Abstract
Purpose
The aim of this study was to evaluate the prevalence of comorbid bipolar disorder (BD) among migraineurs and the impact of migraine–BD comorbidity on disease characteristics.
Patients and methods
A total of 120 adult patients diagnosed with migraine at a single tertiary care center were included in this cross-sectional study. Data on sociodemographic and migraine-related characteristics, family history of psychiatric diseases, comorbid psychiatric diseases, and first-episode characteristics were recorded. Mood Disorders Diagnosis and Patient Registration Form (SCIP-TURK), Mood Disorder Questionnaire (MDQ), and Hypomania Checklist-32-Revised (HCL-32-R) were applied to all patients by experienced clinicians, and clinical diagnoses were confirmed using Structured Clinical Interview for DSM-IV Axis I Disorders (SCID-I). Migraine Disability Assessment Scale (MIDAS) was used to evaluate the headache-related disability. Study parameters were compared between migraineurs with and without comorbid BD.
Results
The diagnosis of comorbid BD was confirmed in 19.2% of migraineurs. A significantly higher percentage of patients with comorbid BD than those without comorbid BD had family history of BD (39.1% vs 6.2%, P<0.001), suicide attempt (30.4% vs 5.2%, P<0.001), and physical abuse (52.2% vs 26.8%, P=0.019). MIDAS scores were significantly higher (50.6 [43.2] vs 33.8 [42.7], P=0.0422) in migraineurs with comorbid BD than in those without comorbid BD. Multivariate logistic regression model revealed that a positive family history of type I BD (odds ratio [OR], 14.42; 95% confidence interval [CI], 2.94–70.73; P=0.001) and MIDAS scores >30 (OR, 3.69; 95% CI, 1.12–12.19; P=0.032) were associated with 14.42 times and 3.69 times increased likelihood of BD, respectively.
Conclusion
Our findings revealed comorbid BD in a remarkable percentage of migraineurs and a higher likelihood of having BD in case of a positive family history of type I BD and MIDAS scores >30. Comorbid BD was associated with a higher rate for a family history of BD, suicide attempt, and childhood physical abuse as well as aggravated migraine-related disability among migraineurs. Migraineurs with and without comorbid BD showed similar sociodemographic and migraine disease characteristics as well as similar high rates for comorbid anxiety and first-episode depression.
Keywords: migraine, bipolar disorder, comorbidity, suicide attempt, MIDAS, depression
Introduction
Migraine is a common episodic incapacitating neurological disorder and a significant public health problem characterized by unilateral throbbing headaches accompanied often with nausea, phonophobia, and/or photophobia.1–3 Migraine is frequently associated with several psychiatric comorbidities, while timely recognition of these comorbidities is important given their adverse effects on migraine course along with the similar impact of poorly treated migraine on the outcome of comorbid psychiatric illness.4–7
Bipolar disorder (BD) is a chronic mood disorder that affects 2%–4% of the general population and is characterized by cyclic occurrence of elevated (manic: type I or hypomanic: type II) and depressed mood states.8,9 While most of the previous studies focused on the comorbidity of migraine with depression or anxiety disorders, recent epidemiologic and clinical evidence indicates the possibility of migraine–BD comorbidity with threefold increase in migraine prevalence shown among patients with BDs.10–14
Migraine is suggested to have an impact on the clinical course of BD, leading to earlier onset of BD, higher prevalence of comorbid anxiety disorders, and atypical symptoms of depression.10,12,15,16 However, limited data are available on the prevalence of BD among migraineurs and the impact of BD on the clinical course of migraine disease.14,17,18
The present study was therefore designed to evaluate the prevalence of comorbid BD among migraineurs and the impact of migraine–BD comorbidity on disease characteristics.
Patients and methods
Study population
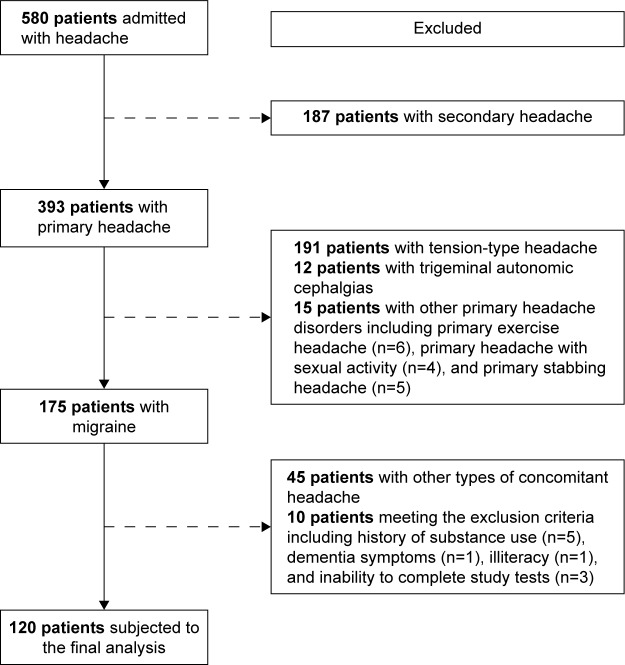
A total of 120 adult patients diagnosed with migraine based on the International Classification of Headache Disorders, third revision (ICHD-3; beta) diagnostic criteria (2) upon their admission to the Neurology and Headache Outpatient Clinic at Erenköy Mental and Neurological Diseases Training and Research Hospital, Istanbul were included in this cross-sectional study. Being illiterate; presence of any neurologic disorders other than headache and any disorder that may cause secondary headache including trauma, infection, cerebrovascular disease, glaucoma, sinusitis, tumor, and craniocervical dystonia; past history of sequela from a neurologic disease or head trauma; and presence of dementia or other organic mental disorders, mental retardation, and substance dependence or substance abuse were the exclusion criteria. As shown in the study flowchart (Figure 1), of 580 patients admitted to the outpatient clinic with a complaint of headache between January 2015 and August 2015, 393 patients had primary headache and 175 out of 393 patients had migraine. Of 175 patients with migraine enrolled into the study, 45 patients were excluded due to comorbidity of other headache types and 10 patients were excluded since they met the exclusion criteria, including history of substance use (n=5), dementia symptoms (n=1), illiteracy (n=1), and inability to complete study tests (n=3). Accordingly, 120 patients with migraine were subjected to the final analysis.
Figure 1.
Study flowchart.
Written informed consent was obtained from each subject following a detailed explanation of the objectives and protocol of the study, which was conducted in accordance with the ethical principles stated in the “Declaration of Helsinki” and approved by the ethics committee of Erenköy Mental and Neurological Diseases Training and Research Hospital.
