Abstract
Respiratory syncytial virus (RSV) infection activates protein kinase C (PKC), but the precise PKC isoform(s) involved and its role(s) remain to be elucidated. On the basis of the activation kinetics of different signaling pathways and the effect of various PKC inhibitors, it was reasoned that PKC activation is important in the early stages of RSV infection, especially RSV fusion and/or replication. Herein, the role of PKC-α during the early stages of RSV infection in normal human bronchial epithelial cells is determined. The results show that the blocking of PKC-α activation by classical inhibitors, pseudosubstrate peptides, or the overexpression of dominant-negative mutants of PKC-α in these cells leads to significantly decreased RSV infection. RSV induces phosphorylation, activation, and cytoplasm-to-membrane translocation of PKC-α. Also, PKC-α colocalizes with virus particles and is required for RSV fusion to the cell membrane. Thus, PKC-α could provide a new pharmacological target for controlling RSV infection.
Respiratory syncytial virus (RSV), a pneumovirus, is an important respiratory pathogen that produces an annual epidemic of respiratory illness which is seen primarily in infants, but also in adults, worldwide (39). Effective vaccines against RSV are not currently available. The development of antivirals requires a comprehensive molecular understanding of the early events of virus-host interaction necessary for virus fusion and entry into cells, an understanding which is lacking. RSV activates multiple signaling pathways (1, 2, 8, 13), including those involving protein kinase C (PKC), which appear to play a role in acute inflammation (1, 29). However, the role of PKC in RSV infection is poorly understood.
PKC comprises a family of serine/threonine kinases with at least 12 members. In general, PKC has a catalytic domain, which also contains the ATP binding site, and a regulatory domain containing the phospholipid and diacylglycerol (DAG) binding site. On the basis of structure and activation requirements, the PKC family can be divided into the following major subclasses: (i) classical PKCs, comprising the α, βI, βII, and γ isozymes that are Ca2+ dependent and DAG sensitive; (ii) novel PKCs, comprising the δ, ɛ, η, θ, and κ isozymes that are Ca2+ independent and DAG sensitive; (iii) atypical PKCs, comprising the ζ and ι/λ isozymes that are Ca2+ independent and DAG insensitive; and (iv) the PKC-μ isozyme that is similar to the atypical isozymes but contains an additional specific signal peptide transmembrane domain (28, 32). PKCs play an important role in infection of mammalian cells by different viruses (9, 10, 30, 31, 33, 38). PKC inhibitors reduce the entry of intracellular mature vaccinia virus by affecting Rac1 activation and actin assembly (44). PKC is also important in the formation of caveolae, which a number of viruses such as human immunodeficiency virus type 1, filoviruses (Ebola), and simian virus 40 (11) utilize to evade the phagolysosomal pathway, thus allowing them to thrive inside cells by indirectly evading the immune system (11). A number of other viruses also use the caveolae pathway to infect the cells.
RSV activates several isozymes of PKC in the alveolar type, carcinoma non-small-cell line A549, and the activation of the atypical isozyme PKC-ζ, in particular, conveys activation of ERK-2 during the early stages of RSV infection (29). The A549 cell line may, however, differ from normal human epithelial cells in its response to RSV infection. Also, these studies utilized RSV from a culture supernatant, which usually contains cytokines and chemokines, some of which may override RSV-induced PKC activation. In addition, this study did not address the role of PKC in early stages of infection.
It was previously reported that RSV requires functional MEK-ERK1/2 and Stat1 pathways for successful infection (18, 19). Because of the potential link between PKC and ERK (21) and since PKC plays a central role in signaling events leading to changes in the cell membrane and cytoskeleton, including caveolae and lipid raft formation (27), we hypothesized that PKC activation plays a role in the early stages of RSV infection, especially RSV fusion. To test this hypothesis, we examined the role of PKC during the early stages of RSV infection in normal human bronchial epithelial (NHBE) cells. The results show that PKC-α is activated upon RSV contact of the cell and that it translocates to the cell membrane, where it plays an important role in the fusion process which is pivotal to successful RSV infection.
MATERIALS AND METHODS
Cell culture.
NHBE cells (Cambrex Bio Science, Walkersville, Md.) were grown at 37°C at 5% CO2 in basal epithelial growth medium supplemented with bovine pituitary extract, insulin, hydrocortisone, human epithelial growth factor, epinephrine, transferrin, retinoic acid, and triiodothyronine for two passages before they were collected, frozen, and stored in liquid nitrogen. Cells were used at passage 4 or 5, and exposure to RSV or the different drugs was done in medium without supplementation.
Production and purification of RSV.
RSV was produced in HEp-2 cells and purified by pelleting through a glycerol layer (6). HEp-2 cells at 50 to 60% confluence were infected with RSV at a multiplicity of infection (MOI) of 0.1 to 0.2 in OptiMEM (Invitrogen, Carlsbad, Calif.) for 2 h at 37°C at 5% CO2. The flasks were each gently rocked for 15 min. The medium was replaced with OptiMEM plus 2% fetal bovine serum (FBS), and the infection was allowed to progress until cytopathological effects were evident. Cells were scraped off, put into 50-ml conical tubes, and vortexed at half speed for 10 s and then centrifuged at 2,100 × g for 10 min at 4°C to separate virus from cells. Supernatants were mixed with 0.1 volume of sterile 1 M MgSO4, layered onto 30% glycerol in 50 mM HEPES (pH 7.5) with 1 M MgSO4, and centrifuged at 24,000 × g with an SW28 rotor for 3 h at 4°C. The viral pellet was gently rinsed with OptiMEM, resuspended in 750 μl of buffer at 4°C (0.22-μm-pore-filtered solution of 50 mM HEPES [pH 7.5], 0.1 M MgSO4, and 150 mM NaCl), aliquoted, and stored in liquid nitrogen.
Western blots.
NHBE cells at 80% confluence were infected with purified RSV (MOI of 3) in basal medium. Viral binding to cell membranes was synchronized by incubation at 4°C for 1 h, and infection was allowed to proceed for various times. Cells were then washed with phosphate-buffered saline (PBS) at 4°C, and membrane proteins were extracted by using a subcellular proteome kit (Calbiochem, San Diego, Calif.) according to the guidelines of the manufacturer and precipitated (protein concentrate kit; Chemicon, Temecula, Calif.). Equal amounts of protein were separated on a sodium dodecyl sulfate-12% polyacrylamide gel, blotted onto a nitrocellulose membrane, and incubated overnight at 4°C with rabbit anti-phospho-PKC-α antibody (Cell Signaling, Beverly, Mass.) in 10 mM Tris (pH 8)-0.15 M NaCl-5% milk. After washing, membranes were incubated for 1 h at room temperature (RT) with horseradish peroxidase-linked secondary antibody, and protein bands were visualized by enhanced chemiluminescence (Cell Signaling).
Single-cycle immunofluorescence assays.
NHBE cells at 80% confluence were exposed to one of the following inhibitors at different concentrations for 30 min at 37°C before infection with RSV at 1 MOI for 2 h: calphostin C, chelerythrine, or myristoylated PKC-α/β pseudosubstrate (myr-PKC) peptide (Calbiochem). Cells were then incubated for 16 h (approximately a single viral replication cycle), fixed with 100% ethanol for 20 min at room temperature, and stained with fluorescein isothiocyanate (FITC)-anti-RSV N protein antibody (Chemicon) for 30 min at 37°C. Infected cells were detected and counted by fluorescence microscopy.
