Abstract
Soft-tissue invasive fungal infections are increasingly recognized as significant entities directly contributing to morbidity and mortality. They complicate clinical care, requiring aggressive surgical debridement and systemic antifungal therapy. To evaluate new topical approaches to therapy, we examined the antifungal activity and cytotoxicity of Manuka Honey (MH) and polyhexamethylene biguanide (PHMB). The activities of multiple concentrations of MH (40%, 60%, 80%) and PHMB (0.01%, 0.04%, 0.1%) against 13 clinical mould isolates were evaluated using a time-kill assay between 5 min and 24 h. Concentrations were selected to represent current clinical use. Cell viability was examined in parallel for human epidermal keratinocytes, dermal fibroblasts and osteoblasts, allowing determination of the 50% viability (LD50) concentration. Antifungal activity of both agents correlated more closely with exposure time than concentration. Exophiala and Fusarium growth was completely suppressed at 5 min for all PHMB concentrations, and at 12 and 6 h, respectively, for all MH concentrations. Only Lichtheimia had persistent growth to both agents at 24 h. Viability assays displayed concentration-and time-dependent toxicity for PHMB. For MH, exposure time predicted cytotoxicity only when all cell types were analyzed in aggregate. This study demonstrates that MH and PHMB possess primarily time-dependent antifungal activity, but also exert in vitro toxicity on human cells which may limit clinical use. Further research is needed to determine ideal treatment strategies to optimize antifungal activity against moulds while limiting cytotoxicity against host tissues in vivo.
Keywords: Topical, polyhexanide, polyhexamethylene biguanide, manuka honey, cytotoxicity, Mucorales
Introduction
Soft-tissue invasive fungal infections (IFIs) occurring after combat-related wounds, in patients who are immunosuppressed, and as sequelae of natural disasters are increasingly recognized as significant complications with direct contribution to morbidity and mortality.1–5 Infections associated with Mucorales, Aspergillus, and Fusarium species have been attributed a mortality rate as high as 25–38%.6 While the mainstay of therapy remains aggressive surgical debridement, this is not always feasible due to occurrence in remote areas, during a natural disaster where infrastructure is destroyed, or when a qualified surgeon is not immediately available. Additionally, achieving surgical control of the infection may require highly morbid limb amputations. Systemic antifungals are used adjunctively in an effort to stem the extent of infection, although clinical data proving efficacy for these infrequent infections are lacking. Systemic antifungals also have significant potential toxicities and drug interactions that limit their use.7 Recent reports of soft-tissue IFIs, and recognition of the therapeutic limitations of systemic antifungal agents, has prompted us to investigate topical therapeutic agents that can be safely applied to open wounds. Previous studies investigating this approach have shown a potential for antifungal activity, but with some evidence of cytotoxicity.8,9 Topical antifungal agents which are safe and effective could be a useful adjunct to systemic antifungals and surgery to further reduce morbidity and mortality from these infections.
Honey has been used in wound care for centuries but fell out of favor after the advent of typical antimicrobials.10,11 In recent years, the development of extensive antimicrobial resistance has led to a resurgence of research into alternative potential therapies, including a reassessment of applications for honey.12,13 Proposed mechanisms of action, which are not completely understood, include osmotic effect, hydrogen peroxide content, acidity, and potential immunomodulatory effect.14–21 Honey has antibacterial properties against antibiotic-resistant bacteria, as well as several fungi including Candida spp. and Aspergillus spp.12,13,22–34 Potential concerns for the topical use of honey on wounds include the introduction of spores or bacterial contaminants, and the standardization of antibacterial activity in each batch.22,26,35–37 To mitigate these concerns, the commercial marketplace has produced a standardized, FDA-approved formulation of honey (known as Manuka Honey [MH]), sterilized with gamma irradiation, which has been incorporated into various commercially available wound dressings.
Polyhexamethylene biguanide (PHMB) is a composite mixture of cationic polyamines commonly employed in contact lens antiseptic solutions, cosmetic preservatives, and swimming pool sanitizers since the 1950s. Biguanides such as PHMB intercalate into bacterial cell membranes and increase cell permeability, leading to eventual loss of membrane function and cell death.38In vitro studies have demonstrated activity of PHMB against Escherichia coli, Staphylococcus aureus, Fusarium solani, Acanthamoeba sp., Listeria monocytogenes, Aspergillus flavus, Pseudomonas aeruginosa and Proteus vulgaris.39–45 PHMB has been used clinically for the treatment of amoebic keratitis.46 Against filamentous fungi, PHMB has previously only been tested in strains recovered from ocular infections and domestic pets.47–53 In addition to its use in cosmetics and ocular products, several FDA-approved wound cleansing products are currently marketed featuring PHMB as the primary active ingredient. Given the severe morbidity associated with soft-tissue IFI, the consequences and constraints of currently available conventional therapies, and availability of several FDA approved compounds for topical use, we examined the antifungal activity PHMB and MH against a panel of moulds isolated from clinical soft-tissue invasive fungal infections occurring in our facility. In addition, we conducted in vitro cytotoxicity testing against human cell types representing severe deep wounds (i.e., traumatic amputation) to gauge the potential suitability of these compounds for use in open wounds.