Assessments
Data on sociodemographic characteristics (age, gender, marital status, educational level, employment, socioeconomic status), caffeine intake, smoking and alcohol consumption, migraine characteristics (disease duration [months], episode frequency, episode duration [hours], severity score, aura, prodromal symptoms, change in side, triggering factors, accompaniments), family history of psychiatric diseases, comorbid psychiatric diseases, and first-episode characteristics were recorded in each patient. Mood Disorders Diagnosis and Patient Registration Form (SCIP-TURK), Mood Disorder Questionnaire (MDQ), and Hypomania Checklist-32-Revised (HCL-32-R) were applied to all patients by experienced physicians, and clinical diagnoses were confirmed by the same psychiatry specialist in all patients via Structured Clinical Interview for DSM-IV Axis I Disorders (SCID-I). Detailed information about characteristics of the first episode was obtained retrospectively during assessment with questionnaires and clinical interview in each patient. Migraine Disability Assessment Scale (MIDAS) was used to evaluate the headache-related disability. Study parameters were compared between migraineurs with and without comorbid BD. In addition, estimators of having bipolar disease were analyzed via univariate and mutually adjusted multivariate logistic regression analyses.
SCID-I
The SCID-I is a semi-structured interview developed by First et al19 in 1997, comprising six structured modules to diagnose both current and lifetime Axis-I psychiatric disorders based on the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (DSM-IV) criteria along with the duration of disorder, family history, and the presence of physical diseases. The Turkish adaptation and reliability of SCID-I was performed by Ozkurkcugil et al20 in 1999.
Diagnostic and Monitoring Form for Mood Disorders (SCIP-TURK)
SCIP-TURK is a 111-item semi-structured scale developed by Ozerdem et al21 in 2004 and consists of four modules that provide data on patient characteristics (sociodemographic features, medical background, family history, smoking, alcohol, and drug use) and characteristics of BD (age at onset and the type and severity of the first episode [postpartum onset, seasonality, or depression subtype], the number and duration of episodes, suicide attempts, social support and stories of childhood trauma or abuse, and the treatments).
MDQ
MDQ is a self-report instrument developed by Hirschfeld et al22 in 2000 to screen lifetime history of manic and/or hypomanic symptoms. MDQ includes 13 yes/no questions mainly derived from DSM-IV in the first sub-item, and the second sub-item asks whether several of any reported manic or hypomanic symptoms or behaviors were experienced during the same period of time or associated with functional impairment related. The total score is the sum of overall scores for each item marked as yes (1 point) or no (0 point). The Turkish version of the MDQ has been validated by Konuk et al23 in 2007, and an optimal cut-off point was reported to be a score of ≥7 as associated with best scores of sensitivity (0.64) and specificity (0.77).
HCL-32-R
HCL-32 is an eight-item self-report instrument to screen lifetime history of manic and/or hypomanic symptoms and to identify BD-sensitive individuals. Participants are requested to focus on “the high periods” and to indicate whether specific thoughts or emotions were present during this state. HCL-32 assesses symptoms in greater detail than DSM-IV-based structured clinical interview, including questions on general well-being (first two items) and 32 yes/no questions on manic and/or hypomanic symptoms (third item), effect of symptoms on life (fourth item) and other people (fifth item), duration of symptoms (sixth item), and the presence of (seventh item) and days per year (eighth item) with such mood period. The optimal cutoff point for HCL-32 was reported to be a score of ≥14 for the original form developed by Angst et al24 in 2005. The Turkish version of HCL-32 was adapted by Vahip et al25 in 2016, and the optimal cutoff point was reported to be a score of 14/15.
MIDAS
The MIDAS, a five-question tool to quantitatively evaluate the headache-related disability in terms of the number of days in the past 3 months and activity limitations due to migraine, was developed by Stewart et al26 in 2001 and validated and checked for reliability for Turkish by Ertas et al27 in 2004. The score obtained is graded based on the number of days with >50% loss in productivity as follows: grade I (0–5 days), indicative of little or no disability; grade II (6–10 days), mild disability; grade III (11–20 days), moderate disability; and grade IV (>21 days), severe disability.
Statistical analysis
Statistical analysis was performed using IBM SPSS Statistics (IBM Corp. Released 2012, IBM SPSS Statistics for Windows, Version 20.0; IBM Corporation, Armonk, NY, USA). Chi-square (χ2) test and Fisher’s exact or Fisher–Holton–Freeman test were used for the comparison of categorical data, while Mann–Whitney U test was used for the analysis of numerical data. The estimators of having bipolar disease were evaluated by using univariate logistic regression; the ones with significant effect were evaluated using mutually adjusted multivariate logistic regression. Data were expressed as median (interquartile range [IQR]) and percent (%) where appropriate. P<0.05 was considered statistically significant.
Results
Patient characteristics
The diagnosis of comorbid BD was confirmed in 23 patients (19.2%; in 18 [78.3%] females and 5 [21.7%] males). No significant difference was noted in migraine patients with (median [IQR] age: 38.0 [15.0] years; 78.3% were females) and without (median [IQR] age: 41.0 [15.0] years; 84.5% were females) comorbid BD in terms of demographic characteristics. With no significant difference between patients with versus without comorbid BD, most of the migraineurs were university graduates (52.2% and 43.3%, respectively), employed (60.9% and 50.5%, respectively), and married (52.2% and 67.0%, respectively) subjects with moderate-to-high socioeconomic status (69.6% and 60.8%). The rates for smoking (60.9% vs 49.5%) and alcohol consumption (60.9% vs 46.4%) were also similar among migraine patients with respect to the presence of comorbid BD (Table 1).
Table 1.
Characteristics of migraineurs with and without comorbid BD
| Patient characteristics | Migraineurs
|
P-value | |
|---|---|---|---|
| With BD (n=23) | Without BD (n=97) | ||
| Age (years), median (IQR) | 38.0 (15.0) | 41.0 (15.0) | 0.377a |
| Gender (female), n (%) | 18 (78.3) | 82 (84.5) | 0.534b |
| Educational status, n (%) | |||
| Primary school | 7 (30.4) | 28 (28.9) | 0.809c |
| Secondary school | 4 (17.4) | 27 (27.8) | |
| University | 12 (52.2) | 42 (43.3) | |
| Employment, n (%) | |||
| Employed | 14 (60.9) | 49 (50.5) | 0.371c |
| Unemployed | 9 (39.1) | 48 (49.5) | |
| Marital status, n (%) | |||
| Married | 12 (52.2) | 65 (67.0) | 0.182c |
| Single/divorced/widowed | 11 (47.8) | 32 (33.0) | |
| Socioeconomic status, n (%) | |||
| Low–moderate | 7 (30.4) | 38 (39.2) | 0.436c |
| Moderate–high | 16 (69.6) | 59 (60.8) | |
| Caffeine intake, n (%) | 7 (30.4) | 12 (12.4) | 0.052b |
| Smoking, n (%) | 14 (60.9) | 48 (49.5) | 0.326c |
| Alcohol consumption, n (%) | 14 (60.9) | 45 (46.4) | 0.212c |
Notes:
Mann–Whitney U test.
Fisher’s exact test.
χ2 test.
Abbreviations: BD, bipolar disorder; IQR, interquartile range.
Migraine characteristics
No significant difference was noted among migraineurs with and without comorbid BD in terms of median disease duration (180.0 vs 132.0 months), latest (4.0 vs 3.0 episodes) and previous month (5.0 vs 4.0 episodes) episode frequency, episode duration (10.0 vs 14.0 hours) and severity score (8.0 for each; Table 2).
