in trials with incompatible flanker-target associations. Such a clear relation between conflict processing and N450 amplitude has previously been shown to be present in adolescents (Killikelly and Szűcs, 2013) and young adults (Passow et al., 2014, West, 2004). Overall, the control group examined in the present study processes cognitive conflict through a developing conflict recognition via the N2. This is accompanied by a selectively enhanced N450 activation in the case of previously perceived flanker-target conflicts. As, on average, the response took place before the occurrence of the N450 component, this conflict-related enhancement might be beneficial in regards to preparation for the subsequent trial.
In contrast to healthy controls, the N2 amplitude in patients with NF1 was significantly and selectively reduced in trials with incompatible flanker-target associations. Thus, an existing flanker-target conflict did not lead to an appropriate enhancement of N2 amplitude. Source localisation procedures suggest that these differences are due to activation modulations in the ACC (BA24) and orbitofrontal areas (BA11). As all earlier ERPs reflecting stimulus perception and attentional selection were not different to those measured in the healthy control group, it is clear that the observed N2 changes do not represent an aftereffect of an earlier deficit. Indeed, a similar lack of an N2 enhancement in high-conflict conditions has previously been reported in other disorders related to dopaminergic dysfunctions like Parkinson's Disease (Willemssen et al., 2011) and Huntington's Disease (Beste et al., 2008). Clear links between NF1 and dopaminergic transmission have been demonstrated on the molecular level in the rodent model (Brown et al., 2010, Brown et al., 2011, Diggs-Andrews et al., 2013, Diggs-Andrews and Gutmann, 2013, van der Voet et al., 2016) and clinically via significant improvements of cognitive symptoms through methylphenidate, a dopamine reuptake inhibitor (Lidzba et al., 2014, Mautner et al., 2002). Along these lines, the lack of an N2 enhancement in patients with NF1 observed in the current study can also be compared to that in patients with ADHD, where conflict monitoring has also been shown to be deficient (Albrecht et al., 2008). Consequently, our results add strong neurophysiological support to the hypothesis of a dopaminergic dysfunction in patients with NF1 as a putative root of the deficient conflict-dependent N2 enhancement. So far, it is unclear how this dopaminergic dysfunction in patients with NF1 changes as they move through adolescence into adulthood. Cognitive problems, however, have been shown to persist into adulthood and to continue to be highly impairing (Mautner et al., 2015).
Furthermore, no compatibility-dependent amplitude distinctions were found in regards to N450 in patients with NF1. However, when considering the waveforms more closely, it becomes apparent that N450 amplitudes in this group are always of a similarly high magnitude as it is the case in incompatible trials in the control group. This suggests that in patients with NF1, the lack of a conflict-related N450-enhancement may be compensated via a generally higher investment of cognitive resources at this stage of information processing. Source localisation suggests for these differences to originate in the ACC (BA24). While healthy controls reactively shift between states of high and low cognitive control depending on the perceived stimuli, patients with NF1 maintain a consistently higher level of engaged cognitive control resources. Interestingly, a very similar pattern concerning N450 amplitudes has previously been found in children with learning disabilities (Liu et al., 2014). Since the behavioural results suggest that cognitive conflicts can in fact be resolved by patients with NF1, the adaptive reliance on a continuously heightened flanker-target compatibility processing via the N450 indeed seems to be an efficient strategy of dealing with conflicts. The disadvantage of this reliance on a mechanism occurring somewhat later during information processing seems to be the generally slower reaction time in the patient group. The mechanisms underlying the intact (and even compensatory) functioning of the N450 in patients with NF1 cannot be fully explained, as the neurobiological underpinnings of the N450 have not yet been clearly defined. Overall, patients with NF1 seem to achieve successful conflict monitoring performance through a consistently enhanced N450 activation, which may aid the preparation of conflict processing in the subsequent trial. This may compensate for dysfunctions in earlier conflict processing mechanisms (N2) which are most likely caused by the dopaminergic deficit inherent to the disorder.