Materials and methods
Thirteen clinical mould isolates from the San Antonio Military Medical Center (SAMMC) Infectious Disease Molecular Epidemiology repository were selected for use in this study.8,54 All clinical isolates were collected from patients during the course of patient care. Individual strains were identified by mould morphology, phenotypic characteristics, and DNA sequence analysis, in accordance with published morphology guides and experience-based optimal laboratory practice at the Fungus Testing Laboratory, University of Texas Health Sciences Center, San Antonio, Texas, USA.55–57 Once identified, they were stored in the strain repository for future research. All mould isolates were separately stored at −80°C in tryptic soy broth with 15% glycerol. The mould isolates included one Lichtheimia sp., three Aspergillus flavus, one Aspergillus fumigatus, one Aspergillus terreus, two Mucor circinelloides, one Fusarium oxysporum, one Exophiala sp., one Apophysomyces sp., and two Actinomucor elegans.
Fungal time-kill assay
A time-kill assay was performed to determine the antifungal activity of MH (Links Medical Products, Inc, Irvine, CA) and PHMB (BOC Sciences, Creative Dynamics Inc., Shirley, NY).8,54 Three different concentrations of MH (40%, 60%, 80%) and PHMB (0.01%, 0.04%, 0.1%) were tested, along with water as a control. Concentrations of MH were chosen based on previous studies documenting minimum inhibitory concentrations of honey, as well as cytotoxicity.7,24
Concentrations of PHMB were selected to represent clinically available wound irrigation and cleansing products, or a ten-fold dilution thereof (0.01%) representing a lower boundary against which to compare potential toxicity. The mould isolates were grown on potato agar slants (Remel, Lenexa, KS) at 35°C for 7 to 10 days. Standard conidia/hyphae suspensions of 5 × 104 CFU/ml were prepared according to the Clinical and Laboratory Standards Institute (CLSI) guidelines54 and aliquots of the suspensions were exposed to the different topical solutions and dilutions for 24 h. At different time points (0, 5 min, 15 min, 30 min, 1 h, 1.5 h, 3 h, 6 h, 12 h, and 24 h) aliquots of 100-fold dilutions were plated onto potato flake agar plates. The predetermined time increments were selected based on recommendations for use of commercially available medical grade honey products. The plates were incubated at 35°C and colony counts determined after incubation for 24 h, or as appropriate for slow growing moulds (2–3 days until appearance of countable colonies). The assay was performed in duplicate.
Cell toxicity assay
Human dermal keratinocytes (HEK-001; ATCC CRL 2404; American Tissue Type Collection, Manassas, VA) were grown in keratinocyte serum-free medium (GIBCO, Grand Island, NY) with 5 ng/ml human recombinant Epidermal Growth Factor and 2 mM L-Glutamine. Human dermal fibroblasts and osteoblasts (PromoCell, Heidelberg, Germany) were grown in Dulbecco modified Eagle medium supplemented with 10% fetal bovine serum, 10 U/ml penicillin, and 10 μg/ml streptomycin. All cell lines were grown and maintained at 37°C in 5% carbon dioxide. The toxicity of MH and PHMB to the various cell lines was evaluated by performing cellular viability assays as previously described.8,54 In brief, confluent monolayers of cells seeded into 96-well plates were exposed to the same concentrations utilized for the time-kill assays for 5 min, 15 min, 30 min, 1 h, 1.5 h, 3 h, 6 h, and 24 h. Following exposure, the cells were washed and resuspended in phosphate buffered saline, pH 7.4 (PBS, Sigma-Aldrich, St. Louis, MO, USA). Cell viability following exposure was evaluated using the CellTiter Fluor assay (Promega, Madison, WI) as recommended by the manufacturer. As a negative control, sterile PBS was used. Cell viability was reported as a percentage compared to the control group. Experiments were performed in triplicate.
