Abstract
Background
To determine the effect of retroperitoneal (RP) exploration on progression-free (PFS) and overall survival (OS) in epithelial ovarian cancer (EOC) patients with stage IIIC disease who underwent optimal debulking surgery.
Methods
Data were collected from GOG-182 records of stage IIIC EOC patients cytoreduced to no gross residual disease (R0) or minimal gross residual (<1cm) disease (MGRD) at primary surgery. Those with stage IIIC disease by intraperitoneal (IP) tumor were included and divided into 3 groups: (1) >2cm IP tumor without lymph node involvement (IP/RP-); (2) >2cm IP tumor with lymph node involvement (IP/RP+); and (3) >2cm IP tumor with no RP exploration (IP/RP?). The effects of disease distribution and RP exploration on PFS and OS were assessed using Kaplan-Meier and Proportional Hazards methods.
Results
There were 1,871 stage IIIC patients in GOG-182 who underwent optimal primary debulking surgery. Of these, 689 (36.8%) underwent RP exploration with removal of lymph nodes from at least 1 para-aortic site and 1,182 (63.2%) did not. There were 269 in the IP/RP- group, 420 in the IP/RP+ group, and 1,182 in the IP/RP? group. Improved PFS (18.5 vs. 16.0 mo, p<0.0001) and OS (53.3 vs. 42.8 mo, p<0.0001) were associated with RP exploration vs. no exploration. In patients with MGRD there was improved PFS (16.8 vs. 15.1 mo, p=0.0108) and OS (44.9 vs. 40.5 mo, p=0.0076) vs. no exploration.
Conclusions
RP exploration at the time of primary surgery in patients with optimally debulked stage IIIC EOC is associated with a survival benefit.
Keywords: ovarian cancer, retroperitoneal exploration, lymphadenectomy, disease burden, surgical debulking
Introduction
Epithelial ovarian cancer (EOC) is the most lethal gynecologic malignancy.1 Presence of lymph node metastases is an important prognostic factor in EOC, and the International Federation of Gynecology and Obstetrics (FIGO) staging system for ovarian cancer has included lymph node status since 1986.2-7 According to the previous FIGO staging system, lymph node metastasis was considered stage IIIC disease even if the primary tumor is confined to the ovary or pelvis.6 As a result, approximately 10-25% of patients with presumed early stage disease confined to the ovaries or pelvis are upstaged to III due to retroperitoneal (RP) lymph node metastasis identified during thorough surgical staging.8-10
While retroperitoneal and intraperitoneal (IP) disease burden are both prognostically important, patients with advanced stage disease by positive lymph nodes alone have improved survival compared to those with bulky peritoneal disease.2-5,11-22 It is unclear whether performing a RP exploration or dissection at the time of primary cytoreductive surgery impacts survival in stage IIIC patients with IP disease. Lymph node assessment is not always performed at the time of primary debulking surgery, particularly if the patient meets other criteria for stage IIIC disease. This surgical practice has been based on data showing no survival advantage in performing lymph node assessment. In a large randomized trial comparing systematic lymphadenectomy and selective resection of only bulky lymph nodes, systematic lymphadenectomy was associated with improved PFS but not OS in optimally debulked ovarian cancer. Of the 427 patients in this trial, 75% had stage IIIC disease, but only 39% received adjuvant platinum and taxane based chemotherapy.23 Two additional ongoing randomized trials are currently being conducted to answer this question.
In the current absence of updated randomized data, our objective was to determine the effect of retroperitoneal exploration, defined as removal of nodal tissue from at least one pelvic or para-aortic lymph node site, on the PFS and OS in EOC patients with stage IIIC disease who underwent optimal debulking surgery in a large multi-institutional trial.24
Patients and Methods
All patient data for this study was abstracted from GOG-182, a large, multi-institutional clinical trial.24 All GOG-182 patients were diagnosed with FIGO stage III or IV histologically confirmed EOC or primary peritoneal cancer (PPC). All patients underwent primary cytoreductive surgery to either optimal (≤1 cm) or suboptimal residual disease before being randomized to 1 of 5 platinum and paclitaxel-based chemotherapy regimens. No statistically significant treatment effects on PFS or OS were found. Further details of eligibility criteria and results from the original study have been published.24 GOG-182 was activated in January 2001, and closed in September 2004.
This current study focused on those GOG-182 patients who had FIGO stage IIIC and underwent cytoreductive surgery with <1cm residual. Stage IIIC patients who underwent surgical cytoreduction to no gross residual disease (R0) or minimal gross residual disease (MGRD) of ≤1 cm at primary debulking surgery were divided into groups based on whether a RP exploration was performed and by distribution of disease. RP exploration was defined as removal of nodal tissue from at least one pelvic or para-aortic lymph node site, as defined by the GOG Surgical Procedures Manual.25 GOG defines pelvic lymphadenectomy as removal of nodal tissue from the distal half of the common iliac arteries, the anterior and medial aspect of the proximal half of the external iliac artery and vein, and the distal half of the obturator fat pad anterior to the obturator nerve. Para-aortic lymph node dissection is described as removal of nodal tissue over the distal inferior vena cava from the level of the inferior mesenteric artery to the mid right common iliac artery and removal of the nodal tissue between the aorta and left ureter from the mid inferior mesenteric artery to the mid left common iliac artery. An adequate nodal resection requires that lymphatic tissue be excised pathologically from each side (right and left), but no specific nodal counts are required.25 Thus, some practitioners may have opted for selective lymph node sampling rather than a full dissection.
Those patients who were stage IIIC by RP disease with positive lymph nodes only were excluded. By definition, these patients had <2 cm of IP disease outside of the pelvis and are now considered stage IIIA based on 2014 FIGO staging.22 Those patients with stage IIIC disease with ≥2 cm of IP tumor were included and divided into groups based on whether a RP exploration was performed. The RP exploration group included: those with ≥2 cm of IP tumor with negative lymph nodes (the IP/RP- group); and those with ≥2 cm of IP tumor with positive lymph nodes (the IP/RP+ group). Systematic RP exploration was defined as removal of nodal tissue from bilateral pelvic and para-aortic sites; selective RP exploration was defined as removal of nodal tissue from at least one but not all pelvic and/or para-aortic nodal sites. The no RP exploration group included those with ≥2 cm of IP tumor with no RP exploration (the IP/RP? group).
Patient demographics and tumor characteristics including age, race, performance status, tumor grade, and histology were extracted from GOG databases. Information describing surgical procedures and preoperative extent of disease based on 56 anatomic locations was abstracted from GOG surgical reporting forms and diagrams and stratified into groups based on a disease score (DS) and surgical complexity score (CS), as previously defined.26 Anatomic locations reported for nodal sampling included 10 sites: right/left para-aortic, right/left common iliac, right/left external iliac, right/left obturator, and right/eft internal iliac. Operative notes and pathology reports were also reviewed to obtain accurate descriptions of disease and surgical extent. Baseline performance status (PS) was defined according to GOG criteria as 0 for normal activity, 1 for symptomatic and fully ambulatory, and 2 for symptomatic and in bed less than 50% of the time. The reported 95% confidence intervals describe the plausible range of values for the true (unobserved) HR in the population as supported by the data.
