Abstract
FK506 binding protein 51(FKBP5) is a co-chaperone of heat shock protein 90 and significantly influences glucocorticoid receptor sensitivity. Single nucleotide polymorphisms (SNPs) in the FKBP5 gene are associated with altered hypothalamus-pituitary-adrenal (HPA) axis function, changes in the structure and function of several cognitive brain areas, and increased susceptibility to post-traumatic stress disorder, major depression, bipolar disorder, and suicidal events. The mechanisms underlying these associations are largely unknown, but it has been speculated that the influence of these SNPs on emotional memory systems may play a role. In the present study, 112 participants were exposed to the socially evaluated cold pressor test (stress) or control (no stress) conditions immediately prior to learning a list of 42 words. Participant memory was assessed immediately after learning (free recall) and 24 h later (free recall and recognition). Participants provided a saliva sample that enabled the genotyping of three FKBP5 polymorphisms: rs1360780, rs3800373, and rs9296158. Results showed that stress impaired immediate recall in risk allele carriers. More importantly, stress enhanced long-term recall and recognition memory in non-carriers of the risk alleles, effects that were completely absent in risk allele carriers. Follow-up analyses revealed that memory performance was correlated with salivary cortisol levels in non-carriers, but not in carriers. These findings suggest that FKBP5 risk allele carriers may possess a sensitized stress response system, perhaps specifically for stress-induced changes in corticosteroid levels, which might aid our understanding of how SNPs in the FKBP5 gene confer increased risk for stress-related psychological disorders and their related phenotypes.
Keywords: stress, cortisol, learning, memory, FKBP5
Graphical Abstract
Exposure to brief stress immediately before learning enhanced long-term memory in non-carriers, but not carriers, of minor alleles for three different FKBP5 polymorphisms. These findings suggest that carriers of such “risk” alleles for FKBP5 may possess a sensitized stress response system, which might aid in our understanding of how SNPs in the FKBP5 gene confer increased risk for stress-related psychological disorders.
INTRODUCTION
The effects of stress on learning and memory are profound, yet complex. Research over the past several decades has shown that stress can enhance, impair, or have no effect on learning and memory, depending on several factors (e.g., sex, stage of learning/memory affected by stress, emotional nature of the learned information, etc.) (Diamond et al., 2007; Joels et al., 2011; Schwabe et al., 2012). It has been well-documented that post-learning stress enhances long-term memory consolidation and pre-retrieval stress impairs recall, both effects being attributable to an interaction between corticosteroid and noradrenergic mechanisms in the amygdala and hippocampus (Roozendaal et al., 2009). Pre-learning stress effects on long-term memory are not as well understood and are more inconsistent in the literature. One factor that has emerged in the past decade as a major determinant of pre-learning stress effects on long-term memory is the timing of stress relative to learning (Diamond et al., 2007; Joels et al., 2011; Schwabe et al., 2012). When a brief stressor is administered immediately before learning, long-term memory is generally enhanced (e.g., Diamond et al., 2007; Zoladz et al., 2011; Quaedflieg et al., 2013; Zoladz et al., 2014b; Vogel & Schwabe, 2016). However, when the same stressor is temporally separated from learning (e.g., 30 min before learning), long-term memory is generally impaired (e.g., Zoladz et al., 2011; Quaedflieg et al., 2013; Zoladz et al., 2013). Investigators have contended that these time-dependent effects of pre-learning stress are attributable to a biphasic influence of stress-induced amygdala activation on hippocampal synaptic plasticity, as well as the temporal profiles of stress-induced noradrenergic and corticosteroid activity (Akirav & Richter-Levin, 2002; Diamond et al., 2007; Joels et al., 2011; Schwabe et al., 2012). Specifically, brief stress experienced immediately before learning enhances long-term memory via the rapid increase in norepinephrine and non-genomic effects of slowly rising corticosteroids exerting excitatory influences on hippocampal synaptic plasticity. In contrast, stress that is temporally separated from learning results in long-term memory impairment due to, at least in part, rising corticosteroid levels exerting gene-dependent, inhibitory influences on hippocampal function.
Our laboratory has been using the pre-learning stress model as a method for better understanding susceptibility factors for stress-induced alterations of learning and memory (Zoladz et al., 2011; Zoladz et al., 2013; Zoladz et al., 2014a; Zoladz et al., 2014b). Because stress-induced alterations of learning and memory are associated with multiple psychological disorders, developing a better understanding of susceptibility factors for stress-induced enhancements or impairments of learning and memory may lend important insight into the mechanisms underlying such illnesses. One susceptibility factor that has garnered a significant amount of attention is the FKBP5 gene, which codes for FK506 binding protein 51 (FKBP5). FKBP5 is a co-chaperone of heat shock protein 90, which binds to the glucocorticoid receptor (GR). This complex reduces GR nuclear translocation and sensitivity to corticosteroids, thus resulting in reduced negative feedback inhibition of the hypothalamus-pituitary-adrenal (HPA) axis (Binder, 2009). Research over the past two decades has shown that single nucleotide polymorphisms (SNPs) in the FKBP5 gene are associated with greater risk for PTSD (Binder et al., 2008; Xie et al., 2010; Boscarino et al., 2011; Mehta et al., 2011; Sarapas et al., 2011; Watkins et al., 2016), major depression (Zobel et al., 2010; Appel et al., 2011), bipolar disorder (Willour et al., 2009), and suicidal events (Brent et al., 2010; Roy et al., 2010; Menke et al., 2013), particularly upon interaction with environmental risk factors, such as early life stress (Binder et al., 2008; Roy et al., 2010; Xie et al., 2010; Appel et al., 2011; Watkins et al., 2016). The mechanism by which these SNPs confer increased susceptibility to such illnesses is still largely unknown. However, researchers have shown that these SNPs are associated with altered HPA axis function (e.g., changes in GR sensitivity, dexamethasone-induced suppression of cortisol, recovery of stress-induced increases in cortisol) and significant changes in the volume and connectivity of several cognitive brain areas (e.g., hippocampus, amygdala, frontal cortex), which are characteristic features of stress-related psychological disorders (Binder et al., 2008; Ising et al., 2008; Zobel et al., 2010; Mehta et al., 2011; Sarapas et al., 2011; Fani et al., 2013; Menke et al., 2013; Fujii et al., 2014a; Fujii et al., 2014c; Fani et al., 2016; Hirakawa et al., 2016).
Given the well-established role of the HPA axis, hippocampus, amygdala, and frontal cortex in stress-memory interactions and the association between stress-memory interactions and stress-related psychological disorders, we predicted that SNPs in the FKBP5 gene might influence how stress affects learning and memory. Investigators have speculated that FKBP5 polymorphisms promote sensitization of the stress response, thus influencing emotional memory formation (Binder et al., 2008; Binder, 2009; Fani et al., 2013; Cheung & Bryant, 2015; Holz et al., 2015), and some have shown that carriers of FKBP5 polymorphisms demonstrate an attentional bias to threat (Fani et al., 2013), greater amygdala responses to emotional stimuli (White et al., 2012; Holz et al., 2015), higher levels of intrusive memories in a laboratory setting (Cheung & Bryant, 2015), and impaired cognition in aged individuals (Fujii et al., 2014b). Thus, we exposed participants to brief stress immediately before learning and hypothesized that three SNPs in the FKBP5 gene, chosen from previous research (Binder et al., 2008), might prevent the commonly observed stress-induced enhancement of long-term memory via an exaggerated physiological stress response.
MATERIALS AND METHODS
Participants
One hundred and twelve healthy undergraduate students (44 males, 68 females; age: M = 19.79, SD = 1.59), predominantly Caucasian (85%), from Ohio Northern University volunteered to participate in the experiment. Individuals were excluded from participating if they met any of the following conditions: diagnosis of Raynaud’s or peripheral vascular disease; presence of skin diseases, such as psoriasis, eczema or scleroderma; history of syncope or vasovagal response to stress; history of any heart condition or cardiovascular issues (e.g., high blood pressure); history of severe head injury; current treatment with psychotropic medications, narcotics, beta-blockers, steroids or any other medication that was deemed to significantly affect central nervous or endocrine system function; mental or substance use disorder; regular use of recreational drugs; regular nightshift work. Participants were asked to refrain from drinking alcohol or exercising extensively for 24 h prior to the experimental sessions and to refrain from eating or drinking anything but water for 2 h prior to the experimental sessions. Participants were awarded class credit and $20 cash upon completion of the study. All of the methods for the experiment were undertaken with the understanding and written consent of each participant, carried out in accordance with the Declaration of Helsinki, and approved by the Institutional Review Board at Ohio Northern University; all experimental procedures took place between 1000 and 1700 hours. The overall sample size was based on previous work reporting physiological / behavioral effects associated with the minor alleles for the FKBP5 polymorphisms (e.g., Ising et al., 2008; Menke et al., 2013; Cheung & Bryant, 2015) and an a priori power analysis (G*Power 3.1.9.2; University of Kiel, Germany) indicating that in order to attain adequate power (i.e., 1 – β = 0.80) to detect moderate effect sizes (i.e., Cohen’s f = 0.25–0.30) for the stress x genotype interactions, assuming minor allele frequencies ranging from 0.42–0.49 (Binder et al., 2008), we would need a total sample of approximately 90–130 participants.
Socially Evaluated Cold Pressor Test (SECPT)
Following completion of a short demographics survey and the collection of baseline measures (see below), participants were asked to submerge their non-dominant hand in a bath of water for 3 min. Participants who had been randomly assigned to the stress condition (n = 56; 20 males, 36 females) placed their hand in a bath of ice cold (0–2°C) water, while participants who had been randomly assigned to the control condition (n = 56; 24 males, 32 females) placed their hand in a bath of warm (35–37°C) water. The water was maintained at the appropriate temperature by a circulating water bath (Cole-Parmer; Vernon Hills, IL). If a participant found the water bath too painful, he or she was allowed to remove his or her hand from the water and continue with the experiment. Based on previous work (Schwabe et al., 2008b), a social evaluative component was added to the cold pressor manipulation. Participants in the stress condition were misleadingly informed that they were being videotaped during the procedure for subsequent evaluation of their facial expressions, and throughout the water bath, they were asked to keep their eyes on a camera that was located on the wall of the laboratory.
