Abstract
After ginseng ingestion, the bioavailability of its parent compounds is low and enteric microbiota plays an important role in parent compound biotransformation to their metabolites. Diet type can influence the enteric microbiota profile. When human subjects on different diets ingest ginseng, their gut microbiota's different profile may influence the metabolism of ginseng parent compounds. In this study, the effects of different diet type on gut microbiota metabolism of American ginseng saponins were investigated. We recruited six healthy adults who regularly consume different diet type. These subjects received 7 days oral American ginseng, and their biological samples were collected for LC-Q-TOF-MS analysis. We observed significant ginsenoside Rb1 (a major parent compound) and compound K (a major active metabolite) level differences in the samples from the subjects consuming different diet. Subjects on Asian diet have much higher Rb1 levels but much lower compound K levels compared to those on Western diet. Since compound K possesses much better cancer chemoprevention potential, our data suggested that consumers on Western diet should obtain better cancer prevention effects with American ginseng intake compared to those on Asian diet. Ginseng compound levels could be enhanced or reduced via gut microbiota manipulation for clinical utility.
Keywords: American ginseng, ginsenoside Rb1, compound K, enteric microbiota, biological sample, diet type
Introduction
American ginseng is one of the most commonly used botanicals in the U.S., and it has many reported pharmacological effects (Yuan et al., 2010; Xie et al., 2015). It is generally accepted that active constituents of ginseng are a group of saponins called ginsenosides (Attele et al., 1999; Qi et al., 2011). To date, over 80 ginsenosides have been identified from this botanical which can be divided into two major groups: protopanaxadiol group (e.g., Rb1, Rc) and protopanaxatriol group (e.g., Re, Rg1) (Qi et al., 2011). The multiple constituents of ginseng support ginseng's multiple pharmacological activities (Attele et al., 1999).
Similar to most herbal medicines, American ginseng is nearly always taken orally. When ingested orally, the bioavailability of ginseng parent compounds is low due to their incomplete absorption and the conversion to their metabolites by the enteric microbiota (Liu et al., 2009; Wang et al., 2011a and 2011b; Hu et al., 2013). After ginseng ingestion, ginsenoside Rb1 can be biotransformed in the gut into compound K, a reported major active metabolite that reach the systemic circulation (Wang et al., 2011a). Using human enteric microbiota treated ginseng in vitro, the conversion from Rb1 to compound K has been demonstrated (Wan et al., 2013). Compound K, not Rb1, has very significant cancer chemoprevention effects (Wang et al., 2012; Kang et al., 2013).
It has been reported that diet type influences the enteric microbiota (Underwood et al., 2009; Mushref and Srinivasan, 2013; Simpson and Campbell, 2015). When human subjects on different diet received oral ginseng, the individuals’ gut microbiota profile may differ (Ingerslev et al., 2014; Simpson and Campbell, 2015). If this diet-induced microbiota profile change subsequently alters the biotransformation of compound K in ginseng compound metabolism, ginseng's cancer chemoprevention outcome in individuals on different diet will be diverse.
In this study, we recruited six healthy male subjects in Chicago area. Three of these subjects regularly consume standard Asian diet, and the remaining three subjects are regularly consuming standard Western diet. All these six subjects received 7 consecutive days oral American ginseng treatment. Subjects’ biological samples (i.e., plasma, urine and feces) were collected for analysis of both ginseng parent compounds and their metabolites using liquid chromatography coupled with quadrupole time-of-flight mass spectrometry (LC-Q-TOF-MS). Special attention has been paid to the levels of ginsenoside Rb1, a major parent compound, and compound K, its major active metabolite. Comparisons have been made to the levels of these two ginseng compounds in relation to the different diet type consumption.
Experimental
Chemicals and reagents
HPLC grade acetonitrile (ACN), methanol and formic acid were purchased from Merck (Darmstadt, Germany). Standards of ginsenoside Rb1 and compound K (purity ≥ 98%) were supplied from Jilin University (Changchun, China), and structures of these two ginseng compounds are shown in Fig. 1C. The internal standard (IS) digoxin (purity ≥ 98%) was obtained from Sigma-Aldrich (St. Louis, USA). Deionized water (18 MΩ•cm−1) was prepared by a Milli-Q water purification system (Millipore, Milford, MA, USA).
