Abstract
1.1. Objectives
Bicuspid aortic valve (BAV) patients can develop thoracic aortic aneurysms (TAA) and therefore require serial imaging to monitor aortic growth. This study investigates the reliability of contrast-enhanced MR angiography (CEMRA) volumetry compared to two-dimensional diameter measurements to identify TAA growth.
1.2. Materials and Methods
A retrospective, IRB approved and HIPAA compliant study was conducted on 20 BAV patients (45±8.9 years old, 20% women) who underwent serial CEMRA with a minimum imaging follow-up of 11 months. MRI was performed at 1.5 T with ECG-gated time-resolved CEMRA. Independent observers measured the diameter at the sinuses of Valsalva (SOV) and mid-ascending aorta (MAA) as well as ascending aorta volume between the aortic valve annulus and innominate branch. Intra/inter-observer coefficient of variation (COV) and intraclass correlation coefficient (ICC) were computed to assess reliability. Growth rates were calculated and assessed by Student’s t-test (p<0.05 significant). The diameter of maximal growth (DMG), defined as the diameter at SOV or MAA with the faster growth rate, was recorded.
1.3. Results
The mean time of follow-up was 2.6±0.82 years (y). The intraobserver COV was 0.01 for SOV, 0.02 for MAA, and 0.02 for volume (interoberserver COV: 0.02, 0.03, 0.04, respectively). The ICC was 0.83 for SOV, 0.86 for MAA, 0.90 for DMG, and 0.95 for volume. Average aortic measurements at baseline and (follow-up) were 42±3 mm (42±3 mm, p=0.11) at SOV, 46±4 mm (47±4 mm, p<0.05) at MAA, and 130±23 mL (144±24 mL, p<0.05). Average size changes were 0.2±0.6 mm/y (1%±2%) at SOV, 0.5±0.8 mm/y (1%±2%) at MAA, 0.7±0.7 mm/y (2%±2%) at DMG, and 6±3 mL/y (4%±3%) with volumetry.
1.4. Conclusions
3D CEMRA volumetry exhibited a larger effect when examining percentage growth, a better ICC, and a marginally lower COV. Volumetry may be more sensitive to growth and possibly less affected by error than diameter measurements.
Keywords: Contrast-enhanced MR Angiography Volumetry, Bicuspid Aortic Valve, Thoracic Ascending Aorta, Ascending Aorta Aneurysm
2. Introduction
Thoracic aortic aneurysms (TAA) and dissections have mortality rates of up to 70% and are responsible for more than 15,000 deaths in the United states annually, making it the 15th most common cause of death in individuals greater than 65 years old (1–3). The pathophysiology of aortic aneurysms is generally described as a loss of elastic fibers and an accumulation of proteoglycan in the aortic media layer leading to weakening of the aortic wall (4, 5). TAA are typically asymptomatic and incidentally identified on imaging studies and thus generally present with acute signs of pain consistent with impending rupture or dissection (5). Fortunately, new advances in technology and imaging modalities in the last decades have enabled physicians to reliably screen TAA noninvasively, treat accordingly, and prevent emergent scenarios from developing (6).
Patients with bicuspid aortic valves (BAV) are a select group known to have an increased risk of ascending aorta dilatation with dissection or rupture. These patients require serial monitoring with imaging from transthoracic echocardiography (TTE), computed tomographic angiography (CTA), or magnetic resonance angiography (MRA) for appropriate timing of surgical repair of the aortic valve and ascending aorta (7–9). Published guidelines recommend elective surgery in BAV patients based on diametric size (>55 mm) and growth rate (>5 mm/year) at the ascending aorta or aortic sinus (4, 10–12). Patients with BAV are particularly difficult to monitor due to several reasons. Firstly, BAV is a congenital disease that is followed with frequent imaging after diagnosed. Therefore, since CTA exposes patients to ionizing radiation and TTE is diagnostically inadequate in 10%–15% of patients, MRA is becoming a mainstay for imaging patients with BAV (8). Secondly, patients with BAV have slow evolving TAA with reported growth rates of less than 1 mm/ year, making the detection of growth difficult with current measurement approaches (7, 8, 13). Recent literature has shown maximal diameter measurements in the aorta may be insensitive to focal aneurysmal changes (14).
To date, three-dimensional (3D) volume reconstructions have been utilized to study abdominal aortic aneurysms and to track growth kinetics of tumors as a more reliable and sensitive measure of treatment response (1, 14–16). From these studies, we hypothesize that 3D segmentation is a comprehensive measurement of the entire volume of interest that allows capture of focal interval changes in vessel caliber that may be missed with cross-sectional two-dimensional (2D) diameter measurements, which are highly dependent on slice selection and angulation (14). As such, 3D volume analysis may be better suited to guide risk stratification and surgical management of patients with BAV. Therefore, the purpose of this study is to assess the inter-and inta-observer reliability and sensitivity of CEMRA volumetry compared to 2D diameter measurements of the ascending aorta in patients with BAV.
