Summary
Objectives
Interictal epileptiform discharges (IEDs) have been linked to memory impairment, but the spatial and temporal dynamics of this relationship remain elusive. In the present study, we aim to systematically characterize the brain areas and times at which IEDs affect memory.
Methods
Eighty epilepsy patients participated in a delayed free recall task while undergoing intracranial EEG monitoring. We analyzed the locations and timing of IEDs relative to the behavioral data in order to measure their effects on memory.
Results
Overall IED rates did not correlate with task performance across subjects (r = 0.03, p = 0.8). However, at a finer temporal scale, within-subject memory was negatively affected by IEDs during the encoding and recall periods of the task but not during the rest and distractor periods (p < 0.01, p < 0.001, p = 0.3, and p = 0.8 respectively). The effects of IEDs during encoding and recall were stronger in the left hemisphere than in the right (p < 0.05). Out of six brain areas analyzed, IEDs in the inferior temporal, medial temporal, and parietal areas significantly affected memory (false discovery rate < 0.05).
Significance
These findings reveal a network of brain areas sensitive to IEDs with key nodes in temporal as well as parietal lobes. They also demonstrate the time-dependent effects of IEDs in this network on memory.
Keywords: Interictal Spikes, Memory, Electrocorticography, Epilepsy, Brain Mapping
Introduction
In 1984, Aarts et al. showed that focal and generalized epileptiform discharges observed in scalp EEG were associated with transient impairment in spatial and word sequence memory tasks.1 More recently, interictal epileptiform discharges (IEDs) in intracranial recordings have been linked to reduced memory performance. Specifically, Krauss et al. demonstrated performance decreases in six of eight patients in a recognition working memory task during trials with medial temporal IEDs.2 In a study of ten patients, Kleen et al. found that hippocampal IEDs impaired performance in a Sternberg working memory experiment if they occurred during the maintenance or recognition stage of the task.3 They concluded that IEDs may cause impairment only if they occur in certain locations and when relevant cognitive processes take place.3,4 Others hypothesize that these results may reflect disruption of a larger, distributed memory network.5 However, both past intracranial and scalp EEG studies have provided limited spatial resolution to address these questions. They either focused on a few brain areas2,3,6 or only distinguished between hemispheres,1,7 leaving spatial patterns in the effect of IEDs on memory to be determined. Meanwhile, intracranial studies of changes in spectral power have highlighted widespread, time-dependent cortical networks supporting human memory.8,9
A major goal of the present study was to map the places and times at which IEDs disrupt memory as a step toward potential therapies and to gain insight into the relevant cognitive processes. Memory impairment is a common complaint among epilepsy patients10 and a well documented deficit.11,12 While the etiology of memory loss is likely multifactorial,13 IEDs have been proposed as one factor and as potential targets for treatment.14 Knowing when and where IEDs are most likely to disrupt memory would be an important step toward the development of interventions.
We hypothesized that IEDs would have the strongest effects during active memory use and in brain areas that support episodic memory. Studies of scalp EEG have found IEDs to be disruptive during memory encoding.1 Meanwhile, studies of intracranial EEG have found hippocampal IEDs to be disruptive during memory retrieval.3 We aimed to study such patterns using a single measurement modality.
Materials and Methods
Subjects
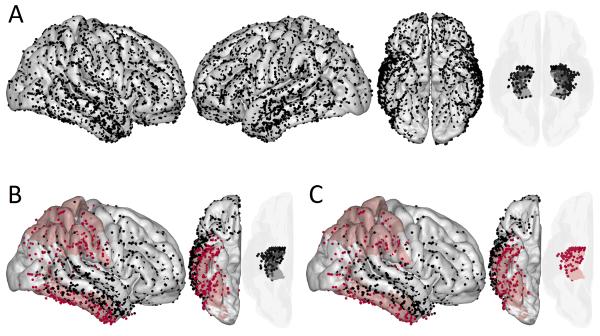
80 patients with intractable epilepsy participated in the study while undergoing intracranial EEG monitoring (36 male, 44 female; 63 right-handed, 11 left-handed, 6 ambidextrous; ages 19-58, median age 34). Only patients with Full Scale IQ estimates of 70 or above were considered for the study. Figure 3 depicts the combined electrode coverage from all patients. Individual patients had between 14 and 181 artifact free channels each (median 102).
Figure 3. Spatial Patterns in the Effect of IEDs.
