Abstract
BACKGROUND
Exercise training is associated with elevations in mood in patients with various chronic illnesses and disabilities. However, little is known regarding the effect of exercise training on acute and chronic mood changes in those with traumatic brain injury (TBI).
OBJECTIVE
The purpose of this study was to examine the time course of mood alterations in response to a vigorous, 12-week aerobic exercise training regimen in ambulatory individuals with chronic TBI (>6 months post-injury).
METHODS
Changes in mood were measured before and after acute bouts of aerobic exercise using the Profile of Mood States Short Form and across a 12-week regimen of vigorous aerobic exercise training.
RESULTS
Ten subjects with non-penetrating TBI (6.6±6.8 years after injury) completed the training regimen. A significant improvement in overall mood was found across the course of the study (p=0.04), with moderate to large effect sizes observed for acute mood improvements following individual bouts of exercise.
CONCLUSIONS
Specific improvements in long term mood state and acute mood responses during individual exercise sessions were observed in these individuals with TBI. The largest improvement in overall mood was observed at 12 weeks of exercise training, with improvements emerging as early as four weeks into the training regimen.
Keywords: affect, brain injuries, rehabilitation, exercise therapy
INTRODUCTION
The annual incidence for traumatic brain injury (TBI) in the USA is estimated at 1.7 million people1,2. Approximately one in 100 US civilians are thought to have long-term or permanent disability resulting from TBI3,4 and the prevalence increases dramatically when military injuries are included5. Survivors of TBI experience emotional, physical, and cognitive distress in addition to physical and social barriers, which make them prone to secondary and chronic conditions6. The prevalence of depression and anxiety disorders is approximately 25% higher in those who have had a TBI than in the general population7. Symptoms such as fatigue, anxiety, and depression8 are exacerbated in patients with TBI as are behaviors like hostility, and/or confusion9. These overall mood characteristics have been associated with low physical activity participation in other clinical and non-clinical populations10, suggesting participation in exercise as a possible modulator of improvements in mood disturbance. Indeed, evidence in individuals with TBI suggests that aerobic exercise training may be associated with decreases in depressive symptoms11–13 and mood disturbances14.
Previous studies have also suggested that for individuals with TBI, participation in aerobic exercise training may result in significant increases in cardiorespiratory fitness15–20, reduced fatigue16,19, and improved cognition21,22. Individual (acute) exercise bouts elicit both physiological23 and emotional responses24 but these effects appear to be transient. Further evidence suggests that routine participation in exercise may result in emotional effects that accrue in a cumulative manner25, however this adaptation has not been substantiated in those who have TBI. A recent investigation demonstrated that a single session of exercise had a positive effect on the affective responses of individuals with TBI26, but the cumulative effects were not investigated.
In this study, changes in mood to an acute exercise bout and overall mood state were characterized over a 12-week regimen of vigorous aerobic exercise training in a small group of individuals with non-penetrating TBI. Exercise-induced increases in cardiorespiratory capacity and improvements in cognitive function were recently reported in this group of subjects16,21. We hypothesized that a significant improvement in overall mood state would also be observed over the training regimen.
METHODS
Approval was obtained from the respective institutional review boards of the participating institutions including National Institutes of Health, NIH 11-CC-0088; Uniformed Services University of the Health Sciences, USUHS G192HI-H; and George Mason University, GMU 7286. This study was registered on clinicaltrials.gov (NCT01294332) before enrollment of subjects and written consent was obtained from all subjects prior to participation in this study.
Participants
Subjects were ambulatory adults with chronic non-penetrating TBI, recruited in the Washington, DC metropolitan area. Information regarding exercise training-induced improvements in cardiorespiratory capacity16 and cognitive function21 was previously reported in these subjects that participated in this research project.
Subjects were sedentary (not currently participating in an aerobic exercise-training regimen), non-smokers and able to walk unassisted on a motorized treadmill. Subjects with a history of cardiovascular, pulmonary, neurological, or metabolic conditions or a medication regimen that would limit the ability to participate in aerobic exercise, achieve an exercise training adaptation, or make participation unsafe were excluded. Female participants were not pregnant at the time of enrollment and regular monthly pregnancy screenings were performed throughout the study. Meeting the inclusion criteria and the absence of an exclusion criterion were determined by physician evaluation prior to enrollment. All subjects had a diagnosis of TBI, which was confirmed during a comprehensive history and physical with a board certified physical medicine and rehabilitation physician with extensive clinical and research experience in TBI. Injury severity was classified by the enrolling physician according to the Department of Veterans Affairs/Department of Defense Clinical Practice Guidelines27,28. Participants were withdrawn from the study if there was emergence of one of the exclusion criteria or failure to participate in at least 80% (24 of the 36) of the aerobic exercise-training sessions.
Study design
The mood states of enrolled subjects were measured by self-report using the Profile of Mood States-Short Form (POMS-SF) questionnaire29,30. The POMS-SF was administered before and after the first exercise training session of week 1 (baseline), week 4, week 8, and week 12 (conclusion of the intervention). Therefore, a total of 8 measures of the POMS-SF were obtained for each subject. The exercise sessions were supervised and performed three times per week for 30 minutes each session, at an intensity ranging between 70% and 80% of the subjects’ heart rate reserve31. Sessions were conducted one-on-one by research staff as sessions were performed according to the subjects’ schedule. The target heart rate range for each subject was determined from the maximum treadmill exercise test to volitional exhaustion, performed at enrollment. An additional 5 to 10 minutes were included for warm up before and cool down after each training session. The warm up and cool down periods did not count towards the 30 minutes of aerobic exercise training periods. During the training sessions, heart rate was monitored continuously and maintained within the target range by adjusting the speed and/or grade of the treadmill.