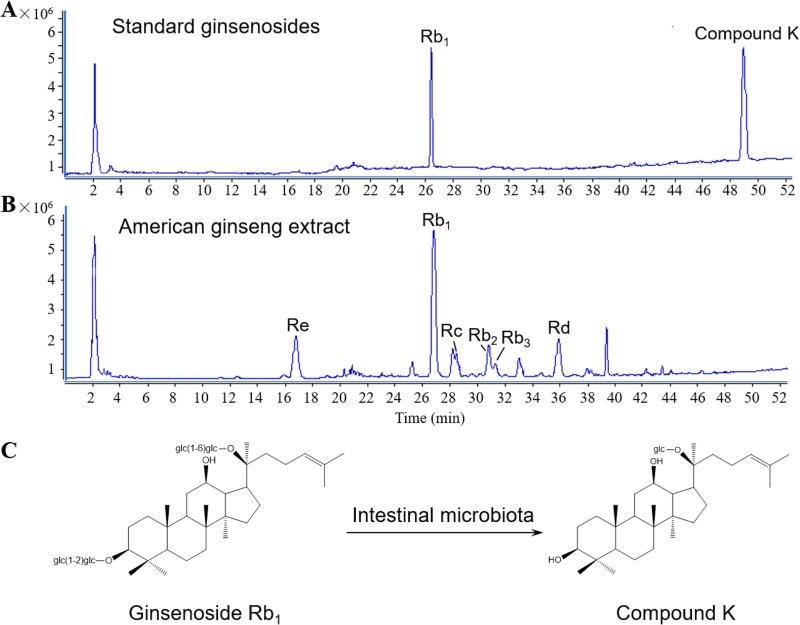
Figure 1.
Ginseng saponins detected in the negative ion mode by LC-Q-TOF-MS analysis. (A) Total ion chromatogram (TIC) of selected standards, ginsenoside Rb1 and compound K. (B) TIC of American ginseng extract. Ginsenosides Re, Rc, Rb2/Rb3 and Rd are detected. (C) Chemical structures of ginsenoside Rb1 and compound K. Intestinal microbiota is critical in the biotransformation (Wan et al., 2013).
Plant materials
Dried roots of American ginseng (Panax quinquefolius L.) were purchased from Roland Ginseng, LLC (Wausau, WI, USA). The voucher samples were authenticated by Dr. Chong-Zhi Wang and deposited at the Tang Center for Herbal Medicine Research at the University of Chicago (Chicago, IL, USA). The air-dried roots of American ginseng were powdered to a homogeneous size, and sieved through a 40 mesh screen.
Subjects and study protocol
The study protocol was approved by the Institutional Review Board at the University of Chicago. At the beginning of the study, subjects received a physical examination and signed their written informed consent for participation in this trial. Six healthy male volunteers (three on Asian diet and three on Western diet) without taking any other medications, participated in the study. They were aged between 18 and 45 years. Each subject was asked to fast overnight before receiving powder of American ginseng next day. Their blood, urine and feces samples were collected as the baseline control. All subjects received an oral treatment with 2 g American ginseng powder (in capsules, swallowed with tap water) per day for 7 consecutive days. At the end of the study, subjects’ biological samples (i.e., blood, urine and feces) were obtained for analysis. The blood samples collected in the heparin tubes were centrifuged at 4000 rpm for 5 min, and the resulting plasma fractions were transferred to Eppendorf tubes and frozen at −80°C until analysis.
Biological sample preparation
To avoid matrix effects and ion suppression, all biological samples were pretreated by solid phase extraction (SPE) before LC-Q-TOF-MS analysis. Waters Oasis HLB columns (1 cc, 30 mg, 30 μm, Waters, Milford, MA, USA) were first preconditioned with methanol (2 × 1 mL), followed by deionized water (2 × 1 mL).
Each plasma sample (200 μL) was vortexed with 20 μL of 2% acetic acid for 30 sec and diluted with 1 mL of physiological saline. The homogenate was loaded onto the preconditioned HLB column. The cartridge was washed with deionized water (2 × 1 mL) before the analyte was recovered by using 1 mL of methanol as eluent. The methanol fraction was evaporated to dryness under nitrogen gas at 20°C, and the residue was then re-dissolved in 100 μL of methanol and centrifuged at 13,000 rpm for 10 min before assay. Each urine sample was prepared in a similar manner.