Subjective and Objective Stress Response Measures
The Positive and Negative Affect Schedule (PANAS) and State Anxiety Inventory (SAI)
Immediately before and approximately 10 min after the water bath manipulation, participants completed the PANAS (Watson et al., 1988) and the SAI (state portion of the State-Trait Anxiety Inventory) (Spielberger et al., 1983). This allowed for a pre-post analysis of stress-induced changes in affect and anxiety, respectively.
Subjective Pain and Stress Ratings
Participants rated the painfulness and stressfulness of the water bath at 1-min intervals on 11-point scales ranging from 0–10, with 0 indicating a complete lack of pain or stress and 10 indicating unbearable pain or stress.
Cardiovascular Analysis
Heart rate (HR) was measured continuously from approximately 1 min before the water bath until its completion via a BioNomadix pulse transducer (Biopac Systems, Inc.; Goleta, CA) placed on the ring finger of participants’ dominant hand. The pulse transducer was connected to the PPG module of the MP150 BIOPAC hardware. Average baseline HR (average HR before water bath) and water bath HR (average of HR during water bath) were calculated for statistical analyses.
Cortisol Analysis
On Day 1, saliva samples were collected from participants immediately before and 25 min after the water bath to analyze salivary cortisol levels. On Day 2, saliva samples were collected from participants immediately before and 25 min after the free recall assessment to analyze salivary cortisol levels. Saliva samples were collected in a Salivette saliva collection device (Sarstedt, Inc., Newton, NC). The samples were stored at −20°C until being thawed and extracted by low-speed centrifugation. Salivary cortisol levels were determined by enzyme immunoassay (Cayman Chemical Co., Ann Arbor, MI) according to the manufacturer’s protocol.
Learning and Memory Task
Immediately following exposure to the water bath, participants were presented with a list of 42 words, which were selected from the Affective Norms for English Words (Bradley & Lang, 1999). Based on standardized valence and arousal ratings, we chose 14 neutral, 14 positive, and 14 negative words (7 arousing and 7 non-arousing per category), which, across emotional valence and arousal categories, were balanced for word length and word frequency. As per previous methodology (Zoladz et al., 2011; Zoladz et al., 2013; Zoladz et al., 2014a; Zoladz et al., 2014b), participants were instructed to read each word aloud and rate its emotional valence on a scale from −4 (very negative) to +4 (very positive) and its arousal level on a scale of 0 (not arousing) to 8 (very highly arousing), with the aid of self-assessment manikins, on a sheet of paper containing the list of words.
Immediately following word list encoding, participants were given 5 min to write down as many words as they could remember from the list of words they just studied (immediate recall). The next day, participants returned to the laboratory to have their memory for the list of words assessed. Participants were again given 5 min to write down as many words as they could remember from the list of words that they studied on the previous day (delayed recall). Fifteen minutes later, participants were given a recognition test. They were presented with a list of words containing 42 “old” words (i.e., words presented on the previous day) and 42 “new” words (i.e., words not presented on the previous day) and were instructed to label each word as “old” or “new.” The “new” words were matched to the “old” words on emotional valence, arousal level, word length and word frequency. To assess participants’ ability to discriminate between “old” and “new” words, we calculated a sensitivity index (d’ = z[p(hit) – p(false alarm)]) for each category of word to be used for statistical analysis.
Genotyping
On Day 2, during the 15-min delay between free recall and recognition testing, a saliva sample was collected from participants via the OGR-500 Oragene (DNA Genotek, Inc.; Ottawa, ON, Canada). The sample was stored at room temperature, until shipped to DNA Genotek, Inc. for genotyping of polymorphisms rs1360780, rs3800373, and rs9296158 in the FKBP5 gene. DNA was extracted from 700 µL of saliva, and quantity and quality control procedures were performed before undergoing TaqMan® assay with PCR amplification for genotype. Primers and probes were obtained through Life Technologies, Inc. (Foster City, CA). The call rate for rs1360780 and rs9296158 was 100%, and the call rate for rs3800373 was 99%.
Statistical Analyses
Based on previous work establishing an association between particular FKBP5 polymorphism alleles, psychological disorders and alterations in the physiological stress response (Binder et al., 2004; Binder et al., 2008; Ising et al., 2008; Binder, 2009; Willour et al., 2009; Brent et al., 2010; Roy et al., 2010; Xie et al., 2010; Zobel et al., 2010; Appel et al., 2011; Boscarino et al., 2011; Mehta et al., 2011; Sarapas et al., 2011; Mahon et al., 2013; Menke et al., 2013; Fujii et al., 2014a; Watkins et al., 2016), we divided participants into “risk allele” carriers [heterozygous, homozygous carriers of the T (rs1360780), C (rs3800373), and A (rs9296158) alleles] and non-carriers [wild type (homozygous for the C (rs1360780), A (rs3800373), and G (rs9296158) alleles] for the purpose of data analysis. Demographic data for each polymorphism can be found in Table 1. The data for each polymorphism were analyzed separately with mixed-model ANOVAs. The between-subjects factors utilized in these analyses were stress (stress, no stress), sex, and polymorphism genotype (risk allele carrier, risk allele non-carrier), and the within-subjects factors were word valence and arousal (for recall and recognition data) or time point [for physiological (heart rate, cortisol) and self-report (PANAS, SAI) data]. Outlier data points that were 3 standard deviations beyond the exclusive group mean were removed from statistical analyses; only 3 data points (1 immediate recall data point from a stressed female risk allele carrier; 1 delayed recall data point from a stressed male risk allele non-carrier; 1 recognition memory data point from a non-stressed female risk allele non-carrier) were classified as outliers. If the assumption of sphericity was violated, Greenhouse-Geisser corrections were employed, with reduced degrees of freedom reported in the analyses. Genotype-independent effects were reported based on the analysis including rs1360780, but were consistent across all three polymorphisms. Alpha was set at .05 for all analyses, and Bonferroni-corrected post hoc tests were employed when the omnibus F indicated the presence of a significant effect. All statistical analyses were performed in SPSS (version 22.0; SPSS, Inc).
Table 1.
Participant Demographics based on FKBP5 Polymorphism Genotype
| rs1360780 | rs3800373 | rs9296158 | ||||
|---|---|---|---|---|---|---|
| Demographic Measure | Carriers (n = 58) |
Non-Carriers (n = 54) |
Carriers (n = 56) |
Non-Carriers (n = 55) |
Carriers (n = 59) |
Non-Carriers (n = 53) |
| Age (years ± SEM) | 19.88 (0.23) |
19.70 (0.20) |
19.93 (0.23) |
19.69 (0.19) |
19.88 (0.22) |
19.70 (0.20) |
| Weight (kg ± SEM) | 72.51 (2.27) |
74.94 (2.40) |
73.98 (2.26) |
73.77 (2.42) |
72.85 (2.26) |
74.60 (2.42) |
| Height (m ± SEM) | 1.72 (0.01) |
1.72 (0.01) |
1.72 (0.01) |
1.72 (0.01) |
1.72 (0.01) |
1.72 (0.01) |
| Education (years ± SEM) | 15.14 (0.16) |
15.24 (0.14) |
15.16 (0.16) |
15.24 (0.14) |
15.15 (0.15) |
15.23 (0.15) |
| Sex (N, %) | ||||||
| Males | 22 (38%) | 22 (41%) | 22 (39%) | 22 (40%) | 22 (37%) | 22 (42%) |
| Females | 36 (62%) | 32 (59%) | 34 (61%) | 33 (60%) | 37 (63%) | 31 (59%) |
| Race (N, %) | ||||||
| American Indian / Alaska Native | 1 (2%) | 0 (0%) | 1 (2%) | 0 (0%) | 1 (2%) | 0 (0%) |
| Asian | 5 (9%) | 3 (6%) | 5 (9%) | 3 (6%) | 5 (9%) | 3 (6%) |
| Black / African American | 1 (2%) | 7 (13%) | 2 (4%) | 6 (11%) | 2 (3%) | 6 (11%) |
| White | 51 (88%) | 44 (82%) | 48 (86%) | 46 (84%) | 51 (86%) | 44 (83%) |
RESULTS
Genotype Characteristics
Chi-square goodness-of-fit analyses revealed that there was no significant deviation from the Hardy-Weinberg equilibrium for any of the polymorphisms: rs1360780 (χ21 = 0.21 (n = 112), P = 0.65), rs3800373 (χ21 = 0.82 (n = 111), P = 0.27), and rs9296158 (χ21 = 0.35 (n = 112), P = 0.55). However, previous work has reported that these SNPs are in linkage disequilibrium (Binder et al., 2004; Binder et al., 2008; Ising et al., 2008; Zobel et al., 2010); thus, the current findings likely reflect one functional effect in the gene.
Subjective and Objective Stress Response Measures
PANAS and SAI
Overall, positive affect decreased and levels of anxiety increased after the water bath manipulation (affect – effect of time point: F1,104 = 12.10, P = 0.001; anxiety – F1,104 = 10.54, P = 0.002). Stressed participants reported lower positive affect than controls (effect of stress: F1,104 = 4.66, P = 0.03; Table 2). Carriers of the FKBP5 risk alleles for rs1360780 (F1,104 = 5.52, P = 0.02), rs3800373 (F1,103 = 5.64, P = 0.02), and rs9296158 (F1,104 = 6.46, P = 0.01) exhibited significantly lower positive affect than non-carriers. Females (affect: 14.74 ± 0.46; anxiety: 39.07 ± 1.10) reported greater levels of negative affect and anxiety than males (affect: 13.18 ± 0.59; anxiety: 34.34 ± 1.42) (affect – effect of sex: F1,104 = 4.28, P = 0.04; anxiety – effect of sex: F1,104 = 6.89, P = 0.01).
Table 2.