3. Materials and Methods
3.1. Study Design
This study was approved by our institutional board review and performed in compliance with Health Insurance Portability and Accountability Act regulations. A retrospective, single center study was conducted on 20 patients with BAV who underwent a baseline and follow-up contrast enhanced MRA (CEMRA) exam between September 1st, 2009 and March 5th, 2014 at a tertiary care, university hospital in Chicago, IL, USA. Patients with a history of known aortic dissection or a history of cardiac surgery or surgery on the aorta or aortic valve before or between two serial scans were excluded from the study. Clinical data including age, gender, and study dates were collected for demographic information.
3.2. Cardiac Magnetic Resonance
Images were acquired on a 1.5T (Magnetom Avanto, Siemens Medical Systems, Erlangen, Germany). All subjects underwent a standard of care cardiovascular MRI, including ECG gated time-resolved cine cardiac MRI for evaluation of cardiac function and valve morphology, as well as contrast enhanced MR angiography (CEMRA) for quantification of aortic dimensions. Briefly, the vendor CE-MRA product sequence was carried out during the intravenous injection of 0.2 mmol/kg of Gadolinium-DTPA (Magnevist, Bayer Pharmaceuticals) in a sagittal oblique orientation with a 3D fast low angle shot (FLASH) sequence. The following imaging parameters were used: repetition time/echo time (TR/TE): 2.5/1.2 ms; flip angle: 25 degrees; field of view: 300 × 400 mm; matrix: 320 × 512; slice: 1.1 mm; voxel size: 0.9 × 0.8 × 1.1 mm; GRAPPA acceleration × 2; 6/8 partial Fourier in x-y and z axes; acquisition time: 18–20 s. K-space was acquired with elliptic centric encoding to mitigate respiratory noncompliance and the acquisition was gated to diastole to minimize motion artifact at the aortic root. Pre-contrast and post-contrast 3D data sets were acquired for automatic image subtraction to remove background signal. The unsubtracted 2D images were used for diameter measurements and the subtracted 3D images were used for volume measurements.
3.3. Diameter Measurements
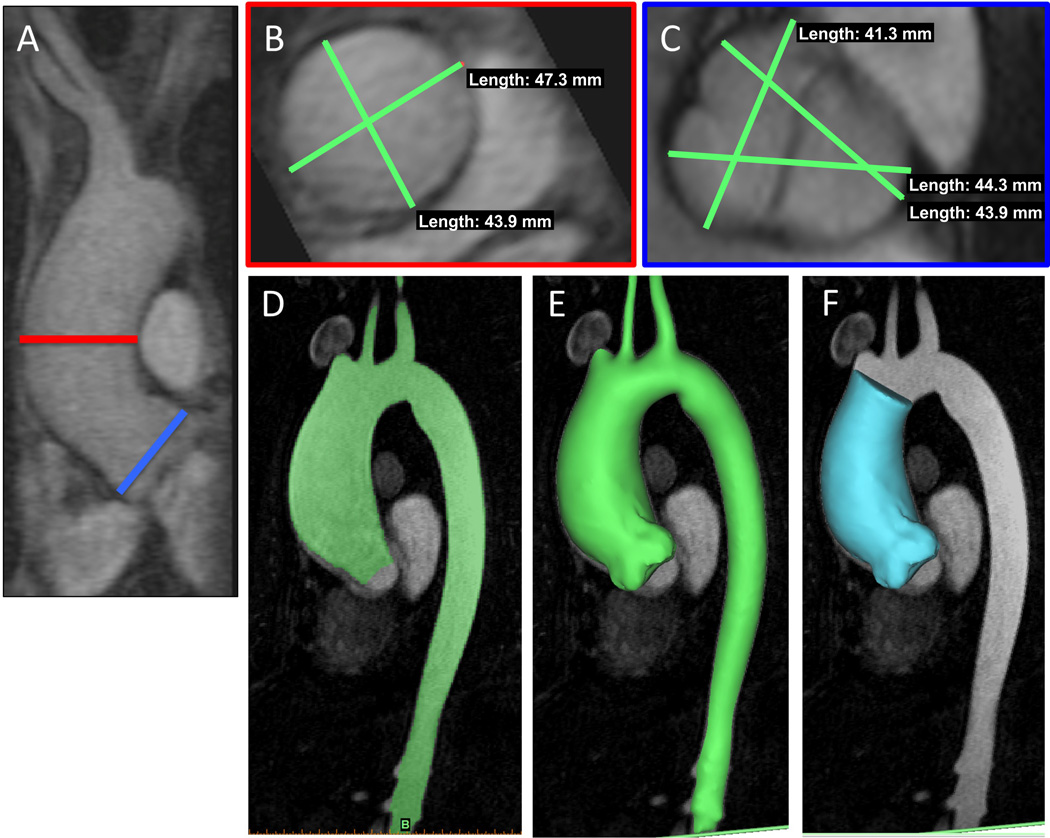
Baseline and follow-up diameter measurements were obtained at the sinuses of Valsalva (SOV) and mid-ascending aorta (MAA) using Horos, a free, open source medical image viewer, by independent observers (MPFB, BT, OR) (see Figure 1A–C). Two observers (BT, OR) repeated the diameter measurements at the SOV and MAA for intra-observer evaluations. Three diameter measurements were taken at the SOV from sinus to sinus, including the external walls; the maximum value of these three measurements was considered the final SOV diameter. At the mid-ascending aorta, two orthogonal diameter measurements were taken orthonormal to the vessel, including the external walls; the higher value was considered the MAA diameter. SOV and MAA growth rates were calculated from changes in the maximal SOV and changes in the maximal MAA from baseline to follow-up scans, respectively. The observers performed these measurements independently without knowledge of prior measurements. Given that surgical intervention for TAA is recommended based on the fastest growing diameter at SOV or MAA (12), the diameter of maximal growth (DMG), which we defined as the SOV or MAA with the faster growth rate, was also recorded for every patient to replicate the clinical setting.