Statistical methods
To determine whether time or concentration best predicted toxicity to human or fungal cells, multiple linear regression was performed using the generalized linear model to fit time, concentration and viability data to the exponential distribution using the reciprocal link function. From the resulting model, the time to 50% viability (i.e., the LD50) was determined. Medians and 25–75% interquartile ranges (IQR) were determined. JMP version 9.0.0 (SAS Institute Inc., Cary, NC) was used for all statistical calculations, and median-IQR plots were generated using GraphPad Prism (GraphPad Software Inc., San Diego, CA), version 5.01.
Results
Fungal time-kill assay
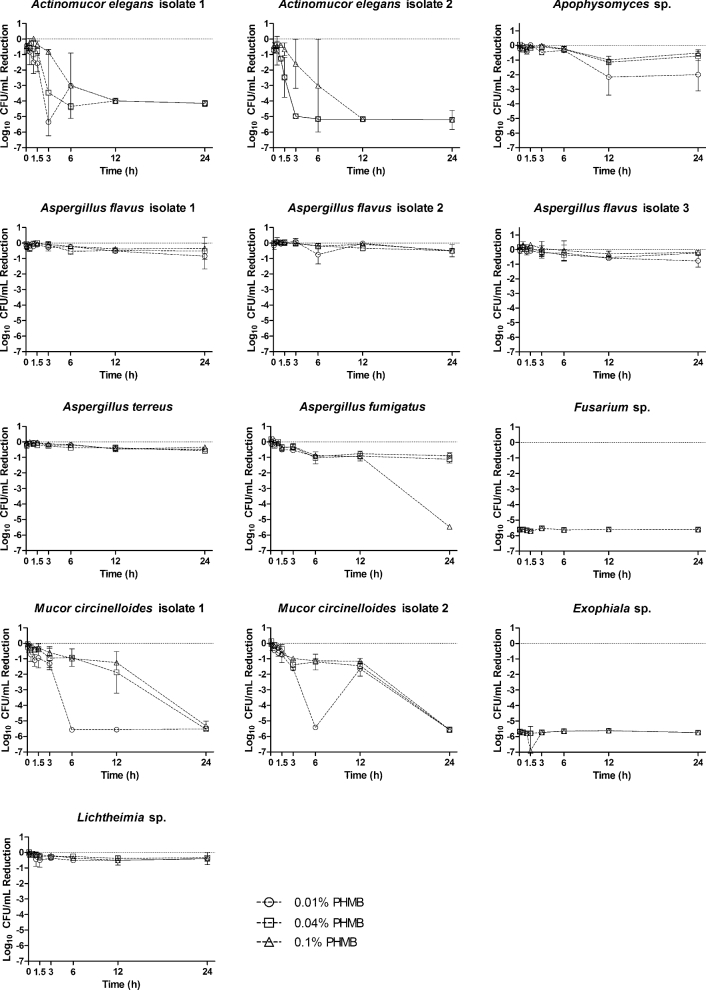
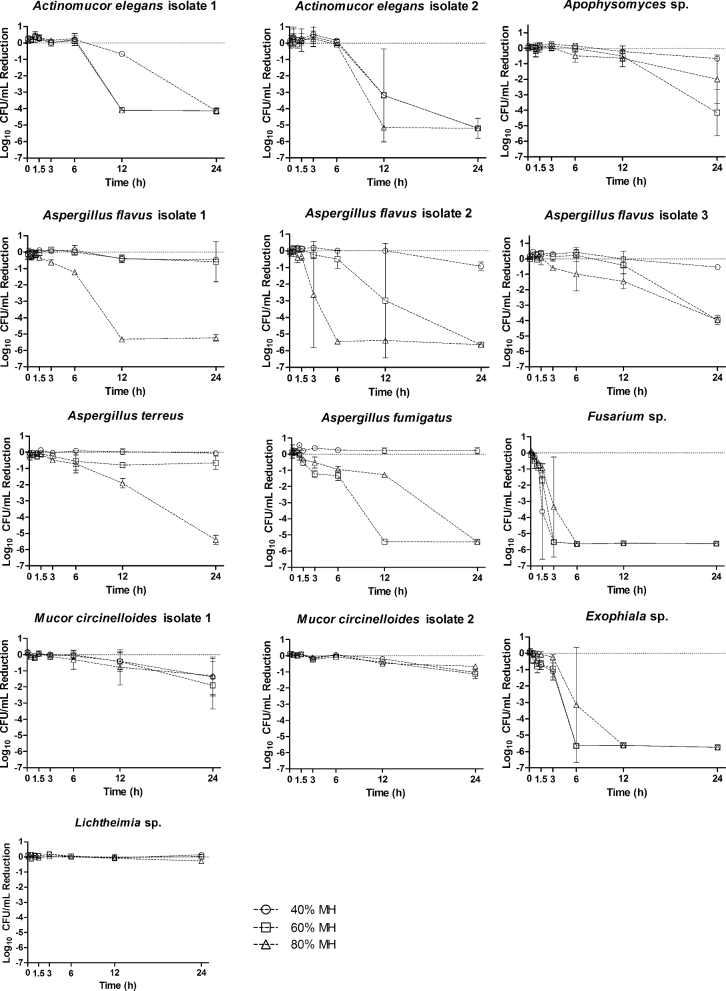
The time-kill assay results differed based on the fungal isolate, exposure time, and topical concentration. Visual inspection of time-kill assays (Figures 1, 2) demonstrated the predominantly time-dependent antifungal activity of both agents. The majority of isolates tested displayed a reduction in CFUs after the 3-hour time point. Only the Exophiala sp. and Fusarium oxysporum isolates displayed a substantial reduction in CFUs prior to the 3-hour time point. In these latter isolates, all concentrations of PHMB were able to completely suppress CFU growth by the 5-minute time point. Similar reductions in CFU by MH were not observed until the 3-hour and 6-hour time point for Fusarium oxysporum and Exophiala sp. respectively. Apophysomyces displayed only a 2-log reduction for both agents, except for 60% MH, for which a 5-log reduction was observed. In this case, CFUs were not substantially decreased until the final 24-hour time point. We observed significant differences in the antifungal potency of the topical agents tested in this study. The Aspergillus isolates displayed greater susceptibility to MH than PHMB. A. flavus isolates 2 and 3 displayed a ≥ 3-log reduction when exposed to 60% and 80% MH. A. flavus isolate 1 and A. terreus displayed a ≥ 3-log reduction when exposed to 80% MH. For all the A. flavus and A. terreus isolates, 40% MH performed comparably to PHMB, with less than 1-log reduction at 24 hours. Conversely, the Mucor circinelloides isolate 2 appeared to be more susceptible to PHMB at 24 hours but relatively resistant to MH. All concentrations of MH resulted in a 2-log reduction at the 24-hour time point, whereas all PHMB concentrations demonstrated antifungal activity as early as 6 hours. M. circinelloides was completely suppressed by 24 hours at all concentrations of PHMB, whereas MH was only able to achieve a 1-log reduction against this mould at 24 hours. The concentration of the topicals was especially important against the two Actinomucor elegans isolates. For both isolates, exposure to each topical agent resulted in a 4-log reduction in growth. However, the lower concentrations of PHMB were particularly active against the isolates. A. elegans isolate 2 was reduced by 5-log units at 3 hours by the 0.01% and 0.04% PHMB concentrations. Paradoxically, only a 1-log reduction was observed with the 0.1% PHMB, and no decrease was observed at 3 hours for any concentration of MH. MH did exert fungicidal activity at 12 hours for the 80% concentration, and at 24 hours for the 40% and 60% concentrations. Lichtheimia proved to be the least susceptible of the fungal isolates to PHMB and MH, demonstrating persistent growth without a single log reduction across a 24-hour exposure.
Figure 1.
