Highlights
-
•
Pituitary involvement is a rare recognized complication of Wegener’s granulomatosis.
-
•
Central diabetes insipidus and varying hypopituitarism is a common presentation.
-
•
Radiological abnormalities occur in greater than 90% of cases.
-
•
Surgery is indicated to confirm diagnosis or when compressive symptoms develop.
-
•
Long-term prognosis of patients with pituitary WG is unknown.
Keywords: Wegener’s granulomatosis, Pituitary gland, Central diabetes insipidus, Case report
Abstract
Introduction
Wegener’s granulomatosis (WG) is a systemic vasculitis that can affect a variety of organs including ear, nose and throat, lungs and kidneys. However WG is unusual in the pituitary and rare in the central nervous system.
Presentation of case
A 56-year-old male with likely WG presented with polyuria and polydipsia despite six months of conservative medical management. MRI scanning revealed an enlarging heterogeneously enhancing pituitary gland. Following endoscopic transsphenoidal pituitary biopsy and debulking, final tissue pathology was diagnostic for WG in the pituitary gland.
Discussion
Diagnosis remains difficult but most patients present with central diabetes insipidus (CDI) as well as varying degrees of hypopituitarism on a background of disease activity in other organs. Clinical judgment needs to balance the need for invasive surgical tissue diagnosis with increasing immunosuppressive therapy.
Conclusion
It is important to consider this rare complication of WG to ensure timely diagnosis and management.
1. Introduction
Wegener’s granulomatosis (WG) is an anti-neutrophil cytoplasmic antibody (ANCA)-associated multi-system disorder characterized by necrotizing granulomatous vasculitis of small and medium sized vessels [1], [2], [3]. The disorder of unknown aetiology typically affects a combination of ear, nose and throat, lungs and kidneys along with other organs including joints, skin and eyes [1], [2], [4]. Pituitary involvement in WG is exceedingly rare. This work has been reported in line with SCARE criteria [5].
2. Presentation of case
A 56-year-old male was referred to our institution for right otomastoiditis with facial nerve palsy on a background of 18 months of chronic sinusitis and otitis media. He was unresponsive to conservative treatment and underwent cortical mastoidectomy, facial nerve decompression and ossicular chain reconstruction. Mastoid lining histopathology showed actively inflamed granulation and fibrous tissue. There was insufficient tissue for further histopathological assessment. Post-operatively inflammatory markers remained raised (CRP 55, ESR 49) with strong c-ANCA/proteinase 3 positivity, prompting a likely diagnosis of WG. The patient also reported polyuria 6–7 times/night and polydipsia 3-4 L/day. Urine microscopy, culture and sensitivities and renal tract ultrasound were normal. Computed tomography of the chest showed non-specific bilateral apical lung nodules. Despite medical management with prednisolone, methotrexate and folic acid, polyuria and polydipsia persisted. HbA1c was 6. Serum and urine osmolality and water deprivation tests were consistent with central diabetes insipidus (CDI) in the setting of normal pituitary hormone tests. Contrast magnetic resonance imaging (MRI) of the pituitary revealed an enlarged, heterogeneously enhancing pituitary gland, which could have represented a pituitary macroadenoma – although unusual for his age. Other differentials included metastasis, lymphoma, hypophysitis or rarely, vasculitis (Fig. 1).
Fig. 1.
Sagittal post contrast T1 weighted fat saturated image showing an enlarged, heterogeneously enhancing pituitary gland. A small cyst-like component seen centrally within the gland resolved on subsequent imaging.
Despite 6 months of conservative management with monthly IV cyclophosphamide, polydipsia increased to 8 L/day and repeat contrast MRI showed interval increase in pituitary gland size, resulting in mild superior displacement of the optic chiasm (Fig. 2). There was no evidence of hypopituitarism and visual field testing was normal. Desmopressin was commenced and due to previous non-conclusive histology and worsening symptomatology now requiring further immunosuppression, endoscopic transsphenoidal pituitary biopsy and debulking was performed. Histology revealed necrotising inflammation within the anterior pituitary associated with histiocytes and scattered multinucleated giant cells (Fig. 3). Vasculitis was not evident in examined tissue and there was no evidence of fungal hyphae or mycobacterium on special stains. Given the supportive clinical data and past history of WG, it was considered diagnostic for pituitary involvement by WG.