Figure 1.
Diameter and Volume Measurement Pictures: (A) Sagittal view of the ascending aorta (AA). MAA measurements taken at the level of the red line. SOV measurements taken at the level of the blue line. (B) MAA measurements at the luminal cross-section of the AA. (C) SOV measurements at the aortic annulus. (D) Preprocessed AA. (E) 3D reconstruction of AA. (F) AA segmented from aortic annulus to first innominate branch.
The growth at SOV, MAA, and DMG, were recorded as both units (mm) and as percentages (%) in order to compare the differences in measured effect size. Diameter percentages were calculated from the measured difference between baseline and follow-up scans divided by the baseline measurements. Growth rates were reported as units per time and percentage per time.
3.4. Volume Measurements
Baseline and follow-up volumetric measurements of the ascending aorta were calculated from the same CEMRA data using a 3D segmentation software platform (Mimics, Materialize, Leuven, Belgium) by two independent observers (ID, OR). A single observer (OR) repeated the segmentation and volumetric measurements for intra-observer evaluations. The ascending aorta was segmented starting from the boundary of the aortic annulus to the orthogonal aorta cross-section occurring immediately at the takeoff for the brachiocephalic artery. Segmentation was achieved using the CEMRA images (Figure 1D–F). Briefly, segmentation was performed by manually thresholding the images to best delineate the aorta lumen and wall. The vessel was manually edited to add lumen regions with low signal and the aortic borders were separated from surrounding vasculature. A point was subsequently selected within the aorta and region growing was performed to separate the aorta structure from surrounding tissue. Volumetric growth rates were calculated based on the difference between the baseline and follow-up versions of the 3D segmented data. Observers performed measurements independently without knowledge of prior measurements.
The volumetric growth was recorded as both units (mL) and as percentages (%) in order to compare the differences in measured effect size. Volumetric percentages were calculated from the difference in volume between baseline and follow-up scans divided by the baseline volume. Growth rates were reported as units per time and percentage per time.
3.5. Statistical Analysis
Statistical analysis was performed using commercially available software, Matlab (Mathworks, Natick MA) and R (R Development Core Team). All continuous variables were recorded as mean and standard deviation. Binary data points were recorded as absolute frequencies. Differences between baseline and follow-up exams were assessed by two-tailed, paired Student’s t-test. A p-value of <0.05 was considered statistically significant. All baseline to follow-up changes in measurements were included in calculations, even those with negative changes. The inclusion of all measurements was done as an effort to dampen the effects of intrinsic measurement errors.
Coefficients of variability (COV) were calculated for inter-observer and intra-observer measurements at SOV, MAA, and with volumetry. The standard deviation (σ) and mean (µ) of the differences in measurement values between two observations were calculated for every patient at SOV, MAA, and volumetry. The COV was then reported as an average of these ratios (σ/µ) at SOV, MAA and volumetry. The intraclass correlation coefficient (ICC) was computed using a two-way ANOVA model to determine observer reliability based on agreement. Bland Altman analysis with limits of agreement and bias were calculated to assess inter-observer agreement.
4. Results
Four (20%) of the 20 patients with BAV were women. The mean age of all patients at the time of their baseline scan was 45.2±8.9 years old. The mean time for follow up was 2.6±0.8 years (y). The mean aortic measurements at baseline and follow-up at SOV, MAA, and with volumetry are listed in detail in Table 1. Growth was determined to be significant at the MAA, with DMG, and with volumetry (p<0.05).
Table 1.