A map of electrodes aggregated across subjects in a common Talairach coordinate system (A). Brain areas in which IEDs predicted impaired memory with the false discovery rate controlled at 0.05 are colored red for encoding (B) and recall (C). Rows (B) and (C) show only those electrodes that captured one or more IEDs and were from subjects included in the generalized linear mixed models. Data from both hemispheres were pooled in order to obtain adequate sample sizes. Cortical electrodes were snapped to an average brain surface. The semi-transparent plots show medial temporal depth electrodes.
The data were collected as part of the Restoring Active Memory project, funded by DARPA (Defense Advanced Research Projects Agency), coordinated by researchers at the University of Pennsylvania, and including sites at the Dartmouth-Hitchcock Medical Center, Emory University Hospital, Hospital of the University of Pennsylvania, Mayo Clinic, Thomas Jefferson University Hospital, University of Texas Southwestern Medical Center, Columbia University Medical Center, Washington University Medical Center, and National Institute of Neurological Disorders and Stroke. Electrodes were placed based on clinical needs determined by patient care teams at the respective institutions. All patients gave informed consent to participate in the study, which was approved by the institutional review boards at each hospital.
Neuropsychological Data
We included neuropsychological scores in the study as baseline data for the effect of IED rates. 47 patients had Full Scale IQ scores from either version III or IV of the Wechsler Adult Intelligence Scale test. The California Verbal Learning Test was administered to 26 subjects. Measures from the test included Short- and Long-Delay Free Recall.
Intracranial EEG Data
Intracranial recordings were sampled at rates ranging from 500 to 2000 Hz (Natus Medical Inc., Nihon Kohden Inc., Grass Technologies Corp.). In order to eliminate non-physiological artifacts such as those due to electrode impedance, we excluded recording channels for which the standard deviation of the raw signal was greater than twice the median value across channels. We validated this criteria with respect to manually-marked channels as described in the Supplementary Material. Afterward, we re-referenced the data to an average referential montage excluding the artifactual channels. We resampled all data to 200 Hz and notch filtered for line noise before further processing.15,16
Electrode Localization
Intracranial electrodes were localized using a combination of automated labeling and expert annotation. For localizing depth electrodes implanted in the medial temporal lobe, subregions were segmented in high-resolution T2-weighted pre-implant MRI using the Automatic Segmentation of Hippocampal Subfields (ASHS) multi-atlas segmentation method.17 For localizing subdural electrodes to cortical regions, Freesurfer18 was used to extract the cortical surface in pre-implant T1-weighted whole brain MRI. A neuroradiologist identified each electrode contact based on a post-implant CT scan. MRI and CT scans were then co-registered using Advanced Normalization Tools.19 An additional step was performed for subdural electrodes to account for possible brain shift due to surgery.20 The neuroradiologist visually confirmed the output of the automated pipeline and added additional detail on localization within the subregions. For visualization purposes, electrode coordinates were also transformed to a common space by registering individual CT scans with an average MRI and projecting the electrodes to the average brain surface.
Task
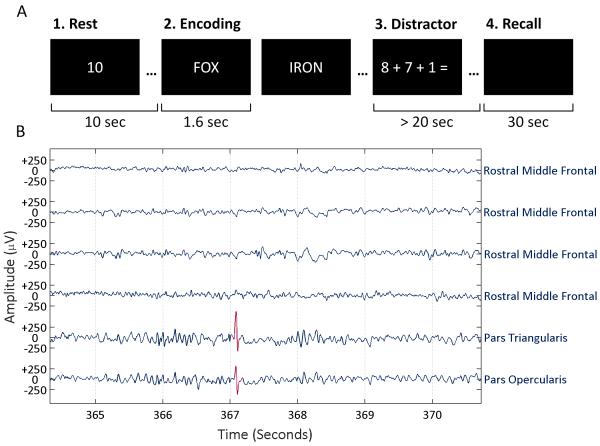
We chose a delayed free recall task for our study because it entails associative processing crucial to episodic memory21 and is a more clinically relevant task than the recognition memory paradigms that have been the focus of previous IED studies.2,3 The task design was similar to those used in other studies.9,15 It consisted of the four parts illustrated in Fig. 1 (A). The experiment presented 12 words per trial and up to 25 word lists per session. If a subject recalled no words on three consecutive trials or showed signs of tiring, the experimenter discontinued the session midway. On average, 1.9 sessions were administered per subject. In total, our study included over 45,000 word presentations from 3,830 trials. Average recall ranged between 0.4 and 7.5 words per list (median 3.4 words).
Figure 1. Study Design.