In the current study, similar compensatory mechanisms could not be found in any of the other examined ERPs, with components reflecting early perceptual and attentional processing (flanker/target P1 and N1) and top-down response inhibition (P3) not differing between the two groups. Compensating for the reduced conflict-related N2 enhancement via an increased early sensory processing would have been an inefficient compensatory strategy, as it would also have increased conflict perception, thus resulting in even higher conflict processing demands. Concerning the P3, we found a trend level amplitude reduction in the patient group. This can probably also be explained by the dopaminergic dysfunction in the patient group, as the P3 has been shown to be significantly modulated by dopamine (Beste et al., 2010, Polich, 2007, Ratsma et al., 2001). Consequently, response selection processes reflected by the P3 could not be used as a compensatory mechanism in this context. This lack of changes in other processing stages supports the specificity of our findings concerning the two different pathways towards successful conflict monitoring in healthy controls and patients with NF1.
Overall, conflict processing is indeed not per se deficient in patients with NF1. However, the dopamine deficiency in this group likely leads to a reduced ability to process conflicts via the N2 ERP. To compensate for this, an alternative route via a constantly enhanced N450 is used to achieve successful conflict processing in this group. The price for this is a general response slowing. Clinically, precisely this slower response speed may be the main contributor to the observed attentional problems and ADHD-like symptoms frequently reported in patients with NF1 (Kayl and Moore, 2000, Mautner et al., 2015, North et al., 1997, Pride et al., 2012). In fact, we found a similar pattern of reduced reaction times and somewhat altered neurophysiological processing mechanisms in patients with NF1 in a GoNogo task (Bluschke et al., in press). Here we could show that, although there are clear similarities on the clinical level, neurophysiological processes clearly differ between patients with NF1 and those with ADHD. To assess and classify these deficits further, the N450 may prove to be a useful biomarker in the future and may thus be used as a target parameter when assessing treatment approaches. Here, it would be particularly interesting to examine how conflict monitoring in general and possible compensatory mechanisms in particular are affected by pharmacological interventions. As the N2 is mainly dopaminergically modulated, an amplitude normalisation could be expected to occur as a result of the treatment with methylphenidate (Acosta et al., 2006, Brown et al., 2010, Mautner et al., 2002), whereas no clear hypothesis can be derived regarding the N450. Specifically, the examination of such compensatory mechanisms may add significant value to the understanding of the heterogeneous cognitive deficits in juvenile and adult patients with NF1.
Competing financial interests
A.B., M.v.d.H and K.P. declare no competing or potential conflicts of interest. V.R. has received payment for consulting and writing activities from Lilly, Novartis, and Shire Pharmaceuticals, lecture honoraria from Lilly, Novartis, Shire Pharmaceuticals, and Medice Pharma, and support for research from Shire and Novartis. He has carried out (and is currently carrying out) clinical trials in cooperation with the Novartis, Shire, and Otsuka companies. C.B. has received payment for consulting from GlaxoSmithKline, and Teva.
Acknowledgements
This work was supported by a grant by the Else Kröner-Fresenius Stiftung (2014_A46) to CB and VR and by a grant by the Friede-Springer Stiftung (033/2017). We thank all participants and Benjamin Teufert for his help with the figures. We acknowledge support by the German Research Foundation and the Open Access Publication Funds of the TU Dresden.