Our primary analysis examined whether the long-term clinical outcomes in stage IIIC EOC/PPC patients with optimal residual disease depended on whether a RP exploration was performed. Outcomes for the primary analysis were PFS and OS. PFS was defined as the time (in months) from date of entry on GOG-182 to documentation of disease progression or death, whichever came first. OS was defined as the time (in months) from date of entry on GOG-182 to death from any cause. Patients who were still alive were censored for OS and PFS, respectively, at the date of last follow up. Differences in the time to event outcomes across the groups were described using adjusted hazard ratio (HR) estimates and 95% confidence intervals.
Multivariate analysis was performed to identify relationships of RP with several factors: PFS, OS, age, and R0. Adjusted HR estimates were obtained from multivariate proportional hazards model. The potentially confounding factors, shown to be statistically associated with the RP exploration groups and the time to event outcomes in univariate analysis, were retained in the multivariate model regardless of statistical significance. The models did not account for possible confounding from significant differences in histology because of the small number of patients in the non-serous group. The primary multivariate results were supported by subgroup-specific Kaplan-Meier estimates of the PFS and OS distributions. Log-rank tests for the null hypotheses of no association between subgroups and OS or PFS outcomes were also provided. Differences in baseline covariates among the stage IIIC subgroups were tested using the Kruskal-Wallis or Pearson Chi square tests as appropriate.
In all cases, p values <0.05 were considered statistically significant. The significance level was not adjusted to control for the effects of multiple testing on the overall Type I error rate. All patients signed an approved informed consent and authorization permitting release of personal health information prior to enrollment. All institutions required approval by their local Institutional Review Board before trial initiation.
Results
GOG-182 assessed 4,312 women with FIGO stage III or IV EOC or PPC. A total of 1,871 patients had stage IIIC disease with ≥2 cm of IP tumor and underwent primary cytoreductive surgery to R0 or MGRD and were included in this analysis, with a median follow up of 79 months. Of these, 689 (36.8%) underwent RP exploration with removal of nodal tissue from at least one pelvic or para-aortic lymph node sites and 1,182 (63.2%) did not. There were 269 in the IP/RP- group, 420 in the IP/RP+ group, and 1,182 in the IP/RP? group.
Of all 689 patients who underwent RP exploration, 158 (22.9%) had a systematic RP exploration performed with removal tissue at bilateral pelvic and para-aortic lymph node sites, while 531 (77.1%) had a selective RP exploration with removal of nodal tissue from at least one site. There were 92 (13.4%) patients with resection of nodal tissue from only 1 site, and 597 (86.6%) patients with resection of nodal tissue from more than 1 site. A total of 231 (33.5%) underwent resection at all 10 nodal sites, while 374 (54.3%) underwent resection of at least 6 sites. When examining the groups who underwent RP exploration, the IP/RP- group was more likely to have more lymph node sites sampled, with a median of 8 sites compared with 5 (p<0.001) in IP/RP+, have a bilateral sampling performed at 84.4% vs. 74.8% (p=0.002), and have both pelvic and para-aortic sites sampled at 57.9% vs. 41.9% (p=0.015), respectively. This data suggests a lymph node sampling in the majority of patients in both groups rather than just resection of bulky nodes.
Table 1 shows the clinical and pathologic characteristics of these patients. The groups were balanced for prognostic factors including race, BMI, PS, histology and tumor grade. Patients in RP exploration group were more likely to be younger (p<0.001), have ovarian rather than PPC (p<0.001), no ascites (p=0.007), and no gross residual disease (p<0.001). The preoperative extent of disease was more likely to be low or moderate in the RP exploration group (p<0.001), and the complexity of surgical procedures was higher (p<0.001). There were no significant differences in the type of adjuvant chemotherapy received or the reasons for discontinuing therapy.
Table 1.
Patient characteristics by group.
| RP exploration (n = 689) | No exploration (n = 1,182) | p | |
|---|---|---|---|
| Age group (years) | |||
|
| |||
| <55 | 294 (42.7%) | 391 (33.1%) | <0.001 |
| 55-64 | 226 (32.8%) | 388 (32.8%) | |
| 65+ | 169 (24.5%) | 403 (34.1%) | |
|
| |||
| Race | |||
|
| |||
| White | 644 (93.5%) | 1,077 (91.1%) | 0.084 |
| Black | 17 (2.5%) | 49 (4.1%) | |
| Other | 28 (4.0%) | 56 (4.8%) | |
|
| |||
| Performance status | |||
|
| |||
| 0 | 346 (50.2%) | 558 (47.2%) | 0.271 |
| 1 | 301 (43.7%) | 527 (44.6%) | |
| 2 | 25 (3.6%) | 67 (5.7%) | |
| Missing | 17 (2.5%) | 30 (2.5%) | |
|
| |||
| Site | |||
|
| |||
| EOC | 623 (90.4%) | 988 (83.6%) | <0.001 |
| PPC | 66 (9.6%) | 194 (16.4%) | |
|
| |||
| Histology | |||
|
| |||
| Serous | 584 (84.8%) | 1,001 (84.7%) | 0.984 |
| Endometrioid | 32 (4.6%) | 45 (3.8%) | |
| Clear cell | 16 (2.3%) | 27 (2.3%) | |
| Mucinous | 6 (0.9%) | 12 (1.0%) | |
| Mixed epithelial | 37 (5.4%) | 69 (5.8%) | |
| Other | 14 (2.0%) | 28 (2.4%) | |
|
| |||
| Grade | |||
|
| |||
| 1 | 27 (4.7%) | 40 (4.1%) | 0.693 |
| 2 | 119 (20.8%) | 217 (22.3%) | |
| 3 | 427 (74.5%) | 716 (73.6%) | |
|
| |||
| Ascites | |||
|
| |||
| Yes | 484 (72.8%) | 902 (78.4%) | 0.007 |
| No | 181 (27.2%) | 249 (21.6%) | |
|
| |||
| Residual disease | |||
|
| |||
| NGRD | 228 (33.1%) | 198 (16.8%) | <0.001 |
| MGRD | 461 (66.9%) | 984 (83.2%) | |
|
| |||
| Preoperative extent of disease | |||
|
| |||
| DS-low | 20 (2.9%) | 7 (0.6%) | <0.001 |
| DS-moderate | 223 (32.4%) | 304 (25.7%) | |
| DS-high | 446 (64.7%) | 871 (73.7%) | |
|
| |||
| Surgical complexity score | |||
|
| |||
| CS-low | 49 (7.1%) | 261 (22.1%) | <0.001 |
| CS-moderate | 429 (62.3%) | 789 (66.8%) | |
| CS-high | 211 (30.6%) | 132 (11.2%) |
Abbreviations: RP- retroperitoneal; EOC- epithelial ovarian cancer; PPC- primary peritoneal cancer; NGRD- no gross residual disease; MGRD- minimal gross residual disease; DS- disease score; CS- surgical complexity score.