Measures
The POMS-SF consists of thirty-seven adjectives describing mood rated on a 5-point Likert scale, ranging from “not at all” to “extremely” for the presence of each. Six mood subscales are assessed, with 5 subscales reflecting negative affect [Anger-Hostility (A-H), Tension-Anxiety (T-A), Fatigue-Inertia (F-I), Depression-Dejection (D-D), and Confusion-Bewilderment (C-B)], and one subscale reflecting positive affect (Vigor-Activity (V-A)). Adjectives for the negative mood subscale include “annoyed”, “tense”, “worn out”, “miserable”, and “uncertain”, while the positive mood subscale uses adjectives such as “energetic” and “lively”. The summary score for POMS-SF is the total mood disturbance (TMD) score, which is calculated by adding together the five negative affect subscales (A-H, T-A, F-I, D-D, C-B) and then subtracting the one positive affect subscale (V-A)32. Therefore, higher scores on TMD indicate a more negative overall mood state. Mood changes across the 12 week training period were assessed using the form administered before the exercise training sessions, while acute mood responses were measured as the difference between the scores obtained before and after an individual bout of exercise. Negative changes suggest improvement of negative affect scales, while positive changes reflect improvement in the positive affect (V-A) scale.
The POMS-SF has strong psychometric properties with correlations between the POMS-SF and the full POMS scale on TMD and subscale scores exceeding 0.95.29 The subscales have strong internal consistency ranging from 0.63 (C-B) to 0.96 (D-D).29 This scale was selected for use in this population for several reasons. First, the POMS is the most frequently used questionnaire to assess mood changes in response to exercise and the POMS-SF has the same psychometric properties as the full scale, but is shorter in length to reduce participant burden. In addition, this scale is sensitive to acute changes in mood, allowing evaluation of changes across a single bout of exercise. Further, the POMS-SF is a questionnaire without confusing instructions or complicated questions. The subject simply responds to a list of adjectives pertinent to the subscale, by rating the degree to which each adjective applies to them..
Statistical analysis
Data were analyzed using SPSS, version 21.0 (IBM Corp, Armonk, New York). A 4×2 repeated measures two-way ANOVA was used to assess POMS-SF scores at week 1 (baseline), week 4, week 8 and week 12 (conclusion of the intervention) as the first effect. The second effect was mood assessed as a function of time before and after the conclusion of acute bouts of exercise. Post-hoc analyses of pairwise differences were performed for statistically significant main and interaction effects. Statistical significance was set at p<0.05 and data are reported as mean ± standard deviation. Effect sizes are reported for all post-hoc tests using Cohen’s d and all figures report 95% confidence intervals.
RESULTS