Table 2.
Migraine characteristics in migraineurs with and without comorbid BD
| Migraine characteristics | Migraineurs
|
P-valuea | |
|---|---|---|---|
| With BD (n=23)
|
Without BD (n=97)
|
||
| Median (IQR) |
Median (IQR) |
||
| Disease duration (months) | 180.0 (156.0) | 132.0 (168.0) | 0.669 |
| Episode frequency | |||
| Latest | 4.0 (5.0) | 3.0 (4.0) | 0.973 |
| Previous month | 5.0 (7.0) | 4.0 (5.0) | 0.149 |
| Episode duration (hours) | 10.0 (18.0) | 14.0 (44.0) | 0.486 |
| Severity score | 8.0 (2.0) | 8.0 (2.0) | 0.326 |
| n (%) | n (%) | P-valueb | |
| Migraine with aura | 17 (73.9) | 53 (54.6) | 0.092 |
| Aura-related clinical difference | 11 (47.8) | 37 (38.1) | 0.394 |
| Type of aura | |||
| Visual | 15 (65.2) | 43 (44.3) | 0.072 |
| Auditory | 5 (21.7) | 13 (13.4) | 0.335 |
| Sensory | 1 (4.3) | 19 (19.6) | 0.118 |
| Motor | 4 (17.4) | 12 (12.4) | 0.506 |
| Other | 1 (4.3) | 1 (1.0) | 0.348 |
| Prodromal symptoms | |||
| Any | 18 (78.3) | 59 (60.8) | 0.117 |
| Psychic | 12 (52.2) | 42 (43.3) | 0.442 |
| Motor | 1 (4.3) | 2 (2.1) | 0.475 |
| Gastrointestinal | 6 (26.1) | 18 (18.6) | 0.399 |
| Othersc | 2 (8.7) | 16 (16.5) | 0.520 |
| Change in side | 16 (69.6) | 70 (72.2) | 0.804 |
| Triggering factors | |||
| Emotional stress | 23 (100.0) | 91 (93.8) | 0.594 |
| Physiologic tiredness | 19 (82.6) | 64 (66.0) | 0.121 |
| Seasonal alteration | 19 (82.6) | 75 (77.3) | 0.780 |
| Hunger | 18 (78.3) | 87 (89.7) | 0.162 |
| Menstruation | 17 (73.9) | 61 (62.9) | 0.319 |
| Alcohol | 11 (47.8) | 23 (23.7) | 0.021 |
| Posture changes | 5 (21.7) | 16 (16.5) | 0.549 |
| Others | 12 (52.2) | 55 (56.7) | 0.694 |
| Accompaniments | |||
| Nausea | 22 (95.7) | 86 (88.7) | 0.458 |
| Vomiting | 16 (69.6) | 62 (63.9) | 0.610 |
| Phonophobia | 21 (91.3) | 94 (96.9) | 0.244 |
| Photophobia | 23 (100.0) | 91 (93.8) | 0.594 |
| Dizziness | 21 (91.3) | 86 (88.7) | 1.000 |
| Insomnia | 21 (91.3) | 81 (83.5) | 0.520 |
| Vertigo | 9 (39.1) | 32 (33.0) | 0.577 |
| Osmophobia | 17 (73.9) | 48 (49.5) | 0.035 |
| Allodynia | 19 (82.6) | 69 (71.1) | 0.263 |
| Others | 1 (4.3) | 4 (4.1) | 1.000 |
Notes:
Mann–Whitney U test.
χ2 test and Fisher’s exact test.
Yawning, chills, polyuria, and somnolence. Values in bold indicate statistical significance (P<0.05).
Abbreviations: BD, bipolar disorder; IQR, interquartile range.
No significant difference was noted in the rate of migraine with aura (73.9% vs 54.6%), presence of aura-related clinical difference (47.8% vs 38.1%), type of aura (visual in 65.2% vs 44.3%), presence (78.3% vs 60.8%) and type (psychic in 52.2% vs 43.3%) of prodromal symptoms, and change in side (69.6% vs 72.2%) with respect to comorbid BD (Table 2).
Emotional stress (100.0% and 93.8%) was the most common triggering factor, while photophobia (100.0% and 93.8%), phonophobia (91.3% and 96.9%), and nausea (95.7% and 88.7%) were the most commonly observed accompaniments similarly in patients with and without BD. Apart from significantly higher rates of alcohol consumption (47.8% vs 23.7%, P=0.021) as a trigger and osmophobia (73.9% vs 49.5%, P=0.035) as an accompaniment in patients with comorbid BD than in those without comorbid BD, the two groups were similar in terms of migraine triggers and accompaniments (Table 2).
Past history and comorbid psychiatric diseases
Migraineurs with versus without comorbid BD were similar in terms of family history of psychiatric disease in first-degree relatives (73.9% vs 74.2%), while a significantly higher percentage of patients with comorbid BD than those without co-morbid BD had a family history of BD (39.1% vs 6.2%, P<0.001), type I BD (34.8% vs 4.1%, P<0.001) in particular (Table 3).
Table 3.
Psychiatric diseases in migraineurs with and without comorbid BD
| Psychiatric diseases | Migraineurs
|
P-value | |
|---|---|---|---|
| With BD (n=23) | Without BD (n=97) | ||
|
| |||
| n (%) | n (%) | ||
| Family history of psychiatric disease | |||
| Any relative | 18 (78.3) | 81 (83.5) | 0.549 |
| First-degree relatives | 17 (73.9) | 72 (74.2) | 0.975 |
| BD | 9 (39.1) | 6 (6.2) | <0.001 |
| Type I | 8 (34.8) | 4 (4.1) | <0.001 |
| Type II | 1 (4.3) | 2 (2.1) | 0.475 |
| Unipolar depression | 13 (56.5) | 62 (63.9) | 0.510 |
| Suicide attempt | 2 (8.7) | 17 (17.5) | 0.524 |
| Comorbid psychiatric disorder | |||
| Any | 21 (91.3) | 79 (81.4) | 0.358 |
| Cyclothymia | 0 (0.0) | 0 (0.0) | 1.000 |
| Dysthymia | 0 (0.0) | 4 (4.1) | 1.000 |
| Schizophrenia | 0 (0.0) | 0 (0.0) | 1.000 |
| Panic disorder | 4 (17.4) | 12 (12.4) | 0.506 |
| Alcohol use disorder | 2 (8.7) | 3 (3.1) | 0.244 |
| Suicide attempt | 7 (30.4) | 5 (5.2) | 0.002 |
| Eating disorder | 1 (4.3) | 0 (0.0) | 0.192 |
| Social phobia | 2 (8.7) | 10 (10.3) | 1.000 |
| Obsessive compulsive disorder | 4 (17.4) | 7 (7.2) | 0.219 |
| Hypochondriasis | 1 (4.3) | 3 (3.1) | 0.578 |
| Posttraumatic stress disorder | 2 (8.7) | 6 (6.2) | 0.648 |
| Dissociative disorder | 4 (17.4) | 12 (12.4) | 0.506 |
| Specific phobia | 5 (21.7) | 37 (38.1) | 0.138 |
| Generalized anxiety disorder | 0 (0.0) | 13 (13.4) | 0.071 |
| Somatoform disorder | 0 (0.0) | 8 (8.2) | 0.351 |
| Anxiety disorder | 10 (43.5) | 61 (62.9) | 0.089 |
| History of childhood abuse | |||
| Any | 17 (73.9) | 69 (71.1) | 0.790 |
| Sexual | 4 (17.4) | 20 (20.6) | 1.000 |
| Physical | 12 (52.2) | 26 (26.8) | 0.019 |
| Emotional | 13 (56.5) | 64 (66.0) | 0.395 |
| Legal | 5 (21.7) | 13 (13.4) | 0.335 |
Notes: χ2 test–Fisher’s exact test. Values in bold indicate statistical significance (P<0.05).