(A) Depiction of the four main parts of a trial. First, subjects rested as a timer counted down ten seconds. Second, subjects studied a series of 12 words, which were presented for 1.6 seconds each with an inter-stimulus interval of 0.8 to 1.2 seconds. Third, subjects solved math problems as a distractor for at least 20 seconds. Finally, subjects had 30 seconds to speak any words they recalled from the list while audio was recorded and synchronized to the intracranial EEG recordings. (B) Example intracranial recordings with an IED detection highlighted in red.
Spike Detection
Human annotations of IEDs in intracranial EEG exhibit significant inter-rater variability.22,23 To avoid potential inconsistencies in human review while marking a large amount of data, we chose to use an automated IED detector after validating it relative to multiple clinicians.24 Fig. 1 (B) shows an example IED detection. Intracranial recordings eliminate some of the issues and artifacts that can make automated detection difficult for scalp EEG.
Our automated detector used template-matching based on a previously published and validated method.16 Recently, template-matching spike detection has been used more routinely for research.25 The original algorithm was modified based on observations from a dataset of hippocampal recordings and then validated on two independent datasets, each annotated by three neurophysiologists.24 Furthermore, the detector performed with a similar receiver operating characteristic to that of another published detector.23 In the present dataset, we excluded 10 electrodes across 5 subjects in which the detector mistakenly marked sharp time-locked signals. The Supplementary Material contains further details about the exclusion of these time-locked events.
Statistical Analysis
Code written in MATLAB (R2014b, The MathWorks Inc.) was used for all analyses.
We investigated whether overall free recall performance and average IED rates correlated with neuropsychological test scores across subjects as a baseline for the later analyses. The IED rate for each subject corresponded to the average rate across electrodes from all experiment sessions. We performed pairwise tests using Spearman's rho.
We next tested whether IEDs have an impact on memory when their timing is taken into account. We evaluated whether the fraction of words recalled for each word list was significantly related to IED rates, averaged across channels, during the four corresponding periods of the experiment (rest, encoding, distractor, recall) using a generalized linear mixed model (GLMM). The model consisted of fixed effects for the IED rates per minute during the four periods and random effects by subject (both slopes and intercepts). The response variable was the fraction of words recalled in each trial, corresponding to a binomial distribution with 12 trials. Thus, a logit link function was used. The model was fit with MATLAB's fitglme function using maximum pseudo likelihood. Effect sizes are reported as odds ratios (OR) for unit increases in IED rate. Odds ratios and 95% confidence intervals (CI) were obtained from exponentiating the regression coefficients and confidence intervals.
We analyzed IEDs during the encoding and recall periods further to test for interactions between them and for lateralization effects. To determine whether joint increases in IED rates during encoding and recall augment or reduce their impact, we fit a GLMM with fixed effects for IED rates during encoding and recall and their interaction. As before and in all subsequent analyses, the model included random effects by subject for all predictor terms. To assess effects of laterality, we fit a GLMM with fixed effects for IED rates in the left and right hemispheres during encoding and recall as well as interaction terms between encoding and recall for both hemispheres and between left and right for both periods. 54 subjects had electrode coverage of both hemispheres. We assessed significant differences in the effect of IEDs between hemispheres using Wald F-tests26 (MATLAB's coefTest) with the null hypothesis that the coefficients for the two sides were equal. Two contrasts were performed: one for encoding and one for recall. Tests of individual regression coefficients had the false discovery rate controlled at 0.05 with the Benjamini–Hochberg procedure.30
We studied spatial patterns in the effect of IEDs on memory on a word-by-word basis. For the encoding periods, we gave each word presentation two binary labels. One indicated whether one or more IEDs occurred in a given brain region during the corresponding 1.6 second time window. The other indicated whether the word was subsequently recalled or forgotten. For the recall periods, we created 1 second windows just prior to valid vocalizations and matched them to surrogate windows at similar delays in other trials, which were at least 3 seconds away from any vocalizations as in previously published work.9 The surrogate windows provided a baseline of non-recall IED prevalence.