In multivariate modeling, the probability of having a RP exploration at the time of surgical debulking decreased with increasing age in 10-year increments (OR 0.79 (95% CI 0.72-0.87), p<0.001). Those with low or moderate preoperative extent of disease were significantly more likely to have a RP exploration compared with those with high disease burden (OR 7.15 (95% CI 2.88-17.72), p<0.001 and OR 1.95 (95% CI 1.54-2.48), p<0.001), respectively. Those who underwent more complex surgical procedures were also more likely to undergo a RP exploration (OR 3.54 (95% CI 2.73-4.59), p<0.001). Multivariate regression revealed that RP exploration was associated with significantly improved PFS (HR 0.85, 95% CI 0.76-0.95, p=0.004) and OS (HR 0.85, 95% CI 0.75-0.96, p=0.009) compared to the no RP exploration group (Table 2). In multivariable proportional hazards modeling, there was no difference in survival between those who underwent systematic RP exploration compared with those who underwent a selective RP exploration (PFS: HR 0.95 (95% CI 0.74-1.23), p=0.71, and OS: HR 1.11 (95% CI 0.89-1.38), p=0.37).
Table 2.
Predictors of OS and PFS.
| PFS | OS | ||||||
|---|---|---|---|---|---|---|---|
|
|
|||||||
| Variable | HR* | 95% CI | P | HR* | 95% CI | P | |
| RP exploration (ref, no RP exploration) | 0.85 | 0.76 to 0.95 | 0.004 | 0.85 | 0.75 to 0.96 | 0.009 | |
| Residual disease (ref, MGRD) | 0.73 | 0.64 to 0.85 | <0.001 | 0.74 | 0.63 to 0.87 | <0.001 | |
| Disease group | |||||||
| DS low (ref, DS-High) | 1.29 | 0.85 to 1.96 | 0.223 | 1.34 | 0.86 to 2.09 | 0.195 | |
| DS mod (ref, DS-High) | 0.76 | 0.67 to 0.87 | <0.001 | 0.72 | 0.62 to 0.83 | <0.001 | |
| Surgical score group | |||||||
| CS High (ref, CS mod) | 1.08 | 0.95 to 1.24 | 0.251 | 0.98 | 0.84 to 1.13 | 0.745 | |
| CS Low (ref, CS mod) | 0.90 | 0.79 to 1.04 | 0.153 | 0.91 | 0.78 to 1.06 | 0.234 | |
| No ascites (ref, yes) | 0.83 | 0.73 to 0.94 | 0.003 | 0.79 | 0.69 to 0.92 | 0.002 | |
| Age (per 1-year increase) | 1.03 | 0.98 to 1.08 | 0.205 | 1.11 | 1.05 to 1.17 | <0.001 | |
Abbreviations: CS, surgical complexity score; DS, disease score; HR, hazard ratio; MGRD, minimal gross residual disease; OS, overall survival; PFS, progression free survival; R0, no gross residual disease; RP, retroperitoneal; ref, reference.
HR estimates obtained from multivariable proportional hazards models.
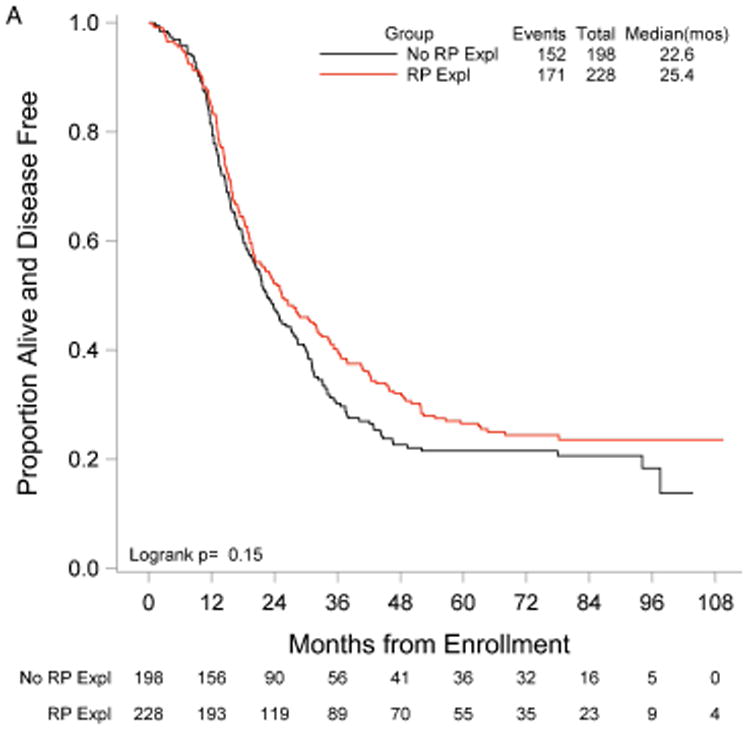
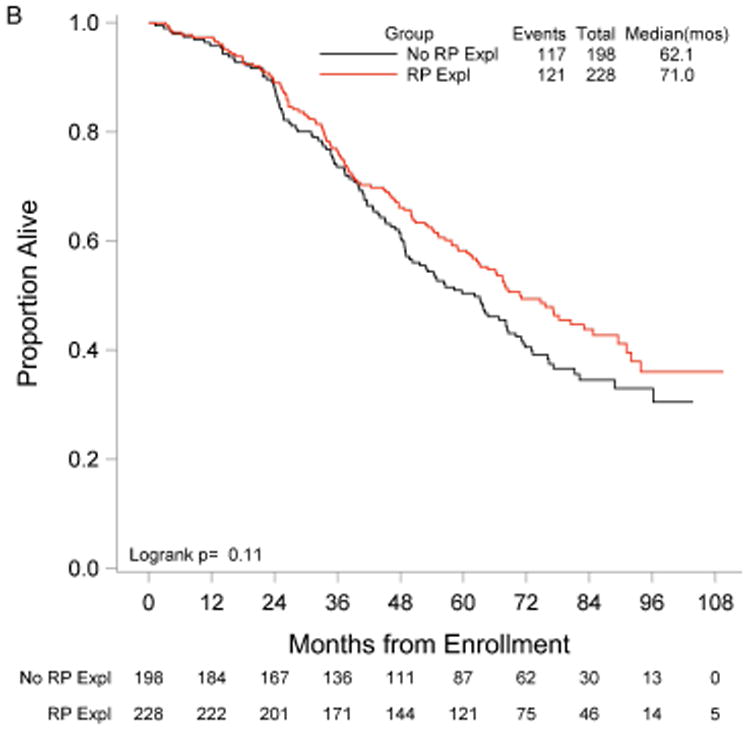
RP exploration was associated with significantly improved PFS, 18.5 compared to 16.0 months in the no RP exploration group (p<0.01) (Fig. 1A). Median OS was 53.3 and 42.8 months in the RP exploration and no RP exploration groups, respectively (p<0.01) (Fig. 1B). This trend was noted in both the R0 (Fig. 2A and Fig. 2B) and MGRD patients but only reached statistical significance in the latter group with longer PFS (16.8 vs. 15.1 months, p=0.0108, Fig. 3A) and OS (44.9 vs. 40.5 months, p=0.0076, Fig. 3B) vs. no exploration. The IP/RP- group had better PFS (22.1 vs. 16.0 months, p<0.01, Fig. 4A) and OS (63.1 vs. 42.8 months, p<0.01, Fig. 4B) vs. IP/RP? group. Among those with RP exploration, the IP/RP- group had better PFS (22.1 vs. 17.2 months, p<0.01, Fig. 5A) and OS (63.1 vs. 45.9 months, p<0.01, Fig. 5B) vs. IP/RP+, respectively.
