Abstract
A long-standing paradigm posits that hypothalamic corticotropin-releasing hormone (CRH) regulates neuroendocrine functions such as adrenal glucocorticoid release, while extra-hypothalamic CRH plays a key role in stressor-triggered behaviors. Here we report that hypothalamus-specific Crh knockout mice (Sim1CrhKO mice, created by crossing Crhflox with Sim1Cre mice) have absent Crh mRNA and peptide mainly in the paraventricular nucleus of the hypothalamus (PVH) but preserved Crh expression in other brain regions including amygdala and cerebral cortex. As expected, Sim1CrhKO mice exhibit adrenal atrophy as well as decreased basal, diurnal and stressor-stimulated plasma corticosterone secretion and basal plasma ACTH, but surprisingly, have a profound anxiolytic phenotype when evaluated using multiple stressors including open field, elevated plus maze, holeboard, light-dark box, and novel object recognition task. Restoring plasma corticosterone did not reverse the anxiolytic phenotype of Sim1CrhKO mice. Crh-Cre driver mice revealed that PVHCrh fibers project abundantly to cingulate cortex and the nucleus accumbens shell, and moderately to medial amygdala, locus coeruleus, and solitary tract, consistent with the existence of PVHCrh-dependent behavioral pathways. Although previous, nonselective attenuation of CRH production or action, genetically in mice and pharmacologically in humans, respectively, has not produced the anticipated anxiolytic effects, our data show that targeted interference specifically with hypothalamic Crh expression results in anxiolysis. Our data identify neurons that express both Sim1 and Crh as a cellular entry point into the study of CRH-mediated, anxiety-like behaviors and their therapeutic attenuation.
INTRODUCTION
The stress response consists of a complex array of neuroendocrine, physiologic and behavioral adaptive/maladaptive changes that are initiated as a means of restoring stability in the face of challenge, and may lead to anxiety and depression.1 Stress-stimulated corticotropin-releasing hormone (CRH) release is essential for coordinating the neuroendocrine and behavioral responses to stress.2–5 Stressors activate the release of CRH from medial parvocellular paraventricular nucleus (mpPVH) neurons, which triggers pituitary release of adrenocorticotropic hormone (ACTH) and resultant secretion of glucocorticoids.6, 7 Stress, and stress-associated dysfunction of CRH neuronal circuitries and the HPA axis in particular, have been implicated in the onset and maintenance of specific psychiatric disorders such as major depression and anxiety disorders.8–10
It has become increasingly feasible to explore the mechanisms underlying anxiety disorder using gene-manipulation strategies in animals.11, 12 Global Crh-deficient mice confirmed the importance of Crh in neuroendocrine,13 but not behavioral stress responses14, 15 while mice with generalized overproduction of Crh, including in brain, exhibit heightened anxiety-like behaviors.16 Crhr1-deficient mice demonstrated a robust role for Crh or a related ligand to mediate anxiety-like behaviors17, 18 whereas a role for Crhr2 in decreased anxiety behaviors has been described,19 but is less certain.12
Although CRH arising in the PVH has been assumed to play an essential role in neuroendocrine regulation of the HPA axis, its role in stress-related behaviors has not been fully studied, despite the PVH being the major brain site of CRH synthesis, with projections to many extra-hypothalamic sites involved in behavior.20–22 We therefore selectively deleted PVH Crh in mice, by generating mice with Crh flanked by LoxP sites (Crhflox mice), and breeding these to Sim1Cre driver mice to create Sim1CrhKO mice.23 Sim1 is expressed predominantly within the PVH, the lateral olfactory tract, the supraoptic and posterior hypothalamic nuclei, with limited expression in some other hypothalamic areas and amygdala.23, 24 Within the PVH, we found that Crh expression was completely abolished in Sim1CrhKO mice, but that in other areas such as amygdala, cerebral cortex and hippocampus, CRH expression was maintained, allowing us to dissect the roles of PVH Crh in HPA axis and stress behavior regulation. We found that hypothalamic Crh has a major role not only in the circuits controlling HPA axis responsiveness, but also in the regulation of anxiety behaviors. The anxiolytic behaviors caused by disruption of PVHCrh in Sim1CrhKO mice persisted after restoration of corticosterone, indicating that these behaviors were not due to the reduction in glucocorticoids in these mice. These data provide a cellular entry point into the study of Crh-mediated anxiety and its therapeutic attenuation.
MATERIALS AND METHODS
Creation of Crhflox (Crhfl/fl) mice
Crhflox mice were generated using previously published methods.25 We used homologous recombination/recombineering in embryonic stem (ES) cells to generate a modified Crh allele (Crhflox) in which exon 2, coding for Crh protein domain, is flanked by two LoxP sites (Fig. 1). Incorporation of the targeting vector into the proper genomic locus in transfected ES cells was validated using a long PCR strategy (5′ incorporation) or Southern blot analysis (3′ incorporation) (Fig. S1a, b). Incorporation-verified targeted ES cells (neo positive) were micro injected into C57BL/6 blastcysts, which were surgically implanted into pseudo-pregnant ICR foster female mice. Chimeric animals were mated with Flpe-expressing mice to remove the neo cassette in vivo. Crhflox mice were crossed with mice that expressed Cre recombinase under the control of either the EIIa promoter (B6. FVB-Tg [EIIa-Cre] C5379Lmgd/J, #003724, Jackson Laboratories), or the Sim1 promoter (B6.FVB (129×1)-Tg [sim1-cre] Lowl/J, #006451, Jackson laboratories, which we termed Sim1CrhWT) to create either germline homozygous global CrhKO (Crhdl/dl) mice or PVH-specific Sim1CrhKO mice, respectively. Excision of exon 2 of Crh was confirmed using PCR. In behavioral experiments, either wildtype (WT), heterozygous Sim1CrhWT, or homozygous Crhflox male mice were used as controls, as indicated. All mice were on a C57BL/6 background, and were between 12 and 40 weeks of age. Animals in different groups of each experiment were within 2 weeks of age.
Figure 1.
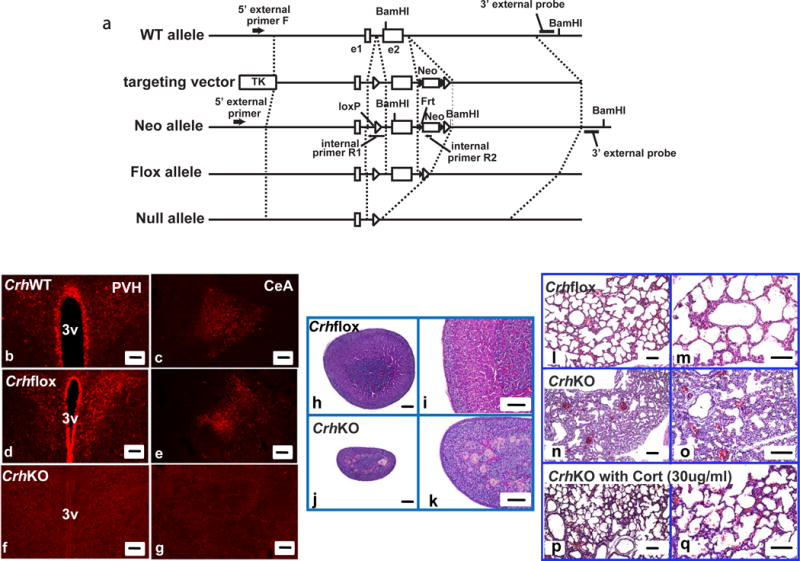
Generation of Crhflox mouse and Crh null mouse (a). Schematic diagram of mouse Crh gene targeting. Wild-type (WT) Crh allele, targeting vector, Neo allele, flox allele, null allele are drawn. Broken lines indicate corresponding locations on each allele or the vector. The targeting vector has two artificial insertions, of which the upstream insertion contains one loxP sequence between exon1 (e1) and exon2 (e2), while the downstream insertion located downstream of exon2 contains the neomycin resistant gene (Neo) flanked by two frt sequences accompanied by an adjacent downstream loxP sequence. The location of two internal BamHI sites and one artificial BamHI site are indicated. Thymidine kinase (TK) is a negative selection marker. Characterization of Crhflox (Crhfl/fl) and CrhKO (Crhdl/dl) mice (b–q). (b–g) Crh-immuno-reactive neurons and fibers were observed in the paraventricular nucleus of hypothalamus (PVH) and central amygdala (CeA) in CrhWT (b, c) and Crhflox (d, e) but not in CrhKO mice (f, g) (scale bar, 100um); (h–k) Adrenal hypoplasia in CrhKO mice. Adrenals from adult male Crhflox (h, i) and CrhKO (j, k) were haematoxylin-eosin stained after paraformaldehyde fixation. CrhKO mice revealed thinner zona fasciculata. Left panel, low magnification (scale bar, 100um); right panel, high magnification (scale bar, 100um); (l–q) Confirmation of fetal lung dysplasia in CrhKO which was rescued by in utero corticosterone replacement. Crhflox fetus revealed normal lung development (l, m) while deletion of Crh cause hypercellular lungs with thick alveolar septae and a paucity of air spaces (n, o). The deficiency of lung development was rescued by corticosterone administration (30ug/ml in drinking water) to female pregnant CrhKO mice (p, q). Left panel, low magnification (scale bar, 100um); right panel, high magnification (scale bar, 100um);
All the required plasmids (PL253, PL452, and PL451) and recombinogenic bacteria strains (EL350) were obtained from Dr. David Conner (Harvard Medical School Department of Genetics). The 129Sv BAC clone (bMQ-297H8) containing the mouse Crh gene (accession# NM_205769) in DH10B was obtained from Sanger Institute (http://www.sanger.ac.uk/). BAC DNA was extracted and transferred to recombinogenic E.coli (EL350). Primers were designed to create miniarms for PL253 to retrieve the mouse CRH gene and its flanking regions (PL253_44705FNOT/PL253_45104RXBA and PL253_57935FXBA/PL253_58229RBAM), miniarms for PL452 for the upstream loxP insertion (PL452_49460FSAL/PL452_49859RECO and PL452_49860FBAM/PL452_50259RNOT) and miniarms for PL451 for the downstream FRT-Neo-FRT-LoxP insertion (PL451_51214FSAL/PL451_51613RECO and PL451_51614FBAM/PL451_52103RNOT). To utilize endogenous XbaI sites (TCTAGA) for retrieval, the XbaI site on PL253 plasmid was destroyed using DNA polymerase I, Large (Klenow) Fragment (New England Biolabs, MA) before ligation, which was named‘PL253delXba’. Two PCR-amplified, subcloned, and sequence-verified miniarm fragments for (PL253delXba, PL452, and PL451) were ligated with the designated vector at (NotI/XbaI/BamHI, SalI/EcoRI/BamHI/NotI, and SalI/EcoRI/BamHI/NotI), respectively. PL253delXba with miniarms was digested with XbaI, underwent electroporation into the BAC in EL350 and was selected with ampicillin to retrieve a 13.5 kb genomic DNA fragment containing the mouse Crh gene. PL452 with miniarms was digested with SalI/NotI/ScaI to obtain a 2.7 kb fragment, which underwent electroporation into EL350 containing the retrieved vector and was spread on LB kanamycin plates to introduce the floxed Neomycin cassette. The PL253delXba fused with PL452 was introduced into Arabinose-treated EL350 to remove the floxed Neomycin cassette. In a like manner, PL451 with miniarms was digested with SalI/NotI/ScaI to obtain a 2.7 kb fragment, which underwent electroporation into EL350 containing the upstream-loxP introduced vector and was spread on LB kanamycin plate to introduce the second loxP site to obtain the final targeting vector. The final targeting vector was verified by functional testing (Flpe induction in EL250 or Cre induction in EL350), restriction enzyme digestion, and DNA sequencing. The final targeting vector was linearized with NotI, electroporated into 129Sv ES cells and selected with G418 (Invitrogen).