Determination of RSV titer.
NHBE cells were incubated with or without the PKC inhibitor myr-PKC peptide for 30 min. A 0.1 MOI of rgRSV (a recombinant virus encoding green fluorescent protein, which was kindly given by M. E. Peeples) was then allowed to bind to the cells for 2 h (14). Cells were washed with PBS, fresh growth medium was added, and infection was allowed to proceed for 60 h before the supernatant was collected. RSV titer was calculated by a modified method (42) which was more accurate than the plaque assay. Fourfold dilutions (100 μl) of each supernatant were added to 80% confluent HEp-2 cells grown in 48-well plates. After 2 h of adsorption and rocking every 15 min, the inocula were replaced with 500 μl of growth medium (10% FBS in modified Eagle's medium and Earle's salts) and infected for 16 h. Viral titer was calculated by counting the number of green cells per well.
Immunofluorescence and confocal microscopy.
Immunofluorescence-based confocal microscopy was used to study the virus entry into the cells in the presence or absence of PKC inhibitors and to determine whether PKC-α or its phosphorylated form colocalizes with viral particles. For these assays, NHBE cells were grown on 8-well chamber slides to 80% confluency and washed with HEPES-buffered saline solution, and myr-PKC peptide added as described above. Cells were equilibrated for 10 min with inhibitor at 4°C and then infected with 20 MOI of rgRSV for 1 h at 4°C to synchronize viral fusion and penetration.
The confocal microscopy protocol for virus entry has been previously described (44-46). Cells were incubated with FITC-conjugated wheat germ agglutinin (FITC-WGA; Vector Laboratories, Burlingame, Calif.) to label cell membranes and then washed and fixed with 4% paraformaldehyde (Electron Microscopy Sciences, Hatfield, Pa.) for 20 min at 4°C and for 40 min at RT. Cells were washed twice with PBS and incubated with permeabilization buffer (1% glycine, 0.1% saponin, and 3% goat serum in PBS) for 20 min at RT. To detect RSV, cells were incubated for 1 h at RT with anti-RSV N protein antibody (Chemicon) followed by rhodamine-conjugated secondary antibody (Chemicon). Washed cells were fixed again with 4% paraformaldehyde (10 min at RT), washed, air dried, and covered with Vectashield antifade-DAPI (4′,6′-diamidino-2-phenylindole) mounting medium (Vector Laboratories).
In the PKC-α-RSV colocalization experiments, NHBE cells were seeded onto 8-well chamber slides. After synchronizing viral binding for 1 h at 4°C, cells were put back at 37°C and the infection was allowed to proceed for 10 min, 30 min, or 1 h. Fixation, permeabilization, and washing were performed as described above. Cells were incubated for 1 h at RT with anti-PKC-α (Transduction Laboratories, San Diego, Calif.) or anti-phospho-PKC-α (Cell Signaling) followed by Alexa-488 secondary antibody (1 h and RT), and with anti-RSV antibody (Chemicon) followed by Alexa-555 secondary antibody (1 h at RT). The final fixation step was performed as described above.
Confocal laser scanning microscopy was performed with an LSM 510 system (Zeiss). The pinhole, scanning time, and magnification were kept constant for all image analyses and adjusted to avoid bleed-through between channels.
Flow cytometry.
In order to determine whether peptide impairs RSV binding, NHBE cells at 80% confluence were incubated with the myr-PKC peptide (Calbiochem) or the nonmyristoylated PKC-α/β pseudosubstrate peptide (Bachem, King of Prussia, Pa.) at different concentrations for 1 h at 37°C and then infected with RSV at 0.1 MOI for 16 h at 37°C (one viral replication cycle). Cells were detached with trypsin-EDTA, pelleted by centrifugation at 400 × g for 5 min at 4°C, and washed twice with staining buffer (2% FBS in PBS). Cells were fixed for 20 min at 4°C with 4% paraformaldehyde, washed, and permeabilized for 30 min at 4°C with 0.2% Triton X-100 in staining buffer and then incubated for 1 h at 4°C with FITC-anti-RSV N protein antibody (Chemicon). An isotype control, FITC-labeled mouse immunoglobulin G2a (BD Pharmingen, San Diego, Calif.), was included to determine the specificity of antibody binding. Flow cytometry was performed with a FACScan (BD Immunocytometry Systems, San Jose, Calif.).
To test for nonspecific effects due to the myristoyl moiety of the peptide, the myr-autocamtide-2-related inhibitory peptide (Calbiochem) was used as a control. Cells were incubated with myr-PKC peptide or myr-autocamtide for 30 min at 37°C before infection with 1 MOI of rgRSV encoding green fluorescent protein for 16 h. Infected cells were identified by flow cytometry.
To determine the role of PKC-α during RSV infection, NHBE cells at 60% confluence were transfected with pDN-PKC-α (40) (kindly provided by J. W. Soh) or the empty vector pcDNA3.1 (Invitrogen) by using Lipofectin (Invitrogen). Cells were also cotransfected with pDsRed2N1, encoding red fluorescent protein, as a transfection efficiency control. Transfected cells were infected with 1 MOI of rgRSV for 16 h, processed as described above, and analyzed by flow cytometry.
To test for RSV binding at 4°C, an immunofluorescence assay was used in which differences in the geometric mean could be detected in series of fourfold dilutions of RSV from 5.6 × 107 to 2.2 × 105 PFU/ml. This approach was validated by Martinez and Melero (24) and Budge et al. (5). NHBE cells (2 × 105 cells per tube) were exposed to various RSV dilutions at 4°C for 30 min, washed, and incubated with Zenon Alexa-488 (Molecular Probes, Eugene, Oreg.)-labeled anti-RSV F antibody (Chemicon). Cells were fixed with 2% paraformaldehyde. To test the effect of the myr-PKC peptide on RSV binding, detached cells were incubated with myr-PKC peptide (0, 12.5, 25, or 50 μM) at 37°C for 30 min followed by exposure to 1.4 × 107 PFU of RSV/ml at 4°C for 30 min and stained for RSV as described above.
ELISA.
For testing of RSV internalization, a sandwich enzyme-linked immunosorbent assay (ELISA) was used to measure RSV N protein in the cytosol. Sixty percent confluent NHBE cells were incubated in the absence or presence of 0, 12.5, 25, and 50 μM myr-PKC peptide for 30 min before infection with 1.7 × 106 PFU of RSV (approximately 5 MOI) for 2 h at 37°C. Different subcellular fractions were obtained by using a subcellular proteome kit (Calbiochem) according to the guidelines of the manufacturer. Cytoplasmic fractions were analyzed by sandwich ELISA. One hundred microliters of cytoplasmic fraction was added to each well of a 96-well plate that had been coated with anti-RSV antibody (AB1128; Chemicon) and blocked with 1% bovine serum albumin in 1× PBS. The plate was incubated for 30 min at RT with continuous mixing and then incubated for 1 h at 37°C. After washing, horseradish peroxidase (Molecular Probes)-labeled secondary antibody was added at 37°C for 30 min. Tetramethylbenzidine (TMB) substrate was then added, and the plate was read at 450 nm.
RSV labeling and evaluation of fusion by fluorescence microscopy.