Pre-Post Changes (± SEM) in Day 1 Affect and Anxiety and Day 2 Cortisol
| Measure/Condition | Pre | Post |
|---|---|---|
| Day 1 Positive Affect (PANAS) | ||
| Stress | ||
| Carriers (n = 25) | 24.37 (1.50)*,β | 22.61 (1.59)*,β |
| Non-carriers (n = 31) | 29.59 (1.22)* | 26.94 (1.30)* |
| No Stress | ||
| Carriers (n = 33) | 29.41 (1.17)β | 26.64 (1.25)β |
| Non-carriers (n = 23) | 29.61 (1.43) | 28.67 (1.53) |
| Day 1 Negative Affect (PANAS) | ||
| Stress | ||
| Carriers (n = 25) | 13.87 (0.90) | 13.54 (0.99) |
| Non-carriers (n = 31) | 13.77 (0.74) | 14.04 (0.81) |
| No Stress | ||
| Carriers (n = 33) | 14.09 (0.71) | 13.81 (0.77) |
| Non-carriers (n = 23) | 14.22 (0.86) | 14.34 (0.95) |
| Day 1 Anxiety (SAI) | ||
| Stress | ||
| Carriers (n = 25) | 34.42 (2.24) | 40.02 (2.31) |
| Non-carriers (n = 31) | 36.43 (1.83) | 38.86 (1.89) |
| No Stress | ||
| Carriers (n = 33) | 35.57 (1.76) | 36.21 (1.81) |
| Non-carriers (n = 23) | 34.20 (2.15) | 37.92 (2.21) |
| Day 2 Salivary Cortisol (nmol/l) | ||
| Stress | ||
| Carriers (n = 25) | 5.81 (0.66) | 6.01 (0.57) |
| Non-carriers (n = 31) | 5.26 (0.54) | 5.44 (0.47) |
| No Stress | ||
| Carriers (n = 33) | 4.78 (0.52) | 5.57 (0.45) |
| Non-carriers (n = 23) | 5.72 (0.64) | 5.83 (0.55) |
Note: the data for rs1360780 are presented as a representative example of all FKBP5 SNP effects. PANAS = Positive and Negative Affect Scale; SAI = State Anxiety Inventory;
main effect of stress p < 0.05 relative to no stress;
main effect of genotype p < 0.05 relative to non-carriers.
Subjective Pain and Stress Ratings
Overall, pain ratings increased throughout the water bath manipulation (effect of time point: F1.44,149.61 = 4.05, P = 0.03). Stressed participants (PAIN: 6.41 ± 0.21; STRESS: 5.67 ± 0.24) rated the water bath as more painful (effect of stress: F1,104 = 444.63, P < 0.001) and more stressful (effect of stress: F1,104 = 263.71, P < 0.001) than controls (PAIN: 0.23 ± 0.20; STRESS: 0.33 ± 0.23) (p’s < 0.001). Stressed females (PAIN: 7.15 ± 0.25; STRESS: 6.71 ± 0.28) also rated the water bath as more painful (effect of sex: F1,104 = 6.68, P = 0.01; Stress x Sex interaction: F1,104 = 6.01, P = 0.02) and more stressful (effect of sex: F1,104 = 11.49, P = 0.001; Stress x Sex interaction: F1,104 = 8.53, P = 0.004) than stressed males (PAIN: 5.67 ± 0.35; STRESS: 4.64 ± 0.39).
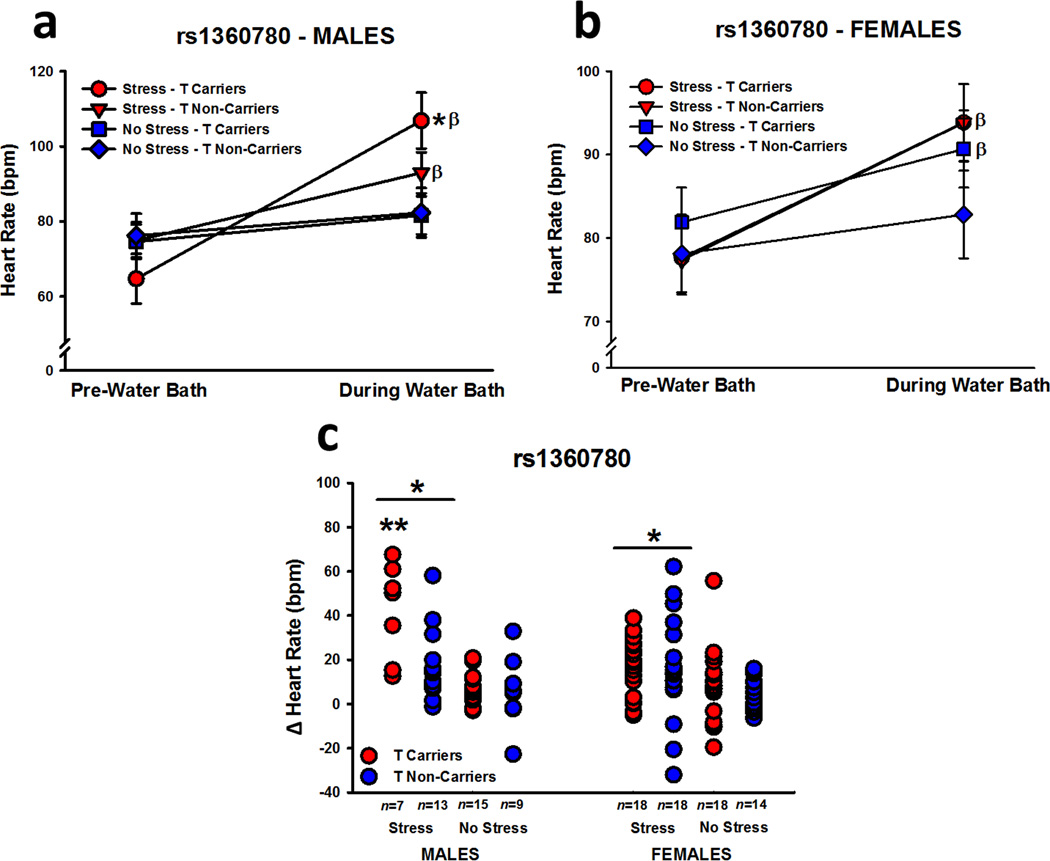
Heart Rate
Stressed participants, particularly males, exhibited significantly greater HR following the water bath, relative to controls (effect of time point: F1,104 = 89.19, P < 0.001; Stress x Time Point interaction, F1,104 = 27.50, P < 0.001; Sex x Time Point interaction: F1,104 = 4.58, P = 0.04; Stress x Sex x Time Point interaction: F1,104 = 4.83, P = 0.03; Figure 1a and 1b). The analysis of HR also showed that carriers of the risk alleles for rs1360780 (Genotype x Time Point interaction: F1,104 = 5.19, P = 0.03; Stress x Sex x Genotype x Time Point interaction: F1,104 = 4.74, P = 0.03), rs3800373 (Genotype x Time Point interaction: F1,103 = 4.19, P = 0.04; Stress x Sex x Genotype x Time Point interaction: F1,103 = 6.78, P = 0.01), and rs9296158 (Genotype x Time Point interaction: F1,104 = 4.23, P = 0.04; Stress x Sex x Genotype x Time Point interaction: F1,104 = 5.79, P = 0.02) exhibited greater HR following the water bath manipulation than non-carriers, which appeared to be driven mainly by a greater stress-induced increase in HR in male risk allele carriers, relative to all other groups. To verify the induction of a stress response in all stressed participants, we analyzed HR change scores (average of HR during water bath – average of pre-water bath HR) with a 3-way (stress, sex, genotype) ANOVA. This analysis revealed a greater increase in HR in stressed participants than controls (effect of stress: F1,104 = 27.50, p < 0.001; Figure 1c). Stressed males did exhibit a greater stress-induced increase in HR than females (effect of sex: F1,104 = 4.58, P = 0.04; Stress x Sex interaction: F1,104 = 4.83, P = 0.03), which was driven by the greatest change in HR being observed in stressed male risk allele carriers (rs1360780 – effect of genotype: F1,104 = 5.19, P = 0.03; Stress x Sex x Genotype interaction: F1,104 = 4.74, P = 0.03; rs3800373 – effect of genotype: F1,103 = 4.19, P = 0.04; Stress x Sex x Genotype interaction: F1,103 = 6.78, P = 0.01; rs9296158 – effect of genotype: F1,104 = 4.23, P = 0.04; Stress x Sex x Genotype interaction: F1,104 = 5.79, P = 0.02).
Figure 1.
Heart rate before and during the water bath manipulation. Stressed male (a) and female (b) participants exhibited significant increases in HR during the SECPT, but stressed male risk allele carriers were the only participants to exhibit significantly greater HR after the water bath relative to non-stressed controls. Upon analysis of HR change data (during water bath – pre-water bath), we confirmed that stress led to a greater increase in HR than non-stressed conditions and that this change was greatest in stressed male risk allele carriers (c). Data in insets a and b are expressed as means ± SEM, and the data from rs1360780 are used as a representative example of the effects observed for all FKBP5 SNPs. * p < 0.05 relative to no stress; ** P < 0.01 relative to all other groups; β P < 0.05 relative to pre-water bath.
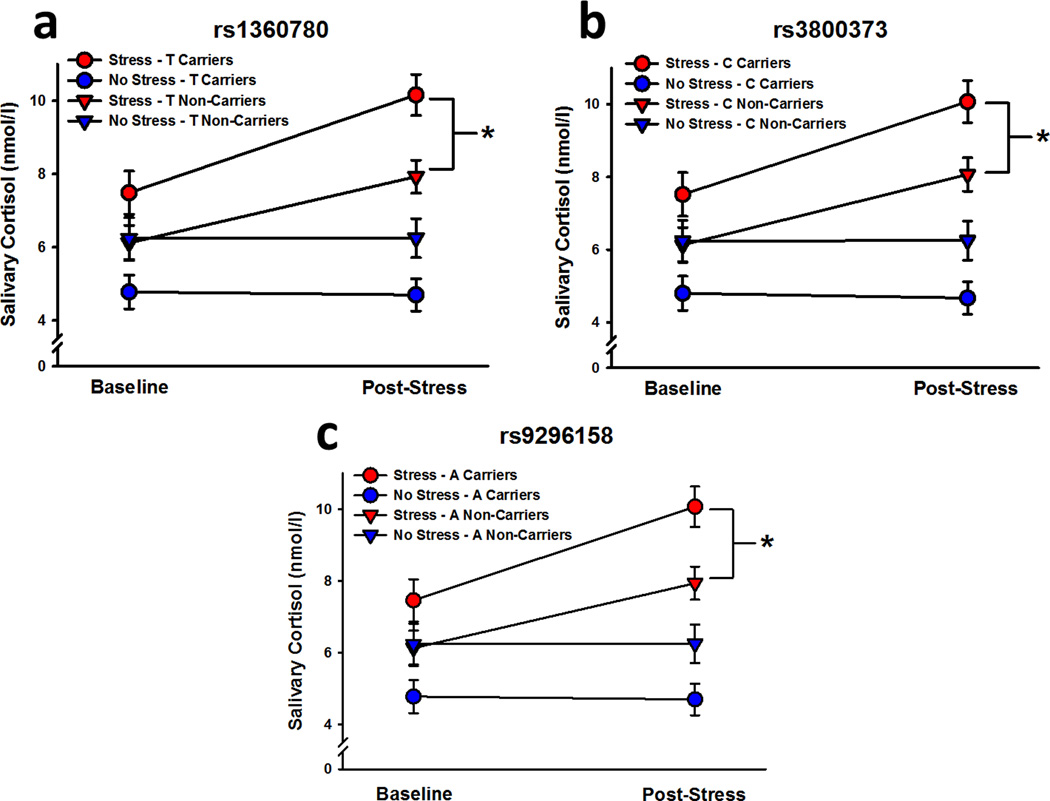
Cortisol
On Day 1, stressed participants exhibited significantly greater salivary cortisol levels than controls following the water bath (effect of stress: F1,103 = 25.28; effect of time point: F1,103 = 40.42; Stress x Time Point interaction: F1,103 = 43.22; P’s < 0.001; Figure 2). Across both time points, risk allele carriers in the stress condition exhibited greater salivary cortisol levels than risk allele carriers in the non-stressed condition (rs1360780 – Stress x Genotype interaction: F1,103 = 11.69; rs3800373 – Stress x Genotype interaction: F1,102 = 10.66; rs9296158 – Stress x Genotype interaction: F1,103 = 11.12; P’s = 0.001). There was no significant influence of stress or genotype on Day 2 salivary cortisol levels (Table 2).