Incorporation of targeting vectors into the proper genomic loci in transfected ES cells was validated using a long PCR strategy (5′ incorporation) or Southern blot analysis (3′ incorporation) (Fig. S1a, b). Briefly, G418 resistant ES cell lines were isolated and analyzed with long PCR using LA Taq (TAKARA BIO INC. Otsu, Japan), external primer and internal primer at both 5′ and 3′ ends to confirm both 5′ and 3′ incorporation of the construct into the proper genomic loci. Long PCR products were 6443 bp using CRH5PextF1_LA and PL452whole98bpR, and 8492 bp using CRH5PextF1_LA and NeoRev3. Long PCR products were cloned into TOPO-XL vector (Invitrogen) and verified with sequencing. Southern blot analysis was carried out using a 32P-labeled 3′probe and genomic DNA extracted from ES cells. Briefly, the 3′ probe was designed for the specific region of DNA external to the construct to confirm site-specific genomic targeting. BamHI digested genomic DNA was electrophoresed on 0.75 % agarose gel in 1xTBE buffer, transferred to a Nylon membrane, and hybridized with probe. After the membrane was washed, bands were visualized using a phosphor screen.
Primer sequences
- Constructing primers
- PL253_44705FNOT 5′-GCGGCCGCATTTGCCCTGGAGGGAAAGGAG-3′
- PL253_45104RXBA 5′-TCTAGAACTATATCATCATAAAAT-3′
- PL253_57935FXBA 5′-TCTAGAAGCTAAGTGAAATG-3′
- PL253_58229RBAM 5′-GGATCCAAACCAAGTTGTGCCAGGGA-3′
- PL452_49460FSAL 5′-GTCGACTTGAGAGACTGAAGAGAAAG-3′
- PL452_49859RECO 5′-GAATTCTGGGATGCAATAGGGGAGCC-3′
- PL452_49860FBAM 5′-GGATCCTGTCCCCAAGCAAACGGAGT-3′
- PL452_50259RNOT 5′-GCGGCCGCAGGTCGGGGGAGAGAGAAGG-3′
- PL451_51214FSAL 5′-GTCGACCATTCTTGAGGGGTGGCTAG-3′
- PL451_51613RECO 5′-GAATTCTTTAGAGGTGGGAGGAACCTC-3′
- PL451_51614FBAM 5′-GGATCCACTTGATCACAGTGGAAATAAC-3′
- PL451_52013RNOT 5′-GCGGCCGCGCAAACGTATTAAAATTAGAAG-3′
- Long PCR primers
- CRH5PextF1_LA 5′-CCAGAGGAGTGGGGCTTGTCAGAGAACTGGACACA-3′
- PL452whole98bpR 5′-GGATCCCCTCGAGGGACCTAATAACTTCGTATAGCATACATTATACGAAGTTATATTAAGGGTTATTGAATATGATCGGAATTGGGCTGCAGGAATTC-3′
- NeoRev3 5′-GGGAACTTCCTGACTAGGGGAGGAGTAGAAGGTGG-3′
- Primers for southern probe
- 3P external_F 5′-AAGCAGAAATGTGCTTGTGTG-3′
- 3P external_R 5′-AGGGGCTGCTGAGTTAACTC-3′
- Genotyping primers
-
4.1)Downstream genotyping
- CRH_51359_F 5′-CAAAGGTTGCTGTGGCTTTATTTTTCCTCTTCA-3′
- CRHNeo_F 5′-ATGGCTTCTGAGGCGGAAAGAACCA-3′
- CRH_51706_R 5′-TTGTCCTCTGACCTCCACCCACTTC-3′
-
4.2)Flpe genotyping (Flpe 725 bp, internal control WT 324 bp)
- Flpfwd (IMR1348) 5′-CACTGATATTGTAAGTAGTTTGC-3′
- Flprev (IMR1349) 5′-CTAGTGCGAAGTAGTGATCAGG-3′
- IMR0042 5′-CTAGGCCACAGAATTGAAAGATCT-3′
- IMR0043 5′-GTAGGTGGAAATTCTAGCATCATCC-3′
-
4.3)Upstream genotyping
- CRH_49667_F 5′-CCAGCTGCCCATGTGCTGGA-3′
- CRH_49959_R 5′-CGCACACCCTAATCGCCCCC-3′
-
4.1)
Corticosterone treatment
Because loss of PVHCrh reduces plasma corticosterone, which might independently affect behavior in Sim1CrhKO mice, some animals were administered corticosterone in their drinking water to restore the plasma corticosterone towards normal (Sim1CrhKOCort). Corticosterone (Sigma-Aldrich, #C2505) was initially dissolved in ethyl alcohol and diluted to a final concentration of 5 or 10 ug/ml in water (0.2% ethyl alcohol final concentration). Control Crhflox mice were randomly assigned to receive either drinking water with 0.2% ethyl alcohol (CrhfloxCon), or drinking water with corticosterone (CrhfloxCort).
Tail blood collection
We collected tail blood (~10ul) in the morning (7–9 am) or evening (5–7 pm) as previously described.26 Within 60 seconds after removal from the home cage, blood was collected from a small ventral incision near the tail tip by gently milking the tail to deliver ~10ul blood into EDTA-coated microvette tubes. Mice were immediately released back to their home cages after bleeding.
Behavioral tests and data analysis
Adult male mice were housed individually. Animals were maintained on a 12 h light/dark cycle in a temperature- and humidity- controlled vivarium, with lights on from 7 am to 7 pm. Animals were maintained in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals (1996). All animal procedures were approved by the animal research facility at Boston Children’s Hospital and Harvard Medical School. All behavioral tests were performed in our neurodevelopmental behavior core (http://core.iddrc.org/neurodevelopmental-behavioral) laboratory in an isolated test room under low light conditions (≈30 lumens). Animal’s behaviors were recorded with an overhead Panasonic WVBP334 digital camera, and video tracking was done with Noldus Information Technology Ethovision XT9 software (Noldus Information Technology, Wageningen, Netherlands). Only one behavior test was performed per day starting at 9 am. Between sessions, the maze/field/arena was rinsed with water and dried with paper towels. All behavioral measurements were calculated using the Ethovision program XT9 and manually analyzed by an observer blinded to genotype and treatment.
Shirpa test
Shirpa test screening was conducted to determine whether Sim1CrhKO mice have normal physiological functions prior to performing anxiety testing. This screen covers a variety of physiological and behavioral assessments including muscle and lower motor neuron, spinocerebellar, and sensory functions.27 Animals received a score for each measured behavior and position, and total scores were analyzed. Animals were scored in a viewing jar for body position (0=active; 1=inactive; 2=excessive activity), tremor (0=present; 1=absent), palpebral closure (0=present; 1=absent), coat appearance (0=normal; 1=abnormal), whisker (0=present; 1=absent), lacrimation (0=present; 1=absent), and defecation (0=present; 1=absent). Mice were then transferred to an arena, where we scored transfer arousal (0=extended freeze; 1=brief freeze; 2=immediate movement), gait (0=fluid movement; 1=lack of fluidity), tail elevation (0=dragging; 1=horizontal extension; 2=elevated tail), startle response (0=prayer reflex; 1=absent; 2=reaction in addition to reflex), touch escape (0=none; 1=response to touch; 2=flees prior to touch), positional passivity (0=struggles when held by tail; 1=struggles when scruffed; 2=struggles when laid supine; 3=no struggle), skin color (0=pale; 1=pink; 2=red), trunk curl (0=present; 1=absent), limb grasping (0=present; 1=absent), visual placing (0=present; 1=absent), righting reflex (0=present; 1=absent), pinnal reflex (0=present; 1=absent), contact righting reflex (0=present; 1=absent), corneal reflex (0=present; 1=absent), biting (0=present; 1=absent), vocalization (0=present; 1=absent), morphology (0=normal; 1=abnormal), tail pinch (0=response; 1=no response), and pupillary light reflex (0=present; 1=absent).
Restraint
Restraint was performed by confining mice in ventilated conical tubes (Corning, NY) to inhibit movement for 30 minutes.28 Tail blood was collected prior to restraint (0 minutes), immediately after the termination of restraint (30 minutes), and at 3 later time points (60, 120, and 240 minutes from the initiation of restraint).
Open Field Test
The open field test was modified as described.29 In this test, the time spent in each zone (center, neutral and wall) and distance traveled in these areas were calculated. Our open-field apparatus consisted of a 45cm diameter circular arena, which was divided into 3 zones (center, neutral and wall), each defined as 1/3 the diameter of the arena (15cm). Each mouse was placed toward the wall at the 6 o’clock position of the open field. Each trial lasted for 15min, with one trial per mouse. Time spent in the different zones, latency and frequency entering into different zones, and total traveled distance were analyzed.
Holeboard Test
The holeboard apparatus consists of 16 (4 × 4) holes in a square arena (42 ×42 cm) with 16 × 16 photo beams for locomotor activity and 1 photo beam in each hole for exploratory behavior. Mice were placed gently at the 6 o’clock position to allow them to explore the arena for 15 minutes. The number of center area entries, distance moved in the periphery and center, and time spent in the periphery and center area were calculated. Fine movement, and head/body stretch and movement (which are considered as subtle risk assessment measures) were scored. Fine movement was calculated as the number of single beam breaks the animal made repeatedly (e.g., moving its head from left to right and back again).30
Elevated Plus Maze (EPM)
The EPM test was modified as previously described.31 The apparatus consisted of a black melamine central square platform (76 × 76 cm) from which four black melamine arms radiated: two oppositely positioned open arms (76×7cm) and two oppositely positioned enclosed arms (76×7cm). The maze was elevated to a height of 74cm from the floor and indirectly illuminated (light intensity in open arms: 25–30 lux). Mice were placed individually into the central platform facing toward an open arm and allowed 6 min of free exploration of the apparatus. An arm entry was defined as the entry of all four paws into one arm. The following dependent variables were measured: time spent in open/closed arms and center, percentage of time spent in each area, number of open/closed arm entries, total distance traveled and velocity.