Twelve individuals were enrolled in the study however two subjects were withdrawn; one was withdrawn at week 10 due to a confirmed positive pregnancy, and another was withdrawn at week 2 due to non-compliance with the exercise-training sessions. Therefore, 10 subjects successfully completed the program and were included in data analyses (participant characteristics are presented in table 1). Subjects had an average of 16.8 ± 1.9 years of education, with 7 of the 10 subjects engaged in full/part-time employment or full-time school at study enrollment. Nine of the 10 subjects either drove themselves or used public transportation to attend the training sessions, while one subject was provided transportation (i.e. taxi service) to/from the training site. The maximum number of exercise training sessions was 36 and subjects participated in 33.3 ± 1.5 of the sessions on average. Training heart rate was sustained at an average of 155.1 ± 6.6 beats per minute, representing a range of 75.6% to 80.2% of the overall heart rate reserve.
Table 1.
Participant characteristics (n=10)
| Age (years) | 32.9 (6.5) |
| Female, n (%) | 6 (60%) |
| Body Mass Index (kg/m2) | 23.8 (4.4) |
| Time Since Injury (years) | 6.6 (6.8) |
| Level of Education at enrollment (years) | 16.8 (1.9) |
| Employment Status (full/part time or full-time studies), n (%) | 7 (70%) |
| TBI Severity* | |
| Mild, n (%) | 5 (50%) |
| Moderate, n (%) | 4 (40%) |
| Severe, n (%) | 1 (10%) |
| Cause of Injury | |
| Falls, n (%) | 7 (70%) |
| Motor Vehicle Accidents, n (%) | 2 (20%) |
| Pedestrian-Motor Vehicle Accident, n (%) | 1 (10%) |
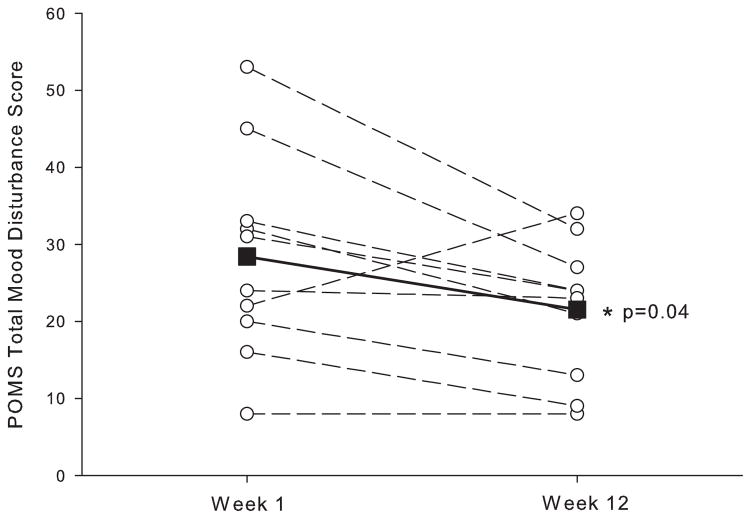
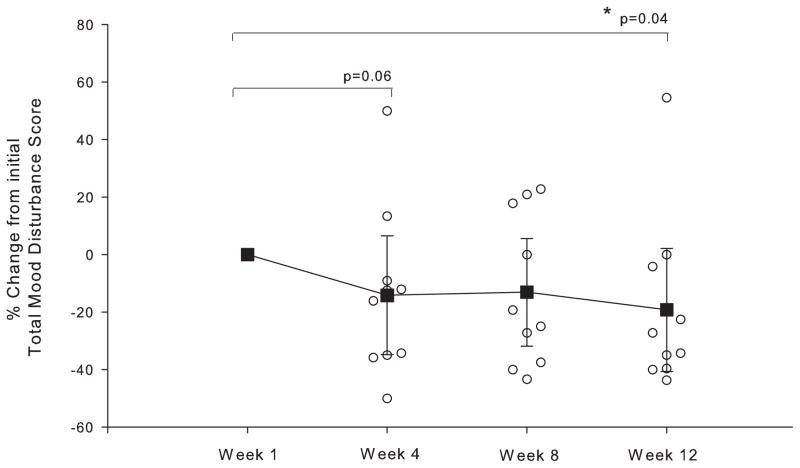
Figure 1 depicts the TMD scores measured before exercise at Week 1 (baseline) and Week 12 of the exercise regimen. Significantly lower TMD scores (−6.9 ± 9.3, p=0.04, d=0.74) were observed at Week 12 compared to Week 1 of exercise training, with 8 of the 10 subjects reporting less mood disturbance at Week 12. Improvement in the TMD scores primarily reflects the significant reduction in the Fatigue-Inertia (F-I) subscale (−2.5 ± 2.6, p=0.01, d=0.98) and to a lesser degree the reduced Anger-Hostility (A-H) subscales (−1.9 ± 3.1, p=0.09, d=0.61). TMD scores before exercise trended downward throughout the 12-week exercise training program with a 14% reduction observed at Week 4 (p=0.06, d=0.68), reaching 19% by Week 12 (p=0.04, d=0.74) (figure 2).
Figure 1.
Subjects overall mood from the Total Mood Disturbance score of the Profile of Mood States – Short Form measured before exercise at week-1 and week-12 of the aerobic exercise-training program. Open circles represent individual participant data and the closed squares represent the group mean at week-1 and week-12. * indicate significant (p<0.05) difference between week-1 and week-12.
Figure 2.
The percent change from initial Total Mood Disturbance score (week 1) for the subsequent weeks of assessment. TMD scores are measured before exercise. Open circles represent individual participant data and closed squares represent the group mean at each time point. Error bars depict 95% confidence interval. * indicate significant (p<0.05) difference from week-1.
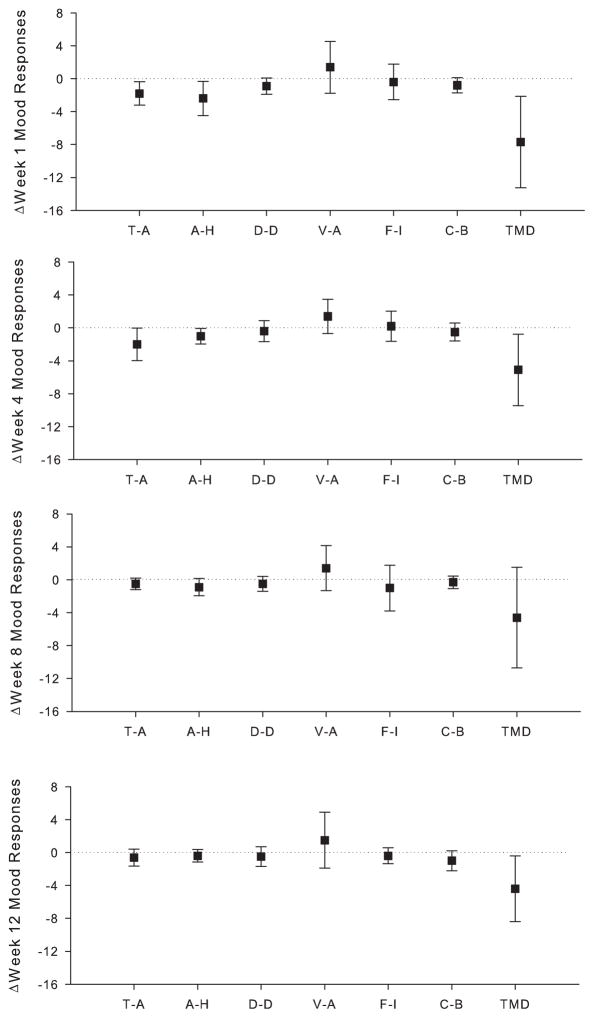
Table 2 provides TMD scores reported by each subject, before and after exercise, for each time point. There was a large effect for acute changes in mood in response to a single exercise bout, with lower TMD scores observed at Week 1 (d=0.99), Week 4 (d=0.84) and Week 12 (d=0.79) (figure 3). A moderate to large effect was also observed for acute reductions at Week 1 for T-A (d=0.90), A-H (d=0.82), D-D (d=0.66) and C-B (d=0.61), at Week 4 for T-A (d=0.73) and A-H (d=0.75), Week 8 for T-A (d=0.51) and A-H (d=0.62), and Week 12 for C-B (d=0.59) (figure 3).
Table 2.