Figure 1.
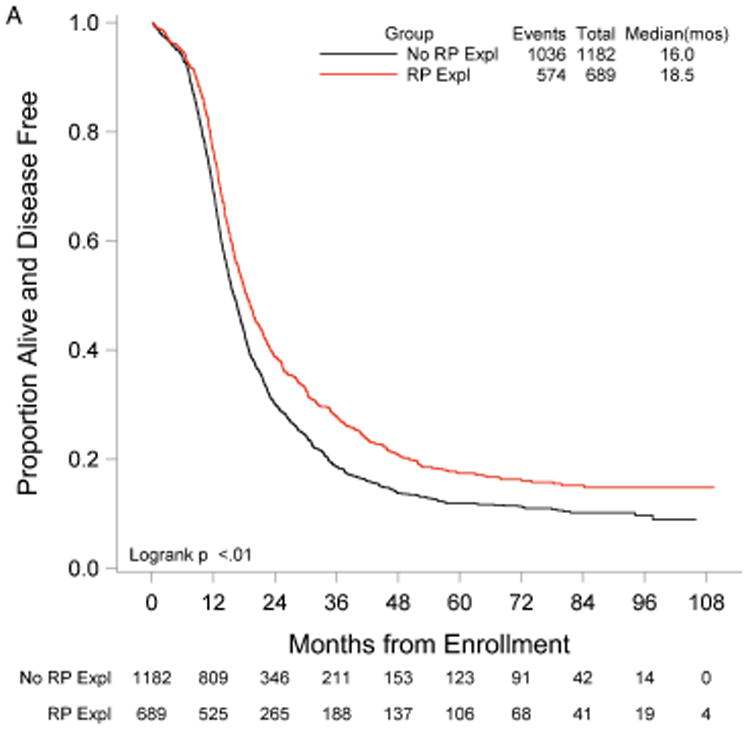
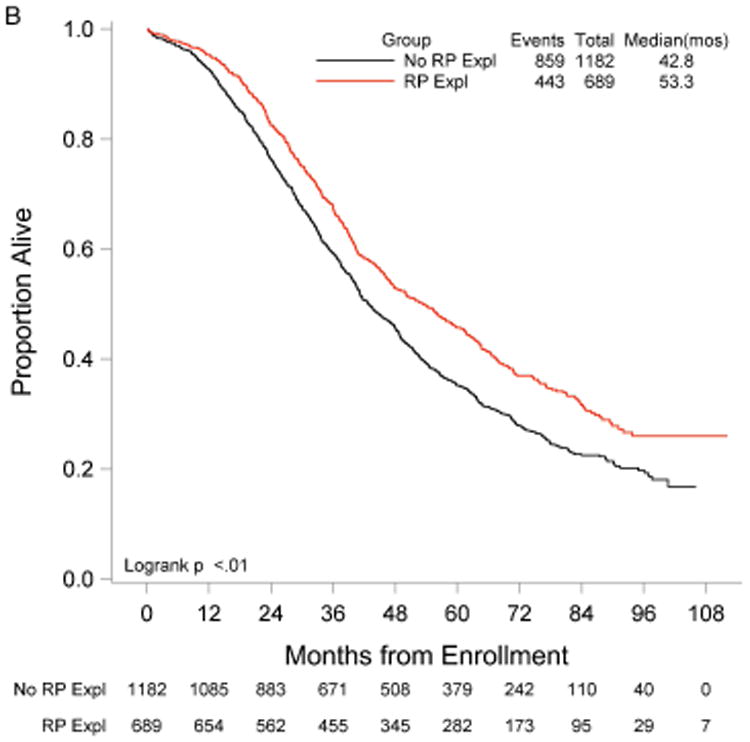
Progression-free (A) and overall (B) survival for patients with (RP Expl) and without (No RP Expl) retroperitoneal exploration at primary cytoreductive surgery.
Figure 2.
Progression-free (A) and overall (B) survival for patients with no gross residual disease with (RP Expl) and without (RP Expl) retroperitoneal exploration at primary cytoreductive surgery.
Figure 3.