Each feces sample (0.5 g) was ultrasonically extracted with 3 mL of 80% methanol for 15 min and then centrifuged (4,000 rpm, 10 min). The 200 μL supernatant was loaded onto the preconditioned HLB column. The cartridge was washed with deionized water (2 × 1 mL) before the analyte was recovered by using 1 mL of methanol as eluent. The methanol fraction was evaporated to dryness under nitrogen gas at 20°C, and the residue was then re-dissolved in 100 μL of methanol and centrifuged at 13,000 rpm for 10 min before assay.
Liquid chromatography coupled with quadrupole time-of-flight mass spectrometry (LC-Q-TOF-MS) analysis
Chromatographic analysis was performed on an Agilent 1290 Series (Agilent, Santa Clara, CA, USA). LC system was equipped with a binary pump, a micro degasser, an auto sampler, and a thermostatically controlled column compartment. Sample separation was carried out at 25°C on an Agilent Zorbax Extend-C18 column (4.6 mm × 250 mm, 5 μm). The mobile phase consisted of 0.1% formic acid water (A) and 0.1% formic acid acetonitrile (B). The optimized elution conditions as follows was employed: 21% B at 0-15 min, from 21-30% B at 15-18 min, from 30-33% B at 18-30 min, 33% B at 30-34 min, from 33-45% B at 34-40 min, from 45-60% B at 40-50 min, from 60-80% B at 50-55 min, from 80-100% B at 55-60 min, and finally maintained at 100% B for 5 min at a flow rate of 1 ml/min. The injection volume of plasma, urine, and feces samples was set at 10, 5 and 10 μL, respectively.
Detection was carried out by a 6530 Q-TOF mass spectrometer (Agilent) with a Dual ESI interface. The parameters of operation were as follows: drying gas N2 flow rate, 10.0 L/min; temperature, 320°C; nebulizer, 35 psig; capillary, 3500 V; OCT RFV, 750 V; and fragmentor voltage, 120 V. Each sample was analyzed in both the positive and negative modes. Mass spectra were recorded across the range m/z 100-3000. The operations and acquisition of data were controlled by Agilent LC-Q-TOF-MS MassHunter Acquisition Software (Version B.05.00). The sample levels of ginsenoside Rb1 and compound K of individual subjects were calculated based on a combined approach of previous publications (Wang et al., 2011b; Wan et al., 2013). A standard curve was used to calculate the compound concentration in the samples, which plots the concentration of the standard (10, 100, 1000 and 10000 ng/ml) against the area ratio of tested compound/internal standard. The area ratio of the samples was applied to the curve and to the level from the known concentration for determining the calculated concentration.
Data analysis
The determined compound levels were presented in mean ± S.D. Data analysis was operated with Agilent MassHunter Workstation software. By comparing the theoretical mass of molecular and/or fragment ions, accurate mass measurements were obtained to gain an empirical molecular formula. The errors were required less than 5 ppm. A one-way ANOVA was used to determine whether the results had statistical significance (p < 0.05) when applicable.
Results and discussions
Ginsenoside Rb1 and compound K
With the standards of ginsenoside Rb1 and compound K mixed in methanol and injected into LC under the optimized conditions, the total ion chromatogram (TIC) of the two reference saponins is shown in Fig. 1A by LC-Q-TOF-MS in the negative ion mode. American ginseng used in this study was analyzed to confirm the authenticity and quality. Fig. 1B shows the typical TIC of the ginseng extract in the negative ion mode. From the MS spectrum, it was observed that as a major ginseng saponin of American ginseng, ginsenoside Rb1 was in a large proportion, and compound K was not found in the original plant. Enteric microbiota can biotransform ginsenoside Rb1 to compound K (Fig. 1C) (Wang et al., 2012; Wan et al., 2013; Kang et al., 2013). It has been reported that enteric microbiota can further biotransform compound K to protopanaxadiol (PPD) (Wang et al., 2011b).