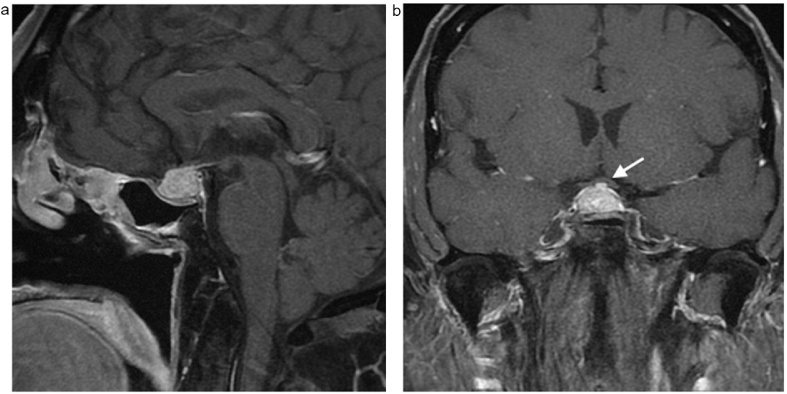
Fig. 2.
a & b: Sagittal (a) and coronal (b) post contrast T1 weighted fat saturated images show interval increase in size of the pituitary gland resulting in superior displacement of the optic chiasm (white arrow).
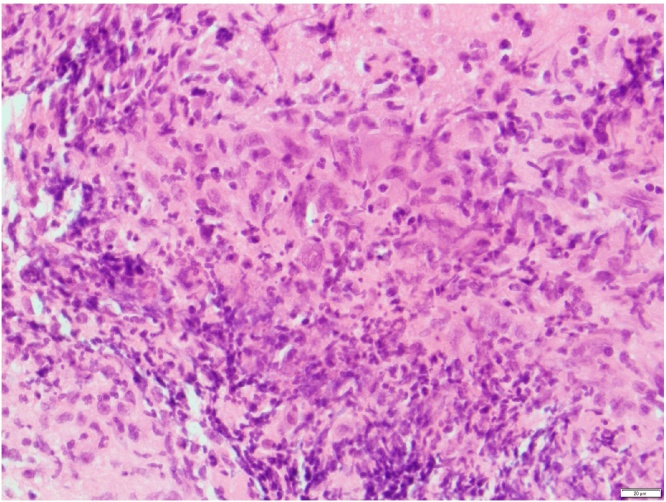
Fig. 3.
Haematoxylin and eosin stained sections at ×400 magnification of mature pituitary sections showing a necrotising inflammatory process, in association with histiocytes and multinucleated giant cells.
Post-operatively he was treated with 4 weeks of methylprednisolone and rituximab pulsing with maintenance six-monthly IV rituximab, weaning prednisolone and ongoing desmopressin. 1 year post-operatively the patient was asymptomatic with normal inflammatory markers (CRP < 3, ESR 11) and pituitary hormones tests. Repeat contrast MRI showed no evidence of residual or recurrent disease. He remains in remission with ongoing maintenance immunosuppressive therapy and desmopressin, as well as follow-up with yearly MRI scans.
3. Discussion
WG in the pituitary is exceedingly rare with less than 50 cases reported in the English literature since 1953 [3], [6]. The majority of cases present with CDI and varying involvement of anterior and posterior pituitary has been described [2], [3]. Of the cases reported, there is almost always evidence of disease activity in other organs before pituitary involvement [1], [3].
The proposed pathophysiology of pituitary WG includes direct granulomatous invasion from nearby anatomic areas namely paranasal sinuses or orbits, in-situ pituitary granuloma formation and/or vasculitis of central nervous system blood vessels, including those in the pituitary gland [2], [7]. Diagnosing pituitary involvement in WG is difficult however, our case highlights that it is an important differential diagnosis to consider. The presence of CDI or hypopituitarism in association with symptoms including nasal congestion or collapse, epistaxis, dyspnoea or known WG, should raise suspicion of WG as a cause [1].
Tissue diagnosis to confirm vasculitis is important due to the toxic nature of medical treatment and possibly invasive surgical management [1]. However histological confirmation of pituitary WG is rarely necessary and should only be conducted if pituitary disease is the sole disease manifestation or, as in our case, pituitary disease is unresponsive to immunosuppression [3]. Radiological abnormalities occur in greater than 90% of cases. MRI frequently reveals diffuse pituitary enlargement with heterogenous or homogenous pattern, infundibular thickening, loss of signal intensity in the adenohypophysis and, rarely, cystic pituitary changes [1], [3], [4], [8].
Cyclophosphamide-based regimens are recommended in the treatment of pituitary WG on the basis of lower relapse rates [1]. Recently, large studies have shown rituximab-based therapy to be beneficial for severe cases of ANCA-associated WG refractory to cyclophosphamide therapy and isolated case reports support its use in pituitary involvement [9], [10]. Our experience would contribute to this evidence. Long-term prognosis of patients with pituitary WG is unknown due to the small number of patients reported in the literature. However, the course of pituitary involvement in WG is generally not parallel to the one of the general disease. Despite systemic therapy and good disease control elsewhere, few patients experience full recovery of pituitary function [1], [3]. With the few cases reported it is unclear if persistent pituitary dysfunction post treatment is a reflection of ‘subclinical disease’ or permanent hypothalamopituitary system damage [1], [2]. Furthermore, follow-up MRI is not always concordant with clinical improvement and its use should be in conjunction with functional pituitary assessment in monitoring recovery [1], [3].
