Abstract
Treatment of primary effusion lymphoma cells latently infected by Kaposi's sarcoma-associated herpesvirus (KSHV; human herpesvirus-8 [HHV-8]) with agents such as 12-O-tetradecanoylphorbol-13-acetate (TPA) induces a lytic viral replication cycle, with an ordered gene expression program. Initial studies of the KSHV expression program following TPA induction using viral microarrays yielded useful information concerning the viral expression program, but precise kinetic assignments for some genes remained unclear. Classically, late herpesvirus genes require viral DNA replication for maximal expression. We used cidofovir (CDV), a nucleotide-analogue KSHV DNA polymerase inhibitor, to dissect KSHV expression into two components: genes expressed without viral DNA replication and those requiring it. The expression of known immediate-early or early genes (e.g., open reading frames [ORFs] 50, K8 bZIP, and 57) serving lytic regulatory roles was relatively unaffected by the presence of CDV, while known late capsid and tegument structural genes (e.g., ORFs 25, 26, 64, and 67) were CDV sensitive. Latency-associated transcript ORF 73 was unaffected by the presence of TPA or CDV, suggesting that it was constitutively expressed. Expression of several viral cellular gene homologs, including K2 (vIL-6), ORF 72 (vCyclin), ORF 74 (vGPCR), and K9 (vIRF-1), was unaffected by the presence of CDV, while that of others, such as K4.1 (vMIP-III), K11.1 (vIRF-2), and K10.5 (LANA2, vIRF-3), was inhibited. The results distinguish KSHV genes whose full expression required viral DNA replication from those that did not require it, providing additional insights into KSHV replication and pathogenesis strategies and helping to show which viral cell homologs are expressed at particular times during the lytic process.
Kaposi's sarcoma-associated herpesvirus (KSHV), or human herpesvirus 8 (HHV-8), has been associated with Kaposi's sarcoma (KS) (14), primary effusion lymphomas (PEL) (or body cavity-based lymphomas [BCBL]), and some forms of multicentric Castleman's disease (MCD) (10, 72). KSHV, a member of the lymphotrophic gammaherpesvirus family, is distantly related to Epstein-Barr virus and more closely related to herpesvirus saimiri and rhesus monkey rhadinovirus. KSHV has a double-stranded DNA genome of about 145 kb organized into at least 88 open reading frames (ORFs) (9, 48, 55, 61, 68, 69, 83). Some transcripts are spliced to produce alternative message species, such as ORF K8 and K8.1 (13, 44). Some KSHV genes, such as K1 or K15, show distinctive geographically associated variations (64, 85). KSHV has many conserved genes with homologies to other herpesviruses, such as those encoding viral transactivators, genes involved with viral DNA replication, and viral structural proteins. In addition, KSHV also contains a large complement of cellular accessory gene homologs, many of which are involved in potentially oncogenic processes, such as cell cycle regulation (vCyclin), promotion of cell growth or angiogenesis (vIL-6, vIL-8R or vGPCR, vMIP-I, and vMIP-III), and inhibition of programmed cell death (vFLIP and vBcl-2). PEL cells have not been found to carry cellular gene mutations associated with other B-cell neoplasms. Although contributions from cellular genetic alterations cannot be ruled out entirely as the responsible cause of the neoplastic phenotype, the expression of certain KSHV genes is believed to be responsible for the neoplastic phenotype of this and other KSHV-associated neoplasms.
During an infection, after KSHV enters the nucleus, the virus can enter a latent state, with the viral DNA existing as an episome, expressing only a few latency-associated genes. The in vivo events that trigger the entry of latently infected cells into the lytic cycle are unclear, but in PEL cell lines, KSHV lytic replication can be induced with phorbol esters or sodium butyrate (52, 67). Like other herpesviruses, KSHV lytic replication follows a carefully ordered gene expression program that leads to mature virion production (34, 58, 63). While the expression patterns of some genes have been clearly described, the expression patterns of others are less clear. However, several approaches, including the use of agents that block the viral replication cycle at distinct stages, offer the opportunity to more precisely define the viral gene expression program.
Classic studies, initially performed on herpes simplex virus, were able to identify several temporally distinct classes of genes that were expressed during the replication cycle: (i) the alpha class or immediate-early genes, which mainly regulate viral gene expression and do not require de novo protein synthesis for full expression; (ii) the beta class or early-middle genes, which typically mediate viral DNA replication and require de novo protein synthesis for expression but whose expression is not suppressed by drugs that inhibit viral DNA replication; and (iii) the gamma class or the late genes, which mainly include virion structural genes and which require viral DNA replication for maximal expression (28, 29).
Here, we defined one aspect of the KSHV gene expression program by use of an inhibitor of KSHV DNA replication, cidofovir (CDV) (35), and a newly designed and constructed long oligonucleotide KSHV microarray, which offers several advantages over previous KSHV expression profiling technologies, including the more specific detection of transcripts and the ability to detect certain polymorphic and variant transcripts. The viral DNA replication inhibitor allowed the clear definition of the late viral transcripts that depend on viral DNA replication from the other transcripts. This clearer delineation of KSHV replication kinetics yielded some notable insights concerning the expression patterns of the viral accessory cellular gene homologs, including those with putative oncogenic activity. The expression of several genes with oncogenic and angiogenic activity, including vGPCR (3, 4, 81), vMIP-I (7), and vIL-6 (2), was not inhibited by CDV treatment, suggesting that these viral cellular gene homologs are expressed early during the viral replication cycle to prepare the host cell for the subsequent stages of viral replication and to blunt the host response to infection. The results also indicate that the oncogenic KSHV genes do not require viral DNA replication for their maximal expression, providing additional insights into the oncogenic properties of KSHV genes and suggesting strategies that may or may not be helpful in treating KSHV-related neoplasms.
MATERIALS AND METHODS
Cell culture, TPA induction, and CDV treatment.
BCBL-1 cells (67) were obtained from Michael McGrath and Don Ganem through the AIDS Research and Reference Reagent Program, Division of AIDS, National Institute of Allergy and Infectious Diseases, National Institutes of Health (NIH). BCBL-1 cells were cultured in growth medium containing RPMI 1640 (BioSource, Camarillo, Calif.) with 10% fetal bovine serum (HyClone, Logan, Utah), 2 mM l-glutamine (Sigma Co., St. Louis, Mo.), penicillin (Sigma) (100 U/ml), streptomycin (Sigma) (100 μg/ml), and 5 × 10−5 M 2-mercaptoethanol (Sigma) (BCBL-1 medium) at 37°C with 5% CO2. Cells were maintained at densities between 2.5 × 105 and 3.0 × 105 cells/ml and split every 2 to 3 days. Cells were seeded the day prior to induction at a density of ∼2.5 × 105 cells/ml in BCBL-1 medium or BCBL-1 medium containing CDV {1-[(S)-3-hydroxy-2-(phosphonomethoxy)propyl] cytosine dihydrate; Vistide} (Gilead Sciences, Inc., Foster City, Calif.). KSHV was induced into lytic replication by adding 12-o-tetradecanoylphorbol-13-acetate (TPA) (Sigma) to a final concentration of 20 ng/ml. One hour after TPA addition, cells were washed with BCBL-1 medium with or without CDV and incubated at 37°C. For the microarray experiments, the cells were pretreated with 100 μM CDV and samples were collected at 0, 6, 8, 10, 12, 24, 36, 48, 72, and 96 h postinduction (hpi) and harvested by centrifugation followed by two washes with ice-cold phosphate-buffered saline. Supernatant (5 ml) was sampled at each time point prior to cell harvesting for subsequent quantitation of supernatant KSHV DNA.
CDV titration assay.
To determine the optimal CDV concentration for suppression of postreplication KSHV transcripts, BCBL-1 cells were seeded at a density of 2.5 × 105 cells/ml in 2 ml of growth medium containing 0, 10, 25, 50, 75, or 100 μM CDV in triplicate wells of 12-well plates and were allowed to incubate at 37°C with 5% CO2 for 14 h prior to induction. TPA was added to achieve a final concentration of 20 ng/ml, and cells were incubated for 1 h at 37°C, washed with prewarmed medium, and replaced in 2 ml of fresh medium with CDV. The cells were incubated at 37°C for 72 h prior to harvesting. Triplicate wells were pooled, and total RNA was isolated using an RNeasy Miniprep kit (QIAGEN, Valencia, Calif.) following the manufacturer's instructions. Residual DNA carryover was eliminated by two DNase I treatments (4 units/treatment) and removal using a DNA-free kit (Ambion, Austin, Tex.) following the manufacturer's instructions.
Isolation of KSHV virion-associated DNA.
Virion-associated DNA was isolated from KSHV particles in a 400-μl aliquot of supernatant collected at each time point on the basis of the protocol of Nishimura et al. (62). Briefly, supernatant was centrifuged at 5,000 × g for 5 min, followed by addition of DNase I (Promega Corp., Madison, Wis.) to achieve a final concentration of 100 U/ml and incubated at 37°C for 1 h to digest unprotected DNA. EDTA was added to achieve a final concentration of 10 mM, and the mixture was incubated at 65°C for 30 min to inactivate the DNase I. Sodium dodecyl sulfate (SDS) was added to achieve a final concentration of 0.5%, proteinase K (Promega) was added to achieve a final concentration of 200 μg/ml, and the mixture was incubated at 65°C for 2 h to remove the protective virion protein capsid and lipid coat. The deprotected viral DNA was isolated by extraction with 1:1 volumes of phenol-chloroform-isoamylalcohol (Invitrogen, Carlsbad, Calif.) (25:24:1) and centrifugation at 10,000 × g for 2 min in a heavy phase-lock gel tube (Eppendorf, Hamburg, Germany). The aqueous layer containing the viral DNA was collected and ethanol precipitated with 0.3 M sodium acetate, air dried, and solubilized in 40 μl of 10 mM Tris-HCl-1 mM EDTA (TE).
RNA preparation.
After harvesting, the cells were processed and poly(A)+ RNA was isolated using a Fast Track 2.0 kit (Invitrogen) following the manufacturer's instructions.
RNA and DNA quantitation by real-time PCR.
All real-time PCR assays were carried out using an ABI Prism 7000 sequence detection instrument (Applied Biosystems, Foster City, Calif.). The standard program parameters were 50°C for 2 min, 95°C for 10 min, and 40 cycles of 95°C for 15 s and 60°C for 1 min. For each treatment condition or time point, samples were amplified in triplicate wells with a total reaction volume of 50 μl per well in 96-well optical reaction plates (Applied Biosystems). Averaged cycle threshold (Ct) results were normalized against endogenous control glyceraldehyde-3-phosphate dehydrogenase (GAPDH), which was amplified in parallel for each condition or time point by use of an assay system from Applied Biosystems. For KSHV RNA quantitation, 400 ng of DNA-free total RNA was reverse transcribed for 1 h at 42°C in 20 μl of reaction buffer containing 200 units of Superscript II (SSII) Maloney murine leukemia virus reverse transcriptase (RT), 1× SSII buffer, 10 mM dithiothreitol (Invitrogen), 2.5 μM random hexamers, 0.5 mM deoxynucleoside triphosphates, and 10 U of SUPERase-In RNase inhibitor (Ambion). The RT reaction was heat inactivated at 70°C for 15 min prior to real-time PCR quantitation. For a single PCR, 20 ng of starting cDNA was used as a starting template in a reaction buffer containing 1× TaqMan master mix (Applied Biosystems), 300 nM forward and reverse ORF 17 gene-specific primers, and 200 nM gene-specific TaqMan sequence detection probe (Applied Biosystems). The sequences for primers and TaqMan probes (with linked fluors in italic characters) were as follows: for forward primer 17RT-F5, 5′-TGGGCTGGACACTGGGTCTATTTC-3′; for reverse primer 17RT-B5, 5′-AGATTTTTCACGGGGGCTCTGG-3′; and for TaqMan probe labeled with 6-carboxyfluorescein succinimidyl ester (6-FAM) and carboxy-tetramethyl rhodamine (TAMRA) 17TM, 5′-6FAM-TTCTGCACCGGAGCCATCACGTC-TAMRA-3′.