Purified RSV was labeled with the lipid probe octadecyl rhodamine B (R18; Molecular Probes) (35). RSV (140 μg of total protein) was incubated for 1 h with 5 μg of R18 at RT and then separated from free R18 by gel filtration on a G-25 Sephadex microspin column (Harvard Apparatus Inc., Holliston, Mass.).
NHBE cells at 80% confluence were incubated with myr-PKC-α/β pseudosubstrate peptide at different concentrations for 1 h at 37°C, followed by ∼5,000 R18-labeled RSV particles per cell for 30 min at 37°C. Unattached and nonfused viral particles were removed by washing the cells with PBS, and RSV was imaged by fluorescence microscopy.
RESULTS
PKC inhibitors block RSV infection.
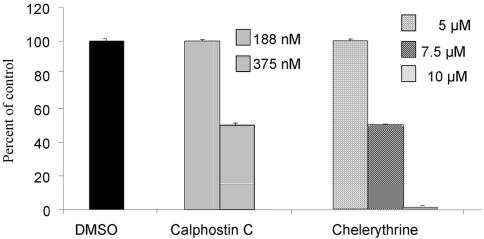
To determine the role of PKC in RSV infection of NHBE cells, the PKC inhibitors calphostin C and chelerythrine were used. Cells were preincubated with different concentrations of these inhibitors and then infected with RSV. Both inhibitors decreased the number of infected cells in a dose-dependent manner (Fig. 1), and 50% inhibition was reached at concentrations of 375 nM for calphostin C and 7.5 μM for chelerythrine. The concentrations of the inhibitors used in these assays did not exhibit cytotoxic activity as they were tested by both crystal violet toxicity assay and 3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide (MTT) assay. These results suggested that PKC is involved in early events of RSV infection.
FIG. 1.
Inhibition of RSV infection of NHBE cells by PKC inhibitors. Confluent NHBE cells were treated with PKC inhibitors at different doses for 30 min before they were infected with RSV at an infectious dose of 1 MOI. The infection was allowed to proceed for 16 h, and infected cells were detected by single-cell immunofluorescence assays. The percentage of inhibition was calculated with respect to the control (dimethyl sulfoxide [DMSO]). The values are means ± standard deviations of three different experiments.
PKC-α/β pseudosubstrate peptide decreases RSV infection.
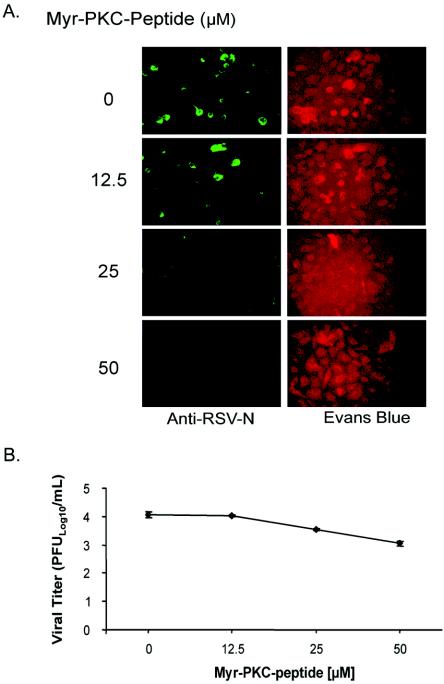
To confirm whether RSV infection of NHBE cells involved classical PKCs, myr-PKC peptide was used. The myristoylated moiety allows the peptide to enter into the cells. NHBE cells treated with myr-PKC peptide prior to RSV infection showed a dose-dependent reduction of the number of infected cells, as determined by single-cycle cell fluorescence assays (Fig. 2A). Also, a fluorescence-based viral titration method using rgRSV was utilized to determine the effect of myr-PKC peptide on virus production. The results showed an inhibitor concentration-dependent reduction in the virus titer (Fig. 2B). A 1-log reduction of virus titer was seen with 50 μM myr-PKC peptide. These results suggest that the activation of classical PKCs plays a role in RSV infection.
FIG. 2.
Inhibition of classical PKC isozymes impairs RSV infection and reduces virus production. (A) Confluent NHBE cells were treated with the myristoylated PKC-α/β pseudosubstrate peptide at the indicated concentrations for 30 min before being infected with RSV at an infectious dose of 1 MOI. The infection was allowed to proceed for 16 h. Infected cells were detected by single-cell immunofluorescence assays (magnification, ×200). (B) NHBE cells grown in a 12-well plate configuration were incubated with different concentrations of the myr-PKC peptide 30 min prior to being infected with 0.1 MOI of rgRSV. Infection was allowed to proceed for 60 h. Culture medium from each treatment condition was collected, and the viral titer was measured. The results are the averages ± standard deviations of three different experiments per experimental condition.
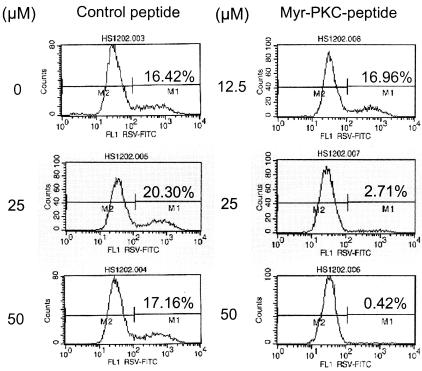
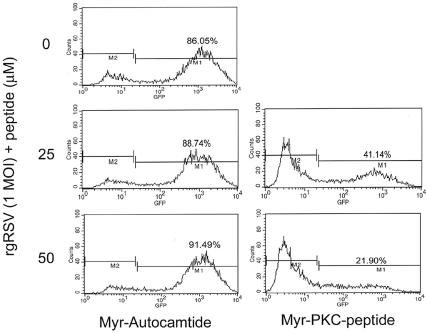
To further verify these results, RSV infection was measured by flow cytometry analysis. A nonmyristoylated peptide with the same pseudosubstrate amino acid sequence did not block RSV infection (Fig. 3), suggesting that the peptide does not interfere with RSV binding to the cell. We also used the myristoylated peptide autocamtide as a control. This peptide acts as a highly specific and potent inhibitor of calmodulin-dependent protein kinase II and has been used as a control for PKC activity assays (26, 30). The results show that 25 and 50 μM of myr-PKC peptide reduced the number of infected cells by 50 and 75%, respectively, compared to the control, myr-autocamtide, which did not reduce the number of infected cells (Fig. 4). These results indicate that the myristoylated moiety is not responsible for the myr-PKC peptide inhibitory effect in RSV infection and that the inhibition of RSV infection is due to inhibition of PKC.
FIG. 3.
PKC-α/β pseudosubstrate peptide does not interfere with the RSV binding to NHBE cells. Confluent NHBE cells were treated with either the PKC pseudosubstrate peptide (control, nonmyristoylated) or the myristoylated PKC pseudosubstrate peptide (myr-PKC peptide) at the indicated concentrations for 30 min before being infected with RSV at an infectious dose of 1 MOI. The infection was allowed to proceed for 16 h. Cell culture monolayers were then detached by trypsin treatment, and single-cell suspensions were processed for analysis by fluorescence-activated cell sorter. The infected cells were detected by an FITC-labeled mouse monoclonal anti-RSV N protein antibody.
FIG. 4.