Time-kill curves for fungal clinical isolates following exposures to polyhexamethylene biguanide (0.01%, 0.04% and 0.1%) from 5 min to 24 h. Reported values are the mean of two independent assays and are reported as the log reduction of treatment groups relative to a nontreated control group, water. Circles: 0.01% PHMB; squares 0.04% PHMB; triangles 0.1% PHMB.
Figure 2.
Time-kill curves for fungal clinical isolates following exposures to Manuka honey (40%, 60%, and 80%) from 5 min to 24 h. Reported values are the mean of two independent assays and are reported as the log reduction of treatment groups relative to a nontreated control group, water. Circles: 40% MH; squares 60% MH; triangles 80% MH.
Cell toxicity assay
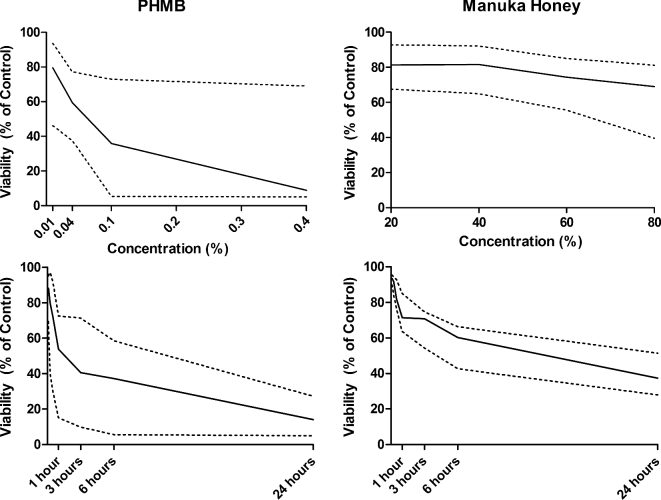
For MH, viability assays did not demonstrate a statistically significant dependency on concentration or exposure time when cell lines were considered monotypically (e.g., only keratinocytes). However, when the cell lines were considered in aggregate, time emerged as a statistically significant predictor of toxicity in the multivariate analysis (Figure 3). For PHMB, cell viability assays for cell lines considered individually, and in aggregate, demonstrated both concentration- and time-dependent cytotoxicity. An exception was fibroblasts, for which only time was a statistically significant predictor of cytotoxicity (Table 1).
Figure 3.
Median lethal dose 50 (LD50, solid lines) and 25–75% interquartile ranges (dotted lines) for human cell lines following exposure to various concentrations of PHMB and MH (top graphs) and over time (bottom graphs) in vitro. Multiple linear regression analysis was used to determine whether topical concentration or exposure time best predicted loss of cell viability in the three human cell lines. Note: Solid lines within each graph depict the mean with dotted lines above and below representing one standard deviation from the mean.
Table 1.
Cell viability of fibroblasts, keratinocytes and osteoblasts exposed to varying concentrations of Manuka honey (MH) and polyhexamethylene biguanide (PHMB).
| Fibroblasts | Keratinocytes | Osteoblasts | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0.5 h | 3 h | 6 h | 24 h | 0.5 h | 3 h | 6 h | 24 h | 0.5 h | 3 h | 6 h | 24 h | ||
| MH | 40% | 98 | 70 | 65 | 50 | 87 | 71 | 64 | 51 | 82 | 62 | 66 | 31 |
| 60% | 96 | 74 | 59 | 46 | 76 | 74 | 59 | 43 | 77 | 51 | 45 | 28 | |
| 80% | 81 | 71 | 41 | 25 | 68 | 43 | 37 | 27 | 70 | 44 | 30 | 12 | |
| PHMB | 0.01% | 99 | 66 | 33 | 28 | 83 | 69 | 56 | 39 | 88 | 35 | 25 | 18 |
| 0.04% | 62 | 46 | 30 | 25 | 62 | 44 | 36 | 27 | 59 | 36 | 38 | 23 | |
| 0.1% | 43 | 15 | 6 | 9 | 44 | 14 | 5 | 8 | 24 | 4 | 5 | 5 | |
Note: Data are presented as percent viability relative to negative control (saline).
Abbreviations: MH, Manuka honey; PHMB, polyhexamethylene biguanide.
Discussion
Soft-tissue invasive fungal infections following traumatic injuries can greatly complicate the clinical course, requiring aggressive surgical debridement and systemic antifungal therapy. Due to the invasive nature and associated mortality of IFIs, aggressive surgical debridement remains the mainstay of therapy and can require highly morbid amputations such as hip disarticulation for lower extremity wound infections. In light of the unsatisfactory status of currently available therapies, the aim of this study was to evaluate two clinically available, FDA-approved compounds, PHMB and MH, as potential adjuvant topical therapies, with the aim of decreasing the need for systemic antifungals and serial surgical debridement. We found that PHMB and MB were active, albeit to varying degrees, against a wide variety of mould isolates. The mechanism of action by which MH exerts its antimicrobial effects remains an area of active study. However, research completed to date may help inform the slower onset of action. Kwakman et al. investigated the antibacterial properties of individual constituents of MH, noting that MH had a higher concentration of methylglyoxal (MGO), which is slowly bactericidal. This was postulated to also be an underlying reason for the slower bactericidal activity of MH against Methicillin-resistant Staphylococcus aureus (MRSA).14 They also found that MH had lower concentrations of hydrogen peroxide and bee defensin-1, both of which were found to have faster rates of bactericidal activity in other monofloral honeys tested. It is possible that the antimicrobial properties of MH responsible for its activity against MRSA are likewise contributing to the antifungal activity observed in this study. If the time-kill assays were extended for a longer duration, perhaps a greater reduction in fungal isolates would be observed. This latency prior to the onset of antimicrobial activity may, in the 24-hour time periods examined in this study, have resulted in our observation of predominantly time-dependent antifungal activity for MH.