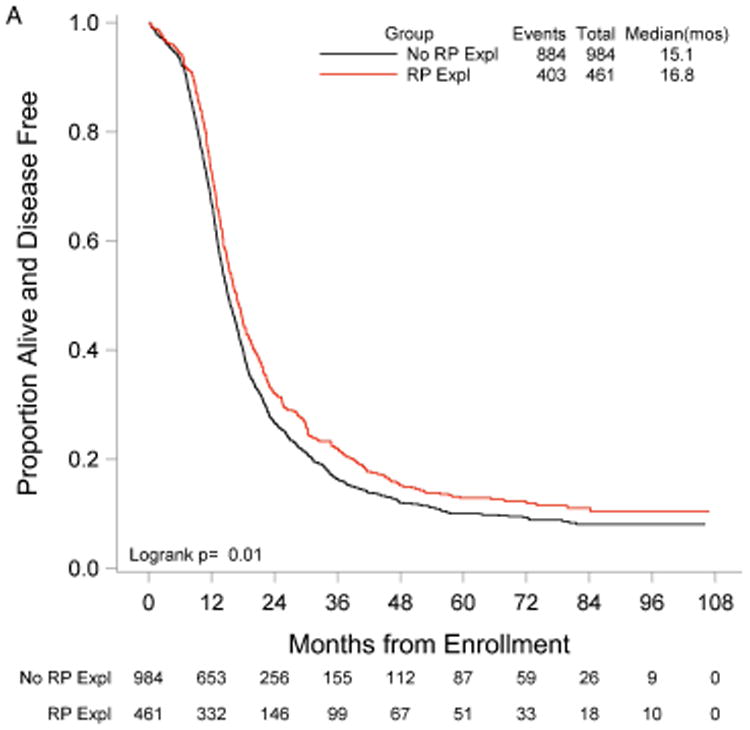
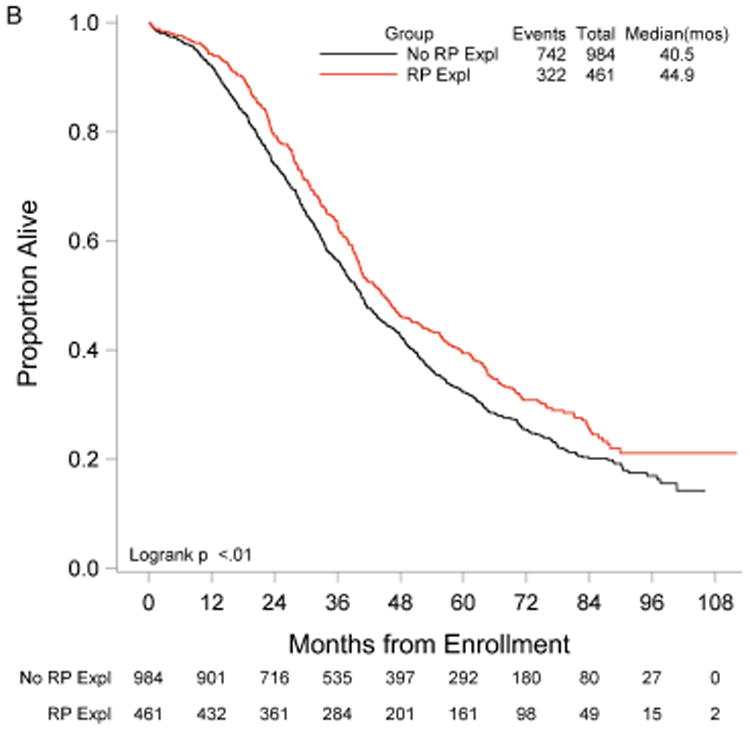
Progression-free (A) and overall (B) survival for patients with minimal gross residual disease with (RP Expl) and without (RP Expl) retroperitoneal exploration at primary cytoreductive surgery.
Figure 4.
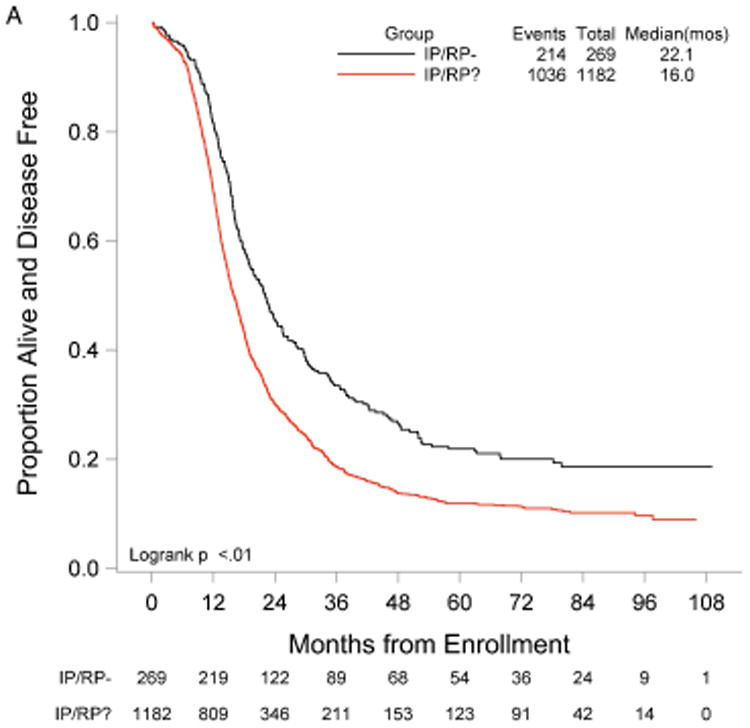
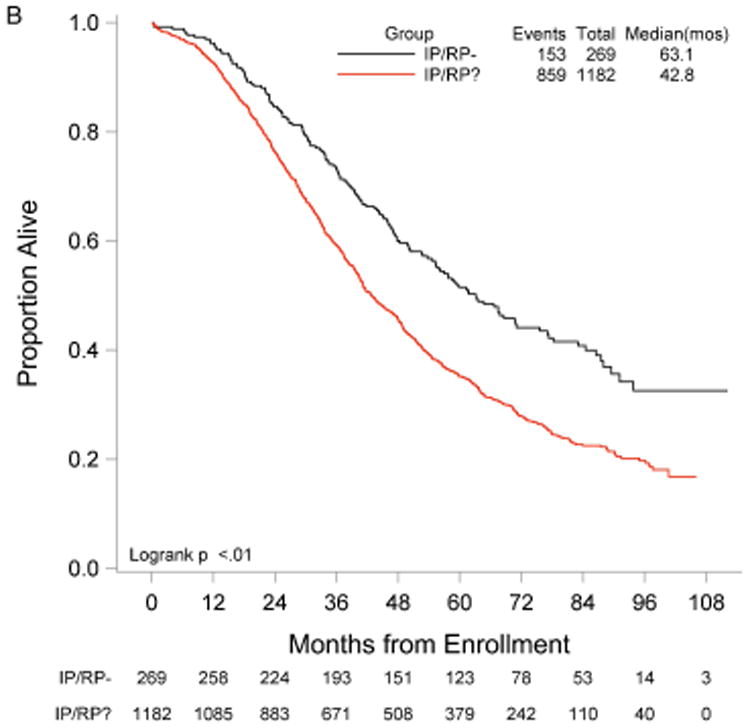
Progression-free (A) and overall (B) survival for patients with a retroperitoneal exploration at primary cytoreductive surgery and negative lymph nodes (IP/RP-) vs. patients without a retroperitoneal exploration (IP/RP?).
Figure 5.