Abbreviation: BD, bipolar disorder.
Comorbid psychiatric diseases were evident in majority of migraineurs with no significant difference between patients with and without comorbid BD (91.3% vs 81.4%). Anxiety disorder was the most common comorbid psychiatric disorder similarly in migraineurs with and without BD (43.5% and 62.9%), while suicide attempt (30.4% vs 5.2%, P=0.002) and history of physical abuse (52.2% vs 26.8%, P=0.019) were significantly more common among migraineurs with comorbid BD than in those without comorbid BD (Table 3).
First-episode characteristics
Apart from a higher prevalence of associated psychotic characteristics (21.7% vs 4.3%, P=0.021) and higher likelihood of spontaneous relief (34.8% vs 31.4%, P=0.022) in patients with comorbid BD than in those without comorbid BD, no significant difference was noted in first-episode characteristics with respect to comorbid BD among migraineurs. Similarly, in migraineurs with and without comorbid BD, first-episode depression (91.4% and 72.2%, respectively), presence of a life event (87.0% and 94.3%, respectively), moderate episode severity (52.2% and 51.4%, respectively), and management of episode via use of antidepressants (47.8% and 67.2%, respectively) were the prominent first-episode characteristics (Table 4).
Table 4.
First-episode characteristics in migraineurs with and without comorbid BD
| Characteristics | Migraineurs
|
P-value | |
|---|---|---|---|
| With BD (n=23) | Without BD (n=97) | ||
|
| |||
| n (%) | n (%) | ||
| First episode | |||
| Mania | 1 (4.3) | 0 (0.0) | 0.059 |
| Mixed | 1 (4.3) | 0 (0.0) | |
| Depression | 21 (91.4) | 70 (72.1) | |
| Presence of a life event | 20 (87.0) | 66 (94.3) | 0.358 |
| Severity of episode | |||
| Mild | 2 (8.7) | 9 (12.9) | 0.944 |
| Moderate | 12 (52.2) | 36 (51.4) | |
| Severe | 9 (39.1) | 25 (35.7) | |
| Associated characteristics | |||
| Postpartum | 2 (8.7) | 4 (5.7) | 0.635 |
| Catatonic | 0 (0.0) | 2 (2.9) | 1.000 |
| Melancholic | 3 (13.0) | 10 (14.3) | 1.000 |
| Atypical | 4 (17.4) | 11 (15.7) | 1.000 |
| Psychotic | 5 (21.7) | 3 (4.3) | 0.021 |
| Relieved | |||
| Any type | 23 (100.0) | 70 (100.0) | |
| Spontaneously | 8 (34.8) | 22 (31.4) | 0.022 |
| With mood stabilizer | 2 (8.7) | 1 (1.4) | |
| With antidepressant | 11 (47.8) | 47 (67.2) | |
| With combined therapy | 2 (8.7) | 0 (0.0) | |
Notes: χ2 test–Fisher’s exact test–Fisher–Holton–Freeman test. Values in bold indicate statistical significance (P<0.05).
Abbreviation: BD, bipolar disorder.
MDQ, HSL-32-R, and MIDAS scale scores
MDQ and HSL-32-R confirmed the likelihood of comorbid BD in 91.3% and 100.0% of patients with BD, while albeit in a significantly lower percentage of patients, they also revealed positive results in 2.1% (P<0.001) and 70.1% (P=0.003) of patients without BD, respectively. Median (IQR) MIDAS scores were significantly higher (37.0 [61.0] vs 19.0 [28.0], P=0.042) in migraineurs with comorbid BD than in those without comorbid BD (Table 5).
Table 5.
MDQ, HSL-32-R, and MIDAS scores in migraineurs with and without comorbid BD
| Scores | Migraineurs
|
P-value | |
|---|---|---|---|
| With BD (n=23) | Without BD (n=97) | ||
| Likelihood of BD, n (%) | |||
| By MDQ | 21 (91.3) | 2 (2.1) | <0.001a |
| By HSL-32-R | 23 (100.0) | 68 (70.1) | 0.003a |
| MIDAS, median (IQR) | 37.0 (61.0) | 19.0 (28.0) | 0.042b |
Notes:
χ2 test–Fisher’s exact test.
Mann–Whitney U test.
Abbreviations: MDQ, Mood Disorder Questionnaire; HSL-32-R, Hypomania Checklist-32-Revised; MIDAS, Migraine Disability Assessment Scale; BD, bipolar disorder; IQR, interquartile range.
Univariate and multivariate logistic regression analyses for estimators of having bipolar disease
Univariate analysis revealed caffeine intake (P=0.039), alcohol as trigger (P=0.025), osmophobia as accompaniment (P=0.025), family history of type I BD (P<0.001), previous suicide attempt (P=0.001), physical abuse in childhood (P=0.022), and MIDAS scores >30 (P=0.014) to be significantly associated with increased likelihood of having BD among migraineurs (Table 6). Mutually adjusted multivariate logistic regression model revealed that a positive family history of type I BD (odds ratio [OR], 14.42; 95% confidence interval [CI], 2.94–70.73; P=0.001) and MIDAS scores >30 (OR, 3.69; 95% CI, 1.12 to 12.19; P=0.032) were significant estimators of having bipolar diseases among migraineurs, leading to 14.42 times and 3.69 times increased likelihood of BD, respectively (Table 6).
Table 6.