For extracellular KSHV particle quantitation, 1 μl of KSHV DNA supernatant preparation or reference standards with 101, 102, 103, 104, or 106 copies of an 828-bp ORF 57 PCR fragment (63) were used as a template along with 1× TaqMan master mix, 300 nM forward and reverse primers for ORF 57, and 200 nM ORF 57 TaqMan sequence detection probe and were run on a single 96-well plate. The ORF 57 primer sequences were as follows: for forward primer 57RT-F5, 5′-TTTGTGACCAGCCGCCATAATCAAG-3′; for reverse primer 57RT-B5, 5′-TCATTTGTTCCTCCACGAAAGCCCC-3′; and for TaqMan probe 57TM, 5′-6FAM-AGAAACCGCAGCCGCCGGAG-TAMRA-3′.
Analysis of de novo viral DNA synthesis.
The native DNA agarose gel assay for the linear and circular herpesvirus DNA was first described by Gardella et al. (21) and applied to KSHV studies by Renne et al. (66). Briefly, approximately 5 × 105 BCBL-1 cells were collected at serial time points following TPA induction of lytic KSHV replication in the presence or absence of CDV. The cells were pelleted, resuspended in 100 μl of sample buffer A containing 15% Ficoll, 40 μg of RNase A/ml, and 0.01% bromophenol blue in 1× Tris-borate-EDTA, and loaded into 20- by 10-mm slots of a 110-mm-long × 140-mm-wide × 3-mm-thick vertical 0.8% SeaKem Gold (Cambrex, Rockland, Maine) agarose gel. A total of 100 μl of lysis buffer containing 5% Ficoll, 1% SDS, 1 mg of pronase/ml, and 0.05% xylene cyanol was carefully overlaid on the cell suspension, and the gel was electrophoresed at 0.8 V/cm for 3 h and then at 4.5 V/cm for 12 h at 4°C by use of 1× Tris-borate-EDTA running buffer. After electrophoresis, the gel was stained with ethidium bromide, treated with 0.25 M HCl for 8 min followed by treatment with 0.5 M NaOH and 1.5 M NaCl, and then neutralized with 0.5 M Tris and 3 M NaCl. The DNA was transferred with 10× SSC (1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate) to a Nytran membrane and hybridized with a KSHV-specific probe.
RNA blot analysis.
A total of 3 μg of poly(A)+ RNA was mixed with 1:1 volumes of glyoxal denaturation-loading buffer (Ambion), incubated at 50°C for 30 min, and electrophoresed on 1% agarose at 4 V/cm. The fractionated RNA was then capillary transferred to a Nytran nylon membrane (Schleicher and Schuell, Keene, N.H.) overnight by use of 10× SSC. The membrane was washed briefly in 5× SSC and UV cross-linked at 120 mJ with a Stratalinker 2400 system (Stratagene, La Jolla, Calif.).
The probes used in the hybridization reactions were PCR-amplified products of KSHV ORF 17 or 57 (63) labeled with [α-32P]dCTP (Amersham, Piscataway, N.J.) (3,000 Ci/mmol) by the random-primed method (Rediprime II; Amersham) and purified with MicroQuant G50 columns (Amersham). Blots were prehybridized and hybridized overnight at 68°C in PerfectHyb Plus (Sigma) with 106 cpm/ml of labeled probe. The hybridized membranes were washed at room temperature in 2× SSC-0.1% SDS followed by washes at 68°C with 0.5× SSC-0.1% SDS and 0.1× SSC-0.1% SDS wash buffers. The filters were exposed to storage phosphor screens (GP; Molecular Dynamics, Amersham) (20 by 25 cm), scanned with a Storm 840 PhosphorImager (Molecular Dynamics, Amersham), and quantitated with ImageQuant software (Molecular Dynamics, Amersham). The blots were then stripped with 1% SDS-0.1× SSC at 80°C. The stripped blots were quantitated for RNA loading by probing for GAPDH.
KSHV DNA oligonucleotide array design.
A total of 84 65mer DNA oligomer probes were custom designed on the basis of available genomic or cDNA sequence information (9, 11, 12, 14, 23, 27, 40, 56, 57, 60, 61, 68, 69, 74, 75, 77, 82, 83). An additional 10 oligonucleotides, 60 to 65 bases in length, were designed on the basis of GenBank cDNA submissions and/or available published information concerning alternatively spliced transcripts. The oligonucleotide sequences were selected using the primer design tools of Compugen (http://www.labonweb.com) and, in some cases, using MacVector (Accelrys, San Diego, Calif.) or manual inspection to design additional primers. These oligonucleotides included the following: K8 type I (T1), which detects the message encoding the functional K8 bZIP protein containing exons I to IV; K8 T2 and T3, which detect the K8 Δ bZIP alternative transcripts with a premature stop codon prior to the activation domain in exon III; K8 T3, which detects the Δ bZIP transcript containing exon I-intron I-exon II-intron II-exon III-exon IV (44); K8.1A, which detects the 752-bp product lacking a 95-bp internal sequence; K8.1B, which detects a shorter 569-bp product lacking a 277-bp sequence (13); and oligonucleotides detecting ORF K1 sequences representing geographic or strain-based polymorphisms (Asia subgroup D, Africa-South America subgroup B, and a universal K1 oligonucleotide that serves as a detector for conserved sequences found in the N terminus) (85). Two oligonucleotides were designed to differentiate the P (predominant) and M (minor) expressed alleles of ORF K15 (64). A probe for human CCL-18 (SCYA-18/MIP-4/DC-CK-1/PARC) (26) was also present on the array as a positive control for TPA induction. (TPA induction was found to increase up to 14-fold at 6 h post-TPA addition, regardless of the CDV treatment regimen employed). The oligonucleotides are listed in Table 1. All oligonucleotides were sequence verified using BLAST (http://www.ncbi.nlm.nih.gov/BLAST/). The KSHV elements were printed along with a set of probes for 96 human “housekeeping” (HK) genes (catalog no. HUMLIBTST; Compugen [http://www.labonweb.com]). The HK elements were used for normalization; generally, three HK elements were printed for each KSHV element. Array elements were spotted in duplicate on two separate regions of the slide, and the highest of the duplicate background-subtracted median signal intensities was used for subsequent analyses.
TABLE 1.
Sequences of oligonucleotides used to construct the arraya
| Array element* | Oligonucleotide detector (5′ to 3′ sense) sequence | Accession (or reference no.) | Oligonucleotide genomic start location | Oligonucleotide genomic stop location | Polarity |
|---|---|---|---|---|---|
| K1 (conserved)* | CGGCCCTTGTGTAAACCTGTCTTTCAGACCTTGTTGGACATCCTGTACAATCAAGATGTTCCTGT | AF133038; (85) | 50 | 114 | + |
| K1 (subgroup B)* | GGACTCAGCTTCACCGAATAACGGCGTCTAACCTAACTGTTTCTTCGCTCACCTGCAATTTTACT | AF133040; (85) | 265 | 329 | + |
| K1 (subgroup D)* | GAACTCGGCTTCTTCGAATTACTGGGGCAACACTGACTATTCCTTGCCTTACCGGCAATTTTACT | AF133043; (85) | 265 | 329 | + |
| ORF 4 | GCGTCTACACCCACTTCCCAAGATGATGCTACGCCTTCAATACCTAGTGTACAGACACCCAATTA | U75698 | 2294 | 2358 | + |
| ORF 6 | ATACAGGGAGCAACGGGAACACAAACGTCTTTCACTGTGCAAACCTGGGATACTTCTCGGGGAGA | U75698 | 5985 | 6050 | + |
| ORF 7 | GCCCAGCATAGAGCCGAAGGACTGGATAGAGCCCAACTTCAACCCAGTTCTATAGCTTTGAGAATC | U75698 | 8355 | 8419 | + |
| ORF 8 | AAAAGTACACCCTCGGTGTTTCAGCGTACCGCAAACGGCCTTCGTCAGCGTCTGAGAGGATATAA | U75698 | 11126 | 11190 | + |
| ORF 9 | AAGCTACAAGCTAGACAGGAGGAGCTTCCACAGATACACGACAGAATCCCCTACGTGTTCGTCGA | U75698 | 14129 | 14193 | + |
| ORF 10 | GCAAAGTAACCGTTTACAACACCCATTCGACAGCATGCAAGAAGGCCCGTGTTCGTTTCGTCTAC | U75698 | 15198 | 15262 | + |
| ORF 11 | GAATGGCGCCCAAACAAGCCAGCACCCCTGAAACTGGTGAACACGAGTGATCATCCCGTCATATT | U75698 | 16774 | 16838 | + |
| K2 | GACATACAGGAAGAGCTCAATAAGCTGACTAAGACGCACTACAGTCCACCCAAATTTGACCGCGG | U75698 | 17727 | 17663 | − |
| ORF 2 | TTTTAACCCGGGTTTTACATGACTTTGCGTGTGACGTGTTTCTCTCGCATGATAGCTTGGCTGCG | U75698 | 18385 | 18321 | − |
| K3 | TAGTGTCTCCTGCCATAATAAGGCTGGACCCTCCTCTCTAGTTGATATCCTTCCACAGGGTTTGC | U75698 | 19535 | 19471 | − |
| ORF 70 | TGACGGTGAGCTTTCCTGTCAGCTGTATCAGAGGTCGGGAGACATGGGTTTGGGAGTTCCTTTTA | U75698 | 20838 | 20774 | − |
| K4 | TCCTGGCATAGACCGGACAAGTGCTGTCTCGGTTACCAGAAAAGACCATTACCACAGGTGCTTCT | U75698 | 21537 | 21573 | − |
| K4.1 | GATAAGCTGATATGCGGGTGGTACTGGACGTCCACCGTGTATTGTCGCCAGAAGGCAGTCATTTT | U93872 | 22290 | 22226 | − |
| K4.2 | TTTTGGACCGTGGGAAATGGGACCGGTGTCCTCTCTATGACCTTTTCAGTCACCTTGCCACCTCG | U93872 | 22715 | 22651 | − |
| K5 | ACGAGTCATCTGAAGGAGACGTCGCCTCTGGAGACAAAGAACGTGACGGTTCATCCGGAGACGAG | AF117253 | 26380 | 26308 | − |
| K6 | CGTCCACGTTTTATGCTGCGTTAGCGTACTGCTTGCCACGTTTTACCTGACGCCCACAGAAAGCG | U50138 | 27209 | 27145 | − |
| K7 | ATTGCGTCGCTTTTGGCAATATACCCATCCTGGCTTTCGGCTAGGTTTTCCGTCCTACTTTTCCC | U50139 | 28716 | 28780 | + |
| ORF 16 | AGCCCTGGCTATACTGACCTTTGGCAGTTTTGTGGCCCAGAAGTTATCCAACGAACCTCACCTGC | U75698 | 30402 | 30466 | + |
| ORF 17 | TGTCAATTCCCGACAATGAAGGAGCACGGAGGAACCTACGTACACCCACCCATTTACGTGCAGGC | U75698 | 32181 | 32117 | − |