Summary of the Total Mood Disturbance scores obtained before and after the first exercise session of Week 1, 4, 8 and 12
| Subject | Gender | Week 1
|
Week 4
|
Week 8
|
Week 12
|
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Before | After | Δ | Before | After | Δ | Before | After | Δ | Before | After | Δ | ||
| 1 | Male | 32 | 14 | −18 | 21 | 19 | −2 | 24 | 26 | 2 | 21 | 24 | 3 |
| 2 | Female | 20 | 14 | −6 | 13 | 8 | −5 | 12 | 5 | −7 | 13 | 7 | −6 |
| 3 | Male | 31 | 24 | −7 | 26 | 23 | −3 | 25 | 31 | 6 | 24 | 22 | −2 |
| 4 | Male | 8 | 6 | −2 | 12 | 10 | −2 | 8 | 8 | 0 | 8 | 4 | −4 |
| 5 | Female | 16 | 7 | −9 | 8 | 4 | −4 | 10 | 9 | −1 | 9 | 4 | −5 |
| 6 | Female | 53 | 34 | −19 | 34 | 27 | −7 | 30 | 29 | −1 | 32 | 25 | −7 |
| 7 | Female | 45 | 35 | −10 | 51 | 32 | −19 | 53 | 30 | −23 | 27 | 26 | −1 |
| 8 | Male | 33 | 23 | −10 | 29 | 23 | −6 | 24 | 24 | 0 | 24 | 21 | −3 |
| 9 | Female | 24 | 32 | 8 | 21 | 26 | 5 | 29 | 16 | −13 | 23 | 22 | −1 |
| 10 | Female | 22 | 18 | −4 | 20 | 12 | −8 | 27 | 18 | −9 | 34 | 16 | −18 |
Figure 3.
Acute mood responses to a bout of exercise performed at week 1, week 4, week 8, and week 12 of the intervention as measured by the subscales of the Profile of Mood States – Short Form. Data are shown as group mean with error bars depicting 95% confidence interval. Dotted lines indicate no change in mood in response to the bout of exercise. Negative values demonstrate improvements in negative mood subscales (T–A, A-H, D-D, F-I, and C-B), while positive values indicate improvements in positive subscale (V-A). Abbreviations: T-A, tension-anxiety; A-H, anger-hostility; D-D, depression-dejection; V-A, vigor-activity; F-I, fatigue-inertia; C-B, confusion-bewilderment; TMD, total mood disturbance.
DISCUSSION
The current study demonstrated improvements in overall mood as well as acute mood responses in a small group of people who have non-penetrating TBI, following a supervised aerobic exercise-training program. These observations were in agreement with previous studies in a variety of patient subsets 24,33–38. Extending these previous observations, the reduction in mood disturbance emerged as early as four weeks into the exercise-training regimen and was sustained over the 12-week study duration. In addition, improvements in acute overall mood responses to individual bouts of exercise were detected after a single exercise bout in week-1 of training, as well as at weeks-4 and -12. The acute mood responses to exercise over the course of an exercise intervention in individuals with TBI have not previously been investigated. Specific acute reductions in tension/anxiety and anger/hostility were also observed in the first 8 weeks of exercise training. While the durability of these effects remains unstudied, a potential modulating influence of exercise participation on overall mood and acute mood responses was identified.
Several investigations have examined the relationship between mood and exercise participation, all demonstrating positive effects in individuals with TBI11,12,14,26,39,40. However, depression was the focus in the majority of these studies11,12,39,40 while the current investigation used a broader conceptualization of mood that incorporates both positive and negative dimensions of mood. Furthermore, acute mood responses to exertional perturbation have been reported by only one other study26, while mood reactivity changes over the course of an exercise-training program remain unexamined. Thus, the current study expands upon prior work by measuring a wider spectrum of mood, with exercise performed at a greater intensity than previously used, and assessment of acute mood responses to individual exercise sessions throughout the course of an exercise-training intervention.
The current study suggests that sustained exercise participation may be an effective strategy for improving the overall mood state for those with TBI, while single bouts of exercise might possibly be utilized for improving mood in acute scenarios. For example, it has been documented that anxiety among those with TBI can be as high as 70%41, with many subjects experiencing increased levels of anger appropriate for therapeutic intervention42. The current study demonstrated that anger/hostility and tension/anxiety levels were diminished immediately following an acute exercise session in agreement with the finding of a previous investigation 26. Thus, encouraging those with TBI to exercise when experiencing feelings of anger or anxiety may be an effective management strategy. The observation that the acute response to exercise was blunted as overall mood improved, further elucidated the effectiveness of exercise for reducing negative mood states. This suggests that exercise participation may be most important when negative mood reactivity is more severe, and implicated the importance of exercise participation for long term maintenance of low anger and anxiety in patients with TBI.
The POMS is a frequently used measure of mood domains and has been utilized to measure mood changes following exercise in the general population24,43,44, and in those with TBI14,45. In the current study, the shortened version of POMS was used as it has the same psychometric properties as the original29, with fewer adjectives that minimizes subject burden and has successfully been used in other investigations46–50. Participants in this study had baseline POMS-SF scores that were comparable with healthy control values51,52 (with the exception of the V-A subscale51), suggesting a lack of mood disturbance in this group of individuals with TBI. However despite these participants scoring within a relatively normal range, scores for overall mood state still improved following exercise training. In previous work it has been demonstrated that individuals with higher levels of mood disturbance will have greater benefits in response to an exercise intervention53. Therefore, results of the current study might have been even more pronounced had these subjects demonstrated abnormal mood patterns at baseline. Nonetheless, the improvements in overall mood were observed without baseline mood abnormalities and underscore the proof of principle regarding the effectiveness of exercise participation as a possible intervention strategy or adjunct therapy for mood disturbance.