Structural characterization of reference ginseng saponins
Diverse ginseng saponins with different structures have been reported (Li et al., 2010; Qi et al., 2012). Fig. 2 shows the typical Q-TOF-MS/MS spectrum of ginsenoside Rb1 and compound K in both positive and negative ion modes. Owing to the presence of formic acid in the mobile phase, the typical solvent adducts [M+HCOO]− (m/z 1153.6022 for Rb1, m/z 667.4426 for compound K) and deprotonated molecules [M-H]− (m/z 1107.5964 for Rb1, m/z 621.4370 for compound K) can be usually observed in the negative mode. In addition, in negative MS/MS ion modes with a collision energy at 50 V, losses of sugar moieties are commonly produced as shown in Fig. 2A. For ginsenoside Rb1, the successive losses of 162 Da corresponding to the -Glc was observed, until the appearance of [M+HCOO]− m/z 459.3823 of PPD-type sapogenin. Meanwhile, a series of low mass ions in the range of m/z 100-400 with a high abundance was found referring to sugar residue ions. Fig. 2B shows positive MS spectrum of ginsenoside Rb1 and compound K with a fragmentor at 120 V. In the positive ion mode, the mother skeleton can be rapidly assigned by the abundant aglycone ion as well as by a series of dehydrated ions from the aglycone. Characteristic fragment ions of PPD-type aglycone at m/z 443.38, 425.37 and 407.36 are shown in the positive MS spectrum of ginsenoside Rb1 and compound K.
Figure 2.
The typical quadrupole TOF-MS/MS spectrum of ginsenoside Rb1 and compound K in both positive and negative ion modes. (A) The negative MS/MS spectrum of ginsenoside Rb1 and compound K with a collision energy at 50 V. (B) The positive MS spectrum of ginsenoside Rb1 and compound K with a fragmentor at 120 V.
Detection of ginsenoside Rb1 and compound K in three biological samples
Ginsenoside Rb1 and compound K in human plasma, urine and feces samples after oral ginseng administration were determined. The typical TIC of a plasma sample was shown in Fig. 3A. Ginsenoside Rb1 and its metabolite compound K were further confirmed from the plasma sample with a narrow mass window of 0.01 Da to restructure the extracted ion chromatograms (EICs), which were also shown in Fig. 3A.
Figure 3.
LC-Q-TOF-MS analysis of ginsenoside Rb1 and compound K in human plasma, urine and feces samples after 7-day oral American ginseng administration. The typical TICs of plasma sample, urine sample and feces sample extracted ion chromatograms (EICs) of ginsenoside Rb1 and compound K are shown in (A), (B) and (C), respectively.
LC-MS data of solvent adducts [M+HCOO]− of the two ginseng compounds identified in plasma samples was listed in Table 1. Ginsenoside Rb1 and compound K were detected in all subjects’ plasma, and compound K, as a metabolite, entered the systemic circulation. The TIC and EICs of ginsenoside Rb1 and compound K in typical urine and feces samples were shown in Fig. 3B and Fig. 3C, respectively.
Table 1.
LC-MS data of ginsenoside Rb1 and compound K identified in plasma after oral administration of American ginseng.
| Subject | Ginsenoside Rb1 | Compound K | |||||
|---|---|---|---|---|---|---|---|
| m/z | Calc m/z | Diff (ppm) | m/z | Calc m/z | Diff (ppm) | ||
| Asian-diet | 1 | 1153.6023 | 1153.6011 | −1.04 | 667.4439 | 667.4427 | −1.97 |
| 2 | 1153.6018 | 1153.6011 | −0.59 | 667.4420 | 667.4427 | 1.08 | |
| 3 | 1153.6010 | 1153.6011 | 0.13 | 667.4413 | 667.4427 | 2.20 | |
| Western-diet | 4 | 1153.6025 | 1153.6011 | −1.22 | 667.4424 | 667.4427 | 0.44 |
| 5 | 1153.5993 | 1153.6011 | 1.66 | 667.4435 | 667.4427 | −1.33 | |
| 6 | 1153.6037 | 1153.6011 | −2.31 | 667.4432 | 667.4427 | −0.85 | |
The typical solvent adducts [M+HCOO]− observed in the negative mode of both compounds.
Calc m/z, calculated m/z; Diff (ppm), difference between m/z and Calc m/z in ppm.
Significant level differences of ginsenoside Rb1 and compound K in subjects consuming different diet
The relative abundance of ginsenoside Rb1 and compound K was detected in the plasma, urine and feces samples of three Asian-diet and three Western-diet subjects. Fig. 4A shows the signal intensity of ginsenoside Rb1 and compound K detected in individual subject biological samples by peak area with a 0.01 Da mass window. It was observed that the abundance of these two compounds in feces differed considerately compared to those in plasma and urine.