Figure 2.
Salivary cortisol levels before and after water bath exposure. Stressed participants, independent of genotype for each of the three FKBP5 SNPs (a, b, c), exhibited significant increases in salivary cortisol levels, relative to control (i.e., non-stressed) participants. Stressed risk allele carriers for each polymorphism exhibited greater salivary cortisol levels, overall, than all other groups. Data are expressed as means ± SEM. * P < 0.001 relative to no stress.
Valence and Arousal Ratings of Learned Words
Valence Ratings
As expected, participants rated negative words more negatively than neutral words, which were rated more negatively than positive words (effect of valence: F1.38,143.62 = 1618.61, P < 0.001). Participants also rated arousing words more negatively than non-arousing words, especially females (effect of arousal: F1,104 = 63.50; Sex x Arousal interaction: F1,104 = 19.05; P’s < 0.001).
Arousal Ratings
As expected, participants rated arousing words as more arousing than non-arousing words (effect of arousal: F1,104 = 185.74, P < 0.001), and this effect was more pronounced in non-stressed participants (Stress x Arousal interaction: F1,104 = 5.98, P = 0.02). Participants also rated positive words as more arousing than negative words, which were rated as more arousing than neutral words (effect of valence: F1.40,145.59 = 97.19, P < 0.001). Overall, females rated words as more arousing than males, especially positive words (effect of sex: F1,104 = 5.73, P = 0.02; Valence x Arousal interaction: F1.85,192.06 = 12.16, P < 0.001; Sex x Valence x Arousal interaction: F1.85,192.06 = 3.35, P = 0.04), and carriers of the risk alleles for rs1360780 (F1,104 = 10.78, P = 0.001), rs3800373 (F1,104 =6.40, P = 0.01), and rs9296158 (F1,104 = 9.47, P = 0.003) rated words as less arousing than non-carriers.
Memory Testing
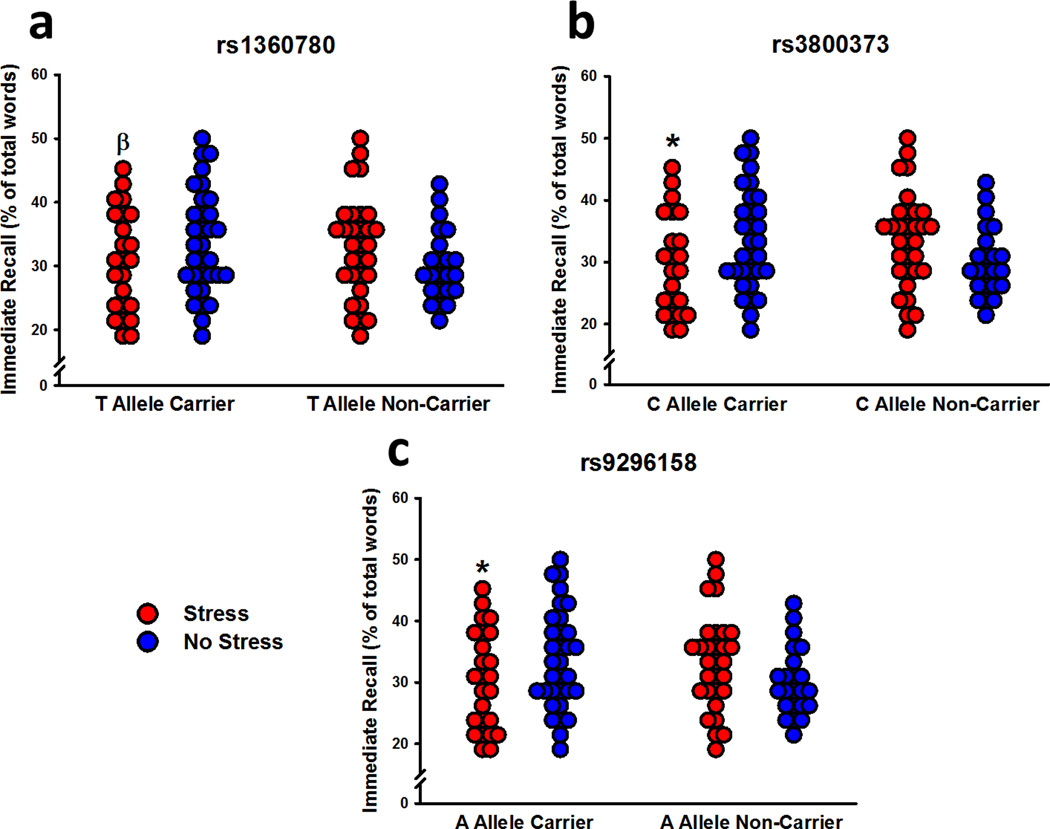
Immediate Free Recall
Participants recalled more positive and negative words than neutral words (effect of valence: F2,206 = 30.88, P < 0.001). They also recalled more arousing words than non-arousing words, particularly when the words were positive (effect of arousal: F1,103 = 62.17; Valence x Arousal interaction: F2,206 = 50.80; P’s < 0.001). There was a significant Sex x Arousal interaction (F1,103 = 6.72, P = 0.01) that was dependent on FKBP5 genotype. Specifically, male risk allele carriers recalled fewer non-arousing words than male non-carriers, while female risk allele carriers recalled more non-arousing words than female non-carriers (rs1360780 – Sex x Genotype interaction: F1,103 = 10.25, P = 0.002; Sex x Genotype x Arousal interaction: F1,103 = 4.89, P = 0.03; rs3800373 – Sex x Genotype interaction: F1,102 = 7.46, P = 0.007; Sex x Genotype x Arousal interaction: F1,102 = 3.52, P = 0.06; rs9296158 – Sex x Genotype interaction: F1,103 = 8.94, P = 0.003; Sex x Genotype x Arousal interaction: F1,103 = 5.35, P = 0.02). The effect of stress on immediate recall was dependent on genotype for rs1360780 (Stress x Genotype interaction: F1,103 = 5.45, P = 0.02; Figure 3a), rs3800373 (Stress x Genotype interaction: F1,102 = 7.61, P = 0.007; Figure 3b), and rs9296158 (Stress x Genotype interaction: F1,103 = 6.45, P = 0.01; Figure 3c). In all three cases, pre-learning stress selectively impaired immediate recall in risk allele carriers.
Figure 3.
Immediate free recall performance organized by stress and carriers of the risk allele for the three FKBP5 SNPs. For each of the three SNPs (a, b, c), exposure to pre-learning stress impaired recall in risk allele carriers. β P < 0.05 relative to non-stressed carriers and P = 0.068 relative to stressed non-carriers; * P < 0.05 relative to non-stressed carriers and stressed non-carriers.