Univariate and mutually adjusted multivariate logistic regression analyses for estimators of having BD
| Variables | Univariate logistic regressiona
|
Multivariate logistic regressionb
|
||||||
|---|---|---|---|---|---|---|---|---|
| OR | 95% CI
|
P-value | OR | 95% CI
|
P-value | |||
| LB | UB | LB | UB | |||||
| Caffeine intake | 0.039 | |||||||
| Negative | Reference | Reference | ||||||
| Positive | 3.10 | 1.06 | 9.07 | 1.30 | 0.30 | 5.61 | 0.726 | |
| Migraine with aura | 0.098 | |||||||
| Negative | Reference | N/A | ||||||
| Positive | 2.35 | 0.85 | 6.48 | |||||
| Visual aura | 0.076 | |||||||
| Negative | Reference | |||||||
| Positive | 2.35 | 0.91 | 6.07 | |||||
| Alcohol (migraine trigger) | 0.025 | |||||||
| Negative | Reference | Reference | ||||||
| Positive | 2.95 | 1.15 | 7.57 | 2.99 | 0.85 | 10.54 | 0.088 | |
| Osmophobia (migraine accompaniment) | 0.040 | |||||||
| Negative | Reference | Reference | ||||||
| Positive | 2.89 | 1.05 | 7.96 | 2.36 | 0.64 | 8.71 | 0.199 | |
| Family history of BD | <0.001 | |||||||
| Negative | Reference | N/Ac | ||||||
| Positive | 9.75 | 3.01 | 31.61 | |||||
| Family history of type 1 BD | <0.001 | |||||||
| Negative | Reference | Reference | ||||||
| Positive | 12.40 | 3.32 | 46.34 | 14.42 | 2.94 | 70.73 | 0.001 | |
| Previous suicide attempt | 0.001 | |||||||
| Negative | Reference | Reference | ||||||
| Positive | 8.05 | 2.27 | 28.51 | 2.72 | 0.55 | 13.43 | 0.220 | |
| Comorbid generalized anxiety disorder | 0.519 | |||||||
| Negative | Reference | N/A | ||||||
| Positive | 1.75 | 0.32 | 9.66 | |||||
| Comorbid anxiety disorder | 0.093 | |||||||
| Negative | Reference | |||||||
| Positive | 0.45 | 0.18 | 1.14 | |||||
| Physical abuse in childhood | 0.022 | |||||||
| Negative | Reference | Reference | ||||||
| Positive | 2.98 | 1.17 | 7.58 | 0.109 | 2.91 | 0.84 | 10.06 | 0.092 |
| MIDAS score, numeric | 1.01 | 1.00 | 1.02 | |||||
| MIDAS score, subgroup | 0.014 | |||||||
| ≤30 | Reference | Reference | ||||||
| >30 | 3.32 | 1.28 | 8.61 | 3.69 | 1.12 | 12.19 | 0.032 | |
Notes:
Variables with P-value ≤0.10 for comparison between study groups were evaluated in univariate logistic regression.
Variables with statistical significance in univariate logistic regression were included in mutually adjusted multivariate logistic regression model,
while family history of BD was omitted due to low OR. N/A, not applicable based on lack of significance in univariate analysis. Values in bold indicate statistical significance (P<0.05).
Abbreviations: BD, bipolar disorder; OR, odds ratio; CI, confidence interval; LB, lower boundary of 95% CI; UB, upper boundary of 95% CI; MIDAS, Migraine Disability Assessment Scale.
Albeit not significant based on 5% type 1 error level, parameters including alcohol as a trigger (OR, 2.99; 95% CI, 0.85–10.54; P=0.088) and physical abuse in childhood (OR, 2.91; 95% CI, 0.84–10.06; P=0.092) may also be considered among the estimators of having bipolar diseases, associated with 2.99 times and 2.91 times increased likelihood of having BD, respectively (Table 6).
Discussion
Our findings revealed the presence of comorbid BD in 19.2% of migraineurs. No significant difference was noted between migraineurs with and without comorbid BD in terms of sociodemographic, migraine disease, and first-episode characteristics, while migraineurs with comorbid BD than those without comorbid BD had significantly higher MIDAS scores, higher rate for family history of BD, and past history of suicide attempt and physical abuse. Mutually adjusted multivariate logistic regression model revealed that a positive family history of type I BD (OR, 14.42; 95% CI, 2.94–70.73; P=0.001) and MIDAS scores >30 (OR, 3.69; 95% CI, 1.12–12.19; P=0.032) were significant estimators of having bipolar diseases associated with 14.42 times and 3.69 times higher likelihood of BD among migraineurs, respectively.
Our findings indicate a high prevalence of BD among migraineurs that exceeds the lifetime prevalence of type I and type II BDs estimated to be 0.6% and 0.4%, respectively, in population-based studies.28 Likewise, comorbid BD was reported to be the second most prevalent comorbid psychiatric disorder in migraineurs as preceded by major depressive disorder.14 Other studies addressing the prevalence of BD among migraineurs indicated twofold increase in BD in patients with migraine than in those without migraine,17 while BD was reported only in 1.2%–1.3% of migraineurs in these studies.17,18 A family history of BD, type I BD in particular, was more common in migraineurs with comorbid BD than in those without comorbid BD in our cohort. This supports the reported increase in the risk for having BD from 0.4%–1.6% in the general population to 3%–8% in case of presence of BD in a first-degree relative.29–31 A family history of mood disorder and migraine in first-degree relatives was also reported to be associated with an increased risk of comorbidity.32 Besides, parental migraine is suggested to be an independent risk factor for BD in the offspring, even in the absence of parental BD.33 Given the association of a family history of type I BD with 14.42 times higher likelihood of having BD in a patients with migraine, our findings emphasize that a positive family history of BD may refer to a stronger risk factor for BD development in the presence of migraine. The presence of a positive family history of type I BD in one-third of migraineurs seems therefore to be associated with a high prevalence of BD in our cohort.
The findings from analysis adjusted for sociodemographics in migraineurs in a past study revealed the association of past-year migraine with increased risk of past-year psychiatric diseases, including agoraphobia without panic disorder (OR, 3.21), dysthymia (OR, 3.17), and BD (OR, 2.78), but not with social phobia, generalized anxiety disorder (GAD), and alcohol or drug abuse/dependence.4 This seems consistent with the identification of comorbid psychiatric diseases in a majority of migraineurs with the identification of BD in almost 20% of migraineurs in our cohort.
Also, none of the migraineurs with comorbid BD in our cohort had GAD or somatoform disorder, while alcohol use disorder and hypochondriasis were similarly rare and anxiety disorder was similarly common among migraineurs with and without comorbid BD.
Depression and anxiety have been consistently reported to be common psychiatric comorbidities among migraineurs, occurring at higher rates compared with the general population.32,34,35 Besides, migraine has been suggested to alter clinical course of BD among migraineurs by increasing frequency of depressive episodes and comorbid anxiety disorders.12,16,36 This seems consistent with high rates for anxiety disorder and first-episode depression in our migraineurs regardless of comorbid BD.
Increased likelihood of index and overall episodes of depression rather than mania in BD patients with migraine than in those without migraine has been suggested to increase the risk of BD patients to receive improper antidepressant monotherapies instead of mood stabilizers.11 First-episode depression was shown to be relieved by prescription of antidepressants per se in 47.8% and mood stabilizers only in 8.7% of migraineurs with comorbid BD in our cohort. This seems notable given the increased likelihood of pain-killer medication abuse, poor treatment adherence, and poor therapeutic outcome and even an increased risk of suicidality in case of misdiagnosing bipolar characteristics in migraine–BD comorbidity.11,37–39
Almost sixfold increase in suicide attempt in migraineurs with comorbid BD than those without comorbid BD in our cohort seems consistent with consideration of BD as a leading cause of premature mortality from suicide40–43 with ~20–30 times higher suicide risk than the general population with no psychiatric disorder.44,45 Besides, comorbid migraine has been considered as an important marker of severity of both type I and type II BDs (20) and to be an independent risk factor for the course of BD,13 while also shown to be associated with an increased risk of suicidal behavior per se.16 Hence, our findings indicate careful consideration of migraineurs with comorbid BD in terms of potential risk of suicide attempts.