The spatial analysis assessed the effects of IEDs in six brain areas using one GLMM for encoding and one for recall. The areas were defined as follows with neocortical labels drawn from the Desikan-Killiany atlas.27
Parietal lobe: precuneus, superior parietal gyrus, inferior parietal gyrus, supramarginal gyrus, postcentral gyrus
Superolateral temporal cortex: transverse temporal gyrus, superior temporal gyrus, banks of the superior temporal sulcus, middle temporal gyrus
Inferior temporal cortex: inferior temporal gyrus, fusiform gyrus
Medial temporal lobe: perirhinal cortex, entorhinal cortex, parahippocampus, hippocampus, amygdala
Precentral gyrus: precentral gyrus, paracentral gyrus
Prefrontal cortex: superior frontal gyrus, middle frontal gyrus, inferior frontal gyrus, lateral orbitofrontal gyrus, medial orbitofrontal gyrus, frontal pole
The occipital lobe and cingulate were not included because of sparse coverage relative to the other areas. 28 subjects had coverage of all six regions of interest, which was necessary for a multiple regression. The GLMM had fixed effects for each area, which reflected whether an IED occurred on a given trial, and random effects for subjects. The response variable was binary: word recalled or not. We controlled the false discovery rate of the coefficient significance tests at 0.05 with the Benjamini–Hochberg procedure.28
A second spatial analysis tested whether IEDs were more or less impactful when they occurred in electrodes overlying epileptogenic cortex. Such electrodes were identified by clinicians when reviewing recordings of seizures. Seizure onset localization notes were available for 60 of the subjects. The GLMM for this analysis had a binary predictor to indicate whether IEDs occurred in any electrode and an interaction term to capture whether or not any of these IEDs occurred in epileptogenic cortex.
Results
We found that free recall correlated significantly with Full Scale IQ (Spearman's r45 = 0.58, p < 0.001) and Short- and Long-Delay Free Recall from the California Verbal Learning Test (r24 = 0.45, p = 0.02 and r24 = 0.47, p = 0.01 respectively). These high correlations indicate that the free recall task relates closely to clinical measures of memory, including Long-Delay Free recall, which assesses longer-term memory: typically recall after 20-30 minutes. Meanwhile, average IED rates did not correlate significantly with free recall (r78 = 0.03, p = 0.8), Full Scale IQ (r45 = 0.21, p = 0.1), Short-Delay Free Recall (r24 = 0.18, p = 0.4), or Long-Delay Free Recall (r24 = −0.16, p = 0.4 respectively). That is, average IED rates alone did not explain differences in memory performance.
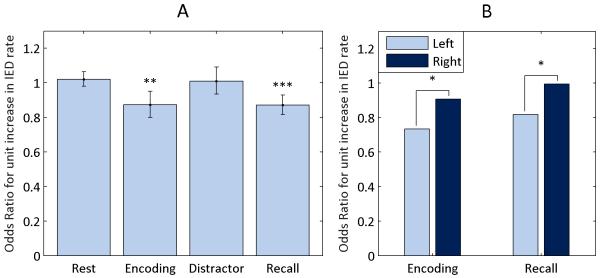
Increases in IED rates during the encoding and recall periods reduced recall performance (OR 0.87, CI 0.80–0.95 and OR 0.87, CI 0.82–0.93 respectively) while increases during the rest and distractor periods did not (OR 1.02, CI 0.98–1.0 and OR 1.01, CI 0.93–1.09 respectively). Fig. 2 (A) depicts these results. The negative finding for the rest and distractor periods suggest that IEDs have a transient impact on recall, only disrupting memory processes ongoing during the discharge. IEDs in the encoding and recall periods had an antagonistic interaction effect (OR 1.08, CI 1.03–1.12). That is, elevated IED rates in one of the periods lessens the impact of elevated rates in the other.
Figure 2. Influence of Task Period and Hemisphere.
IED rates during the four task periods were evaluated as predictors of word recall with a generalized linear mixed model (GLMM). Plot (A) shows the effect size for each predictor with 95% confidence intervals. A GLMM was also used to compare the effects of IED rates depending on hemisphere for encoding and recall (B). Contrasts were evaluated with Wald F-tests. Interaction terms are not shown.
* P < 0.05, ** P < 0.01, *** P < 0.001
IEDs in the left hemisphere had a greater impact on memory than IEDs in the right hemisphere during both encoding (OR 0.73 vs. 0.91, p = 0.04, CI 0.63–0.86 vs. 0.79–1.04) and recall (OR 0.82 vs. 0.99, p = 0.02, CI 0.73–0.91 vs. 0.89–1.11). Fig. 2 (B) illustrates the different odds ratios. The interaction terms between hemispheres were not significant, meaning simultaneous increases in IED rates in both hemispheres did not have an effect significantly different from the combination of the individual effects of left and right hemisphere IED rates. An antagonistic interaction was observed between IED rates from encoding and recall in the left hemisphere (OR 1.16, CI 1.09–1.23) though not the right (OR 1.01, CI 0.97–1.02).