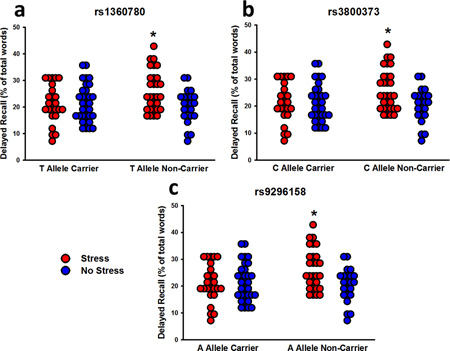
Delayed Free Recall
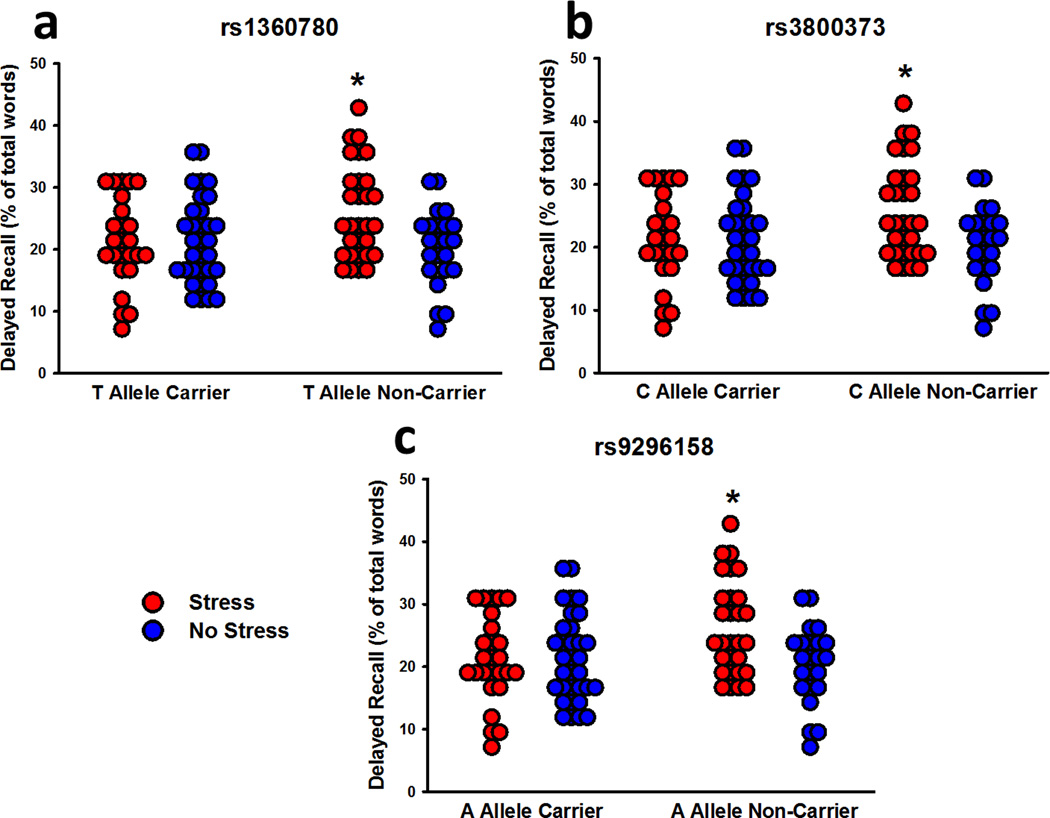
Twenty-four hours following learning, participants recalled more positive and negative words than neutral words (effect of valence: F2,206 = 12.64, P < 0.001). They also recalled more arousing words than non-arousing words, particularly when the words were positive (effect of arousal: F1,103 = 84.34; Valence x Arousal interaction: F2,206 = 56.11; P’s < 0.001). Females recalled more words than males (effect of sex – F1,103 = 4.49, P = 0.04), particularly female risk carriers for rs1360780 (Sex x Genotype interaction: F1,103 = 5.39, P = 0.02), rs3800373 (Sex x Genotype interaction: F1,102 = 5.56, P = 0.02), and rs9296158 (Sex x Genotype interaction: F1,103 = 4.93, P = 0.03). Similar to immediate recall, the effects of stress on delayed recall depended on genotype for rs1360780 (Stress x Genotype interaction: F1,103 = 7.09, P = 0.01; Figure 4a), rs3800373 (Stress x Genotype interaction: F1,102 = 6.21, P = 0.01; Figure 4b), and rs9296158 (Stress x Genotype interaction: F1,103 = 7.71, P = 0.007; Figure 4c). Pre-learning stress led to a long-term enhancement of recall in non-carriers of the FKBP5 risk alleles only. That is to say, pre-learning stress exerted no effect on long-term memory in FKBP5 risk allele carriers.
Figure 4.
Delayed free recall performance organized by stress and carriers of the risk allele for the three FKBP5 SNPs. For each of the three SNPs (a, b, c), stress enhanced long-term recall in non-carriers of the risk allele, but had no effect on carriers. * P < 0.05 relative to all other groups.
Recognition
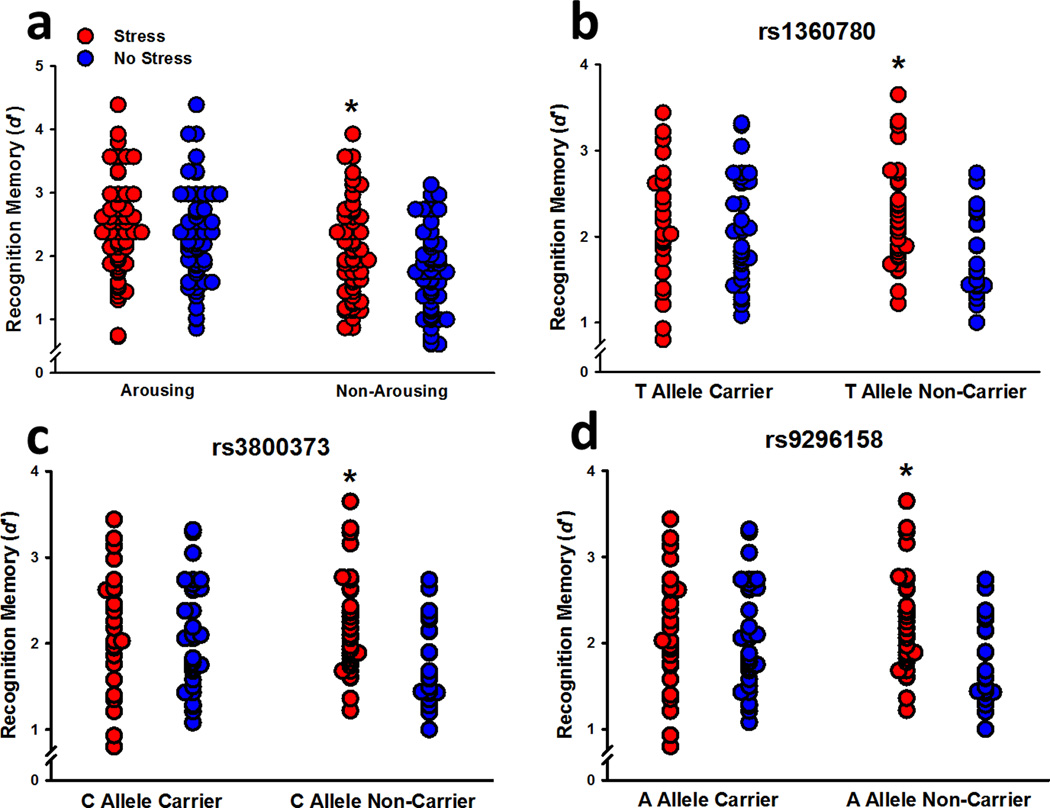
Participants recognized more positive and neutral words than negative words (effect of valence: F2,206 = 35.76, P < 0.001). They also recognized more arousing words than non-arousing words, particularly when the words were negative or neutral (effect of arousal: F1,103 = 36.05, P < 0.001; Valence x Arousal interaction: F2,206 = 3.71; P = 0.03). Females recognized more positive words than males (Sex x Valence interaction: F2,206 = 3.85, P = 0.02), and male risk allele carriers were the only participants to not show greater recognition memory for arousing words relative to non-arousing words (rs1360780: Sex x Genotype x Arousal interaction: F1,103 = 4.78, P = 0.03; rs3800373: Sex x Genotype x Arousal interaction: F1,102 = 4.46, P = 0.04; rs9296158: Sex x Genotype x Arousal interaction: F1,103 = 4.19, P = 0.04). Stress enhanced long-term recognition of non-arousing words, relative to controls, but had no significant impact on the recognition of arousing words, possibly due to a ceiling effect (Stress x Arousal interaction: F1,103 = 6.48, P = 0.01; Figure 5a). Once again, the effect of stress was dependent on the genotype for rs1360780 (Stress x Genotype interaction: F1,103 = 5.16, P = 0.03; Figure 5b), rs3800373 (Stress x Genotype interaction: F1,102 = 5.50, P = 0.02; Figure 5c), and rs9296158 (Stress x Genotype interaction: F1,103 = 6.04, P = 0.02; Figure 5d). Similar to the effects observed for delayed recall, pre-learning stress enhanced long-term recognition memory in non-carriers of the FKBP5 risk alleles; this stress-induced enhancement was not observed in FKBP5 risk allele carriers.
Figure 5.
Delayed recognition memory organized by stress and carriers of the risk allele for the three FKBP5 SNPs. Stress selectively enhanced long-term recognition memory for non-arousing words (a). For each of the three SNPs (b, c, d), stress enhanced long-term recognition memory in non-carriers of the risk allele, relative to non-stressed non-carriers, but had no effect on carriers. * P < 0.01 relative to non-arousing word recognition in non-stressed participants or overall recognition memory in non-stressed non-carriers.