Figure 4.
Levels of ginsenoside Rb1 and compound K in plasma, urine and feces samples in three Asian-diet consuming subjects and three Western-diet consuming subjects using LC-Q-TOF-MS analysis. (A) Signal intensity of ginseng compounds detected in the individual subjects’ biological samples. (B) Mean intensity of ginseng compounds in Asian-diet subjects (or A) and Western-diet subjects (or W). *, p < 0.05 between A and W. Note different ordinate scales in plasma, urine and feces plots.
Fig. 4B shows the mean intensity of ginsenoside Rb1 and compound K in the subjects. The calculated average plasma, urine and feces levels for ginsenoside Rb1 were 11.3, 41.7 and 232.5 ng/mL in Asian-diet subjects, while 5.1, 71.9 and 340.4 ng/mL in Western-diet subjects, respectively. Moreover, the calculated average plasma, urine and feces levels for compound K were 47.7, 96.6 and 4998.4 ng/mL in Asian-diet subjects, while 65.7, 122.1 and 15137.6 ng/mL in Western-diet subjects, respectively. Compared with Asian-diet subjects, Western-diet subjects had significantly higher compound K levels in plasma (37.7% increase), urine (26.4% increase) and feces (202.8% increase) (all p < 0.05). The much higher compound K level in feces is likely due to the long duration of ginseng-microbiota interactions in the colon without compound absorption.
Asian diet contains different vegetables and large amounts of starch from rice; a Western diet has high in fat and animal protein. These different daily diet types may alter the enteric microbiota population, affecting herbal compound gut metabolism and absorption (Moco et al., 2012; Genton et al., 2015; Janssen et al., 2015; Simpson and Campbell, 2015). Thus, significant level differences of Rb1 and compound K in biological samples observed in our study can be contributed to the consumption of two different types of diet and subsequently induced enteric microbiota profile change.
Data obtained from this study showed that compared with subjects consuming Asian diet, subjects consuming Western diet have much lower levels of Rb1 but much higher levels of compound K, suggesting the different diet types affect subjects’ enteric microbiota population. Since compound K possesses significant higher cancer chemoprevention potential than that of Rb1, our data suggested that consumers on Western diet would achieve better cancer prevention effects after American ginseng intake compared to those on Asian diet (Wang et al., 2012; Yu et al., 2015a and 2015b).
Asian ginseng and notoginseng are very commonly used ginseng species in oriental countries, and they have somewhat similar ginsenoside profile compared to American ginseng (Attele et al., 1999; Wang et al., 2006). Thus, it appears that the diet type on enteric microbiota would also influence their stated effects (Sun et al., 2011; Liu et al., 2015; Huang et al., 2015). The reported herbal medication effects could be enhanced by enteric microbiota (Xiao et al., 2015; Wang et al., 2015). On the other hand, enteric microbiota were also responsible for the adverse events from diet compounds (Zhang et al., 2013).
Detection of ginsenoside Rh2 in feces samples
Ginsenoside Rh2 was also detected in the biological samples, especially from the feces. We observed that the average relative abundance of ginsenoside Rh2 was higher in feces samples (0.903 × 106 ± 0.186 × 106) compared to that in plasma (0.378 × 104 ± 0.078 × 104). In the feces samples, both two stereoisomeric forms of Rh2 were observed as 20S- and 20R-, and these isomers may have different pharmacological effects (Zhang et al., 2012; Chio et al., 2013). Similar to compound K, we observed that average Rh2 amount from feces is higher in subjects consuming Western diet (1.525 × 106 ± 0.216 × 106) compared to those consuming Asian diet (0.279 × 106 ± 0.159 × 106).