Clinical Data
| Baseline | Follow- Up | ||||
|---|---|---|---|---|---|
| Mean | SD | Mean | SD | P-Value | |
| Age (Years) | 45.2 | 8.9 | 47.8 | 8.9 | 0.36 |
| Sex (Females) | 4 (20%) | -- | 4 (20%) | -- | -- |
| Sinus of Valsalva Diameter (mm) | 42 | 3 | 42 | 3 | 0.11 |
| Mid Ascending Aorta Diameter (mm) | 46 | 4 | 47 | 4 | 0.004 |
| Ascending Aorta Volume (mL) | 130 | 23 | 144 | 24 | <0.001 |
SD: standard deviation
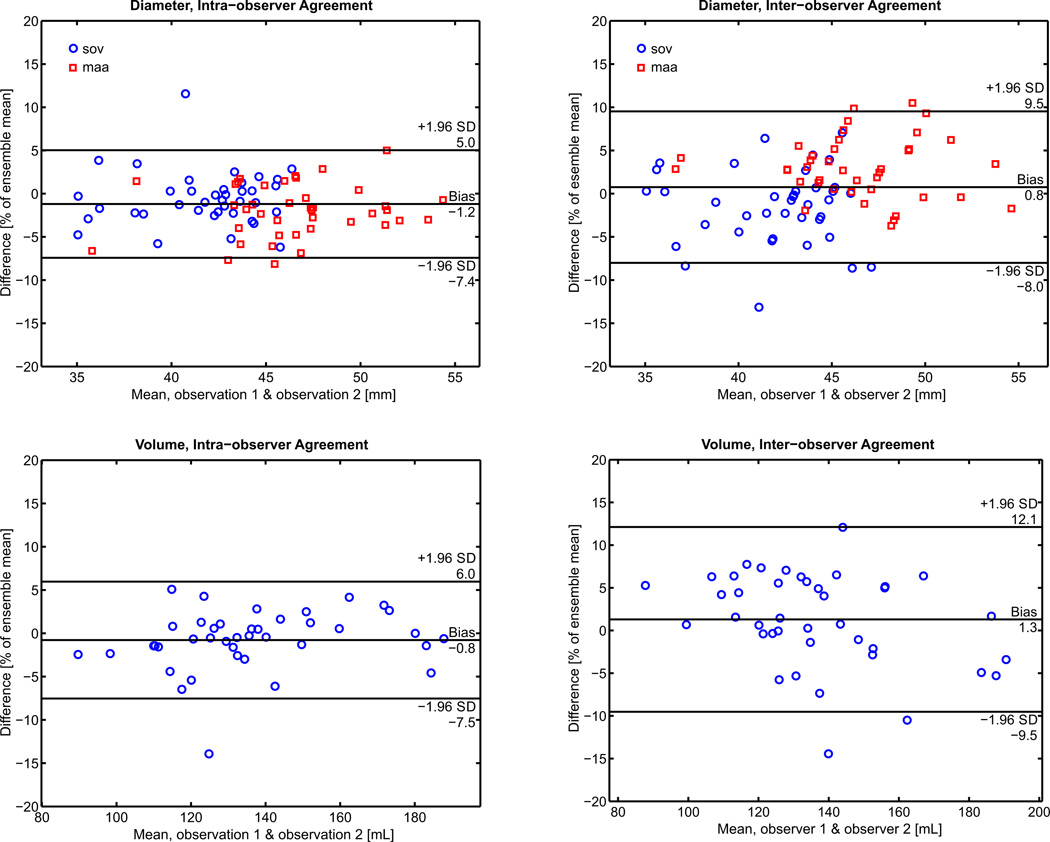
Inter-observer and intra-observer COV for SOV, MAA, and volumetry are listed in detail in Table 3. The ICC was 0.83 for SOV measurements, 0.86 for MAA, and 0.95 for volume measurements (Table 2). Bland-Altman analyses of all diameter and volume measurements for inter-observer and intra-observer agreement are displayed with bias and limits of agreement represented as a function of the percent of the ensemble mean in Figure 2 (to enable comparison between the different units of diameter and volume). In terms of absolute values, the bias and limits of agreement for the intra-observer measurements of SOV and MAA were −0.2±1.4mm and 0.9±1.3mm, respectively. The bias and limits of agreement for the absolute inter-observer measurements of SOV and MAA were −0.6±1.9mm and 1.3±1.6mm, respectively. The bias and limits of agreement for the absolute intra-observer and inter-observer measurements of volume were −1.1±9.1 mL and 1.8±14.8 mL.
Table 3.
Growth Rate
| Units / year | % / year | Total Growth | Total Growth (%) | |||||
|---|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | |
| Sinus of Valsalva (mm) | 0.2 | 0.6 | 0.6% | 2% | 1 | 2 | 1% | 4% |
| Mid-Ascending Aorta Diameter (mm) | 0.5 | 0.8 | 1% | 2% | 1 | 2 | 3% | 4% |
| Diameter of Maximal Growth (mm) | 0.7 | 0.7 | 2% | 2% | 2 | 2 | 4% | 4% |
| Ascending Aorta Volume (mL) | 6 | 3 | 4% | 3% | 14 | 9 | 11% | 7% |
SD: Standard deviation
Table 2.
Reliability
| Interobserver COV |
Intraobserver COV |
Intraclass Correlation Coefficient [CI] |
|
|---|---|---|---|
| Sinus of Valsalva | 0.02 | 0.01 | 0.83 [0.69–0.91] |
| Mid-Ascending Aorta Diameter | 0.03 | 0.02 | 0.86 [0.50–0.95] |
| Ascending Aorta Volume | 0.04 | 0.02 | 0.95 [0.91–0.97] |
COV: Coefficient of variation
CI: Confidence Interval
Figure 2.
Interobserver and intraobserver Bland Altman analysis of diameter measurements and volume measurements. Limits of agreement and bias are reported on the horizontal bars as a function of the percent of the ensemble mean. Note: the values for the limits of agreement of the individual MAA and SOV measurements are reported in the results section.