Myristoylated moiety did not block RSV binding to cells. Confluent NHBE cells were treated with either the myr-autocamtide or the myr-PKC peptide at the indicated concentrations for 30 min before being infected with rgRSV at an infectious dose of 1 MOI. The infection was allowed to proceed for 16 h. Cell culture monolayers were then detached by trypsin treatment, and single-cell suspensions were processed for analysis by fluorescence-activated cell sorter. Infected cells were detected by the fluorescence emitted by enhanced green fluorescent protein produced by rgRSV.
Lack of PKC-α leads to decreased RSV infection.
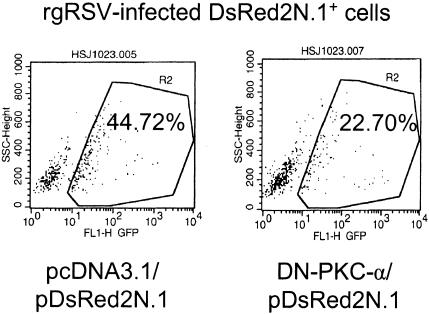
NHBE cells express PKC-α, -βII, -γ, -δ, -ɛ, -θ, -ι, and -λ and a Western blot analysis showed that PKC-α levels decreased at 1 h and consequently increased by 2 h in cells postinfection, while the levels of all other PKCs remained unaltered (our unpublished results). Since a decrease of PKC levels is correlated with their previous activation (22, 32), a dominant-negative mutant construct of PKC-α was used to examine the role of PKC-α in rgRSV infection of NHBE cells. The dominant-negative construct of PKC-α is a kinase-resistant mutant resulting from a K368→R point mutation in the ATP binding site. Dominant-negative PKC-α (DN-PKC-α) competes with the wild-type form for binding to the so-called receptors for activated C kinase to where PKC is translocated to exert its biological function. In addition, DN-PKC-α sequesters the potential substrates from the wild-type PKC-α. Thus, cells were transiently transfected with either pDN-PKC-α or the background plasmid pcDNA3.1. Along with these plasmids, pDsRed2N1, which encodes red fluorescent protein, was also added to the transfection mixture in order to identify the transfected cells. Subsequently, the percentage of transfected cells that were infected by rgRSV was determined by flow cytometry. Infection in DN-PKC-α-transfected cells decreased by 50% compared with that seen in pcDNA3.1-transfected cells (Fig. 5). These results show that PKC-α plays an important role in facilitating RSV infection of normal human bronchial epithelial cells.
FIG. 5.
Expression of DN-PKC-α reduced the number of RSV-infected cells. Approximately 8 × 105 NHBE cells grown in 6-well plates were transfected with 1 μg of either pDN-PKC-α or pcDNA3.1. Along with these plasmids, 100 ng of pDsRed2N1 was also cotransfected to visualize transfected cells by flow cytometry. Twenty-four hours later, cells were infected with rgRSV, and infection was indicated by the expression of enhanced green fluorescence protein. The percentage of infection in red fluorescent cells (infection in transfected cells) was then determined by flow cytometry.
RSV infection induces activation of PKC-α and its translocation to the cell membrane.
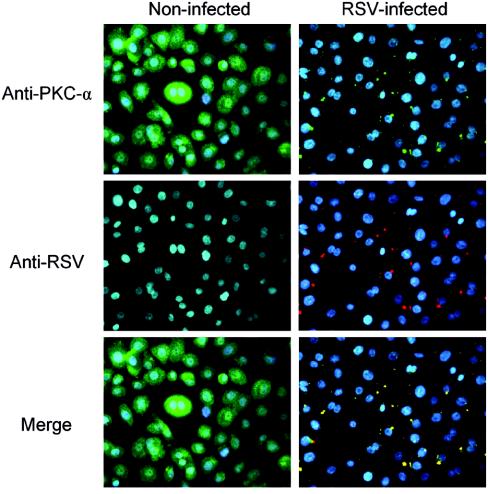
To determine whether RSV infection activates PKC-α in the infected cell, the translocation of PKC-α was examined by immunocytofluorescence and confocal microscopy in NHBE cells exposed to RSV at an infectious dose of 20 MOI. PKC-α translocates from the cytoplasm to the cell plasma membrane and colocalizes with viral particles as early as 10 min (at 37°C) after a viral binding synchronization step at 4°C for 1 h (Fig. 6). In addition, PKC-α colocalizes with the viral particles for up to 1 h at the cell membrane (data not shown).
FIG. 6.
PKC-α colocalizes with RSV at early stages of infection. Confluent NHBE cells grown on 8-well chamber slides were incubated with 20 MOI of RSV for 1 h at 4°C to allow viral binding synchronization. Slides were then exposed at 37°C for 10 min to allow virus penetration. Later, cells were processed for immunocytofluorescence. Cells were fixed with 4% paraformaldehyde, permeabilized with 0.1% saponin, and stained with mouse monoclonal anti-PKC-α antibody (green), goat polyclonal anti-RSV antibody (red), and DAPI (blue, nucleus staining). Fluorescence images (magnification, ×400) were taken by cooled camera device under respective dual-filter mode (either green-blue or red-blue) and triple-filter mode (merge).
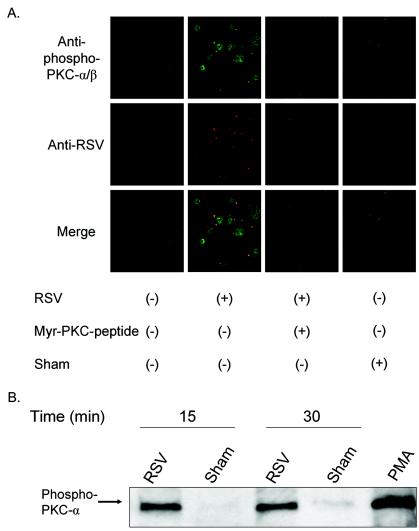
Translocation of PKC involves an autophosphorylation event that allows PKC-α to migrate to the cell membrane for further signaling events. There are four potential phosphorylation sites in PKC-α, Thr-250, Thr-497, Thr-638, and Ser-657, that are phosphorylated in activated PKC-α (32, 34). The phosphorylation status of the translocated PKC-α was determined by using an anti-phospho-Thr-638 PKC-α antibody. Confocal microscopy showed an increase of phospho-PKC-α that is associated with those viral particles contacting the cells as early as 10 min (at 37°C) after a viral binding synchronization step (Fig. 7A). Such colocalization at the cell membrane is also seen 1 h after virus exposure.
FIG. 7.
Activated PKC-α colocalizes with RSV particles on the cell membrane. (A) Confluent NHBE cells grown on 8-well chamber slides were exposed to RSV at an infectious dose of 20 MOI for 10 min at 37°C following viral binding synchronization for 1 h at 4°C. As negative controls, cells were either pretreated with a PKC-α/β pseudosubstrate inhibitor peptide at 50 μM for 30 min before infection or exposed to a sham treatment (Centricon filtrate obtained from purified RSV). NHBE cells were fixed with 4% paraformaldehyde, permeabilized with 0.1% saponin, and stained with rabbit polyclonal anti-phospho-Thr-638 PKC-α antibody (green), goat polyclonal anti-RSV antibody (red), and DAPI (blue, nucleus staining). Confocal images (magnification, ×630) were taken by using laser excitation sources for Alexa-488 (green) or Alexa-555 (red) and assembled with Adobe Photoshop software version 7.01. (B) NHBE cells grown in T-25 flasks were infected with purified RSV at an infectious dose of 3 MOI for 15 or 30 min at 37°C following a viral binding synchronization step with incubation at 4°C for 1 h. Membrane fractions of each experimental condition were obtained, equal amounts of protein (8 μg) of the membrane fractions were analyzed by western blot, and the phospho-Thr-638 PKC-α was probed by the specific antibody.