| ORF 18 | AAGTGAGATGCTGCTGTGTGAGGCATACCGGGACAGCCTCTGGATGCACTTGAACGATAAGGTGG | U75698 | 32705 | 32769 | + |
| ORF 19 | GGATTGTTGCGCTGGAGGTGTTTATCCTCGCGTATGGATTGCTGGAGTTTGGGCAAGTCGCGCGA | U75698 | 34333 | 34269 | − |
| ORF 20 | TTCTGGTATCGGGTGGGCATCAGCCCGTATGTTACGTTGTAGAGCTCAAGACTTGTCTGAGTCAC | U40377 | 35093 | 35029 | − |
| ORF 21 | TCGTACATATACGACGTGCCCACCGTCCCGACTAGCAAGCCGTGGCATTTAATGCACGACAACTC | U75698 | 35566 | 35630 | + |
| ORF 22 | TTATTTGTGGCTGAGGGACAACGGGACCGTAGTGGAGATAAGGGGCATGTATAGAAGACGCGCAG | U75698 | 39155 | 39219 | + |
| ORF 23 | CGAACTGATATGTGACGGCAATCCACTTTCTGAGGTGCTCGGATTTCTTGCCAAGTATATGCCCA | U75698 | 40460 | 40396 | − |
| ORF 24 | AAGTGTAACCTAATCCCGAAAATCTATGCCCGAAACAAGAAGACCAGGCTAGACCAGTTGGGCCG | U75698 | 42597 | 42533 | − |
| ORF 25 | ACTACAGACCTCCAGTACGTCGTGGTCAACGGTACAGACGTGTTTTTGGACCAGCCTTGCCATAT | U75698 | 46677 | 46741 | + |
| ORF 26 | AATGTGTCATTTATGGGGCGCACATATCGTCTGGACGTAGACAACACGGATCCACGTACTGCCCT | U75698 | 47530 | 47594 | + |
| ORF 27 | TACCCCTTGCGACGTGTCGTGGGAAGATATCTACAACGGGACTTACCTAGCTCGGCCTGGAAACT | U93872 | 48787 | 48851 | + |
| ORF 28 | TGGTGCGAATGGCCACCAAGCCTCCCGTGATTGGTCTTATAACAGTGCTCTTCCTCCTAGTCATA | U40377 | 49047 | 49111 | + |
| ORF 29B | GCAGTTGTCATGGCCCATTTTATGGCTACCGATGATAGACACATGTACAAGCCCATATCCCCACA | U93872 | 50413 | 50349 | − |
| ORF 30 | ATGGGTGAGCCAGTGGATCCTGGACATGTGGTGAATGAGAAAGATTTTGAGGAGTGTGAACAATT | U75698 | 50623 | 50687 | + |
| ORF 31 | TGACAGGGACGCATATCACGGGATGCTAGCGTGTCTGAAACGGGACATTGTGCGGTATTTGCAGA | U75698 | 51077 | 51141 | + |
| ORF 32 | CTATTAAAGGCCGCTATAGGGCGTCGAAGGAGGATCTGGTGTTCATTCGAGGCCGCTATGGCTAG | U75698 | 52704 | 52766 | + |
| ORF 33 | GCGACCTGACCAATTGCACTATGGGTCTCGAATTCAGGAATGTGAACCCTTTTGTTTGGCTCGGG | U93872 | 53200 | 53264 | + |
| ORF 34 | CAGGTCACATGTACGCTCCCAAACGGGATCTTTTGTCGTTCGTTAATCATGCCCTGAAGTACACC | U75698 | 55243 | 55307 | + |
| ORF 35 | ATTAAGTCGGCTCTGGAGGCCAACATCAACAGGAGGGCAGCTGTATCGCTATTTGATCGTTTTGG | U75698 | 55669 | 55733 | + |
| ORF 36 | AGGTATTGGACCAACCGTACCCTATCAGCCCTAACATGGGACTGACCATCGACATGTCCTCGTTG | U75698 | 56835 | 56899 | + |
| ORF 37 | TTATCAGGTATTGCTGCAGAGTTCGATCGTCGAGGAGTACATTGGCCTAGATAGCGGCATTCCTC | U75698 | 58394 | 58458 | + |
| ORF 38 | GACCCCATTCGCGTTTCAGAAAAGGGCATGTTGCTTGAGCAATCGCAATCCCCATATCCCGCATT | U75698 | 61772 | 61836 | + |
| ORF 39 | CCCCGAGGACCCAATACCAGTCCGACCATGAAAGTGACAGTGAAATCGACGAAACGCAAATGATA | U75698 | 60169 | 60105 | − |
| ORF 40 | ACAGTGTTTGGGTACGACTCCCTGGCCATTTCAAGGGAATGTGAAGATCAGTATGTGTGGCCCAC | U75698 | 61280 | 61344 | + |
| ORF 41 | TACCTGGCTGCAAAGCTTAACCACATGCATTGAACGAGCCCTAAACATGCCTCCCGACACTTCCT | U75698 | 62045 | 62109 | + |
| ORF 42 | CATAACCGAGCTCTGCTGCCTGTTATCGATGCTCGAGAACTGTCGAGACATGTCACCAACGTTTT | U75698 | 62862 | 62798 | − |
| ORF 43 | GTCACCTATTCTCAGGACACCATATCCATCCTGCTTGGTCCATTCACGTATGTGATCGCGGACCT | U75698 | 64673 | 64609 | − |
| ORF 44 | TGACGCCCAGTCCCCATCCTCAAAGTACATCATCAAAGCCCTATGCAACCCCAAGACTACTCTGA | U75698 | 67186 | 67250 | + |
| ORF 45 | AAAGGGTCACCTCCCAACCCAATCTCCCAGTACTTCCGCCCACTCGATTTCATCAGGAAGCACAA | U75698 | 68478 | 68414 | − |
| ORF 46 | GCTCACACGAGGGACTTGGCTGGGATTGGTTCACGAGTTTCATCATCAGTAGCATATCCTCAAAG | U75698 | 69170 | 69106 | − |
| ORF 47 | CGAAGTTTGACGGCCTATACTGTAGGTTTTAACGCGACCACTGCAGATAGCTCTATTCACAACGT | U75698 | 69797 | 69733 | − |
| ORF 48 | CACCCCGGCATATGGAGTAGTTCTAGAGTGTGCTGATGATTCCGATGATTCATTGGATGACTTTT | U75698 | 71331 | 71267 | − |
| ORF 49 | CCCTGACACCCGACTGTTCGGACGTAGAGCTTGGCGAACTCTGCTCCCACCTACACCATTGTAAA | U75698 | 72514 | 72450 | − |
| ORF 50 | CGGAGTCTCCGGCGGATATACCGTCACCTTCTGGTGGAGAGTATACGCAACTGCAACCGGTCAGG | U71367 | 74478 | 74542 | + |
| K8 (bZIP, T1)* | CGTGTCATCGAAAGCATACACAAGACAGCTGCAGCAGGCATTAGAAGAAAAGGATGCACAACTAT | AF072865: (44) | (75529-75564/splice) | (splice/75646-75673) | + |
| K8 (T2 + T3)* | GGAAACAGGTGTCTATCTTGGCCGGCTGGTTACTCAAATGGGAACAATGGCGCCACCTTG | 44 | 75573 | 75633 | + |
| K8 (T3)* | GCGTTCAACGCCCAGCACAGCCCACACATGTCCTGTTTTCTCCTGTTTTTGTCTCTTTAA | 44 | 75410 | 75470 | + |
| K8 1A* | CCAAATGTCTCCGTATCTGTCGAAGATACGTCTGCCTCTGGGTCTGGAGAAGATGCAATAGATGA | AF068829; (13) | 76164 | 76228 | + |
| K8, 1B* | TAAACGGGACCAGACTAGCAGCTGGATCTCCGTCGAGATCATATTCATCTGGGGAACCAT | AF068830; (13) | (76120-75154/splice) | (splice/76432-75456) | + |
| K8 2 | GCTGGGACTTGTCCTTATACTTTACCTGTGCGTTCCACGATGCCGGCGTAAGAAACCCTACATAG | U71367; AF091348 | 76626 | 76690 | + |
| ORF 52 | CAGCAAGGTTGTAACACAAAAGCAAGTGGACGATGCCCTGAAGGGACTTTCGCTTAGAATCGACG | U75698 | 77099 | 77035 | − |
| ORF 53 | TACTAGGTCACTGCTGGGTTACGGCCAACTCGACAGGTGTCGCATCATCTACAGAGCGTTCTAGT | U75698 | 77440 | 77376 | − |
| ORF 54 | AAATAGCGTCCGAAAACATACCCACGAAGACAACCCCGTCCACGAACCCAACGTAGCCACCGCTT | U75698 | 78512 | 78576 | + |
| ORF 55 | TCAGACGACTCGGTAATATGGGCCTCTGAGATCAGCCACTCCTTGTCGGAACCCACTTCTGTATT | U75698 | 79294 | 79230 | − |
| ORF 56 | GATAGAGACCCTATCCGGTCGTTCAATAGAGGACTGGCTACACTCGGCCGTTTGGGATAAAGCAT | U75698 | 81604 | 81668 | + |
| ORF 57 | ATGGGAAACCGCTTAGTAGAGGCATGTAACCTTCTTGGCGAGGTCAAGCTTAACTTCAGGGGAGG | U93872 | 83794 | 83858 | + |
| K9 | AGCTATGCGATACGCTGGACGCATGTGCAAAAGGCATTCTGCTGACTAGCTCTTGTAATGGCATA | U75698 | 84879 | 84815 | − |
| K10 | ATACGTTGGGGCACCTATGTCAGAGTTTCGTACCAGAACTGCTTCGCATACCGCGTCTTACAGTC | U75698 | 87792 | 87728 | − |
| K10.5 | ATGTTCCCAGAACCCACTGGCAGACATTAGCCACTCTTGCTTGCATTCGCGCAAAGGGTTAAGAG | AY008303 | 90931 | 90867 | − |
| K11 | CCACGAGATCCAACAAGCTTTTGACGTGGAGCGACATAATCGAGAACCTGAAGGGTCCCGGTACG | U75698 | 93266 | 93202 | − |
| K11.1 | CGCTGGATTATGACACCTAGGCCATACAAGGGATGTGAAGGATGTCTTGTGTACTTGACGCAGGA | AF045550 | 94018 | 93954 | − |
| ORF 58 | GCGAAAACTCCACAGGGCACTTAACGCTCCACAGATGGTATTGGCCCTATGCACGGTTGGAAATT | U75698 | 95497 | 95433 | − |
| ORF 59 | AACTAAGGACAGCACAAAGAGGCCTCACAAGAGGCGCTCAGACTCGAGCCAGTCCAGGGATCGTG | U75698 | 96701 | 96637 | − |
| ORF 60 | TGTTAACGTGGTGGACATCCGGAGGTTCCTGGAAGCCACCGCTGACAGGATTCTGCGTGATATTT | U75698 | 97656 | 97592 | − |
| ORF 61 | GACAGAGTCAGGAATGCGAGCTATCTTAGGGACCTGCTGCTACATGGATACAGGCTTGGTCTAAA | U75698 | 100013 | 99949 | − |
| ORF 62 | GAAGGGCTTTGCGGAGGTTGTGGCCATGATAAAGGATCACTTTACGGATGTAATCCGGACCAAGT | U75698 | 101009 | 100945 | − |
| ORF 63 | ATCTTAACAGACTCAACCACCACATTCTCAGGATTCCCTTCCCACAGGACGCCCTTTCTGAACTC | U75698 | 103456 | 103520 | + |
| ORF 64 | ATAAATCGCCTCTTCCCAACCTCGTAGAGAGATACGCGCGGGGTTTCCTGGACACGCCCTCTGTA | U75698 | 111591 | 111655 | + |
| ORF 65 | TATCTGAGGAGGATGGGTGGAATTCAAAGGCGGGATCATCTGCAGACCCTTAGGGATCAGAAACC | U75698 | 112175 | 112111 | − |
| ORF 66 | TCTACTGTTCTTACTGTGGCAGCGAACATATGAGGGTGTATCCCCTGTGCGATATTACCGGACGC | U75698 | 113549 | 113485 | − |
| ORF 67 | GGTGTTCTCAAAGAACCTGGCCGTGTACAACTCCATGGTAATATGCCGTACCTACTTCACGGACT | U75698 | 114173 | 114109 | − |
| ORF 67.5 | TCATACTCTGGTGTTGGAGAGCAAGGTGGACACGGTAAGGCAGGTCCTGCGCAAGATCGTGAGCA | U93872 | 114767 | 114703 | − |
| ORF 68 | ACAGCTCCTCGATGCCAACACCCTGTTCGACTGCGAGGTCGTGCAGACTTTGGTCTTTCTCTTTA | U75698 | 116213 | 116277 | + |
| ORF 69 | TAGACTGCACCGACGAGATGCGGGATGACATTCAAAAGGGAACCGCACTTGTCAACGCCCTATAA | AF148805 | 117513 | 117577 | + |
| K12 | GAAATTGTGGCACTGACTTCGGCAGACGCCAAGTGGTGGATATAGAACCACCTTTCATGGCAGTA | AF148805 | 118052 | 117988 | − |
| K13 | GCATGACAGGGAAGTGGTATTGTTCCTCCTAAACGTGTTCATACCTCAACCCACACTCCCCCAAT | AF148805 | 122402 | 122338 | − |
| ORF 72 | TGTTAGTGGCCAGTAAGCTCAGAAGCCTCACGCCTATTTCTACCAGTTCACTTTGCTATGCCGCG | U40667 | 123157 | 123093 | − |
| ORF 73 | GAGGATGACGATGATGAGGACAATGAGGACGAGGAGGATGACGAGGAGGAGGACAAGAAGGAGGA | U52064 | 125080 | 125016 | − |
| K14 | CACCCCAAGTGAGGGAATATGTCTCATCACCTGGGGAAATGAGAGCATATCAATCCCGGCTTCTA | AF148805 | 128699 | 128763 | + |
| ORF 74 | CCTGGGATCCCTCTTTAGGCAGAGGATGTACGGTCTCTTCCAAAGCCTCAGGCAGTCTTTCATGT | AF148805 | 130319 | 130383 | + |
| ORF 75 | GGCCATGGCGTTCCAGTCAATCTACCTATGGAGCGTCAAGAAGATCCAACGACCACCACTAAACAT | U40394 | 134440 | 134375 | − |
| K15m* | AGATTACCAATATATACACCACACGACACACCACATGCTCATGCAGGCAGGATATGTCCCGATGT | AF156885; (64) | 136439 | 136375 | − |
| K15p* | GAGAAGGCGGCGCATATACACGCGGGACCAGAACTTACACCACAATGACAACCACCTTGGCAATA | U85269; (64) | 136422 | 136358 | − |
| hu CCL-18* | CCTCCTAACCAAGAGAGGCCGGCAGATCTGTGCTGACCCCAATAAGAAGTGGGTCCAGAAATACA | NM_002988; (26) | N/A | N/A | N/A |
All detector elements were designed by the Compugen lab on web software except those denoted by an asterisk. The target sequence designs were developed on the basis of the RNA sense (+) strand, which anneals to the anti-sense strand of labeled cDNA probe. Also shown are the accession numbers or reference numbers in parentheses and the genomic locations of the oligonucleotide detectors and their polarity or directionality.