While association of BD and migraine is suggested to occur only among migraineurs with aura,46 our findings revealed no significant difference between migraineurs with versus without comorbid BD in terms of presence and type of aura as well as alterations in clinical course attributable to migraine with aura. In addition, our findings revealed no impact of comorbid BD on migraine disease characteristics, including disease duration, frequency, duration and severity of episodes, and prodromal symptoms. Alcohol was a more common triggering factor for migraine in case of comorbid BD, while similarly, low alcohol consumption rates were noted among migraineurs with and without comorbid BD in our cohort. The low rate of alcohol abuse among BD patients was also reported in another study30 from Turkey and attributed to the overall low alcohol consumption rate in our country, particularly among females.30,47
This seems notable given the 2.99 times increased likelihood of having BD among our migraineurs in whom alcohol is a triggering factor, albeit not reached statistical significance in our analysis probably due to already low consumption rates.
Comorbid BD was associated with an increase in MIDAS scores in our cohort, indicating BD to aggravate migraine-associated disability and deterioration in the functionality among migraine patients. Besides, MIDAS scores >30 were associated with 3.69 times increased likelihood of having BD in migraineurs. Accordingly, our findings seem to emphasize the potential of screening for comorbid BD among migraineurs, particularly in those with disability and poor functionality, to implement adequate treatment strategy and limit potential adverse outcome.12,33,48 Notably, an increase in MIDAS scores and a significant deterioration in the functionality were also reported in a recent study among Turkish migraineurs, in case of comorbid personality disorders.48
Our findings support the potential association of migraine–BD comorbidity with adverse health-related outcomes,12,33 considering not only the migraine-related alteration in course of BD but also BD-related aggravation in migraine-related disability.12 Hence, our findings emphasize the likely role of earlier detection and proper treatment of each disease in prevention of adverse outcomes associated with migraine–BD comorbidity.12,33
A history of childhood physical abuse was more prevalent in migraineurs with comorbid BD than in those without comorbid BD in our cohort. In addition, albeit not significant a past history of physical abuse in childhood was associated with 2.91 times increased likelihood of having BD in migraineurs. While a twofold rise in migraine prevalence is suggested in patients with a history of childhood abuse than in those without,49 physical abuse has also been considered a predictive risk factor for mania.50 Hence, our findings are in agreement with data indicating a significant association of child adversity score with the total number of medical comorbidities in BD patients, including migraine headaches.51
Supporting lower specificity and higher sensitivity of HSL-32 compared with MDQ in diagnosing BD,52,53 our findings indicate HSL-32 and MDQ to reveal the likelihood of comorbid BD in 100.0% and 91.3% of patients, respectively, with confirmed diagnosis, while HSL-32 also revealed positive results in 70.1% of patients without BD. In fact, unipolar depressed patients have been suggested to have clinical features that resemble the type II BD patients in the presence of migraine, possibly indicating that migraine in depressed patients is a bipolar spectrum trait.54 Notably, 72.1% of migraineurs without comorbid BD in our cohort presented with unipolar depression and HCL-32 revealed possible BD in 70.1% of patients in this group. This seems also notable given the high accuracy of HCL-32 in the detection of hypomanic components and thus “softer” BD cases.24,55
Certain limitations to this study should be considered. First, due to the cross-sectional design, establishing any cause and effect relationship is impossible. Second, due to the small sample size and recruiting participants from a single tertiary care center, generalizing our findings to the overall patient population is difficult along with the likelihood of certain regional differences to be ignored. Third, lack of a group without migraine in the study population is another limitation, which otherwise would extend the knowledge achieved in the current study. Nevertheless, despite these certain limitations, given the limited data available on this subject, our findings represent a valuable contribution to the literature.
Conclusion
Our findings revealed a high prevalence of comorbid BD in migraineurs along with an increased likelihood of having BD in case of a positive family history of type I BD and MIDAS scores >30. Comorbid BD was associated with a higher rate for a family history of BD, more common suicide attempt and childhood physical abuse as well as aggravated migraine-related disability among migraineurs. Migraineurs with and without comorbid BD showed similar sociodemographic and migraine disease characteristics as well as similar high rates for anxiety, depression, and first-episode depression. Our findings indicate the association of migraine–BD comorbidity with the likelihood of alteration in the clinical course of both diseases along with an increased risk of BD development in patients with a positive family history and severe migraine-related disability. Thus, our findings emphasize enhanced attention and early recognition of migraine–BD comorbidity in the routine clinical practice to minimize and appropriately manage potential adverse health outcomes in the long term. Longitudinal prospective studies addressing the migraine–BD comorbidity in terms of actual prevalence and risk status of each disease and potential adverse impact of comorbidity on the clinical course of each disease per se are needed for elucidating potential mechanisms underlying co-occurrence of migraine and BD and for a better therapeutic outcome.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Lipton RB, Bigal ME, Steiner TJ, Silberstein SD, Olesen J. Classification of primary headaches. Neurology. 2004;63(3):427–435. doi: 10.1212/01.wnl.0000133301.66364.9b. [DOI] [PubMed] [Google Scholar]