References
- Acosta M.T., Gioia G.A., Silva A.J. Neurofibromatosis type 1: new insights into neurocognitive issues. Curr. Neurol. Neurosci. Rep. 2006;6:136–143. doi: 10.1007/s11910-996-0036-5. [DOI] [PubMed] [Google Scholar]
- Albrecht B., Brandeis D., Uebel H., Heinrich H., Mueller U.C., Hasselhorn M., Steinhausen H.-C., Rothenberger A., Banaschewski T. Action monitoring in boys with attention-deficit/hyperactivity disorder, their nonaffected siblings, and normal control subjects: evidence for an endophenotype. Biol. Psychiatry. 2008;64:615–625. doi: 10.1016/j.biopsych.2007.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anastasaki C., Woo A.S., Messiaen L.M., Gutmann D.H. Elucidating the impact of neurofibromatosis-1 germline mutations on neurofibromin function and dopamine-based learning. Hum. Mol. Genet. 2015;24:3518–3528. doi: 10.1093/hmg/ddv103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beste C., Saft C., Andrich J., Gold R., Falkenstein M. Stimulus-response compatibility in Huntington's disease: a cognitive-neurophysiological analysis. J. Neurophysiol. 2008;99:1213–1223. doi: 10.1152/jn.01152.2007. [DOI] [PubMed] [Google Scholar]
- Beste C., Willemssen R., Saft C., Falkenstein M. Response inhibition subprocesses and dopaminergic pathways: basal ganglia disease effects. Neuropsychologia. 2010;48:366–373. doi: 10.1016/j.neuropsychologia.2009.09.023. [DOI] [PubMed] [Google Scholar]
- Beste C., Ness V., Falkenstein M., Saft C. On the role of fronto-striatal neural synchronization processes for response inhibition—evidence from ERP phase-synchronization analyses in pre-manifest Huntington's disease gene mutation carriers. Neuropsychologia. 2011;49:3484–3493. doi: 10.1016/j.neuropsychologia.2011.08.024. [DOI] [PubMed] [Google Scholar]
- Beste C., Stock A.-K., Epplen J.T., Arning L. Dissociable electrophysiological subprocesses during response inhibition are differentially modulated by dopamine D1 and D2 receptors. Eur. Neuropsychopharmacol. 2016;26:1029–1036. doi: 10.1016/j.euroneuro.2016.03.002. [DOI] [PubMed] [Google Scholar]
- Bluschke A., Chmielewski W.X., Roessner V., Beste C. Intact context-dependent modulation of conflict monitoring in childhood ADHD. J. Atten. Disord. 2016 doi: 10.1177/1087054716643388. [DOI] [PubMed] [Google Scholar]
- Bluschke A., von der Hagen M., Papenhagen K., Roessner V., Beste C. Response inhibition in attention deficit disorder and neurofibromatosis type 1 – clinically similar, neurophysiologically different. Sci. Rep. 2017 doi: 10.1038/srep43929. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown J.A., Emnett R.J., White C.R., Yuede C.M., Conyers S.B., O’Malley K.L., Wozniak D.F., Gutmann D.H. Reduced striatal dopamine underlies the attention system dysfunction in neurofibromatosis-1 mutant mice. Hum. Mol. Genet. 2010;19:4515–4528. doi: 10.1093/hmg/ddq382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown J.A., Xu J., Diggs-Andrews K.A., Wozniak D.F., Mach R.H., Gutmann D.H. PET imaging for attention deficit preclinical drug testing in neurofibromatosis-1 mice. Exp. Neurol. 2011;232:333–338. doi: 10.1016/j.expneurol.2011.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chmielewski W.X., Mückschel M., Roessner V., Beste C. Expectancy effects during response selection modulate attentional selection and inhibitory control networks. Behav. Brain Res. 2014;274:53–61. doi: 10.1016/j.bbr.2014.08.006. [DOI] [PubMed] [Google Scholar]
- Chmielewski W.X., Roessner V., Beste C. Predictability and context determine differences in conflict monitoring between adolescence and adulthood. Behav. Brain Res. 2015;292:10–18. doi: 10.1016/j.bbr.2015.05.054. [DOI] [PubMed] [Google Scholar]