KSHV oligonucleotide array fabrication.
Oligonucleotides were lyophilized in 96-well plates. The KSHV oligonucleotide master plate was solubilized to an initial 5× concentration of 1 μg/μl in TE buffer. DNA (25 μl) from each well of the master plate was then transferred to duplicate daughter U-bottom plates at the same original well position. A total of 100 μl of 3× SSC was added to each daughter plate to achieve a final 1× printing concentration of 10 μM or 200 ng/μl, and the mixture was sealed and stored at −80°C until ready for printing. The control human oligonucleotide housekeeping plate was directly solubilized to a 10 μM printing concentration with 3× SSC, sealed, and stored at −80°C until ready for printing. All microarray printing was performed using an OmniGrid arrayer (Gene Machines, San Carlos, Calif.) at the Advanced Technologies Center, Center for Cancer Research, National Cancer Institute. The 96-well storage plates for the cellular housekeeping and KSHV genes were robotically transferred to a 384-well printing plate. The housekeeping genes were transferred into triplicate wells. The oligonucleotides in the 384-well plate were spotted in duplicate onto poly-l-lysine-coated slides by use of SMP-3 spotting pins (Telechem International, Sunnyvale, Calif.), generating a total of eight subarrays containing 2 × 3 subarrays of housekeeping genes and 2 × 1 subarrays of viral genes. (An additional set of 384 human genes related to cell cycle control was also printed on the arrays but was not analyzed for this study.) The printed slides were allowed to dry for 1 to 3 days, UV cross-linked at 600 mJ, and blocked according to the protocol of Massimi et al. (50).
DNA microarray probe labeling and cohybridization.
The indirect-labeling method was performed according to J. Hasseman's standard operating procedure for aminoallyl labeling of RNA for microarrays from The Institute For Genomic Research (www.tigr.org). The hybridization method was performed according to the protocol described by Ideker et al. (31). The hybridized slides were then washed briefly at room temperature with 0.5× SSC and 0.01% SDS to remove the coverslip, followed by three successive room temperature washes with 0.5× SSC, 0.1× SSC, and 0.01× SSC for 2 min for each wash. Slides were dried by centrifugation and immediately scanned. Three independent time-course experiments were performed. The data obtained from experimental repeats were consistent.
Oligonucleotide microarray analysis.
The slides were scanned with a GenePix 4000 scanner (Axon Instruments, Inc., Foster City, Calif.) as previously described (63) and analyzed using Genepix Pro 4.0 (Axon Instruments) and a custom designed gene array list file generated by the Bioinformatics and Molecular Analysis Section (BIMAS) at the Center for Information Technology (CIT), NIH. The localized raw expression ratios were calculated following background subtraction, and the expression ratios were normalized using the expression ratios obtained for the control cellular housekeeping genes to obtain a calibrated expression ratio (CalRatio) (6, 16). CalRatio data were subsequently uploaded into the Center for Cancer Research Microarray Data Base (mAdb) (http://nciarray.nci.nih.gov/) for spot filtering and for clustering analysis using M. Eisen's Cluster and TreeView software (http://rana.lbl.gov/EisenSoftware.htm). The spot filtering criteria were a median target signal-over-background ratio of at least 1.5 and a spot diameter of at least 90 microns. A simple data set extraction was performed in which only the highest signal intensity ratio for each duplicate DNA spot was used in the subsequent analyses. There were 90 ORF detectors that passed initial spot filtering criteria and were used for statistical analysis.
Microarray data analysis and statistical considerations.
CalRatios were log2 transformed and clustered using Pearson's correlation coefficient with the hierarchical clustering algorithm and Cluster and TreeView in the mAdb analysis toolset. Graphical summaries of the data were prepared. For some analyses, results were normalized to the maximum CalRatio value for each ORF. To test the null hypothesis that CDV has no effect on an ORF's CalRatio, we used paired t tests on all the data collected in three separate biological replicates. In some cases, therefore, when the analysis included data from all time points, up to 30 data points per KSHV gene were used in the analysis. When either the early (up to 24 h) or the late (24 to 96 h) time points were used, up to 15 data points for each KSHV gene were included in the analysis. For some of these calculations, the JMP 5.0.1a statistical package (SAS Institute, Cary, N.C.) was used. All tests were two-sided. We first computed t tests for all time points (0 to 96 h) for each ORF. Since CDV is a viral DNA replication inhibitor and since the late lytic genes should show the largest difference from CDV results, we then split the samples and separately computed t statistics for the first half (early time points, 0 to 12 h) and the second half (late time points, 24 to 96 h) of the time course.
While there were 88 different ORF probes included in the array, there is a high correlation among the expression patterns of many of these ORFs. Some of them exist in polycistronic transcripts, some are controlled by the same regulatory element(s), and some may be controlled by functionally similar regulatory elements. Herpesviruses frequently have been considered to have three or four principal temporal expression classes. To control for multiple comparisons in our analysis, we adjusted the prechosen alpha level of 0.05 by the number of independent expression groups, which we conservatively estimated to be no larger than 10. We thus chose the significance level to be 0.005 for all our tests.
The data discussed in this paper have been deposited in the National Center for Biotechnology Information (NCBI) Gene Expression Omnibus (GEO) (www.ncbi.nlm.nih.gov/geo) and are accessible through GEO accession number GSE1640.
RESULTS
Effects of CDV on KSHV DNA and RNA synthesis.
CDV, a cytidine nucleotide analogue inhibitor of herpesvirus DNA polymerases, has been shown to effectively inhibit KSHV DNA polymerase and KSHV replication (35). We first sought to determine the concentrations of CDV that would effectively inhibit KSHV DNA replication and late gene expression in our system. BCBL-1 cells were treated with various concentrations of CDV for 14 h, and then KSHV was induced into lytic replication with TPA. The cells were harvested after 72 h, and total RNA was isolated from the cells. The relative amount of ORF 17 RNA was determined with a real-time RT-PCR assay. CDV decreased ORF 17 RNA production in a dose-dependent manner; CDV at 75 μM reduced mean ORF 17 expression to 19% (± 2% standard error) of the level in untreated cells (Fig. 1A). The presence of CDV at up to 100 μM had no effect on BCBL-1 cell viability (Fig. 1B).
FIG. 1.
The effect of CDV on KSHV RNA synthesis, cell viability, and virion production. (A) Real-time RT-PCR assay for KSHV ORF 17 RNA. RNA was harvested at 72 h after the induction of lytic replication from BCBL-1 cells pretreated with 0, 10, 25, 50, 75, and 100 μM CDV. The TPA-induced and CDV-treated ORF 17 cycle threshold (Ct) values were normalized against endogenous GAPDH Ct (dCt) values, and the Ct value for the latent background non-CDV-treated samples was subtracted to determine the net expression (ddCt). Data values were calculated as the amplification efficiency of the ORF 17 probe, one doubling per cycle, raised to the power of −ddCT (2−ddCt) to determine the change (n-fold) in expression. Data values from three independent experiments are plotted as the average percentages for the maximal TPA-induced, non-CDV-treated (0 μM) conditions set at 100% expression. (B) BCBL-1 cells with 100 μM CDV pretreatment (indicated with a white X on a black circle and a stippled line) and non-CDV-treated cells (black diamond with solid line) were induced with 20 ng of TPA/ml and grown in parallel with untreated cells (open triangle with dashed lines). Samples were collected at serial time points, and viability was determined with trypan blue staining. Data shown are the averages of three separate experiments. The presence of CDV appeared to have no effect on BCBL-1 cell viability. (C) Real-time PCR assay for virion DNA. Supernatant was collected at the indicated time points from untreated (open bar), TPA-induced (black bar), and TPA-induced and 100 μM CDV-treated (gray-shaded bar) cell culture samples and assayed for KSHV DNA after an initial DNase treatment, followed by SDS-proteinase K treatment and DNA extraction. CDV suppresses late KSHV replication in a dose-dependent manner and inhibits free virion DNA to near-baseline levels.
We tested the ability of CDV to inhibit KSHV virion production by use of an assay for non-DNase-sensitive KSHV DNA in the cell culture supernatant and found that 100 μM CDV blocked KSHV DNA replication, decreasing the amount of KSHV DNA produced following TPA induction to near-baseline levels (Fig. 1C).
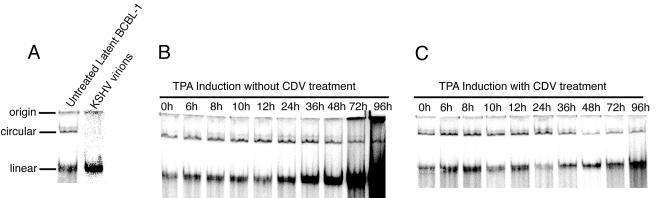
We assessed the effect of CDV treatment on KSHV viral DNA replication by use of a native agarose gel electrophoresis assay (21, 66) that distinguishes between linear viral DNA, presumably the result of viral DNA replication, and circular KSHV DNA, presumably representing the episomal version of the viral DNA. We found that the first increases in TPA-induced, KSHV DNA replication were seen by 24 h (Fig. 2), indicating that initiation of viral replication had occurred some time between 12 and 24 h of TPA addition. When the cells were treated with CDV, there were no observed increases in linear KSHV DNA for up to 96 h post-TPA addition, indicating that CDV had completely inhibited KSHV DNA replication.
FIG. 2.
The effect of CDV on de novo KSHV DNA replication in BCBL-1 cells. Approximately 5 × 105 BCBL-1 cells under all treatment conditions were resuspended in sample buffer A, loaded onto a 0.8% native vertical agarose gel, overlaid with lysis buffer, and electrophoresed initially at 0.8 V/cm for 3 h and then at 4.5 V/cm for 12 h at 4°C. Gels were subsequently blotted for KSHV DNA with a KSHV-specific probe. (A) Control lanes show the baseline KSHV DNA profile in untreated latent BCBL-1 cells and in KSHV virions, which show only the linear form. Latent BCBL-1 cells show both the episomal circular KSHV DNA form and the linear KSHV DNA form, an indication that viral replication had occurred. This observation is probably due to spontaneous lytic reactivation in a small percentage of the population of cells. (B) BCBL-1 cells induced with TPA in the absence of CDV treatment. The first onset of actively induced, de novo KSHV replication occurs some time between 12 and 24 h after TPA addition, as the linear KSHV DNA form steadily increases in amount from 24 to 96 h. (C) BCBL-1 cells induced with TPA in the presence of CDV. Active de novo KSHV replication is inhibited by CDV after TPA induction. Only the carryover background linear viral DNA still remains from the small percentage of cells that had spontaneously reactivated prior to CDV treatment and TPA addition, in similarity to the untreated latent KSHV DNA profile.
The effect of CDV on the KSHV gene expression program.