Light-Dark Box
The light-dark box apparatus consisted of 2 rectangular compartments (26 × 26 cm) connected by a door.32 One compartment is white and illuminated (80 lux), and the other is black and dark (0 lux). Mice were placed gently in the center of the dark area facing the door for 5 minutes, the latency to the first entry in the light area, the number of entries, and the percentage of time spent in the light area, total moved distance and velocity were measured.
Novel Object Recognition Task (NORT)
The NORT test has been introduced into anxiety testing recently, although it was originally developed to assess non-spatial working memory.33–36 In the present study, the modified protocol consisted of 2 sessions. In the training session, mice were presented with two identical objects (2 small white p.v.c pipes with screws) in the square arena (W20 × L20 × D10 cm) and allowed to explore them for 5 min (same object trial). Animals were returned to their home cages for a 5 min break. Then, one of the two familiar objects was replaced with a novel object (1 blue glass cylinder for novel object) and the mice were again allowed to explore them for 5 min (choice trial). The novel object was randomly placed into either the left or right test arena. The animal was considered to be “investigating” an object when its nose-point, detected by Noldus Information Technology Ethovision XT9 tracking software, entered into the designated zone containing the object. The cumulative duration for familiar object, novel object, frequency to either object, total distance traveled and velocity were calculated by the program.
All behavioral tests were performed in three different animal models. First, the behavioral effects of Sim1Cre and Crhflox were assessed by comparing CrhWT, Sim1CrhWT, and Crhflox mice. Next, the effect of removing Crh from Sim1 neurons on anxiety behaviors was investigated in Sim1CrhKO compared with Crhflox mice. Finally, to differentiate the effect of loss of PVHCrh from reduced corticosterone in Sim1CrhKO mice, behavioral testing was performed in Sim1CrhKO and Crhflox animals treated with corticosterone (5–10ug/ml) or vehicle in drinking water, as described above.
Radioimmunoassays (RIA)
Plasma concentration of corticosterone and ACTH were determined by radioimmunoassay using 125I RIA kits from MP Biomedicals.37 All corticosterone plasma samples were analyzed in duplicate, and plasma corticosterone measures from a single experiment were performed in the same RIA analysis to avoid interassay variability. For ACTH, single plasma samples were analyzed due to the large required volume (50 ul of plasma/mouse). We performed several experiments using different cohorts of mice with similar results in all experiments.
In situ hybridization (ISH)
In situ hybridization was performed as described previously.37, 38 Brains were collected with isopentane on dry ice and stored at −80°C. Targeted brain areas (from Bregma −0.10 to Bregma −1.70) were sectioned (12um) onto coated slides from coronal direction according to the Mouse Brain in Stereotaxic Coordinates.39 Antisense cRNA probes complementary to mouse Crh exon 2 (578 bp) were used for in situ hybridization. The Crh DNA construct was subcloned into pGEM4Z vector (Promega), linearized with HindIII, and transcribed with SP6 RNA polymerase. The probe was labeled by in vitro transcription using [35S] UTP, with prehybridization, hybridization, and post-hybridization protocols conducted as described previously.37, 38 Hybridized slides were exposed on Kodak Biomax MR-2 film (Eastman Kodak) for 5 days. Images were captured from epson scanner (epson perfection V700 photo; Epson, USA). CrhKO mice were included as specificity negative controls. Assessment of PVH Crh mRNA expression was performed using the Mouse Brain in Stereotaxic Coordinates.39
Semi-quantitative analysis was conducted on every sixth brain section containing the anatomical region of interest using NIH Image software (http://imagej.nih.gov/ij/). The average gray level (signal) was quantified bilaterally in the PVH by subtracting the gray level signal over a non-hybridized area of tissue (white matter) and expressed as the corrected gray level. The mean corrected gray level values were calculated for each animal and used in the statistical analysis in a blinded manner.
Q-RT-PCR
Animals were euthanized by cervical dislocation and brains were rapidly removed. PVH and brainstem were collected using a cooled mouse brain matrix with 0.5mm section dividers (ASI Instruments Inc., Warren, MI). Using the Palkovits punch technique (Electron Microscopy Science, PA), PVH was dissected using the fornix, optic tracts, and third ventricle as landmarks (Bregma, around −0.22mm to −1.70mm). Samples from different animals of the same genotype were pooled together to extract RNA. NTS-enriched brainstem was isolated from the anterior (rostral) margin (Bregma −6.12mm), to the posterior (caudal) margin (Bregma −8.12) using obex (bregma, −3.8mm) and the fourth ventricle as previously published.40 Whole hypothalamus was isolated by block dissection according as previously described.41 The landmarks including the fornix (Bregma, −0.22mm), optic tracts and mammillary nuclei (Bregma, −2.54mm) were used to dissect the hypothalamic region. Collected tissues from animals were transferred into RNAlater, and total RNA was extracted using RNeasy Plus Universal kits (Qiagen). cDNAs were synthesized (SuperScriptTM III First-Strand Synthesis System; Invitrogen) according to the manufacturer’s instruction. Q-RT-PCR was performed as previously described.37 Crh primer sequences were designed using Primer3 software42 and synthesized by IDT. Q-RT-PCR analysis was performed in the iCycler iQ Multi-Color Real Time PCR Detection System (Bio-Rad). cDNA amounts present in each sample were determined using iQ SYBR Green Supermix (Bio-Rad). Threshold cycle readings for each of the unknown samples were used, and the results were calculated in Excel using the ΔΔCt method, using L32 as a house-keeping reference gene.43 Negative RT samples were included to rule out genomic DNA contamination.
Q RT-PCR primers:
CRH_F 5′- TCTGCAGAGGCAGCAGTGCGGG -3′
CRH_R 5′- CGGATCCCCTGCTGAGCAGGGC -3′
L-32_F 5′- GCCAGGAGACGACAAAAA -3′
L-32_R 5′- AATCCTCTTGCCCTGATCC -3′
Immunohistochemistry (IHC)
Free-floating sections were incubated with a rabbit anti-Crh antibody (diluted 1:5000; a generous gift from the late Dr. Wylie Vale’s lab) overnight at 4°C and Crh IHC staining was performed as described previously.37 Sections were rinsed five times for 5 min in 50mMKPBS and subsequently incubated in biotinylated goat anti-rabbit secondary antibody (diluted 1:500; Vector Laboratories) for 1 h. Following five, 5 min rinses in KPBS, sections were incubated in avidin-biotin complex (diluted 1:1000; Vector Laboratories) for 1 additional hour. Sections were rinsed five times for 5 min in KPBS, incubated in biotin-labeled tyramide (diluted 1:250; PerkinElmer Life Sciences), rinsed five times for 5 min, and incubated in Cy3-conjugated streptavidin (diluted 1:500; Jackson ImmunoResearch) for 30 min. All sections in the series were then examined by fluorescence microscopy to identify positively labeled cells and fibers. Images of the PVH were captured from low to high magnification by using a Nikon E800 microscope and Nikon Elements software.
PVHCrh projection tracing
To track PVHCrh projections in the brain, we stereotaxically microinjected 40 nl of AAV8-FLEX-hsyn-ChR2-mCherry (UNC Vector Core) into the unilateral PVH (from bregma: A/P 0.26, M/L 0.76, D/V −4.8) of adult Crh-ires-Cre mice44 (a gift from Dr. Bradford Lowell) using a glass micropipette and an air pressure injection system. Mice were perfused 7 weeks later, the brains were removed and stored in 10% formalin overnight followed by transfer into 20% sucrose. Sectioned brain tissue (30um) was quenched in 0.3% H2O2 for 30 minutes, and then incubated overnight with rabbit polyclonal antiserum against mCherry (1:5000; Clontech #632496). Between this incubation and each subsequent incubation, tissue was washed with 1XPBS for 5 min × 5 times. On the second day, sections were incubated with biotinylated-conjugated donkey anti-rabbit antiserum (1:500, Jackson#712065152) for 2 h, followed by avidin-biotin complex (ABC) solution for 1h. Sections were incubated in DAB (3, 3′-Diaminobenzidine), TBS, H2O2 for less than 5 minutes. Sections were mounted and coverslipped (Cytoseal, Thermoscientific). Images were captured with a Zeiss AxioCam HRc microscope and AxioVision Rel software (v4.8).
Hematoxylin and Eosin staining (HE staining)
Adrenals were collected into 10% formalin and tissues were sectioned at 6um thickness from paraffin-embedded blocks and stained with hematoxylin and eosin in the Rodent Histopathology Core lab, Harvard Medical School (http://www.dfhcc.harvard.edu/research/core-facilities/rodent-histopathology/). Images were taken and analyzed using a Nikon E800 microscope and Nikon Elements software.
Statistical analyses
Data are expressed as mean ± SEM. Data were analyzed using two-tailed Student t test for samples with similar variances, ANOVA, and repeated measures ANOVA as appropriate (Statview, SAS Institute; GB Stat version 9.0, Dynamic Microsystems). Significant main effects were analyzed by the PLSD post hoc test. Significance for all statistical analyses was set at P ≤ 0.05. All sample sizes were determined by prior pilot studies to determine the minimum number of animals required to achieve statistical significance. No animals were excluded from analysis in any experiment.
RESULTS
Characterization of Crhflox and CrhKO mice
Crhflox mice (Crhfl/fl) were crossed with EIIaCre mice to create global CrhKO mice (Crhdl/dl) (Fig. 1a; Fig. S1a, b). Crhflox and CrhWT mice revealed a similar expression pattern and comparable fluorescent density for Crh, which indicates that the insertion of loxP sites flanking Crh exon 2 did not affect Crh protein expression. Compared to these animals, CrhKO revealed the expected complete loss of Crh peptide in the PVH and CeA (Fig. 1b–g). Crhflox mice had normal adrenal morphology (Fig. 1h, i), whereas CrhKO mice had a markedly atrophic appearance of the zona fasciculate of the adrenal gland, the area primarily responsible for basal as well as stress-triggered corticosterone release (Fig. 1j, k). Homozygous Crhflox mice revealed normal food intake and bodyweight (Fig. S2a, b). CrhKO mice born from Crhdl/+ heterozygous mothers had normal growth, viability and fertility without a requirement for glucocorticoid replacement. However, all offspring of homozygous CrhKO mothers died within the first several hours of delivery after a normal 19–20 days’ gestation. These offspring showed lung dysplasia in comparison to Crhflox neonates at 2 h of life. CrhKO neonates showed marked hypercellularity of the lungs, increased alveolar thickness and paucity of air spaces in comparison to those neonates from Crhflox mice (Fig. 1l–q). Administration of corticosterone (30ug/ml in drinking water) to pregnant CrhKO mothers fully reversed CrhKO neonates’ death with resulting normal lung morphology (Fig. 1p, q).