In this study, fungicidal activity was observed at all three concentrations of PHMB. The growth of approximately half of the fungal isolates investigated was reduced by > 2-log CFUs after exposure to PHMB, and the rate of kill was not further increased in proportion to the PHMB concentration. This is not unexpected, given its current use as an antiseptic in contact lens solutions to prevent fungal growth leading to ocular infection.50 PHMB was most potent against the Fusarium and Exophiala isolates but had minimal activity against A. flavus and A. terreus isolates. This is consistent with the role of A. flavus as a cause of ocular fungal infection. As we did not demonstrate concentration-dependent fungicidal activity, further research is needed for additional fungal characteristics that may serve as indicators of clinical susceptibility to PHMB.
Toxic cellular effects of the agents that could potentially limit clinical use were evaluated with cellular toxicity assays. When all three human cell lines were examined in aggregate, MH was found to have time-dependent cellular toxicity at later time points. The underlying cause for this is unclear. While this may reflect the greater statistical power achieved by more observations, this may also reflect the osmotic effect of honey, which is known to be part of its antibacterial properties.15,17,58 This has previously been noted as the primary means of bactericidal activity for honey against Helicobacter pylori.58 While our study found that MH was toxic to human cells, studies exposing human tissue explants to MH suggest epidermal cytocompatibility, with modest early stimulation of epidermal keratinocytes and fibroblasts compared to control.59 Similarly, honey-impregnated dressings were best suited to healing partial thickness burns 4 to 5 days faster than conventional dressings.36 Use of medical grade honey to accelerate healing for chronic venous stasis wounds and pressure ulcers was not supported.36
On the basis of cellular viability assays, we found PHMB tested at or below concentrations currently used in human wound care to be toxic to cultured human cells, in a time-dependent and concentration-dependent manner. These findings contrast with previous studies examining PHMB cellular toxicity.39–44 In one study in which PHMB was applied to murine fibroblasts and E. coli, antibacterial effects exceeded cytotoxic effects.39 A caveat to this study is that the cell cultures were only exposed to PHMB long enough for bacterial counts to undergo a 3-log reduction. However, studies using PHMB as a component of wound dressings39 found that the dressings maintained antimicrobial efficacy without damaging viable tissue. Notably, the extent to which in vitro cytotoxicity testing of cultured cells (which lack a basement membrane or higher tissue architecture) accurately predicts in vivo human tissue toxicity is unclear. The use of PHMB in ophthalmologic medicine further suggests a degree of clinical safety.46
Despite evidence of cytotoxicity in cultured cells, there may still be a role for the use of MH and PHMB. Buffered sodium hypochlorite (Dakin's solution) has also displayed toxicity in studies using cultured human cells. A study by Kozol et al. in 1988 evaluated the effect of Dakin's solution on neutrophil migration in fibroblasts and endothelial cells in a rabbit model to serve as a surrogate marker of cellular viability. They also evaluated direct cellular toxicity using electron microscopy and tryptan blue.60 In their study, all concentrations of Dakin's solution were cytotoxic, despite being lower than currently used in clinical practice. Similarly, cellular toxicity was found on exposure of human keratinocytes to Dakin's solution, as evidenced by decreased uptake of neutral red dye. Decreased uptake of neutral red was interpreted as a loss of cellular integrity, as it was only taken up into the lysosomes of intact cells.61 A study by Homeyer et al. likewise displayed toxicity of both Dakin's solution as well as Melaleuca alternifolia (tea tree) oil when exposed to human cell cultures at concentrations currently employed in clinical use.9 It is important to note that both MH and PHMB displayed overall greater activity against fungal growth than did M. alternifolia oil when exposed to similar filamentous fungi. Thus, despite multiple studies providing evidence of cellular damage of components of cells important to wound healing, Dakin's solution continues to be used as a topical adjunct in clinical practice. Its use has been credited with decreased use of systemic antibiotics, decrease in overall microbial burden and practical application to wounds not otherwise amenable to dressings.62
Future studies should examine the safety of PHMB and MH at lower concentrations. Repeating the study with lower concentrations of MH may potentially decrease toxicity to human cell lines, while allowing for the investigation of fungistatic properties of the agent. PHMB displayed a concentration-dependent cell toxicity and a binary, “all-or-nothing” antifungal effect. Against more susceptible isolates, PHMB was uniformly fungicidal, while having little activity against less susceptible isolates. Testing at lower concentrations than used here could better define concentrations which optimize antifungal activity and host cellular cytotoxicity. In addition, potential fungistatic properties inherent to the agent may be revealed at lower concentrations. Likewise, exposing cell cultures to both PHMB and MH, either simultaneously or in succession, may better define the tolerability of human cells to these agents and better characterize their potential clinical efficacy. Medical grade honey has been shown to be effective against multiple bacterial biofilms as well as Candida biofilms. To our knowledge no research has been conducted on the efficacy of honey on biofilms of filamentous fungi. Further investigation of these properties may be of clinical benefit.63,64
Limitations of our study included the utilization of only one type of medical grade honey. Previous studies have found that different commercially available and widely used medical grade honeys possess different mechanisms of action for achieving bacterial eradication.14 However, we opted to utilize the only FDA-approved medical grade honey that could therefore easily be implemented in clinical care. A practical limitation of the study was evaluating the effects of MH and PHMB on single strains of the fungal species. Not testing all known fungal strains allows for a limited understanding of the agent's antifungal efficacy. Different isolates of the same species could potentially possess different sensitivity profiles against MH and PHMB. Attempts to minimize this effect were made with the testing of multiple strains of A. flavus, Actinomucor sp. and Mucor sp. However, among those fungal species with multiple strains, all strains behaved similarly when tested. Additional limitations included the use of cell cultures rather than live tissue for the toxicity assays, as previously mentioned.