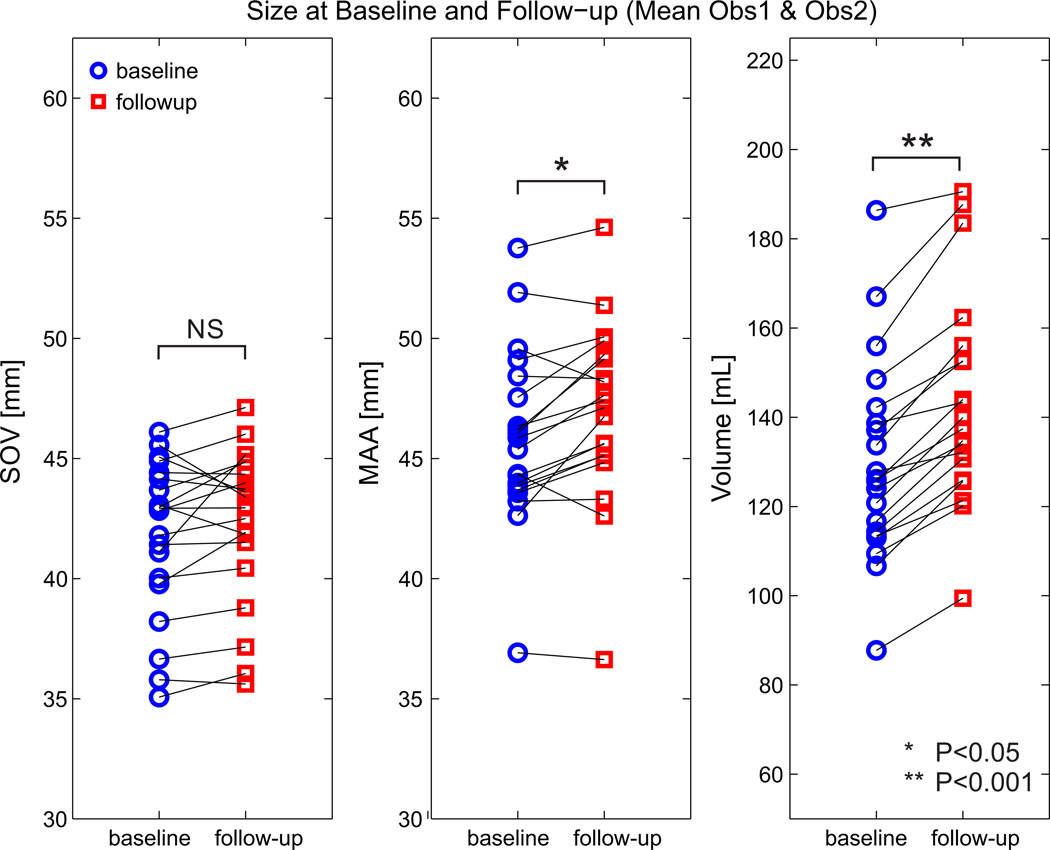
Average size changes between baseline and follow-up were 0.2±0.6 mm/y (1±2 %/y) at SOV (p>0.05), 0.5±0.8 mm/y (1±2 %/y) at MAA (p<0.05), and 0.7±0.7 mm/y (2±2 %/y) at DMG (p<0.05) compared to 6±3 mL/y (4±3 %/y) with volumetry (p<0.05) (Figure 3). Growth rates over the entire follow-up period (mean of 2.6 years) are also displayed in Table 3. Over the entire follow up period, the percent growth of the volume measurement was 3.7 times greater than the MAA percentage growth (11% vs 3%, p<0.001) and 2.8 times greater than the DMG percentage growth (11% vs 4%, p<0.001) (Table 3).
Figure 3.
Changes in growth as a function of diameter measurement location or volumetry.
5. Discussion
Patients with bicuspid aortic valves are known to be at increased risk for aortic pathology including aortic dilatation, dissection, and rupture. Serial follow-up imaging is essential for appropriate timing of surgical intervention (17). Current guidelines from 2014 recommend that patients with BAV should have an initial transthoracic echocardiogram (TTE) study for assessment of their valve morphology and aortic annulus, sinus, sinotubular junction, and mid-ascending aorta diameter measurements. MRA or CTA studies are recommended at baseline if TTE images of the ascending aorta are inadequate (12). Patients with aortic diameters greater than 40 mm are recommended to begin serial follow up scans with TTE, MRA or CTA. With new advances in MR technology and its increased accessibility associated with faster acquisition times, MRA is becoming the preferred modality for follow up, as it does not suffer from the limited field of view of TTE and it does not utilize ionizing radiation as with CTA (12, 18, 19).
Despite cardiac magnetic resonance being an accepted modality for monitoring aortic growth in patients with BAV, there is no standardized methodology for diameter measurements at the Sinus of Valsalva or mid-ascending aorta (20). Diameter measurements can be affected by plane angulation, inclusion or exclusion of wall thickness, whether measurements are taken at the cusp or commissure, and the phase of the cardiac cycle (21). These sources of variability may affect reproducibility among users and limit the reliability of investigating thoracic aneurysms with focal or minimal growth (20). Furthermore, Merrit et al showed aortic dilatation morphology in BAV patients widely differed, suggesting that a single diameter measurement may not accurately portray growth along the entire aorta (22).