Crhflox mice showed comparable morning and evening plasma corticosterone compared to CrhWT mice indicating a normal glucocorticoid diurnal rhythm. Loss of Crh in CrhKO mice severely reduced basal plasma corticosterone (Fig. S3a). Crhflox mice had a normal glucocorticoid stress response to an acute 30 min restraint challenge, which, as in WT mice, declined over time following the termination of restraint (Fig. S3b). Under these same conditions, CrhKO mice had reduced glucocorticoid release (Fig. S3a, b). These results are consistent with the adrenal atrophy demonstrated in CrhKO mice (Fig. 1h–k). In all of these ways, this CrhKO mouse line is a phenocopy of a previously-created line of global CrhKO mice,13 which demonstrates that the Cre-loxP strategy using EIIaCre targeting Crh exon 2 results in the complete loss of Crh expression and function.
Characterization of Sim1CrhKO mice
Analysis of Crh mRNA by in situ hybridization revealed different Crh expression patterns in Crhflox and Sim1CrhKO mice: as expected, Sim1CrhKO mice had markedly reduced Crh mRNA in hypothalamus, particularly in the PVH region from bregma −0.58 to bregma −1.06, consistent with co-expression of Crh and Sim1Cre in this area (Fig. 2, a–d). In contrast, Crh mRNA expression was no different between Sim1CrhKO and Crhflox mice in other areas such as central amygdala and cerebral cortex, indicating successful PVH deletion of Crh by Sim1Cre. Global CrhKO mice did not show any Crh mRNA signals in the brain (Fig. 2e, f). Semi-quantitation of Crh mRNA expression by in situ hybridization confirmed that Sim1Cre efficiently deleted 70~80% of Crh in PVH (Fig. 2g). Using Q-RT-PCR to measure Crh mRNA expression in Sim1CrhKO mice, we found a 60~70% reduction in PVH-enriched hypothalamic punches (Fig. 2h), but only a 30% reduction in whole hypothalamus (Fig. S4), and no decrease in brainstem (Fig. 2h). Sim1CrhKO mice revealed deceased expression of Crh-immunoreactivity in PVH compared to Crhflox mice (Fig. 2 i, j), consistent with the decrease in Crh mRNA (Fig. 2c, d). In contrast, Crh-immunoreactivity in CeA was similar between the two groups (Fig. 2k, l).
Figure 2.
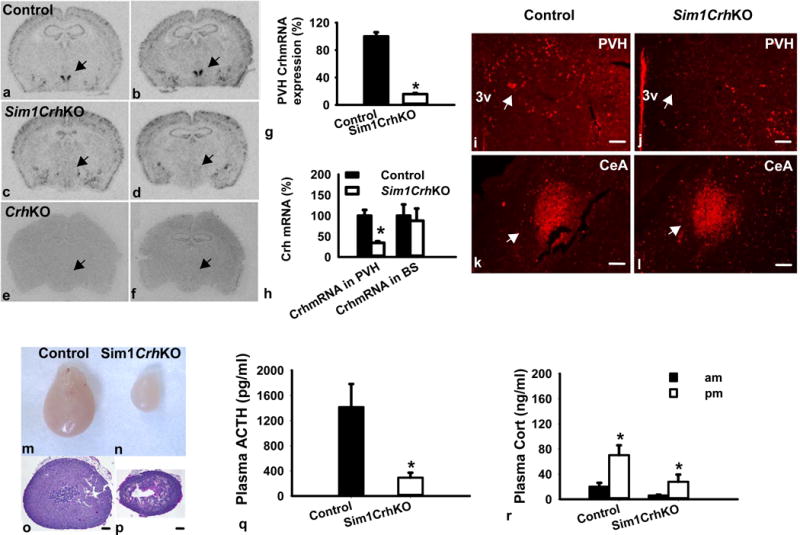
Characterization of Sim1CrhKO mice. Crhflox mice were crossed with Sim1Cre mice to delete the floxed Crh gene in Sim1-expressing tissues; (a–f) Representative images for Crh mRNA expression in the PVH by in situ hybridization (Bregma −0.70mm to Bregma −1.06mm). Top row, Crhflox (control); middle row, Sim1CrhKO; bottom row, CrhKO. Arrows point to PVH; (g) Semi-quantitative densitometry of film images showed that Crh was deleted in the PVH area of Sim1CrhKO mice, (Sim1CrhKO versus Crhflox, n=8 versus 8); (h) Q RT-PCR analysis revealed that Crh mRNA expression decreased in the PVH but not in the brainstem in Sim1CrhKO mice compared to the control (Sim1CrhKO versus Crhflox, n=5 versus 4; Control, filled bars; Sim1CrhKO, open bars); (i–l) Crh immunohistochemistry staining in the PVH and CeA. Sim1CrhKO showed less Crh-ir positive neurons and fibers in the PVH but comparable amount of Crh-ir positive staining in CeA area. Arrows point to PVH and CeA, scale bar: 100um; PVH, paraventricular nucleus of the hypothalamus; CeA, central nucleus of the amygdala; (m–r) Physiological characterization of Sim1CrhKO mice; (m–p) Adrenal hypoplasia in Sim1CrhKO versus Crhflox control mice: Whole adrenals and haematoxylin-eosin stained adrenal sections from the widest diameter, respectively, of Crhflox (m, o) and Sim1CrhKO (n, p) male mice; (q) Plasma ACTH was lower in male Sim1CrhKO (7) versus Crhflox (10) mice; (r) Plasma corticosterone was lower in Sim1CrhKO versus Crhflox mice at diurnal morning (9–10am) and evening (5–6pm) in males (n = 7 versus 10); *p<0.05 versus control.
Loss of PVH Crh affects adrenal development and HPA responsiveness
Sim1CrhKO mice revealed striking adrenal atrophy, particularly in the zona fasciculata, the primary corticosterone-producing area (Fig. 2 m–p). Both adrenal weight, adjusted adrenal weight [adrenal weight (milligrams)/bodyweight (grams) × 100] and adrenal cross-sectional area were decreased by deletion of Crh in Sim1 neurons (Fig. S5a).
As expected, disruption of Crh expression in PVH affected downstream pituitary ACTH secretion, as manifested by the significantly decreased plasma ACTH in Sim1CrhKO mice compared to control mice despite their having lower plasma corticosterone (Fig. 2q, r). Introduction of Sim1Cre did not affect basal plasma corticosterone (Fig. S5b). In addition to reduced basal plasma corticosterone in Sim1CrhKO animals (Fig. 2r), loss of PVH Crh significantly attenuated stress-induced plasma corticosterone release (about 2/3 less than control) in Sim1CrhKO animals (Fig. S5d).
General Health Characterization of Sim1CrhKO mice
Before analyzing more subtle behaviors, all animals were screened by a modified Shirpa test protocol for functional and physiological behavior assessment.27 There were no differences in total Shirpa scores between control (Crhflox) and Sim1CrhKO mice, suggesting that disruption of Crh in Sim1 neurons does not affect overall physical and physiological characteristics (Fig. S6).
Attenuation of anxiety behaviors in Sim1CrhKO mice
The role of PVH CRH in neuroendocrine HPA axis regulation is clear,13 but the site of CRH involved in the regulation of stress-associated behaviors such as anxiety is less so. Moreover, global knockout of Crh in mice,14, 15 and pharmacological antagonism of CRH in humans,45 has not produced anxiolysis. We therefore evaluated anxiety behaviors in Sim1CrhKO mice. In the open field paradigm, Sim1CrhKO mice had increased cumulative duration in the center (Fig. 3a, b). Sim1CrhKO animals showed shorter latency into both center and neutral areas compared to control mice, indicating that disruption of Crh in Sim1 neurons significantly decreased emotional anxiety behaviors (Fig. 3c). Consistent with this, deletion of Crh in Sim1 neurons increased the frequency of entering the center area while there were no differences for entering neutral and wall areas (Fig. 3d). Both Sim1CrhKO and control mice increased their time spent in the center during the last 5 minutes of testing, but Sim1CrhKO mice showed striking increment at the beginning (0min–5min) and middle (5min–10min) period (Fig. S7a). Sim1CrhKO mice more frequently entered the center area during the first 10 minutes, although eventually control mice did so as well (Fig. S7b). No differences were found in total distance travelled and velocity between Sim1CrhKO and Crhflox control mice (Fig. S7c).
Figure 3.
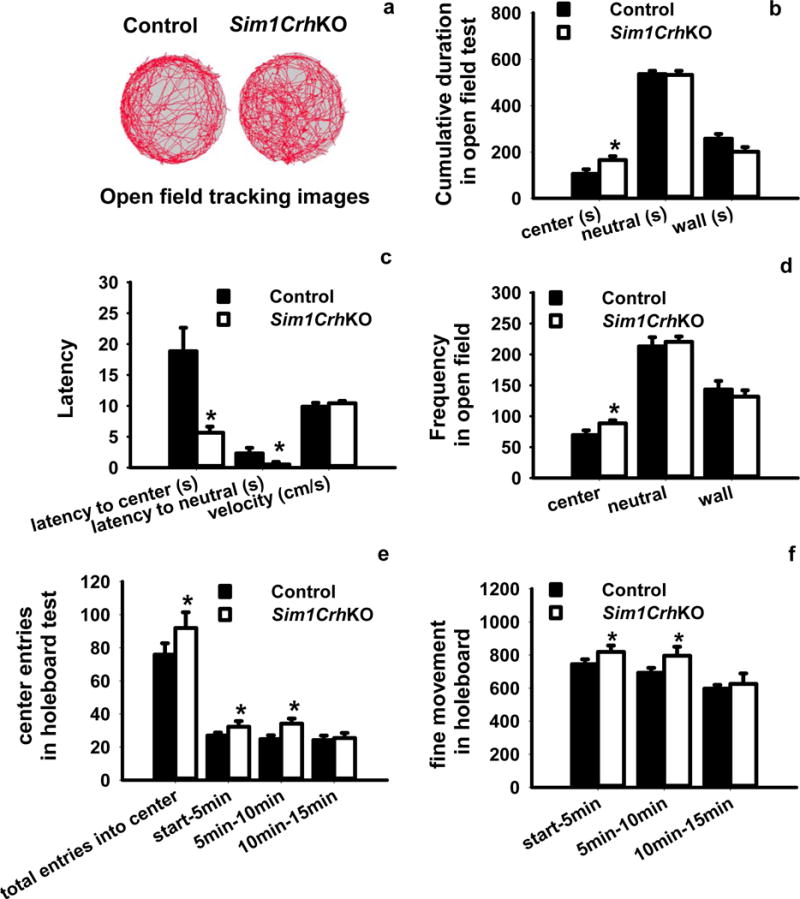
Analysis of anxiety-like behaviors by the open field test and holeboard test. (a, b) Compared to Crhflox control mice, Sim1CrhKO mice spent more time in the center area and less time in wall areas while both groups spent equivalent amounts of time in neutral areas; c) Sim1CrhKO mice revealed decreased latency of first entry into center and neutral areas compared with control mice, whereas there were no differences for velocity; d) Sim1CrhKO mice entered into center more frequently compared to control animals; e) Holeboard analysis revealed Sim1CrhKO mice had significantly increased entrance frequency into the center compared to Crhflox controls, and that this occurred during the first and second 5 minute periods; f) Fine movement was significantly increased in Sim1CrhKO compared to Crhflox mice. *P<0.05, Sim1CrhKO versus Crhflox control mice (n = 7 versus 10).