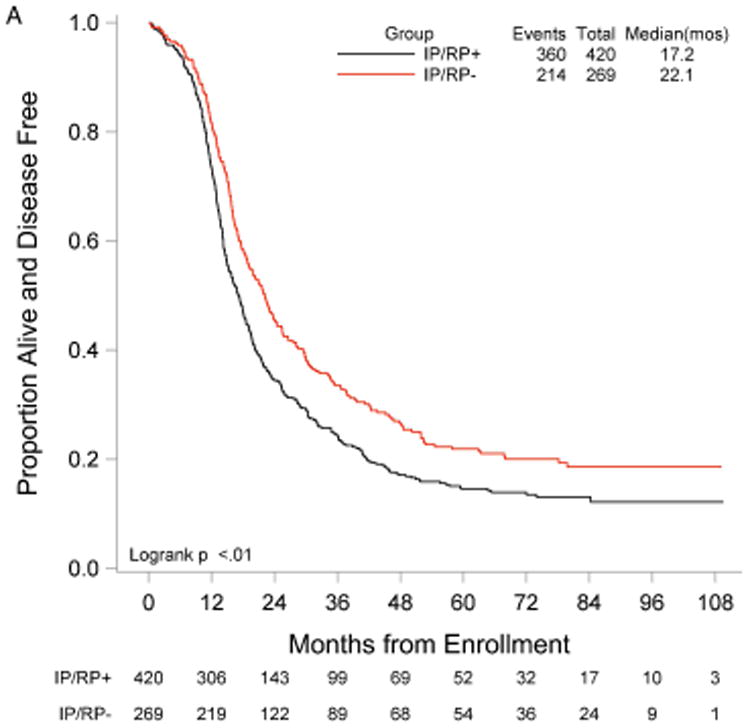
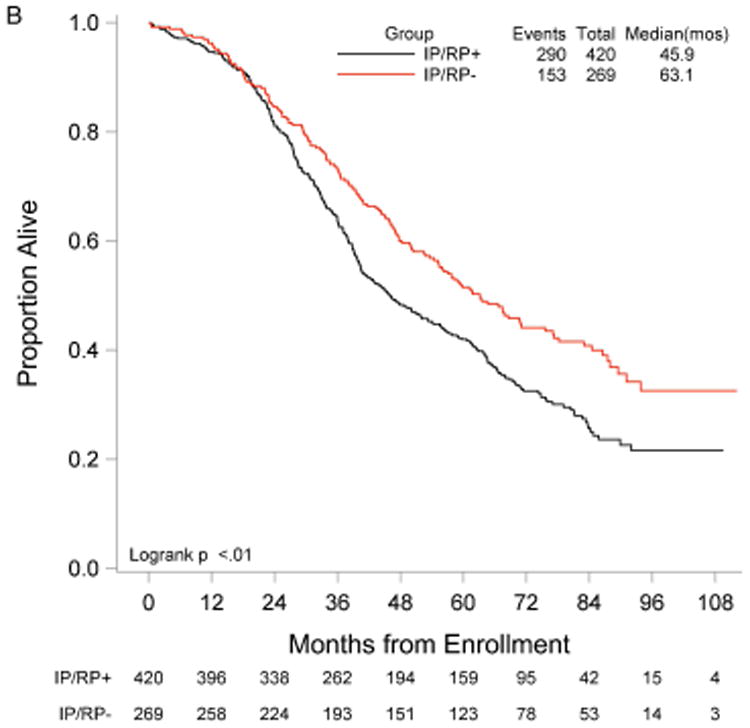
Progression-free (A) and overall (B) survival for patients with a retroperitoneal exploration at primary cytoreductive surgery and negative lymph nodes (IP/RP-) vs. positive lymph nodes (IP/RP+).
Patients who underwent a retroperitoneal exploration were more likely to obtain R0 (OR 2.02 (95% CI 1.54-2.65), p<0.001). The effects of RP exploration and R0 at cytoreduction were independently associated with improved PFS and OS. RP exploration was significantly associated with improved PFS (HR 0.85 (95% CI 0.76-0.95), p=0.004) and OS (HR 0.85 (95% CI 0.75-0.96), p=0.009). R0 at cytoreduction was associated with significantly improved PFS (HR 0.73 (95% CI 0.64-0.85), p<0.001) and OS (HR 0.74 (95% CI 0.63-0.87), p<0.001).
Discussion
Among stage IIIC EOC patients who underwent optimal cytoreduction, our data was associated with a significant improvement in PFS and OS for patients who underwent a RP exploration at the time of surgical debulking compared to those who did not. For the RP exploration and no RP exploration groups, PFS was 18.5 vs. 16.0 months, respectively, and OS was 53.3 vs. 42.8 months, respectively. In patients with MGRD, PFS and OS were significantly improved in those who underwent a RP exploration compared to those who did not, suggesting a survival advantage in this subgroup when a thorough surgical exploration is performed at cytoreduction.
Maximal cytoreductive effort at primary debulking surgery is a significant prognostic factor in EOC, but it is still unclear what role systematic lymphadenectomy plays in prognosis and therapy. Chan et al. retrospectively examined SEER data for 13,918 women with stage III-IV EOC and found that the extent of lymphadenectomy is associated with improved survival.27 Panici et al. published the first multicenter randomized trial comparing systematic lymphadenectomy and selective resection of bulky nodes.23 Systematic lymphadenctomy was associated with significantly improved PFS but not OS. An exploratory data analysis of three prospective randomized trials, reported by du Bois et al., compared complete, incomplete and no nodal dissection in advanced ovarian cancer debulking surgery. They concluded that lymphadenectomy may offer a survival benefit to patients with complete intraperitoneal debulking to treat advanced stage disease.28 These results need to be confirmed in the current prospective randomized trial AGO-LION (NCT007712218), which is comparing systematic vs. no lymphadenectomy in stage IIB-IV EOC optimally debulked patients with complete intraperitoneal tumor resection, with a primary end-point of overall survival.29 Similarly, the results of the CARACO trial (NCT0128490) are still pending, which is a randomized phase III multicenter French trial comparing complete vs. bulky lymphadenectomy in stage III-IV EOC patients undergoing optimal debulking surgery.30 The primary objective of the CARACO trial is 5-year survival without recurrence.
In the current absence of definitive randomized clinical trial data, this study represents the largest set of well-annotated data on the extent of disease and surgical effort in EOC from a large, multi-institutional trial. In contrast to previous studies, our group of patients received equivalent adjuvant treatments with similar PFS and OS across treatment arms. Because they were enrolled in a clinical trial with strictly enforced eligibility criteria, this cohort is relatively uniform compared to other studies investigating this topic. All patients were treated with modern platinum/taxane-based regimens with no statistically significant difference in prognosis across the five arms. These factors tend to reduce the possibility of confounding and enhance the reliability of the prognostic effects we have estimated. Inclusion of patients with removal of nodal tissue from at least one lymph node served as a surrogate marker for RP exploration and assessment even if systematic lymphadenectomy was not performed.