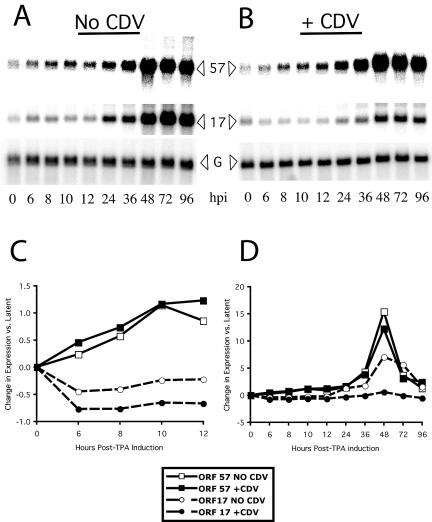
We used a newly designed and fabricated KSHV long oligonucleotide array to obtain genome-wide quantitative information about KSHV gene expression. The array contained 89 KSHV-specific detector elements, including detectors for essentially all the KSHV ORFs, with specific detectors for certain alternatively spliced RNA species and geographic variants (see Materials and Methods). The array also included a set of housekeeping genes for normalization purposes. To determine the effects of CDV on the KSHV gene expression program we collected samples in parallel at serial times (0, 6, 8, 10, 12, 24, 36, 48, 72, and 96 h) from uninduced BCBL-1 cells, from BCBL-1 cells treated with TPA to induce lytic KSHV replication, and from BCBL-1 cells treated with TPA in the presence of CDV. We isolated RNA from the samples and initially assessed RNA quality and the effectiveness of the TPA induction by use of RNA blots for an early KSHV gene, ORF 57, and a late KSHV gene, ORF 17 (Fig. 3). To assess the changes in ORF 57 and ORF 17 expression more quantitatively, we evaluated the signal intensity of the bands by use of a phosphor storage screen imager. The results are plotted in Fig. 3C (by use of an expanded scale for the early time points) and Fig. 3D (with plotting of all of the time points). We found that in the absence of CDV, ORF 57 began to show an increase in expression at relatively early times, first showing an increase in RNA levels by 6 h and continuing to increase in abundance later in the time course, while ORF 17 showed increases in expression at later times, first showing an increase at 24 h and peaking in abundance by 48 to 96 h. CDV had modest effects on ORF 57 expression but profoundly reduced the expression of ORF 17, as assessed using the RNA blots.
FIG. 3.
The effect of CDV on KSHV early and late gene expression. RNA was isolated from BCBL-1 cells induced with TPA in either the absence (A) or the presence (B) of CDV and assayed for the expression of a prototypical early KSHV gene, ORF 57, or a prototypical late KSHV gene, ORF 17, by use of RNA blotting. The blots were reprobed with GAPDH as a loading control (G). In the absence of CDV, ORF 57 showed increases in expression at early time points, while the expression of ORF 17 increased only later. CDV decreased the expression of ORF 17 but had little effect on ORF 57. The signal due to each band was quantitated using a phosphor storage screen imager and normalized to the signal due to GAPDH. The changes in expression for each gene at each time point were calculated by first normalizing the expression at each time point to the signal due to a GAPDH loading control and dividing by the background expression observed for the gene in uninduced, latent cells and then comparing the expression of the gene at each specified time point to the normalized expression of the gene at the 0 time point. (C) Data from the early time points (0 to 12 h after induction) in the time course, plotted on an expanded scale, show that the expression of ORF 57 increased before that of ORF 17 and that CDV had no effect on ORF 57. (D) The data from the entire time course show that in the absence of CDV the expression of ORF 17 increased later than the expression of ORF 57 and that the presence of CDV led to a marked decrease in the expression of ORF 17.
After establishing the effects of CDV on the expression of prototype early and late genes and on KSHV viral replication, we examined the effect of CDV on the global pattern of KSHV gene expression. Figure 4 shows the calibrated expression ratios (CalRatios) for KSHV genes after the induction of lytic replication in the absence or presence of CDV. These CalRatios compare the KSHV gene expression in the BCBL-1 cells induced into lytic replication (with and without CDV) to the expression in uninduced, latent reference BCBL-1 cells. (Fig. 4 also presents the results of additional statistical analyses discussed below.)
FIG. 4.
Statistical analysis of KSHV gene expression in response to the presence of CDV. Three independent experiments were performed. The results for each ORF were combined and grouped prior to calculations performed using a paired t test. Results for each ORF are displayed, ordered according to the physical location of the ORF in the KSHV genome. The calibrated expression ratios (CalRatio) are shown for each ORF time point, as taken from one experiment. The CalRatio is the normalized, background-subtracted median target signal intensity for the ORF from the induced cells divided by the normalized, background-subtracted median target signal intensity for the ORF in uninduced cells maintained and harvested in parallel. Statistical analyses were performed on all time points (0 to 96 hpi), the early time points (0 to 12 hpi), and the late time points (24 to 96 hpi). The table shows t ratios and associated P values. Levels of significance are color coded (blue, P ≥ 0.005; orange, 0.005 > P ≥ 0.001; red, 0.001> P > 0.0001; pink, P ≤ 0.0001). *, KSHV ORF descriptions or functional homologies are as generally described by Russo et al. (69) or Neipel et al. (61). See references 18, 19, 22, 30, 32, 33, 37, 38, 39, 41, 42, 43, 46, 59, 71, 73, 78, 79, and 84 for specific information concerning the ORFs, as indicated. S indicates CDV-sensitive genes; I indicates CDV-insensitive genes, as assessed using the t statistic (P ≤ 0.005) for the late data points.
In an initial effort to describe the effect of CDV on the KSHV gene expression program, we used hierarchical clustering analysis, Pearson's correlation coefficient, and a pair-wise average linkage algorithm (17) to group genes with similar expression patterns (Fig. 5). In the absence of CDV, the KSHV transcription program following induction of lytic replication by TPA closely resembled the patterns observed in previous studies, in which lytic replication was induced either by TPA or by the overexpression of the KSHV ORF 50 (Rta) (25, 49, 51, 58, 63, 75), with known immediate-early genes showing increases in expression relatively early after induction and known late genes, such as the virion structural genes, showing increases in expression later in the lytic program (Fig. 5A). For cells that were treated with CDV prior to TPA induction, the dendrogram is notably rearranged and shows marked changes in the pattern of KSHV gene expression (Fig. 5B). Some genes continue to be expressed at high levels in the presence of CDV; these genes are grouped in a large cluster near the top of the clustergram. After induction into lytic replication, the expression of a substantial number of genes, including many virion structural genes, is greatly inhibited by CDV, as evidenced by the many genes with black-colored blocks (indicating no change in expression compared to the uninduced reference cells) or green-colored blocks (indicating a reduction in expression compared to that of the uninduced reference cells) in the presence of CDV. This is particularly notable in comparison with the pattern seen in the absence of CDV, where there are many red-colored blocks (indicating an increase in expression compared to uninduced reference cells) and essentially no black- or green-colored squares at the later times. These CDV-sensitive genes were grouped together near the bottom in two separate clusters.
FIG.5.
The effect of CDV on KSHV global gene expression profiles assessed by hierarchical clustering. The column headings indicate the time after TPA induction of lytic replication. Each row shows the expression pattern of a single KSHV ORF element expressed as a log2-transformed expression ratio. Similar expression patterns, determined by Pearson's correlation coefficient, were clustered by an average-linkage algorithm. Expressed intensity is represented by the indicated color grid (see panel C legend). Green indicates lower expression compared to the mean, black denotes expression equal to the mean, and bright red indicates very high expression compared to the mean. (A) No CDV treatment. Most of the early genes are found near the bottom of the clustergram; late genes tend to be located near the top. (B) After 100 μM CDV treatment. The clustergram grouping of genes is substantially rearranged, with CDV-sensitive genes closely grouped together. Arrow 1 points at a node of a large cluster of CDV-insensitive KSHV genes. Genes expressed earlier tended to be less affected by the drug and are found near the top of the clustergram. Note the relative location of ORF 50 among a small group of genes that showed very early expression starting at 6 h and continued to be expressed throughout the time course. Arrows 2 and 3 indicate clusters that show genes that are sensitive and highly sensitive to CDV treatment, respectively. Note expression is reduced at the late time points. Although present in this figure, ORF elements 20a, 31, 43, 56, and K8.1B failed later quality-filtering criteria and were removed from further analyses. (C) Legend for color key. The top scale indicates the log2-transformed expression ratio. The bottom scale indicates the calibrated expression ratio, where the calibrated ratio is the expression in TPA-induced cells in the absence or presence of CDV treatment divided by the expression observed in uninduced latent reference cells.
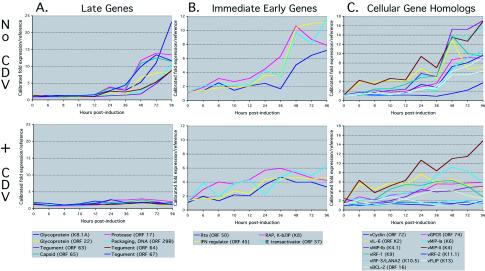
To illustrate the effect of CDV on selected classes of genes that would likely show either sensitivity to CDV or a lack of sensitivity to CDV, we chose 12 genes that included ORFs encoding putative viral structural proteins or putative immediate-early viral regulatory genes and transactivators (Fig. 6A and B). Since some of the cellular homolog accessory genes are believed to be critically involved in the pathogenesis of the cancers related to KSHV, we also show in Fig. 6C the effect of CDV on the expression of 11 selected cell homolog accessory genes related to cell signaling and growth, immune evasion, antiapoptosis, and angiogenesis. The presence of CDV dramatically suppressed the expression of all the KSHV late structural genes, indicating that, as expected, KSHV DNA replication is required for the expression of these genes. When we examined the effect of CDV on the expression pattern of the putative immediate-early genes and transactivators (36, 44, 49, 83), we found that all of these genes showed only modest decreases with CDV treatment. The modest decrease or plateau in expression of the immediate-early genes observed with CDV treatment after 24 h may result from the inhibition of KSHV DNA replication in the dividing BCBL-1 cells during the course of the experiment, causing a loss of newly accumulated viral genomic templates for further transcription.
FIG. 6.
CDV sensitivity of selected classes of KSHV genes. The graphs plot the ratio of the expression of the gene after induction to the expression of the gene without induction. (A) Late KSHV transcripts. Various known late genes coding for viral structural and assembly proteins show peak expression at 72 to 96 h (top graph). The presence of CDV completely abolishes the expression of these genes. (B) Selected immediate-early genes. The top graph shows the expression profiles of several ORFs: 50 (RTA), K8 b-ZIP (RAP), 45, and 57 (MTA). The expression of these genes is insensitive to the presence of CDV. (C) Selected KSHV accessory cellular homolog genes in the absence (top) or presence (bottom) of CDV treatment. Certain cellular gene homologs were sensitive to CDV (vFLIP, vIRF-2, vIRF-3/LANA2, and vMIP-III [vMIP-1b]). Other cellular gene homologs were insensitive (vMIP-1 [vMIP-1a], vMIP-11, vCyclin, vGPCR, vIL-6, vIRF-1, and vBcl-2). See also Fig. 4.
Some of the KSHV accessory cellular gene homologs, including vCyclin, vIL-6, vGPCR, vMIPs-I, -II, and -III, vFLIP, vBcl-2, and vIRF-1, vIRF-2, and vIRF-3/LANA2, likely play important roles in KSHV pathogenesis and the pathogenesis of the cancers related to KSHV (1-5, 7, 8, 20, 24, 47, 54, 68, 70, 81). Since the expression patterns for some of the accessory cellular gene homologs were unclear in previous studies and since a careful determination of the accessory cellular gene homolog expression patterns may offer additional insights into their role in viral replication and the pathogenesis of KSHV-related diseases, we paid particular attention to the expression patterns of these genes and to their sensitivity to CDV (Fig. 4, 6C, and 7). We defined CDV sensitivity as a statistically significant (P ≤ 0.005) change in the expression of the gene between CDV-treated and non-CDV-treated cells at the 24- through 96-h time points after TPA induction, the time when increases of viral linear DNA were observed (Fig. 2). The accessory cellular gene homologs that showed sensitivity to CDV treatment were vFLIP, vIRF-2, vIRF-3/LANA-2, and vMIP-III. The cellular gene homologs that were insensitive to CDV treatment included vMIP-I, vMIP-II, vGPCR, vIL-6, vIRF-1, vBcl-2 and vCyclin (CDV insensitivity was defined as being represented by a P value greater than 0.005).