The holeboard task also measures anxiety behaviors due to the reluctance of rodents to enter the center of an open arena. It provides a more subtle assessment of anxiety which may be missed in other behavioral tests because this task does not evoke a high level of arousal or fear.46 In agreement with the open field testing, the total entries into the center of the holeboard were significantly greater in Sim1CrhKO mice, with differences observed during the test’s first 10 minutes (Fig. 3e). Sim1CrhKO animals significantly increased their fine movement behaviors compared to Crhflox control mice in the first 10 minutes of the test, suggesting that loss of PVH Crh attenuated subtle anxiety behaviors, with the animals showing more exploratory tendency and risky behaviors in their new environment (Fig. 3f). We did not observe any genotype differences in the time spent in both center and periphery area of the holeboard arena (Fig. S8a, b). Sim1CrhKO mice moved more into the center, although this difference did not reach statistical significance (Fig. S8c).
In the EPM test, we found a significant effect of genotype. The frequency of entry into the open arms was not different between genotypes but Sim1CrhKO animals spent less time in the closed arms, suggesting that they had increased exploratory behaviors outside of the closed arms. Sim1CrhKO animals significantly increased the time spent in the open arms (Fig. 4 a–c). However, we did not observe differences for duration in center or duration in closed arms on the EPM apparatus (Fig. S9). In another traditional anxiety test, the light-dark box test, Sim1CrhKO animals showed decreased latency to enter the lit compartment compared to the littermate siblings (Fig. 4d).
Figure 4.
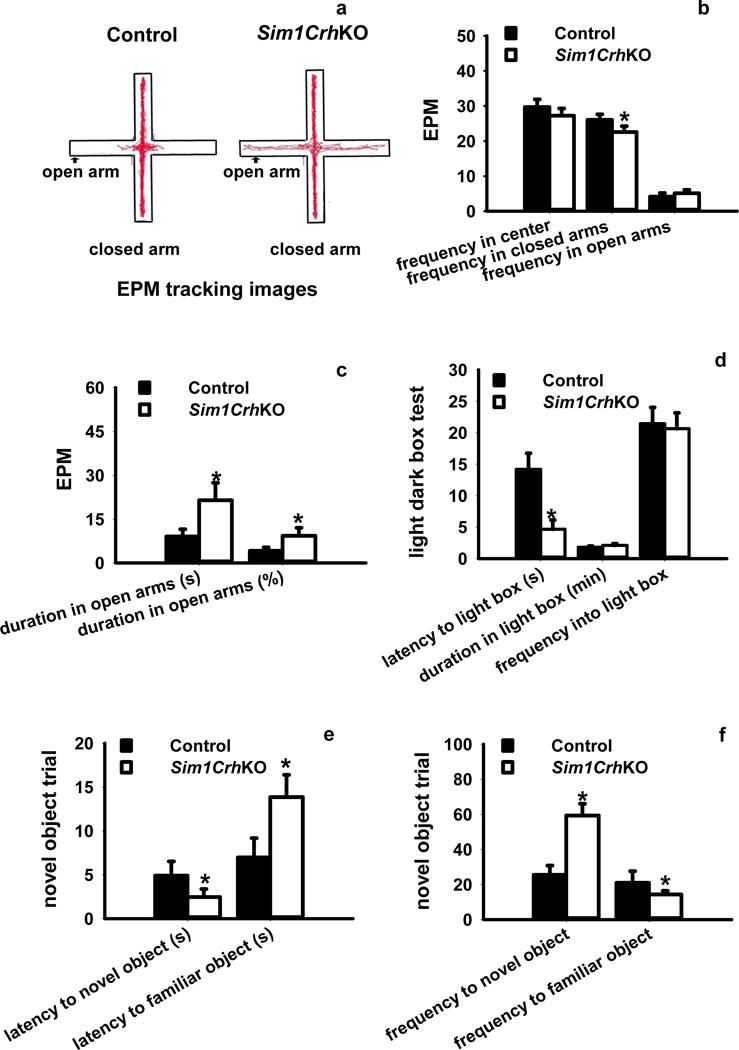
Analysis of anxiety-like behaviors by the elevated plus maze (EPM), light-dark box and novel object recognition test (NORT). a, b) EPM analysis revealed that deletion of Crh in Sim1Cre neurons significantly decreased the entrance frequency into the closed arms compared to Crhflox control mice; c) Sim1CrhKO mice spent significantly more time in the open arms in term of duration and percentage compared to control mice; d) Sim1CrhKO compared with Crhflox mice had a shorter latency to emerge from darkness into the light box. However, there were no differences in other measured anxiety parameters (time spent in the light part, frequency into light box. d) There were no significant differences for duration in center and closed arms between control and Sim1CrhKO mice; e) Deletion of Crh from Sim1 neurons significantly decreased latency to novel objects while increased latency to familiar objects; f) Sim1CrhKO mice exhibited significantly increased touching frequency for novel objects while decreased exploring behaviors for familiar objects; *P<0.05, Sim1CrhKO versus Crhflox control mice (n = 7 versus 10).
The novel object recognition test (NORT) assesses general anxiety behaviors by using familiar and novel object stimuli. Sim1CrhKO mice showed significant preference for approaching a novel versus a familiar object, indicating that Crh expressed in Sim1 neurons is critical to regulate anxiety activities towards new stimuli. Sim1CrhKO mice also exhibited decreased latency to approaching novel versus familiar objects (Fig. 4e), greater frequency of approach to novel versus familiar objects (Fig. 4f). Sim1CrhKO mice showed longer exploratory duration around a novel object (Fig. S10a), suggesting that Sim1CrhKO mice have lower anxiety toward exploring novel objects. Animals did not show any position preference in either familiar or novel objects trials, since they had similar touching/recognizing behaviors for familiar objects between groups during both same and novel object trials regardless the object positions (Fig. S10b). In contrast, we observed that Sim1CrhKO and Crhflox control mice travelled overall similar distances around novel and familiar objects (Fig. S10c), again suggesting that loss of PVH Crh did not affect their general locomotion behaviors.
As we expected, deletion of hypothalamic Crh attenuated stress-stimulated plasma corticosterone during the open field test (Fig. S11a), holeboard test (Fig. S11b), EPM test (Fig. S11c), light-dark-box test (Fig. S11d) and NORT behavior assessment (Fig. S11e). These data support the important role of PVH Crh in regulating the responsiveness of the HPA axis.
The results of behavioral assays were similar among CrhWT, Crhflox, and Sim1CrhWT mice in the open field (Fig. S12a), EPM (Fig. S12b), light-dark box (Fig. S12c), holeboard (Fig. S12d), and NORT tests (Fig. S12e). This indicates that Sim1Cre itself is not involved in the anxiolytic phenotype of Sim1CrhKO mice.
Anxiolytic behavior in Sim1CrhKO mice is not due to reduced plasma corticosterone
Because loss of PVHCrh reduces plasma corticosterone, which might independently affect behavior in Sim1CrhKO mice, we performed behavioral testing in these mice after administration of corticosterone in their drinking water to restore the plasma corticosterone towards normal (Sim1CrhKOCort mice). With their nocturnal drinking pattern, Sim1CrhKOCort mice exhibited a quasi-normal diurnal rhythm in plasma corticosterone, being high when measured in the early morning (after drinking during the night) and low when measured in the early evening (after not drinking during the daytime) (Fig. 5a). Compared to CrhfloxCon and CrhfloxCort mice, SimCrhKOCort animals spent more time in the center of the open field (Fig. 5b). Compared to CrhfloxCon and CrhfloxCort mice, Sim1CrhKOCort animals entered the open arms of the EPM more frequently and for a longer duration (Fig. 5c), anxiolytic behaviors similar to those of untreated SimCrhKO mice (Fig. 4 a–c). Interestingly, CrhfloxCort animals showed decreased time in the EPM open arms, suggesting an anxiogenic effect of supplemental corticosterone in these mice (Fig. 5c). In the light-dark box, Sim1CrhKOCort animals spent more time in light dark box although the results did not reach statistical significance (Fig. 5d). Similar to the Sim1CrhKO mice in the holeboard test (Fig. 3f), Sim1CrhKOCort mice had significantly increased fine movement (Fig. 5e). There was a nonsignificant trend of increased cumulative duration for novel object interaction in Sim1CrhKOCort (Fig. 5f). These results suggest that the anxiolytic behavior in Sim1CrhKO mice is not due to reduced plasma corticosterone.
Figure 5.
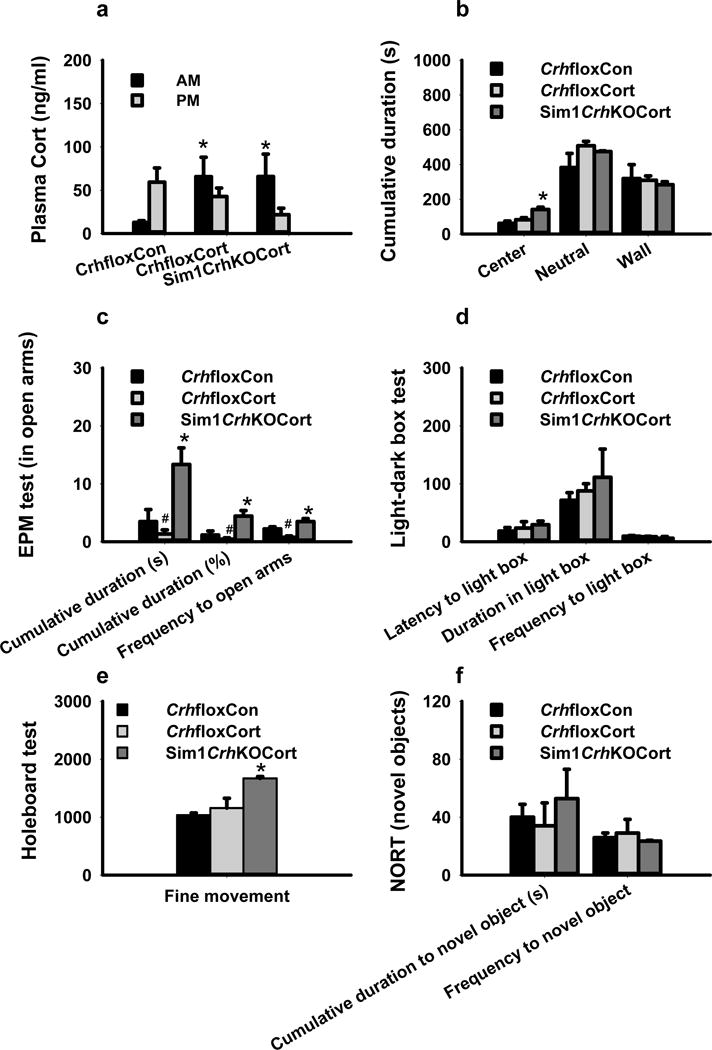
Corticosterone supplementation did not reverse anxiolytic behaviors in Sim1CrhKO mice. a) Morning (7–8am) plasma corticosterone increased in CrhfloxCort and Sim1CrhKOCort animals during administration of corticosterone (5ug/ml) in drinking water compared to CrhfloxCon mice; b) Sim1CrhKOCort mice spent more time in the center of open field test compared to CrhfloxCon and CrhfloxCort mice; c) During EPM testing, Sim1CrhKO mice with corticosterone supplementation (Sim1CrhKOCort), had significantly increased cumulative duration and the entrance frequency into the open arms compared to Crhflox control mice, while CrhfloxCort animals had decreased time in the open arms; d) During lightbox testing, Sim1CrhKOCort mice showed a non-significant trend of more time in the light area; e) During holeboard testing, Sim1CrhKOCort mice showed increased fine movement compared to CrhfloxCon and CrhfloxCort animals; f) During novel object testing, Sim1CrhKOCort mice showed a non-significant trend towards increased cumulative duration for novel objects; *P<0.05, Sim1CrhKOCort and CrhfloxCort versus CrhfloxCon; #P<0.05, CrhfloxCort versus CrhfloxCon. For all tests, Sim1CrhKOCort (n = 4), CrhfloxCon (n = 5) and CrhfloxCort (n = 4).
