Abstract
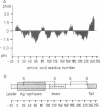
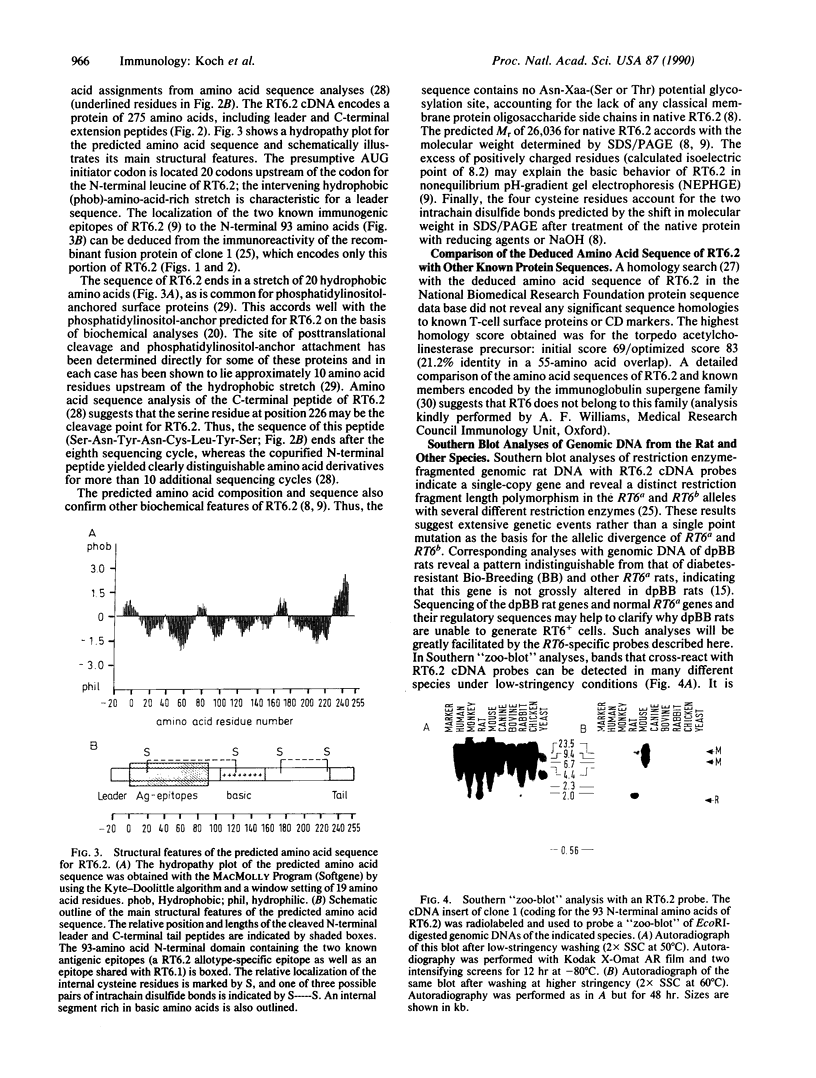
RT6 is an unusual cell membrane protein that is expressed exclusively by postthymic T cells. The inherent defect in its expression has been correlated to lymphopenia and genetically determined susceptibility for insulin-dependent diabetes mellitus in the rat. We report here the primary structure of the RT6.2 alloantigen as deduced from the cDNA sequence. The predicted amino acid sequence of RT6.2 begins with a conventional leader of 20 amino acids and ends in a hydrophobic C-terminal extension peptide of 29 amino acids as is common for phosphatidylinositol-anchored proteins. Native RT6.2 is predicted to comprise 226 amino acids, with a calculated Mr of 26,036. Four cysteine residues account for two intrachain disulfide bonds. The sequence lacks potential N-glycosylation sites and contains an excess of positively charged residues. Homology searches in protein sequence data banks suggest that RT6.2 is not encoded by a member of the immunoglobulin supergene family. Moreover, these analyses did not reveal any close homologies of RT6.2 to known proteins: the highest homology found was 21.2% identity in a 52-amino acid overlap to the torpedo acetylcholinesterase precursor. Southern blot analyses indicate that RT6.2 is the product of a single-copy gene and provide evidence for closely related genes in the mouse and other species. The corresponding gene products remain to be identified.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Butcher G. W., Clarke S., Tucker E. M. Close linkage of peripheral T-lymphocyte antigen A (PtaA) to the hemoglobin variant Hbb on linkage group I of the rat. Transplant Proc. 1979 Sep;11(3):1629–1630. [PubMed] [Google Scholar]
- DeWitt C. W., MCCullough M. Ag-F: serological and genetic identification of a new locus in the rat governing lymphocyte membrane antigens. Transplantation. 1975 Apr;19(4):310–317. [PubMed] [Google Scholar]
- Ferguson M. A., Williams A. F. Cell-surface anchoring of proteins via glycosyl-phosphatidylinositol structures. Annu Rev Biochem. 1988;57:285–320. doi: 10.1146/annurev.bi.57.070188.001441. [DOI] [PubMed] [Google Scholar]
- Greiner D. L., Handler E. S., Nakano K., Mordes J. P., Rossini A. A. Absence of the RT-6 T cell subset in diabetes-prone BB/W rats. J Immunol. 1986 Jan;136(1):148–151. [PubMed] [Google Scholar]
- Greiner D. L., Mordes J. P., Handler E. S., Angelillo M., Nakamura N., Rossini A. A. Depletion of RT6.1+ T lymphocytes induces diabetes in resistant biobreeding/Worcester (BB/W) rats. J Exp Med. 1987 Aug 1;166(2):461–475. doi: 10.1084/jem.166.2.461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard J. C., Scott D. W. The identification of sera distinguishing marrow-derived and thymus-derived lymphocytes in the rat thoracic duct. Immunology. 1974 Nov;27(5):903–922. [PMC free article] [PubMed] [Google Scholar]
- Jackson R. A., Buse J. B., Rifai R., Pelletier D., Milford E. L., Carpenter C. B., Eisenbarth G. S., Williams R. M. Two genes required for diabetes in BB rats. Evidence from cyclical intercrosses and backcrosses. J Exp Med. 1984 Jun 1;159(6):1629–1636. doi: 10.1084/jem.159.6.1629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kashan A., Buck F., Haag F., Koch F., Thiele H. G. A single-step purification procedure and partial amino acid sequence analysis of picomole amounts of the rat T cell alloantigen RT6.2. Immunol Lett. 1989 Dec;23(2):133–137. doi: 10.1016/0165-2478(89)90125-9. [DOI] [PubMed] [Google Scholar]