- 2.Headache Classification Subcommittee of the International Headache Society The international classification of headache disorders: 3rd edition (beta version) Cephalalgia. 2013;33:629–808. doi: 10.1177/0333102413485658. [DOI] [PubMed] [Google Scholar]
- 3.Vecchia D, Pietrobon D. Migraine: a disorder of brain excitatory-inhibitory balance? Trends Neurosci. 2012;35:507–520. doi: 10.1016/j.tins.2012.04.007. [DOI] [PubMed] [Google Scholar]
- 4.Ratcliffe GE, Enns MW, Jacobi F, Belik SL, Sareen J. The relationship between migraine and mental disorders in a population-based sample. Gen Hosp Psychiatry. 2009;31(1):14–19. doi: 10.1016/j.genhosppsych.2008.09.006. [DOI] [PubMed] [Google Scholar]
- 5.Rathnasiri Bandara SM. Migraine and psychiatric disorders co-morbidity explained by sinus hypoxic nitric oxide theory – a new hypothesis on the Sino rhinogenic theory. Med Hypotheses. 2014;82(3):257–265. doi: 10.1016/j.mehy.2013.11.045. [DOI] [PubMed] [Google Scholar]
- 6.Minen MT, Tanev K. Influence of psychiatric comorbidities in migraineurs in the emergency department. Gen Hosp Psychiatry. 2014;36(5):533–538. doi: 10.1016/j.genhosppsych.2014.05.004. [DOI] [PubMed] [Google Scholar]
- 7.Baskin SM, Smitherman TA. Migraine and psychiatric disorders: comorbidities, mechanisms, and clinical applications. Neurol Sci. 2009;30(suppl 1):S61–S65. doi: 10.1007/s10072-009-0071-5. [DOI] [PubMed] [Google Scholar]
- 8.Ketter TA. Diagnostic features, prevalence, and impact of bipolar disorder. J Clin Psychiatry. 2010;71(6):e14. doi: 10.4088/JCP.8125tx11c. [DOI] [PubMed] [Google Scholar]
- 9.American Psychiatric Association . Diagnostic and Statistical Manual of Mental Disorders. 5th ed. Arlington, VA: American Psychiatric Association; 2013. [Google Scholar]
- 10.McIntyre RS, Konarski JZ, Wilkins K, Bouffard B, Soczynska JK, Kennedy SH. The prevalence and impact of migraine headache in bipolar disorder: results from the Canadian Community Health Survey. Headache. 2006;46(6):973–982. doi: 10.1111/j.1526-4610.2006.00469.x. [DOI] [PubMed] [Google Scholar]
- 11.Fornaro M, De Berardis D, De Pasquale C, et al. Prevalence and clinical features associated to bipolar disorder-migraine comorbidity: a systematic review. Compr Psychiatry. 2015;56:1–16. doi: 10.1016/j.comppsych.2014.09.020. [DOI] [PubMed] [Google Scholar]
- 12.Brietzke E, Moreira CL, Duarte SV, et al. Impact of comorbid migraine on the clinical course of bipolar disorder. Compr Psychiatry. 2012;53(6):809–812. doi: 10.1016/j.comppsych.2011.10.006. [DOI] [PubMed] [Google Scholar]
- 13.Ibiloglu AO, Caykoylu A. The comorbidity of migraine in bipolar disorder. JMOOD. 2011;1:25–33. [Google Scholar]
- 14.Leo RJ, Singh J. Migraine headache and bipolar disorder comorbidity: a systematic review of the literature and clinical implications. Scand J Pain. 2016;11:136–145. doi: 10.1016/j.sjpain.2015.12.002. [DOI] [PubMed] [Google Scholar]
- 15.Low NC, Du Fort GG, Cervantes P. Prevalence, clinical correlates, and treatment of migraine in bipolar disorder. Headache. 2003;43(9):940–949. doi: 10.1046/j.1526-4610.2003.03184.x. [DOI] [PubMed] [Google Scholar]
- 16.Ortiz A, Cervantes P, Zlotnik G, et al. Cross-prevalence of migraine and bipolar disorder. Bipolar Disord. 2010;12(4):397–403. doi: 10.1111/j.1399-5618.2010.00832.x. [DOI] [PubMed] [Google Scholar]
- 17.Merikangas KR, Angst J, Isler H. Migraine and psychopathology. Results of the Zurich cohort study of young adults. Arch Gen Psychiatry. 1990;47(9):849–853. doi: 10.1001/archpsyc.1990.01810210057008. [DOI] [PubMed] [Google Scholar]
- 18.Semiz M, Şentürk IA, Balaban H, Yağız AK, Kavakçı Ö. Prevalence of migraine and co-morbid psychiatric disorders among students of Cumhuriyet University. J Headache Pain. 2013;14:34. doi: 10.1186/1129-2377-14-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV Clinical Version (SCID-I/CV) Washington, DC: American Psychiatric Press; 1997. [Google Scholar]
- 20.Ozkurkcugil A, Aydemir Ö, Yıldız M, Danacı E, Köroğlu E. Adaptation and reliability study of structured clinical interview for DSM-IV axis I disorders. Turk J Drug Ther. 1999;12:233–236. [Google Scholar]
- 21.Ozerdem A, Yazici O, Oral ET, et al. The Mood Disorders Study Group Psychiatric Association of Turkey Establishment of a registry program for bipolar illness in Turkey. International Society of Affective Disorders 2nd Biennial Conference-Cancun, Mexico. J Affect Disord. 2004;78(suppl 1):86. [Google Scholar]
- 22.Hirschfeld RM, Williams JB, Spitzer RL, et al. Development and validation of a screening instrument for bipolar spectrum disorder: the Mood Disorder Questionnaire. Am J Psychiatry. 2000;157(11):1873–1875. doi: 10.1176/appi.ajp.157.11.1873. [DOI] [PubMed] [Google Scholar]
- 23.Konuk N, Kıran S, Tamam L, Karaahmet E, Aydın H, Atik L. Validation of the Turkish version of the “Mood Disorder Questionnaire” for the screening of bipolar disorders. Türk Psikiyatri Derg. 2007;18:147–154. [PubMed] [Google Scholar]
- 24.Angst J, Adolfsson R, Benazzi F, et al. The HCL-32: towards a self-assessment tool for hypomanic symptoms in outpatients. J Affect Disord. 2005;88(2):217–233. doi: 10.1016/j.jad.2005.05.011. [DOI] [PubMed] [Google Scholar]
- 25.Vahip S, Aydemir O, Akkaya C, et al. Reliability and validity study of the Turkish version of hypomania checklist-32-revised. Türk Psikiyatri Derg. 2016;27:1–10. [PubMed] [Google Scholar]
- 26.Stewart WF, Lipton RB, Dowson AJ, Sawyer J. Development and testing of the Migraine Disability Assessment (MIDAS) Questionnaire to assess headache-related disability. Neurology. 2001;15(6 suppl 1):20–28. doi: 10.1212/wnl.56.suppl_1.s20. [DOI] [PubMed] [Google Scholar]
- 27.Ertas M, Siva A, Dalkara T, et al. Turkish MIDAS Group Validity and reliability of the Turkish Migraine Disability Assessment (MIDAS) questionnaire. Headache. 2004;44(8):786–793. doi: 10.1111/j.1526-4610.2004.04146.x. [DOI] [PubMed] [Google Scholar]
- 28.Merikangas KR, Jin R, He JP, et al. Prevalence and correlates of bipolar spectrum disorder in the world mental health survey initiative. Arch Gen Psychiatry. 2011;68(3):241–251. doi: 10.1001/archgenpsychiatry.2011.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yazici O. Bipolar-I and bipolar-II disorders. In: Köroglu E, Gülec C, editors. Essentials Book of Psychiatry. 2nd ed. Ankara: HYB Press; 2007. p. 273. [Google Scholar]
- 30.İbiloğlu AO, Cayköylü A. Distinctive sociodemographic, clinical and temperament characteristics of bipolar-I, bipolar-II and major depressive disorders. Turk Psikiyatri Derg. 2011;22:159–165. [PubMed] [Google Scholar]