3D reconstruction volume measurements have been documented as a reliable method for tracking abdominal aorta aneurysmal (AAA) growth and tumor necrosis quantification (15, 23). Multiple studies have demonstrated volume measurements are more sensitive to abdominal aortic aneurysm growth change since maximal diameters do not always change with aortic dilatation (24–26). Kontopodis et al showed 3D reconstructions from CT scans are more predictive than 2D measurements for determining need for surgical intervention in patients with AAA (23). Uribe et al demonstrated 3D cardiac ventricular volume calculations at MR had less intra- and inter-observer variability than traditional 2D measurements (27). From these studies, we extrapolated that 3D volume calculations of TAA on CEMRA should similarly produce reliable and more robust analysis than diameter measurements.
The reported average growth rates for ascending aortas in patients with BAV range widely from 0.2 mm/y to 2 mm/y (28) with rates increasing exponentially over time; larger aortic diameters (>50 mm) are associated with faster diameter growth (>3.6mm/y) (29). Reassuringly, the diameter growth rates measured in our study corresponded well to the measurements reported in these prior studies. To our knowledge, there have been no reports of volume growth rates at the ascending aorta for patients with BAV, thus all growth rates in this study were converted to percentage of change compared to baseline scans, allowing us to compare growth rates between techniques despite different units (mm vs mL).
We hypothesized that volume is more sensitive than diameter to detect growth due to the exponential relationship between diameter and volume. For example, if we assume the ascending aorta is a cylinder of diameter d and height h, as the diameter increases by a factor of c the volume increases by a factor of c2 (Appendix 1). So given an average aortic diameter of 48 mm and a slow growing TAA of 0.5 mm, the volume would theoretically increase by 2.1% while diameter increases by 1.0%. With a more concerning growth of 7 mm, the volume would theoretically increase by 31% while the diameter increases by 15%. However, a cylindrical approximation assumes the best-case scenario, as the simple exercise used here assumes that diameter growth occurs uniformly along the entire aorta. In actual patients, there will more likely be non-uniform growth along the aorta that may only be captured with volumetry, rather than conventional two-dimensional measurements at specific locations in the aorta. From these calculations, we expected the growth rate percentages from volume measurements to be greater than twice the diameter measurements. Notably, if an exponential relationship is the sole reason for the greater growth rates found with the volume measurement, then it is expected that the standard deviation as a percent of the volume growth would also be equal to- or greater than those found with the diameter measurements. This was not the case. For example, the SOV diameter measurement had a SD 283% of the yearly growth (Table 2, ~0.6/0.2), MAA had a SD 166% of the yearly growth, DMG had a SD 107% of the yearly growth, and Volume had a SD 58% of the yearly growth. We hypothesize that the lower standard deviation (as a percent of the volume measurement) when compare to the diameter measurements are due to the comprehensive nature of the measurement, that is, focal regions of growth were not potentially ‘missed’ as is the risk with a single diameter measurement.
Despite volumetry being a novel measurement technique that requires knowledge of specialized software, the high ICC and low COV suggest volumetry is as reliable among different users as 2D measurements in the ascending aorta. Of note, the limits of agreement were highest for the interoberserver measurements of volume. Quantitatively, the degree of variability for the interobserver analysis works out to be 4.6% of the mean value between observers (i.e. abs[(obs1-obs2)/observer mean]*100). This value was deemed acceptable in relation to the other measurements of variability: 2.5% for intraobserver volume, 2.7% for intraobserver diameter, and 3.5% for interobserver diameter measurements. We anticipate with increased software familiarity and further development of a standardized protocol, volumetry reliability will continue to improve. Additionally, the comprehensive nature of volumetry measurements offers less risk for a region of focal dilation going undetected. While the growth reported here was small regarding the absolute change, the results here suggest that volumetry will more sensitive to focal changes within the ascending aorta and less affected by intrinsic measurement variability than 2D diameter measurements.
Although the sample size is limited and measurements are from a single center, this study has very encouraging results. Volumetry provides morphologic assessment of the ascending aorta, is more sensitive to ascending aorta size changes, and demonstrates good reproducibility among different observers. Current guidelines for timing of surgical intervention are solely based on criteria from 2D measurements – at the location of the maximum diameter. We replicated the maximal diameter measurements longitudinally to compute diameter growth rates. Notably, we did not compute averages or minimums of cross-sectional diameter measurements, as this is not recommended in the guidelines. With further research, volumetry may supplement or even replace 2D measurements as a clinical tool for monitoring TAA in patients with BAV. The increased sensitivity seen with volumetry has potential to improve risk stratification and may serve as a reliable guide for timing of surgical intervention in patients with BAV.