Identification of efferent projections of PVHCrh-responsive neurons
Using an AAV reporter in a mouse expressing Cre from a Crh promoter driver,44 we traced the efferent projections of PVHCrh neurons in Crh-ires-Cre mice (Fig. S13). As expected, there were intense projections of PVH Crh to the median eminence (Fig. S14i). We observed moderate to dense fiber projections to several brain areas that may be involved in anxiety behaviors, including the cerebral cortex, amygdala, septum, bed nucleus of the stria terminalis (BNST), accumbens nucleus (Acb), hypothalamus, midbrain, and brainstem, including nucleus of the solitary tract (Sol) and locus coeruleus (Fig. S14). The anatomical distribution of these and other PVH efferent projecting fibers was qualitatively analyzed (Table S1).
DISCUSSION
In this report, we have found that selective Crh deficiency in Sim1 neurons causes, as expected, 1) decreased basal ACTH and glucocorticoid plasma concentrations; 2) attenuated glucocorticoid secretion following variable stress challenges; and 3) adrenal gland atrophy, particularly in the zona fasciculata, the region responsible for corticosterone production. Unexpectedly, Crh deficiency in Sim1 neurons also causes markedly attenuated stressor-induced anxiety-like behaviors evaluated by open field,47 holeboard,30 elevated plus maze,31 light-dark-box,32 and novel object recognition task,35 effects that were not reversed by corticosterone supplementation of Sim1CrhKO mice. Moreover, we found that PVHCrh fibers project widely to areas that are potentially involved in behavior, including BNST, MeA, CeA, NTS and LC. The consistency and robustness of the behavioral findings across these different test modalities that address different aspects of anxiety48 indicate that Crh expressed in Sim1 neurons, likely acting as a neuromodulator, has a major role in the manifestation of anxiety-like behaviors. This may have important implications for how to best target CRH expression or action as a potential therapeutic approach to the clinical treatment of anxiety disorders in humans.
Validation of Crh floxed mice to create selective Crh deficiency
We first showed that the Crhflox mouse we created by flanking the second, coding exon of Crh with LoxP sites is functionally capable of selectively deleting Crh. We deleted Crh in gametes to create global CrhKO mice by crossing Crhflox mice with EIIaCre mice, in which Cre is expressed in the early mouse embryo.49 By this method we obtained germline homozygous Crh-deficient mice that completely recapitulated the phenotype of the Crh knockout mice we had previously created by a different targeted Crh deletion strategy in a line of mixed 129/C57BL/6 background that retained expression of Pgkneo,13 including neonatal lethal lung dysplasia when born from Crh-deficient homozygous mothers which is rescued by prenatal glucocorticoid therapy,50 absence of Crh peptide and mRNA from brain, atrophic adrenal glands with preserved adrenomedullary tissue, markedly attenuated basal diurnal corticosterone secretion,51 and severely attenuated stimulated corticosterone following restraint stress.52 These actions of Crh in mice are mediated by Crhr1, global knockout of which phenocopies the neuroendocrine deficits of CrhKO mice.17, 18 Deletion of Crh specifically in Sim1 neurons does not cause as profound a reduction in basal or stress-induced plasma corticosterone concentrations as does global deletion of Crh, possibly because Sim1Cre may not delete all PVH Crh. Supporting this, 1) Crh mRNA in the PVH region of Sim1CrhKO mice was reduced by 70–80% measured by in situ hybridization, by 60~70% in PVH-enriched hypothalamic punches measured by Q-RT-PCR (Fig. 2g,h), but only by 30% in whole hypothalamus (Fig. S4), and 2) Crh peptide was absent from the PVH but detectable in some neurons lateral to the PVH in Sim1CrhKO mice (Fig. 2j).
Contribution of PVH Crh to anxiety-like behaviors
The reduced anxiety phenotype of Sim1CrhKO mice is very similar to the behavior of Crhr1 knockout mice,17, 18 likely because this receptor transduces these Crh-mediated behaviors, a conclusion also supported by the ability of Crhr1-specific antagonists to reverse anxiety-like behaviors,53 including, surprisingly, in CrhKO mice.14 The observed anxiolytic behaviors in Sim1CrhKO mice are due neither to the presence of Sim1Cre nor to reduced plasma corticosterone, and are consistent with the existence of a PVHCrh-specific anxiogenic pathway. This conclusion is supported by findings in mice with postnatal deletion of Pomc, which have elevated hypothalamic Crh mRNA, markedly reduced plasma corticosterone, and a robust increase in anxiety-like behaviors.54
The different anxiety phenotypes observed between Sim1CrhKO and previous global CrhKO mice13 suggest that a difference in the method, extent, or timing of Crh gene deletion may affect these behaviors. One difference between the two models is that only exon 2 (which includes the entire coding region) of Crh is deleted in Sim1CrhKO mice, whereas all of exon 1 and only the coding portion of exon 2 is deleted in the global CrhKO mouse.55 Also, in the first,13 but not in Sim1CrhKO mice, the phosphoglycerine kinase promoter, Pgk, drives expression of the neomycin resistance gene, neo, in most cells. Further, the behavioral studies in the global CrhKO mouse were performed in mice on a mixed 129/C57BL/6 background,17, 18 whereas Sim1CrhKO mice are on a pure C57BL/6 background. Finally, Sim1CrhKO mice manifest Crh deficiency only after e10.5, when Sim1 expression in mouse brain is first detected,24 whereas global Crh knockout mice lack Crh from the time of conception. The earlier deletion in global CrhKO mice might trigger different compensatory processes, such as urocortin being able to substitute for the loss of Crh.53
The target neurons responsible for reduced anxiety in Sim1CrhKO mice are not known. Using Crh-ires-Cre/AAV reporter mice, we found that cerebral cortex, BNST, septum, amygdala, dorsolateral hypothalamus, NTS and LC receive efferent fiber projections from PVHCrh neurons. It is possible that PVHCrh might evoke stress-induced behavioral responses via these pathways, since the BNST receives information from stress-regulatory limbic regions,56–58 and the amygdala mediates responses to stress by altering hindbrain neuroendocrine, autonomic and behavioral pathways.59 Although the amygdala is a behavioral target for glucocorticoids,9–11, 60–62 we consider it unlikely that reduced corticosterone in Sim1CrhKO mice causes their anxiolytic behavior, since corticosterone supplementation does not reverse it. PVHCrh neurons project to NTS and LC and other regions of the caudal brainstem and spinal cord, such that if autonomic responses to stress were blunted in Sim1CrhKO mice, decreased interoceptive transmission could contribute to reduced anxiety behaviors. We could not answer whether Sim1CrhKO mice have reduced Crh autonomic projections using Crh-ires-Cre/AAV reporter mice (which express Crh in all its endogenous sites), but we did not observe any reduction in Crh mRNA expression in the brainstem of Sim1CrhKO animals.
Anxiety disorders are among the most common of all psychiatric diseases.63 Although a link between CRH and anxiety is less clear in humans than in animals, compelling data exist.45 Single nucleotide polymorphisms (SNPs) in CRH and CRHR1 are associated with phenotypes linked to anxiety,64 depression,65, 66 and anti-depressant treatment responses.67, 68 Postmortem studies of depressed persons reveal increased numbers of CRH-expressing neurons in the hypothalamus,69 decreased CRH binding in frontal cortex,70 and increased CRH content of cerebral spinal fluid.71 Nonetheless, of the eight completed phase II and III drug trials of CRHR1 nonpeptide antagonists in subjects with either depression or anxiety, six have been largely negative72–75 and findings of the other two have not been disclosed.45 How can our results in Sim1CrhKO mice be reconciled with these negative CRHR1 antagonist trial outcomes? Griebel and Holsboer have offered several plausible explanations ranging from the preclinical use of inappropriate animal models to the clinical phase II/III use of heterogeneous trial subjects.45 We offer another explanation based upon the anxiolytic effect of selective Crh deficiency in Sim1CrhKO mice. Rather than targeting CRH receptors throughout the brain, perhaps more focused inhibition of CRH production or release from neurons that co-express CRH and SIM1 would be more effective in the treatment of anxiety and depression disorders. Although this will be technically challenging, CRH originating in SIM1 neurons may be a promising therapeutic target for the treatment of psychiatric diseases.
Supplementary Material
Acknowledgments
We thank Drs. Clifford B. Saper and Nick Andrews for their helpful discussion. We acknowledge the BCH Neurodevelopmental Behavior Core (IDDRC, P30 HD 18655) and the Rodent Histopathology Core lab, Harvard Medical School for their technical support. We thank Dr. Bradford Lowell for the kind gift of Crh-ires-Cre mice. This study was supported by National Institutes of Health Grants 5K01MH096148-03 (to R.Z), T32DK007699-30 (to J.A.M), and NSFC 31271095 (to R.Z.).
Footnotes
SUPPLEMENTARY INFORMATION
Supplementary information is available at the Molecular Psychiatry website.
CONFLICT OF INTEREST
The authors declare no conflict interest.