Physiological Correlates of Memory Effects
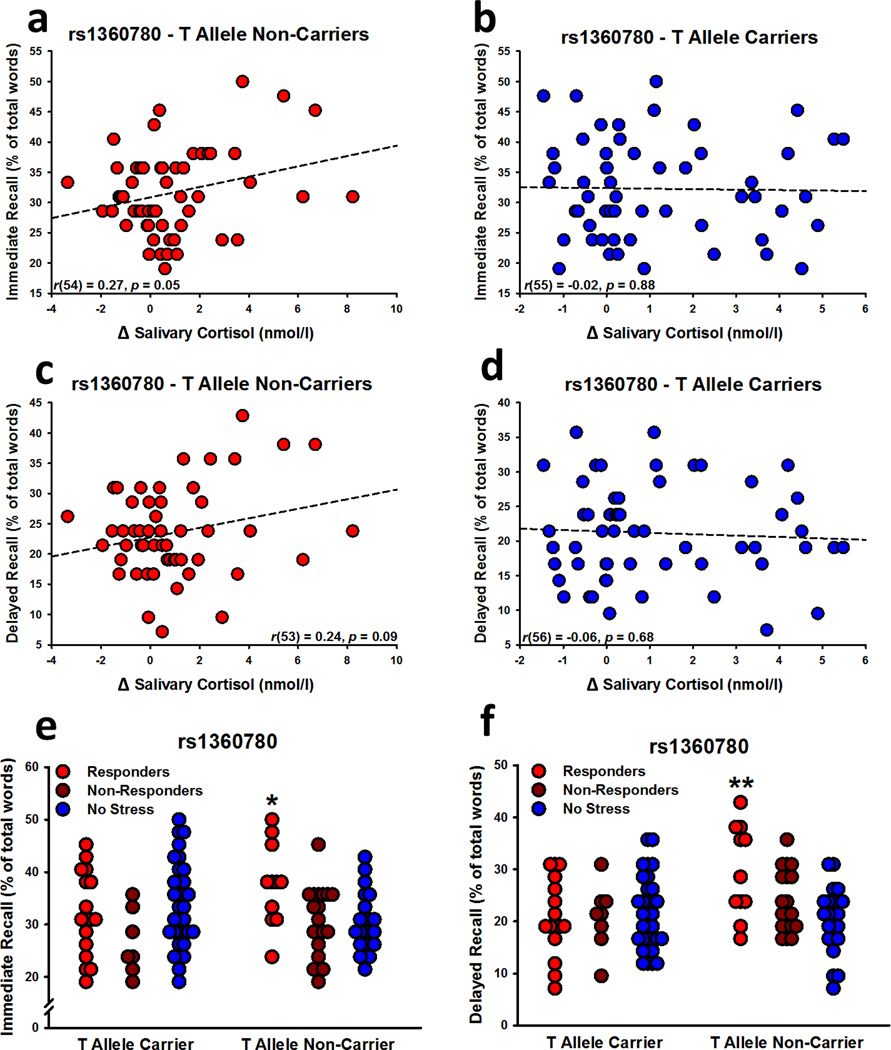
In order to probe potential physiological correlates of the observed Stress x Genotype interactions, we performed bivariate correlations between our physiological measures (HR and salivary cortisol levels) and memory performance. These analyses revealed significant or marginally significant positive correlations between changes in salivary cortisol levels and recall performance in non-carriers of the risk alleles for rs1360780 (Immediate Recall: r54 = 0.27, P = 0.05; Delayed Recall: r53 = 0.24, P = 0.09), rs3800373 (Immediate Recall: r55 = 0.30, P = 0.02; Delayed Recall: r54 = 0.20, P = 0.15), and rs9296158 (Immediate Recall: r53 = 0.27, P = 0.05; Delayed Recall: r52 = 0.24, P = 0.09) (Figures 6a and 6c), while no significant relationships were observed between such measures in risk allele carriers for rs1360780 (Immediate Recall: r55 = −0.02, P = 0.88; Delayed Recall: r56 = −0.06, P = 0.68), rs3800373 (Immediate Recall: r53 = −0.06, P = 0.66; Delayed Recall: r54 = −0.05, P = 0.75), and rs9296158 (Immediate Recall: r56 = −0.01, P = 0.92; Delayed Recall: r57 = −0.06, P = 0.69) (Figures 6b and 6d). Based on these findings, we divided stressed participants into cortisol responders (stressed participants exhibiting an increase in salivary cortisol of at least 2 nmol/l) and non-responders (stressed participants exhibiting a change in salivary cortisol less than 2 nmol/l), similar to previous work from our laboratory (Zoladz et al., 2011; Zoladz et al., 2013; Zoladz et al., 2014a) and that of others (Schwabe et al., 2008a), and then performed mixed-model ANOVAs on memory performance with responder (responder, non-responder, no stress) as a between-subjects factor in place of stress. These analyses revealed significant Responder x Genotype interactions for immediate recall and delayed recall for rs1360780 (Immediate Recall: F2,98 = 3.97, P = 0.02; Delayed Recall: F2,98 = 5.55, P = 0.01; Figures 6e and 6f), rs3800373 (Immediate Recall: F2,97 = 5.56, P = 0.01; Delayed Recall: F2,97 = 4.74, P = 0.01), and rs9296158 (Immediate Recall: F2,98 = 4.27, P = 0.02; Delayed Recall: F2,98 = 5.58, P = 0.01), revealing that pre-learning stress enhanced short- and long-term recall in non-carriers of the FKBP5 risk alleles who exhibited greater cortisol responses to the stress (i.e., responders). Pre-learning stress had no impact on short- or long-term memory in non-carriers who exhibited a blunted cortisol response to the stress (i.e., non-responders) or in stressed risk allele carriers overall.
Figure 6.
Relationship between changes in salivary cortisol levels and recall performance, using the rs1360780 polymorphism of FKBP5 as a representative example. Changes in salivary cortisol levels were positively correlated with immediate (a) and delayed recall (c) in non-carriers of the risk allele for rs1360780. No such relationship was observed in carriers of the risk allele (b, d). Analyses of immediate and delayed recall based on cortisol response to the stress revealed that non-carriers who exhibited a greater cortisol response to the stress had enhanced memory; this effect was no observed in risk allele carriers. * P < 0.05 relative to responders carrying the risk allele; ** P < 0.01 relative to non-stressed non-carriers and responders carrying the risk allele.
DISCUSSION
Previous work has shown that SNPs in the FKBP5 gene are associated with altered HPA axis function, changes in the structure and function of several cognitive brain areas, and increased risk for stress-related psychological disorders (Binder et al., 2004; Binder et al., 2008; Ising et al., 2008; Binder, 2009; Willour et al., 2009; Brent et al., 2010; Roy et al., 2010; Xie et al., 2010; Zobel et al., 2010; Appel et al., 2011; Boscarino et al., 2011; Mehta et al., 2011; Sarapas et al., 2011; Mahon et al., 2013; Menke et al., 2013; Fujii et al., 2014a; Fujii et al., 2014c; Fani et al., 2016; Hirakawa et al., 2016; Watkins et al., 2016). Because investigators have speculated that these SNPs may alter emotional memory systems (Binder et al., 2008; Binder, 2009; Fani et al., 2013; Cheung & Bryant, 2015; Holz et al., 2015), thus conferring greater risk for stress-related psychopathology, we examined the influence of three commonly studied SNPs in the FKBP5 gene on stress-induced alterations of learning and memory. Our results revealed that pre-learning stress impaired short-term memory in carriers of the FKBP5 risk alleles. More importantly, pre-learning stress enhanced long-term recall and recognition memory in non-carriers of the FKBP5 risk alleles, while having no long-term effect on risk allele carriers. Follow-up analyses revealed that memory performance was correlated with salivary cortisol levels in non-carriers, but not in carriers. These findings support the notion that FKBP5 polymorphisms result in a sensitized stress response system, perhaps specifically for stress-induced changes in corticosteroid levels, which could lend insight into how such SNPs are linked to psychological illness.
Risk Allele Carriers Exhibit Hypersensitivity to Stress
Brief stress administered immediately before learning impaired short-term memory in FKBP5 risk allele carriers and selectively enhanced long-term memory in non-carriers of the risk alleles. The stress-induced enhancement of long-term memory in non-carriers is consistent with our previous work and, as we (Diamond et al., 2007; Zoladz et al., 2011; Zoladz et al., 2013; Zoladz et al., 2014a; Zoladz et al., 2014b; Zoladz et al., 2014c) and others (Joels et al., 2011; Schwabe et al., 2012) have speculated, results from a rapid increase in noradrenergic activity interacting with slowly rising corticosteroid levels that exert non-genomic, excitatory influences on the hippocampus, an area significantly influenced by stress-induced amygdala activity and densely packed with corticosteroid receptors. Partial support for this hypothesis comes from the finding that the stress-induced enhancement of recall in non-carriers was associated with salivary cortisol levels. Specifically, non-carriers exhibiting a greater cortisol response to the stress demonstrated better short- and long-term recall. This association suggests that cortisol exerted excitatory effects on cognitive processing in non-carriers and resulted in a “memory formation” mode that promoted consolidation processes (Schwabe et al., 2012).
Instead of exhibiting behavior consistent with a rapid, stress-induced excitatory phase (and enhanced long-term memory), risk allele carriers demonstrated long-term recall and recognition performance that was unaffected by pre-learning stress, and their short-term memory was actually impaired. We would contend that risk allele carriers retain a sensitized stress response system; thus, the temporal dynamics of stress effects on learning are theoretically shifted, resulting in a much more transient, or potentially absent, excitatory phase of cognitive processing following stress exposure (see Diamond et al., 2007 for graphical illustration of the temporal dynamics model). Specifically, risk allele carriers may exhibit an abnormally intense response that biases them toward an inhibitory phase for learning and memory. The fact that the association between cortisol and memory observed in non-carriers was not observed in carriers implies that the stress-induced change in cortisol had a much different, potentially more adverse, impact in risk allele carriers. Others have also shown that risk allele carriers exhibit a prolonged cortisol response to stress (Ising et al., 2008), which could have taken place in the present study and resulted in a greater likelihood of GR-dependent, gene-mediated inhibitory effects on hippocampal function, thus preventing the long-term enhancing effects of stress. Differential responses to corticosteroids between risk allele carriers and non-carriers could result from altered FKBP5 activity. Previous work has associated FKBP5 polymorphisms with increased transcription of the FKBP5 gene and elevated levels of the FKBP5 protein (Binder et al., 2004; Binder, 2009; Klengel et al., 2013), which result in GR resistance, increased levels of circulating cortisol (Reynolds et al., 1999; Ising et al., 2008) and negative influences on GR activity and GR-mediated synaptic plasticity (Bennett & Lagopoulos, 2014; Young et al., 2015). Unfortunately, we did not assess the recovery of salivary cortisol levels, FKBP5 mRNA levels, or FKBP5 protein levels in participants, so we cannot verify this hypothesis. Still, our findings support the notion that FKBP5 risk allele carriers exhibit altered effects of stress on cognition, possibly as a result of differential sensitivity to stress-induced changes in corticosteroid levels.
Our finding that acute stress impaired short-term memory in risk allele carriers and enhanced long-term memory in non-carriers only is in contrast to the findings of Cheung and Bryant (2015), who reported no FKBP5 genotype-dependent differences in memory for images. This inconsistency could relate to methodological differences between the two studies, as we employed a list of words, rather than images, as the learning stimulus, and Cheung and Bryant (2015) assessed delayed (48-hr), but not immediate, recall. One interesting, and potentially relevant, finding reported by Cheung and Bryant (2015) is that FKBP5 risk allele carriers exhibited elevated levels of salivary alpha-amylase, which is an indicator of noradrenergic / sympathetic nervous system (SNS) activity (Nater & Rohleder, 2009). Cheung and Bryant (2015) speculated that FKBP5 risk allele carriers may have exhibited greater salivary alpha-amylase levels due to a heightened sensitivity to anticipatory stress. In the present study, risk allele carriers exhibited greater HR than non-carriers following the water bath manipulation, providing further support for the possibility of a sensitized stress response system in these individuals. The association between noradrenergic / SNS activity and stress-induced alterations of learning and memory is well-documented in the literature (Roozendaal et al., 2009); however, we did not observe any significant associations between HR and memory performance in risk allele carriers or non-carriers.
A surprising observation was that FKBP5 risk allele carriers rated the studied words as less arousing than non-carriers. It is possible that this difference contributed to our observed effects, given the well-established finding of superior memory for emotionally arousing material. Specifically, carriers may have been more susceptible to the effects of stress on memory for the word lists because, given the lower arousal ratings, they were already less likely to recall as many words as non-carriers. However, the data observed in non-stressed carriers does not appear to support this line of reasoning, as they exhibited immediate and delayed memory performance that was superior, albeit not significantly, or statistically equivalent to that of non-stressed non-carriers for every memory measure. Another interesting observation in the present study was the sex-dependent effects of FKBP5 polymorphisms on physiology and behavior, which, to our knowledge, has received minimal attention in previous work. We observed stress-induced changes in HR that were particularly selective to male risk allele carriers, and we observed differential recall of arousing and non-arousing words in male versus female risk allele carriers. Collectively, these findings support the possibility that the effects of FKBP5 polymorphisms are influenced by sex differences and the perception of emotionally arousing material.