4. Conclusion
Pituitary involvement in WG is an exceedingly rare complication of this disease. This case report highlights the need to consider this differential diagnosis in patients with CDI in the setting of relevant associated symptoms and disease background. Timely diagnosis is important to ensure best possible management so as to minimize the risk of irreversible pituitary function loss, especially considering long-term prognosis is largely unknown.
Consent
Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal on request.
Conflict of interest statement
The authors have no conflict of interest to declare.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Ethical approval
Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal on request.
Author contribution
Samantha M Baird: data collection, manuscript preparation and revision, study design.
Upasna Pratap: study concept and design, surgeon who performed the operation.
Catriona McLean: participation in drafting and critically revising article.
Candice P Law: participation in drafting and critically revising article.
Nicholas Maartens: surgeon who performed the operation.
Registration of research studies
researchregistry1900.
Guarantor
Samantha M Baird.
Contributor Information
Samantha M. Baird, Email: smbaird@iinet.net.au.
Upasna Pratap, Email: upasna.pratap@gmail.com.
Catriona McLean, Email: c.mclean@alfred.org.au.
Candice P. Law, Email: c.law@alfred.org.au.
Nicholas Maartens, Email: n.maartens@alfred.org.au.
References
- 1.Yong T.Y., Li J.Y.Z., Amato L., Mahadevan K., Phillips P.J., Coates P.S. Pituitary involvement in Wegener’s granulomatosis. Pituitary. 2007;11:77–84. doi: 10.1007/s11102-007-0021-2. [DOI] [PubMed] [Google Scholar]
- 2.Santoro S.G., Guida A.H., Furioso A.E., Glikman P., Rogozinski A.S. Panhypopituitarism due to Wegener’s granulomatosis. Arq. Bras. Endocrinol. Metab. 2011;55:481-–485. doi: 10.1590/s0004-27302011000700008. [DOI] [PubMed] [Google Scholar]
- 3.Kapoor E., Cartin-Ceba R., Specks U., Leavitt J., Erickson B., Erickson D. Pituitary dysfunction in granulomatosis with polyangiitis: the mayo clinic experience. J. Clin. Endocrinol. Metab. 2014;99:3988–3994. doi: 10.1210/jc.2014-1962. [DOI] [PubMed] [Google Scholar]
- 4.Pereira E.A.C., Plaha P., Hofer M., Karavitaki N., Cudlip S.A. Hypophyseal Wegener’s granulomatosis presenting by visual field constriction without hypopituitarism. Clin. Neurol. Neurosurg. 2013;115:762–764. doi: 10.1016/j.clineuro.2012.06.041. [DOI] [PubMed] [Google Scholar]
- 5.Agha R.A., Fowler A.J., Saetta A., Barai I., Rajmohan S., Orgill D.P., for the SCARE Group The SCARE statement: consensus-based surgical case report guidelines. Int. J. Surg. 2016 doi: 10.1016/j.ijsu.2016.08.014. [DOI] [PubMed] [Google Scholar]
- 6.Ahlstrom C., Liedholm K., Truedsson E. Respirato-renal type of polyarteritis nodosa. Acta Med. Scand. 1953:323–332. doi: 10.1111/j.0954-6820.1953.tb15703.x. [DOI] [PubMed] [Google Scholar]
- 7.Špíšek R., Kolouchová E., Jenšovský J., Rusina R., Fendrych P., Plas J. Combined CNS and pituitary involvement as a primary manifestation of Wegener granulomatosis. Clin. Rheumatol. 2005;25:739–742. doi: 10.1007/s10067-005-0065-5. [DOI] [PubMed] [Google Scholar]
- 8.Fujisawa I. Magnetic resonance imaging of the hypothalamic-Neurohypophyseal system. J. Neuroendocrinol. 2004;16:297–302. doi: 10.1111/j.0953-8194.2004.01183.x. [DOI] [PubMed] [Google Scholar]
- 9.Hughes J., Barkhoudarian G., Ciarlini P., Laws E., Mody E., Inzucchi S. Refractory pituitary granulomatosis with polyangiitis (Wegener’s) treated with rituximab. Endocr. Pract. 2015;19:1–7. doi: 10.4158/EP12181.CR. [DOI] [PubMed] [Google Scholar]
- 10.Stone J.H., Merkel P.A., Spiera R., Seo P., Langford C.A. Rituximab versus cyclophosphamide for ANCA-associated vasculitis. N. Engl. J. Med. 2010;363:221–232. doi: 10.1056/NEJMoa0909905. [DOI] [PMC free article] [PubMed] [Google Scholar]