In conclusion, we evaluated the antifungal activity of two agents proposed for adjunctive topical therapy for soft tissue invasive fungal infections: Manuka Honey and polyhexamethylene biguanide. We exposed a variety of filamentous fungi to these agents, finding variable activity. We also observed significant cytotoxicity of these agents to cultured human cell lines. Toxicity was predominantly time-dependent for MH, and both time-dependent and concentration-dependent for PHMB. Whether cellular toxicity at the concentrations tested and longer exposure times limits clinical benefit remains unknown. The use of these agents with similar concentrations in commercially available products supports the hypothesis that clinical use would not result in cell toxicity. Topical application of one or both agents on traumatic wounds and surgical sites may still be possible. Further research is needed to evaluate the cytotoxicity of both agents using a more clinically relevant system such as ex vivo tissue explants, in vivo animal models and eventually human trials before widespread clinical use can be recommended.
Acknowledgments
Support for this work (IDCRP-024) was provided by the Infectious Disease Clinical Research Program (IDCRP), a Department of Defense program executed through the Uniformed Services University of the Health Sciences, Department of Preventive Medicine and Biostatistics. This project has been funded by the Department of Defense Global Emerging Infections Surveillance and Response System (GEIS) [HT9404-12-1-0011], a division of the Armed Forces Health Surveillance Center, National Institute of Allergy and Infectious Diseases, National Institutes of Health, under Inter-Agency Agreement Y1-AI-5072, and the Department of the Navy under the Wounded, Ill, and Injured Program [HU001-10-1-0014].
The views expressed are those of the authors and do not necessarily reflect the official views of the Uniformed Services University of the Health Sciences, the Henry M. Jackson Foundation for the Advancement of Military Medicine, Inc., the National Institutes of Health or the Department of Health and Human Services, Brooke Army Medical Center, the US Army Institute of Surgical Research, the US Army Medical Department, the US Army Office of the Surgeon General, the Department of Defense or the Departments of the Army, Navy, or Air Force. Mention of trade names, commercial products, or organizations does not imply endorsement by the US Government.
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and the writing of the paper.
References
- 1. Neblett Fanfair R, Benedict K, Bos J et al. Necrotizing cutaneous mucormycosis after a tornado in Joplin, Missouri, in 2011. N Engl J Med 2012; 367: 2214–2225. [DOI] [PubMed] [Google Scholar]
- 2. Warkentien T, Rodriguez C, Lloyd B et al. Invasive mold infections following combat-related injuries. Clin Infect Dis 2012; 55: 1441–1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Murray C, Wilkins K, Molter N et al. Infections complicating the care of combat casualties during Operations Iraqi Freedom and Enduring Freedom. J Trauma 2011; 71(supplement): S62–S73. [DOI] [PubMed] [Google Scholar]
- 4. Paolino K, Henry J, Hospenthal D et al. Invasive fungal infections following combat-related injury. Mil Med 2012; 177: 681–685. [DOI] [PubMed] [Google Scholar]
- 5. Murray C, Obremskey W, Hsu J et al. Prevention of infections associated with combat-related extremity injuries. J Trauma 2011; 71(supplement): S235–S257. [DOI] [PubMed] [Google Scholar]
- 6. Lloyd B, Weintrob A, Rodriguez C et al. Effect of early screening for invasive fungal infections in U.S. service members with explosive blast injuries. Surg Infect 2014; 15: 619–626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lewis R. Current concepts in antifungal pharmacology. Mayo Clin Proc 2011; 86: 805–817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Barsoumian A, Sanchez C, Mende K et al. In vitro toxicity and activity of Dakin's solution, mafenide acetate, and amphotericin B on filamentous fungi and human cells. J Orthop Trauma 2013; 27: 428–436. [DOI] [PubMed] [Google Scholar]
- 9. Homeyer D, Sanchez C, Mende K et al. In vitro activity of Melaleuca alternifolia (tea tree) oil on filamentous fungi and toxicity to human cells. Med Mycol 2015; 53: 285–294. [DOI] [PubMed] [Google Scholar]
- 10. Sipos P, Gyory H, Hagymasi K et al. Special wound healing methods used in ancient Egypt and the mythological background. World J Surg 2004; 28: 211–216. [DOI] [PubMed] [Google Scholar]
- 11. Sheikh D, Zaman S, Naqvi S et al. Studies on the antimicrobial activity of honey. Pak J Pharm Sci 1995; 8: 51–62. [PubMed] [Google Scholar]
- 12. Langemo D, Hanson D, Anderson J et al. Use of honey for wound healing. Adv Skin Wound Care 2009; 22: 113–118. [DOI] [PubMed] [Google Scholar]
- 13. Lee D, Sinno S, Khachemoune A. Honey and wound healing. Am J Clin Dermatol 2011; 12: 181–190. [DOI] [PubMed] [Google Scholar]