- Cisek P., Kalaska J.F. Neural correlates of reaching decisions in dorsal premotor cortex: specification of multiple direction choices and final selection of action. Neuron. 2005;45:801–814. doi: 10.1016/j.neuron.2005.01.027. [DOI] [PubMed] [Google Scholar]
- Diggs-Andrews K.A., Gutmann D.H. Modeling cognitive dysfunction in neurofibromatosis-1. Trends Neurosci. 2013;36:237–247. doi: 10.1016/j.tins.2012.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diggs-Andrews K.A., Tokuda K., Izumi Y., Zorumski C.F., Wozniak D.F., Gutmann D.H. Dopamine deficiency underlies learning deficits in neurofibromatosis-1 mice. Ann. Neurol. 2013;73:309–315. doi: 10.1002/ana.23793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dippel G., Beste C. A causal role of the right inferior frontal cortex in implementing strategies for multi-component behaviour. Nat. Commun. 2015;6:6587. doi: 10.1038/ncomms7587. [DOI] [PubMed] [Google Scholar]
- Falkenstein M., Hoormann J., Hohnsbein J. ERP components in Go/Nogo tasks and their relation to inhibition. Acta Psychol. 1999;101:267–291. doi: 10.1016/s0001-6918(99)00008-6. [DOI] [PubMed] [Google Scholar]
- Falkenstein M., Hoormann J., Hohnsbein J. Inhibition-related ERP components: variation with modality, age, and time-on-task. J. Psychophysiol. 2002;16:167–175. [Google Scholar]
- Folstein J.R., Van Petten C. Influence of cognitive control and mismatch on the N2 component of the ERP: a review. Psychophysiology. 2008;45:152–170. doi: 10.1111/j.1469-8986.2007.00602.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs M., Kastner J., Wagner M., Hawes S., Ebersole J.S. A standardized boundary element method volume conductor model. Clin. Neurophysiol. Off. J. Int. Fed. Clin. Neurophysiol. 2002;113:702–712. doi: 10.1016/s1388-2457(02)00030-5. [DOI] [PubMed] [Google Scholar]
- Herrmann C.S., Knight R.T. Mechanisms of human attention: event-related potentials and oscillations. Neurosci. Biobehav. Rev. 2001;25:465–476. doi: 10.1016/s0149-7634(01)00027-6. [DOI] [PubMed] [Google Scholar]
- Huijbregts S.C.J., de Sonneville L.M.J. Does cognitive impairment explain behavioral and social problems of children with neurofibromatosis type 1? Behav. Genet. 2011;41:430–436. doi: 10.1007/s10519-010-9430-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huijbregts S., Swaab H., de Sonneville L. Cognitive and motor control in neurofibromatosis type I: influence of maturation and hyperactivity-inattention. Dev. Neuropsychol. 2010;35:737–751. doi: 10.1080/87565641.2010.508670. [DOI] [PubMed] [Google Scholar]
- Kayl A.E., Moore B.D. Behavioral phenotype of neurofibromatosis, type 1. Ment. Retard. Dev. Disabil. Res. Rev. 2000;6:117–124. doi: 10.1002/1098-2779(2000)6:2<117::AID-MRDD5>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- Killikelly C., Szűcs D. Asymmetry in stimulus and response conflict processing across the adult lifespan: ERP and EMG evidence. Cortex. 2013;49:2888–2903. doi: 10.1016/j.cortex.2013.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein P.-A., Petitjean C., Olivier E., Duque J. Top-down suppression of incompatible motor activations during response selection under conflict. NeuroImage. 2014;86:138–149. doi: 10.1016/j.neuroimage.2013.08.005. [DOI] [PubMed] [Google Scholar]
- Koini M., Rombouts S.A.R.B., Veer I.M., Van Buchem M.A., Huijbregts S.C.J. White matter microstructure of patients with neurofibromatosis type 1 and its relation to inhibitory control. Brain Imaging Behav. 2016 doi: 10.1007/s11682-016-9641-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ladouceur C.D., Dahl R.E., Carter C.S. Development of action monitoring through adolescence into adulthood: ERP and source localization. Dev. Sci. 2007;10:874–891. doi: 10.1111/j.1467-7687.2007.00639.x. [DOI] [PubMed] [Google Scholar]