There was no increase in PKC-α phosphorylation when NHBE cells were exposed to the sham control, i.e., filtrate resulting from centrifugation of the RSV suspension through a Centricon YM-100 (Fig. 7A). The exposure of cells to PKC-α pseudosubstrate peptide (at 50 μM) resulted in a reduction of phospho-PKC-α and a concomitant reduction in the number of RSV particles contacting the cells. UV-irradiated RSV (2 hits of UV radiation at 1,800 mJ) showed a colocalization pattern similar to that of the live infectious virus (data not shown). To confirm PKC activation, membrane proteins of cells exposed to RSV were analyzed by Western blot analysis with an antibody to phospho-PKC-α (Fig. 7B). The results showed that the membrane fraction of cells exposed to RSV, not sham, showed anti-phospho-PKC-α antibody binding. Together, these results indicate that RSV particles induce the translocation and activation of PKC-α when they contact NHBE cells.
PKC-α activation is required for RSV fusion.
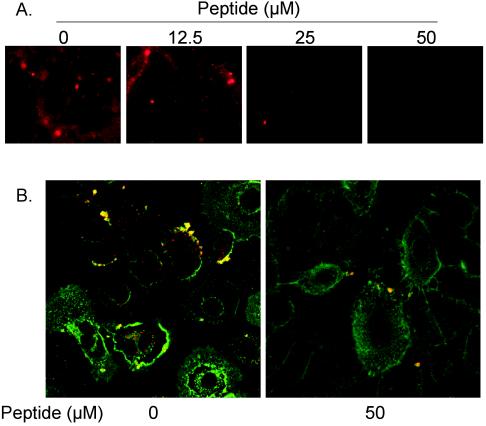
To determine whether PKC-α activation plays a role in the fusion of RSV with the NHBE cell membrane, a fluorescence-dequenching method described previously (35) was used to examine RSV fusion. The fusion of RSV (labeled with octadecyl rhodamine R18 at a self-quenching concentration) with unlabeled NHBE cells was directly observed by fluorescence microscopy. The viral fusion results in an increase in the quantum yield of R18 due to membrane fusion events and leads to a dye dilution in the merged membrane. RSV fusion was significantly inhibited when NHBE cells were pretreated with PKC-α/β pseudosubstrate peptide at 25 μM and was virtually absent when cells were pretreated with peptide inhibitor at 50 μM (Fig. 8A).
FIG. 8.
(A) Inhibition of PKC-α activity blocks viral fusion. Confluent NHBE cells seeded onto 8-well chamber slides were preincubated with a PKC-α/β pseudosubstrate peptide for 30 min at the indicated concentrations before cells were exposed to octadecyl rhodamine B (R18)-labeled RSV (5,000 RSV particles/cell). The infection was allowed to proceed for 30 min at 37°C. After removal of the unattached virus, cells were imaged (magnification, ×200) by using a fluorescence microscope. (B) Myr-PKC-α/β pseudosubstrate peptide significantly reduces the number of viral cores inside NHBE cells. NHBE cells were infected on ice with purified RSV (20 MOI) and then incubated at 37°C for 1 h. Cells were then washed with ice-cold PBS containing FITC-WGA for the staining of the plasma membrane, as described above. Cells were again washed with ice-cold PBS, fixed with 3.7% paraformaldehyde, and permeabilized with 0.1% saponin. After washing with PBS, samples were treated for indirect immunofluorescence. Viral cores were probed by mouse monoclonal anti-RSV N protein antibodies and were revealed by rhodamine-labeled goat anti-mouse antibody. The pictures (magnification, ×630) represent single optical sections perpendicular to the z axis of a confocal microscope.
The inhibition of viral fusion was further confirmed by a viral entry assay utilizing confocal microscopy (44-46). At 1 h postinfection, some of the viral particles, as detected by rhodamine-labeled anti-RSV N antibody, were localized at the perinuclear region, whereas others still remained close to the plasma membrane (FITC-WGA) (Fig. 8B). The number of viral cores seen inside NHBE cells after 1 h of infection significantly decreased when the cells had been exposed to myr-PKC peptide.
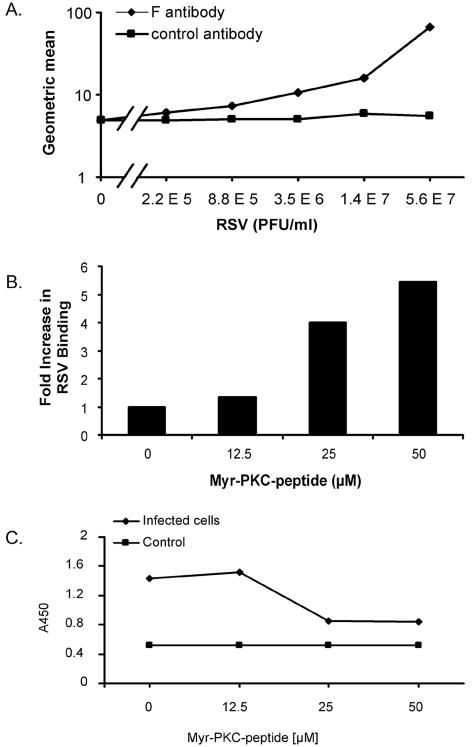
In order to determine whether PKC inhibition impairs either viral binding or fusion, the virus signal was analyzed at 4°C for binding and at 37°C for viral penetration. Whether PKC inhibition reduces the number of virus bound to the cell plasma membrane at 4°C was analyzed by flow cytometry. This assay was able to detect differences in the geometric means of a series of fourfold dilutions of the RSV viral particles encompassing the dynamic range from 5.6 × 107 to 2.2 × 105 PFU/ml (Fig. 9A). The results indicate that PKC inhibition did not impair binding of the viral particles to the cells at 4°C. Moreover, exposure to 25 and 50 μM of myr-PKC peptide induces an increase in the number of viral particles bound to the cells by 4- and 5.4-fold, respectively, compared with those not exposed to the myr-PKC peptide (Fig. 9B).
FIG. 9.
Quantitation of viral attachment and penetration. (A) NHBE cells in suspension were incubated on ice with serial fourfold dilutions of RSV to allow viral attachment. After 30 min, cells were washed three times with ice-cold staining buffer, and bound virus was detected with Zenon Alexa-488 anti-RSV F monoclonal antibody. Geometric mean fluorescence values were obtained and graphed. The curve for the isotype control antibody is also shown. (B) A total of 106 NHBE cells per ml in suspension were placed in the absence or presence of different concentrations of the myr-PKC peptide for 30 min at 37°C. Cells were washed with ice-cold basal medium, and 106 cells/ml were exposed to 1.4 × 107 PFU of RSV/ml for 30 min on ice. Unbound virus was removed by washing in ice-cold staining buffer, and bound virus was detected by flow cytometry with Zenon Alexa-488 anti-RSV F monoclonal antibody. (C) NHBE cells grown on 6-well plates were incubated in the absence or presence of different concentrations of the myr-PKC peptide for 30 min before the cells were infected with 1.7 × 106 PFU/well (approximately 5 MOI) for 2 h at 37°C. Cytoplasmic fractions were then isolated from each experimental condition, and a sandwich ELISA was conducted to probe the presence of RSV N protein in these fractions. TMB substrate was then added, and the plate was read at 450 nm (A450). The cytoplasmic fractions of noninfected cells were used as a control. These assays were duplicated with similar results.