Several mechanisms could explain the improvements in mood seen in this group of subjects. These mechanisms fall within two main categories: psychological and physiological54. Although the mechanisms are discussed as separate entities, it is likely that a combination of various modulators might be responsible for the mood changes. Psychological contributors may include increased self-esteem55, self-efficacy56, self-image57, social interaction with research staff58 and distraction from worries, anxiety, and depressing thoughts59. Potential physiological mediators include increased cerebral blood flow60, neurogenesis61, vascular endothelial growth factor62, brain-derived neurotrophic factor63, serotonin61, core body temperature64, analgesia65, and attenuation of stress responsiveness66.
Limitations of the current study include a small sample size that may not be representative of the TBI population in general. Findings from this study should be taken as a proof of principle that aerobic exercise training may positively affect overall mood state in select individuals with TBI. Mood changes were also assessed utilizing a shortened version of the POMS, which has not been specifically validated in individuals with TBI. Therefore, more research is needed for generalization of these results to the TBI population at large, and validation of the POMS-SF for mood changes in persons with TBI. The present investigation also did not include a control group or delayed entry condition, making it difficult to account for natural fluctuation in mood over time. However, the observed improvement in mood both acutely and across the 12-week training program suggests that mood changes were likely related to exercise participation, rather than random mood fluctuations. Closer examination of our results revealed an improvement in mood for female subjects only following exercise at Week 8 (Table 2), suggesting a gender difference for mood changes with chronic exercise. However our sample size was small, and sub-group analyses could not be conducted with sufficient power. Future research should consider gender as a possible mediating variable in the mood reactions to exercise. The research staff that supervised the participants’ exercise sessions also administered the mood assessments. Therefore, it is possible that participants responded more positively to their mood state in an effort to “please” the research staff. Separate staff conducting the exercise bouts and mood assessments may be necessary for future studies. In addition, our sample did not demonstrate the same level of negative mood that is normally seen in individuals with TBI, thereby limiting the generalizability of these findings. The high level of participation required for the exercise training sessions (i.e, 3 visits per week for 12 weeks to a specific location) likely limited our subjects to those with high levels of functioning that were independent in community living and instrumental activities of daily living. Participants were also included based on their ability to walk unassisted on the treadmill and perform vigorous exercise. Therefore, findings of this study may not be generalizable to those with significant functional deficits, gait impairments or those unable to perform aerobic exercise at a vigorous intensity.
CONCLUSION
The current investigation demonstrated that, in these individuals with TBI, overall mood state was improved following vigorous aerobic exercise training, with improvements beginning to emerge as early as four weeks into the intervention. Exercise is economical, generally accessible and safe for most individuals. These results suggest that aerobic exercise training could be useful for improving mood in people who have TBI, in addition to the previously identified benefits of improved cardiorespiratory function, cognition, exertional tolerance, fatigue, and health related quality of life. Further work is necessary before the findings suggested in this study can be generalized to the TBI population at large. An understanding of the mechanisms through which the improvements in mood state occur may substantiate a wider application of findings including individuals who have primary mood disturbances, as well as mood disorders secondary to TBI.
Acknowledgments
Support for this work included funding from Department of Defense in the Center for Neuroscience and Regenerative Medicine (grant no. G192HI-H) and the Intramural Research Program of the National Institutes of Health, Clinical Center (Rehabilitation Medicine Department intramural funds).
Footnotes
Conflicts of Interest Statement:
The authors report no conflicts of interest.
References
- 1.Faul M, Xu L, Wald M, Coronado VG. Traumatic Brain Injury in the United States: Emergency Department Visits, Hospitalizations and Deaths 2002–2006. Atlanta, GA: National Center for Injury Prevention and Control, Centers for Disease Control and Prevention; 2010. p. 74. [Google Scholar]
- 2.Ma VY, Chan L, Carruthers KJ. Incidence, prevalence, costs, and impact on disability of common conditions requiring rehabilitation in the United States: stroke, spinal cord injury, traumatic brain injury, multiple sclerosis, osteoarthritis, rheumatoid arthritis, limb loss, and back pain. Arch Phys Med Rehabil. 2014;95(5):986–995. doi: 10.1016/j.apmr.2013.10.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zaloshnja E, Miller T, Langlois JA, Selassie AW. Prevalence of long-term disability from traumatic brain injury in the civilian population of the United States, 2005. J Head Trauma Rehabil. 2008;23(6):394–400. doi: 10.1097/01.HTR.0000341435.52004.ac. [DOI] [PubMed] [Google Scholar]