- Lamm C., Zelazo P.D., Lewis M.D. Neural correlates of cognitive control in childhood and adolescence: disentangling the contributions of age and executive function. Neuropsychologia, Advances in Developmental Cognitive Neuroscience. 2006;44:2139–2148. doi: 10.1016/j.neuropsychologia.2005.10.013. [DOI] [PubMed] [Google Scholar]
- Lammert M., Friedman J.M., Kluwe L., Mautner V.F. Prevalence of neurofibromatosis 1 in German children at elementary school enrollment. Arch. Dermatol. 2005;141:71–74. doi: 10.1001/archderm.141.1.71. [DOI] [PubMed] [Google Scholar]
- Larson M.J., Clayson P.E., Clawson A. Making sense of all the conflict: a theoretical review and critique of conflict-related ERPs. Int. J. Psychophysiol. 2014;93:283–297. doi: 10.1016/j.ijpsycho.2014.06.007. [DOI] [PubMed] [Google Scholar]
- Larson M.J., Clayson P.E., Primosch M., Leyton M., Steffensen S.C. The effects of acute dopamine precursor depletion on the cognitive control functions of performance monitoring and conflict processing: an event-related potential (ERP) study. PLoS One. 2015;10 doi: 10.1371/journal.pone.0140770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S.-C., Passow S., Nietfeld W., Schröder J., Bertram L., Heekeren H.R., Lindenberger U. Dopamine modulates attentional control of auditory perception: DARPP-32 (PPP1R1B) genotype effects on behavior and cortical evoked potentials. Neuropsychologia. 2013;51:1649–1661. doi: 10.1016/j.neuropsychologia.2013.04.005. [DOI] [PubMed] [Google Scholar]
- Lidzba K., Granstroem S., Leark R.A., Kraegeloh-Mann I., Mautner V.-F. Pharmacotherapy of attention deficit in neurofibromatosis type 1: effects on cognition. Neuropediatrics. 2014;45:240–246. doi: 10.1055/s-0034-1368117. [DOI] [PubMed] [Google Scholar]
- Liu C., Yao R., Wang Z., Zhou R. N450 as a candidate neural marker for interference control deficits in children with learning disabilities. Int. J. Psychophysiol. 2014;93:70–77. doi: 10.1016/j.ijpsycho.2014.05.007. [DOI] [PubMed] [Google Scholar]
- Loitfelder M., Huijbregts S.C.J., Veer I.M., Swaab H.S., Van Buchem M.A., Schmidt R., Rombouts S.A. Functional connectivity changes and executive and social Problems in neurofibromatosis type I. Brain Connect. 2015;5:312–320. doi: 10.1089/brain.2014.0334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marco-Pallarés J., Grau C., Ruffini G. Combined ICA-LORETA analysis of mismatch negativity. NeuroImage. 2005;25:471–477. doi: 10.1016/j.neuroimage.2004.11.028. [DOI] [PubMed] [Google Scholar]
- Mautner V.-F., Kluwe L., Thakker S.D., Leark R.A. Treatment of ADHD in neurofibromatosis type 1. Dev. Med. Child Neurol. 2002;44:164–170. doi: 10.1017/s0012162201001876. [DOI] [PubMed] [Google Scholar]
- Mautner V.-F., Granström S., Leark R.A. Impact of ADHD in adults with neurofibromatosis type 1: associated psychological and social problems. J. Atten. Disord. 2015;19:35–43. doi: 10.1177/1087054712450749. [DOI] [PubMed] [Google Scholar]
- Mazziotta J., Toga A., Evans A., Fox P., Lancaster J., Zilles K., Woods R., Paus T., Simpson G., Pike B., Holmes C., Collins L., Thompson P., MacDonald D., Iacoboni M., Schormann T., Amunts K., Palomero-Gallagher N., Geyer S., Parsons L., Narr K., Kabani N., Le Goualher G., Boomsma D., Cannon T., Kawashima R., Mazoyer B. A probabilistic atlas and reference system for the human brain: International Consortium for Brain Mapping (ICBM) Philos. Trans. R. Soc. Lond. Ser. B Biol. Sci. 2001;356:1293–1322. doi: 10.1098/rstb.2001.0915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mückschel M., Stock A.-K., Beste C. Psychophysiological mechanisms of interindividual differences in goal activation modes during action cascading. Cereb. Cortex N. Y. N. 2014;1991(24):2120–2129. doi: 10.1093/cercor/bht066. [DOI] [PubMed] [Google Scholar]