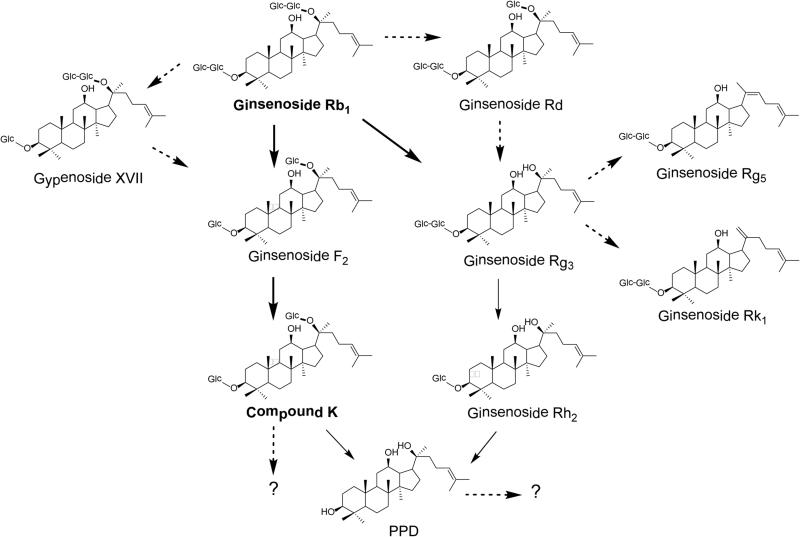
Fig. 5 shows major and minor metabolic routes of ginsenoside Rb1. For the major route, from ginsenoside Rb1 to ginsenoside F2, the conversion selective eliminates C-3 and C-20 sugar moieties. From ginsenoside F2 to compound K is a C-3 sugar moiety elimination, while from compound K to PPD is a C-20 sugar moiety elimination. It is possible that compound K and PPD can be further converted to other metabolites. The potential metabolites should be identified in future studies and their pharmacological activities also should be investigated. For the minor route, ginsenoside Rh2, which was detected mainly in the feces samples in our study, is transformed by the elimination of C-20 and C-3 sugar moieties, respectively. Fig. 5 also indicated that ginsenosides Rk1 and Rg5 are the metabolites in the minor route via dehydration from Rg3.
Figure 5.
Major and minor metabolic routes of ginsenoside Rb1 (Qi et al., 2011; Wan et al., 2013). Compound K, a major metabolite of ginsenoside Rb1, can be further biotransformed to protopanaxadiol (PPD) or possibly other compounds. Ginsenoside Rh2, including both 20S- and 20R- stereoisomeric forms, was detected in feces samples as a minor metabolite via another metabolic pathway from ginsenoside Rb1 and Rg3.
Conclusions
In this study, ginsenoside Rb1 and its major metabolite, compound K, were determined in biological samples from human subjects consuming different types of diet. We observed significant ginsenoside Rb1 and compound K level differences in the test samples from subjects consuming Asian diet and Western diet. The ginseng compound biotransformation difference is likely linked to diet-induced gastrointestinal microbiota population difference. Further studies are needed to enlarge the samples size, to characterize ginseng compound metabolic pathways, and to identify which enteric microbiota population is affected by which diet type. The ginseng compound changes we observed in this study should be enhanced or reduced by gut microbiota manipulation for clinical utility.
Acknowledgments
This work was supported in part by grants from the NIH/NCCAM AT004418 and AT005362, the National Science Foundation of China (No. 81603378), the Natural Science Foundation of Jiangsu Province (BK20160545), the Senior Talent Cultivation Program of Jiangsu University (15JDG069), and the Priority Academic Program Development of Jiangsu Higher Education Institution (PAPD).