To determine virus internalization, a sandwich ELISA was used to probe the presence of RSV N protein in the cytoplasmic fractions of RSV-infected NHBE cells previously exposed to different concentrations of myr-PKC peptide. NHBE cells showed a significant decrease (50%) of viral penetration, as measured by the optical density at 450 nm upon incubation with 25 and 50 μM of myr-PKC peptide (Fig. 9C). There was no impairment of the viral fusion when the cells were treated with 12.5 μM of myr-PKC peptide, and no change in optical density was seen in uninfected cells. Together, these data indicate that PKC activity is required for RSV to fuse with the cell membranes and internalize into the cells.
DISCUSSION
The results of these studies demonstrate that (i) the inhibition of PKC activity by classical and pseudosubstrate peptides impairs RSV infection; (ii) PKC-α, in particular, is involved in this process; (iii) RSV causes PKC-α translocation to the cell membrane, which is accompanied with an increase in PKC-α phosphorylation; and (iv) PKC-α activation plays an important role in early events of RSV infection, particularly in the fusion and internalization of the virus to the host cell membrane.
A major finding of this study is the identification of PKC, and PKC-α in particular, as an important player in the early processes of RSV infection such that blocking PKC significantly impairs RSV infection. The following evidence supports the role of PKCs in RSV infection. Studies in which inhibitors such as calphostin C, chelerythrine, Ro-318220 (data not shown), and myristoylated PKC-α/β pseudosubstrate peptide are used demonstrate that inhibition of PKCs can significantly attenuate RSV infection. Calphostin C is a perylenequinone metabolite that targets classical and novel PKC isoforms and inhibits (at a 50% inhibitory concentration [IC50] of 50 to 100 nM) both phorbol ester binding and phosphotransferase activity of PKC by binding to the regulatory domain (17). Chelerythrine is a benzophenanthridine that is a potent, reversible, selective antagonist of classical and novel PKCs that targets the catalytic domain with an IC50 of 660 nM (16). Both calphostin C and chelerythrine have been used to inhibit PKC-α (7, 37, 50, 52).
NHBE cells were exposed to the inhibitors for the same period of time as when they were infected with RSV. In addition, the range of concentrations of the inhibitors used in the present study were the same as those reported in previous studies that have used human bronchial epithelial cells as targets (20, 43). These results are also consistent with those of other studies which used these inhibitors and showed a role for PKC in viral infection by several enveloped viruses, such as the vesicular stomatitis virus, herpes simplex I virus, turkey herpes virus, vaccinia virus, Sindbis virus, human herpesvirus 8, and adenovirus type 2 (9, 30, 31). In addition, productive entry of the influenza virus is inhibited when cells are exposed to a specific inhibitor of PKC, bisindolylmaleimide I (36), which acts early in the infectious cycle but does not affect viral binding to the cells.
A specific role for PKC-α became clear from experiments involving the use of (i) the inhibitor myristoylated PKC-α/β pseudosubstrate peptide and (ii) the dominant-negative mutant for PKC-α. Myr-PKC peptide both reduces the number of infected cells and impairs the virus production from infected cells. The amino acid sequence for myr-PKC peptide was derived from the pseudosubstrate domain whose function is to keep these kinases in their inactive state; thus, it is a very specific competitive inhibitor of PKC-α and -β. The N-myristoyl modification permits the entry of the PKC pseudosubstrate peptide into the intracellular milieu, presumably by membrane insertion, in such a way that PKC is inhibited. The doses of this peptide found to be effective in inhibiting PKC activity and RSV infection are in agreement with the IC50 of myr-PKC peptide (range, 8 to 20 μM), and even specificity is seen with a maximal (98%) inhibitory concentration of 100 μM (12, 15). Moreover, the decline in the infection is due to the blocking of PKC and not to a nonspecific impairment of the binding of RSV to the cells, which was evident from the inactivity of both nonmyristoylated PKC peptide and the myr-autocamtide-2. On the other hand, myr-PKC peptide was previously shown to inhibit the transport of adenovirus type 2 through the actin cortex, which suggests a role of PKC in this process (30).
Furthermore, the above-mentioned evidence in combination with the analysis of protein expression of PKC isoforms implied specific involvement of PKC-α in RSV infection, which was confirmed by using a pDN-PKC-α construct. The results of this study showed that pDN-PKC-α-transfected cells exhibit significantly decreased RSV infection. These results are consistent with those of studies that have used DN-PKC-α to probe the role of PKC-α during infection with enteropathogenic Escherichia coli or exposure to its outer membrane proteins (23). The partial reduction of virus infection upon DN-PKC-α expression is consistent with previous reports in the literature showing the ability of this construct to modulate PKC activity and the signaling pathways dependent on such activation. For example, DN-PKC-α reduced the expression of luciferase dependent on the transcriptional activation of a promoter driven by the serum response element by 50% (40). In addition, other signaling molecules reportedly activated during RSV infection (18, 19) might be acting in concert with PKC during the initial infection process.
The demonstration of the involvement of PKC-α in the early RSV infection process prompted a detailed analysis of the activation of PKC-α. The results showed that PKC-α translocates to the cell membrane colocalizing with the viral particles. In addition to translocation, there is a concurrent increase of PKC-α phosphorylation, which is another feature of PKC activation. Although the usual pattern of translocation after treatment with phorbol myristate acetate is a homogeneous distribution along the cell membrane (48), it has also been shown that activated PKC isozymes can cluster in specific locations at the cell membrane (47). Recently, activated PKC-α was shown to cluster in the cell membrane of human brain microvascular endothelial cells at the entry site of E. coli K1 (41). The result that myr-PKC peptide prevents the translocation of PKC-α to the membrane indicates the requirement of membrane-associated active PKC for successful RSV infection.
While PKC-α activation and translocation is required for successful infection, the mechanism of how activated PKC facilitates infection remained unclear. Membrane translocation of the activated PKC-α suggested that it might play a role in the very fundamental process of fusion of RSV to plasma membrane and internalization. The results of this study demonstrate that inhibition of PKC-α activity impairs viral fusion. This finding is evident from (i) the reduction in the fluorescence signal due to the dequenching of R18-labeled RSV upon treatment of cells with myr-PKC peptide, (ii) the reduction in the number of viral cores in the cytoplasm of those cells exposed to 50 μM myr-PKC peptide, (iii) the fact that the viral binding at 4°C is not impaired but actually increases when PKC is inhibited, and (iv) the fact that the number of virus fusion events is reduced by 50% as demonstrated by ELISA. Nevertheless, the precise mechanism of PKC inhibition of RSV membrane fusion is unknown. The PKC-α isozyme plays an important role during the formation of the caveolae (27, 34, 49), which is a key system involved in both RSV infection and maturation (3, 4, 25, 51). Previously, RSV has been shown to bind to caveolae during the early stages of interaction with bovine dendritic cells (51), although it is not known whether the same mechanism holds for epithelial cells. Whether rafts or caveolae are required for RSV internalization is under investigation.