FIG. 7.
Physical map of the KSHV genome, with corresponding expression profiles of each ORF. The colored ORF maps display the location of the ORFs; the genes are color coded according to putative functional or structural grouping (some genes may fall into more than one functional class). The expression profile for each ORF during the time course, in the presence and absence of CDV, is plotted as a bar graph and connected to the ORF's map location by a thin line. The expression of each ORF was normalized to the maximum expression of that gene. The colored bars represent the expression of the gene in the absence of CDV. Black bars represent the expression of the gene in the presence of CDV. The color of the bar represents the statistical significance of the difference between CDV-treated and non-CDV-treated cell results, as assessed using a paired two-tailed t test. Blue bars indicate no significant difference due to treatment. Yellow-to-pink bars represent increasing levels of significant negative difference as a result of CDV treatment (P < 0.005 to P ≪ 0.005) (see figure color key).
The values obtained from the statistical analysis, utilizing a paired Student's t test applied to the combined calibrated expression ratio results from three biological replicate experiments on the basis of 15 data points for tests applied to either only the late (24 to 96 h) or early (0 to 12 h) time points or on the basis of the complete set of 30 data points per ORF, are listed in Fig. 4 and are color coded by level of significance, as also shown in Fig. 7. The genes are displayed in Fig. 4 according to their physical order in the genome, beginning with ORF K1 and ending with ORF K15, with any alternative transcripts assigned the same order number. Although for Fig. 4 we made an overall classification of each gene as being CDV sensitive or CDV insensitive on the basis of the t statistic by the use of the late (24 to 96 h) data points, separate examinations of the early (0 to 12 h) data points and of all (0 to 96 h) data points also proved informative. When the early (0 to 12 h) time points were examined separately, it became apparent that CDV treatment was associated with a small but statistically significant increase in expression for some KSHV genes, including ORFs 10, K2, 70, and K4. These genes are located in close physical proximity to each other in the viral genome. For the late (24 to 96 h) time points, CDV strongly inhibited the expression of many genes, including known structural genes such as ORFs 17, 19, 22, 25, and 26, which are located closely together. Other genes physically located together that were sensitive to CDV included ORFs K10.5, K11, and K11.1 and ORFs 64 to 67. With the exception of one gene, ORF 56, which did not pass our data quality filters, none of the replication-related genes such as ORF 6, 9, 40, 41, 44, or 59 were significantly affected by CDV.
The analysis showed that all of the known virion structural genes expressed with late kinetics were significantly inhibited by CDV. In contrast, the expression of the immediate-early genes ORFs 50, 57, 45, and K8 Kb-ZIP did not show statistically significant inhibition by CDV, as expected. The more detailed assessment of the accessory cellular gene homologs showed that most of them were insensitive to CDV, indicating that these genes were expressed with immediate-early to early expression kinetics. These included vBcl-2, vCyclin (ORF 72), vMIP-I (ORF K6), vMIP-II (ORF K4), vGPCR (ORF 74), vIL-6 (ORF K2), and vIRF-1 (ORF K9). A few accessory cellular gene homologs, including two of the viral interferon regulatory factors, vIRF-2 (ORF K11.1) and vIRF-3/LANA2 (ORF K10.5) and angiogenesis-related chemokine vMIP-III (ORF K4.1), which exhibited late-expression kinetics, were significantly inhibited by CDV. The viral FLICE inhibitory protein homolog, vFLIP (ORF K13), was sensitive to CDV, as assessed at the late 24- to 96-h time points. Interestingly, four accessory cell homolog genes, vMIP-II, vGPCR, vIL-6, and vIRF-1, showed statistically significant increases in expression with CDV treatment during the early time period (0 to 12 h).
DISCUSSION
In the 1970s, several herpesvirus molecular virologists (28, 65, 76, 80) showed that herpesvirus gene expression followed a clear, temporally ordered program; these virologists used drugs that block herpesvirus replication at different stages to carefully delineate the expression program, showing which genes required viral DNA replication or protein synthesis for expression. Those studies provided crucial information concerning the replication and pathogenesis strategies of herpesviruses, showing, for example, that regulatory and trans-acting genes were expressed early, that genes involved in viral DNA replication were expressed in the middle of the replication cycle, and that genes encoding virion structural proteins were expressed at the end of the replication cycle. The advent of array technology now allows for the careful description of the expression patterns of the complete complement of KSHV genes, information that offers much helpful information concerning the replication and pathogenesis strategies of the virus and may guide efforts at devising therapies for the diseases caused by the virus (34, 63).
The KSHV genome map (Fig. 7) shows that there are regions of the viral genome where most of the ORFs exhibit CDV sensitivity or insensitivity. For example, the leftmost region (1 to ∼18 kb) contained primarily non-CDV-sensitive genes: ORF K1 to ORF 11. Further along the genome, from approximately positions 31 to 53 kb, there is a cluster of ORFs, from ORF 17 to ORF 33, transcribed in both directions, all exhibiting strong CDV sensitivity. These observations suggest that the regions containing ORFs with similar patterns of expression kinetics and sensitivity to CDV may be controlled in a coordinate fashion by common regulatory elements. The expression of some ORFs, however, is not closely coordinated with the expression of surrounding ORFs. For example, the lytic late gene ORF K8.1 (positions 76214 to 76508) is sensitive to CDV but is surrounded by non-CDV-sensitive genes. This suggests that its expression may be controlled by systems distinct from those controlling neighboring genes.
The use of CDV, together with that of the KSHV array-based assays, provides helpful information distinguishing the temporal expression patterns of those genes that depend on viral DNA replication for maximal transcription from those that do not, thus providing new or additional insights into the regulation of KSHV gene expression, particularly including the expression of the KSHV genes that may mediate the pathogenesis of the diseases associated with KSHV. Most of the genes sensitive to CDV were virion structural proteins. With the exception of ORF 63, all the genes encoding tegument proteins were sensitive to CDV. A total of 75% of the genes encoding capsid proteins were CDV sensitive. Interestingly, only ∼25% of genes with glycoprotein homology were CDV sensitive. The remaining ∼75%, which were CDV-insensitive and were expressed early in the lytic cycle, may be required by virus at a relatively early point in the replication cycle and so may not have a strictly structural function or may need to be expressed earlier in the replication cycle to allow for posttranslational modification or targeting to the required location within the cell by the appropriate time. Of the KSHV genes thought to be involved in viral DNA replication, 87% were CDV-insensitive, a finding that was anticipated. These genes are required for the replication of KSHV DNA, so it would be expected that they would be expressed before the time at which CDV acts. As expected, all of the major early regulators of transcription and replication, such as ORFs 50, 57, and K8 bZIP, were insensitive, with K8 showing small but significant early increases in expression after CDV treatment.
Roughly 40% of the KSHV accessory genes homologous to cellular genes are CDV sensitive, suggesting that these genes may be regulated so that they function during the later stages of KSHV replication. The CDV-insensitive accessory cellular gene homologs, expressed with early expression kinetics, likely prepare the host cell for the later stages of viral replication. Some examples include genes involved in activities directed at blocking host cell apoptosis during viral replication, genes aimed at thwarting the host immune response to protect the host cell against the immune response while the virus is replicating, genes that may increase blood supply to regions in which the host cell is located, or genes that may increase the likelihood of viral spread.
Four KSHV genes showed small, but statistically significant, early increases in expression with CDV: vGPCR has been shown to cause Kaposi sarcoma-like tumors in mice (4, 54); vIL-6, which can be activated by alpha interferon (IFN-α), antagonizes the IFN pathway and bypasses the gp80 subunit of interleukin-6R (IL-6R) to bind directly to the gp130 transducer, aiding in cell proliferation and survival (15, 53); vMIP-II, related to the RANTES CC chemokine, has been shown to promote angiogenesis in chick chorioallantoic membrane assays (7); and finally, vIRF-1 has been shown to inhibit transduction of IFN-β and downstream inducibility of p21WAF1/CIP1, leading to NIH3T3 cell foci formation and tumorigenesis in nude mice (20). The cause of the increases in early expression seen for these genes in the presence of CDV is unknown. Possibly they are subject to negative regulation by a late gene whose expression is suppressed by CDV. Perhaps more interesting, however, is the clinical significance of these findings. After KSHV was identified as an agent associated with KS (14) and CDV was found to strongly inhibit KSHV replication (35), CDV was initially considered as a candidate therapeutic agent for KS. However, a clinical trial of the use of CDV to treat KS showed that while CDV could decrease KSHV viral load, it failed to produce an improvement in the KS lesions and, instead, was associated with worsening KS disease, including the enlargement of existing lesions and the development of new lesions (45). All four genes that showed increases in expression with CDV (vGPCR, vIL-6, vIRF-1, and vMIP-II) have been implicated in the pathogenesis of KS or KS-like tumors, offering a rationale for the failure of CDV in the treatment of the disease. Thus, a careful study of the kinetics of KSHV gene expression and the systems that control viral replication and gene expression not only can yield interesting insights into the basic biology of the virus but also can produce information that can aid in the development of effective therapies for KSHV-associated disease.
Acknowledgments
We thank R. Little and V. Krishnan (HIV and AIDS Malignancy Branch, National Cancer Institute, NIH) for critical reading of the manuscript. We thank J. Powell, E. Asaki, and BIMAS, CIT, NIH, for providing us with informatics help with the viral gene array lists and web-based tools on mAdb for our analysis and uploading the datasets into the Gene Expression Omnibus Database. We also thank H. Yan and S. Zhao for assisting us with the robotic plate transfers and preparation of microarrays and the two anonymous reviewers for their helpful comments.
This work was supported in part by the NIH Intramural AIDS Targeted Antiviral Program.