- 14. Kwakman P, Zaat S. Antibacterial components of honey. IUBMB Life 2012; 64: 48–55. [DOI] [PubMed] [Google Scholar]
- 15. Kwakman P, te Velde A, de Boer L et al. How honey kills bacteria. FASEB J 2010; 24: 2576–2582. [DOI] [PubMed] [Google Scholar]
- 16. Al-Waili N, Salom K, Butler G et al. Honey and microbial infections: a review supporting the use of honey for microbial control. J Med Food 2011; 14: 1079–1096. [DOI] [PubMed] [Google Scholar]
- 17. Israili Z. Antimicrobial properties of honey. Am J Ther 2014; 21: 304–323. [DOI] [PubMed] [Google Scholar]
- 18. Molan P. Potential of honey in the treatment of wounds and burns. Am J Clin Dermatol 2001; 2: 13–19. [DOI] [PubMed] [Google Scholar]
- 19. Olaitan PB, Adeleke OE, Ola IO. Honey: a reservoir for microorganisms and an inhibitory agent for microbes. Afr Health Sci 2007; 7: 159–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lusby P, Coombes A, Wilkinson J. Honey: a potent agent for wound healing? J Wound Ostomy Continence Nurs 2002; 29: 295–300. [DOI] [PubMed] [Google Scholar]
- 21. Al-Waili N, Salom K, Al-Ghamdi A. Honey for wound healing, ulcers, and burns: data supporting its use in clinical practice. Sci World J 2011; 11: 766–787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Blair S, Cokcetin N, Harry E et al. The unusual antibacterial activity of medical grade Leptospermum honey: antibacterial spectrum, resistance and transcriptome analysis. Eur J Clin Microbiol Infect Dis 2009; 28: 1199–1208. [DOI] [PubMed] [Google Scholar]
- 23. Maeda Y, Loughrey A, Earle J et al. Antibacterial activity of honey against community-associated methicillin-resistant Staphylococcus aureus (CA-MRSA). Complement Ther Clin Pract 2008; 14: 77–82. [DOI] [PubMed] [Google Scholar]
- 24. George N, Cutting K. Antibacterial honey: in vitro activity against clinical isolates of MRSA, VRE, and other multiresistant gram-negative organisms. Wounds 2007; 19: 231–236. [PubMed] [Google Scholar]
- 25. Kwakman P, Van den Akker J, Guclu A et al. Medical‐grade honey kills antibiotic‐resistant bacteria in vitro and eradicates skin colonization. Clin Infect Dis 2008; 46: 1677–1682. [DOI] [PubMed] [Google Scholar]
- 26. AL-Waili N, Al Ghamdi A, Ansari M et al. Differences in composition of honey samples and their impact on the antimicrobial activities against drug multiresistant bacteria and pathogenic fungi. Arch Med Res 2013; 44: 307–316. [DOI] [PubMed] [Google Scholar]
- 27. Kuncic MK, Jaklic D, Lapanje A et al. Antibacterial and antimycotic activities of Slovenian honeys. Br J Biomed Sci 2012; 69: 154. [PubMed] [Google Scholar]
- 28. Wellford T, Eadie T, Llewellyn G. Evaluating the inhibitory action of honey on fungal growth, sporulation, and aflatoxin production. Z Lebensm Unters Forsch 1978; 166: 280–283. [DOI] [PubMed] [Google Scholar]
- 29. Irish J, Carter D, Shokohi T et al. Honey has an antifungal effect against Candida species. Med Mycol 2006; 44: 289–291. [DOI] [PubMed] [Google Scholar]
- 30. Koc A, Silici S, Ercal B et al. Antifungal activity of Turkish honey against Candida spp. and Trichosporon spp: an in vitro evaluation. Med Mycol 2009; 47: 707–712. [DOI] [PubMed] [Google Scholar]
- 31. Boukraa L, Bouchegrane S. Additive action of honey and starch against Candida albicans and Aspergillus niger. Rev Iberoam Micol 2007; 24: 309–311. [DOI] [PubMed] [Google Scholar]
- 32. Obaseiki-Ebor E, Afonya T. In vitro evaluation of the anticandidiasis activity of honey distillate (HY−1) compared with that of some antimycotic agents. J Pharm Pharmacol 1984; 36: 283–284. [DOI] [PubMed] [Google Scholar]
- 33. Koc A, Silici S, Kasap F et al. Antifungal activity of the honeybee products against Candida spp. and Trichosporon spp. J Med Food 2011; 14: 128–134. [DOI] [PubMed] [Google Scholar]
- 34. Mulu A, Diro E, Tekleselassie H et al. Effect of Ethiopian multiflora honey on fluconazole-resistant Candida species isolated from the oral cavity of AIDS patients. Int J STD AIDS 2010; 21: 741–745. [DOI] [PubMed] [Google Scholar]
- 35. Snowdon JA, Cliver DO. Microorganisms in honey. Int J Food Microbiol 1996; 31: 1–26. [DOI] [PubMed] [Google Scholar]
- 36. Jull AB, Walker N, Deshpande S. Honey as a topical treatment for wounds. Cochrane Database Syst Rev 2015; 6: CD005083. [DOI] [PubMed] [Google Scholar]
- 37. Alzahrani H, Alsabehi R, Boukraa L et al. Antibacterial and antioxidant potency of floral honeys from different botanical and geographical origins. Molecules 2012; 17: 10540–10549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. McDonnell G, Russell D. Antiseptics and disinfectants: activity, action and resistance. Clin Microbiol Rev 1999; 12: 147–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Muller G, Kramer A. Biocompatibility index of antiseptic agents by parallel assessment of antimicrobial activity and cellular cytotoxicity. J Antimicrob Chemother 2008; 61: 1281–1287. [DOI] [PubMed] [Google Scholar]