In summary, our results demonstrate for the first time that classical PKC, particularly PKC-α, plays an important role in successful RSV infection. The results also show that exposure to RSV induces rapid activation of PKC-α and its translocation to the membrane, where it appears to facilitate RSV fusion to the host cell membrane. A comprehensive understanding of the role of PKC-α in RSV membrane fusion and internalization may lead to the effective targeting of PKC-α activity-related pathways and may provide new pharmacological means of attenuating RSV infection.
Acknowledgments
H.S.-J.-V. is the recipient of the Colciencias-Fulbright-LASPAU Scholarship and an American Heart Association grant. This study was supported by the funds of a VA Merit Review Award, by NIH support grant 1 RO1 HL071101-01A2 to S.S.M., and by the Joy McCann Culverhouse Endowment to the University of South Florida Division of Allergy and Immunology Airway Disease Research Center.
REFERENCES
- 1.Bitko, V., and S. Barik. 1998. Persistent activation of RelA by respiratory syncytial virus involves protein kinase C, underphosphorylated IκBβ, and sequestration of protein phosphatase 2A by the viral phosphoprotein. J. Virol. 72:5610-5618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bitko, V., A. Velazquez, L. Yang, Y.-C. Yang, and S. Barik. 1997. Transcriptional induction of multiple cytokines by human respiratory syncytial virus requires activation of NF-κB and is inhibited by sodium salicylate and aspirin. Virology 232:369-378. [DOI] [PubMed] [Google Scholar]
- 3.Brown, G., J. Aitken, H. W. Rixon, and R. J. Sugrue. 2002. Caveolin-1 is incorporated into mature respiratory syncytial virus particles during virus assembly on the surface of virus-infected cells. J. Gen. Virol. 83:611-621. [DOI] [PubMed] [Google Scholar]
- 4.Brown, G., H. W. Rixon, and R. J. Sugrue. 2002. Respiratory syncytial virus assembly occurs in GM1-rich regions of the host-cell membrane and alters the cellular distribution of tyrosine phosphorylated caveolin-1. J. Gen. Virol. 83:1841-1850. [DOI] [PubMed] [Google Scholar]
- 5.Budge, P. J., Y. Li, J. A. Beeler, and B. S. Graham. 2004. RhoA-derived peptide dimers share mechanistic properties with other polyanionic inhibitors of respiratory syncytial virus (RSV), including disruption of viral attachment and dependence on RSV G. J. Virol. 78:5015-5022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carpenter, L. R., J. N. Moy, and K. A. Roebuck. 2002. Respiratory syncytial virus and TNF alpha induction of chemokine gene expression involves differential activation of RelA and NF-kappa B1. BMC Infect. Dis. 2:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chao, M. D., I. S. Chen, and J. T. Cheng. 1998. Inhibition of protein kinase C translocation from cytosol to membrane by chelerythrine. Planta Med. 64:662-663. [DOI] [PubMed] [Google Scholar]
- 8.Chen, W., M. M. Monick, A. B. Carter, and G. W. Hunninghake. 2000. Activation of ERK2 by respiratory syncytial virus in A549 cells is linked to the production of interleukin 8. Exp. Lung Res. 26:13-26. [DOI] [PubMed] [Google Scholar]
- 9.Constantinescu, S. N., C. D. Cernescu, and L. M. Popescu. 1991. Effects of protein kinase C inhibitors on viral entry and infectivity. FEBS Lett. 292:31-33. [DOI] [PubMed] [Google Scholar]
- 10.Dangoria, N. S., W. C. Breau, H. A. Anderson, D. M. Cishek, and L. C. Norkin. 1996. Extracellular simian virus 40 induces an ERK/MAP kinase-independent signalling pathway that activates primary response genes and promotes virus entry. J. Gen. Virol. 77:2173-2182. [DOI] [PubMed] [Google Scholar]
- 11.Duncan, M. J., J. S. Shin, and S. N. Abraham. 2002. Microbial entry through caveolae: variations on a theme. Cell. Microbiol. 4:783-791. [DOI] [PubMed] [Google Scholar]
- 12.Eichholtz, T., D. B. de Bont, J. de Widt, R. M. Liskamp, and H. L. Ploegh. 1993. A myristoylated pseudosubstrate peptide, a novel protein kinase C inhibitor. J. Biol. Chem. 268:1982-1986. [PubMed] [Google Scholar]
- 13.Gower, T. L., M. E. Peeples, P. L. Collins, and B. S. Graham. 2001. RhoA is activated during respiratory syncytial virus infection. Virology 283:188-196. [DOI] [PubMed] [Google Scholar]
- 14.Hallak, L. K., D. Spillmann, P. L. Collins, and M. E. Peeples. 2000. Glycosaminoglycan sulfation requirements for respiratory syncytial virus infection. J. Virol. 74:10508-10513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Harris, T. E., S. J. Persaud, T. Saermark, and P. M. Jones. 1996. A myristoylated pseudosubstrate peptide inhibitor of protein kinase C: effects on glucose- and carbachol-induced insulin secretion. Mol. Cell. Endocrinol. 121:133-141. [DOI] [PubMed] [Google Scholar]
- 16.Herbert, J. M., J. M. Augereau, J. Gleye, and J. P. Maffrand. 1990. Chelerythrine is a potent and specific inhibitor of protein kinase C. Biochem. Biophys. Res. Commun. 172:993-999. [DOI] [PubMed] [Google Scholar]
- 17.Kobayashi, E., H. Nakano, M. Morimoto, and T. Tamaoki. 1989. Calphostin C (UCN-1028C), a novel microbial compound, is a highly potent and specific inhibitor of protein kinase C. Biochem. Biophys. Res. Commun. 159:548-553. [DOI] [PubMed] [Google Scholar]
- 18.Kong, X., H. San Juan, A. Behera, M. E. Peeples, J. Wu, R. F. Lockey, and S. S. Mohapatra. 2004. ERK-1/2 activity is required for efficient RSV infection. FEBS Lett. 559:33-38. [DOI] [PubMed] [Google Scholar]
- 19.Kong, X., H. San Juan, M. Kumar, A. K. Behera, A. Mohapatra, G. R. Hellermann, S. Mane, R. F. Lockey, and S. S. Mohapatra. 2003. Respiratory syncytial virus infection activates STAT signaling in human epithelial cells. Biochem. Biophys. Res. Commun. 306:616-622. [DOI] [PubMed] [Google Scholar]
- 20.Krunkosky, T. M., B. M. Fischer, N. J. Akley, and K. B. Adler. 1996. Tumor necrosis factor alpha (TNF alpha)-induced ICAM-1 surface expression in airway epithelial cells in vitro: possible signal transduction mechanisms. Ann. N. Y. Acad. Sci. 796:30-37. [DOI] [PubMed] [Google Scholar]
- 21.Liebmann, C. 2001. Regulation of MAP kinase activity by peptide receptor signalling pathway: paradigms of multiplicity. Cell. Signal. 13:777-785. [DOI] [PubMed] [Google Scholar]
- 22.Lu, Z., D. Liu, A. Hornia, W. Devonish, M. Pagano, and D. A. Foster. 1998. Activation of protein kinase C triggers its ubiquitination and degradation. Mol. Cell. Biol. 18:839-845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Malladi, V., B. Shankar, P. H. Williams, and A. Balakrishnan. 2004. Enteropathogenic Escherichia coli outer membrane proteins induce changes in cadherin junctions of Caco-2 cells through activation of PKCα. Microbes Infect. 6:38-50. [DOI] [PubMed] [Google Scholar]