REFERENCES
- 1.An, J., Y. Sun, R. Sun, and M. B. Rettig. 2003. Kaposi's sarcoma-associated herpesvirus encoded vFLIP induces cellular IL-6 expression: the role of the NF-kappaB and JNK/AP1 pathways. Oncogene 22:3371-3385. [DOI] [PubMed] [Google Scholar]
- 2.Aoki, Y., E. S. Jaffe, Y. Chang, K. Jones, J. Teruya-Feldstein, P. S. Moore, and G. Tosato. 1999. Angiogenesis and hematopoiesis induced by Kaposi's sarcoma-associated herpesvirus-encoded interleukin-6. Blood 93:4034-4043. [PubMed] [Google Scholar]
- 3.Arvanitakis, L., E. Geras-Raaka, A. Varma, M. C. Gershengorn, and E. Cesarman. 1997. Human herpesvirus KSHV encodes a constitutively active G-protein-coupled receptor linked to cell proliferation. Nature 385:347-350. [DOI] [PubMed] [Google Scholar]
- 4.Bais, C., B. Santomasso, O. Coso, L. Arvanitakis, E. G. Raaka, J. S. Gutkind, A. S. Asch, E. Cesarman, M. C. Gershengorn, E. A. Mesri, and M. C. Gerhengorn. 1998. G-protein-coupled receptor of Kaposi's sarcoma-associated herpesvirus is a viral oncogene and angiogenesis activator. Nature 391:86-89. [DOI] [PubMed] [Google Scholar]
- 5.Bellows, D. S., B. N. Chau, P. Lee, Y. Lazebnik, W. H. Burns, and J. M. Hardwick. 2000. Antiapoptotic herpesvirus Bcl-2 homologs escape caspase-mediated conversion to proapoptotic proteins. J. Virol. 74:5024-5031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bittner, M., P. Meltzer, Y. Chen, Y. Jiang, E. Seftor, M. Hendrix, M. Radmacher, R. Simon, Z. Yakhini, A. Ben-Dor, N. Sampas, E. Dougherty, E. Wang, F. Marincola, C. Gooden, J. Lueders, A. Glatfelter, P. Pollock, J. Carpten, E. Gillanders, D. Leja, K. Dietrich, C. Beaudry, M. Berens, D. Alberts, and V. Sondak. 2000. Molecular classification of cutaneous malignant melanoma by gene expression profiling. Nature 406:536-540. [DOI] [PubMed] [Google Scholar]
- 7.Boshoff, C., Y. Endo, P. D. Collins, Y. Takeuchi, J. D. Reeves, V. L. Schweickart, M. A. Siani, T. Sasaki, T. J. Williams, P. W. Gray, P. S. Moore, Y. Chang, and R. A. Weiss. 1997. Angiogenic and HIV-inhibitory functions of KSHV-encoded chemokines. Science 278:290-294. [DOI] [PubMed] [Google Scholar]
- 8.Burysek, L., and P. M. Pitha. 2001. Latently expressed human herpesvirus 8-encoded interferon regulatory factor 2 inhibits double-stranded RNA-activated protein kinase. J. Virol. 75:2345-2352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Burysek, L., W. S. Yeow, and P. M. Pitha. 1999. Unique properties of a second human herpesvirus 8-encoded interferon regulatory factor (vIRF-2). J. Hum. Virol. 2:19-32. [PubMed] [Google Scholar]
- 10.Cesarman, E., Y. Chang, P. S. Moore, J. W. Said, and D. M. Knowles. 1995. Kaposi's sarcoma-associated herpesvirus-like DNA sequences in AIDS-related body-cavity-based lymphomas. N. Engl. J. Med. 332:1186-1191. [DOI] [PubMed] [Google Scholar]
- 11.Cesarman, E., P. S. Moore, P. H. Rao, G. Inghirami, D. M. Knowles, and Y. Chang. 1995. In vitro establishment and characterization of two acquired immunodeficiency syndrome-related lymphoma cell lines (BC-1 and BC-2) containing Kaposi's sarcoma-associated herpesvirus-like (KSHV) DNA sequences. Blood 86:2708-2714. [PubMed] [Google Scholar]
- 12.Cesarman, E., R. G. Nador, F. Bai, R. A. Bohenzky, J. J. Russo, P. S. Moore, Y. Chang, and D. M. Knowles. 1996. Kaposi's sarcoma-associated herpesvirus contains G protein-coupled receptor and cyclin D homologs which are expressed in Kaposi's sarcoma and malignant lymphoma. J. Virol. 70:8218-8223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chandran, B., C. Bloomer, S. R. Chan, L. Zhu, E. Goldstein, and R. Horvat. 1998. Human herpesvirus-8 ORF K8.1 gene encodes immunogenic glycoproteins generated by spliced transcripts. Virology 249:140-149. [DOI] [PubMed] [Google Scholar]
- 14.Chang, Y., E. Cesarman, M. S. Pessin, F. Lee, J. Culpepper, D. M. Knowles, and P. S. Moore. 1994. Identification of herpesvirus-like DNA sequences in AIDS-associated Kaposi's sarcoma. Science 266:1865-1869. [DOI] [PubMed] [Google Scholar]
- 15.Chatterjee, M., J. Osborne, G. Bestetti, Y. Chang, and P. S. Moore. 2002. Viral IL-6-induced cell proliferation and immune evasion of interferon activity. Science 298:1432-1435. [DOI] [PubMed] [Google Scholar]
- 16.Chen, Y., V. Kamat, E. R. Dougherty, M. L. Bittner, P. S. Meltzer, and J. M. Trent. 2002. Ratio statistics of gene expression levels and applications to microarray data analysis. Bioinformatics 18:1207-1215. [DOI] [PubMed] [Google Scholar]
- 17.Eisen, M. B., P. T. Spellman, P. O. Brown, and D. Botstein. 1998. Cluster analysis and display of genome-wide expression patterns. Proc. Natl. Acad. Sci. USA 95:14863-14868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Endres, M. J., C. G. Garlisi, H. Xiao, L. Shan, and J. A. Hedrick. 1999. The Kaposi's sarcoma-related herpesvirus (KSHV)-encoded chemokine vMIP-I is a specific agonist for the CC chemokine receptor (CCR)8. J. Exp. Med. 189:1993-1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Feng, P., J. Park, B. S. Lee, S. H. Lee, R. J. Bram, and J. U. Jung. 2002. Kaposi's sarcoma-associated herpesvirus mitochondrial K7 protein targets a cellular calcium-modulating cyclophilin ligand to modulate intracellular calcium concentration and inhibit apoptosis. J. Virol. 76:11491-11504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gao, S. J., C. Boshoff, S. Jayachandra, R. A. Weiss, Y. Chang, and P. S. Moore. 1997. KSHV ORF K9 (vIRF) is an oncogene which inhibits the interferon signaling pathway. Oncogene 15:1979-1985. [DOI] [PubMed] [Google Scholar]
- 21.Gardella, T., P. Medveczky, T. Sairenji, and C. Mulder. 1984. Detection of circular and linear herpesvirus DNA molecules in mammalian cells by gel electrophoresis. J. Virol. 50:248-254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Glaunsinger, B., and D. Ganem. 2004. Lytic KSHV infection inhibits host gene expression by accelerating global mRNA turnover. Mol. Cell 13:713-723. [DOI] [PubMed] [Google Scholar]
- 23.Glenn, M., L. Rainbow, F. Aurade, A. Davison, and T. F. Schulz. 1999. Identification of a spliced gene from Kaposi's sarcoma-associated herpesvirus encoding a protein with similarities to latent membrane proteins 1 and 2A of Epstein-Barr virus. J. Virol. 73:6953-6963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Godden-Kent, D., S. J. Talbot, C. Boshoff, Y. Chang, P. Moore, R. A. Weiss, and S. Mittnacht. 1997. The cyclin encoded by Kaposi's sarcoma-associated herpesvirus stimulates cdk6 to phosphorylate the retinoblastoma protein and histone H1. J. Virol. 71:4193-4198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gradoville, L., J. Gerlach, E. Grogan, D. Shedd, S. Nikiforow, C. Metroka, and G. Miller. 2000. Kaposi's sarcoma-associated herpesvirus open reading frame 50/Rta protein activates the entire viral lytic cycle in the HH-B2 primary effusion lymphoma cell line. J. Virol. 74:6207-6212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Guan, P., A. H. Burghes, A. Cunningham, P. Lira, W. H. Brissette, K. Neote, and S. R. McColl. 1999. Genomic organization and biological characterization of the novel human CC chemokine DC-CK-1/PARC/MIP-4/SCYA18. Genomics 56:296-302. [DOI] [PubMed] [Google Scholar]
- 27.Haque, M., J. Chen, K. Ueda, Y. Mori, K. Nakano, Y. Hirata, S. Kanamori, Y. Uchiyama, R. Inagi, T. Okuno, and K. Yamanishi. 2000. Identification and analysis of the K5 gene of Kaposi's sarcoma-associated herpesvirus. J. Virol. 74:2867-2875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Honess, R. W., and B. Roizman. 1974. Regulation of herpesvirus macromolecular synthesis. I. Cascade regulation of the synthesis of three groups of viral proteins. J. Virol. 14:8-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Honess, R. W., and B. Roizman. 1975. Regulation of herpesvirus macromolecular synthesis: sequential transition of polypeptide synthesis requires functional viral polypeptides. Proc. Natl. Acad. Sci. USA 72:1276-1280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hu, J., A. C. Garber, and R. Renne. 2002. The latency-associated nuclear antigen of Kaposi's sarcoma-associated herpesvirus supports latent DNA replication in dividing cells. J. Virol. 76:11677-11687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ideker, T., S. Ybarra, and S. Grimmond. 2003. Hybridization and posthybridization washing, p. 228-239. In D. Bowtell and J. Sambrook (ed.), DNA microarrays, 1st ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 32.Ishido, S., C. Wang, B. S. Lee, G. B. Cohen, and J. U. Jung. 2000. Downregulation of major histocompatibility complex class I molecules by Kaposi's sarcoma-associated herpesvirus K3 and K5 proteins. J. Virol. 74:5300-5309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Izumiya, Y., S. F. Lin, T. J. Ellison, A. M. Levy, G. L. Mayeur, C. Izumiya, and H. J. Kung. 2003. Cell cycle regulation by Kaposi's sarcoma-associated herpesvirus K-bZIP: direct interaction with cyclin-CDK2 and induction of G1 growth arrest. J. Virol. 77:9652-9661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jones, P. C., and B. Roizman. 1979. Regulation of herpesvirus macromolecular synthesis. VIII. The transcription program consists of three phases during which both extent of transcription and accumulation of RNA in the cytoplasm are regulated. J. Virol. 31:299-314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kedes, D. H., and D. Ganem. 1997. Sensitivity of Kaposi's sarcoma-associated herpesvirus replication to antiviral drugs. Implications for potential therapy. J. Clin. Investig. 99:2082-2086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kirshner, J. R., D. M. Lukac, J. Chang, and D. Ganem. 2000. Kaposi's sarcoma-associated herpesvirus open reading frame 57 encodes a posttranscriptional regulator with multiple distinct activities. J. Virol. 74:3586-3597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kledal, T. N., M. M. Rosenkilde, F. Coulin, G. Simmons, A. H. Johnsen, S. Alouani, C. A. Power, H. R. Luttichau, J. Gerstoft, P. R. Clapham, I. Clark-Lewis, T. N. Wells, and T. W. Schwartz. 1997. A broad-spectrum chemokine antagonist encoded by Kaposi's sarcoma-associated herpesvirus. Science 277:1656-1659. [DOI] [PubMed] [Google Scholar]
- 38.Kliche, S., W. Nagel, E. Kremmer, C. Atzler, A. Ege, T. Knorr, U. Koszinowski, W. Kolanus, and J. Haas. 2001. Signaling by human herpesvirus 8 kaposin A through direct membrane recruitment of cytohesin-1. Mol. Cell 7:833-843. [DOI] [PubMed] [Google Scholar]
- 39.Lee, B. S., X. Alvarez, S. Ishido, A. A. Lackner, and J. U. Jung. 2000. Inhibition of intracellular transport of B cell antigen receptor complexes by Kaposi's sarcoma-associated herpesvirus K1. J. Exp. Med. 192:11-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Li, M., H. Lee, D. W. Yoon, J. C. Albrecht, B. Fleckenstein, F. Neipel, and J. U. Jung. 1997. Kaposi's sarcoma-associated herpesvirus encodes a functional cyclin. J. Virol. 71:1984-1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Li, M., J. MacKey, S. C. Czajak, R. C. Desrosiers, A. A. Lackner, and J. U. Jung. 1999. Identification and characterization of Kaposi's sarcoma-associated herpesvirus K8.1 virion glycoprotein. J. Virol. 73:1341-1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Liao, W., Y. Tang, S. F. Lin, H. J. Kung, and C. Z. Giam. 2003. K-bZIP of Kaposi's sarcoma-associated herpesvirus/human herpesvirus 8 (KSHV/HHV-8) binds KSHV/HHV-8 Rta and represses Rta-mediated transactivation. J. Virol. 77:3809-3815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lin, C. L., H. Li, Y. Wang, F. X. Zhu, S. Kudchodkar, and Y. Yuan. 2003. Kaposi's sarcoma-associated herpesvirus lytic origin (ori-Lyt)-dependent DNA replication: identification of the ori-Lyt and association of K8 bZip protein with the origin. J. Virol. 77:5578-5588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lin, S. F., D. R. Robinson, G. Miller, and H. J. Kung. 1999. Kaposi's sarcoma-associated herpesvirus encodes a bZIP protein with homology to BZLF1 of Epstein-Barr virus. J. Virol. 73:1909-1917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Little, R. F., F. Merced-Galindez, K. Staskus, D. Whitby, Y. Aoki, R. Humphrey, J. M. Pluda, V. Marshall, M. Walters, L. Welles, I. R. Rodriguez-Chavez, S. Pittaluga, G. Tosato, and R. Yarchoan. 2003. A pilot study of cidofovir in patients with kaposi sarcoma. J. Infect. Dis. 187:149-153. [DOI] [PubMed] [Google Scholar]