- Mückschel M., Gohil K., Ziemssen T., Beste C. The norepinephrine system and its relevance for multi-component behavior. NeuroImage. 2016 doi: 10.1016/j.neuroimage.2016.10.007. [DOI] [PubMed] [Google Scholar]
- NIH National Institutes of Health Consensus Development Conference Statement: neurofibromatosis. Bethesda, Md., USA, July 13–15, 1987. Neurofibromatosis. 1988;1(3):172–178. https://www.ncbi.nlm.nih.gov/pubmed/3152465 [PubMed] [Google Scholar]
- North K.N., Riccardi V., Samango-Sprouse C., Ferner R., Moore B., Legius E., Ratner N., Denckla M.B. Cognitive function and academic performance in neurofibromatosis. 1: consensus statement from the NF1 Cognitive Disorders Task Force. Neurology. 1997;48:1121–1127. doi: 10.1212/wnl.48.4.1121. [DOI] [PubMed] [Google Scholar]
- Nunez P.L., Pilgreen K.L. The spline-Laplacian in clinical neurophysiology: a method to improve EEG spatial resolution. J. Clin. Neurophysiol. Off. Publ. Am. Electroencephalogr. Soc. 1991;8:397–413. [PubMed] [Google Scholar]
- Oades R.d., Dmittmann-Balcar A., Zerbin D. Development and topography of auditory event-related potentials (ERPs): mismatch and processing negativity in individuals 8–22 years of age. Psychophysiology. 1997;34:677–693. doi: 10.1111/j.1469-8986.1997.tb02143.x. [DOI] [PubMed] [Google Scholar]
- Ocklenburg S., Güntürkün O., Beste C. Lateralized neural mechanisms underlying the modulation of response inhibition processes. NeuroImage. 2011;55:1771–1778. doi: 10.1016/j.neuroimage.2011.01.035. [DOI] [PubMed] [Google Scholar]
- Pascual-Marqui R.D. Standardized low-resolution brain electromagnetic tomography (sLORETA): technical details. Methods Find. Exp. Clin. Pharmacol. 2002;(24 Suppl. D):5–12. [PubMed] [Google Scholar]
- Passow S., Westerhausen R., Hugdahl K., Wartenburger I., Heekeren H.R., Lindenberger U., Li S.-C. Electrophysiological correlates of adult age differences in attentional control of auditory processing. Cereb. Cortex. 2014;24:249–260. doi: 10.1093/cercor/bhs306. [DOI] [PubMed] [Google Scholar]
- Plasschaert E., Van Eylen L., Descheemaeker M.-J., Noens I., Legius E., Steyaert J. Executive functioning deficits in children with neurofibromatosis type 1: the influence of intellectual and social functioning. Am. J. Med. Genet. B Neuropsychiatr. Genet. 2016;171:348–362. doi: 10.1002/ajmg.b.32414. [DOI] [PubMed] [Google Scholar]
- Polich J. Updating P300: an integrative theory of P3a and P3b. Clin. Neurophysiol. Off. J. Int. Fed. Clin. Neurophysiol. 2007;118:2128–2148. doi: 10.1016/j.clinph.2007.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pride N.A., Payne J.M., North K.N. The impact of ADHD on the COgnitive and academic functioning of children with NF1. Dev. Neuropsychol. 2012;37:590–600. doi: 10.1080/87565641.2012.695831. [DOI] [PubMed] [Google Scholar]
- Ratsma J.E., van der Stelt O., Schoffelmeer A.N.M., Westerveld A., Boudewijn Gunning W. P3 event-related potential, dopamine D2 receptor A1 allele, and sensation-seeking in adult children of alcoholics. Alcohol. Clin. Exp. Res. 2001;25:960–967. [PubMed] [Google Scholar]
- Ribeiro M.J., Violante I.R., Bernardino I., Edden R.A.E., Castelo-Branco M. Abnormal relationship between GABA, neurophysiology and impulsive behavior in neurofibromatosis type 1. Cortex J. Devoted Study Nerv. Syst. Behav. 2015;64:194–208. doi: 10.1016/j.cortex.2014.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowbotham I., Pit-ten Cate I.M., Sonuga-Barke E.J.S., Huijbregts S.C.J. Cognitive control in adolescents with neurofibromatosis type 1. Neuropsychology. 2009;23:50–60. doi: 10.1037/a0013927. [DOI] [PubMed] [Google Scholar]