This study had several limitations. It is a single center retrospective review of clinical scans and the non-randomization of patients may have introduced bias unidentified by clinical characteristics. Although all exams were diagnostic in our sample cohort (because of sufficient ECG gating and the elliptic-centric k-space reordering approach), errors can occur due to cardiac and respiratory motion. Due to the lack of a gold standard for BAV follow-up, the diameter and volume measurement accuracies could not be validated (e.g. with CTA). 3D segmentation was time-consuming using this research software (approximately 15 minutes per subject) which requires both a trained user and a consensus on definitions for the distal and proximal boundaries of the ascending aorta. Furthermore, volumetry can be performed with modalities other than CEMRA, but the influence of imaging technique on volume measurements is unknown. Lastly, no follow up data regarding clinical outcomes such as frequency of surgeries or complications were tracked. Future studies should aim to collect follow-up data regarding clinical outcomes to correlate volume changes with risk of dissection or rupture and potentially develop volumetry as a prognostic as well as diagnostic tool.
In conclusion, 3D CEMRA volumetric analysis exhibited a larger percentage growth, better ICC, and good inter and intra-observer COV when measuring ascending thoracic aorta size changes. Volumetric analysis may provide a reliable method to more comprehensively detect ascending aortic growth and guide appropriate therapy for better BAV outcomes.
Supplementary Material
Acknowledgments
Source of Funding: NIH K25HL119608
Footnotes
Disclosures: None
Conflicts of Interest: None declared
References
- 1.Geisbusch S, Stefanovic A, Schray D, et al. A prospective study of growth and rupture risk of small-to-moderate size ascending aortic aneurysms. J Thorac Cardiovasc Surg. 2014;147(1):68–74. doi: 10.1016/j.jtcvs.2013.06.030. [DOI] [PubMed] [Google Scholar]
- 2.Bickerstaff LK, Pairolero PC, Hollier LH, et al. Thoracic aortic aneurysms: a population-based study. Atherosclerosis. 1982;15:29. [PubMed] [Google Scholar]
- 3.Clouse WD, Hallett JW, Schaff HV, et al. Mayo Clinic Proceedings. Elsevier; 2004. Acute aortic dissection: population-based incidence compared with degenerative aortic aneurysm rupture; pp. 176–180. [DOI] [PubMed] [Google Scholar]
- 4.Hiratzka LF, Bakris GL, Beckman JA, et al. 2010 ACCF/AHA/AATS/ACR/ASA/SCA/SCAI/SIR/STS/SVM Guidelines for the Diagnosis and Management of Patients With Thoracic Aortic Disease: Executive Summary. Journal of the American College of Cardiology. 2010;55(14):1509–1544. doi: 10.1016/j.jacc.2010.02.015. [DOI] [PubMed] [Google Scholar]
- 5.Zhang L, Wang HH. The Genetics and Pathogenesis of Thoracic Aortic Aneurysm Disorder and Dissections. Clin Genet. 2015 doi: 10.1111/cge.12713. [DOI] [PubMed] [Google Scholar]
- 6.Goldstein SA, Evangelista A, Abbara S, et al. Multimodality imaging of diseases of the thoracic aorta in adults: from the American Society of Echocardiography and the European Association of Cardiovascular Imaging: endorsed by the Society of Cardiovascular Computed Tomography and Society for Cardiovascular Magnetic Resonance. J Am Soc Echocardiogr. 2015;28(2):119–182. doi: 10.1016/j.echo.2014.11.015. [DOI] [PubMed] [Google Scholar]
- 7.Michelena HI, Della Corte A, Prakash SK, et al. Bicuspid aortic valve aortopathy in adults: Incidence, etiology, and clinical significance. Int J Cardiol. 2015;201:400–407. doi: 10.1016/j.ijcard.2015.08.106. [DOI] [PubMed] [Google Scholar]
- 8.Michelena HI, Prakash SK, Della Corte A, et al. Bicuspid aortic valve: identifying knowledge gaps and rising to the challenge from the International Bicuspid Aortic Valve Consortium (BAVCon) Circulation. 2014;129(25):2691–2704. doi: 10.1161/CIRCULATIONAHA.113.007851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Evangelista A. Imaging aortic aneurysmal disease. Heart. 2014;100(12):909–915. doi: 10.1136/heartjnl-2013-305048. [DOI] [PubMed] [Google Scholar]
- 10.Svensson LG, Adams DH, Bonow RO, et al. Aortic valve and ascending aorta guidelines for management and quality measures. Ann Thorac Surg. 2013;95(6 Suppl):S1–S66. doi: 10.1016/j.athoracsur.2013.01.083. [DOI] [PubMed] [Google Scholar]
- 11.Oladokun D, Patterson BO, Sobocinski J, et al. Systematic Review of the Growth Rates and Influencing Factors in Thoracic Aortic Aneurysms. Eur J Vasc Endovasc Surg. 2016 doi: 10.1016/j.ejvs.2016.01.017. [DOI] [PubMed] [Google Scholar]