- 46.Lorenzo, M. E., J. U. Jung, and H. L. Ploegh. 2002. Kaposi's sarcoma-associated herpesvirus K3 utilizes the ubiquitin-proteasome system in routing class major histocompatibility complexes to late endocytic compartments. J. Virol. 76:5522-5531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lubyova, B., M. J. Kellum, A. J. Frisancho, and P. M. Pitha. 2003. Kaposi's sarcoma-associated herpesvirus-encoded vIRF-3 stimulates the transcriptional activity of cellular IRF-3 and IRF-7. J. Biol. Chem. 279:7643-7654. [DOI] [PubMed] [Google Scholar]
- 48.Lubyova, B., and P. M. Pitha. 2000. Characterization of a novel human herpesvirus 8-encoded protein, vIRF-3, that shows homology to viral and cellular interferon regulatory factors. J. Virol. 74:8194-8201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lukac, D. M., J. R. Kirshner, and D. Ganem. 1999. Transcriptional activation by the product of open reading frame 50 of Kaposi's sarcoma-associated herpesvirus is required for lytic viral reactivation in B cells. J. Virol. 73:9348-9361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Massimi, A., T. Harris, G. Childs, and S. Somerville. 2003. Printing on glass slides, p. 71-78. In D. Bowtell and J. Sambrook (ed.), DNA microarrays, 1st ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 51.Miller, G., L. Heston, E. Grogan, L. Gradoville, M. Rigsby, R. Sun, D. Shedd, V. M. Kushnaryov, S. Grossberg, and Y. Chang. 1997. Selective switch between latency and lytic replication of Kaposi's sarcoma herpesvirus and Epstein-Barr virus in dually infected body cavity lymphoma cells. J. Virol. 71:314-324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Miller, G., M. O. Rigsby, L. Heston, E. Grogan, R. Sun, C. Metroka, J. A. Levy, S. J. Gao, Y. Chang, and P. Moore. 1996. Antibodies to butyrate-inducible antigens of Kaposi's sarcoma-associated herpesvirus in patients with HIV-1 infection. N. Engl. J. Med. 334:1292-1297. [DOI] [PubMed] [Google Scholar]
- 53.Molden, J., Y. Chang, Y. You, P. S. Moore, and M. A. Goldsmith. 1997. A Kaposi's sarcoma-associated herpesvirus-encoded cytokine homolog (vIL-6) activates signaling through the shared gp130 receptor subunit. J. Biol. Chem. 272:19625-19631. [DOI] [PubMed] [Google Scholar]
- 54.Montaner, S., A. Sodhi, A. Molinolo, T. H. Bugge, E. T. Sawai, Y. He, Y. Li, P. E. Ray, and J. S. Gutkind. 2003. Endothelial infection with KSHV genes in vivo reveals that vGPCR initiates Kaposi's sarcomagenesis and can promote the tumorigenic potential of viral latent genes. Cancer Cell 3:23-36. [DOI] [PubMed] [Google Scholar]
- 55.Moore, P. S., and Y. Chang. 2001. Kaposi's sarcoma-associated herpesvirus, p. 2803-2833. In D. M. Knipe and P. M. Howley (ed.), Fields virology, 4th ed., vol. 2. Lippincott Williams & Wilkins, Philadelphia, Pa. [Google Scholar]
- 56.Moore, P. S., and Y. Chang. 1995. Detection of herpesvirus-like DNA sequences in Kaposi's sarcoma in patients with and without HIV infection. N. Engl. J. Med. 332:1181-1185. [DOI] [PubMed] [Google Scholar]
- 57.Moore, P. S., S. J. Gao, G. Dominguez, E. Cesarman, O. Lungu, D. M. Knowles, R. Garber, P. E. Pellett, D. J. McGeoch, and Y. Chang. 1996. Primary characterization of a herpesvirus agent associated with Kaposi's sarcomae. J. Virol. 70:549-558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Nakamura, H., M. Lu, Y. Gwack, J. Souvlis, S. L. Zeichner, and J. U. Jung. 2003. Global changes in Kaposi's sarcoma-associated virus gene expression patterns following expression of a tetracycline-inducible Rta transactivator. J. Virol. 77:4205-4220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Nealon, K., W. W. Newcomb, T. R. Pray, C. S. Craik, J. C. Brown, and D. H. Kedes. 2001. Lytic replication of Kaposi's sarcoma-associated herpesvirus results in the formation of multiple capsid species: isolation and molecular characterization of A, B, and C capsids from a gammaherpesvirus. J. Virol. 75:2866-2878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Neipel, F., J. C. Albrecht, A. Ensser, Y. Q. Huang, J. J. Li, A. E. Friedman-Kien, and B. Fleckenstein. 1997. Human herpesvirus 8 encodes a homolog of interleukin-6. J. Virol. 71:839-842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Neipel, F., J. C. Albrecht, and B. Fleckenstein. 1997. Cell-homologous genes in the Kaposi's sarcoma-associated rhadinovirus human herpesvirus 8: determinants of its pathogenicity? J. Virol. 71:4187-4192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Nishimura, K., K. Ueda, S. Sakakibara, K. Ishikawa, J. Chen, T. Okuno, and K. Yamanishi. 2001. Functional analysis of Kaposi's sarcoma-associated herpesvirus RTA in an RTA-depressed cell line. J. Hum. Virol. 4:296-305. [PubMed] [Google Scholar]
- 63.Paulose-Murphy, M., N. K. Ha, C. Xiang, Y. Chen, L. Gillim, R. Yarchoan, P. Meltzer, M. Bittner, J. Trent, and S. Zeichner. 2001. Transcription program of human herpesvirus 8 (Kaposi's sarcoma-associated herpesvirus). J. Virol. 75:4843-4853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Poole, L. J., J. C. Zong, D. M. Ciufo, D. J. Alcendor, J. S. Cannon, R. Ambinder, J. M. Orenstein, M. S. Reitz, and G. S. Hayward. 1999. Comparison of genetic variability at multiple loci across the genomes of the major subtypes of Kaposi's sarcoma-associated herpesvirus reveals evidence for recombination and for two distinct types of open reading frame K15 alleles at the right-hand end. J. Virol. 73:6646-6660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Powell, K. L., D. J. Purifoy, and R. J. Courtney. 1975. The synthesis of herpes simplex virus proteins in the absence of virus DNA synthesis. Biochem. Biophys. Res. Commun. 66:262-271. [DOI] [PubMed] [Google Scholar]
- 66.Renne, R., M. Lagunoff, W. Zhong, and D. Ganem. 1996. The size and conformation of Kaposi's sarcoma-associated herpesvirus (human herpesvirus 8) DNA in infected cells and virions. J. Virol. 70:8151-8154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Renne, R., W. Zhong, B. Herndier, M. McGrath, N. Abbey, D. Kedes, and D. Ganem. 1996. Lytic growth of Kaposi's sarcoma-associated herpesvirus (human herpesvirus 8) in culture. Nat. Med. 2:342-346. [DOI] [PubMed] [Google Scholar]
- 68.Rivas, C., A. E. Thlick, C. Parravicini, P. S. Moore, and Y. Chang. 2001. Kaposi's sarcoma-associated herpesvirus LANA2 is a B-cell-specific latent viral protein that inhibits p53. J. Virol. 75:429-438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Russo, J. J., R. A. Bohenzky, M. C. Chien, J. Chen, M. Yan, D. Maddalena, J. P. Parry, D. Peruzzi, I. S. Edelman, Y. Chang, and P. S. Moore. 1996. Nucleotide sequence of the Kaposi sarcoma-associated herpesvirus (HHV8). Proc. Natl. Acad. Sci. USA 93:14862-14867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Sarid, R., T. Sato, R. A. Bohenzky, J. J. Russo, and Y. Chang. 1997. Kaposi's sarcoma-associated herpesvirus encodes a functional bcl-2 homologue. Nat. Med. 3:293-298. [DOI] [PubMed] [Google Scholar]
- 71.Sharp, T. V., H. W. Wang, A. Koumi, D. Hollyman, Y. Endo, H. Ye, M. Q. Du, and C. Boshoff. 2002. K15 protein of Kaposi's sarcoma-associated herpesvirus is latently expressed and binds to HAX-1, a protein with antiapoptotic function. J. Virol. 76:802-816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Soulier, J., L. Grollet, E. Oksenhendler, P. Cacoub, D. Cazals-Hatem, P. Babinet, M. F. d'Agay, J. P. Clauvel, M. Raphael, L. Degos, et al. 1995. Kaposi's sarcoma-associated herpesvirus-like DNA sequences in multicentric Castleman's disease. Blood 86:1276-1280. [PubMed] [Google Scholar]
- 73.Stine, J. T., C. Wood, M. Hill, A. Epp, C. J. Raport, V. L. Schweickart, Y. Endo, T. Sasaki, G. Simmons, C. Boshoff, P. Clapham, Y. Chang, P. Moore, P. W. Gray, and D. Chantry. 2000. KSHV-encoded CC chemokine vMIP-III is a CCR4 agonist, stimulates angiogenesis, and selectively chemoattracts TH2 cells. Blood 95:1151-1157. [PubMed] [Google Scholar]
- 74.Sun, R., S. F. Lin, L. Gradoville, and G. Miller. 1996. Polyadenylylated nuclear RNA encoded by Kaposi sarcoma-associated herpesvirus. Proc. Natl. Acad. Sci. USA 93:11883-11888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Sun, R., S. F. Lin, L. Gradoville, Y. Yuan, F. Zhu, and G. Miller. 1998. A viral gene that activates lytic cycle expression of Kaposi's sarcoma-associated herpesvirus. Proc. Natl. Acad. Sci. USA 95:10866-10871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Swanstrom, R. I., and E. K. Wagner. 1974. Regulation of synthesis of herpes simplex type 1 virus mRNA during productive infection. Virology 60:522-533. [DOI] [PubMed] [Google Scholar]
- 77.Thome, M., P. Schneider, K. Hofmann, H. Fickenscher, E. Meinl, F. Neipel, C. Mattmann, K. Burns, J. L. Bodmer, M. Schroter, C. Scaffidi, P. H. Krammer, M. E. Peter, and J. Tschopp. 1997. Viral FLICE-inhibitory proteins (FLIPs) prevent apoptosis induced by death receptors. Nature 386:517-521. [DOI] [PubMed] [Google Scholar]
- 78.Unal, A., T. R. Pray, M. Lagunoff, M. W. Pennington, D. Ganem, and C. S. Craik. 1997. The protease and the assembly protein of Kaposi's sarcoma-associated herpesvirus (human herpesvirus 8). J. Virol. 71:7030-7038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Wang, S. E., F. Y. Wu, Y. Yu, and G. S. Hayward. 2003. CCAAT/enhancer-binding protein-alpha is induced during the early stages of Kaposi's sarcoma-associated herpesvirus (KSHV) lytic cycle reactivation and together with the KSHV replication and transcription activator (RTA) cooperatively stimulates the viral RTA, MTA, and PAN promoters. J. Virol. 77:9590-9612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ward, R. L., and J. G. Stevens. 1975. Effect of cytosine arabinoside on viral-specific protein synthesis in cells infected with herpes simplex virus. J. Virol. 15:71-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Yang, T. Y., S. C. Chen, M. W. Leach, D. Manfra, B. Homey, M. Wiekowski, L. Sullivan, C. H. Jenh, S. K. Narula, S. W. Chensue, and S. A. Lira. 2000. Transgenic expression of the chemokine receptor encoded by human herpesvirus 8 induces an angioproliferative disease resembling Kaposi's sarcoma. J. Exp. Med. 191:445-454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Zhong, W., H. Wang, B. Herndier, and D. Ganem. 1996. Restricted expression of Kaposi sarcoma-associated herpesvirus (human herpesvirus 8) genes in Kaposi sarcoma. Proc. Natl. Acad. Sci. USA 93:6641-6646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Zhu, F. X., T. Cusano, and Y. Yuan. 1999. Identification of the immediate-early transcripts of Kaposi's sarcoma-associated herpesvirus. J. Virol. 73:5556-5567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Zhu, F. X., S. M. King, E. J. Smith, D. E. Levy, and Y. Yuan. 2002. A Kaposi's sarcoma-associated herpesviral protein inhibits virus-mediated induction of type I interferon by blocking IRF-7 phosphorylation and nuclear accumulation. Proc. Natl. Acad. Sci. USA 99:5573-5578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Zong, J. C., D. M. Ciufo, D. J. Alcendor, X. Wan, J. Nicholas, P. J. Browning, P. L. Rady, S. K. Tyring, J. M. Orenstein, C. S. Rabkin, I. J. Su, K. F. Powell, M. Croxson, K. E. Foreman, B. J. Nickoloff, S. Alkan, and G. S. Hayward. 1999. High-level variability in the ORF-K1 membrane protein gene at the left end of the Kaposi's sarcoma-associated herpesvirus genome defines four major virus subtypes and multiple variants or clades in different human populations. J. Virol. 73:4156-4170. [DOI] [PMC free article] [PubMed] [Google Scholar]