During encoding, IEDs in the parietal and inferior temporal cortex predicted impaired memory on a word-by-word basis. During recall, IEDs in the parietal, inferior temporal, and medial temporal areas predicted impaired memory on a word-by-word basis. Table 1 summarizes the results and Fig. 3 highlights electrodes in the significant brain areas. Also, if IEDs occurred in epileptogenic cortex they had a greater impact on memory, which was significant during encoding (OR 0.88, CI 0.78–0.98) though not recall (OR 0.90, CI 0.75–1.09).
Table 1. Impact of IEDs on Memory by Brain Area.
The effects of IEDs in different brain areas on word recall estimated with one generalized linear mixed model for encoding and one for recall. Odds ratios (OR) less than one indicate reduced probability of recall with the presence of IEDs.
| Encoding | Recall | |||
|---|---|---|---|---|
|
| ||||
| Location | OR | P | OR | P |
|
| ||||
| Parietal | 0.84 | 0.02* | 0.63 | 0.01* |
| Superolateral Temporal | 0.96 | 0.6 | 0.82 | 0.1 |
| Inferior Temporal | 0.82 | 0.01* | 0.74 | 0.02* |
| Medial Temporal | 0.87 | 0.05 | 0.67 | 0.001* |
| Precentral | 1.24 | 0.2 | 0.61 | 0.1 |
|
| ||||
| Prefrontal | 0.92 | 0.5 | 0.85 | 0.4 |
Significant with the false discovery rate controlled at a level of 0.05
Discussion
Our findings associate IEDs and impaired memory in a free recall task. Furthermore, the results show that this association depends on the timing and location of the IEDs. The patterns in the effect suggest a connection to underlying cognitive processes. IEDs only significantly reduced performance during the encoding and recall periods of the task, i.e. when memory processes were active. Furthermore, this effect was significantly stronger for left-hemisphere IEDs than right-hemisphere ones, paralleling the left dominance of language.
The spatial results also parallel cognitive processes as mapped with fMRI. The effect of IEDs in parietal and inferior temporal areas during encoding match fMRI studies of episodic memory encoding29–31 as well as language.32 Similarly, the significant results for parietal, inferior temporal, and medial temporal areas during recall are consistent with existing fMRI results.30,33 One notable difference between the IED and imaging findings is the non-significant prefrontal result for IEDs. Prefrontal areas were frequently identified as significant in the fMRI studies.30–32 One potential explanation for the non-significant prefrontal result is that the effect could not be estimated as precisely because IEDs were 41% less prevalent in the prefrontal cortex than the temporoparietal areas on average. Comparing IED and fMRI results naturally raises the limitation that we needed IEDs to be present to estimate their effect.
Another notable exception to the parallel between IED results and cognitive processes is the medial temporal lobe. IEDs in the medial temporal lobe significantly predicted reduced memory performance during recall but not encoding. This finding is unexpected given evidence of memory encoding processes in the hippocampus.34 One potential explanation is that the hippocampus is more important for consolidation after a word has been viewed35 and so the analysis of IEDs during the presentation windows missed the effect. Another explanation is that IEDs more strongly disrupted task-related retrieval processes than encoding processes in the hippocampus. This interpretation is consistent with studies of hippocampal IEDs that showed effects on memory during maintenance and retrieval but not encoding.3,4
Clinical and objective memory is associated with many variables.13 One consequent limitation is that we cannot estimate the clinical effect of our findings, although statistically significant, without accounting for the many other factors. We anticipated large effect sizes, odds ratios between 0.5 and 0.7, based on the existing literature3 but observed milder effects, odds ratios around 0.8 to 0.9. Although lower odds ratios were observed for IEDs during recall, these ratios cannot be compared with the others because the numbers of surrogate windows and recall events were approximately balanced,9 creating an artificial starting ratio of 0.81 recall to surrogate windows on average. Furthermore, the antagonistic interaction of IEDs in the encoding and recall periods supports the possibility that other factors, such as antiepileptic drugs, could reduce the impact of IEDs by impairing memory themselves. Consequently, the results of this study may not generalize well beyond refractory epilepsy patients in a perioperative setting, and the clinical relevance of IEDs to memory remains uncertain.
In exchange for the advantage of consistent and deterministic behavior, automated IED detection introduced some limitations. Specifically, it was not possible to account for different IED types. Existing evidence suggests that the slow-wave component of IEDs may be more relevant to cognitive disruption than the spike.36 Our automated detector did not distinguish between the two, so the present results leave the possibility open that either or both components could have contributed to the observed impairment. High frequency oscillations (HFO) are another epileptiform signal that could be informative in future studies.