- 40. Butcher M. PHMB: an effective antimicrobial in wound bioburden management. Br J Nurs 2012; 21: S16–S21. [DOI] [PubMed] [Google Scholar]
- 41. Wiegand C, Bauer M, Hipler U et al. Poly(ethyleneimines) in dermal applications: biocompatibility and antimicrobial effects. Int J Pharm 2013; 456: 165–174. [DOI] [PubMed] [Google Scholar]
- 42. Chadeau E, Dumas E, Adt I et al. Assessment of the mode of action of polyhexamethylene biguanide against Listeria innocua by Fourier transformed infrared spectroscopy and fluorescence anisotropy analysis. Can J Microbiol 2012; 58: 1353–1361. [DOI] [PubMed] [Google Scholar]
- 43. Behrens-Baumann W, Seibold M, Hofmuller W et al. Benefit of polyhexamethylene biguanide in fusarium keratitis. Ophthalmic Res 2012; 48: 171–176. [DOI] [PubMed] [Google Scholar]
- 44. Sibbald R, Coutts P, Woo K. Reduction of bacterial burden and pain in chronic wounds using a new polyhexamethylene biguanide antimicrobial foam dressing: clinical trial results. Adv Skin Wound Care 2011; 24: 78–84. [DOI] [PubMed] [Google Scholar]
- 45. Rohrer N, Widmer A, Waltimo T et al. Antimicrobial efficacy of 3 oral antiseptics containing octenidine, polyhexamethylene biguanide, or citroxx: can chlorhexidine be replaced? Infect Control Hosp Epidemiol 2010; 31: 733–739. [DOI] [PubMed] [Google Scholar]
- 46. Kumar R, Lloyd D. Recent advances in the treatment of Acanthamoeba keratitis. Clin Infect Dis 2002; 35: 434–441. [DOI] [PubMed] [Google Scholar]
- 47. Panda A, Ahuja R, Biswas N et al. Role of 0.02% Polyhexamethylene biguanide and 1% povidone iodine in experimental Aspergillus keratitis. Cornea 2003; 22: 138–141. [DOI] [PubMed] [Google Scholar]
- 48. Messick C, Pendland S, Moshirfar M et al. In-vitro activity of polyhexamethylene biguanide (PHMB) against fungal isolates associated with infective keratitis. J Antimicrob Chemother 1999; 44: 297–298. [DOI] [PubMed] [Google Scholar]
- 49. Fiscella R, Moshifar M, Messick C et al. Polyhexamethylene biguanide (PHMB) in the treatment of experimental Fusarium keratomycosis. Cornea 1997; 16: 447–449. [PubMed] [Google Scholar]
- 50. Xu Y, He Y, Zhou L et al. Effects of contact lens solution disinfectants against filamentous fungi. Optom Vis Sci 2014; 91: 1440–1445. [DOI] [PubMed] [Google Scholar]
- 51. Kilvington S, Lam A, Nikolic M et al. Resistance and growth of Fusarium species in contact lens disinfectant solutions. Optom Vis Sci 2013; 90: 430–438. [DOI] [PubMed] [Google Scholar]
- 52. Rebong R, Santaella R, Goldhagen B et al. Polyhexamethylene biguanide and calcineurin inhibitors as novel antifungal treatments for Aspergillus keratitis. Invest Opthalmol Vis Sci 2011; 52: 7309. [DOI] [PubMed] [Google Scholar]
- 53. Banovic F, Bozic F, Lemo N. In vitro comparison of the effectiveness of polihexanide and chlorhexidine against canine isolates of Staphylococcus pseudintermedius, Pseudomonas aeruginosa and Malassezia pachydermatis. Vet Dermatol 2013; 24: 409–413. [DOI] [PubMed] [Google Scholar]
- 54. Clinical and Laboratory Standards Institute Reference method for broth dilution antifungal susceptibility testing of filamentous fungi, Approved Standard Second Ed CLSI document M38-A2. Wayne, PA: CLSI; 2008. [Google Scholar]
- 55. Larone D. Medically Important fungi: a guide to identification, 5th edition, Washington, DC: ASM Press, 2011. [Google Scholar]
- 56. Sutton D, Fothergilol A, Rinaldi M. Guide to clinically significant fungi. Philadelphia: Williams & Wilkins, 1998. [Google Scholar]
- 57. De Hoog G. Atlas of clinical fungi, Second edition The Netherlands: Centraalbureau voor Schimmelcultures, 2000. [Google Scholar]
- 58. Osato M, Reddy S, Graham D. Osmotic effect of honey on growth and viability of Helicobacter pylori. Dig Dis Sci 1999; 44: 462–464. [DOI] [PubMed] [Google Scholar]
- 59. Du Toit D, Page B. An in vitro evaluation of the cell toxicity of honey and silver dressings. J Wound Care 2009; 18: 383–389. [DOI] [PubMed] [Google Scholar]
- 60. Kozol R. Effects of sodium hypochlorite (Dakin's solution) on cells of the wound module. Arch Surg 1988; 123: 420. [DOI] [PubMed] [Google Scholar]
- 61. Cooper M, Laxer J, Hansbrough J. The cytotoxic effects of commonly used topical antimicrobial agents on human fibroblasts and keratinocytes. J Trauma 1991; 31: 775–784. [DOI] [PubMed] [Google Scholar]
- 62. Cornwell P, Arnold-Long M, Barss S et al. The use of Dakin's solution in chronic wounds. J Wound, Ostomy Continence Nurs 2010; 37: 94–104. [DOI] [PubMed] [Google Scholar]
- 63. Ramage G, Robertson S, Williams C. Strength in numbers: antifungal strategies against fungal biofilms. Int J Antimicrob Agents 2014; 43: 114–120. [DOI] [PubMed] [Google Scholar]
- 64. Ansari M, Al-Ghamdi A, Usmani S et al. Effect of jujube honey on Candida albicans growth and biofilm formation. Arch Med Res 2013; 44: 352–360. [DOI] [PubMed] [Google Scholar]