- 4.Corrigan JD, Selassie AW, Orman JA. The epidemiology of traumatic brain injury. J Head Trauma Rehabil. 2010;25(2):72–80. doi: 10.1097/HTR.0b013e3181ccc8b4. [DOI] [PubMed] [Google Scholar]
- 5.Helmick KM, Spells CA, Malik SZ, Davies CA, Marion DW, Hinds SR. Traumatic brain injury in the US military: epidemiology and key clinical and research programs. Brain Imaging Behav. 2015;9(3):358–366. doi: 10.1007/s11682-015-9399-z. [DOI] [PubMed] [Google Scholar]
- 6.Driver S, Ede A, Dodd Z, Stevens L, Warren AM. What barriers to physical activity do individuals with a recent brain injury face? Disabil Health J. 2012;5(2):117–125. doi: 10.1016/j.dhjo.2011.11.002. [DOI] [PubMed] [Google Scholar]
- 7.Seel RT, Rosenthal M, Hammond FM, Corrigan JD, Black K. Depression after traumatic brain injury: a National Institute on Disability and Rehabilitation Research Model Systems multicenter investigation. Arch Phys Med Rehabil. 2003;84(2):117–184. doi: 10.1053/apmr.2003.50106. [DOI] [PubMed] [Google Scholar]
- 8.Archer t. Influence of Physical Exercise on Traumatic Brain Injury Deficits: Scaffolding Effect. Neurotox Res. 2012;21(4):418–434. doi: 10.1007/s12640-011-9297-0. [DOI] [PubMed] [Google Scholar]
- 9.Hassmen P, Koivula N, Uutela A. Physical exercise and psychological well-being: a population study in Finland. Prev Med. 2000;30(1):17–25. doi: 10.1006/pmed.1999.0597. [DOI] [PubMed] [Google Scholar]
- 10.Berger BG, Motl RW. Exercise and mood: A selective review and synthesis of research employing the profile of mood states. J Appl Sport Psychol. 2000;12(1):69–92. doi: 10.1080/10413200008404214. [DOI] [Google Scholar]
- 11.Bellon K, Kolakowsky-Hayner S, Wright J, et al. A home-based walking study to ameliorate perceived stress and depressive symptoms in people with a traumatic brain injury. Brain Inj. 2014;29(3):1–7. doi: 10.3109/02699052.2014.974670. [DOI] [PubMed] [Google Scholar]
- 12.Schwandt M, Harris JE, Thomas S, Keightley M, Snaiderman A, Colantonio A. Feasibility and effect of aerobic exercise for lowering depressive symptoms among individuals with traumatic brain injury: a pilot study. J Head Trauma Rehabil. 2012;27(2):99–103. doi: 10.1097/HTR.0b013e31820e6858. [DOI] [PubMed] [Google Scholar]
- 13.Wise EK, Hoffman JM, Powell JM, Bombardier CH, Bell KR. Benefits of exercise maintenance after traumatic brain injury. Arch Phys Med Rehabil. 2012;93(8):1319–1323. doi: 10.1016/j.apmr.2012.05.009. [DOI] [PubMed] [Google Scholar]
- 14.Driver S, Ede A. Impact of physical activity on mood after TBI. Brain Inj. 2009;23(3):203–212. doi: 10.1080/02699050802695574. [DOI] [PubMed] [Google Scholar]
- 15.Bhambhani Y, Rowland G, Farag M. Effects of circuit training on body composition and peak cardiorespiratory responses in patients with moderate to severe traumatic brain injury. Arch Phys Med Rehabil. 2005;86(2):268–276. doi: 10.1016/j.apmr.2004.04.022. [DOI] [PubMed] [Google Scholar]
- 16.Chin LMK, Chan L, Woolstenhulme JG, Christensen EJ, Shenouda CN, Keyser RE. Improved Cardiorespiratory Fitness With Aerobic Exercise Training in Individuals With Traumatic Brain Injury. J Head Trauma Rehabil. 2015;30(6):382–390. doi: 10.1097/HTR.0000000000000062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Driver S, O’Connor J, Lox C, Rees K. Evaluation of an aquatics programme on fitness parameters of individuals with a brain injury. Brain Inj. 2004;18(9):847–859. doi: 10.1080/02699050410001671856. [DOI] [PubMed] [Google Scholar]
- 18.Hunter M, Tomberlin J, Kirkikis C, Kuna ST. Progressive exercise testing in closed head-injured subjects: comparison of exercise apparatus in assessment of a physical conditioning program. Phys Ther. 1990;70(6):363–371. doi: 10.1093/ptj/70.6.363. [DOI] [PubMed] [Google Scholar]
- 19.Jankowski LW, Sullivan SJ. Aerobic and neuromuscular training: effect on the capacity, efficiency, and fatigability of patients with traumatic brain injuries. Arch Phys Med Rehabil. 1990;71(7):500–504. [PubMed] [Google Scholar]
- 20.Wolman RL, Cornall C, Fulcher K, Greenwood R. Aerobic training in brain-injured patients. Clin Rehabil. 1994;8(3):253–257. doi: 10.1177/026921559400800311. [DOI] [Google Scholar]
- 21.Chin LMK, Keyser RE, Dsurney J, Chan L. Improved Cognitive Performance Following Aerobic Exercise Training in People With Traumatic Brain Injury. Arch Phys Med Rehabil. 2015;96(4):754–759. doi: 10.1016/j.apmr.2014.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Grealy MA, Johnson DA, Rushton SK. Improving cognitive function after brain injury: the use of exercise and virtual reality. Arch Phys Med Rehabil. 1999;80(6):661–667. doi: 10.1016/s0003-9993(99)90169-7. [DOI] [PubMed] [Google Scholar]
- 23.Thompson PD, Crouse SF, Goodpaster B, Kelley D, Moyna N, Pescatello L. The acute versus the chronic response to exercise. Med Sci Sports Exerc. 2001;33(6 Suppl):S438–S445. doi: 10.1097/00005768-200106001-00012. discussion S452–S453. [DOI] [PubMed] [Google Scholar]
- 24.Yeung RR. The acute effects of exercise on mood state. J Psychosom Res. 1996;40(2):123–141. doi: 10.1016/0022-3999(95)00554-4. [DOI] [PubMed] [Google Scholar]
- 25.Haskell WL. Health consequences of physical activity: understanding and challenges regarding dose-response. Med Sci Sports Exerc. 1994;26(6):649–660. doi: 10.1249/00005768-199406000-00001. [DOI] [PubMed] [Google Scholar]
- 26.Rzezak P, Caxa L, Santolia P, et al. Affective responses after different intensities of exercise in patients with traumatic brain injury. Front Psychol. 2015;6:839. doi: 10.3389/fpsyg.2015.00839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kay T, Harrington DE, Adams A, Andersen T, Berrol S, Cicerone K. Definition of mild traumatic brain injury. J Head Trauma Rehabil. 1993;8(3):86–87. [Google Scholar]