References
- 1.McEwen BS. Central effects of stress hormones in health and disease: Understanding the protective and damaging effects of stress and stress mediators. European journal of pharmacology. 2008;583(2–3):174–185. doi: 10.1016/j.ejphar.2007.11.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Toth M, Gresack JE, Bangasser DA, Plona Z, Valentino RJ, Flandreau EI, et al. Forebrain-specific CRF overproduction during development is sufficient to induce enduring anxiety and startle abnormalities in adult mice. Neuropsychopharmacology. 2014;39(6):1409–1419. doi: 10.1038/npp.2013.336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bale TL, Vale WW. CRF and CRF receptors: role in stress responsivity and other behaviors. Annu Rev Pharmacol Toxicol. 2004;44:525–557. doi: 10.1146/annurev.pharmtox.44.101802.121410. [DOI] [PubMed] [Google Scholar]
- 4.McEwen BS. Physiology and neurobiology of stress and adaptation: central role of the brain. Physiol Rev. 2007;87(3):873–904. doi: 10.1152/physrev.00041.2006. [DOI] [PubMed] [Google Scholar]
- 5.Dallman MF. Modulation of stress responses: how we cope with excess glucocorticoids. Exp Neurol. 2007;206(2):179–182. doi: 10.1016/j.expneurol.2007.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vale W, Spiess J, Rivier C, Rivier J. Characterization of a 41-residue ovine hypothalamic peptide that stimulates secretion of corticotropin and beta-endorphin. Science. 1981;213(4514):1394–1397. doi: 10.1126/science.6267699. [DOI] [PubMed] [Google Scholar]
- 7.Widmaier EP, Dallman MF. The effects of corticotropin-releasing factor on adrenocorticotropin secretion from perifused pituitaries in vitro: rapid inhibition by glucocorticoids. Endocrinology. 1984;115(6):2368–2374. doi: 10.1210/endo-115-6-2368. [DOI] [PubMed] [Google Scholar]
- 8.Bao AM, Swaab DF. Corticotropin-releasing hormone and arginine vasopressin in depression focus on the human postmortem hypothalamus. Vitam Horm. 2010;82:339–365. doi: 10.1016/S0083-6729(10)82018-7. [DOI] [PubMed] [Google Scholar]
- 9.Choleris E, Devidze N, Kavaliers M, Pfaff DW. Steroidal/neuropeptide interactions in hypothalamus and amygdala related to social anxiety. Prog Brain Res. 2008;170:291–303. doi: 10.1016/S0079-6123(08)00424-X. [DOI] [PubMed] [Google Scholar]
- 10.Rodrigues SM, LeDoux JE, Sapolsky RM. The influence of stress hormones on fear circuitry. Annu Rev Neurosci. 2009;32:289–313. doi: 10.1146/annurev.neuro.051508.135620. [DOI] [PubMed] [Google Scholar]
- 11.Arnett MG, Kolber BJ, Boyle MP, Muglia LJ. Behavioral insights from mouse models of forebrain–and amygdala-specific glucocorticoid receptor genetic disruption. Mol Cell Endocrinol. 2011;336(1–2):2–5. doi: 10.1016/j.mce.2010.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Finn DA, Rutledge-Gorman MT, Crabbe JC. Genetic animal models of anxiety. Neurogenetics. 2003;4(3):109–135. doi: 10.1007/s10048-003-0143-2. [DOI] [PubMed] [Google Scholar]
- 13.Muglia L, Jacobson L, Dikkes P, Majzoub JA. Corticotropin-releasing hormone deficiency reveals major fetal but not adult glucocorticoid need. Nature. 1995;373(6513):427–432. doi: 10.1038/373427a0. [DOI] [PubMed] [Google Scholar]
- 14.Weninger SC, Dunn AJ, Muglia LJ, Dikkes P, Miczek KA, Swiergiel AH, et al. Stress-induced behaviors require the corticotropin-releasing hormone (CRH) receptor, but not CRH. Proc Natl Acad Sci U S A. 1999;96(14):8283–8288. doi: 10.1073/pnas.96.14.8283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dunn AJ, Swiergiel AH. Behavioral responses to stress are intact in CRF-deficient mice. Brain Res. 1999;845(1):14–20. doi: 10.1016/s0006-8993(99)01912-5. [DOI] [PubMed] [Google Scholar]
- 16.van Gaalen MM, Stenzel-Poore MP, Holsboer F, Steckler T. Effects of transgenic overproduction of CRH on anxiety-like behaviour. Eur J Neurosci. 2002;15(12):2007–2015. doi: 10.1046/j.1460-9568.2002.02040.x. [DOI] [PubMed] [Google Scholar]
- 17.Smith GW, Aubry JM, Dellu F, Contarino A, Bilezikjian LM, Gold LH, et al. Corticotropin releasing factor receptor 1-deficient mice display decreased anxiety, impaired stress response, and aberrant neuroendocrine development. Neuron. 1998;20(6):1093–1102. doi: 10.1016/s0896-6273(00)80491-2. [DOI] [PubMed] [Google Scholar]
- 18.Timpl P, Spanagel R, Sillaber I, Kresse A, Reul JM, Stalla GK, et al. Impaired stress response and reduced anxiety in mice lacking a functional corticotropin-releasing hormone receptor 1. Nat Genet. 1998;19(2):162–166. doi: 10.1038/520. [DOI] [PubMed] [Google Scholar]
- 19.Bale TL, Contarino A, Smith GW, Chan R, Gold LH, Sawchenko PE, et al. Mice deficient for corticotropin-releasing hormone receptor-2 display anxiety-like behaviour and are hypersensitive to stress. Nature genetics. 2000;24(4):410–414. doi: 10.1038/74263. [DOI] [PubMed] [Google Scholar]
- 20.Kita I, Seki Y, Nakatani Y, Fumoto M, Oguri M, Sato-Suzuki I, et al. Corticotropin-releasing factor neurons in the hypothalamic paraventricular nucleus are involved in arousal/yawning response of rats. Behavioural brain research. 2006;169(1):48–56. doi: 10.1016/j.bbr.2005.12.003. [DOI] [PubMed] [Google Scholar]
- 21.Rodaros D, Caruana DA, Amir S, Stewart J. Corticotropin-releasing factor projections from limbic forebrain and paraventricular nucleus of the hypothalamus to the region of the ventral tegmental area. Neuroscience. 2007;150(1):8–13. doi: 10.1016/j.neuroscience.2007.09.043. [DOI] [PubMed] [Google Scholar]
- 22.Hsu DT, Price JL. Paraventricular thalamic nucleus: subcortical connections and innervation by serotonin, orexin, and corticotropin-releasing hormone in macaque monkeys. The Journal of comparative neurology. 2009;512(6):825–848. doi: 10.1002/cne.21934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Balthasar N, Dalgaard LT, Lee CE, Yu J, Funahashi H, Williams T, et al. Divergence of melanocortin pathways in the control of food intake and energy expenditure. Cell. 2005;123(3):493–505. doi: 10.1016/j.cell.2005.08.035. [DOI] [PubMed] [Google Scholar]
- 24.Fan CM, Kuwana E, Bulfone A, Fletcher CF, Copeland NG, Jenkins NA, et al. Expression patterns of two murine homologs of Drosophila single-minded suggest possible roles in embryonic patterning and in the pathogenesis of Down syndrome. Molecular and cellular neurosciences. 1996;7(1):1–16. doi: 10.1006/mcne.1996.0001. [DOI] [PubMed] [Google Scholar]
- 25.Liu P, Jenkins NA, Copeland NG. A highly efficient recombineering-based method for generating conditional knockout mutations. Genome Res. 2003;13(3):476–484. doi: 10.1101/gr.749203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vahl TP, Ulrich-Lai YM, Ostrander MM, Dolgas CM, Elfers EE, Seeley RJ, et al. Comparative analysis of ACTH and corticosterone sampling methods in rats. Am J Physiol Endocrinol Metab. 2005;289(5):E823–828. doi: 10.1152/ajpendo.00122.2005. [DOI] [PubMed] [Google Scholar]
- 27.Rogers DC, Fisher EM, Brown SD, Peters J, Hunter AJ, Martin JE. Behavioral and functional analysis of mouse phenotype: SHIRPA, a proposed protocol for comprehensive phenotype assessment. Mamm Genome. 1997;8(10):711–713. doi: 10.1007/s003359900551. [DOI] [PubMed] [Google Scholar]
- 28.Solomon MB, Furay AR, Jones K, Packard AE, Packard BA, Wulsin AC, et al. Deletion of forebrain glucocorticoid receptors impairs neuroendocrine stress responses and induces depression-like behavior in males but not females. Neuroscience. 2012;203:135–143. doi: 10.1016/j.neuroscience.2011.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Marriott AS, Smith EF. An analysis of drug effects in mice exposed to a simple novel environment. Psychopharmacologia. 1972;24(3):397–406. doi: 10.1007/BF00402534. [DOI] [PubMed] [Google Scholar]
- 30.Boissier JR, Simon P. The exploration reaction in the mouse Preliminary note. Therapie. 1962;17:1225–1232. [PubMed] [Google Scholar]
- 31.Pellow S, Chopin P, File SE, Briley M. Validation of open:closed arm entries in an elevated plus-maze as a measure of anxiety in the rat. J Neurosci Methods. 1985;14(3):149–167. doi: 10.1016/0165-0270(85)90031-7. [DOI] [PubMed] [Google Scholar]
- 32.Crawley J, Goodwin FK. Preliminary report of a simple animal behavior model for the anxiolytic effects of benzodiazepines. Pharmacol Biochem Behav. 1980;13(2):167–170. doi: 10.1016/0091-3057(80)90067-2. [DOI] [PubMed] [Google Scholar]
- 33.Bevins RA, Besheer J, Palmatier MI, Jensen HC, Pickett KS, Eurek S. Novel-object place conditioning: behavioral and dopaminergic processes in expression of novelty reward. Behav Brain Res. 2002;129(1–2):41–50. doi: 10.1016/s0166-4328(01)00326-6. [DOI] [PubMed] [Google Scholar]
- 34.Ennaceur A, Michalikova S, Bradford A, Ahmed S. Detailed analysis of the behavior of Lister and Wistar rats in anxiety, object recognition and object location tasks. Behav Brain Res. 2005;159(2):247–266. doi: 10.1016/j.bbr.2004.11.006. [DOI] [PubMed] [Google Scholar]
- 35.Silvers JM, Harrod SB, Mactutus CF, Booze RM. Automation of the novel object recognition task for use in adolescent rats. J Neurosci Methods. 2007;166(1):99–103. doi: 10.1016/j.jneumeth.2007.06.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kutlu MG, Gould TJ. Nicotine modulation of fear memories and anxiety: Implications for learning and anxiety disorders. Biochem Pharmacol. 2015;97(4):498–511. doi: 10.1016/j.bcp.2015.07.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhang R, Jankord R, Flak JN, Solomon MB, D’Alessio DA, Herman JP. Role of glucocorticoids in tuning hindbrain stress integration. J Neurosci. 2010;30(44):14907–14914. doi: 10.1523/JNEUROSCI.0522-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Asai M, Ramachandrappa S, Joachim M, Shen Y, Zhang R, Nuthalapati N, et al. Loss of function of the melanocortin 2 receptor accessory protein 2 is associated with mammalian obesity. Science. 2013;341(6143):275–278. doi: 10.1126/science.1233000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Paxinos G, Franklin K. The mouse brain. Elsevier. 2012 [Google Scholar]