Surgical effort and tumor biology interact to affect patient outcomes. Even when surgical effort results in R0, initial disease burden is a significant prognostic indicator, as demonstrated in a recent study. Patients with the highest preoperative disease burden had significantly poorer PFS and OS, and this was maintained in R0 patients.31 RP exploration may be a proxy for a more thorough surgical effort in these patients rather than tumor biology alone driving outcomes. In a prospective single-institution trial, 100 consecutive patients with stage III and IV EOC underwent RP lymph node dissection during primary cytoreductive surgery. Lymph nodes were examined and reported to be palpably macroscopically positive before or after the RP space was opened or during the nodal dissection. Of the 66% of patients with positive lymph nodes, 92% were macroscopically positive, but only 31% were palpable before RP dissection. The authors concluded that the decision not to perform lymphadenectomy for optimally debulked patients may result in unrecognized macroscopic residual disease.32 Our data would further support a therapeutic advantage of RP exploration particularly among patients with substantial IP disease (high DS) in which the risk of RP disease may be increased compared to patients with minimal IP disease (low DS). Our data did not demonstrate a statistical advantage for RP exploration among those patients that had otherwise achieved R0 status. Given that the PFS and OS survival curves converge with almost 200 patients in each group (RP exploration versus no RP exploration), our observations are not likely attributable to Type II error.
Surgeon discretion is also a potential factor that may affect patient outcomes. It is conceivable that the surgeon has impressions or information about patient prognosis that influences the RP exploration decision, based on unmeasured indicators of disease burden, vitality of the patient, etc. Given the small but significant survival differences and the large sample size of this study, it is possible that these survival advantages are to some degree indicative of these unmeasured factors, or the “accuracy” of the surgeon's impression, and not completely about the act of exploration.
In conclusion, there is evidence that RP exploration at the time of primary debulking surgery of patients with IP stage IIIC optimally debulked epithelial ovarian cancer is associated with a survival benefit. The multivariable analysis shows that when evaluating for clinical and surgical factors such as surgical complexity and preoperative disease burden, the PFS and OS benefit remain in the group who underwent a RP exploration. This benefit is particularly seen in those patients with minimal gross residual disease of <1 cm. Therefore, even if preoperative imaging is negative for lymphadenopathy, our data suggests that when minimal or no gross residual is achieved, a RP nodal exploration should be performed based on a potential survival benefit that may be conferred by the performance of the dissection.
Acknowledgments
This study was supported by National Cancer Institute grants to the Gynecologic Oncology Group (GOG) Administrative Office (CA 27469), the Gynecologic Oncology Group Statistical Office (CA 37517), NRG Oncology (1U10 CA180822) and NRG Operations (U10CA180868).
Footnotes
Conflict of interest disclosures: Authors have no conflicts of interest with regards to this research
The following Gynecologic Oncology Group member institutions participated in the primary treatment studies: University of Alabama at Birmingham, Oregon Health Sciences University, Duke University Medical Center, Abington Memorial Hospital, University of Rochester Medical Center, Walter Reed Army Medical Center, Wayne State University, University of Minnesota Medical School, University of Southern California at Los Angeles, University of Mississippi Medical Center, Colorado Gynecologic Oncology Group P.C., University of California at Los Angeles, University of Washington, University of Pennsylvania Cancer Center, University of Miami School of Medicine, Milton S. Hershey Medical Center, Georgetown University Hospital, University of Cincinnati, University of North Carolina School of Medicine, University of Iowa Hospitals and Clinics, University of Texas Southwestern Medical Center at Dallas, Indiana University School of Medicine, Wake Forest University School of Medicine, Albany Medical College, University of California Medical Center at Irvine, Tufts-New England Medical Center, Rush-Presbyterian-St. Luke's Medical Center, University of Kentucky, Eastern Virginia Medical School, The Cleveland Clinic Foundation, Johns Hopkins Oncology Center, State University of New York at Stony Brook, Eastern Pennsylvania GYN/ONC Center, P.C., Southwestern Oncology Group, Washington University School of Medicine, Memorial Sloan-Kettering Cancer Center, Columbus Cancer Council, University of Massachusetts Medical School, Fox Chase Cancer Center, Medical University of South Carolina, Women's Cancer Center, University of Oklahoma, University of Virginia Health Sciences Center, University of Chicago, University of Arizona Health Science Center, Tacoma General Hospital, Eastern Collaborative Oncology Group, Thomas Jefferson University Hospital, Case Western Reserve University, and Tampa Bay Cancer Consortium.
References
- 1.Siegel R, Ma J, Zou Z, Jemal A. Cancer Statistics, 2014. CA Cancer J Clin. 2014;64:9–29. doi: 10.3322/caac.21208. [DOI] [PubMed] [Google Scholar]
- 2.Knapp RC, Friedman EA. Aortic lymph node metastases in early ovarian cancer. Am J Obstet Gynecol. 1974;119:1013–1017. doi: 10.1016/0002-9378(74)90251-8. [DOI] [PubMed] [Google Scholar]
- 3.Chen SS. Survival of ovarian carcinoma with or without lymph node metastasis. Gynecol Oncol. 1987;27:368–372. doi: 10.1016/0090-8258(87)90260-5. [DOI] [PubMed] [Google Scholar]
- 4.Spirtos NM, Gross GM, Freddo JL, Ballon SC. Cytoreductive surgery in advanced epithelial cancer of the ovary: the impact of aortic and pelvic lymphadenectomy. Gynecol Oncol. 1995;56:345–352. doi: 10.1006/gyno.1995.1061. [DOI] [PubMed] [Google Scholar]
- 5.Burghardt E, Giardi F, Lahousen M, Tamussino K, Stettner H. Patterns of pelvic and para-aortic lymph node involvement in ovarian cancer. Gynecol Oncol. 1991;40:103–106. doi: 10.1016/0090-8258(91)90099-q. [DOI] [PubMed] [Google Scholar]
- 6.FIGO cancer committee. Staging announcement. Gynecol Oncol. 1986;25:383–385. [Google Scholar]