- 12.Nishimura RA, Otto CM, Bonow RO, et al. 2014 AHA/ACC Guideline for the Management of Patients With Valvular Heart DiseaseA Report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Journal of the American College of Cardiology. 2014;63(22):e57–e185. doi: 10.1016/j.jacc.2014.02.536. [DOI] [PubMed] [Google Scholar]
- 13.Detaint D, Michelena HI, Nkomo VT, et al. Aortic dilatation patterns and rates in adults with bicuspid aortic valves: a comparative study with Marfan syndrome and degenerative aortopathy. Heart. 2014;100(2):126–134. doi: 10.1136/heartjnl-2013-304920. [DOI] [PubMed] [Google Scholar]
- 14.Renapurkar RD, Setser RM, O'Donnell TP, et al. Aortic volume as an indicator of disease progression in patients with untreated infrarenal abdominal aneurysm. Eur J Radiol. 2012;81(2):e87–e93. doi: 10.1016/j.ejrad.2011.01.077. [DOI] [PubMed] [Google Scholar]
- 15.Galizia MS, Tore HG, Chalian H, et al. MDCT necrosis quantification in the assessment of hepatocellular carcinoma response to yttrium 90 radioembolization therapy: comparison of two-dimensional and volumetric techniques. Acad Radiol. 2012;19(1):48–54. doi: 10.1016/j.acra.2011.09.005. [DOI] [PubMed] [Google Scholar]
- 16.Scheske JA, Chung JH, Abbara S, Ghoshhajra BB. Computed Tomography Angiography of the Thoracic Aorta. Radiol Clin North Am. 2016;54(1):13–33. doi: 10.1016/j.rcl.2015.08.004. [DOI] [PubMed] [Google Scholar]
- 17.Wojnarski CM, Svensson LG, Roselli EE, et al. Aortic Dissection in Patients With Bicuspid Aortic Valve–Associated Aneurysms. The Annals of Thoracic Surgery. 2015;100(5):1666–1674. doi: 10.1016/j.athoracsur.2015.04.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dudzinski DM, Isselbacher EM. Diagnosis and Management of Thoracic Aortic Disease. Curr Cardiol Rep. 2015;17(12):106. doi: 10.1007/s11886-015-0655-z. [DOI] [PubMed] [Google Scholar]
- 19.Mongeon F-P, Marcotte F, Terrone DG. Multimodality Noninvasive Imaging of Thoracic Aortic Aneurysms: Time to Standardize? Canadian Journal of Cardiology. 2016;32(1):48–59. doi: 10.1016/j.cjca.2015.09.025. [DOI] [PubMed] [Google Scholar]
- 20.Asch FM, Yuriditsky E, Prakash SK, et al. The Need for Standardized Methods for Measuring the Aorta: Multimodality Core Lab Experience From the GenTAC Registry. JACC Cardiovasc Imaging. 2016;9(3):219–226. doi: 10.1016/j.jcmg.2015.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Burman ED, Keegan J, Kilner PJ. Aortic root measurement by cardiovascular magnetic resonance: specification of planes and lines of measurement and corresponding normal values. Circ Cardiovasc Imaging. 2008;1(2):104–113. doi: 10.1161/CIRCIMAGING.108.768911. [DOI] [PubMed] [Google Scholar]
- 22.Merritt B, Turin A, Markl M, Carr J. Association between leaflet fusion pattern and thoracic aorta morphology in patients with bicuspid aortic valve. Journal of Cardiovascular Magnetic Resonance. 2012;14(Suppl 1):M4-M. doi: 10.1002/jmri.24376. [DOI] [PubMed] [Google Scholar]
- 23.Kontopodis N, Lioudaki S, Pantidis D, et al. Advances in determining abdominal aortic aneurysm size and growth. World Journal of Radiology. 2016;8(2):148–158. doi: 10.4329/wjr.v8.i2.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.van Keulen JW, van Prehn J, Prokop M, et al. Potential value of aneurysm sac volume measurements in addition to diameter measurements after endovascular aneurysm repair. J Endovasc Ther. 2009;16(4):506–513. doi: 10.1583/09-2690.1. [DOI] [PubMed] [Google Scholar]
- 25.Kritpracha B, Beebe HG, Comerota AJ. Aortic diameter is an insensitive measurement of early aneurysm expansion after endografting. J Endovasc Ther. 2004;11(2):184–190. doi: 10.1583/03-976.1. [DOI] [PubMed] [Google Scholar]
- 26.Kauffmann C, Tang A, Therasse E, et al. Measurements and detection of abdominal aortic aneurysm growth: Accuracy and reproducibility of a segmentation software. Eur J Radiol. 2012;81(8):1688–1694. doi: 10.1016/j.ejrad.2011.04.044. [DOI] [PubMed] [Google Scholar]
- 27.Uribe S, Tangchaoren T, Parish V, et al. Volumetric Cardiac Quantification by Using 3D Dual-Phase Whole-Heart MR Imaging. Radiology. 2008;248(2):606–614. doi: 10.1148/radiol.2482071568. [DOI] [PubMed] [Google Scholar]
- 28.Tadros TM, Klein MD, Shapira OM. Ascending aortic dilatation associated with bicuspid aortic valve: pathophysiology, molecular biology, and clinical implications. Circulation. 2009;119(6):880–890. doi: 10.1161/CIRCULATIONAHA.108.795401. [DOI] [PubMed] [Google Scholar]
- 29.Shimada I, Rooney SJ, Pagano D, et al. Prediction of thoracic aortic aneurysm expansion: validation of formulae describing growth. Ann Thorac Surg. 1999;67(6):1968–1970. doi: 10.1016/s0003-4975(99)00435-x. discussion 79–80. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.