Relevance for Understanding Psychological Illness
Several SNPs in the FKBP5 gene have been linked to increased susceptibility for psychological disorders. The SNPs in the FKBP5 gene studied here have been reported to result in altered GR sensitivity and HPA axis function, and extensive work has shown that individuals with stress-related psychological disorders, such as PTSD and major depression, exhibit altered baseline levels of cortisol, GR density and GR sensitivity (de Kloet et al., 2006; Pariante & Lightman, 2008; Yehuda, 2009). The presently-studied SNPs may confer greater susceptibility to such psychological illness or to disorder-related phenotypes by altering HPA axis and SNS activity, thereby influencing cognitive function. If carriers of the FKBP5 risk alleles possess a sensitized stress response system, they may be more prone to stress-induced cognitive impairments or the fragmentation of memories that are stored following stress exposure (Cheung & Bryant, 2015). This could ultimately help explain traumatic memory formation and cognitive impairments observed in multiple stress-related psychological disorders.
Limitations and Conclusions
We have shown that three commonly studied SNPs in the FKBP5 gene influence how pre-learning stress affects long-term memory. Our sample size was large enough to detect moderate effect sizes, but it may not have been large enough to detect smaller effects. On the other hand, it is important to point out that the observed effects were evident for three different assessments of memory across two days. Some limitations of our study include the use of a word list as our learning material and the lack of racial/ethnic diversity in our sample, both of which reduce the external validity of our findings. It is also important to note that, because of the nature of the present study, we are unable to make any causal conclusions regarding the neurobiological mechanisms underlying the observed effects. Finally, the effects observed for these SNPs likely reflect one functional influence, given the high amount of linkage disequilibrium between them. Notwithstanding these limitations, our results may facilitate future endeavors aiming to understand the link between FKBP5 and psychopathology.
Acknowledgments
The research reported in this publication was supported by the National Institute of Mental Health of the National Institutes of Health under award number R15MH104836. The National Institutes of Health had no further role in the study design; in the collection, analysis, and interpretation of the data; in the writing of the manuscript; or in the decision to submit the manuscript for publication. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
COMPETING INTERESTS
The authors have no conflicts of interest or competing financial interests to report.
AUTHOR CONTRIBUTIONS
PRZ designed the study, analyzed the data, and wrote the first draft and revision of the manuscript. BRR ran the cortisol assays and provided feedback on the first draft and revised manuscript. AMD, HEN, MKF, BEM, CMB, TJD, ARS, and MBE completed all data collection and provided feedback on the first draft and revised manuscript.
DATA ACCESSIBILITY
All data collected in the reported experiment have been submitted to European Journal of Neuroscience and deposited in Figshare.
References
- Akirav I, Richter-Levin G. Mechanisms of amygdala modulation of hippocampal plasticity. J Neurosci. 2002;22:9912–9921. doi: 10.1523/JNEUROSCI.22-22-09912.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Appel K, Schwahn C, Mahler J, Schulz A, Spitzer C, Fenske K, Stender J, Barnow S, John U, Teumer A, Biffar R, Nauck M, Volzke H, Freyberger HJ, Grabe HJ. Moderation of adult depression by a polymorphism in the FKBP5 gene and childhood physical abuse in the general population. Neuropsychopharmacology. 2011;36:1982–1991. doi: 10.1038/npp.2011.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett MR, Lagopoulos J. Stress and trauma: BDNF control of dendritic-spine formation and regression. Progress in neurobiology. 2014;112:80–99. doi: 10.1016/j.pneurobio.2013.10.005. [DOI] [PubMed] [Google Scholar]
- Binder EB. The role of FKBP5, a co-chaperone of the glucocorticoid receptor in the pathogenesis and therapy of affective and anxiety disorders. Psychoneuroendocrinology. 2009;34(Suppl 1):S186–S195. doi: 10.1016/j.psyneuen.2009.05.021. [DOI] [PubMed] [Google Scholar]
- Binder EB, Bradley RG, Liu W, Epstein MP, Deveau TC, Mercer KB, Tang Y, Gillespie CF, Heim CM, Nemeroff CB, Schwartz AC, Cubells JF, Ressler KJ. Association of FKBP5 polymorphisms and childhood abuse with risk of posttraumatic stress disorder symptoms in adults. Jama. 2008;299:1291–1305. doi: 10.1001/jama.299.11.1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binder EB, Salyakina D, Lichtner P, Wochnik GM, Ising M, Putz B, Papiol S, Seaman S, Lucae S, Kohli MA, Nickel T, Kunzel HE, Fuchs B, Majer M, Pfennig A, Kern N, Brunner J, Modell S, Baghai T, Deiml T, Zill P, Bondy B, Rupprecht R, Messer T, Kohnlein O, Dabitz H, Bruckl T, Muller N, Pfister H, Lieb R, Mueller JC, Lohmussaar E, Strom TM, Bettecken T, Meitinger T, Uhr M, Rein T, Holsboer F, Muller-Myhsok B. Polymorphisms in FKBP5 are associated with increased recurrence of depressive episodes and rapid response to antidepressant treatment. Nature genetics. 2004;36:1319–1325. doi: 10.1038/ng1479. [DOI] [PubMed] [Google Scholar]
- Boscarino JA, Erlich PM, Hoffman SN, Rukstalis M, Stewart WF. Association of FKBP5, COMT and CHRNA5 polymorphisms with PTSD among outpatients at risk for PTSD. Psychiatry research. 2011;188:173–174. doi: 10.1016/j.psychres.2011.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley MM, Lang PJ. Affective norms for English words (ANEW): instruction manual and affective ratings. Technical Report C-1, The Center for Research in Psychophysiology, University of Florida. 1999 [Google Scholar]
- Brent D, Melhem N, Ferrell R, Emslie G, Wagner KD, Ryan N, Vitiello B, Birmaher B, Mayes T, Zelazny J, Onorato M, Devlin B, Clarke G, DeBar L, Keller M. Association of FKBP5 polymorphisms with suicidal events in the Treatment of Resistant Depression in Adolescents (TORDIA) study. The American journal of psychiatry. 2010;167:190–197. doi: 10.1176/appi.ajp.2009.09040576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung J, Bryant RA. FKBP5 risk alleles and the development of intrusive memories. Neurobiology of learning and memory. 2015;125:258–264. doi: 10.1016/j.nlm.2015.09.008. [DOI] [PubMed] [Google Scholar]
- de Kloet CS, Vermetten E, Geuze E, Kavelaars A, Heijnen CJ, Westenberg HG. Assessment of HPA-axis function in posttraumatic stress disorder: pharmacological and non-pharmacological challenge tests, a review. Journal of psychiatric research. 2006;40:550–567. doi: 10.1016/j.jpsychires.2005.08.002. [DOI] [PubMed] [Google Scholar]
- Diamond DM, Campbell AM, Park CR, Halonen J, Zoladz PR. The temporal dynamics model of emotional memory processing: a synthesis on the neurobiological basis of stress-induced amnesia, flashbulb and traumatic memories, and the Yerkes-Dodson law. Neural plasticity. 2007;2007:60803. doi: 10.1155/2007/60803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fani N, Gutman D, Tone EB, Almli L, Mercer KB, Davis J, Glover E, Jovanovic T, Bradley B, Dinov ID, Zamanyan A, Toga AW, Binder EB, Ressler KJ. FKBP5 and attention bias for threat: associations with hippocampal function and shape. JAMA psychiatry. 2013;70:392–400. doi: 10.1001/2013.jamapsychiatry.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fani N, King TZ, Shin J, Srivastava A, Brewster RC, Jovanovic T, Bradley B, Ressler KJ. Structural and Functional Connectivity in Posttraumatic Stress Disorder: Associations with Fkbp5. Depression and anxiety. 2016;33:300–307. doi: 10.1002/da.22483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujii T, Hori H, Ota M, Hattori K, Teraishi T, Sasayama D, Yamamoto N, Higuchi T, Kunugi H. Effect of the common functional FKBP5 variant (rs1360780) on the hypothalamic-pituitary-adrenal axis and peripheral blood gene expression. Psychoneuroendocrinology. 2014a;42:89–97. doi: 10.1016/j.psyneuen.2014.01.007. [DOI] [PubMed] [Google Scholar]
- Fujii T, Ota M, Hori H, Hattori K, Teraishi T, Matsuo J, Kinoshita Y, Ishida I, Nagashima A, Kunugi H. The common functional FKBP5 variant rs1360780 is associated with altered cognitive function in aged individuals. Scientific reports. 2014b;4:6696. doi: 10.1038/srep06696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujii T, Ota M, Hori H, Hattori K, Teraishi T, Sasayama D, Higuchi T, Kunugi H. Association between the common functional FKBP5 variant (rs1360780) and brain structure in a non-clinical population. Journal of psychiatric research. 2014c;58:96–101. doi: 10.1016/j.jpsychires.2014.07.009. [DOI] [PubMed] [Google Scholar]
- Hirakawa H, Akiyoshi J, Muronaga M, Tanaka Y, Ishitobi Y, Inoue A, Oshita H, Aizawa S, Masuda K, Higuma H, Kanehisa M, Ninomiya T, Kawano Y. FKBP5 is associated with amygdala volume in the human brain and mood state: A voxel-based morphometry (VBM) study. International journal of psychiatry in clinical practice. 2016;20:106–115. doi: 10.3109/13651501.2016.1144772. [DOI] [PubMed] [Google Scholar]