References
- Attele AS, Wu JA, Yuan CS. Ginseng pharmacology: multiple constituents and multiple actions. Biochemical Pharmacology. 1999;58:1685–1693. doi: 10.1016/s0006-2952(99)00212-9. [DOI] [PubMed] [Google Scholar]
- Choi WY, Lim HW, Lim CJ. Anti-inflammatory, antioxidative and matrix metalloproteinase inhibitory properties of 20(R)-ginsenoside Rh2 in cultured macrophages and keratinocytes. Journal of Pharmacy and Pharmacology. 2013;65:310–316. doi: 10.1111/j.2042-7158.2012.01598.x. [DOI] [PubMed] [Google Scholar]
- Genton L, Cani PD, Schrenzel J. Alterations of gut barrier and gut microbiota in food restriction, food deprivation and protein-energy wasting. Clinical Nutrition. 2015;34:341–349. doi: 10.1016/j.clnu.2014.10.003. [DOI] [PubMed] [Google Scholar]
- Hu ZY, Yang JL, Cheng C, Huang YH, Du FF, Wang FQ, Niu W, Xu F, Jiang RR, Gao XM, Li C. Combinatorial metabolism notably affects human systemic exposure to ginsenosides from orally administered extract of Panax notoginseng roots (Sanqi). Drug Metabolism and Disposition. 2013;41:1457–1469. doi: 10.1124/dmd.113.051391. [DOI] [PubMed] [Google Scholar]
- Huang XP, Ding H, Lu JD, Tang YH, Deng BX, Deng CQ. Effects of the combination of the main active components of Astragalus and Panax notoginseng on inflammation and apoptosis of nerve cell after cerebral ischemia-reperfusion. American Journal of Chinese Medicine. 2015;43:1419–1438. doi: 10.1142/S0192415X15500809. [DOI] [PubMed] [Google Scholar]
- Ingerslev HC, Strube ML, Jørgensen L, Dalsgaard I, Boye M, Madsen L. Diet type dictates the gut microbiota and the immune response against Yersinia ruckeri in rainbow trout (Oncorhynchus mykiss). Fish and Shellfish Immunology. 2014;40:624–633. doi: 10.1016/j.fsi.2014.08.021. [DOI] [PubMed] [Google Scholar]
- Janssen AWF, Kersten S. The role of the gut microbiota in metabolic health. FASEB Journal. 2015;29:3111–3123. doi: 10.1096/fj.14-269514. [DOI] [PubMed] [Google Scholar]
- Kang KA, Piao MJ, Kim KC, Zheng J, Yao CW, Cha JW, Kim HS, Kim DH, Bae SC, Hyun JW. Compound K, a metabolite of ginseng saponin, inhibits colorectal cancer cell growth and induces apoptosis through inhibition of histone deacetylase activity. International Journal of Oncology. 2013;43:1907–1914. doi: 10.3892/ijo.2013.2129. [DOI] [PubMed] [Google Scholar]
- Liu F, Bai X, Ding RB, Hu YJ, Su H, Wan JB. UPLC/Q-TOFMS-based metabolomics studies on the protective effect of Panax notoginseng saponins on alcoholic liver injury. American Journal of Chinese Medicine. 2015;43:695–714. doi: 10.1142/S0192415X15500433. [DOI] [PubMed] [Google Scholar]
- Moco S, Martin FP, Rezzi S. Metabolomics view on gut microbiome modulation by polyphenol-rich foods. Journal of Proteome Research. 2012;11:4781–4790. doi: 10.1021/pr300581s. [DOI] [PubMed] [Google Scholar]
- Mushref MA, Srinivasan S. Effect of high fat-diet and obesity on gastrointestinal motility. Annals of Translational Medicine. 2013;1:14. doi: 10.3978/j.issn.2305-5839.2012.11.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi LW, Wang CZ, Yuan CS. Isolation and analysis of ginseng: advances and challenges. Natural Product Reports. 2011;28:467–495. doi: 10.1039/c0np00057d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi LW, Wang HY, Zhang H, Wang CZ, Li P, Yuan CS. Diagnostic ion filtering to characterize ginseng saponins by rapid liquid chromatography with time-of-flight mass spectrometry. Journal of Chromatography A. 2012;1230:93–99. doi: 10.1016/j.chroma.2012.01.079. [DOI] [PubMed] [Google Scholar]