- Koch F., Haag F., Kashan A., Thiele H. G. Construction of a rat T-cell hybridoma cDNA expression library and isolation of a cDNA clone for the rat T-cell differentiation marker RT6.2. Immunology. 1989 Jul;67(3):344–350. [PMC free article] [PubMed] [Google Scholar]
- Koch F., Kashan A., Thiele H. G. Production of a rat T cell hybridoma that stably expresses the T cell differentiation marker RT6.2. Hybridoma. 1988 Aug;7(4):341–353. doi: 10.1089/hyb.1988.7.341. [DOI] [PubMed] [Google Scholar]
- Koch F., Kashan A., Thiele H. G. The rat T-cell differentiation marker RT6.1 is more polymorphic than its alloantigenic counterpart RT6.2. Immunology. 1988 Oct;65(2):259–265. [PMC free article] [PubMed] [Google Scholar]
- Koch F., Thiele H. G., Low M. G. Release of the rat T cell alloantigen RT-6.2 from cell membranes by phosphatidylinositol-specific phospholipase C. J Exp Med. 1986 Oct 1;164(4):1338–1343. doi: 10.1084/jem.164.4.1338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipman D. J., Pearson W. R. Rapid and sensitive protein similarity searches. Science. 1985 Mar 22;227(4693):1435–1441. doi: 10.1126/science.2983426. [DOI] [PubMed] [Google Scholar]
- Low M. G., Saltiel A. R. Structural and functional roles of glycosyl-phosphatidylinositol in membranes. Science. 1988 Jan 15;239(4837):268–275. doi: 10.1126/science.3276003. [DOI] [PubMed] [Google Scholar]
- Lubaroff D. M., Greiner D. L., Reynolds C. W. Investigations of T-lymphocyte subpopulations in the rat using alloantigenic markers. Transplant Proc. 1979 Mar;11(1):1092–1094. [PubMed] [Google Scholar]
- Matsumoto K., McCafferty E., Neilson E. G., Gasser D. L. Mapping of the genes for tubular basement membrane antigen and a submaxillary gland protease in the rat. Immunogenetics. 1984;20(2):117–123. doi: 10.1007/BF00364483. [DOI] [PubMed] [Google Scholar]
- Miller C., DeWitt C. Rat alloantibody responses against strong and weak histocompatibility antigens. J Immunol. 1972 Nov;109(5):919–926. [PubMed] [Google Scholar]
- Nadeau J. H., Reiner A. H. Linkage and synteny homologies in mouse and man. Curr Top Microbiol Immunol. 1988;137:39–40. doi: 10.1007/978-3-642-50059-6_6. [DOI] [PubMed] [Google Scholar]
- Saiki R. K., Gelfand D. H., Stoffel S., Scharf S. J., Higuchi R., Horn G. T., Mullis K. B., Erlich H. A. Primer-directed enzymatic amplification of DNA with a thermostable DNA polymerase. Science. 1988 Jan 29;239(4839):487–491. doi: 10.1126/science.2448875. [DOI] [PubMed] [Google Scholar]
- Stutman O. Postthymic T-cell development. Immunol Rev. 1986 Jun;91:159–194. doi: 10.1111/j.1600-065x.1986.tb01488.x. [DOI] [PubMed] [Google Scholar]
- Thiele H. G., Arndt R., Stark R., Wonigeit K. Detection and partial molecular characterization of the rat T-lymphocyte surface protein L21 by allo-(anti-RT-Ly-2.2) and xeno-(anti-RT-LN-LyIg) sera. Transplant Proc. 1979 Sep;11(3):1636–1638. [PubMed] [Google Scholar]
- Thiele H. G., Koch F., Haag F., Wurst W. Evidence for normal thymic export of lymphocytes and an intact RT6a gene in RT6 deficient diabetes prone BB-rats. Thymus. 1989;14(1-3):137–143. [PubMed] [Google Scholar]
- Thiele H. G., Koch F., Hamann A., Arndt R. Biochemical characterization of the T-cell alloantigen RT-6.2. Immunology. 1986 Oct;59(2):195–201. [PMC free article] [PubMed] [Google Scholar]
- Williams A. F., Barclay A. N. The immunoglobulin superfamily--domains for cell surface recognition. Annu Rev Immunol. 1988;6:381–405. doi: 10.1146/annurev.iy.06.040188.002121. [DOI] [PubMed] [Google Scholar]
- Wonigeit K. Characterization of the RT-Ly-1 and RT-Ly-2 alloantigenic systems by congenic rat strains. Transplant Proc. 1979 Sep;11(3):1631–1635. [PubMed] [Google Scholar]