- 40.Huo L, Grill HJ, Bjorbaek C. Divergent regulation of proopiomelanocortin neurons by leptin in the nucleus of the solitary tract and in the arcuate hypothalamic nucleus. Diabetes. 2006;55(3):567–573. doi: 10.2337/diabetes.55.03.06.db05-1143. [DOI] [PubMed] [Google Scholar]
- 41.Munzberg H, Flier JS, Bjorbaek C. Region-specific leptin resistance within the hypothalamus of diet-induced obese mice. Endocrinology. 2004;145(11):4880–4889. doi: 10.1210/en.2004-0726. [DOI] [PubMed] [Google Scholar]
- 42.Rozen S, Skaletsky H. Primer3 on the WWW for general users and for biologist programmers. Methods Mol Biol. 2000;132:365–386. doi: 10.1385/1-59259-192-2:365. [DOI] [PubMed] [Google Scholar]
- 43.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25(4):402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 44.Krashes MJ, Shah BP, Madara JC, Olson DP, Strochlic DE, Garfield AS, et al. An excitatory paraventricular nucleus to AgRP neuron circuit that drives hunger. Nature. 2014;507(7491):238–242. doi: 10.1038/nature12956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Griebel G, Holsboer F. Neuropeptide receptor ligands as drugs for psychiatric diseases: the end of the beginning? Nat Rev Drug Discov. 2012;11(6):462–478. doi: 10.1038/nrd3702. [DOI] [PubMed] [Google Scholar]
- 46.Oades RD, Isaacson RL. The development of food search behavior by rats: the effects of hippocampal damage and haloperidol. Behav Biol. 1978;24(3):327–337. doi: 10.1016/s0091-6773(79)90184-6. [DOI] [PubMed] [Google Scholar]
- 47.Christmas AJ, Maxwell DR. A comparison of the effects of some benzodiazepines and other drugs on aggressive and exploratory behaviour in mice and rats. Neuropharmacology. 1970;9(1):17–29. doi: 10.1016/0028-3908(70)90044-4. [DOI] [PubMed] [Google Scholar]
- 48.Crawley J. What’s Wrong With My Mouse?: Behavioral Phenotyping of Transgenic and Knockout Mice. 2nd. Wiley-Liss; 2007. [Google Scholar]
- 49.Lakso M, Pichel JG, Gorman JR, Sauer B, Okamoto Y, Lee E, et al. Efficient in vivo manipulation of mouse genomic sequences at the zygote stage. Proc Natl Acad Sci U S A. 1996;93(12):5860–5865. doi: 10.1073/pnas.93.12.5860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Muglia LJ, Bae DS, Brown TT, Vogt SK, Alvarez JG, Sunday ME, et al. Proliferation and differentiation defects during lung development in corticotropin-releasing hormone-deficient mice. Am J Respir Cell Mol Biol. 1999;20(2):181–188. doi: 10.1165/ajrcmb.20.2.3381. [DOI] [PubMed] [Google Scholar]
- 51.Muglia LJ, Jacobson L, Weninger SC, Luedke CE, Bae DS, Jeong KH, et al. Impaired diurnal adrenal rhythmicity restored by constant infusion of corticotropin-releasing hormone in corticotropin-releasing hormone-deficient mice. J Clin Invest. 1997;99(12):2923–2929. doi: 10.1172/JCI119487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jacobson L, Muglia LJ, Weninger SC, Pacak K, Majzoub JA. CRH deficiency impairs but does not block pituitary-adrenal responses to diverse stressors. Neuroendocrinology. 2000;71(2):79–87. doi: 10.1159/000054524. [DOI] [PubMed] [Google Scholar]
- 53.Waters RP, Rivalan M, Bangasser DA, Deussing JM, Ising M, Wood SK, et al. Evidence for the role of corticotropin-releasing factor in major depressive disorder. Neurosci Biobehav Rev. 2015;58:63–78. doi: 10.1016/j.neubiorev.2015.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Greenman Y, Kuperman Y, Drori Y, Asa SL, Navon I, Forkosh O, et al. Postnatal ablation of POMC neurons induces an obese phenotype characterized by decreased food intake and enhanced anxiety-like behavior. Mol Endocrinol. 2013;27(7):1091–1102. doi: 10.1210/me.2012-1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Muglia LJ, Jenkins NA, Gilbert DJ, Copeland NG, Majzoub JA. Expression of the mouse corticotropin-releasing hormone gene in vivo and targeted inactivation in embryonic stem cells. J Clin Invest. 1994;93(5):2066–2072. doi: 10.1172/JCI117201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Geerling JC, Shin JW, Chimenti PC, Loewy AD. Paraventricular hypothalamic nucleus: axonal projections to the brainstem. J Comp Neurol. 2010;518(9):1460–1499. doi: 10.1002/cne.22283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Cullinan WE, Herman JP, Watson SJ. Ventral subicular interaction with the hypothalamic paraventricular nucleus: evidence for a relay in the bed nucleus of the stria terminalis. J Comp Neurol. 1993;332(1):1–20. doi: 10.1002/cne.903320102. [DOI] [PubMed] [Google Scholar]
- 58.Dong HW, Petrovich GD, Watts AG, Swanson LW. Basic organization of projections from the oval and fusiform nuclei of the bed nuclei of the stria terminalis in adult rat brain. J Comp Neurol. 2001;436(4):430–455. doi: 10.1002/cne.1079. [DOI] [PubMed] [Google Scholar]
- 59.Rinaman L. Hindbrain noradrenergic A2 neurons: diverse roles in autonomic, endocrine, cognitive, and behavioral functions. Am J Physiol Regul Integr Comp Physiol. 2011;300(2):R222–235. doi: 10.1152/ajpregu.00556.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ahima RS, Harlan RE. Charting of type II glucocorticoid receptor-like immunoreactivity in the rat central nervous system. Neuroscience. 1990;39(3):579–604. doi: 10.1016/0306-4522(90)90244-x. [DOI] [PubMed] [Google Scholar]
- 61.Fuxe K, Agnati LF. Receptor-receptor interactions in the central nervous system. A new integrative mechanism in synapses. Med Res Rev. 1985;5(4):441–482. doi: 10.1002/med.2610050404. [DOI] [PubMed] [Google Scholar]
- 62.Makino S, Gold PW, Schulkin J. Corticosterone effects on corticotropin-releasing hormone mRNA in the central nucleus of the amygdala and the parvocellular region of the paraventricular nucleus of the hypothalamus. Brain Res. 1994;640(1–2):105–112. doi: 10.1016/0006-8993(94)91862-7. [DOI] [PubMed] [Google Scholar]
- 63.Kessler RC, Chiu WT, Demler O, Merikangas KR, Walters EE. Prevalence, severity, and comorbidity of 12-month DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry. 2005;62(6):617–627. doi: 10.1001/archpsyc.62.6.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Smoller JW, Rosenbaum JF, Biederman J, Kennedy J, Dai D, Racette SR, et al. Association of a genetic marker at the corticotropin-releasing hormone locus with behavioral inhibition. Biol Psychiatry. 2003;54(12):1376–1381. doi: 10.1016/s0006-3223(03)00598-5. [DOI] [PubMed] [Google Scholar]
- 65.Liu Z, Zhu F, Wang G, Xiao Z, Wang H, Tang J, et al. Association of corticotropin-releasing hormone receptor1 gene SNP and haplotype with major depression. Neurosci Lett. 2006;404(3):358–362. doi: 10.1016/j.neulet.2006.06.016. [DOI] [PubMed] [Google Scholar]
- 66.Polanczyk G, Caspi A, Williams B, Price TS, Danese A, Sugden K, et al. Protective effect of CRHR1 gene variants on the development of adult depression following childhood maltreatment: replication and extension. Arch Gen Psychiatry. 2009;66(9):978–985. doi: 10.1001/archgenpsychiatry.2009.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Licinio J, O’Kirwan F, Irizarry K, Merriman B, Thakur S, Jepson R, et al. Association of a corticotropin-releasing hormone receptor 1 haplotype and antidepressant treatment response in Mexican-Americans. Mol Psychiatry. 2004;9(12):1075–1082. doi: 10.1038/sj.mp.4001587. [DOI] [PubMed] [Google Scholar]
- 68.Papiol S, Arias B, Gasto C, Gutierrez B, Catalan R, Fananas L. Genetic variability at HPA axis in major depression and clinical response to antidepressant treatment. J Affect Disord. 2007;104(1–3):83–90. doi: 10.1016/j.jad.2007.02.017. [DOI] [PubMed] [Google Scholar]
- 69.Purba JS, Hoogendijk WJ, Hofman MA, Swaab DF. Increased number of vasopressin-and oxytocin-expressing neurons in the paraventricular nucleus of the hypothalamus in depression. Arch Gen Psychiatry. 1996;53(2):137–143. doi: 10.1001/archpsyc.1996.01830020055007. [DOI] [PubMed] [Google Scholar]
- 70.Nemeroff CB, Owens MJ, Bissette G, Andorn AC, Stanley M. Reduced corticotropin releasing factor binding sites in the frontal cortex of suicide victims. Arch Gen Psychiatry. 1988;45(6):577–579. doi: 10.1001/archpsyc.1988.01800300075009. [DOI] [PubMed] [Google Scholar]
- 71.Arato M, Banki CM, Bissette G, Nemeroff CB. Elevated CSF CRF in suicide victims. Biol Psychiatry. 1989;25(3):355–359. doi: 10.1016/0006-3223(89)90183-2. [DOI] [PubMed] [Google Scholar]
- 72.Binneman B, Feltner D, Kolluri S, Shi Y, Qiu R, Stiger T. A 6-week randomized, placebo-controlled trial of CP-316,311 (a selective CRH1 antagonist) in the treatment of major depression. Am J Psychiatry. 2008;165(5):617–620. doi: 10.1176/appi.ajp.2008.07071199. [DOI] [PubMed] [Google Scholar]
- 73.Coric V, Feldman HH, Oren DA, Shekhar A, Pultz J, Dockens RC, et al. Multicenter, randomized, double-blind, active comparator and placebo-controlled trial of a corticotropin-releasing factor receptor-1 antagonist in generalized anxiety disorder. Depress Anxiety. 2010;27(5):417–425. doi: 10.1002/da.20695. [DOI] [PubMed] [Google Scholar]
- 74.Kirchhoff VD, Nguyen HT, Soczynska JK, Woldeyohannes H, McIntyre RS. Discontinued psychiatric drugs in 2008. Expert Opin Investig Drugs. 2009;18(10):1431–1443. doi: 10.1517/13543780903184591. [DOI] [PubMed] [Google Scholar]
- 75.Zobel AW, Nickel T, Kunzel HE, Ackl N, Sonntag A, Ising M, et al. Effects of the high-affinity corticotropin-releasing hormone receptor 1 antagonist R121919 in major depression: the first 20 patients treated. J Psychiatr Res. 2000;34(3):171–181. doi: 10.1016/s0022-3956(00)00016-9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