Differing IED frequencies across brain regions and subjects has been cited before as a limiting factor.2 The greater number of subjects available (80) than in previous studies helped to alleviate this problem and obtain good brain coverage despite variable, clinically-determined electrode placements. The multiple regression approach and random effects by subject were chosen to further address variability. The same subset of subjects contributed to all brain areas in the spatial analysis. However, overall, some areas had more electrodes or IEDs than others, which could lead to unequal likelihood of type II errors between the areas studied. Additionally, it is possible that the finding that IEDs impaired memory more significantly in the seizure onset zone than outside it is due to overlap between epileptogenic areas and areas important for memory such as the temporal lobe, which contained 68% of the electrodes marked epileptogenic. The finding regarding epileptogenic areas is difficult to interpret and has not been identified in other studies.3,6
Matsumoto et al. posed an alternative interpretation of the relationship between IEDs and task performance. In a visual recognition memory task, they showed IED rates to decrease relative to baseline during correct encoding trials, but not incorrect trials.6 They take this as evidence that the mental processes active during successful visual memory encoding suppress IEDs. This interpretation could also apply to our results for free recall. We found decreased recall performance with IEDs, which corresponds to decreased IED rates during successful memory encoding and retrieval. With either interpretation our findings map the memory network involved. Nevertheless, the question of causality warrants further investigation with tasks designed to distinguish between the explanations. IEDs have been found to occur more frequently during periods of drowsiness or inattention,37 so a third possible explanation is that a drowsiness reduced memory and increased IEDs, thereby creating the association observed.
Ultimately, the goal is to better understand memory impairment in epilepsy and inform strategies for developing treatments. One fundamental limitation of the current analysis is that it cannot prove causality, i.e. whether the IEDs disrupt cognition or are simply electrophysiological indicators of some underlying mechanism. This limitation also allows for the alternative explanations of the effect discussed in the last paragraph. Induced models of IEDs in rats without seizures are one approach to begin decoupling the effects of IEDs from other features of epilepsy.38 A potential approach for humans would be to use electrical brain stimulation to modulate IEDs. Work has been done which suggests that some stimulation protocols can reduce IEDs39 and some can improve memory in epilepsy patients.40 It would be valuable to determine whether a memory effect is mediated by suppression of IEDs.
Supplementary Material
Key Point Box.
IEDs impaired recall memory in multiple brain areas, including inferior, medial temporal, and parietal ones
The timing of IEDs modulated their impact on memory
The patterns in the effect of IEDs reflected the timing and location of memory processes
Acknowledgements
This work was supported by the National Institute of Health (Grant R01-NS074450) and by the Defense Advanced Research Projects Agency (DARPA) Restoring Active Memory (RAM) program (Cooperative Agreement N66001-14-2-4032). The views, opinions, and/or findings contained in this material are those of the authors and should not be interpreted as representing the official views or policies of the Department of Defense or the U.S. Government.
We thank Blackrock Microsystems for providing neural recording equipment. We also wish to thank the clinical and research personnel who helped collect data for the Restoring Active Memory (RAM) project.
Footnotes
Disclosures
MS states that he is the PI of research contracts with UCB Pharma, Eisai, Pfizer, Glaxo, Accorda, Neurelis, Upsher Smith, Sunovion, SK Life Sciences, Medtronics, Marinus, Brain and Sentinel, though none directly relate to this study. RG states that he has contracts with Medtronic, St. Jude Medical, MRI Interventions, Neuralstem, SanBio, and Neuropace, though none directly pertain to this research either. BJ has research contracts with Neuropace, Inc. not relating to this research. That is, none of the authors has any conflict of interest.