- Simpson HL, Campbell BJ. Review article: dietary fibre-microbiota interactions. Alimentary Pharmacology and Therapeutics. 2015;42:158–179. doi: 10.1111/apt.13248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun S, Qi LW, Du GJ, Mehendale SR, Wang CZ, Yuan CS. Red notoginseng: higher ginsenoside content and stronger anticancer potential than Asian and American ginseng. Food Chemistry. 2011;125:1299–1305. doi: 10.1016/j.foodchem.2010.10.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Underwood MA, Salzman NH, Bennett SH, Barman M, Mills DA, Marcobal A, Tancredi DJ, Bevins CL, Sherman MP. A randomized placebo-controlled comparison of 2 prebiotic/probiotic combinations in preterm infants: impact on weight gain, intestinal microbiota, and fecal short-chain fatty acids. Journal of Pediatric Gastroenterology and Nutrition. 2009;48:216–225. doi: 10.1097/MPG.0b013e31818de195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan JY, Liu P, Wang HY, Qi LW, Wang CZ, Li P, Yuan CS. Biotransformation and metabolic profile of American ginseng saponins with human intestinal microflora by liquid chromatography quadrupole time-of-flight mass spectrometry. Journal of Chromatography A. 2013;1286:83–92. doi: 10.1016/j.chroma.2013.02.053. [DOI] [PubMed] [Google Scholar]
- Wang CZ, McEntee E, Wicks S, Wu JA, Yuan CS. Phytochemical and analytical studies of Panax notoginseng (Burk.) F.H. Chen. Journal of Natural Medicines. 2006;60:97–106. [Google Scholar]
- Wang HY, Qi LW, Wang CZ, Li P. Bioactivity enhancement of herbal supplements by intestinal microbiota focusing on ginsenosides. American Journal of Chinese Medicine. 2011a;39:1103–1115. doi: 10.1142/S0192415X11009433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang CZ, Kim KE, Du GJ, Qi LW, Wen XD, Li P, Bauer BA, Bissonnette MB, Musch MW, Chang EB, Yuan CS. Ultra-performance liquid chromatography and time-of-flight mass spectrometry analysis of ginsenoside metabolites in human plasma. American Journal of Chinese Medicine. 2011b;39:1161–1171. doi: 10.1142/S0192415X11009470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang CZ, Du GJ, Zhang Z, Wen XD, Calway T, Zhen Z, Musch MW, Bissonnette M, Chang EB, Yuan CS. Ginsenoside compound K, not Rb1, possesses potential chemopreventive activities in human colorectal cancer. International Journal of Oncology. 2012;40:1970–1976. doi: 10.3892/ijo.2012.1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang CZ, Qi LW, Yuan CS. Cancer chemoprevention effects of ginger and its active constituents: Potential for new drug discovery. American Journal of Chinese Medicine. 2015;43:1351–1363. doi: 10.1142/S0192415X15500767. [DOI] [PubMed] [Google Scholar]
- Xiao HT, Zhong L, Tsang SW, Lin ZS, Bian ZX. Traditional Chinese medicine formulas for irritable bowel syndrome: from ancient wisdoms to scientific understandings. American Journal of Chinese Medicine. 2015;43:1–23. doi: 10.1142/S0192415X15500019. [DOI] [PubMed] [Google Scholar]
- Xie GX, Wang CZ, Yu CH, Qiu YP, Wen XD, Zhang CF, Yuan CS, Jia W. American ginseng significantly reduced the progression of high-fat-diet-enhanced colon carcinogenesis in Apc(Min/+) mice. Journal of Proteome Research. 2015;14:3336–3347. [Google Scholar]
- Yu C, Wen XD, Zhang Z, Zhang CF, Wu XH, Martin A, Du W, He TC, Wang CZ, Yuan CS. American ginseng attenuates azoxymethane/dextran sodium sulfate-induced colon carcinogenesis in mice. Journal of Ginseng Research. 2015a;39:14–21. doi: 10.1016/j.jgr.2014.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu C, Wen XD, Zhang Z, Zhang CF, Wu X, He X, Liao Y, Wu N, Wang CZ, Du W, He TC, Yuan CS. American ginseng significantly reduced the progression of high-fat-diet-enhanced colon carcinogenesis in Apc (Min/+) mice. Journal of Ginseng Research. 2015b;39:230–237. doi: 10.1016/j.jgr.2014.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan CS, Wang CZ, Wicks SM, Qi LW. Chemical and pharmacological studies of saponins with a focus on American ginseng. Journal of Ginseng Research. 2010;34:160–167. doi: 10.5142/jgr.2010.34.3.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang JW, Lu M, Zhou F, Sun HP, Hao G, Wu XL, Wang GJ. Key Role of nuclear factor-kappa B in the cellular pharmacokinetics of adriamycin in MCF-7/Adr cells: The potential mechanism for synergy with 20(S)-ginsenoside Rh2. Drug Metabolism and Disposition. 2012;40:1900–1908. doi: 10.1124/dmd.112.045187. [DOI] [PubMed] [Google Scholar]
- Zheng X, Zhao A, Xie G, Chi Y, Zhao L, Li H, Wang C, Bao Y, Jia W, Luther M, Su M, Nicholson JK, Jia W. Melamine-induced renal toxicity is mediated by the gut microbiota. Science Translational Medicine. 2013;5:172ra22. doi: 10.1126/scitranslmed.3005114. [DOI] [PubMed] [Google Scholar]