- 31.Chiaroni P, Hantouche EG, Gouvernet J. Hyperthymic and depressive temperaments study in controls, as a function of their familial loading for mood disorders. Encephale. 2004;30:509–514. doi: 10.1016/s0013-7006(04)95464-4. [DOI] [PubMed] [Google Scholar]
- 32.Franchini L, Bongiorno F, Dotoli D, Rainero I, Pinessi L, Smeraldi E. Migraine headache and mood disorders: a descriptive study in an outpatient psychiatric population. J Affect Disord. 2004;81(2):157–160. doi: 10.1016/S0165-0327(03)00164-2. [DOI] [PubMed] [Google Scholar]
- 33.Sucksdorff D, Brown AS, Chudal R, Heinimaa M, Suominen A, Sourander A. Parental and comorbid migraine in individuals with bipolar disorder: a nationwide register study. J Affect Disord. 2016;206:109–114. doi: 10.1016/j.jad.2016.07.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Victor TW, Hu X, Campbell J, White RE, Buse DC, Lipton RB. Association between migraine, anxiety and depression. Cephalalgia. 2010;30(5):567–575. doi: 10.1111/j.1468-2982.2009.01944.x. [DOI] [PubMed] [Google Scholar]
- 35.Louter MA, Pijpers JA, Wardenaar KJ, et al. Symptom dimensions of affective disorders in migraine patients. J Psychosom Res. 2015;79(5):458–463. doi: 10.1016/j.jpsychores.2015.09.014. [DOI] [PubMed] [Google Scholar]
- 36.Dilsaver SC, Benazzi F, Oedegaard KJ, Fasmer OB, Akiskal HS. Is a family history of bipolar disorder a risk factor for migraine among affectively ill patients? Psychopathology. 2009;42(2):119–123. doi: 10.1159/000204762. [DOI] [PubMed] [Google Scholar]
- 37.Fornaro M, De Berardis D, Iasevoli F, et al. Treatment adherence towards prescribed medications in bipolar-II acute depressed patients: relationship with cyclothymic temperament and “therapeutic sensation seeking” in response towards subjective intolerance to pain. J Affect Disord. 2013;151:596–604. doi: 10.1016/j.jad.2013.07.004. [DOI] [PubMed] [Google Scholar]
- 38.Fornaro M, Martino M, De Pasquale C, Moussaoui D. The argument of antidepressant drugs in the treatment of bipolar depression: mixed evidence or mixed states? Expert Opin Pharmacother. 2012;13:2037–2051. doi: 10.1517/14656566.2012.719877. [DOI] [PubMed] [Google Scholar]
- 39.Fornaro M, Stubbs B. A meta-analysis investigating the prevalence and moderators of migraines among people with bipolar disorder. J Affect Disord. 2015;178:88–97. doi: 10.1016/j.jad.2015.02.032. [DOI] [PubMed] [Google Scholar]
- 40.Subramaniam M, Abdin E, Vaingankar JA, Chong SA. Prevalence, correlates, comorbidity and severity of bipolar disorder: results from the Singapore Mental Health Study. J Affect Disord. 2013;146(2):189–196. doi: 10.1016/j.jad.2012.09.002. [DOI] [PubMed] [Google Scholar]
- 41.Roshanaei-Moghaddam B, Katon W. Premature mortality from general medical illnesses among persons with bipolar disorder: a review. Psychiatr Serv. 2009;60(2):147–156. doi: 10.1176/ps.2009.60.2.147. [DOI] [PubMed] [Google Scholar]
- 42.Laursen TM, Munk-Olsen T, Gasse C. Chronic somatic comorbidity and excess mortality due to natural causes in persons with schizophrenia or bipolar affective disorder. PLoS One. 2011;6:e24597. doi: 10.1371/journal.pone.0024597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chang JC, Chen HH, Yen AM, Chen SL, Lee CS. Survival of bipolar depression, other type of depression and comorbid ailments: ten-year longitudinal follow-up of 10,922 Taiwanese patients with depressive disorders (KCIS no PSY1) J Psychiatr Res. 2012;46:1442–1448. doi: 10.1016/j.jpsychires.2012.07.014. [DOI] [PubMed] [Google Scholar]
- 44.Pompili M, Gonda X, Serafini G, et al. Epidemiology of suicide in bipolar disorders: a systematic review of the literature. Bipolar Disord. 2013;15(5):457–490. doi: 10.1111/bdi.12087. [DOI] [PubMed] [Google Scholar]
- 45.Berkol TD, İslam S, Kırlı E, Pınarbaşı R, Özyıldırım İ. Suicide attempts and clinical features of bipolar patients. Saudi Med J. 2016;37(6):662–667. doi: 10.15537/smj.2016.6.12776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Breslau N, Davis GC, Andreski P. Migraine, psychiatric disorders, and suicide attempts: an epidemiologic study of young adults. Psychiatry Res. 1991;37(1):11–23. doi: 10.1016/0165-1781(91)90102-u. [DOI] [PubMed] [Google Scholar]
- 47.Oztürk MO. Mental Health and Disorders. Ankara: Nobel Medical Bookstore; 2002. pp. 292–428. [Google Scholar]
- 48.Kayhan F, Ilik F. Prevalence of personality disorders in patients with chronic migraine. Compr Psychiatry. 2016;68:60–64. doi: 10.1016/j.comppsych.2016.04.007. [DOI] [PubMed] [Google Scholar]
- 49.Fuller-Thomson E, Baker TM, Brennenstuhl S. Investigating the association between childhood physical abuse and migraine. Headache. 2010;50(5):749–760. doi: 10.1111/j.1526-4610.2010.01626.x. [DOI] [PubMed] [Google Scholar]
- 50.Levitan RD, Parikh SV, Lesage AD, et al. Major depression in individuals with a history of childhood physical or sexual abuse: relationship to neurovegetative features, mania, and gender. Am J Psychiatry. 1998;155:1746–1752. doi: 10.1176/ajp.155.12.1746. [DOI] [PubMed] [Google Scholar]
- 51.Post RM, Altshuler LL, Leverich GS, et al. Role of childhood adversity in the development of medical co-morbidities associated with bipolar disorder. J Affect Disord. 2013;147(1–3):288–294. doi: 10.1016/j.jad.2012.11.020. [DOI] [PubMed] [Google Scholar]
- 52.Meyer TD, Bernhard B, Born C, et al. The hypomania checklist-32 and the Mood Disorder Questionnaire as screening tools – going beyond samples of purely mood-disordered patients. J Affect Disord. 2011;128(3):291–298. doi: 10.1016/j.jad.2010.07.003. [DOI] [PubMed] [Google Scholar]
- 53.Chou CC, Lee IH, Yeh TL, et al. Comparison of the validity of the Chinese versions of the Hypomania Symptom Checklist-32 (HCL-32) and Mood Disorder Questionnaire (MDQ) for the detection of bipolar disorder in medicated patients with major depressive disorder. Int J Psychiatry Clin Pract. 2012;16(2):132–137. doi: 10.3109/13651501.2011.644563. [DOI] [PubMed] [Google Scholar]
- 54.Oedegaard KJ, Fasmer OB. Is migraine in unipolar depressed patients a bipolar spectrum trait? J Affect Disord. 2005;84(2–3):233–242. doi: 10.1016/j.jad.2003.11.007. [DOI] [PubMed] [Google Scholar]
- 55.Lee K, Oh H, Lee EH, Kim JH, Kim JH, Hong KS. Investigation of the clinical utility of the hypomania checklist 32 (HCL-32) for the screening of bipolar disorders in the non-clinical adult population. BMC Psychiatry. 2016;16:124. doi: 10.1186/s12888-016-0831-8. [DOI] [PMC free article] [PubMed] [Google Scholar]