- 24.Martinez, I., and J. A. Melero. 2000. Binding of human respiratory syncytial virus to cells: implication of sulfated cell surface proteoglycans. J. Gen. Virol. 81:2715-2722. [DOI] [PubMed] [Google Scholar]
- 25.McCurdy, L. H., and B. S. Graham. 2003. Role of plasma membrane lipid microdomains in respiratory syncytial virus filament formation. J. Virol. 77:1747-1756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Meier, O., K. Boucke, S. V. Hammer, S. Keller, R. P. Stidwill, S. Hemmi, and U. F. Greber. 2002. Adenovirus triggers macropinocytosis and endosomal leakage together with its clathrin-mediated uptake. J. Cell Biol. 158:1119-1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mineo, C., and R. G. Anderson. 2001. Potocytosis. Robert Feulgen lecture. Histochem. Cell Biol. 116:109-118. [DOI] [PubMed] [Google Scholar]
- 28.Mochly-Rosen, D., and A. S. Gordon. 1998. Anchoring proteins for protein kinase C: a means for isozyme selectivity. FASEB J. 12:35-42. [PubMed] [Google Scholar]
- 29.Monick, M., J. Staber, K. Thomas, and G. Hunninghake. 2001. Respiratory syncytial virus infection results in activation of multiple protein kinase C isoforms leading to activation of mitogen-activated protein kinase. J. Immunol. 166:2681-2687. [DOI] [PubMed] [Google Scholar]
- 30.Nakano, M. Y., K. Boucke, M. Suomalainen, R. P. Stidwill, and U. F. Greber. 2000. The first step of adenovirus type 2 disassembly occurs at the cell surface, independently of endocytosis and escape to the cytosol. J. Virol. 74:7085-7095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Naranatt, P. P., S. M. Akula, C. A. Zien, H. H. Krishnan, and B. Chandran. 2003. Kaposi's sarcoma-associated herpesvirus induces the phosphatidylinositol 3-kinase-PKC-ζ-MEK-ERK signaling pathway in target cells early during infection: implications for infectivity. J. Virol. 77:1524-1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Parekh, D. B., W. Ziegler, and P. J. Parker. 2000. Multiple pathways control protein kinase C phosphorylation. EMBO J. 19:496-503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pelkmans, L., D. Puntener, and A. Helenius. 2002. Local actin polymerization and dynamin recruitment in SV40-induced internalization of caveolae. Science 296:535-539. [DOI] [PubMed] [Google Scholar]
- 34.Prevostel, C., V. Alice, D. Joubert, and P. J. Parker. 2000. Protein kinase Cα actively downregulates through caveolae-dependent traffic to an endosomal compartment. J. Cell Sci. 113:2575-2584. [DOI] [PubMed] [Google Scholar]
- 35.Razinkov, V., A. Gazumyan, A. Nikitenko, G. Ellestad, and G. Krishnamurthy. 2001. RFI-641 inhibits entry of respiratory syncytial virus via interactions with fusion protein. Chem. Biol. 8:645-659. [DOI] [PubMed] [Google Scholar]
- 36.Root, C. N., E. G. Wills, L. L. McNair, and G. R. Whittaker. 2000. Entry of influenza viruses into cells is inhibited by a highly specific protein kinase C inhibitor. J. Gen. Virol. 81:2697-2705. [DOI] [PubMed] [Google Scholar]
- 37.Rosales, O. R., C. Isales, M. Nathanson, and B. E. Sumpio. 1992. Immunocytochemical expression and localization of protein kinase C in bovine aortic endothelial cells. Biochem. Biophys. Res. Commun. 189:40-46. [DOI] [PubMed] [Google Scholar]
- 38.Sieczkarski, S. B., H. A. Brown, and G. R. Whittaker. 2003. Role of protein kinase C βII in influenza virus entry via late endosomes. J. Virol. 77:460-469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Simoes, E. A. 1999. Respiratory syncytial virus infection. Lancet 354:847-852. [DOI] [PubMed] [Google Scholar]
- 40.Soh, J. W., E. H. Lee, R. Prywes, and I. B. Weinstein. 1999. Novel roles of specific isoforms of protein kinase C in activation of the c-fos serum response element. Mol. Cell. Biol. 19:1313-1324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sukumaran, S. K., and N. V. Prasadarao. 2002. Regulation of protein kinase C in Escherichia coli K1 invasion of human brain microvascular endothelial cells. J. Biol. Chem. 277:12253-12262. [DOI] [PubMed] [Google Scholar]
- 42.Techaarpornkul, S., N. Barretto, and M. E. Peeples. 2001. Functional analysis of recombinant respiratory syncytial virus deletion mutants lacking the small hydrophobic and/or attachment glycoprotein gene. J. Virol. 75:6825-6834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Urbach, V., N. Helix, B. Renaudon, and B. J. Harvey. 2002. Cellular mechanisms for apical ATP effects on intracellular pH in human bronchial epithelium. J. Physiol. 543:13-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Vanderplasschen, A., M. Hollinshead, and G. L. Smith. 1998. Intracellular and extracellular vaccinia virions enter cells by different mechanisms. J. Gen. Virol. 79:877-887. [DOI] [PubMed] [Google Scholar]
- 45.Vanderplasschen, A., and G. L. Smith. 1997. A novel virus binding assay using confocal microscopy: demonstration that the intracellular and extracellular vaccinia virions bind to different cellular receptors. J. Virol. 71:4032-4041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Vanderplasschen, A., and G. L. Smith. 1999. Using confocal microscopy to study virus binding and entry into cells. Methods Enzymol. 307:591-607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Villalba, M., K. Bi, F. Rodriguez, Y. Tanaka, S. Schoenberger, and A. Altman. 2001. Vav1/Rac-dependent actin cytoskeleton reorganization is required for lipid raft clustering in T cells. J. Cell Biol. 155:331-338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wagner, S., C. Harteneck, F. Hucho, and K. Buchner. 2000. Analysis of the subcellular distribution of protein kinase Cα using PKC-GFP fusion proteins. Exp. Cell Res. 258:204-214. [DOI] [PubMed] [Google Scholar]
- 49.Wanaski, S. P., B. K. Ng, and M. Glaser. 2003. Caveolin scaffolding region and the membrane binding region of SRC form lateral membrane domains. Biochemistry 42:42-56. [DOI] [PubMed] [Google Scholar]
- 50.Wang, Y., A. Sostman, R. Roman, S. Stribling, S. Vigna, Y. Hannun, J. Raymond, and J. G. Fitz. 1996. Metabolic stress opens K+ channels in hepatoma cells through a Ca2+- and protein kinase cα-dependent mechanism. J. Biol. Chem. 271:18107-18113. [DOI] [PubMed] [Google Scholar]
- 51.Werling, D., J. C. Hope, P. Chaplin, R. A. Collins, G. Taylor, and C. J. Howard. 1999. Involvement of caveolae in the uptake of respiratory syncytial virus antigen by dendritic cells. J. Leukoc. Biol. 66:50-58. [DOI] [PubMed] [Google Scholar]
- 52.Wyatt, T. A., H. Ito, T. J. Veys, and J. R. Spurzem. 1997. Stimulation of protein kinase C activity by tumor necrosis factor-α in bovine bronchial epithelial cells. Am. J. Physiol. 273:L1007-L1012. [DOI] [PubMed] [Google Scholar]