- 28.Management of Concussion/mTBI Working Group. VA/DoD Clinical Practice Guideline for Management of Concussion/Mild Traumatic Brain Injury. J Rehabil Res Dev. 2009;46(6):CP1–CP68. [PubMed] [Google Scholar]
- 29.Curran SL, Andrykowski MA, Studts JL. Short Form of the Profile of Mood States (POMS-SF): Psychometric information. Psychol Assess. 1995;7(1):80–83. doi: 10.1037/1040-3590.7.1.80. [DOI] [Google Scholar]
- 30.Shacham S. A shortened version of the Profile of Mood States. J Pers Assess. 1983;47(3):305–306. doi: 10.1207/s15327752jpa4703_14. [DOI] [PubMed] [Google Scholar]
- 31.Karvonen MJ, Kentala E, Mustala O. The effects of training on heart rate; a longitudinal study. Ann Med Exp Biol Fenn. 1957;35(3):307–315. [PubMed] [Google Scholar]
- 32.McNair DM, Lorr M, Droppleman LF. Manual for the Profile of Mood States. San Diego, CA: Educational and Industrial Testing Services; 1971. [Google Scholar]
- 33.Cramer SR, Nieman DC, Lee JW. The effects of moderate exercise training on psychological well-being and mood state in women. J Psychosom Res. 1991;35(4–5):437–449. doi: 10.1016/0022-3999(91)90039-q. [DOI] [PubMed] [Google Scholar]
- 34.DiLorenzo TM, Bargman EP, Stucky-Ropp R, Brassington GS, Frensch PA, LaFontaine T. Long-term effects of aerobic exercise on psychological outcomes. Prev Med. 1999;28(1):75–85. doi: 10.1006/pmed.1998.0385. [DOI] [PubMed] [Google Scholar]
- 35.Guszkowska M. Effects of exercise on anxiety, depression and mood. Psychiatr Pol. 2004;38(4):611–620. [PubMed] [Google Scholar]
- 36.Stanton JM, Arroll B. The effect of moderate exercise on mood in mildly hypertensive volunteers: a randomized controlled trial. J Psychosom Res. 1996;40(6):637–642. doi: 10.1016/0022-3999(95)00643-5. [DOI] [PubMed] [Google Scholar]
- 37.Stark R, Schöny W, Kopp M. Acute effects of a single bout of moderate exercise on psychological well-being in patients with affective disorder during hospital treatment. Neuropsychiatr Klin Diagn Ther Rehabil Organ Ges Österr Nervenärzte Psychiater. 2012;26(4):166–170. doi: 10.1007/s40211-012-0033-7. [DOI] [PubMed] [Google Scholar]
- 38.Unick JL, Michael JC, Jakicic JM. Affective responses to exercise in overweight women: Initial insight and possible influence on energy intake. Psychol Sport Exerc. 2012;13(5):528–532. doi: 10.1016/j.psychsport.2012.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hoffman JM, Bell KR, Powell JM, et al. A randomized controlled trial of exercise to improve mood after traumatic brain injury. PM R. 2010;2(10):911–919. doi: 10.1016/j.pmrj.2010.06.008. [DOI] [PubMed] [Google Scholar]
- 40.Gordon WA, Sliwinski M, Echo J, McLoughlin M, Sheerer MS, Meili TE. The benefits of exercise in individuals with traumatic brain injury: a retrospective study. J Head Trauma Rehabil. 1998;13(4):58–67. doi: 10.1097/00001199-199808000-00006. [DOI] [PubMed] [Google Scholar]
- 41.Rao V, Lyketsos CG. Psychiatric aspects of traumatic brain injury. Psychiatr Clin North Am. 2002;25(1):43–69. doi: 10.1016/s0193-953x(03)00052-2. [DOI] [PubMed] [Google Scholar]
- 42.Bailie JM, Cole WR, Ivins B, et al. The experience, expression, and control of anger following traumatic brain injury in a military sample. J Head Trauma Rehabil. 2015;30(1):12–20. doi: 10.1097/HTR.0000000000000024. [DOI] [PubMed] [Google Scholar]
- 43.Leunes A, Burger J. Profile of Mood States Research in Sport and Exercise Psychology: Past, Present, and Future. J Appl Sport Psychol. 2000;12(1):5–15. doi: 10.1080/10413200008404210. [DOI] [Google Scholar]
- 44.Szabo A. Acute Psychological Benefits of Exercise Performed at Self-Selected Workloads: Implications for Theory and Practice. J Sports Sci Med. 2003;2(3):77–87. [PMC free article] [PubMed] [Google Scholar]
- 45.Smith RB, Tiberi A, Marshall J. The use of cranial electrotherapy stimulation in the treatment of closed-head-injured patients. Brain Inj BI. 1994;8(4):357–361. doi: 10.3109/02699059409150986. [DOI] [PubMed] [Google Scholar]
- 46.McCabe MP, Firth L, O’Connor E. A comparison of mood and quality of life among people with progressive neurological illnesses and their caregivers. J Clin Psychol Med Settings. 2009;16(4):355–362. doi: 10.1007/s10880-009-9168-5. [DOI] [PubMed] [Google Scholar]
- 47.Schrepf A, Clevenger L, Christensen D, et al. Cortisol and inflammatory processes in ovarian cancer patients following primary treatment: relationships with depression, fatigue, and disability. Brain Behav Immun. 2013;30(Suppl):S126–S134. doi: 10.1016/j.bbi.2012.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Van Schaik P, Blake J, Pernet F, Spears I, Fencott C. Virtual Augmented Exercise Gaming for Older Adults. Cyberpsychol Behav. 2008;11(1):103–106. doi: 10.1089/cpb.2007.9925. [DOI] [PubMed] [Google Scholar]
- 49.Hay EL, Diehl M. Reactivity to Daily Stressors in Adulthood: The Importance of Stressor Type in Characterizing Risk Factors. Psychol Aging. 2010;25(1):118–131. doi: 10.1037/a0018747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lutgendorf SK, Garand L, Buckwalter KC, Reimer TT, Hong S-Y, Lubaroff DM. Life Stress, Mood Disturbance, and Elevated Interleukin-6 in Healthy Older Women. J Gerontol A Biol Sci Med Sci. 1999;54(9):M434–M439. doi: 10.1093/gerona/54.9.M434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cunningham LL, Andrykowski MA, Wilson JF, McGrath PC, Sloan DA, Kenady DE. Physical symptoms, distress, and breast cancer risk perceptions in women with benign breast problems. Health Psychol. 1998;17(4):371–375. doi: 10.1037//0278-6133.17.4.371. [DOI] [PubMed] [Google Scholar]
- 52.McGuirk M. Mood, Anxiety, Rpe, and %Mhr after a Single 15, 30 and 60 Minute Session of Vinyasa Yoga. 2012 May; http://digitalcommons.georgiasouthern.edu/etd/131.