Ethical Publication Statement
We confirm that we have read the Journal’s position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
References
- 1.Aarts JHP, Binnie CD, Smit AM, et al. Selective cognitive impairment during focal and generalized epileptiform EEG activity. Brain. 1984;107:293–308. doi: 10.1093/brain/107.1.293. [DOI] [PubMed] [Google Scholar]
- 2.Krauss GL, Summerfield M, Brandt J, et al. Mesial temporal spikes interfere with working memory. Neurology. 1997;49:975–80. doi: 10.1212/wnl.49.4.975. [DOI] [PubMed] [Google Scholar]
- 3.Kleen JK, Scott RC, Holmes GL, et al. Hippocampal interictal epileptiform activity disrupts cognition in humans. Neurology. 2013;81:18–24. doi: 10.1212/WNL.0b013e318297ee50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kleen JK, Scott RC, Holmes GL, et al. Hippocampal interictal spikes disrupt cognition in rats. Ann Neurol. 2010;67:250–7. doi: 10.1002/ana.21896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kucewicz MT, Worrell GA, Gotman J. Pathologic brain network activity. Neurology. 2013;81:12–3. doi: 10.1212/WNL.0b013e318297ef3c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Matsumoto JY, Stead M, Kucewicz MT, et al. Network oscillations modulate interictal epileptiform spike rate during human memory. Brain. 2013;136:2444–56. doi: 10.1093/brain/awt159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Binnie CD. Cognitive impairment during epileptiform discharges: is it ever justifiable to treat the EEG? Lancet Neurol. 2003;2:725–30. doi: 10.1016/s1474-4422(03)00584-2. [DOI] [PubMed] [Google Scholar]
- 8.Burke JF, Long NM, Zaghloul KA, et al. Human intracranial high-frequency activity maps episodic memory formation in space and time. NeuroImage. 2014;85(Part 2):834–43. doi: 10.1016/j.neuroimage.2013.06.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Burke JF, Sharan AD, Sperling MR, et al. Theta and high-frequency activity mark spontaneous recall of episodic memories. J Neurosci. 2014;34:11355–65. doi: 10.1523/JNEUROSCI.2654-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McAuley JW, Elliott JO, Patankar S, et al. Comparing patients’ and practitioners’ views on epilepsy concerns: a call to address memory concerns. Epilepsy Behav. 2010;19:580–3. doi: 10.1016/j.yebeh.2010.09.001. [DOI] [PubMed] [Google Scholar]
- 11.Bell B, Lin JJ, Seidenberg M, et al. The neurobiology of cognitive disorders in temporal lobe epilepsy. Nat Rev Neurol. 2011;7:154–64. doi: 10.1038/nrneurol.2011.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hermann B, Seidenberg M, Lee E-J, et al. Cognitive phenotypes in temporal lobe epilepsy. J Int Neuropsychol Soc. 2007;13:12–20. doi: 10.1017/S135561770707004X. [DOI] [PubMed] [Google Scholar]
- 13.Mazarati A. Epilepsy and forgetfulness: one impairment, multiple mechanisms. Epilepsy Curr. 2008;8:25–6. doi: 10.1111/j.1535-7511.2007.00224.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Holmes GL. EEG abnormalities as a biomarker for cognitive comorbidities in pharmacoresistant epilepsy. Epilepsia. 2013;54:60–2. doi: 10.1111/epi.12186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Long NM, Burke JF, Kahana MJ. Subsequent memory effect in intracranial and scalp EEG. NeuroImage. 2014;84:488–94. doi: 10.1016/j.neuroimage.2013.08.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nonclercq A, Foulon M, Verheulpen D, et al. Spike detection algorithm automatically adapted to individual patients applied to spike-and-wave percentage quantification. Neurophysiol Clin Clin Neurophysiol. 2009;39:123–31. doi: 10.1016/j.neucli.2008.12.001. [DOI] [PubMed] [Google Scholar]
- 17.Yushkevich PA, Pluta JB, Wang H, et al. Automated volumetry and regional thickness analysis of hippocampal subfields and medial temporal cortical structures in mild cognitive impairment. Hum Brain Mapp. 2015;36:258–87. doi: 10.1002/hbm.22627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fischl B, Kouwe A, van der, Destrieux C, et al. Automatically parcellating the human cerebral cortex. Cereb Cortex. 2004;14:11–22. doi: 10.1093/cercor/bhg087. [DOI] [PubMed] [Google Scholar]