- 7.FIGO Committee on Gynecologic Oncology. Current FIGO staging for cancer of the vagina, fallopian tube, ovary, and gestational trophoblastic neoplasia. Int J Gynaecol Obstet. 2009;105:3–4. doi: 10.1016/j.ijgo.2008.12.015. [DOI] [PubMed] [Google Scholar]
- 8.Benedetti-Panici P, Greggi S, Maneschi F, et al. Anatomical and pathological study of retroperitoneal nodes in epithelial ovarian cancer. Gynecol Oncol. 1993;51:150–154. doi: 10.1006/gyno.1993.1263. [DOI] [PubMed] [Google Scholar]
- 9.Petru E, Lahousen M, Tamussino K, et al. Lymphadenectomy in stage I ovarian cancer. Am J Obstet Gynecol. 1994;170:656–662. doi: 10.1016/s0002-9378(94)70244-6. [DOI] [PubMed] [Google Scholar]
- 10.Baiocchi G, Raspagliesi F, Grosso G, et al. Early ovarian cancer: Is there a role for systematic pelvis and para-aortic lymphadenectomy? Int J Gynecol Cancer. 1998;8:103–108. [Google Scholar]
- 11.Hacker NF, Berek JS, Lagasse LD, Nieberg RK, Elashoff RM. Primary cytoreductive surgery for epithelial ovarian cancer. Obstet Gynecol. 1983;61:413–420. [PubMed] [Google Scholar]
- 12.Einhorn N, Nilsson B, Sjoall K. Factors influencing survival in carcinoma of the ovary. Study from a well-defined Swedish population. Cancer. 1985;55:2019–2025. doi: 10.1002/1097-0142(19850501)55:9<2019::aid-cncr2820550932>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 13.Hoskins WJ, Bundy BN, Thigpen JT, Omura GA. The influence of cytoreductive surgery on recurrence-free interval and survival in small volume stage III epithelial ovarian cancer: a Gynecologic Oncology Group study. Gynecol Oncol. 1992;47:159–166. doi: 10.1016/0090-8258(92)90100-w. [DOI] [PubMed] [Google Scholar]
- 14.Farias-Eisner R, Teng F, Oliveria M, et al. The influence of tumor grade, distribution, and extent of carcinomatosis in minimal residual stage III epithelial ovarian cancer after optimal primary cytoreductive surgery. Gynecol Oncol. 1994;55:108–110. doi: 10.1006/gyno.1994.1257. [DOI] [PubMed] [Google Scholar]
- 15.Carino F, Fuda G, Ciccone G, et al. Significance of lymph node sampling in epithelial carcinoma of the ovary. Gynecol Oncol. 1997;65:467–472. doi: 10.1006/gyno.1997.4633. [DOI] [PubMed] [Google Scholar]
- 16.Onda T, Yoshikawa H, Yasugi T, et al. Patients with ovarian carcinoma upstaged to stage III after systematic lymphadenectomy have similar survival to stage I/II patients and superior survival to other stage III patients. Cancer. 1998;83:1555–1560. [PubMed] [Google Scholar]
- 17.Kanazawa K, Suzuki T, Tokashiki M. The validity and significance of substage IIIC by node involvement in epithelial ovarian cancer: impact of nodal metastasis on patient survival. Gynecol Oncol. 1999;73:237–241. doi: 10.1006/gyno.1999.5349. [DOI] [PubMed] [Google Scholar]
- 18.Cliby WA, Aletti GD, Wilson TO, Podratz KC. Is it justified to classify patients to stage IIIC epithelial ovarian cancer based on nodal involvement only? Gynecol Oncol. 2006;103:797–801. doi: 10.1016/j.ygyno.2006.08.047. [DOI] [PubMed] [Google Scholar]
- 19.Ferrandina G, Scambia G, Legge F, Petrillo M, Salutari V. Ovarian cancer patients with “node-positive-only” stage IIIC disease have a more favorable outcome than stage IIIA/B. Gynecol Oncol. 2007;107:154–156. doi: 10.1016/j.ygyno.2007.05.016. [DOI] [PubMed] [Google Scholar]
- 20.Baek SJ, Park JY, Kim DY, et al. Stage IIIC epithelial ovarian cancer classified solely by lymph node metastasis has a more favorable prognosis than other types of stage IIIC epithelial ovarian cancer. J Gynecol Oncol. 2008;19:223–228. doi: 10.3802/jgo.2008.19.4.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rungruang B, Miller A, Richard SD, et al. Should stage IIIC ovarian cancer be further stratified by intraperitoneal vs. retroperitoneal disease?: a Gynecologic Oncology Group study. Gynecol Oncol. 2012;124:53–58. doi: 10.1016/j.ygyno.2011.09.024. [DOI] [PubMed] [Google Scholar]
- 22.Prat J FIGO Committee on Gynecologic Oncology. Staging classification for cancer of the ovary, fallopian tube, and peritoneum. Int J Gynaecol Obstet. 2014;124:1–5. doi: 10.1016/j.ijgo.2013.10.001. [DOI] [PubMed] [Google Scholar]
- 23.Panici PB, Maggioni A, Hacker N, et al. Systemic aortic and pelvic lymphadenectomy versus resection of bulky nodes only in optimally debulked advanced ovarian cancer: a randomized clinical trial. J Natl Cancer Inst. 2005;20:560–566. doi: 10.1093/jnci/dji102. [DOI] [PubMed] [Google Scholar]
- 24.Bookman MA, Brady MF, McGuire WP, et al. Evaluation of New Platinum-Based Treatment Regimens in Advanced-Stage Ovarian Cancer: A Phase III Trial of the Gynecologic Cancer Intergroup. J Clin Oncol. 2009;27:1419–1425. doi: 10.1200/JCO.2008.19.1684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gynecologic Oncology Group. Surgical Procedures Manual. Buffalo, NY: Gynecologic Oncology Group; 2007. http://www.gog.org. [Google Scholar]
- 26.Rodriguez N, Miller A, Richard SD, et al. Upper abdominal procedures in advanced stage ovarian or primary peritoneal carcinoma patients with minimal or no gross residual disease: an analysis of Gynecologic Oncology Group (GOG) 182. Gynecol Oncol. 2013;130:487–492. doi: 10.1016/j.ygyno.2013.06.017. [DOI] [PubMed] [Google Scholar]
- 27.Chan JK, Urban R, Hu JM, et al. The potential therapeutic role of lymph node resection in epithelial ovarian cancer: a study of 13,918 patients. BR J Cancer. 2007;96:1817–1822. doi: 10.1038/sj.bjc.6603803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.du Bois A, Reuss A, Harter P, et al. Potential role of lymphadenectomy in advanced ovarian cancer: a combined exploratory analysis of three prospectively randomized phase III multicenter trials. J Clin Oncol. 2010;28:1733–1739. doi: 10.1200/JCO.2009.25.3617. [DOI] [PubMed] [Google Scholar]
- 29.Lymphadenectomy In Ovarian Neoplasms (LION) https://clinicaltrials.gov/ct2/show/NCT00712218.
- 30.Pelvic and Aortic-cava Lymphadenectomy Randomized for Ovarian Cancer (CARACO) https://clinicaltrials.gov/ct2/show/record/NCT01218490.
- 31.Horowitz NS, Miller A, Rungruang B, et al. Does aggressive surgery improve outcomes? Interaction between preoperative disease burden and complex surgery in patients with advanced-stage ovarian cacner: an analysis of GOG 182. J Clin Oncol. 2015;333:937–43. doi: 10.1200/JCO.2014.56.3106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Eisenkop SM, Spirtos NM. The clinical significance of occult macroscopically positive retroperitoneal nodes in patients with epithelial ovarian cancer. Gynecol Oncol. 2001;82:143–149. doi: 10.1006/gyno.2001.6232. [DOI] [PubMed] [Google Scholar]