- 53.Wegner M, Helmich I, Machado S, Nardi AE, Arias-Carrion O, Budde H. Effects of exercise on anxiety and depression disorders: review of meta-analyses and neurobiological mechanisms. CNS Neurol Disord Drug Targets. 2014;13(6):1002–1014. doi: 10.2174/1871527313666140612102841. [DOI] [PubMed] [Google Scholar]
- 54.Weinstein AA, Lydick SE, Biswabharati S. Exercise and its relationship to psychological health and well-being. In: Gomes R, Resende R, Albuquerque A, editors. Positive Human Functioning from a Multidimensional Perspective, Volume 2: Promoting Healhy Lifestyles. Hauppauge, NY: Nova Science Publishers, Inc; 2014. pp. 147–166. [Google Scholar]
- 55.Ryan MP. The antidepressant effects of physical activity: Mediating self-esteem and self-efficacy mechanisms. Psychol Health. 2008;23(3):279–307. doi: 10.1080/14768320601185502. [DOI] [PubMed] [Google Scholar]
- 56.Bodin T, Martinsen EW. Mood and self-efficacy during acute exercise in clinical depression. A randomized, controlled study. J Sport Exerc Psychol. 2004;26(4):623–633. [Google Scholar]
- 57.Monshouwer K, ten Have M, van Poppel M, Kemper H, Vollebergh W. Possible mechanisms explaining the association between physical activity and mental health: Findings From the 2001 Dutch Health Behaviour in School-Aged Children Survey. Clin Psychol Sci. 2012 Sep; doi: 10.1177/2167702612450485. [DOI] [Google Scholar]
- 58.North TC, McCullagh P, Tran ZV. Effect of exercise on depression. Exerc Sport Sci Rev. 1990;18:379–415. [PubMed] [Google Scholar]
- 59.Paluska SA, Schwenk TL. Physical activity and mental health: Current concepts. Sports Med. 2000;29(3):167–180. doi: 10.2165/00007256-200029030-00003. [DOI] [PubMed] [Google Scholar]
- 60.Ide K, Secher N. Cerebral blood flow and metabolism during exercise. Prog Neurobiol. 2000;61(4):397–414. doi: 10.1016/S0301-0082(99)00057-X. [DOI] [PubMed] [Google Scholar]
- 61.Ernst C, Olson AK, Pinel JPJ, Lam RW, Christie BR. Antidepressant effects of exercise: evidence for an adult-neurogenesis hypothesis? J Psychiatry Neurosci JPN. 2006;31(2):84–92. [PMC free article] [PubMed] [Google Scholar]
- 62.Fabel K, Fabel K, Tam B, et al. VEGF is necessary for exercise-induced adult hippocampal neurogenesis. Eur J Neurosci. 2003;18(10):2803–2812. doi: 10.1111/j.1460-9568.2003.03041.x. [DOI] [PubMed] [Google Scholar]
- 63.Cotman CW, Berchtold NC. Exercise: A behavioral intervention to enhance brain health and plasticity. Trends Neurosci. 2002;25(6):295–301. doi: 10.1016/s0166-2236(02)02143-4. [DOI] [PubMed] [Google Scholar]
- 64.Martinsen DEW. Benefits of exercise for the treatment of depression. Sports Med. 1990;9(6):380–389. doi: 10.2165/00007256-199009060-00006. [DOI] [PubMed] [Google Scholar]
- 65.Wolfe GI, Trivedi JR. Painful peripheral neuropathy and its nonsurgical treatment. Muscle Nerve. 2004;30(1):3–19. doi: 10.1002/mus.20057. [DOI] [PubMed] [Google Scholar]
- 66.Rimmele U, Seiler R, Marti B, Wirtz PH, Ehlert U, Heinrichs M. The level of physical activity affects adrenal and cardiovascular reactivity to psychosocial stress. Psychoneuroendocrinology. 2009;34(2):190–198. doi: 10.1016/j.psyneuen.2008.08.023. [DOI] [PubMed] [Google Scholar]