- 19.Avants BB, Epstein CL, Grossman M, et al. Symmetric diffeomorphic image registration with cross-correlation: evaluating automated labeling of elderly and neurodegenerative brain. Med Image Anal. 2008;12:26–41. doi: 10.1016/j.media.2007.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dykstra AR, Chan AM, Quinn BT, et al. Individualized localization and cortical surface-based registration of intracranial electrodes. NeuroImage. 2012;59:3563–70. doi: 10.1016/j.neuroimage.2011.11.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Merkow MB, Burke JF, Stein JM, et al. Prestimulus theta in the human hippocampus predicts subsequent recognition but not recall. Hippocampus. 2014;24:1562–9. doi: 10.1002/hipo.22335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Barkmeier DT, Shah AK, Flanagan D, et al. High inter-reviewer variability of spike detection on intracranial EEG addressed by an automated multi-channel algorithm. Clin Neurophysiol Off J Int Fed Clin Neurophysiol. 2012;123:1088–95. doi: 10.1016/j.clinph.2011.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Janca R, Jezdik P, Cmejla R, et al. Detection of interictal epileptiform discharges using signal envelope distribution modelling: application to epileptic and non-epileptic intracranial recordings. Brain Topogr. 2015;28:172–83. doi: 10.1007/s10548-014-0379-1. [DOI] [PubMed] [Google Scholar]
- 24.Horak P, Meisenhelter S, Testorf M, et al. Implementation and evaluation of an interictal spike detector. Proceedings of SPIE; 2015; 96000N1–11. [Google Scholar]
- 25.Karoly PJ, Freestone DR, Boston R, et al. Interictal spikes and epileptic seizures: their relationship and underlying rhythmicity. Brain. 2016;139:1066–78. doi: 10.1093/brain/aww019. [DOI] [PubMed] [Google Scholar]
- 26.Korn EL, Graubard BI. Simultaneous testing of regression coefficients with complex survey data: use of Bonferroni t statistics. Am Stat. 1990;44:270–6. [Google Scholar]
- 27.Desikan RS, Ségonne F, Fischl B, et al. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. NeuroImage. 2006;31:968–80. doi: 10.1016/j.neuroimage.2006.01.021. [DOI] [PubMed] [Google Scholar]
- 28.Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc Ser B Methodol. 1995;57:289–300. [Google Scholar]
- 29.Uncapher MR, Wagner AD. Posterior parietal cortex and episodic encoding: insights from fMRI subsequent memory effects and dual-attention theory. Neurobiol Learn Mem. 2009;91:139–54. doi: 10.1016/j.nlm.2008.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kim H. Neural activity that predicts subsequent memory and forgetting: a meta-analysis of 74 fMRI studies. NeuroImage. 2011;54:2446–61. doi: 10.1016/j.neuroimage.2010.09.045. [DOI] [PubMed] [Google Scholar]
- 31.Spaniol J, Davidson PSR, Kim ASN, et al. Event-related fMRI studies of episodic encoding and retrieval: meta-analyses using activation likelihood estimation. Neuropsychologia. 2009;47:1765–79. doi: 10.1016/j.neuropsychologia.2009.02.028. [DOI] [PubMed] [Google Scholar]
- 32.Binder JR, Frost JA, Hammeke TA, et al. Human brain language areas identified by functional magnetic resonance imaging. J Neurosci. 1997;17:353–62. doi: 10.1523/JNEUROSCI.17-01-00353.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Perrone-Bertolotti M, Girard C, Cousin E, et al. NEREC, an effective brain mapping protocol for combined language and long-term memory functions. Epilepsy Behav EB. 2015;53:140–8. doi: 10.1016/j.yebeh.2015.09.017. [DOI] [PubMed] [Google Scholar]
- 34.Fernández G, Effern A, Grunwald T, et al. Real-time tracking of memory formation in the human rhinal cortex and hippocampus. Science. 1999;285:1582–5. doi: 10.1126/science.285.5433.1582. [DOI] [PubMed] [Google Scholar]
- 35.Nadel L, Moscovitch M. Memory consolidation, retrograde amnesia and the hippocampal complex. Curr Opin Neurobiol. 1997;7:217–27. doi: 10.1016/s0959-4388(97)80010-4. [DOI] [PubMed] [Google Scholar]
- 36.Shewmon DA, Erwin RJ. Focal spike-induced cerebral dysfunction is related to the after-coming slow wave. Ann Neurol. 1988;23:131–7. doi: 10.1002/ana.410230205. [DOI] [PubMed] [Google Scholar]
- 37.Leung LW. Hippocampal interictal spikes induced by kindling: relations to behavior and EEG. Behav Brain Res. 1988;31:75–84. doi: 10.1016/0166-4328(88)90160-x. [DOI] [PubMed] [Google Scholar]
- 38.Hernan AE, Alexander A, Lenck-Santini P-P, et al. Attention deficit associated with early life interictal spikes in a rat model is improved with ACTH. PLoS ONE. 2014;9:e89812. doi: 10.1371/journal.pone.0089812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yamamoto J, Ikeda A, Satow T, et al. Low-frequency electric cortical stimulation has an inhibitory effect on epileptic focus in mesial temporal lobe epilepsy. Epilepsia. 2002;43:491–5. doi: 10.1046/j.1528-1157.2002.29001.x. [DOI] [PubMed] [Google Scholar]
- 40.Suthana N, Haneef Z, Stern J, et al. Memory enhancement and deep-brain stimulation of the entorhinal area. N Engl J Med. 2012;366:502–10. doi: 10.1056/NEJMoa1107212. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.