- Holz NE, Buchmann AF, Boecker R, Blomeyer D, Baumeister S, Wolf I, Rietschel M, Witt SH, Plichta MM, Meyer-Lindenberg A, Banaschewski T, Brandeis D, Laucht M. Role of FKBP5 in emotion processing: results on amygdala activity, connectivity and volume. Brain structure & function. 2015;220:1355–1368. doi: 10.1007/s00429-014-0729-5. [DOI] [PubMed] [Google Scholar]
- Ising M, Depping AM, Siebertz A, Lucae S, Unschuld PG, Kloiber S, Horstmann S, Uhr M, Muller-Myhsok B, Holsboer F. Polymorphisms in the FKBP5 gene region modulate recovery from psychosocial stress in healthy controls. The European journal of neuroscience. 2008;28:389–398. doi: 10.1111/j.1460-9568.2008.06332.x. [DOI] [PubMed] [Google Scholar]
- Joels M, Fernandez G, Roozendaal B. Stress and emotional memory: a matter of timing. Trends in cognitive sciences. 2011;15:280–288. doi: 10.1016/j.tics.2011.04.004. [DOI] [PubMed] [Google Scholar]
- Klengel T, Mehta D, Anacker C, Rex-Haffner M, Pruessner JC, Pariante CM, Pace TW, Mercer KB, Mayberg HS, Bradley B, Nemeroff CB, Holsboer F, Heim CM, Ressler KJ, Rein T, Binder EB. Allele-specific FKBP5 DNA demethylation mediates gene-childhood trauma interactions. Nature neuroscience. 2013;16:33–41. doi: 10.1038/nn.3275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahon PB, Zandi PP, Potash JB, Nestadt G, Wand GS. Genetic association of FKBP5 and CRHR1 with cortisol response to acute psychosocial stress in healthy adults. Psychopharmacology. 2013;227:231–241. doi: 10.1007/s00213-012-2956-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta D, Gonik M, Klengel T, Rex-Haffner M, Menke A, Rubel J, Mercer KB, Putz B, Bradley B, Holsboer F, Ressler KJ, Muller-Myhsok B, Binder EB. Using polymorphisms in FKBP5 to define biologically distinct subtypes of posttraumatic stress disorder: evidence from endocrine and gene expression studies. Archives of general psychiatry. 2011;68:901–910. doi: 10.1001/archgenpsychiatry.2011.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menke A, Klengel T, Rubel J, Bruckl T, Pfister H, Lucae S, Uhr M, Holsboer F, Binder EB. Genetic variation in FKBP5 associated with the extent of stress hormone dysregulation in major depression. Genes, brain, and behavior. 2013;12:289–296. doi: 10.1111/gbb.12026. [DOI] [PubMed] [Google Scholar]
- Nater UM, Rohleder N. Salivary alpha-amylase as a non-invasive biomarker for the sympathetic nervous system: current state of research. Psychoneuroendocrinology. 2009;34:486–496. doi: 10.1016/j.psyneuen.2009.01.014. [DOI] [PubMed] [Google Scholar]
- Pariante CM, Lightman SL. The HPA axis in major depression: classical theories and new developments. Trends in neurosciences. 2008;31:464–468. doi: 10.1016/j.tins.2008.06.006. [DOI] [PubMed] [Google Scholar]
- Quaedflieg CW, Schwabe L, Meyer T, Smeets T. Time dependent effects of stress prior to encoding on event-related potentials and 24h delayed retrieval. Psychoneuroendocrinology. 2013;38:3057–3069. doi: 10.1016/j.psyneuen.2013.09.002. [DOI] [PubMed] [Google Scholar]
- Reynolds PD, Ruan Y, Smith DF, Scammell JG. Glucocorticoid resistance in the squirrel monkey is associated with overexpression of the immunophilin FKBP51. The Journal of clinical endocrinology and metabolism. 1999;84:663–669. doi: 10.1210/jcem.84.2.5429. [DOI] [PubMed] [Google Scholar]
- Roozendaal B, McEwen BS, Chattarji S. Stress, memory and the amygdala. Nature reviews. 2009;10:423–433. doi: 10.1038/nrn2651. [DOI] [PubMed] [Google Scholar]
- Roy A, Gorodetsky E, Yuan Q, Goldman D, Enoch MA. Interaction of FKBP5, a stress-related gene, with childhood trauma increases the risk for attempting suicide. Neuropsychopharmacology. 2010;35:1674–1683. doi: 10.1038/npp.2009.236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarapas C, Cai G, Bierer LM, Golier JA, Galea S, Ising M, Rein T, Schmeidler J, Muller-Myhsok B, Uhr M, Holsboer F, Buxbaum JD, Yehuda R. Genetic markers for PTSD risk and resilience among survivors of the World Trade Center attacks. Disease markers. 2011;30:101–110. doi: 10.3233/DMA-2011-0764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwabe L, Bohringer A, Chatterjee M, Schachinger H. Effects of pre-learning stress on memory for neutral, positive and negative words: Different roles of cortisol and autonomic arousal. Neurobiology of learning and memory. 2008a;90:44–53. doi: 10.1016/j.nlm.2008.02.002. [DOI] [PubMed] [Google Scholar]
- Schwabe L, Haddad L, Schachinger H. HPA axis activation by a socially evaluated cold-pressor test. Psychoneuroendocrinology. 2008b;33:890–895. doi: 10.1016/j.psyneuen.2008.03.001. [DOI] [PubMed] [Google Scholar]
- Schwabe L, Joels M, Roozendaal B, Wolf OT, Oitzl MS. Stress effects on memory: An update and integration. Neuroscience and biobehavioral reviews. 2012;36:1740–1749. doi: 10.1016/j.neubiorev.2011.07.002. [DOI] [PubMed] [Google Scholar]
- Spielberger CD, Gorsuch RL, Lushene RD, Vagg PR, Jacobs GA. Manual for the State-Trait Anxiety Inventory. Palo Alto, CA: Consulting Psychologist Press; 1983. [Google Scholar]
- Vogel S, Schwabe L. Stress in the zoo: Tracking the impact of stress on memory formation over time. Psychoneuroendocrinology. 2016;71:64–72. doi: 10.1016/j.psyneuen.2016.04.027. [DOI] [PubMed] [Google Scholar]
- Watkins LE, Han S, Harpaz-Rotem I, Mota NP, Southwick SM, Krystal JH, Gelernter J, Pietrzak RH. FKBP5 polymorphisms, childhood abuse, and PTSD symptoms: Results from the National Health and Resilience in Veterans Study. Psychoneuroendocrinology. 2016;69:98–105. doi: 10.1016/j.psyneuen.2016.04.001. [DOI] [PubMed] [Google Scholar]
- Watson D, Clark LA, Tellegen A. Development and validation of brief measures of positive and negative affect: the PANAS scales. Journal of personality and social psychology. 1988;54:1063–1070. doi: 10.1037//0022-3514.54.6.1063. [DOI] [PubMed] [Google Scholar]
- White MG, Bogdan R, Fisher PM, Munoz KE, Williamson DE, Hariri AR. FKBP5 and emotional neglect interact to predict individual differences in amygdala reactivity. Genes, brain, and behavior. 2012;11:869–878. doi: 10.1111/j.1601-183X.2012.00837.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willour VL, Chen H, Toolan J, Belmonte P, Cutler DJ, Goes FS, Zandi PP, Lee RS, MacKinnon DF, Mondimore FM, Schweizer B, DePaulo JR, Jr, Gershon ES, McMahon FJ, Potash JB. Family-based association of FKBP5 in bipolar disorder. Molecular psychiatry. 2009;14:261–268. doi: 10.1038/sj.mp.4002141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie P, Kranzler HR, Poling J, Stein MB, Anton RF, Farrer LA, Gelernter J. Interaction of FKBP5 with childhood adversity on risk for post-traumatic stress disorder. Neuropsychopharmacology. 2010;35:1684–1692. doi: 10.1038/npp.2010.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yehuda R. Stress hormones and PTSD. In: Shiromani PJ, Keane TM, LeDoux JE, editors. Post-traumatic stress disorder: Basic science and clinical practice. New York, NY: Humana Press; 2009. pp. 257–275. [Google Scholar]
- Young KA, Thompson PM, Cruz DA, Williamson DE, Selemon LD. BA11 FKBP5 expression levels correlate with dendritic spine density in postmortem PTSD and controls. Neurobiology of stress. 2015;2:67–72. doi: 10.1016/j.ynstr.2015.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zobel A, Schuhmacher A, Jessen F, Hofels S, von Widdern O, Metten M, Pfeiffer U, Hanses C, Becker T, Rietschel M, Scheef L, Block W, Schild HH, Maier W, Schwab SG. DNA sequence variants of the FKBP5 gene are associated with unipolar depression. The international journal of neuropsychopharmacology / official scientific journal of the Collegium Internationale Neuropsychopharmacologicum (CINP) 2010;13:649–660. doi: 10.1017/S1461145709991155. [DOI] [PubMed] [Google Scholar]
- Zoladz PR, Clark B, Warnecke A, Smith L, Tabar J, Talbot JN. Pre-learning stress differentially affects long-term memory for emotional words, depending on temporal proximity to the learning experience. Physiology & behavior. 2011;103:467–476. doi: 10.1016/j.physbeh.2011.01.016. [DOI] [PubMed] [Google Scholar]
- Zoladz PR, Kalchik AE, Hoffman MM, Aufdenkampe RL, Burke HM, Woelke SA, Pisansky JM, Talbot JN. Brief, pre-retrieval stress differentially influences long-term memory depending on sex and corticosteroid response. Brain and cognition. 2014a;85:277–285. doi: 10.1016/j.bandc.2014.01.010. [DOI] [PubMed] [Google Scholar]
- Zoladz PR, Kalchik AE, Hoffman MM, Aufdenkampe RL, Lyle SM, Peters DM, Brown CM, Cadle CE, Scharf AR, Dailey AM, Wolters NE, Talbot JN, Rorabaugh BR. ADRA2B deletion variant selectively predicts stress-induced enhancement of long-term memory in females. Psychoneuroendocrinology. 2014b;48:111–122. doi: 10.1016/j.psyneuen.2014.06.012. [DOI] [PubMed] [Google Scholar]
- Zoladz PR, Peters DM, Kalchik AE, Hoffman MM, Aufdenkampe RL, Woelke SA, Wolters NE, Talbot JN. Brief, pre-learning stress reduces false memory production and enhances true memory selectively in females. Physiology & behavior. 2014c;128:270–276. doi: 10.1016/j.physbeh.2014.02.028. [DOI] [PubMed] [Google Scholar]
- Zoladz PR, Warnecke AJ, Woelke SA, Burke HM, Frigo RM, Pisansky JM, Lyle SM, Talbot JN. Pre-learning stress that is temporally removed from acquisition exerts sex-specific effects on long-term memory. Neurobiology of learning and memory. 2013;100:77–87. doi: 10.1016/j.nlm.2012.12.012. [DOI] [PubMed] [Google Scholar]