- Seer C., Lange F., Georgiev D., Jahanshahi M., Kopp B. Event-related potentials and cognition in Parkinson's disease: an integrative review. Neurosci. Biobehav. Rev. 2016;71:691–714. doi: 10.1016/j.neubiorev.2016.08.003. [DOI] [PubMed] [Google Scholar]
- Sekihara K., Sahani M., Nagarajan S.S. Localization bias and spatial resolution of adaptive and non-adaptive spatial filters for MEG source reconstruction. NeuroImage. 2005;25:1056–1067. doi: 10.1016/j.neuroimage.2004.11.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stock A.-K., Steenbergen L., Colzato L., Beste C. The system neurophysiological basis of non-adaptive cognitive control: inhibition of implicit learning mediated by right prefrontal regions. Hum. Brain Mapp. 2016;37:4511–4522. doi: 10.1002/hbm.23325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szűcs D., Soltész F. Functional definition of the N450 event-related brain potential marker of conflict processing: a numerical stroop study. BMC Neurosci. 2012;13:35. doi: 10.1186/1471-2202-13-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tandonnet C., Garry M.I., Summers J.J. Selective suppression of the incorrect response implementation in choice behavior assessed by transcranial magnetic stimulation. Psychophysiology. 2011;48:462–469. doi: 10.1111/j.1469-8986.2010.01121.x. [DOI] [PubMed] [Google Scholar]
- Taylor P.C.J., Nobre A.C., Rushworth M.F.S. Subsecond changes in top down control exerted by human medial frontal cortex during conflict and action selection: a combined transcranial magnetic stimulation electroencephalography study. J. Neurosci. 2007;27:11343–11353. doi: 10.1523/JNEUROSCI.2877-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Veen V., Carter C.S. The anterior cingulate as a conflict monitor: fMRI and ERP studies. Physiol. Behav. 2002;77:477–482. doi: 10.1016/s0031-9384(02)00930-7. [DOI] [PubMed] [Google Scholar]
- Verleger R., Kuniecki M., Möller F., Fritzmannova M., Siebner H.R. On how the motor cortices resolve an inter-hemispheric response conflict: an event-related EEG potential-guided TMS study of the flankers task. Eur. J. Neurosci. 2009;30:318–326. doi: 10.1111/j.1460-9568.2009.06817.x. [DOI] [PubMed] [Google Scholar]
- van der Voet M., Harich B., Franke B., Schenck A. ADHD-associated dopamine transporter, latrophilin and neurofibromin share a dopamine-related locomotor signature in Drosophila. Mol. Psychiatry. 2016;21:565–573. doi: 10.1038/mp.2015.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West R. The effects of aging on controlled attention and conflict processing in the Stroop task. J. Cogn. Neurosci. 2004;16:103–113. doi: 10.1162/089892904322755593. [DOI] [PubMed] [Google Scholar]
- Willemssen R., Falkenstein M., Schwarz M., Müller T., Beste C. Effects of aging, Parkinson's disease, and dopaminergic medication on response selection and control. Neurobiol. Aging. 2011;32:327–335. doi: 10.1016/j.neurobiolaging.2009.02.002. [DOI] [PubMed] [Google Scholar]
- Wozniak D.F., Diggs-Andrews K.A., Conyers S., Yuede C.M., Dearborn J.T., Brown J.A., Tokuda K., Izumi Y., Zorumski C.F., Gutmann D.H. Motivational disturbances and effects of l-dopa administration in neurofibromatosis-1 model mice. PLoS One. 2013;8 doi: 10.1371/journal.pone.0066024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang T., Wang C., Tan F., Mou D., Zheng L., Chen A. Different relationships between central dopamine system and sub-processes of inhibition: spontaneous eye blink rate relates with N2 but not P3 in a Go/Nogo task. Brain Cogn. 2016;105:95–103. doi: 10.1016/j.bandc.2016.04.003. [DOI] [PubMed] [Google Scholar]